Research on the Damage of the Central Nervous System Lymphoma to the Nervous System
Jairo Hernandez1, Caroline Davidson1, Thomas Reilly1, Seif Hanbali1, Hussam Abou-Al-Shaar2, Ghaidaa Ebrahim1, Andrew Nguyen1, Brandon Lucke-Wold1*
1Department of Neurosurgery, University of Florida, Gainesville, USA
2Department of Neurosurgery, University of Pittsburgh Medical Center, Pittsburgh, USA
*Correspondence to: Brandon Lucke-Wold, PhD, Neurosurgery Resident, Department of Neurosurgery, University of Florida, 2046 NE Waldo Rd Ste 1150, Gainesville, FL 32609, USA; Email: Brandon.Lucke-Wold@neurosurgery.ufl.edu
Abstract
Management of central nervous system lymphoma requires multidisciplinary care. The disease can manifest in the context of immunocompromised states or in the context of chronic infections. Nervous system damage from this lymphoma has highly variable presentation that is dependent on the location of the tumor lesions. Damage from disease progression can lead to lasting neurologic deficits and even death. However, some lesions are a consequence of radiation-induced neurotoxicity. This review discusses the sources of and consequences of brain damage due to tumor damage and the associated effect of clinical therapies. We discuss workup, management, and treatments. These include chemotherapy and radiation techniques. We discuss potential complications and avoidance strategies. The review will serve as a user-friendly resource for clinicians.
Keywords: lymphoma, evaluation, complications, management
1 INTRODUCTION
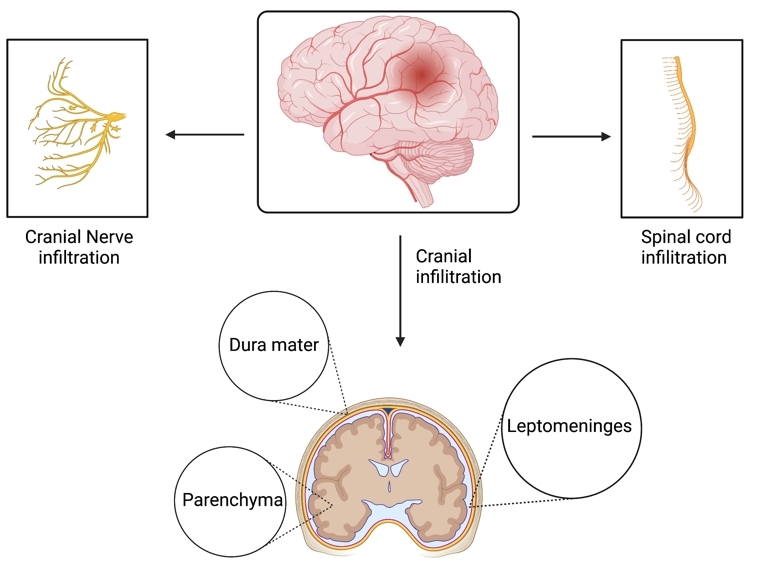
Lymphoma of the central nervous system (CNS) represents an aggressive and rare subset of non-Hodgkin lymphoma. The World Health Organization defines primary CNS lymphoma (PCNSL) as those cases confined to the CNS dura, parenchyma, cranial nerves, spinal cord, and leptomeninges (Figure 1). Secondary CNS lymphoma refers to systemic non-Hodgkin’s lymphoma that has metastasized to the CNS[1]. In the United States, PCNSL has an annual incidence of ~1400 cases, comprising 1% of non-Hodgkin lymphoma cases and 3-5% of all primary brain tumors[2-4]. Up to 80% of PCNSL patients present with focal neurological deficits and the symptoms usually correlate with lesion location[5]. Neuropsychiatric changes and intracranial pressure are present in 32% to 43% of these patients[6]. Ocular involvement of retina, vitreous, or choroid manifest as blurred vision and/or floaters; these can be isolated (10%) or with cerebral symptoms (10-20%)[7]. Nervous system damage from CNS lymphoma has a variety of presentations depending on the location of the lesion, and some lesions are a consequence of radiation-induced neurotoxicity. This review aims to explore the etiology and prognosis, pathophysiology and genetic abnormalities, and the diagnostic and treatment strategies for this condition.
|
Figure 1. Common locations of primary central nervous system lymphoma include the dura mater, parenchyma, and leptomeninges[6,8]. Additionally, this form of lymphoma may also attack the cranial nerves and spinal cord[6].
2 ETIOLOGY AND PROGNOSIS
There are two scoring systems for determining the prognosis for PCNSL. The first is the International Extranodal Lymphoma Study Group Score, which considers five risk factors. These include age older than 60 and the patient’s ability to carry out activities of daily living per their Eastern Cooperative Oncology Group Performance Score, as well as certain markers of disease progression, including elevated lactate dehydrogenase levels, elevated CSF protein concentrations, and extent of brain involvement, especially in the periventricular regions, basal ganglia, brainstem, and cerebellum[6,9]. This system predicts a two-year survival rate of 80% for patients with zero to one of these risk factors, 48% for two to three, and 15% for four to five[6,9]. The second is the Memorial Sloan Kettering Cancer Center (MSKCC) Prognostic Score. It stratifies patients into three groups according only to age and their Karnofsky Performance Status (KPS), a metric for measuring quality of life and disability similar to the Eastern Cooperative Oncology Group Performance Score, where a score closer to 100 predicts a favorable outcome[6].
Past these metrics, there are other factors that affect prognosis. Among the most significant is any immunodeficiency, either congenital or acquired, such that immunodeficient patients typically present with PCNSL in their third to fourth decades of life, versus immunocompetent patients in the fifth to seventh[3]. Conditions include Wiskott-Aldrich, ataxia-telangiectasia, severe-combined or common-variable immunodeficiency, organ transplant, or HIV/AIDS. Of note, PCNSL is an AIDS-defining illness with CD4+ T-cell counts less than 50 cells/µL, secondary to infection with Epstein-Barr virus in nearly 100% of cases[9]. A second factor affecting prognosis is treatment; High-dose methotrexate (HD-MTX)-based chemotherapy with or without radiation results in long-term survival in roughly 15-20% of patients, whereas untreated PCNSL results in an overall survival of roughly 1.5 months[10]. A final factor to consider in determining prognosis is the variant of PCNSL. Most cases of PCNSL are diffuse large B-cell lymphoma (DLBCL), with smaller percentages of Burkitt, lymphoblastic, marginal zone, and T-cell lymphomas[11]. Other atypical etiologies include primary meningeal lymphoma and lymphomatosis cerebri[3]. Low-grade B-cell lymphoma generally presents with better outcomes, whereas intravascular large B-cell lymphoma, so-called for its preferential growth within small vessels, presents with poorer outcomes, likely because it presents similarly to other conditions (CNS vasculitis and amyloid angiopathy) and may go untreated for some time[5]. Taken together with the two scoring systems, these three considerations (immunodeficiency, treatment with methotrexate, and the variant of PCNSL) improve the accuracy of a patient’s prognosis. Of note, biomarkers like BCL-6, which may bear on prognosis in other contexts, do not necessarily correlate in PCNSL[11].
3 PATHOPHYSIOLOGY AND GENETIC ABNORMALITIES
Most cases of PCNSL fall under the category of DLBCL. Specifically, the B-cells implicated in PCNSL most likely mutate in their late germinal center or early post-germinal center stage of development when the cell is undergoing somatic hypermutation[11]. What remains unknown, however, is whether the B-cells that ultimately become cancerous begin their lives elsewhere in the body then reach the CNS via circulation, only to accrue more mutations that may confer advantages in survival and proliferation in the environment of the CNS, or in the CNS itself[11]. Although overall understanding of the pathogenesis is poor, some of the mutations involved have been outlined. The most frequent mutation is activation of the proto-oncogene MYD88, specifically by replacing leucine at position 265 with proline, which presents in 94.4% of cases[12,13]. This activates the transcription factor NFkB, which in turn causes cell proliferation, angiogenesis, and ultimately cancer[9]. Other mutations that induce NFkB include activation of CD79B and CARD11, as well as deletions in TNFAIP3 and TLR1XR1[11]. Although this seems to be the main mechanism of pathogenesis, other mechanisms have been described, such as the hypermethylation of the tumor suppressors DAPK, CDKN2A, MGMT, and RFC as well as PRDM1 and PTPRK[14]. BCL-6 translocations, SHP1 promoter hypermethylation, and HLA genes have also shown involvement in various studies[15-17]. In double-expressor-lymphoma, the overexpression of BCL-2 and C-MYC are characteristic; however, patients with PCNSL may also experience greater quantities of these proteins[18]. One study reported that immunocompromised PCNSL patients especially show an overexpression of BCL-2 related proteins[19].
Despite our expanding understanding (Table 1), the complete pathogenesis is unknown and warrants further investigation.
Table 1. Genetic Abnormalities of Primary Central Nervous System Lymphoma
Study |
Study Type and Size |
Mutation and Presence |
Mechanism of Action |
Yamada et al.[13] |
Cohort analysis of 18 PCNSL patients |
MYD88 (94%) and CD79B (61%) somatic mutations |
Activation of NFkB, which leads to angiogenesis, proliferation, and ultimately cancer. |
Deckert et al.[14] |
Cohort analysis of 25 PCNSL patients |
DAPK (84%), CDKN2A (75%), MGMT (52%), and RFC (30%) hypermethylation |
Tumor suppressor genes from different pathways are co-inactivated through promoter hypermethylation. |
Schwindt et al.[15] |
Cohort analysis of 37 PCNSL patients |
BCL-6 translocations (38%) |
BCL6 gene regulates lymphocyte differentiation, cell-cycle control, and is targeted in somatic hypermutation. Deregulation of these processes are hypothesized to contribute to PCNSLs. |
Liu et al.[16] |
Cohort analysis of 33 PCNSL patients |
SHP1 promoter hypermethylation (88%) |
SHP1 promoter hypermethylation contributes to activation of JAK/STAT signaling pathway, which has been shown to participate in the pathogenesis of PCNSL. |
Schwindt et al.[17] |
Cohort analysis of 19 PCNSL patients |
Most frequent abnormalities were loses in 6p21.32 (74%) and gains in 18q21 (43%) |
The former region harbors HLA genes, which can lead to a lack of MHC class II antigen expression by PCNSL tumor cells. |
4 DIAGNOSIS AND TREATMENT
4.1 Diagnosis
Diagnosis of PCNSL can be difficult due to the highly variable clinical presentations; however, it always begins with neural imaging. An magnetic resonance imaging (MRI) is the best strategy for visualizing lymphoma lesions. In instances where patients are unable to undergo MRI, CT scans can also provide clear representations of PCNSL damage[20]. Other imaging strategies like PET scans have not yet been established[8]. A definitive diagnosis can be made with a stereotactic brain tissue biopsy[21]. It should be noted that corticosteroids are disruptive to the lesions due to their lymphotoxin nature. Therefore, they must be avoided in order to preserve the integrity of pathology results except in cases of life-threatening edema[20]. Additionally, in order to complete staging, a lumbar punctuation is required to assess leptomeningeal involvement which is common in PCNSL patients[20]. Ultimately, every patient should complete an ophthalmologic assessment with a slit lamp as dysfunction of visual systems are also common[20,21]. While surgery is required to biopsy tissue for diagnosis, complete resection of lymphoma lesions is not recommended. PCNSL is usually multifocal and involves internal neuroanatomy. Therefore, surgical removal of lesions is avoided to minimize unnecessary suffering and damage to important brain structures[10]. Studies have demonstrated that surgical intervention in PCNSL shows very little benefit compared to other therapies[22]. However, one more recent systematic review showed that PCNSL patients who underwent surgical resection actually showed higher overall survival when compared to peers who had a biopsy alone[23]. The most optimal treatments have yet to be defined, but there are multiple therapeutic strategies for targeting PCNSL including radiotherapy, induction chemotherapy and consolidative therapy[10].
4.2 Whole Brain Radiotherapy (WBRT)
WBRT has been historically implemented in the care of PCNSL patients because of the lymphoma’s particular sensitivity to radiation[10]. Due to the invasive, multifocal nature of PCNSL, the whole brain field is used to encompass all potential lesions. The lateral fields are equally weighted and most commonly, 6-10mV photons are utilized[24]. The anterior temporal lobes and cribriform plate should not be excluded from the radiation field. Additionally, coverage of the optic nerve and retina should be ensured[24]. Some studies have recommended using a hyper-fractionated radiation schedule in order to reduce neurotoxicity[25]. Due to the high incidence of leptomeningeal involvement, studies have encouraged craniospinal irradiation (CSI) in addition to WBRT. However, other evidence shows supplemental CSI leads to toxicity[26,27].
While early uses of WBRT showed a high response rate, relapse was observed in many patients[24]. WBRT has been historically useful for treating PCNSL; however, there are significant drawbacks when it is employed alone. WBRT is not a specific technique as it lacks the ability to locally target lesions. Also, there is evidence to show that this technique can disseminate radiographically-occult lymphoma cells beyond the field of radiation[21]. Finally, delayed neurotoxicity commonly manifests in patients aged older than 60 years. These symptoms include cognitive impairment, incontinence, memory dysfunction, and gait disturbances[10,21,24]. Other therapies have proven to be more effective than WBRT. For example, patients who were given WBRT in combination with HD-MTX based chemotherapy did not show greater quality of life compared to patients who were only administered chemotherapy[28,29]. Due to these limitations, WBRT’s place as a primary treatment option for PCNSL should be challenged.
4.3 High-dose Methotrexate (HD-MTX)-based Induction Chemotherapy & Autologous Stem Cell Transplantation
HD-MTX-based induction chemotherapy is quickly becoming the new standard for early diagnosis of PCNSL. Its use has been demonstrated in studies from as early as the 1970s[30]; however, the optimal dose of HD-MTX has yet to be defined. One more recent study of thirty-one patients found that HD-MTX administered every 14 days can be used to treat CNS lymphomas and cerebral spinal fluid cancer (leptomeningeal disease) after a minimum of six treatment cycles[21,31]. Some studies pair this form of chemotherapy with WBRT as a consolidative radiation technique[32]. Though, this should be considered carefully as the there appears to be a much higher incidence of delayed neurotoxicity amongst PCNL patients who undergo treatment plans that included WBRT[10,29]. Also, some longitudinal data has provided substantial support for the argument that patients that receive dose-intensive chemotherapy, without WBRT, still experience long-term-progression free survival[21,33]. Particularly regarding elderly patients with PCNSL, it is agreed that HD-MTX chemotherapy should be the first treatment considered[10]. Another promising alternative to whole brain radiation therapy has been found in high-dose chemotherapy followed by autologous stem cell transplantation (HD-ASCT)[34,35]. One study concluded that in young patients with a relatively new diagnosis of PCNSL, HD-ASCT shows lower neurotoxicity and lower rates of relapse[34]. However, in resource-constrained settings, these chemotherapy techniques have proven to be very difficult to implement[36]. The best consolidation strategy following HD-MTX in order to minimize relapse risk has yet to be defined. However, a new systematic review found no difference in overall survival and progression-free survival between patients who underwent HD-ASCT versus WBRT after their HD-MTX treatment[37]. Therefore, these treatment regimens are worth continuing to explore and optimize.
4.4 Novel Strategies
While PCNSL outcomes have improved with the introduction and spread of high-dose-methotrexate, approximately 50% of patients experience remission of some kind[20]. There is absolutely an unmet need for therapies aimed at relapsed patients[38]. Nevertheless, with an improved understanding of PCNSL pathology, novel and precise therapies have been considered for improved treatment of the lymphoma. In these common cases of relapse or recurrent lymphomas, novel pharmaceutical agents like Bruton tyrosine kinase (BTK) inhibitors or immunomodulatory drugs could fulfill this need[8,20]. Ibrutinib is a BTK inhibitor that disrupts the B-cell receptor pathway. Its use has shown responses similar to those of radiographic methods[39,40]. An 840mg oral dose daily shows an overall response rate of 77%; this response rate increased to 80% when combined with chemotherapy pharmaceuticals like methotrexate or rituximab[41]. Ibrutinib does especially show promise in cases of refractory or relapsing PCNSL[39,42,43]. One systemic literature review concluded that when used in combination therapy with HD-MTX or other chemotherapies, ibrutinib was well-tolerated and beneficial for patients with refractory or relapsing PCNSL[43]. However, CARD11 mutations, a well-known ibrutinib resistance mechanism, proved to be a barrier as PCNSL patients possessing this mutation did not respond to ibrutinib treatment[39,44].
Regarding immunomodulatory drugs, pomalidomide and lenalidomide have shown promise in patients with relapsed or refractory PCNSL[45,46]. Both therapeutic agents bind endogenous cereblon, a universally expressed ligase protein, resulting in antiproliferative activity within cancer cells[47]. Patients administered a 5mg daily dose of pomalidomide for three to four weeks showed significant penetration in the cerebral spinal fluid and CNS in preclinical testing with optimistic outcomes in the majority of subjects; this study concluded that treatment with pomalidomide is practical and maintains a low toxicity[45]. There is also growing support for the use of lenalidomide as a PCNSL treatment. While the mechanism of action is still being explored, lenalidomide has proved to have a multitude of important effects in tumor cells including activating G0/G1 arrest[47]. A pre-clinical study found that lenalidomide is well tolerated by patients without any observed toxicities; they concluded the overall response rate to lenalidomide within 14 patients was 64%[38]. With such diverse treatment avenues to be explored, outcomes for patients with PCNSL will likely continue to improve.
5 RADIATION-INDUCED NEUROTOXICITY
While radiation-focused treatments have proven to be successful in prolonging disease progression, the potential neurotoxicity from these modalities complicates treatment. Omuro et al. performed a retrospective analysis of 183 PCNSL patients undergoing whole-brain radiotherapy and HD-MTX-based treatments. The 5-year incidence of neurotoxicity was 24% (43/183), and presented as subcortical dementia, behavioral changes, incontinence, and gait ataxia. On imaging, patients revealed cortical-subcortical atrophy and diffuse white matter disease[48]. Early radiation-induced neurotoxicity presents with cognitive dysfunctions followed by autonomic and motor pathologies, thereby presenting in patients with an overall profile resembling various types of subcortical dementias and arteriosclerotic encephalopathy[49,50]. One mechanism associated with these radiation-induced subcortical diseases is the disruption of the parallel segregated circuits that link the frontal lobe to subcortical structures. Each circuit has a distinct behavioral syndrome, thereby resulting in the presentation of various cognitive dysfunctions[51]. Disruption of the basal-ganglionic circuits has also been shown to result in motor dysfunctions[52]. Functional MRI has shown success in recognizing radiation-induced neurotoxicity through decreased neuronal activity and neural activations while attempting motor and sensory tasks in the months following treatment[53,54]. Spectroscopy has also found success in identifying acute radiation injury even in tissues distant from the irradiated area[55].
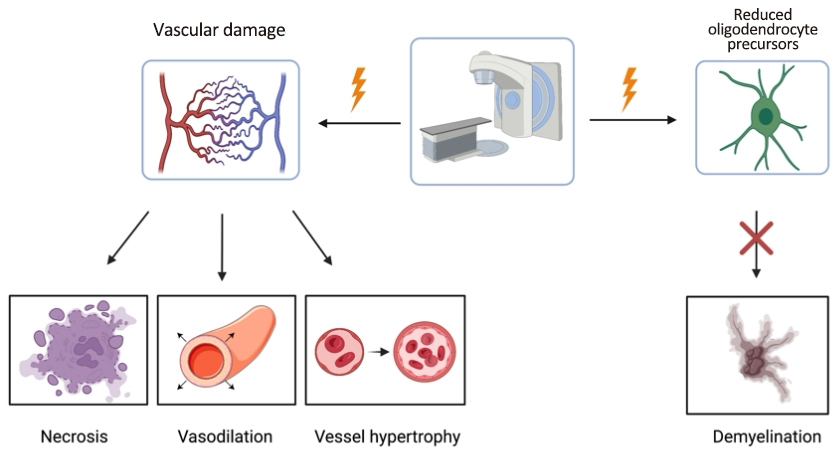
Oligodendrocytes and vasculature are considered the primary targets of radiation therapy[56]. Radiation-induced vascular injury induces dilation, necrosis, and hypertrophy of vessels[57]. Progressive demyelination is seen due to the diminished production of the oligodendrocyte precursors, which decrease the net production of myelin (Figure 2)[58]. A retrospective analysis concluded that radiation-induced neurotoxicity is progressive and irreversible, therefore prevention is essential[48]. One proposed method of prevention is to defer or eliminate radiotherapy by developing improved chemotherapy regimens, which has been the recent trend in clinical trials[48,59-62].
|
Figure 2. The primary targets of radiation therapy for PCNSL patients include the associated vascular damage and lack of oligodendrocyte precursors. However, damage due to the radiation induces necrosis, vasodilation, vessel hypertrophy and demyelination[56,57].
6 DAMAGE ON THE CENTRAL NERVOUS SYSTEM
Having accrued mutations, B-cells will begin proliferating. In immunocompetent patients, this typically presents as a single, supratentorial mass within the periventricular white matter with subependymal infiltration and edema, whereas in immunocompromised patients, there are likely multiple lesions with this presentation[9]. Damages incurred from PCNSL typically manifests in lesions located in brain structures[63,64]. PCNSL presentation varies based on the size and location of the mass and relationship to adjacent structures[65]. It can appear as a single lesion in approximately 60% of patients but can also present as multiple lesions in others[66,67]. The lesion(s) can be found in the cerebral hemispheres in 38% of patients, thalamus/basal ganglia in 16%, corpus callosum in 14%, periventricular region in 12%, cerebellum in 9%, eyes in 5%, and isolated spinal cord in less than 1% of patients[68,69]. Interestingly, leptomeningeal lymphoma in the absence of an intracranial mass presents in less than 5% of all PCNSLs[5,68]. The cancer cells of PCNSL exhibit a special predilection for perivascular growth, proliferating in blood vessels, although the reason for this angiotropism remains unknown. Regardless of growth pattern, inflammation ensues, with the proliferation of T-cells, macrophages, and astrocytes at the site of the cancer[11].
As expected, functions associated with the damaged regions are often those effected in patients with PCNSL (Table 2). For example, in the case of a left cerebellar lesion, a 70-year-old woman experienced worsening dexterity and clumsiness in her left hand[20]. However, even in cases where patients show damage to similar neural regions, there can be huge variety in the symptoms presented. A 78-year-old woman with a right cerebellar lesion displayed progressive right-sided ataxia[70]. A 63-year-old man with PCNSL masses in the frontal and parietal cortex who was admitted for left hemiplegia following a seizure[71]. Another elderly woman with progressive dementia had PCNSL manifest in the corpus callosum[72]. A patient who suffered multifocal, extensive lesions in the cerebral hemispheres and the corpus callosum presented with severe numbness throughout her left face, arm, and leg[73]. In one rare isolated intraventricular PCNSL, an 85-year-old man presented with memory deficits, altered sensorium, and progressive vision loss[74]. Visual disturbances are common in PCNSL. One 53-year-old man presented with wide retinal whitening who’s PCNSL presented as migrating retinal lesions[75]. Finally, in a case of PCNSL limited to the intramedullary spinal cord, the patient had tremendous lower back pain and weakness in his lower extremities[76]. With such variety in symptom presentation, it can be difficult to definitively diagnosis PCNSL. However, with greater investigation into the process of diagnosis, tremendous progress in diagnosis accuracy has been made.
Table 2. Case Reports of PCNSL
Study |
Age (years) |
Sex |
Location |
Presentation |
Kim et al.[73] |
70 |
F |
Corpus callosum |
Numbness in arm, face, leg |
Kanazawa et al.[72] |
73 |
F |
Corpus callosum |
Progressive dementia |
Kim et al.[73] |
70 |
F |
Cerebral hemisphere |
Numbness in arm, face, leg |
Goldbrunner et al.[71] |
63 |
M |
Cerebral hemisphere |
Left sided hemiparesis, local motor seizure |
Grommes[20] |
70 |
F |
Cerebellum |
Reduced dexterity and clumsiness |
Galarza et al.[70] |
78 |
F |
Cerebellum |
Progressive right sided ataxia |
Zeng et al.[77] |
52 |
F |
Paraventricular |
Weakness in limbs and mental decline |
Haddad et al.[78] |
72 |
F |
Third ventricle |
Acute memory loss and confusion |
Khanna et al.[74] |
85 |
M |
Lateral/third ventricle |
Memory deficits, vision loss, altered sensorium |
Tan et al.[75] |
53 |
M |
Both eyes |
Retinal whitening and unilateral visual disturbance |
Guzzetta et al.[76] |
82 |
M |
Spinal cord |
Weakness in lower extremities and back pain |
Methods of locating and assessing the damage from lymphoma on the CNS include nuclear MRI, FDG-PET, diffusion tensor imaging, proton MR spectroscopy, and DSC-MRI[79-84]. Using advanced imaging systems allows for differentiation between lesions caused by PCNSL and masses such as glioblastoma multiforme or other brain metastases[82]. The number of lesions caused by PCNSL is usually 1, however, multiple lesions can be detected within patients who have PCNSL[6]. The size of the lesion varies considerably between brain structures with a diameter that ranges between 2-70mm[85]. Lesions located in the corpus callosum typically have the largest lesions in diameter (approximately 45mm) on average compared to other lesions located in brain structure[85]. The average diameter of a lesion is approximately 24mm. In addition to lesions, accompanying symptoms such as neurological defects and increased intracranial pressure are found in PCNSL patients[64,86].
PCNSL typically presents with headache, confusion, and lethargy related to increased intracranial pressure, focal neurological deficits and seizures related to tumor location as well as neuropsychiatric and cognitive symptoms[5,65,67,68,87,88]. PCNSL can also involve the vitreous of the eye and in some instance may affect vision or ocular motility[7,67,87,89]. Interestingly, the occurrence of B symptoms (fever, weight loss, and night sweats) is uncommon and can precede neurological manifestations in 7-15% of patients[87,90]. However, some patients can be entirely asymptomatic, especially in isolated leptomeningeal involvement, and can be diagnosed sometimes with cerebrospinal fluid analysis with a lumbar puncture[67,68]. It is also important to remember that PCNSL in immunocompromised individuals can present with signs and symptoms related to their immunocompromised status or related opportunistic infections or cancers[65].
7 CONCLUSION
CNS lymphoma is a rare form of non-Hodgkin lymphoma that can be isolated to the central nervous space or secondary in the setting of disseminated disease. Most patients present with focal neurological deficits which correlate with the location of the lesion, along with other symptoms like behavioral changes and seizures. Treatments include HD-MTX followed by consolidation therapy, although more than half of patients with CNS lymphoma experience a relapse and long-term survivors experience neurotoxicity. The role of WBRT and other emerging techniques in PCNSL treatment regimens continues to be debated. Recent advancements in the pathophysiology of this disease will hopefully guide the development of future treatments. With this updated review, we hope to provide clinicians with information about CNS lymphoma damage on the CNS, along with updated information on the etiology, prognosis, pathophysiology, genetic abnormalities, diagnostic procedures, and the current and developing treatment strategies.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
Conceptualization, Lucke-Wold B; methodology, Lucke-Wold B and Hernandez J; writing-original draft preparation, Hernandez J, Davidson C, Reilly T, Hanbali S, Abou-Al-Shaar H, Ebrahim G, Nguyen A; writing-review and editing, Hernandez J, Davidson C, Lucke-Wold B; supervision, Lucke-Wold B. All authors have read and agreed to the published version of the manuscript.
Abbreviation List
BTK, Bruton tyrosine kinase
CNS, Central nervous system
CSI, Craniospinal irradiation
DLBCL, Diffuse large B-cell lymphoma
HD-ASCT, High-dose chemotherapy followed by autologous stem cell transplantation
HD-MTX, High-dose methotrexate
KPS, Karnofsky Performance Status
MRI, Magnetic resonance imaging
MSKCC, Memorial Sloan Kettering Cancer Center
PCNSL, Primary CNS lymphoma
WBRT, Whole brain radiotherapy
References
[1] Green K, Hogg JP. Central Nervous System Lymphoma. StatPearls Publishing: Florida, USA, 2022.
[2] Lukas RV, Stupp R, Gondi V et al. Primary Central Nervous System Lymphoma: PART 1: Epidemiology, Diagnosis, Staging, and Prognosis. Oncology, 2018; 32: 17-22.
[3] Bathla G, Hegde A. Lymphomatous involvement of the central nervous system. Clin Radiol, 2016; 71: 602-609.[DOI]
[4] Villano JL, Koshy M, Shaikh H et al. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer, 2011; 105: 1414-1418.[DOI]
[5] Giannini C, Dogan A, Salomão DR. CNS lymphoma: a practical diagnostic approach. J Neuropathol Exp Neurol, 2014; 73: 478-494.[DOI]
[6] Grommes C, DeAngelis LM. Primary CNS Lymphoma. J Clin Oncol, 2017; 35: 2410-2418.[DOI]
[7] Chan CC, Rubenstein JL, Coupland SE et al. Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist, 2011; 16: 1589-1599.[DOI]
[8] Schaff LR, Grommes C. Primary central nervous system lymphoma. Blood, 2022; 140: 971-979.[DOI]
[9] Rubenstein JL, Gupta NK, Mannis GN et al. How I treat CNS lymphomas. Blood, 2013; 122: 2318-2330.[DOI]
[10] Han CH, Batchelor TT. Diagnosis and management of primary central nervous system lymphoma. Cancer, 2017; 123: 4314-4324.[DOI]
[11] Yang XL, Liu YB. Advances in Pathobiology of Primary Central Nervous System Lymphoma. Chin Med J (Engl), 2017; 130: 1973-1979.[DOI]
[12] Fukumura K, Kawazu M, Kojima S et al. Genomic characterization of primary central nervous system lymphoma. Acta Neuropathol, 2016; 131: 865-875.[DOI]
[13] Yamada S, Ishida Y, Matsuno A et al. Primary diffuse large B-cell lymphomas of central nervous system exhibit remarkably high prevalence of oncogenic MYD88 and CD79B mutations. Leuk Lymphoma, 2015; 56: 2141-2145.[DOI]
[14] Deckert M, Montesinos-Rongen M, Brunn A et al. Systems biology of primary CNS lymphoma: from genetic aberrations to modeling in mice. Acta Neuropathol, 2014; 127: 175-188.[DOI]
[15] Schwindt H, Akasaka T, Zühlke-Jenisch R et al. Chromosomal translocations fusing the BCL6 gene to different partner loci are recurrent in primary central nervous system lymphoma and may be associated with aberrant somatic hypermutation or defective class switch recombination. J Neuropathol Exp Neurol, 2006; 65: 776-782.[DOI]
[16] Liu J, Wang Y, Sun X et al. Promoter methylation attenuates SHP1 expression and function in patients with primary central nervous system lymphoma. Oncol Rep, 2017; 37: 887-894.[DOI]
[17] Schwindt H, Vater I, Kreuz M et al. Chromosomal imbalances and partial uniparental disomies in primary central nervous system lymphoma. Leukemia, 2009; 23: 1875-1884.[DOI]
[18] Hatzl S, Posch F, Deutsch A et al. Immunohistochemistry for c-myc and bcl-2 overexpression improves risk stratification in primary central nervous system lymphoma. Hematol Oncol, 2020; 38: 277-283.[DOI]
[19] Camilleri-Broët S, Camparo P, Mokhtari K et al. Overexpression of BCL-2, BCL-X, and BAX in primary central nervous system lymphomas that occur in immunosuppressed patients. Mod Pathol, 2000; 13: 158-165.[DOI]
[20] Grommes C. Central Nervous System Lymphomas. Continuum, 2020; 26: 1476-1494.[DOI]
[21] Wang CC, Carnevale J, Rubenstein JL. Progress in central nervous system lymphomas. Br J Haematol, 2014; 166: 311-325.[DOI]
[22] Reni M, Ferreri AJM, Garancini MP et al. Therapeutic management of primary central nervous system lymphoma in immunocompetent patients: results of a critical review of the literature. Ann Oncol, 1997; 8: 227-234.[DOI]
[23] Chojak R, Koźba-Gosztyła M, Polańska K et al. Surgical resection versus biopsy in the treatment of primary central nervous system lymphoma: a systematic review and meta-analysis. J Neurooncol, 2022; 160: 753-761.[DOI]
[24] Milgrom SA, Yahalom J. The role of radiation therapy in the management of primary central nervous system lymphoma. Leuk Lymphoma, 2015; 56: 1197-1204.[DOI]
[25] Shah GD, Yahalom J, Correa DD et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol, 2007; 25: 4730-4735.[DOI]
[26] Citterio G, María Ferreri AJ, Reni M. Current uses of radiation therapy in patients with primary CNS lymphoma. Expert Rev Anticancer Ther, 2013; 13: 1327-1337.[DOI]
[27] Cruz-Sanchez FF, Artigas J, Cervos-Navarro J et al. Brain lesions following combined treatment with methotrexate and craniospinal irradiation. J Neuro-oncol, 1991; 10: 165-171.[DOI]
[28] Herrlinger U, Schäfer N, Fimmers R et al. Early whole brain radiotherapy in primary CNS lymphoma: negative impact on quality of life in the randomized G-PCNSL-SG1 trial. J Cancer Res Clin Oncol, 2017; 143: 1815-1821.[DOI]
[29] Thiel E, Korfel A, Martus P et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol, 2010; 11: 1036-1047.[DOI]
[30] Skarin AT, Zuckerman KS, Pitman SW et al. High-dose methotrexate with folinic acid in the treatment of advanced non-Hodgkin lymphoma including CNS involvement. Blood, 1977; 50: 1039-1047.
[31] Wieduwilt MJ, Valles F, Issa S et al. Immunochemotherapy with intensive consolidation for primary CNS lymphoma: a pilot study and prognostic assessment by diffusion-weighted MRI. Clin Cancer Res, 2012; 18: 1146-1155.[DOI]
[32] Morris PG, Correa DD, Yahalom J et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol, 2013; 31: 3971-3979.[DOI]
[33] Rubenstein JL, Hsi ED, Johnson JL et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol, 2013; 31: 3061-3068.[DOI]
[34] Gritsch D, Mrugala MM, Marks LA et al. Is Autologous Stem Cell Transplantation a Safe and Effective Alternative to Whole Brain Radiation as Consolidation Therapy in Patients With Primary Central Nervous System Lymphoma? A Critically Appraised Topic. Neurologist, 2021; 26: 137-142.[DOI]
[35] Liu J, Guo J, Sun X et al. Efficacy and Safety of Autologous Stem-Cell Transplantation as Part of First-Line Treatment for Newly Diagnosed Primary Central Nervous System Lymphoma: A Systematic Review and Meta-Analysis. Front Oncol, 2021; 11: 799721.[DOI]
[36] de Pádua Covas Lage LA, Araújo Soares V, Meneguin TD et al. The role of whole-brain radiotherapy (WBRT) in primary central nervous system lymphoma: is it an alternative to ASCT for consolidation following HD-methotrexate based induction in low-income settings? Radiat Oncol, 2022; 17: 171.[DOI]
[37] Epperla N, Reljic T, Chowdhury SM et al. Autologous hematopoietic cell transplantation versus whole-brain radiotherapy consolidation in primary central nervous system lymphoma: A systematic review and meta-analysis. Hematol Oncol, 2022.[DOI]
[38] Rubenstein JL, Geng H, Fraser EJ et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv, 2018; 2: 1595-1607.[DOI]
[39] Grommes C, Pastore A, Palaskas N et al. Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov, 2017; 7: 1018-1029.[DOI]
[40] Lionakis MS, Dunleavy K, Roschewski M et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell, 2017; 31: 833-843.e5.[DOI]
[41] Grommes C, Tang SS, Wolfe J et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood, 2019; 133: 436-445.[DOI]
[42] Yu H, Kong H, Li C et al. Bruton's tyrosine kinase inhibitors in primary central nervous system lymphoma-evaluation of anti-tumor efficacy and brain distribution. Transl Cancer Res, 2021; 10: 1975-1983.[DOI]
[43] Nepal G, Khurana M, Bucheli DH et al. Ibrutinib in Refractory or Relapsing Primary Central Nervous System Lymphoma: A Systematic Review. Neurol Int, 2022; 14: 99-108.[DOI]
[44] Bartlett NL, Costello BA, LaPlant BR et al. Single-agent ibrutinib in relapsed or refractory follicular lymphoma: a phase 2 consortium trial. Blood, 2018; 131: 182-190.[DOI]
[45] Tun HW, Johnston PB, DeAngelis LM et al. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood, 2018; 132: 2240-2248.[DOI]
[46] Calimeri T, Steffanoni S, Gagliardi F et al. How we treat primary central nervous system lymphoma. ESMO Open, 2021; 6: 100213.[DOI]
[47] Lopez-Girona AEA, Mendy D, Ito T et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia, 2012; 26: 2326-2335.[DOI]
[48] Omuro AM, Ben-Porat LS, Panageas KS et al. Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol, 2005; 62: 1595-1600.[DOI]
[49] Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery, 2001; 49: 1166-1186.[DOI]
[50] Román GC, Erkinjuntti T, Wallin A et al. Subcortical ischaemic vascular dementia. Lancet Neurol, 2002; 1: 426-436.[DOI]
[51] Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol, 1993; 50: 873-880.[DOI]
[52] Tullberg M, Fletcher E, DeCarli C et al. White matter lesions impair frontal lobe function regardless of their location. Neurology, 2004; 63: 246-253.[DOI]
[53] Håberg A, Kvistad KA, Unsgård G et al. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with primary brain tumors: clinical application and outcome. Neurosurgery, 2004; 54: 902-915.[DOI]
[54] Lehéricy S, Duffau H, Cornu P et al. Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: comparison with intraoperative stimulation in patients with brain tumors. J Neurosurg, 2000; 92: 589-598.[DOI]
[55] Lakshmi RJ, Kartha VB, Murali Krishna C et al. Tissue Raman spectroscopy for the study of radiation damage: brain irradiation of mice. Radiat Res, 2002; 157: 175-182.[DOI]
[56] Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res, 2000; 153: 357-370.[DOI]
[57] Lai R, Abrey LE, Rosenblum MK et al. Treatment-induced leukoencephalopathy in primary CNS lymphoma: a clinical and autopsy study. Neurology, 2004; 62: 451-456.[DOI]
[58] van der Maazen RWM, Kleiboer BJ, Verhagen I et al. Repair capacity of adult rat glial progenitor cells determined by an in vitro clonogenic assay after in vitro or in vivo fractionated irradiation. Int J Radiat Biol, 1993; 63: 661-666.[DOI]
[59] Batchelor T, Carson K, O'Neill A et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol, 2003; 21: 1044-1049.[DOI]
[60] Herrlinger U, Schabet M, Brugger W et al. German Cancer Society Neuro-Oncology Working Group NOA-03 multicenter trial of single-agent high-dose methotrexate for primary central nervous system lymphoma. Ann Neurol, 2002; 51: 247-252.[DOI]
[61] Pels H, Schmidt-Wolf IGH, Glasmacher A et al. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol, 2003; 21: 4489-4495.[DOI]
[62] Hoang-Xuan K, Taillandier L, Chinot O et al. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: a multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol, 2003; 21: 2726-2731.[DOI]
[63] Bühring U, Herrlinger U, Krings T et al. MRI features of primary central nervous system lymphomas at presentation. Neurology, 2001; 57: 393-396.[DOI]
[64] Bellinzona M, Roser F, Ostertag H et al. Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: a series of 33 cases. EJSO, 2005; 31: 100-105.[DOI]
[65] Nader R. Neurosurgery Case Review: Questions and Answers, 2nd ed. Thieme Medical Publishers: Stuttgart, German, 2020.
[66] Küker W, Nägele T, Thiel E et al. Primary central nervous system lymphomas (PCNSL): MRI response criteria revised. Neurology, 2005; 65: 1129-1131.[DOI]
[67] Korfel A, Schlegel U. Diagnosis and treatment of primary CNS lymphoma. Nat Rev Neurol, 2013; 9: 317-327.[DOI]
[68] Ferreri AJM, Marturano E. Primary CNS lymphoma. Best Pract Res Clin Haematol, 2012; 25: 119-130.[DOI]
[69] Ferreri AJM, Reni M, Pasini F et al. A multicenter study of treatment of primary CNS lymphoma. Neurology, 2002; 58: 1513-1520.[DOI]
[70] Galarza Fortuna GM, Dvir K, Febres-Aldana C et al. Primary Central Nervous System Lymphoma in an Immunocompetent Patient Presenting as Multiple Cerebellar Lesions: A Case Report and Review of Literature. J Investig Med High Impact Case Rep, 2019; 7: 2324709619893548.[DOI]
[71] Goldbrunner R, Warmuth-Metz M, Tonn JC et al. Primary Ki-1-positive T-cell lymphoma of the brain--an aggressive subtype of lymphoma: case report and review of the literature. Surg Neurol, 1996; 46: 37-41.[DOI]
[72] Kanazawa T, Karatsu K, Kuramae T et al. Microscopic biopsy after unsuccessful endoscopic biopsy for primary central nervous system lymphoma of the corpus callosum: A case report. Ann Med Surg (Lond), 2021; 69: 102746.[DOI]
[73] Kim YJ, Kim SK, Jung TY et al. Inflammatory Brain Lesions as Omen of Primary Central Nervous System Lymphoma: A Case Report and Literature Review. Brain Sci, 2021; 11: 191.[DOI]
[74] Khanna G, Ahlawat S, Garg N et al. A Rare Case of Isolated Intraventricular Primary Central Nervous System Lymphoma in an 85-Year-Old Man. Asian J Neurosurg, 2021; 16: 623-625.[DOI]
[75] Tan SZ, Steeples LR, Chhabra R et al. An unusual case report of primary vitreoretinal lymphoma. BMC Ophthalmol, 2018; 18: 223.[DOI]
[76] Guzzetta M, Drexler S, Buonocore B et al. Primary CNS T-cell lymphoma of the spinal cord: case report and literature review. Lab Med, 2015; 46: 159-163.[DOI]
[77] Zeng X, Lu X, Li X et al. A case report of primary central nervous system lymphoma. J Int Med Res, 2020; 48: 300060520937839.[DOI]
[78] Haddad R, Alkubaisi A, AI Bozom I et al. Solitary Primary Central Nervous System Lymphoma Mimicking Third Ventricular Colloid Cyst-Case Report and Review of Literature. World Neurosurg, 2019; 123: 286-294.[DOI]
[79] Kawai N, Okubo S, Miyake K et al. Use of PET in the diagnosis of primary CNS lymphoma in patients with atypical MR findings. Ann Nucl Med, 2010; 24: 335-343.[DOI]
[80] Yamashita K, Yoshiura T, Hiwatashi A et al. Differentiating primary CNS lymphoma from glioblastoma multiforme: assessment using arterial spin labeling, diffusion-weighted imaging, and ¹⁸F-fluorodeoxyglucose positron emission tomography. Neuroradiology, 2013; 55: 135-143.[DOI]
[81] Toh CH, Castillo M, Wong AMC et al. Primary cerebral lymphoma and glioblastoma multiforme: differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am J Neuroradiol, 2008; 29: 471-475.[DOI]
[82] Kickingereder P, Sahm F, Wiestler B et al. Evaluation of microvascular permeability with dynamic contrast-enhanced MRI for the differentiation of primary CNS lymphoma and glioblastoma: radiologic-pathologic correlation. AJNR Am J Neuroradiol, 2014; 35: 1503-1508.[DOI]
[83] Chawla S, Zhang Y, Wang S et al. Proton magnetic resonance spectroscopy in differentiating glioblastomas from primary cerebral lymphomas and brain metastases. J Comput Assist Tomogr, 2010; 34: 836-841.[DOI]
[84] Lu SS, Kim SJ, Kim HS et al. Utility of proton MR spectroscopy for differentiating typical and atypical primary central nervous system lymphomas from tumefactive demyelinating lesions. AJNR Am J Neuroradiol, 2014; 35: 270-277.[DOI]
[85] Küker W, Nägele T, Korfel A et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol, 2005; 72: 169-177.[DOI]
[86] Liu Y, Yao Q, Zhang F. Diagnosis, prognosis and treatment of primary central nervous system lymphoma in the elderly population (Review). Int J Oncol, 2021; 58: 371-387.[DOI]
[87] Bataille B, Delwail V, Menet E et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg, 2000; 92: 261-266.[DOI]
[88] Gerstner ER, Batchelor TT. Primary central nervous system lymphoma. Arch Neurol, 2010; 67: 291-297.[DOI]
[89] Grimm SA, McCannel CA, Omuro AMP et al. Primary CNS lymphoma with intraocular involvement: International PCNSL Collaborative Group Report. Neurology, 2008; 71: 1355-1360.[DOI]
[90] Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg, 1988; 68: 835-853.[DOI]
Copyright © 2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.




 Copyright ©
Copyright ©