Comparison of the Efficacy, Immune Function and Survival Rate of Sorafenib and Apatinib in the Treatment of Advanced Hepatocellular Carcinoma
Weihong Mu1, Dongqing Mi1*
1Prenatal Diagnosis Center, Shijiazhuang Obstetrics and Gynecology Hospital, Shijiazhuang, Hebei Province, China
*Correspondence to: Dongqing Mi, PhD, Associate Professor, Prenatal Diagnosis Center, Shijiazhuang Obstetrics and Gynecology Hospital, Shijiazhuang 050011, Hebei Province, China; Email: mdq85@126.com
Abstract
Objective: To explore the influence of sorafenib combined with apatinib in treating advanced hepatocellular carcinoma (HCC) and its influence on immune function.
Methods: One hundred and ninety-nine patients with advanced HCC who received treatment in our hospital were collected. Ninety-eight patients were treated with sorafenib and included in the control group (CG). One hundred and one patients received sorafenib combined with apatinib and they were included in the study group (SG). After therapy, the therapeutic effect, immune function and other indexes were compared between the two groups.
Results: After therapy, the total effective rate (ORR) and disease control rate (DCR) in SG were obviously higher than those in CG (P<0.05). After therapy, the serum interleukin -10 (IL-10) and interleukin -18 (IL-18) were obviously declined in both groups (P<0.05), and the decline of IL-10 and IL-18 in SG was obviously greater than that in CG (P<0.05). After therapy, vascular endothelial growth factor (VEGF) declined obviously in both groups (P<0.05), and the reduction of VEGF in SG was obviously greater than that in CG (P<0.05). After therapy, the number of alpha fetal protein (AFP) decreased in SG was obviously higher than that in CG (P<0.05). In SG, it could obviously ameliorate the progression free survival (PFS) and overall survival (OS) of patients with advanced HCC (P = 0.01, P<0.05), and there were no obvious differences in the incidence rate of adverse reactions in both groups (P>0.05).
Conclusion: Sorafenib combined with apatinib can obviously improve the therapeutic effect, immune function and survival rate in treating patients with advanced HCC, and it is safe and worthy of clinical promotion.
Keywords: sorafenib, apatinib, advanced hepatocellular carcinoma, efficacy, immune function, survival rate
1 Introduction
Liver cancer (LC) is the sixth most prevalent carcinoma and the fourth primary cause of carcinoma-related death in the world[1], and its morbidity and mortality are still on the rise in many countries[2,3]. Early primary hepatic carcinoma can be treated by surgery or ablation techniques, but advanced LC is still a difficult problem for clinicians[4]. The most common type of primary hepatic carcinoma is Hepatocellular carcinoma (HCC)[5], which is also the sixth most malignant tumour, and ranks the third in mortality among all tumours[6], so it is one of the deadliest cancers in the world[7]. HCC is also in the forefront of tumour morbidity and mortality in China[8]. It has no typical symptoms in the early stage and has made rapid progress. Although some progress has been made in clinical treatment, the efficacy of immunotherapy for HCC is not as expected due to the complexity of the immune microenvironment of HCC[9]. Most patients were in the middle and late stage when they visited the hospital, and they were often accompanied by liver dysfunction and liver cirrhosis of different degrees, with low excision rate and high recurrence rate[10]. The choice of treatment depends on the potential liver dysfunction and cancer staging. The treatment plan includes routine arterial treatment and systemic treatment for patients with advanced diseases, such as sorafenib[11].
Sorafenib is an oral tyrosine kinase inhibitor[12], which is approved as the first-line chemotherapy drug for HCC and can effectively ameliorate the survival rate of patients with advanced LC[13]. Many clinical studies have revealed that some patients with LC are insensitive to sorafenib. In fact, the number of patients who have significantly benefited from sorafenib therapy is limited[14]. As a novel inhibitor of vascular endothelial growth factor (VEGF) receptor 2 tyrosine kinase, apatinib has definite anti-tumour effect on a variety of solid tumours[15], and it is a safe and promising treatment for HCC[16].
In this research, the effects of sorafenib combined with apatinib on sufferers with advanced HCC were investigated by comparing the therapeutic effects, immune functions and other indexes in both groups.
2 Materials and methods
2.1 Baseline Data
One hundred and ninety-nine patients with advanced HCC who received treatment in Shijiazhuang Obstetrics and Gynecology Hospital were collected. Ninety-eight patients were treated with sorafenib and included in the Control group (CG), including 59 men and 39 women, with a mean age of (62.54±3.13) years old. A total of 101 patients received sorafenib combined with apatinib and were included in the study group (SG), including 64 males and 37 females, with a mean age of (63.27±3.24) years old.
Inclusion criteria: The patient was escorted to the hospital by family members; Clinicopathological data were complete; All of them were confirmed by puncture and pathological examination; They were in line with stage B or C of BCLC stage[17]; The estimated survival time was ≥3 months.
Exclusion criteria were as below: those who had a history of mental sickness and family history of mental sickness, other malignant tumours, drug dependence, allergy to therapeutic drugs, and those who had communication difficulties due to aphasia, dysphoria, unconsciousness and were unable to cooperate with the examination.
The test was ratified by the ethics Committee of our hospital, and the patients and their families affixed informed consent after understanding the experimental process.
2.2 Therapeutic Methods
In CG, patients took sorafenib (AmyJet Scientific Inc., SIH-476-10MG) orally on an empty stomach twice a day, 0.4g/time. In SG, patients were given apatinib (Jiangsu Hengrui Pharmaceutical Co., Ltd., H20140103) on the basis of the CG, once a day, 500mg/time, for 4 weeks. If the patients could tolerate it, the dosage was maintained. If the patients could not tolerate it, it could be reduced to 250mg/time, once a day, or the medication was suspended. At the same time, symptomatic support treatment was given, and the medication was continued after remission. In both groups, patients were treated continuously for 3 months.
2.3 Outcome Measures
After treatment for 4 weeks, the curative effect and the changes of laboratory indexes [serum interleukin-10 (IL-10), interleukin-18 (IL-18), VEGF, Alpha Fetal Protein (AFP) and liver and kidney function] were observed in both groups, and the survival and adverse reactions of patients were followed up. The efficacy was evaluated via MRI enhanced scanning: All target lesions were not enhanced, indicating complete response (CR). The sum of lesion diameters was declined by ≥30%, indicating partial response (PR). The sum of lesion diameter was declined by 20-29%, indicating stable disease (SD). The sum of lesion diameter was increased by ≥20%, indicating progression of disease (PD). Total effective rate (ORR) = (CR+PR)/total×100%. Disease control rate (DCR) = (CR+PR+SD)/total×100%. The levels of IL-10, IL-18 and VEGF were tested by enzyme-linked immunosorbent assay. AFP and liver and kidney functions were tested by hospital biochemical laboratory. A decrease of 50% or more indicated a significant decrease in AFP. The patients were followed up by telephone, outpatient or inpatient, and the changes of patient’s condition in both groups were regularly followed up until the patients were lost to follow-up, progressed or died. Progression free survival (PFS) is the time from the beginning of therapy to tumour progression or death. Overall survival (OS) is the time from the beginning of treatment to the patient’s death or the last follow-up.
2.4 Statistical Analysis
All statistical analysis of the experimental results were carried out via SPSS20.0 (IBM Corp, Armonk, NY, United States). All graphs were plotted by GraphPad Prism 7 (GraphPad Software Co., Ltd., San Diego, United States). Counting data were represented by n (%). Chi-square test was used to compare between groups. Measured data were represented by mean±SD. T test was used to compare the two groups. Kaplan-Meier was applied to draw the survival curve of patients in both groups. The difference was statistically significant with P<0.05.
3 Results
3.1 Comparison of Baseline Data
By comparing the general information such as gender, age and body mass index of patients in both groups (Table 1), there were no obvious differences between the two groups (P>0.05).
Table 1. Comparison of Baseline Data between the Two Groups
|
SG (n=101) |
CG (n=98) |
t/χ2 |
P |
Age (years), mean±SD |
63.27±3.24 |
62.54±3.13 |
1.62 |
0.11 |
Gender, n (%) |
|
|
0.21 |
0.65 |
Male |
64 (63.37) |
59 (60.20) |
|
|
Female |
37 (36.63) |
39 (39.80) |
|
|
Body mass index (kg/m2), mean±SD |
20.53±2.11 |
20.49±2.13 |
0.13 |
0.89 |
BCLC staging, n (%) |
|
|
0.00 |
0.97 |
B |
26 (25.74) |
25 (25.51) |
|
|
C |
75 (74.26) |
73 (74.49) |
|
|
Child-Pugh grading, n (%) |
|
|
0.03 |
0.86 |
0.86A |
72 (71.29) |
71 (72.45) |
|
|
B |
29 (28.71) |
27 (27.55) |
|
|
Lesion size (cm), mean±SD |
7.34±2.02 |
7.29±2.13 |
0.17 |
0.87 |
Number of intrahepatic lesions, n (%) |
|
|
0.13 |
0.72 |
1-3 |
27 (26.73) |
24 (24.49) |
|
|
>3 |
74 (73.27) |
74 (75.51) |
|
|
3.2 Comparison of Clinical Curative Effect between the Two Groups
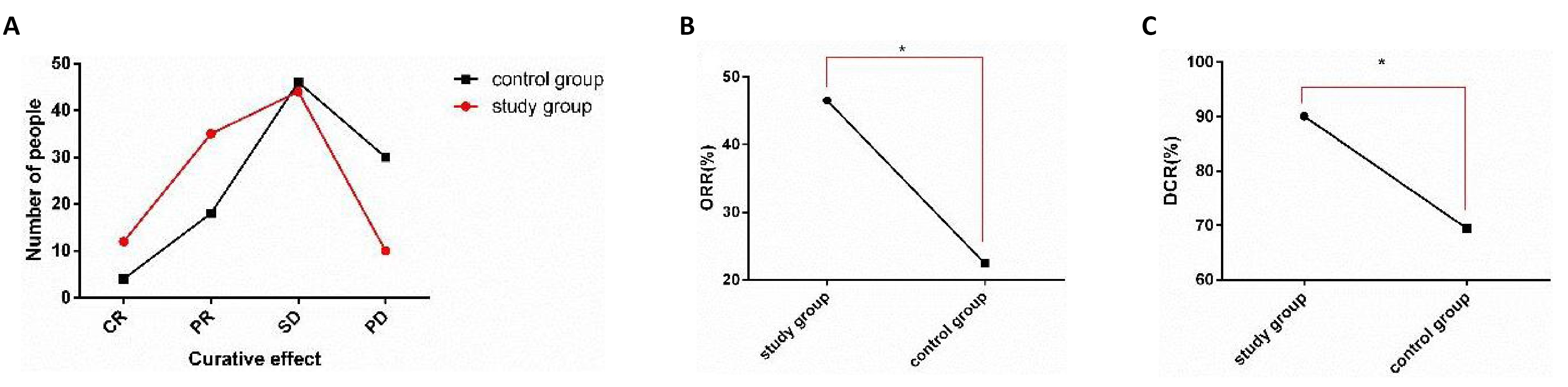
The curative effects were compared in both groups after therapy (Figure 1). After therapy, the ORR in SG (46.53%) was obviously higher than that in CG (22.45%) (P<0.05), and the DCR in SG (90.10%) was also obviously higher than that in CG (69.39%) (P<0.05).
|
Figure 1. Comparison of clinical efficacy between two groups. A: After therapy, the number of CR and PR in SG was obviously higher than that in CG, and the number of PD was obviously less than that in CG; B: The ORR in SG was obviously higher than that in CG; C: The DCR in SG was obviously higher than that in CG. Note: *indicates the comparison between the two groups; *P<0.05.
3.3 Comparison of IL-10 and IL-18 between the Two Groups before and after Therapy
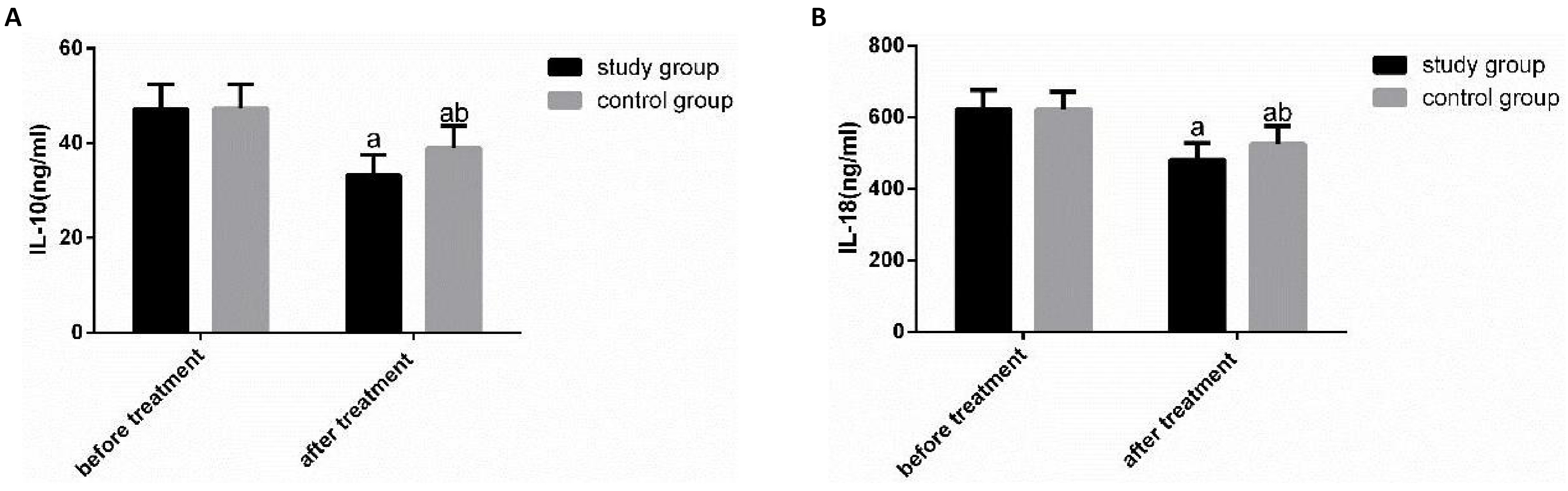
The expression levels of IL-10 and IL-18 were compared in both groups before and after therapy (Figure 2). Before therapy, there was no obvious difference in IL-10 and IL-18 between the two groups (P>0.05). After treatment, IL-10 and IL-18 were obviously declined in both groups (P<0.05), and the reduction of IL-10 and IL-18 in SG was obviously greater than that in CG (P<0.05).
|
Figure 2. Comparison of IL-10 and IL-18 expression levels between the two groups before and after therapy. A: There was no obvious difference in IL-10 between the two groups before therapy, but it declined obviously after therapy, and the SG was obviously lower than the CG; B: There was no significant difference in IL-18 between the two groups before therapy, but it declined obviously after therapy, and the SG was obviously lower than the CG. Notes: a represents the comparison between the same group before and after treatment; b represents the comparison with the SG after treatment.
3.4 Comparison of VEGF between the Two Groups before and after Therapy
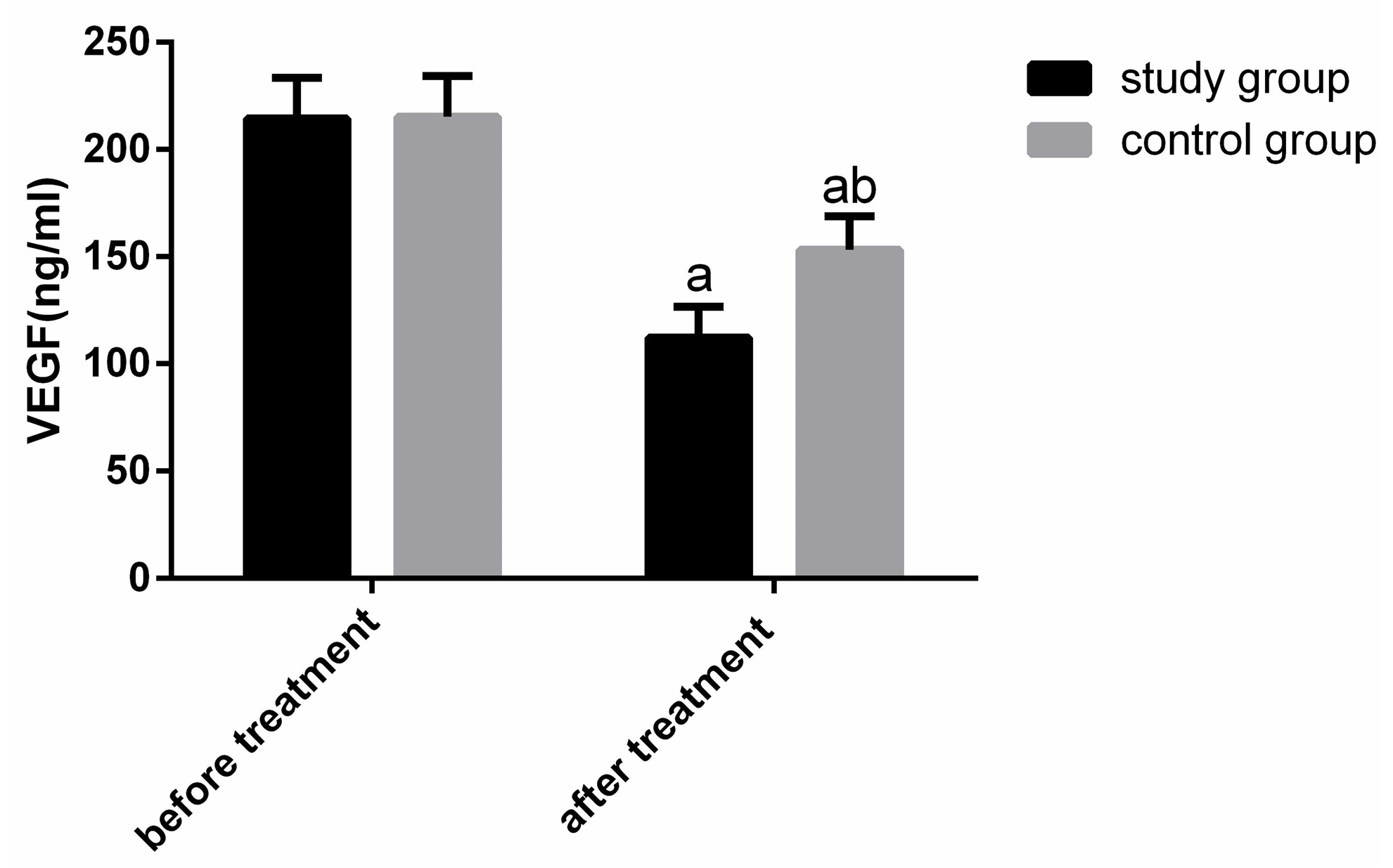
The changes of VEGF expression were compared between the two groups before and after therapy (Figure 3). Before therapy, there were no obvious differences in VEGF in both groups (P>0.05). After therapy, VEGF were obviously declined (P<0.05), and the reduction of VEGF in SG was obviously greater than that in CG (P<0.05).
|
Figure 3. Comparison of VEGF expression between the two groups before and after treatment. There was no obvious difference in VEGF between the two groups before treatment, but it declined obviously in both groups after treatment, and the patients in SG were obviously lower than those in CG. Notes: a represents the comparison between the same group before and after treatment; b represents the comparison with the SG after treatment.
3.5 Changes of AFP between the Two Groups before and after Treatment
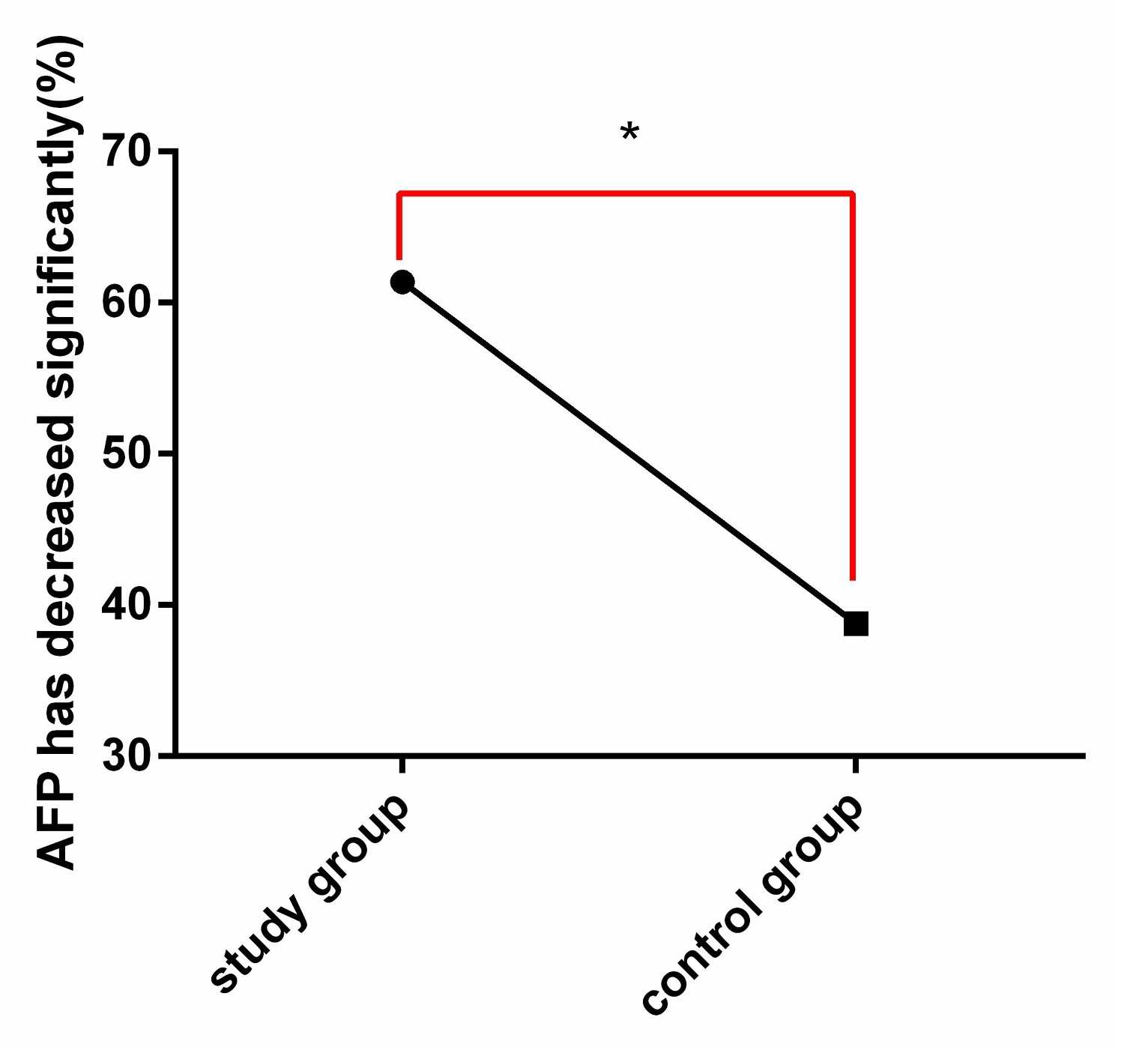
The changes of AFP were compared between the two groups before and after therapy (Figure 4). After therapy, there were 62 patients in SG whose AFP decreased by ≥50%, which was obviously higher than 38 patients in CG (P<0.05).
|
Figure 4. Comparison of AFP changes between the two groups after therapy. After therapy, the reduction rate of AFP in SG was obviously higher than that in CG. Note: * indicates the comparison between the two groups, *P<0.05.
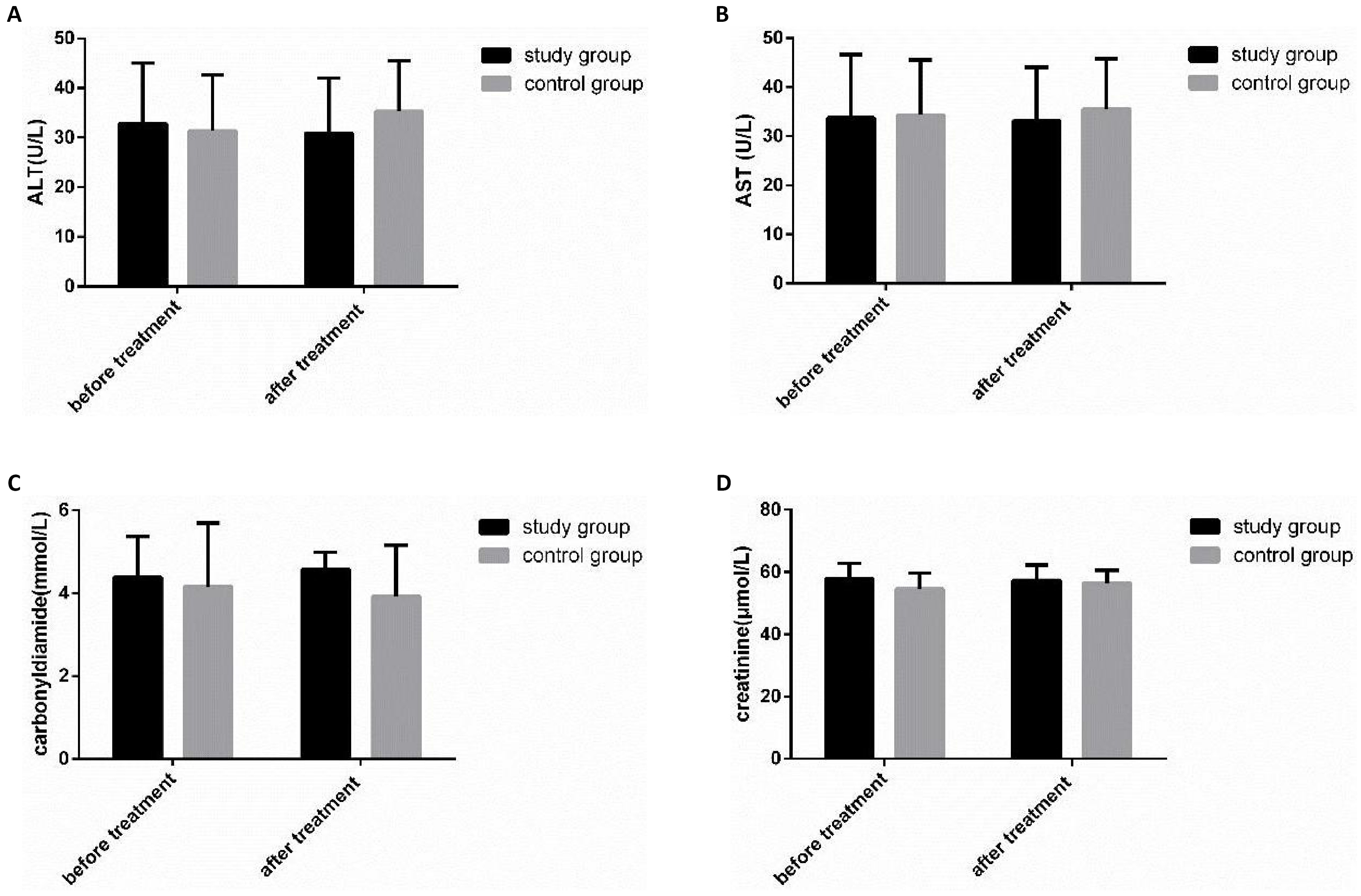
3.6 Comparison of Hepatic and Renal Function between the Two Groups before and after Therapy
The changes of hepatic and renal function were compared in both groups before and after therapy (Figure 5). There was no obvious change in hepatic and renal function in both groups before and after therapy (P>0.05).
|
Figure 5. Comparison of liver and kidney function changes between the two groups before and after therapy. A: There was no obvious change in ALT before and after therapy in both groups; B: There was no obvious change in AST before and after therapy in both groups; C: There was no obvious change in urea before and after therapy in both groups; D: There was no obvious change in creatinine in both groups before and after therapy.
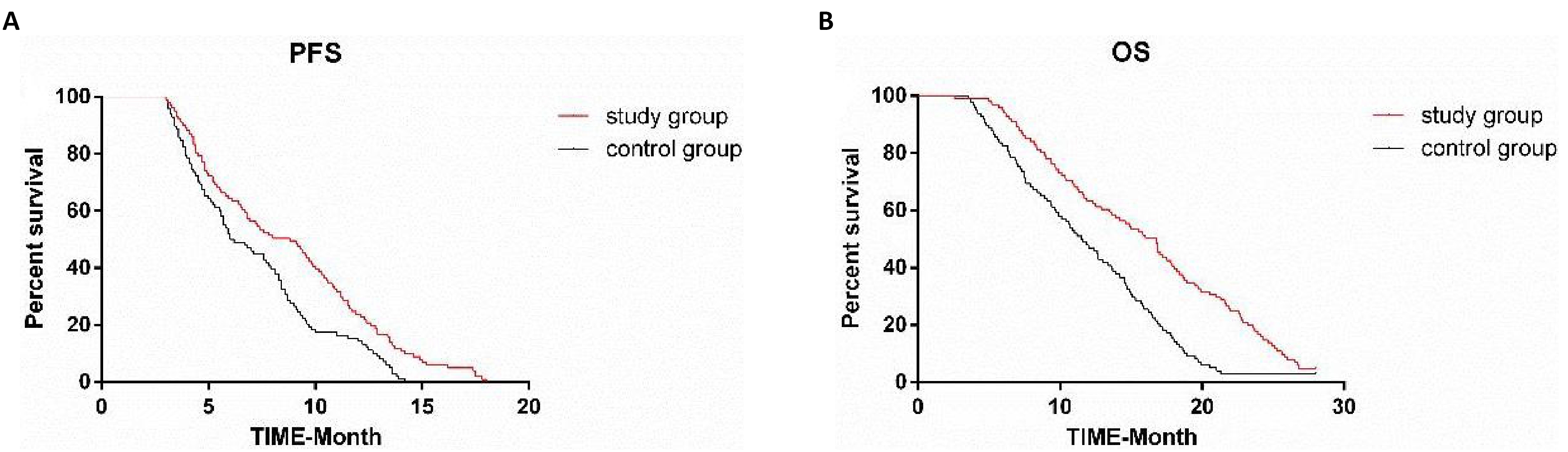
3.7 Comparison of Survival Rate between the Two Groups
In both groups, patients were followed up for 28 months. One hundred and ninety-nine patients were followed up, and there were no missing cases. The median follow-up time was 14 months. The median PFS and OS in CG were 6.1 months and 11.5 months respectively. The median PFS and OS in SG were 8.8 months and 16.8 months respectively. Sorafenib combined with apatinib could significantly ameliorate PFS and OS in patients with advanced HCC (P = 0.01, P<0.05) (Figure 6).
|
Figure 6. Comparison of survival rate between the two groups after follow-up. A: PFS was compared between the two groups, and it was found that the SG was obviously longer than the CG; B: OS was compared between the two groups, and it was found that the SG was obviously longer than the CG.
3.8 Comparison of Adverse Reactions between the Two Groups
The incidence rate of adverse reactions were compared in both groups during treatment (Table 2). The symptoms of discomfort in both groups were relieved after symptomatic treatment, and there were no obvious differences in the incidence rate of adverse reactions in both groups (P>0.05).
Table 2. Comparison of Adverse Reactions between the Two Groups, n (%)
|
Nausea and Vomiting |
Abdominal Pain |
Hand-foot-skin Reaction |
Diarrhea |
Headache and Dizziness |
Incidence of Adverse Reactions |
SG (n=101) |
2 (1.98) |
2 (1.98) |
15 (14.85) |
6 (5.94) |
3 (2.97) |
28 (27.72) |
CG (n=98) |
3 (3.06) |
2 (2.04) |
14 (14.29) |
5 (5.10) |
3 (3.06) |
27 (27.55) |
χ2 |
- |
- |
- |
- |
- |
0.00 |
P |
- |
- |
- |
- |
- |
0.98 |
4 Discussion
HCC is the most common primary hepatic carcinoma, and its main risk factor is the disease that causes liver cirrhosis, such as alcoholic hepatic disease, non-alcoholic steatohepatitis or chronic hepatitis B or C[18]. The 5-year survival rate of patients with early LC who are diagnosed to meet the conditions of surgical resection or transplantation can reach over 60%[19]. For most patients who are later diagnosed as unresectable advanced LC, the one-year survival rate was less than 40%[20]. These data reveal that it is necessary to find a more suitable treatment method, so this research was designed to explore the effect of combined medication on patients with advanced HCC by treating patients in both groups with different treatment methods and observing related indicators.
A large number of studies have revealed that sorafenib is the standard therapy for advanced HCC, and it has shown efficacy in advanced HCC[21,22]. However, the treatment of patients with advanced HCC is extremely complicated in clinical practice[23]. As a treatment standard, sorafenib can indeed prolong the OS of patients, but its curative effect is limited due to its unsatisfactory objective efficacy and low survival rate[24]. However, the combination of drugs can exert the synergistic effect of drugs[25], so sorafenib combined with apatinib was used to treat patients in this research. Studies have shown that the abnormal expression of VEGF2 is bound up with the development of HCC, so it is an effective target for anti-cancer treatment[26]. Apatinib is a small molecule inhibitor of VEGF2 tyrosine kinase, which has shown strong antineoplastic activity in a variety of tumours. It can effectively suppress tumour growth and induce apoptosis of HCC[27], and has therapeutic potential as a radiosensitizer in LC[28]. In this research, ORR and DCR in SG were markedly higher than those in CG after therapy, which indicated that sorafenib combined with apatinib had better clinical effect in treating advanced HCC, and it could significantly ameliorate the indicators of patients. Because of the close relationship between LC and inflammation, previous studies have revealed the tumour-promoting and anti-tumour effects of various immune cell types and mediators[29]. IL-10 is a cytokine that can regulate the immune activity of antigen presenting cells, T cells and other immune cells[30]. Studies have shown that patients with advanced HCC show higher serum levels of IL-10[31], and IL-10 can lead to poor prognosis, poor tumour staging and low anti-tumour immunity in patients with unresectable HCC[32]. IL-18 was originally known as interferon-γ inducer, and its typical function is to promote the production of interferon γ by a variety of immune cells, mainly CD4, T cells and NK cells[33]. Studies have shown that serum IL-18 level in patients with LC is obviously enhanced[34], and the increase of circulating IL-18 level is bound up with poor prognosis of HCC[35]. Therefore, the expression levels of IL-10 and IL-18 in both groups were detected after therapy in this research, and it was concluded that the levels of IL-10 and IL-18 were obviously declined in both groups after therapy, and the reduction of IL-10 and IL-18 in SG was obviously greater than that in CG, indicating that the two medication methods could effectively ameliorate the immune function of patients, but the clinical performance of combined medication was better. HCC is a highly vascularized tumour, so it is very important to study its angiogenesis[36]. In this research, the expression of VEGF was tested in both groups before and after treatment, and it was concluded that VEGF declined obviously in both groups after treatment, and the reduction of VEGF in SG was obviously greater than that in CG, which indicated that the combined medication could improve the expression level of VEGF in patients more effectively, thus affecting the clinical efficacy. AFP is the most important tumour marker of LC, and some studies have shown that the prognosis of patients with advanced HCC is poor due to the increase of AFP concentration[37]. Combined with this research, the AFP reduction rate of patients in SG was higher than that in CG after treatment, which indicated that combined medication could improve the AFP expression level of patients more effectively, thus ameliorating the prognosis. Tyrosine kinase inhibitors have completely changed the treatment of carcinoma. Many researches have revealed that sorafenib can effectively improve the median OS of patients with HCC[38]. In this research, patients were followed up for 28 months in both groups, and all 199 patients were followed up. It was concluded that the median PFS and OS were 6.1 months and 11.5 months respectively in CG. The median PFS and OS were 8.8 months and 16.8 months respectively in SG. The results indicated that sorafenib combined with apatinib could obviously improve PFS, OS and survival of patients with advanced HCC. In terms of safety, patients in both groups showed symptoms of discomfort in this research, which were relieved after symptomatic treatment. There were no obvious differences in the incidence rate of adverse reactions in both groups, and there was no obvious change in the liver and kidney function in both groups before and after therapy. It could be seen that the combined medication had little effect on the liver and kidney function of patients with advanced HCC, and did not aggravate the damage of liver and kidney function or increased the total incidence of adverse reactions.
This research was mainly designed to discuss the effect of sorafenib combined with apatinib on patients with advanced HCC by comparing the curative effect, survival rate and other relevant indexes in both groups after treatment, but there are still some limitations in this study. In this study, the indicators were only detected after treatment, and tracking studies were not conducted. At the same time, it is still necessary to further analyse the indicators of patients with advanced HCC treated with different doses of sorafenib combined with apatinib, so as to provide a better basis for the therapy of advanced HCC in the future.
5 Conclusion
To sum up, sorafenib combined with apatinib can significantly improve the therapeutic effect, immune function and survival rate in treating patients with advanced HCC, and it is safe and worthy of clinical promotion.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
Mu W designed this study and wrote the article; Mi D collected the data and performed the statistical analysis; Mu W and Mi D revised the papers for important intellectual content; all authors approved the final version.
Abbreviation List
AFP, Alpha fetal protein
CG, Control group
CR, Complete response
DCR, Disease control rate
HCC, Hepatocellular carcinoma
IL-10, Interleukin-10
IL-18, Interleukin-18
LC, Liver cancer
ORR, Total effective rate
OS, Overall survival
PD, Progression of disease
PFS, Progression free survival
PR, Partial response
SD, Stable disease
SG, Study group
VEGF, Vascular endothelial growth factor
References
[1] Deng Z, Xu XY, Yunita F et al. Synergistic anti-liver cancer effects of curcumin and total ginsenosides. World J Gastrointest Oncol, 2020; 12: 1091-1103. DOI: 10.4251/wjgo.v12.i10.1091
[2] Cocker F, Chien YK, Palmer AJ et al. Increasing incidence and mortality related to liver cancer in Australia: Time to turn the tide. Aust N Z J Public Health, 2019; 43: 267-273. DOI: 10.1111/1753-6405.12889
[3] Merabishvili VM, Gurkalo VK, Merabishvili EN. Morbidity, mortality and reliability of accounting of patients of different age groups with liver cancer in Russia. Adv Gerontol, 2020; 33: 240-245.
[4] Villanueva A. Selected summary for the 2015 Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE). Hepat Oncol, 2016; 3: 5-8. DOI: 10.2217/hep.15.37
[5] Shuaichen L, Guangyi W. Bioinformatic analysis reveals CYP2C9 as a potential prognostic marker for HCC and liver cancer cell lines suitable for its mechanism study. Cell Mol Biol (Noisy-le-grand), 2018; 64: 70-74.
[6] Wei X, Yang W, Zhang F et al. PIGU promotes hepatocellular carcinoma progression through activating NF-κB pathway and increasing immune escape. Life Sci, 2020; 260: 118476. DOI: 10.1016/j.lfs.2020.118476
[7] Czauderna C, Schmidtmann I, Koch S et al. High pre-treatment static and dynamic alpha-fetoprotein values predict reduced overall survival in hepatocellular carcinoma. United European Gastroenterol J, 2021; 9: 388-397. DOI: 10.1177/2050640620972611
[8] An L, Zeng H, Zheng R et al. Liver cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi, 2019; 41: 721-727.
[9] Wang Q, Huang J, Zhang H et al. Identification and analysis of immune-related subtypes of hepatocellular carcinoma. Exp Biol Med (Maywood), 2020; 246: 667-677. DOI: 10.1177/1535370220970130
[10] Hsu CC, Hsieh PM, Chen YS et al. Axl and autophagy LC3 expression in tumors is strongly associated with clinical prognosis of hepatocellular carcinoma patients after curative resection. Cancer Med, 2019; 8: 3453-3463. DOI: 10.1002/cam4.2229
[11] Walton M, Wade R, Claxton L et al. Selective internal radiation therapies for unresectable early-, intermediate- or advanced-stage hepatocellular carcinoma: systematic review, network meta-analysis and economic evaluation. Health Technol Assess, 2020; 24: 1-264. DOI: 10.3310/hta24480
[12] Ruanglertboon W, Sorich MJ, Logan JM et al. The effect of proton pump inhibitors on survival outcomes in advanced hepatocellular carcinoma treated with sorafenib. J Cancer Res Clin Oncol, 2020; 146: 2693-2697. DOI: 10.1007/s00432-020-03261-3
[13] Wu J, Chai H, Li F et al. SETD1A augments sorafenib primary resistance via activating YAP in hepatocellular carcinoma. Life Sci, 2020; 260: 118406. DOI: 10.1016/j.lfs.2020.118406
[14] Cheng Z, Wei-Qi J, Jin D. New insights on sorafenib resistance in liver cancer with correlation of individualized therapy. Biochim Biophys Acta Rev Cancer, 2020; 1874: 188382. DOI: 10.1016/j.bbcan.2020.188382
[15] Yang Z, Chen G, Cui Y et al. The safety and efficacy of TACE combined with apatinib on patients with advanced hepatocellular carcinoma: a retrospective study. Cancer Biol Ther, 2019; 20: 321-327. DOI: 10.1080/15384047.2018.1529099
[16] Liu J, Xie S, Duan X et al. Assessment of efficacy and safety of the transcatheter arterial chemoembolization with or without apatinib in the treatment of large hepatocellular carcinoma. Cancer Chemother Pharmacol, 2020; 85: 69-76. DOI: 10.1007/s00280-019-04004-z
[17] Heinrich S, Sprinzl M, Schmidtmann I et al. Validation of prognostic accuracy of MESH, HKLC, and BCLC classifications in a large German cohort of hepatocellular carcinoma patients. United European Gastroenterol J, 2020; 8: 444-452. DOI: 10.1177/2050640620904524
[18] El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology, 2014; 60: 1767-1775. DOI: 10.1002/hep.27222
[19] Heimbach JK, Kulik LM, Finn RS et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology, 2018; 67: 358-380. DOI: 10.1002/hep.29086
[20] Park JW, Chen M, Colombo M et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int, 2015; 35: 2155-2166. DOI: 10.1111/liv.12818
[21] Wada Y, Takami Y, Matsushima H et al. The safety and efficacy of combination therapy of sorafenib and radiotherapy for advanced hepatocellular carcinoma: A retrospective study. Intern Med, 2018; 57: 1345-1353. DOI: 10.2169/internalmedicine.9826-17
[22] Tanaka T, Kuzuya T, Ishigami M et al. Efficacy and safety of sorafenib in unresectable hepatocellular carcinoma with bile duct invasion. Oncology, 2020; 98: 621-629. DOI: 10.1159/000507051
[23] Bertacco A, Vitale A, Mescoli C et al. Sorafenib treatment has the potential to downstage advanced hepatocellular carcinoma before liver resection. Per Med, 2020; 17: 83-87. DOI: 10.2217/pme-2018-0114
[24] Kim BK, Kim DY, Byun HK et al. Efficacy and safety of liver-directed concurrent chemoradiotherapy and sequential sorafenib for advanced hepatocellular carcinoma: A prospective phase 2 trial. Int J Radiat Oncol Biol Phys, 2020; 107: 106-115. DOI: 10.1016/j.ijrobp.2020.01.027
[25] Afifi AM, El-Husseiny AM, Tabashy RH et al. Sorafenib- taurine combination model for hepatocellular carcinoma cells: Immunological aspects. Asian Pac J Cancer Prev, 2019; 20: 3007-3013. DOI: 10.31557/APJCP.2019.20.10.3007
[26] Shigeta K, Datta M, Hato T et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology, 2020; 71: 1247-1261. DOI: 10.1002/hep.30889
[27] Xin M, Xie Q, Ma L et al. Synergistic anti-tumour effects of Clostridium butyricum in combination with apatinib in CT26 colorectal tumour-bearing mice. Anticancer Drugs, 2019; 30: 991-997. DOI: 10.1097/CAD.0000000000000817
[28] Liao J, Jin H, Li S et al. Apatinib potentiates irradiation effect via suppressing PI3K/AKT signaling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res, 2019; 38: 454. DOI: 10.1186/s13046-019-1419-1
[29] Ouyang Y, Tang Y, Fu L et al. Exosomes secreted by chronic hepatitis B patients with PNALT and liver inflammation grade ≥A2 promoted the progression of liver cancer by transferring miR-25-3p to inhibit the co-expression of TCF21 and HHIP. Cell Prolif, 2020; 53: e12833. DOI: 10.1111/cpr.12833
[30] Gopinathan U, Ovstebo R, Brusletto BS et al. Transcriptomic data from two primary cell models stimulating human monocytes suggest inhibition of oxidative phosphorylation and mitochondrial function by N. meningitidis which is partially up-regulated by IL-10. BMC Immunol, 2017; 18: 46. DOI: 10.1186/s12865-017-0229-5
[31] Hayashi T, Yamashita T, Terashima T et al. Serum cytokine profiles predict survival benefits in patients with advanced hepatocellular carcinoma treated with sorafenib: A retrospective cohort study. BMC Cancer, 2017; 17: 870. DOI: 10.1186/s12885-017-3889-x
[32] Chan SL, Mo FK, Wong CS et al. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer, 2012; 118: 3984-3992. DOI: 10.1002/cncr.26726
[33] Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: Back to the future. Immunity, 2013; 39: 1003-1018. DOI: 10.1016/j.immuni.2013.11.010
[34] Iliaz R, Akyuz U, Tekin D et al. Role of several cytokines and adhesion molecules in the diagnosis and prediction of survival of hepatocellular carcinoma. Arab J Gastroenterol, 2016; 17: 164-167. DOI: 10.1016/j.ajg.2016.10.002
[35] Markowitz GJ, Yang P, Fu J et al. Inflammation-dependent IL18 signaling restricts hepatocellular carcinoma growth by enhancing the accumulation and activity of tumor-infiltrating lymphocytes. Cancer Res, 2016; 76: 2394-2405. DOI: 10.1158/0008-5472.CAN-15-1548
[36] Huang B, Huang M, Li Q. Cancer-associated fibroblasts promote angiogenesis of hepatocellular carcinoma by VEGF-mediated EZH2/VASH1 pathway. Technol Cancer Res Treat, 2019; 18: 1533033819879905. DOI: 10.1177/1533033819879905
[37] Zhu AX, Kang YK, Yen CJ et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol, 2019; 20: 282-296. DOI: 10.1016/S1470-2045(18)30937-9
[38] Amanuma M, Nagai H, Igarashi Y. Sorafenib might induce sarcopenia in patients with hepatocellular carcinoma by inhibiting carnitine absorption. Anticancer Res, 2020; 40: 4173-4182. DOI: 10.21873/anticanres.14417
Copyright © 2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.





 Copyright ©
Copyright ©