Influence of Palm Fruit Fibe and High Alumina Cement on Omifun Kaolin for Furnace Insulation
Stephen Saturday Onosemudiana1, Oladayo Olaniran1*, Davies Oladayo Folorunso1, Henry Kayode Talabi1
1Metallurgical & Materials Engineering Department, Federal University of Technology, Akure, Nigeria
*Correspondence to: Oladayo Olaniran, PhD, Associate Professor, Metallurgical & Materials Engineering Department, Federal University of Technology, PMB 704, Akure, Nigeria; Email: ooladayolaniran@futa.edu.ng
Abstract
Objective: This work is designed to enhance the insulating properties of Omifun kaolin from Ose Local Government of Ondo State, Nigeria for furnace insulation by reinforcing it with some additives such as high alumina cement and oil palm fruit fiber. This was aimed at developing local technology for the production of insulating bricks in such a way as to reduce the sole dependency on importations of insulating bricks to service the available furnaces in the country.
Methods: Sieve analysis was performed on the Omifun kaolin. Characterization by X-ray Fluorescence (XRF), X-ray Diffraction (XRD), and Scanning Electron Microscopy/ Energy Dispersive Spectroscopy (SEM/EDS). Subsequently, the samples of kaolin reinforced with pulverized palm fruit fiber (1,2,3,4, and 5%) were prepared and tested for permeability. The sample with the best potential was further mixed with varying weight percent (3, 6, 9, 12, 15, 18, 21, and 24%) of high alumina cement, fired within 800-1200℃, and further tested for bulk density, compressive strength, thermal shock resistance (TSR), and linear expansion.
Results: The characterization revealed, in the acceptable limits, the relative quantities of the desired oxides (SiO2, Al2O3, and CaO) with phases (kaolinite, quartz, and plagioclase) expected of good insulating bricks. From the samples containing fixed 3% palm fruit fiber and varying percentages (3, 6, 9, 15, 18, 21, and 24%) of high alumina cement, the sample containing 3% palm fruit fiber and 9% addition of high alumina cement exhibited the most promising qualities desired of good material for furnace insulation applications.
Conclusion: The characterization techniques adopted in this study; XRD, XRF, and SEM/EDS revealed, in the acceptable limits, the relative quantities of the desired oxides (SiO2, Al2O3, and CaO) and phases (kaolinite, quartz, and plagioclase) expected of good insulating bricks are present in Omifun kaolin. With permeability of 44.69%, crushing strength of 54.09KN/cm2, linear expansion of -0.39mm, TSR of 24 cycles, and bulk density of 1.93g/cm3 at firing temperature range 800-1200℃, Omifun kaolin can compete and successfully replace imported insulating bricks.
Keywords: palm fruit fiber, insulation, permeability, compressive strength, alumina
1 INTRODUCTION
Clay is a geo-material consisting of different natural compounds aggregated together, used extensively for industrial applications for its cost efficiency, chemical inertness, and stability[1]. Clay is a fine-grained soil material that contains aggregates of earth minerals with metal oxides and natural matter. It has an average particle size of less than 2µm which is responsible for the various physicochemical properties such as plasticity and other rheological properties. Clays are categorized based on their relative plasticity or malleability, green or dry strength, shrinkage properties, and vitrification range. Processing routes determine the properties of the final products. When clay is fired at elevated temperatures, its resistance to physical and thermal corrosion and melting (volume stability) after losing its moisture content and plasticity determine its efficiency[2].
Kaolin is a chalk-like, lightweight, soft sedimentary rock category of clay found in nature with a chemical composition of Al2Si2O5(OH)4, it contains about 90% of mineral kaolinite with impurities such as Na2O, CaO, K2O, fluxes and silicates. It is a common constituent of the earth lithosphere utilized as raw materials for traditional ceramics[3]. Kaolinite is mostly utilized because of its availability, stability, cheaper cost, environmental compatibility, re-usability, acid-activation ability, non-corrosiveness and structural characteristics[4]. Kaolinite was termed a 1:1 type layer silicate with a tetrahedral sheet of silica (SiO2) linked with an oxygen atom and an octahedral sheet of alumina (Al2O3). Kaolinite possesses high chemical stability and low thermal expansion[5]. Kaolin clay is averagely valued at $162.79 per ton and it needs little or no chemical processing but has high recyclability for industrial use[6]. It is utilized in the production of insulating fire brick (IFB), paper, rubber, paint, drying agents, and numerous other products. It has medical advantages such as its anti-diarrheal properties. Kaolin is naturally non-harmful to humans unless with long exposure which results in silicosis and lung cancer.
Insulating fire bricks are shaped refractory materials with an application temperature above 800℃ and a porosity higher than 45%. Fire clay has two major components which are alumina and silica. The percentage of silica ranges between 60 to 70% while alumina on the other hand ranges from 25 to 35%. A refractory material can withstand high temperatures without breaking or deforming. Other characteristic features of refractory materials include; low thermal conductivity, reduced heat storage, quality insulating properties, powerful thermal shock resistance (TSR), and high level of heat resistance and durability[7]. Refractory products are used wherever high temperatures are required and this includes refractory bricks for furnace linings, insulating firebricks for backup insulation in furnace lining, tubes for electric furnaces, crucibles, thermocouple sheaths, and refractory cements, among others. The classifications of refractory materials according to their chemical nature are basic, neutral, and acid refractories[8].
Refractory materials are separated into subclasses based on the chemical composition (acid, base, and special), method of implementation (shaped and unshaped), method of manufacture (fused and sintered), and porosity content (porous and dense). Refractory materials possess novel properties such as high mechanical and thermal properties, resistance to corrosion and abrasion from fluids, and TSR[9]. They are also characterized by an excellent capability to retain their dimensional and chemical integrity at elevated temperatures. Refractories are used in furnace linings, kilns, incinerators, and reactors[10].
Kaolin refractory clays are distinguished from fire clays because of their whiter colour, coarse particles, lower plasticity, and fewer impurities. It has a melting temperature between 740℃ and 1785℃ and a relative density of 2.65g/cm3. Kaolin is non-soluble in water and has been used as insulating bricks for industrial kilns or furnaces. Insulating bricks are known for their high porosity levels, lightweight, low thermal conductivity, and good thermal resistance. Porosity is induced in refractories by aggregating pore-forming agents such as oil palm fiber, sawdust, wheat straw, rice husk, and many other organic particulates[11].
Although a substantial amount of research has been carried out in the area of production and development of good refractory materials for over two centuries, this has resulted in a variety of refractory materials available in the world market today[12]. Most developing nations that are consumers of refractory materials still have to spend their hard-earned foreign reserves on the importation of these materials, such that their needs are met. There has therefore been a continuous rise of interest in such a way of looking inward, to develop good refractories using locally sourced materials.
Production of insulating firebricks can take many routes such as burning out prior incorporated organic matter in the firebricks and preservation of bubble-like structures formed in the insulating refractories[11]. Prior researchers have utilized different organic wastes such as fluted pumpkin stem waste, macadamia nutshell, coconut shell, oil palm fiber, sawdust, and waste glass[11-14]. A major factor that determines the final properties exhibited by insulating refractory materials is the four-step manufacturing route: raw material processing, forming, firing and final processing[12].
2 MATERIALS AND METHOD
2.1 Materials
The materials for this research included Kaolin from Omifun deposit in Ose Local Government of Ondo State, high alumina cement, and oil palm fiber.
2.2 Methodology
The method used in this research was based on the extensive literature review of various past research in the field of the development of refractories as listed below:
2.2.1 Preparation of the Raw Kaolin for Characterization
The raw kaolin after being mined from the deposit was washed in water and the deleterious particles in it were removed by decantation. X-ray fluorescence (XRF) analyzer, Scanning Electron Microscopy (SEM), and X-ray Diffraction (XRD) analyses were used in the determination of the elemental compositions, morphological characterization, and phase identification of the kaolin.
2.2.2 Development of the Kaolin Based Hybrid Composites
Samples of kaolin with admixtures of varying percentages of pulverized oil palm fiber (1, 2, 3, 4, 5%) were prepared and tested for permeability. The composition with the best potential was further mixed with high alumina cement at varying quantities (3, 6, 9, 12, 15, 18, 21 and 24%), according to the appropriate American Standards of Testing and Materials (ASTM) standard procedure as reported by Folorunso and Bello[14] and, Folorunso and Akinwande[11]), These specimens were then mold, fired in the temperature range of 800-1200℃ and subjected to some selected insulation properties tests.
2.2.3 Evaluation of the Thermal Insulation Properties
The following insulation property tests were then carried out on composites produced from (2.2.2) to obtain the composition that will conform to the standard value for furnace insulation operations.
Bulk density: It was evaluated by using Equation (1):
|
Where,
Mf is the mass of fired sample.
Msat is the mass of saturated sample immersed in water.
Msus is the mass of suspended mass of sample in water.
Apparent porosity: It was evaluated by using Equation (2):
|
where,
D is the weight of fired samples.
W is the weight of samples soaked in water for 6 hours.
S is the weight of samples immersed in water.
Permanent linear shrinkage: It was evaluated by using Equations (3) and (4)
|
Where,
T1 is the thickness before drying
T2 is the thickness after sun drying.
T3 is the thickness after firing.
Compressive strength: It was evaluated by using Equation (5):
|
TSR tests were performed following the ASTM C133 standard[15]. Cylindrical test specimens were heated in a furnace maintained at 1100°C for 30min and then removed to cool in air for 10min. After this, the test specimen was examined for the formation of cracks on the surface. The specimens were then returned to the furnace, heated for 10min, air-cooled again for 10min and the surface was examined for cracks. This cycle of heating and cooling was repeated until surface cracks were observed. The number of cycles required to produce cracks is regarded as the TSR.
3 RESULTS
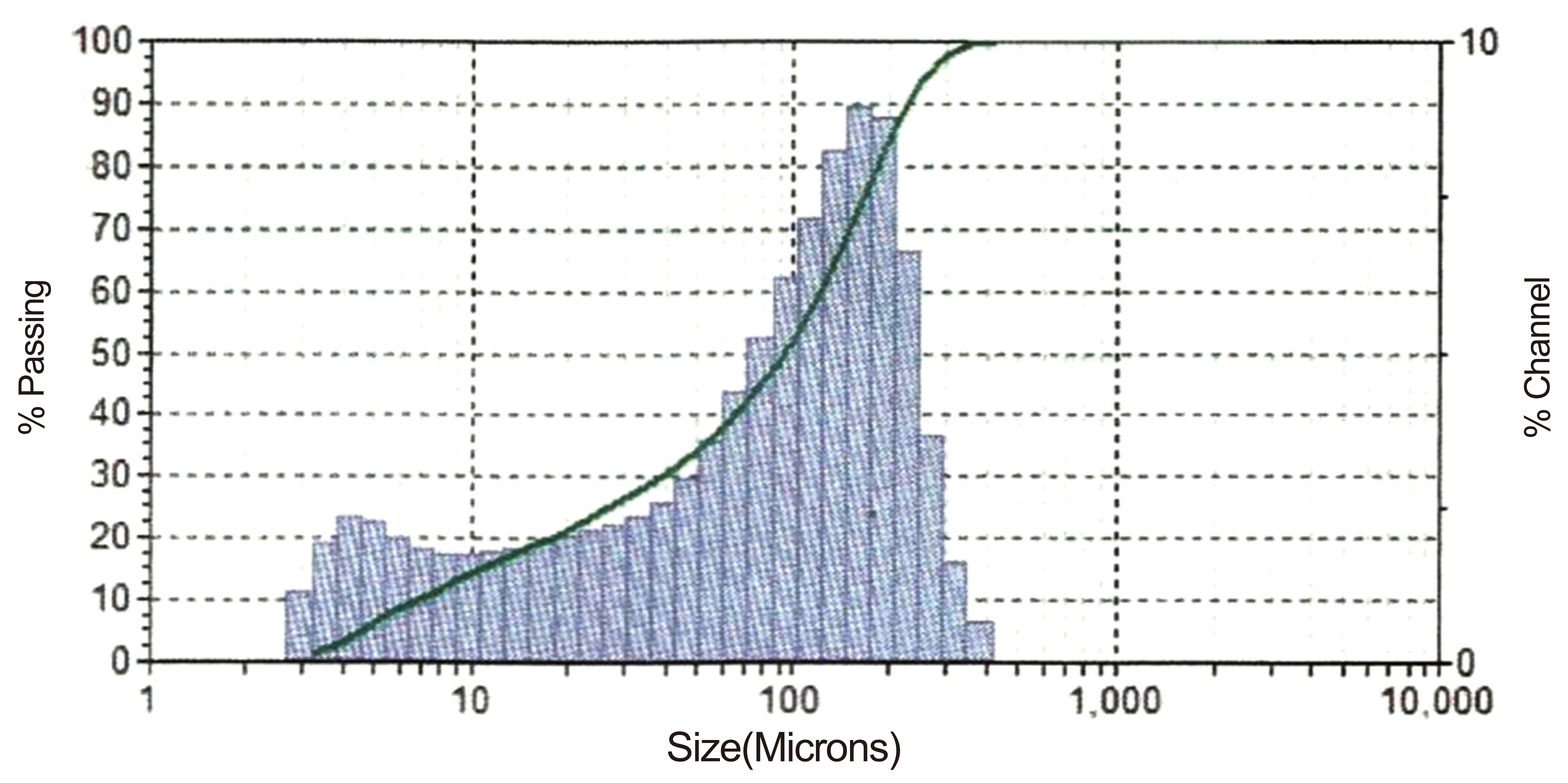
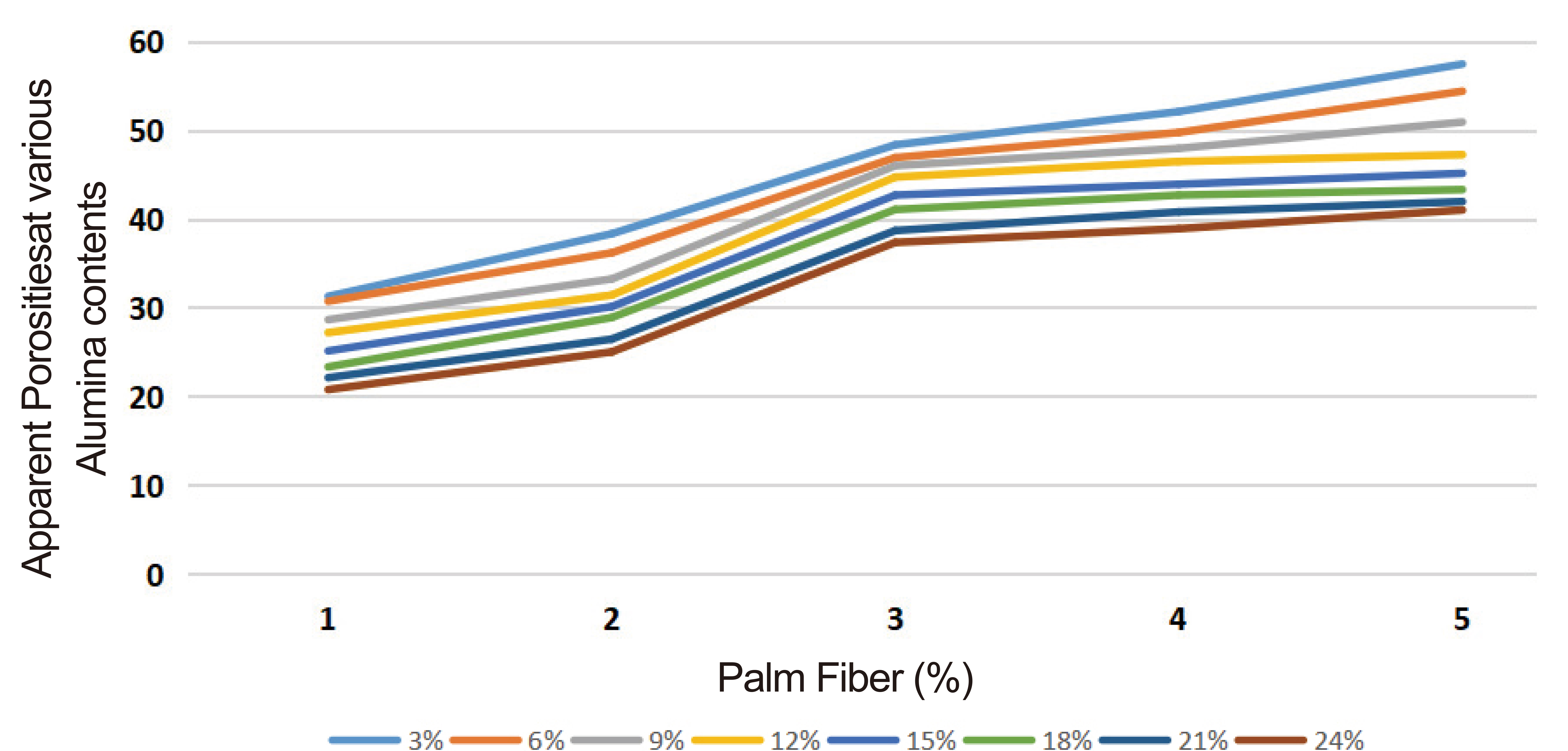
The results obtained during the research are presented as follows: the result of the particle size analysis of the pulverized Omifun kaolin is represented in Figure 1. Table 1 is the representation of the XRF qualitative analysis on the kaolin. The results show the percentages of all the major (Al2O3, SiO2 and CaO with other negligible constituents. Table 2 represents the calculated oxide contents of the kaolin from the XRD quantitative analysis. This also shows the dominance of SiO2 and Al2O3 in the composition. The result of apparent porosity test of varying composition of palm fruit fibers and Alumina cement is presented in Figure 2.
|
Figure 1. Sieve analysis of Omifun kaolin.
Table 1. XRF Quantitative Analysis of Omifun Kaolin
Constituents |
Omifun Kaolin (%) |
Al2O3 |
36.619 |
SiO2 |
41.788 |
Fe2O3 |
2.447 |
K2O |
2.065 |
MgO |
1.099 |
TiO2 |
1.691 |
CaO |
4.016 |
Na2O |
2.066 |
MnO |
1.007 |
P2O5 |
1.004 |
SrO |
2.38 |
ZnO |
3.188 |
Cu |
0 |
BaO |
0 |
Ni |
0.003 |
SO3 |
0.061 |
ZrO2 |
0.036 |
Total |
99.47 |
Table 2. Calculated Oxide Content of Omifun Kaolin from XRD Quantitative Analysis
Constituents |
Al2O3 |
SiO2 |
Na2O |
2O |
TiO2 |
ZnO |
SrO |
% Oxide Content |
37.244 |
46.925 |
0.22 |
0.041 |
1.728 |
3.245 |
2.375 |
|
Figure 2. Apparent porosities at various oil palm fruit fibers and alumina cement contents.
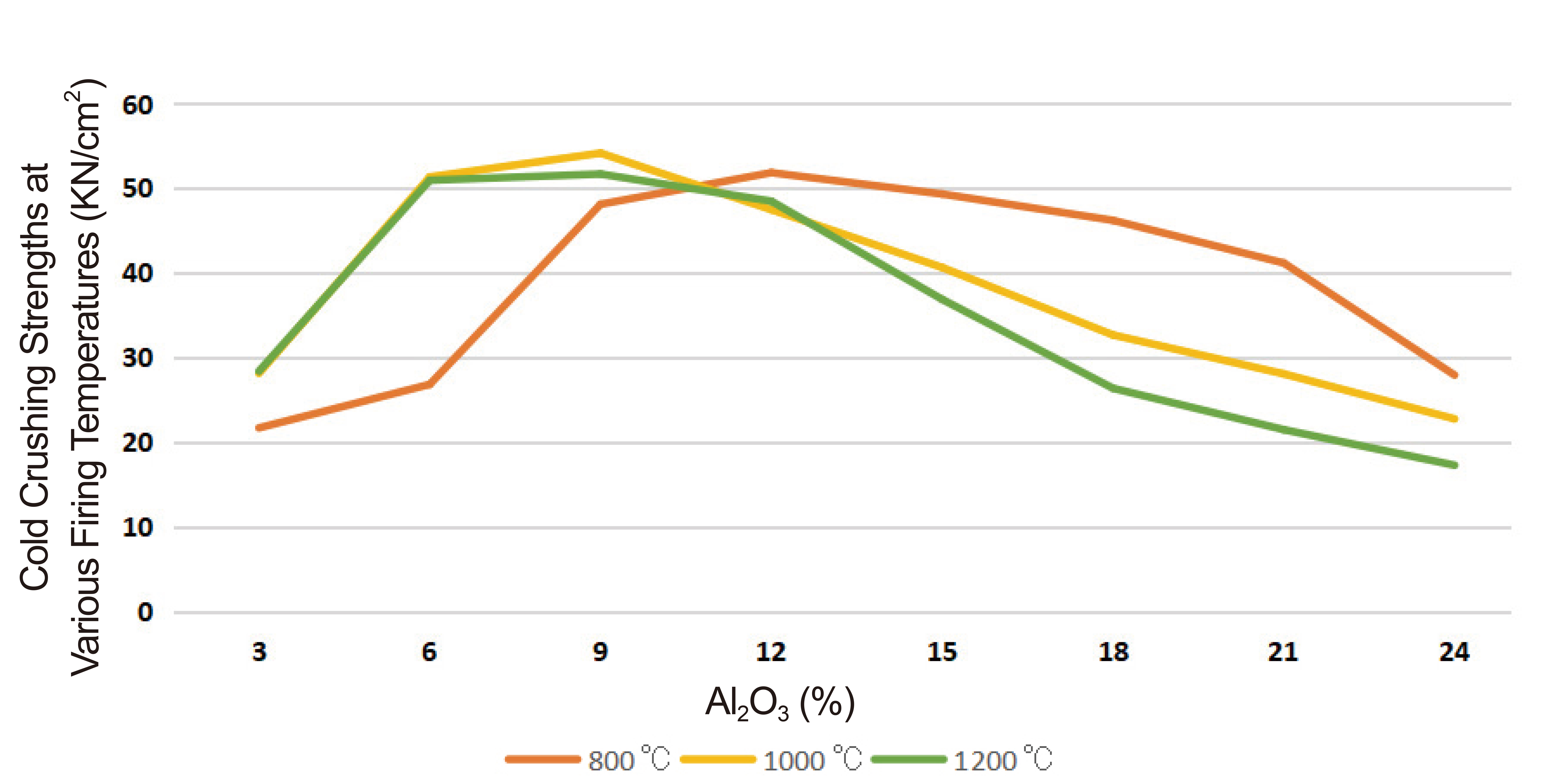
The results of crushing strength of various firing temperature and permanent linear changes at different firing temperatures are presented in Figures 3 and 4 respectively. Other results of analysis performed on the Omifun kaolin include; TSR that is represented in Figure 5, and Bulk densities at varying firing temperatures as presented in Figure 6.
|
Figure 3. Crushing strengths at various firing temperatures.
|
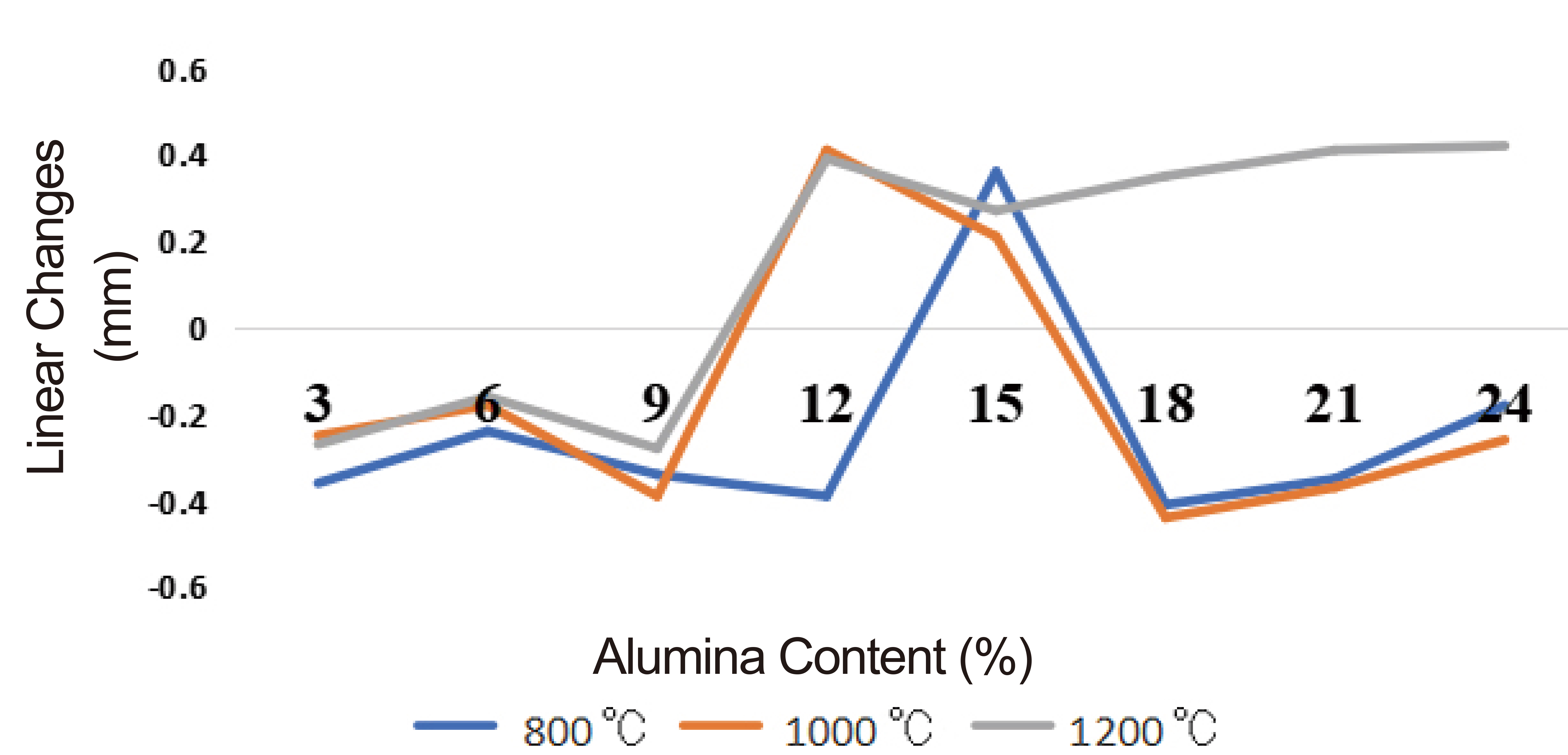
Figure 4. Permanent linear changes at different firing temperatures.
|
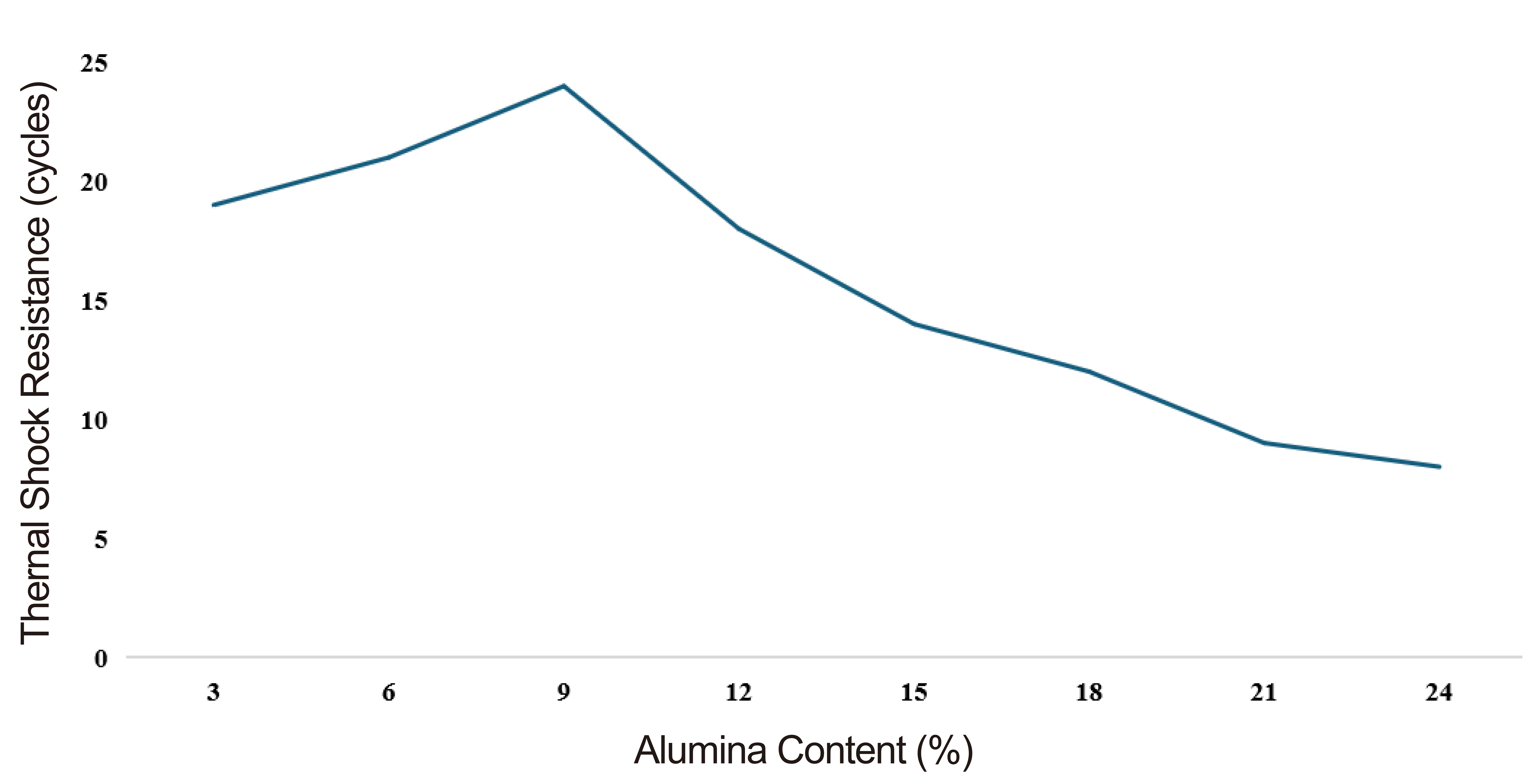
Figure 5. TSR at various alumina contents.
|
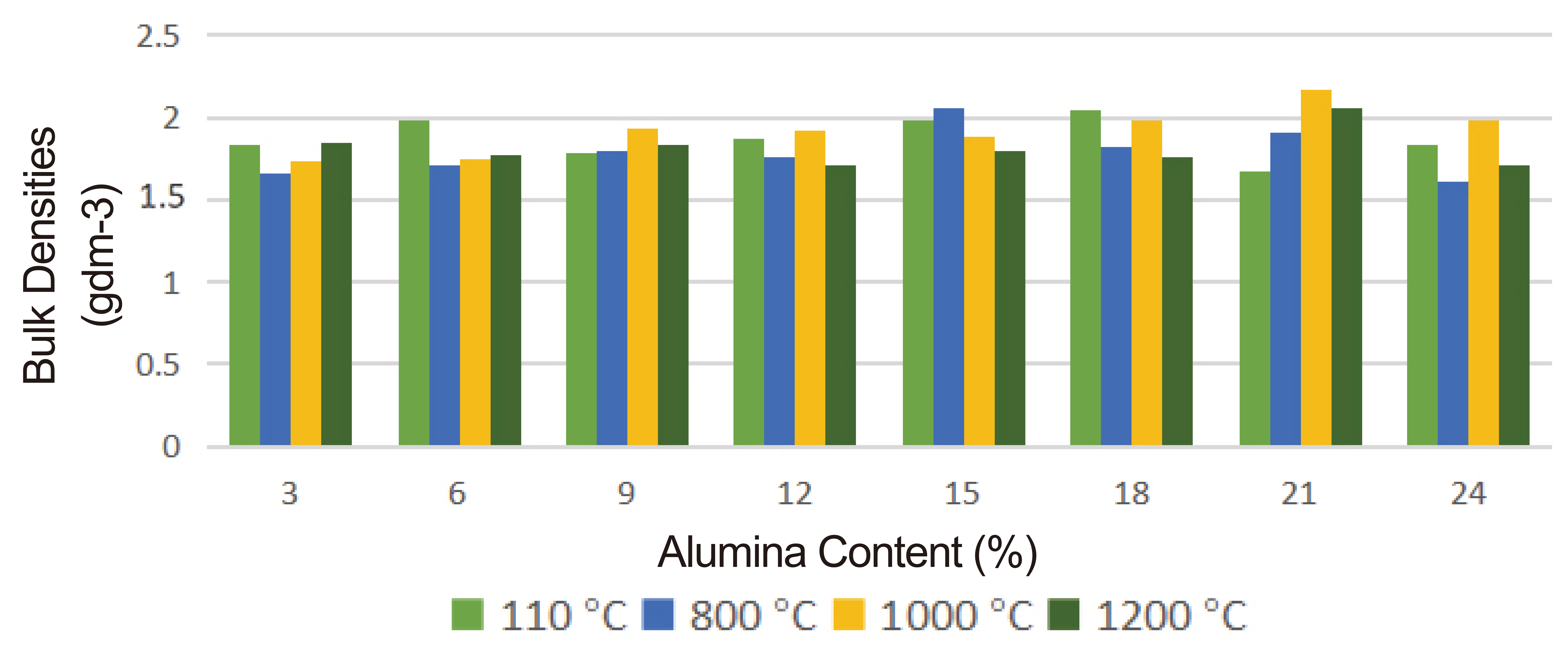
Figure 6. Bulk densities at drying and various firing temperatures.
3.1 Discussion
The result of the sieve analysis as shown in Figure 1 revealed an average particle size of 100µm. The bulk of the kaolin and palm fruit fibre were therefore sieved to 100µm for subsequent works.
The XRF analysis, as expressed in Table 1 clearly shows the various oxide phases present in the Omifun kaolin. The desirable oxide phases (Al2O3, SiO2 and CaO) are present in appreciable quantities while the remaining and less essential ones are all in very insignificant proportions.
Table 2 shows the result of the calculated oxide content of the kaolin sample from the XRD analysis. The dominant presence of Al2O3 and SiO2 further confirms the applicability of Omifun Kaolin for refractory application, especially in furnace insulation. These two phases (Al2O3 and SiO2) are very important in determining the thermal conductivity, compressive strength, and wear resistance of insulating blocks. These is in agreement with Folorunso and Akinwande[13].
Table 3 shows the result of mineralogical analysis carried out to identify and determine the relative amounts of minerals contained in the kaolin. The types and quantities of minerals present in any clay usually have a very dominant influence on its refractory performance. For the purpose of insulation, kaolinite and muscovite are the most desirable because they significantly enhance refractoriness of refractory materials[16]. The abundance of kaolinite in the result attested to the usefulness of Omifun kaolin for insulation works.
Table 3. Relative Phases (Weight%) Present in Omifun Kaolin
Phases |
Kaolinite |
Quartz |
Anatase |
Muscovite |
Plagioclase |
Weight (%) |
91.72 |
4.78 |
1.76 |
0.39 |
1.31 |
The results of the apparent porosities at different composition of palm fiber and alumina cement additions are presented in Figure 2. Porosity will be generated by the burning-off of the palm fruit fiber at the application of firing at high temperature. This means that the higher the percentage composition of the palm fruit fiber present, the more the porosity value will be. However, there is a standard acceptable value of this porosity for insulating bricks. The results revealed that 3% addition of palm fiber exhibited the most promising compliance to standard: ≥45% porosity (ASTMC 210[17], at all levels of alumina cement additions. 3% palm fiber addition was therefore made constant for further tests at all firing temperatures. Even though 4 and 5% addition of oil palm fibre also showed compliance with ASTMC 210[17], but they were not considered because too much porosity will eventually reduce the strength of the insulating composite. This was due to the fact that, when porosity was increased, the water adsorption will also increase, and this will have a negative impact on the strength of the insulating bricks. This agrees with the findings of Aramide and Oke[14].
The variation of the cold crushing strengths of the formed composites at various alumina cement concentrations and firing temperatures is presented in Figure 3. It was observed that the strength increased initially with alumina content addition and later decreased as the quantity of alumina increased. This was common to all the three temperatures (800, 1000 and 1200℃) considered. Similarly, the strength was observed to be on the increase up to a limited firing temperature and later reduced as the temperature goes higher. These trends can be attributed to the fact that the low melting oil palm fibre burnt off completely at higher firing temperature thereby reducing the cohesiveness of kaolin grains and consequently the adhesion of the grains to alumina cement as well. The reductions in the cohesive and adhesive forces could lead to the reductions in the crushing strengths.
The results of the variations of permanent linear changes with alumina contents and firing temperatures are shown in Figure 4. It was observed that all the samples initially suffered linear contractions at less than 9% alumina content but again experienced linear expansions at higher alumina contents (9-15%) for all the firing temperatures (800, 1000 and 1200°C). Further addition of alumina (above 15%) again resulted in linear contraction. This behaviour may be indicative of change in the allotropic form, variations in the clay-alumina chemical reactions and, or liquid phase formative sintering reactions at different alumina contents and firing temperatures. The variations were however, within the range; -0.4-+0.4% recommended for good furnace insulation materials[18,19], which confirms the usefulness of the prepared composite for furnace insulations.
Results of the TSR of the samples at various alumina contents and firing temperatures are presented in Figure 5. TSR above 30 cycles are classified as ‘excellent’, those in the range 25-30 cycles as ‘good’, 20-25 cycles as ‘fair’ 15-20 cycles as acceptable, 10-15 cycles as ‘poor’ while less than 10 cycles are classified as ‘very poor’[20]. The sample containing 9% alumina content with 24 cycles therefore exhibited the most promising compliance as it ranges above acceptable and close to good.
The results of the bulk densities at the drying and firing temperatures are presented in Figure 6. Very close variations were noticed in the various samples tested. The variations could be attributed to void formation resulting from the burning-off of oil palm fibers, being organic matters, from the brick samples during firing. These organic matters are usually not uniformly distributed in the kaolin matrix and hence another possible reason for the variation. More so, firing at high temperatures could cause void closure as a result of softening or liquid phase formation which could also cause bulk density variation.
However, as expected of a good insulating refractory, the bulk density should range between 1.6 and 2.0gcm-3[13-15,20]. This range of values is exhibited by virtually all the samples tested. Hence the composites so prepared are good for insulation purposes.
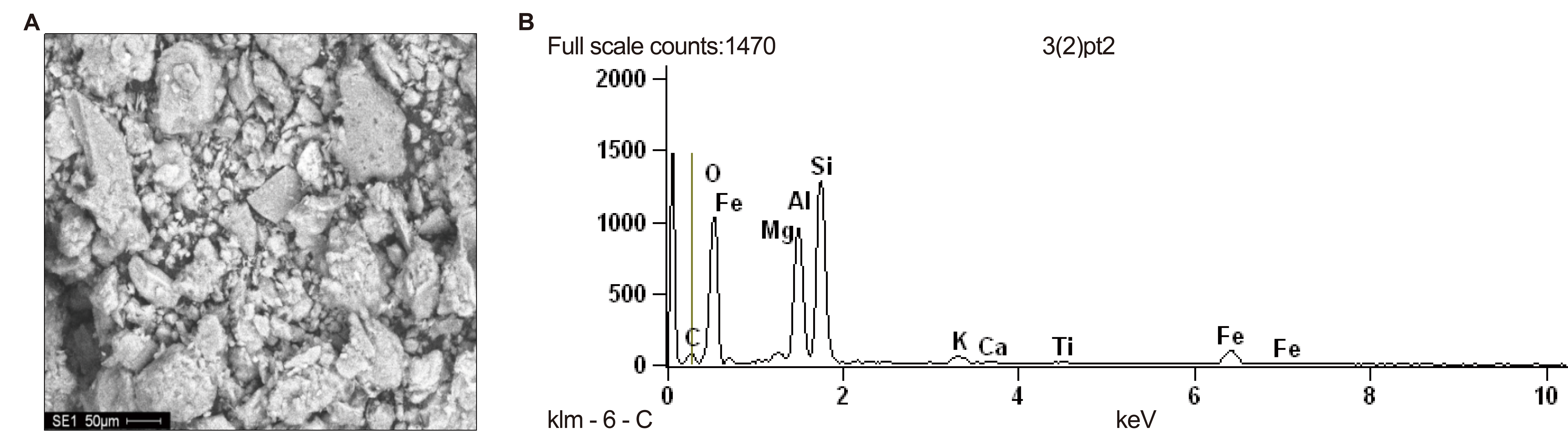
Figure 7 shows the result of the SEM/ Energy Dispersive Spectroscopy (EDS) analysis for the Kaolin. Figure 7A is the SEM micrograph showing the relative sizes of the clay particles at X 500 magnification. The arrangement comprises of bigger sizes but flat irregular shapes of Al2O3 structure. Whereas the SiO2 consist of uneven oval shaped structure while the CaO is also made up of irregular and uneven oval shaped but bigger average size compared with that of Al2O3 structure. Figure 7B represents the spectra depicting the peaks of the elements present in the Omifon kaolin. The spectra clearly confirmed sufficient presence of the desirable oxides (SiO2 and Al2O3) which were also shown by the SEM analysis. These are the essential components required for insulation applications as reported in previous works[13-15].
|
Figure 7. A: SEM micrograph (X500); B: EDS pattern for Omifun kaolin sample.
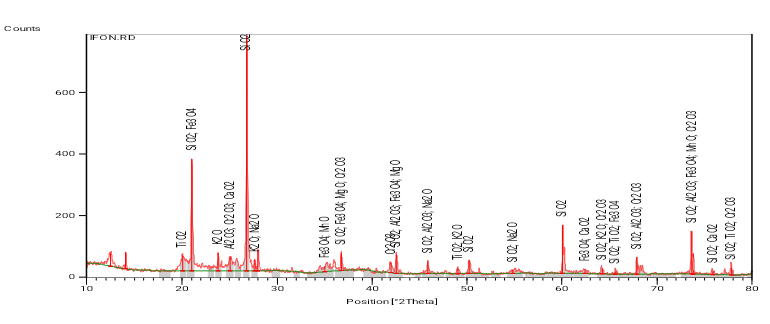
Qualitative analysis of the Omifun clay was done and presented in Figure 8 as the XRD pattern of Omifun kaolin in the raw state. The pattern shows sharp high peaks for the essential oxides; SiO2 and Al2O3 but sharp and shorter peaks for the less essential; Fe3O4, K2O, MgO, MnO, Na2O and CaO. Some other oxide phases which are present are in very negligible proportions.
|
Figure 8. XRD pattern for Omifun kaolin.
The properties of a good brick must include but not limited to; low thermal conductivity, light weight, and sufficient resistance to temperature which enables them to be successfully used as insulation in the hot part of the furnace wall. The ability of the produced Omifun kaolin refractory material to have all these mentioned refractory properties will allow its successful application in the hot side of furnace. This will allow the use of thin walls of low thermal conductivity and low heat content. The low heat content will be particularly valuable in saving fuel and time on heating up and allows rapid changes in temperature to be made and will permits rapid cooling. All this will add to the economic viability and performance of any furnace where it will be applied.
4 CONCLUSION
The characterization techniques adopted in this study; XRD, XRF and SEM/EDS) revealed, in the acceptable limits, the relative quantities of the desired oxides (SiO2, Al2O3 and CaO) and phases (kaolinite, quartz and plagioclase) expected of good insulating bricks.
The tests conducted also showed clearly that kaolin, when fortified with palm fruit fibre and high alumina cement in the appropriate quantities would serve successfully in furnace insulation applications.
After thorough evaluations and comparison of results obtained from the various tests conducted with the existing standard values, it was observed that samples containing 3% addition of palm fruit fibre and 9% addition of high alumina cement exhibited the most promising qualities desired of good material for furnace insulation applications which compare with the standard; the bulk density ranges between 1.6-2.0gcm3, porosity was above 45%, crushing strength was above 50KN/cm2, linear expansion was between -0.4 and +0.4%, and thermal shock is 24 which was above acceptable but in the fair zone.
Acknowledgements
The study was supported by the Federal University of Technology for providing an enabling environment for this research.
Conflicts of Interest
The authors declared there is no conflict.
Author Contribution
Onosemudiana SS was responsible for data analysis and writing the original draft, Olaniran O was responsible for material sourcing, data analysis, reviewing and editing the manuscript, Talabi HK was responsible for material sourcing, Folorunso DO was responsible for conceptualization, research methodology.
Abbreviation List
ASTM, American Standards of Testing and Materials
EDS, Energy Dispersive Spectroscopy
SEM, Scanning Electron Microscopy
TSR, Thermal shock resistance
XRD, X-ray Diffraction
XRF, X-ray fluorescence
References
[1] Hadi EM, Hussein SI. A sustainable method for porous refractory ceramic Manufacturing from kaolin by adding of burned and raw wheat straw. Energy Procedia, 2019; 157: 241-253.[DOI]
[2] Titiladunayo IF, Fapetu OP. Selection of Appropriate Clay for Furnace Lining in a Pyrolysis Process. J Emerg Trends Eng Appl Sci, 2011; 2: 938-945.[DOI]
[3] Vandana A, Anand D, Ashwin W. Fabrication and Characterization of Micro-Porous Ceramic Membrane Based on Kaolin and Alumina. Indian J Chem Techn, 2017; 24: 367-373.
[4] Mamudu A, Emetere M, Okocha D et al. Parametric Investigation of Indigenous Nigeria Mineral Clay (Kaolin and Bentonite) as a Filler in the Fluid Catalytic Cracking Unit (FCCU) of a Petroleum Refinery. Alex Eng J, 2020; 59: 5207-5217.[DOI]
[5] Uddin MK. A Review on the Adsorption of Heavy Metals by Clay Minerals, with Special Focus on the Past Decade. Chem Eng J, 2016; 308: 438-462.[DOI]
[6] Komadel P, Madejova J. Acid Activation of Clay Minerals. Dev Clay Sci, 2006; 6: 263-287.[DOI]
[7] A complete guide to light weight insulating fire brick. Available at:[Web]
[8] Borode JO, Onyemaobi OO, Omotoyinbo JA. Suitability of Some Nigeria Clays as Refractory Raw Materials. Nigerian J Eng Manag, 2000; 3: 14-18.
[9] Sadik C, El-Amrani I, Albizane A. Recent Advances in Silica-Alumina Refractory: A Review. J Asian Ceram Soc, 2014; 2: 83-96.[DOI]
[10] Mokwa JB, Lawal SA, Abolarin MS et al. Characterization and Evaluation of Selected Kaolin Clay Deposits in Nigeria for Furnace Lining Application. Nigerian J Techn, 2019; 38: 936-946.
[11] Zaidan SA, Hamood AF, Ibrahim SN. Effect of Porosity on Thermal Conductivity and Flexural Strength of Ceramic Foam. Eng Techn J, 2016; 34: 610-620.[DOI]
[12] Aribo S, Olaniran O. Performance evaluation of insulating firebricks produced from hydrometallurgically purified termite hill clay reinforced with alumina. Am J Eng Res, 2015; 4: 1-7.[DOI]
[13] Folorunso DO. Influence of Wood Saw Dust and Waste Glass Admixture on Selected Properties of Fired Clay Bricks for Masonry. J Eng Eng Techn, 2021; 15: 104-116.[DOI]
[14] Aramide F, Oke SR. Production and Characterization of Clay Bonded Carbon Refractory from Carbonized Palm Kernel Shell. Acta Techn Cornvinensis-Bull Eng Tome, 2014; 7: 133-140.[DOI]
[15] Folorunso DO, Akinwande AA, Balogun O. Property Evaluation and Compliance Level of Glass Waste Reinforced Ceramic Composite for Masonry Applications. Inter J Adv Acad Res, 2021; 7: 1-14.[DOI]
[16] Folorunso DO, Bello OS. Enhancement of the Mechanical and Thermal Integrity of Ijapo Clay for Thermal Insulation. J Environ Te, 2021; 2: 30-38.[DOI]
[17] Standard test methods for Cold Crushing Strength, thermal shock resistance and Modulus of Rupture of refractory brick and shape. American Society for Testing and Materials. Pennsylvania, 1984.
[18] Folorunso DO. Characterization and Value Enhancement of Some Nigerian Refractory Materials for Thermal Insulation. IOSR J Mech Civil Eng, 2018;15: 79-86.
[19] ASTM C 201-86 Standard test method for thermal conductivity of Refractories. American Society for Testing and Materials. Pennsylvania, 1986. Available at:[Web]
[20] Hassan SB. Refractory Properties of Bauchi and Onibode Clays of Nigeria Furnace Lining. African J Sci Techn, 2000; 11: 33-41.[DOI]
Copyright © 2024 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.

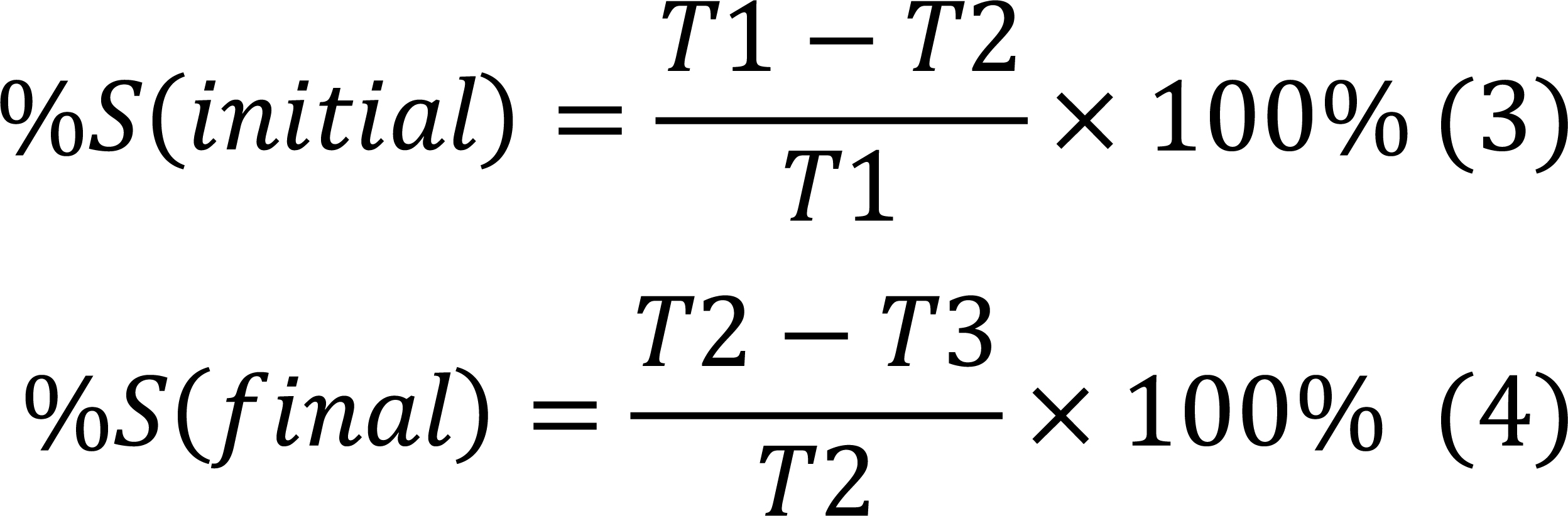



 Copyright ©
Copyright ©