Observing the Effects of Probiotics Combined with Rexine and Peptisonide Enteral Nutrition Guidance in Postoperative Intracerebral Hemorrhage Patients
Fei Liu1, Ya-nan Zhao1, Li-qian Zhang1, Nai-kun Gao1, Zhi-fei Qiao1, Jie Yao2*
1Intensive Care Medicine, The First Affiliated Hospital of Hebei North University, Zhangjiakou, Hebei Province, China
2Department of anesthesiology, The First Affiliated Hospital of Hebei North University, Zhangjiakou, Hebei Province, China
*Correspondence to: Jie Yao, Department of anesthesiology, The First Affiliated Hospital of Hebei North University, Changqing Road, Zhangjiakou 075000, Hebei Province, China; Email: jingjie1229@163.com
Abstract
Objective: To investigate the therapeutic effects of probiotics combined with Rexine or Peptisonide enteral nutrition guidance in postoperative intracerebral hemorrhage (ICH) patients.
Methods: Clinical data of 60 ICH patients treated at our hospital from May 2022 to January 2024 were retrospectively analyzed. The patients were divided into an observation group (OG) (probiotics combined with Peptisonide) and a control group (CG) (probiotics combined with Rexine), with 30 cases in each group. The comparison of neurological function scores, immunoglobulin levels, inflammation and infection markers, and adverse reactions between the two groups was conducted during the treatment process.
Results: Before treatment, the two groups were comparable in terms of The National Institutes of Health Stroke Scale (NIHSS) scores, Immunoglobulin A (IgA), Immunoglobulin G (IgG), Immunoglobulin M (IgM), D-lactic acid, and Procalcitonin (PCT) levels (P>0.05). After treatment, the NIHSS scores significantly decreased in both groups, with a more pronounced reduction observed in the OG (P<0.05). Levels of IgA, IgG, and IgM significantly increased, with a more substantial rise in the OG (P<0.05). The D-lactic acid levels significantly decreased, and both the D-lactic acid and PCT levels were lower in the OG (P<0.05). The incidence of vomiting, gastric retention, and gastrointestinal bleeding did not differ significantly between the two groups (P>0.05), but the incidence of diarrhea and constipation was notably lower in the OG compared to the CG (P<0.05).
Conclusion: The probiotic combined with Peptisonide enteral nutrition scheme significantly improves neurological function, enhances immunity, reduces inflammatory response, and decreases adverse reactions.
Keywords: probiotics, Rexine, Peptisonide, enteral nutrition, ICH, therapeutic effect
1 INTRODUCTION
In the United States, approximately 10% of all stroke cases are intracerebral hemorrhage (ICH)[1]. ICH is a disease caused by the rupture of cerebral blood vessels, leading to a substantial amount of blood seeping into the brain tissue and forming a hematoma, resulting in brain tissue necrosis. This condition is often associated with a very high mortality rate[2]. ICH predominantly occurs in the elderly population and typically presents with severe symptoms. It is closely related to factors such as cerebrovascular diseases, hypertension, and smoking[3]. Without timely and effective treatment, patients are at a significant risk of mortality[4]. While surgical treatment is a common approach, it may lead to postoperative complications such as swallowing difficulties and temporary loss of eating ability[5,6]. Therefore, enteral nutrition therapy is required but can cause complications including diarrhea and bloating, which can lead to gut microecological imbalance and malnutrition[7,8]. In summary, early postoperative enteral nutrition support is crucial.
Probiotics can aid in regulating gastrointestinal function and immune response by altering the activity of the patient’s gut microbiota. They help maintain microbial balance in the intestine, improve nutrient absorption, enhance immune system function, and promote intestinal health recovery[9]. Enteral nutrition formulas provide the essential nutrients and energy required by the body, including amino acids, essential fatty acids, and vitamins[10]. Rexine uses whole protein as a nitrogen source and contains a comparable amount of fat[11]. Peptisonide is a short peptide enteral nutrition formula that can quickly provide energy and protect the mucosa for those with impaired gastrointestinal function[11,12]. Currently, probiotics have shown efficacy in treating diarrhea symptoms and improving nutritional status in patients[13]. However, there is a lack of studies on the combined application of probiotics and nutritional formulas.
Therefore, this study selects the clinical data of 60 ICH patients treated at our hospital from May 2022 to January 2024 to investigate the effects of probiotics combined with Rexine and Peptisonide enteral nutrition guidance in postoperative ICH patients.
2 MATERIALS AND METHODS
This retrospective study reviewed the clinical records of 60 ICH patients who received treatment at our hospital between May 2022 and January 2024. The patients were categorized into two groups based on their treatment methods: an observation group (OG) and a control group (CG), with 30 patients in each group. Inclusion criteria: (1) meeting the Chinese Guidelines for the Diagnosis and Treatment of ICH (2019)[14]; (2) complete clinical data. Exclusion criteria: (1) patients with gastrointestinal bleeding; (2) individuals with psychiatric conditions; (3) individuals with cancer. This research received approval from our hospital's medical ethics committee, and informed consent was obtained from both the patients and their families.
3 METHODS
Both the CG and the OG received probiotic combined treatment. The treatment regimen included oral administration of Bifid Triple Viable Capsules (Shanghai Sine Pharmaceutical Co., Ltd., National Drug Approval Number S10950032), 1.5 grams three times a day, diluted in an appropriate amount of drinking water, and administered through a nasogastric tube for 1 week.
The CG received probiotics combined with Rexine enteral nutrition treatment, administered through a nasogastric tube with Rexine enteral nutrition solution (Huarui Pharmaceutical Co., Ltd., National Drug Approval Number J20140075). The energy density of this solution is 1kcal/ml. The initial dose was 10ml (equivalent to 10kcal)/kg on the first day, with an infusion rate of 20ml/h, gradually increasing to 25-30ml per kg of body weight. The rate was increased by 20ml/h daily, with a maximum infusion rate of 70-80ml/h. The OG received probiotics combined with Peptisonide enteral nutrition treatment, administered through a nasogastric tube with Peptisonide enteral nutrition solution (Nutricia Pharmaceutical Co., Ltd., National Drug Approval Number H20010285). The administration method and dosage for Peptisonide and Rexine were the same. During the treatment, doctors regularly assessed the patients' nutritional status and gastrointestinal function. Based on these monitoring results, nutritional formulas and infusion rates were adjusted accordingly. Both groups were treated continuously for 1 week.
4 OBSERVATION INDICATORS
4.1 Neurological Function Score
The National Institutes of Health Stroke Scale (NIHSS)[15] was used to evaluate the degree of neurological impairment in ICH patients. The scale ranges from 0 to 42 points. Higher scores indicate more severe neurological impairment, while lower scores indicate less severe impairment.
4.2 Changes in Immunoglobulin Levels
Venous blood samples (3-5mL) were collected from both groups before and after treatment to measure immunoglobulin levels, including Immunoglobulin G (IgG), Immunoglobulin A (IgA) and Immunoglobulin M (IgM), using immunoturbidimetry.
4.3 Changes in Inflammation and Infection Indicators
Venous blood samples (2-5mL) were collected from both groups before and after treatment to measure Procalcitonin (PCT) levels using immunofluorescence, and D-lactate levels using colorimetry.
4.4 Adverse Reactions
During the treatment period, medical staff closely monitored the patients for symptoms and signs of adverse reactions, recording and managing occurrences of vomiting, gastric retention, diarrhea, gastrointestinal bleeding, and constipation.
4.5 Statistical Analysis
Using SPSS 22.0 software, statistical analysis was conducted, and GraphPad Prism 9 software was employed for image processing. Measurement data were described as mean ± standard deviation (x̄±s) and analyzed between groups using the t-test. For categorical data, represented as numbers and percentages (%), comparisons between groups were assessed using the χ² test. A significance level of P<0.05 was considered statistically significant.
5 RESULTS
5.1 Comparison of General Information between the Two Groups
Between the two groups, comparison of general information revealed no statistically significant differences (P>0.05), indicating comparability (Table 1).
Table 1. Comparison of General Information between the Two Groups (x̄±s)
Group |
|
CG |
OG |
t/χ² |
P |
Case(n) |
|
30 |
30 |
|
|
Gender |
Male |
14 |
15 |
0.0667 |
0.7962 |
|
Female |
16 |
15 |
|
|
Maximum |
66 |
65 |
0.0007 |
0.9789 |
|
Minimum |
45 |
44 |
|||
Average |
55.93±4.69 |
55.88±4.71 |
0.0412 |
0.9673 |
|
Hemorrhage position |
Brainstem |
5 |
6 |
0.2793 |
0.9639 |
Cerebellum |
9 |
8 |
|||
Cerebral hemisphere |
6 |
7 |
|||
Basal ganglia |
10 |
9 |
5.2 Comparison of NIHSS Scores between the Two Groups
Before treatment, there were no significant differences in NIHSS scores between the two groups (P>0.05). After treatment, both groups showed significant reductions, with the OG demonstrating a significantly greater decrease compared to the CG (P<0.05). See Table 2.
Table 2. Comparison of NIHSS Scores Between the Two Groups (x̄±s)
Group |
Case |
Before Treatment |
After Treatment |
CG |
30 |
8.16±2.03 |
2.23±0.78 |
OG |
30 |
8.26±2.15 |
3.17±0.56 |
t |
|
0.1852 |
5.3620 |
P |
|
0.8537 |
0.0001 |
5.3 Comparison of Changes in Immunoglobulin Levels Between the Two Groups
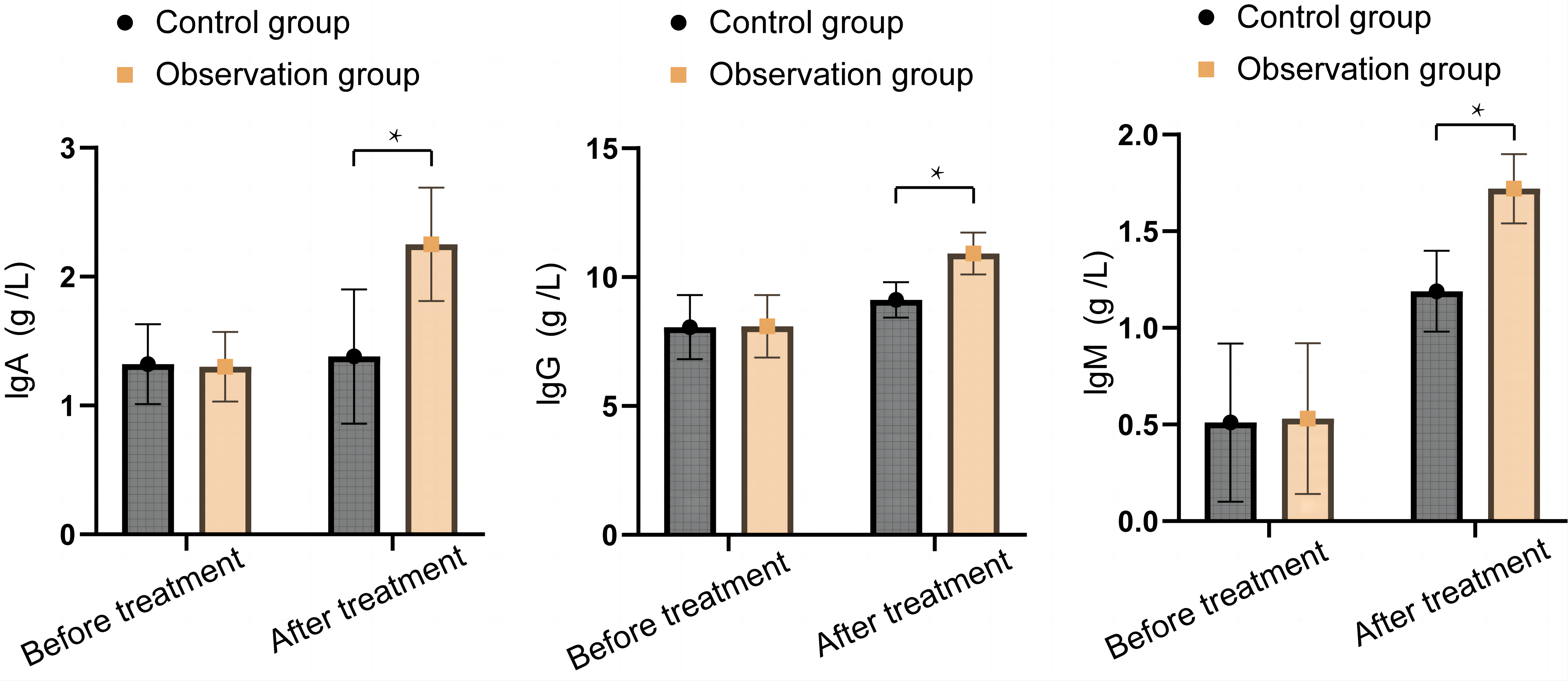
Figure 1 illustrates that before treatment, there were no significant differences in IgA, IgG, and IgM levels between the two groups (P>0.05). After treatment, both groups showed significant increases in these levels, with the OG exhibiting notably higher levels than the CG (P<0.05).
|
Figure 1. Comparison of changes in immunoglobulin levels between the two groups. * Show that P<0.05.
5.4 Comparison of Inflammation and Infection Indicators Between the Two Groups
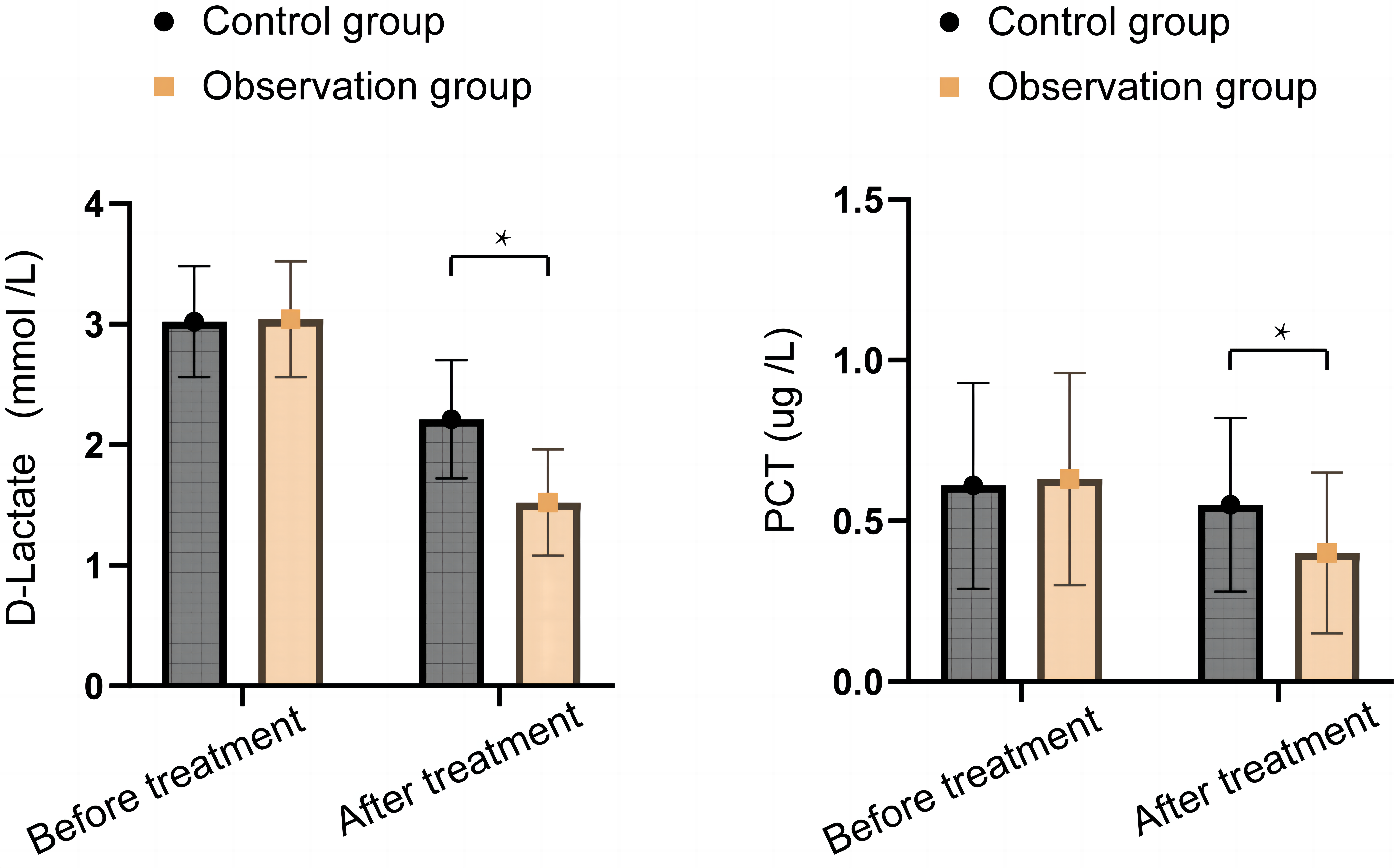
As shown in Figure 2, there were no significant differences in D-lactate and PCT levels between the two groups before treatment (P>0.05). After treatment, D-lactate levels significantly decreased in both groups, and the levels of D-lactate and PCT in the OG were significantly lower than those in the CG (P<0.05).
|
Figure 2. Comparison of changes in inflammation and infection indicators between the two groups. * Show that P<0.05.
5.5 Comparison of Adverse Reaction Incidence Between the Two Groups
The incidence of vomiting, gastric retention, and gastrointestinal bleeding did not differ significantly between the two groups (P>0.05). However, the OG exhibited significantly lower rates of diarrhea and constipation compared to the CG (P<0.05). See Table 3.
Table 3. Comparison of Adverse Reaction Incidence Between the Two Groups (%)
Group |
Case |
Vomiting |
Gastric Retention |
Diarrhea |
Gastrointestinal Bleeding |
Constipation |
CG |
30 |
4 |
2 |
11 |
5 |
10 |
OG |
30 |
2 |
1 |
2 |
4 |
2 |
χ2 |
|
0.1228 |
0.0008 |
5.2002 |
0.0022 |
4.5000 |
P |
|
0.7260 |
0.9774 |
0.0226 |
0.9626 |
0.0339 |
6 DISCUSSION
ICH is a common and high-risk acute cerebrovascular disease characterized by complex and rapidly progressing pathology, with high rates of disability and mortality[16]. Due to changes in diet and lifestyle, the incidence of ICH is gradually increasing[17]. ICH causes multiple organ damage, leading to neuroendocrine dysfunction and metabolic disorders, resulting in malnutrition and affecting prognosis[18]. Therefore, stronger nutritional support is required. In this study, we explored the effects of probiotic combined with Rexine (CG) and probiotic combined with Peptisonide (OG) on neurological impairment, immunoglobulin levels, inflammatory and infection markers, and adverse reactions in 60 ICH patients. NIHSS scores indicate that the treatment with probiotics combined with Peptisonide is more effective than that with probiotics combined with Rexine. Overall, the enteral nutrition regimen can improve neurological function and reduce adverse reactions.
After treatment, NIHSS scores significantly decreased in both groups, with the OG showing a greater reduction than the CG. This indicates that probiotics combined with Peptisonide are more effective in improving neurological impairment in ICH patients compared to probiotics combined with Rexine. Zhang[19] reported that providing sufficient energy helps maintain normal physiological functions, meet nutritional needs, and enhance neurological function. Additionally, before treatment, there were no significant differences in IgA, IgG, and IgM levels between the two groups. However, after treatment, the levels of IgA, IgG, and IgM significantly increased in both groups, with the OG showing greater improvement. This suggests that probiotics combined with Peptisonide help enhance the immune function of patients. Immunoglobulins are antibodies, including IgG, IgM, and IgA. IgA plays a role in the mucosal defense system[20,21]. IgG is crucial for long-term regulation in primary immune responses[22], while IgM is the earliest antibody to appear, mainly used for early disease diagnosis and initial defense[23]. However, further research is needed on the changes and significance of IgA, IgG, and IgM in ICH.
Our results show that before treatment, there were no significant differences in D-lactic acid and PCT levels between the two groups. After treatment, D-lactic acid levels significantly decreased in both groups, and the levels of D-lactic acid and PCT in the OG were lower than those in the CG. This suggests that probiotics combined with Peptisonide can reduce serum D-lactic acid and PCT levels in ICH patients. D-lactic acid can enter the bloodstream during infection or intestinal mucosal damage. When intestinal inflammation occurs, increased permeability leads to the release of PCT into the bloodstream, rapidly raising its concentration[24]. Probiotics aid in nutrient absorption, inflammation suppression, and maintenance of the intestinal mucosa. Peptisonide can be directly absorbed by the intestinal mucosa, protecting the gut barrier and reducing infections[25].
There were no significant differences in the incidence of vomiting, gastric retention, and gastrointestinal bleeding between the two groups. However, the incidence of diarrhea and constipation in the OG was significantly lower than in the CG, indicating that probiotics combined with Peptisonide can effectively reduce adverse reactions in ICH patients. Studies have shown that probiotics combined with low-fat enteral nutrition can effectively improve the nutritional status and reduce diarrhea in patients with ICH or cerebral infarction[26].
In conclusion, the combination of probiotics and Peptisonide enteral nutrition provides significant therapeutic benefits for postoperative ICH patients. It can improve neurological and immune functions, reduce infections, and lower the incidence of adverse reactions, thus having important implications for improving patient outcomes.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest.
Ethical Statement
The protocol was approved by the ethics committee of The First Affiliated Hospital of Hebei North University. All Patients and guardians of the patients were informed about the study and voluntarily signed informed consent forms. All the methods were carried out in accordance with the Declaration of Helsinki.
Author Contribution
Liu F was responsible for the design and writing of the paper. Zhao Y designed the experiments. Zhang L was involved in data analysis. Gao N, Qiao Z, and Yao J contributed to the literature search.
Abbreviation List
CG, Control group
ICH, Intracerebral hemorrhage
IgA: Immunoglobulin A
IgG: Immunoglobulin G
IgM: Immunoglobulin M
NIHSS, The National Institutes of Health Stroke Scale
OG, Observation group
PCT, Procalcitonin
Reference
[1] Tsao CW, Aday AW, Almarzooq ZI et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation, 2022; 145: e153-e639.[DOI]
[2] Peng J, Volbers B, Sprügel MI et al. Influence of Early Enteral Nutrition on Clinical Outcomes in Neurocritical Care Patients With Intracerebral Hemorrhage. Front Neurol, 2021; 12: 665791.[DOI]
[3] Cheng X, Ru W, Du K et al. Association between enteral nutrition support and neurological outcome in patients with acute intracranial haemorrhage: A retrospective cohort study. Sci Rep, 2019; 9: 16507.[DOI]
[4] Cai Z, Zhao K, Li Y et al. Early Enteral Nutrition Can Reduce Incidence of Postoperative Hydrocephalus in Patients with Severe Hypertensive Intracerebral Hemorrhage. Med Sci Monit, 2022; 28: e935850.[DOI]
[5] Mizuma A, Netsu S, Sakamoto M et al.. Effect of early enteral nutrition on critical care outcomes in patients with acute ischemic stroke. J Int Med Res, 2021; 49: 3000605211055829.[DOI]
[6] Wang J, Yu K, Zeng Y. Early enteral nutrition intervention promotes multiple functional recovery in patients with traumatic intracerebral hemorrhage: A prospective randomized controlled study. Clin Neurol Neurosurg, 2023; 234: 108010.[DOI]
[7] Zheng J, Li H, Guo R et al. Neuroprotection of nalmefene for postoperative patients with spontaneous intracerebral hemorrhage. Int J Neurosci, 2015; 125: 918-923.[DOI]
[8] Nishimura K, Koga M, Minematsu K et al. Intracerebral hemorrhage in patients after heart valve replacement. J Neurol Sci, 2016; 363: 195-199.[DOI]
[9] Yuan F, Yang F, Zhang W et al. Optimizing early enteral nutrition in severe stroke (OPENS): protocol for a multicentre randomized controlled trial. BMC Neurol, 2019; 19: 24.[DOI]
[10] Reintam Blaser A, Starkopf J, Alhazzani W et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med, 2017; 43: 380-398.[DOI]
[11] Choi YK, Kim HJ, Ahn J et al. Impact of early nutrition and feeding route on clinical outcomes of neurocritically ill patients. PLoS One, 2023; 18: e0283593.[DOI]
[12] Chao K, Wang D, Yang H et al. Beneficial Effect of Immune-Enhanced Enteral Nutrition on Immune Function in Patients With Severe Neurological Diseases: A Single-Center Randomized Controlled Trial. Front Nutr, 2021; 8: 685422.[DOI]
[13] Gong L, Wang Y, Shi J. Enteral nutrition management in stroke patients: a narrative review. Ann Palliat Med, 2021; 10: 11191-11202.[DOI]
[14] Raciti L, Raciti G, Pulejo G et al. Neurogenic Dysphagia and Nutrition in Disorder of Consciousness: An Overview with Practical Advices on an “Old” but Still Actual Clinical Problem. Medicines Basel, 2022; 9: 16.[DOI]
[15] Suzuki K, Onodera H, Sugiyama R et al. The randomized study of enteral nutrition with rapid versus conventional administration in acute stroke patients; the protocol of rapid EN trial. Front Neurol, 2024; 15: 1393345.[DOI]
[16] Suzuki K, Sugiyama R, Katano T et al. The safety of rapid administration of enteral nutrition in acute stroke patients. J Neurol Sci, 2022; 437: 120270.[DOI]
[17] Webb AJ, Avramovska S, Qualls S et al. Impact of an enteral nutrition holding guideline on daily nutrition goals in patients taking phenytoin. Nutr Clin Pract, 2023; 38: 1334-1342.[DOI]
[18] Crider K, Williams J, Qi YP et al. Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Cochrane Database Syst Rev, 2022; 2: CD014217.[DOI]
[19] Zanley E, Shah ND, Craig C et al. Guidelines for gastrostomy tube placement and enteral nutrition in patients with severe, refractory hypogclycemia after gastric bypass. Surg Obes Relat Dis, 2021; 17: 456-465.[DOI]
[20] Martinez EE, Melvin P, Callif C et al. Postpyloric vs gastric enteral nutrition in critically ill children: A single-center retrospective cohort study. JPEN J Parenter Enteral Nutr, 2023; 47: 494-500.[DOI]
[21] Liauchonak S, Hamilton S, Franks JD et al. Impact of implementing an evidence-based definition of enteral nutrition intolerance on nutrition delivery: A prospective, cross-sectional cohort study. Nutr Clin Pract, 2023; 38: 376-385.[DOI]
[22] Oratz T, Bate C, Smith A et al. Nutritional Support: Enteral Nutrition Pathway for Children Undergoing Hematopoietic Stem Cell Transplantation. Clin J Oncol Nurs, 2022; 26: 651-658.[DOI]
[23] Rungsattatharm L, Kongkiattikul L, Samransamruajkit R et al. Achievement of nutritional goals after a pediatric intensive care unit nutrition support guideline implementation. Clin Nutr ESPEN, 2022; 50: 277-282.[DOI]
[24] Barra ME, Phillips KM, Chung DY et al. A Novel Correction Equation Avoids High-Magnitude Errors in Interpreting Therapeutic Drug Monitoring of Phenytoin Among Critically Ill Patients. Ther Drug Monit, 2020; 42: 617-625.[DOI]
[25] Górska M, Kudzin J, Borkowska A et al. Continuous Glucose Monitoring in Enterally Fed Children with Severe Central Nervous System Impairment. Nutrients, 2023; 15: 513.[DOI]
[26] Wilfred PM, Mathew S, Chacko B et al. Estimation of Free Phenytoin Concentration in Critically Ill Patients with Hypoalbuminemia: Direct-measurement vs Traditional Equations. Indian J Crit Care Med, 2022; 26: 682-687.[DOI]
Copyright © 2024 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.




 Copyright ©
Copyright ©