Effect of Humidity on the Inactivation of Bacillus Subtilis Spore by Non-thermal Atmospheric Air Plasma
Haiyu Li1, Zhenyu Zhou1, Dongping Liu1, Yunqiu Cui1, Yuan Sun1, Jiaxin Li1, Yiming Wang1, Zhishang Wang1, Zhiguo Zhao1, Na Lu1*
1School of Electrical Engineering, Dalian University of Technology, Dalian, Liaoning Province, China
*Correspondence to: Na Lu, PhD, Professor, School of Electrical Engineering, Dalian University of Technology, No. 2 Linggong Road, Dalian 116024, Liaoning Province, China; E-mail: luna@dlut.edu.cn
Abstract
Objective: If a medical device is not properly disinfected, it will pose a potential danger to patients. Spore is an experimental strain to verify the effect of disinfection on medical devices. It has a complex structure and is somewhat resistant to conventional disinfection techniques. Based on the advantages of simple structure and abundant reactive oxygen and nitrogen species (RONS), surface dielectric barrier discharge (SDBD) has been used to deal with inactivate spores, which provided experimental support for the application of sterilization spores in medical devices.
Methods: The sterilization efficiency of the plasma was evaluated by calculating the number of viable colonies on Tryptose Soya Agar. RONS including ozone, nitrogen oxides and other reactive species produced in SDBD were measured by ozone analyzer, Fourier infrared spectrometer and chemical probes, respectively. Ultraviolet-visible spectrophotometer was used to determine the concentration of protein and nucleic acid.
Results: After 20min of SDBD treatment, the number of inactivated spores could reach more than 4 log at high humidity (>6g/m3), while the spores were hardly inactivated at low humidity (<6g/m3). Ozone production was inhibited with increasing humidity while OH radicals and peroxynitrous acid increased with increasing humidity. The optical density values of protein and nucleic acid released from spores treated with SDBD was detected and a strong correlation was found with the spore inactivation trend. The protein shell and membrane structure of the spores were destroyed by SDBD, and more leakage and destruction of proteins and nucleic acids in the spores were obtained under the high humidity.
Conclusion: With the increase of air humidity (from 0.15g/m3 to 70.16g/m3), stable SDBD is obtained showing a typical filamentous discharge pattern. Compared with low humidity, high humidity is more favorable to SDBD inactivation of spores.
Keywords: surface dielectric barrier discharge, disinfection, humidity, reactive oxygen and nitrogen species, spores
1 INTRODUCTION
Nowadays, advances in health care have made it necessary for hospitals to repeatedly disinfect reusable medical instruments such as scalpels, hemostatic forceps and endoscopes. These devices, which come into direct contact with patient tissue, may retain microbes that can cause inflammation and even disease if they are not thoroughly disinfected. Among these microorganisms, the spores of some pathogenic bacteria are the most difficult to eliminate. The existing medical instruments are mainly sterilized by heat, γ-radiation and ethylene oxide method. However, parts of some medical instruments are not resistant to high temperature and the joints of different parts are not easily processed by γ-radiation. The ethylene oxide method usually suffers from leakage risk[1]. Therefore, it is of great significance to develop mild and green disinfection techniques for medical devices.
Non-thermal plasma operating at atmospheric pressure and room temperature has been extensively studied as a safe and effective method of bactericide[2-4]. Dielectric barrier discharge (DBD) has become the main point of research due to its simple device structure, high concentration of the active substance and good disinfection efficiency[5-8]. Moldgy et al.[9,10] found that DBD is more effective at disinfecting wet samples than dry samples and hypothesized that the involvement of water would improve the plasma’s ability to disinfect. Kogelheide et al.[11] found that spore inactivation by DBD was strongly correlated with the humidity content in the feed gas, which implied that reactive species formed either directly or indirectly from water molecules were strong mediators of spore inactivation. Patil et al.[12] found that gas type and relative humidity levels were significant factors in the inactivation of spores by DBD. Studies have shown that humidity is an important parameter in plasma disinfection, which results in a different plasma chemistry compared to air dry discharge plasma and enhances the sporicidal ability of DBD.
Surface dielectric barrier discharge (SDBD), as a type of DBD, has the advantage of generating uniform discharge easily in ambient air. Alternating current (AC) can produce large-area homogeneous SDBD plasma starting from voltage amplitudes of only a few kV. Furthermore, the specific surface area of the plasma region of SDBD is usually larger than that of DBD[13-15]. Wang et al.[16] found that water droplets-tuned SDBD could significantly contribute to the generation of reactive species associated with water, thus leading to the rapid inactivation of E. coli. However, until now, there has been a lack of systematic studies on how the role of humidity in SDBD leads to spore inactivation.
In this study, the effect of humidity on SDBD inactivated Bacillus subtilis spores is studied. The humidity of the plasma working gas is determined by changing the volume ratio of moist to dry air at the intake. To assess the effect of humidity on the disinfection capacity in SDBD, the spores of Bacillus subtilis are used as a bioindicator and a genetic model system for treatment. Ozone analyzer, Fourier infrared spectrometer and chemical probes are used to measure the effect of air humidity on the active components produced by SDBD. The mechanism of inactivation of Bacillus subtilis spore by SDBD is analyzed by detecting the optical density (OD) values of protein and nucleic acid in solutions of Bacillus subtilis spore treated with SDBD using an ultra-visible spectrophotometer.
2 MATERIALS AND METHODS
2.1 Experimental Setup
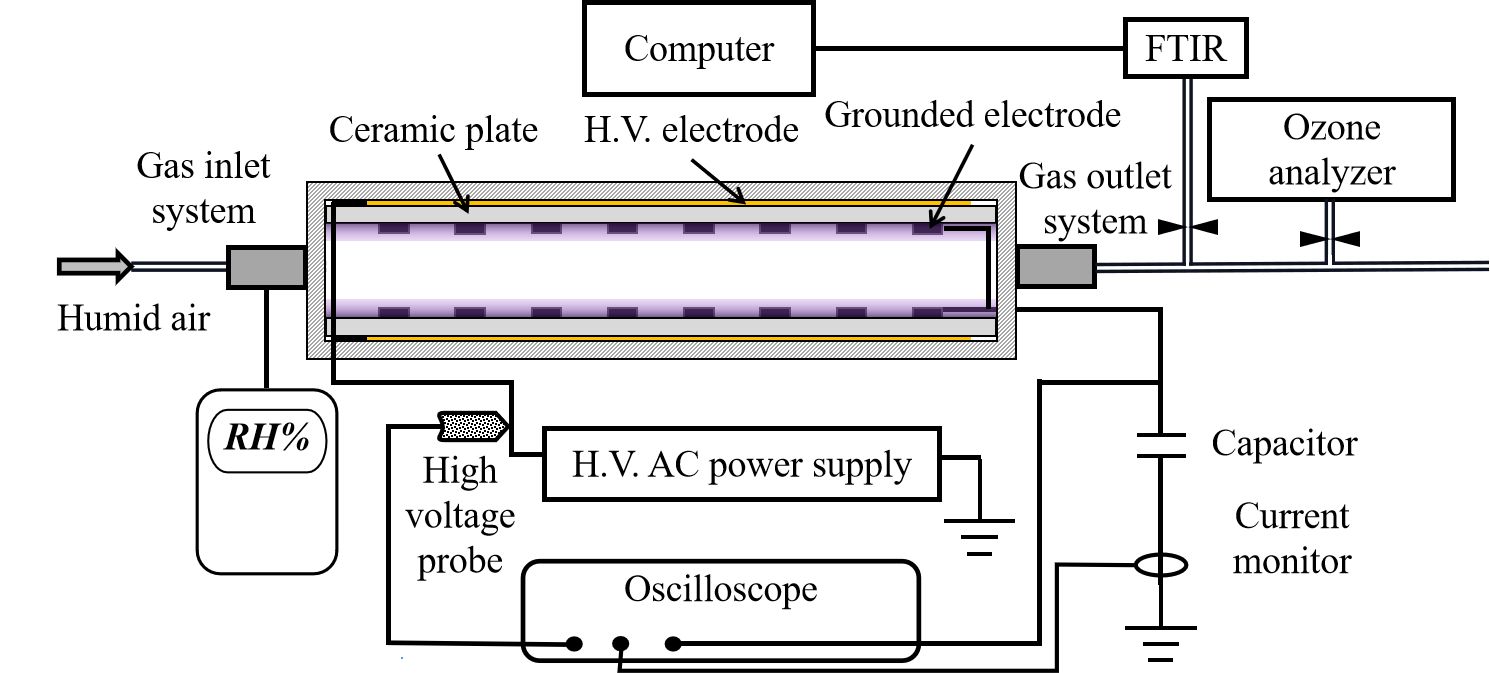
Figure 1 shows the experimental setup consisting of an SDBD reactor, an adjustable sinusoidal high voltage (HV) AC power supply, air humidification system and diagnostic system. The SDBD reactor contains two identical discharging cells, which are connected in parallel to the circuit. For each discharging cell, a thin layer of copper foil (130mm long and 130mm wide) is used as HV electrode, and the grounded electrodes are 18 metal strips (each 120mm long and 0.5mm high), which are separated by a ceramic plate with 155mm long, 155mm wide and 2.5mm thick. In order to prevent redundant discharges, the edges of the HV electrodes are covered with insulation.
|
Figure 1. Schematic diagram of the experimental setup.
The two discharging cells are placed on the upper and lower surfaces of the inner chamber, respectively. The dimensions of the chamber are 160mm long, 160mm wide and 70mm high. An air inlet and an outlet are connected on both sides of the chamber, respectively. In this study, different humidity, namely 0.15, 2.30, 4.61, 8.44, 11.51 and 70.16g/m3, are chosen as process gasses. The humidity above 6g/m3 is defined as high humidity and the humidity below 6g/m3 is defined as low humidity. Compressed air is used as the process gas. Washer cylinder and nebulizer are used to provide humidification below and over 15g/m3, respectively. The compressed air is delivered via two gas paths. One of the gas routes is directly connected to the mixing cylinder. The other gas path is humidified by the washing cylinder or sprayer and hooked up to a mixing cylinder. The humidity of the gas in the mixing cylinder can be adjusted by the volume ratio between dry and moist air. Then, the gas is conveyed to the plasma region through the gas flow tube. At the air inlet, a humidity tester (DT-625) is used to monitor the humidity of the gas entering the sealed space.
A sinusoidal AC power supply (Yija PVM500, China) is associated with the HV electrodes. The applied voltage and current are measured by using a 1:1000 HV probe (Tektronix P6015A) and a current monitor (Pearson 4100) respectively, and the measured signal is displayed on a digital oscilloscope (Tektronix MDO3104). Unless stated otherwise, all experiments in this research are carried out with an AC repetition frequency of 10kHz and a peak-to-peak voltage of 14kV. The discharge power is calculated from the voltage across the integrating capacitor (2.2μF) and the current flowing through the circuit. An infrared thermometer (DS-2TPH10-3AUF) is used to measure the temperature in a sealed space.
2.2 Sample Preparation of Bacterial Cells and Treatment
Bacillus subtilis spores (ATCC 6633) were from Beijing Microbial Culture and Collection Center. Bacteria was extracted from a single colony and cultured in Tryptone Soya Broth liquid medium with shaking for 4 to 5h at 37°C, which were then cultured on the Tryptose Soya Agar (TSA) medium in a Roche bottle at 37°C until abundant spores are observed under the microscope (at least 7 days). A total of 15mL sterile water was added to the Roche bottle and the bacterial colonies are gently scraped off using an L-shaped stick. The spores were placed in a water bath at 45°C for 24h so that the chain dissolved and dispersed into a single spore. Then the spore solution was centrifuged for 30min (3000r/min), the supernatant was discarded, and distilled water was added for centrifugal cleaning again, which was repeated three times. The washed spore solution was placed in a water bath at 80°C for 10min to kill the remaining bacterial propagules. After cooling to room temperature, it was stored in a refrigerator at 4°C. The spores were diluted to 5.5×106 CFU/mL with sterile water. And quantifiable colonies were obtained on the control plate. A total of 10μL diluted spore solution was evenly spread on the surface of a steel plate with a diameter of 12mm and a thickness of 1.2mm. Then it was placed in a sterilized incubator at 37°C for 10min until the surface of the steel sheet was dry. Finally, the steel sheet was placed on the Teflon feed spline. The sampler was inserted into the chamber, ensuring that the steel sheet is face-up and located near the central point of the chamber.
After plasma treatment, the steel sheets were placed into a centrifugal tube containing 2mL sterile water and subjected to ultrasonic treatment for 5min. The steel sheet stained with spore was untreated as the control. After ultrasonic treatment, 300μL bacterial suspension was dispersed on the TSA plate and incubated overnight at 37°C. The sterilization efficiency of the plasma was evaluated by calculating the number of viable colonies on the TSA.
2.3 Measurement of Reactive Oxygen and Nitrogen Species (RONS) Concentrations
O3 is measured by a portable ozone analyzer (Thermo Scientific 6700). The gas in the chamber is firstly mixed with air to adjust the concentration of O3 within the measuring range and then pumped into the ozone analyzer. Finally, depending on the dilution ratio, the obtained concentration is converted to the actual concentration.
Nitrogen oxides are measured by Fourier infrared spectrometer (FTIR, Nicolet 6700). During the measurement, the gas in the chamber is mixed with the carrier gas (nitrogen) at a volume ratio of 1:6. The mixed gas is then transported into the sample chamber via an air pump. The constant flow rate of the air pump is 0.5L/min. The FTIR spectra are recorded in the wavenumber range from 500 to 2500cm-1. The following vibration bands were used for the diagnosis of active species: N2O at 2235cm-1; NO at 1900cm-1; NO2 at 1630 and 2916cm-1; N2O5 at 1720cm-1; O3 at 1055cm-1[17].
In this study, oxidative capacity of RONS produced by SDBD in air are determined by two chemical probes [terephthalic acid (TA) and 2’,7’-dichlorodihydrofuorescein diacetate (H2DCFDA)][18-20]. The strength of the RONS oxidation reflects the concentration of strongly oxidizing species, such as OH radicals (‧OH) and Peroxynitrous acid (ONOOH), generated by the SDBD TA can be oxidized into 2-hydroxyterephthalic acid (HTA) by the RONS in NaOH solution. After the deacetylation of H2DCFDA in the NaOH solution, the non-fluorescent form DCFH can be oxidized into the highly fluorescent form 2’,7’-dichlorofluorescein (DCF) by the RONS from the gas phase. The initial concentrations of TA and NaOH are 4 and 10nmol/L, respectively. A total of 3mL TA solution is spread onto the petri dish (64cm2) to capture the RONS from the gas phase. The HTA can be measured by the fluorescence intensity detected by a fluorescence spectrometer (Cary Eclipse 2018A43C)[18]. When the HTA solution is irradiated by UV light (=310nm), HTA molecules emit light at =425nm.
The process of deacetylation is provided by hydrolysis with NaOH by mixing 0.5mL of stock H2DCFDA solution (1mM prepared in ethanol, Shyuanye Company, Shanghai, China) with 2mL of NaOH solution (0.1mol/L). The reaction is stopped after 30min incubation at room temperature by adding 7.5mL of phosphate buffer (0.1mol/L)[19]. By this procedure, 3mL of working solutions of DCFH (50µmol/L) is obtained and spread onto the petri dish (64cm2) to capture the RONS from the gas phase. DCF measurement is also performed with a fluorescence spectrometer. The working solution without plasma treatment is used as a control.
2.4 Measurement of Protein and Nucleic Acid Concentrations
The proteins and nucleic acids in the spore fluid are derived from spore rupture and leakage after SDBD treatment. Ultraviolet-visible spectrophotometer (UVS) is used to determine the concentration of protein and nucleic acid. The treated steel sheets are placed in a centrifuge tube containing 2mL sterile water for 5min of ultrasonic processing to obtain the treated spore solution. The OD of the supernatant is determined by the UVS method. The detection wavelength of protein is 280nm, and the detection wavelength of nucleic acid is 260nm. The OD value is directly proportional to the concentration of the detected element. Therefore, it can reflect the detected concentration indirectly[21-23].
2.5 Statistical Analysis
All the experiments are repeated three times. All percentage and relative values of the data set are represented as the mean±SD deviation of the indicated number of replicates (n≥3). Statistical analyses of the data are performed with the student’s t-test to establish the significance between data points. Significant differences are based on P<0.05.
3 RESULTS AND DISCUSSION
3.1 Measurements of SDBD Power
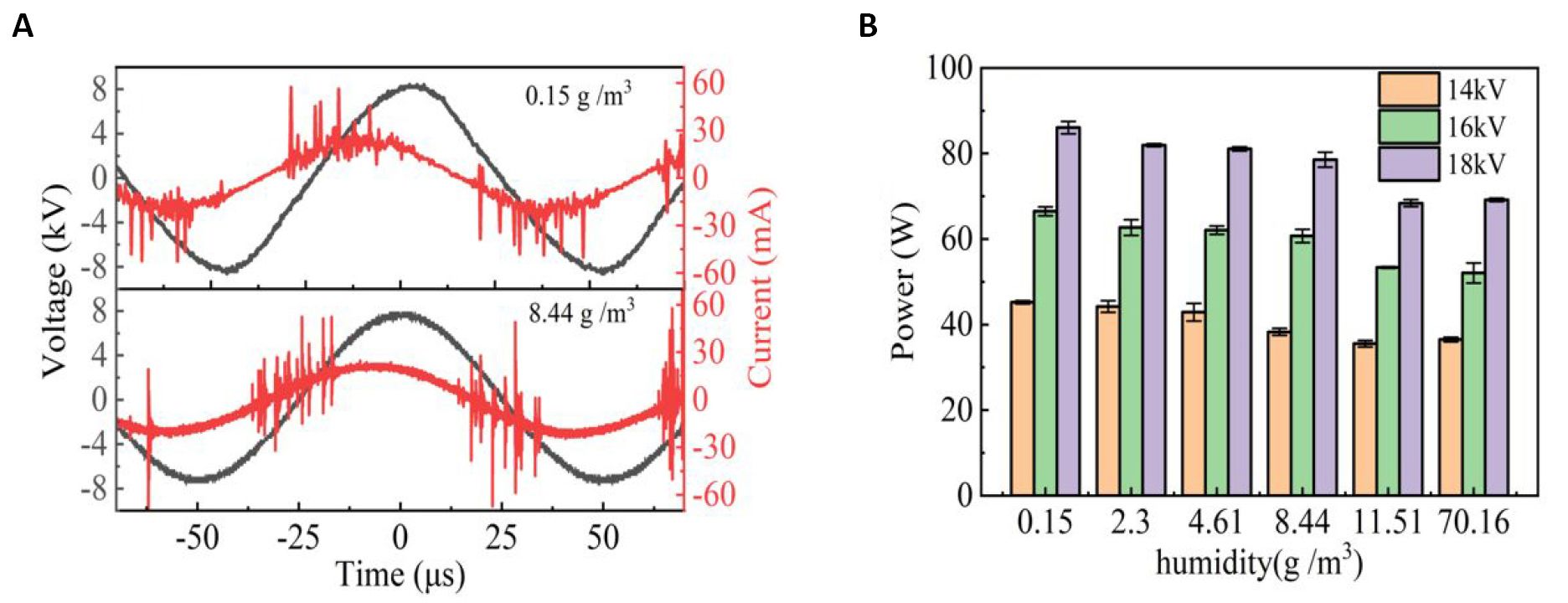
Figure 2A shows the waveforms of applied voltage and discharge current, discharge power and internal chamber temperature of the SDBD plasma, respectively. The results show that the SDBD is a typical filamentous discharge state under humidity conditions of 0.15g/m3 and 8.44g/m3. According to Figure 2B, the discharge power of SDBD decreases with the increase of humidity. Because of the electronegativity of water molecules, water molecules tend to absorb electrons, thus reducing the number of high-energy electrons, leading to a decrease in the discharge power. However, more highly oxidizing RONS, such as ‧OH, H2O2, HO and ONOOH, are produced by SDBD attributing to water molecule introduction, which are key to SDBD disinfection.
Figure 2. Effect of humidity on voltage and current waveforms and discharge power for SDBD plasma. A: The applied voltage is 16kV, and the humidity is 0.15g/m3 and 8.44g/m3; B: SDBD plasma discharge power as a function of air humidity at different applied voltages.
3.2 Bacillus Subtilis Spore Inactivation by Surface Microdischarge Plasma
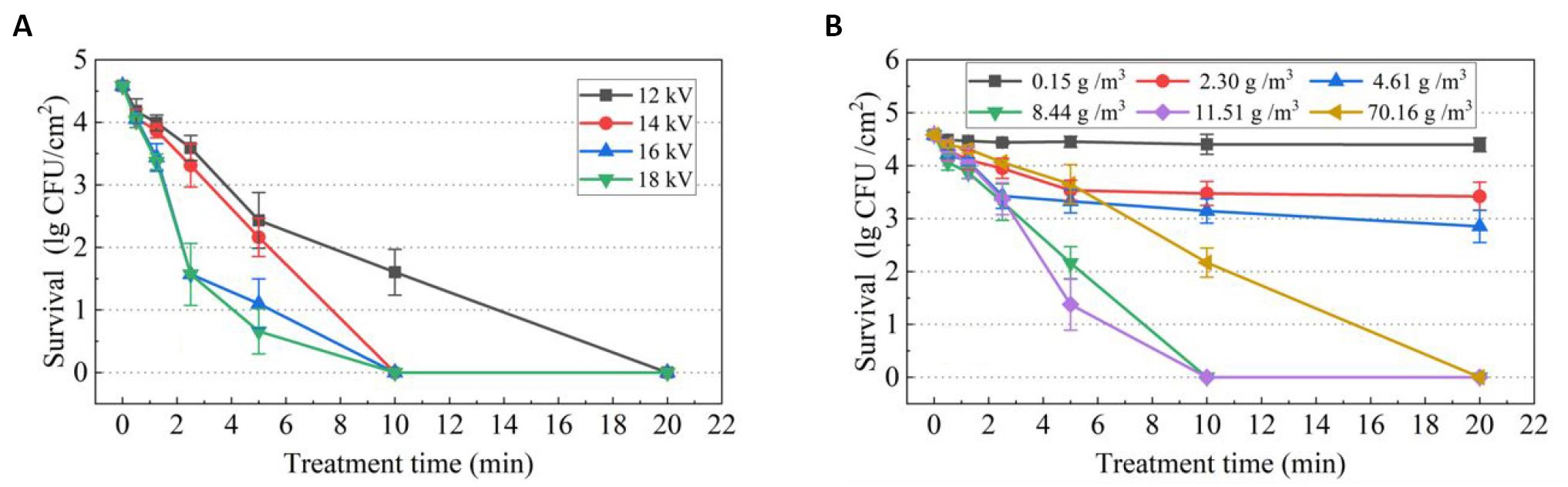
Figure 3 shows the effects of applied voltage and humidity on the survival of Bacillus subtilis spores treated with SDBD plasma. It can be seen from Figure 3A that with the increase of applied voltage, the number of spores surviving decreases. The spores are completely inactivated at 5min under 18kV applied voltage discharge, but the internal temperature of the SDBD reactor is found to exceed 80oC, which may cause thermal damage to some medical devices. Under the applied voltage of 14kV, spores are completely killed at 10min. At this time, the internal temperature of the SDBD reactor is not above 55oC. Influence of humidity on the sporicidal effect under applied voltage of 14kV is investigated, as shown in Figure 3B. As the humidity increases, the efficacy of spore inactivation first increases and then decreases. Spores cannot be completely inactivated when humidity is lower than 8.44g/m3. After 5min of discharge, further prolongation of the treatment time has little effect on spore inactivation. Increasing the humidity to 11.51g/m3, SDBD is able to inactivate 4 log of spores at the treatment time of 10min. When the humidity is further increased to 70.16g/m3, it takes 20min to reach 4 log of spore inactivation. Therefore, humidity of 11.51g/m3 and applied voltage of 14kV are determined as the optimal sterilization condition in this study.
|
Figure 3. The survival of spores as a function of SDBD plasma treatment time. A: Varying applied voltage at a fixed humidity of 8.44g/m3; B: varying humidity at fixed applied voltage of 14kV.
3.3 Analysis of Reactive Species from SDBD
Figure 4 shows the concentration of O3 as a function of discharge time at different humidity. The major pathways for O3 formation by plasma are given by:
|
|
|
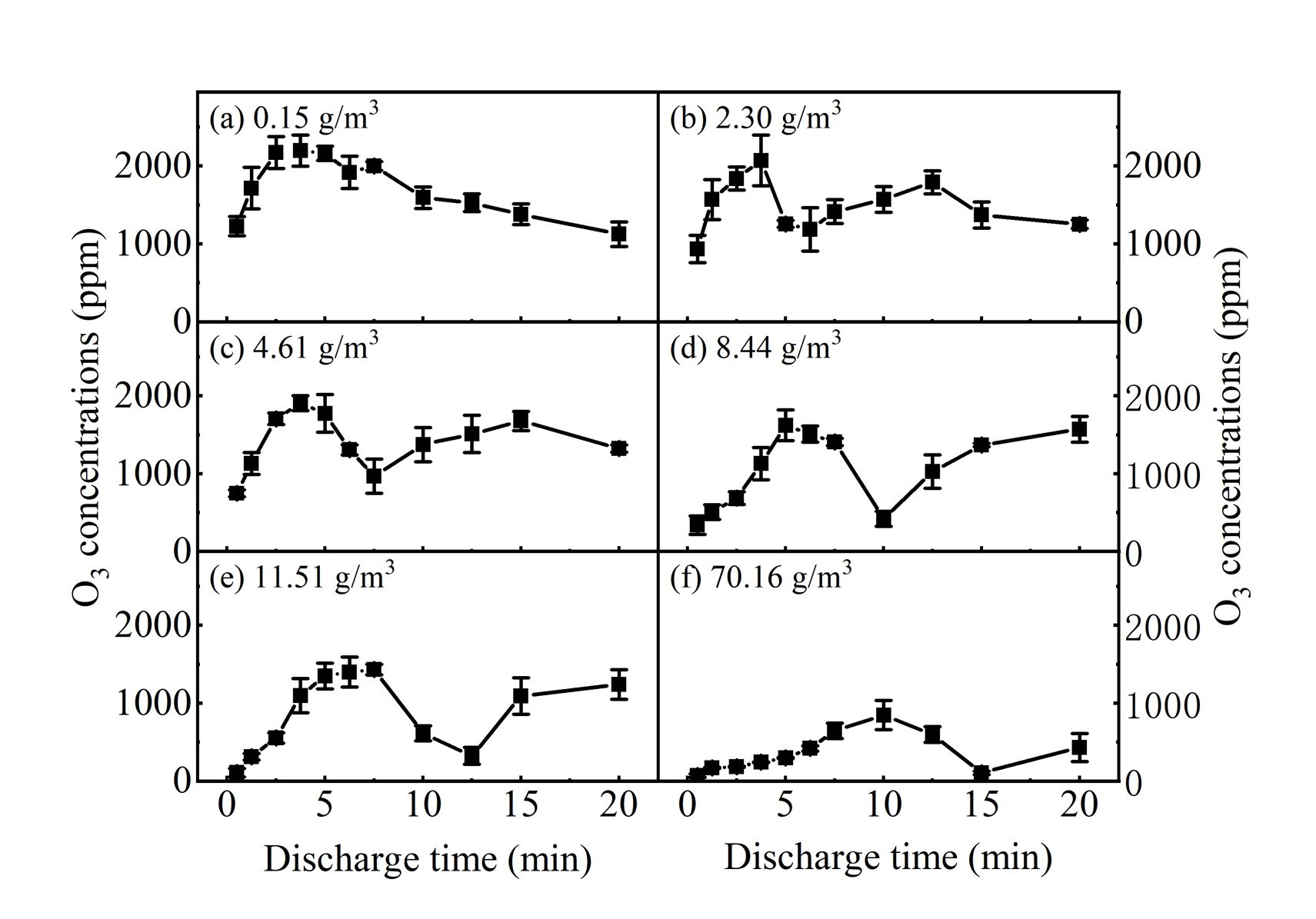
Under the humidity of 0.15g/m3, the O3 concentration first increases and then decreases with increasing discharge time, reaching the maximum concentration of 2154ppm after 2.5min treatment. With the increase of humidity, the maximum O3 concentration gradually decreases and the time when the maximum occurs is gradually delayed, indicating that the increase of humidity will inhibit the generation of O3. Upon increasing humidity, electron density would decrease due to the electronegativity of water, resulting in a drop of the formation of O atoms (Equation (1)), which further reduces the O3 generation[24]. Furthermore, nitrogen and oxygen excited species also play an important role in the formation of O atoms. Higher humidity can accelerate the relaxation rate of the vibration-to-translation of N2 and O2[25], which decreases the concentrations of both nitrogen and oxygen excited species[26], and thus inhibits O3 generation. As shown in Equation (3), the generated ‧OH reacts readily with O3 to form HO2[26,27]. Accordingly, humidity is found to affect the production of O3 in the present study.
|
|
Figure 4. The concentration of O3 as a function of discharge time.
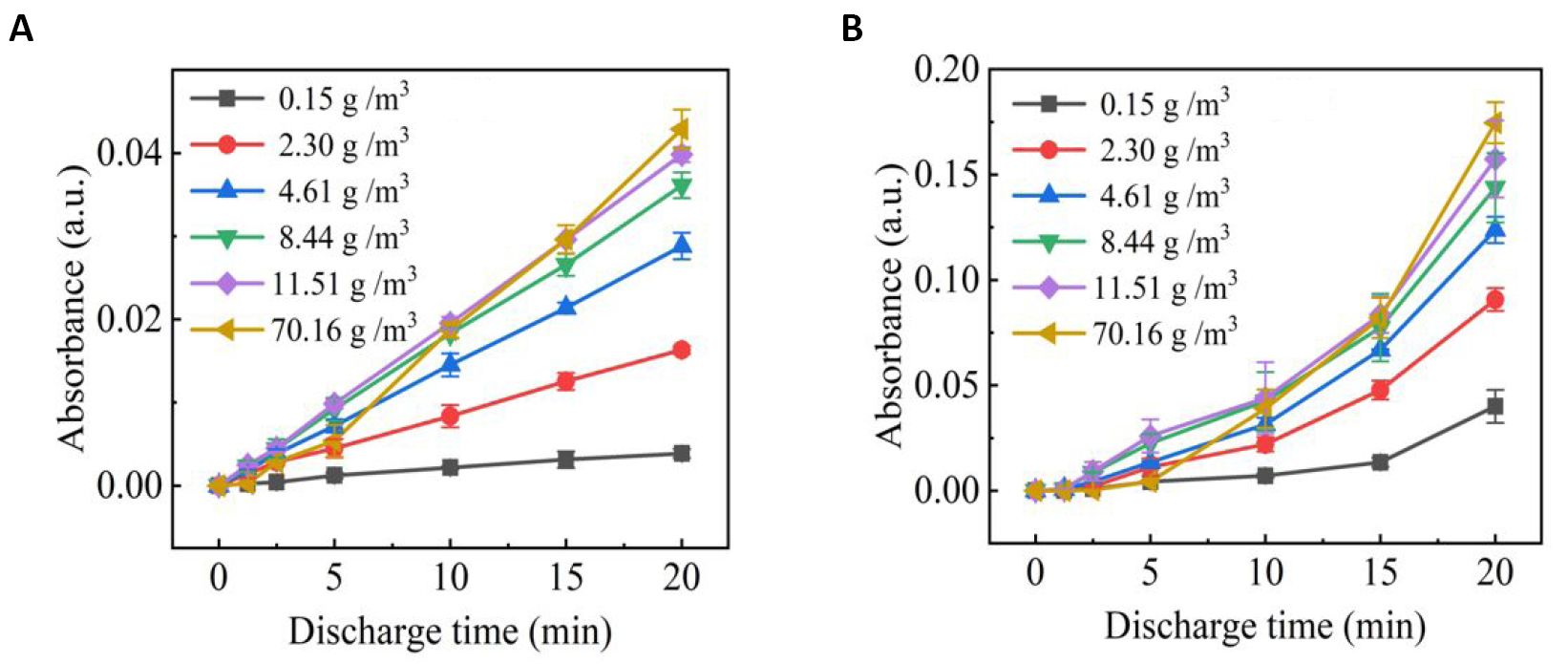
The components of gaseous RONS produced by SDBD for discharge times of 5min and 20min under different humidity contents are detected by FTIR (Figure 5). More nitrogen oxides accumulated in the chamber at 20min of discharge than at 5min of discharge. It is found that nitrogen oxides are the main RONS besides O3. The influences of humidity and discharge time on the absorbance of NO (1900cm-1) and NO2 (1630cm-1) are shown in Figure 6. It can be seen that the concentrations of NO and NO2 increase with the discharge time. In addition, the increase of humidity also promotes the production of NO and NO2 by SDBD, which are produced in large quantities mainly through the oxidation of excited nitrogen by O2 and O3. And the addition of water molecules promotes the formation of water-based compounds, such as ‧OH and HO2, which could also oxidize N atoms to form NO and NO2[28-30].
|
|
|
|
|
|
|
|
|
|
|
It should be noted that the growth rate of NO decreases with the increase of humidity under the condition high humidity, suggesting that the NO and NO2 could be consumed through their reactions with the OH to generate HNO2 and HNO3[24,28]:
|
|
|
|
Figure 5. Gas phase FTIR spectra of SDBD plasma at different humidity with discharge times of (A) 5min and (B) 20min.
|
Figure 6. Absorbance of (A) NO (1900cm-1) and (B) NO2 (1630cm-1) in the FTIR spectrum.
Figure 7 shows the concentrations of HTA and DCF chemical probes as a function of discharge time. It can be found that the concentration of HTA reaches saturation after a certain time accumulation, which is delayed with the increase of the humidity of the working gas. Under the low humidity, the concentration of HTA reaches saturation within 10min. However, at high humidity, it does not reach saturation until 20min after discharge. Figure 7B shows that working gas humidity can significantly increase the fluorescence intensity of DCF. The maximum concentration of DCF is 4.65nmol/L at the humidity of 70.16g/m3 after 20min discharge. Overall, humidity has a significant effect on the generation of RONS. The water molecules absorb energy and form ‧OH[29,30]. ‧OH can react with nitrogen-containing acids to form ONOOH, which occurs primarily in clusters of water in the gas phase, but can also form directly in the gas phase[30,31].
|
|
|
|
|
|
Figure 7. The concentrations of HTA (A) and DCF (B) as a function of discharge time for varying humidity.
3.4 Analysis of Protein and Nucleic Acid Concentrations
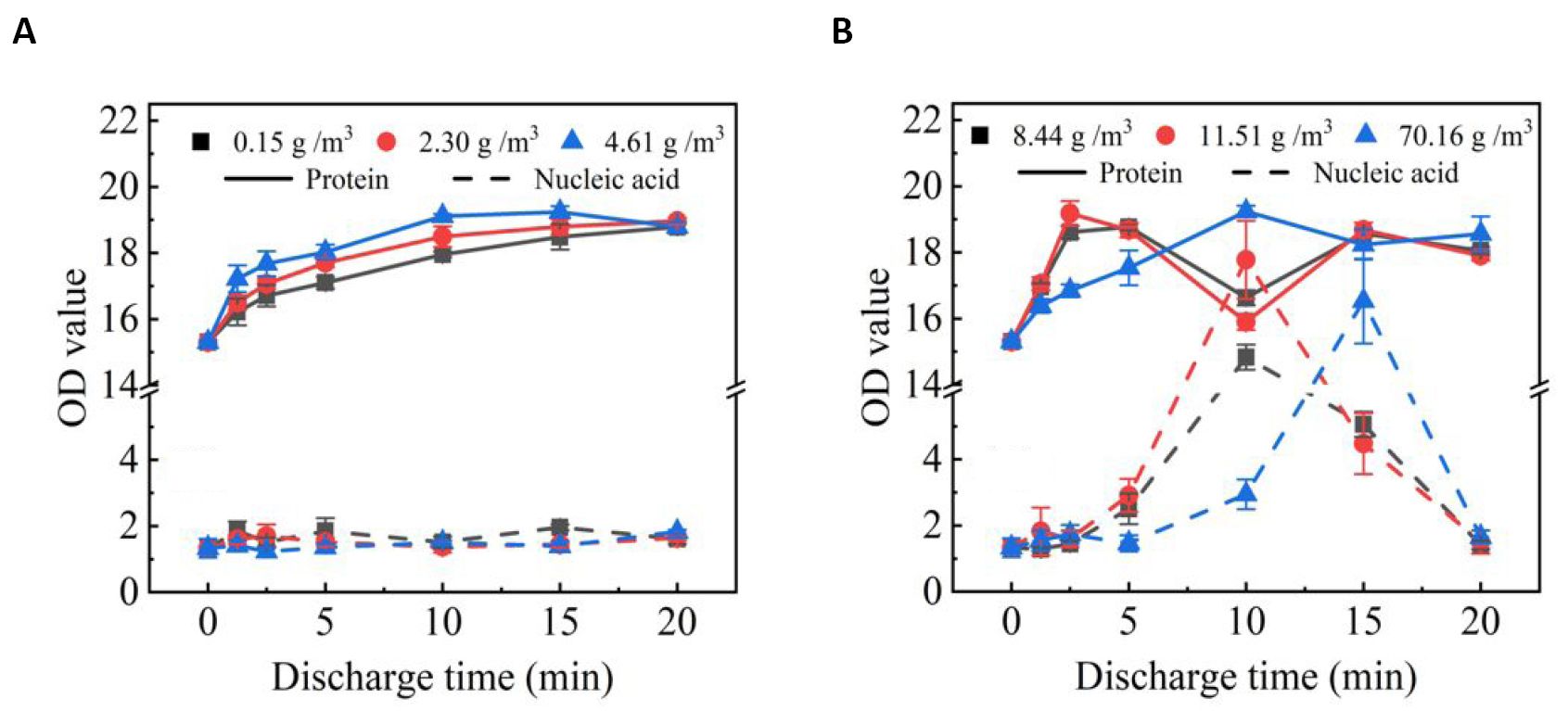
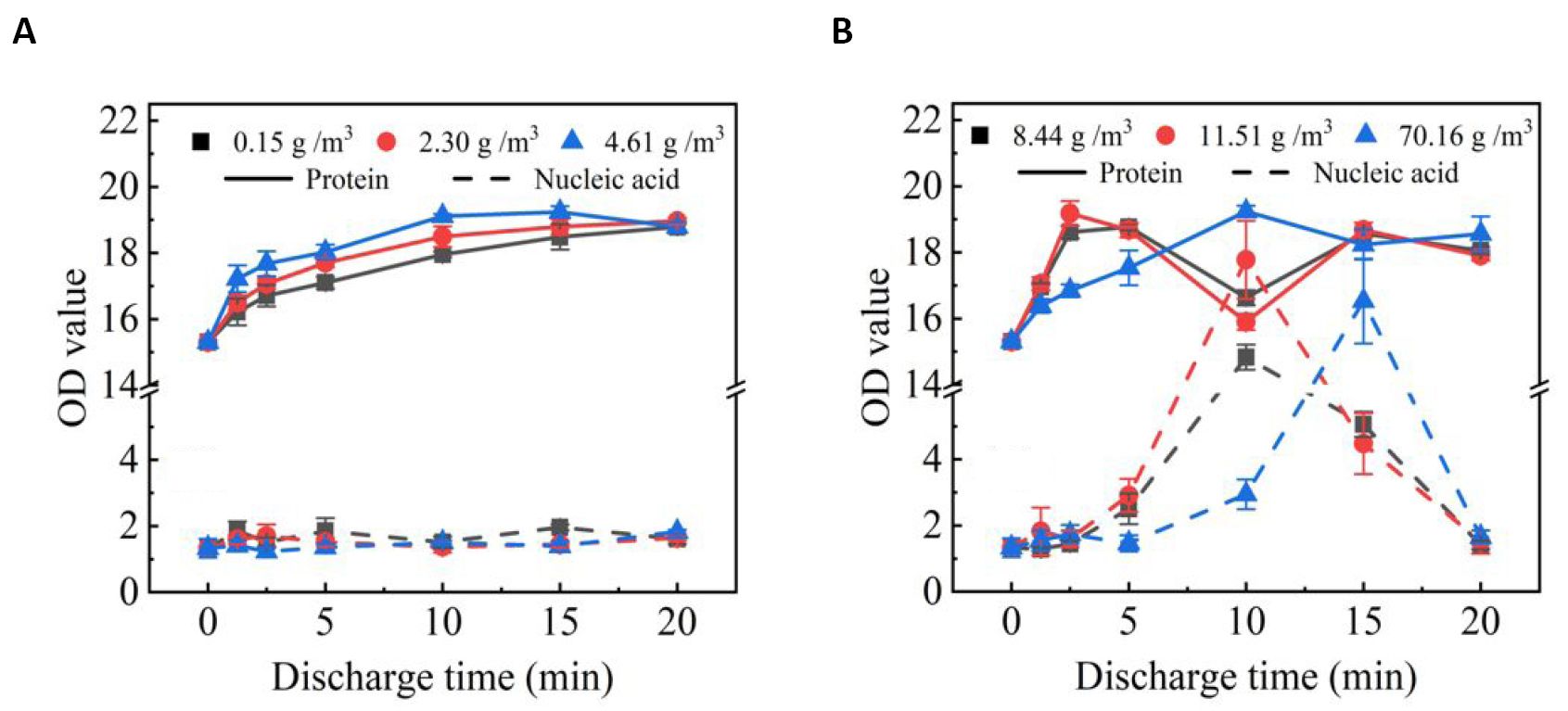
Figure 8 shows the OD values of protein and nucleic acid in spore solution after SDBD plasma treatment as a function of treatment time. It can be seen that at low humidity, the OD value of protein in spore solution increases first and then decreases slightly after SDBD plasma treatment. And at high humidity the OD value of protein shows two peaks. The OD value of nucleic acid did not increase significantly at low humidity. After SDBD treatment for a period of time, there is a peak in the OD value of nucleic acid in the spore fluid at high humidity and then drops rapidly.
|
Figure 8. OD values of the spore fluid versus plasma treatment time at low humidity (A) and at high humidity (B).
The structure of spores has seven layers from inside to outside, namely core, inner membrane, germ cell wall, cortex, out membrane, outer / inner shell, and crus[32]. In the spore core are stored nucleic acids that record genetic information and proteins involved in spore germination, growth and reproduction. Studies have found that the main mechanism of spore inactivation by plasma is RONS damage to the spore structure, causing internal material such as proteins and nucleic acids to leak out, resulting in the death of the spore[32-35]. In Figure 8, at low humidity, no significant leakage of nucleic acids was observed. This could mean that after the treatment of SDBD, at low humidity the OD peak of the protein alone may simply be caused by the exfoliation of the outer protein shell of the envelope, which dose not result in a large leakage of enzymes and nucleic acids within the spores. In contrast, after treatment with SDBD, the apparent leakage of nucleic acids at high humidity indicates that spore integrity has been lost. The second OD peak of the protein, which appears together with the OD peak of the nucleic acid, should be caused by the leakage of information transmitters associated with spore germination and enzymes associated with life activity within the spore[33,34].
4 CONCLUSION
In this paper, the inactivation of Bacillus subtilis spores by SDBD plasma generated at different air humidity is investigated. Humidity plays an important factor in the inactivation of spores by SDBD. Under the high humidity (>6g/m3), the number of inactivated spores reaches more than 4 log after 20min of SDBD plasma treatment. However, under the low humidity (<6g/m3), the spores cannot be completely inactivated. For the measurement of gaseous RONS, the results suggest that low humidity is beneficial to the production of O3 which has little sporicidal effect, while high humidity is more conducive to the production of other RONS, such as ‧OH, H2O2, HO2 and ONOOH. Nitrogen oxides play an influential role in the formation of these strongly oxidizing RONS, and may dominate the sterilization process based on the oxidation properties. The protein shell and membrane structure of the spores are oxidized and destroyed by RONS, resulting in the ejection of the contents and inactivation of the spore. This study provides an analysis of the effect of humidity on the inactivated spores of SDBD, which can be further used for surface sterilization of medical devices.
Acknowledgements
The study was financially supported by Project 12275042 of the China Natural Science Foundation.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
Liu D and Li H provided the conception and design of the study. Li H wrote the main manuscript text. Zhou Z and Li H prepared Figure 1, Sun Y, Li J, Wang Y, Wang Z and Li H prepared Figure 3 and Figure 5, Li H prepared Figure 2, Figure 4, and Figures 6-8. Lu N, Liu D, Cui Y and Zhao Z critically revised the important knowledge content of this article. All authors reviewed the manuscript.
Abbreviation List
AC, Alternating current
DBD, Dielectric barrier discharge
DCF, 2',7'-dichlorofluorescein
H2DCFDA, 2',7'-dichlorodihydrofuorescein diacetate
HTA, 2-hydroxyterephthalic acid
HV, High voltage
OD, Optical density
‧OH, OH radicals
ONOOH, Peroxynitrous acid
RONS, Reactive oxygen and nitrogen species
SDBD, Surface dielectric barrier discharge
TA, Terephthalic acid
TSA, Tryptose Soya Agar
UVS, Ultraviolet-visible spectrophotometer
References
[1] O’Connor N, Cahill O, Daniels S et al. Cold atmospheric pressure plasma and decontamination. Can it contribute to preventing hospital-acquired infections? J Hosp Infect, 2014; 88: 59-65. [DOI]
[2] Guo L, Yao Z, Yang L et al. Plasma-activated water: An alternative disinfectant for S protein inactivation to prevent SARS-CoV-2 infection. Chem Eng J, 2021; 421: 127742. [DOI]
[3] Laroussi M, Bekeschus S, Keidar M et al. Low-temperature plasma for biology, hygiene, and medicine: Perspective and roadmap. IEEE T Radiat Plasma, 2021; 6: 127-157. [DOI]
[4] Feizollahi E, Misra NN, Roopesh MS. Factors influencing the antimicrobial efficacy of Dielectric Barrier Discharge (DBD) Atmospheric Cold Plasma (ACP) in food processing applications. Crit Rev Food Sci, 2021; 61: 666-689. [DOI]
[5] Matsui K, Ikenaga N, Sakudo N. Effects of humidity on sterilization of Geobacillus stearothermophilus spores with plasma-excited neutral gas. Jpn J Appl Phys, 2015; 54: 06GD02. [DOI]
[6] Lukes P, Dolezalova E, Sisrova I et al. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci T, 2014; 23: 015019. [DOI]
[7] Xi W, Wang W, Liu Z et al. Mode transition of air surface micro-discharge and its effect on the water activation and antibacterial activity. Plasma Sources Sci T, 2020; 29: 095013. [DOI]
[8] Salmon A, Stancu GD, Laux CO. Decontamination of endospores by plasma sources on dried surfaces: A review of key parameters and inactivation results. Front Phys-Lausanne, 2021; 9: 677971. [DOI]
[9] Moldgy A, Nayak G, Aboubakr HA et al. Inactivation of virus and bacteria using cold atmospheric pressure air plasmas and the role of reactive nitrogen species. J Phys D Appl Phys, 2020; 53: 434004. [DOI]
[10] Moldgy A, Aboubakr H, Nayak G et al. Comparative evaluation of the virucidal effect of remote and direct cold air plasmas with UV‐C. Plasma Process Polym, 2020; 17: 1900234. [DOI]
[11] Kogelheide F, Voigt F, Hillebrand B et al. The role of humidity and UV-C emission in the inactivation of B. subtilis spores during atmospheric-pressure dielectric barrier discharge treatment. J Phys D Appl Phys, 2020; 53: 295201. [DOI]
[12] Patil S, Moiseev T, Misra NN et al. Influence of high voltage atmospheric cold plasma process parameters and role of relative humidity on inactivation of Bacillus atrophaeus spores inside a sealed package. J Hosp Infect, 2014; 88: 162-169. [DOI]
[13] Brandenburg R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci T, 2017; 26: 053001. [DOI]
[14] Morfill GE, Shimizu T, Steffes B et al. Nosocomial infections - a new approach towards preventive medicine using plasmas. New J Phys, 2009; 11: 115019. [DOI]
[15] Isbary G, Shimizu T, Li Y et al. Cold atmospheric plasma devices for medical issues. Expert Rev Med Devic, 2013; 10: 367-377. [DOI]
[16] Wang X, Qi Z, Zhao Z et al. Rapid Inactivation of E. coli by Water Droplet-Tuned Surface Micro-Discharges. Plasma Chem Plasma P, 2022; 42: 1291-1310. [DOI]
[17] Machala Z, Tarabová B, Sersenová D et al. Chemical and antibacterial effects of plasma activated water: Correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. J Phys D Appl Phys, 2018; 52: 034002. [DOI]
[18] Kanazawa S, Furuki T, Nakaji T et al. Application of chemical dosimetry to hydroxyl radical measurement during underwater discharge. J Phys Conf Ser, 2013; 418: 012102. [DOI]
[19] Tarabová B, Lukeš P, Hammer MU et al. Fluorescence measurements of peroxynitrite/peroxynitrous acid in cold air plasma treated aqueous solutions. Phys Chem Chem Phys, 2019; 21: 8883-8896. [DOI]
[20] Ki SH, Masur K, Baik KY et al. Effects of humidity on room disinfection by dielectric barrier discharge plasma. J Phys D Appl Phys, 2019; 52: 425204.
[21] Ma Y, Zhang G , Shi X et al. Chemical mechanisms of bacterial inactivation using dielectric barrier discharge plasma in atmospheric air. IEEE T Plasma Sci, 2008; 36: 1615-1620. [DOI]
[22] Liang Q, Li Y. A rapid and accurate method for determining protein content in dairy products based on asynchronous - injection alternating merging zone flow-injection spectrophotometry. Food Chem, 2013; 141: 2479-2485. [DOI]
[23] Deng S, Cheng C, Ni G et al. The interaction of an atmospheric pressure plasma jet using argon or argon plus hydrogen peroxide vapour addition with Bacillus subtilis. Chinese Phys B, 2010; 19: 105203.
[24] Abdelaziz AA, Ishijima T, Osawa N et al. Quantitative analysis of ozone and nitrogen oxides produced by a low power miniaturized surface dielectric barrier discharge: Effect of oxygen content and humidity level. Plasma Chem Plasma P, 2019; 39: 165-185. [DOI]
[25] Ono R, Teramoto Y, Oda T. Effect of humidity on gas temperature in the afterglow of pulsed positive corona discharge. Plasma Sources Sci T, 2009; 19: 015009.
[26] McKay K, Liu D, Rong M et al. Generation and loss of reactive oxygen species in low-temperature atmospheric-pressure RF He+O2+H2O plasmas. J Phys D Appl Phys, 2012; 45: 172001.
[27] Lukes P, Clupek M, Babicky V et al. Generation of ozone by pulsed corona Sdischarge over water surface in hybrid gas - liquid electrical discharge reactor. J Phys D Appl Phys, 2005; 38: 409.
[28] Moiseev T, Misra NN, Patil S et al. Post-discharge gas composition of a large-gap DBD in humid air by UV-Vis absorption spectroscopy. Plasma Sources Sci T, 2014; 23: 065033.
[29] Park S, Choe W, Jo C. Interplay among ozone and nitrogen oxides in air plasmas: Rapid change in plasma chemistry. Chem Eng J, 2018; 352: 1014-1021. [DOI]
[30] Liu X, Wang Z, Li J et al. Inactivation of E. coli, S. aureus, and Bacteriophages in Biofilms by Humidified Air Plasma. Int J Mol Sci, 2022; 23: 4856. [DOI]
[31] Abida O, Mielke LH, Osthoff HD. Observation of gas-phase peroxynitrous and peroxynitric acid during the photolysis of nitrate in acidified frozen solutions. Chem Phys Lett, 2011; 511: 187-192. [DOI]
[32] McKenney PT, Driks A, Eichenberger P. The Bacillus subtilis endospore: assembly and functions of the multilayered coat. Nat Rev Microbiol, 2013; 11: 33-44. [DOI]
[33] Zhu Z, Bassey AP, Huang T et al. The formation, germination, and cold plasma inactivation of bacterial spore. Food Chem Adv, 2022; 1: 100056. [DOI]
[34] Kuzminova A, Kretková T, Kylián O et al. Etching of polymers, proteins and bacterial spores by atmospheric pressure DBD plasma in air. J Phys D Appl Phys, 2017; 50: 135201.
[35] Huang Y, Ye X, Doona CJ et al. An investigation of inactivation mechanisms of Bacillus amyloliquefaciens spores in non‐thermal plasma of ambient air. J Sci Food Agr, 2019; 99: 368-378. [DOI]
Copyright © 2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.





 Copyright ©
Copyright ©