Research Progress of Nanoscale Zero-valent Iron in Removal of Heavy Metals From Groundwater
Yanqi Wu1,2, Yuhong Guan2,3, Song Li4, Nongyue He3,5, Zhishan Yuan6, Hui Chen4*
1State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, Macau, China
2Shenzhen LemnisCare Medical Technology Co., Ltd., Shenzhen, Guangdong Province, China
3Hunan Key Laboratory of Biomedical Nanomaterials and Devices, Hunan University of Technology, Zhuzhou, Hunan Province, China
4Institute of Future Science, University of South China, Hengyang, Hunan Province, China
5State Key Laboratory of Bioelectronics, National Demonstration Center for Experimental Biomedical Engineering Education, School of Biological Science and Medical Engineering, Southeast University, Nanjing, Jiangsu Province, China
6School of Electromechanical Engineering, Guangdong University of Technology, Guangzhou, Guangdong Province, China
*Correspondence to: Hui Chen, Institute of Future Science, University of South China, No. 28 Changsheng West Road, Hengyang 421099, Hunan Province, China; Email: puchenlinyimv@163.com
DOI: 10.53964/jmn.2023003
Abstract
Groundwater heavy metal pollution has become increasingly serious, receiving widespread attention. Permeable reactive barrier (PRB) is one of the commonly used in situ remediation technologies for groundwater heavy metal pollution. Currently, it has been widely used in engineering practice. Nanoscale zero-valent iron (nZVI) is an emerging remediation material with broad application prospects for groundwater heavy metal pollution. This article summarizes different types of PRB and methods for preparing nZVI. It also introduces the different modifications and effects of nZVI, and elaborates on the relevant problems that need to be overcome in the engineering application of nZVI. This study believes that seeking more affordable modification methods and environmentally friendly modification materials are key issues that need to be addressed in the future.
Keywords: nanoscale zero-valent iron, nanomaterial, modification, heavy metal, permeable reactive barrier, groundwater pollution
1 INTRODUCTION
With the rapid development of industrialization and urbanization, groundwater heavy metal pollution has become an increasingly serious environmental problem worldwide. Heavy metals have strong toxicity and bioaccumulation characteristics, causing serious harm to human health and ecological systems[1]. Therefore, the remediation of heavy metal pollution in groundwater has become a hot topic in the field of environmental protection.
Groundwater heavy metal remediation technologies mainly include ex-situ remediation and in-situ remediation[2]. Ex-situ remediation mostly refers to pumping and treating technologies, while in-situ remediation includes groundwater aeration technology, chemical agent oxidation, bioremediation technology, and permeable reactive barrier (PRB)[3]. Nowadays, in-situ remediation technologies are mainly used for groundwater heavy metal remediation. PRB is one of the most commonly used in-situ remediation technologies for groundwater heavy metal remediation. It involves setting specific materials along the groundwater flow path to capture, absorb or convert harmful substances in groundwater into harmless substances[4]. Compared with traditional technologies, PRB has the advantages of low cost, easy operation, no need for large-scale land acquisition, and no damage to the original ecological environment[5,6]. Therefore, in engineering practice, it has gradually replaced traditional technologies and has been widely used.
Nanoscale zero-valent iron (nZVI) filler is an emerging remediation material with high activity and good reduction capability, which can effectively remove heavy metal pollutants from groundwater[7]. Compared with traditional remediation materials, nZVI filler has a larger specific surface area and better permeability, which gives it a higher remediation efficiency and a relatively smaller impact on the environment[8]. Therefore, nZVI is considered as a groundwater heavy metal pollution control material with broad application prospects. However, nZVI is prone to aggregation due to its high surface energy and magnetism, and it often encounters clogging and other issues during use[9]. In addition, the activity of nZVI filler is higher than that of Fe filler, making it more susceptible to oxidation during use. As the proportion of oxidized nZVI increases, the proportion of nZVI that can perform reduction decreases, leading to a continuous decrease in removal efficiency[10]. To address these issues such as aggregation, clogging, and oxidation, this paper summarizes the structural types of PRBs, the preparation and modification methods of nanoscale iron fillers, and discusses the relevant issues that need to be overcome during the engineering application of nZVI, in order to provide some reference for future research.
2 STRUCTURE TYPES OF PRB
There are mainly two types of PRB structure based on different designs, continuous reaction wall type and funnel-gate type PRB.
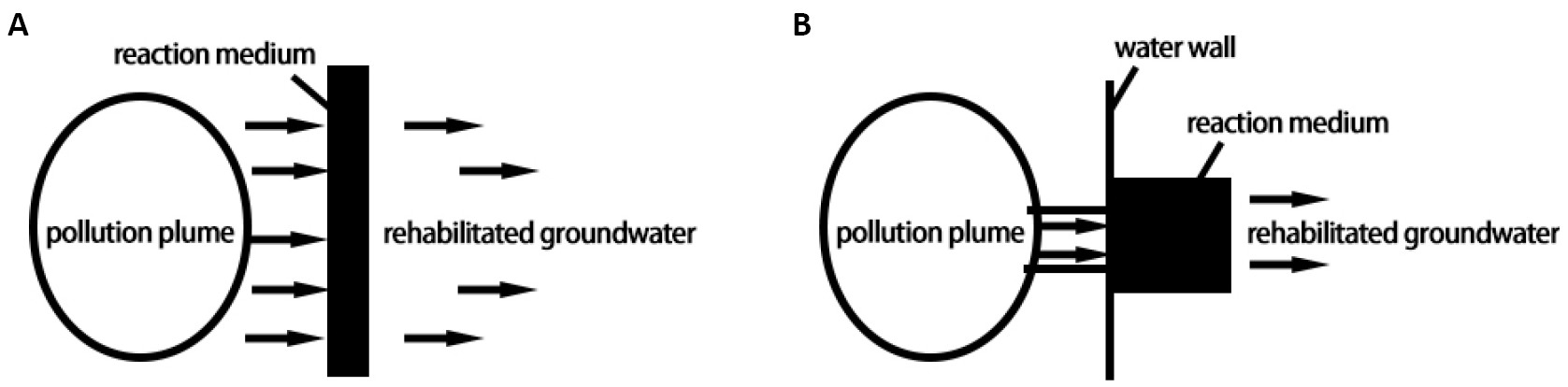
The continuous reaction wall type PRB is the most widely used type of PRB as shown in Figure 1A. It refers to a fully continuous anti-seepage barrier formed by sealing the underground layer or excavating deep trenches and filling them with pollutant removal agents in the direction of contaminated groundwater flow[11]. This structure often uses materials such as steel sheet piles, concrete troughs or HDPE as isolation layers, and fills materials such as zero-valent iron, activated carbon, biomass, etc., as reaction media inside the isolation layer to remove pollutants[12]. The advantage of the continuous reaction wall type PRB is its good anti-seepage effect, which can effectively prevent the migration of pollutants. Meanwhile, the reaction rate and removal efficiency can be adjusted by increasing the width or length of the reaction media. However, its disadvantage is the high construction cost, requiring sufficient space and design work, and thus it is suitable for handling larger underground water pollution areas.
|
Figure 1. Schematic diagram of the working principle of PRB intercepting contaminated plumes. A: Continuous Reactive Wall PRB; B: Funnel-gate PRB.
Funnel-gate type PRB consists of a water-resistant funnel, a water gate, and a treatment unit (reactive material), as shown in Figure 1B. The funnel-gate type PRB refers to a method of guiding groundwater flow into the reaction area by setting up a funnel and water gate in the direction of contaminated groundwater flow, and then removing pollutants by filling reaction media[13]. This structure often uses concrete funnels and pipes as water guides and fills zero-valent iron, biomass, and other reaction media around the funnel or inside the pipe[14]. The advantage of the funnel-gate type PRB is its relatively low construction cost, which can achieve efficient treatment in a small space. At the same time, the reaction rate and removal efficiency can be controlled by adjusting the size and position of the funnel and water gate. However, its disadvantage is that the anti-seepage effect is not as good as that of the continuous reaction wall type PRB, and it requires regular maintenance and replacement of reaction media.
In summary, both the continuous reaction wall type PRB and the funnel-gate type PRB can effectively address heavy metal pollution in groundwater according to different design methods. The choice of which type of PRB structure to use should be based on specific circumstances, such as the size, nature, topography, and geological conditions of the contaminated area.
3 REMOVAL MECHANISM AND PREPARATION OF nZVI
3.1 Removal Mechanism of nZVI
nZVI is a highly reactive nanomaterial that exhibits both high reactivity and adsorption capacity, enabling it to react with many organic and inorganic compounds for their removal[15]. The following explains the mechanisms of nZVI for removing pollutants: (1) Reduction: nZVI has high reducibility and can partially or completely reduce pollutants into their original states, such as reducing organics containing chlorides into harmless methane[16]. (2) Adsorption: nZVI has numerous active sites on its surface, which can physically or chemically adsorb pollutants, thus reducing their concentrations[17]. (3) Oxidation: nZVI contains a significant amount of Fe(0) and Fe(II) on its surface that can react with oxygen in water to form highly oxidizing Fe(III), consequently oxidizing some of the hard-to-reduce pollutants (such as toxic organic compounds) into more easily removable compounds[18]. (4) Passivation: nZVI can easily form a passive layer on its surface, which can prevent further dissolution of iron and release of pollutants, thus extending the lifespan of nZVI[19]. In summary, the removal mechanism of nZVI for pollutants is a complex process involving reduction, adsorption, oxidation, and passivation, where these mechanisms interact and cooperate with each other to ultimately remove pollutants.
3.2 Preparation Methods of nZVI
The preparation methods for nZVI can be broadly classified into physical and chemical synthesis methods. Physical methods include sputtering, high-energy ball milling, inert gas condensation, and mixed plasma methods. The preparation methods and corresponding characteristics are summarized in Table 1. Chemical synthesis methods mainly include chemical reduction method, thermal decomposition of iron carbonyl, microemulsion method, electrochemical deposition method, and green synthesis method. The preparation methods and corresponding characteristics are summarized in Table 2.
Table 1. Physical Preparation Methods of nZVI
Method |
Brief Description |
Features |
Sputtering method[20] |
By means of sputtering, Al2O3 is used to isolate Fe, and Fe and Al2O3 are sputtered onto the same substrate at the same time, thereby making nZVI |
The purity of the product is high, but the corresponding cost is also high, and the energy consumed is large |
High energy ball milling method[21] |
Run the metal iron powder in the high-energy ball mill for a long time and squeeze it repeatedly to make it into dispersed ultrafine particles |
The process is simple, but the preparation cycle is longer and the purity is lower |
Inert gas condensation method[22] |
In an inert gas environment, Fe is evaporated, after which nitrogen gas containing a small amount of O2 is passed through for passivation treatment to obtain nZVI |
The product has high purity and good dispersion, but it has high requirements for equipment and it is difficult to reach the required high temperature |
Hybrid plasma method[23] |
Evaporate Fe into a vapor in an inert protective gas, perform sufficient energy and charge exchange, and then rapidly cool it to form local supersaturation, thereby forming nZVI particles |
The particle size of the product is small and uniform, but the equipment cost is high |
Table 2. Chemical Preparation Methods of nZVI
Method |
Brief Description |
Features |
Chemical reduction method |
- |
- |
Gas phase reduction method[24] |
Preparation of nZVI by reduction of iron salt precursors by reducing gasses such as H2 at high temperature |
The product has high purity and small particle size, but requires high equipment precision |
Liquid phase reduction method[25] |
In the liquid phase system, use NaBH4 and other strong reducing substances to chemically reduce Fe3+ or Fe2+ to synthesize nZVI |
Easy to operate in the laboratory, but the produced product is easy to agglomerate and oxidize |
Solid phase reduction method[26] |
By Fe2+ and matrix composition Al2O3 through the sol-gel method (sol-gel method), and then processed by heat treatment and H2 reduction to prepare nZVI |
Suitable for large-scale production, but the produced product has problems of uneven particles and easy agglomeration |
Pyrolysis carbonyl iron method[27] |
In an environment of noble gasses, nano zero-valent iron (nZVI) is prepared by decomposing iron pentacarbonyl Fe(CO)5 through thermal decomposition |
The process flow is simple and easy to operate, but the product is easy to oxidize and agglomerate |
Microemulsion method[28] |
Microemulsion is prepared using octane, CTAB, butanol, and water. Then, encapsulated nano zero-valent iron (nZVI) was synthesized by reducing Fe3+ with NaBH4 in the microemulsion system. |
The particle distribution of the product is uniform and the dispersion is good, but the process is complex and the operation requirements are high |
Electrochemical deposition method[29] |
nZVI is prepared by pulse electrodeposition in an electrolyte containing (NH4)2Fe(SO4)2, sodium citrate, citric acid, and boric acid by controlling pulse parameters |
The product has high density and small porosity, but impurities are easily introduced during the process |
Green synthesis[30] |
Using effective active ingredients in natural plants, nano zero-valent iron (nZVI) with a particle size of 10-20nm is successfully synthesized by reducing Fe3+ with plant extract |
The physical and chemical properties of the product are stable and can avoid pollution to the environment, but the synthesis efficiency is low |
Preparation methods for nZVI are well-established. In physical preparation methods, inert gas condensation and mixed plasma methods yield the highest purity of nZVI, but the difficulty of their operations is relatively high. High-energy ball milling is widely used in industrial production. Among chemical synthesis methods, gas-phase reduction and electrochemical deposition methods yield the most ideal results. Green synthesis method has become popular in recent years due to its environmentally friendly features. Liquid-phase reduction is the simplest and most feasible operation for preparing and modifying nano zero-valent iron, making it the most commonly used method in laboratory research.
4 MODIFICATION OF nZVI
After solid particle size reduction, the proportion of surface atoms increases as the particle size decreases, which enhances the surface free energy and activity of the particles. This makes the surface atoms of the nano-particles more active and reactive with other atoms, which can lead to surface passivation[31]. This phenomenon of activation of nano-particles limits the development of nZVI in heavy metal removal from groundwater to a certain extent. Therefore, modifying and optimizing nZVI is an effective method to improve the degradation efficiency of pollutants. In terms of heavy metal removal from groundwater, the modified nZVI composite materials mainly include: metal-doped nZVI, loaded nZVI (carbon-loaded nZVI, inorganic clay-loaded nZVI), surfactant-encapsulated nZVI, and polyelectrolyte-encapsulated nZVI. In the following section, we will review the modification methods and effects of these nZVI composites, and discuss the relevant issues that need overcoming for their engineering applications.
4.1 Metal-doped nZVI
Metal-doped nZVI is a modification method that involves doping the surface of nZVI with relatively stable metal elements to enhance its catalytic performance. Metals commonly used for doping include copper (Cu), nickel (Ni), iron (Fe), etc. This method can improve catalytic activity, increase electron transfer rate, and enhance stability[32]. Metal-doped nZVI can also be further utilized for treating organic and inorganic pollutants such as chlorinated hydrocarbons, benzene series pollutants, heavy metals, etc. Zhu et al.[33] synthesized Fe-Ni and Fe-Cu bimetallics separately using FeCl3•6H2O, NiSO4•7H2O and FeCl3•6H2O, CuSO4 respectively through a series of steps in NaBH4. These synthesized bimetallics were then used to treat Cr6+. Under optimal experimental conditions, all synthesized bimetallics achieved a removal rate of 99% for Cr6+. Scanning electron microscopy (SEM) observation of Fe-Ni bimetallic ions found an aggregation phenomenon, but the chain-like structure increased adsorption space, improved reduction efficiency, reduced aggregation impact, and achieved the expected removal effect. The authors then used Langmuir first-order kinetic model for fitting and found that the time was proportional to the adsorption amount. The thermodynamic equation proved that the removal of Cr6+ by Fe-Ni and Fe-Cu was a spontaneous endothermic reaction. Another study by Qu et al.[34] involved the oxidation treatment of sulfate on Fe-Cu bimetallics, which not only effectively removed heavy metals but also had a certain effect on organic matter removal. Dong et al.[35] evaluated the short-term and long-term operational differences of Fe-Ni bimetallic particles in water. The results showed that the short-term performance of Fe-Ni bimetallic particles in water was ideal, but after long-term operation, the main effective product was Fe3O4. Long-term operation caused Ni to enter the solution, resulting in particle passivation, no longer mitigating particle aggregation and oxidation, thus continuously reducing removal efficiency. Based on the above research, it can be seen that bimetallic particles have a faster reaction rate than pure nZVI particles and overcome the disadvantages of easy aggregation. However, in practical commercial use, bimetallic particles still face many problems, such as: (1) catalytic activity affects the life of bimetallic particles; (2) poor long-term performance of bimetallic particles; (3) the manufacturing process of bimetallic particles is more complicated than that of pure nZVI technology; (4) the price of doped precious metals such as Ag and Au is high; (5) the use of bimetallic particles will cause secondary pollution to the environment. These problems need to be addressed in the commercial use of bimetallic particles.
4.2 Loaded nZVI
Loaded nZVI refers to a material form that enhances its performance by encapsulating or adsorbing other materials on its surface. This method can remove pollutants such as organic pollutants and heavy metals in water, while overcoming some of the disadvantages of nZVI itself, such as agglomeration, surface oxidation, and low sedimentation rate[36]. Specifically, nZVI is loaded onto materials such as clay, diatomaceous earth, iron oxide, alumina, and activated carbon. These materials can provide support and surface area to fix nZVI, and in some cases, they have the ability to remove pollutants themselves. The advantages of loaded nZVI include improving the dispersion and stability of nZVI, making it easier to act in water; enhancing the removal ability of different types of pollutants; being able to exist stably in water for a long time; and being flexible to use in different application scenarios.
4.2.1 Carbon Material Loaded nZVI
Porous material loaded nZVI refers to the use of porous materials with high specific surface area and numerous pores to encapsulate nZVI, in order to achieve efficient removal of pollutants. nZVI has excellent catalytic reduction performance and can reduce various pollutants into non-toxic and harmless substances[37]. However, nZVI tends to aggregate and stack in water, which is not conducive to long-term stable applications[38]. To solve this problem, researchers have loaded nZVI on porous materials to increase its dispersion and stability, as well as enhance its reaction activity. Common porous materials include iron oxide nanoparticles, silica gel, activated carbon, ZSM, etc. These porous materials have the advantages of high porosity, large specific surface area, good chemical stability, and controllable pore structure, which can effectively fix and stabilize nZVI and increase its reduction efficiency. Among porous materials, carbon materials are widely studied and applied due to their superior physical and chemical properties. Li et al.[39] investigated the treatment of Pb2+ by wrapping nZVI with hydrophilic biochar (BC) made from acid-peroxidized corn straw. The results showed that the adsorption capacity of the absorbent for Pb2+ can reach 135.4mg/g at a concentration of 50mg/L, and the maximum adsorption capacity can reach 480.9mg/g at a concentration of 200mg/L. Hou et al.[40] prepared amino-modified BC loaded nZVI to remove Cr6+ from water, using PEI as a functional monomer and corn straw BC as a carrier. Compared with unmodified nZVI, the removal efficiency of amino-modified BC loaded nZVI increased by about 3%, and after 28 days of aging, the removal effect of modified nZVI did not show significant changes. These results indicate that amino-modified BC loaded nZVI has better removal performance and stability than unmodified nZVI. Chen et al.[41] attempted to prepare BC from hyacinth, and then used BC and chitosan (C) to repair nZVI, forming C-nZVI-BC. C-nZVI-BC can absorb 82.2mg/g of Cr2+ at pH=2. This study effectively utilized invasive alien species such as hyacinth, and further modified it with C to enhance its adsorption capacity and environmental friendliness.
Although the above-mentioned materials have many advantages, their preparation process requires the production of BC first, followed by modification and reduction under anaerobic conditions, making the preparation procedure relatively complex. However, in the study by Fan et al.[42], the authors improved the complex preparation process by using one-step pyrolysis to prepare composite materials of BC and nZVI, making the large-scale application of carbon material loaded nZVI possible.
4.2.2 Inorganic Clay Loaded nZVI
Inorganic clay loaded nZVI is a technique that loads nZVI onto the surface of high adsorption inorganic clays to promote the dispersion, stability and catalytic performance of nZVI. Common inorganic clays include bentonite, montmorillonite, and zeolite, which have many functional groups such as hydroxyl, silicate, oxide, etc. These functional groups can interact with active functional groups on the surface of nZVI such as carbonyls, hydroxyls, etc., promoting nZVI adsorption and fixation[43]. At the same time, the high porosity and large specific surface area of inorganic clays can also increase the dispersion and stability of nZVI. Inorganic clay loaded nZVI can be widely used in soil, groundwater, wastewater treatment and other fields, mainly by the catalytic reduction of nZVI to remove organic matter and heavy metal pollutants. Due to the adsorption capacity and selectivity of inorganic clays, efficient removal of different types of pollutants can be achieved[44]. Meanwhile, they have advantages such as wide natural distribution and easy availability of raw materials. Li et al.[45] used zeolite to load nZVI and simultaneously adsorb As3+, Cd2+ and Pb2+ from aqueous solution. Due to the formation of multiphase compounds between zeolite and nZVI, synergy and competitive effects occurred among heavy metals. The maximum adsorption capacities of As3+, Cd2+ and Pb2+ were 11.52, 48.63 and 85.37mg/g, respectively. Gao et al.[46] used high-silicon ZSM-5 zeolite molecular sieve as a carrier to load nZVI for the removal of Pb2+ from wastewater. ZSM-5 has a stable framework and pore structure that can prevent nZVI from aggregating in the pores. Moreover, the structure of ZSM-5 is not easily changed. In practical experiments, the removal rate of Pb2+ was as high as 95.74%.
Compared with single nZVI, composite materials can increase the adsorption strength and removal efficiency. However, the adsorption rate of composite materials is usually related to the environmental conditions. If there are multiple ions in the environment, competitive adsorption will occur, which will reduce the removal of target pollutants. This is also a problem that needs to be overcome when applying composite materials to practical engineering.
4.3 Surfactant-encapsulated nZVI
Surfactants are a type of chemical with amphiphilic properties, meaning they have a hydrophilic end and a hydrophobic end. Due to this property, surfactants can encapsulate the surface of nZVI and prevent its aggregation[47]. Additionally, due to the hydrophilic nature of surfactants, the encapsulated nZVI can be more easily dispersed in water, increasing its reactivity and environmental remediation efficiency[48]. It is worth noting that when using surfactants to encapsulate nZVI, the dosage and type of surfactant should be carefully considered. Excessive use of surfactants may hinder the reactivity of nZVI and cause side effects. Therefore, appropriate surfactant dosage and type should be selected based on the category of target pollutants and water characteristics. Kang et al.[49] used a β-cyclodextrin cross-linked polymer (β-CDP) to encapsulate nZVI for the removal of Cd2+. Cyclodextrins have internal hydrophobic and external hydrophilic characteristics, and SEM was used to investigate the β-CDP-encapsulated nZVI, which had abundant pores to promote mass transfer and enhance pollutant removal. The encapsulated material removed Cd2+ in 40% NaOH medium with a removal efficiency of up to 98.9%, and after being placed for one month, the removal efficiency remained at 90.5%. Li et al.[50] used PVP and NaOA to modify nZVI simultaneously for the removal of Cr6+, and found that the two had a synergistic effect in eliminating Cr6+, with a removal efficiency of up to 99.5% and a maximum removal capacity of 231.75mg/g.
Due to the excellent dispersibility and transportability of surfactant-encapsulated nZVI, its application range has been greatly enhanced. In addition to continuous reaction walls, suspended solutions can also be formed by injection for purifying groundwater[51]. However, the impact of nZVI’s dispersibility on pollutant removal is still unknown, and in the complex environment of groundwater, surfactants can quickly desorb from nZVI, causing nZVI to become exposed, which may result in secondary pollution and weaken the removal efficiency.
4.4 Polymeric Electrolyte Encapsulation of nZVI
Polymeric electrolyte encapsulation is another common method for stabilizing and dispersing nZVI in water while enhancing its reactivity. Polymeric electrolytes are high molecular weight compounds that exhibit ionization properties[52]. When in contact with ions in water, they form a charged protective layer that can stably encapsulate nZVI. This is crucial for enhancing the reactivity of nZVI because stable nZVI particles can more easily interact with pollutants and quickly remove them[53]. When using polymeric electrolyte encapsulation of nZVI, it is also important to choose the appropriate type and quality ratio of polymeric electrolyte. Excessive amounts of polymeric electrolyte may affect the conductivity and solubility of the water, which in turn can affect the reactivity and stability of nZVI. Therefore, the appropriate type and quality ratio of polymeric electrolyte should be chosen based on the characteristics of the target pollutants and water to ensure the best environmental restoration effect. Jiemvarangkul et al.[54] investigated the migration of nZVI encapsulated in three types of polymeric electrolytes, PV3A, PAA, and soy protein, and found that both PV3A and PAA could increase the migration rate by reducing the particle size and carrying negative charge on the surface of nZVI. Among these three materials, PV3A-stabilized nZVI showed the best transport performance. The experiment was conducted under the injection concentration of nZVI at 10g/L, which is significant for establishing effective remediation in aquifers. Liu et al.[55] studied and analyzed the effects of two different polymeric electrolytes, APAM and CMC, encapsulating nZVI, and showed that nZVI encapsulated with CMC had better dispersibility in suspended solution. In addition, the nZVI modified by this polymeric electrolyte achieved a Ni2+ removal efficiency of over 80% within 2h.
The advantage of using polymeric electrolyte encapsulation to stabilize nZVI lies in reducing aggregation and oxidation while enhancing transport performance. CMC, compared to other polymeric electrolytes, can form complexes with heavy metals for enhanced removal efficiency. In addition, CMC exhibits electrostatic repulsion and other factors that can effectively reduce the aggregation of nZVI. However, the high cost of environmental remediation, unstable encapsulation effects, and potential pollution are challenges that need to be overcome when applying CMC to practical engineering.
5 CONCLUSION AND OUTLOOK
The application of PRB in situ groundwater remediation technology is gaining increasing popularity. nZVI, as a unique material with a core-shell structure, exhibits excellent reducibility and adsorption capacity, making it an ideal choice for converting high-valent metals into low-valent metals. Despite various modification studies conducted in the laboratory, practical engineering applications still face several challenges. Firstly, the complexity of the groundwater environment is a significant consideration. Groundwater contains various pollutants and environmental interference factors, such as natural organic matter, dissolved oxygen, and microorganisms, which may affect the removal efficiency and stability of nZVI. Therefore, future research needs to address these environmental interferences and improve the applicability of nZVI in complex groundwater environments. Secondly, the aggregation and migration abilities of nZVI need further improvement. In groundwater, nZVI particles tend to aggregate, leading to a decrease in their migration capability and limiting their effective transport and remediation performance in contaminated source areas. Thus, future research should focus on enhancing the dispersion stability of nZVI, reducing particle aggregation, and increasing its migration ability in groundwater. Additionally, considering the risk of secondary contamination to groundwater, caution must be exercised in the selection of non-biodegradable dispersants and chemical synthesis methods when using nZVI. Some commonly used dispersants and synthesis methods may introduce new pollutants, posing potential risks to the groundwater environment. Therefore, future research should aim to develop more environmentally friendly synthesis methods and dispersants for nZVI to minimize the risk of secondary groundwater pollution.
In conclusion, to make nZVI more feasible and sustainable in practical engineering, future research should concentrate on the following aspects: overcoming environmental interferences, improving material selectivity, enhancing the migration ability of nZVI, developing convenient and environmentally friendly synthesis methods, reducing costs, extending operation cycles, and establishing effective migration models for nZVI. By addressing these issues, further advancement and application of nZVI technology in groundwater remediation can be promoted.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
Wu Y was responsible for writing, and original-draft. Guan Y, Li S, He N, and Yuan Z were responsible for reviewing. Chen H was responsible for supervision. All authors contributed to the manuscript and approved the final version.
Abbreviation List
BC, Biochar
C, Chitosan
nZVI, Nanoscale zero-valent iron
PRB, Permeable reactive barrier
SEM, Scanning electron microscopy
β-CDP, β-cyclodextrin cross-linked polymer
References
[1] Luo Z, Zhu J, Yu L et al. Heavy metal remediation by nano zero-valent iron in the presence of microplastics in groundwater: Inhibition and induced promotion on aging effects. Environ Pollut, 2021; 287: 117628.[DOI]
[2] Ismanto A, Hadibarata T, Widada S et al. Groundwater contamination status in Malaysia: level of heavy metal, source, health impact, and remediation technologies. Bioproc Biosyst Eng, 2023; 46: 467-482.[DOI]
[3] Zhu F, Tan X, Zhao W et al. Efficiency assessment of ZVI-based media as fillers in permeable reactive barrier for multiple heavy metal-contaminated groundwater remediation. J Hazard Mater, 2022; 424: 127605.[DOI]
[4] Budania R, Dangayach S. A comprehensive review on permeable reactive barrier for the remediation of groundwater contamination. J Environ Manage, 2023; 332: 117343.[DOI]
[5] Maamoun I, Eljamal O, Falyouna O et al. Multi-objective optimization of permeable reactive barrier design for Cr(VI) removal from groundwater. Ecotox Environ Saf, 2020; 200: 110773.[DOI]
[6] Miller E, Menashe O, Dosoretz CG. A tailored permeable reactive bio-barrier for in situ groundwater remediation: removal of 3-chlorophenol as a case study. Environ Technol, 2022; 43: 1200-1210.[DOI]
[7] Suazo-Hernández J, Sepúlveda P, Cáceres-Jensen L et al. nZVI-Based Nanomaterials Used for Phosphate Removal from Aquatic Systems. Nanomaterials (Basel), 2023; 13: 399.[DOI]
[8] Wang S, Zhao M, Zhou M et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. J Hazard Mater, 2019; 373: 820-834.[DOI]
[9] Tarekegn MM, Hiruy AM, Dekebo AH. Nano zero valent iron (nZVI) particles for the removal of heavy metals (Cd2+, Cu2+ and Pb2+) from aqueous solutions. RSC Adv, 2021; 11: 18539-18551.[DOI]
[10] Sun P, Wang Z, An S et al. Biochar-supported nZVI for the removal of Cr(VI) from soil and water: Advances in experimental research and engineering applications. J Environ Manage, 2022; 316: 115211.[DOI]
[11] Vaezihir A, Bayanlou MB, Ahmadnezhad Z et al. Remediation of BTEX plume in a continuous flow model using zeolite-PRB. J Contam Hydrol, 2020; 230: 103604.[DOI]
[12] Romero-Fernández M, Moreno-Perez S, Orrego AH et al. Designing continuous flow reaction of xylan hydrolysis for xylooligosaccharides production in packed-bed reactors using xylanase immobilized on methacrylic polymer-based supports. Bioresour Technol, 2018; 266: 249-258.[DOI]
[13] Schwarz A, Pérez N. Long-term operation of a permeable reactive barrier with diffusive exchange. J Environ Manage, 2021; 284: 112086.[DOI]
[14] Chien SC, Li Y, Liu C. Permeable reactive barrier of waste sludge from wine processing utilized to block a metallic mixture plume in a simulated aquifer. Water Sci Technol, 2021; 84: 2472-2485.[DOI]
[15] Baldermann A, Kaufhold S, Dohrmann R et al. A novel nZVI-bentonite nanocomposite to remove trichloroethene (TCE) from solution. Chemosphere, 2021; 282: 131018.[DOI]
[16] Long Y, Liang J, Xue Y. Ultrasound-assisted electrodeposition synthesis of nZVI-Pd/AC toward reductive degradation of methylene blue. Environ Sci Pollut R, 2021; 28: 67098-67107.[DOI]
[17] Liu J, Liu A, Guo J et al. Enhanced aggregation and sedimentation of nanoscale zero-valent iron (nZVI) with polyacrylamide modification. Chemosphere, 2021; 263: 127875.[DOI]
[18] Yang J, Tian H, Guo J et al. 3D porous carbon-embedded nZVI@Fe2O3 nanoarchitectures enable prominent performance and recyclability in antibiotic removal. Chemosphere, 2023; 331: 138716.[DOI]
[19] Huang P, Zhang P, Wang C et al. P-doped biochar regulates nZVI nanocracks formation for superefficient persulfate activation. J Hazard Mater, 2023; 450: 130999.[DOI]
[20] Pan C, Hou D, Zhang M. Preparation and particle size calculation of iron nanoparticles by sputtering. Inform Rec Mater, 1999; 17: 8-9.
[21] Bianco LD, Hernado A, Navarro E et al. Structural configuration and magnetic effects in as-milled and annealed nanocrystalline iron. J Phy IV France, 1998; 8: 2-107.[DOI]
[22] Li F, Yang W, Xue D. Preparation and study of iron nanoparticles. J Lanzhou Univ (Nat Sci), 1994; 30: 144-146.
[23] Yang L, Wang D, Zhang Y et al. Preparation of iron nanoparticles by plasma system and experimental study. Nucl Fusion Plasmas Phys, 2011; 31: 372-378.
[24] Luo J, Zhang Z, Zhang S. New progress in preparation of iron nanoparticles by gas phase method. Mater Rev, 2007; 21: 130-133.
[25] Wang C, Zhang W. Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ Sci Technol, 1997; 31: 2154-2156.[DOI]
[26] Santos A, Ardisson JD, Tambourgi EB et al. Synthesis of granular Fe-Al2O3 by the sol-gel method. J Magn Magn Mater, 1998; 177-181: 247-248.[DOI]
[27] Liu X, Xu J, Liu S et al. Preparation of nano-scale metallic iron particles. Powder Metall Technol, 1996; 14: 26-29.
[28] Li F, Vipulanandan C, Mohanty KK. Microemulsion and solution approaches to nanoparticle iron production for degradation of trichloroethylene. Colloid Surface A, 2003; 223: 103-112.[DOI]
[29] Natter H, Schmelzer M, Löffler MS et al. Grain-Growth Kinetics of Nanocrystalline Iron Studied in Situ by Synchrotron Real-Time X-ray Diffraction. J Phys Chem B, 2000; 104: 2467-2476.[DOI]
[30] Machado S, Pinto SL, Grosso JP et al. Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci Total Environ, 2013; 445-446: 1-8.[DOI]
[31] Khurshid H, Mustafa MRU, Isa MH. Adsorption of chromium, copper, lead and mercury ions from aqueous solution using bio and nano adsorbents: A review of recent trends in the application of AC, BC, nZVI and MXene. Environ Res, 2022; 212: 113138.[DOI]
[32] Li Q, Chen Z, Wang H et al. Removal of organic compounds by nanoscale zero-valent iron and its composites. Sci Total Environ, 2021; 792: 148546.[DOI]
[33] Zhu F, Li L, Ren W et al. Effect of pH, temperature, humic acid and coexisting anions on reduction of Cr(Ⅵ) in the soil leachate by nZVI/Ni bimetal material. Environ Pollut, 2017; 227: 444-450.[DOI]
[34] Qu G, Chu R, Wang H et al. Simultaneous removal of chromium(Ⅵ) and tetracycline hydrochloride from simulated wastewater by nanoscale zero-valent iron/copper-activated persulfate. Environ Sci Pollut R, 2020; 27: 40826-40836.[DOI]
[35] Dong H, Jiang Z, Deng J et al. Physicochemical transformation of Fe/Ni bimetallic nanoparticles during aging in simulated groundwater and the consequent effect on contaminant removal. Water Res, 2018; 129: 51-57.[DOI]
[36] Yao B, Liu Y, Zou D. Removal of chloramphenicol in aqueous solutions by modified humic acid loaded with nanoscale zero-valent iron particles. Chemosphere, 2019; 226: 298-306.[DOI]
[37] Fan J, Chen X, Xu Z et al. One-pot synthesis of nZVI-embedded biochar for remediation of two mining arsenic-contaminated soils: Arsenic immobilization associated with iron transformation. J Hazard Mater, 2020; 398: 122901.[DOI]
[38] Liu P, Wang X, Ma J et al., Highly efficient immobilization of nZVI onto bio-inspired reagents functionalized polyacrylonitrile membrane for Cr(VI) reduction. Chemosphere, 2019; 220: 1003-1013.[DOI]
[39] Li S, Yang F, Li J et al. Porous biochar-nanoscale zerovalent iron composites: Synthesis, characterization and application for lead ion removal. Sci Total Environ, 2020; 746: 141037.[DOI]
[40] Hou S, Tian H, Huang C et al. Removal of Cr(Ⅵ) from aqueous solution by amino-modified biochar supported nano zero-valent iron. Acta Scientiae Circumstantiae, 2020; 40: 3931-3938.
[41] Chen X, Li F, Xie X et al. Nanoscale Zero-valent Iron and Chitosan Functionalized Eichhornia Crassipes Biochar for Efficient Hexavalent Chromium Removal. Int J Environ Res Public Health, 2019; 16: 3046.[DOI]
[42] Fan J, Chen X, Xu Z et al. One-pot synthesis of nZVI-embedded biochar for remediation of two mining arsenic-contaminated soils: Arsenic immobilization associated with iron transformation. J Hazard Mater, 2020; 398: 122901.[DOI]
[43] Fang Y, Wen J, Zeng G et al. From nZVI to SNCs: development of a better material for pollutant removal in water. Environ Sci Pollut R, 2018; 25: 6175-6195.[DOI]
[44] Zhou W, Liu F, Yi S et al. Simultaneous stabilization of Pb and improvement of soil strength using nZVI. Sci Total Environ, 2019; 651: 877-884.[DOI]
[45] Li Z, Wang L, Meng J et al. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(Ⅱ), Pb(Ⅱ), and As(Ⅲ) in aqueous solution and soil. J Hazard Mater, 2018; 344: 1-11.[DOI]
[46] Gao D, Zheng A, Zhang X et al. Mercaptosilane-assisted synthesis of sub-nanosized Pt particles within hierarchically porous ZSM-5/SBA-15 materials and their enhanced hydrogenation properties. Nanoscale, 2015; 7: 10918-10924.[DOI]
[47] Li S, Feng D, Liu Q et al. Surfactant-enhanced reduction of soil-adsorbed nitrobenzene by carbon-coated nZVI: Enhanced desorption and mechanism. Sci Total Environ, 2023; 856: 159186.[DOI]
[48] Tian H, Liang Y, Yang D et al. Characteristics of PVP-stabilised nZVI and application to dechlorination of soil-sorbed TCE with ionic surfactant. Chemosphere, 2020; 239: 124807.[DOI]
[49] Kang H, Yang Z, Wan Y. Removal performance of Cd2+ using nanoscale zero-valent iron embedded by β-cyclodextrin. Environ Eng, 2015; 33: 122-125.
[50] Li J, Fan M, Li M et al. Cr(Ⅵ) removal from groundwater using double surfactant-modified nanoscale zero-valent iron (nZVI): Effects of materials in different status. Sci Total Environ, 2020; 717: 137112.[DOI]
[51] Khurshid H, Mustafa MRU, Isa MH. Adsorption of chromium, copper, lead and mercury ions from aqueous solution using bio and nano adsorbents: A review of recent trends in the application of AC, BC, nZVI and MXene. Environ Res, 2022; 212: 113138.[DOI]
[52] Zhou T, Huang X, Ding N et al. Porous polyelectrolyte frameworks: synthesis, post-ionization and advanced applications. Chem Soc Rev, 2022; 51: 237-267.[DOI]
[53] Bediako JK, Ouardi YE, Mouele ESM et al. Polyelectrolyte and polyelectrolyte complex-incorporated adsorbents in water and wastewater remediation - A review of recent advances. Chemosphere, 2023; 325: 138418.[DOI]
[54] Jiemvarangkul P, Zhang W, Lien HL. Enhanced transport of polyelectrolyte stabilized nanoscale zero-valent iron (nZVI) in porous media. Chem Eng J, 2011; 170: 482-491.[DOI]
[55] Liu J, Liu A, Zhang W. The influence of polyelectrolyte modification on nanoscale zero-valent iron (nZVI): Aggregation, sedimentation, and reactivity with Ni(Ⅱ) in water. Chem Eng J, 2016; 303: 268-274.[DOI]
Copyright © 2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.




 Copyright ©
Copyright ©