Prevalence of Intestinal Parasitosis Caused by Nematopsis sp (Protozoa: Apicomplexa) in Litopenaeus vannamei and its Relationship with Water Turbidity, in a Fattening Farm
Edison Pascal1*, Ysaías Alvarado1, Helimar Vásquez2
1Center for Molecular Biomedicine, Venezuelan Institute of Scientific Research (IVIC), Maracaibo, Venezuela
2Faculty of Veterinary Sciences, University of Zulia (LUZ), Maracaibo, Venezuela
*Correspondence to: Edison Pascal, PhD, Postdoctoral Researcher, Molecular Biomedicine Center, Venezuelan Institute of Scientific Research (IVIC), 92XM+6WP, Pan-American Highway, Macarao Parish, 1204, Venezuela; Email: edisonpascal@gmail.com
Abstract
Objective: This research aims to calculate the prevalence of intestinal parasitosis caused by Nematopsis sp (Protozoa: Apicomplexa) on white shrimp (Litopenaeus vannamei) and relate it to water turbidity, in a fattening farm.
Methods: The work was carried out on the western coast of the state of Falcón (Venezuela), where the animals (for clinical and laboratory analysis) and the turbidity data of the pool water were obtained. For the turbidity analysis, the Secchi disk was used, which allowed the measurement to be recorded in centimeters of transparency. The shrimp were captured by random sampling in the lagoons and immediately transferred to the laboratory for clinical study, subsequently data on prevalence, correlation (R), and coefficient of determination (R2) were obtained.
Results: Once the analyses were carried out, a correlation coefficient (R) of 0.319951628 was obtained, indicating the low dependence (weak positive connection) between the related variables (the prevalence of diseases and the levels of turbidity in the water). In the evaluation of the R2, the value of 0.102369044 was obtained, which indicated that in only 10.23% of the infected animals, there was an influence of turbidity on the prevalence of infected animals.
Conclusion: The prevalence values obtained in this study are consistent with those reported in other research in Latin America. In turn, the related data demonstrated that water turbidity could be related (weakly) to the presence of the parasitic protozoan Nematopsis sp, however, their statistical relationship is very scarce.
Keywords: prevalence, parasites, shrimp, gregarines, turbidity
1 INTRODUCTION
Shrimp farming represents one of the fastest-growing aquaculture sectors in Latin America, Asia, and Africa, contributing 8% of total fishery production. The shrimp aquaculture industry has had a significant boom; in 1980, shrimp farms produced 2% of the world's demand, and in 1990 they generated a record harvest of a total of 663,000 metric tons of shrimp, which shows an increase in the 12% compared to previous years. One million hectares of water produced an average of more than 630Kg/Ha, that is, demand increased by 25% at that time, and fishermen supplied the remaining 75% of demand, producing 2.6 million metric tons[1].
Crustaceans are aquatic organisms that can be affected by a variety of diseases caused by different infectious agents, including viruses, bacteria, fungi, and parasites. Among these agents, parasites represent one of the main threats to the health of crustaceans, and protozoa, in particular, play a crucial role as causative agents of diseases in these aquaculture species of interest[2].
These Apicomplexans, already mentioned, are endoparasitic protists with a complex life cycle, which use arthropods, mollusks, or annelids as definitive or intermediate hosts[3]. They obtain their food by osmosis in the cavity of the host, a defined membrane covers the body of the gregarines and their cytoplasm is differentiated ectoplasm and endoplasm, the ectoplasm presents myonemes, which allow the organism to make sliding movements[4].
According to the morphology of the trophozoite, sporozoans can be divided into three groups; the cephaline gregarines (Cephaline), where the trophozoite is divided into 3 parts, the acephaline gregarines (Acephaline) with only two parts and the paraophioidines, which in the adult state appear as a single elongated cell with a single nucleus in the middle zone. Morphologically, the smallest anterior part is the protomerite and the posterior part is the deuteromerite, which has a nucleus. The protomerite can be anterior, it is called epimerite expands freely, and can be used as a movable sucker to attach to the intestine of the shrimp (thigmotactio)[5].
This parasitic disease can occur due to a high planting density, and deterioration of water quality concerning physicochemical parameters: Low oxygen concentration, increase in the concentration of ammonia, or nitrites, changes in salinity, turbidity in the water, among others, and other alterations of the environment that develop, to the infection of a pathogen and the quality of the food supplied. The synergistic effect between the stress caused by alterations in environmental factors and the presence of a pathogenic organism increases the possibility of losses in shrimp farming[6].
Based on this premise, the present study aims to compare the relationship between a physicochemical parameter, specifically turbidity, and the prevalence of intestinal parasitosis caused by the apicomplexan protozoan Nematopsis sp in white shrimp (Litopenaeus vannamei) in a fattening farm. The choice of turbidity as a variable of interest is based on its potential impact on the health of crustaceans, as it has been documented that water quality influences susceptibility to various infections.
2 MATERIALS AND METHODS
2.1 Geographic Area of the Study
The research was conducted along the western coast of Falcón state in Venezuela, specifically in the coastal region of Mauroa municipality within the Gulf of Venezuela, in the far western part of the country. This area is home to several farms that L. vannamei. The particular farm examined in this study is located near the town of Casigua, with coordinates 11º 02' 59'' N 71º 04' 12'' W. The animals for the study were sourced from the fattening farm in the study area during a cultivation cycle that lasted from May to October 2022.
2.2 Water Turbidity Analysis
During sampling in each lagoon or pool, where the animals were obtained, this equipment was used to measure turbidity, which allowed the measurement to be recorded in centimeters of transparency. Significantly, it was observed that as transparency in centimeters increased, turbidity decreased, suggesting an inverse relationship between both parameters. This information is essential to better understand the environmental conditions in which animals develop and, in particular, to evaluate the impact of turbidity on the health and well-being of shrimp. The ponds have a maximum depth of two meters[7,8].
2.3 Sampling and Clinical Analysis of Organisms
The shrimp were collected using a 2-meter diameter net, with random casts made at different locations in the lagoon or pools. The captured specimens were placed in plastic containers and subsequently transported to the laboratory for pathological analysis. Additionally, particular attention was given to specific clinical signs that might suggest the presence of parasitosis caused by gregarines, which is crucial for gaining a better understanding of shrimp health and assessing the potential impact of infections.
To detect the presence of gregarines, the diagnostic method based on fresh mounting was used, using optical microscopy with 10x and 40x objectives. This procedure does not require any type of staining for the identification of the protozoan, making it a quick and efficient method to evaluate the presence of these parasites in shrimp samples. The identification of gregarines is crucial to understanding the dynamics of infection and developing appropriate management strategies to prevent and control these parasites in shrimp farming systems[9]. For each pool or lagoon sampled, a sample composed of 100 individuals was analyzed, to determine the number of sick animals and calculate the prevalence of gregarine infection. This sample size is representative and allows obtaining a reliable estimate of the health situation in each crop unit.
2.4 Parasitic Disease Prevalence Calculation
Prevalence is a fundamental epidemiological parameter that indicates the proportion of affected animals in a given population, and its calculation is essential to evaluate the impact of the disease and make informed decisions about the management and control of parasitosis in shrimp farming.
To calculate the prevalence of the parasitic disease, the equation proposed by Morales et al.[10] and by Peña and Varela[11] was used, which is widely used in epidemiological studies and allows for determining the proportion of affected individuals in a given population. This formula is essential to quantify the impact of parasitosis and monitor the evolution of the disease over time, which is crucial to implement effective prevention and control strategies in shrimp farming.
|
In which:
P: Prevalence
N: Number of hosts with parasites
Nt: Cumulative total of hosts
2.5 Statistical Examination of the Relationship between the Prevalence of Parasitic Diseases and Water Turbidity
To assess the association between the number of infected animals and its connection with water turbidity, Linear Regression analysis will be employed[12]. This method will enable calculating the line value in a scatter plot using the applicable equation:
|
In which:
y: Represents the dependent variable.
a: Is the intercept on the Y axis (also known as “y-intercept”).
b: Represents the slope of the line, which indicates the inclination of the line. It is the change in “y” for each unit change in “x”.
x: Is the independent variable, which is the value manipulated or introduced into the equation to calculate “y”.
This equation is fundamental in linear regression, where the goal is to fit a line to a set of data to model the relationship between the variables[12].
Furthermore, the Correlation Coefficient R will be computed using the relevant equation. This will facilitate evaluating the strength and direction of the association between the number of infected animals and the water’s physical parameters.
|
In which:
R: This is the Pearson correlation coefficient, which measures the strength and direction of the linear relationship between two variables.
![]() : This symbol represents the sum of the terms indicated below, from i =1 to “n”, where n is the total number of data pairs.
: This symbol represents the sum of the terms indicated below, from i =1 to “n”, where n is the total number of data pairs.
xi: This is the value of the independent variable in the i -th data pair.
![]() : This is the mean (average) of all values of the independent variable “x”.
: This is the mean (average) of all values of the independent variable “x”.
yi: This is the value of the dependent variable in the i -th data pair.
![]() :This is the mean (average) of all values of the dependent variable “y”.
:This is the mean (average) of all values of the dependent variable “y”.
![]() : This represents the deviation of each value “x_i” from the mean “
: This represents the deviation of each value “x_i” from the mean “![]() ”.
”.
![]() : This represents the deviation of each value “y_i” from the mean “
: This represents the deviation of each value “y_i” from the mean “![]() ”.
”.
![]() : This part of the formula calculates the square root of the sum of the squares of the deviations of “x”.
: This part of the formula calculates the square root of the sum of the squares of the deviations of “x”.
![]() : Similar to the previous one, this part calculates the square root of the sum of the squares of the deviations of “y”.
: Similar to the previous one, this part calculates the square root of the sum of the squares of the deviations of “y”.
After calculating the prevalence of the parasitic disease, the Coefficient of Determination (R2) is calculated using the corresponding equation:
|
In which:
Ssreg: Sum of Squares Regression
Sstot: Cumulative sum of squares
Ssres: The sum of Squares of Residuals
This statistical parameter is essential to evaluate the strength and direction of the relationship between the variables studied, thus allowing a better understanding of the dynamics of the disease and its relationship with environmental, epidemiological, and management factors in shrimp farming.
3 RESULTS AND DISCUSSION
3.1 Identification of Clinical Signs and Laboratory Analysis for detecting Nematopsis sp.
During the field sampling, a thorough analysis of the clinical signs present in the sampled organisms was carried out to determine the presence of Gregarines. One of the most relevant indicators of this infection is the appearance of a yellowish coloration in the intestinal thread area, which may suggest an alteration in the health of the affected organism. However, it is important to highlight that, throughout the observations made in the field, no clinical manifestations were recorded that could be associated with an infection caused by these protozoa. This absence of symptoms suggests that, under the evaluated conditions, the sampled organisms did not exhibit evident signs of being affected by Gregarines, which could indicate a relatively stable health status in the analyzed population.
In contrast, the situation was different in the laboratory's Pathological Biology study, where positively infected animals were observed. For the fresh analysis, the intestinal contents of L. vannamei were extracted, and the presence or absence of Nematopsis sp. was determined, considering the number of infected animals in each sample (Figure 1).
|
Figure 1. Trophozoites of the protozoan Nematopsis sp. were extracted from the intestinal contents of L. vannamei that were analyzed during the test.
The trophozoite of Nematopsis sp. exhibits distinct morphological features, with the protomerite located at the posterior end of the parasite and the deuteromerite at the lower part, where a clearly defined nucleus can be observed (Figure 1). Similarly, in the early linear syzygy of two trophozoites, the septum tends to vanish, and the nucleus of the satellite is positioned toward the basal region.
Likewise, in this research (to identify Nematopsis sp) the syzygy itself, formed by the trophozoites of this parasite, was taken into account, which can be early, late, and linear, of two individuals or multiple[6].
Gregarine trophozoites of the genus Nematopsis sp. were commonly found in the midgut of L. vannamei; however, these parasites were also occasionally detected in the stomach and hepatopancreas, though less frequently.
Apicomplexans of the genus Nematopsis sp are generally located in the intestine (midgut) or rectum; unlike Cephalolobus sp which could be located (generally) in the mouthparts of L vannamei, or the stomach of these crustaceans[13].
3.2 Statistical Analysis of the Relationship between Prevalence of Parasitic Diseases and Water Turbidity
3.2.1 Prevalence
The data presented in Table 1 indicate that the prevalence of parasitosis caused by the protozoan showed an upward trend during the trial, peaking in September at 35.5% and concluding in October at 32%.
Table 1. Total Average of the Prevalence and Turbidity of the Water During the Trial, for Each Month
Month |
Prevalence (%) |
Turbidity (cm) |
May |
20 |
50 |
June |
25 |
53.75 |
July |
25.55 |
54.44 |
August |
26.66 |
50.83 |
September |
35.5 |
49 |
October |
32 |
45 |
However, other literature sources indicate a maximum prevalence of 20% for gregarines in shrimp farms across Latin America[10]. Similarly, in Venezuela, Nematopsis sp. was recorded in juvenile Penaeus schmitti from the Gulf of Cariaco (Sucre state), with average prevalence values of 82.3%, yet no intestinal damage was reported. Only Nematopsis penaei has been identified as the sole species responsible for damage in Penaeus aztecus, Penaeus duorarum, and Penaeus setiferus in various locations in the United States. However, these authors observed hyperplasia with the formation of folds in the midgut and damage to the stomach epithelium.
In this study, a peak prevalence of 35.5% was recorded in September, which then declined to 32% in October. It is noteworthy that the highest prevalence reported for the region (Latin America) is 20%[10], which suggests that the prevalences obtained in this study are high, however, Laboratory analysis did not observe hyperplasia or other damage at the intestinal level. It is important to consider that the prevalence of parasitosis caused by gregarines can fluctuate based on the region, as well as environmental and sanitary conditions.
3.2.2 Turbidity
Regarding turbidity in the water, Boyd[14] mentions that, if the visibility of the Secchi disk is less than 30cm, it is due to suspended materials that can interfere with some processes in the culture lagoon and cause potential problems.
In this case, we can note that the highest transparency was found in July (55.44cm), denoting little turbidity, which is expressed as low algal growth, and few solids or organic matter in suspension[14]. However, their values tended to decrease from September to October (45cm), indicating a slight increase in turbidity due to a modest rise in phytoplankton productivity and a greater presence of suspended solids in the lagoon waters.
From this perspective, turbidity in lagoons or shrimp farming ponds results from the blooming of algae and soil particles or suspended organic matter. Both types of turbidity restrict the penetration of light into the aquatic system, and the decrease in this at the bottom of the pool or pond does not allow the growth of filamentous algae and macrophyte aquatic plants, undesirable at the bottom[15].
The quality of the water in the lagoons is a true critical point in the shrimp production and fattening process and must be controlled in physical, chemical, and biological parameters. These must be adequate and maintained within acceptable ranges for the good development of the shrimp. If not, the crop population could experience low growth, proliferation of pathogens with disease outbreaks, eventual mortality, and low product quality[16].
Maintaining water quality is a crucial factor for the optimal development of the immune system in crustaceans. From this viewpoint, farmed animals are better equipped to handle pathogenic agents present in their external environment. Turbidity of the water, caused by the excessive presence of suspended particles, could indicate unsanitary conditions in the shrimp’s habitat. High turbidity can be a sign of contamination by organic matter, sediments, or microorganisms (viruses, bacteria, and parasites), which can create a stressful and unfavorable environment for the development and health of farmed shrimp[15].
3.2.3 Analysis of the Relationship between Prevalence and Turbidity
The graph (Figure 2) shows a slight but significant trend between parasitic disease and water turbidity. Parasitosis caused by gregarines appears to increase slightly as water turbidity decreases.
|
Figure 2. Prevalence (in percentage) versus water turbidity (in centimeters), during the experiment run time.
This finding could indicate that there is a relationship, albeit weak, between water turbidity and the presence of the parasite Nematopsis sp. This correspondence suggests that water turbidity may have an impact on the spread of the disease associated with this parasite. However, it is important to note that, although turbidity may be an influencing factor, it cannot necessarily be considered the primary cause of the infection. Water turbidity can alter environmental conditions, affecting the health of aquatic organisms and consequently facilitating the proliferation of the parasite. Additionally, other factors such as water quality, temperature, and the presence of other contaminants may also play a crucial role in the dynamics of the disease.
In shrimp culture systems, the presence of high concentrations of suspended particles due to water turbidity has negative effects on light penetration, the biological productivity of the system, and the overall quality of the habitat for the cultured organisms. Scientific research has shown that after 3 weeks of exposure to high turbidity levels, the mortality rate in farmed white shrimp Litopenaeus vannamei (also known as Penaeus vannamei) can increase by up to 20%. Additionally, turbidity also decreases the shrimp’s tolerance to fluctuations in water salinity and reduces their consumption and efficiency in utilizing dissolved oxygen[16].
In this study, generally high-water transparency values were observed, indicating that turbidity levels were low for most of the time. Transparency is an indirect measure of turbidity, since the greater the transparency, the lower the amount of particles in suspension that obstruct the passage of light through the water. Therefore, when high transparencies are reported, as was the case in this research, it can be inferred that turbidity remained at reduced values compared to other aquatic systems.
3.2.4 Linear Regression Study, Calculation of the Correlation Coefficient and the Determination Coefficient
This time, a scatter plot was created (Figure 3), and linear regression mathematical tests were conducted to calculate the Correlation Coefficient and the Determination Coefficient. This index allows us to evaluate whether “Y” actually increases as a result of an increase in “X”, therefore, it can be considered as the analysis of two related issues[12].
|
Figure 3. Scatter diagram that relates prevalence to water turbidity.
To prepare the graph, the straight-line equation formula was used:
|
Concerning the Correlation Coefficient (R), a value of 0.319951628 was obtained, indicating a low level of dependence (weak positive relationship) between the variables (X and Y), that is, the physicochemical parameter analyzed (turbidity) does not have a marked influence on the prevalence of parasitosis caused by the apicomplexan protozoan, these variables being weakly related.
Once the calculation was performed to obtain the coefficient of determination R², a value of 0.102369044 was obtained. This result indicates that only 10.23% of the infected animals showed a significant influence of turbidity on the prevalence of the disease. In contrast, 89.77% of the studied crustaceans showed no evidence that water turbidity influenced the prevalence of the parasitic disease. This finding suggests that although water turbidity can have an effect in some cases, its impact is limited and is not a determining factor in most of the observed infections. The low proportion of turbidity influence could indicate that other environmental, biological, or even genetic factors play a more important role in the susceptibility of crustaceans to parasitic disease.
It is important to highlight that the data obtained suggest that water turbidity may be related to the presence of gregarines, which cause parasitosis, but the weak positive connection and the low coefficient of determination indicate that the relationship between both variables is not strong. Furthermore, these values do not allow us to establish a weak causal relationship, between the turbidity of the water and the prevalence of parasitosis caused by these protozoans, since there may be other factors that influence the presence of the Apicomplexans that cause parasitosis[17].
The previous studies by Saavedra et al.[6], have shown that the presence of gregarines is closely related to stable conditions of some physicochemical parameters. However, in our analysis, we did not find significant evidence of a direct association between the number of gregarines per intestine and the average of this physicochemical parameter of the water of the pools or ponds. This suggests that other factors, such as water quality, management of culture systems, and shrimp resistance, may play a more important role in the dynamics of gregarine parasitosis[18].
In a previous study carried out by Guzmán et al.[19], researchers concluded that infection by the parasite Nematopsis sp. in farmed shrimp of the species L. vannamei hurts the development of these crustaceans, which translates into a decrease in productivity and economic profits in shrimp farms. However, when comparing the results of that study with those obtained in the present investigation, it is observed that the degree of infection by this parasite was higher in the work carried out by Guzmán et al., compared to the infection levels found. in the current study.
From a broader approach, this study can be very useful to understand the dynamics of parasitic infections in farmed white shrimp. This information is valuable for the development and implementation of effective strategies for the control and prevention of parasitosis in the field of aquaculture[20].
4 CONCLUSIONS
The prevalence values obtained in this study are consistent with those reported in other investigations in Latin America on the presence of Nematopsis sp. in shrimp. Likewise, no intestinal damage was observed in the hosts. The related data demonstrated that water turbidity could be related to the presence of the parasitic protozoan Nematopsis sp, however, their statistical relationship is very scarce. Despite the prevalence detected, no epidemiological problem occurred on the farm during the study.
Acknowledgments
The researchers appreciate the support of the following institutions and companies: Venezuelan Institute of Scientific Research (IVIC), specifically the Center for Molecular Biomedicine (CBM), as well as the Faculty of Veterinary Sciences of the University of Zulia (LUZ), and the Lamar Group (Falcón Division), which served as a column and support, to carry out this investigation.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
Pascal and Vásquez carried out the clinical analysis, bibliographic analysis, and writing of the document, and Alvarado carried out the epidemiological calculations and bibliographic analysis.
Abbreviation List
L. vannamei, Litopenaeus vannamei
P, Prevalence
R, Pearson correlation coefficient
R2, Coefficient of determination
References
[1] Villalón J. Practical Manual for Semi-Intensive Commercial Production of Marine Shrimp. Texas A & M University, Sea Grant College Program: Galveston, Texas, USA, 1991.
[2] Pascal E, Vásquez H, Sandrea Y et al. Prevalence of parasitosis in shrimp (Penaeus vannamei) on two farms in Falcón State, Venezuela [In Spanish]. REDIELUZ, 2022; 12: 94-98.
[3] Figueredo A, Conroy D, Esteve M et al. Intestinal gregarinosis in juveniles of Farfantepenaeus brasiliensis from La Restinga, Margarita Island (Venezuela) [In Spanish]. AquaTIC, 2016; 44: 1-11.
[4] Prado C. Diagnosis, treatment and prevention of infestations caused by gregarines in Penaeus vannamei shrimp, using medicated diet [In Spanish]. undergraduate thesis. ESPOL, Guayaquil, Ecuador, 1996.
[5] Hernández R. Description of the intestinal infestation by gregarines of white shrimp Litopenaeus vannamei cultivated in San Blas, Nayarit [In Spanish]. Master Thesis. Xalisco, Nayarit, 2020.
[6] Saavedra-Bucheli M, Álvarez-León R, Rey-Carrasco I. Analysis of the incidence of gregarines in commercial crops of Litopenaeus vannamei and L stylirostris in the southern Colombian Caribbean [In Spanish]. Arq Cien Mar Fortaleza, 2008; 41: 9-23.
[7] Quijandria S. Correlation between Secchi disk and turbidimetry [In Spanish]. R-Chemical S. A. Lima, Peru, 2023. Available at:[Web]
[8] Pascal E, Vásquez H, Rodríguez R et al. Calculation of the incidence on parasitosis caused by Nematopsis sp in farmed shrimp [In Spanish]. Congress REDIELUZ 2022. Academic Vice-Rectorate of the University of Zulia (LUZ), 2022.
[9] Rodríguez M, Peña M. Prevalence of gregarines (Protozoa) in marine shrimp (Litopenaeus vannamei Boone, 1931) on four farms in El Salvador [In Spanish]. undergraduate thesis. Faculty of Agronomic Sciences, El Salvador University, 2017.
[10] Morales MS, Ruiz A, Pereira A et al. Disease prevalence of white shrimp (Litopenaeus vannamei) cultured in eight regions of Latin America [In Spanish]. Sci Mag, Fac Vet Sci Univ Zulia, 2011; 21: 434-446.
[11] Peña-Navarro N, Vargas-Cordero R, Varela-Mejías A. Natural products as immune system stimulators in Litopenaeus vannamei infected with Vibrio parahaemolyticus [In Spanish]. Agron Mesoam, 2013; 24: 133-147.[DOI]
[12] Copete C. Introduction to linear regression analysis [In Spanish]. Benemérita Autonomous University of Puebla, 2007.
[13] Lotz L, Overstreet R. Parasites and predators of peneid shrimp [In Spanish]. Editorial McGraw-Hill, Mexico, 1989.
[14] Boyd C. Considerations on Water and Soil Quality in Shrimp Farming. Department of Fisheries and Allies, Aquacultures. Auburn University, Alabama, USA, 2004.
[15] Mayer E. Monitoring of pond water quality to improve shrimp and fish production [In Spanish]. IAF (International Aquafeed): Spain, 2012. Available at:[Web]
[16] Rojas A, Haws M, Cabanillas J. Good management practices for shrimp farming. The David and Lucile Packard Foundation [In Spanish]. United States Agency for International Development (Cooperative Agreement No. PCE-A-00-95-0030-05), 2005.
[17] Morales V, Cuéllar-Anjel J. Pathology and immunology of penaeidos shrimps (Second edition) [In Spanish]. OIRSA: Panama, 2014.
[18] Zavala O, Hernández R, Valdez F et al. Intestinal gregarine infestation in cultured white shrimp Penaeus vannamei in Nayarit, Mexico [In Spanish]. Bio Cienc J, 2022; 9: e1277.[DOI]
[19] Guzmán F, Pérez R, Gutiérrez G et al. Impact of gregarine parasitosis (Nematopsis sp) on shrimp culture Litopenaeus vannamei [In Spanish]. Ra Ximhai, 2014; 6: 10.
[20] Pascal E, Portillo E, Méndez A et al. Relationship between infection caused by the apicomplex protozoan Nematopsis sp and the weight of white shrimp Litopenaeus vannamei in a cultivation system. J Biomed Res Environ Sci, 2023; 4: 1405-1411.[DOI]
Copyright © 2024 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.

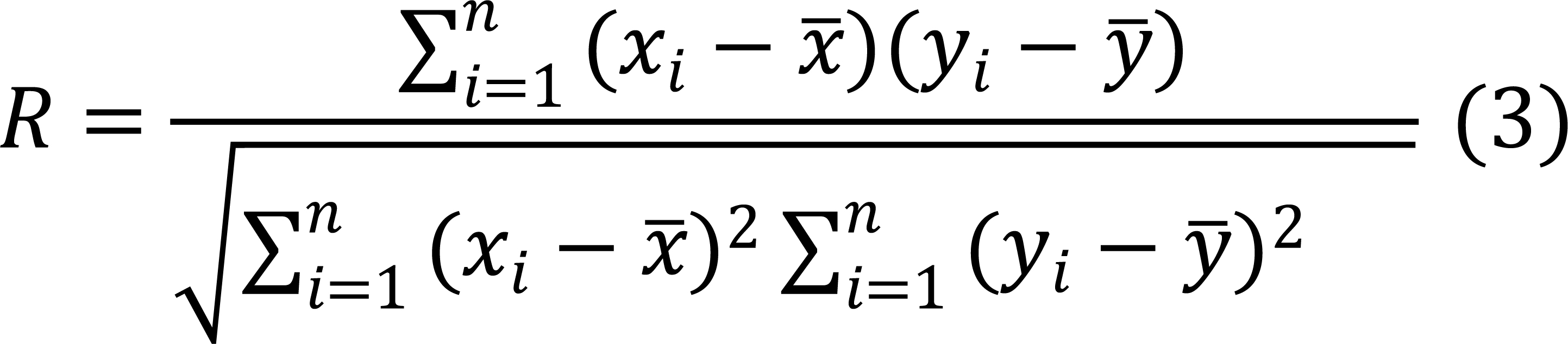






 Copyright ©
Copyright ©