Environmental, Thermal, and Mechanical Studies of SBR-Nano Aluminum Oxide Composites
Wasudeo B Gurnule1*, Rashmi R Dubey1, Devidas S Bhagat2, Yashpal Rathod3, Bhim P Kafle4
1Department of Chemistry, Kamla Nehru Mahavidyalaya, Nagpur, India
2Department of Forensic Chemistry and Toxicology, Government Institute of Forensic Science, Aurangabad, India
3Department of Chemistry, J. M. Patel College, Bhandara, India
4Department of Chemical Science and Engineering, Kathmandu University, Kathmandu, Nepal
*Correspondence to: Wasudeo B Gurnule, PhD, Professor, Department of Chemistry, Kamla Nehru Mahavidyalaya, 44G7+X33, Sakkardara Rd, Raghuji Nagar, Nagpur 440024, India; Email: wbgurnule@gmail.com
DOI: 10.53964/jmn.2022010
Abstract
Objective: The present study looked at the abrasion characteristics of two-part matrix nanocomposites made of styrene butadiene rubber (SBR) and nano aluminium oxide. The wear characteristics of various forms of polymeric matrix have been sporadically studied. In the present investigation, our main aim was to synthesize rubber/nano aluminium oxide nanocomposites as possible materials in industrial applications and to evaluate environmental, thermal and mechanical properties of the composite.
Methods: The formulations of the styrene-butadiene rubber compounds were reinforced with aluminum oxide nanoparticles. Mixing was performed at room temperature using two-roll mixing mill with a rotor friction ratio of 1:1.25. After the coagulation of rubber composites, rubber matrix was washed with water and dried at 70°C. SBR-nanocomposite was synthesized by emulsion polymerization method.
Results: Elemental analysis results were in good agreement with the calculated values. The increased crosslink density, which resulted in increased hardness and modulus ultimately, produced the enhancement of abrasion resistance. The abrasion resistance is an essential property for the application of tire and belt. The properties were enhanced because of the higher polymer filler interactions than filler-filler interactions. The functional groups were incorporated into the polymer, and the stress is much more efficiently transferred from the polymer matrix to the inorganic filler, resulting in an increase in tensile properties. The enhanced degree of filler-polymer interaction is known for its ability to restrict the swelling ratio. The order of activation energies for SBR-nano aluminum oxide nanocomposites was parallel to the order of their thermal stability. The order of reaction (n) of SBR-nano aluminum oxide composite was 0.40.
Conclusion: All the nanocomposites are potential candidates to prepare SBR rubber hybrid composites. SBR rubber nanocomposites are promising materials given their excellent mechanical, ozone resistance, flame resistance, and good thermal properties.
Keywords: thermal, synthesis, environmental, Raman spectroscopy, nano aluminum oxide, mechanical properties
1 INTRODUCTION
Polymer materials possess a wide range of applications, especially when utilized as a matrix for reinforced composites[1-7]. The intense application of composite forms has been driven by the commercial value of polymers in various industries. There are different types of these composites in these materials depending on the size of the additives. Nanocomposites refer to composites whose additives are less than 100nm in size and are fabricated based on polymeric materials. Recently, interest in polymeric-based nanocomposites materials has grown[8-12]. Nanostructured materials have become increasingly important in the last decade due to their numerous potential uses in various fields. An alternative to macroscopically packed polymers is a polymer nanocomposite.
Nanocomposites exhibit significantly better properties compared to pure polymers or conventional composites because of their particle size[13,14]. This include improved strength and modulus, reduced gas permeability, and reinforced solvent and heat resistance[15,16]. Polymer-based nanocomposites are special ideal arrangements to analyze the dynamics and structure of polymers in constrained settings in addition to their potential applications[17-19]. One of the areas that have drawn attention in recent years is the blending of rubber through polymeric and non-polymeric materials. The two-part matrix that makes up thermoplastic or thermoset has been frequently described literature. Due to their unique qualities, combined rubber and resin matrices have been produced in several works. Nano clay was used by Bonnia and his colleagues to manufacture the natural rubber/polyester nanocomposite materials[20].
Boukerrou and his colleagues worked with organo clay and rubbery epoxy resin[21]. The morphological, mechanical, and viscoelastic characteristics of this ternary nanocomposite were reported, and it was demonstrated that the rubbery epoxy resin matrix effectively dispersed the nanoclay. Additionally, it has been shown that addition of nanoclay could improve their mechanical characteristics. Zhao et al.[22] conducted research on the acrylonitrile butadiene and phenol-hindered rubber compounds and found that this mixture was available to create a high-performance dampening material. Elasticity in rubber materials draws attention, and resin materials provide excellent mechanical and thermal characteristics. To enhance the wear qualities of epoxy resin, Yu et al.[23] synthesised nano-rubber particles. The activated carbon powder (PAC)/iron (Fe)/silicon (Si)/zinc (Zn) nanocomposite was successfully synthesized using a co-precipitation method, in which Fe, Si and Zn were loaded on the PAC. The sulfonated covalent organic frameworks prepared with melamine and terephthalaldehyde, followed by sulfonation, were used as acidic porous catalysts. A simple and efficient method for the synthesis of pyrazolopyranopyrimidines under solvent-free has been developed[24-26].
As part of this work, we looked at the abrasion characteristics of two-part matrix nanocomposites made of styrene butadiene rubber (SBR) and nano aluminum oxide. The wear characteristics of various forms of the polymeric matrix have been marginally studied. To this end, the current research looked into how SBR compounds behave when exposed to nanoparticles of aluminium oxide.
2 MATERIALS AND METHODS
2.1 Materials
We bought nanocomposites from the Center Scientific Company in Nagpur, India. We procured tetramethyl thiuram disulfide, 2,2'-dithiobis, stearic acid, and N,N'-diphenyl P-phenylene diamine from the Central Scientific Company in Nagpur, India, zinc and sulphur oxides (from Post Graduate Department of chemistry Kamla Nehru Mahavidyalaya, Nagpur, India) from Shree Radha, with SBR latex as the polymer.
2.2 Preparation of SBR-nanocomposite
Initially, the nano-alumina was vigorously rotated in toluene to form a suspension of nano-alumina. SBR latex was added to the suspension of nano aluminum oxide for thorough mixing. At room temperature, the mixture began to coagulate and was repeatedly cleaned in water and dried for 12h at 70°C to produce the SBR-nanocompound.
2.3 Compounding of Rubber
SBR-nano compound was combined with substances listed in Table 1. At 150°C, the SBR-nanocompound was vulcanised. After mixing the SBR-nanocompound mixture for 15min on a two-roll mill, all other materials, which are specified in Table 1, were added and mixed for 10min.
Table 1. Formulation of Rubber Compound
Ingredients |
Phr |
|||
1 |
2 |
3 |
4 |
|
SBR |
100 |
100 |
100 |
100 |
Aluminum oxide |
0 |
2 |
10 |
12 |
Stearic acid |
2 |
2 |
2 |
2 |
Zinc oxide |
5 |
5 |
5 |
5 |
2,2’-dithiobis |
0.5 |
0.5 |
0.5 |
0.5 |
Tetramethyl thiuram disulfide (TMTD) |
0.2 |
0.2 |
0.2 |
0.2 |
N,N’-Diphenyl P-phenylene diamine |
1 |
1 |
1 |
1 |
Sulphur |
2 |
|
2 |
2 |
2.4 Characterizations
2.4.1 Elemental Analysis
The study was carried out at the Sophisticated Analytical Instrumentation Facility (SAIF), Cochin, using standard tools. The percentage of different elements, such as carbon (C), hydrogen (H), nitrogen (N), sulphur (S), and oxygen (O), contained in the rubber nanocomposite was calculated using elemental analysis.
2.4.2 Fourier-transform Infrared Spectroscopy (FTIR)
An IR-affinity spectrophotometer from Shimadzu was used to determine the FTIR spectra of SBR-nanocomposites (from the Department of Chemistry Kamla Nehru Mahavidyalaya, Nagpur, India).
2.4.3 Raman Spectroscopy
A Jobin-Yvon T 64000 spectrometer was used for the Raman scattering tests. SBR and SBR with filler were illuminated with an argon-krypton ion laser (Coherent model Innova 70C) at the 647.1nm wavelength to reduce the fluorescence impact (red line). A confocal microscope (Olympus BX40) with 100× magnification objectives was utilized to concentrate the laser beam (from National Chemical Laboratory Pune, India). To eliminate electronic peaks, each spectrum was recorded over 60s with 10 accumulations in the frequency of 400-3,500cm-1.
2.5 Mechanical Tests
To evaluate the tensile strength, elongation at break, and tear resistance properties of the material, a tensile Monsanto T10 Tensometer conforming to ASTM D412 was used. It has two jaws, one of which is stationary and the other moves rapidly at a rate of 500mm per minute (from Mumbai University).
2.5.1 Abrasion Resistance
The test was carried out using the HZ50 Wallace Test Equipment in accordance with ASTM D2228. The equipment was powered at 250 watts, and the sample was rotated 500 times in 5min (from University of Mumbai) after which the weight difference before and after the test was calculated.
2.5.2 Swelling Study
With toluene as the solvent, the equilibrium swelling method was utilized to estimate the crosslinking density of all the vulcanized samples. The weight change between before and after the test was then estimated. The conserved samples with known weights were given seven days at room temperature to swell in fresh toluene. Fresh toluene was used as the solvent replacement after every 24h. After 7 days, the samples were removed, the adhering liquid was quickly wiped with a paper towel, and then the samples were immediately weighed. Vacuum drying was done at 80°C until a consistent weight was achieved. Flory-equation Rehner's was used to calculate the crosslinking density Ve, which is expressed as the number of elastically active chains per unit volume (1):
|
Where,
Vr is the volume percentage of rubber in the swollen network,
Ve is the network chain density,
V1 is the molar volume of toluene (106.3cm3mol-1),
X1 is the Flory-Huggins rubber-solvent interaction parameter.
2.5.3 Thermal Studies
The non-isothermal thermogravimetric analysis was performed in an air atmosphere with a heating rate of 10°C min-1 from a temperature range of RT to 700°C using Perkin Elmer thermogravimetric analysis (TGA) analyzer. The thermograms were recorded at SAIF, Sophisticated Test and Instrumentation Centre (STIC), Cochin University, Cochin. The thermal stability of rubber nanocomposites, based on the initial decomposition temperature, was used to define their relative thermal stability, irrespective of the degree of decomposition. A brief account of thermal behavior of these rubber nanocomposites was given in results and discussion. Thermal stability of the SBR-nano aluminum oxide nanocomposites was determined by TGA. To obtain the relative thermal stability of the rubber nanocomposites, the method described by Sharp-Wentworth(SW) and Freeman-Carroll(FC) was adopted. The thermoanalytical data and the decomposition temperatures were determined for different stages.
3 RESULTS AND DISCUSSION
3.1 Elemental Analysis
All four rubber nanocomposites were tested at the SAIF, STIC, Cochin University, Cochin, for C, H, N, and S. Using messenger approach, empirical formulas were calculated for each of the four SBR- aluminum oxide (2phr), SBR-aluminum oxide (10phr), and SBR-aluminum oxide (12phr). A good agreement was observed between the estimated values and the observed results. The empirical formula was used to calculate the empirical weight of a single repeating unit[27]. Analytical details for each of the four SBR-Al2O3 nanocomposite materials are provided in Table 2. Table 2 contains information on the styrene-butadiene rubber and nano aluminum oxide composites' elemental analyses.
Table 2. Information on the Styrene-Butadiene Rubber and Nano aluminum Oxide Composites' Elemental Analyses
Rubber Samples |
% of C Observed (Cal.) |
% of H Observed (Cal.) |
% of N Observed (Cal.) |
% of O Observed (Cal.) |
% of S Observed (Cal.) |
Empirical Formula |
Empirical Weight |
SBR |
83.44 |
8.11 |
2.56 |
5.85 |
2.34 |
C38H44 N1O2S |
546.993 |
(81.44) |
(8.05) |
(2.31) |
(5.08) |
(1.39) |
|||
SBR-aluminum oxide (2phr) |
83.44 |
8.11 |
0.26 |
5.85 |
2.34 |
C38H44 NO2S |
546.993 |
(82.42) |
(8.10) |
(0.20) |
(4.80) |
(2.30) |
|||
SBR-aluminum oxide (10phr) |
83.90 |
9.15 |
0.24 |
5.59 |
1.12 |
C40H52NO2S |
572.667 |
(82.90) |
(8.15) |
(0.20) |
(4.59) |
(1.10) |
|||
SBR-aluminum oxide (12phr) |
80.88 |
9.16 |
1.20 |
6.70 |
2.30 |
C40H54N1O4S1 |
644.935 |
(79.78) |
(8.15) |
(0.20) |
(6.20) |
(1.88) |
3.2 FTIR Spectroscopy for SBR-nano Aluminum Oxide Composites
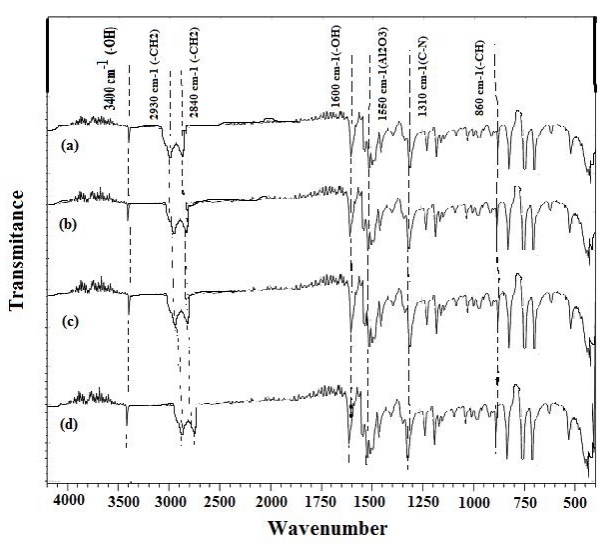
Figure 1 displays the FTIR spectra of various nano aluminium oxide loading levels. The large peak at 3,400cm-1 in each of these spectra is attributed to stretching vibrations of the -OH group in addition to the adsorbed water. Peaks at 1,040cm-1 and 600cm-1 are caused by the transitional phase of alumina's Al-O stretching vibration. Both asymmetric and symmetric stretching accounts for the peak at 1,100cm-1 and 470cm-1.
In addition to the absorption bands that correspond to the vibration of specific functional groups, all SBR-filler spectra show two distinct peaks at 2,930cm-1 and 2,840cm-1, as well as one peak at around 1,450cm-1. These two tiny peaks, measuring around 2,930cm-1 and 2,840cm-1, are the distinguishing bands of the symmetric and antisymmetric stretching vibrations of the -CH2 group, respectively. The bending vibration frequency of the -CH2 group is 1,450cm-1. The spectra of SBR-nanocomposites exhibit each of these peaks in Figure 1, which is supported by the band peculiar to the alkene group at 1,080cm-1. The peak at 1,600cm-1 is related to the free -OH group's existence. Al2O3 nanoparticle variations in surface chemistry are attributed to the peak at 1,550cm-1. The existence of characteristic signals of C-N stretching is the cause of the peaks at 1,310cm-1 and 1,180cm-1. Al-O stretching vibrations are 566cm-1 and 828cm-1, while the Al-O-Al stretching vibration is 756cm-1. The C-C bond's stretching vibration falls between 1,040 and 1,020cm-1. Asymmetrical deformation of CH3 and scissoring deformation of CH2 both correspond to 1,462cm-1[28].
|
Figure 1. Fourier-transform infrared spectroscopy (FTIR) spectra of SBR-nanocomposite. a: Unfilled composites; b: 2phr aluminum oxide in SBR; c: 10phr aluminum oxide in SBR; d: 12phr aluminum oxide in SBR.
3.3 Raman Spectroscopy for SBR-Nano Aluminum Oxide Nanocomposites
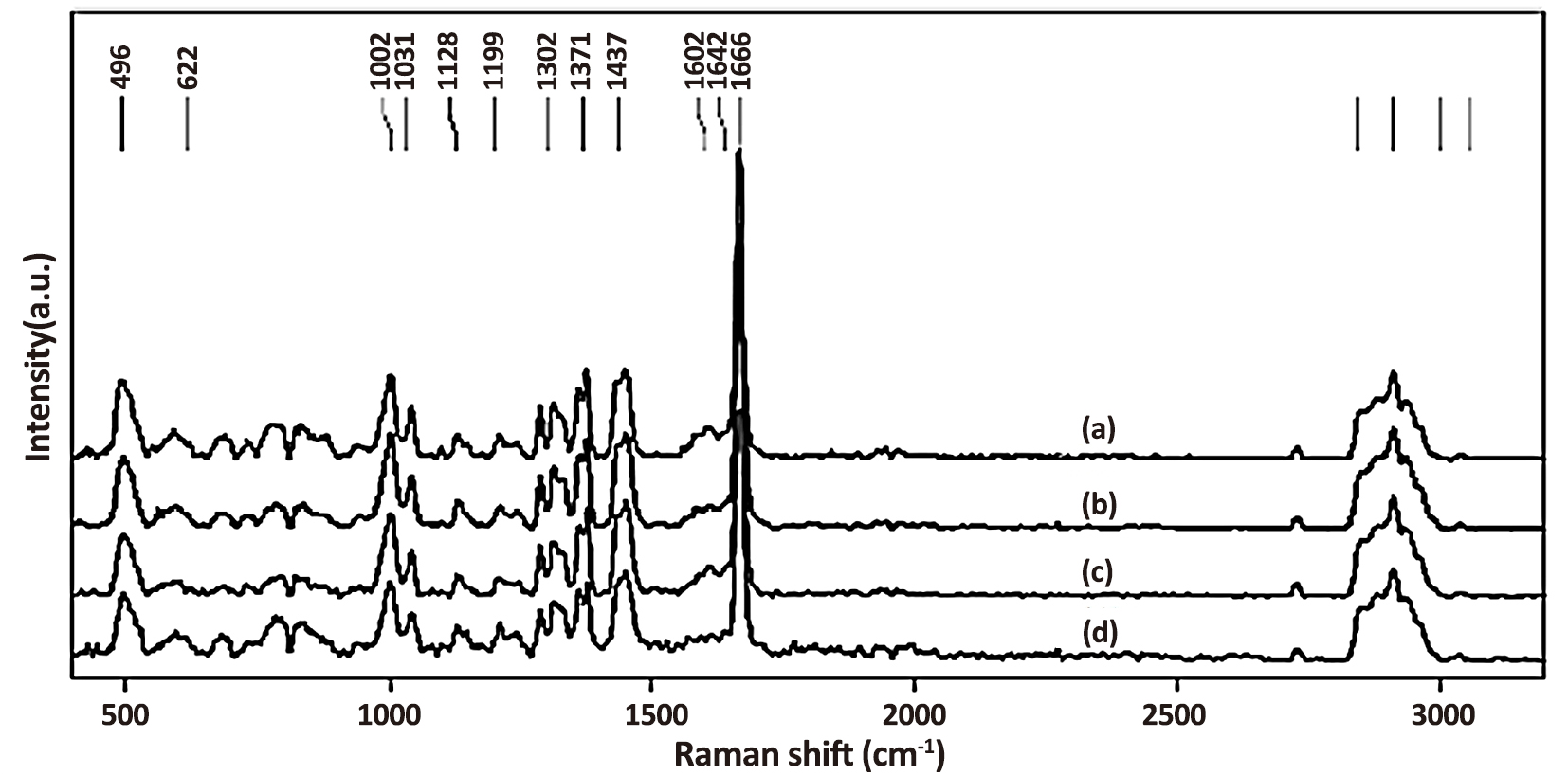
The presence of impurities and the addition of crosslinking agents, fillers, and other rubber ingredients are the primary causes of fluorescence parasite signals in the Raman scattering spectrum, particularly with the laser excitation lines in the visible region. Due to the markedly higher quantum yield of the fluorescence process than that of the Raman process, the primary spectroscopic information is overlapped. To clearly identify the different bands of SBR and SBR-nanocomposites, Raman spectroscopy was used on the coagulated rubber without nano aluminium oxide. As shown in Figure 2, the spectra of SBR and SBR-nano aluminium oxide composites were examined, and band assignments were conducted in accordance with the comparison to the spectra of published works. In the literature only some of the Raman peaks of SBR have been assigned. The Raman analysis of polystyrene and butadiene rubber in the current study provides thorough attribution. Stretching vibrations of -CH2 and -CH3 that are both symmetric and asymmetric were frequently observed between 2,800 and 3,000cm-1. At 1,666 and 1,668cm-1, C=C stretching vibrations of SBR were seen. Moreover, differentiation between the contributions of SBR and SBR-nano aluminium oxide composites was considered challenging due to the overlapped signals. Hence, only the distinguishing peaks were emphasized. There are examples of Raman spectra of unfilled SBR samples at different mix ratios in Figure 2. The findings demonstrated that the blend mix affects the strength of Raman peaks. As the nano aluminium oxide concentration of the SBR rubber increased, the intensities of the isolated characteristic bands of SBR at 1,371, 1,128, and 496cm-1 tended to diminish, while the intensities of the distinctive signals at 1,002, 1,302, 1,602 and 3,058cm-1 tended to increase. The internal standard was the peak at 1666cm-1, which was independent of the SBR ratio. By graphing the intensity ratio of the distinctive signals of each rubber against the mix ratio, it is feasible to determine the composition in the blends from this association. It should be mentioned that a calibration curve was built using the typical signals for SBR at 1,371 and 1,302cm-1, respectively, for -CH3 asymmetric deformation and =C-H in-plane deformation. Owing to its best resolution among the selected peaks, the characteristic peak at 1,371cm-1, which is not overlapped, could be observed. The Raman characteristic signals of SBR showed no significant alterations following the addition of nano aluminum oxide. This observation is most likely the outcome of the interplay between the poor Raman intensity of the amorphous nanosized aluminium oxide particles and the low quantum efficiency with a frequency of 647.1nm. The characteristic bands of nano aluminum oxide are surprisingly feeble when the material is stimulated at 647.1nm. Furthermore, these signals are not improved by increasing the scan count or accumulation duration. When the excitation wavelength was adjusted to 514.5nm, the nano aluminium oxide sample displayed a Raman spectrum with substantial signals at 456, 796, and 994cm-1 corresponding to Al-O-Al and Al-OH stretching vibration. Unfortunately, the SBR's peak frequencies at 496 and 1,000cm-1, which correspond to =CC2 rocking and -CH2 stretching, respectively, were in the same frequency range. As a result, differentiation between the SBR and nano aluminium oxide Raman signals was considered challenging.
|
Figure 2. Raman spectra of SBR-nanocomposite. a: unfilled composites; b: 2phr aluminum oxide in SBR; c:10phr aluminum oxide in SBR; d: 12phr aluminum oxide in SBR.
3.4 Mechanical Testing
3.4.1 Tensile Strength
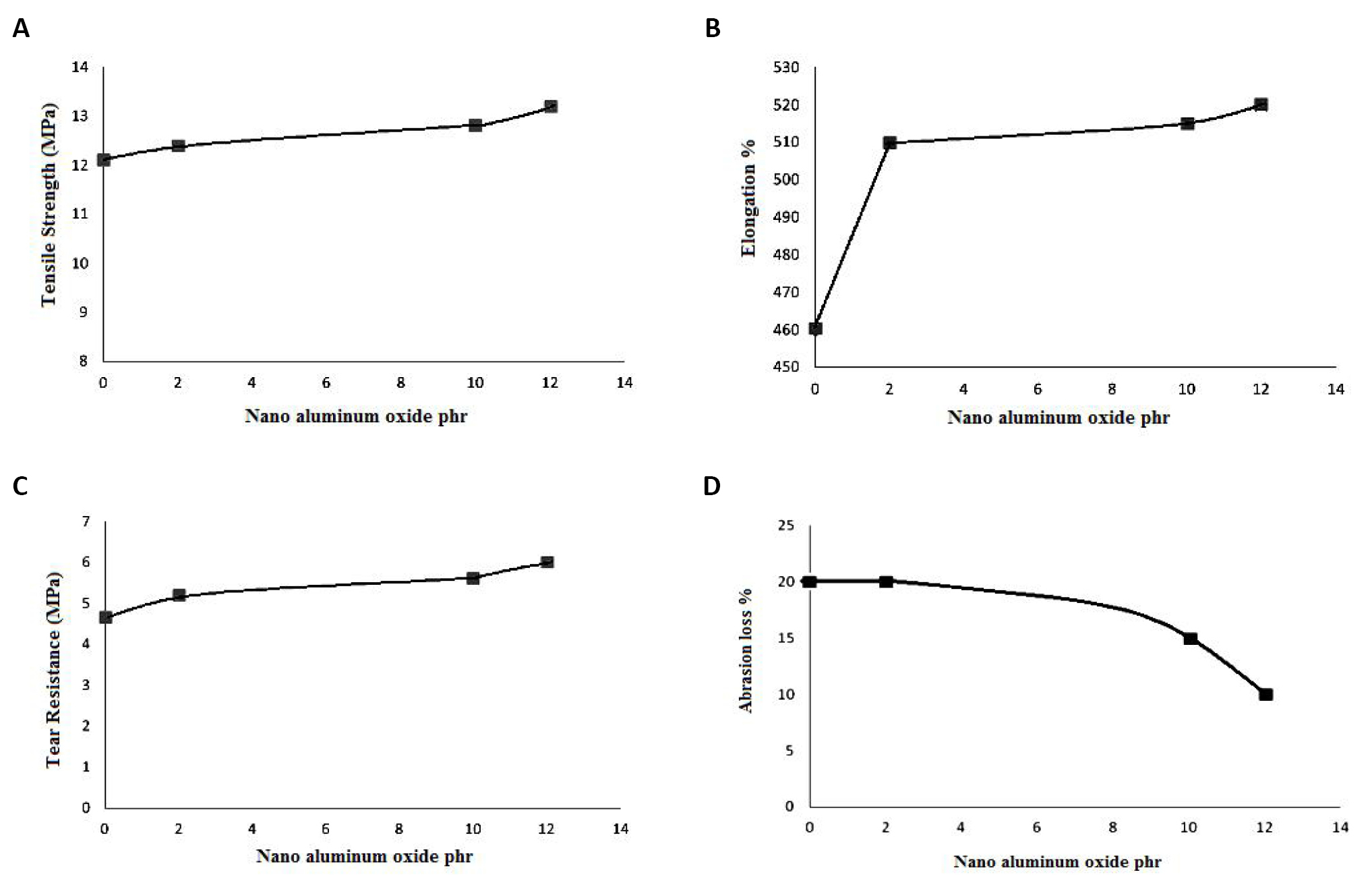
Alumina nanoparticle addition (Figure 3A) demonstrated an increase in tensile strength. Tensile strength increased with concentration. Such behavior may be ascribed to the presence of nanoparticles in the gaps among rubber chains, which resulted in a stiff structure with increased tensile strength (13.2MPa). Table 3 displays the tensile strength values. Rubber chains intercalated into the layers increased the tensile strength. Higher filler-polymer interactions than filler-filler interactions provided the improved characteristics. As a result of the incorporation of the functional groups, the stress was transferred from the polymer matrix to the inorganic filler considerably more effectively, which enhanced the tensile characteristics. Strong hydrogen bonds served as the intercalation's driving force. The tensile strength of nano aluminum oxide and SBR rubber has increased.
Table 3. Mechanical Properties of SBR Unfilled and Filled Nano Aluminum Oxide Composites
Compounds |
1 |
2 |
3 |
4 |
σ100% (MPa) |
2.3 |
2.6 |
2.8 |
2.9 |
σ200% (MPa) |
2.5 |
2.9 |
3.2 |
3.2 |
σ300% (MPa) |
2.8 |
3.0 |
3.4 |
3.5 |
Tensile strength, Mpa |
12.1 |
12.4 |
12.8 |
13.2 |
Elongation at break, % |
460 |
510 |
515 |
520 |
Tear resistance, MPa |
4.7 |
5.2 |
5.6 |
6 |
Abrasion resistance, % |
20 |
20 |
15 |
10 |
Cross-link density ×10-4 |
2.63 |
3.05 |
3.31 |
3.33 |
|
Figure 3. The effects of nano alumina. A: The effect of nano alumina on tensile strength; B: The effect of nano alumina on elongation property; C: The effect of nano alumina on tear resistance property; D: The effect of nano alumina on abrasion resistance property.
3.4.2 Elongation Property
The elongation properties increase through the accumulation of alumina nanoparticles, as in Figure 3B. This is due to the diffusion of extremely small alumina nanoparticles into the rubber chains, allowing for increased stretching and thus elongation. The extreme elongation at break values is listed in Table 3. The elongation at break (%) curves of nanocomposites with varied percentages of nano aluminium oxide is shown in Figure 3B. As the weight percentage of filler increases, all systems' elongation at break increases. The rise at higher loads is caused by an increase in material stiffness, probably associated with a decrease in tensile crystallization. All systems displayed a similar trend. The utmost uses require a huge early strengthening, and the vital straining is not practically critical[29,30].
3.4.3 Tear Resistance Property
The particles will fill the crevices between rubber chains and strengthen the mechanical bond between them for the same reason described in the case of the tensile property, which will improve tear resistance. This feature is linked to the tensile property, and as shown in Figure 3C, tear resistance improves with increasing amounts of nano aluminium oxide, reaching a maximum of 6MPa at 12pphr. The system's tear strength curves are displayed in relation to the weight percentage of the concentration of nano aluminum oxide in Figure 3C. SBR rubber loaded with nano aluminium oxide offers improved tear strength, a sign of the resistance provided by SBR rubber to facilitate the spread of cracks and the super reinforcing of nano aluminium oxide. Most importantly, the thin rubber layers change the route of the rip, which gives nanocomposites a high tear strength.
3.4.4 Abrasion Resistance Property
Figure 3D demonstrates that the weight loss of rubber is constant by the adding nano aluminium oxide to the SBR matrix at 2pphr, but drops at 10pphr because the nano aluminium oxide functions as a filler and is not exposed to the abrasion load. Due to the restriction of weight loss by nanoparticles, rubber weight loss in 12pphr again falls till it reaches 10%. Abrasion loss, a measure of reinforcing, decreases as the hardness increases,as in Figure 3D. The improvement in abrasion resistance was the result of the high crosslink density, which also leads to enhanced hardness and modulus. There also existed a decrease in abrasion loss. The increased rubber-filler interaction also accounted for the improved abrasion resistance in the SBR-nano aluminium oxide filled composites. The intercalation and exfoliation of the modified silicate increased the surface area of the filler, resulting in more contact between the filler and the matrix[31].
3.5 Thermal Degradation Studies
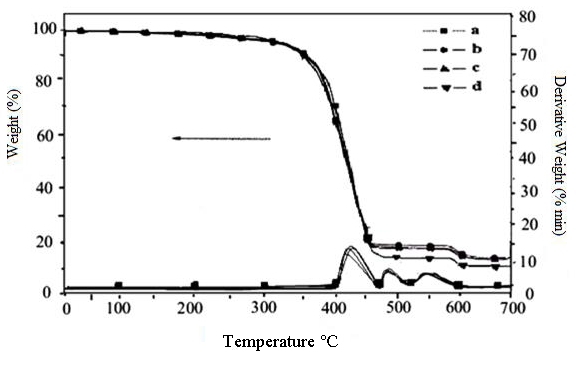
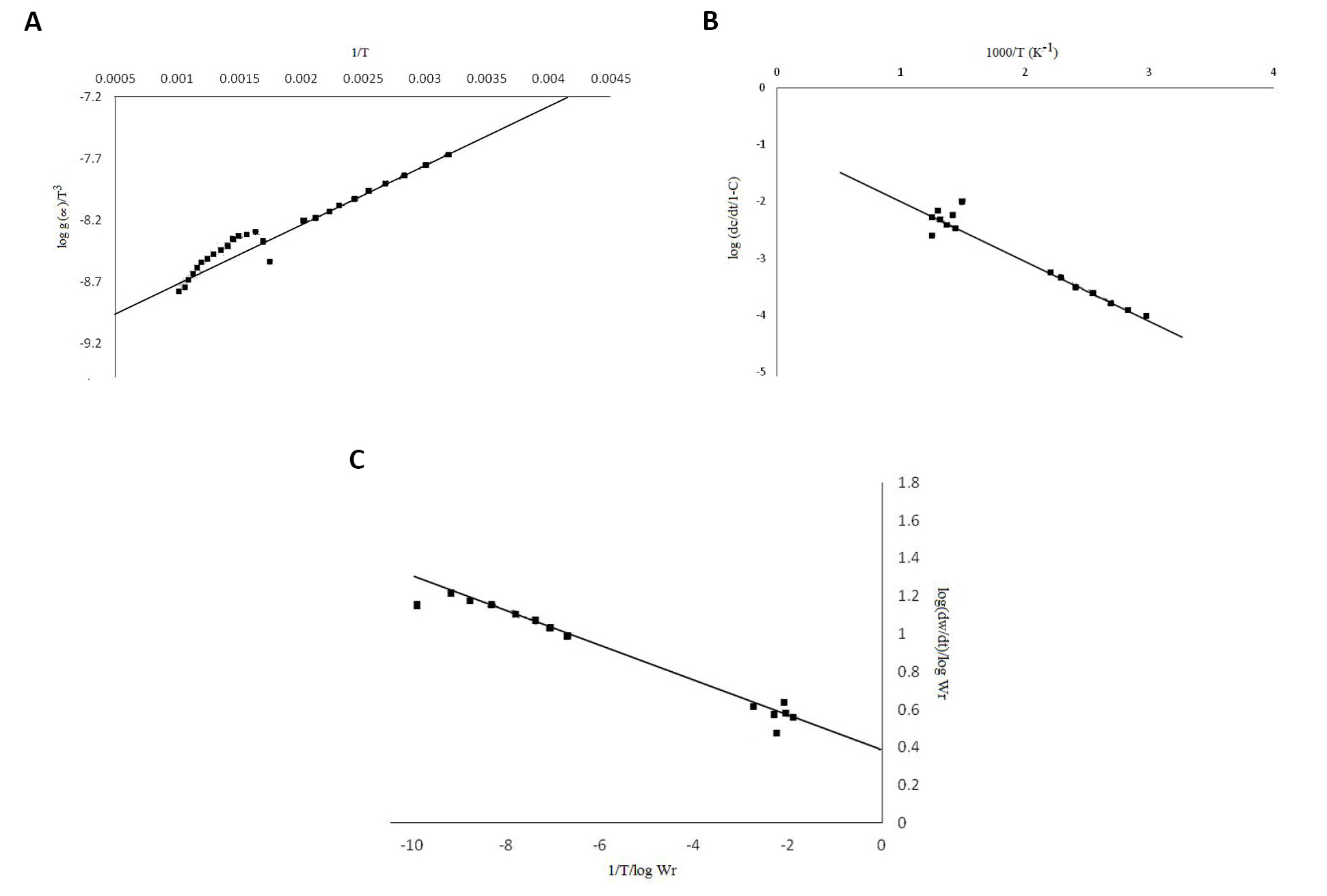
The thermal stability of the SBR-nano aluminum oxide nanocomposites was determined by TGA. The thermograms of the rubber nanocomposites are shown in Figure 4, and their percentage of weight loss at various temperatures is observed. To obtain the relative thermal stability of the rubber nanocomposites, the method described by SW and FC was adopted. The thermoanalytical data and the decomposition temperatures were determined for different stages[32-37]. The ‘average Ea’ calculated by FC and ‘average Ea’ by SW is nearly same.
|
Figure 4. Thermogravimetric curves of SBR-nanocomposite. a: Unfilled composites; b: 2phr aluminum oxide in SBR; c: 10phr aluminum oxide in SBR; d: 12phr aluminum oxide in SBR.
Thermogravimetric analysis was performed to evaluate the thermal stability of the composites. The amount of aluminum oxide content in the rubber composites is determined from the residual weight percentage of particular composites. The differential thermogravimetric curves of samples showed three peaks. The first weight loss observed at the temperature range 350°C to 450°C was ascribed to the degradation of rubber component. The second weight loss appeared at the temperature range of 500°C to 560°C. The next weight loss seen at the temperature range of 600°C to 680°C was due to the decomposition of carbonaceous residue. Temperature at maximum weight loss is given by the peak of derivative thermogravimetric curve. All the composites showed similar thermal stability, and the Tmax was found to be 400°C and 460°C, respectively, for all composites. With the help of thermogravimetric data, the thermal activation energies were 3.45 by FC and 4.43 by SW methods, and the order of reaction was found to be 0.40 (Figure 5).
|
Figure 5. SW plot (A), thermal activation energy plot (B) and FC plot (C) of SBR-aluminum oxide nanocomposites.
From the knowledge of activation energy using FC method, it is possible to calculate the values of various kinetic parameters. The Ea values calculated by SW and FC methods were in good agreement with each other. The order of activation energies for SBR-nano aluminum oxide nanocomposites were parallel to the order of their thermal stability. The order of reaction (n) of SBR-nano aluminum oxide composite was 0.40[38-49]. From the above results, it is quite clear that the decomposition reaction does not obey the exact first order kinetics.
4 CONCLUSION
SBR compositions were successfully prepared both with and without nanoscale aluminium oxide particles. Abrasion results were obtained by fabricating rubber nanocomposites using two mills for mechanical mixing and ASTM D2228 equipment for enhanced spectral properties. The abrasion data showed the SBR sample's volumetric mass loss content. The rubber base compound comprised of styrene butadiene has a high abrasion content, which proves that SBR contains more abrasive properties. With the addition of nano aluminium oxide to the SBR sample, the samples' abrasion content also decreased, which may be attributed to the beneficial effects of nano aluminium oxide on the mechanical and physical properties of SBR.
Acknowledgements
For the results of the elemental analysis, the authors are grateful to the Sophisticated Analytical Instruments Facility, SAIF, in Cochin. For conducting the mechanical tests of the rubber nanocomposites, the authors are also grateful to Mumbai University in Mumbai.
Conflicts of Interest
The authors declared no conflict of interest, financial or otherwise.
Author Contribution
Gurnule WB and Dubey RR designed the experiment. Bhagat DS, Gurnule WB and Kafle BP supervised the work. Rathod Y and Gurnule WB performed the data analysis. Rathod Y and Gurnule WB drafted the manuscript. All the authors contributed to writing the article, read and approved its submission.
Abbreviation List
C, Carbon
FC, Freeman-Carroll
Fe, Iron
FTIR, Fourier-transform infrared spectroscopy
H, Hydrogen
N, Nitrogen
O, Oxygen
PAC, Activated carbon powder
S, Sulphur
SAIF, Sophisticated Analytical Instrumentation Facility
SBR, Styrene butadiene rubber
Si, Silicon
STIC, Sophisticated test and instrumentation centre
SW, Sharp-Wentworth
TGA, Thermogravimetric analysis
Zn, Zinc
References
[1] Hesabi ZR, Kouchi A, Beni YT et al. Modeling fatigue behavior of quasiisotropic laminates. Procedia Eng, 2011; 10: 3764-3768. DOI: 10.1016/j.proeng.2011.04.615
[2] Abadyan M, Khademi V, Bagheri R et al. Use of rubber modification technique to improve fracture-resistance of hoop wound composites. Mater Design, 2009; 30: 1976-1984. DOI: 10.1016/j.matdes.2008.09.001
[3] Abadyan M, Bagheri R, Haddadpour H et al. Investigation of the fracture resistance in hoop wound composites modified with two different reactive oligomers. Mater Design, 2009; 30: 3048-3055. DOI: 10.1016/j.matdes.2008.12.012
[4] Abadyan M, Khademi V, Bagheri R et al. Loading rate induced transition in toughening mechanism of rubber modified epoxy. J Macromol Sci Part B Phys, 2010; 49: 602-614. DOI: 10.1080/00222341003595253
[5] Abadyan M, Bagheri R, Kouchakzadeh MA. Study of fracture toughness of a hybrid rubber modified epoxy: Part I: Synergistic toughening. Appl Polym Sci, 2012; 125: 2467-2475. DOI: 10.1002/app.35367
[6] Abadyan M, Kouchakzadeh MA, Bagheri R. Study of fracture toughness of a hybrid rubber modified epoxy: Part II. Effect of loading rate. Appl Polym Sci, 2012; 125: 2476-2483. DOI: 10.1002/app.35379
[7] Abadyan M, Bagheri R, Kouchakzadeh MA et al. Exploring the tensile strain energy absorption of hybrid modified epoxies containing soft particles. Mater Design, 2011; 32: 2900-2908. DOI: 10.1016/j.matdes.2010.12.003
[8] Salehi Vaziri H, Abadyan M, Nouri M et al. Investigation of the fracture mechanism and mechanical properties of polystyrene/silica nanocomposite in various silica contents. J Mater Sci, 2011; 46: 5628-5638. DOI: 10.1007/s10853-011-5513-9
[9] Vaziri HS, Omaraei IA, Abadyan M et al. Thermophysical and rheological behavior of polystyrene/silica nanocomposites: Investigation of nanoparticle content. Mater Design, 2011; 32: 4537-4542. DOI: 10.1016/j.matdes.2011.01.022
[10] Pandey JK, Reddy KR, Kumar AP et al. An overview on the degradability of polymer nanocomposites. Polym Degrad Stab, 2005; 88: 234-250. DOI: 10.1016/j.polymdegradstab.2004.09.013
[11] Huang ZM, Zhang YZ, Kotaki M et al. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol, 2003; 63: 2223-2253. DOI: 10.1016/S0266-3538(03)00178-7
[12] Giannelis EP. Polymer-layered silicate nanocomposites: Synthesis, properties and applications. Appl Organomet Chem, 1998; 12: 675-680. DOI: 10.1002/(SICI)1099-0739(199810/11)12:10/11<675::AID-AOC779>3.0.CO;2-V
[13] Koo CM, Ham HT, Choi MH et al. Characteristics of polyvinylpyrrolidone-layered silicate nanocomposites prepared by attrition ball milling. Polym, 2003; 44: 681-689. DOI: 10.1016/S0032-3861(02)00803-0
[14] Jordan J, Jacob KI, Tannenbaum R et al. Experimental trends in polymer nanocomposites-a review. Mater Sci Eng R Rep, 2005; 393: 1-11. DOI: 10.1016/j.msea.2004.09.044
[15] Munusamy Y, Ismail H, Mariatti M et al. Effect of electron beam irradiation on the properties of ethylene-(vinyl acetate) copolymer/natural rubber/organoclay nanocomposites. J Vinyl Addit Technol, 2009; 15: 39-46. DOI: 10.1002/vnl.20174
[16] Tai Y, Miao J, Qian J et al. An effective way to stabilize silicon nitride nanoparticles dispersed in rubber matrix by a one-step process. Mater Chem Phys, 2008; 112: 659-667. DOI: 10.1016/j.matchemphys.2008.06.074
[17] Lai SM, Chen CM. Preparation, structure, and properties of styrene-ethylene-butylenestyrene block copolymer/clay nanocomposites: Part II fracture behaviors. Eur Polym J, 2008; 44: 3535-3547. DOI: 10.1016/j.eurpolymj.2008.09.002
[18] Zhao S, Schadler LS, Duncan R et al. Mechanisms leading to improved mechanical performance in nanoscale alumina filled epoxy. Compos Sci Technol, 2008; 68: 2965-2975. DOI: 10.1016/j.compscitech.2008.01.009
[19] Liu X, Zhao S. Study on structure and properties of SSBR/SiO2 co-coagulated rubber and SSBR filled with nanosilica composites. J Appl Polym Sci, 2008; 109: 3900-3907. DOI: 10.1002/app.28621
[20] Bonnia NN, Ahmad SH, Surip SN et al. Mechanical properties and environmental stress cracking resistance of rubber toughened polyester/clay composite. Adv Mater Res, 2012; 576: 318-321. DOI: 10.4028/www.scientific.net/AMR.576.318
[21] Boukerrou A, Duchet J, Fellahi S et al. Morphology and mechanical and viscoelastic properties of rubbery epoxy/organoclay montmorillonite nanocomposites. J Appl Polym Sci, 2007; 103: 3547-3552. DOI: 10.1002/app.24727
[22] Zhao XY, Xiang P, Tian M et al. Nitrile butadiene rubber/hindered phenol nanocomposites with improved strength and high damping performance. Polym, 2007; 48: 6056-6063. DOI: 10.1016/j.polymer.2007.08.011
[23] Yu S, Hu H, Ma J et al. Tribological properties of epoxy/rubber nanocomposites. Tribology International, 2008; 41: 1205-1211. DOI: 10.1016/j.triboint.2008.03.001
[24] Farsi R, Mohammadi MK, Saghanezhad SJ. Sulfonamide-functionalized covalent organic framework (COF-SO3H): An efficient heterogeneous acidic catalyst for the one-pot preparation of polyhydroquinoline and 1,4-dihydropyridine derivatives, Res Chem Intermed. 2021; 47: 1161-1179. DOI: 10.1007/s11164-020-04322-5
[25] Honari M, Sanaeishoar H, Kiasat AR et al. Efficient synthesis of pyrazolopyranopyrimidines using DBU-based nanomagnetic catalyst. Res Chem Intermed, 2021; 47: 1829-1841. DOI: 10.1007/s11164-021-04397-8
[26] Mehrdoost A, Jalilzadeh Yengejeh R, Mohammadi MK et al. Comparative analysis of UV-assisted removal of azithromycin and cefixime from aqueous solution using PAC/Fe/Si/Zn nanocomposite. J Health Sci Surveill Syst, 2021; 9: 39-49.
[27] Mankar R, Gurnule W. Synthesis, characterization, environmental properties and mechanical studies of SBR-nano aluminum oxide composites. Current Appl Polym Sci, 2019; 3: 1-15. DOI: 10.2174/2452271603666190614164629
[28] Mankar RV, Gurnule WB, Vajpai KS. Evaluation of thermal and mechanical properties of styrene-butadiene rubber-nanocomposite by using tin oxide as filler. Mater Today Proc, 2019; 15: 371-379. DOI: 10.1016/j.matpr.2019.04.096
[29] Mankar RV, Gurnule WB. Study of environmental properties of rubber-nanocomposites derived from styrene-butadiene rubber and nano carbon black. Saudi J Eng Technol, 2019; 4: 36-44.
[30] Mankar R, Gurnule WB. Synthesis and characterization of SBR-nanocomposite with carbon black nanoparticle. Res J Pharm Biol Chem Sci, 2018; 9: 791-799.
[31] Singru RN, Zade AB, Gurnule WB. Synthesis, characterization and thermal degradation studies of terpolymer resins derived from p-cresol, melamine and formaldehyde. J Appl Polym Sci, 2008; 109: 859-868. DOI: 10.1002/app.28197
[32] Chakole SP, Rathod YU, Pandit VU et al. Synthesis, charaterization of and thermal behavior of 2, 2’-dihydroxybiphenyl-formaldehyde-phenylenediamine copolymer. Mat Today Proc, 2022; 53: 96-100. DOI: 10.1016/j.matpr.2021.12.395
[33] Rahangdale SS, Chakole SP, Gurnule WB. Thermal degradation studies of 2,2’-dihydroxybiphenyl-ethylenediamine-formaldehyde copolymer. Res J Pharm Biol Chem Sci, 2021; 12: 451-460. DOI: 10.48175/IJARSCT-2443
[34] Gurnule WB, Rathod YU. Synthesis, characterization and thermal behaviour studies of terpolymer resin derived from 8-hydroxyquinoline-5-sulphonic acid and anthranilic acid. Current Appl Polym Sci, 2021; 4: 47-54. DOI: 10.2174/2452271604666200116101454
[35] Rathod YU, Zanje SB, Gurnule WB. Hydroxyquinoline copolymers synthesis, characterization and thermal degradation studies. J Phys Conf Ser, 2021; 1913: 1-8. DOI: 10.1088/1742-6596/1913/1/012061
[36] Gurnule WB, Das NC, Vajpai S et al. Synthesis, characterization and thermal degradation study of copolymer resin-II: Resulting from 2-hydroxy4-methoxybenzophenone, 1,5-diaminonaphthalene and formaldehyde. Int J Res Biosci Agric Tech, 2021; 8: 194-204.
[37] Gurnule WB, Rathod YU. Synthesis, characterization and thermal behaviour studies of terpolymer resin derived from 8-Hydroxyquinoline-5-sulphonic acid and anthranilic acid. Curr Appl Polym Sci, 2021; 4: 47-54. DOI: 10.2174/2452271604666200116101454
[38] Gurnule WB, Das N. Kinetic study of non-isothermal decomposition of copolymer resin derived from 2, 4-dihydroxypropiophenone, 1, 5-diaminonaphthalene and formaldehyde. Mater Today Proc, 2019; 15: 611-619. DOI: 10.1016/j.matpr.2019.04.128
[39] Mahant RD, Gurnule WB. Synthesis and thermal degradation studies of aromatic based epoxy resins bearing salicyladehyde and epichorohydrin group. Int J Pharm, Biol Chem Sci, 2019; 10: 1643-1649.
[40] Singru RN, Zade AB, Gurnule WB. Synthesis, characterization and thermal degradation studies of copolymer derived from 2, 4-dihydroxy propiophenone and 4-pyridylamine. Int J Recent Sci Res, 2019; 10: 31772-31778.
[41] Das NC, Gurnule WB. Thermal degradation studies of copolymer derived from 2-hydroxy, 4-methoxybenzophenone, 1,5-diaminonaphthalene and formaldehyde. Int J Curr Eng Sci Res, 2019; 6: 1414-1425.
[42] Mahant RD, Kongre N, Bhagat DS et al. Synthesis, characterization and thermal conductivity of diglycidyl monomer bearing thiourea and salicyladehyde group epoxy resin. Int J Curr Eng Sci Res, 2009; 6: 1303-1308.
[43] Kamdi DD, Gurnule WB. Non-isothermal kinetic studies of novel o-TMF copolymer. Int J Curr Eng Sci Res, 2019; 6: 1297-1302.
[44] Kohad CG, Gurnule WB. Synthesis and thermal degradation studies of P-toludine, ethylenediamine, and formaldehyde copolymer resin. Int J Curr Eng Sci Res, 2019; 6: 503-512.
[45] Gurnule WB, Khobragade J. Thermal degradation studies of copolymer resin-iii derived from 8-hydroxyquinoline 5-sulphonic acid-thiosemicarbazide-formaldehyde. Int J Curr Eng Sci Res, 2019; 6: 116-122.
[46] Gurnule WB, Khobragade J, Ahamed M. Thermal degradation studies of high performance copolymer resin derived from 8-hydroxyquinoline 5-sulphonic acid, semicarbazide and formaldehyde. Der Pharm Chem, 2014; 6: 334-342.
[47] Nandekar KA, Dontulwar JR, Gurnule WB. Thermogravimetric studies and kinetics of newly synthesized copolymer derived from p-hydroxy benzoic acid and semicarbazide. Rasayan J Chem, 2012; 5: 261-268.
[48] Butoliya SS, Zade AB, Gurnule WB. Terpolymer resin VIII: chelation ion exchanger properties of 2,4-dihydroxybenzophenone-oxamide-formaldehyde terpolymer resins. J Appl Polym Sci, 2009; 113: 1-9. DOI: 10.1002/app.29769
[49] Tarase MV, Zade AB, Gurnule WB. Kinetics of thermal degradation studies of some new terpolymers derived from 2,4-dihydroxypropiophenone, oxamide and formaldehyde. J Appl Polym Sci, 2010; 116: 619-627. DOI: 10.1002/app.30844
Copyright © 2022 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.





 Copyright ©
Copyright ©