Raffia hookeri Fiber: Effect of Alkali Treatment on Morphology, Composition and Technological Application Properties
Beckley Victorine Namondo1*, Ekane Peter Etape1, Josepha Foba-Tendo1
1Department of Chemistry, Faculty of Science, University of Buea, Buea, Cameroon
*Correspondence to: Beckley Victorine Namondo, PhD, Senior Lecturer, Department of Chemistry, Faculty of Science, University of Buea, Buea P.O. Box 63, Cameroon; Email: namondo31@gmail.com
Abstract
Background: The presence of natural fiber in composites has shown a positive influence on the resilience of the reinforced polymer composites but this influence shows variability with Natural fiber Surface treatment, concentration of treatment solution and the length of treatment time. 5wt.% of sodium hydroxide (NaOH) alkali solution concentration has shown to be effective and efficient in dissolving the hemicellulose and the lignin portions while preserving the cellulose part of the fiber. There is enough literature on the chemical, physical, mechanical and thermal properties of Natural fibers but there is no comprehensive study on the chemical composition, morphology and concentration of the chemical compositions, water absorption behavior and thermal properties of Raffia hookeri fibers.
Objective: Given that these properties vary with both the plant type and Botanical species, we are taking this advantage to study in detailed the morphological and composition of Raffia hookeri fiber and to Characterize the fiber for the evaluation of Mechanical Properties for Technological application.
Methods: Raffia hookeri fiber was extracted and the basic technological application properties such as moisture and water adsorption, morphology, chemical, physical and thermal properties were analyzed using, chemical composition analysis, scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS), Fourier transformed Infrared (FTIR), X-ray diffraction (XRD) and Thermo gravimetric analysis (TGA) while tensile test was used to evaluate the mechanical behavior of the fibers.
Results: Chemical analysis revealed that the fiber was made up of cellulose (40wt.%), hemicellulose (20wt.%), lignin (33wt.%) and extractives 7wt.%, Thermal analysis indicated thermal stability up to 220oC, while the SEM/EDS results revealed that the effect of treatment on the fiber surface increased with the treatment time and reached maximum after 10h. This variability inferred on the morphological composition which recorded modifying effects both on the fiber-polymer matrix and the mechanical properties of the treated fibers. The FT-IR and XRD results indicated modification of the functional groups on the fiber morphology which improved on the mechanical, moisture and water adsorption capacity of the treated fibers.
Conclusion: The alkali treatment has modifying effects on Raffia hookeri fiber morphology and chemical composition which affect its technological application Properties.
Keywords: natural fiber, Raffia hookei, microstructure, morphological composition, application properties
1 INTRODUCTION
Natural evolution of the environment such as climate have embarrassed the world with untold damage on infrastructure and health. Some of these embarrassments have been initiated by shortfalls experienced by mechanical structures especially cement composites. Efforts to solve this problem has shifted interest to reinforcement of composites with polymeric materials such as fiber. The presence of synthetic fiber in composites has shown a positive influence on the resilience of the reinforced polymer composites but to restrain the advancement of synthetic polymer composites is the dependence of performance on sudden and adverse mechanical stress. The stress which is not expressed on the surface but may lead to degradation complexities in the internal structure of the material leading to undetectable residual damage following failure. However, even in cases where there exist the possibility of testing, it is very expensive and unaffordable. This problem has caused an insurgence in the quest for alternative techniques to improve on the reliability of composite materials. Natural problems can be solved using natural solutions. Thus industrial application of Nano lignocellulosic fibers present a new avenue for valorization of plant based biomass residues, especially in tropical African countries such as Cameroon where forestry, food, and cash crop production are important economic activities that generate a diverse range of residues. The motivating characteristic properties of natural fiber include: low cost and low density, high specific strength and rigidity, and ease of processing attributed to their nonabrasive nature. Several studies have reported on the use of natural fibers such as raffia[1-4], Urena lobate[5], flax[6,7], sisal[8-10], abaca[11-13], banana[14,15], coir[16,17] and pineapple[18] to manufacture polymer composites but very little attention has been given to the potential use of raffia palm fibers in polymer composites[4] and the few cases reported have limited attention on defects and distortions that occur during the pulping process of fiber which affect mechanical properties such as resistance and breaking energy. There is the need to look into the effects of fiber on both the composite mechanical properties and the correlation between the fibers inorganic composition and the compositional effects on the matrix of cement composites. Generally, significant changes in surface morphology are observed after alkali treatment. The removal of surface impurities, non-cellulosic materials, inorganic substances and waxes result in rougher surfaces and better fiber separation due to alkali treatments enhancing the mechanical properties of the fiber such as the strength which relates positively to defibrillation[19]. Rough surfaces in fiber are usually attributed to silica bodies but there has been a little controversy in analyzing the effect of these silica bodies. Pinheiro et al.[20] reported that “fibers can be used for diverse applications, such as reinforcement in polymeric matrix composites or to produce glucose for second-generation ethanol and in both applications, surface properties are essential, and the presence of silica bodies is usually harmful” while Buson et al.[21] reported that the presence of silica bodies’ increased surface roughness which contributes to the best fiber/matrix adhesion in composites. Elzubair et al.[22] in a study on piassava fiber reported on the presence of an ordered array of silica rich spiny protrusions and reentrant cells which could interact with the polymer matrix in a composite. He further used energy-dispersive X-ray spectroscopy (EDS) analysis to confirm silica in these protrusions. Other literature reports have postulated that these spiny protrusions act as anchors and enhances the mechanical interlocking at the fiber matrix interface[23-27]. Fedele et al.[28], indicated that the tensile strength of raffia fibers do not change significantly with fiber length and crosshead speed but have a numerical value lower than those of flax, jute, hemp and sisal fibers but exhibit chemical, thermal, physical and elongation properties in the range found in several natural fibers that are used as reinforcements for polymers. Tran et al.[29] after investigating the microstructure coir fibers reported that the fiber comprise of several elemental fibrillates which are tightly packed together with each having its own lumen comprising essentially of three distinct regions: a porous inner region consisting of large lumens, a more compact middle region and a very thin outer surface (epidermis) and between the two lies the inner core. The middle lamella glues the elementary fibers together and, it is made up of lignin and hemicellulose[30,31]. Synchrotron-based Fourier-transform infrared spectromicroscopy of the cross-section of the fiber showed that lignin is concentrated mostly on the outside while cellulose and pectin are concentrated in the mid-section of the fibers[32]. However, literature on the fiber morphology and physicochemical composition has failed to indicate the proportional presence of minerals in different concentrations at various portions of the fiber microstructure[33], while studying the microstructures of ceramic composite containing concrete as the matrix and raffia palm fibers at different percentages by weight as reinforcement was studied, attributed thermal structural changes to atomic vibration. His observation is an indication that addition of raffia palm fibers to the matrix leads to changes in the morphology which are related to other mineral atoms present and postulation was strengthen by Raymond et al.[34] who reported that raffia fiber treatment reduced the polyester matrix adhesion. Raffia fiber is mainly constituted of carbon (C) and oxygen (O) with a percentage of mass, respectively, in the intervals 51-57% and 41-48%, and composites can be made based on fibers by observing the bulk density and Young’s modulus of the matrix which are comparable to polymer in order to make better choices of matrix[9,35]. This variability in density and Young’s modulus of the matrix have a strong correlation with the masses of all the elements including the metals introduced into the matrix by the fibers and, the proportion and type of minerals are a function of the treatment method, time, species and even the botanical origin of the plant.
Fiber treatment with sodium hydroxide solution also known as mercerization is not only economical but effective in improving the interfacial incompatibility between the matrix and the fiber, reduce water uptake of fibers by removing the hydroxyl groups associated with hemicellulose. It improves the adhesive characteristics of the fiber surface by removing natural waxy materials, hemicellulose and artificial impurities[28]. However, the effect of NaOH treatment varies with concentration of the alkaline solution, treatment temperature and the length treatment time. The removal of hemicellulose and lignin is known to improve the tensile strength as well as the modulus of elasticity of the composite produced with the treated fiber, in comparison with those containing untreated fibers. Elenga et al.[34] studied the effects of alkali treatment on the surface chemistry and properties of raffia fiber by treating the fiber with different concentrations (2.5, 5 and 10 wt.%) of sodium hydroxide (NaOH) so as to preserve the cellulose part of the fiber but dissolving the hemicellulose and the lignin portions. The results showed that higher concentration of sodium hydroxide attacked the cellulose, leading to fiber weakening.
Portland cement which is one of the basic materials for the fabrication of cement composites is a blend of inorganic minerals in defined proportions. Introduction of any other impurity material in the matrix may interfere with the original formulation values which may directly or indirectly infer compositional effects on the matrix but authors have failed to evaluate and / or indicate the variability in fiber morphology with the concentration of Alkali with respect to the mineral compositional effect of fiber morphology on the host cement matrix. There is enough literature on the chemical, physical, mechanical and thermal properties of Natural fibers but there is no comprehensive study on the chemical composition, morphology and concentration of the chemical compositions, water absorption behavior and thermal properties of Raffia hookeri fibers. Given that these properties vary with both the plant type and Botanical species, we are taking this advantage to study in detailed the morphological and composition of Raffia hookeri fiber and to Characterize the fiber for the evaluation of Mechanical Properties for Technological application.
2 MATERIALS AND METHODS
2.1 Materials
The raffia fibers used in this study shown in Figure 1 were harvested from the Raffia hookeri species from Bangem, Kupe Muanenguba Division, South West Region of Cameroon. Sodium hydroxide (analytical grade), deionized water, fresh water and all basic laboratory equipment were used.
|
Figure 1. Raffia fibers used in this study. A: Raffia hookeri bamboo; B: Raw fiber as harvested from the stem; C: Fiber samples after pretreatment.
2.2 Methodology
Raffia fibers used in this study shown in Figure 1, were harvested from the Raffia hookeri species, at Bangem, Kupe Muanenguba Division, South West Region of Cameroon. The fibers were taken from an adult Raffia hookeri bamboo plants, with dry sticks, mature bamboo fronts (aged 4 to 7 years) from which they were extracted manually with the aid of a kitchen knife: Figure 1B, while the fiber samples obtained were washed in warm fresh water at 50±2°C using the vertical autoclave, and rinsed in deionized water at room temperature then oven dried at 80°C for 24h are shown in Figure 1C. This pretreatment was to ensure the ease of workability, processing and characterization by eliminating binders and oily substances as well as other impurities on the fiber surface. The dried fibers were subsequently air-cooled to room temperature and preserved in a desiccator for further use. To treat the fiber, they were immersed in 5% aqueous sodium hydroxide solution for varied periods: (0.5h, 2h, 4h and 10h), under gentle agitation. The fibers were then washed first in tap water followed by deionized water to stabilize the pH to 7 as that of the deionized water used. Furthermore, the fibers were oven dried again at 60±2°C for 24h, cool down to room temperature (25°C) and preserved in the desiccator for further use.
2.2.1 Moisture Content
The moisture content test of the Raffia hookeri bamboo fibers is carried out based on the loss of water mass by drying in an oven until a constancy of weight while the moisture content test is done by weighing approximately 1.0g of each type of fiber using an analytical balance. The samples in triplicates are introduced into a humidity generator maintained at 25°C then 50°C in separate runs. The samples are subjected to this regime within 4h intervals of removal and weighing until the change in weight turned to zero. The moisture adsorption of the fiber is determined using Equation (1):
|
Wo and Wa are the weights of the dry fiber and the moist fibers respectively.
In addition, to determining the water adsorption capacity, 1.0g of the dry sample is immersed in a beaker with deionized water initially at room temperature 25°C and the sample fibers removed at intervals of 2h, drained from the water, dried on surface with the aid of paper towels and reweighed within 20-30s to avoid any loss in weight by evaporation then replaced into the bath. This cycle is repeated until the change in weight turned to zero. This procedure is done in triplicates and the weight percent of moisture content calculated according to Equation (2):
|
Wa - water absorption (%),
Wf - final weight after immersed in deionized water (g),
Wi - dry fiber initial weight (g).
2.2.2 Tensile Test
Tensile tests are performed using a TA.XT texture analyzer (Stable Micro Systems Ltd., UK) following the preparation method in literature[36] and repeated by Omar et al[37]. The test configuration consist of a “C-shaped” paper attached to the fiber with cyanoacrylate adhesive to ensure the fiber alignment as the sample is loaded into the analyzer by clamping them on both ends with the help of a specialized tensile grip. Once the sample is in place (between the grip), the paper is cut off to start the tests and the fiber is pulled in opposite directions at a constant speed. A load cell of 1kN is employed while the sample deformation is obtained from the cross head displacement of the texture analyzer. A total of 10 samples is used during testing. From the force and displacement results obtained from the tensile tests, the true stress (), is determined using Equation (3),
|
Given that, F is the load obtained from the texture analyzer, D is the diameter of the sample and, l and lo, the deformed and original lengths of the sample, respectively. The real strain (log strain), was determined using Equation (4):
|
2.2.3 Characterization
The structural and functional group of the samples were studied at the Rhodes University, Grahams town, South Africa by Fourier Transform Infrared (FTIR) spectroscopy (Perkin Elmer, Model: spectrum one FT-IR spectrometer). FTIR analysis was carried out by using an FTIR Varian 600-IR, equipped with a Mercury Cadmium Telluride (MCT) detector and attached with Attenuated Total Reflectance (ATR) unit (PIKE MIRacle). Air-dried fiber samples (in powder form) are put into the sample compartment of the ATR and pressed against the diamond crystal. Similar pressure is applied for all measurement by using the pressure applicator attached with a torque knob. The wave number range is chosen in the 4000-600cm-1 range with a 150scan and resolution of 4cm-1. The spectra are detected in absorption mode. Background scanning and correction are performed before running new sample. The relative peak absorbance is normalized (the intensity of highest absorbance peak normalized to unity) for all the IR bands of each sample aiming to analyze the chemical composition of the fibers before and after the treatments. The thermo gravimetric (TG) analysis is also carried out at the Cape Town University, Cape Town South Africa, by TG [NETZSCH STA (Model No 409C)] in nitrogen atmosphere, at a heating rate of 10°C/min, using α-Al2O3 as a reference material. The fiber samples were studied by X-ray diffraction technique (Philips PAN analytical, Netherland) using Cu Kα radiation. The instrument is run at power settings of 35kV and 25mA and the samples are scanned in 2θ ranges from 15 to 80° with the step size of 0.02° and a count time of 4s / step. Peak separations are carried out using Gaussian deconvolution. The sample morphology and chemical composition are done by scanning electron microscopy (SEM) and EDS. The samples are carbon coated (fine carbon tape) to study the morphology. The phase constitution after equilibration is characterized by SEM both in the back scattered and secondary electron imaging mode (HITACHI, Model No.S-3400N). The bulk composition of the equilibrated samples as well as the chemical composition of all the phases observed under the scanning electron microscope were determined using an Electron Diffraction X ray (EDX) analyzer (Thermo Electron Noran System (NSS-300) EDS) attached to the SEM. The EDX analyzer could also detect the oxygen concentration.
3 RESULTS
3.1 Moisture Adsorption and Water Absorption
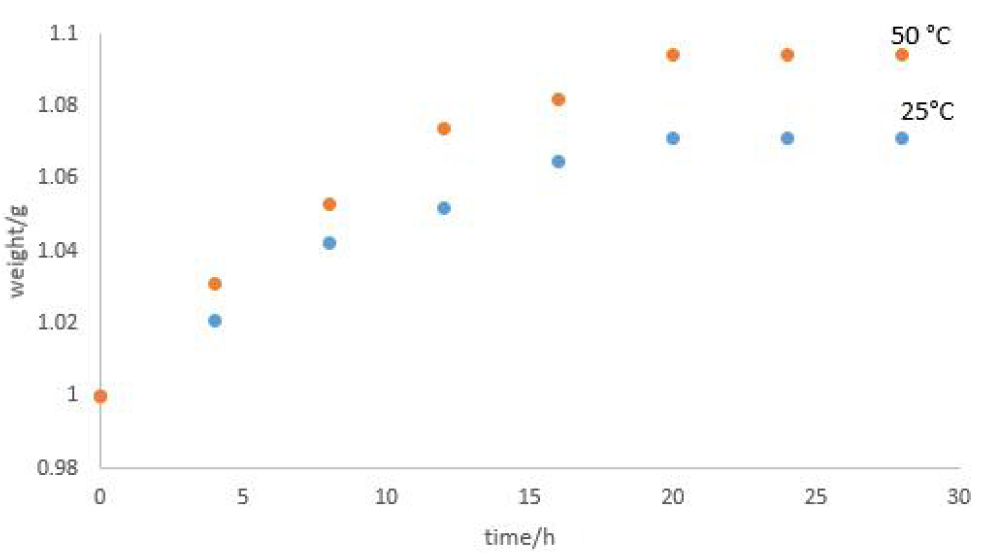
The variation of % moisture adsorbed by Raffia hookeri fibers with respect to soaking time in water at room temperature and 50°C is illustrated in Figure 2. The profile shows that moisture adsorption increased steadily within the first 12h of exposure under both test conditions (25 and 50°C), then stabilized, indicating that the equilibrium saturation stage was reached. The average equilibrium moisture content values obtained for the raw Raffia hookeri at 25 and 50°C are 7.1wt.% and 9.4wt.% respectively.
|
Figure 2. Variation of % moisture adsorbed by Raffia hookeri fibers as a function of time at room temperature.
These results suggested that, both the environmental temperature and time of exposure influence the moisture adsorption behavior of the fiber. However, after treatment with the alkali for 10h, the values obtained increased to 8.72% and 10.98% at room temperature and 50°C respectively. This observation is attributed to the removal of extractives such as waxes, low molecular weight lignin and oils. Also the water adsorption capacity values of the fibers increased with alkaline treatment from 113.21±4.76 to 147.62±6.83. This increase is justified by the increase in the number of hydroxyls groups resulting from the Alkalization process.
3.2 Morphological and Compositional Studies
There is a strong relationship between the morphology and the chemical compositional effects of treatment
3.2.1 Microstructure of Raffia hookeri
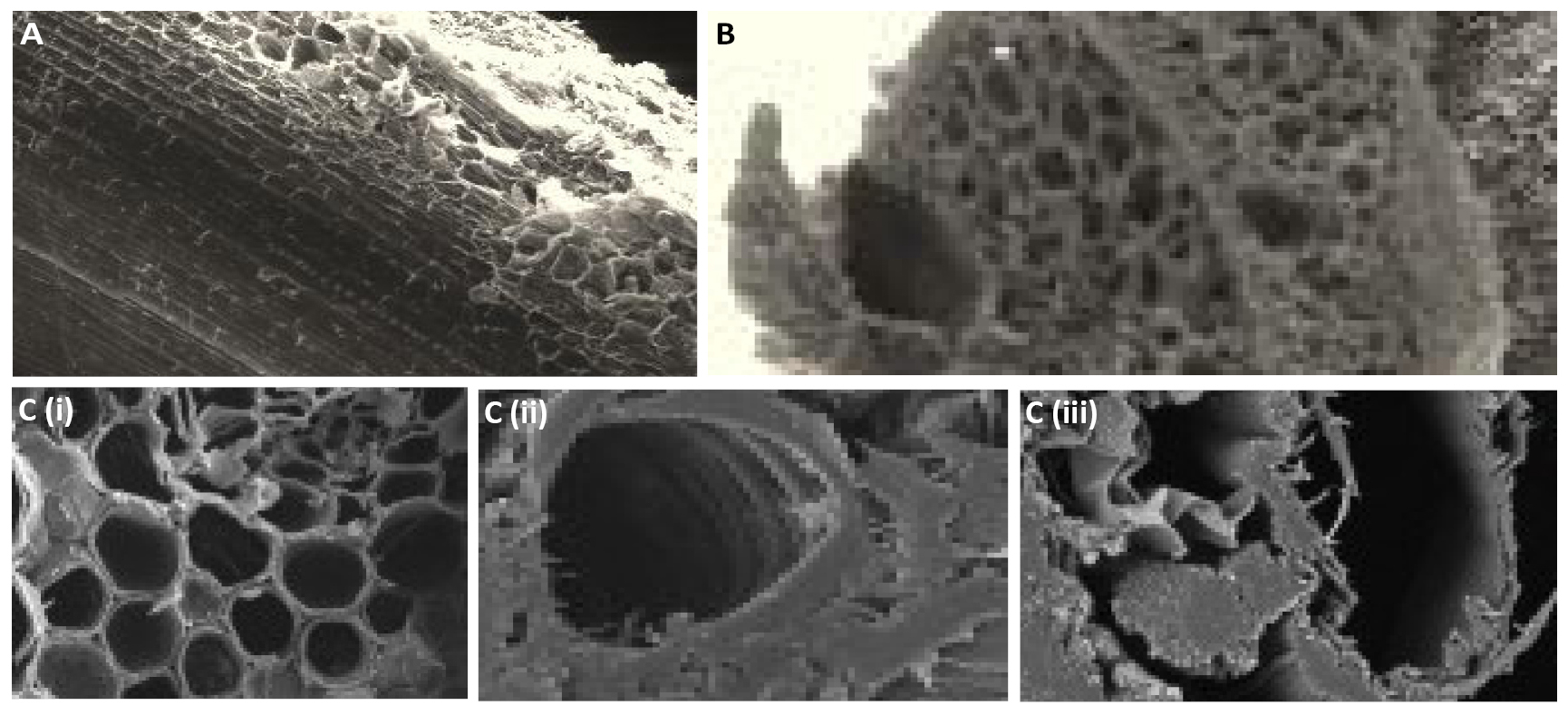
Figure 3 shows the SEM micrographs for: untreated Longitudinal section, untreated cross section and treated cross sections of the sampled fibers. The images reveal a hard core sandwiched between a fragile honey-combed regular hexagonal cell structured periphery and an inner layers each presenting cell walls of thicknesses about 6μm and cell sizes of 5 to 25µm. Furthermore, the structure and elemental composition of the raw and treated fibers are presented on Figures 4-7.
|
Figure 3. SEM micrographs. A: Untreated longitudinal section; B: Untreated cross section; C: Treated cross section with (i) fibrillated structure, (ii) leached lumen after 4h of treatment, and (iii) after 10h of treatment.
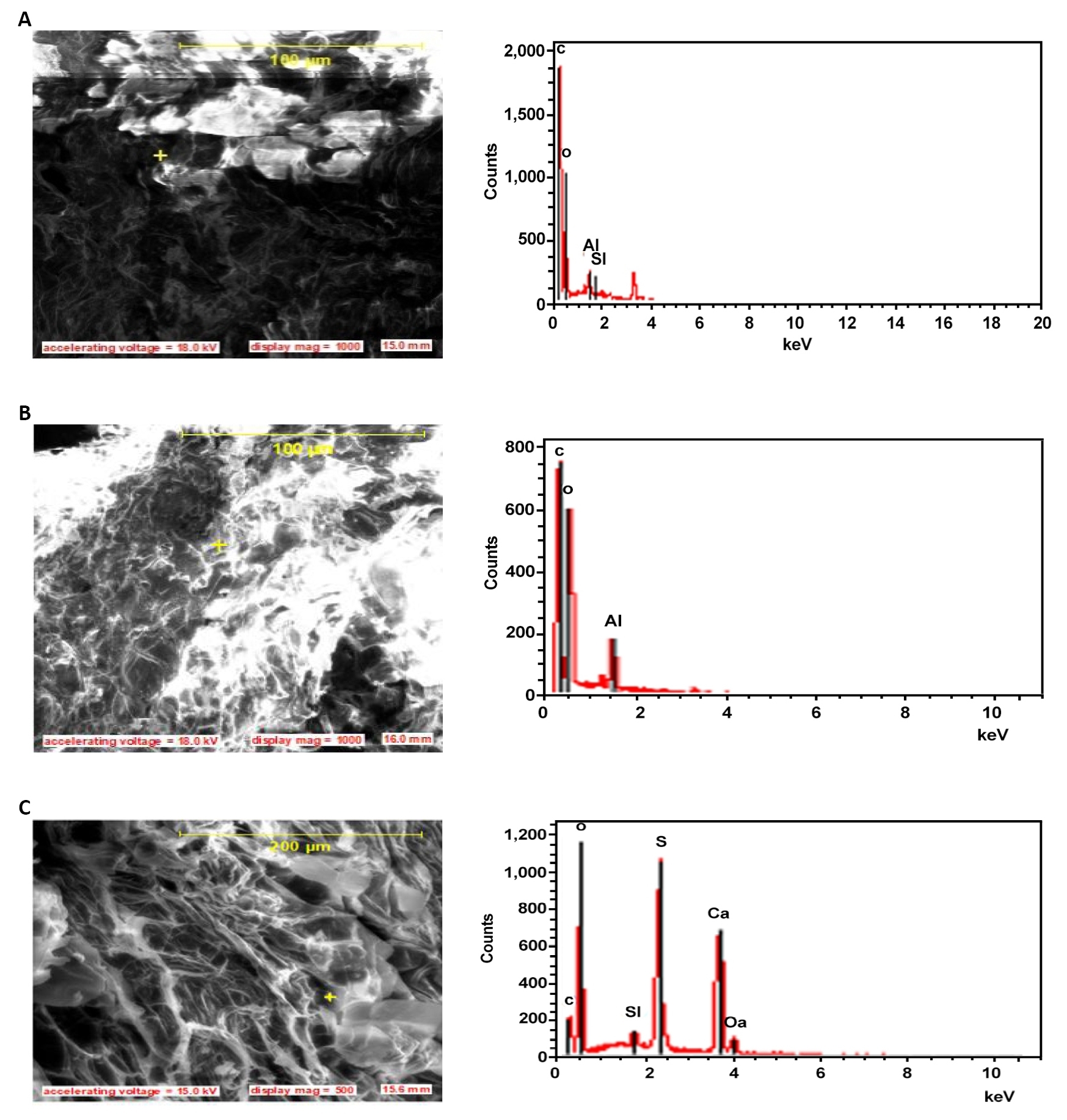
Figure 4. SEM pictures and EDS showing peripheral structure and elemental composition of the (A) Epidermis, (B) Middle lamella, (C) Fibrillate of the raw fibers.
|
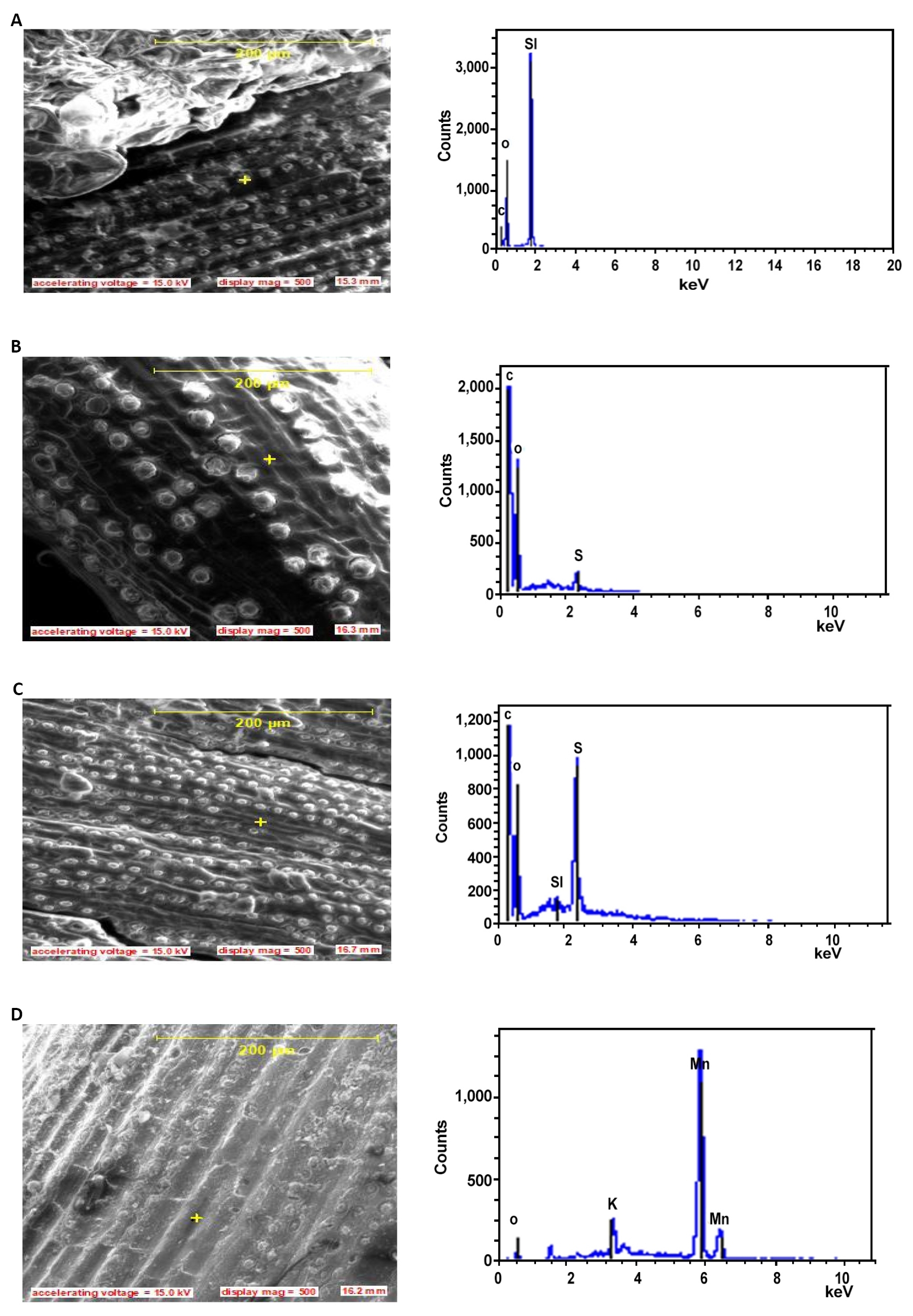
Figure 5. SEM pictures and EDS showing microstructure and elemental composition of the 5% Alkali treated fibers for (A) 0.5h, (B) 2h, (C) 4h, and (D) 10h.
|
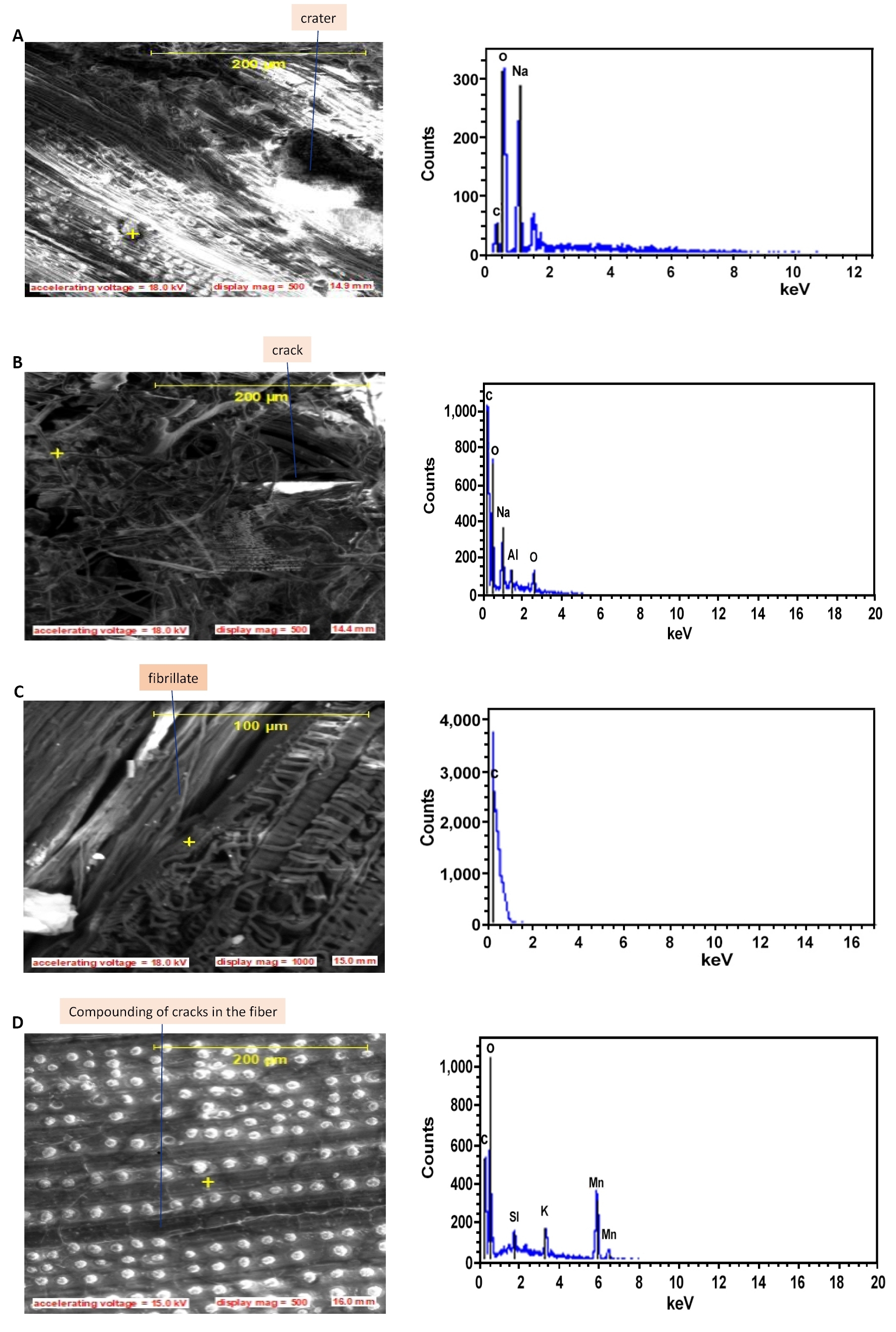
Figure 6. SEM pictures and EDS showing elemental composition and microstructure (A) Crater, (B) Crack, (C) fibrillates, (D) compounding of cracks in the 5% Alkali treated fibers.
|
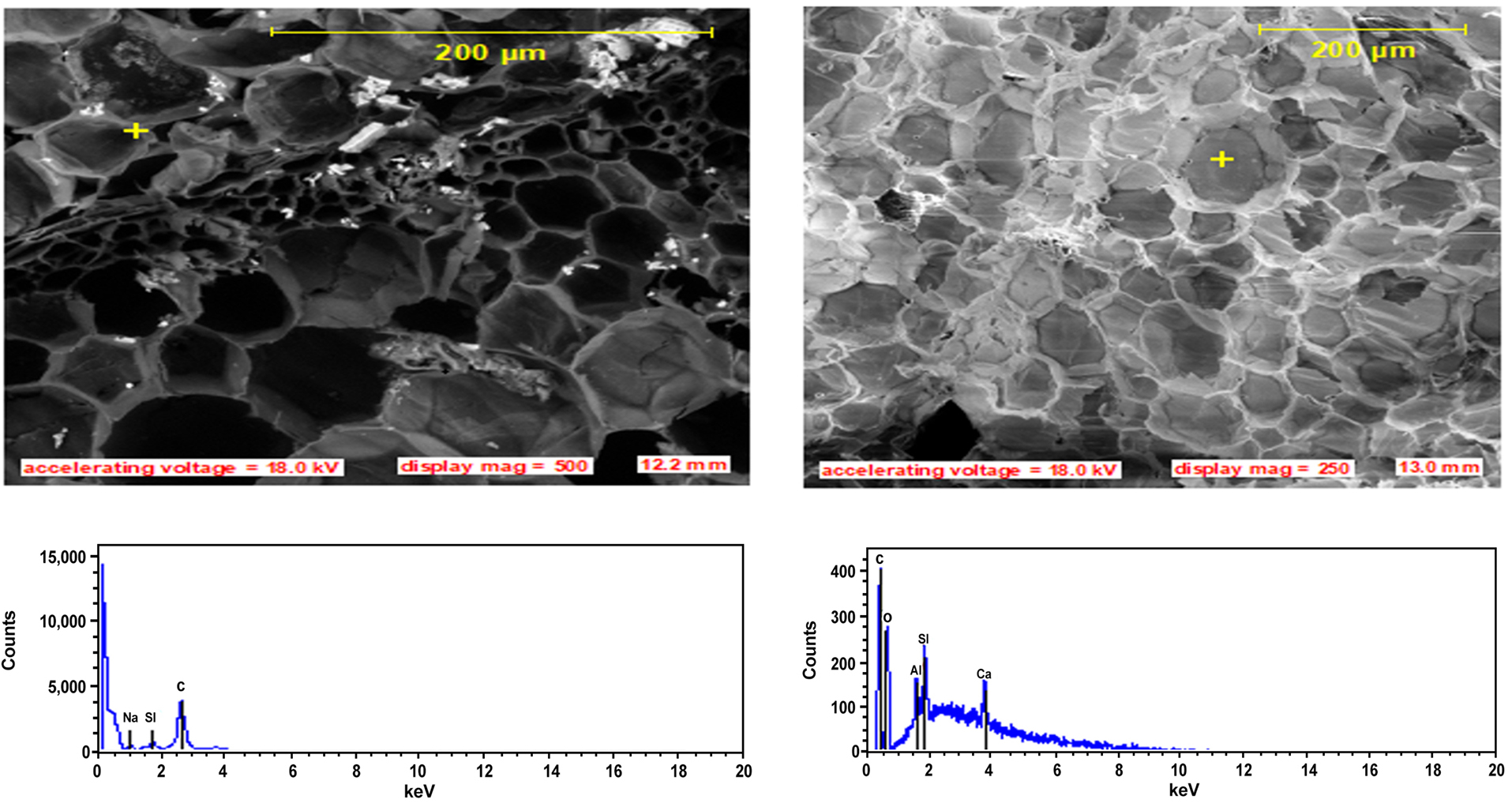
Figure 7. SEM pictures and EDS profiles showing the morphology and elemental composition of the fibrillate cores and Lumens.
3.2.2 Chemical Composition and Possible Compositional Effect on the Polymer -Matrix Interface
The chemical composition of the fiber is the prime variable of evaluation as far as fiber treatment is concerned. The results of chemical composition (dry matter basis) of Raffia hookeri obtained in wt.% cellulose, hemicellulose and extractives (pectin, wax, minerals and ash) are compared with literature values from Raffia farinifera in Table 1. The results obtained in the present study comprise of cellulose (40wt.%), hemicellulose (20wt.%), lignin (33wt.%) and extractives 7%. The literature values reveal a difference from those of the species under investigation which recorded a lower percentage cellulose and extractive content but higher wt.% of lignin and hemicellulose.
Table 1. Chemical Composition of Raffia hookeri Compared to Raffia Farinifera
Cellulose (wt.%) |
Hemicellulose (wt.%) |
Lignin (wt.%) |
Other (pectin, wax, ash and minerals) wt.% |
Reference / Raffia Species |
53.0±0.9 |
13.0±1.2 |
24.0±2.8 |
10.0 |
Fadele et al.[28], Raffia farinifera |
40.0±1.6 |
20.1±0.9 |
33.0±3.1 |
7.0 |
Present study, Raffia hookeri |
3.2.3 X-ray Diffraction Results of the Raffia hookeri Fiber
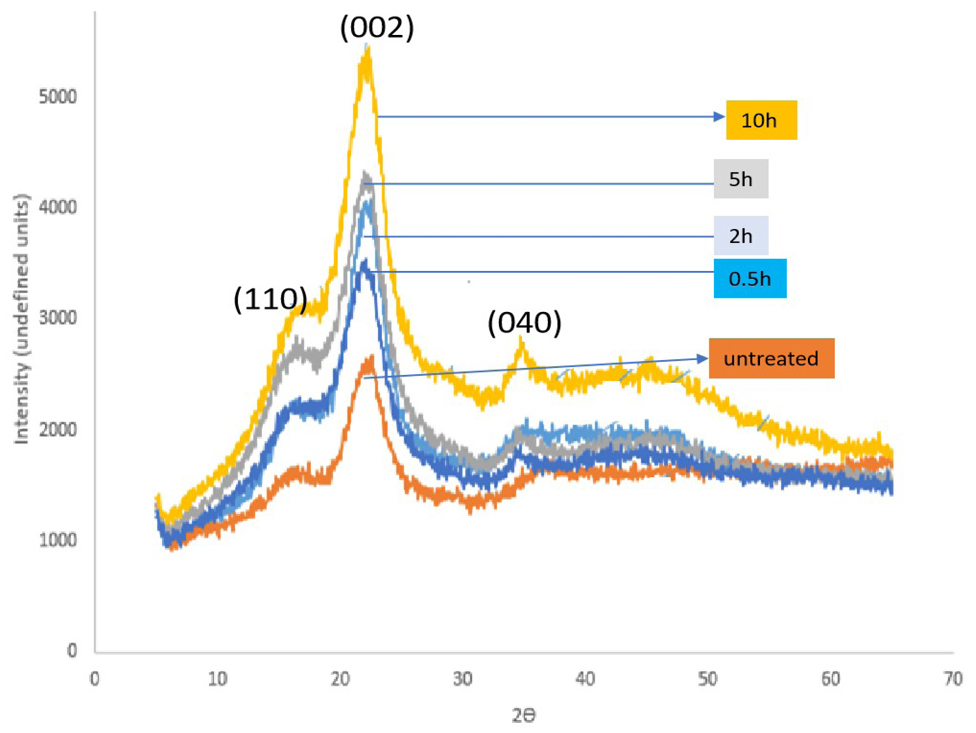
The XRD profiles peaks shown on Figure 3 characterize the crystalline monoclinic Iβ cellulose[38]. The fiber samples show five crystalline peaks 2θ: 17.83, 19.33, 23.3, 29.4 and 34.5, corresponding to lattice planes; (101, 021, 002, 130 and 040). The diffraction peaks in the ranges; 2θ, 15.7°-16.30° reflection assigned to the (110) crystallographic plane and 18.30°-18.40° 2θ reflection assigned (021) are attributed to the amorphous phase while the 21.90°-22.20° 2θ reflection were assigned to the (002) crystallographic plane of cellulose I[34]. The treated fiber were more crystalline and the crystalinity increased with the length of soaking time.
|
Figure 8. X-ray profile of Raffia hookerie as a function of treatment time in 5.0% Alkali solution.
3.3 FTIR Analysis
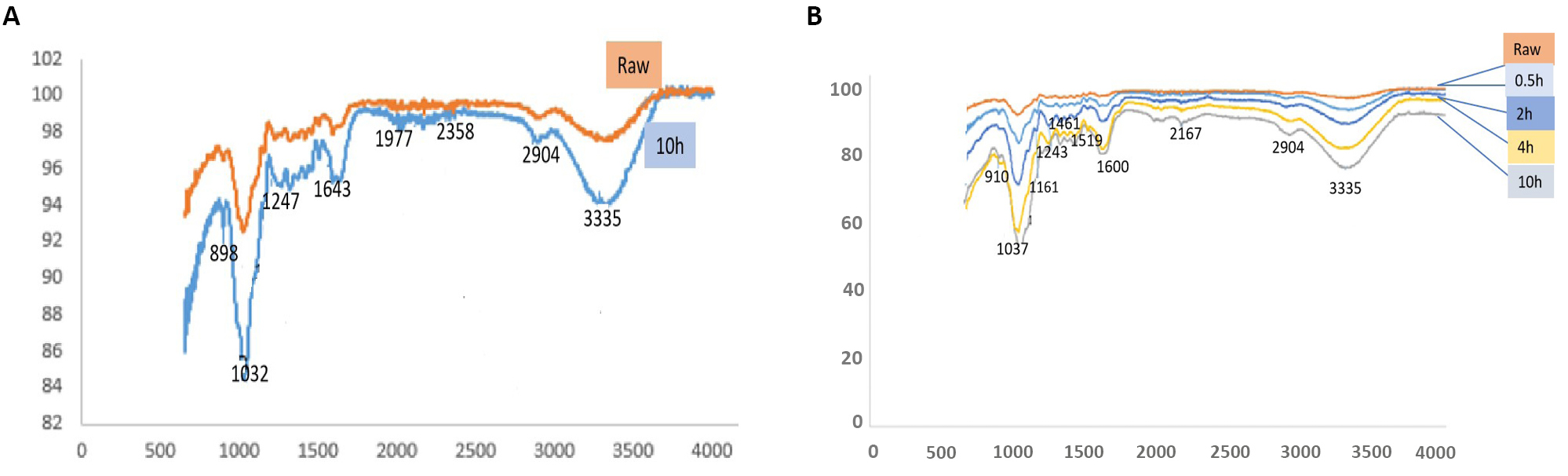
The FTIR profile presents spectra for untreated and 5% Alkali treated fiber over various soaking times. The spectra reflect characteristic lignocellulosic bands of Raffia fiber. The results as shown in Figure 9A and 9B, present variability in band peak intensity and broadness with treatment. The large absorption band in the region of 3400-3200cm-1 with peak at 3335cm-1 and the band at 1600cm-1 are attributed to the v-OH groups and signify the hydrophilicity of the natural fiber[39]. The peak intensity and broadness increased with treatment, indicating that treated fiber contain more water than raw fiber which confirm the observation from moisture content and water adsorption study and similar observations were reported in literature[34].
|
Figure 9. The FTIR profile presents spectra for untreated and 5% Alkali treated fiber over various soaking times. A: FT-IR profiles of untreated and 5% Alkali treated Raffia hookeri, fibers for 10h; B: FT-IR profiles of as a function of treatment time: 0, 0.5h, 2h, 4h and 10h.
The absorption peak detected at 1733cm-1 is attributed to v(C=O) stretching of methyl ester and carboxylic acid in pectin[40]. This band gently disappeared with treatment indicating that the leaching action of the sodium hydroxide removed the extractives which is in conformity with the XRD results. The absorbance peak in the region of 1610cm−1 that overlapped with the 1600cm-1 band is characteristic of natural fibers and attributed to v(C=C) stretching vibrations indicating the presence of fatty acids[1]. The weak absorption peak at 1243cm-1 detected in raw fiber but which gradually disappeared with treatment is attributed to v(C=C) stretching in lignin and the gradual disappearance with treatment confirm the fact that lignin is gradually leached by treatment. The absorbance peak detected around 1037cm-1 is attributed to the v(C=O) and v(C-O-C) stretching respectively[1]
3.4 Thermal Analysis
The thermal analysis as shown on Figure 10, revealed that the treated fiber are more thermally stable compared to raw Raffia hookeri fibers.
|
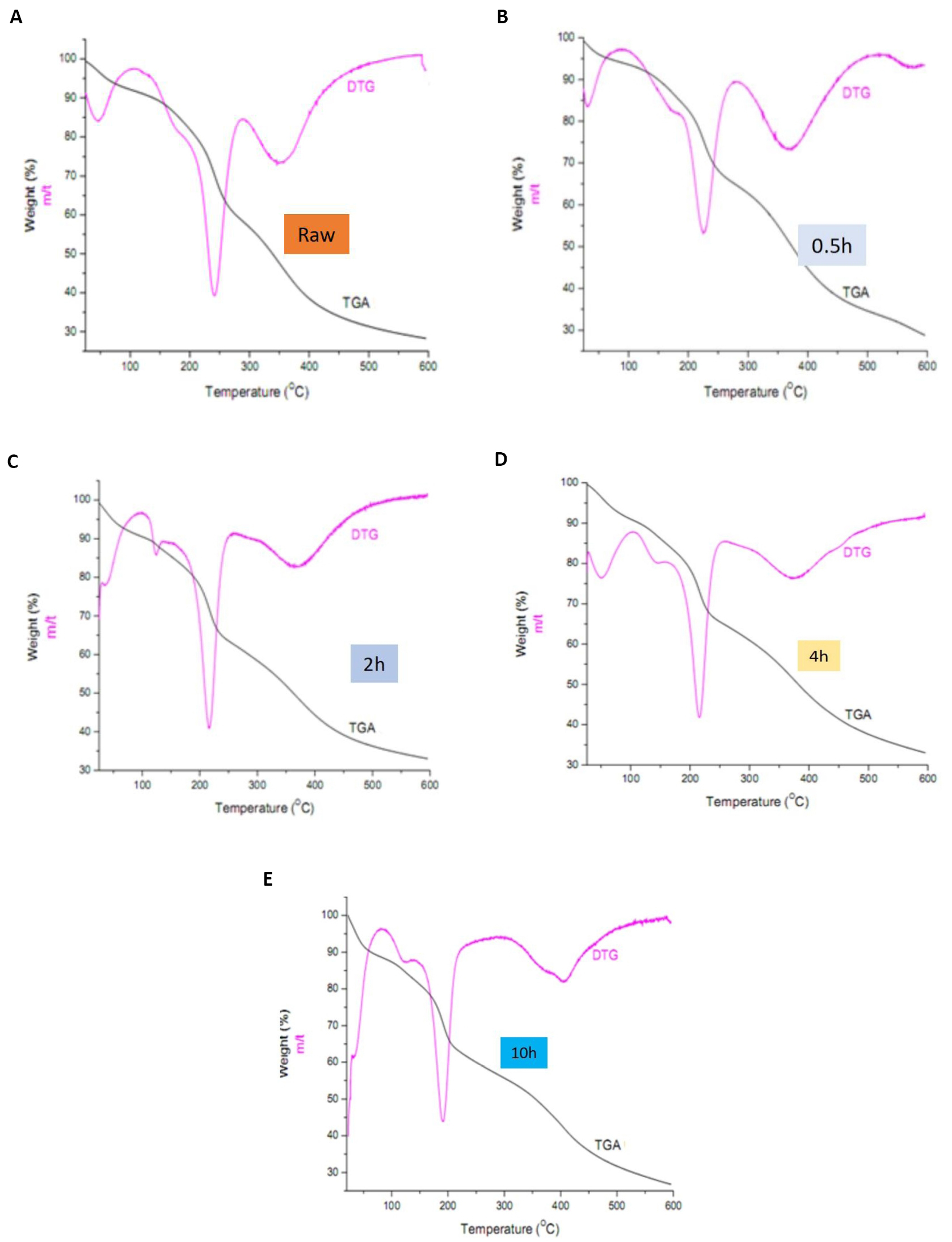
Figure 10. TGA/DTG profiles for (A) raw fiber, (B) 0.5h treated, (C) 2h treated, (D) 4h treated, and (E) 10h treated Raffia hookeri fibers.
3.5 Mechanical Properties
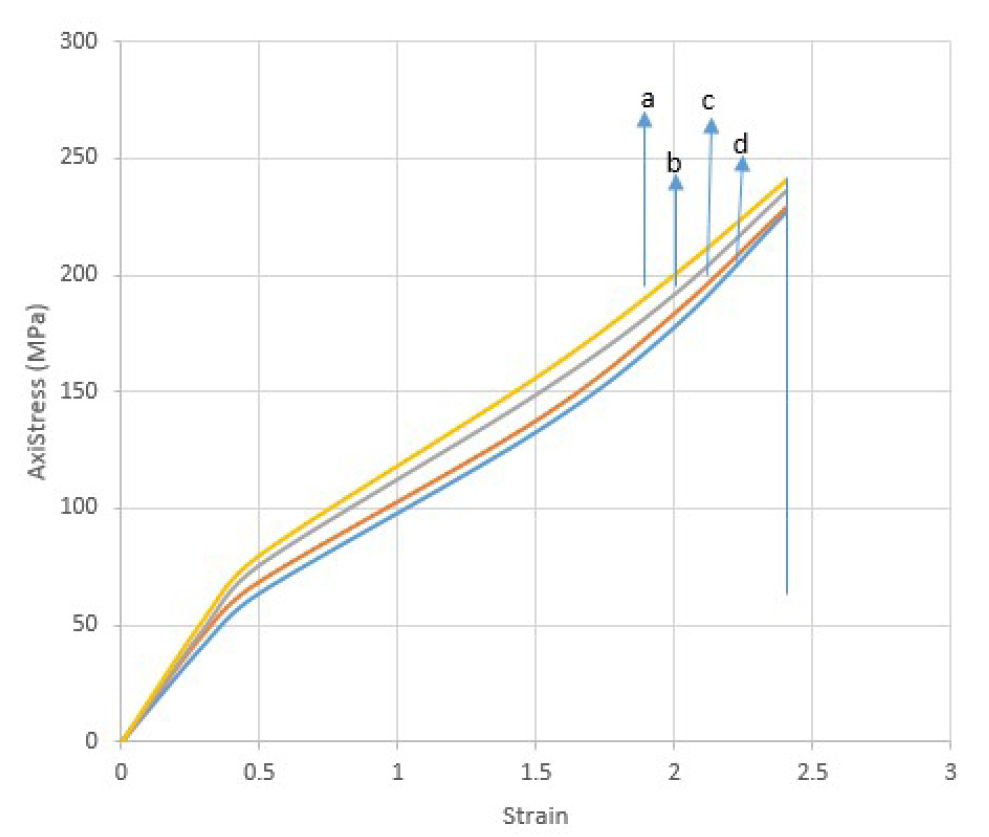
A typical stress-strain curve obtained for cleaned untreated RPH is shown in Figure 11. The sample fiber initially deformed inelastically after which it deformed elastically until the maximum stress when it breaks.
|
Figure 11. Stress-strain curve obtained for raw (a), Alkali treated Raffia hookeri fiber for (b) 2h, (c) 4h, (d) 10h at room temperature.
4 DISCUSSION
The surface of the core as presented on the longitudinal views of Figure 5 shows partly embedded silica bodies in linearly arranged circular craters. The silica bodies are of uniform sizes and shapes, and present linear filamentous arrays. Other literatures have reported either random, serial or both arrangements for various fibers[24-26]. During the soaking period, the colorless sodium hydroxide solution was observed to turn reddish-brown and the intensity of the color increased with the increase in the soaking time. This observation is attributed to the induced leaching effect over the impurities such as wax and some fragments of lignin from the untreated fibers surfaces as shown on Figure 4 and the effect weakens the boundary layers of the fiber surfaces which then disintegrate, exposing the underlying core. The details of the chemical reactions involved in this process has been reported in literature[41]. As the soaking time increased, the leaching effect also reveals the existence of numerous elementary fibers as shown in Figures 5D and 6C, each having a separate lumen also shown in Figure 7. This observation is recorded because, the lignin and hemicellulose middle layer which hold the elementary fibers together is gently dissolved by the leaching action of the alkaline solution. The maximum effect was observed after 10h of soaking time when the fibers are completely cleaned from silica bodies (as shown in Figure 6D), exposing a necked fiber cores with craters (Figure 6A), cracks (Figure 6B), a radial network of phloem and xylem vessels (which are two pathways for transportation of water and sugars between the core of the fiber and the surrounding medium and a large lumen) (Figure 6C). Similar cracks have been reported for Raffia farinifera[28], Coir[16], Sisal[8], abaca[11] and flax[6] fibers to affect the fracture behavior of natural fibers and such defects as kinks observed in this study have resulted in a longitudinal splitting of the fiber over a large area (Figure 6D). As the treatment time increased, there is a possibility that, defects may continue until they encountered other defect along the fiber length leading to the spread of defects and compounding failure in the material. The presence of individual lumens in fibrillates serves as voids which may ensure insulator properties in heat and sounds, making this fiber a good candidate for use in acoustics. The high presence of silica bodies and the craters when the silica bodies are removed, both serve as holdfast points in composites as they enhance interlocking at the fiber-matrix interface, improving the mechanical strength of the frame work[27]. Increase in cleaning of the fiber surface reduces the fiber size and increase the lumen diameter, these two changes lead to a surface volume increase enhancing crucially the surface properties which influence agglomeration of the fibrillates and physical properties associated with small size effects. These properties with the specificity of the spherical shape, micro size and specific surface area of the fiber is expected to modify polymer matrix properties such as phase behavior and thermal stability.
The untreated Raffia hookeri fibers in this study is expected to have a hydrophilicity advantage over untreated Raffia farinifera fibers which have a higher wt.% of cellulose and will be a better reinforcement feed in thermoplastics. The lower cellulose content associated with higher fractions of lignin and extractives is also indicative of the fact that they will degrade at lower temperatures compared to the Raffia farinifera[28]. This behavior has been attributed to the higher wt.% of hemicellulose which is the fiber components responsible for the initial thermal degradation behavior and correlates positively with moisture content.
Portland cement which is one the prime materials for the fabrication of composites is an aggregate of inorganic minerals in defined proportions as presented on Table 2. The Table also presents the minerals present in the Raffia hookeri fibers and the various wt.% content. Introducing fiber into the composite matrix directly may deviates the compositional formulation of the cement and the effect of the impurity mineral could be positive or negative depending on its concentration and the threshold of formulation in the parent cement. The minerals in the raw fiber are located at different parts of the fiber (as shown on the EDS profiles on Figures 4-7), and their possible presence in the polymer- composite matrix depends on treatment time. After the first 30mins and 1h soaking time as shown on Figure 5A and 5B, the peripheral components of the fiber are removed and the presence of Magnesium, Silicon, Iron, Alkali, Aluminum and Calcium are revealed. At high temperature and in the presence of Al and Ca, the Iron oxide has a possibility of forming tricalcium aluminoferrites which will improve the impact strength and hardening of the composite whereas excess Aluminum in the matrix will act as a flux to weaken the cement. Also, excess calcium in the presence of Sulphur Oxides can form Calcium Sulfate which will increases the setting time of the cement. The Alkali, sodium oxide and potassium oxide in excess can react with the aggregates to cause disintegration of the concrete so reducing the gain strength. Other literature also reported that, the alkali content of Portland cement has a significant effect on expansion of concrete[42]. The presence of silica bodies on the fiber surface may serve as holdfast position in the polymer fiber composites but as soaking time increased, after 4h as shown on Figure 5C, some silica bodies become loose and can be liberated into the matrix where due to formation of calcium silicate in the matrix may cause slow setting. This explains why Pinheiro et al.[20] indicated that the presence of silica bodies is usually harmful. The possible presence of magnesia at the later stage of soaking is inevitable and even in small amounts imparts hardness and color to the cement though may also make cement unsound at deviant concentrations. Therefore the microstructure compositional effect of fiber are improved with treatment but depend on the treatment time.
Table 2. Fiber Morphological Compositional Elements with Possible Interference On Portland Cement Matrix
Portland Cement |
|
Raffia hookeri |
|||||
Element |
Form |
Formulation % |
Upper Limit |
Wt.% |
Position on Fiber Morphology |
Possible Compositional Effects on Cement Matrix |
|
Carbon (C) |
|
NA |
NA |
|
|
|
|
Oxygen (O) |
|
NA |
NA |
|
|
|
|
Magnesium(Mg) |
MgO |
2% |
|
0.02±0.01% |
|
|
|
Aluminum(Al) |
Al2O3 |
5% |
|
0.47±0.03% |
All positions |
Quick setting |
|
Silicon (Si) |
SiO2 |
22% |
|
2.42±1.04% |
Attached on the core and core |
Impact strength |
|
Calcium (Ca) |
CaO |
4% |
|
0.26±0.23% |
All positions |
Impacts on setting time |
|
Sulphur (S) |
SO3 |
1% |
3.6% |
0.26±0.18% |
Predominantly at the periphery |
Performance and durability |
|
Iron(Fe) |
Fe2O3 |
1% |
|
0.24±0.12% |
More in the Lumen and core |
Impact strength and hardness |
|
Alkali( Na/K) |
Na2O/K2O |
1% |
|
0.095±0.014% |
More in the Lumen and core |
Concrete expansion |
|
Manganese (Mn) |
Mn2O3 |
|
|
0,087±0.043% |
Lumen |
|
|
Titanium (Ti) |
TiO2 |
|
|
0.06±0.04% |
Periphery and Lumen |
Hydration effects and pore development in the matrix |
|
The peak intensities and peak width at half maximum for (002) plane are greater for treated fiber and the observation increases with increase in soaking time in 5wt.% sodium hydroxide solution. Basing implication on the direct proportionality between crystal size and peak width at half Maximum, this is an indication that the crystal size and crystallinity increased with treatment time. The increase in peak intensity of the (002) and (040) peaks is attributed to the elimination of the amorphous components of the fiber such as hemicellulose and lignin by the leaching action of the alkaline solution as observed from the decrease in peak intensity at the (110, and 021) planes. Similar observations were reported in literature[34] while working on Raffia textile fiber. The degree of cellulose crystallinity is one of the most important crystalline structural parameters and CI in this study was estimate using the equation proposed by Segal et al.[43] (Equation (5))
|
Where I002 corresponds to the (002) lattice reflection peak (the maximum intensity) at an angle of 2θ (around 22°). Iam corresponds to the height of the minimum peak position between (002) and the (110) peaks, and is attributed to the amorphous fraction (minimum intensity) located at about 18°. The crystallinity index (CI) of the Raffia hookeri is estimated to be 66. Which compares with 66.6% reported for Raffia farinifera[28] and similar to 64% in other literature[34]. The value difference between the treated and untreated sample fiber in the present study indicated an increase in CI of 35.6% though all the XRD peaks exhibited by the raw and treated Raffia hookeri fiber are similar and attributed to the crystallinity of cellulose I, with a CI of 67%.
The FTIR peak at 898cm-1 detected in the sample after 10h soaking time, as shown on Figure 9A, but which is absent in the raw fiber, indicates the proportionate increase in the amount of cellulose. This peak is a parameter of comparison or reference band for cellulose[44] though observed at 894cm-1 in other literature[1].
The thermal stability of the sample fiber increases with both treatment with 5wt.% NaOH and the length of treatment time. The degradation profile from TGA¥DTG shows multiple steps reflected by weight loss portions: The initial weight loss portion is observed in the temperatures range 30-100°C for all fibers analyzed (with shifts to higher temperatures as treatment time increases) and could be attributed to the hydrophilicity of the fiber[45]. The intensity of the peak increased with treatment time confirming the fact that, the treated fiber is more hydrophilic since hydrophilicity increases the amount of water adsorbed by fibers. Further thermal degradation takes place in three steps with the first step, occurring between the temperatures of 150-200°C and could be attributed to the degradation of hemicellulose[46] and extractives. The intensity of this peak reduced with increase in length of treatment time confirming the fact that hemicellulose and extractives such as wax and pectin which decompose at lower temperatures, had been remove from the fiber surfaces with treatment and the effect increased with increase in the length of treatment time. The next weight loss that occurred around 220°C, is attributed to the main degradation of cellulose[47] and the peak intensity increases with treatment. Finally, the weight loss portion that occurred between 250 and 600°C, with peak intensities decreasing for treated fibers, was attributed to the slow degradation of lignin. Similar trends were reported in literature for different fibers: Raffia farinifera[28], raffia fiber textile[34] hemp and kenaf[48]. The increase in the cellulose peak intensity and decrease in lignin peak intensity with treatment and length of treatment time from the thermal studies are in conformity with the FTIR and XRD result. The beginning of thermal degradation between 150-200°C is lower than the average value for plant fiber (219°C)[46] as well as 256°C[34] but this increased with treatment time. This is an indication that degradation temperature is surely a variable of the plant species and/or the Botanical origin of the plant. Similar tensile result to that expressed in Figure 11 for the present study have been reported for other natural fiber: abaca[11] and sisal[8]. The non-linear deformation behavior is attributed to a collapse of the weak primary cell walls and delamination between the elemental fibers in the fiber bundle. The initial non-linear deformation is attributed to the collapse of the weak primary cell walls as well as on the delamination between fibrillates in the fiber bundle. However, the variability is positive with treatment time and the maximum mean stress obtained improves from 148±28MPa to 274±43MPa. This average fracture strength value obtained in this study is quite different to the breaking strength value reported for Raphia textilis from Congo reported by Elenga et al. (average value of 500±97MPa)[4] and Raphia farinifera from Madagasca reported by Sandy and Bacon (average value of 500±80MPa)[49]. The difference in the Botanical species and Geographical location of literature raffia fibers in comparison to Raffia hookeri in this study could account for the difference in the reported average strengths. The effect of chemical treatment on the tensile properties of RPH is presented in Figure 11 and the tensile strength of alkaline treated fibers decreases with treatment and length of treatment time. Similar result was reported by Mahjoub et al.[50] on the tensile properties of kenaf fibers. He reported a decrease in the tensile strength and modulus of kenaf fiber due to increasing concentration (5, 7, 10 and 15%) of NaOH solution and immersion time. As a result of treatment, the fibers assume specific properties such as the spherical shape, micro size and specific surface area which are likely to modify their mechanical properties such as tensile strength. Alkaline treatment has a modifying effect on the mechanical properties of fiber such as tensile stress but the degree of modification depends on the length of the treatment time.
5 CONCLUSION
For the very first time a detailed morphological and chemical compositional Study, effect of 5wt.% Sodium hydroxide treatment, and Characterization of the physical, mechanical and thermal properties of Raffia hookeri fiber for the evaluation of properties for technological application has been carried out. Within the limit of the data obtained in this study, the following conclusions could be drawn:
The results obtained in the present study comprise of cellulose (40wt.%), hemicellulose (20wt.%), lignin (33wt.%) and extractives 7%. The results in comparison with literature values from other species reveal a significant difference. The species under investigation recorded a lower percentage cellulose and extractive content but higher wt.% of lignin and hemicellulose. The fibers in the present study also exhibited lower thermal stability 150-200°C relative to the average value for plant fiber (219°C)[46] as well as 256°C[34] but this increased with treatment time. This is an indication that degradation temperature is surely a variable of the plant species and / or the Botanical origin of the plant.
● The detailed morphological study has confirmed the presence of natural protrusion (silica bodies) which improve on the structural integrity of raffia and other fibers in which they are present but it is worth noting that the liberation of free silica bodies in the polymer composite matrix may be harmful due to the introduction of excess silicon dioxide (silica bodies) in the matrix.
● Alkaline treated Raffia hookeri exhibits morphological, thermal and physical properties in the range found in several natural fibers and therefore constitute a new source of natural fibers for reinforcements in polymers and has great potential for use in technological applications. However, to ensure maximum output, the chemical compositional effect on the fiber matrix and the length of treatment time must be given due consideration for fibers used in low cost and lightweight cement composites.
Acknowledgements
We wish to express our gratitude to Mbuagbaw Helen Mbuagbaw Biochemistry teacher and Vice principal at Government High school Limbe, Fako, South West region, Cameroon, who participated in the collection and botanical identification of the samples. We are saddened by the fact that she died even before the project was completed. May the soul of this our dearest one rest in perfect peace.
Conflicts of Interest
This manuscript is not under consideration in any other journal and the authors declare no conflict of interest.
Author Contribution
All authors have participated sufficiently in all the phases of the work and agree to take public responsibility for the content, including participation in the concept, design, analysis, writing and/or revision of the manuscript.
Abbreviation List
ATR, Attenuated total reflectance
CI, Crystallinity index
DTG, Differential thermo gravimetric
EDS, Energy-dispersive X-ray spectroscopy
EDX, Electron diffraction X-ray
FTIR, Fourier transform infrared
KN, Kilo Neutron
MCT, Mercury cadmium telluride
RPH, raffia Hookeri
SEM, Scanning electron microscopy
TG, Thermogravimeteric
TGA, Thermo gravimetric analysis
XRD, X-ray diffraction
References
[1] Namondo BV, Foba-Tendo J, Etape EP. Potential of blended biomass feedstock from some species of raffia palm (Raffia farinifera, Raffia hookeri and Raffia vinifera) and Oil Palm Empty Fruit Bunch (OPEFB) from Cameroon. Afr J Pure Appl Chem, 2018; 12: 25-33. DOI: 10.5897/AJPAC2018.0753
[2] Stanislas TT, Tendo JF, Ojo EB et al. Production and characterization of pulp and nanofibrillated cellulose from selected tropical plants. J Nat Fibers, 2022; 19: 1592-1608. DOI: 10.1080/15440478.2020.1787915
[3] Mohammed L, Ansari MNM, Pua G et al. A review on natural fiber reinforced polymer composite and its applications. Int J Polym Sci, 2015; 2015: 243947. DOI: 10.1155/2015/243947
[4] Elenga RG, Dirras GF, Maniongui JG et al. On the microstructure and physical properties of untreated raffia textilis fiber. Compos Part A-Appl S, 2009; 40: 418-422. DOI: 10.1016/j.compositesa.2009.01.001
[5] Kengoh JB, Peter Etape E, Namondo BV et al. Influence of Urena lobata Fibre Treatment on Mechanical Performance Development in Hybrid Urena lobata: Fibre/Gypsum Plaster Composites. Adv Mater Sci Eng, 2021; 2021: 1-10. DOI: 10.1155/2021/5514525
[6] Aslan M, Chinga-Carrasco G, Sørensen BF et al. Strength variability of single flax fibres. J Mater Sci, 2011; 46: 6344-6354. DOI: 10.1007/s10853-011-5581-x
[7] Li X, Panigrahi S, Tabil LG. A study on flax fiber-reinforced polyethylene biocomposites. Appl Eng Agric, 2009; 25: 525-531. DOI: 10.13031/2013.27454
[8] Silva FA, Chawla N, Filho RDT. Tensile behavior of high performance natural (sisal) fibers. Compos Sci Technol, 2008; 68: 3438-3443. DOI: 10.1016/j.compscitech.2008.10.001
[9] Bledzki AK, Gassan J. Composites reinforced with cellulose based fibres. Prog Polym Sci, 1999; 24: 221-274. DOI: 10.1016/S0079-6700(98)00018-5
[10] Li Y, Mai YW, Ye L. Sisal fibre and its composites: a review of recent developments. Compos Sci Technol, 2000; 60: 2037-2055. DOI: 10.1016/S0266-3538(00)00101-9
[11] Cai M, Takagi H, Nakagaito AN et al. Influence of alkali treatment on internal microstructure and tensile properties of abaca fibers. Ind Crops Prod, 2015; 65: 27-35. DOI: 10.1016/j.indcrop.2014.11.048
[12] Faruk O, Bledzki AK, Fink HP et al. Biocomposites reinforced with natural fibers: 2000-2010. Prog Polym Sci, 2012; 37: 1552-1596. DOI: 10.1016/j.progpolymsci.2012.04.003
[13] Punyamurthy R, Sampathkumar D, Bennehalli B et al. Abaca fiber reinforced epoxy composites: evaluation of impact strength. Int J Sci Basic Appl Res, 2014; 18: 305-317.
[14] Pujari S, Ramakrishna A, Kumar MS. Comparison of jute and banana fiber composites: A review. Int J Curr Eng Technol, 2014; 2: 121-126. DOI: 10.14741/ijcet/spl.2.2014.22
[15] Aseer JR, Sankaranarayanasamy K, Jayabalan P et al. Morphological, physical, and thermal properties of chemically treated banana fiber. J Nat Fibers, 2013; 10: 365-380. DOI: 10.1080/15440478.2013.824848
[16] Mathura N, Cree D. Characterization and mechanical property of Trinidad coir fibers. J Appl Polym Sci, 2016; 133: 43692. DOI: 10.1002/app.43692
[17] Mir SS, Hasan M, Hasan SMN et al. Effect of chemical treatment on the properties of coir fiber reinforced polypropylene and polyethylene composites. Polym Compos, 2017; 38: 1259-1265. DOI: 10.1002/pc.23690
[18] Arib RMN, Sapuan SM, Ahmad M et al. Mechanical properties of pineapple leaf fibre reinforced polypropylene composites. Mater Des, 2006; 27: 391-396. DOI: 10.1016/j.matdes.2004.11.009
[19] Saha P, Manna S, Chowdhury SR et al. Enhancement of tensile strength of lignocellulosic jute fibers by alkali-steam treatment. Bioresource Technol, 2010; 101: 3182-3187. DOI: 10.1016/j.biortech.2009.12.010
[20] Pinheiro FGC, Leitão RC, Frollini E. Removing silica from oil palm mesocarp fibers. Biomass Convers. Biorefin, 2021; 1-13. DOI: 10.1007/s13399-021-02065-w
[21] Buson RF, Melo LFL, Oliveira MN et al. Physical and mechanical characterization of surface treated bamboo fibers. Sci Technol Adv Mater, 2018; 30: 67-73. DOI: 10.1016/j.stmat.2018.03.002
[22] Elzubair A, Bonelli CMC, Rademaker H, Miguez Suarez JC, Mano EB. 2002. NATAL, 09 A13 DE NOVEMRO DE, 2002.
[23] Hanipah SH, Omar FN, Talib AT et al. Effect of silica bodies on oil palm fibre-polyethylene composites. BioResources, 2020; 15: 360-367. DOI: 10.15376/biores.15.1.360-367
[24] Bahrin EK, Baharuddin AS, Ibrahim MF et al. Physicochemical property changes and enzymatic hydrolysis enhancement of oil palm empty fruit bunches treated with superheated steam. BioResources, 2012; 7: 1784-1801.
[25] Ishola MM, Millati R, Syamsiah S et al. Structural changes of oil palm empty fruit bunch (OPEFB) after fungal and phosphoric acid pretreatment. Molecules, 2012; 17: 14995-15012. DOI: 10.3390/molecules171214995
[26] Shamsudin S, Shah UKM, Zainudin H et al. Effect of steam pretreatment on oil palm empty fruit bunch for the production of sugars. Biomass Bioenergy, 2012; 36: 280-288. DOI: 10.1016/j.biombioe.2011.10.040
[27] Pai AR, Jagtap RN. Surface morphology & mechanical properties of some unique natural fiber reinforced polymer composites-A review. J Mater Environ Sci, 2015; 6: 902-917.
[28] Fadele O, Oguocha INA, Odeshi A et al. Characterization of raffia palm fiber for use in polymer composites. J Wood Sci, 2018; 64: 650-663. DOI: 10.1007/s10086-018-1748-2
[29] Tran LQN, Minh TN, Fuentes CA et al. Investigation of microstructure and tensile properties of porous natural coir fibre for use in composite materials. Ind Crops Prod, 2015; 65: 437-445. DOI: 10.1016/j.indcrop.2014.10.064
[30] Fidelis MEA, Pereira TVC, Gomes OFM et al. The effect of fiber morphology on the tensile strength of natural fibers. J Mater Res Technol, 2013; 2: 149-157. DOI: 10.1016/j.jmrt.2013.02.003
[31] Wang F, Shao J, Keer LM et al. The effect of elementary fibre variability on bamboo fibre strength. Mater Des, 2015; 75: 136-142. DOI: 10.1016/j.matdes.2015.03.019
[32] Odeyemi SO, Giwa ZT. Thermal resistance of raffia palm reinforced concrete. Rev Constr, 2021; 20: 5-14. DOI: 10.7764/rdlc.20.1.5
[33] Tagne NRS, Mbou TE, Harzallah O et al. Physicochemical and mechanical characterization of raffia vinifera pith. Adv Mater Sci Eng, 2020; 2020: 1-10. DOI: 10.1155/2020/8895913
[34] Elenga RG, Djemia P, Tingaud D et al. Effects of Alkali Treatment on the Microstructure, Composition, and Properties of the Raffia textilis Fiber. BioResources, 2013; 8: 2934-2949.
[35] Nam TH, Ogihara S, Tung NH et al. Effect of alkali treatment on interfacial and mechanical properties of coir fiber reinforced poly (butylene succinate) biodegradable composites. Composites, Part B, 2011; 42: 1648-1656. DOI: 10.1016/j.compositesb.2011.04.001
[36] Yusoff MZM, Salit MS, Ismail N. Tensile properties of single oil palm empty fruit bunch (OPEFB) fibre. Sains Malays, 2009; 38: 525-529.
[37] Omar FN, Mohammed MAP, Baharuddin AS. Effect of silica bodies on the mechanical behaviour of oil palm empty fruit bunch fibres. BioResources, 2014; 9: 7041-7058.
[38] Sathitsuksanoh N, Zhu Z, Wi S et al. Cellulose solvent-based biomass pretreatment breaks highly ordered hydrogen bonds in cellulose fibers of switchgrass. Biotechnol Bioeng, 2011; 108: 521-529. DOI: 10.1002/bit.22964
[39] Tibolla H, Pelissari FM, Menegalli FC. Cellulose nanofibers produced from banana peel by chemical and enzymatic treatment. Lwt-Food Sci Technol, 2014; 59: 1311-1318. DOI: 10.1016/j.lwt.2014.04.011
[40] Ouajai S, Shanks RA. Composition, structure and thermal degradation of hemp cellulose after chemical treatments. Polymer degradation and stability, 2005; 89: 327-335. DOI: 10.1016/j.polymdegradstab.2005.01.016
[41] Zhou Y, Fan M, Chen L. Interface and bonding mechanisms of plant fibre composites: An overview. Composites, Part B, 2016; 101: 31-45. DOI: 10.1016/j.compositesb.2016.06.055
[42] Shehata MH, Thomas MDA. The role of alkali content of Portland cement on the expansion of concrete prisms containing reactive aggregates and supplementary cementing materials. Cement Concrete Res, 2010; 40: 569-574. DOI: 10.1016/j.cemconres.2009.08.009
[43] Segal L, Creely JJ, Martin Jr AE et al. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J, 1959; 29: 786-794. DOI: 10.1177/004051755902901003
[44] Oh SY, Yoo DI, Shin Y et al. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydrate Res, 2005; 340: 417-428. DOI: 10.1016/j.carres.2004.11.027
[45] Célino A, Gonçalves O, Jacquemin F et al. Qualitative and quantitative assessment of water sorption in natural fibres using ATR-FTIR spectroscopy. Carbohydrate Polym, 2014; 101: 163-170. DOI: 10.1016/j.carbpol.2013.09.023
[46] Yao F, Wu Q, Lei Y et al. Thermal decomposition kinetics of natural fibers: activation energy with dynamic thermogravimetric analysis. Polym Degrad Stabil, 2008; 93: 90-98. DOI: 10.1016/j.polymdegradstab.2007.10.012
[47] Teixeira FP, Gomes OFM, de Andrade Silva F. Degradation mechanisms of curaua, hemp, and sisal fibers exposed to elevated temperatures. BioResources, 2019; 14: 1494-1511.
[48] Oliveira AKF, d'Almeida JRM. Characterization of ubuçu (Manicaria saccifera) natural fiber mat. Polym Renew Resour, 2014; 5: 13-28. DOI: 10.1177/204124791400500102
[49] Sandy M, Bacon L. Tensile testing of raffia. J Mater Sci Lett, 2001; 20: 529-530.
[50] Mahjoub R, Yatim JM, Sam ARM et al. Tensile properties of kenaf fiber due to various conditions of chemical fiber surface modifications. Constr Build Mater, 2014; 55: 103-113. DOI: 10.1016/j.conbuildmat.2014.01.036
Copyright © 2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.





 Copyright ©
Copyright ©