A General Review on Poly Butylene Succinate Fiber in the Global Textile Industry
Ömer Fırat Turşucular1*
1Department of Hatin Textile, Hatin Tex Weaving Companies, Dosab, Turkey
*Correspondence to: Ömer Fırat Turşucular, MD, R&D in Chief, Department of Hatin Textile, Hatin Tex Weaving Companies, Dosab, 16245, Turkey; Email: omerfirattursucular@gmail.com
Abstract
It was the technical explanation of various physical, chemical, thermal, mechanical, and biological changes of Poly Butylene Succinate (PBS) fiber with both theoretical, and experimental studies. It was also aimed to lead technically to various future experimental studies in this scientific field thanks to sustainable, more harmless and more ecological approaches. PBS fiber is produced first in the form of chips as a result of the polycondensation reaction. Then, in the form of fiber by applying the melt spinning method. It is increasingly used in application areas such as sheets, filaments, non-woven fabrics, textiles, flame retardant (FR), UV protection, antimicrobial, clothing, filters, laminates, molded foam products, injection molded, packaging, antenna, electronics, agriculture, forestry, construction products, orthopedic implant, bone treatment, and biomedical fields thanks to PBS, which is a sustainable, ecological, environmentally friendly, and biodegradable fiber. Firstly, PBS polymer chips must be produced by the chemical synthesis method (transesterification). Secondly; drying, mixing, extrusion, solidification, drawing, lubrication, and winding processes should be applied thanks to the melt drawing method, respectively. Particularly effective factors in PBS yarn production are extruder (L/D) ratio, number of extruder screws, extruder length, extruder diameter, inside the extruder before drying, and then melting, temperature, time, pressure, and mixing speed, enthalpy, average molecular weight, MFR, and PDI values, and noble gas type (N2), dosing pressure, number of nozzle holes, nozzle cross-section, solidification (cooling) distance, temperature, air flow rate (volume flow rate), rotation of godets attention should be paid to speeds, temperature values, draft rates, lubricating chemical type, and concentration, and winding speed values. Moreover, various experimental studies should be carried out.
Keywords: PBS fiber, Its chemical structure, Its manufacturing process, Its general properties, Its applications
1 INTRODUCTION
In the first main part of this theoretical review study included that the history of PBS fiber, and its global commercial market were examined from a technical perspective. In the second main part included that it was examined from a technical perspective from the chemical structure, and manufacturing process of PBS fiber. In the third main part included that four different chemical synthesis methods of PBS fiber, and melt spinning production method were examined technically. In the last main part included that the general properties, and applications of PBS fiber were examined from a technical perspective. The importance of PBS fiber that the PBS fiber is a type of PES fiber, which has a semi-aromatic, and crystalline chemical structure. It has a mean and significant Mw ranges of 60,000g/mol to 100,000g/mol[1-4]. It can be produced by the chemical reaction of succinic acid, and 1,4-butanediol, using various catalysts, and the melt spinning method[1,2,4-45]. PBS fiber can also be produced by adding co-polymers[6,46,27-37]. The elastic modulus (E), tensile strength, maximum breaking percentage elongation (%), thermal stability, MFR, PDI, crystallinity, Tg, Tc, and Tm values can be changed thanks to the added co-polymer in the production of PBS fiber[4,28,33,35,40,42,43,46,47]. The PBS fiber has lower energy consumption in terms of both temperature (°C), and pressure (Pa) values compared to PET fiber. Thus, it is sustainable and ecological fiber type[1-9,13-36,48,50]. It is a more rigid, and brittle type of PES fiber compared to PET fiber[2,11,46]. The PBS fiber has sustainability, ecological production, environmental friendliness, high biodegradability, high chemical resistance, high mechanical properties, high thermal stability, and easy processability thanks to its easy melting compared to other aliphatic chemical structured polyester (PES) fiber types[1,4,5,7,11,15,16,22,25,28,31,35-37,46,49]. The application areas of PBS fiber are sheets, filaments, non-woven fabrics, textiles, flame retardant (FR), UV protection, antimicrobial, clothing, filters, laminates, molded foam products, injection molding, packaging, antenna, electronics, agriculture, forestry, construction products. orthopedic implant, bone treatment and biomedical fields[1,2,4,28-32,36-44,46,47,51,52]. The producing countries of PBS fiber in the world textile industry; They are China, Japan, Korea, Germany, and the USA[1]. The main purpose of this theoretical review study was to explain that the various physical, chemical, thermal, mechanical, and biological changes of PBS fiber with both theoretical, and experimental studies. It was aimed to technically lead various experimental studies to be conducted in the future in this scientific field thanks to sustainable, more harmless and more ecological approaches.
1.1 The Historical Background, and Global Commercial Market of PBS Fiber
PBS fiber is a type of polyester (PET) with a semi-aliphatic, and semi aromatic structure, and is biodegradable. It has excellent mechanical properties[1]. The synthesis of PBS was synthesized by Carothers in 1931, but its molecular weight (Mw) was less than 5,000, resulting in a weak and brittle mechanical property behavior. As a solution to this situation, the Mw, or Mn has been increased since the 1990s[1,2,9,10,13].
The first serious study was carried out in 1993 so that the first semi-commercial PBS fiber was produced by the Japanese Showa High Polymer Company under the trade name Bionelle, with an annual capacity of 3,000 tons, by using diisocyanate chemicals, increasing the chain length, synthesizing them by the polycondensation method and applying the melt spinning method, respectively. The first produced commercial PBS fiber had a Mn in the range of 20,000g/mol to 200,000g/mol, and a mean Mw of 40,000g/mol to 1,000,000g/mol[1,46]. The average Mn and Mw of PBS fiber generally ranges from 60,000g/mol to 100,000g/mol[3,4]. Japanese Mitsubishi Chemicals Company produced the first commercial PBS fiber with an ecological approach and biodegradable structure under the trade name GS Pla in April 2003 with a capacity of 3,000 tons[1,46]. In addition, in 2006, thanks to the consultancy of Tsinghua University, commercial PBS fiber was produced by the direct melt spinning method by the Chinese Hexing Chemical Company with an annual production capacity of 3,000 tons in Beijing, China. Moreover, by the end of 2009, the annual production capacity was increased to 10,000 tons[1,46]. In October 2007, commercial PBS fiber was produced by the Chinese Xinfu Pharmactical Company, synthesized by the one-step polycondensation method and melt spinning method, in Hangzhou, China, with the consultancy of the Chinese Academy of Science and the Institute of Physics, and the annual production capacity was increased to 20,000 tons[1,46]. In general, PBS fiber production was produced commercially, and successfully with low production capacities for the first time, with the collaboration the scientific and technical studies of Chinese scientists and industrialists. Moreover, other countries with high quality in science such as Japan, Korea, Germany, and the USA have shown interest in this issue both scientifically and commercially during the time so that they have become its producer countries (especially are Germany and the USA) with more serious annual production capacities. The major commercial producers of PBS fiber, and its co-polymer fibers in the world were presented in Table 1[1].
Table 1. The Major Commercial Producers of PBS Fiber, and Its Co-polymer Fibers in the World (1)
Manufacturer, and Country |
Product |
Monomers |
Production Capacity (Ton/ annual) |
Hexing Chemical, China |
PBS and its copolymers |
Succinic acid, butanediol, branched alkanedicarboxylic acid |
10.000 |
Xinfu Pharmaceutical, China |
PBS, PBSA |
Succinic acid, adipic acid, butanediol |
3.000 |
Jinfa Tech, China |
PBSA |
Succinic acid, adipic acid, butanediol |
300 |
BASF, Germany |
Ecoflex |
Adipic acid, terephthalic acid, butanediol |
14.000 |
Eastmann, USA |
East Bio |
Adipic acid, terephthalic acid, butanediol |
15.000 |
Showa, Japan |
Bionolle |
Succinic acid, adipic acid, butanediol |
5.000 |
Mitsubishi Chemical, Japan |
GS Pla |
Succinic acid, lactic acid, butanediol |
3.000 |
Mitsubishi Gas Chemicals, Japan |
Iupec |
Succinate, carbonate, butanediol |
|
Nippon Shokubai, Japan |
Lunare |
Succinic acid, adipic acid, ethylene glycol |
|
Ube, Japan |
ETERNACOLL 3050 |
Decanedicarboxylic acid, ethylene glycol |
|
Ire Chemical, Korea |
Enpol |
Succinic acid, adipic acid, terephthalic acid, butanediol |
|
SK Chemicals, Korea |
Skygreen |
Succinic acid, adipic acid, butanediol, ethylene glycol |
|
1.2 The Chemical Structure, and Manufacturing Process of PBS Fiber
PBS is a renewable, and petroleum-based polymer[1-55]. PBS can be produced as a result of the polycondensation of the chemical components succinic acid, and 1,4-butanediol[1-14,22,28-31,33,34,41,45,51]. PBS fiber is a type of thermoplastic PES fiber with a semi-aliphatic, semi-aromatic and semi-crystalline chemical structure[5,10,46,48-50]. The characteristic bond of PBS fiber is ester bonds. Moreover, it has a chemical structure with polar carbonyl and non-polar groups[24,49]. While the 1,4-butanediol segment in the chemical structure of the PBS fiber provides softness, easy processability, and high maximum percent elongation at break (%) values, the succinic acid segment provides rigidity, high modulus of elasticity, high maximum breaking force, high tensile strength, and high impact strength values[13,48]. Added to chemical synthesis as a co-polymer in the production of PBS fiber include carbon and its compounds, carbon black, carbon nanotube, Mg(OH)2, cellulose nanocrystal, CaCO3, ammonium polyphosphate, diethyl aluminum phosphinate, butylene di-methyldi-glycolate, ethylene. succinate, hexamethylene succinate, shell flour, dibutyl adipate, adipic acid, or chemicals such as fumaric acid (in the concentration range of 5% to 20% by volume) reduce its rheological, thermal, and mechanical values[4,8,9,12,14,16,25,26,28-31,33,35,38-40,42]. Basalt, jute, lignin, organo montmorillonite, epoxidized linseed oil, epoxidized soybean oil, ZnO2, and PMMA increase its mechanical properties[4,29,41,43,45,47]. PBS fiber production is a type of PES fiber that has a higher cost due to the higher cost of its chemical components compared to PA, PE, PS, and PET[46]. The density of PBS fiber ranges from 1.22g/cm3 to 1.23g/cm3[1,25]. The glass transition temperature (Tg) of PBS fiber ranges from -44.3°C to -27°C. (average is -37°C). The crystallization temperature (Tc) of PBS fiber varies between 68 °C and 89°C. (average is 80°C)[1,3,4,13,15-31,33,34,38,41,42,46,47,49]. The melting temperature (Tm) of PBS fiber is specifically 115°C for an average Mn value of 70,000g/mol[2]. The degree of crystallinity (%) of PBS fiber varies between 35% and 45%[1,13,46,49]. The melt flow rate values for PBS fiber are between 5g to 34g mass for 2kg to 2.16kg mass over 10 minutes and at temperatures from 140°C to 190°C[1,10,22,30,34,40,46,47,49]. It’s the polydispersity index ratio (Mw/Mn) ranges from 1.47 to 2.3[6,22,52]. Its relative viscosity values (dL/g) range from 0.75 to 1.53. Its corresponding average Mn ranges from 21,000g/mol to 63,000g/mol[2].
In addition, it melts in a shorter time thanks to its lower Tm compared to most other PES fiber types, and can be combined with other polymers (co-polymer chemical structures such as lignin, palm oil fiber, flax, jute, tapioca). It can be processed more easily by mixing natural, or synthetic polymers such as starch, PDA, PFN, PLA, PLLA, hydroxyl, and vinyl[6,27-37,46]. Moreover, it is more rigid and brittle than most PES fiber types[2,11,46]. There is a degradable ester group in the chemical structure of PBS. Thus, it can transform into low Mw polymers when exposed to water. In an aqueous environment, as temperature, and time increase, the degradation (hydrolysis) rate also increases. Thus, its tensile strength, bending strength, and impact strength values also decrease[21,27,30,34,44,46]. It has a low crystallization rate, and low melt viscosity. For this reason, partial difficulties may be encountered in fiber production. As a solution to this situation, processability, and maximum percent elongation at break (%) values can be increased by adding some additional chemical structures (co-polymer) in the extrusion process. The main reason for this situation is that as the shear rate decreases, the melt viscosity increases[46]. The closed chemical formula of PBS fiber is -[O–(CH2)m-O-CO-(CH2)n-CO]n[20,46,53]. While m value is 4, n value is 2. Moreover, it has 2 different crystal polymorphs (monoclinic crystal lattice structures), α, and β. The β crystal polymorph of PBS is observed under high pressure[20,44,46]. For the α-monoclinic lattice structure of PBS fiber has a=0.523nm, b=0.908nm, c=1.079nm, β=124°, for the β-monoclinic lattice structure of PBS fiber has a=0.584nm, b=0.832nm, c=1.186nm, b=132°[34]. The chemical structure of PBS fiber was presented in Figure 1[1]. When the chemical structure of PBS fiber was examined, it was determined from Figure 1 that it had methylene, and carbonyl groups and had also O atoms, too[1].
|
Figure 1. The Chemical Structure of PBS Fiber[1].
1.3 The Chemical Synthesis Methods, and Production Method for Chips, and Fiber of PBS
PBS chips are synthesized by 4 different synthesis methods. These are transesterification polymerization, direct polymerization of succinic acid, and butanediol, condensation-polymerization followed by chain-extension, and lipase-catalyzed synthesis, respectively[1,46].
1.3.1 The Transesterification Polymerization of PBS Chips
Transesterification polymerization is the most widely used synthesis method in the synthesis of PBS chips, and their production by melt spinning, as in polyethylene terephthalate (PET), which is the most commonly produced PES type today. In this method: polymerization starting with stoichiometric amounts of succinic acid, and 1,4-butanediol in concentrations ranging from 10% to 15% by volume.
It can be synthesized by using a catalyst such as ammonium phosphate, ammonium salt, phosphonium salt, and organic peroxide, dicumyl peroxide, but especially tetra-n-butyl-titanate, or tetraisopropyl titanate, and is produced by the melt spinning method[1-45]. Moreover; this method has a two-step polymerization like transesterification, and polycondensation[1-45].
Before the reaction, the chemical reactor is filled with nitrogen (N2) gas, a noble gas with chemical inert properties (THF, TPP, HCl, H2SO4, ethanol, chloroform, dichloromethane, diethylether, or dichlorobenzene chemicals can also be used in liquid form in the concentration range of 1.5% to 2% by volume) at room temperature, or approximately 40°C to remove air, and moisture and prevent oxidation for transesterification. They are dried at a temperature range of 100°C, (average is 80°C) with stirring at a stirring speed ranging from n=8 rpm to 1000rpm (average is n=150rpm)[1-45,48,49]. Then, under a temperature ranging from 140°C to 190°C (average is 30K/min for the heating rate), the chemical reaction system is carried out in a chemical reactor made of stainless steel for a period of 1 hour to 24 hours (average is 2 hours), and under 2mbar pressure at mixing speed ranging from n=150rpm to 1000rpm (average is n=150rpm) to initiate transesterification, and melted[1-45,48]. Afterwards; most of the methanol, and water produced by transesterification are heated at a higher temperature than by polycondensation, that is, in the temperature range of 200°C to especially 230°C (but depending on the time, the temperature is reduced to 50°C at the end of the process), in the range of 4 hours to 9 hours (but only when Mw, and Mn is desired). may occur within 24 hours). α-hydroxy acids, Sb2O3, GeO2, or a mixture of metals or, in particular, Ti, and its compounds, which are the most effective catalyst compounds (examples are titanium (IV) isopropoxide, titanium tetraisopropoxide, titanium (IV) isobutoxide, or titanium (IV) n-butoxide) but using a catalyst chemical such as Ti(OBu)4, or Ti(OC4H9)4, which is the most effective Ti compound chemical Nitrogen (N2), a noble gas with chemical inert properties, is formed in the reaction under vacuum in 2 hours, in the pressure ranges of 40Pa to 80Pa, or in a pressure range that is gradually reduced from 2bar to 0.7mbar depending on the time (average of 2 hours) so that it is distilled to remove 1,4-butanediol and polymerize oligomers[1-11,13-36,48,50]. The main reason for oligomer formation is succinic acid[6]. In another source, the polycondensation process can produce average Mn ranges 5,000g/mol to 12,000g/mol for over 50 hours. The COOH/OH molar ratio is r=0.95[5]. The succinic acid/1,4-butanediol percent molar mass ratio is generally 95%/5%[5,50]. Moreover, when ammonium, and/or phosphonium chemical compounds (salt chemical structures) are used as catalysts, the average Mn increases, but the polycondensation process takes a very long time, ranging from 24 hours to 80 hours[5,7]. Additionally, after 24 hours, PBS chips with an average Mn of 30,000g/mol are present[5]. Through transesterification polymerization, PBS chips with an average Mn of 59,500g/mol, and an average Mw of 104,100g/mol can be synthesized[1]. The chemical reaction equation of transesterification synthesis of PBS chips was presented in Figure 2[1]. When the chemical reaction equation of the transesterification synthesis of PBS chips was examined, it was determined from Figure 2 that the ester (PBS) structure (main product), and methyl alcohol (by-product) emerged as a result of the chemical reaction of carboxylic acid, and dialcohol structure[1].
|
Figure 2. The Chemical Reaction Equation of Transesterification Synthesis of PBS Chips[1].
1.3.2 The Direct Polymerization of Succinic Acid, and 1,4-Butanediol of PBS Chips
The average Mw of PBS fiber produced by direct polymerization of PBS fiber with succinic acid, and 1,4-butanediol is 100,000g/mol[2].
1.3.2.1 The Direct Melt Polymerization of Succinic Acid, and 1,4-Butanediol of PBS Chips
It is a direct polymerization process of PBS starting from dicarboxyl acid, and alkyl diol without a chain extension step[1]. Direct melt polymerization of PBS can be carried out in two ways. The first is the completion of polymerization in succinic acid, and 1,4-butanediol solution. This polymerization is called "direct melt polymerization". The second is the completion of the solution of the raw materials, that is, the solution polymerization[1-45]. Direct melt polymerization is simple and can produce high average Mn, or Mw PBS chips. Thus, it is considered the most widely used production process for the production of PBS, considering the economic considerations, and its potential applications in food contact packaging. In direct melt polymerization, PBS chips are prepared using a two-step process. In the first step; the esterification chemical reaction, it takes place at a temperature ranging from 150°C to 200°C under atmospheric pressure or under low vacuum pressure. In the second step, polycondensation takes place under high vacuum pressure and at a higher temperature, between 220°C and 240°C thanks to the process called deglycolization. Both esterification and polycondensation must be carried out in a nitrogen (N2) gas atmosphere to prevent oxidation. For the production of PBS chips with high average Mw, or Mn the following points should be taken into consideration: (1) All of the water formed during the esterification stage should be removed from the environment. The amount of water formed should reach a value in the range of 85% to 90%. Otherwise, the polycondensation time will be too long and PBS chips with the desired high average Mw, or Mn will not be produced. Moreover, by-products such as tetrahydrofuran must be removed from the environment. (2) Esterification must be carried out at the appropriate temperature. The esterification reaction rate improves as the temperature is increased, but causes more byproducts to be formed. The main by-product is tetrahydrofuran, formed due to dehydration of 1,4-butanediol. Tsinghua University, and Hexing Chemical Company have developed a unique esterification technique that suppresses the formation of the byproduct tetrahydrofuran. The final tetrahydrofuran content in PBS was less than 0.1% thanks to this technique. (3) A sufficiently high vacuum pressure must be applied to remove water, and 1,4-butanediol particles formed during the polycondensation process.Technical Institute, Department of Physics, and Chemistry, Chinese Academy of Sciences, has developed a technique called "cold trap" technique in the flow line of PBS chips production, enabling the removal of water, and by-products formed during the polycondensation stage. In this technique, it is possible to condense, and remove water or by-products with small average Mw, or Mn formed under a stable vacuum pressure, and a temperature of -120°C during the polycondensation process. Thus, PBS chips with an average Mw, or Mn of more than 200,000g/mol can be produced. (4) Catalysts with high chemical reactivity, and hydrolysis resistance should be selected to increase the average Mw, or Mn of PBS chips, and shorten the reaction time. A thermal, and chemical stabilizer should be also incorporated into the chemical reactor to complete the polycondensation in order to reduce degradation throughout the polycondensation process,too. Many scientists have carried out experimental studies on the type of catalyst chemical, and catalysis reaction to be used in the polycondensation process for the production of PBS chips. In conclusion, they determined the use of catalyst chemicals such as Sc(CF3SO)3, Sc(NTf2)3, titanium tetrabutoxide, titanium(IV) isopropoxide, phosphate acid, and titanium, and tin compounds as appropriate. The most active catalyst chemicals to be used in the polycondensation process for the production of PBS chips are antimony, tin, and titanium compounds. Among these catalyst chemicals, titanium tetrabutoxide (or (tetrabutyl titanate), or titanium (IV) isopropoxide chemicals are often used. In one of the experimental results, PBS chips with an average up to 59,000g/mol as Mn were produced by using tetra-n-butoxy titanate as a catalyst chemical, and polyphosphoric acid chemicals as a chemical and thermal stabilizer. Tetra-n-butoxy germanium is an effective chemical catalyst for the production of high Mw of PBS chips. By using titanium (IV) isopropoxide catalyst chemical, PBS chips with average of 77,000g/mol as their Mn, and 141,000g/mol as their Mw were produced[1-45].
1.3.2.2 The Solution Polymerization of Succinic Acid, and 1,4-Butanediol of PBS Chips
Raw materials are dissolved in a solvent such as xylene or decahydronaphthalene. This method is a modified method of direct melt polymerization, and is called solution polymerization. It ensures the removal of small molecular water or by-products formed during the polycondensation process, in the esterification reaction and the condensation reaction in the solvent. Thus, it can prevent the oxidation of PBS chips that will occur during the PBS chips polycondensation, and is carried out at a lower temperature. Moreover, the disadvantages of this method are its relatively higher cost, and the fact that the polycondensation process takes longer. PBS chips with a 4-Å molecular size were produced during the polycondensation process, using SnCl2, a catalyst chemical, and dimethyl benzene, a new water lifter chemical to produce PBS chips with an average Mn of 24,800g/mol at the end of the 70-hour polycondensation process. In another experimental study; a two-phase solvent, water lifter (decalin), and a polymer mixture of succinic acid, and 1,4-butanediol in the molten system enabled the production of PBS chips as a single-stage polycondensation process with the distannoxane catalyst chemical at atmospheric pressure, and under azeotropic conditions. PBS chips with an average of 117,000 g/mol as their Mn and 277,000g/mol as their Mw were produced in the presence of 0.001 mol% 1-chloro-3-hydroxy-1,1,3,3-tetrabutyldistannoxane at the end of the 72-hour polycondensation process.
In another experimental study, PBS chips were produced by solution polymerization using SnCl2, Ti(OiPr)4, Ti(OBu)4, Sn(Oct)2, Zn(Ac)2, and p-toluenesulfonate (p-TS) catalyst chemicals. When SnCl2 catalyst chemical was used among these catalysts, PBS chips with an average Mw of 79,000g/mol were produced as a result of the polycondensation process lasting 12 hours[1]. The chemical equations of esterification and polycondensation in the direct melt method of PBS chips was presented in Figure 3[1]. When the chemical equations of esterification, and polycondensation in the direct melting method of PBS chips were examined, it was determined from Figure 3 that the polycondensation reaction occurred reversibly, and gradually, releasing water first. Subsequently, it was determined that the ester (PBS) structure (main product) emerged depending on the degree of polymerization (m and n) of the carboxylic acid, and dialcohol monomers used[1].
|
Figure 3. The Chemical Equations of Esterification and Polycondensation in the Direct Melt Method of PBS Chips[1].
1.3.3 The PBS Condensation Polymerization, and Subsequent Chain Extension of PBS Chips
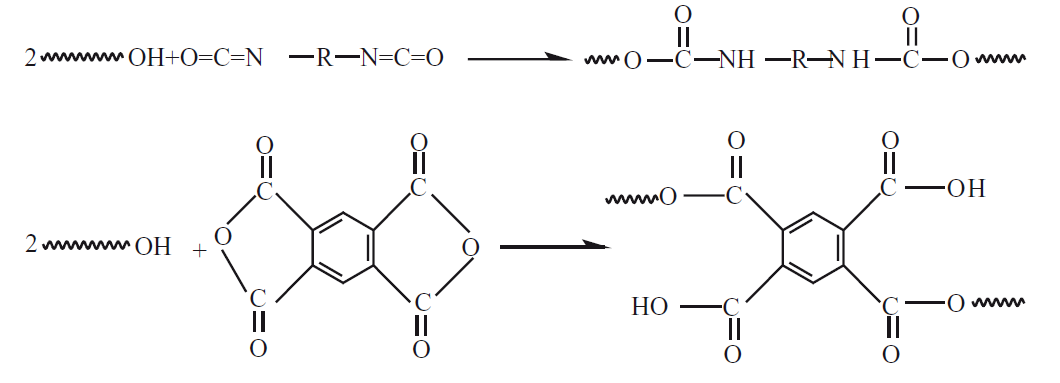
The chain-extension method is another method of producing high average Mw PBS chips. An additional chain-extension is achieved compared to direct melt condensation polymerization. Thus, the average Mw of PBS chips can be increased. The chain-extender chemical molecule can chemically react with two different functional terminal groups of PBS chips, namely –OH, and -COOH. Ideally, a chain-extender chemical molecule will bond two PBS chips with their chemical chains. In this process, polycondensation conditions are not as critical as well as direct melt polycondensation. On the other hand, the disadvantage of this method is that the biodegradability, and biosafety of the produced PBS chips will decrease due to the inclusion of chain-extending chemical molecules. When the chain-extender chemical molecules used in the production of PBS chips with this method were investigated, it was determined that diisocyanate, oxazoline, anhydride, and epoxy compounds were used. While diisocyanate, and anhydride, -OH-terminated PBS chips are suitable for chain-extension, oxazoline, and epoxy -COOH-terminated PBS chips are suitable for chain-extension. While producing high Mw of PBS fiber (Bionolle), the Japanese Showa Denko Company used the chemical hexamethylene diisocyanate as a chain-extender chemical molecule in the polycondensation reaction of 1,4-butanediol with aliphatic dicarboxylic succinic acid. PBS chips as HO-PBS-OH can be produced with a relatively higher Mw thanks to the hexamethylene diisocyanate chain-extender chemical molecule.
The produced PBS chips were reported to have an approximate average of 300,000g/mol as their Mw, and an average of 200,000g/mol as their Mn[1]. Organometallic components can easily degrade at high chemical reaction temperatures (especially: 190°C and above). In this case, PBS changes the color of the chips, and causes them to have a lower average Mw of PBS chips[46]. Some chain-extender chemical molecules used in the production of PBS chips that PBS chips have effects on chemical, thermal, and mechanical properties. Hexamethylene increases its crystallinity rate, and degradation rate. Ethylene decreases its crystallinity rate, and increases its surface tension, and degradation rate. 1,6-hexanediamine increases its wettability. Thiodiglycolate decreases its average Mw, and increases its hydrophobicity, and thermal stability. Thiodiethylene succinate increases its crystallinity rate, and degradation rate. Terephthalate increases its tensile strength, and thermal stability[46]. Organo montmorillonite increases its elastic modulus (E), tensile strength, thermal stability, and hydrophobicity[28]. Carbon black, Mg(OH)2, cellulose nanocrystal, and CaCO3 decrease the elasticity modulus (E), tensile strength, and thermal stability[28]. It decreases Tg, Tc, and tensile strength, but increases the MFR value, elasticity modulus (E), crystallinity rate, and Tm, as the PA 11 ratio (%) increases (up to 20% concentration by volume)[4]. In PLLA/PBS (99/1 to 90/10) structures; the maximum percent elongation at break (%), and crystallinity rate increase as the PBS ratio (%) increases. Moreover, if it is in PLLA/PBS (99/1) structure; the highest elasticity modulus (E), and tensile strength values are determined[33]. It increases the elastic modulus (E), and tensile strength values, but decreases the maximum percent elongation at break (%), as the lignin ratio (%) increases[35]. It increases the modulus of elasticity (E) and tensile strength values, but reduces the maximum percent elongation at break (%) and impact strength, as the diethyl phosphinate ratio (%) increases (up to 20% concentration by volume)[40]. In PLA/PBS mixed structures: it increases the viscosity (Pa.s), maximum percent elongation at break (%), and impact strength, but decreases the modulus of elasticity (E), tensile strength, crystallinity rate, Tg, and Tm, as the PBS ratio (%) increases[42]. In PBS/jute mixed structures: it increases the modulus of elasticity (E), and tensile strength, but reduces the maximum percent elongation at break (%), as the jute ratio (%) increases (up to 20% concentration by volume)[43]. It increases the crystallinity rate, modulus of elasticity (E), and tensile strength values, but decreases Tg, Tc, water vapor permeability, oxygen permeability, maximum percent elongation at break (%), shear strength, and impact strength, as the ZnO2 ratio (%) increases (up to 10% concentration by volume). Moreover, it did not change the Tm[47]. It increases the elasticity modulus (E), tensile strength, bending strength, and impact strength values, as the basalt ratio (%) increases (up to 10% concentration by volume)[45]. The chemical reaction equation of the chain-extender chemical molecule hexamethylene diisocyanate of PBS chips was presented in Figure 4[1]. When the chemical reaction equation of hexamethylene diisocyanate, the chain-extending chemical molecule of PBS chips was examined, it was determined from Figure 4 that the polycondensation reaction occurred non-reversibly, and gradually, so that the N=C double bond was broken and was turned into a N-C single bond. After it was determined that it transformed into aromatic (ring) structures. In addition, it was determined that the carbonyl functional end groups were formed in the first stage, and carbonyl groups were transformed into the chemical structure form containing hydroxyl groups, and O atoms in the second stage.
Moreover, it was determined that the molecular weight (g/mol) of the chemical structure increased[1].
|
Figure 4. The Chemical Reaction Equation of the Chain-Extender Chemical Molecule Hexamethylene Diisocyanate of PBS Chips[1].
Bizoxazoline is a new type of chain-extending chemical molecule and can bind low-molecular PBS chemical molecules during production processes such as chemical synthesis by polycondensation of PBS fiber, chemical reaction extrusion, and fiber spinning. Chain extension of PBS with bisoxazoline is an innovative method that enables gelation, and does not result in the production of low average Mw of PBS fiber, and is suitable for industrial applications. Its average Mn increased from 111,700g/mol to 189,500g/mol thanks to bisoxazoline used as a chain-extender[1]. The chemical reaction equation of the bisoxazoline chain-extender chemical molecule of PBS chips was presented in Figure 5[1]. When the chemical reaction equation of the bisoxazoline chain extender chemical molecule of PBS chips was examined, it was determined from Figure 5 that the polycondensation reaction occurred non-reversibly, and gradually, so that it was determined that aromatic (ring) structures were broken and were transformed into long-chain aliphatic structured chemical form. It was also determined that carbonyl, and hydroxy functional end groups were present in the chemical structure, too[1].
|
Figure 5. The Chemical Reaction Equation of the Bisoxazoline Chain-extender Chemical Molecule of PBS Chips[1].
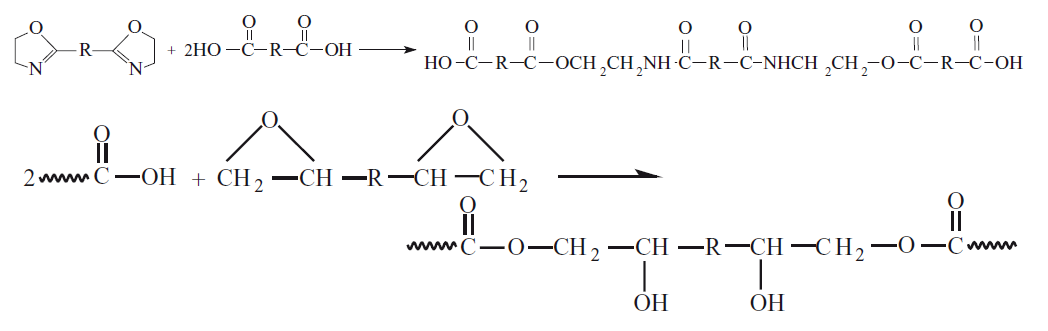
1.3.4 The Lipase-Catalyzed Synthesis of PBS Chips
Lipase-catalyzed synthesis of PBS chips is a recently developed method with no residual metal salts under milder conditions. In this method, chemical reaction mixtures of diethyl succinate, and 1,4-butanediol catalyzed the chemical synthesis of lipase B, monophasic PBS chips based on Candida rugosa, or Candida Antarctica enzymes[1,10,23]. The purpose of using the lipase catalyst is to reduce energy consumption in the production process of PBS chips, but at the same time the production rate decreases[6,23]. Enzymatic degradation has two stages. It is first the absorption of lipase and then the hydrolysis of PBS chips[23]. The chemical C₆H₅CH₃ is also widely used as an environmental conditioning chemical[6]. In determining the average Mn of PBS chips, the reaction temperature played an important role. After polymerization, the average Mn was 2,000g/mol, 4,000g/mol, 8,000g/mol, and 7,000g/mol, respectively, in diphenyl ether for 24 hours, and the temperature values were 60°C, 70°C, respectively. PBS chips were synthesized at temperature conditions of 80°C, and 90°C. Due to the low Mn, after between 5 and 10 hours of polymerization, the polymer chain length stopped, and precipitation occurred due to the limitation in the growth of the crystalline regions in the polymer chain. By increasing the polymerization temperature from 80°C to 95°C, the monophasic reaction mixture was maintained after 21 hours. Moreover, PBS chips production with an average the Mw of 38,000g/mol, and a PDI (Mw/Mn) of 1.39 was achieved[1]. In an experimental study with lipase: as a result of various concentrations of lipase enzyme added to PBS chips (from 100/0 to 30/70); crystallinity rate (%), from 45% to 32%, Tg from -34°C to -49°C, Tc from 87°C to 17°C, the Tm decreased from 112°C to 40°C, and the PDI index decreased from 1.74 to 1.71[23]. In an experimental study with lipase, for the production of PBS chips, the average Mn was 36,000 g/mol in 95°C for 6 hours, while the average Mn was 73,000g/mol in 95 °C for 36 hours[6]. A pH value of 7 is recommended for enzyme applications[13].
PBS chips synthesized by lipase-catalysis have a narrower PDI. Metal chemicals are no longer present in the chemical structure of PBS chips thanks to this method. This method enables the production of low average Mw of PBS chips. Moreover, it can cause problems in the thermal process. The solution to the problem in this thermal process is the use of immobilized lipase-chemical as a catalyst. It can also be applied for ring-opening polymerization of cyclic monomers, succinic anhydride, and synthesis of tetrahydrofuran for synthesized PHB chips. PBS chips with an average of Mn of 12,400g/mol were produced with 49% efficiency thanks to the triflate catalyzed by aluminum at 100°C for 48 hours[1]. The lipase-catalyzed synthesis process of PBS chips was presented in Figure 6[6]. When the lipase-catalyzed synthesis process of PBS chips was examined, it was determined from Figure 6 that the polycondensation reaction occurred non-reversibly, and gradually, so that it was determined that aromatic (ring) structure was broken and was transformed into long-chain aliphatic structured chemical form. It was also determined that the presence of carbonyl functional end groups and O atoms were detected as a result of polymerization thanks to the lipase catalyst chemical addition. Moreover, it was determined that the molecular weight (g/mol) of the chemical structure decreased[6].
|
Figure 6. The Lipase-catalyzed Synthesis Process of PBS Chips[6].
1.3.5 The Production Process of PBS Fiber by Melt Spinning Method
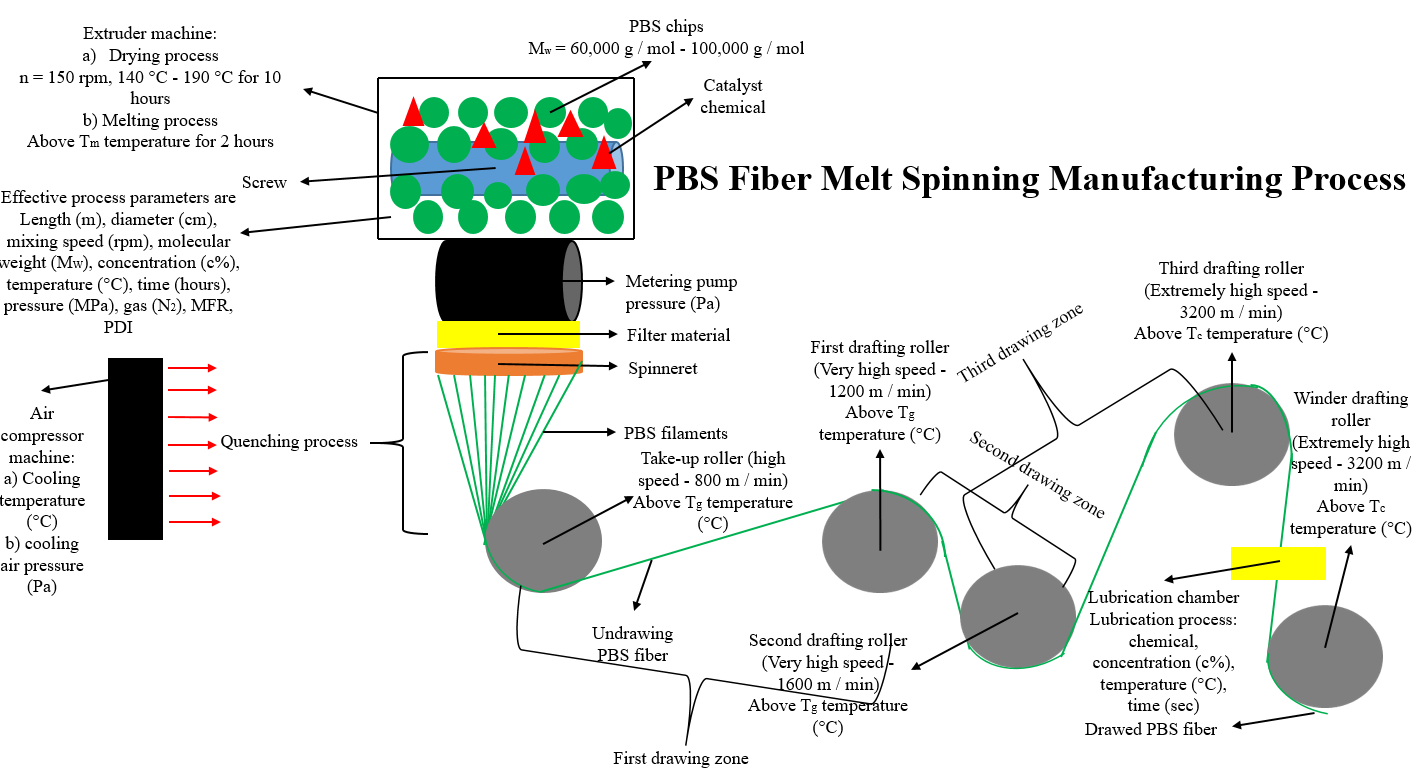
The average draft ratio in the production of PBS fiber by the melt spinning method is between 1 and 4 ratios. The drafting rate is the ratio of the output roller rotation speed to the feed roller rotation speed. (Vout/Vsupply). In the extrusion process, temperature (°C) values are generally gradually increased in the extruder[3,11,15-22]. The production process of PBS fiber by melt spinning method was presented in Figure 7.
When the production of PBS fiber by the direct production method of the melt spinning method was examined, it was determined that PBS chips could be produced by drying, mixing, melting, qunching, drawing, lubrication, and winding processes, respectively.
|
Figure 7. The Production Process of PBS Fiber by Melt Spinning Method.
In an experimental study for the production of PBS fiber, they were dried in an extruder at 60°C for 12 hours. Afterwards, the extrusion temperatures (°C) are 210°C, 240°C, and 270°C, respectively. The spinneret hole diameter was 0.5mm. MFR value was 0.63g/min. The solidification temperature was room temperature. The solidification distance was 330cm=3.3m. Afterwards, yarn pulling was performed. Drafting speeds were 0.5km/min, 1 km/min, 1.5km/min, and 2km/min, respectively. According to experimental results, for 20mm inter-jaw distance, 20mm/min drafting speed, and 10 repeated tests, as the drafting speed increased, the tensile strength increased in tensile strength test. Additionally, necking behavior was not observed for fibers such as PES types at the initial solidification distance of 150cm=1.5m. This means that depending on the drawing speed, there was no transition from the amorphous structure to the crystalline structure in the internal structure of the PBS fiber. Shortly, the internal structure orientation of the PBS yarn to be produced was not fully observed. Because the filaments that will enable the production of molten, and viscous PBS yarn have maintained their high adhesion (stickiness) to each other.
In this case, it prevented the filaments from being drawn properly, that was, the production of PBS yarn. Moreover, after the solidification distance of 150cm=1.5m, the crystallinity behavior increased non-linearly, and suddenly depending on the gravity speed. In addition, as the gravity speed increased, the non-linear, and sudden crystallization behavior increased with a very serious acceleration. The tensile strength values of PBS fiber with an extrusion temperature of 210°C are while it was 130MPa for 1 km/min draft speed, it increased to 190MPa for 2km/min draft speed, respectively. The tensile strength values of PBS fiber with an extrusion temperature of 240°C are while it was 55MPa for 1km/min drafting speed, it increased to 150MPa for 2km/min drafting speed, respectively. The elasticity modulus values of PBS fiber with an extrusion temperature of 210°C are while it was 0.82GPa for 1km/min drafting speed, it increased to 1.4GPa for 2km/min drafting speed, respectively. The elasticity modulus values of PBS fiber with an extrusion temperature of 240°C are while it was 0.28GPa for 1km/min drafting speed, it increased to 0.88GPa for 2km/min drafting speed, respectively. Moreover, its Tm temperature was 115°C[15]. In an experimental study for the production of PBS/PLA fiber, samples were produced in 10 increments from 100/0 to 0/100. They were dried in the extruder at 65°C for 24 hours. Afterwards, extrusion temperatures (°C) are 165°C, 180°C, and 190°C, respectively. Extruder (L/D) ratio was 20/1. The spinneret hole diameter was 0.5mm. Extruder length was 40 mm. Extruder diameter was 20mm. The speed value was 30rpm. Afterwards, yarn pulling was performed. The Tc of pure PBS was 48.1°C. The Tm of pure PBS was 94.9°C. The Tc of pure PLA was 108.2°C. The Tm of pure PLA was 170.5°C. It was observed that PBS had higher crystallization compared to PLA. Moreover, it was observed that PBS increased the Tc temperature value of PLA. While the crystallinity rate of pure PBS was 43.3%, the crystallinity rate of pure PLA was 20%, respectively. (The heating rate is 10K/min). While the elasticity modulus (E) value of pure PBS at room temperature was 285MPa, when the PLA ratio was 20%, the elasticity modulus (E) value of PBS/PLA (80/20) at room temperature increased to 540MPa. The elastic modulus (E) value of PBS/PLA (60/40) at room temperature increased to 946MPa. The elasticity modulus (E) value of PBS/PLA (40/60) at room temperature increased to 1405MPa. Shortly, as the PLA ratio increased in PBS/PLA blended structures, the elasticity modulus (E) increased. Moreover, it was concluded that PBS, and PLA fibers did not fully mix with each other. In SEM images up to PBS/PLA (60/40), it was determined that PLA was well dispersed in PBS. It was determined that PLA had a fibrillar structure between PBS/PLA (60/40) and PBS/PLA (40/60)[16]. In an experimental study for the production of PLA/PBS fiber, samples from 100/0 to 88/12 were produced in 3 increments. They were dried in the extruder at 80°C for 12 hours. Afterwards, they were mixed, and dried in the extruder ranged 160°C to 175°C, respectively, and at the frequency of the screw speed was 5.9Hz. Moreover, they continued to be dried in the extruder at 80°C for 48 hours before the fiber spinning process. Afterwards, yarn was drawn with a spinneret hole diameter of 0.3mm, and a drafting speed of 0.8km/min. The drafting ratio was between 3.6 and 4. The yarn counts (dtex) of the PBS/PLA blended yarns produced ranged from 114.96 dtex to 120dtex. The Tm of pure PLA was 167.13°C. As PBS was added to PLA, the Tm of PLA/PBS blended yarns was 169.18°C. It was observed that PBS had higher crystallization compared to PLA. The Tg of pure PLA, and PLA/PBS blended yarns remained the same at 63°C.
The reason for this was the fact that they had a single Tg and that their amorphous phases could mix homogeneously with each other. Moreover, it was determined that as the PBS ratio (%) increased in PLA/PBS blended yarns, the degree of fiber orientation of the produced yarns increased. Thus, the crystallinity ratio (%) increased. As the PBS ratio increased in PLA/PBS blended yarns, the elasticity modulus (E), tensile strength values, and shrinkage (%) behavior ratio decreased, but the maximum percent elongation at break (%) value, and moisture recovery (%) behavior ratio increased. As the PBS ratio increased for elastic modulus (E) values, it decreased from 80.43cN/dtex to 44.00cN/dtex. As the PBS ratio increased for tensile strength values, it decreased from 3.77cN/dtex to 2.28cN/dtex. As the PBS ratio increased for maximum percent elongation at break (%) values, it increased from 18% to 30.36%. Shrinkage (%) behavior decreased from 32% to 10% as the PBS ratio increased. Moisture recovery (%) behavior increased from 0.035% to 0.055% as the PBS ratio increased[17]. In an experimental study for the production of PLA/PBS fiber, samples were produced in 10 increments from 90/10 to 50/50. They were dried in the extruder at 80°C for 12 hours. Afterwards, they were mixed, and dried in extrusion temperatures (°C) ranged 140°C to 165°C, respectively, and speed (n was 80rpm). Moreover, they continued to be dried in the oven at 80°C for 12 hours before the fiber spinning process. In the extrusion process, temperature values between 190°C and 210°C, respectively, and speed values (n was 8 rpm) were carried out. Afterwards, yarn pulling was performed. The Tm of pure PBS was 113°C. The Tm of pure PLA was 171°C. As PBS was added to PLA (from PLA/PBS-100/0 to PLA/PBS-50/50), the Tc of PLA/PBS decreased from 92.8°C to 89°C. As PBS was added to PLA (from PLA/PBS-100/0 to PLA/PBS-50/50), the Tm of PLA/PBS decreased from 171.5°C to 169.3°C. As PBS was added to PLA (from PLA/PBS-100/0 to PLA/PBS-50/50), the crystallinity ratio of PLA in the PLA/PBS crystallinity ratio increased from 40.7% to 50.8%. As PBS was added to PLA (from PLA/PBS-100/0 to PLA/PBS-50/50), the crystallinity ratio of PBS in the PLA/PBS crystallinity ratio increased from 49.3% to 63.8%. Because it was observed that PBS had higher crystallization compared to PLA. The elasticity modulus (E) value of pure PLA was 2.1GPa. The elasticity modulus (E) value of pure PBS was 0.5GPa. As PBS was added to PLA (from PLA/PBS-100/0 to PLA/PBS-50/50), the PLA/PBS elasticity modulus (E) value decreased from 2.1GPa to 1.1GPa. The tensile strength of pure PLA was 49MPa. The elasticity modulus (E) value of pure PBS was 20MPa. As PBS was added to PLA (from PLA/PBS-100/0 to PLA/PBS-50/50), the PLA/PBS tensile strength value decreased from 49MPa to 28MPa. The highest tensile strength value was determined as 51MPa in the sample with PLA/PBS 90/10 ratio[18]. In an experimental study for the production of PBS/nano-sized wood pulp fiber, samples from 100/0 to 85/15 were produced in 5 increments. They were dried in a vacuum oven at 60 °C for 8 hours under a pressure ranged of 5mbar to 20mbar. Moreover, they continued to be dried in the extruder at 70rpm, 70°C for 24 hours before the fiber spinning process. In the extrusion process, temperature values between 130°C, and 160°C, respectively, and speed values (n was 70rpm) were carried out. Afterwards, yarn pulling was performed. The Tc of pure PBS was 75°C. The Tm of pure PBS was 114°C. As nano-sized wood pulp was added to PBS (from PBS/nano-sized wood pulp-100/0 to PBS/nano-sized wood pulp-85/15), the Tc of PBS/nano-sized wood pulp increased from 75.0°C to 77.8°C.
As nano-sized wood pulp was added to PBS (from PBS/nano-sized wood pulp-100/0 to PBS/nano-sized wood pulp-50/50), the Tm of PBS/nano-sized wood pulp increased to 114.0°C from 116.0°C. The nano-sized wood pulp ratio (%) provided maximum values of 5% for Tc, and 10% for Tm. The elasticity modulus (E) value of pure PBS was 0.28GPa. As nano-sized wood pulp was added to PBS, the elasticity modulus (E) value of PBS/nano-sized wood pulp increased from 0.28GPa to 0.46GPa (from PBS/nano-sized wood pulp-100/0 to PBS/nano-sized wood pulp-85/15). The tensile strength of pure PBS was 31MPa. As nano-sized wood pulp was added to PBS (from PBS/nano-sized wood pulp-100/0 to PBS/nano-sized wood pulp-85/15), the tensile strength value of PBS/nano-sized wood pulp decreased from 31MPa to 28MPa. The maximum percent elongation (%) value of pure PBS was 30. As nano-sized wood pulp was added to the PBS (from PBS/nano-sized wood pulp-100/0 to PBS/nano-sized wood pulp-85/15), the maximum percent breaking elongation (%) value of PBS/nano-sized wood pulp decreased from 30 to 12%[21]. In an experimental study for PBS fiber production, the 1-4-bitanediol/succinic acid molar mass ratio (%) was 7/3. It was dried in the extruder at 110°C for 24 hours. Afterwards, the extrusion temperature (°C) was 225°C. The number of spinneret holes was 36. The spinneret hole diameter was 0.32mm. Afterwards, yarn pulling was performed. Drafting speeds are 2.0km/min, 2.5km/min, 3.0km/min, 3.5km/min, and 4km/min, respectively. According to experimental results, as the drafting speed increased from 2.0 to 4.0; crystalline domain orientation (%) from 82.9% to 87%, crystallinity ratio from 38.4% to 60.1%, tensile strength value from 236.4MPa to 418.2MPa, and standard deviation (%CV) value increased from 9.4% to 9.9%. for tensile strength. Moreover, the value of maximum percent elongation at break (%) decreased from 60.5% to 24.1%, and the standard deviation (%CV) value for maximum percent elongation at break (%) decreased from 3.8% to 0.9%. The main reason for this situation was that as the drafting ratio increased, the orientation, and crystallization ratios in the internal structure of the PBS yarn increased[20]. In an experimental study conducted for the production of PBS fiber, it was subjected to a pre-treatment process with lipase enzyme. Thereafter, extrusion temperatures (°C) ranged from 105°C to 135°C. Monofilament diameter was 250μm. Afterwards, yarn pulling was performed. Extruder screw speed was 8.7m/min. Drafting speed was 25.9m/min. Drafting ratio was 3. According to experimental results, the crystallinity rate (%) was determined as 43%, the elasticity modulus (E) was 0.44GPa, the tensile strength value was 250MPa, and the maximum percent elongation at break (%) value was 29% for drafting ratio 3[3]. In an experimental study for the production of PBS chips, stabilizer chemical as well as pure PBS fiber was added, and a large number of samples were produced. They were dried in an extruder at 80°C for 5 hours. Afterwards, were mixed, and melted at 190°C, 200°C, and 210°C, respectively, and the number of revolutions is (n=100rpm). Extruder length was 400 mm. Extruder diameter was 16 mm. Extruder L/D ratio was 25:1. Its average Mw was 173,000g/mol, and its average Mn was 75,000g/mol. The MFR value was 1.2dl/g. The PDI index was 2.3. According to experimental results, as the stabilizer chemical concentration increased (from 0.1% to 0.5%), the average Mw, and PDI index increased, while the average Mn value decreased. Moreover, the crystallinity (%) increased from 46% to 58%, and Tc increases from 75.1°C to 86.6°C, but the Tm remained the same at 114°C[21]. In an experimental study for the production of LDPE/PBS fiber, samples were produced in 20 increments from 100/0 to 40/60.
They were dried in the extruder at 75°C for 4 hours. Afterwards, they were mixed, and melted at the screw speed in the extruder at a temperature range of 140°C to 160°C, and a frequency of 5.9Hz. Extruder length was 924mm. Extruder diameter was 22mm. Extruder L/D ratio was 42:1. MFR value of LDPE chips was 2.17g/10min. MFR value of PBS chips was 25g/10min. In LDPE/PBS blended structures, as the PBS ratio (%) increased, the viscosity, and maximum percent elongation at break (%) values decreased. The MFR value of LDPE increased as the extrusion rate increased. While the LDPE/PBS MFR value increased at PBS ratio values between 20% and 40%, the extrusion ratio was maximum at 40%. The PBS rate increased slightly again to 60%. After the PBS ratio was 60%, the MFR value decreased significantly. Thus, a cooling effect was observed and the extrusion rate increased. Moreover, LDPE/PBS mixed chips structures turned into a more fluid, and easy processabiliy as in yarn form, but the LDPE ratio began to slowly melt[22].
1.4 The General Properties, and Applications of PBS Fiber
The applications of PBS fiber are sheets, filaments, non-woven fabrics, textiles, FR, UV protection, antimicrobial, clothing, filters, laminates, molded foam products, injection molded, packaging, antenna, electronics, agriculture, forestry, construction products, orthopedic implant, bone treatment and biomedical fields[1,2,4,24-27,36-44,46,47,51,53-55]. The biodegradation time of PBS structured films is approximately 6 months[35]. The tensile strength of PBS fiber ranges from 20MPa to 34MPa (average is 22MPa)[1,3,4,15-22,28,29,35,46]. The tensile strength values of PBS fiber are almost the same as the tensile strength values of LDPE, HDPE, PE, and PP fibers[1,41,43,45,47]. The flexural modulus (modulus of elasticity (E)) of PBS fiber is 2.5GPa[29]. The bending strength of PBS fiber is 32MPa[29]. The tensile modulus (modulus of elasticity (E)) of PBS fiber varies between values of 30MPa and 707MPa. (average is 500 MPa)[3,4,15-28,35,46]. The maximum percent breaking elongation (%) value of PBS fiber varies between 126% and 560% (average is 126%)[1,29,35]. The impact strength of PBS fiber is 300J/m[1]. PBS fiber compared to other aliphatic chemical structured PES fiber types that it has high biodegradability, high chemical resistance, high mechanical properties, high thermal stability, and easy processability thanks to its easy melting[4,5,7,11,13,16,22,25,28,35-42,46,49]. When produced at higher Mw of PBS fiber, its mechanical properties such as elasticity modulus (E), and tensile strength, and its thermal properties such as Tg, Tc, and Tm increase[3,15-22,46]. In an experimental study included that PBS fiber with an average of 40,000g/mol to 50,000g/mol as their Mn had its highest tensile strength, but at an average of 60,000g/mol as its Mn, it had the highest degradation (hydrolysis) rate was found. Moreover, as its average Mn increased up to the average value of 40,000g/mol as its Mn, the crystallinity ratio (%), Tg, crystallization (Tc), and melting (Tm) temperatures increased[2]. In addition, PBS fiber is widely used, especially in food applications, thanks to its high biodegradability in an easy and short time. It is a special type of PES fiber with a compostable certificate[46]. Biodegradable, and biocomposite materials can be produced with palm oil fibre, flax, and jute. Moreover, tensile, and impact strengths are increasing so that they can be produced the production of sustainable, recyclable, and ecological materials.
The main reason for this situation is the high cross-linking capacity of polymers with each other. It can also be combined with urethane[4,46]. In the UV blocking application of PBS fiber, it was determined that it blocked UV light better and transmitted visible light better, the elasticity modulus (E), and tensile strength increased and the maximum percent elongation at break (%) decreased thanks to the lignin chemical[35]. In the environmentally friendly resistive switching memory device application of PBS fiber, it was determined that it provided high photoluminescence, high on/off current ratio, low threshold voltage, and extremely high memory stabilite thanks to PFN, and AgNW chemicals[36]. In the bio-based intumescent FR application of PBS fiber, it was determined that the pHRR, THR, and mass loss were decreased thanks to phytic acid, and guanosine chemicals[37]. In the bone tissue healing in the tissue skeleton application of PBS fiber, it was determined that it reduced cellular adhesion at the tissue-bone interface, and differentiate gene expression thanks to the diglycolate chemical[54]. In the FR application of PBS fiber, it was determined that the pHRR, and THR were decreased thanks to ammonium polyphosphate and aluminum AlPi chemicals[38]. In the bio-based FR application of PBS fiber, it was determined that the pHRR, and THR were decreased, and charring behavior was developed.thanks to lignin chemical[39]. In the FR application of PBS fiber, it was determined that the thermal stability, and pHRR value of the PBS fiber were decreased and its elasticity modulus (E), and tensile strength were increased, and adhesion behavior was also improved thanks to Aluminum Diethylphosphinate chemical[40]. In the biocomposite structures with various lignocellulosic fibers applications of PBS fiber, it was determined that the the elasticity modulus (E), and tensile strength values were increased, and water absorbency was decreased and was improved the surface morphology thanks to the high chemical adhesion of sisal and curaua fibers. It was also determined that the water absorbency increased thanks to coconut, and sugarcane bagasse fibers, too[41]. In the biocomposite structures with PLA fiber applications of PBS fiber, it was determined that the elastic modulus (E), and tensile strength values were increased, but impact strength was decreased as PLA fiber add-on increased[42]. In the biocomposite structures with jute fiber applications of PBS fiber, it was determined that the bending modulus, bending strength, elasticity modulus (E), tensile strength values were increased, and the maximum percent elongation at break value was decreased as jute fiber add-on increased[43]. In the food packaging application of PBS fiber, it was determined that the elastic modulus (E), and tensile strength values were increased, but tear strength was decreased as ZnO chemical add-on increased. It was improved the antibacterial activity as ZnO chemical add-on increased. It was increased the degree of crystallinity, and were decreased Tg, and Tc temperatures as ZnO chemical add-on increased. Tm was maintained its temperature as ZnO chemical add-on increased[47]. In the food packaging application of PBS fiber, it was determined that the gas permeability was increased in ethanol, and water environments, but the gas permeability was decreased in acetic acid, and isooctane environments[55]. In the bio-based antenna application of PBS fiber, it was determined that the values had appropriate frequency, and resonance band gap thanks to the silica aerogel nanoparticles[51]. In the antenna application of PBS fiber, it was determined that the values had appropriate frequency, and resonance band gap thanks to its chemical structure[44].
In the biocomposite structures with basalt fiber applications of PBS fiber, it was determined that the elasticity modulus (E), tensile strength, flexural modulus, flexural strength, impact strength, thermal stability values were increased, and was also improved the surface morphology thanks to the basalt fibers, too[45].
2 CONCLUSIONS
2.1 The General Technical Specifications of PBS Fiber
2.1.1 The Historical Background, and Global Commercial Market of PBS Fiber
PBS polymer chips were first synthesized chemically in 1931, but they were weak, and brittle due to their extremely low average of 5000 as their Mw. As a solution to this negative situation, commercial forms with their average Mw ranging from 60,000g/mol to 100,000g/mol have been produced since the 1990s, by the joint, and technical work of Chinese and Japanese scientists and their engineers. Today they are commercially available to the entire global textile industry. It is actively sold.
2.1.2 The Chemical Structure, and Manufacturing Process of PBS Fiber
PBS is a renewable and petroleum-based polymer. PBS chips can be produced as a result of the polycondensation of the chemical components succinic acid and 1,4-butanediol. PBS fiber is a type of thermoplastic PES fiber with a semi-aliphatic, semi-aromatic, and semi-crystalline chemical structure. The characteristic bond of PBS fiber is ester bonds. Moreover, it has a chemical structure with polar carbonyl and non-polar groups. While the 1,4-butanediol segment in the chemical structure of the PBS fiber provides softness, easy processability, and high maximum percent elongation at break (%) values, the succinic acid segment provides rigidity, high modulus of elasticity, high maximum breaking force, high tensile strength, and high impact strength values. The closed chemical formula of PBS fiber is -[O–(CH2)m-O-CO-(CH2)n-CO]n. While m value is 4, n value is 2. Moreover, it has 2 different crystal polymorphs (monoclinic crystal lattice structures), α, and β.
For the α-monoclinic lattice structure dimensional values a=0.523nm, b=0.908nm, c=1.079nm, β=124°, for the β-monoclinic lattice structure dimensional values a=0.584 nm, b=0.832nm, c=1.186nm, b=132°. The degree of crystallinity (%) of PBS fiber varies between 35%, and 45%. MFR values for PBS fiber are 5g to 34g mass for 2kg to 2.16kg mass over 10 minutes and at temperatures from 140°C to 190°C. The PDI index ratio (Mw/Mn) ranges from 1.47 to 2.3. The average Mw of PBS fiber ranges from 60,000g/mol to 100,000g/mol.
2.1.3 The Thermal Structure of PBS Fiber
The density of PBS fiber ranges from 1.23g/cm3 to 1.26g/cm3 (average is 1.23g/cm3). The Tg of PBS fiber ranges from -44.3°C to -27°C (average is -37°C). The Tc of PBS fiber ranges from 68 °C to 89 °C (average is 80 °C). The Tm of PBS fiber ranges from 109 °C to 132°C (average is 115°C).
2.1.4 The Mechanical Structure of PBS Fiber
The biodegradation time of PBS-structured films is approximately 6 months. The tensile strength of PBS fiber ranges from 20MPa to 34MPa (average is 22MPa). The tensile strength values of PBS fiber are almost the same as the tensile strength values of LDPE, HDPE, PE, and PP fibers.
The flexural modulus (modulus of elasticity (E)) of PBS fiber is 2.5GPa. The bending strength of PBS fiber is 32MPa. The tensile modulus (modulus of elasticity (E)) of PBS fiber varies between values of 30MPa and 707MPa. (average is 500MPa). The maximum percent breaking elongation (%) value of PBS fiber varies between 126% and 560% (average is 126%). The impact strength (J/m) of PBS fiber is 300. PBS fiber compared to other aliphatic chemical structured PES fiber types that it has high biodegradability, high chemical resistance, high mechanical properties, high thermal stability, and easy processability thanks to its easy melting.
2.1.5 The General Applications of PBS Fiber
The applications of PBS fiber are sheets, filaments, nonwovens, textiles, FR, UV protection, antimicrobial, garments, filters, laminates, molded foam products, injection molded, packaging, antenna, electronics, agriculture, forestry, construction products, orthopedic implant, bone treatment, and biomedical fields.
2.2 The Effective Factors in the Production of PBS Polymer Chips
The effective factors in the polymer chips production (chemical synthesis) process, which is the first step in producing PBS fiber in its yarn form: They depend on the type of co-polymer and catalyst chemicals added, their chemical structure, functional terminal end groups, their concentration by volume (%), average Mw, or Mn, MFR value, PDI (Mw/Mn) value, pressure, temperature, time, noble (N2) gas or liquid or aqueous medium and mixing speed (rpm) values. Because, PBS fiber is affected by the granule size of the polymer chips, the number of granules, the ratio of amorphous, and crystalline regions in the chemical internal structure, and the orientation of the amorphous region.
These effective factors are biodegradability rate, Tg, Tc, Tm, crystallinity rate, enthalpy value, vibrations, breaks or formations in its chemical bond structures, elasticity modulus (E), maximum breaking force, maximum percent breaking elongation (%), tensile strength, bending strength, impact strength, shear strength, creep, strength, fatigue strengths, shrinkage behavior, water vapor permeability, oxygen permeability and fiber morphology, when the PBS fiber is drawn in yarn form.
The effective factors in the production process of polymer in yarn form (melt spinning method), which is the second and last stage for the production of PBS fiber in yarn form: They are extruder (L/D) ratio, number of extruder screws, extruder length, extruder diameter, temperature inside the extruder before drying, and then melting, time, pressure, and mixing speed, enthalpy, average Mn, or Mw, MFR, and PDI values, and noble gas type (N2), dosing pressure, number of nozzle holes, nozzle cross-section, solidification (cooling) distance, temperature, air flow rate (volume flow rate), rotation of godets speeds (draft speeds), temperature values, draft rates, lubricating chemical type, and concentration, and winding speed.
2.3 The Optimized and Recommended Production Process to Produce PBS Fiber in Yarn
2.3.1 Drying, and Mixing Process
PBS chips can be dried, and mixed in the ratio of succinic acid (concentration by volume 90%), and 1-4-butanediol (concentration by volume 10%), with the catalyst chemical in the ratio of Ti(OBu)4 (concentration by volume 5%) in the extruder at a mixing speed of 150rpm, 2mbar under pressure in N2 gas, at an average temperature of 80°C for 12 hours.
Purpose is to removal of air, and moisture, and to prevent of oxidation of PBS structure.
2.3.2 Extrusion Process
Afterwards, it must be melted under 2mbar pressure in N2 gas, 150rpm stirring speed, an average temperature of 230°C for 4 hours again in the extruder. Purpose is to produce the melt, and viscous PBS structure.
2.3.3 Solidification Process
The solidification distance should be between 2 m and 3 meters. The solidification temperature should be at room temperature. Tthe solidification speed should be carried out 0.55 m/second in air. Purpose is to produce solid phase multifilaments that will form the yarn.
2.3.4 Drawing, Lubrication and Winding Processes
Feed roller speed is 800m/min (+10°C above Tg temperature), (first pre-draft) roller speed is 1200m/min (+10°C above Tg temperature), (second pre-draft) roller speed is 1600m/min (+10°C above Tg temperature), third (main draft) roller speed is 3200m/min. min (+10°C above Tc temperature) and the total draw value should be 4. Purpose is to produce the yarn in a single main filament (yarn) structure with multifilaments in the solid phase. The lubricating chemical should be any type of oil (2% volume concentration) that can wet the PBS. Purpose is to improve the wettability of the PBS yarn and the filaments that make up the yarn to stay together and make it easier to wind it on the bobbin. The winding cylinder speed should be 3200m/min. Purpose is to wind the final PBS yarn onto the empty bobbin. It is then packaged and made ready to be sold.
In the production of PBS fiber, PBS polymer (in the form of chips) is used, and various co-polymer chemicals based on natural polymers can be used. When its production as yarn form certain production process parameters are changed such as concentration, temperature, time, pressure, drafting speed and drafting rate, etc. Thus, the physical, mechanical, chemical, thermal, and biological properties of PBS fiber should be determined in terms of technical results by applying various experimental studies in the future, thanks to sustainable, more harmless and more ecological approaches.
Acknowledgments
Not applicable.
Conflicts of Interest
The author declares that he has no known competing financial interests, or personal relationships that could have appeared to influence the work reported in this paper.
Data Avalability
No additional data are available.
Copyright and Permissions
Copyright © 2025 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Author Contribution
Turşucular ÖF contributed to all conceptual developments of the study, made technical inferences, and compiled the general study.
Abbreviation List
FR, flame retardant
PES, polyester
PBS, Poly Butylene Succinate
PBSA, Poly(Butylene Succinate-co-butylene Adipate)
PET, Poly Ethylene Terephthalate
Tg, glass transition temperature
Tc, crystallization temperature
Tm, melting temperature
References
[1] Xu J, Guo BH. Microbial succinic acid, its polymer poly (butylene succinate), and applications. Plastics from bacteria: Natural functions and applications, Springer Berlin, Heidelberg, 2010; 347-388.[DOI]
[2] Jin T, Zhou M, Hu S et al. Effect of molecular weight on the properties of poly (butylene succinate). Chinese J Polym Sci, 2014; 32: 953-960.[DOI]
[3] Nakano S, Salmawy AEL, Nakamura T et al. Properties and Enzymatic Degradability of Melt-Spun Fibers of Poly (butylene succinate) and its Various Dertivatives. Sen'i Gakkaishi, 2002; 58: 209-215.[DOI]
[4] Nanni A, Messori M. Thermo-mechanical properties and creep modelling of wine lees filled Polyamide 11 (PA11) and Polybutylene succinate (PBS) bio-composites. Compos Sci Technol, 2020; 188: 107974.[DOI]
[5] Jacquel N, Freyermouth F, Fenouillot F et al. Synthesis and properties of poly (butylene succinate): Efficiency of different transesterification catalysts. J Polym Sci Pol Chem , 2011; 49: 5301-5312.[DOI]
[6] Ren L, Wang Y, Ge J et al. Enzymatic Synthesis of High‐Molecular‐Weight Poly (butylene succinate) and its Copolymers. Macromol Chem Phys, 2015; 216: 636-640.[DOI]
[7] Bautista M, de Ilarduya AM, Alla A et al. Cationic poly (butylene succinate) copolyesters. Eur Polym J, 2016; 75: 329-342.[DOI]
[8] Kim DJ, Kim WS, Lee DH et al. Modification of poly (butylene succinate) with peroxide: crosslinking, physical and thermal properties, and biodegradation. J Appl Polym Sci, 2001; 81: 1115-1124.[DOI]
[9] Ihn KJ, Yoo ES, Im SS. Structure and morphology of poly (tetramethylene succinate) crystals. Macromolecules, 1995; 28: 2460-2464.[DOI]
[10] Di Lorenzo ML, Androsch R, Righetti MC. Low-temperature crystallization of poly (butylene succinate). Eur Polym J, 2017; 94: 384-391.[DOI]
[11] Yao SF, Chen XT, Ye HM. Investigation of structure and crystallization behavior of poly (butylene succinate) by fourier transform infrared spectroscopy. J Phys Chem B, 2017; 121: 9476-9485.[DOI]
[12] Xu Y, Xu J, Guo B et al. Crystallization kinetics and morphology of biodegradable poly (butylene succinate‐co‐propylene succinate) s. J Polym Sci Pol Phys, 2007; 45: 420-428.[DOI]
[13] Xu J, Guo BH. Poly (butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol J, 2010; 5: 1149-1163.[DOI]
[14] Nikolic MS, Poleti D, Djonlagic J. Synthesis and characterization of biodegradable poly (butylene succinate-co-butylene fumarate) s. Eur Polym J, 2003; 39: 2183-2192.[DOI]
[15] Shi XQ, Takasaki M, Ito H et al. Structural Development and Properties of Melt Spun Poly (butylene succinate) and Poly (butylene terephthalate-co-succinate-co-adipate) Biodegradable Fibers. Int Polym Proc, 2006; 21: 64-69.[DOI]
[16] Tan L, Qu J. Characterization of poly (butylene succinate)/poly (lactic acid) blends with in-situ sub-micron fibers and intercalation structure manufacturing by volumetric pulsating elongation flow. Polym Test, 2019; 77: 105889.[DOI]
[17] Hassan EAM, Elarabi SE, Wei Y et al. Biodegradable poly (lactic acid)/poly (butylene succinate) fibers with high elongation for health care products. Text Res J, 2018; 88: 1735-1744.[DOI]
[18] Jompang L, Thumsorn S, On JW et al. Poly (lactic acid) and poly (butylene succinate) blend fibers prepared by melt spinning technique. Energy Procedia, 2013; 34: 493-499.[DOI]
[19] Platnieks O, Sereda A, Gaidukovs S et al. Adding value to poly (butylene succinate) and nanofibrillated cellulose-based sustainable nanocomposites by applying masterbatch process. Ind Crop Prod, 2021; 169: 113669.[DOI]
[20] Luo S, Li F, Yu J et al. Aggregation structure development and mechanical properties of biodegradable poly (butylene succinate‐co‐terephthalate) fibers. J Appl Polym Sci, 2012; 125: 2426-2432.[DOI]
[21] Georgousopoulou IN, Vouyiouka S, Dole P et al. Thermo-mechanical degradation and stabilization of poly (butylene succinate). Polym Degrad Stabil, 2016; 128: 182-192.[DOI]
[22] Yang J, Liang JZ, Li FJ. Melt strength and extensional viscosity of low-density polyethylene and poly (butylene succinate) blends using a melt-spinning technique. J Macromol Sci B, 2012; 51: 1715-1730.[DOI]
[23] Bi S, Tan B, Soule JL et al. Enzymatic degradation of poly (butylene succinate-co-hexamethylene succinate). Polym Degrad Stabil, 2018; 155: 9-14.[DOI]
[24] Chrissafis K, Paraskevopoulos KM, Bikiaris DN. Thermal degradation mechanism of poly (ethylene succinate) and poly (butylene succinate): Comparative study. Thermochim Acta, 2005; 435: 142-150.[DOI]
[25] Gan Z, Abe H, Kurokawa H et al. Solid-state microstructures, thermal properties, and crystallization of biodegradable poly (butylene succinate)(PBS) and its copolyesters. Biomacromolecules, 2001; 2: 605-613.[DOI]
[26] Gigli M, Lotti N, Gazzano M et al. Novel eco-friendly random copolyesters of poly (butylene succinate) containing ether-linkages. React Funct Polym, 2012; 72: 303-310.[DOI]
[27] Kanemura C, Nakashima S, Hotta A. Mechanical properties and chemical structures of biodegradable poly (butylene-succinate) for material reprocessing. Polym Degrad Stabil, 2012; 97: 972-980.[DOI]
[28] Karakehya N. Comparison of the effects of various reinforcements on the mechanical, morphological, thermal and surface properties of poly (butylene succinate). Int J Adhes Adhes, 2021; 110: 102949.[DOI]
[29] Li H, Chang J, Cao A et al. In vitro evaluation of biodegradable poly (butylene succinate) as a novel biomaterial. Macromol Biosci, 2005; 5: 433-440.[DOI]
[30] Chinnasamy S, Ramachandran M, Soundharaj S. Mechanical and Thermal Properties of poly butylene succinate (PBS) Nano Composites. REST J Emerg Trends Modell Manuf, 2022; 8: 58-67.[DOI]
[31] Liminana P, Garcia-Sanoguera D, Quiles-Carrillo L et al. Development and characterization of environmentally friendly composites from poly (butylene succinate)(PBS) and almond shell flour with different compatibilizers. Compos Part B-Eng, 2018; 144: 153-162.[DOI]
[32] Rizzarelli P, Carroccio S. Thermo-oxidative processes in biodegradable poly (butylene succinate). Polym Degrad Stabil, 2009; 94: 1825-1838.[DOI]
[33] Shibata M, Inoue Y, Miyoshi M. Mechanical properties, morphology, and crystallization behavior of blends of poly (L-lactide) with poly (butylene succinate-co-L-lactate) and poly (butylene succinate). Polym, 2006; 47: 3557-3564.[DOI]
[34] Wang X, Zhou J, Li L. Multiple melting behavior of poly (butylene succinate). Eur Polym J, 2007; 43: 3163-3170.[DOI]
[35] Zhang Y, Zhou S, Fang X et al. Renewable and flexible UV-blocking film from poly (butylene succinate) and lignin. Eur Polym J, 2019; 116: 265-274.[DOI]
[36] Hsieh HC, Wu N, Chuang TH et al. Eco-friendly polyfluorene/poly (butylene succinate) blends and their electronic device application on biodegradable substrates. ACS Appl Polym Mater, 2020; 2: 2469-2476.[DOI]
[37] Chen S, Wu F, Hu Y et al. A fully bio-based intumescent flame retardant for poly (butylene succinate). Mater Chem Phys, 2020; 252: 123222.[DOI]
[38] Dumazert L, Rasselet D, Pang B, et al. Thermal stability and fire reaction of poly (butylene succinate) nanocomposites using natural clays and FR additives. Polym Advan Technol, 2018; 29: 69-83.[DOI]
[39] Ferry L, Dorez G, Taguet A et al. Chemical modification of lignin by phosphorus molecules to improve the fire behavior of polybutylene succinate. Polym Degrad Stabil, 2015; 113: 135-143.[DOI]
[40] Wang Y, Jiang D, Wen X et al. Investigating the effect of aluminum diethylphosphinate on thermal stability, flame retardancy, and mechanical properties of poly (butylene succinate). Front Mater, 2021; 8: 737749.[DOI]
[41] Frollini E, Bartolucci N, Sisti L et al. Poly (butylene succinate) reinforced with different lignocellulosic fibers. Ind Crop Prod, 2013; 45: 160-169.[DOI]
[42] Hassan E, Wei Y, Jiao H, et al. Dynamic mechanical properties and thermal stability of poly (lactic acid) and poly (butylene succinate) blends composites. J Fiber Bioeng Inform, 2013; 6: 85-94.[DOI]
[43] Liu L, Yu J, Cheng L et al. Mechanical properties of poly (butylene succinate)(PBS) biocomposites reinforced with surface modified jute fibre. Compos Part A-Appl S, 2009; 40: 669-674.[DOI]
[44] Tai HJ. Dielectric spectroscopy of poly (butylene succinate) films. Polym, 2007; 48: 4558-4566.[DOI]
[45] Zhang Y, Yu C, Chu PK et al. Mechanical and thermal properties of basalt fiber reinforced poly (butylene succinate) composites. Mater Chem Phys, 2012; 133: 845-849.[DOI]
[46] Rafiqah SA, Khalina A, Harmaen AS et al. A review on properties and application of bio-based poly (butylene succinate). Polym, 2021; 13: 1436.[DOI]
[47] Petchwattana N, Covavisaruch S, Wibooranawong S et al. Antimicrobial food packaging prepared from poly (butylene succinate) and zinc oxide. Measurement, 2016; 93: 442-448.[DOI]
[48] Huang CL, Jiao L, Zhang JJ et al. Poly (butylene succinate)-poly (ethylene glycol) multiblock copolymer: Synthesis, structure, properties and shape memory performance. Polymer Chemistry, 2012; 3: 800-808.[DOI]
[49] Frollini E, Bartolucci N, Sisti L, et al. Biocomposites based on poly (butylene succinate) and curaua: Mechanical and morphological properties. Polym Test, 2015; 45: 168-173.[DOI]
[50] Oishi A, Zhang M, Nakayama K et al. Synthesis of poly (butylene succinate) and poly (ethylene succinate) including diglycollate moiety. Polym J, 2006; 38: 710-715.[DOI]
[51] Habib Ullah M, Mahadi WNL, Latef TA. Aerogel poly (butylene succinate) biomaterial substrate for RF and microwave applications. Scientific reports, 2015; 5: 12868.[DOI]
[52] Ye HM, Chen XT, Liu P et al. Preparation of poly (butylene succinate) crystals with exceptionally high melting point and crystallinity from its inclusion complex. Macromolecules, 2017; 50: 5425-5433.[DOI]
[53] Ishikawa H, Omori S, Ohki Y. Dielectric properties of polybutylene succinate and polybutylene succinate adipate[C]/2007 IEEE International Conference on Solid Dielectrics. Winchester, UK, 8-13 July 2007: 635-638.[DOI]
[54] Cristofaro F, Gigli M, Bloise N et al. Influence of the nanofiber chemistry and orientation of biodegradable poly (butylene succinate)-based scaffolds on osteoblast differentiation for bone tissue regeneration. Nanoscale, 2018; 10: 8689-8703.[DOI]
[55] Siracusa V, Lotti N, Munari A et al. Poly (butylene succinate) and poly (butylene succinate-co-adipate) for food packaging applications: Gas barrier properties after stressed treatments. Polym Degrad Stabil, 2015; 119: 35-45.[DOI]

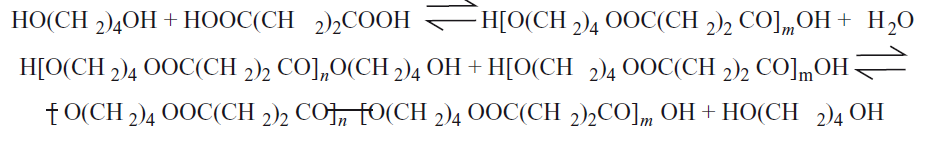



 Copyright ©
Copyright ©