Adriamycin-loaded gelatin microspheres in the treatment of bone tumors
Shaolong Zhou1#, Xiujiang Shi1#*, Yi Chen1, Li Li1, Chen Chen1
1School of Chemical and Biological Engineering, Yichun University, Yichun, Jiangxi Province, China.
#Both authors contributed equally to this manuscript.
*Correspondence to: Xiujiang Shi, School of Chemical and Biological Engineering, Yichun University, No. 576 Xuefu Road, Yichun 336000, Jiangxi Province, China; Email: ycsxjiang@126.com
Abstract
Objective: This study harnessed adriamycin-loaded gelatin microspheres (ADM-GMS) to examine their properties and in vitro release characteristics, and explore their effect on human osteosarcoma U-20s cell strain and its mechanism.
Methods: ADM-GMS was prepared using the emulsification-crosslinking method. The scanning electron microscope was employed to observe the shape of microspheres, and particle size and distribution were measured using a laser particle size device. The drug loading rate and encapsulation rate were calculated by ultraviolet spectrophotometry, and the drug release performance of the microspheres to adriamycin (ADM) was evaluated. The cell counting kit-8 (CCK-8) method was used to evaluate the anti-tumor activity of ADM-GMS on human osteosarcoma U-20s cell strain in vitro.
Results: We determined an optimal material ratio of 1:10 for ADM-GMS, with which the microspheres showed a round shape and excellent dispersity. The average particle size with the optimal material ratio was 14.02±1.67μm, with a drug loading rate of 6.05±0.26% and an encapsulation rate of 84.27±3.10%. ADM-GMS had excellent sustained-release properties and a significant inhibitory effect on the growth of human osteosarcoma U-20s.
Conclusion: ADM-GMS, prepared with a material ratio of 1:1, has a promising slow-release ability and an anti-bone tumor effect with a lower Semi-inhibitory concentration. Thus, this ADM gelatin delivery system is worthy of further clinical research. However, the detection method in this study is simple and weakly supported by clinical trials, and more investigations are required for further verification.
Keywords: adriamycin-loaded gelatin microspheres, bone tumors, effect
1 INTRODUCTION
Osteosarcoma is the most common malignant bone tumor in clinical practice[1]. It mostly occurs in adolescents, with a propensity to lung metastasis in the early stage and a somber prognosis[2]. In the 1970s, patients with osteosarcoma were mostly treated with amputation surgery, which is associated with a poor postoperative quality of life[3]. With medical advances, the use of chemotherapy provides significant survival benefits for patients with surgery. Chemotherapy after surgical resection is important for limb saving. Clinically, the most common chemotherapy regimen is high-dose methotrexate, adriamycin (ADM), and cisplatin[4,5]. ADM is an efficacious drug for almost all malignant tumors and osteosarcomas[6].
While chemotherapy has achieved good results, there are serious side effects such as depletion of granulocytes and platelets and infection. Due to potential irreversible heart failure induced by ADM toxicity, maximum ADM dose levels are limited[7]. In addition, it has been reported that ADM is associated with poor specificity and multiple side effects, such as gastrointestinal reactions, cardiotoxicity, and bone marrow suppression, and that long-term medication of ADM may result in drug tolerance[8,9]. To reduce these side effects, previous studies have shown that ADM can be released more slowly and safely by using microspheres as drug carriers[10]. Therefore, it is essential to develop a novel drug that improves the bioavailability and specificity of ADM for targeting bone tumors, reduces drug dosage, and minimizes toxic side effects.
Because bones are rich in vascular leakage, microspheres cannot be used as effective arterial embolic agents for osteosarcoma, such as those used in the treatment of lung and liver cancer. But studies have demonstrated that embedding doxorubicin in biodegradable gelatin microspheres ensures slow drug release and increases local drug concentration[11-13]. Therefore, we designed slow-releasing microspheres to deliver doxorubicin (ADM), investigated its physical and chemical properties, and examined the effects of drug-loaded microspheres on human osteosarcoma U-20s cell lines. The report is as follows.
2 MATERIALS AND METHODS
2.1 Instruments and Materials
ADM was purchased from Shenzhen Main Luck Pharmaceutical Inc. The medical gelatin was purchased from Beijing Chemical Reagent Company. The human osteosarcoma cell strain U-20s was purchased from the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. Fetal bovine serum was purchased from Nanjing Shenghang Biochannel Co., Ltd. UV2550 UV spectrophotometer was purchased from Shimadzu Corporation, Japan. D-800 smart drug dissolution apparatus was purchased from Tianjin University Radio Factory. S-3000N scanning electron microscope was purchased from Hitachi, Japan; RW20 electric mixer was purchased from IKA. Electric heating constant temperature water bath box was purchased from Sushazhou Medical Equipment Factory.
2.2 Methods
2.2.1 Preparation of Adriamycin-loaded Gelatin Microspheres (ADM-GMS)
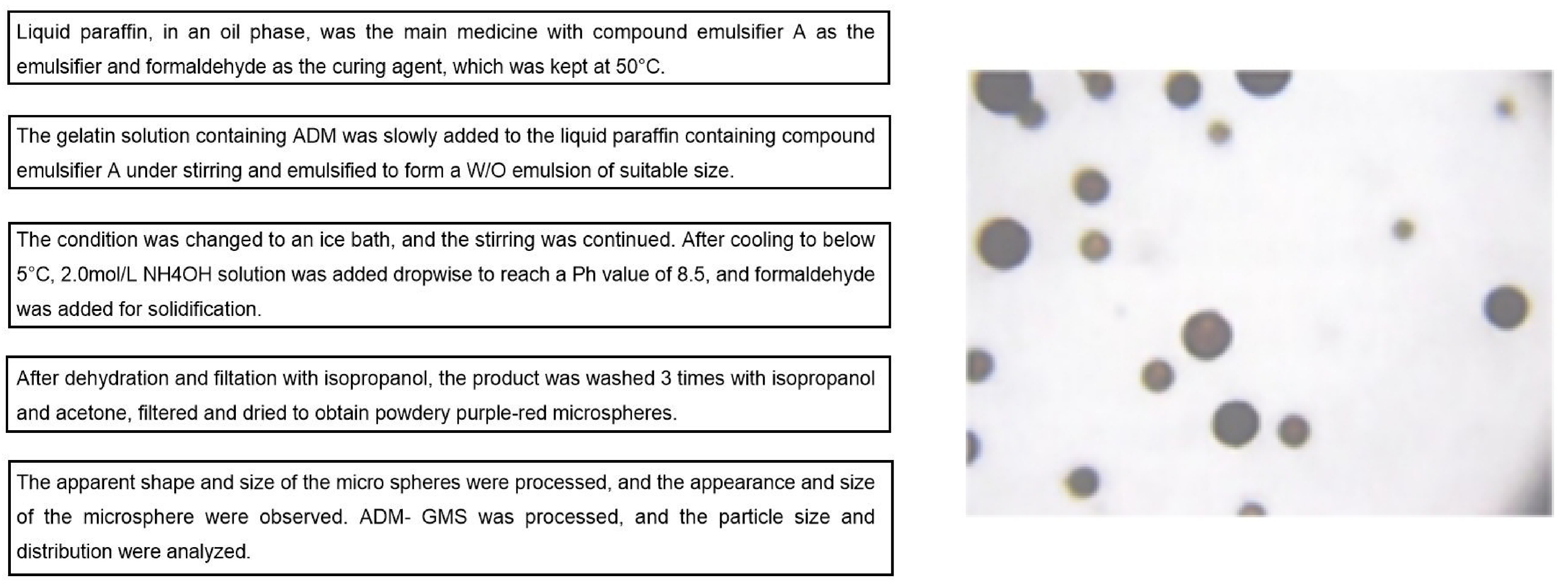
The emulsification-crosslinking method was used to prepare ADM-GMS. The medical gelatin with a concentration of 25% was heated in a water bath at 50°C until it was completely dissolved, and doxorubicin hydrochloride was added and mixed thoroughly in a vortex mixer. An appropriate amount of Span-80 was added to liquid paraffin and placed in a three-necked bottle. Using liquid paraffin in the oil phase as the main drug, compound emulsifier A as the emulsifier, and formaldehyde as the curing agent, with the temperature maintained at 50°C, gelatin solution containing ADM was slowly added to liquid paraffin containing compound emulsifier A under stirring and emulsified to form a W/O emulsion of suitable size. After changing to an ice bath and continuing to stir until it cools below 5°C, a 2.0mol/L NH4OH solution was added to increase the pH value to 8.5, followed by the addition of formaldehyde for curing. The product was dehydrated and filtered with isopropyl alcohol, washed 3 times with isopropyl alcohol and acetone, filtered, and dried to obtain powdered purple-red microspheres (Figure 1).
|
Figure 1. Optimization process and results of ADM-GMS.
The XS-18 optical biological microscope was used to observe and analyze the apparent shape and size of the microspheres. Then, the typical samples were dissolved in absolute ethanol, dispersed by ultrasonic, and gilded, and the appearance and size of the microsphere were observed with a scanning electron microscope. ADM-GMS was dissolved in 0.9% NaCl solution, ultrasonically dispersed for 6min, and analyzed for particle size and distribution using a laser particle size meter (Mastersizer 2000 analyzer). For the 722-grating spectrophotometer, 482nm was used as the measurement wavelength, with the regression equation of: C = 67.2706A-0.6881, where C is the concentration of ADM (pg/mL), A is the absorbance value and r = 0. 9991(n=3).
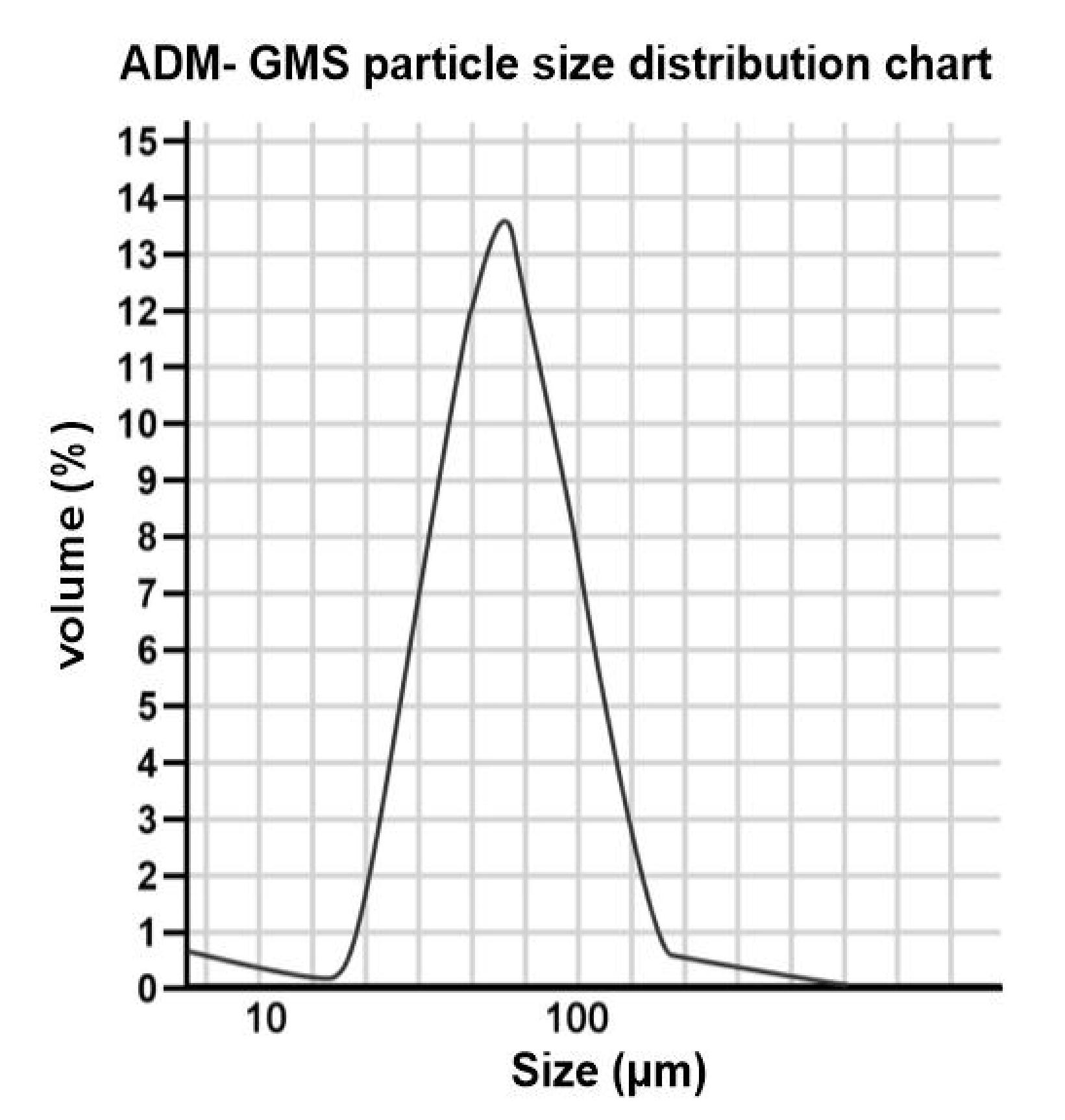
The in vitro release determination of ADM-GMS was performed as follows. The precisely weighed ADM-GMS was dispersed in 200mL of phosphate buffer with a pH of 7.4, and the water bath was maintained at 37°C with a stirring speed of 100r/min. 10mL of solution was sampled periodically while supplementing with the same volume of phosphate buffer. The sample solution was centrifugated at high speed for 2min, and 5mL of the supernatant was withdrawn to maintain a constant volume. The gelatin microsphere solution operated by the same method was used as a blank control. The absorbance was measured at 482nm, and the standard regression equation was used to calculate the cumulative release percentage of the microspheres. The results showed that the particle size of the microspheres had a good normal distribution. The average particle size of each sample was 69.154μm, of which 87.32% of the total particle size was in the range of 50-120μm. The above test results suggested that ADM-GMS met the requirements of arterial embolization for the treatment of tumors with a passive targeting function (Figure 2).
|
Figure 2. ADM-GMD particle size distribution chart.
2.2.2 Characterization and Particle Size Detection of ADM-GMS
A few microspheres were placed on a glass slide at room temperature and observed under the optical biological microscope and scanning electron microscope after adding an appropriate amount of liquid paraffin. It could be seen that the microspheres are purplish red in color, with smooth and round surfaces, good dispersion, and relatively uniform size. The ball diameter of 500 microspheres was detected under the microscope to obtain the arithmetic mean diameter. The particle size of the microspheres to be measured = The length when the eyepiece micrometer coincides with the stage micrometer / The number of grids occupied when the eyepiece micrometer and the stage micrometer coincide × The number of grids of the eyepiece micrometer occupied by the microspheres to be measured.
2.2.3 Detection of Drug Content and Encapsulation Rate in Microspheres
The configuration of standard products was as follows. 10mg of ADM reference substance was weighed and placed in a 100mL volumetric flask to prepare a series of concentration solutions, with normal saline as a blank control. The elevation rate of pH value was slightly faster at the beginning when NH4OH was used as the alkali source. After the pH value reached about 7, the elevation rate slowed down; therefore, the pH value of the final reaction could be stably controlled. Ultraviolet spectrophotometry was used to determine the concentration of each solution at 570nm. Linear regression analysis was performed, and a standard curve was drawn to calculate the regression equation, which was employed as the basis for determining the drug content.
The drug content and encapsulation efficiency in microspheres were determined as follows: 20mg of ADM drug-loaded microspheres was weighed and placed in 50mL of physiological saline, 50mL of acetic acid pepsin solution was added, with the temperature at 37°C without light, and placed in a constant temperature shaker for 72h. 1mL of the supernatant was diluted to 10mL and measured by ultraviolet spectrophotometry. According to the standard curve equation, the content and encapsulation efficiency of the contained drugs were calculated. Encapsulation rate = ADM contained in ADM-GMS microspheres/total amount of ADM input × 100%.
2.2.4 In Vitro Release Curve Detection
20mg of ADM-GMS was put into the sample bottle of the drug dissolution apparatus at a temperature of 37°C and completely immersed in 100mL of physiological saline. 1mL was sampled at certain intervals, with 1mL of physiological saline for replenishment. Ultraviolet spectrophotometry was used to determine the drug spectrophotometric value of each tube, and the release amount was calculated according to the standard curve.
2.2.5 Cytotoxicity Test of ADM-GMS
The cell counting kit-8 (CCK-8) method was used to determine the cell-killing effect of ADM-GMS and naked drug ADM. Human osteosarcoma U-20s cells were inoculated, at a density of 5×103cells/well, into 96-well plates with DMEN high-glycemic culture medium containing 10% newborn calf serum. The cells were then placed in an incubator containing 5% CO2 and 37°C for 24h at full humidity. A blank control group was set up, with ADM-GMS as the experimental group, ADM naked drug as the control group. The experimental group was cultured for 24, 48, and 72h after adding a certain dose of drugs, and then the cell survival rate was measured by the CCK-8 method. The absorbance of each well was measured at 490nm with a microplate reader.
2.3 Statistical Processing
SPSS 22.0 statistical software was used to analyze the data of this experiment. The measurement data were expressed as mean±SD and analyzed using t-test. P<0.05 indicates that the difference was statistically significant.
3 RESULTS
3.1 Optimal Preparation of ADM-GMS
We set 4 groups of experiments with different material ratios to explore the optimal preparation of ADM-GMS. The physical and chemical properties rates of different material ratios of 1:5, 1:10, 1:15, and 1:20 were 72.52±3.26%, 84.45±2.77%, 86.70±2.12%, 89.74±2.96% respectively. The analysis of the characteristics of the microspheres prepared with different material ratios in each group showed that the best material ratio of ADM-GMS was 1:10 (drug: carrier) (Table 1).
Table 1. Physical and Chemical Properties of ADM-GMS
Groups |
Ratios |
Form |
Rate (%) |
1 |
1:5 |
Irregular, crystalline |
72.52±3.26 |
2 |
1:10 |
Smooth and round |
84.45±2.77 |
3 |
1:15 |
Smooth and round |
86.70±2.12 |
4 |
1:20 |
Smooth and round |
89.74±2.96 |
3.2 Detection of Drug Loading Rate and Encapsulation Rate of ADM-GMS
In this study, the microspheres prepared with a material ratio of 1:10 were spherical after being dried and pelletized, with a relatively uniform shape and an average particle size of (14.02±1.67)μm, which was larger than the average particle size of (12.11±1.71)μm in the blank microspheres. The measured drug loading rate was about 6%, and the encapsulation rate exceeded 80%, whereas blank microspheres showed no measured drug loading rate or encapsulation rate (Table 2).
Table 2. Physical and Chemical Properties of ADM-GMS
Microspheres |
Average Particle Size (μm) |
Polydispersity |
Drug Loading Rate (%) |
Encapsulation Rate (%) |
Blank microspheres |
12.11±1.71 |
0.15±0.02 |
NA |
NA |
ADM-GMS microspheres |
14.02±1.67 |
0.15±0.04 |
6.05±0.26 |
84.27±3.10 |
3.3 In Vitro Release of ADM-GMS
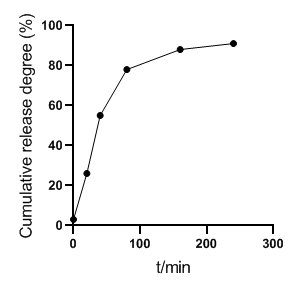
The in vitro release results of ADM-GMS showed that ADM released about 26% in 20min, about 55% in 40min, about 78% in 80min, about 88% in 160min, and more than 90% in 240min, as shown in Figure 3. It confirmed that the microspheres have an obvious slow-release effect.
|
Figure 3. In vitro release curve of ADM-GMS.
3.4 Inhibition of ADM-GMS in Vitro
The results demonstrated that both naked drug ADM and ADM-GMS have killing effects on U-20s, and are time- and concentration-dependent. The ADM killing effects on U-20s cells at 5 different concentrations were the strongest at 24h, followed by 48h, and gradually attenuated at 72h. The ADM-GMS killing effects on U-20s cells at 5 different concentrations were the strongest at 24h, followed by 48h, and gradually attenuated at 72h (Tables 3 and 4).
Table 3. Effects of Different Concentrations of ADM on the Growth of U-20s Cells (μg/mL, n=6)
Groups |
24h |
48h |
72h |
0.5 |
91.46±5.35 |
89.89±5.33 |
71.16±5.29 |
1 |
90.27±3.12* |
75.22±4.03* |
37.13±4.26* |
2 |
89.11±2.32* |
50.22±3.38* |
22.26±3.78* |
4 |
80.78±2.40* |
30.70±3.03* |
20.17±3.30* |
8 |
72.82±2.53* |
21.58±2.53* |
13.17±3.10* |
Table 4. Effects of Different Concentrations of ADM-GMS on the Growth of U-20s Cells (μg/mL, n=6)
Groups |
24h |
48h |
72h |
0.5 |
93.46±5.42 |
78.80±4.63 |
42.56±4.35 |
1 |
90.27±3.12* |
58.22±4.13* |
28.09±3.16* |
2 |
82.11±2.32* |
40.56±3.22* |
8.77±2.58* |
4 |
78.64±2.40* |
23.70±2.03* |
8.68±2.09* |
8 |
69.80±2.53* |
20.56±1.97* |
7.18±2.10* |
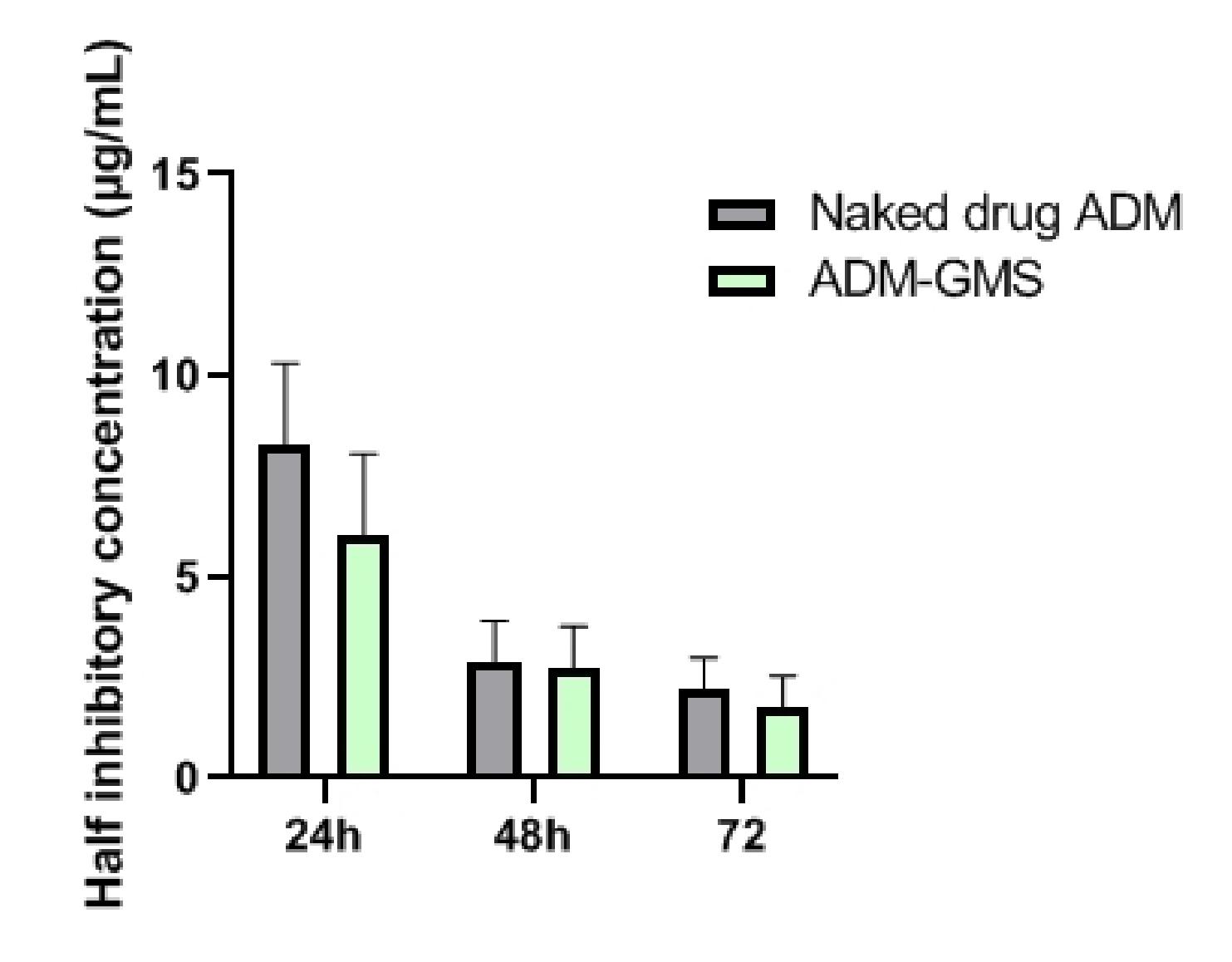
After calculating the half inhibitory concentration (IC50) of the naked drug ADM and ADM-GMS, it was found that the IC50 value of ADM-GMS at each time point was lower than that of the naked drug ADM. In the comparison of IC50 between the two at 24h, naked drug ADM exhibited a higher IC50, as compared to ADM-GMS (P<0.05). No statistical differences were observed at 48h and 72h despite a higher IC50 of Naked drug ADM than that of ADM-GMS (P>0.05) (Table 5 and Figure 4).
Table 5. IC50 Values of Naked Drugs ADM and ADM-GMS at Differents Time Points (μg/mL, n=6)
IC50 |
24h |
48h |
72h |
Naked drug ADM |
8.26±2.05 |
2.86±1.04 |
2.20±0.78 |
ADM-GMS |
6.05±2.01 |
2.73±1.04 |
1.76±0.79 |
t |
2.309 |
0.265 |
1.189 |
P |
0.035 |
0.794 |
0.252 |
|
Figure 4. The IC50 of naked drug ADM and ADM-GMS on U-20s cells.
4 DISCUSSION
Osteosarcoma originates from mesenchymal cells, characterized by early metastasis, high malignancy, rapid disease progression, and a high fatality rate[14,15]. In recent years, with the improvement of surgical techniques and neoadjuvant chemotherapy, the 5-year survival rate of patients with bone tumors has increased by 40% to 70%. However, studies have revealed that the adverse reactions of chemotherapy drugs, bone defects, and tumor recurrence after surgery critically hinder the physical and mental health of patients[16,17].
ADM is an antitumor antibiotic of the anthracycline class, which can prevent the formation of mRNA by interfering with the transcription process and produce a wide range of biochemical reactions in the body, exerting a strong cytotoxic effect[18,19]. Currently, ADM has been frequently used in chemotherapy for various malignant tumors such as osteosarcoma[20]. However, its clinical application is limited due to multiple systemic adverse reactions, such as bone marrow suppression, cardiotoxicity, heart damage, and gastrointestinal adverse reactions. To ameliorate its drug effect, increase its bioavailability, and reduce toxic and side effects, the research of new dosage forms of ADM has exerted a tremendous fascination on researchers.
Drug-loaded microspheres, as a drug sustained-release and controlled-release preparation, are a new type of drug delivery system that has been studied in recent years. The drug offers a long function time and no obvious effect on the normal tissues of the body. Moreover, gelatin is a non-toxic, low antigenic, and biodegradable polymer material. As an embolic agent, the gelatin sponge is an ideal drug carrier that has been widely used in clinical practice. It will gradually degrade and absorb after 2 weeks in the body[21,22]. Microspheres refer to tiny spherical entities formed by dissolving, adsorbing, and dispersing drugs inside the polymer carrier, with a particle size mostly ranging from 1 to 250μm[23]. At present, the biodegradable polymer materials that can be injected into the human body are mainly divided into two categories according to their sources: natural biodegradable polymers and chemically synthesized polymers. Common natural biodegradable polymers include chitosan, proteins, and gelatin[24]. Gelatin is mainly extracted by hydrolysis of collagen in animal skin or bone. Doxorubicin is a sensitive drug against malignant tumors, to which osteosarcoma is particularly sensitive. Hence, it is feasible to combine gelatin and ADM in a certain proportion to prepare drug particles. Furthermore, there are no toxic and side effects in clinical applications as the preparation is thoroughly disinfected and contains no harmful substances. The drug microspheres stay in small arteries with a diameter of less than 100um, which can substantially prevent collateral circulation[25]. Research has demonstrated that chemotherapeutic drugs loaded in microspheres are released locally in the tumor, with a release time as long as 105 days[26]. The high degree of embolism results in severe ischemic necrosis of most of the tumor. Aseptic inflammation triggered by tumor tissue necrosis can stimulate the proliferation of fibrous tissue to ensure a more complete tumor pseudocapsule[27]. Thus, this regimen can significantly abate or eliminate the clinical symptoms. In addition, the gradual degradation of gelatin in the body and the slow release of the contained doxorubicin maintain the concentration of chemotherapeutic agents in the tumor at a consistently high level with minimal systemic distribution[28]. It demonstrates that microparticle embolization of osteosarcoma yields promising outcomes in the development of limb salvage surgery. Therefore, in this study, gelatin was selected as the carrier of doxorubicin microspheres. The physical and chemical properties of the drug-loaded microspheres were determined using different technical methods and the antitumor effect of the drug-loaded microspheres on human osteosarcoma cell lines was investigated.
The ADM-GMS prepared in this research showed a drug loading rate of about 6%, which is considered an excellent drug loading efficiency. Moreover, the encapsulation rate in this study exceeded 80%, which indicates a promising slow-release effect. Through in vitro pharmacodynamic experiments on human osteosarcoma U-20s cells, it has been confirmed that ADM-GMS microspheres yield positive inhibitory effects on osteosarcoma U-20s tumor cells and can reduce the concentration of the drug. The analysis of its anti-tumor mechanism may be related to the slow-release properties of gelatin microspheres, such as low friction coefficient, good biocompatibility, and natural degradation in the body. The biocompatibility of ADM-GMS and its ability to allow rapid diffusion of molecules make them useful for drug delivery, and are based on local delivery of chemotherapy drugs, rather than simply increasing doses (because, as, since……please put your reason but with strong basis). In addition, it may also be related to the method gelatin microspheres enter the cell. Cells ingest drug-loaded gelatin microspheres through high-efficiency endocytosis, and when the concentration remains at a low level, it can reach a relatively high intracellular drug concentration, thereby effectively inducing cell apoptosis[29]. This study preliminarily explored the inhibitory effect of ADM-GMS on the proliferation of osteosarcoma U-20s cell strain. Nonetheless, this study did not conduct in-depth research on the molecular mechanism and pharmacokinetics, and the detection method was comparatively single. The relevant literature references were limited, and more methods are to be required to study the inhibitory effects of ADM-GMS on bone tumor cells. The limitation of this study is that ADM-GMS has not been further studied in clinical trials. Experiments will be expanded to obtain relevant animal experimental data in future studies.
5 CONCLUSION
Finding an effective way to bend osteosarcoma chemotherapy remains a huge challenge. Here, we chose gelatin microspheres to load ADM to achieve local release of the drug. ADM-GMS can significantly increase the concentration of local drugs and exert anti-tumor cell effects. Future studies of animal models of osteosarcoma are needed to investigate whether ADM-GMS system can locally kill osteosarcoma cells and can support local bone tissue regeneration.
Acknowledgements
This study was supported by the Special Youth Project of Clinical Medicine funded by Nantong University (Grant No. 2019LQ019).
Conflicts of Interest
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contribution
Zhou S and Shi X wrote the manuscript. Chen Y, Li L and Chen C contributed to original draft preparation, review and editing. All authors read and approved the manuscript for submission.
Abbreviation List
ADM, Adriamycin
ADM-GMS, Adriamycin-loaded gelatin microspheres
CCK-8, Cell Counting Kit-8
IC50, Half inhibitory concentration
References
[1] Yang C, Tian Y, Zhao F et al. Bone microenvironment and osteosarcoma metastasis. Int J Mol Sci, 2020; 21: 6985.[DOI]
[2] Chen C, Xie L, Ren T et al. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett, 2021; 500: 1-10.[DOI]
[3] Han G, Bi WZ, Xu M et al. Amputation versus limb-salvage surgery in patients with osteosarcoma: A meta-analysis. World J Surg, 2016; 40: 2016-2027.[DOI]
[4] Zang S, Wang J, Wen J et al. Establishment of a dynamic osteosarcoma biobank: Ruijin experience. Cell Tissue Bank, 2020; 21: 447-455.[DOI]
[5] Koch R, Gelderblom H, Haveman L et al. High-dose treosulfan and melphalan as consolidation therapy versus standard therapy for high-risk (metastatic) ewing sarcoma. J Clin Oncol, 2022; 40: 2307-2320.[DOI]
[6] Guma SR, Lee DA, Yu L et al. Natural killer cell therapy and aerosol interleukin-2 for the treatment of osteosarcoma lung metastasis. Pediatr Blood Cancer, 2014; 61: 618-626.[DOI]
[7] Voors AA, Kremer D, Geven C et al. Adrenomedullin in heart failure: Pathophysiology and therapeutic application. Eur J Heart Fail, 2019; 21: 163-171.[DOI]
[8] Lang NW, Kasparek MF, Synak L et al. What sports activity levels are achieved in long-term survivors with modular endoprosthetic humerus reconstruction following primary bone sarcoma resection? Wien klin Wochenschr, 2021; 133: 14-20.[DOI]
[9] Li JJ, Tian DM, Yang L et al. Influence of a metaphyseal sleeve on the stress-strain state of a bone-tumor implant system in the distal femur: An experimental and finite element analysis. J Orthop Surg Res, 2020; 15: 589.[DOI]
[10] Defail AJ, Edington HD, Matthews S et al. Controlled release of bioactive doxorubicin from microspheres embedded within gelatin scaffolds. J Biomed Mater Res A, 2006; 79: 954-962.[DOI]
[11] Zhao X, Wu Q, Gong X et al. Osteosarcoma: A review of current and future therapeutic approaches. Biomed Eng Online, 2021; 20: 24.[DOI]
[12] D'Ambrosio L, Touati N, Blay JY et al. Doxorubicin plus dacarbazine, doxorubicin plus ifosfamide, or doxorubicin alone as a first-line treatment for advanced leiomyosarcoma: A propensity score matching analysis from the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Cancer, 2020; 126: 2637-2647.[DOI]
[13] Dai X, Ma W, Jha RK et al. Adrenomedullin and its expression in cancers and bone. A literature review. Front Biosci(Elite ed), 2010; 2: 1073-80.[DOI]
[14] Brown JM, Matichak D, Rakoczy K et al. Osteosarcoma of the pelvis: clinical presentation and overall survival. Sarcoma, 2021; 2021: 8027314.[DOI]
[15] Parry MC, Laitinen M, Albergo J et al. Osteosarcoma of the pelvis. Bone Joint J, 2016; 98-B: 555-563.[DOI]
[16] Sakkers R, Wijk I. Amputation and rotationplasty in children with limb deficiencies: current concepts. J Child Ortho, 2016; 10: 619-626.[DOI]
[17] Huang L, Guo W, Yang R et al. Proposed scoring system for evaluating neurologic deficit after sacral resection: Functional outcomes of 170 consecutive patients. Spine, 2016; 41: 628-637.[DOI]
[18] Cagel M, Grotz E, Bernabeu E et al. Doxorubicin: Nanotechnological overviews from bench to bedside. Drug Discov Today, 2017; 22: 270-281.[DOI]
[19] Gomes AS, Monteleone PA, Sayre JW et al. Comparison of triple-drug transcatheter arterial chemoembolization (tace) with single-drug tace using doxorubicin-eluting beads: long-term survival in 313 patients. AJR Am J roentgenol, 2017; 209: 722-732.[DOI]
[20] Ahnfelt E, Sjögren E, Hansson P et al. In vitro release mechanisms of doxorubicin from a clinical bead drug-delivery system. J Pharm Sci, 2016; 105: 3387-3398.[DOI]
[21] Haghiralsadat F, Amoabediny G, Sheikhha MH et al. A novel approach on drug delivery: Investigation of a new nano-formulation of liposomal doxorubicin and biological evaluation of entrapped doxorubicin on various osteosarcoma cell lines. Cell J, 2017; 19: 55-65.[DOI]
[22] Pozo-Rodríguez AD, Solinís MA, Rodríguez-Gascón A. Applications of lipid nanoparticles in gene therapy. Eur J Pharm Biopharm, 2016; 109: 184-193.[DOI]
[23] Kim S, Sah H. Merits of sponge-like PLGA microspheres as long-acting injectables of hydrophobic drug. J Biomater Sci Polym Ed, 2019; 30: 1725-43.[DOI]
[24] Stratton S, Shelke NB, Hoshino K et al. Bioactive polymeric scaffolds for tissue engineering. Bioact Mater, 2016; 1: 93-108.[DOI]
[25] Oryan A, Kamali A, Moshiri A et al. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int J Biol Macromol, 2018; 107: 678-688.[DOI]
[26] Li Y, Sun Y, Shan HC et al. Comparative analysis of early follow-up of biologic fixation and cemented stem fixation for femoral tumor prosthesis. Orthop Surg, 2019; 11: 451-459.[DOI]
[27] Liu X, Zhang Y, Wu H et al. A conductive gelatin methacrylamide hydrogel for synergistic therapy of osteosarcoma and potential bone regeneration. Int j biol macromol, 2023; 228: 111-122.[DOI]
[28] Lin Y, Yang Y, Yuan K et al. Multi-omics analysis based on 3D-bioprinted models innovates therapeutic target discovery of osteosarcoma. Bioact Mater, 2022; 18: 459-470.[DOI]
[29] Dahan M, Anract P, Babinet A et al. Proximal femoral osteosarcoma: Diagnostic challenges translate into delayed and inappropriate management. Orthop Traumatol Surg Res, 2017; 103: 1011-1015.[DOI]
Copyright © 2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.




 Copyright ©
Copyright ©