Effect of Accelerated Storage on the Stability of Azoxystrobin Fungicide and It Is Toxicity on Albino Rats
Manal A. A. Abdel razik1![]() , Mohammed Abdallah Saleh2, Khairy A. Ibrahim3, Dyaa M Nassar2*
, Mohammed Abdallah Saleh2, Khairy A. Ibrahim3, Dyaa M Nassar2*
1Pesticides Department, Faculty of Agric, Menoufia University, Shebin El-Kom, Egypt
2Department of Pesticides Analysis Researches, Central Agricultural Pesticides Laboratory, Agricultural Research Center, Dokki, Giza, Egypt
3Department of Mammalian and Aquatic Toxicology, Central Agricultural Pesticides Laboratory, Agricultural Research Center, Dokki, Giza, Egypt
*Correspondence to: Manal A. A. Abdel razik, PhD, Professor, Pesticides Department, Faculty of Agric, Menoufia University, Shebin El-Kom, PO Box 32514, Egypt; Email: new1folder@yahoo.com
Abstract
Objective: This study was conducted to determined the effect accelerated storage procedure on fungicide stability, as well as, to evaluate the impact of sub lethal doses of tested fungicides on reproductive and liver in male albino rats.
Methods: Determine the effect of accelerated storage at 54±2℃ for 3, 7, 14 and 21 days on the stability of azoxystrobin. Investigate the effects of sub chronic exposure on male albino rats. Investigate the effects of sub chronic exposure on histopathological examination of the liver.
Results: Azoxystrobin were relatively stable after accelerated storage at 54±2℃ for 21 days. The mediam lethal dose (LD50) of azoxystrobin was 681.71mg/kg. Azoxytrobin 25% induced a significantly decreased in the sperm count and the motility percent at 1/20 and 1/40 LD50.The serum LH and FSH levels decreased with increasing doses of azoxystrobin. There were no histopathological alteration occurred after treated with azoxystrobin at 1/20 and 1/40 of LD50. The ALT and AST enzymes levels increased to (7.75 &.36.58U/L) and (4.39 &15.23U/L) after treated with 1/20 and 1/40 of LD50, also, the cholesterol and triglycerides levels were increased recoding (116.01 &184.89mg/dL) and (110.18 &138.24mg/dL) after treated with 1/20 and 1/40 of LD50, respectively. The rats treated with 1/20 of LD50 displayed massive inflammatory cells aggregation.
Conclusion: Azoxystrobin were relatively stable after accelerated storage at 54±2℃ for 21 days. The high dose 1/20 of LD50 decreased the serum LH and FSH levels, increased the liver enzymes, and induced histopathological effects of the liver.
Keywords: azoxystrobin, accelerated storage, fertility, sperms, reproductive, liver, enzymes, histology
1 INTRODUCTION
Pesticides are chemicals that are widely applied in protecting plants from pests, insects and infectious diseases as well as foods stored in storage facilities from rodents, insects and other biological pollutants additionally, pesticides are used in order to prevent the spread of pests that are dangerous to both humans and cattle. Rodenticides, insecticides, fungicides and herbicides are types of pesticides used to eradicate or manage various pests including fungi, insects, weeds and rodents[1]. Fungicides are a class of pesticides that include biological agents or biocidal chemicals that are used to eradicate or stop the spread of parasitic fungi or fungal spores[2]. Most plants have fungal illnesses, and when the right environmental factors are present-such as an abundance of irrigation water, a high plant density, and a tardiness in addressing the issue from the begin pathological infection multiplies and gets worse. As a result, the quality and quantity of the crops grown decline[3]. Fungicides have made a significant contribution to raising agricultural output and supplying the expanding human nutrient needs. Despite the fact that these pesticides have many advantages, but also poses a number of risks to people, living things, and the ecosystem as a whole. This is one of the issues that people currently face[4]. Azoxystrobin, a systemic fungicide from the strobilurin family, is applied as a preventive fungicide to protect fruits and vegetables from fungi that cause diseases like (soil fungus, downy mildew, powdery mildew, as well as fruit rots)[5]. In the present day, pesticides are commonly used to get rid of undesired plants, fungi, or insects. It is well recognised that any chemical substance may cause health issues due to its toxicity. Pesticides directly affect both human and animal health[6].
Bartlett DW and Rahul G et al. reported that pyraclostrobin was faster degradation in aqueous solution under the UV photolysis compared with sunlight[7,8]. Zeng LR et al. found that Strobilurin residues stay in the air, soil, or water after field applications[9]. Rodrigues ET et al. reported that amistar fungicide was stable at room temperature for up to 48h[10]. Reddy SN et al. revealed that exposure the azoxystrobin impairs oocyte maturation[11]. Gao W et al. found that azoxystrobin reduction oxidative and metabolic processes and decreased the serum levels of sex hormones, calcium, and total protein in rats and induced distinct histological characteristics[12]. Strobilurins were less toxic to mammals[13].
The aim of this study is determine the effect accelerated storage procedure on fungicide stability, as well as, to evaluate the impact of sub lethal doses of tested fungicides on reproductive and liver in male albino rats.
2 MATERIALS AND METHODS
2.1 Tested Insecticide
Azoxystrobin is Methyl (2E)-2-(2-{[6-(2-cyanophenoxy) pyrimidin-4-yl] oxy} phenyl)-3-methoxyprop-2-enoate (C22H17N3O5). The formulation (Amistar 25% SC) was supplied from Central Agricultural Pesticides Laboratory - Agriculture Research Center (ARC) - Ministry of Agriculture, which obtained from Syngenta AGRO AG - Switzerland or its factories in Hungary.
Accelerated storage procedure According to CIPAC M[14], this procedure was followed. In the a bottle, 50mL of an aqueous suspension concentration were added, the bottles were storage at 54±2℃ for 3, 7, 14, and 21 days with their caps on. On schedule, each bottle was taken out of the oven and allowed to cool to room temperature before having its cap removed. From these bottles, an estimate of the percentage of the active component has been made.
2.2 Determination of Active Ingredient of Azoxystrobin Content
2.2.1 Standard Preparation
Standard of azoxystrobin Figures 1 and 2 were prepared according to the method of Marczewska P et al.[15] with some modification, where 10mg of analytical standard of tested fungicides were weighed in 25mL volumetric flask and filled to the mark with HPLC grade methanol, then placed in an ultrasonic bath for 5min to completed dissolution.
|
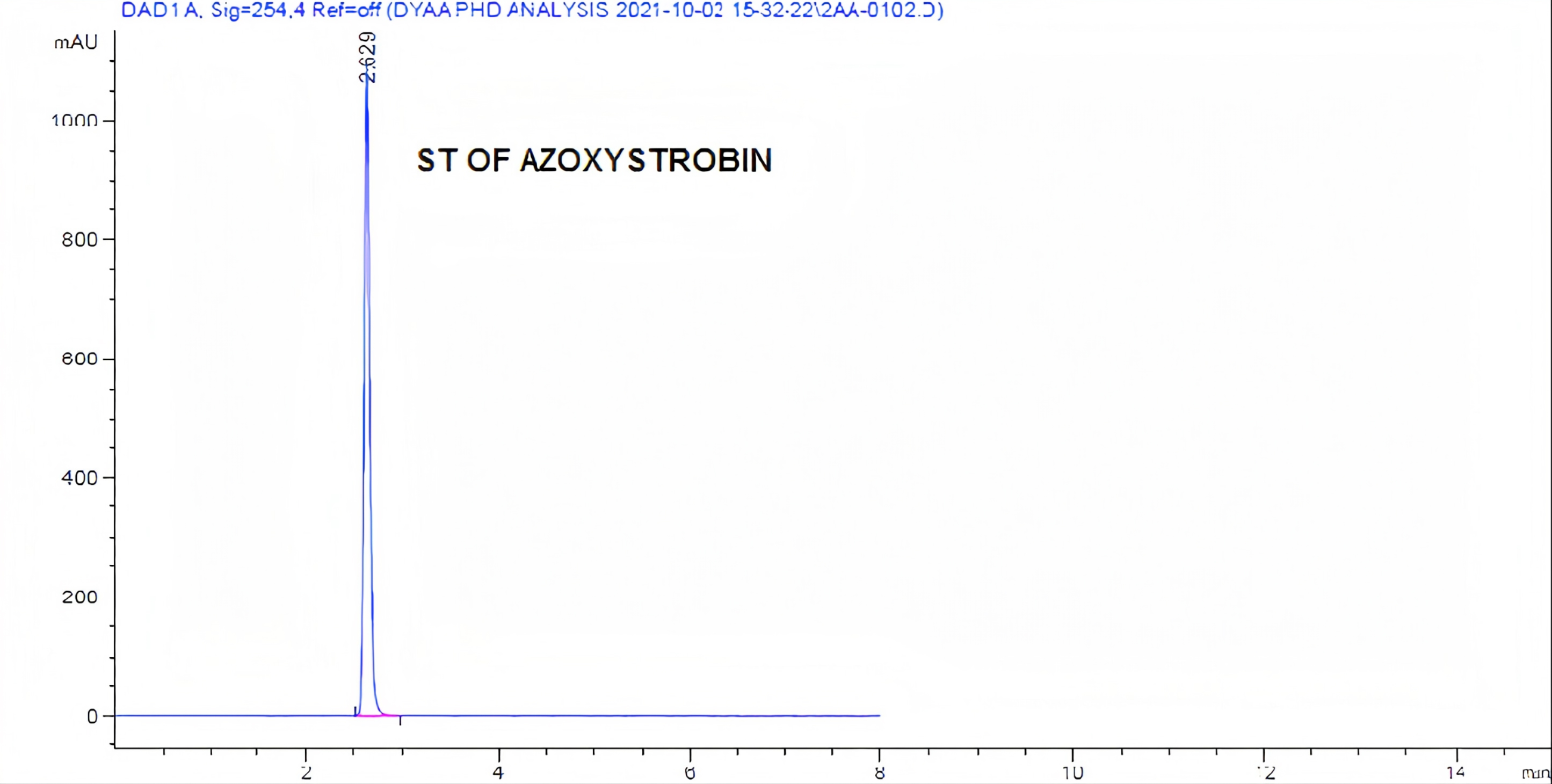
Figure 1. Standard chromatogram of azoxystrobin analysis by HPLC.
|
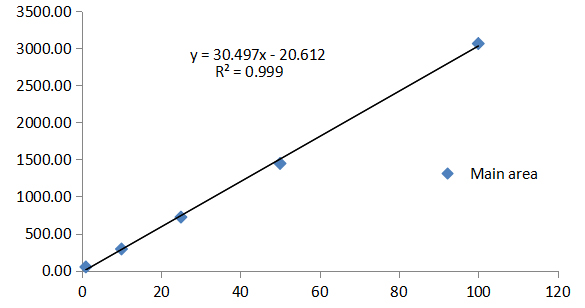
Figure 2. Calibration curve of azoxystrobin.
2.2.2 Determination of Active Ingredient Content of Azoxystrobin by HPLC
The active ingredient percentage for azoxystrobin was determined before and after storage using HPLC high performance liquid chromatography (Table 1), according to the method of Lazic S and Sunjka D[16].
Table 1. Type of HPLC High Performance Liquid Chromatography
HPLC |
Detector |
Column |
Agilent technologies 1,200 series |
DAD* |
Zorbax Eclipse Plus C18 (4.6×250mm×5µm) |
Notes: *DAD: Diode array detector.
With some modification: acetonitrile-methanol (90:10) was used as mobile phase, at the rate 1mL/min, and wavelength 254nm. At this condition the retention time (RT) of azoxystrobin was 2.629min.
2.3 Sub Chronic Effect of Azoxystrobin on Spermatogenesis, Fertility and Liver in Male Albino Rats
2.3.1 Animals and Treatments
A total of 35 Mature male albino rats (Rattus Norvegicus Sprague Dawley strain), body weight ranging from 150-170g were obtained from Helwan farm of Egyptian Organization for Vaccine and Biological preparation (Vaccera). Male albino rats were housed in Central of Excellence in toxicology testing, Central Agricultural Pesticides Laboratory (CAPL), Giza, Dokki, Egypt. These animals were put under good health laboratory conditions and adept for 2 weeks before the experimental periods. The animals were allowed access to water and drinking through specially designed glasses and fed on the standard basal diet consisting of mixture of starch 60%, salt mixture 4%, casein 20%, cotton seed oil 10%, cellulose 5% and vitamin mixture 1%.
2.3.2 Determination of Lethal Dose (LD50)
Twenty male albino rats were divided into four groups and placed in cages. Each group received different oral doses of the fungicide azoxystrobin (444.444, 666.666, 1,000, and 1,500mg/kg body weight), respectively. Deaths were followed up for three days according to Weil CS[17].
|
Where:
Log Da = the log of the lowest of the four dosage levels used
d = the logarithm of the constant ratio between dosage levels
f = it is taken from the table
2.3.3 Experimental Design
Fifteen of Adult male albino rats were divided allocate in to three groups an average of five from them per groups as follows: Group 1 (control): rats were fed normal and given tap water, and rats were not given any doses of pesticides. Group 2: rats given the highest dose of azoxystrobin (1/20 of LD50). Group 3: rats given the lowest dose of azoxystrobin (1/40 of LD50).
2.3.4 Sampling
Weekly animal weights were taken, and the amount of body weight gain was calculated. At the end of the 65-day trial, the animals were scarified under anesthesia, after which the heart was taken out and the animals were dissected. Thru the eye, blood samples were obtained. Blood was drawn into special tubes made for this purpose, allowed to stand at room temperature for 30min, centrifuged for 15min at 3,000rpm to separate the serum, and then stored at -20℃ until fertility hormone tests and liver enzymes were examined. The testicles, liver, and epididymis were taken out. To determine fertility, the epididymis was immediately prepared. For histological analysis under a light microscope, the testes and livers of male albino rats that had been slaughtered were removed, weighed, and put in 10% formalin solution.
2.4 Fertility-related Parameters
2.4.1 Estimation of Motility and Sperm Counts
The right cauda epididymis of each rat was chopped and thoroughly mixed in 10mL of warm (36℃) 0.9% NaCl solution for sperm counts and motility testing soon after sacrifice. This mixture was examined using a light microscope (ZEISS, 40X) on a volume of 20µL. Eight fields were used to count both motile and non-motile sperms, and the percentage of motile spermatozoa was calculated using the formula below:
|
The technique was made according to Ngoula F et al[18]. Sperms counts: 380µL of water was taken for 20µL of mixture, a glass cover was placed over the counting area on the hemocytometer slide and the micropipette was used to introduce 20µL of the mixture diluted with water and examined under a light microscope (ZEISS, 100X) on 5 fields[19].
2.4.2 Sperm Viability Testing
This method is used to tell a live sperm from a dead one. 50µL drop of the eosin stain (1%) was applied to 50µL of stock sperm suspension on a microscope slide, which was then viewed under a microscope after being allowed to stand for 5min at 37℃. While the heads of living spermatozoa were not stained with the eosin stain, the heads of deceased spermatozoa were. Sperm viability was calculated as the proportion of viable sperm to all sperm counted[20].
2.4.3 Hormone Levels Assay
Chemiluminescence Immunoassay (CLIA) system was used to analyze the levels of serum luteinizing hormone (LH), follicule stimulating hormone (FSH), and testosterone (T) using a competitive enzyme linked immunosorbent assay (ELISA) method using a rat kit purchased from kamiya biomedical company[21].
2.5 Liver Enzyme Level Estimation
2.5.1 Determination of AST and ALT Levels
The enzymes alanine aminotransferase (ALT/GPT) and aspartate aminotransferase (AST/GOT) were measured using colorimetric technique. Method for determining AST and ALT: First, 100µL of serum, 0.5mL of reagent 1 (R1 buffer), and the combined contents of the test tubes should be added to the test tube. The mixture of the contents of the tubes should then be added together with 0.5 millilitres of reagent 2, and the mixture should be incubated at 20 to 25℃ for precisely 20min. Finally, combine the contents of the combined tubes with 5 millilitres of sodium hydroxide. The second tube was created using the same steps as the first one, but without the serum to create a reagent blank. After five minutes, compare the specimen’s absorbance at 546nm to a blank using an enzyme analyzer (JASCO V-630 spectrophotometer)[22].
2.5.2 Determination of Total Protein
A colorimetric method was used to calculate the total amount of protein. Calculating total protein involves: First, place 1mL of reagent (R) in test tube (1). Then, place 1mL of reagent (R) and 20µL of standard in test tube (2). There are 20µL of sample in test tube (3) and 1mL of reagent (R) for the sample (serum). All of these test tubes endure a 10min incubation period at room temperature. Determine the absorbance of the sample and the standard against the reagent blank in less than 30min using an enzyme analyzer (JASCO V-630 spectrophotometer) operating at 540nm[23].
Calculation:
|
2.5.3 Determination of Triglycerides
Triglycerides were measured using a colorimetric technique. These steps are used to compute triglycerides: One millilitre of reagent (R) should be placed in the first test tube, one millilitre of reagent (R) and 10µL of standard in the second test tube, and 10µL of sample (serum) and 1mL of reagent (R) in the third test tube. All of these test tubes are incubated at 37℃ for 5min using an enzyme analyzer (JASCO V-630 spectrophotometer) operating at 546nm; determine the absorbance of the sample and the standard against the reagent blank in less than 30min[24].
Calculation:
|
2.5.4 Determination of Cholesterol
A colorimetric method was used to calculate cholesterol levels. To determine cholesterol, follow these steps: The first test tube should include one millilitre of reagent (R), the second test tube should contain one millilitre of reagent (R) and 10µL of standard, and the third test tube should have µL of sample (serum) combined with one millilitre of reagent (R). These test tubes are all incubated for 5min at 37℃. Determine the absorbance of the sample and the standard against the reagent blank using an enzyme analyzer (JASCO V-630 spectrophotometer) operating at 546nm in less than 30min[25].
Calculation:
|
2.6 Histopathological Examination for Testes and livers
Testes and livers were fixed in neutral buffered formalin at 10% for the duration of the histological investigation, and then rehydrated in 70% ethanol. After cutting the tissue and mounting them on slides, the tissue was embedded in paraffin. Hematoxylin and eosin (H&E) stain was used to examine the slide slices under a light microscope[26].
2.7 Statistical Analysis
SPSS 16.0 (SPSS, USA) was used for all statistical comparisons between groups. Each group’s mean value and associated standard deviation (SD) were calculated statistically from the data supplied in this study. One-way analysis of variance (ANOVA) was used to statistically analyze differences between means. If the F-ratio was significant, the statistical significance between the groups was assessed at P<0.05 level of significance using the posthoc least significant difference (LSD) tests for multiple comparisons.
3 RESULTS
3.1 Effect of Accelerated Storage Procedure on Azoxystrobin Stability
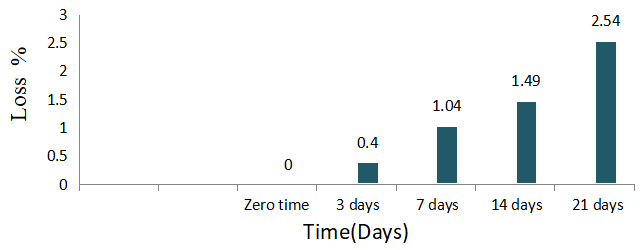
The effect of accelerated storage procedure after 3, 7, 14 and 21 days at 54±2℃ on stability of azoxystrobin fungicide is shown in Table 2 and Figure 3. The obtained results indicated that there were low impact on the stability of azoxystrobin was happened after storage at 54±2℃ for 3,7,14 and 21 days, where the content percent was ranged between (24.79-24.16%) after fungicide storage for 21 days at 54±2℃. The loss percent reached to 2.54% after 21 day from accelerate storage at 54±2℃.
Table 2. Effect of Accelerated Storage Procedure After 3, 7, 14 and 21 days At 54±2℃ on Stability of Azoxystrobin
Time (Days) |
Azoxystrobin |
|
Content (w/v) % |
Loss % |
|
Zero time |
24.79 |
0 |
3 days |
24.69 |
0.4 |
7 days |
24.53 |
1.04 |
14 days |
24.42 |
1.49 |
21 days |
24.16 |
2.54 |
|
Figure 3. Loss percent of azoxystrobin after accelerate storage for 21 days at 54±2℃.
The obtained results are in agreement with FAO[27] which decided that the percentage of active ingredient should not be less than 95%, meaning that the loss rate does not exceed 5%. Also Yao J et al. indicated that the microspheres’ azoxystrobin at 54℃ for 14 days had good physical and chemical stability during the storage test[28].
3.2 Toxicological Effect of Azoxystrobin
3.2.1 The Median LD50 of Azoxystrobin
The obtained results showed that the mediam LD50 of azoxystrobin (Amistar 25%) was 681.71mg/kg. The sub lethal doses 1/20 and 1/40 of LD50 for azoxystrobin were 34.08 and 17.04mg/kg BW, respectively.
3.2.2 Clinical Signs and Body Weight
Azoxystrobin was administered orally to albino rat males for 65 days; it was obvious that no deaths occurred during this time, and no additional indicators of general toxicity were seen. The effect of azoxystrobin in body weight of male albino rats and body weight gain % after administered orally for 65 days were shown in Table 3. The obtained data indicated that there were no significant differences between treated groups and control in body weight. According to the findings, there was no discernible difference in body weights between the exposure groups to azoxystrobin.
Table 3. Effect on Body Weight of Male Albino Rats Exposed to Azoxystrobin, for 65 days
Treatment |
Body Weight (g) |
Body Weight Gain |
||
Initial |
Final |
(g) |
% |
|
Control |
157±3.78a |
348±15.17a |
191 |
100 |
AZX 1/20 |
159±4.58a |
351±24.13a |
192 |
100 |
AZX 1/40 |
158±6.24a |
348±32.92a |
190 |
99 |
Notes: Results are presented as means ± SD. a Indicate not significant difference at P<0.05 compared with the control group, respectively.
3.3 Effect of Azoxystrobin Fertility in Male Albino Rats
3.3.1 Fertility-related Parameters of Azoxystrobin
Table 4 illustrate the effect of azoxystrobin (amistar 25%) at doses 1/20 and 1/40 LD50 on male rats after exposed for 65 days, where, testicular metrics including sperm count, sperm motility, and viable sperms examination are also shown.
Table 4. Effect on Sperms Characteristics Male Rats Exposed to Sublethal Doses Azoxystrobin for 65 Days
Treatment |
Counts (106) |
Sperm Characteristics |
||
Count % |
Motility % |
Viable % |
||
Control |
43.33a±1.15 |
100 |
99.7a±0.006 |
96.7a±0.015 |
AZX 1/20 |
38.66b±1.53 |
89.22 |
89b±0.01 |
89.3b±0.015 |
AZX 1/40 |
40.33c±1.53 |
93.07 |
92.3c±0.015 |
91.7c±0.015 |
Notes: Results are presented as means ± SD. b, c Indicate a significant difference at P<0.05 compared with the control group, respectively.
The obtained data revealed that azoxytrobin 25% induced a significantly decreased in the sperm count at dose levels 1/20 and 1/40 compared with control, and the sperm count percent decreased to 89.22 and 93.07% in rats treated with 1/20 and 1/40 of LD50, respectively. The effect of 1/20 of LD50 dose was more than 1/40 of LD50. As for motility percent, the data in Table 4 clearly indicated that the motility percent decreased significantly two treatments compared with control recording motility percent 89 and 92.3%, for 1/20 and 1/40 of LD50 respectively. The high dose was more effective than low one, suggesting that sperm motility also decreased as the dose increased. As well as, the percentage of viable sperm % declined significantly in two treatments compared with control recording 89.3% and 91.7% after treated with 1/20 and 1/40 of LD50 respectively. Also, the higher dose induced the higher decrease.
3.3.2 Effect of Azoxystrobin on the Sexual Hormones
The levels of concentration LH, FSH, and T serum hormones are displayed in Table 5. The findings demonstrated a statistically significant decrease in serum LH and FSH concentration levels with increasing doses of azoxystrobin. Where, the LH, FSH, and T level were (4.42 and 4.79mlu/mL), (3.97 and 4.04mlu/mL) and (2.93 and 3.23ng/mL), respectively. It was obvious that the high sub LD50 induced the higher decrease in three hormones level.
Table 5. Effect on Serum Hormone Levels in Male Rats Exposed to Azoxystrobin for 65 Days
Treatment |
Serum Hormone Levels |
||
LH (mlu/mL) |
FSH (mlu/mL) |
T (ng/mL) |
|
Control |
5.22a±0.036 |
4.24a±0.030 |
3.34a±0.030 |
AZX 1/20 |
4.42b±0.058 |
3.97b±0.010 |
2.93b±0.035 |
AZX 1/40 |
4.79c±0.025 |
4.04c±0.015 |
3.23c±0.015 |
Notes: Results are presented as means ± SD. b, c Indicate a significant difference at P<0.05 compared with the control group, respectively.
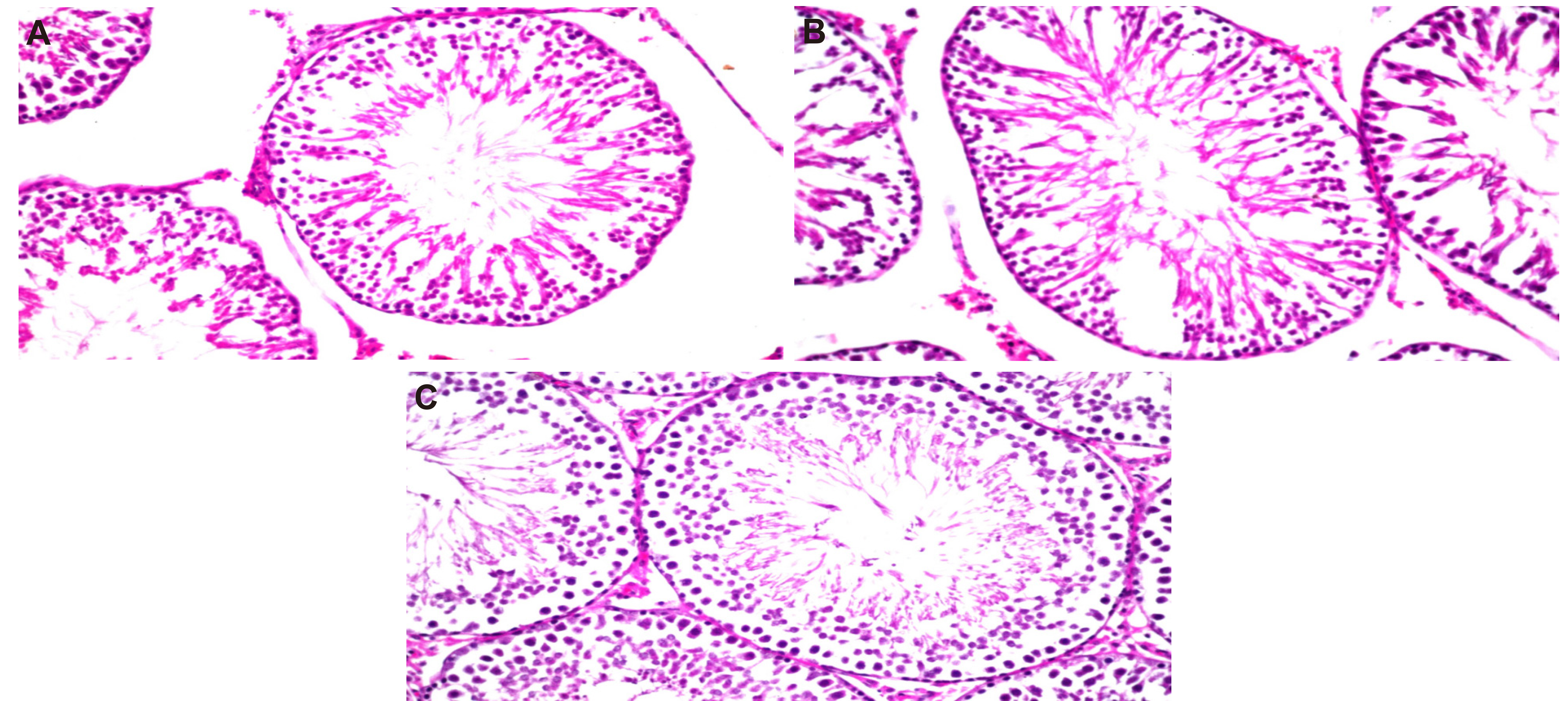
3.3.3 Testicular Histopathology Examination
A dose-dependent effect of azoxystrobin on sperm production was revealed by reproductive histopathology. The control rats displayed there was no histopathological alteration and the normal histologicl structure of the mature active seminiferous tubules with complete spermatogenic series (Figure 4A). The rats treated to azoxystrobin 1/20 and 1/40 of the dosage LD50 showed there were no observed histopathological alteration occurred Figure 4B and C.
|
Figure 4. A photomicrograph of testicular of male rats. A: Control group; B: High dose of azoxystrobin 1/20 LD50; C: Low dose of azoxystrobin 1/40 LD50.
3.4 Effect of Azoxystrobin on Liver in Male Albino Rats
3.4.1 Effect of Azoxystrobin on Enzymes
The data in Table 6 revealed that there were significant differences between treatments and between control in the levels of ALT, AST, cholesterol, total protein, and triglycerides in the blood. The ALT and AST enzymes levels increased to (7.75 & 36.58U/L) and (4.39 & 15.23U/L) after treated with 1/20 and 1/40 of LD50, respectively, compared with control which recorded 3.05 and 10.22U/L. Also, the cholesterol and triglycerides levels were increased in two treatments compared with control, recoding (116.01 & 184.89mg/dL) and (110.18 & 138.24mg/dL) after treated with 1/20 and 1/40 of LD50, respectively. As for total protein, there were significant differences between treatment 1/20 of LD50 and control, while there were no significant differences between treatment 1/40 of LD50 and control.
Table 6. Effect on Serum Enzymes Levels in Male Rats Exposed to Azoxystrobin for 65 Days
Serum Enzymes Levels |
Treatment |
||
Control |
AZX 1/20 |
AZX 1/40 |
|
ALT (U/L) |
3.05a±0.064 |
7.75b±0.392 |
4.39c±0.096 |
AST (U/L) |
10.22a±0.17 |
36.58b±1.39 |
15.23c±0.30 |
Chelestrol (mg/dL) |
62.75a±0.66 |
116.01b±0.72 |
110.18c±1.67 |
Total protein (g/dL) |
5.70a±0.115 |
6.03b±0.180 |
5.89a±0.050 |
Triglycerides (mg/dL) |
106.06a±1.36 |
184.89b±1.97 |
138.24c±4.16 |
Notes: Results are presented as means ± SD. b, c Indicate a significant difference at P<0.05 compared with the control group, respectively.
3.4.2 Histopathological Examination of the Liver
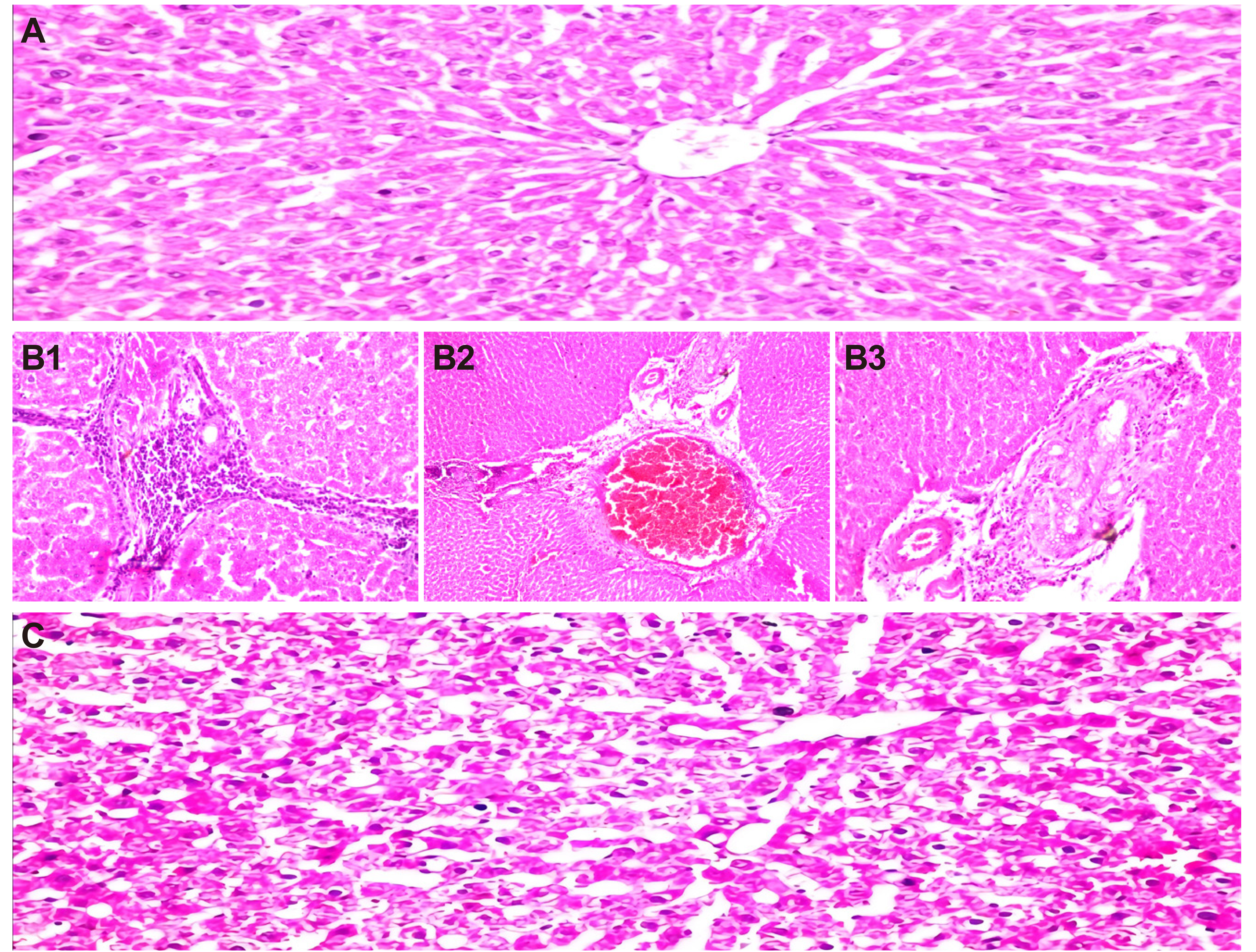
Histopathology analysis in rats demonstrated a dose-dependent effect of azoxystrobin on liver. The normal histologic structure of the central vein and surrounding hepatocytes in the parenchyma were observed in the control rats, which showed no histopathological changes recorded in Figure 5A.
|
Figure 5. A photomicrograph of liver of male rats. A: Control group; B: High dose of azoxystrobin 1/20 LD50; C: Low dose of azoxystrobin 1/40 LD50.
The rats treated to azoxystrobin 1/20 of the dosage LD50 in Figure 5B displayed there was massive inflammatory cells aggregation was observed in the portal area (Figure 5B1). The portal area showed also severs dilatation and congestion in the portal vein (Figure 5B2) as well as per ductal inflammatory cells infiltration surrounding the hyperplastic bile ducts (Figure 5B3). The rats administered to azoxystrobin 1/40 of the dosage LD50 reported that there was sever degeneration in the hepatocytes with dilatation in the central vein and sinusoids in Figure 5C.
4 DISCUSSION
According to the current research, the treated male albino rats did not experience any general harmful effects from azoxystrobin oral doses of 1/20 and 1/40 LD50, and there was no change in body weight. These findings are in line with Fishel FM who claimed that the strobilurin group, to which the compound azoxystrobin belongs, is a group that is safe for mammals and so does not cause body weight loss[29].
The effect of 1/20 of LD50 dose was more than 1/40 of LD50, these results corroborate with those of Kaplan A et al. who discovered that giving male white rats azoxystrobin doses of 1/10 and 1/20 LD50 for two months resulted in a decrease in sperm count and sperm motility[23].
As for the effect of azoxystrobin on the sexual hormones, the obtained results are compatible with Gad EL‑Hak HN et al. who reported that Azoxystrobin exposure caused testicular toxicity due to changes in hormone levels and sperm counts[13]. Azoxystrobin also reduced sperm characteristics, which affected FSH, LH and T levels.
As for the testicular histopathology examination, the obtained results from administering doses of azoxystrobin 25% at rates 1/20 and 1/40 of LD50 are disagree with Ziada RM et al.[30] who determined that the effect of various doses of azoxystrobin at rates of 1/10, 1/20, and 1/40 of LD50 on the germinal epithelium has been measured to prove decrease in spermatogenic cells. All treated groups had substantially different germinal tissue from the control group.
Generally, the tested enzymes levels were increased in two doses and the high dose induced the higher increase in enzyme levels.
The obtained data indicated that, azoxystrobin 1/20 and 1/40 of LD50 increased the concentration of both ALT and AST, and this is consistent with Ziada RM et al.[30] who reported that azoxystrobin increased liver function ALT and AST. We also find that azoxystrobin 1/20 and 1/40 of LD50 increased the concentration of both cholesterol and triglycerides, and this is not consistent with Fang N et al.[31] who explained that kresoxim-methyl belonging to the strobilurins group led to a decrease in the level of cholesterol and triglycerides.
As for the histopathological examination of the liver, these results agree with Gad EL‑Hak HN et al.[13] who showed that liver sections of the treated group with azoxystrobin 1/10 of LD50 dose demonstrated hydropic degeneration. Moderate dose (1/20 of LD50) demonstrated infiltration of mononuclear cells (arrow), hypertrophied, and edoema of portal area. A high dose was shown infiltration of mononuclear cells (arrow), hypertrophied, and edoema of portal area.
5 CONCLUSION
Azoxystrobin was relatively stable after accelerated storage at 54±2℃ for 21 days. Azoxytrobin 25% induced a significantly decreased in the sperm count and the motility percent at 1/20 and 1/40 LD50 compared with control. The serum LH and FSH levels decreased with increasing doses of azoxystrobin. The ALT, AST, cholesterol, total protein, and triglycerides levels were increased in the blood after treated with 1/20 and 1/40 of LD50, also, the cholesterol and triglycerides levels were increased. Azoxystrobin at 1/20 of LD50 displayed massive inflammatory cells aggregation; while at 1/40 of LD50 sever degeneration in the hepatocytes with dilatation in the central vein and sinusoids.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest.
Ethical Statement
Ethics approval and consent to participate.
Author Contribution
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Abdel razik MAA, Saleh MA, Nassar DM and Ibrahim KA. The first draft of the manuscript was written by Abdel razik MAA and Saleh MA commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Abbreviation List
ALT/GPT, Alanine aminotransferase
AST/GOT, Aspartate aminotransferase
LD50, Lethal dose
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Lushchak VI, Matviishyn TM, Husak VV et al. Pesticide toxicity: A mechanistic approach. EXCLI J, 2018; 17: 1101-1136.[DOI]
[2] Dierolf K, Glass C, McKergow M et al. Towards useful organising in the SF world. InterAction, 2012; 4: 5.
[3] Zhu ZQ, Zhou F, Li JL et al. Carbendazim resistance in field isolates of Sclerotinia sclerotiorum in China and its management. Crop Prot, 2016; 81: 115-121.[DOI]
[4] Bentley KS, Kirkland D, Murphy M et al. Evaluation of thresholds for benomyl-and carbendazim-induced aneuploidy in cultured human lymphocytes using fluorescence in situ hybridization. Mutat Res/Gen Tox En, 2000; 464: 41-51.[DOI]
[5] Lentza-Rizos C, Avramides EJ, Kokkinaki K. Residues of azoxystrobin from grapes to raisins. J Agr Food Chem, 2006; 54: 138-141.[DOI]
[6] Mesnage R, Séralini GE. Toxicity of pesticides on health and environment. Front Public Health, 2018; 6: 268.[DOI]
[7] Bartlett DW, Clough JM, Godwin JR et al. The strobilurin fungicides. Pest Manag Sci, 2002; 58: 649-662.[DOI]
[8] Rahul G, Naidu NV, Ramachandra B. Development and validation of stability-indicating RP-HPLC method for the estimation of azoxystrobin in its formulations. Int J Pharm Chem Anal, 2022; 9: 40-49.[DOI]
[9] Zeng LR, Shi LH, Meng XG et al. Evaluation of photolysis and hydrolysis of pyraclostrobin in aqueous solutions and its degradation products in paddy water. J Environ Sci Heam, 2019; 54: 317-325.[DOI]
[10] Rodrigues ET, Lopes I, Pardal MÂ. Occurrence, fate and effects of azoxystrobin in aquatic ecosystems: A review. Environ Int, 2013; 53: 18-28.[DOI]
[11] Reddy SN, Gupta S, Gajbhiye VT. Effect of moisture, organic matter, microbial population and fortification level on dissipation of pyraclostrobin in soils. B Environ Contam Tox, 2013; 91: 356-361.[DOI]
[12] Gao W, Zhang C, Li B et al. Azoxystrobin exposure impairs meiotic maturation by disturbing spindle formation in mouse oocytes. Front Cell Dev Biol, 2022; 10: 1053654.[DOI]
[13] GadEL‑Hak HN, Al‑Eisa RA, Ryad L et al. Mechanisms and histopathological impacts ofacetamiprid andazoxystrobin inmale rats. Environ Sci Pollut R, 2022; 29: 43114-43125.[DOI]
[14] CIPAC. Accelerated storage procedure. In: CIPAC Handbook: Physico-chemical methods for technical and formulated pesticides. Volume F. Collaborative International Pesticides Analytical Council Ltd: Harpenden, England, 1995.
[15] Marczewska P, Płonka M, Rolnik J et al. Determination of azoxystrobin and its impurity in pesticide formulations by liquid chromatography. J Environ Sci Heal B, 2020; 55: 599-603.[DOI]
[16] Lazić S, Šunjka D. Determination of azoxystrobin and difenoconazole in pesticide products. Commun Agr Appl Biol Sci, 2015; 80: 375-380.
[17] Weil CS. Tables for convenient calculation of median-effective dose (LD50 or ED50) and instructions in their use. Biometrics, 1952; 8: 249-263.[DOI]
[18] Ngoula F, Watcho P, Dongmo MC et al. Effects of pirimiphos-methyl (an organophosphate insecticide) on the fertility of adult male rats. Afr Health Sci, 2007; 7: 3.
[19] Mohammad MA, Mohamad MM, Dradka H. Effects of black seeds (Nigella sativa) on spermatogenesis and fertility of male albino rats. Res J Med Med Sci, 2009; 4: 386-390.
[20] Moskovtsev SI, Librach CL. Methods of sperm vitality assessment. Methods Mol Biol, 2013; 927: 13-19.[DOI]
[21] Koivunen ME, Krogsrud R. Principles of immunochemical techniques used in clinical laboratories. Lab Med, 2015; 37: 490-497.[DOI]
[22] Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol, 1957; 28: 56-63.<span dir="ltr"></span>[DOI]
[23] Kaplan A, Szalbo J. Clinical chemistry: Interpretation and techniques, 2nd ed. Williams & Wilkins: Baltimore, USA, 1983; 157.
[24] Mc Gowan MW, Artiss JD, Strandbergh DR et al. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem, 1983; 29: 538-542.<span dir="ltr"></span>[DOI]
[25] Tietz NW, Caraway WT. Fundamentals of clinical chemistry. W. B. Saunders: Philadelphia, USA, 1976.
[26] Bancroft JD, Layton C, Suvarna SK. Bancroft’s theory and practice of histological techniques. Churchill Livingstone Elsevier: Oxford, UK, 2013.
[27] FAO. FAO Specification and Evaluation for Agricultural Pesticides. Azoxystrobin, 2022; 2022: 72-80.
[28] Yao J, Cui B, Zhao X et al. Antagonistic effect of azoxystrobin poly (lactic acid) microspheres with controllable particle size on Colletotrichum higginsianum Sacc. Nanomaterials, 2018; 8: 857.<span dir="ltr"></span>[DOI]
[29] Fishel FM. Pesticide Toxicity Profile: Strobilurin Pesticides: PI-67/PI104, 9/2005. EDIS, 2005; 11: 67.[DOI]
[30] Ziada RM, Abdulrhman SM, Nahas AA. Hepato-nephro-toxicity induced by premium fungicide and protective effect of sesame oil. Egypt J Hosp Med, 2020; 81: 2445-2450.[DOI]
[31] Fang N, Zhang C, Hu H et al. Histology and metabonomics reveal the toxic effects of kresoxim- methyl on adult zebrafish. Chemosphere, 2022; 309: 136739.<span dir="ltr"></span>[DOI]
Copyright © 2024 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.






 Copyright ©
Copyright ©