Production Physiology and Biochemical properties of Crude Thermophile Protease by Mild - Halophilic Bacillus thuringiensis aizawai HD 865
Amira M. Roshdy1*, Nesreen H. Abou - Baker2, Omaima A. Sharaf3
1Department of Microbial Chemistry, Biotechnology Research Institute, National Research Centre, Cairo, Egypt
2Department of Soils and Water Use, Agricultural and Biological Institute, National Research Centre, Cairo, Egypt
3Department of Agricultural Microbiology, Agricultural and Biological Institute, National Research Centre, Cairo, Egypt
*Correspondence to: Amira M. Roshdy, PhD, Associate Professor, Department of Microbial Chemistry, Biotechnology Research Institute, National Research Centre, 33 El-Bohouth Street, Dokki, Cairo, 12622, Egypt; E-mail: amiraroshdy82@yahoo.com
Abstract
Objective: The current study aims to enrich the research library of Bacillus thuringensis, the most famous bacteria as a biopesticide, with a product that may enhance its ability as a biopesticide as well as its many other applications, namely the heat-tolerant alkaline protease.
Methods: The strain Bacillus thuringiensis aizawai HD 865, obtained from HD Culture Collection was grown on a liquid growth medium of nutrient broth and yeast extract, and colonies were counted on agar plates. The zygote is then transferred to a skim milk environment to estimate the enzymatic activity under the different growth conditions of the growth environment (sodium chloride concentration, pH, aeration, fermentation duration), then testing the optimal conditions for enzymatic activity in the enzymatic reaction mixture (sodium chloride concentration, salinity resistance, optimum temperature, thermal stability, optimum pH and stability). Then analyze the data statistically.
Results: Optimum alkaline protease medium production conditions were, 2 % NaCl, so Bacillus thuringiensis aizawai HD865 a mild - halophilic microorganism, aeration level 80%, pH 7 and 8 days of fermentation period. On the other hand, crude alkaline protease properties were found that, the optimum temperature for its activity was relatively high (70ºC), while heat-stabilities after 5 min of heating crude enzyme in boiling water bath then incubated for 15min on 30, 40, 50, 60, 70ºC, were 184.23, 178.07, 164.29, 165.51 and 121.46 Units, respectively; whereas the optimum pH was at pH 7.6 (151.76 Units) with tris buffer then at pH 9.6 with glycine - NaOH buffer; While pH stability was at pH 7.6 with tris buffer then at pH 8.4 with glycine - NaOH buffer. Thus, activities have decreased at different concentrations (0, 1, 2, 3, 4, 5 %) of sodium chloride in reaction mixture with enzyme activities (151.25, 137.35, 135.12, 135, 135.89, 114.38 Units) respectively, also decreased when rinse crude enzyme for 5 min in NaCl (0, 1, 2, 3, 4, 5 %), with (151.04, 175.57, 168.69, 154.61, 155.39 and 143.39 Units) respectively.
Conclusion: Bacillus thuringiensis aizawai HD 865 strain is considered a low - salt tolerant strain, while the alkaline protease extracted from it is heat - resistant.
Keywords: extremozymes, compatible solutes, bio - additive, thermo - stability, pH stability, salt stability
1 INTRODUCTION
Microbial enzymes considered as the most sustainable biocatalyst in comparison with those of plants or animals; because of its economic cost, productivity, rapid and easy processing Ojo-Omoniyi et al[1]. Daraban et al.[2] also compared the limitations of using biological and chemical pesticides Through a database for the period from 2012 to 2022, and by analyzing 24,676 scientific articles on biocides, 4,321 of them were determined to be relevant; 522 scientific articles on bioinsecticides were also analysed, and 474 of them were identified as relevant. They pointed out that the risks of using chemical pesticides on non-target species are exceeded, in addition to their harm to biological diversity of plants, animals, aquatic and terrestrial organisms, and the ecosystem and food system, with immediate effects (such as in cases of eye or skin irritation, dizziness, headache, or nausea) and chronic diseases may occur (such as diabetes, asthma, and cancer), and there are multiple factors to evaluate the risks of chemical pesticides (including the duration of exposure to the pesticide, the amount of pesticide, the extent of toxicity and stability of the pesticide, and other environmental factors), he pointed out that the number of bees decreases when chemical pesticides are used, which leads to crop losses due to lack of pollination. The use of pesticides has also been associated with learning and memory difficulties at realistically critical rates in acute and chronic cases, with neonicotinoids and non-neonicotinoids chemical insecticides. Robertson et al.[3] showed the importance of extremophilic enzymes in industrial applications; because of harsh conditions such as extreme temperature, salinity, ionic strength and pH values; which suggests the microbial source of these enzymes recommended, sufficient and applicable for industrial level production.
Also salinity has an impacted effect of different contaminants and chemical pesticides distribution in different environments, such as aquatic environments[4]. Saeed et al.[5] explained the possibility of green industrial applications of halo-alkaline protease in pharmaceutical, textile, leather (bating and dehairing), detergents (proteins stain cleaning) and food (favors and texture) industries, through the biochemical characterization of the wide range properties; such as pH, temperature and salinity. Studying different microbial strains enzymes, production conditions and biochemical properties, enhances innovative discovery of economically and commercially application of dustrial levels of halo - alkaline proteases all over the world[6]. On the economic side, the research department of Statista stated that the value of the global biopesticides market was estimated at about 1.8 billion US dollars in 2021. It is supposed to reach about 2.3 billion US dollars by 2027. Bayer CropScience and BASF are considered among the leading companies in the global bioindustry (Statista 2023)[7]. According to the Made-In-China website[8], the prices of biological pesticides are in the range of 62-309 US dollars per hectare for application or program with the product, but the cost of chemical pesticide is 73-382 US dollars per hectare, which also shows the economic feasibility of biological alternatives.
As mentioned by Cira-Chávez et al.[9], microorganisms may be classified according to their tolerance to salinity in the growth medium, to low tolerance, as they grow in medium with a sodium chloride concentration of 1-6%, 7-15%, moderate tolerance to salinity, while 15-30% are highly tolerant to salinity. Halophilic microorganisms are known for their ability to live in salty environments to varying degrees and may require salinity to remain alive, extracting enzymes from these extreme - tolerant strains has promising technical applications, such as food, bioremediation of environmental waste, and biosynthesis of compounds industries; Given the eco - friendly practical importance of alkaline protease production, more research must be done on microbial enzymes production, especially since Bacillus is one of the known bacterial strains to produce protease[10]. Halo - tolerant microorganisms also called compatible solutes that it can adapt to salinity stress and accumulate some organic solutes[9].
Asar et al.[11] mentioned many microbial protease production by halophiles such as Pseudoalteromonas CP76, Natrialba magadii, Halobacterium mediterranei and Bacillus clausii, and showed that halophiles compatible solutes applicable in many industries, such as food moisturing and preservating, educational model, cosmetics, biofuel, bioremediation, pharmaceutical and chemical industries.
In addition of many alkaline protease industrial applications, like biofuel and many others, characterizing enzymes properties enhances technological development with sustainability[1]. On the other hand, enzymes profiling with production conditions and biochemical characterization support scientific researches for systematic reviews, to develop performance of technological industrial conditions[12].
So halo-alkaline protease from bio pesticide Bacillus thuringeinsis subspecies aizawai HD 865 can be innovative alternative, which replace chemical pesticide and bio remediate the residual chemical contaminants from environmental field, which make this study part of the library of Bacillus thuringeinsis properties and proposed applications.
2 MATERIALS AND METHODS
2.1 Bacterial Strain
The Bacillus thuringiensis subspecies aizawai HD 865 examined in the present study was obtained from HD (Howard Dulmage Collection, Cotton Insect Research Laboratory, ARS, USDA, Brownsville, Texas, USA) culture collection.
2.2 Inoculum Preparation
The Bacillus thuringiensis aizawai HD 865 inoculum was prepared by inoculating in nutrient broth yeast extract medium[13], at 30℃ for 24h, the colony forming units achieved 23×106 CFU/mL, by plating 10 fold serial dilutions in nutrient broth agar plates[14].
2.3 Halo - alkaline Protease Production Medium
The prepared inoculum was grown in 100mL skim milk liquid medium (10g skim milk and 5g sodium chloride), pH 7[15]. Then, incubated at 30ºC at 100rpm for 24h and were used as inoculum (2mL inoculum / 100mL medium) for experimental flasks. Effect of fermentation conditions, in terms of sodium chloride concentration in medium (1-25%), pH (4.5-8.5), Aeration (5-50mL medium / 100mL conical flasks), and fermentation time (1-7 days) on alkaline protease production by Bacillus thuringiensis aizawai HD 865 were investigated at 35ºC under shaking (100rpm). The thermo, pH and salt stabilities of crude enzyme were studied at temperature range of 30-70℃, 5.6-11.6 pH values, and 0-5% NaCl concentrations, for 15min[11].
2.4 Enzyme Assay
The growth medium was centrifuged at 4,000rpm for 10min at 4℃ to remove the insoluble matters. The clear supernatant was used as crude extract for halo - alkaline protease assay in glycine NaOH buffer at pH 10[11].
2.5 Statistical Analysis
To study the sole impact of NaCl concentration, fermentation period, medium pH and aeration of culture; and salinity, pH and temperature of crude enzyme, the One - Way Randomized Blocks design was applied. We used the CoStat statistical package to analyze the ANOVA test and Duncanʼs Multiple Range Test for mean comparisons at the 0.01 probability level[16].
3 RESULTS AND DISCUSSION
The Bacillus thuringensis is considered the most famous bacteria in the global biocidal market, so the production of compounds with other industrial applications is considered an added market value to it. This enhances the efficiency of its use at the industrial level; especially if this compound is the alkaline protease enzyme with a wide range of industrial applications, and if it has extremophile properties, is considered one of the most promising enzymes in many industries.
As Foda et al.[17] mentioned, the Bacillus thuringiensis aizawai HD 865 strain was tested for the production of alkaline protease in non-salty conditions and it gave 20.83U/mL/min on Nutrient Broth Yeast Extract Standard Medium growth medium, and 26.63U/mL/min on 3% (w/v) soybean as medium and 247 (U/g wheat bran/min) under solid state fermentation conditions.
3.1 Production Physiology of Alkaline Protease by Bacillus thuringiensis aizawai HD 865
3.1.1 Effect of Medium NaCl Concentration on Alkaline Protease Production by Bacillus thuringiensis aizawai HD 865
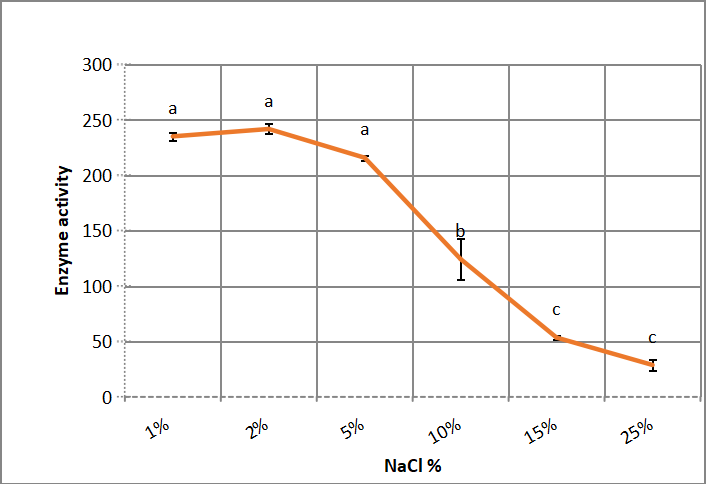
.Figure 1 shows that the best salinity concentrations of Bacillus thuringiensis aizawai HD 865 growth medium are ranged between 1-5% NaCl w/v without significant difference between 1, 2 and 5% NaCl. However, the decline was severe and significant when the salt concentration was raised from 5% to 25%.
|
Figure 1. Effect of medium NaCl concentration on alkaline protease production by Bacillus thuringiensis aizawai HD 865. According to Duncanʼs multiple range test, any two points with the same letters (a, b, c, d and e) are not significant at the 1% level of significance.
So it considers as mild halophilic bacteria according to DasSarma and DasSarma[18] that classified slight halopphiles by growing at 2-5% NaCl growth medium. While considered as moderate halophilic according to[19], who defined optimum growth medium sodium chloride concentration rate from 3 to 15%.
The range of 1-5% NaCl concentration has many suitable industrial applications such as compatible solutes, bacterio-rhodopsin, pigments and surfactant[20].
Asar et al[11] found that the optimum concentration of sodium chloride in growth medium for halo - alkaline protease from Bacillus cereus NRC-1 is 5%. While Daoud et al.[21] found that Bacillus halodurans US193 resist up to 12.5% NaCl, as Benmebarek and Kharroub[10] reported that Idiomarina loihiensis is highly halophilic organism with 22.5% NaCl concentration in growth medium, fungal strain of Asperillus reticulatus was obligate halophile that required 6-9% NaCl in growth medium[22].
3.1.2 Effect of Fermentation Period of Culture on Alkaline Protease Production by Bacillus thuringiensis aizawai HD 865
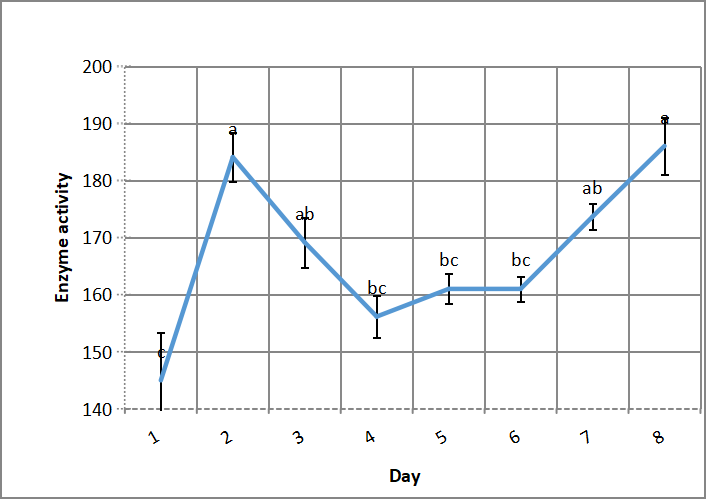
As shown in Figure 2 production of alkaline protease increases significantly in the second day, which thought to be according to the compatible solute strategy of amino acids solutes in/out bacterial cell, and decreases gradually without significant difference to the 6th day, then turns to increase again to reach the maximum in the last tested day (eighth day), that time is needed to resist salinity stress.
|
Figure 2. Effect of fermentation period of culture on alkaline protease production by Bacillus thuringiensis aizawai HD 865. According to Duncanʼs multiple range test, any two points with the same letters (a, b, c, d and e) are not significant at the 1% level of significance.
Cira-Chávez et al.[9] explained this retarding by compatible solutes technique; through accumulate organic compounds such as amino acids, sugars, poly - ol and their derivatives, from environment into the microbial cell to resist salinity stress that these solutes act as stabilizers for many extreme conditions, such as heat, pH and salinity. While Schneegurt[23] showed that slow microbial growth in salty medium needs even up to weeks to adapt to salinity; because of evaporation through thermal shaking, which may precipitate salt in growth medium. However earlier 2-3 days growth may be achieved by fortified growth medium by MgSO4 and KCl with similar or higher growth count values by comparing to native growth medium as reported by[24].
As showed by Asar et al.[11], optimum halo-alkaline protease production by Bacillus cereus NRC-1 was after 5 days, according to salinity resistance it takes more time for growth than original medium; because of evaporation and salt precipitation.
3.1.3 Effect of Medium pH on Alkaline Protease Production by Bacillus thuringiensis aizawai HD 865
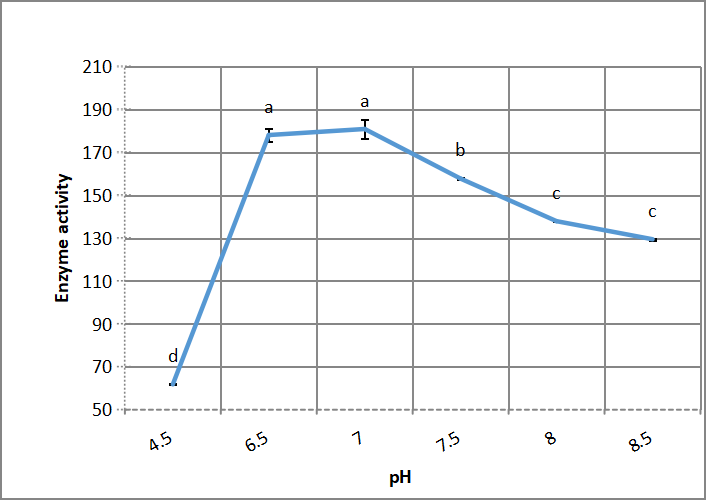
A sharp and significant increase in enzyme activity occurs by increasing the pH value from 4.5 to 6.5 (Figure 3). The optimum pH of growth medium for halo-alkaline protease by Bacillus thuringiensis aizawai HD 865 is neutral (6.5-7.0). Also the drop in enzyme activity was severe and significant by increase medium pH from 7 to 8.
|
Figure 3. Effect of medium pH on alkaline protease production by Bacillus thuringiensis aizawai HD 865. According to Duncanʼs multiple range test, any two points with the same letters (a, b, c, d and e) are not significant at the 1% level of significance.
Asar et al.[11] showed that the neutral pH of growth medium is the optimum. Also Srivastava and Srivastava[25] explained that common extracellular and intracellular pH for bacterial growth is neutral pH; according to structural proteins amino acids charge interactions, which affect by pH through secondary and tertiary proteins structure, then change enzyme protein shape and respectively reactions activity. On the other hand Daoud et al.[21] reported that Bacillus halodurans US193 optimum growth pH was 9.7. Benmebarek and Kharroub[10] also found that pH 10 of growth medium was the optimum growth pH of Idiomarina loihiensis.
3.1.4 Effect of Aeration on Alkaline Protease Production by Bacillus thuringiensis aizawai HD 865
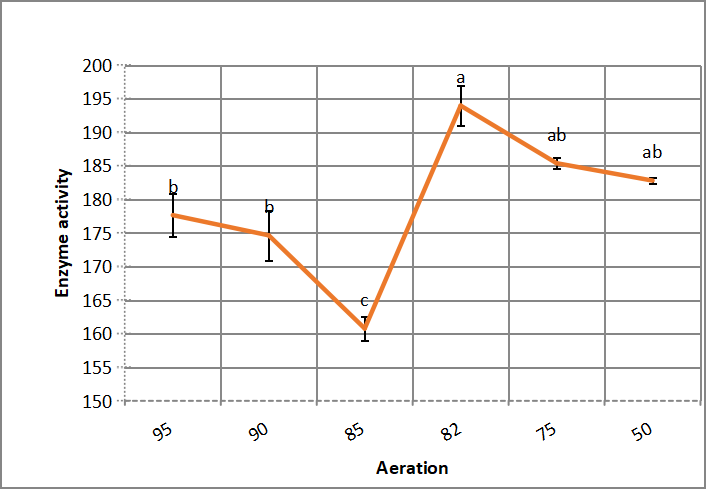
.Figure 4 shows that Bacillus thuringiensis aizawai HD 865 presents maximum halo - alkaline protease production at 82% aeration level. Enzyme activity values decrease gradually as aeration is reduced from 82 to 50%.
|
Figure 4. Effect of aeration on alkaline protease production by Bacillus thuringiensis aizawai HD 865. According to Duncanʼs multiple range test, any two points with the same letters (a, b, c, d and e) are not significant at the 1% level of significance.
In accordance with Asar et al.[11], that reported that Bacillus cereus NRC-1 is an aerobic bacterium, which needs 95% aeration for maximizing enzyme production with 70 alkaline protease activity units, and Robinson et al.[26] which used 25mL medium in 125mL falsks.
3.2 Biochemical Properties of Crude Alkaline Protease in Enzyme Reaction Mixture Produced by the Halo - tolerant Bacillus thuringiensis aizawai HD 865
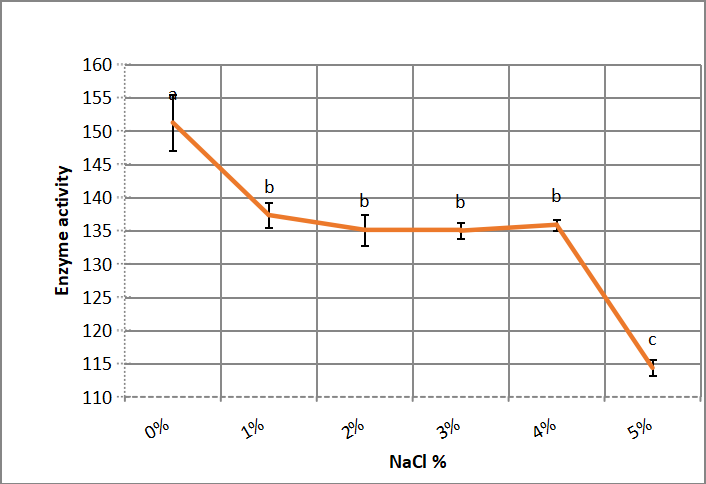
3.2.1 Effect of NaCl Concentration in Enzyme Reaction Mixture
As shown in Figure 5, when enzyme reaction mixture contains (1-5%) NaCl, alkaline protease activity decreased significantly at 1% and without significant difference between 1-4%.
|
Figure 5. Effect of NaCl concentration in enzyme reaction mixture of crude alkaline protease. According to Duncanʼs multiple range test, any two points with the same letters (a, b, c, d and e) are not significant at the 1% level of significance.
In accordance with Asar et al.[11] that Bacillus cereus NRC 1 alkaline protease decreased activity in presence of NaCl and Chung et al.[22], reported that Asperillus reticulatus extracellular protease activity, decreased by NaCl presence. While Daoud et al.[21] illustrated that optimum NaCl concentration for serine protease activity, produced by Bacillus halodurans US193, is 0.25M.
Devi-Rajeswari et al.[27] found that optimum enzyme activity was at 1.5M NaCl. While Salinivibrio showed maximum enzyme activity at 0.5M NaCl concentration[28]. Patel et al.[29] reported reducing activity by increasing NaCl concentration. In contrast, Halogeometricum borinquense tolerate salinity up to 35% NaCl[30].
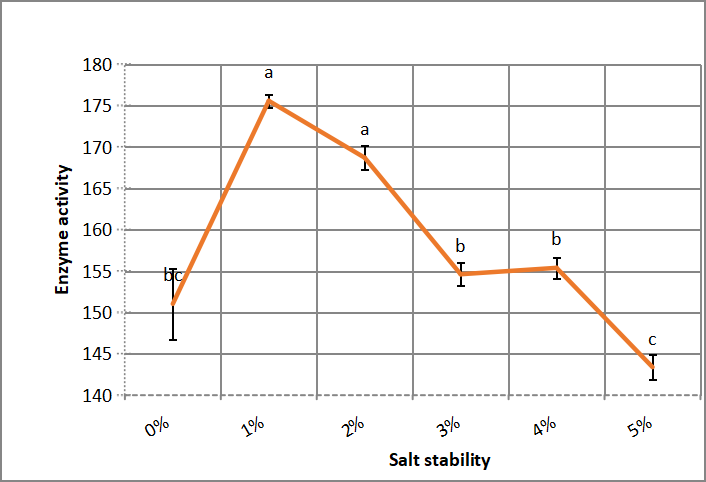
3.2.2 Salt Stability in Enzyme Reaction Mixture
.Figure 6 explains alkaline protease activity when kept crude enzyme in different concentrations of NaCl (1-5), for two hours before starting reaction, that 1% NaCl is the best salt concentration for enzyme stability followed by 2% without significant difference between them.
|
Figure 6. Salt stability of crude alkaline protease. According to Duncanʼs multiple range test, any two points with the same letters (a, b, c, d and e) are not significant at the 1% level of significance.
Chung et al.[22] illustrated that extracellular protease from Asperillus reticulatus has retained 69% of its activity up to 7% NaCl. While Asar et al.[11] reported that alkaline protease of Halophilic Bacillus cereus NRC-1 retained 59% of its activity at 5% NaCl. Sana et al.[31] found that marine bacterium enzyme has optimum salt concentration at 30% NaCl and salt stability up to 35% NaCl.
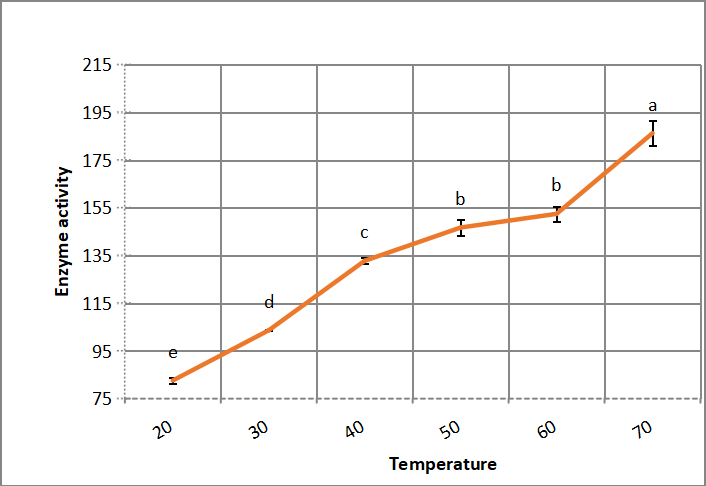
3.2.3 Optimum Temperature of Enzyme Reaction Mixture
Alkaline protease activity increases significantly by increasing temperature up to 70℃, which indicate thermophile alkaline protease activity, as shown in Figure 7.
|
Figure 7. Optimum temperature of crude alkaline protease. According to Duncanʼs multiple range test, any two points with the same letters (a, b, c, d and e) are not significant at the 1% level of significance.
Gupta et al.[32] illustrated that the optimum temperature for alkaline protease extracted from Halobiforma BNMIITR, is 50℃. Chung et al.[22] reported that optimum temperature for extracellur protease from Asperillus reticulatus was 40-50℃. Saeed et al.[5] showed that optimum temperature for metalloalkaline protease activity produced by Bacillus cereus is 60℃. In accordance with Asar et al.[11] that alkaline protease of halophilic Bacillus cereus NRC-1 optimum temperature is 60℃. While Daoud et al.[21] found that Bacillus halodurans haloalkaline protease activity optimum temperature is 70℃. While Devi-Rajeswari et al.[27] and Sana et al.[31] reported that optimum temperature for bacterial enzyme activity was 40℃. Gupta et al.[32] reported optimum temperature at 60℃ for solvent resistant bacterium Pseudomonas aeruginosa.
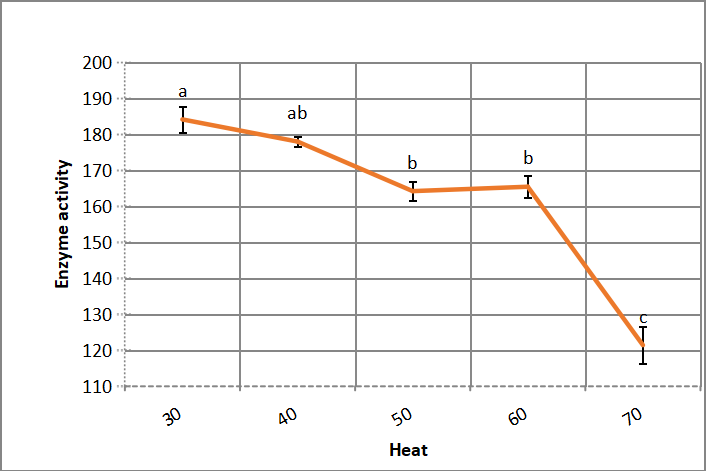
3.2.4 Heat Stability in Enzyme Reaction Mixture
Alkaline protease activity decreases gradually by increasing temperature of incubating enzyme reaction mixture, after heating enzyme in water bath for 5min, as illustrated in Figure 8. The decrease turns to be severe with increasing heat more than 60℃.
|
Figure 8. Heat stability of crude alkaline protease. According to Duncanʼs multiple range test, any two points with the same letters (a, b, c, d and e) are not significant at the 1% level of significance.
According to negative charge of carboxylic groups of halophilic enzymes, it is soluble at salt concentrations, as compatible solutes of Na+ enhance thermal stability and enzymes activity[9]. Asar et al.[11] reported that Bacillus cereus halophilic alkaline protease decline activity to 72% at 70℃. Gupta et al.[32] found that solvent resistant bacterium Pseudomonas aeruginosa produce thermostable enzyme up to 65℃.
3.2.5 Optimum pH of Enzyme Reaction Mixture
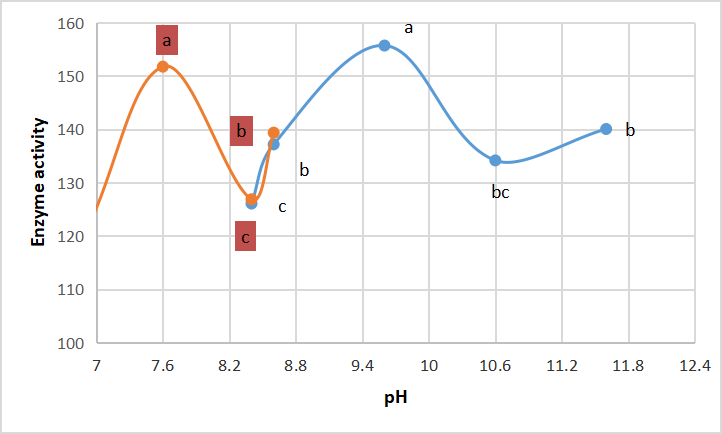
Illustrates that there are two peaks of optimum pH for enzyme activity, peak at 7.6 (by using tris-HCl buffer) and other peak at 9.6 (by using glycine-NaOH buffer) in Figure 9.
|
Figure 9. Optimum pH of crude alkaline protease. According to Duncanʼs multiple range test, any two points with the same letters (a, b, c, d and e) are not significant at the 1% level of significance.
Blandine[33] explained existence of two peaks for optimum pH by existence of aspartic acidic protease at pH 2.79 and serine alkaline protease at pH 9.0. Atrooz and Alomari[34] reported optimum pH for enzyme activity at pH 3 and pH 9, explained that by presence molecular iso enzyme. Whereas, Ma et al.[35] suggest one of three theories, for multiple optimum pHs; either for enzyme purity degree, many isozyme forms, or ionized enzyme-substrate complexes formation.
Chung et al.[22] showed that the optimum pH for alkaline serine protease produced by fungal strain Asperillus reticulatus was 9.5-10.5. Saeed et al.[5] illustrated that optimum pH for activity of metalo-alkaline protease produced by Bacillus cereus is 10, in accordance with Daoud et al.[21]. While Asar et al.[11] found that optimum pH for alkaline protease of Bacillus cereus was 7.6. As Adinarayana et al.[36] found that Bacillus subtilis PE-11 produced enzyme with optimum pH 10.
3.2.6 pH Stability in Enzyme Reaction Mixture
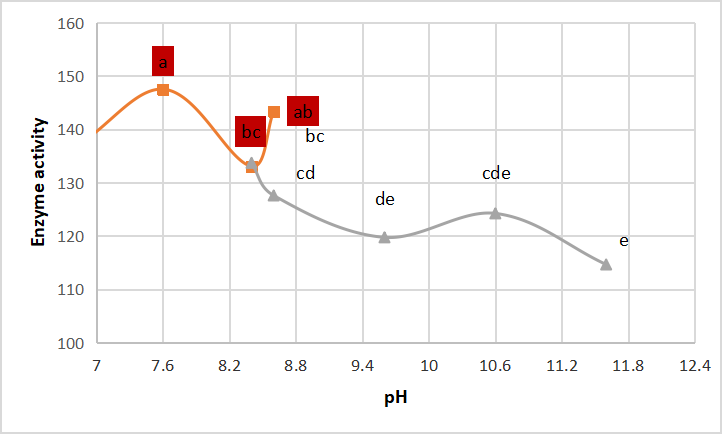
Crude enzyme has many peaks when kept in different buffer pHs (tris-HCl and glycine-NaOH) before incubating, as shown in Figure 10.
|
Figure 10. pH stability of crude alkaline protease. According to Duncanʼs multiple range test, any two points with the same letters (a, b, c, d and e) are not significant at the 1% level of significance.
Gupta et al.[32] reported that Halobiforma BNMIITR protease was stable at pH range 6.0-12.0. Asar et al.[11] illustrated that pH stability of protease activity declined 36% at pH 11.6.
4 CONCLUSION
Table 1 summarized the optimum growth medium conditions for production of alkaline protease by Bacillus thuringiensis aizawai HD 865.
Table 1. Production Physiology of Alkaline Protease by Bacillus thuringiensis aizawai HD 865
Medium Factor |
Optimum Value |
Alkalkine Protease Activity Units |
NaCl % |
2% |
241.76 |
Aeration level |
80% |
193.96 |
pH |
7 |
180.86 |
Fermentation period |
8 days |
188.66 |
So Bacillus thuringiensis aizawai HD 865 a mild-halo-aerophilic microorganism. While Table 2 summarized biochemical properties of crude alkaline protease in enzyme reaction mixture produced by the halo-tolerant Bacillus thuringiensis aizawai HD 865.
Table 2. Biochemical Properties of Crude Alkaline Protease in Enzyme Reaction Mixture Produced by the Halo - tolerant Bacillus thuringiensis aizawai HD 865
Protease Reaction Mixture Factor |
Optimum Value |
Alkalkine Protease Activity Units |
optimum temperature |
70℃ |
186.28 |
heat-stabilities |
30℃ |
184.23 |
optimum pH |
pH 7.6 tris buffer pH 9.6 glycine-NaOH buffer |
151.76 155.71 |
pH stability |
pH 7.6 tris buffer pH 8.4 glycine-NaOH buffer |
147.54 133.71 |
NaCl % |
0% |
151.25 |
NaCl stability |
0% |
151.04 |
So crude alkaline protease produced by the mild-halo-aerophilic Bacillus thuringiensis aizawai HD 865, is thermo-alkaline protease.
Crude alkaline protease produced by the mild - halo - aerophilic Bacillus thuringiensis aizawai HD 865, recommended to further studies for applications such as detergents, pigments, pesticides, leather bating, silver recovery, textiles and agricultural and industrial waste water bioremediation, for example.
Acknowledgements
Not applicable.
Conflicts of Interest
The author declared no conflict of interest.
Author Contribution
M. Roshdy A was responsible for the design and implementation of experiments. M. Roshdy A and A. Sharaf O were responsible for participating in practical experiments, writing, reading, reviewing, and approving manuscripts. H. Abou - Baker N performed statistical analyses and data and wrote statistical analysis reviews in manuscripts.
References
[1] Ojo-Omoniyi OA, Moro DD, Afolab OB. Microbial Proteases: Sources, Significance and Industrial Applications. Int J Curr Microbiol App Sci, 2024; 13: 1-23.[DOI]
[2] Daraban GM, Hlihor RM, Suteu D. Pesticides vs. Biopesticides: From Pest Management to Toxicity and Impacts on the Environment and Human Health. Toxics, 2023; 11: 983.[DOI]
[3] Robertson DE, Mathur EJ, Swanson RV et al. The discovery of new biocatalysts from microbial diversity. SIM News, 1996; 46: 3-4.
[4] Romain SJ, Basirico LM, Hutton S et al. Influence of salinity on the partitioning behaviour of six commonly used pesticides in fish eggs. Environ Toxicol Chem, 2023; 43: 299-306.[DOI]
[5] Saeed K, Riaz S, Adel A et al. Characterization of alkaline metalloprotease isolated from halophilic bacterium Bacillus cereusand its applications in various industrial processes. An Acad Bras Cienc, 2023; 95: e20230014.[DOI]
[6] Razzaq A, Shamsi S, Ali A et al. Microbial Proteases Applications. Front Bioeng Biotechnol, 2019; 7: 110.[DOI]
[7] Statista Research Department. Global bio-pesticides market size 2016-2027. Accessed 20 September 2024. Available at:[Web]
[8] Made-In-China. Shenzhen King Quenson Industry Co., Ltd. Accessed 20 September 2024. Available at:[Web]
[9] Cira-Chávez LA, Guevara-Luna J, Soto-Padilla MY et al. Introduction: Kinetics of Halophilic Enzymes. In: Kinetics of Enzymatic Synthesis. IntechOpen Publishing: London, 2018; 1.
[10] Benmebarek H, Kharroub K. Production Assay and Partial Characterization of a Protease Produced by Idiomarina loihiensis, a Moderately Halophilic Bacterium Strain. Biol Life Sci Forum, 2024; 31: 18.[DOI]
[11] R. Asar A, Abdel-Salam MS, Kahil T. Halotolerant Alkaline Protease Production by New Isolate Halophilic Bacillus cereus NRC-1. Middle East J Appl Sci, 2016; 6: 964-976.
[12] Yao H, Liu S, Liu T et al. Microbial‑derived salt‑tolerant proteases and their applications in high‑salt traditional soybean fermented foods: a review. Bioresour Bioprocess, 2023; 10: 82.[DOI]
[13] Roshdy AM, Shata HM, Ali SM et al. Commercial Extraction of Applicable Alkaline Protease and Chitinase by Bacillus thuringiensis dendrolimus IP 4A/4B. J Mod Agric Biotechnol, 2022; 1: 21.[DOI]
[14] Teng P, Chang C, Huang C et al. Effects of solid-state fermented wheat bran by Bacillus amyloliquefaciens and Saccharomyces cerevisiae on growth performance and intestinal microbiota in broiler chickens. Ital J Anim Sci, 2017; 16: 552-562.[DOI]
[15] Jadhav AG, Jaybhaye A, Musaddiq M. Salt tolerant protease produced by an aerobic species belonging to the Bacillus genus isolated from saline soil. Int J Sci Res Pub, 2013; 3: 1-8.
[16] Anonymous A. Cohort Soft Ware Crop (Costat user manual version 3.03). California, USA, 1989.
[17] S. Foda M, M. Ali S, Kahil T et al. Production Physiology of Alkaline Protease by Bacillus thuringiensis spp. under Solid-State Fermentation Conditions. J Appl Sci Res, 2013; 9: 1975-1984.
[18] DasSarma S, DasSarma P. Halophiles. Wiley Publishing: London, UK, 2005.
[19] Kushner DJ. Life in high salt and solute concentrations: halophilic bacteria. In Microbial Life in Extreme Environments. Academic Press: London, UK, 1978.
[20] DasSarma S, DasSarma P. Introduction: Halophiles. In: Encyclopedia of Life Sciences. Nature Publishing: Germany, 2001; 1-9.
[21] Daoud L, Hmani H, Ben Ali M et al. An Original Halo-Alkaline Protease from Bacillus halodurans Strain US193: Biochemical Characterization and Potential Use as Bio-Additive in Detergents. J Polym Environ, 2018; 26: 23-32.[DOI]
[22] Chung D, Yu WJ, Lim JY et al. Characterization of the Proteolytic Activity of a Halophilic Aspergillus reticulatus Strain SK1-1 Isolated from a Solar Saltern. Microorganisms, 2022; 10: 29.[DOI]
[23] Schneegurt MA. Introduction: Media and Conditions for the Growth of Halophilic and Halotolerant Bacteria and Archaea. In: Advances in Understanding the Biology of Halophilic Microorganisms. Springer Publish: Germany, 2012; 35-38.
[24] Yeannes M, Ameztoy I, Ramirez E et al. Culture alternative medium for the growth of extreme halophilic bacteria in fish products. Food Sci Technol, 2011; 31: 561-566.[DOI]
[25] Srivastava S, Srivastava P. Understanding Bacteria. Kluwer Academic Publish: London, UK, 2003.
[26] Robinson J, Pyzyna B, G. Atrasz R et al. Growth kinetics of extremely halophilic archaea (family Halobacteriaceae) as revealed by Arrhenius Plots. J Bacteriol, 2005; 187: 923-929.[DOI]
[27] Devi-Rajeswari V, Jayaraman G, Sridharan TB. Purification and characterization of extracellular protease from halotolerant bacterium Virgibacillus dokdonensis VITP14. Asian J Biochem, 2012; 7: 123-132.[DOI]
[28] Karbalaei-Heidari H, Ziaee A, Schaller J et al. Purification and characterization of an extracellular haloalkaline protease produced by the moderately halophilic bacterium, Salinivibrio sp. strain AF-2004. Enzym Microbial Technol, 2007; 40: 266-272.[DOI]
[29] Patel RK, Dodia MS, Joshi RH et al. Purification and characterization of alkaline protease from a newly isolated haloalkaliphilic Bacillus sp. Process Biochem, 2006; 41: 2002-2009.[DOI]
[30] Vidyasagar M, Prakash S, Litchfield C et al. Purification and characterization of a thermostable, haloalkaliphilic extracellular serine protease from the extreme halophilic archaeon Halogeometricum borinquense strain TSS101. Archaea, 2006; 2: 51-57.[DOI]
[31] Sana B, Ghosh D, Saha M et al. Purification and characterization of a salt, solvent, detergent and bleach tolerant protease from a new gamma-Proteobacterium isolated from the marine environment of the Sundarbans. Process Biochem, 2006; 41: 208-215.[DOI]
[32] Gupta A, Roy I, Patel RK et al. One-step purification and characterization of an alkaline protease from haloalkaliphilic Bacillus sp. J Chromatogr A, 2005; 1075: 103-108.[DOI]
[33] Blandine M, Serge N, Clergé T. Partial Purification and Characterization of Protease from Abrus precatorius Linn. (Fabaceae) from Cameroon. Adv Enzym Res, 2016; 4: 35-43.[DOI]
[34] Atrooz OM, Nasser AF. Determination of the activity and kinetics parameters of proteases in the crude plant extracts of Mentha piperita L. and Thymus capitatus L. J Appl Biolo Biotechno, 2020; 8: 33-37.[DOI]
[35] Ma Y, Chen Y, Liu P et al. Comparative study of the biochemical properties of membrane-bound and soluble polyphenol oxidase from Prunus mume. Lwt-Food Sci Technol, 2022; 171: 114156.[DOI]
[36] Adinarayana K, Ellaiah P, Prasad DS. Purification and partial characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. AAPS Pharm Sci Technol, 2003; 4: 1-9.[DOI]
Copyright © 2024 The Author. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.




 Copyright ©
Copyright ©