Investigation of The Possible Effects of COVID-19 on HbA1c Levels of Adults without Diabetes: A Retrospective Research
Deniz Ozturk1*![]() , Yılmaz Sahin1
, Yılmaz Sahin1![]() , Seda Askin1
, Seda Askin1![]() , Bugra Kerget2
, Bugra Kerget2![]() , Muhammet Celik2
, Muhammet Celik2![]()
1Health Services Vocational School, Ataturk University, Erzurum, Turkey
2Faculty of Medicine, Ataturk University, Erzurum, Turkey
*Correspondence to: Deniz Ozturk, PhD, Assistant Professor, Health Services Vocational School, Ataturk University, Erzurum 25240, Turkey; Email: d.ozturk@atauni.edu.tr
Abstract
Objective: To retrospectively investigate the possible effects of COVID-19 disease on the hemoglobin A1c (HbA1c) levels of non-diabetic adults.
Methods: This study was carried out by scanning the files of 19 non-diabetic adult men and 15 women diagnosed with COVID-19, aged 18-80, who applied to Faculty of Medicine, Ataturk University between 2018-2022.
Results: After screening, the HbA1c levels of the participants were recorded both before and three months after the diagnosis of COVID-19. The paired samples t-test was used to compare the variations in HbA1c values before and after COVID-19 in both male and female participants. The significance level was evaluated as P<0.05. As a result of statistical analysis, the present study demonstrated that the total HbA1c values of both male and female participants increased significantly after COVID-19 (P<0.05). In addition, it was observed that the total HbA1c value augmented after COVID-19 compared to before the COVID-19 period, regardless of gender.
Conclusion:The HbA1c levels of adults without non-diabetic have increased after COVID-19 disease. It may result in a negative reflection on the glycemic profiles of the participants.
Keywords: adults, non-diabetic, HbA1c, COVID-19, retrospective study
1 INTRODUCTION
COVID-19 is an infection with clinical manifestations that ranges from asymptomatic to severe respiratory symptoms and even death[1]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide as an Omicron variant. This variant is a heavily mutated virus and has been designated an alarming variant by the World Health Organization [2].
Diabetes is recognized as a worldwide public health problem due to its rising prevalence and its association with development of various diseases, including stroke, kidney failure, and heart disease[3]. Glycemic management is even more critical for patients with both diabetes and COVID-19, as diabetes has been reported to be associated with a poor prognosis of COVID-19[4,5]. Evidence shows better glycemic control is closely associated with improved clinical outcomes in COVID-19 patients[6,7]. At the same time,whether COVID-19 contributes to hyperglycemia is still condused[8]. A previous study suggests that the pancreas could be the target of a coronavirus attack since coronavirus was detected in the pancreas[9]. Another study find that the coronavirus damaged the endocrine part of the pancreas. This shows that coronavirus can cause acute insulin-dependent diabetes mellitus[10].
Although many studies indicated that diabetes is a significant risk factor for COVID-19, the impact of COVID-19 infection on glycemic parameters, including blood glucose and glycosylated hemoglobin A1c (HbA1c), remains unclear[11]. HbA1c stands for glycated hemoglobin which occurs when glucose (sugar) in the body attaches to red blood cells.
The rapid worldwide spread of COVID-19 and its Omicron variant as well as the necessity of continually improved knowledge about glycemic management during coronavirus infection prompted us to undertake such a study.
The literature review found there is no study on the quantitative evaluation of HbA1c in individuals diagnosed with COVID-19. This study is to retrospectively investigate the possible effects of COVID-19 on HbA1c levels and, thus, on the glycemic index in adult individuals without a diagnosis of diabetes.
2 MATERIALS AND METHODS
2.1 Design of the Study
The criteria for case file included in the study are as follows: (1) Aged between 18-80 years old; (2) Non-diabetic, with HbA1c level measured before diagnosed with COVID-19; (3) Non-diabetic, measuring HbA1c level 3 months after the diagnosis of COVID-19; (4) Case files without any disease such as diabetes, hypertension, neurodegenerative disease, head trauma, alcohol and drug addiction, epilepsy.
2.2 Population and Sample of the Research
For the 15% difference in sensitivity between 0.80 and 0.95 to be significant in COVID-19 screening, 30 patients were calculated with the NCSS / PASS program at 80% power and 95% confidence level. According to the calculated power analysis, the study involved 34 male and female case files between 18-80 diagnosed with COVID-19 and met the criteria. Case files were divided into before and after COVID-19 diagnosis, HbA1c data were obtained by electronically scanning the files of both chest diseases and the biochemistry department. Some cases, especially those containg HbA1c data before COVID-19, were accessed electronically via the e-pulse data system of the Ministry of Health.
2.3 Ethical Statement
Before the study, permission was obtained from the Atatürk University Faculty of Medicine Clinical Research Ethics Committee (Number: B.30.2.ATA.0.01.00/170). In addition, individuals who agreed to participate in the study were asked to read and sign the informed consent form, which was carried out following the Principles of the Declaration of Helsinki.
2.4 Statistical Analysis
Data were expressed as mean±SD in bar graphs, and individual variations in HbA1c values before and after COVID-19 were shown in scatter plot graphs. First, using the Shapiro-Wilk test to determine whether the data showed a normal distribution. Thethe Shapiro-Wilk test results showed that the data distribution was normal (P>0.05). Therefore, paired samples t-test was used to compare the variations of HbA1c values before and after COVID-19 in both male and female participants. The significance level was evaluated as P<0.05.
3 RESULTS
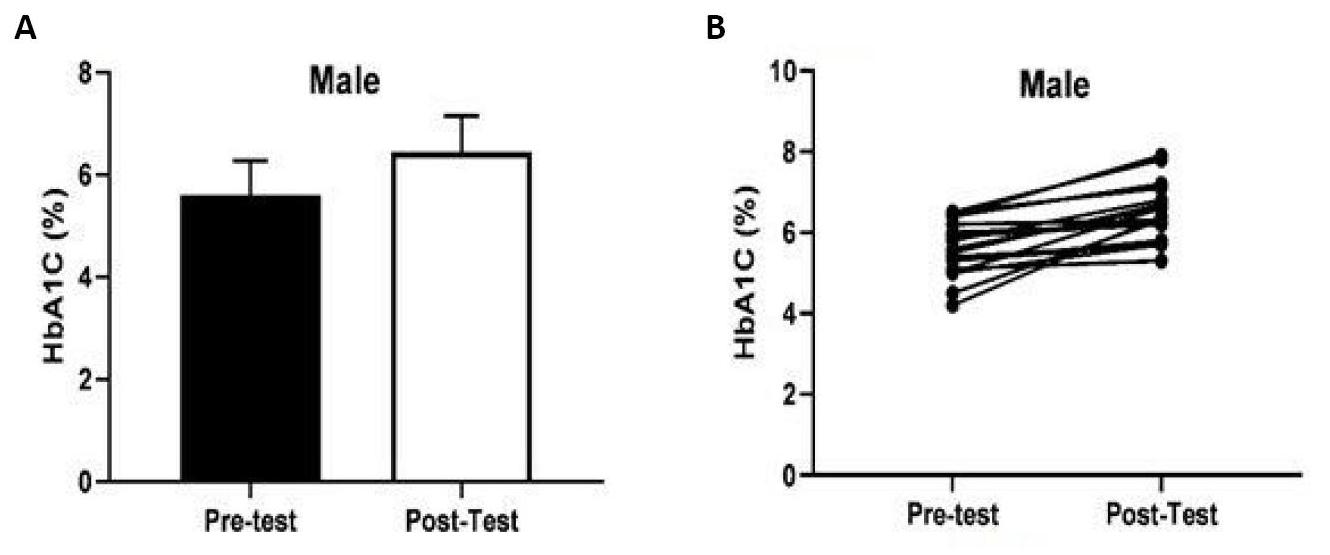
Figure 1A showed the variation on HbA1c value total score (mean±SD) before and after COVID-19 in male participants. Figure 1B showed individual changes in HbA1c values in male participants. It was observed that the total HbA1c values of 19 male participants had significantly increased [t(10)=6.051, P=0.003, ηp2=0.67].
|
Figure 1. Comparison of HbA1c values in male (mean age: 61.00±11.41 years) participants before and after COVID-19.
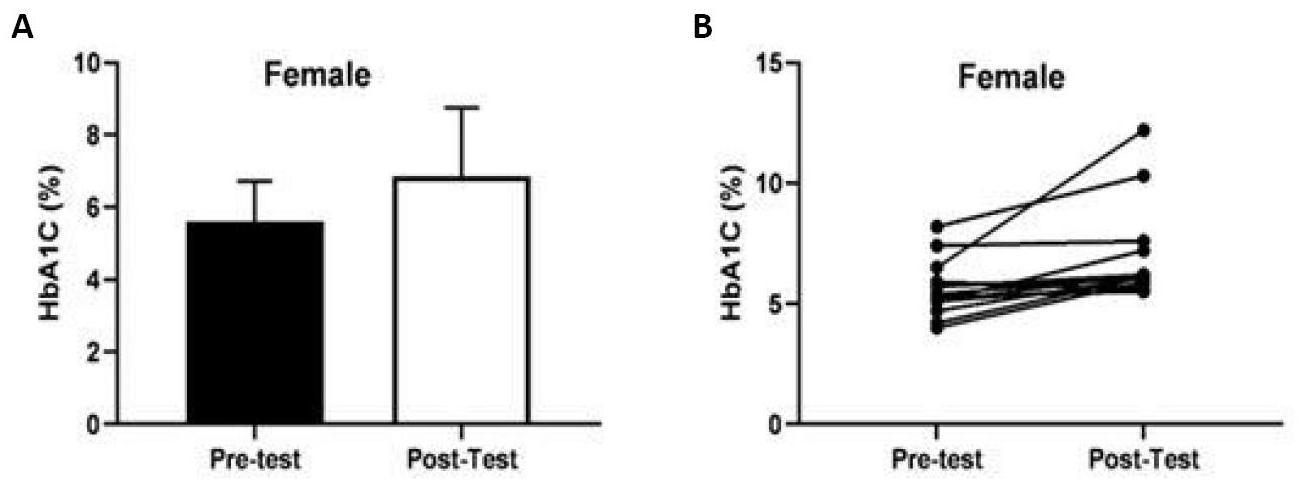
The change in the total score (mean±SD) of the HbA1c value before and after COVID-19 in female participants was shown in Figure 2A, whilet he individual changes in the HbA1c value in female participants were shown in Figure 2B. Compared to before the COVID-19 period, the total HbA1c value of 15 female participants statistically significantly improved after COVID-19 [t(10)=3.331, P=0.004, ηp2=0.44].
|
Figure 2. Comparison of HbA1c values in female participants (mean age: 55.53±10.78 years) before and after COVID-19.
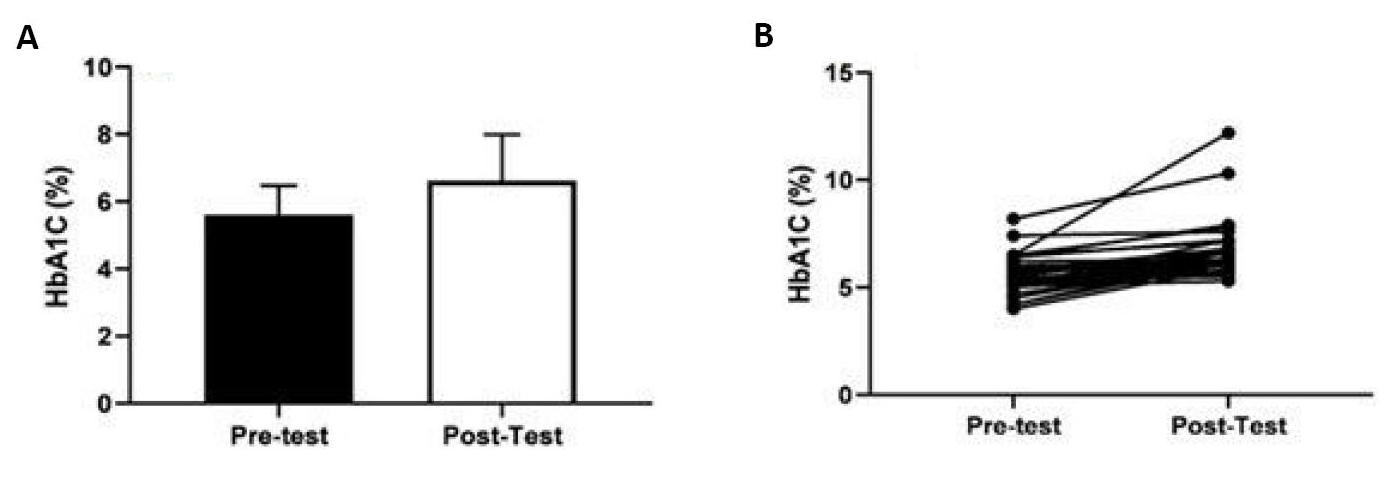
Figure 3A showed the change in the total score (mean±SD) of the HbA1c value before and after COVID-19 and the individual changes in the HbA1c value showed in Figure 3B. According to the figures mentioned above, a statistically significant difference was found in the comparison of the HBA1c (%) value before and after COVID-19, regardless of gender (34 people in total) (t(33)=5.590, P=0.001, ηp2=0.48). Compared to the pre-COVID-19 period, it was observed that the total HbA1c value increased after COVID-19.
|
Figure 3. Comparison of HbA1c value before and after COVID-19 without gender discrimination (mean age: 58.58±11.31 years).
4 DISCUSSION
Our study observed a significant increase in the total HbA1c values of both male and female participants (P<0.05). It has been observed that the total HbA1c value increased after COVID-19 compared to pre-COVID-19, regardless of gender.
HbA1c is a marker formed due to slow and non-enzymatic glycosylation of hemoglobin. It was used to evaluate glycemic control as it reflects the average glucose level during the long term[12]. A study by Yang et al.[10] demonstrated that the coronavirus can damage the pancreas , potentially leading to acute insulin-dependent diabetes mellitus.
Although many studies suggested that diabetes is a significant risk factor for COVID-19, the impact of COVID-19 infection on glycemic parameters, such as blood glucose and HbA1c, remains unclear[13].
To the best of our knowledge, this study is the first to investigate HbA1c levels in adult individuals diagnosed with COVID-19 and without diabetes. According to the outcome data of HbA1c values, our study provides the initial evidence suggesting that COVID-19 may have a positive effect on blood glycemia level.
Similar to our study, many studies investigated a general linear relationship between the increased glucose levels and the severity of COVID-19[14,15]. Some studies also reported that the increase in blood glucose levels (hyperglycemia) might be a cause that increases the body’s susceptibility to pathogenic infection and inflammation in diabetes patients[16].
Hyperglycemia may exacerbated the symptoms of COVID-19, especially in patients without diabetes. In the first studies, an increasing in cytokine levels was detected before inflammation, indicating that this increase may be associated with SARS-CoV-1 infection[17,18].
Furthermore, many studies have reported that hyperglycemia or diabetes was an independent risk factor that worsens the prognosis of the disease and could cause death in patients with many infectious diseases such as SARS and COVID-19[19-24]. Current research showed a high increase in cytokine levels such as IFNγ, IL1β, and IL6 in COVID-19 patients[25,26]. In another study, hyperglycemia was found to elevate levels of inflammatory cytokine and oxidative stress, potentially idsrupting the balance between inflammatory and anti-inflammatory cytokines[27]. Additionally, studies indicated that innate immune responses to infection are altered by acute hyperglycemia, which may partly explain the poor outcomes in COVID-19 patients who develop hyperglycemia[28,29].
4.1 Limitations of the Study
The study had several limitations due to the retrospective scanning of case files and unpredictable pandemic risks. Firstly, because all data were obtained from patient cohorts, HbA1c values were unavailable in all individuals without a history of diabetes before the pandemic. However, when we look at the power analysis of our study, the number we could reach was sufficient. Secondly, the HbA1c values of the patients who were not diagnosed with diabetes but might have prediabetes can be reached. Therefore, we recommend that a study be conducted in larger cohort groups, in which a detailed distinction is made in diabetes and even in diabetes cases are included.
5 CONCLUSION
Based on this study, the positive correlation was identified between COVID-19 patients and HbA1c levels in non-diabetic adults diagnosed with COVID-19 . This suggested that the HbA1c level was elevated in case files who diagnosis of COVID-19. Furthermore, it also raised HbA1c levels and leading to an increasing in glycemia levels.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declared that they have no conflict of interest.
Author Contribution
Ozturk D, Sahin Y, Askin S and Kerget B designed this study. Ozturk D and Sahin Y reviewd literature. The data organization, method determination, and analysis were done by all authors together, and both of them electronically scanned the raw data. Sahin Y and Çelik M reviewed the work, interpreted it, and revised the discussion and the abstract. Askin S and Kerget B edited the raw data through the system and created the open-access dataset. All authors contributed to the manuscript and approved the final version.
Abbreviation List
HbA1c, Hemoglobin A1c
SARS-CoV, Severe acute respiratory syndrome coronavirus
References
[1] Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet, 2020; 395: 1054-1062. [DOI]
[2] Aleem A, Ab AS, Slenker AK. Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). Eur PMC, 2021. Available at: [Web]
[3] Laiteerapong N, Cifu AS. Screening for prediabetes and type 2 diabetes mellitus. JAMA, 2016; 315: 697-698. [DOI]
[4] Katulanda P, Dissanayake HA, Ranathunga I et al. Prevention and management of COVID-19 among patients with diabetes: an appraisal of the literature. Diabetologia, 2020; 63: 1440-1452. [DOI]
[5] Shehav-Zaltzman G, Segal G, Konvalina N et al. Remote Glucose Monitoring of Hospitalized, Quarantined Patients With Diabetes and COVID-19. Diabetes Care, 2020; 43: 75-76. [DOI]
[6] Zhang Y, Li H, Zhang J et al. The clinical characteristics and outcomes of patients with diabetes and secondary hyperglycaemia with coronavirus disease 2019: A single-centre, retrospective, observational study in Wuhan. Diabetes Obes Metab, 2020; 22: 1443-1454. [DOI]
[7] Zhu L, She Z, Cheng X et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab, 2020; 31: 1068-1077. [DOI]
[8] Zhang N, Yun R, Liu L et al. Association of glycosylated hemoglobin and outcomes in patients with COVID-19 and pre-existing type 2 diabetes: A protocol for systematic review and meta-analysis. Medicine (Baltimore), 2020; 99: 23392. [DOI]
[9] Ding Y, He L, Zhang Q et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol, 2004; 203: 622-630. [DOI]
[10] Yang J, Lin S, Ji X et al. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol, 2010; 47: 193-199. [DOI]
[11] Zhu B, Jin S, Wu L et al. J-shaped association between fasting blood glucose levels and COVID-19 severity in patients without diabetes. Diabetes Res Clin Pract, 2020; 168: 108381. [DOI]
[12] ClinLab Navigator. Hemoglobin A1c. Accessed 18 November 2018. Available at: [Web]
[13] Chen J, Wu C, Wang X et al. The Impact of COVID-19 on Blood Glucose: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne), 2020; 11: 574541. [DOI]
[14] Wang S, Ma P, Zhang S et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia, 2020; 63: 2102-2111. [DOI]
[15] Liu R, Ma Q, Han H et al. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease 2019. Clin Chem Lab Med, 2020; 58: 1121-1124. [DOI]
[16] Hodgson K, Morris J, Bridson T et al. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology, 2015; 144: 171-185. [DOI]
[17] Falzarano D, Wit E, Rasmussen AL et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med, 2013; 19: 1313-1317. [DOI]
[18] Wong CK, Lam CW, Wu AK et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol, 2004; 136: 95-103. [DOI]
[19] Singh AK, Khunti K. Assessment of risk, severity, mortality, glycemic control and antidiabetic agents in patients with diabetes and COVID-19: A narrative review. Diabetes Res Clin Pract, 2020; 165: 108266. [DOI]
[20] Guo W, Li M, Dong Y et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev, 2020; 36: 3319. [DOI]
[21] Singh AK, Gupta R, Ghosh A et al. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr, 2020; 14: 303-310. [DOI]
[22] Sardu C, D'Onofrio N, Balestrieri ML et al. Outcomes in Patients With Hyperglycemia Affected by COVID-19: Can We Do More on Glycemic Control?. Diabetes Care, 2020; 43: 1408-1415. [DOI]
[23] Yang JK, Feng Y, Yuan MY et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med, 2006; 23: 623-628. [DOI]
[24] Chen N, Zhou M, Dong X et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet, 2020; 395: 507-513. [DOI]
[25] Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 2020; 395: 497-506. [DOI]
[26] Esposito K, Nappo F, Marfella R et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation, 2002; 106: 2067-2072. [DOI]
[27] Dasu MR, Devaraj S, Zhao L et al. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008; 57: 3090-3098. [DOI]
[28] Jafar N, Edriss H, Nugent K. The Effect of Short-Term Hyperglycemia on the Innate Immune System. Am J Med Sci, 2016; 351: 201-211. [DOI]
Copyright © 2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.




 Copyright ©
Copyright ©