Pain Adverse Events, Bell's Palsy, and Guillain-Barré Syndrome Following Vaccination
Darrell O Ricke1*
1Molecular BioInsights Inc, Winchester, Massachusetts, USA
*Correspondence to: Darrell O Ricke, PhD, Computational Biologist, Molecular BioInsights Inc, 37 Pilgrim Drive, Winchester, Massachusetts 01890, USA; Email: doricke@molecularbioinsights.com
Abstract
Objective: Some individuals (vaccinees) experience pain related adverse events following vaccinations. The majority of these pain related vaccination reactogenicity adverse events resolve within days. Rare adverse events like Bell’s palsy and Guillain-Barré syndrome (GBS) have been associated with some vaccines. Herein, multiple working hypotheses are examined in the context of available characteristics of vaccinees and onset of these pain related adverse events post vaccination.
Methods: The Vaccine Adverse Event Reporting System database was data mined for pain associated vaccine adverse events data by vaccine, age, gender, dose, and onset post vaccination. Results for vaccines with the highest number of pain related adverse events were compared.
Results: For the pain related adverse events examined, the highest number of adverse events are reported within 1 day, roughly half this number the second day, and roughly a quarter this number by the third day. The day of onset for these pain related adverse events approximates a power of two decay pattern for the first three days. This same pattern is observed for all of the vaccines with the highest number of pain related adverse events. The consistency of these day of onset frequency patterns of examined adverse events following vaccinations for multiple unrelated vaccines enables the exclusion of specific vaccine components and excipients as specifically causative entities.
Conclusion: The observed onset occurrences of examined pain associated adverse events are consistent with likely etiology relationship with innate immune responses to vaccinations for multiple vaccines including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) coronavirus disease 2019, influenza, and additional vaccines. Innate immune responses may be contributing to the initial etiology of Bell’s palsy and GBS post SARS-CoV-2 mRNA and adenoviral vaccinations.
Keywords: vaccines, adverse events, innate immune response, histamine
1 INTRODUCTION
Vaccines are designed to protect vaccinees (vaccinated individuals) against viral and bacterial infectious disease. Some vaccinees experience one or more adverse events post vaccination. Vaccine reactogenicity refers to the subset of adverse events that occur soon after vaccination and are physical manifestations of the inflammatory response to vaccination[1]. Most reactogenicity adverse events resolve within days. Other adverse events have persistent symptoms that may last weeks, months, or longer. The etiology of these adverse events remains unknown.
Pain is a common element in a subset of the adverse events reported post vaccination. Some adverse events like “injection site pain” have obvious causal relationship with injection vaccinations. Other rare adverse events like Bell’s palsy and Guillain-Barré syndrome (GBS) can occur with causality difficult to assess with frequencies close to background occurrence frequencies[2,3]. GBS is a rapid-onset muscle weakness caused by the immune system damaging the peripheral nervous system. GBS has been associated with influenza[4] and coronavirus disease 2019 (COVID-19) vaccinations[5]. GBS has been reported following Moderna[6-10], Pfizer BioNTech BNT162b2[11-19], Oxford AstraZeneca ChAdOx1 day[15,20-34] , Johnson & Johnson/Janssen Ad26.CoV2.S[10,35-39], Sinopharm[33,34], and Sputnik V[34,40] COVID-19 vaccines. Many GBS patients are expected as sporadic cases and should not be considered causal[41]. For Pfizer BioNTech BNT162b2 mRNA vaccine, no increased incidence of GBS in a cohort of 3.9 million recipients was detected[42]. A review of GBS incidence in Vaccine Safety Datalink found an increased incidence following Ad.26.COV.2 but not BNT172b2 or mRNA-1273 vaccines[43]. An excess of GBS cases following AstraZeneca-Oxford ChAdOx1-S vaccination has also been identified[44]. An increased risk for GBS after first dose but not second dose of ChAdOx1 nCov-19 vaccination has been reported[45]. Warnings that rare GBS cases may link to J&J and AstraZeneca vaccines have been issued[46]. One etiology model for GBS following COVID-19 vaccination is autoimmune autoantibodies[5]; but, no serum anti-ganglioside antibodies were found in 15 of 17 patients tested[47]. Nearly all GBS patients after COVID-19 vaccinations also had facial weakness or paralysis[43].
Bell’s palsy is a disease characterized by a rapid and unilateral onset of peripheral paresis (paralysis) of the seventh cranial nerve. Bell’s palsy has been reported as an adverse event following immunization for influenza[48] and COVID-19 CoronaVac (Sinovac Biotech, Hong Kong)[49]. Bell’s palsy cases have also been reported following Moderna mRNA-1273[50-54], Pfizer/BioNTech BNT162b2[55-59], Johnson & Johnson/Janssen Ad26.CoV2.S[60,61] COVID-19 vaccinations. Burrows et al.[55] report a patient with sequential contralateral facial nerve palsies following the first and second doses of Pfizer-BioNTech BNT162b2 COVID-19 vaccine. Other studies do not detect an enrichment signal for Bell’s palsy or facial paralysis with COVID-19 vaccines[2,62]. Some cases of facial paralysis may be caused by reactivation of latent herpes simplex virus (HSV)[63] or varicella zoster virus (VSV) in a mechanism similar to Ramsey Hunt syndrome. An increased risk for Bell’s palsy has been observed for concomitant administration of meningococcal conjugate vaccine with another vaccine[64]. A population based study reported 132 cases in 2.6 million vaccinees and 152 cases in 2.4 million vaccinees after first and second doses for BNT162b2 mRNA COVID-19 vaccine[65]. An excess of 1.112 Bell’s palsy reports per 100,000 people who received 2 doses of BNT162b2 has been estimated[66]. Significantly fewer adverse neurological events were reported following BNT162b2 or mRNA-1273 vaccination compared to Ad26.CoV2.S[67].
The Vaccine Adverse Event Reporting System (VAERS) database tracks reported adverse events following vaccinations for the United States. Herein, VAERS is data mined for reports of pain associated adverse events. Multiple working hypotheses[68] are evaluated for pain related adverse events following vaccination leveraging these VAERS data mining results.
2 MATERIALS AND METHODS
The VAERS database (https://vaers.hhs.gov)[69] was data mined for pain associated vaccine adverse events data by vaccine name or vaccine type, age, gender, dose, and onset post vaccination. The downloaded data includes all VAERS reports from 1990 until May 13, 2022. A Ruby program named vaers_slice.rb[70] was used to tally selected reported vaccine adverse events by vaccine. The vaers_slice.rb program takes as input a list of one or more symptoms and outputs a summary of the yearly VAERS Symptoms, Vax, and Data files from 1990 to 2022. The output from vaers_slice.rb consists of five reports: summaries by vaccine, summaries by age of onset of symptoms, summaries by day of onset of symptoms, and two summaries of additional symptoms reported (selected symptoms and all other symptoms). The VAERS adverse events by vaccine name were extracted for Abdominal pain, Abdominal pain lower, Abdominal pain upper, Arthralgia (pain in joint), Asthenia (abnormal physical weakness or lack of energy), Axillary pain, Back pain, Bell’s palsy, Bone pain, Breast pain, Chest pain, Dysphagia (difficulty or discomfort in swallowing), Ear pain, Eye pain, Facial pain, Facial paralysis, Facial paresis, Guillain-Barre syndrome, Hemiparesis, Hypoaesthesia (partial or total loss of sensation), Injection site pain, Lymph node pain, Lymphadenopathy (enlarged lymph nodes), Musculoskeletal chest pain, Musculoskeletal pain, Musculoskeletal stiffness, Myalgia (muscle pain), Neck pain, Neuralgia, Oropharyngeal pain (mouth and pharynx pain), Pain, Pain in extremity, Pain in jaw, Pain of skin, Paraesthesia (an abnormal sensation, typically tingling or pricking), Renal pain, Spinal pain, and Swelling face were extracted. The VAERS adverse events by vaccine type were extracted for Bell’s palsy, Fatigue, Guillain-Barre syndrome, Headache, Miller Fisher syndrome, and Pyrexia. Microsoft Excel was used create figures.
3 RESULTS
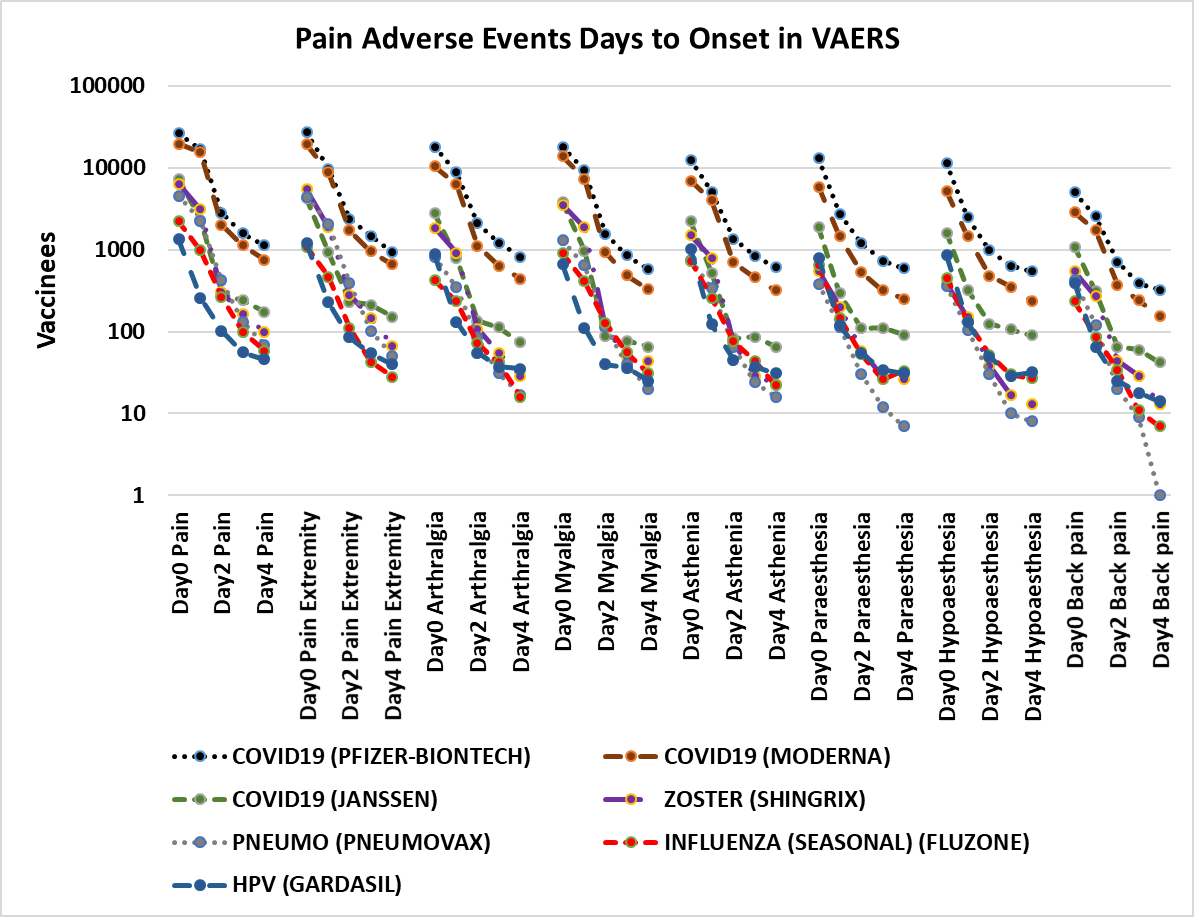
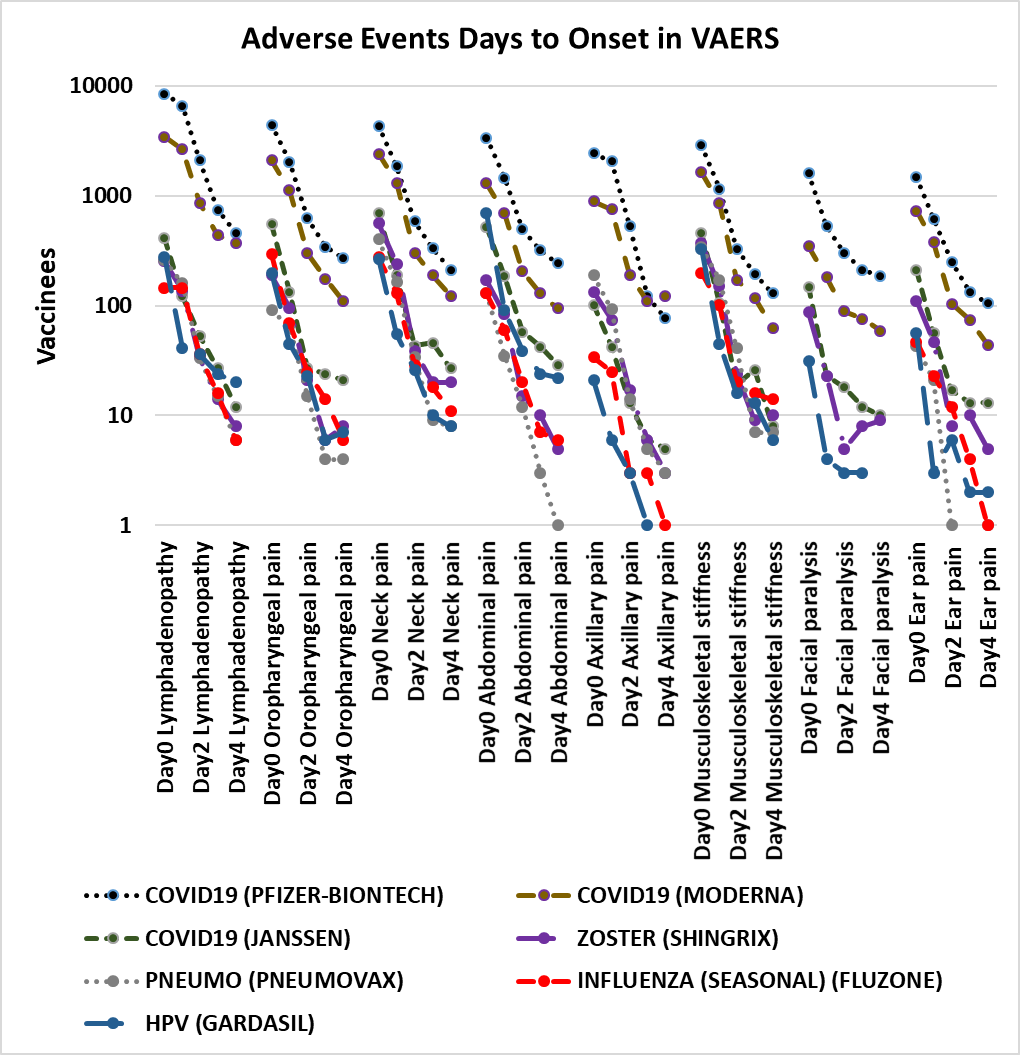
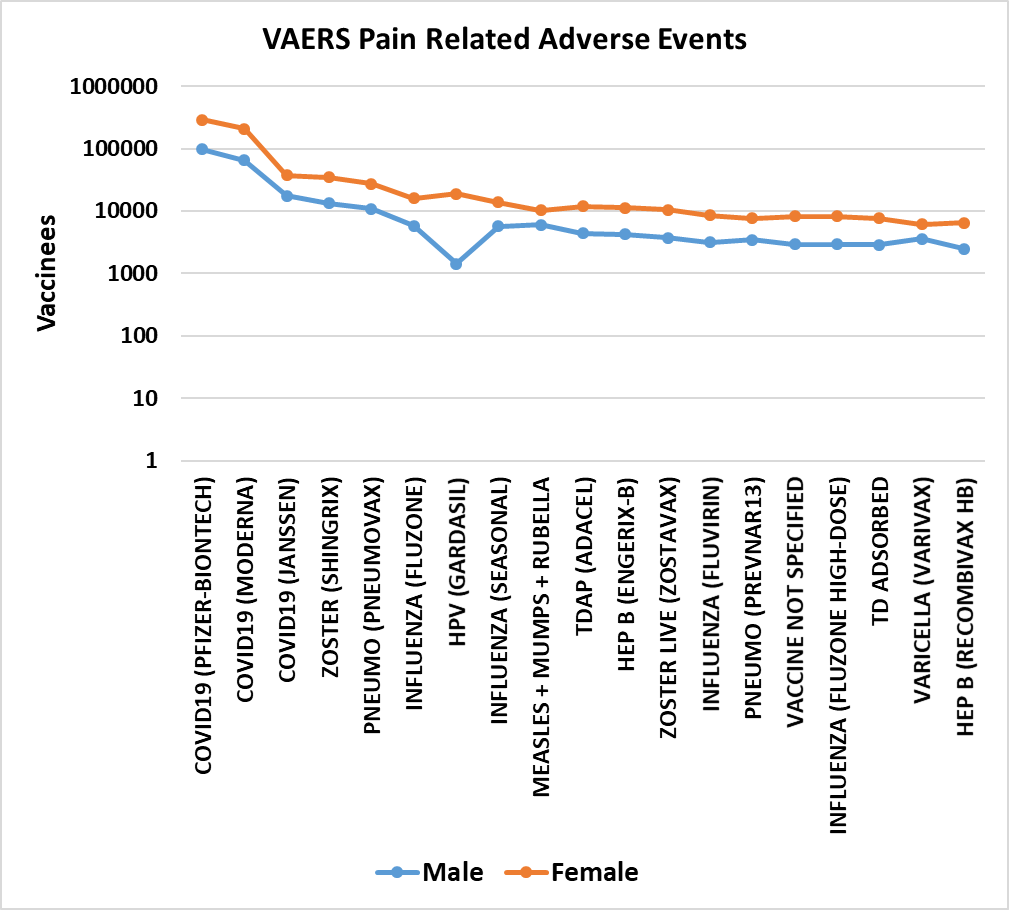
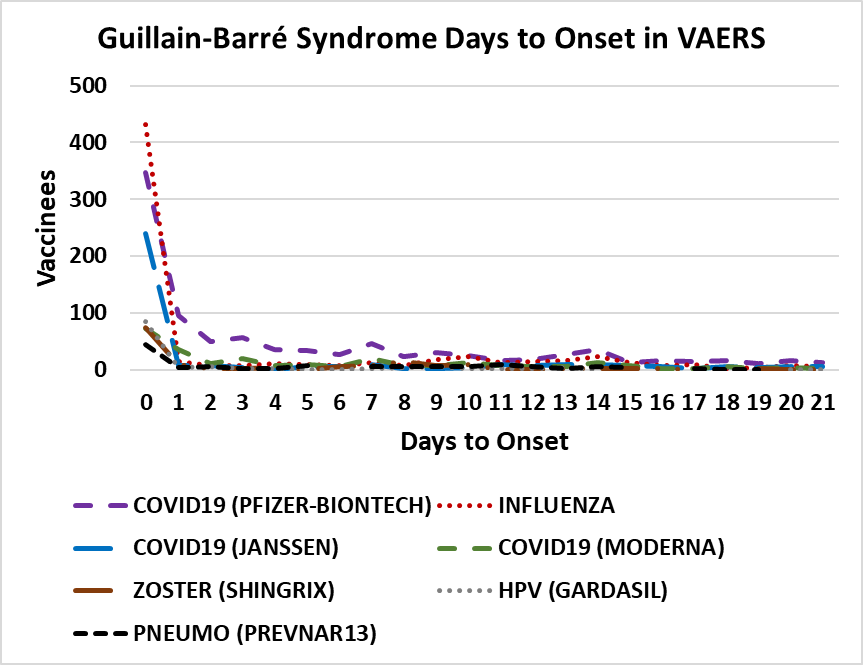
The results include all reports of selected adverse events from 1990 until May 13, 2022. These adverse events share a non-random pattern of onset; Figures 1 and 2 illustrate this onset pattern for 16 pain associated adverse events in VAERS. This onset pattern is also present for GBS and Bell’s palsy (Figures 3 and 4). These adverse events also exhibit excess reports of pain associated adverse events post vaccination for females compared to males for twenty vaccines (Figure 5). Some vaccinees experience more than one adverse event; correlations of reports of multiple pain associated adverse events are summarized in Table 1 for the most frequently reported adverse events for selected pain associated adverse events with all adverse events.
Both GBS and Bell’s palsy are rare adverse events reported post vaccination. The three most commonly reported adverse events for many vaccines are headache, fatigue, and pyrexia (fever). The proportion of GBS and Bell’s palsy reports are compared to these commonly reported adverse events as a comparison metric for unrelated vaccines. Proportional enrichment by vaccine for GBS and Bell’s palsy are calculated for three reactogenicity adverse events (headache, fatigue, and pyrexia/fever) in Tables 2 and 3.
|
Figure 1. Pain adverse events days to onset in VAERS[69]. Vaccines plotted include COVID-19 (Pfizer-BioNTech, Moderna, and Janssen), Influenza (FLUZONE), Shingles Zoster (SHINGRIX), Human papillomavirus HPV (GARDASIL), and Pneumococcal PNEUMO (PREVNAR13) for adverse events pain, pain in extremity, arthralgia (joint pain), myalgia (muscle pain), asthenia (weakness), paraesthesia (tingling sensation), and back pain.
|
Figure 2. Additional pain adverse events days to onset in VAERS[69]. Vaccines plotted include COVID-19 (Pfizer-BioNTech, Moderna, and Janssen), Influenza (seasonal) (FLUZONE), Shingles Zoster (SHINGRIX), Human papillomavirus HPV (GARDASIL), and Pneumococcal PNEUMO (PNEUMOVAX) for adverse events abdominal pain, axillary pain, ear pain, facial paralysis, lymphadenopathy, musculoskeletal stiffness, neck pain, and oropharyngeal pain (mouth and pharynx pain).
|
Figure 3. GBS days to onset in VAERS[69]. Vaccines plotted include COVID-19 (Pfizer-BioNTech, Moderna, and Janssen), Influenza, Shingles Zoster (SHINGRIX), Human papillomavirus HPV (GARDASIL), and Pneumococcal PNEUMO (PREVNAR13).
|
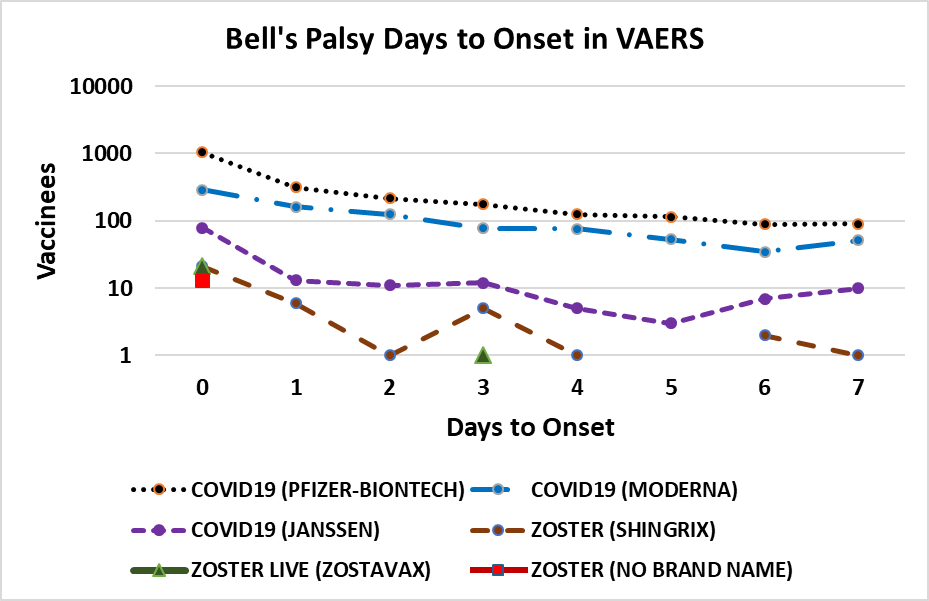
Figure 4. Bell’s palsy days to onset in VAERS[69]. Vaccines plotted include COVID-19 (Pfizer-BioNTech, Moderna, and Janssen) and Shingles Zoster (SHINGRIX, ZOSTAVAX, and no brand name).
|
Figure 5. Pain adverse events by gender in VAERS[69] from 1990 to May 13, 2022.
Table 1. Co-occurrences of Highest Frequency Vaccine Associated Pain Adverse Events from VAERS[69] (1990 to May 13, 2022)
Adverse Event |
Arthralgia |
Asthenia |
Hypoaesthesia |
Myalgia |
Pain |
Pain in Extremity |
Paraesthesia |
Arthralgia |
|
8,315 |
4,195 |
26,645 |
19,818 |
18,744 |
4,982 |
Asthenia |
8,315 |
|
3,895 |
10,336 |
13,827 |
7,990 |
4,674 |
Hypoaesthesia |
4,195 |
3,895 |
|
2,731 |
6,556 |
8,138 |
16,237 |
Myalgia |
26,645 |
10,336 |
2,731 |
|
14,898 |
12,787 |
3,975 |
Pain |
19,818 |
13,827 |
6,556 |
14,898 |
|
28,608 |
7,683 |
Pain in extremity |
18,744 |
7,990 |
8,138 |
12,787 |
28,608 |
|
8,002 |
Paraesthesia |
4,982 |
4,674 |
16,237 |
3,975 |
7,683 |
8,002 |
|
Table 2. Proportional GBS Compared to Reactogenicity Adverse Events Headache, Fatigue, and Pyrexia (Fever); the Proportions were Normalized to Highest Observed Proportion (e.g., FLUX).
Vaccine |
Headache |
Guillain-Barré Syndrome |
Normalized Proportion |
Fatigue |
Normalized proportion |
Pyrexia |
Normalized Proportion |
FLUX |
2,970 |
779 |
92.1% |
1,797 |
100.0% |
5,249 |
100.0% |
FLU3 |
7,118 |
1,386 |
68.4% |
3,576 |
89.4% |
12,757 |
73.2% |
FLU (H1N1) |
966 |
139 |
50.5% |
458 |
70.0% |
1,065 |
87.9% |
FLUX (H1N1) |
421 |
65 |
54.2% |
228 |
65.8% |
581 |
75.4% |
HEPAB |
537 |
84 |
54.9% |
424 |
45.7% |
636 |
89.0% |
FLUC4 |
343 |
48 |
49.1% |
271 |
40.9% |
359 |
90.1% |
HPV2 |
899 |
61 |
23.8% |
394 |
35.7% |
513 |
80.1% |
HIBV |
165 |
47 |
100.0% |
342 |
31.7% |
18,576 |
1.7% |
FLUN3 |
570 |
58 |
35.7% |
245 |
54.6% |
993 |
39.4% |
FLU4 |
2,716 |
297 |
38.4% |
1,794 |
38.2% |
3,956 |
50.6% |
YF |
640 |
50 |
27.4% |
284 |
40.6% |
871 |
38.7% |
PNC13 |
872 |
137 |
55.2% |
1,079 |
29.3% |
7,916 |
11.7% |
IPV |
679 |
81 |
41.9% |
474 |
39.4% |
8,900 |
6.1% |
TYP |
1,266 |
77 |
21.4% |
551 |
32.2% |
1,592 |
32.6% |
TDAP |
4,194 |
267 |
22.3% |
2,178 |
28.3% |
5,935 |
30.3% |
TD |
1,595 |
94 |
20.7% |
518 |
41.9% |
3,596 |
17.6% |
MNQ |
2,802 |
132 |
16.5% |
1,007 |
30.2% |
2,957 |
30.1% |
HEPA |
1,764 |
131 |
26.1% |
1,092 |
27.7% |
5,691 |
15.5% |
HPV4 |
5,049 |
154 |
10.7% |
3,024 |
11.7% |
2,681 |
38.7% |
HEP |
4,351 |
219 |
17.7% |
1,805 |
28.0% |
11,117 |
13.3% |
MMR |
2,336 |
133 |
20.0% |
1,185 |
25.9% |
21,749 |
4.1% |
PPV |
3,543 |
199 |
19.7% |
2,215 |
20.7% |
14,372 |
9.3% |
DTAP |
776 |
52 |
23.5% |
687 |
17.5% |
12,832 |
2.7% |
UNK |
2,750 |
78 |
10.0% |
2,356 |
7.6% |
3,450 |
15.2% |
VARCEL |
1,562 |
55 |
12.4% |
779 |
16.3% |
11,961 |
3.1% |
VARZOS |
12,418 |
270 |
7.6% |
9,752 |
6.4% |
14,461 |
12.6% |
COVID-19 |
182,521 |
2,001 |
3.8% |
154,437 |
3.0% |
153,429 |
8.8% |
Notes: The following vaccines with at least 40 reports of GBS in VAERS[69] were included: COVID-19, DTAP (diphtheria, pertussis, & tetanus), Influenza: FLU(H1N1), FLU3 (trivalent), FLU4 (quadivalent), FLUC4 (Flucelvax quadrivalent), FLUN3 (Flumist), FLUX (Influenza (seasonal) unknown manufacturer), FLUX(H1N1), HEP (hepatitis B), HEPA (hepatitis A), HEPAB (hepatitis B), HIBV (haemophilus), HPV2 (human papillomavirus), HPV4 (human papillomavirus type 4), IPV (inactivated poliovirus), MMR (measles, mumps, & rubella), MNQ (Menigococcal), PNC13 (Pneumococcal conjugate), PPV (Pneumococcal polysaccharide), TD (tetanus & diphtheria), TDAP (diphtheria, pertussis, & tetanus), TYP (typhoid), UNK (unknown), VARCEL (chickenpox Varicella), VARZOS (Herpes Zoster), and YF (yellow fever). Enrichment was normalized to the vaccine (FLUX) with the highest ratio of adverse events: GBS/reactogenicity adverse event for headache, fatigue, and pyrexia.
Table 3. Proportional Bell’s Palsy Compared to Reactogenicity Adverse Events Headache, Fatigue, and Pyrexia (Fever); the Proportions were Normalized to the Highest Observed Proportion (e.g., COVID-19).
Vaccine |
Headache |
Bell’s Palsy |
Enrichment |
Fatigue |
Enrichment |
Pyrexia |
Enrichment |
COVID-19 |
182,521 |
5,711 |
100.0% |
154,437 |
100.0% |
153,429 |
100.0% |
UNK |
2,750 |
49 |
56.9% |
2,356 |
56.2% |
3,450 |
38.2% |
FLU4 |
2,716 |
40 |
47.1% |
1,794 |
60.3% |
3,956 |
27.2% |
VARZOS |
12,418 |
94 |
24.2% |
9,752 |
26.1% |
14,461 |
17.5% |
Notes: The following vaccines with at least 40 reports of Bell’s palsy were included: COVID-19, FLU4 (influenza quadivalent), UNK (unknown), and VARZOS (Herpes Zoster). Enrichment was normalized to the vaccine (COVID-19) with the highest ratio of adverse events: Bell’s palsy/reactogenicity adverse event for headache, fatigue, and pyrexia.
4 DISCUSSION
For all of the pain associated adverse events examined, the highest reports are within 24h of vaccination (day 0). For each pain associated adverse event, the number of reports for day 1 are roughly half that of day 0; likewise, the number of adverse events reported for day 2 are roughly half that of day 1 (Figures 1 and 2). Females report pain associated adverse events between two and three fold more frequently than males (Figure 5). Vaccinees sometimes report more than one pain associated adverse event (Table 1). For adverse events like injection site pain, this is consistent with expectations. Other adverse events reported by vaccinees are nausea, headache, pyrexia, fatigue, chills, and other. The consistency of the frequency patterns of these adverse events following vaccinations for multiple unrelated vaccines enables the exclusion of specific vaccine components and excipients as specifically causative entities; however, these components and excipients are likely the key determinants of the reactogenicity level associated with each vaccine. Possible working hypotheses of the causes of pain, paresis, or paralysis related adverse events following vaccination include innate immune responses, inflammation, latent virus reactivation, and autoimmune antibodies.
Vaccinations are designed to stimulate immune humoral (e.g., antibody) immune responses. Vaccines elicit immediate innate immune responses from vaccinees. These innate immune responses include the release of inflammatory molecules including chemokines, cytokines, interleukins, lymphokines, and monokines from immune cells[71-74]. The blood-nerve barrier is not as tight as the blood-brain barrier; it is possible for T cells and macrophages to leak in at inflamed tissue[75]. Vaccination-induced autoimmune antibody responses would require either primary humoral immune response or memory humoral immune responses; these humoral immune responses would peak roughly 7 to 10 days post vaccination. Hence, autoimmune antibody responses are unlikely associated with the majority of observed immediate onset reactogenicity adverse responses observed (Figures 1 and 2). Miller Fisher syndrome has some presentation overlaps with GBS[76]; like other examined adverse events, immediate onset signals also occur for Miller Fisher syndrome adverse events in VAERS associated with COVID-19 and influenza vaccines. Reactivation of latent viruses has been observed post Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) vaccinations[77,78]; clinical and molecular evidence of reactivation of latent viruses associated with the majority of the reported pain associated adverse events is currently lacking. While reactivation of latent viruses has occurred post vaccinations, the onset timing of 7 to 21 days[77,78] is inconsistent with observed immediate onset of pain associated adverse events. Consistent with the observed immediate onset of reported pain associated adverse events, innate immune response molecules are known to be associated with pain. These innate immune responses include the release of inflammatory molecules, including histamine, interleukin 1β (IL-1β), interleukin 6 (IL-6), monocyte chemoattractant protein, prostaglandin E2 (PGE2), tumor necrosis factor (TNF; formerly TNFα), etc.; these innate immune cells include macrophages, granulocytes including mast cells, T helper cells, and other immune cells[71,72,79,80]. PGE2 is a well-known lipid mediator that contributes to inflammatory, neuropathic, and visceral pain, see[80]. IL-1β, IL-6, and TNF are involved in the process of pathological pain[72]. Histamine is known to be algesic (cause pain) to peripheral nervous system[74]. Type I interferons have been proposed as a potential mechanism linking COVID-19 mRNA vaccines to Bell’s palsy[81].
4.1 GBS
VAERS reports for GBS illustrate a pattern of immediate onset timing associated with seven vaccines (Figure 3). The onset for the majority of the GBS reports are within 24h (day 0), roughly ½ this the next day (day 1), and roughly ¼ this the second day (Figure 3). This onset pattern is too rapid for molecular mimicry, epitope sharing, and autoimmune antibodies to be causative prior to day 7. Similar patterns shared by COVID-19, Influenza, Shingles Zoster, human papillomavirus, and Pneumococcal vaccines support innate immune responses as a major component of disease early etiology. Three of the highest frequencies reactogenicity adverse events shared across the examined pain related adverse events are headache, fatigue, and pyrexia (fever). Examining the frequencies of GBS in proportion to these reactogenicity adverse events illustrates that the frequency of GBS is highest for Influenza vaccines with a lower frequency for COVID-19 vaccines (Table 2). The general consistency of occurrence frequencies across all of the examined unrelated vaccines in Table 2 further supports the hypothesis that reactogenicity responses to vaccination in general are coupled to the frequency of GBS following vaccinations. Clinically, most GBS patients following COVID-19 vaccination showed typical demyelination neuropathy with albumin-cytological dissociation[82]; the timing suggests that demyelination neuropathy and albumin-cytological dissociation might be subsequent events in the disease etiology for patients with immediate onset adverse events. The immediate onset pattern of GBS following vaccination is different from the observed pattern for Zoster vaccines[83]; their reported Zoster vaccine onset pattern is consistent with the development of autoimmune antibodies in contrast to the immediate onset Zoster vaccine records in VAERS (Figure 3). Note that autoantibodies are detected for some GBS patients post COVID-19 vaccination[14,84]; onset of GBS for multiple patients is consistent with the development of autoantibodies[9,13-15,19,22-31].
In one report, nearly all GBS patients after COVID-19 vaccinations also had facial weakness or paralysis[43]. Another report included nine GBS patients with rare subtype known as Bilateral Facial Palsy with paresthesias (BFP) with five vaccinated with Sputnick V and four with ChAdOx1[40]. Of these nine patients, four tested positive with with ganglioside antibody panel (2: anti-GM1, antig-GD1a, and anti-sulfatide)[40].
4.2 Bell’s Palsy
The frequency of Bell’s palsy is highest for COVID-19 and lower for Zoster and Influenza vaccines (Table 3 and Figure 4). The frequencies for non-COVID-19 vaccines is low for vaccines but with enrichment for day 0 onsets for a few vaccines. Onset of Bell’s palsy within 5h of BNT162b2 vaccination[55] and 12h after mRNA-1273 vaccination[51] together with VAERS day 0 onset reports can be leveraged to limit candidate etiology possibilities. The association pattern for immediate onset is consistent with innate immune responses for very high reactogenicity vaccines (COVID-19 mRNA and adenovirus) or concomitant administration of vaccines. The working hypothesis for live Zoster vaccines reactivating latent Herpes family viruses is also consistent with current models for Bell’s palsy[72].
4.3 Persistent Pain Models
Candidate models for persistent pain include autoimmune antibodies, nerve damage and/or demyelination, reactivated latent viruses, immune cells infiltration at blood-never barrier during inflammation (albumin-cytological dissociation seen in GBS), innate immune cells with feedback loops with nerve cells, mast cell and eosinophil paired couplets, and ongoing expression of vaccine protein[85] by innate immune cells. Immediate onset adverse event lymphadenopathy (Figure 2) is consistent with ongoing expression of vaccine protein by innate immune cells. Mast cells and eosinophils are known to form bidirectional interactions resulting in a hyperactivated state, reviewed[86]. Additional research is needed to resolve the pathogenesis model(s) of persistent pain adverse events following vaccinations. Immediate onset of pain related adverse events might suggest that early interventions might lessen the severity of symptoms and possibly even decrease the frequencies of occurrences. Cellular feedback loops are possible between nerve cells and mast cells driving neurogenic inflammation and nociceptive pain[87].
4.4 Histamine
Pain related inflammatory molecules released by innate immune responses include histamine. Histamine is known to be associated with peripheral nerve pain[74,88]. Elevated histamine levels are predicted as drivers of cardiac adverse events including myocarditis and pericarditis[70] and menstrual adverse events[89]. Ongoing vaccine expression in innate immune cells, lasting months[85], may drive localized release of inflammatory molecules including histamine.
4.5 Exploratory Treatment Candidates
Dampening histamine responses from innate immune mast cells may reduce the population frequency and severity of some pain adverse events following vaccinations. Antihistamine treatments exhibiting efficacy in treating COVID-19 patients may target possible granulocytes and mast cells associated with vaccine responses. Candidate treatments for evaluation include high dose famotidine[90-92], cetirizine[93,94], and dexchlorpheniramine[93]. Oral treatment with diamine oxidase may also be beneficial. Alternatively, if mast cell and eosinophil couplets are involved, targeting them with anti-IL-5 (mepolizumab)[95] may be beneficial. Evaluation of these treatments and treatment combinations on vaccinees in case reports, case series, etc. can inform subsequent randomized controlled clinical trials for reducing vaccine pain adverse events.
5 CONCLUSION
Data mining VAERS for pain associated adverse events illustrates likely etiology of innate immune responses driving pain related adverse events post vaccination including rare reports of GBS and Bell’s palsy. The consistency of the frequency patterns of examined adverse events following vaccinations for multiple unrelated vaccines enables the exclusion of specific vaccine components and excipients as specifically causative entities. Identification of likely role of innate immune responses in the etiology of pain related adverse events post vaccination suggest possible candidate treatments for evaluation in clinical studies. Innate immune responses may be contributing to the initial etiology of rare cases of GBS and Bell’s palsy post SARS-CoV-2 mRNA and adenoviral vaccinations.
Acknowledgements
The authors thank Nora Smith for useful discussions.
Conflicts of Interest
The author declared no conflict of interest.
Author Contribution
The author contributed to the manuscript and approved the final version.
Supplementary Data and Availability of Data
Data files summarizing the pain related adverse events summarized from VAERS are available from the Corresponding Author (Dr. Darrell O. Ricke) upon a reasonable request whenever possible.
Abbreviation List
COVID-19, Coronavirus disease 2019
IL-1β, Interleukin 1β
IL-6, Interleukin 6
PGE2, Prostaglandin E2
SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
TNF, Tumor necrosis factor
VAERS, Vaccine Adverse Event Reporting System
[1] Hervé C, Laupèze B, Del Giudice G et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines, 2019; 4: 39. DOI: 10.1038/s41541-019-0132-6
[2] Renoud L, Khouri C, Revol B et al. Association of facial paralysis with mRNA COVID-19 vaccines: A disproportionality analysis using the world health organization pharmacovigilance database. JAMA Intern Med, 2021; 181: 1243-1245. DOI: 10.1001/jamainternmed.2021.2219
[3] Shemer A, Pras E, Einan-Lifshitz A et al. Association of COVID-19 vaccination and facial nerve palsy: A case-control study. JAMA Otolaryngol, 2021; 147: 739-743. DOI: 10.1001/jamaoto.2021.1259
[4] Galeotti F, Massari M, D’Alessandro R et al. Risk of Guillain-Barré syndrome after 2010-2011 influenza vaccination. Eur J Epidemiol, 2013; 28: 433-444. DOI: 10.1007/s10654-013-9797-8
[5] Khan Z, Ahmad U, Ualiyeva D et al. Guillain-Barre syndrome: An autoimmune disorder post-COVID-19 vaccination? Clinical Immunology Communications, 2022; 2: 1-5. DOI: 10.1016/j.clicom.2021.12.002
[6] Dalwadi V, Hancock D, Ballout AA et al. Axonal-Variant Guillian-Barre syndrome temporally associated with mRNA-based moderna SARS-CoV-2 vaccine. Cureus, 2021; 13: e18291. DOI: 10.7759/cureus.18291
[7] George TB, Kainat A, Pachika PS et al. Rare occurrence of Guillain-Barré syndrome after Moderna vaccine. BMJ Case Rep, 2022; 15: e249749. DOI: 10.1136/bcr-2022-249749
[8] Matarneh AS, Al-battah AH, Farooqui K et al. COVID-19 vaccine causing Guillain-Barre syndrome, a rare potential side effect. Clin Case Rep, 2021; 9: e04756. DOI: 10.1002/ccr3.4756
[9] Masuccio FG, Comi C, Solaro C. Guillain-Barrè syndrome following COVID-19 vaccine mRNA-1273: a case report. Acta Neurol Belg, 2021. DOI: 10.1007/s13760-021-01838-4
[10] Sriwastava S, Shrestha AK, Khalid SH et al. Spectrum of neuroimaging findings in post-COVID-19 vaccination: A case series and review of literature. Neurol Int, 2021; 13: 622-639. DOI: 10.3390/neurolint13040061
[11] Hughes DL, Brunn JA, Jacobs J et al. Guillain-Barré syndrome after COVID-19 mRNA vaccination in a liver transplantation recipient with favorable treatment response. Liver Transpl, 2022; 28: 134-137. DOI: 10.1002/lt.26279
[12] Aomar-Millán IF, Martínez de Victoria-Carazo J, Peregrina-Rivas JA et al. COVID-19, Guillain-Barré syndrome, and the vaccine. A dangerous combination. Rev Clín Esp (Engl Ed), 2021; 221: 555-557. DOI: 10.1016/j.rceng.2021.05.002
[13] Bouattour N, Hdiji O, Sakka S et al. Guillain-Barré syndrome following the first dose of Pfizer-BioNTech COVID-19 vaccine: case report and review of reported cases. Neurol Sci, 2022; 43: 755-761. DOI: 10.1007/s10072-021-05733-x
[14] Fukushima T, Tomita M, Ikeda S et al. A case of sensory ataxic Guillain-Barré syndrome with immunoglobulin G anti-GM1 antibodies following the first dose of mRNA COVID-19 vaccine BNT162b2 (Pfizer). QJM-Int J Med, 2022; 115: 25-27. DOI: 10.1093/qjmed/hcab296
[15] Kim JW, Kim YG, Park YC et al. Guillain-Barre syndrome after two COVID-19 vaccinations: Two case reports with follow-up electrodiagnostic study. J Korean Med Sci, 2022; 37: e38. DOI: 10.3346/jkms.2022.37.e58
[16] Kim Y, Zhu Z, Kochar P et al. A pediatric case of sensory predominant Guillain-Barré syndrome following COVID-19 vaccination. Child Neurol Open, 2022; 9: 2329048X221074549. DOI: 10.1177/2329048X221074549
[17] Yamada S, Yamada K, Nishida H. A case of sequential development of polymyalgia rheumatica and Guillain-Barré syndrome following administration of the Pfizer-BioNTech COVID-19 Vaccine. Intern Med, 2022; 0319-22. DOI: 10.2169/internalmedicine.0319-22
[18] Trimboli M, Zoleo P, Arabia G et al. Guillain-Barré syndrome following BNT162b2 COVID-19 vaccine. Neurol Sci, 2021; 42: 4401-4402. DOI: 10.1007/s10072-021-05523-5
[19] Malamud E, Otallah SI, Caress JB et al. Guillain-Barré syndrome after COVID-19 vaccination in an adolescent. Pediatr Neurol, 2022; 126: 9-10. DOI: 10.1016/j.pediatrneurol.2021.10.003
[20] Ogbebor O, Seth H, Min Z et al. Guillain-Barré syndrome following the first dose of SARS-CoV-2 vaccine: A temporal occurrence, not a causal association. IDCases, 2021; 24: e01143. DOI: 10.1016/j.idcr.2021.e01143
[21] Min YG, Ju W, Ha YE et al. Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine: Report of two cases and review of literature. J Neuroimmunol, 2021; 359: 577691. DOI: 10.1016/j.jneuroim.2021.577691
[22] Biswas A, Pandey SK, Kumar D et al. Post coronavirus disease-2019 vaccination Guillain-Barré syndrome. Indian J Public Health, 2021; 65: 422-424. DOI: 10.4103/ijph.ijph_1716_21
[23] Aldeeb M, Okar L, Mahmud SS et al. Could Guillain-Barré syndrome be triggered by COVID-19 vaccination? Clin Case Rep, 2022; 10: e05237. DOI: 10.1002/ccr3.5237
[24] Kanabar G, Wilkinson P. Guillain-Barré syndrome presenting with facial diplegia following COVID-19 vaccination in two patients. BMJ Case Rep, 2021; 14: e244527. DOI: 10.1136/bcr-2021-244527
[25] Introna A, Caputo F, Santoro C et al. Guillain-Barré syndrome after AstraZeneca COVID-19-vaccination: A causal or casual association? Clin Neurol Neurosurg, 2021; 208: 106887. DOI: 10.1016/j.clineuro.2021.106887
[26] McKean N, Chircop C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep, 2021; 14: e244125. DOI: 10.1136/bcr-2021-244125
[27] Kripalani Y, Lakkappan V, Parulekar L et al. A rare case of Guillain-Barré syndrome following COVID-19 vaccination. EJCRIM, 2021; 8: 9. DOI: 10.12890/2021_002797
[28] Hasan T, Khan M, Khan F et al. Case of Guillain-Barré syndrome following COVID-19 vaccine. BMJ Case Rep, 2021; 14: e243629. DOI: 10.1136/bcr-2021-243629
[29] James J, Jose J, Gafoor VA et al. Guillain-Barré syndrome following ChAdOx1 nCoV-19 COVID-19 vaccination: A case series. Neurol Clin Neurosci, 2021; 9: 402-405. DOI: 10.1111/ncn3.12537
[30] da Silva GF, da Silva CF, Oliveira REN da N et al. Guillain-Barré syndrome after coronavirus disease 2019 vaccine: A temporal association. Clin Exp Neuroimmunol, 2022; 13: 92-94. DOI: 10.1111/cen3.12678
[31] Patel SU, Khurram R, Lakhani A et al. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep, 2021; 14: e242956. DOI: 10.1136/bcr-2021-242956
[32] Allen CM, Ramsamy S, Tarr AW et al. Guillain-Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol, 2021; 90: 315-318. DOI: 10.1002/ana.26144
[33] Tabatabaee S, Rezania F, Alwedaie SMJ et al. Post COVID-19 vaccination Guillain-Barre syndrome: three cases. Hum Vacc Immunother, 2022; 18: 2045153. DOI: 10.1080/21645515.2022.2045153
[34] Karimi N, Boostani R, Fatehi F et al. Guillain-Barre syndrome and COVID-19 vaccine: A report of nine patients. BCN, 2021; 12: 703-710. DOI: 10.32598/bcn.2021.3565.1
[35] Thant HL, Morgan R, Paese MM et al. Guillain-Barré syndrome after Ad26.COV2.S vaccination. Am J Case Rep, 2022; 23: e935275. DOI: 10.12659/AJCR.935275
[36] Zubair AS, Bae JY, Desai K. Facial diplegia variant of Guillain-Barré syndrome in pregnancy following COVID-19 vaccination: A case report. Cureus, 2022; 14: e22341. DOI: 10.7759/cureus.22341
[37] Rossetti A, Gheihman G, O’Hare M et al. Guillain-Barré syndrome presenting as facial diplegia after COVID-19 vaccination: A case report. J Emerg Med, 2021; 61: e141-e145. DOI: 10.1016/j.jemermed.2021.07.062
[38] Prasad A, Hurlburt G, Podury S et al. A novel case of bifacial diplegia variant of Guillain-Barré syndrome following Janssen COVID-19 vaccination. Neurol Int, 2021; 13: 404-409. DOI: 10.3390/neurolint13030040
[39] Stefanou MI, Karachaliou E, Chondrogianni M et al. Guillain-Barré syndrome and fulminant encephalomyelitis following Ad26.COV2.S vaccination: double jeopardy. Neurol Res Pract, 2022; 4: 6. DOI: 10.1186/s42466-022-00172-1
[40] Castiglione JI, Crespo JM, Lecchini L et al. Bilateral facial palsy with paresthesias, variant of Guillain-Barré syndrome following COVID-19 vaccine: A case series of 9 patients. Neuromuscul Disord, 2022; 32: 572-574. DOI: 10.1016/j.nmd.2022.05.003
[41] Lunn MP, Cornblath DR, Jacobs BC et al. COVID-19 vaccine and Guillain-Barré syndrome: Let’s not leap to associations. Brain, 2021; 144: 357-360. DOI: 10.1093/brain/awaa444
[42] García-Grimshaw M, Michel-Chávez A, Vera-Zertuche JM et al. Guillain-Barré syndrome is infrequent among recipients of the BNT162b2 mRNA COVID-19 vaccine. Clin Immunol, 2021; 230: 108818. DOI: 10.1016/j.clim.2021.108818
[43] Hanson KE, Goddard K, Lewis N et al. Incidence of Guillain-Barré syndrome after COVID-19 vaccination in the vaccine safety datalink. JAMA Netw Open, 2022; 5: e228879. DOI: 10.1001/jamanetworkopen.2022.8879
[44] Osowicki J, Morgan H, Harris A et al. Guillain-Barré syndrome in an australian state using both mRNA and Adenovirus-Vector SARS-CoV-2 vaccines. Ann Neurol, 2021; 90: 856-858. DOI: 10.1002/ana.26218
[45] Keh RYS, Scanlon S, Datta-Nemdharry P et al. COVID-19 vaccination and Guillain-Barré syndrome: analyses using the National Immunoglobulin Database. Brain, 2022; awac067. DOI: 10.1093/brain/awac067
[46] Dyer O. COVID-19: Regulators warn that rare Guillain-Barré cases may link to J&J and AstraZeneca vaccines. BMJ, 2021; 374: n1786. DOI: 10.1136/bmj.n1786
[47] Caress JB, Castoro RJ, Simmons Z et al. COVID-19-associated Guillain-Barré syndrome: The early pandemic experience. Muscle Nerve, 2020; 62: 485-491. DOI: 10.1002/mus.27024
[48] Po ALW. Non-parenteral vaccines. BMJ, 2004; 329: 62. DOI: 10.1136/bmj.329.7457.62
[49] Wan EYF, Chui CSL, Lai FTT et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis, 2022; 22: 64-72. DOI: 10.1016/S1473-3099(21)00451-5
[50] Poudel S, Nepali P, Baniya S et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg, 2022; 78: 103897. DOI: 10.1016/j.amsu.2022.103897
[51] Cellina M, D’Arrigo A, Floridi C et al. Left Bell’s palsy following the first dose of mRNA-1273 SARS-CoV-2 vaccine: A case report. Clin Imag, 2022; 82: 1-4. DOI: 10.1016/j.clinimag.2021.10.010
[52] Martin-Villares C, Vazquez-Feito A, Gonzalez-Gimeno MJ et al. Bell’s palsy following a single dose of mRNA SARS-CoV-2 vaccine: a case report. J Neurol, 2022; 269: 47-48. DOI: 10.1007/s00415-021-10617-3
[53] Iftikhar H, Noor SMU, Masod M et al. Bell’s palsy after 24 hours of mRNA-1273 SARS CoV-2 vaccine. Cureus, 2021; 13: e15935. DOI: 10.7759/cureus.15935
[54] Sohil Pothiawala. Bell’s palsy after second dose of moderna COVID-19 Vaccine: Coincidence or causation? AML, 2021; 28: 2. DOI: 10.15388/Amed.2021.28.2.7
[55] Burrows A, Bartholomew T, Rudd J et al. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep, 2021; 14: e243829. DOI: 10.1136/bcr-2021-243829
[56] Obermann M, Krasniqi M, Ewers N et al. Bell’s palsy following COVID-19 vaccination with high CSF antibody response. Neurol Sci, 2021; 42: 4397-4399. DOI: 10.1007/s10072-021-05496-5
[57] Mussatto CC, Sokol J, Alapati N. Bell’s palsy following COVID-19 vaccine administration in HIV+ patient. Am J Ophthalmol Case Rep, 2022; 25: 101259. DOI: 10.1016/j.ajoc.2022.101259
[58] Colella G, Orlandi M, Cirillo N. Bell’s palsy following COVID-19 vaccination. J Neurol, 2021; 268: 3589-3591. DOI: 10.1007/s00415-021-10462-4
[59] Gómez de Terreros Caro G, Gil Díaz S, Pérez Alé M et al. Bell’s palsy following COVID-19 vaccination: a case report. Neurología (Engl Ed), 2021; 36: 567-568. DOI: 10.1016/j.nrleng.2021.04.002
[60] Tahir N, Koorapati G, Prasad S et al. SARS-CoV-2 vaccination-induced transverse myelitis. Cureus, 2021; 13: e16624. DOI: 10.7759/cureus.16624
[61] Nishizawa Y, Hoshina Y, Baker V. Bell’s palsy following the Ad26.COV2.S COVID-19 vaccination. QJM-Int J Med, 2021; 114: 657-658. DOI: 10.1093/qjmed/hcab143
[62] Tamaki A, Cabrera CI, Li S et al. Incidence of bell palsy in patients with COVID-19. JAMA Otolaryngol, 2021; 147: 767-768. DOI: 10.1001/jamaoto.2021.1266
[63] McCormick DP. Herpes-Simplex virus as cause of Bell’s palsy. Lancet, 1972; 299: 937-939. DOI: 10.1016/S0140-6736(72)91499-7
[64] Tseng H-F, Sy LS, Ackerson BK et al. Safety of quadrivalent meningococcal conjugate vaccine in 11- to 21-Year-Olds. Pediatrics, 2017; 139: e20162084. DOI: 10.1542/peds.2016-2084
[65] Shibli R, Barnett O, Abu-Full Z et al. Association between vaccination with the BNT162b2 mRNA COVID-19 vaccine and Bell’s palsy: a population-based study. Lancet Reg Health Eu, 2021; 11: 100236. DOI: 10.1016/j.lanepe.2021.100236
[66] Wan EYF, Chui CSL, Ng VWS et al. Messenger RNA coronavirus disease 2019 (COVID-19) vaccination with BNT162b2 increased risk of Bell’s palsy: A nested case-control and self-controlled case series study. Clin Infect Dis, 2022; ciac460. DOI: 10.1093/cid/ciac460
[67] Frontera JA, Tamborska AA, Doheim MF et al. Neurological events reported after COVID-19 vaccines: An analysis of vaccine adverse event reporting system. Ann Neurol, 2022; 91: 756-771. DOI: 10.1002/ana.26339
[68] Chamberlin TC. The method of multiple working hypotheses. Science, 1890; ns-15: 92-96. DOI: 10.1126/science.ns-15.366.92
[69] VAERS. Vaccine Adverse Event Reporting System. U.S. Department of Health & Human Services; 2021. Accessed May 20, 2022. Available at https://vaers.hhs.gov/
[70] Ricke DO. Vaccines associated cardiac adverse events, including SARS-CoV-2 myocarditis, elevated histamine etiology hypothesis. J Virol Viral Dis, 2022; 2: 1-11. DOI: 10.54289/JVVD2200108
[71] Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res, 2013; 6: 803-14. DOI: 10.2147/JPR.S53660
[72] Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin, 2007; 45: 27-37. DOI: 10.1097/AIA.0b013e318034194e
[73] Thacker MA, Clark AK, Marchand F et al. Pathophysiology of peripheral neuropathic pain: Immune cells and molecules. Anesth Analg, 2007; 105: 838-847. DOI: 10.1213/01.ane.0000275190.42912.37
[74] Yu J, Lou GD, Yue JX et al. Effects of histamine on spontaneous neuropathic pain induced by peripheral axotomy. Neurosci Bull, 2013; 29: 261-269. DOI: 10.1007/s12264-013-1316-0
[75] Babazadeh A, Mohseni Afshar Z, Javanian M et al. Influenza vaccination and guillain-barré syndrome: Reality or fear. J Transl Int Med, 2019; 7: 137-142. DOI: 10.2478/jtim-2019-0028
[76] Sejvar JJ, Kohl KS, Gidudu J et al. Guillain-Barré syndrome and Fisher syndrome: Case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine, 2011; 29: 599-612. DOI: 10.1016/j.vaccine.2010.06.003
[77] Agrawal S, Verma K, Verma I et al. Reactivation of herpes zoster virus after COVID-19 vaccination: Is there any association? Cureus, 2022; 14: e25195. DOI: 10.7759/cureus.25195
[78] Plüß M, Mese K, Kowallick JT et al. Case report: Cytomegalovirus reactivation and pericarditis following ChAdOx1 nCoV-19 vaccination against SARS-CoV-2. Front Immunol, 2022; 12: 784145. DOI: 10.3389/fimmu.2021.784145
[79] Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscl Throm Vas, 2011; 31: 986-1000. DOI: 10.1161/ATVBAHA.110.207449
[80] Kawabata A. Prostaglandin E2 and Pain-An Update. Biol Pharm Bull, 2011; 34: 1170-1173. DOI: 10.1248/bpb.34.1170
[81] Soeiro T, Salvo F, Pariente A et al. Type I interferons as the potential mechanism linking mRNA COVID-19 vaccines to Bell’s palsy. Therapie, 2021; 76: 365-367. DOI: 10.1016/j.therap.2021.03.005
[82] Fernandez PEL, Pereira JM, Risso IF et al. Guillain-Barre syndrome following COVID-19 vaccines: A scoping review. Acta Neurol Scand, 2022; 145: 393-398. DOI: 10.1111/ane.13575
[83] Goud R, Lufkin B, Duffy J et al. Risk of Guillain-Barré syndrome following recombinant zoster vaccine in medicare beneficiaries. JAMA Intern Med, 2021; 181: 1623-1630. DOI: 10.1001/jamainternmed.2021.6227
[84] Scendoni R, Petrelli C, Scaloni G et al. Electromyoneurography and laboratory findings in a case of Guillain-Barré syndrome after second dose of Pfizer COVID-19 vaccine. Hum Vacc Immunother, 2021; 17: 4093-4096. DOI: 10.1080/21645515.2021.1954826
[85] Röltgen K, Nielsen SCA, Silva O et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell, 2022; 185: 1025-1040.e14. DOI: 10.1016/j.cell.2022.01.018
[86] Galdiero MR, Varricchi G, Seaf M et al. Bidirectional mast cell-eosinophil interactions in inflammatory disorders and cancer. Front Med, 2017; 4: 103. DOI: 10.3389/fmed.2017.00103
[87] Rosa AC, Fantozzi R. The role of histamine in neurogenic inflammation. Brit J Pharmacol, 2013; 170: 38-45. DOI: 10.1111/bph.12266
[88] Dale HH, Laidlaw PP. The physiological action of beta-iminazolylethylamine. J Physiol, 1910; 41: 318-344. DOI: 10.1113/jphysiol.1910.sp001406
[89] Ricke DO. Etiology model for elevated histamine levels driving high reactogenicity vaccines (including COVID-19) Associated Menstrual Adverse Events. Research Square, 2022; 1-9. DOI: 10.21203/rs.3.rs-1508835/v1
[90] Malone RW, Tisdall P, Fremont-Smith P et al. COVID-19: Famotidine, histamine, mast cells, and mechanisms. Front Pharmacol, 2021; 12: 633680. DOI: 10.3389/fphar.2021.633680
[91] Tomera K, Kittah J. Brief Report: Rapid clinical recovery from severe COVID-19 with High Dose Famotidine and high dose celecoxib adjuvant therapy. Preprints, 2020; 6: 2020080519. DOI: 10.20944/preprints202008.0519.v1
[92] Mather JF, Seip RL, McKay RG. Impact of famotidine use on clinical outcomes of hospitalized patients with COVID-19. Am J Gastroenterol, 2020; 115: 1617-623. DOI: 10.14309/ajg.0000000000000832
[93] Morán Blanco JI, Alvarenga Bonilla JA, Homma S et al. Antihistamines and azithromycin as a treatment for COVID-19 on primary health care - A retrospective observational study in elderly patients. Pulm Pharmacol Ther, 2021; 67: 101989. DOI: 10.1016/j.pupt.2021.101989
[94] Hogan II RB, Hogan III RB, Cannon T et al. Dual-histamine receptor blockade with cetirizine - famotidine reduces pulmonary symptoms in COVID-19 patients. Pulm Pharmacol Ther, 2020; 63: 101942. DOI: 10.1016/j.pupt.2020.101942
[95] Otani IM, Anilkumar AA, Newbury RO et al. Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol, 2013; 131: 1576-1582. DOI: 10.1016/j.jaci.2013.02.042
Copyright ©2022 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.





 Copyright ©
Copyright ©