Polymeric Hydrogels for Energy Devices
1Synthetic Organic Chemistry Research Laboratory, PG and Research Department of Chemistry, Muthurangam Government Arts College (Affiliated to Thiruvalluvar University), Vellore, India
*Correspondence to: Selvam Guhanathan, PhD, Professor, Synthetic Organic Chemistry Research Laboratory, PG and Research Department of Chemistry, Muthurangam Government Arts College (Affiliated to Thiruvalluvar University), Otteri Road, Vellore, 632002, India; Email: profguhanathan12@gmail.com
Abstract
Innovative energy storage devices, viz., supercapacitors and rechargeable batteries are of great awareness owing to their comprehensive claims in flexible electronics and implantations. In simple electronic devices, frequently facing problems like bending, collision, steeping, piercing, and even encountering shearing, fire, water, etc. Hence, a high mandate is needed for the constructive and consistency of energy storage devices. Hydrogels are hydrophilic three-dimensional polymers filled with water which are highly noticeable electrolyte materials due to their high-water permeability and smoothness, which enable them to fulfil the vital necessities for flexible energy storage devices. Hydrogels with intriguing physicochemical assets for justifiable energy storing for water production. The proposal of novel hydrogel electrolytes for supercapacitors and batteries with electrochemical performances for imminent growth. The biopolymeric hydrogel electrolytes can be chosen as substitute materials for supercapacitors due to their decent ionic conductivity, no fluid seepage, and no toxicity. Focus of the review aims to highlight the hydrogels materials combined into batteries and deliver thrilling tailorable architectures for multifunctionalities with amicable applications.
Keywords: hydrogels, energy storage materials, electrolyte, electrode, supercapacitors
1 INTRODUCTION
Energy is a vital requirement for the pecuniary development of any nation. There is a robust mandate for the expansion of resources that can exhibit the probable growth for power sources. Here, a gifted variety of polymeric materials, especially, hydrogels, has been deliberated as an energy storing material. The hydrogels are beneficial, since they have possessed an exclusive combination of carbon-based conductors and conservative polymers. The art of preparation of hydrogels, which includes both conservative synthesis and the novel approaches towards the application of hydrogels as energy storage materials. At last, the imminent opportunities and scenarios in the growth of hydrogels as high-class energy storage devices are described in this review.
Hydrogels are “3D” dimensional polymeric networks in which there are a lot of water loving groups that swell quickly by absorbing large amounts of water which in turn responses to their external environment. A network is made by copolymerizing hydrophilic monomers with crosslinkers. Cross-linking nature makes them into insolubility in water which enable for excellent mechanical strength and physical integrity. Due to these reasons, hydrogels have received significant attention in recent years for biomedical applications, such as drug delivery, tissue engineering, and so on[1].
Hydrogels are a class of cross-linked polymer analogues to biological tissues because they hold water or biological fluids without dissolving. Owing to their biocompatibility, soft properties, and high-water content, hydrogels may be considered alternative materials for biomedical applications[2]. In 1960, researchers introduced the cross-linked polyhydroxyethylmethacrylate and its water-holding capacity. These kinds of materials were found to be potential candidates for human tissue applications. Hydrogels are in fact the first material prepared for utilisation, gelation time, and degradation rate of chitosan-based injectable hydrogels for in situ applications[3]. The complete classifications of hydrogels have also been exploited pictorially in Table 1.
Table 1. Classifications of Hydrogels
Nature |
Categorization |
Origin |
Natural Synthetic |
Polymer composition |
Homopolymeric hydrogels Copolymeric hydrogels Interpenetrating polymer network |
Configuration |
Amorphous Crystalline |
Water content |
Low swelling High swelling Medium swelling Superabsorbent |
Porosity |
Non-porous Micro-porous Macro-porous Super porous |
Types of cross-linking |
Chemically cross-linked Physically cross-linked (Ionic interaction / Hydrogen bonds / Hydrophobic interactions) |
Physical appearance |
Matrix Film Microsphere |
Network electrical charge |
Non-ionic (neutral) Ionic (anionic / cationic) Amphoteric electrolyte Zwitterionic electrolyte |
2 HYDROGEL PROPERTIES AND ELECTROLYTES
2.1 Overview of Hydrogel Properties
Hydrogels are “3D” macromolecular hydrophilic with interpenetrated networks that swallow large amounts of water and biological fluids[4-6]. Hydrogels contain an ionizable acidic or basic pendant groups such as R-SO3H, R-COOH, and R-NH2 groups, are responsible for its water holding capacity[7]. Hydrogels, when fully swollen, possess some unique properties, such as being soft and rubbery with low interfacial tension towards water and biological fluids. The following significant properties of hydrogels have been shown in Figure 1.
|
Figure 1. Significant properties of hydrogels.
Several kinds of stimuli-sensitive polymeric hydrogels that respond to changes in their environments, such as pH, temperature, magnetic field, electric field, glucose, enzyme, light, ultrasonic, and photo, have been developed (Figure 2). The smart polymers undergo an unexpected change in their physical properties in response to environmental stimuli. Such polymers, otherwise called “intelligent polymers”, have the capability to return to their original shape even after the removal of external stimuli[8].
|
Figure 2. “Smart” or “intelligent” hydrogels pathway to energy sources.
The “smart” or “intelligent” hydrogels exhibited changes in the gel structure in response to environmental stimuli. Smart hydrogels, which are different from inert hydrogels in that changes in the environmental conditions such as pH, temperature, light, and magnets respond by altering the degree of swelling. These sensing tendencies are attractive in many biomedical and environmental applications. The volume-changing behaviour of smart hydrogels is particularly useful in drug delivery applications. The degree of swelling depends only on the chemical composition of the polymers, which do not respond to external pH changes for non-ionic hydrogels. However, the swelling depends on the chemical composition of the gel as well as the pH medium[9]. Such intelligent polymeric hydrogels create a pathway to energy resources like hydrogels as electrodes, electrolytes, etc., for battery applications[10].
Functional polymeric hydrogels are an attractive material of dais for energy-storage technologies. Hence, the expansion of hydrogels with enhanced physicochemical properties, such as improved mechanical strength, flexibility, and charge transport capacity offer an innovative chance for next-generation batteries and supercapacitors.
Hydrogels with fascinating physicochemical properties can exhibit a gorgeous material for sustainable energy storage as well as clean water technologies. The researcher, Guo et al.[11] highlighted key aspects of building blocks and synthetic approaches to hydrogel-based materials in a tunable manner from bulk to the molecular scale. Numerous strategies in refining electronic and ionic conductivity, controlling swelling and deswelling behaviours, and integrating multiple functionalities have also been discussed[11].
2.2 Fundamentals and Traditional of Hydrogel Electrolytes
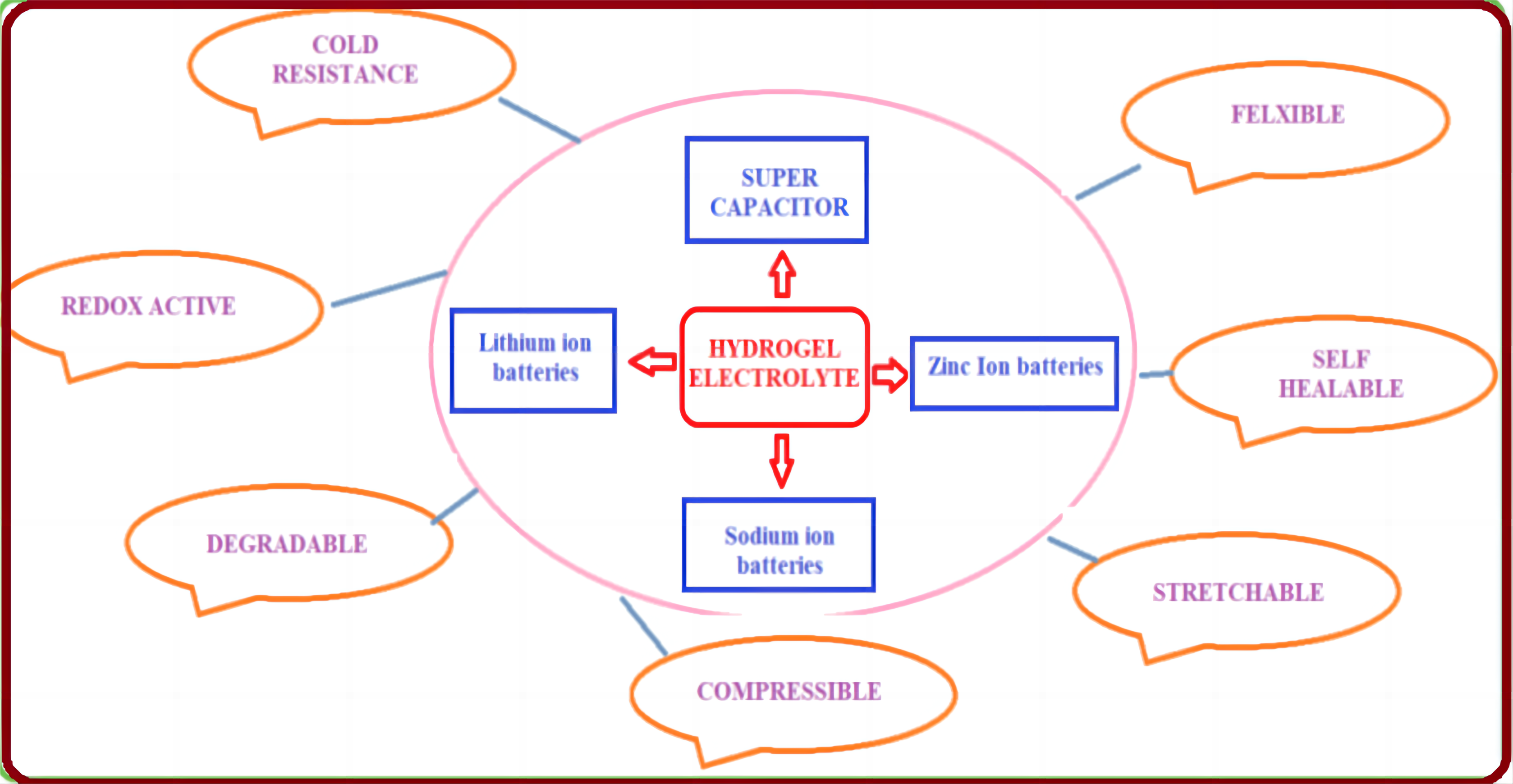
Hydrogel materials are hydrated polymer that puff up with tuneable water. Such a type of hydrogel donates the polymeric environments with unique softness and wetness that covers water. This can be supportive in dissolving the mobile electrolyte ions for electrochemical reactions[12-14]. Nowadays, hydrogel electrolytes have been intensively studied and considered as electrolyte materials for flexible batteries owing to their reputable compatibility with electrodes and high ionic conductivity. Henceforth, it is a promising material for batteries with manifold functions. In the hydrogel medium, the dissolved electrolytic ions can be fascinated and localised by the adjacent charged functional groups on the polymeric chains in the network. In addition to this, the interstitial spaces in the flexible cross-linked network can be filled with a significant amount of water to ensure the elasticity of the hydrogel matrix[15,16]. Such significant features endow the hydrogel materials with an inimitable liquid-like ionic conductivity, and in the meantime, they are booking the dimensional stability of quasi-solid states, making them the perfect candidate for flexible batteries (Figure 3).
|
Figure 3. Traditional hydrogel electrolytes.
2.3 Functional Hydrogel Electrolytes
In the present polymer technology, it is feasible to design hydrogel materials with distinguishable properties. The methodology includes self-assembly, cross-linkable capabilities, the formation of IPN could be employed for specific functions, viz., rubbery stretchability, auto-healing aptitude, thermosensitivity and the biodegradation. In addition, the intrinsic ionic conductivity, interface compatibility and mechanical flexibility of the intended hydrogel electrolytes ought to be considered to determine their electrochemical properties. Researchers fabricated and reported against deformations which contain a super-tough trivalent aluminium ion cross-linked with alginate and polyacrylamide (PAM) hydrogel electrolyte as applied it to a supercapacitor[17-19].
In the 1990s, solution-free electrolytes known as solid polymer electrolytes (SPEs) were made by mixing alkali metal salts into polyethylene oxide as a plasticizer, which was resourcefully added to evade the leakage and evaporation problems of electrolytes and to hold dimensional stability. Thus, this innovation served as an perfect alternative to traditional aqueous electrolytes for Zinc-based batteries. But the inadequate low conductivity (10-7-10-8S∙cm-1) of SPE was found to be a setback. Recently, incorporation of hydrogel electrolytes into aqueous electrolytes became a new method for generating electrolytes for Zn-based batteries while assuaging evaporation and leakage. A literature survey focused on developing hydrogels with improved ionic conductivity, wettability, and mechanical strength for usage as quasi-solid electrolytes in Zn-based batteries[20].
The generations of hydrogels were denoted as conventional single- and double-network hydrogels. The single-network hydrogels are facing poor mechanical properties due to crack propagation. Hence, its application is restricted. However, in the double network hydrogels, there exist both a short chain network and a long chain matrix. The short chain network is pre-stretched, rigid, and brittle, whereas the long chain network, noticed by its coiled strands, is found to be soft. In hard applications, double network hydrogels are preferred because, when stretched, the short chain matrix breaks down by rupturing bonds, dispersing energy. Meanwhile, the long chain network upholds the elasticity and integrity of hydrogels in the deformation process. Hence, while designing the double network hydrogels, a few points must be considered:
(1) Inflexible and fragile polymers are used for the short-chain network, while soft and ductile polymers are employed for the long-chain network.
(2) In short chain networks, the molar concentrations used are almost about 30 times lower than those used in long chain networks.
(3) The construction of an asymmetric double network consists of tightly cross-linked and loosely cross-linked networks[21].
Examples of double network hydrogels:
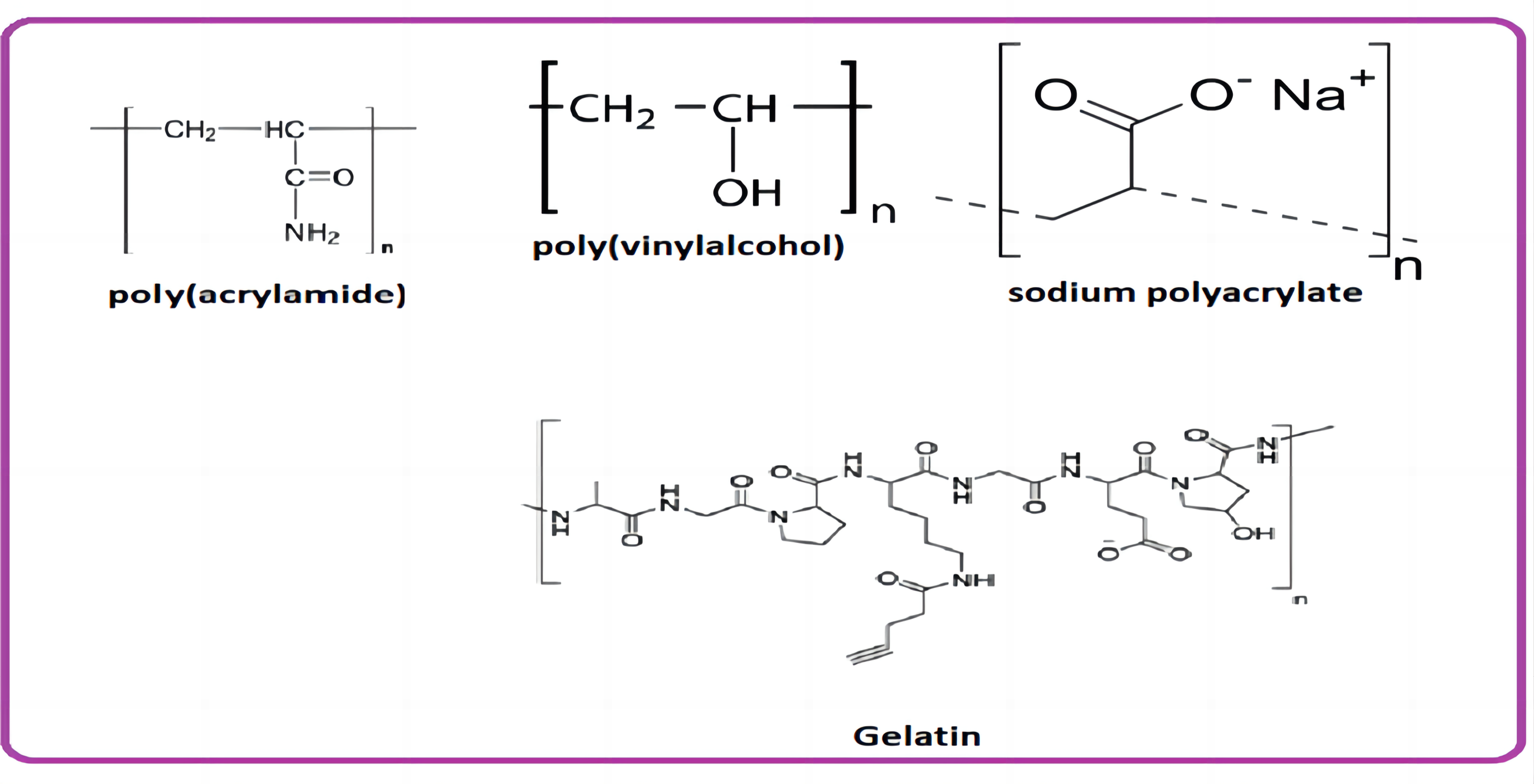
● Alkaline polysaccharides (chitosan) and PAM[22]
● Polyvinyl alcohol (PVA) has been used normally in flexible energy storage electronic devices. PVA hydrogels can be mixed with electrolytic salts to achieve high ionic conductivity[23]. Once physical damage occurs, they automatically repair themselves due to the presence of hydroxy side groups as well as hydrogen bonding.
● For example: Huang et al.[24] have developed a PVA hydrogel with two molar concentration of zinc trifluoromethane sulfonate, creating a sustainable and recross-linkable network for achieving high ionic conductivity.
● Li et al.[25,26] proposed an electrolyte using novel gelatin and PAM. Liu et al.[27] organised a Zn-alginate containing PAM double cross-linked energy-dissipative hydrogel electrolyte framework. Moreover, batteries can be designed into unpredictable shapes under twists and can even survive damage from a car.
Wang et al.[28] planned a nanofibrillated cellulose (NFC) PAM hydrogel composite with robust thickness, exhibiting remarkable mechanical properties and exaggerated polymer channels that enhance ionic conductivity. The zinc-ion batteries, with NFC and PAM as hydrogel electrolytes, were fabricated using sewing techniques. They have demonstrated batteries as clothes for little toys and explored the flexibility and wearability of the battery. In addition to a thermoresponsive property, an anti-freezing character can be introduced into hydrogel while designing a polymer network. Generally, the temperature at sub-zero levels makes it highly difficult to operate it. To broaden the utilisation of batteries under sub-zero conditions, it is essential to design ideal anti-freezing electrolytes with a lower freezing point. To impart an anti-freezing nature to the hydrogel electrolyte, it is necessary to add lipophilic components. However, this requires more complex synthetic buildups. Researchers have introduced dihydric and trihydric alcohols as solutes into the polymeric hydrogel to reduce the freezing point of the water. Accordingly, Mo et al.[29,30] developed dihydric alcohol based non-toxic inhibitors for designing anti-freezing hydrogel electrolytes that can provide tolerance in harsh environments.
3 APPLICATIONS OF HYDROGELS IN ENERGY DEVICES
3.1 Overview of Energy Applications
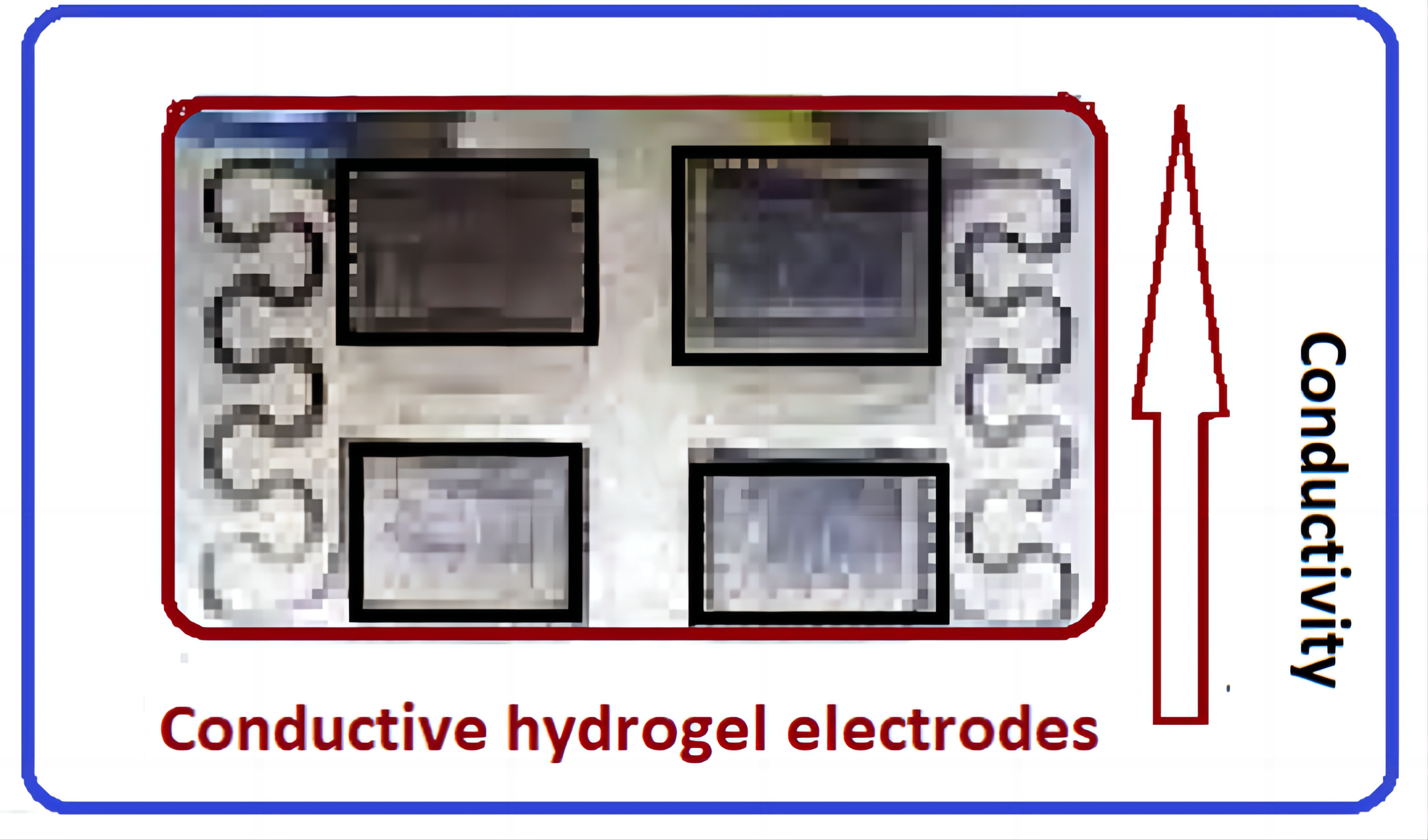
Hydrogels are semi-solid with a high-water content, unlike metals, ceramics, or typical plastics. Hydrogels are usually viscoelastic, shapable, and ionically conductive and can be applied in numerous applications. Conductive hydrogel serves as a coating material for electrodes, improving the performance of conventional electronic devices. It significantly utilized lower amounts of energy compared to metal electrodes, substituting C-cell functions. Conductive hydrogels have been demonstrated as genuine materials that enhance the mechanical properties of an electrode and instantaneously improve its electrical properties (Figure 4). Conductive hydrogels are hybrid materials formed by combining a conductive polymer and a hydrogel, creating a predictable bioelectrode material. Due to the presence of the hydrogel component, conductive hydrogel swells in aqueous surroundings, enabling the access of ions and forming a 3D surface. Ji et al.[31] utilized super-strong, super-stiff, and conductive alginate hydrogels for practical applications, in synthetic hydrogels as non-natural biological tissues, flexible electronics, and conductive membranes for specific applications. Such hydrogels are straightforwardly laminated as a stable gel electrolyte membrane for an aqueous supercapacitor.
|
Figure 4. Conductive hydrogel electrode.
Himori and Sakata[32] have developed flexible conductive polymer hydrogels as an electrode material. Electrochemical biosensors with conductive polymer hydrogels have been developed for highly sensitive nano-structed receptable biosensors for target analytes.
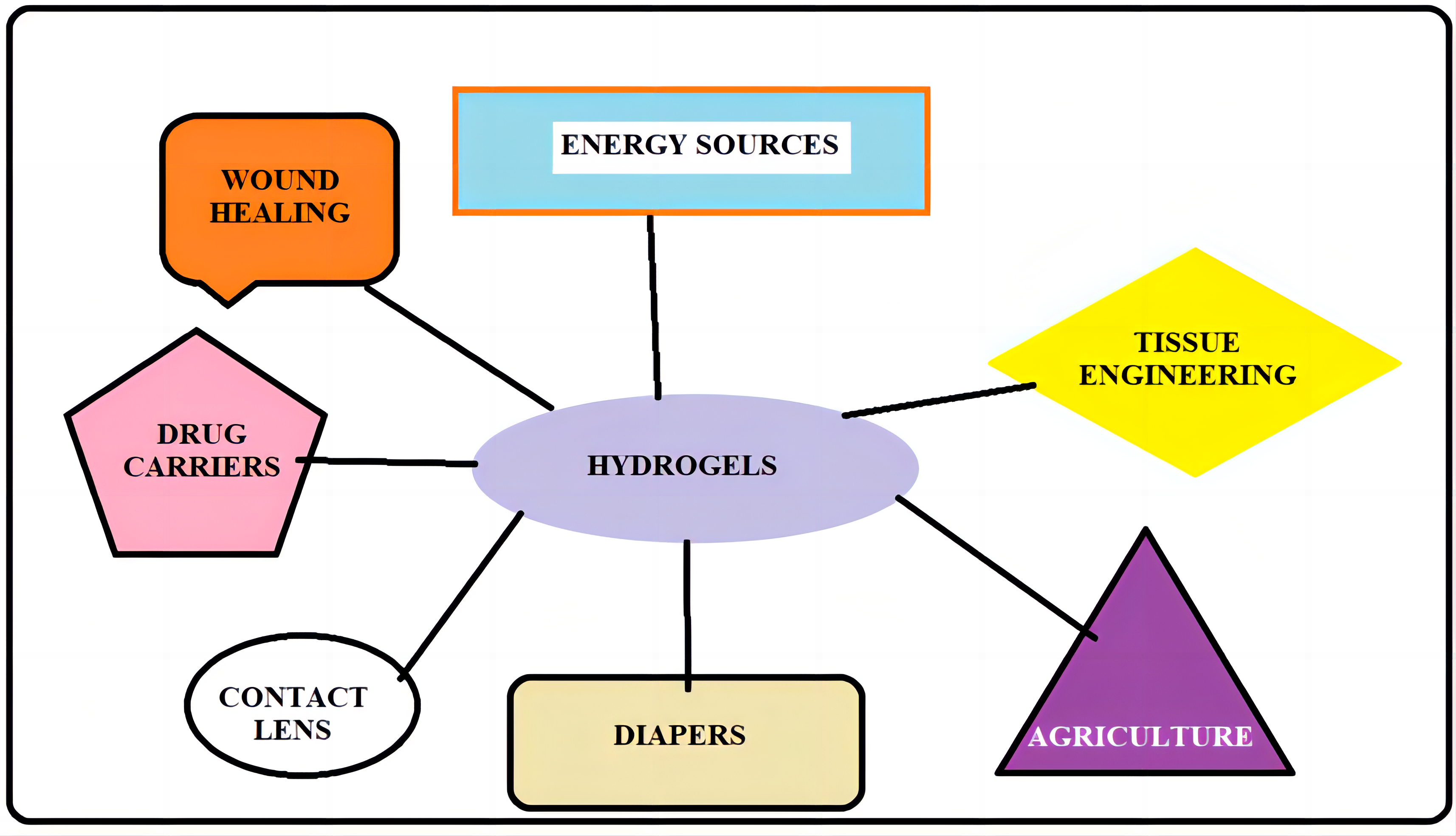
Hydrogels can be applied into enormous application in the various fields like drugs carriers, tissue engineering, leak proof materials, biocompatible medical implants, electrode, and electrolyte materials as regenerative energy devices (Figure 5).
|
Figure 5. Applications of hydrogels.
Guo et al.[33] deliberated polyaniline hydrogels, stating that the combination of conducting polymers and hydrogels holds immense potential in various fields, including electrochemical energy storage, metal corrosion resistance, biological and chemical sensors, etc. Self-cross linkable polyaniline hydrogels have been synthesised using an oxidative coupling reaction in the absence of crosslinkers using persulfate as the oxidising agent. Currently, numerous scientific communities and researchers are incongruent towards the development of energy storage and conversion devices to meet demands in the field of energy. Supercapacitors attracted as energy storage devices due to their marvellous features. However, their low conductivity produces a barricade to their applied applications. This review appraisals the researches rendered by the researchers on the binder-free flexible supercapacitor electrode, based on polypyrrole / fluorinated graphene nanocomposite hydrogel on carbon cloth designed using in situ oxidative polymerization. The resultant composite hydrogel possessed excellent electrochemical performance with a high specific capacitance[34]. Similarly, fluorinated graphene hydrogels are applied as binder- and additive-free electrode materials for supercapacitors. This can be further modified with reduction of graphene oxide and incorporated the fluorine atoms into the graphene matrix[35].
Wearable patch type hydrogels electrodes received much consideration in the biomedical application for bio-signal monitoring perhaps their inferior mechanical properties prevent its utility. Provided, researchers composed biodegradable gelatin based conductive polymers for electrocardiography measurements, thus by incorporating poly(3,4-ethylenedioxythiophene) with poly(sodium 4-styrenesulfonate) into gelatin hydrogels cross-linked by natural crosslinkers like genipin towards their improved mechanical properties and electrical conductivity[36].
3.2 Hydrogels in Batteries, Supercapacitors, and Fuel Cells
In general, batteries contain four main parts: Cathode, anode, electrolyte, and separator, which are the four main components of lithium-ion batteries. Single-use zinc-carbon batteries consist of an electrolyte which is a wet paste of ammonium chloride and / or zinc chloride. The electrolyte paste is sandwiched between a zinc case as the anode and a manganese oxide core as the cathode. Most of the electrolytes used in commercial lithium-ion batteries are non-aqueous solutions containing Lithium hexafluorophosphate salt dissolved in organic carbonates, specifically mixtures of ethylene carbonate with dimethyl carbonate, propylene carbonate, diethyl carbonate, and others[37].
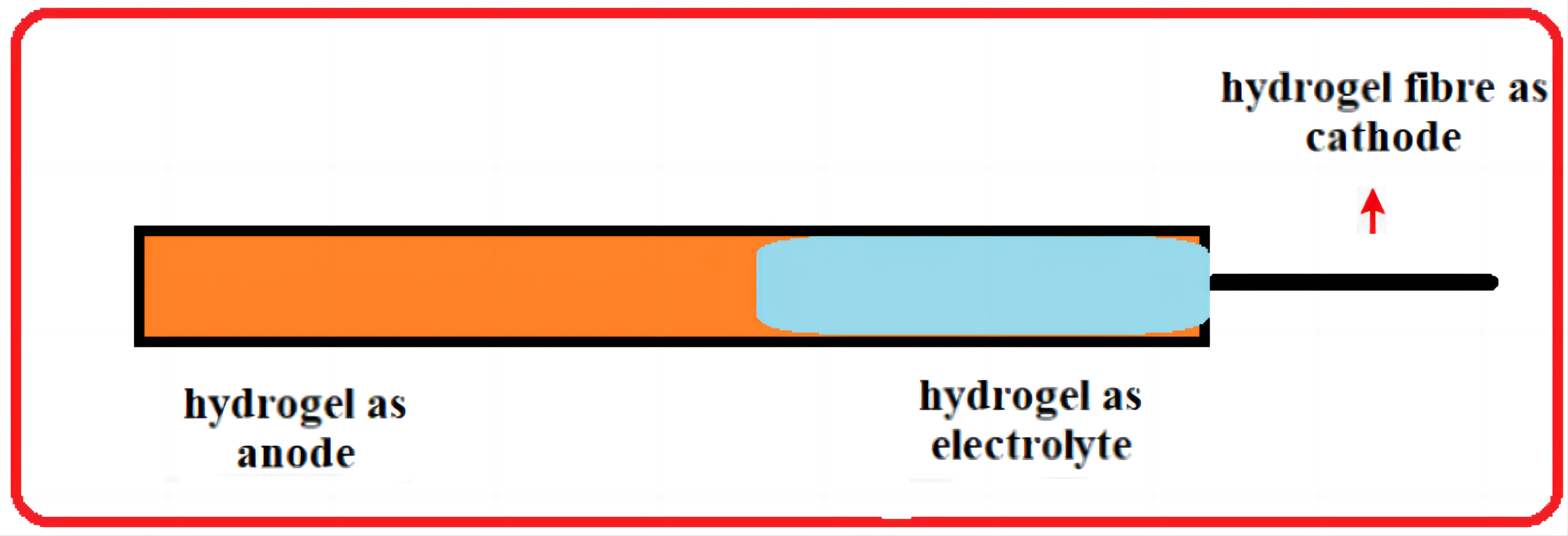
Researchers developed fibre-based batteries as an ideal energy storage device for wearable electronics owing to their superior properties compared to conventional bulk and thin-film batteries. However, the materials currently used in reported fibre batteries are intrinsically rigid or have limited flexibility, which can cause rigidity that might harm damage biological tissues. Henceforth, it is necessary to design ultra soft fibre-based batteries harmonized between mechanical properties with biological tissues (Figure 6).
|
Figure 6. Utilisation of hydrogels in batteries.
To create wearable and implantable bioelectronics that can accommodate dynamic and uneven biological tissues while reducing unwanted immune responses. Nevertheless, batteries cannot achieve the softness of tissues due to the high Young’s moduli of their components. So, the creation of tissue-like soft batteries remains a challenge. This review further summarises the research report of the first ultrasoft batteries totally based on hydrogels[38]. Further, the following table justifies the performances of barriers with an insight into the hydrogel electrolyte materials (Table 2)[25-30,39-48].
Table 2. Performance of Batteries
Battery Type |
Hydrogel Electrolyte |
Performance of Electrolyte |
Zn-MnO2 |
Gelatin-g-PAM electrolyte containing ZnSO4 (2M) and MnSO4 (0.1M)[25] |
Ionic conductivity: 1.76×10-2S∙cm-1, strength: 7.76MPa |
Zn-MnO2 |
PAM-based electrolyte containing ZnSO4 (2M) and MnSO4 (0.1M)[26] |
Tensile strength: 273kPa, stretchability: 3,000% strain, ionic conductivity: 17.3×10-3S∙cm-1 |
Zn-MnO2 |
Zn-alginate / PAM electrolyte containing ZnSO4 (2M) and MnSO4 (0.1M)[27] |
Zn-alginate / PAM electrolyte containing ZnSO4 (2M) and MnSO4 (0.1M) |
Zn-MnO2 |
NFC / PAM electrolyte containing ZnSO4 (2M) and MnSO4 (0.2M)[28] |
Ionic conductivity: 22.8mS∙cm-1, stretchability: 1,400% strain |
Zn-MnO2 |
PNA sol-gel electrolyte containing ZnSO4 (0.3M) and MnSO4 (0.015M)[29] |
Resistance: 18.1MΩ at room temperature; 160.9MΩ at 70℃ |
Zn-MnO2 |
EG-waPUA / PAM based electrolyte containing ZnSO4 (2 M) and MnSO4 (0.1M)[30] |
Ionic conductivity: 16.8mS∙cm-1, ionic conductivity: 14.6mS∙cm-1 at -20℃ |
Zinc-air |
PAM electrolyte containing Zn(CH3COO)2 (0.2M) and KOH (6M)[39] |
Charge-discharge voltage gap: 0.78V at 5mA∙cm-2, power density: 118mW∙cm-2 |
Zn//NiCo and Zn-air |
PANa electrolyte containing Zn(CH3COO)2 (0.2M) and KOH (6M)[40] |
Ionic conductivity: 0.17S∙cm-1 |
Zn-air |
PAM-co-PAA electrolyte containing Zn(CH3COO)2 (0.2M) and KOH (6M)[41] |
Stretchability: 2,700% strain, tensile strength: 102.8KPa, water retention: 68.7% in the air for 10 days |
NiCo//Zn |
PANa electrolyte containing Zn(CH3COO)2 (0.2M) and KOH (6M)[42] |
Stretchability: up to 1,700% strain, compressibility: 80% |
Zi-air |
PANa cross-linked by cellulose chains and MBAA anchors electrolyte containing Zn(CH3COO)2 (0.2M) and KOH (6M)[43] |
Ion conductivity: 0.28S∙cm-1 |
NiCo||Zn |
PANa-Fe3+ electrolyte containing Zn(CH3COO)2 (0.2M) and KOH (6M)[44] |
Stretchability: ~1,000% strain, tensile strength: 205kPa |
Ni ion batteries |
alkaline hydrogel - PEO-KOH-H2O[45] |
high crystallinity and low melting-point of PEO, limited operating temperature-range |
Ni-MH batteries |
Potassium polyacrylate hydrogel electrolytes[46,47]
|
The degree of network formation depends upon the pH and ionic strength of the medium |
PVA |
PVA hydrogel electrolytes[48]
|
Studied Ionic conductivity data |
PVA / PAA |
PVA / polyacrylic acid blend hydrogel electrolytes[48] |
decreases with increase in the proportion of PAA in the blend |
Gelatin |
Gelatin hydrogel electrolytes[48]
|
Application is limited due to its low network rigidity |
Inorganic hydrogels |
Inorganic hydrogel electrolytes[48] |
most promising host materials to design high proton conducting composites - possess ionic conductivity values as high as 102S∙cm-1 under ambient conditions |
Because of their palpable cost and resource advantages, the sodium-ion batteries are seen as a promising technology for the development of a new generation of energy storage devices. Considering current environmental and electronic equipment’s safety concerns, aqueous electrolytes that are flame-resistant, cheap and eco-friendly have garnered significant attention. In recent eras, plentiful enhancements have been noticed on aqueous sodium-ion batteries. This development is nothing but the transition from liquid electrolyte to hydrogel electrolyte for flexible and leak-proof definitions, enthusiastically awaited in the generation of flexible wearable electronics. The low-temperature tolerance of aqueous sodium-ion batteries was reported by Cheng et al.[49], indicating a high challenge. However, these batteries are being considered for large-scale energy storage applications due to their high protection, low cost, and eco-productiveness. The researchers report that, for the first time, a Na2SO4-SiO2 hydrogel-type electrolyte functioned at low temperatures as low as -30℃ (under cryogenic conditions) using fumed silica as the gel matrix and methyl alcohol as the anti-freezing stabilizer[49].
A novel calcium-ion solid polymer electrolyte cross-linked with poly(ethylene glycol) diacrylate has been described by Francielli et al[50,51]. According to their findings as follows:
● Calcium-ion SPEs were fashioned for the first time from poly(ethylene glycol) diacrylate.
● Ionic conductivities from 3.0×10-6S·cm-1 to 3.4×10-4S·cm-1 were achieved.
● All electrolytes remained stable at temperatures of approximately 140℃.
● Calcium-ions were complexed with poly(ethylene glycol) diacrylate in the network.
Ionic conductivity, water retention capability, electrolyte permeability, and electrode-electrolyte interaction are critical characteristics of generic hydrogel electrolytes. One important factor influencing the hydrogel electrolyte’s stability is its water-retaining capability. Strong electrode-electrolyte interaction and superior ionic conductivity can protect the reactions during battery operation. Hydrogels with excellent electrolyte permeability are also required since the concentration of electrolytes affects battery performance. Hydrogel electrolytes are typically endowed with extra functions, including self-healing and mechanical qualities, in order to meet the peculiar requirements of the devices.
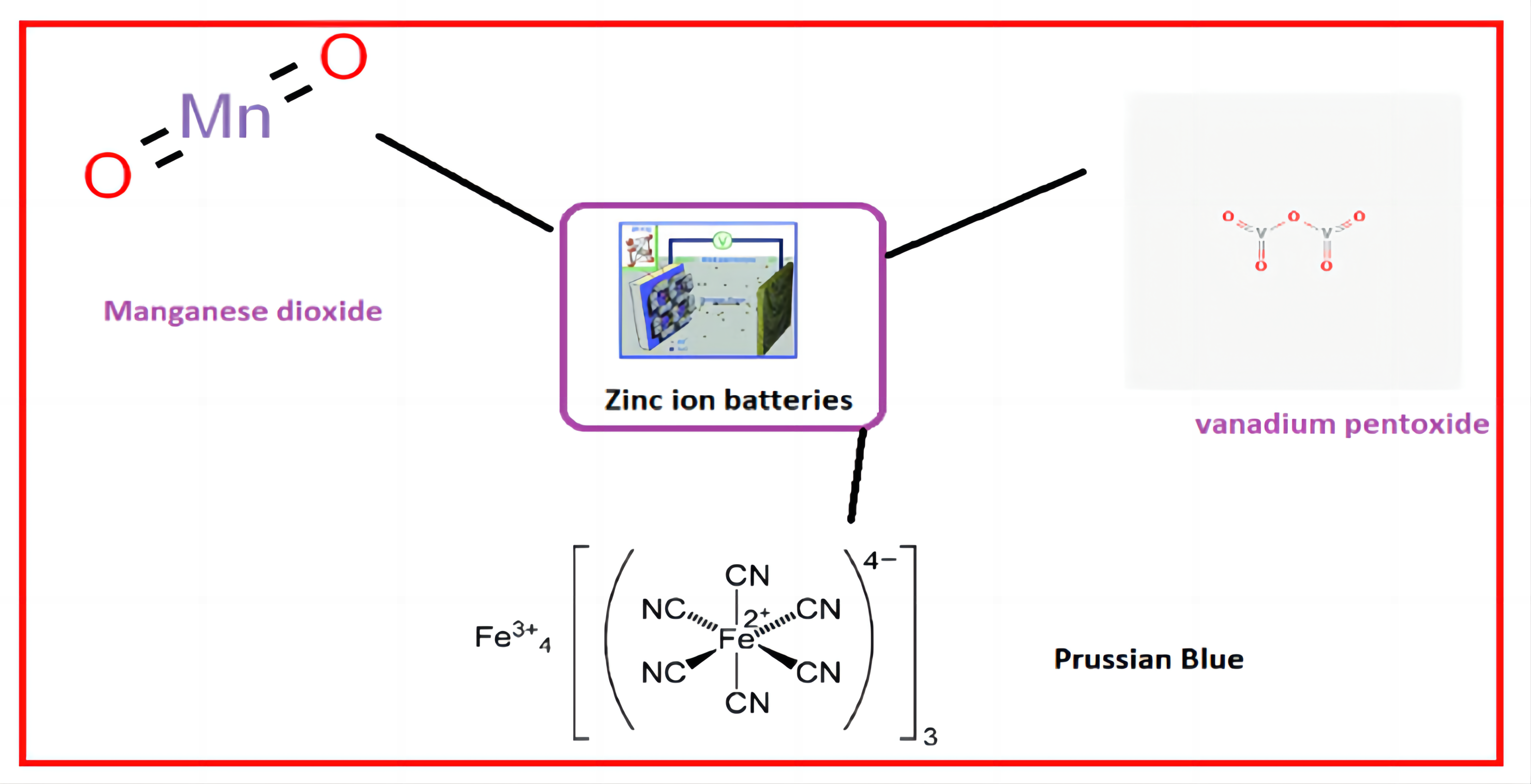
Recently, the cathode materials tailored for Zinc-ion batteries mainly include manganese dioxide, vanadium pentoxide, Prussian blue, and conductive polymers. Zn-MnO2 batteries are highly reversible due to their eco-protectiveness, cheapness, and comfort of fabrication (Figure 7).
|
Figure 7. Cathode materials for zinc-ion batteries.
Due to the complimentary benefits of zinc batteries and capacitors combined, Fu et al.[52] created flexible zinc-ion hybrid capacitors based on hydrogel electrolytes. These capacitors are a new and extremely promising option for possible large-scale energy storage. However, the freezing causes a significant reduction in mechanical and conductivity qualities along with a significant compromise of interfacial adhesion, which significantly hinders the low-temperature applications of these flexible zinc-ion hybrid capacitors. In order to produce self-adhesive polyzwitterionic hydrogels, unparalleled conductivity, strong interfacial adhesion, and exceptionally high mechanical strength over a wide temperature range of 25 to -60℃, a self-catalytic nano-reinforced strategy is engineered. This results in flexible zinc-ion hybrid capacitors with excellent low temperature adaptability.
Liu et al.[53] has suggested that high-safety, inexpensive rechargeable zinc-ion batteries could replace lithium-ion batteries due to the growing need for large-scale energy storage. It is crucial to investigate stable zinc anodes and high-performance cathodes for the significant progress in both areas. The development of energy storage devices based on zinc is still hampered by several fundamental problems. The electrolyte is a crucial component which offers the fundamental operating conditions necessary to guarantee the high compatibility and reversible cycling of every component in the battery.
Aqueous Zn-ion batteries have demonstrated great promise for wearable and flexible electronic devices because of their inherent safety, affordability, and environmental friendliness. However, the inability of flexible zinc-ion batteries to maintain a reliable and long-lasting electrolyte severely limits their practical use in harsh environments on a regular basis. In their work, they reported creating a stable hydrogel electrolyte by combining cotton cellulose nanofiber grafted with a copolymer of xanthan gum-PAM. High ionic conductivity, robust ion adsorption, outstanding adhesion, high mechanical strength, and great cycling stability were all displayed by this hydrogel electrolyte.
To satisfy the expectations of modern devices for ease, efficiency, and quality, electronic devices are increasingly expected to be flexible and multifunctional on a global scale. Therefore, it is imperative to build wearable and adaptable energy storage technologies. Zinc-ion capacitors and aqueous zinc-ion batteries have emerged as two of the most promising options for wearable electronics recently because of their functional controllability, inherent safety, low cost, and superior electrochemical performance. Concurrently, the introduction of polymer hydrogels electrolytes in conventional zinc-ion batteries and capacitors found colourful applications. This has been considered the ideal solution for energy sources incorporated into those cutting-edge wearable electronic devices. In addition to outlining the many fascinating uses for hydrogel electrolytes, an analysis of their underlying concepts has been conducted. In the end, every detail of these cases was condensed, and a thorough assessment of the associated difficulties, useful fixes, and possibilities for working zinc-ion batteries and capacitors was also conducted[53].
Liu et al.[54] employed zinc-ion energy storage devices enriched with hydrogel electrolyte considered a promising candidate for flexible and wearable electronics applications. This is because of their safe nature, low cost, and good mechanical characteristics. However, conventional hydrogel electrolytes face limitations at sub-zero temperatures. Hence, the authors reported an anti-freezing, safe, and non-toxic gel electrolyte based on PVA with zinc-ion and ethylene glycol.
Li et al.[55] assembled electronic storage devices from traditional hydrogel electrolytes and reported inferior nature due to capabilities under low temperature and fail at high temperatures due to water evaporation. Hence, they have prepared organo hydrogels consisting of poly(2-acrylamido-2-methylpropanesulfonic acid) in a binary solvent electrolyte of ethylene glycol and water (10% v/v) along with zinc chloride and ammonium chloride. Owing to the synergistic solvation of zinc chloride, ethylene glycol, and water, the organo hydrogels possess a lower freezing point (lower than -60℃) than the control hydrogel electrolyte.
The resilience of the hydrogel electrolyte is closely correlated with the energy density, power density, and cycle life of batteries. A flexible Zn-based device’s service life can be significantly increased by having a good water retention capacity, especially for semi-open Zn-air batteries where vapour evaporation has a greater impact. Although there have been some reports on increasing the ability of hydrogel electrolytes to retain water, their durability is still insufficient for commercial applications. Additionally, it is anticipated that a suitable structure will be created for Zn-based batteries with hydrogel electrolytes to reduce the amount of exposed surface to the air and lessen the impact of thermal evaporation.
The interaction between various ions and polymers is significantly influenced by the valence state and size of the ionic radii, which further leads to variations in the ionic conductivity of hydrogels. Additionally, there is a close connection between the ionic conductivity of hydrogels and the molecular weight and degree of cross-linking of the polymer. The electrolytic salts could be alkaline or neutral. Therefore, further research into suitable polymers and improved production techniques for hydrogel electrolytes with various pH levels is required.
Investigating hydrogel electrolytes with unique characteristics, such as stretchability and self-healing, requires consideration of varied application contexts. Zn-based batteries that feature smart electrolytes have recently been discovered to offer remarkable properties (rubbery stretchability, self-healing capability, and good durability). Although, after prolonged use, a device’s performance is likely to degrade. Therefore, the most pressing worry is the dependability of Zn-based devices. As an illustration of self-healing, some claim that broken Zn-based batteries can recover on their own without external stimulus. However, healing effectiveness will significantly decline after multiple cycles of cutting and healing. The degree of strain recovery after stress release can still be improved through the creation of a modification approach for the polymer (such as a tiny fraction of permanent crosslinks).
Investigating eco-friendly hydrogel electrolytes are advantageous for the long-term advancement of civilization. Future large-scale production will surely result from the pressing need for power-source devices. Environmental pollution can be significantly reduced by the creation of biodegradable battery materials and green synthesis processes. The use of environmentally safe hydrogel electrolytes in Zn-based devices has received less focus and effort, even though there have already been some investigations into biocompatible hydrogels.
Supercapacitors, sometimes referred to as ultracapacitors, are high-capacity capacitors that have lower voltage limits but far larger capacitance values than ordinary capacitors. Rechargeable batteries and electrolytic capacitors are connected by it. Based on their performances, super capacitors (SCs) are divided into two categories: Pseudo capacitors and double-layer capacitors. By integrating the potential windows of the two electrodes, the connection of redox and carbon-based materials can facilitate the manufacturing of hybrid supercapacitors. This increases the energy density of the SCs by widening the overall voltage of the device. Cellulose, lignin, and chitosan are commonly utilized as biopolymers in the production of capacitors due to their strength, degradability, and longevity[56].
The following list of environmental problems arising due to
● consistently depleting fossil-based resources
● climate change
● their consumption
Hence, there is a convincing need for the development of energy storage devices such as supercapacitors and battery materials. They function as energy storage devices with a longer cycling life and higher power density than batteries. Further, they have also a higher energy density than conventional capacitors.
Hydrogel-based supercapacitors can be a choice of materials over others preferred by the researchers for the following reasons:
● Electrical performances: Ultrahigh ionic conductivity, superior power density, and stable electrochemical performances
● Mechanical performances: Preferred viscosity, stability in solid-state performance, high stretchability
● Life cycle issues: Long-term cycling ability, stable interface electrolytic nature
Furthermore, Table 3 illustrates the performance of polymer electrolyte for super capacitor applications[57-72].
Table 3. Performance of Polymer Electrolyte for Super Capacitor Applications
S. No. |
Polymer Electrolyte |
Feature of Super Capacitor |
1 |
Graphene oxide + starch gel |
Graphene oxide and starch gel as a hybrid binder for environmentally friendly high-performance supercapacitors[57] |
2 |
Melamine foam / carbon electrode |
Nitrogen / oxygen co-doped monolithic carbon electrodes derived from melamine foam for high-performance supercapacitors[58] |
3 |
Agar based electrode / electrolyte |
Agar-based porous electrode and electrolyte for flexible symmetric supercapacitors with ultrahigh energy density[59] |
4 |
Biodegradable gel electrolyte |
A biodegradable gel electrolyte for use in high-performance flexible supercapacitors[60] |
5 |
DNA gel electrolyte |
Flexible supercapacitor with a pure DNA gel electrolyte[61] |
6 |
Carboxylated chitosan hydrogel film |
Biopolymer-based carboxylated chitosan hydrogel film cross-linked by HCl as gel polymer electrolyte for all-solid-sate supercapacitors[62] |
7 |
Graphene and graphene oxide / gelatin |
Differences between graphene and graphene oxide in gelatin based systems for transient biodegradable energy storage applications[63] |
8 |
Starch and gelatin based hydrogels |
Comparison of starch and gelatin hydrogels for non-toxic supercapacitor electrolytes[64] |
9 |
Carbon aerogel / polymer gel |
Flexible solid-state supercapacitors based on carbon aerogel and some electrolyte polymer gels[65] |
10 |
Eco-friendly carbon based electrolyte |
A comparative evaluation of sustainable binders for environmentally friendly carbon-based supercapacitors[66] |
11 |
Gelatin hydrogel electrolyte |
Gelatin hydrogel electrolytes and their application to electrochemical supercapacitors[48] |
12 |
Biopolymer hydrogel electrolyte |
Biopolymer-based hydrogel electrolytes for advanced energy storage / conversion devices: Properties, applications, and perspectives[67] |
13 |
Self healing polymer electrolytes |
Challenge towards next generation of lithium ion batteries[68] |
14 |
Agarose hydrogels |
Natural convection on macroelectrodes to ultramicroelectrodes[69] |
15 |
Hydrogel electrolyte / cellulose-bentonite coordinate interaction |
High performance solid state zinc-ion hybrid super capacitors[70] |
16 |
PAM / EDTA-modified chitosan / graphene oxide hydrogels |
PAM / EDTA / chitosan / graphene oxide for super capacitor applications[71] |
17 |
Cellulose based functional gels |
Applicable to the flexible super capacitors[72] |
To achieve high energy densities,
● SCs require modified electrode construction.
● the electrolyte is important in conversion devices and electrochemical energy storage because it facilitates ionic transport between the two electrodes.
● hydrogel electrolytes are made up primarily of polymer networks and conductive salt dissolved in a solvent.
4 BIOPOLYMERS IN ENERGY STORAGE DEVICES
4.1 Role of Biopolymers in Energy Storage
Energy storage systems are crucial for controlling energy use in many applications, including electronic gadgets and cars. Supercapacitors and batteries, two materials utilized to build these devices, have adjustable lifetimes and levels of efficiency. To achieve the highest level of durability and efficiency, these devices designs and component combinations are essential. Despite their potential for high performance, synthetic materials have a number of disadvantages because of their synthetic origins and environmental problems with synthetic processing and technique. All forms of technology often involve the three processes of production, usage, and disposal. Material disposal is particularly problematic since many materials have negative environmental effects. Biopolymer materials offer remedies to issues produced by synthetic materials because they are naturally occurring[72,73].
4.2 Applications of Biopolymers in Energy Devices
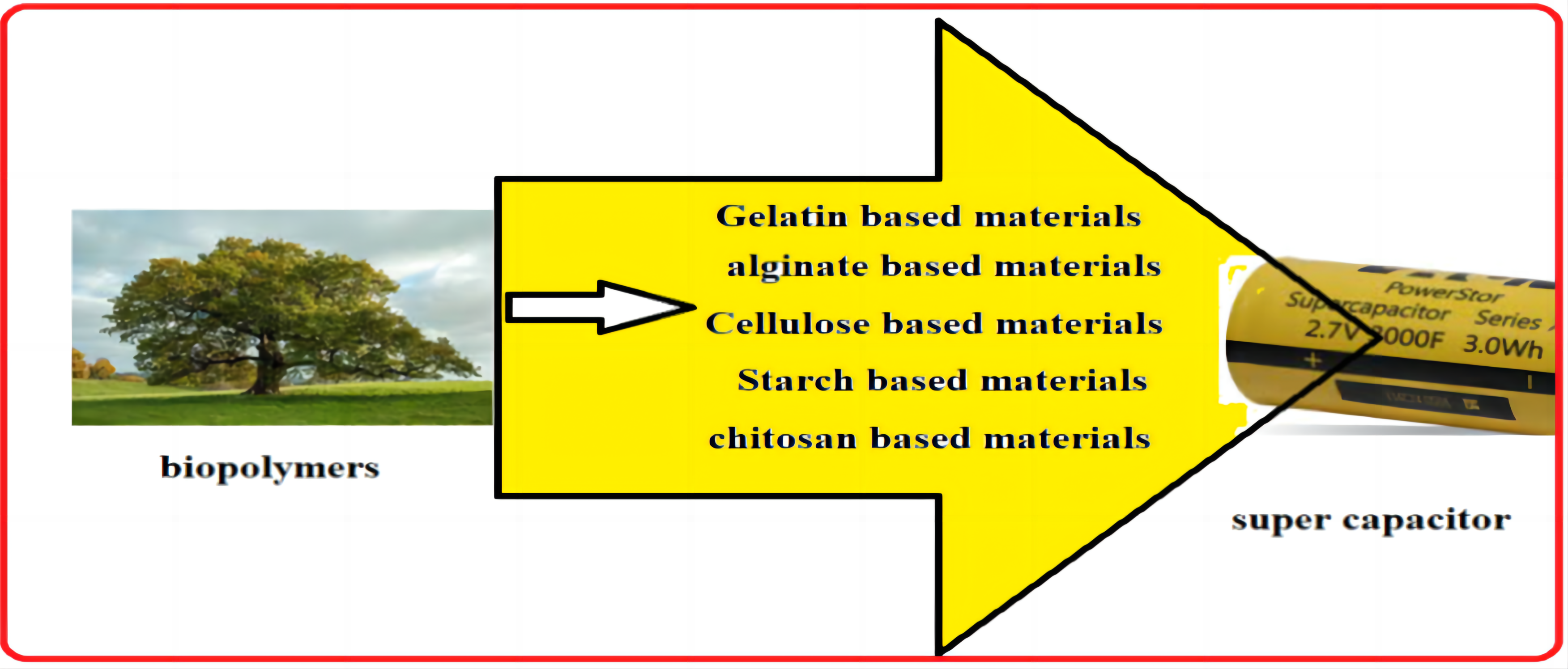
The need to address the growing global pollution and consumption of fossil fuels has made the development of sustainable renewable energy sources and effective energy storage technologies imperative. Supercapacitors have drawn the attention of researchers because they are high-performance energy storage devices that can support the fast expansion of low-power electronics (such as wearable and portable electronics) and high-power military applications (such as guided missile technology and extremely sensitive naval warheads). By combining electrode and electrolyte materials, supercapacitor performance can be expressed in terms of electrochemical properties[74,75]. Supercapacitors with high capacitance and exceptional stability find application in diverse scientific and technological domains (Figure 8).
|
Figure 8. Utilization of biopolymers as supercapacitors.
5 HYDROGELS IN ENERGY DEVICES
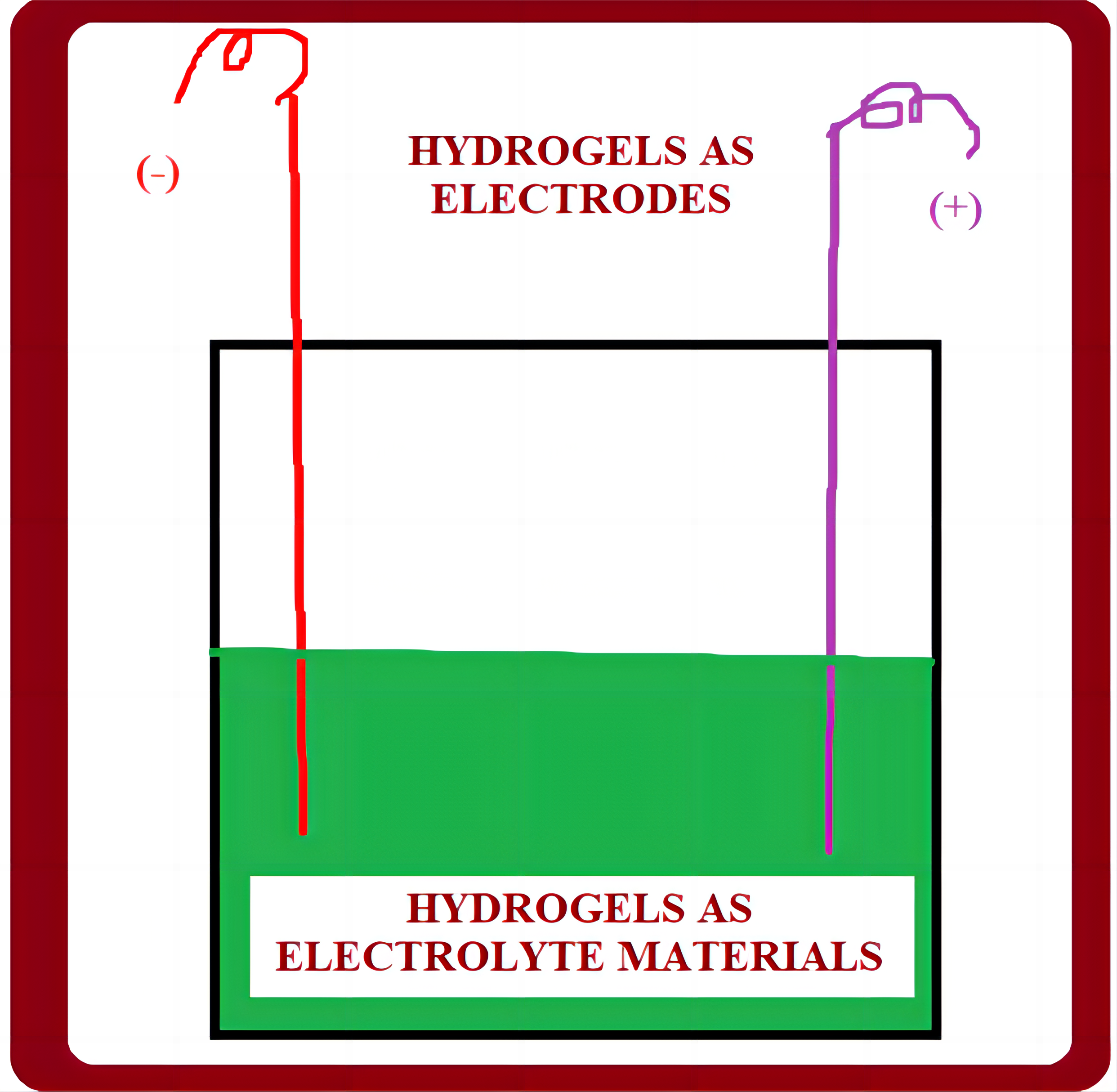
5.1 Hydrogels as Electrolytes
Hydrogel electrolytes have gained popularity due to their functionality, low cost, abundance, flexibility, and environmental friendliness. In addition to biodegradability, the biopolymers aid in ion conduction, allowing for high electrolytic conductivity, mechanical robustness, and thermal stability. The use of gel polymer electrolytes is of great importance to build high-performing rechargeable lithium metal batteries due to their good electrochemical properties and enhanced security (Figure 9). Hydrogel electrolytes can help overcome the limited contact area between the solid-state electrolyte and the electrode material. As with liquid electrolytes, this is accompanied by high ionic mobility and conductivity. Besides, biopolymers, like chitosan, cellulose, alginate, lignin and chitin, have been studied to synthesise operational hydrogel electrolytes. The existence of hydrophilic groups in the biopolymer structures displays a strong ability for special interactions with salt anions, subsequently ministering characteristics of cation transport and salinity solubility[76].
|
Figure 9. Applications of hydrogel electrolytes.
To learn more about the characteristics of the polymer electrolyte, researchers have also examined the cross-linking process of polymer networks for hydrogel materials possessing a variety of functionalities, including stretchability, compressibility, and self-healing[52]. The future application of hydrogel electrolytes in zinc-based batteries built on the benefits of functionalized hydrogel and oxide-based cathodic materials for aqueous rechargeable materials is the main topic of this review. To evaluate the mechanical properties, the electrochemical protocol, and the crucial role of the polymer hydrogel electrolyte in zinc-based batteries, they investigate functionalized hydrogel electrolytes under harsh circumstances. Lastly, there is growing hope for the commercial use of hydrogel electrolytes in energy conversion devices, due to the difficulties they present for zinc-based batteries currently under development[28]. The amount of trapped solvent water can be up to 2,000 times the weight of the polymer chain framework, according to previous reports[17-19]. As a result, hydrogel electrolytes have comparable ionic conductivity to traditional liquid electrolytes because solutes can diffuse or permeate within the hydrogels, and elastic cross-linked polymer chains maintain shape and volume under certain conditions, achieving solid like dimensional stability. Because of their cross-linking network, hydrogels can absorb and retain a large amount of water. More importantly, the degree of cross-linking is closely related to water absorption. Water absorption is inversely related to the degree of cross-linking[4-6].
To achieve solid-state electrochromic devices, an acceptable compromise is to use polymer electrolyte gels, which combine the merits of liquid and solid electrolytes. However, many drawbacks remain, such as inflammability, a sharp decline in ionic conductivity at zero temperature, and interfacial problems with the electrodes. Here, researchers introduced a PAM-based hydrogel electrolyte containing a high concentration of zinc-ions, which delivers an ionic conductivity at room temperature[15]. Furthermore, larger-sized electrochromic devices also show ultra-long cycle stability and attractive anti-freeze properties. Their research report provides an idea for the large-scale fabrication of anti-freeze electrochromic devices with remarkable lifetimes.
Using citric acid as the chemical crosslinker, PVA / hydroxyl ethyl cellulose was synthesized into the hydrogel neutral electrolyte. The supercapacitor was designed to achieve greater cell voltages using sodium sulphate as the neutral electrolyte. To study the flexible supercapacitor’s performance, researchers changed the citric acid content from 0% to 30%. The hydrogel and two constructed activated carbons were the components of the supercapacitor. The results of their investigation reveal the excellent towards the application of the neutral electrolyte. Therefore, it can be concluded that the hydrogel neutral electrolyte of PVA / hydroxyl ethyl cellulose can be utilised as a high-voltage flexible supercapacitor[73-75].
With the increasing mandate for large-scale energy storage, high-safety and low-cost rechargeable zinc-ion batteries have been regarded as potential substitutes for lithium-ion batteries. Exploring high-performance cathodes and a stable zinc anode is important for fruitful achievements. However, many fundamental issues still hinder the development of zinc-based energy storage systems. As a pivotal component, the electrolyte provides the basic operating environment to ensure the high compatibility and reversible cycling of each component in the battery[73-78].
The use of cellulose-based hydrogels as aqueous electrolytes for electrochemical devices has been developed by Gomez et al[79]. According to them, these materials were obtained by cross-linking hydroxyethyl cellulose with divinyl sulfone in the presence of carboxymethyl cellulose, creating a semi-interpenetrating polymer network structure. The small-amplitude oscillatory shear technique revealed that the rheological properties could be conveniently varied by simply changing the gel composition. The ease of synthesis and processing of the hydrogels allowed the assembly of an all-in-one electrochromic device with high transmittance variation, improved switching time, and good colour efficiency. On the other hand, the swelling ability of the hydrogels permits the tuning of the electrolyte to improve the performance of a printed zinc / manganese dioxide primary battery. The results prove the potential of cellulose-based hydrogels as electrolytes for more sustainable electrochemical devices.
5.2 Hydrogels as Electrode Material
Measuring human biomarkers for medical diagnosis is made easier with the use of electrochemical biosensors. Potentiometric or amperometric measurement is the conceptual foundation of electrochemical procedures. Potentiometric biosensors, like field-effect transistors, are suitable for the direct detection of small biomolecules because they allow the direct detection of biomolecular charges without the need for redox reactions, regardless of the size of the molecules. Metal, carbon, oxide films, and other materials are frequently used as electrode materials. However, because of their stiffness, these electrode materials have a problem with flexibility in the human body. As a result, flexible conductive polymer hydrogels are gaining popularity as an alternative electrode material. Furthermore, conductive hydrogels for energy and biomedical applications have recently been developed. Because of their biocompatibility, high conductivity, 3D nanostructure, solvated surface, and enlarged interface, electrochemical biosensors with conductive polymer hydrogels have been developed[32-38].
As an electrode material, flexible conductive polymer hydrogels are gaining popularity due to their biocompatibility, high conductivity, 3D nanostructure, solvated surface, and enlarged interface. Electrochemical biosensors with conductive polymer hydrogels were developed by Himori and Sakata[32]. Target analytes can be detected with extreme sensitivity using conductive polymer hydrogels that have receptor molecules, like enzymes, integrated in their three-dimensional nanostructure. These hydrogels must be created with a supporting substrate since they are too brittle to stand alone. This indicates that their use as flexible biosensors is negatively impacted by mechanical toughness loss. In their research, they suggested a hydrogel electrode that is free-standing, conductive, and coated that is placed on a substrate made of polyaniline, PVA, and phenyl boronic acid. Moreover, the examination of its mechanical characteristics at different glucose concentrations, along with consideration of the hydrogel compositions, validated its electrical responsiveness to glucose[32].
6 HYDROGELS IN BIOELECTRONICS
6.1 Hydrogels in Fuel Cells
Future energy and mobility scenarios heavily rely on electrochemical energy conversion and storage technology. For a range of uses, including stationary power systems and electric cars, fuel cells offer the possibility of clean and effective electricity generation. The performance, dependability, longevity, and cost of fuel cell systems have a significant impact on their ability to compete commercially. Technology can advance in this area with the creation of low-cost ion-conducting membranes for polymer electrolyte fuel cells with tailored architecture and functional features. Radiation-induced graft copolymerization, often known as “radiation grafting”, is a flexible and possibly inexpensive way to change an existing polymer film to add desirable features, such as ion conductivity. Compared to perfluoroalkyl sulfonic acid, which is frequently employed, using inexpensive base polymers and readily available grafting monomers, radiation-grafted proton-conducting membranes, such as Nafion, can be created for use in fuel cells. The key difficulties in this case revolve around establishing concurrent performance and durability characteristics that are competitive with those of perfluoroalkyl sulfonic acid membranes.
An et al.[80] discuss the manufacture and use of a novel, economical, and ecologically friendly agar chemical hydrogel electrode binder in fuel-electrolyte-fed fuel cells. Agar chemical hydrogel is created by a chemical cross-linking process between glutaraldehyde and agar, with acetic acid acting as a catalyst. Fuel-electrolyte-fed fuel cells function better when an agar chemical hydrogel-based electrode is used instead of a traditional Nafion ionomer-based electrode, according to the fuel cell performance characterization. The improved performance is mainly due to the higher mass / charge transmission that agar’s hydrophilic nature and water retention give. According to this research, conventional Nafion ionomers in fuel-electrolyte-fed fuel cells may be replaced with an inexpensive agar chemical hydrogel binder.
● A novel agar chemical hydrogel binder have been synthesized by An et al.[80] for fuel-electrolyte-fed fuel cells.
● The agar chemical hydrogel-based electrode offers better performance than the Nafion-based material.
● A peak power density of 380mW·cm-2 is achieved with the agar chemical hydrogel-based electrode.
● The agar chemical hydrogel binder can replace the Nafion ionomers in fuel-electrolyte-fed fuel cells.
A larger density of bacteria is fixed in the anode of microbial fuel cells (MFCs) that use conductive hydrogel entrapped in polyaniline / phytic acid. This reduces the resistance of the microorganism’s transferring electrons to the electrode and improves the efficiency of electron transfer. The anode of the MFC with conductive hydrogel entrapment has a short start time, a low apparent resistance, and a high output power density. To create the effective MFC that have been established, this innovative method of constructing the anode for MFC offers a useful electrode model[81].
One of the most important unsolved problems in clean energy technology is still MFC, and their features might not be suitable for real-world use. Using polyaniline, phytic acid, and PAM, Xing et al.[82] create a conductive hydrogel in which E. coli is embedded and fixed on carbon paper to create an MFC anode. To create an MFC anode, the researchers have combined polyaniline / phytic conductive hydrogel and microbial embedding technology. This method creates a new type of MFCs anode with high immobilised microorganism density, high microbial activity, and excellent electron transfer from the inside polyaniline material.
● Thus, it is possible to efficiently modify hydrogel electrolytes based on biopolymers to increase mechanical strength over traditional electrolytes by doping them with inorganic filler, blending them, and grafting them.
● Block co-polymerization or mixing the polymer monomers can enhance ionic conductivity even more while decreasing the crystallinity of the biopolymer and enhancing its ductility and toughness.
● Polymer hydrogel electrolytes as wearable electronics with tuneable mechanical characteristics along with biocompatibility and ionic conductivity.
● Ease of construction combined with stretchability and adaptability to intricate work settings.
● When temperatures drop below zero, biopolymeric hydrogels are prone to freezing, which diminishes their mechanical strength and ionic conductivity.
● To create the anti-freezing behaviour of hydrogel electrolyte thereby incorporating the zwitterionic group and salt into the polymeric network.
6.2 Applications of Hydrogels in Bioelectronics
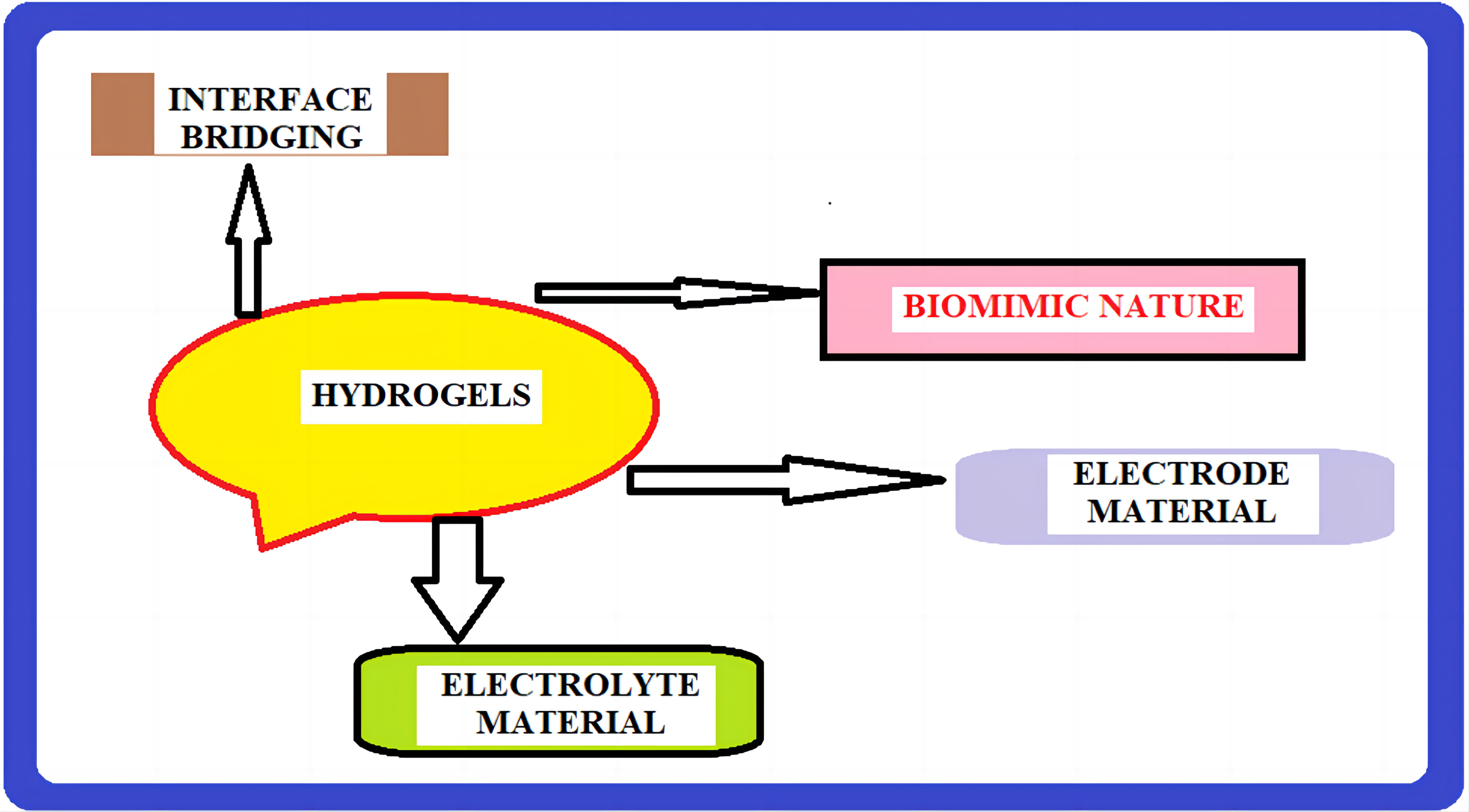
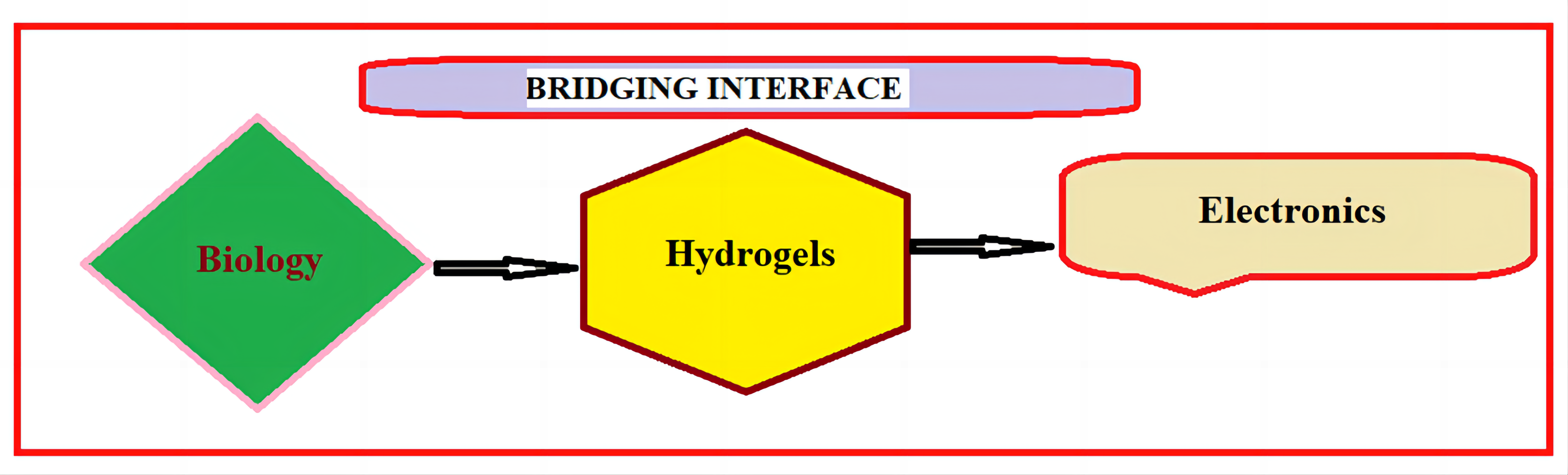
The rapidly expanding fields of neurological science and engineering, diagnosis, therapy, wearable technology, and implantable devices are all based on bioelectronic interfaces. Discovering the connection between disparate materials, such as soft, wet, and live biological systems, and artificial electronic systems is one of science’s most daunting issues. Because of their versatility in electrical, mechanical, and biofunctional engineering, as well as their resemblance to biological tissues, hydrogels have emerged as a viable material candidate for the next generation of bioelectronic interfaces[83] (Figure 10).
|
Figure 10. Hydrogels at the interface between biology and electronics. Hydrogels possess a unique set of properties to bridge the gap between biology and electronics, providing promising opportunities for bioelectronics applications.
7 CONCLUSION
Modern society depends critically on both energy and water, and cutting-edge technologies for sustainable energy conversion and storage as well as water resource management are the subject of extensive research globally. Because of their appealing and customizable physiochemical features, hydrogels are becoming a more desirable material platform for applications linked to energy and water, going beyond their typical biological uses. The use of biopolymeric hydrogels is covered in this chapter, along with important synthetic and practical instruments for the building blocks, crosslinkers, and functional additives. Hydrogels can also be used as trailblazers and models for designing three-dimensional structures made of electrochemically active materials.
Because hydrogels can retain water, they are used in energy storage devices in addition to biomedical, environmental, and organ replacement applications. This review serves as a starting point for future research by outlining important points for researchers to consider. With applications in the energy and environmental domains, this capacity has allowed it to be used as an electrolyte, electrode, and super capacitor. Consequently, wearable electronics and clean, green, and pollution-free transportation are supported by these incredibly effective flexible supercapacitors. The remaining obstacles to adopt hydrogel electrolytes based on biopolymers for sophisticated energy conversion and storage devices, as well as the fundamental strategies to support future advancement and study biopolymer hydrogels are environmentally benign and non-toxic, but they have a low mechanical strength that leads to weak interface bonding, potentially reduced in electrochemical performance under physically demanding conditions, and insufficient cross-linking. Therefore, there are significant restrictions on the electrochemical performance of energy storage devices that use hydrogel electrolytes based on biopolymers. Biopolymers are finding their way into a number of uncharted applications thanks to the drive-in research towards more environmentally friendly materials. Due to their unique blend of mechanical and ionic conductivity, hydrogels are garnering a lot of interest as electrolytes for flexible electrochemical devices. Additionally, the novel conducting biopolymer hydrogels with good electrical and electrochemical behaviour are explored, along with their anti-freezing properties, mechanical tolerance, and conducting behaviour. This makes the hydrogels potentially useful in electronic devices like supercapacitors, biosensors, bioelectronics, solar cells, and memory device applications.
Acknowledgements
The author wishes to acknowledge Muthurangam Government Arts College (Affiliated to Thiruvalluvar University) for providing laboratory facilities.
Conflicts of Interest
The author declared no conflict of interest.
Author Contribution
Guhanathan S solely contributed to the manuscript and approved the final version.
Abbreviation List
MFCs, Microbial fuel cells
NFC, Nanofibrillated cellulose
PAM, Polyacrylamide
PVA, Polyvinyl alcohol
SCs, Super capacitors
SPEs, Solid polymer electrolytes
References
[1] Kopeček J, Vacík J, Lím D. Permeability of membranes containing ionogenic groups. J Polym Sci Part A: Polym Chem, 1971; 9: 2801-2815.[DOI]
[2] Yannas IV, Lee E, Orgill DP et al. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. P Natl Acad Sci, 1989; 86: 933-937.[DOI]
[3] Ganji F, Abdekhodaie MJ, Ramazanisa A. Gelation time and degradation rate of chitosan-based injectable hydrogel. J Sol-Gel Sci Techn, 2007; 42: 47-53.[DOI]
[4] Franklin DS, Guhanathan S. Investigation of citric acid-glycerol based pH-sensitive biopolymeric hydrogels for dye removal applications: A green approach. Ecotox Environ Safe, 2015; 121: 80-86.[DOI]
[5] Franklin DS, Guhanathan S. Performance of silane-coupling agent-treated hydroxyapatite/diethylene glycol-based pH-sensitive biocomposite hydrogels. Iran Polym J, 2014; 23: 809-817.[DOI]
[6] Chitra G, Franklin DS, Guhanathan S et al. Indole-3-acetic acid based tunable hydrogels for antibacterial, antifungal and antioxidant applications. J Macromol Sci A, 2017; 54: 151-163.[DOI]
[7] Lin DC, Yurke B, Langrana NA. Inducing reversible stiffness changes in DNA-crosslinked gels. J Mater Res, 2005; 20: 1456-1464.[DOI]
[8] Stenekes RJH, Talsma H, Hennink WE. Formation of dextran hydrogels by crystallization. Biomaterials, 2001; 22: 1891-1898.[DOI]
[9] Hennink WE, Van Nostrum CF. Novel crosslinking methods to design hydrogels. Adv Drug Deliver Rev, 2001; 54: 13-36.[DOI]
[10] Ye T, Wang J, Jiao Y et al. A Tissue-Like Soft All-Hydrogel Battery. Adv Mater, 2021; 34: 2105120.[DOI]
[11] Guo Y, Fang Z, Yu G. Multifunctional hydrogels for sustainable energy and environment. Polym Int, 2021; 70: 1425-1432.[DOI]
[12] Tinesha S, Veeradasan P, Shing FK et al. The recent development of polysaccharides biomaterials and their performance for supercapacitor applications. Mater Res Bull, 2020; 126: 110839.[DOI]
[13] Kong L, Yuan Z, Sun N et al. Advances in flexible hydrogels for light-thermal-electricity energy conversion and storage. J Energy Storage, 2023; 60: 106618.[DOI]
[14] Mo F, Cui M, He N et al. Recent progress and perspectives on advanced flexible Zn-based batteries with hydrogel electrolytes. Mater Res Lett, 2022; 10: 501-520.[DOI]
[15] Ai X, Zhao Q, Duan Y et al. Zinc polyacrylamide hydrogel electrolyte for quasi-solid-state electrochromic devices with low-temperature tolerance. Cell Rep Phys Sci, 2022; 3: 101148.[DOI]
[16] Cheng Y, Chi X, Yang J et al. Cost attractive hydrogel electrolyte for low temperature aqueous sodium ion batteries. J Energy Storage, 2021; 40: 102701.[DOI]
[17] Sakthivel M, Franklin, DS, Sudarsan S et al. Investigation on pH-switchable (itaconic acid/ethylene glycol/acrylic acid) based polymeric biocompatible hydrogel. RSC Adv, 2016; 6: 106821-106831.[DOI]
[18] Sakthivel M, Franklin, DS, Sudarsan S et al. Investigation on Au-nano incorporated pH-sensitive (itaconic acid/acrylic acid/triethylene glycol) based polymeric biocompatible hydrogels. Mat Sci Eng C, 2017; 75: 517-523.[DOI]
[19] Sudarsan S, Franklin DS, Sakthivel M et al. Non toxic, antibacterial, biodegradable hydrogels with pH-stimuli sensitivity: Investigation of swelling parameters. Carbohyd Polym, 2016; 148: 206-215.[DOI]
[20] Yang H, Liu Y, Kong L et al. Biopolymer-based carboxylated chitosan hydrogel film crosslinked by HCl as gel polymer electrolyte for all-solid-sate supercapacitors. J Power Sources, 2019; 426: 47-54.[DOI]
[21] Li X, Yang Q, Zhao Y et al. Dual physically crosslinked double network hydrogels with high toughness and self-healing properties. Soft Matter, 2017; 5: 911-920.[DOI]
[22] Li SN, Li B, Gong LX et al. Enhanced mechanical properties of polyacrylamide/chitosan hydrogels by tuning the molecular structure of hyperbranched polysiloxane. Mater Design, 2019; 162: 162-170.[DOI]
[23] Wang Z, Li H, Tang Z et al. Hydrogel Electrolytes for Flexible Aqueous Energy Storage Devices. Adv Funct Mater, 2018; 28: 1804560.[DOI]
[24] Huang S, Wan F, Bi S et al. A Self-Healing Integrated All-In-One Zinc-Ion Battery. Angew Chem Int Edit, 2019; 58: 4313-4317.[DOI]
[25] Li H, Han C, Huang Y et al. An extremely safe and wearable solid-state zinc ion battery based on a hierarchical structured polymer electrolyte. Energ Environ Sci, 2018; 4: 941-951.[DOI]
[26] Li H, Liu Z, Liang G et al. Waterproof and Tailorable Elastic Rechargeable Yarn Zinc Ion Batteries By A Cross-Linked Polyacrylamide Electrolyte. ACS Nano, 2018; 12: 3140-3148.[DOI]
[27] Liu Z, Wang D, Tang Z et al. A mechanically durable and device-level tough Zn-MnO2 battery with high flexibility. Energy Storage Mater, 2019; 23: 636-645.[DOI]
[28] Wang D, Li H, Liu Z et al. A Nanofibrillated Cellulose/Polyacrylamide Electrolyte-Based Flexible and Sewable High-Performance Zn-MnO2 Battery with Superior Shear Resistance. Small, 2018; 14: 1803978.[DOI]
[29] Mo F, Li H, Pei Z et al. A smart safe rechargeable zinc ion battery based on sol-gel transition electrolytes. Sci Bull, 2018; 63: 1077-1086.[DOI]
[30] Mo F, Liang G, Meng Q et al. A flexible rechargeable aqueous zinc manganese-dioxide battery working at -20℃. Energ Environ Sci, 2019; 12: 706-715.[DOI]
[31] Ji D, Park JM, Oh MS et al. Superstrong, superstiff, and conductive alginate hydrogels. Nat Commun, 2022; 13: 3019.[DOI]
[32] Himori S, Sakata T. Free-standing conductive hydrogel electrode for potentiometric glucose sensing. RSC Adv, 2022; 12: 5369-5373.[DOI]
[33] Guo H, He W, Lu Y et al. Self-crosslinked polyaniline hydrogel electrodes for electrochemical energy storage. Carbon, 2015; 92: 133-141.[DOI]
[34] Wang Z, Li X, Yang Z et al. Fully transient stretchable fruit‐based battery as safe and environmentally friendly power source for wearable electronics. EcoMat, 2021; 3: e12073.[DOI]
[35] Gupta A, Sardana S, Dahiya S et al. Binder-free polypyrrole/fluorinated graphene nanocomposite hydrogel as a novel electrode material for highly efficient supercapacitors. Appl Surf Sci Adv, 2022; 11: 100297.[DOI]
[36] Lee Y, Yim SG, Lee GW et al. Self-Adherent Biodegradable Gelatin-Based Hydrogel Electrodes for Electrocardiography Monitoring. Sensors, 2020; 20: 5737.[DOI]
[37] Wang J, Ye T, Li Y et al. Ultrasoft all-hydrogel aqueous lithium-ion battery with a coaxial fiber structure. Polym J, 2022; 54: 1383-1389.[DOI]
[38] Yang M, Luo J, Guo X et al. Aqueous Rechargeable Sodium-Ion Batteries: From Liquid to Hydrogel. Batteries, 2022; 8: 180.[DOI]
[39] Ma L, Chen S, Pei Z et al. Single-Site Active Iron-Based Bifunctional Oxygen Catalyst for a Compressible and Rechargeable Zinc-Air Battery. ACS Nano, 2018; 12: 1949-1958.[DOI]
[40] Huang Y, Li Z, Pei Z et al. Solid-State Rechargeable Zn//Nico and Zn-Air Batteries with Ultralong Lifetime and High Capacity: The Role of a Sodium Polyacrylate Hydrogel Electrolyte. Adv Energy Mater, 2018; 8: 1802288.[DOI]
[41] Tang K, Yuan C, Xiong Y et al. Inverse-opal-structured hybrids of N, S-codoped-carbon-confined Co9S8 nanoparticles as bifunctional oxygen electrocatalyst for on-chip all-solid-state rechargeable Zn-air batteries. Appl Catal B-Environ, 2020; 260: 118209.[DOI]
[42] Liu J, Hu M, Wang J et al. An intrinsically 400% stretchable and 50% compressible NiCo//Zn battery. Nano Energy, 2019; 58: 338-346.[DOI]
[43] Ma L, Chen S, Wang D et al. Super-Stretchable Zinc-Air Batteries Based on an Alkaline-Tolerant Dual-Network Hydrogel Electrolyte. Adv Energy Mater, 2019; 9: 1803046.[DOI]
[44] Huang Y, Liu J, Wang J et al. An Intrinsically Self-Healing Nico//Zn Rechargeable Battery with a Self-Healable Ferric-Ion-Crosslinking Sodium Polyacrylate Hydrogel Electrolyte. Angew Chem Int Edit, 2018; 57: 9810-9813.[DOI]
[45] Iwakura C, Furukawa N, Ohishi T et al. Nickel/Metal Hydride Cells Using an Alkaline Polymer Gel Electrolyte Based on Potassium Salt of Crosslinked Poly(acrylic acid). Electrochem, 2001; 69: 659-663.[DOI]
[46] Iwakura C, Nohara S, Furukawa N et al. The possible use of polymer gel electrolytes in nickel/metal hydride battery. Solid State Ionics, 2002; 148: 487-492.[DOI]
[47] Iwakura C, Ikoma K, Nohara S et al. Capacity Retention Characteristics of Nickel/Metal Hydride Batteries with Polymer Hydrogel Electrolyte. Electrochem Solid St, 2005; 8: A45.[DOI]
[48] Choudhury NA, Sampath S, Shukla AK. Gelatin Hydrogel Electrolytes and Their Application to Electrochemical Supercapacitors. J Electrochem Soc, 2008; 155: A74.[DOI]
[49] Cheng Y, Chi X, Yang J et al. Cost attractive hydrogel electrolyte for low temperature aqueous sodium ion batteries. J Energy Storage, 2021; 40: 102701.[DOI]
[50] Lee Y, Yim SG, Lee GW et al. Self-Adherent Biodegradable Gelatin-Based Hydrogel Electrodes for Electrocardiography Monitoring. Sensors, 2020; 20: 5737.[DOI]
[51] Francielli SG, Cameron VB, Saeid B et al. A novel calcium-ion solid polymer electrolyte based on crosslinked poly(ethylene glycol) diacrylate. J Power Sources, 2019; 414: 302-307.[DOI]
[52] Fu Q, Hao S, Meng L et al. Engineering Self-Adhesive Polyzwitterionic Hydrogel Electrolytes for Flexible Zinc-Ion Hybrid Capacitors with Superior Low-Temperature Adaptability. ACS Nano, 2021; 15: 18469-18482.[DOI]
[53] Liu C, Xie X, Lu B et al. Electrolyte Strategies toward Better Zinc-Ion Batteries. Acs Energy Lett, 2021; 6: 1015-1033.[DOI]
[54] Liu J, Ahmed S, Khanam Z et al. Ionic Liquid-Incorporated Zn-Ion Conducting Polymer Electrolyte Membranes. Polymers, 2020; 12: 1755.[DOI]
[55] Li X, Wang H, Sun X et al. Flexible Wide-Temperature Zinc-Ion Battery Enabled by an Ethylene Glycol-Based Organohydrogel Electrolyte. Acs Appl Energ Mater, 2021; 4: 12718-12727.[DOI]
[56] Parvini E, Hajalilou A, Tavakoli M. A bright future of hydrogels in flexible batteries and Supercapacitors storage systems: A review. Int J Energ Res, 2022; 46: 13276-13307.[DOI]
[57] Rapisarda M, Marken F, Meo M et al. Graphene oxide and starch gel as a hybrid binder for environmentally friendly high-performance supercapacitors. Commun Chem, 2021; 4: 169.[DOI]
[58] Zhang R, Jing X, Chu Y et al. Nitrogen/oxygen co-doped monolithic carbon electrodes derived from melamine foam for high-performance supercapacitors. J Mater Chem A, 2018; 6: 17730-17739.[DOI]
[59] Guo Y, Wang T, Chen X et al. Agar-based porous electrode and electrolyte for flexible symmetric supercapacitors with ultrahigh energy density. J Power Sources, 2021; 507: 230252.[DOI]
[60] Moon WG, Kim GP, Lee M et al. A Biodegradable Gel Electrolyte for Use in High-Performance Flexible Supercapacitors. Acs Appl Mater Inter, 2015; 7: 3503-3511.[DOI]
[61] Mitta SB, Harpalsinh R, Kim J et al. Flexible Supercapacitor with a Pure DNA Gel Electrolyte. Adv Mater Interfaces, 2022; 9: 2200133.[DOI]
[62] Yang H, Liu Y, Kong L et al. Biopolymer-based carboxylated chitosan hydrogel film crosslinked by HCl as gel polymer electrolyte for all-solid-sate supercapacitors. J Power Sources, 2019; 426: 47-54.[DOI]
[63] Landi G, Sorrentino A, Iannace S et al. Differences between graphene and graphene oxide in gelatin based systems for transient biodegradable energy storage applications. Nanotechnol, 2017; 28: 054005.[DOI]
[64] Railanmaa A, Lehtimäki S, Lupo D. Comparison of starch and gelatin hydrogels for non-toxic supercapacitor electrolytes. Appl Phys A, 2017; 123: 459.[DOI]
[65] Esawy T, Khairy M, Hany A et al. Flexible solid-state supercapacitors based on carbon aerogel and some electrolyte polymer gels. Appl Phys A, 2018; 124: 566.[DOI]
[66] Landi G, Notte LL, Palma AL et al. A Comparative Evaluation of Sustainable Binders for Environmentally Friendly Carbon-Based Supercapacitors. Nanomaterials-Basel, 2022; 12: 46.[DOI]
[67] Xu T, Liu K, Sheng N et al. Biopolymer-based hydrogel electrolytes for advanced energy storage/conversion devices: Properties, applications, and perspectives. Energy Storage Mater, 2022; 48: 244-262.[DOI]
[68] Marinow A, Katcharava Z, Binder WH. Self-Healing Polymer Electrolytes for Next-Generation Lithium Batteries. Polymers-Basel, 2023; 15: 1145.[DOI]
[69] Han J, Jang S, Kim BK et al. Electrochemical study of agarose hydrogels for natural convection on macroelectrodes and ultramicroelectrodes. J Anal Sci Technol, 2023; 14: 10.[DOI]
[70] Lu J, Lin X, Wang S et al. High ionic conductivity and toughness hydrogel electrolyte for high-performance flexible solid-state zinc-ion hybrid supercapacitors enabled by cellulose-bentonite coordination interactions. Green Chem, 2023; 25: 1635-1646.[DOI]
[71] Shen J, Dai Y, Xia F et al. Polyacrylamide/EDTA-modified chitosan/graphene oxide hydrogels as an adsorbent and supercapacitor for sustainable applications. Sustain Mater Techno, 2023; 36: e00586.[DOI]
[72] Zhu X, Jiang G, Wang G et al. Cellulose-based functional gels and applications in flexible supercapacitors. Resour Chem Mater, 2023; 2: 177-188.[DOI]
[73] Liu C, Wang X, Zhang H et al. Self-Healable, High-Strength Hydrogel Electrode for Flexible Sensors and Supercapacitors. Acs Appl Mater Inter, 2021; 13: 36240-36252.[DOI]
[74] Sundharamurthi S, Sreeraj TR, Amalraj A et al. Chapter 13 - Biopolymer composites in supercapacitor and energy storage devices. Biopolymers Ind Appl, 2021; 13: 305-329.[DOI]
[75] Rosi M, Fatmizal MNZ, Fathona IWF et al. Hydrogel neutral electrolyte of PVA/HEC for flexible supercapacitor. AIP Conf P, 2022; 2708: 070002.[DOI]
[76] Lu K, Jiang T, Hu H et al. Hydrogel Electrolytes for Quasi-Solid Zinc-Based Batteries. Front Chem, 2020; 8: 546728.[DOI]
[77] Mathew V, Sambandam B, Kim S et al. Manganese and Vanadium Oxide Cathodes for Aqueous Rechargeable Zinc-Ion Batteries: A Focused View on Performance, Mechanism, and Developments. ACS Energy Lett, 2020; 5: 2376-2400.[DOI]
[78] Ai X, Zhao Q, Duan Y et al. Zinc polyacrylamide hydrogel electrolyte for quasi-solid-state electrochromic devices with low-temperature tolerance. Cell Rep Phys Sci, 2022; 3: 101148.[DOI]
[79] Gomez I, Alesanco Y, Blázquez JA et al. Room-Temperature Self-Standing Cellulose-Based Hydrogel Electrolytes for Electrochemical Devices. Polymers, 2020; 12: 2686.[DOI]
[80] An L, Zhao TS, Zeng L. Agar chemical hydrogel electrode binder for fuel-electrolyte-fed fuel cells. Appl Energ, 2013; 109: 67-71.[DOI]
[81] Allen RM, Bennetto HP. Microbial fuel-cells. Appl Biochem Biotech, 1993; 39: 27-40.[DOI]
[82] Xing D, Wang H, Li L. Preparation of new anode of microbial fuel cell using Escherichia coli-immobilizing hydrogel: Proceedings of the 4th International Conference on Mechatronics, Materials, Chemistry and Computer Engineering 2015. Xi’an, China, 12-13 December 2015.[DOI]
[83] Yuk H, Lu B, Zhao X. Hydrogel bioelectronics. Chem Soc Rev, 2019; 48: 1642.[DOI]
Copyright © 2024 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.




 Copyright ©
Copyright ©