Study of the Gelatinization, Fatty Acid Profiles and the Conversion of Ergosterol into Vitamin D in Wheat Shorts by Optimization of Low-pressure Radiofrequency Cold Plasma Treatment Conditions
Minghui Zhao1,2, Mengya Wang1,2, Guangbiao Gao1,2, Jianzhang Wu2, Xingjun Li1*, Yonglin Ren3
1Academy of National Food and Strategic Reserves Administration, National Engineering Research Center for Grain Storage and Transportation, Beijing, China
2College of Grain and Strategic Reserves, Henan University of Technology, Zhengzhou, Henan Province, China
3College of Environmental and Life Sciences, Murdoch University, Murdoch, Australia
*Correspondence to: Xingjun Li, Professor, PhD, Institute of Grain Storage and Transportation, Academy of National Food and Strategic Reserves Administration, No. 26, Road Dongbei, Changping District, Beijing, 102209, China; Emai: lxj@ags.ac.cn
Abstract
Objective: Cold plasma (CP) treatment is used to remove contaminants like ergosterol (ERG) in cereal foods.
Methods: The conversion of ERG into vitamin D (VD), gelatinization properties and fatty acid (FA) profiles were evaluated in wheat shorts treated with 120W low-pressure radiofrequency oxygen or helium CP for 90-120s.
Results: Compared with the untreated samples, 46-64% of ERG was degraded and produced a maximum amount of vitamin D2 and Vitamin D3-like compounds in the 120W CP 90-120s treated samples. Oxygen CP had a higher capacity to degrade ERG than helium CP. The peak temperature of gelatinization was increased by both CP treatments, while the other gelatinization properties were not changed. The content of mono-unsaturated free fatty acids (FAs) in wheat shorts had no significant change, 88.3-95% of total FAs, 82.2-89.1% of unsaturated FAs and 80.4-87.2% of polyunsaturated FAs were maintained. Helium CP increased the contents of C18: 0, C18: 1n9c, C18: 1n9t, and C20: 3n6 and saturated FAs, and reduced the C20: 1 and mono-unsaturated FAs content. Oxygen CP did not change C18: 0, C18: 1n9c, C18: 1n9t, and C20: 1 contents, but reduced the C20: 3n6 content. The oxygen CP oxidized polyunsaturated FAs, leading to a decrease in the unsaturated FA content, while helium plasma caused the decomposition of monounsaturated and polyunsaturated FAs, and partially converted some unsaturated FAs into saturated FAs.
Conclusion: CP treatment is useful to change ERG into VD in wheat shorts.
Keywords: cold plasma, wheat shorts, helium cold plasma, oxygen cold plasma, FA profile
1 INTRODUCTION
Cold plasma (CP) is an emerging environmentally-friendly, non-thermal food processing technology. Plasma is a highly energized ionized gas, generally known as the fourth state of matter, that contains a large number of different species, including electrons, ions, free radicals such as reactive oxygen species (ROS), reactive nitrogen species (RNS), and ultraviolet (UV) photons[1,2]. Each year, at least 25% of global crops (over 500 million tonnes) are contaminated with mycotoxins[3]. Over the past decade, CP has been tested for microbial reduction[4] and the degradation of mycotoxins in grain and food samples[5,6]. Because oxygen containing gas plasmas produce active species of photo dissociation of molecular oxygen like ozone, atomic oxygen and other oxide radicals, Basaran et al.[7] used low-pressure air cold plasma to mitigate nearly one-half of total aflatoxin in hazelnuts, peanuts, and pistachio after 20min treat ment time. Atmospheric pressure plasma is a source of ROS and RNS such as oxide radicals, molecular oxygen, ozone, nitrogen dioxide and the radicals of hydroxyl and nitric oxide[8], Iqdiam et al.[9] inoculated peanuts (16% moisture content) with Aspergillus flavus at 30°C for 21 days and then treated 10g samples with an atmospheric pressure plasma jet (650V and 70-90Hz, fed with compressed air at 21°C and a flow rate of 107L/min, with a sample distance of 5cm from the plasma source) for 2min. The mycotoxins were reduced by 23%, with a temperature change of -24°C to 92°C during treatment, compared to a mycotoxin reduction of 38% with shaking treatment and a temperature change of 24°C to 78°C. The CP-treated peanuts exhibited no significant differences in peroxide values, free fatty acids (FAs), acidity, oxidative stability index, and sensor evaluation compared to the untreated samples. However, the effect of CP is dependent on the product being treated, with losses in antioxidants or lipids reported in some studies[10,11]. There are fewer reports on fatty acids profile of cereals and their products after cold plasma treatment.
Fusarium graminearum is an agriculturally-important toxigenic fungus that causes great damage in the food and livestock industries. Its most important metabolite is deoxynivalenol (DON), a mycotoxin that can be lethal to affected animals[12]. Hajnal et al.[13] used surface barrier discharge air plasma (200V and 50Hz, plasma source distance from sample of 6-51mm, gas temperature 40°C, RH 45%) to treat 10g of wheat flour spiked with 100μg/kg each of Fusarium spores, monomethyl ester, and T-2 toxin. The treatment time ranged from 0-180s. The maximum degradation of Fusarium spores, monomethyl ester, and T-2 toxin was achieved at a distance of 6mm from the plasma source and a treatment time of 180s, with degradation rates of 61%, 74%, and 55%, respectively. Chen et al.[14] used dielectric barrier discharge air plasma (50kV, plasma source distance from sample ˂8mm) to treat 5g of wheat grains with 2-2.5mg/kg DON for 8min, with a DON degradation rate of 30% in the 16% moisture content samples. DON-overproof wheat grain is related to the abnormal years. However, few studies have used CP to treat more grams of wheat products like wheat shorts with higher contents of natural DON and Ergosterol (ERG).
Wheat shorts are a by-product (3-5%) of wheat flour milling and are composed of the aleurone layer, endosperm, and a small amount of fine bran[15]. Wheat shorts are frequently used as a feed material for livestock and poultry due to their higher contents of crude protein, crude fibre, lipids, vitamins, and minerals[16]. The CP can improve the functionality of grain and oil food products[17] and can increase their safety by eliminating microorganisms and reducing mycotoxins and pesticide residues[18,19]. ERG commonly serves as an indicator of total fungal biomass[20]. When ERG is detected in cereal grains, it means that the cereal has been contaminated by a fungus, which gives the possibility of mycotoxin contamination. Olsson et al.[21] reported the relationship between the amount of ERG and the concentration of certain mycotoxins such as chratoxin A and DON in barley. According to a report by Pietri et al.[22], where a cereal of ordinary quality should contain less than 3μg/g ERG, while that containing 8μg/g ERG was considered to be of inferior quality. ERG is a precursor of vitamin D (VD) and a component of fungal cell membranes[23]. It is produced by most fungi, but most plants do not produce ERG[24]. ERG is an abundant bioactive compound in mushrooms, and in recent years, many studies have been carried out to convert ERG into vitamin D2 (VD2) in mushrooms and their processing waste under UV irradiation, and then recover VD2[23,25]. However, there is no report dealing with the conversion of ERG in wheat shorts into vitamins.
In this study, low-pressure radio frequency (RF) oxygen and helium plasma were used to treat wheat shorts samples with higher ERG levels. Then, the changes in the content of VD2, gelatinization properties and fatty acid (FA) profiles of the wheat shorts were explored. The aim was to reduce the ERG content of the wheat shorts and provide basic data for the optimization of the CP process.
2 MATERIALS AND METHODS
2.1 Materials
Naturally-infected Fusarium head blight wheat (50kg) was obtained from Pingyuan County, Shandong Province, China in July 2020. Three kilograms of wheat flour were processed from grains using the test roller mill (LRMM-8040-3-D) and a test flour sieve (LFS-30, sieve diameter of 30cm, swivelling speed of 250rpm) from Buler Grain Test Instrument Wuxi Co. Ltd, Wuxi, China, and 1.12 kilograms of wheat shorts and 505 grams of wheat bran were obtained. The wheat shorts fraction was determined to contain 2.38μg/g DON using ROSA FAST5 DON quantitative test on a mycotoxin rapid detection system (Charm Rosa, USA). The wheat shorts sample was stored at -20°C until use.
2.2 Cold Plasma Treatment
The wheat shorts were treated with a cold plasma processor (HD-3N, Changzhou Hanjie Biotechnology Co. Ltd., Jiangsu Province, China). A schematic diagram of this plasma apparatus was illustrated in our previous paper[26]. The electrodes were capacitively coupled to a solid RF power supply with the frequency of 13.56MHz (PSG-II type, working power range 0-1000W with the accuracy of ±1W). The background pressure was ≤5Pa and the working pressure was in the range of 80-180Pa with accuracy of ±1Pa. The plasma source was located 3cm away from the sample. The 70g wheat shorts samples were uniformly spread on the mesh, which was maintained on a glass stand between two electrodes. At first, the system was evacuated to 21Pa with three rotary vacuum pumps to remove any adsorbed gases or water vapour from the sample surface. The untreated sample was also kept under vacuum before analysis. Helium or oxygen was used as the feed gas for plasma generation and the working pressure was then changed to 141Pa using the mass flow controller. The RF power supply was set at 120W, and the matching network was adjusted to get a stable glow discharge for a certain time with a chrono marker. The samples were subjected to helium or oxygen plasma treatment at 120W for 0, 30, 60, 90, 120, 150, and 180s, respectively. After CP treatment, each sample was placed in a sealed plastic bag (200×140×0.04mm, Apple Brand, Shanghai, China) and transferred to the laboratory in a carton box and kept at -20°C for analysis.
2.3 Content of ERG, VD2 and Vitamin D3 (VD3)
Determination of the ERG content in wheat shorts was performed according to the Chinese National Standard GB/T 25221-2010[27], with some modifications. To prepare a standard curve, a standard stock solution (500μg/mL) was prepared by weighing 5mg (accurate to 0.01mg) of ERG standard (Shanghai Aladdin Biochemical Technology Co., Ltd), adding 20 mg of antioxidant BHT, dissolving it in methanol, and diluting it to 10mL with methanol. The stock solution was stored at -20°C. Stock solution was diluted with methanol to prepare a standard series of working solutions of 500, 1000, 1500, 2000, 2500, and 3000ng/mL that were stored at 4°C for a maximum of seven days. The standard curve regression equation was obtained by injecting the series of standard solutions from low to high concentration and detecting the peak area integration value, with the ERG standard working solution concentration as the abscissa axis and the peak area integration value as the ordinate axis. The standard curves of VD2 (high-pressure liquid chromatography (HPLC) grade ˃98%) and D3 (HPLC grade ˃99%, Yuanye Biotechnology LTD. Co, Shanghai, China) were prepared as was ERG.
Sample extraction: the sample (10g), sodium hydroxide (5g), and methanol (40mL) were placed in a 150mL round-bottom flask. The sample was refluxed at a rate of approximately one drop per second in a water bath with a condenser for 60min (80°C). After cooling to room temperature, the sample was passed through filter paper and transferred to a 150mL separatory funnel to which 10mL of 3% potassium chloride aqueous solution was added and mixed before 10mL of n-hexane was added. The mixture was vigorously shaken for 3min and then allowed to separate. Next, 5mL of the n-hexane layer was passed through a Sep-Pak Plus silica gel column (Waters, Milford, USA) that had been pre-treated with 5mL of n-hexane. Methanol (5mL) was used to elute ERG from the column. This methanol solution was used as the sample solution for HPLC.
For HPLC chromatography, an Inertsil ODS-SP silica gel column (column length 150mm, inner diameter 4.6mm, and particle size 5μm) was used. The mobile phase A was methanol-water (8:2) and mobile phase B was methanol-ethanol (7:3). Within the first 5min, the proportion of the mobile phase components was 1:1. Then, the proportion of component B was increased to 70% over the next 5min, and then increased to 90% over the next 3min. This ratio was maintained for 5min. The column temperature was 40°C, the injection volume was 10μL, the flow rate was 1.0mL/min, and the detection wavelength was 282nm.
2.4 Gelatinization Properties
The gelatinization properties of the wheat shorts were determined with a differential scanning calorimeter (DSC; 214 Polyma, Netzsch, Germany) as previously described by Gao et al[6]. A sample (5.0mg) was weighed into an aluminium crucible and distilled water was added to give a water/sample ratio of 2:1. The sealed aluminium crucible was equilibrated at 4°C overnight. The DSC temperature increased from 25°C to 110°C at heating rate of 10°C/min.
2.5 FA Profile Analysis
FA analysis was conducted according to a protocol described by Tao et al[28]. For lipid extraction, 300mg sample were weighed into a 10-mL centrifugal tube and blended with 1mL hexane and 0.25mL of 2mol/L KOH-methanol solution for 30s. When extraction for 40min in a water bath at 30°C with a 300W ultrasonic probe, the sample was cooled to room temperature and mixed with 0.25mL of 2mol/L HCl solution for 1min. Once centrifugation at 7600×g for 10min, the supernatants were used to gas chromatography-mass spectrometry analysis.Each sample had three parallel extracts.
Fatty acid methyl esters (FAME) in a sample were measured with a gas chromatography system (Agilent 8890 GC) equipped with fused-silica column (HP-5MS UI; 30m length ×0.25mm internal diameter ×0.25μm film thickness) coupled with a mass selective detector (MSD; 5977B, Agilent Technologies, Santa Clara, CA, USA). Samples were injected in split mode (0.3μL, split ratio 10) at an initial temperature of 130°C for 3min, then the temperature was raised at 5°C/min to 180°C. After 8min, the temperature was again raised at 5°C/min to 240°C and kept for 12min. Helium was used as a carrier gas at 1.0mL/min and solvent delay time was 1.6min. For MS system, the temperatures at injection port, ionization source, transfer line and quadrupole were 260°C, 230°C, 280°C and 150°C, respectively, and electron-impact mass spectra were recorded at 70eV ionization voltage. The acquisitions were done in full-scan mode.
The MS information of each FAME peak was used to MassHunter Qualitative Analysis V10.0 software (Agilent Technologies, Santa Clara, CA, USA) and compared to the offline MS library of NIST08s and the online NIST Chemistry Web Book, SRD 69 (https://webbook.nist.gov/chemistry/#), taking a minimum similarity value of 80%. The FAME standards were followed and the experimental retention indices of a series of n-alkanes (C7-C30, Sigma-Aldrich Co., St. Louis, MO, USA) in an HP-5MS column were compared with those reported in the reference at the same experimental conditions. The measured concentration (μg/L) was quantified with a external standard method, in which 37 species of FAME (Sigma-Aldrich Co., USA) mixed with different gradients were adopted. The linear ranges of the standard curves were 0.5-400μg/L (R2>0.99).
2.6 Data Analysis
SPSS software (Version 17.0[29]) was used for data analysis. One-way analysis of variance and independent-sample t-tests were used to compare multiple and pairs of means, respectively. To observe the effects of several factors, a General Linear Model-Univariate method (GLM) was used to compare means by least significant difference tests[30]. Statistical significance was declared at a level of P<0.05.
3 RESULTS
3.1 Degradation of ERG and Production of VD in Wheat Shorts by Low-pressure CP
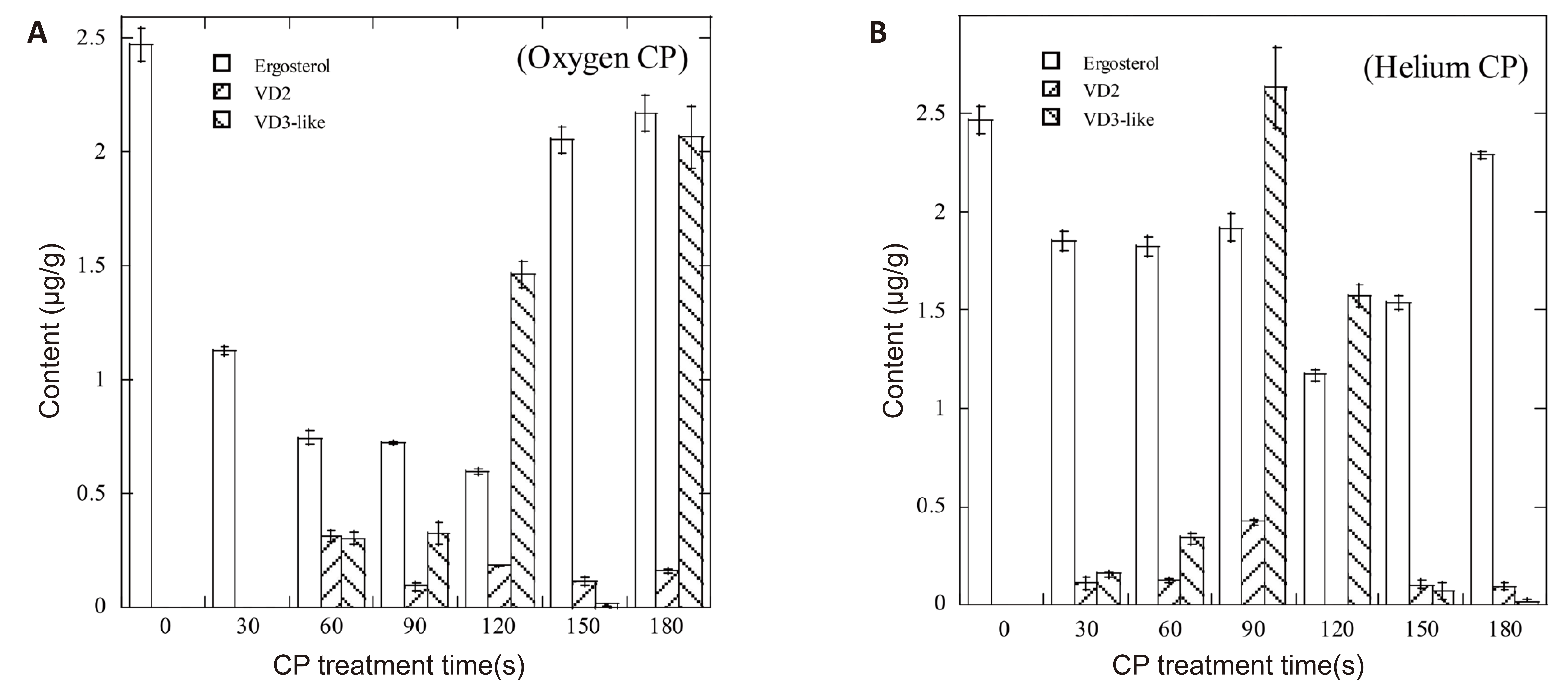
The contents of ERG, VD2 and VD3-like compounds in wheat shorts after low-pressure RF cold plasma treatment were detected by HPLC. Figure 1 shows the degradation of ERG in wheat shorts by 120W oxygen or helium CP treatment. Compared with the untreated sample, both of the CP treatments for 30-180s significantly decreased the ERG content of the wheat shorts, and contents of VD2 and VD3-like were detected. When oxygen CP was adopted, the contents of ERG and VD2 in wheat shorts had a significant negative correlation coefficient (-0.4389). When helium CP was adopted, the contents of ERG and VD3-like had an insignificant negative correlation coefficient (-0.3601). The correlation coefficients between VD2 and VD3-like were 0.3889 and 0.6867 (Table 1). These results suggest that CP treatment can convert ERG into VD2 and VD3-like in wheat shorts.
|
Figure 1. Conversion of ERG into VD in wheat shorts by CP treatments.
Table 1. The Correlation Coefficients Between ERG, VD2 and VD3-like in Wheat Shorts After CP Treatment
Data Sets |
Correlation Coefficients |
|
Oxygen CP |
Helium CP |
|
ERG and VD2 |
-0.4389* |
0.0548 |
ERG and VD3-like |
-0.0141 |
-0.3601 |
VD2 and VD3-like |
0.3889 |
0.6867** |
Notes: **, very significant; *, significant; df=19, P=0.433 for α=0.05, P=0.549 for α=0.01.
GLM analysis shows that the ERG degradation rate of oxygen CP is bigger than that of helium CP when compared with the untreated samples, and both CP treatments produced similar contents of VD2 or VD3-like in wheat shorts. 120W low-pressure RF CP treatment on wheat shorts had the maximum ERG degradation at a treatment time of 120s and the maximum production of VD2 and VD3-like at 90s (Table 2).
Table 2. Effect of Feed Gas and CP Treatments on the Contents of ERG and VD in Wheat Shorts
Factors |
Levels |
ERG (μg/g) |
VD2 (μg/g) |
VD3-like (μg/g) |
CP |
Oxygen |
1.409±0.070d |
0.123±0.021b |
0.595±0.142c |
species |
Helium |
1.867±0.070c |
0.121±0.021b |
0.684±0.142bc |
CP |
0s |
2.468±0.131a |
0.000±0.038d |
0.000±0.266d |
time |
30s |
1.487±0.131d |
0.054±0.038cd |
0.078±0.266d |
|
60s |
1.285±0.131d |
0.217±0.038a |
0.321±0.266d |
|
90s |
1.322±0.131d |
0.258±0.038a |
1.480±0.266a |
|
120s |
0.882±0.131e |
0.093±0.038bc |
1.518±0.266a |
|
150s |
1.794±0.131c |
0.108±0.038bc |
0.040±0.266d |
|
180s |
2.130±0.131b |
0.124±0.038bc |
1.040±0.266ab |
Notes: Means with the different superscript letters in a column are different significantly (P<0.05) among different CP treatment times.
3.2 Effect of Low-pressure CP on the Gelatinization Properties of Wheat Shorts
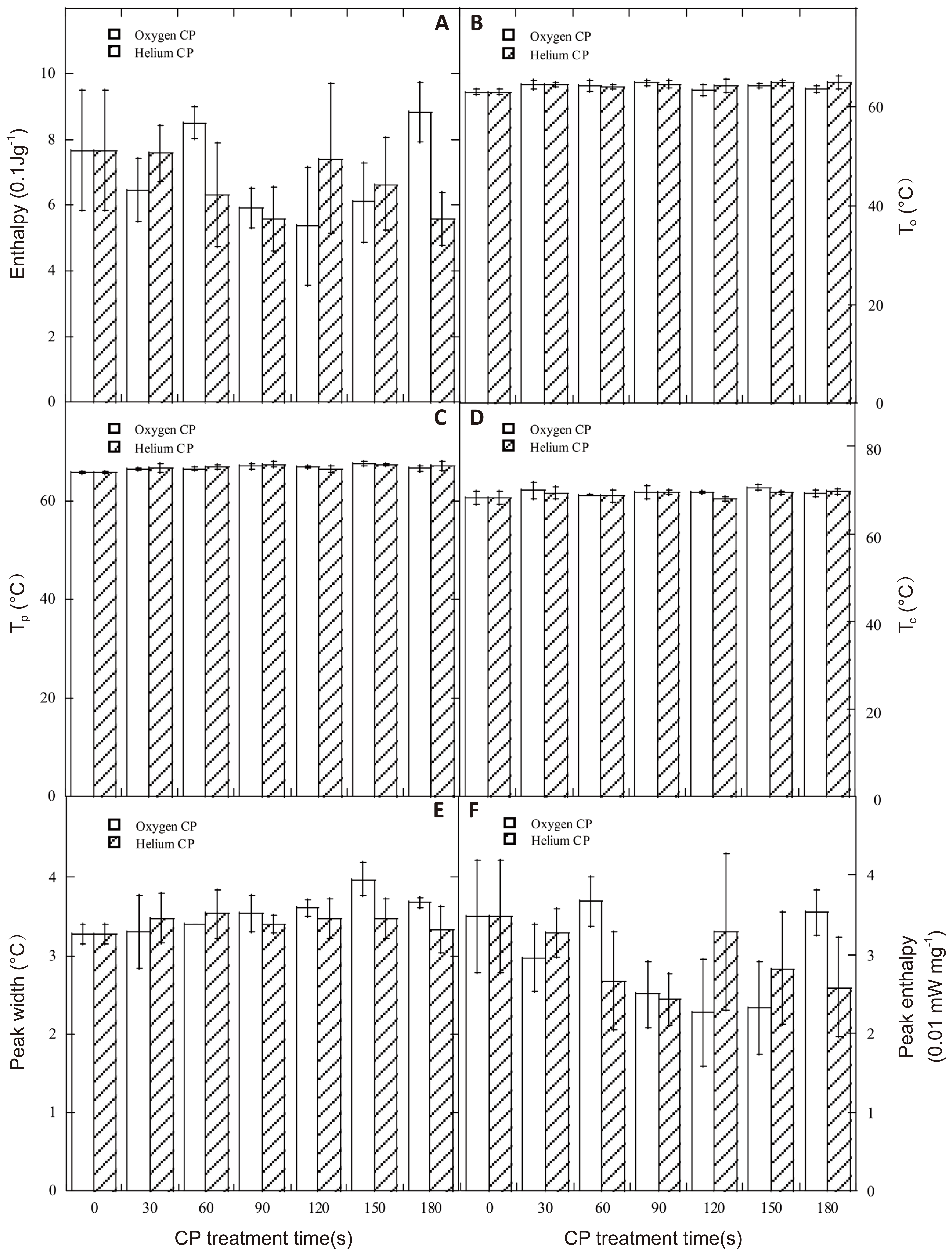
Figure 2 shows the changes in the DSC gelatinization parameters of wheat shorts as a function of CP treatment time. Compared with the untreated samples (0s CP treatment), helium CP treatment tended to decrease the enthalpy of gelatinization (∆H, Figure 2A) and the peak enthalpy (Figure 2F) of wheat shorts with increases in treatment time. However, oxygen CP treatment resulted in the minimal enthalpy of gelatinization and peak enthalpy values at 120s, with increases in these two parameters from 150s to 180s. Both the helium and oxygen CP treatments for 60-180s significantly increased the onset temperature of gelatinization (the onset temperature of gelatinization (To), Figure 2B) and peak temperature (The peak temperature of gelatinization(Tp), Figure 2C), while there were no differences in the final temperature values (the conclusion temperature of gelatinization (Tc), Figure 2D) with the 30-180s treatments, relative to the untreated samples. Helium CP treatment for 30-180s did not significantly change the peak width (Figure 2E) of gelatinization. In contrast, oxygen CP treatment for 30-90s did not significantly change the peak width of gelatinization, but this value was increased with the 120-180s treatments.
|
Figure 2. Changes in a differential scanning calorimeter-determined gelatinization parameters in wheat shorts with the different treatment times of oxygen and helium CP.
Table 3 further shows the effect of feed gas and CP treatment time on the gelatinization properties of wheat shorts. Compared with the untreated samples (0s CP treatment), oxygen and helium plasma treatment did not change the ∆H value, but significantly decreased the peak enthalpy and increased the To and Tc values. Helium plasma also increased the Tp and provided similar a peak width, while oxygen plasma did not change the Tp and increased the peak width. With increases in the CP treatment time, the To, Tp, Tc, and peak width tended to increase, ∆H did not change, and peak enthalpy tended to decrease. These results suggest that the higher peak temperatures of gelatinization were induced in wheat shorts by oxygen and helium plasma, leading to a slower rate of starch gelatinization.
Table 3. Effect of Feed Gas and CP Treatments on the Gelatinization Properties of Wheat Shorts
Factors |
Levels |
ΔH (0.1J/g) |
To (°C) |
Tp (°C) |
Tc (°C) |
Peak Width(°C) |
Peak Enthalpy (0.01mW/mg) |
CP |
Oxygen |
6.97±0.34a |
63.95±0.18b |
66.71±0.11cd |
69.38±0.23abc |
3.53±0.05b |
2.96±0.14bc |
species |
Helium |
6.68±0.34ab |
64.26±0.18ab |
66.79±0.11c |
68.98±0.23bcd |
3.42±0.05cd |
2.93±0.14bc |
CP |
0s |
7.65±0.64a |
63.00±0.34c |
65.80±0.20d |
68.23±0.42e |
3.27±0.10d |
3.48±0.27a |
time |
30s |
7.03±0.64a |
64.45±0.34ab |
66.55±0.20c |
69.62±0.42ab |
3.38±0.10bcd |
3.11±0.27abc |
|
60s |
7.41±0.64a |
64.08±0.34ab |
66.72±0.20c |
68.70±0.42de |
3.47±0.10bcd |
3.17±0.27ab |
|
90s |
5.74±0.64b |
64.67±0.34a |
67.20±0.20ab |
69.53±0.42abcd |
3.47±0.10bcd |
2.46±0.27d |
|
120s |
6.38±0.64ab |
63.82±0.34b |
66.68±0.20c |
68.77±0.42cde |
3.53±0.10abc |
2.77±0.27bcd |
|
150s |
6.37±0.64ab |
64.50±0.34a |
67.43±0.20a |
70.02±0.42a |
3.72±0.10a |
2.57±0.27cd |
|
180s |
7.20±0.64a |
64.22±0.34ab |
66.83±0.20bc |
69.40±0.42abcd |
3.50±0.10bc |
3.06±0.27abc |
Notes: ΔH, enthalpy of gelatinization; To, the onset temperature of gelatinization; Tp, the peak temperature of gelatinization; Tc, the conclusion temperature of gelatinization. Means with the different superscript letters in a column are different significantly (P<0.05) among different CP treatment times.
3.3 Effects of Low-pressure CP Treatment on the FA Profile of Wheat Shorts
Tables 4 and 5 show the changes in the FA profiles of wheat shorts after CP treatment. Among the 32 species of FAs identified in wheat shorts, there were 12 FA species with contents ˃ 20μg/g including palmitic acid (C16: 0), stearic acid (C18: 0), oleic acid (C18: 1n9c), linoleic acid (C18: 2n6c), α-linolenic acid (C18: 3n3), cis-11-eicosenoic acid (C20: 1), cis-4, 7, 10, 13, 16, 19-docosahexaenoic acid (C22: 6n3), cis-8, 11, 14-eicosatrienoic acid (C20: 3n6), lignoceric acid (C24: 0), nervonic acid (24: 1), lauric acid (C12: 0), and cis-13-docosenoic acid (C22: 1n9).
Table 4. Effect of Feed Gas and CP Treatments on the Contents of Main Fatty Acids in Wheat Shorts
|
|
|
|
Fatty |
Acids |
(μg/g) |
|
|
Factors |
Levels |
C10: 0 |
C12: 0 |
C14-0 |
C14: 1 |
C15: 0 |
C15: 1 |
C16: 0 |
CP |
O |
14.0±1.0b |
31.6±0.8bc |
14.5±0.4a |
5.1±0.2b |
16.3±0.3bc |
7.1±0.3ab |
2046±29bc |
Species |
He |
8.3±1.0c |
29.5±0.8d |
13.7±0.4bc |
4.8±0.2b |
16.7±0.3ab |
6.6±0.3bcd |
1995±29c |
CP |
0s |
8.6±1.9c |
33.8±1.4ab |
13.0±0.7c |
4.6±0. 5b |
15.5±0.6c |
5.8±0.5d |
2018±54bc |
time |
30s |
9.1±1.9c |
28.7±1.4de |
13.8±0.7abc |
4.5±0.5b |
16.3±0.6abc |
6.0±0.5cd |
2091±54ab |
|
60s |
8.2±1.9c |
28.9±1.4de |
14.0±0.7abc |
4.7±0.5b |
17.2±0.6ab |
7.4±0.5ab |
2029±54bc |
|
90s |
17.2±1.9a |
30.5±1.4cd |
14.2±0.7abc |
4.7±0.5b |
17.4±0.6a |
7.5±0.5a |
2147±54a |
|
120s |
7.2±1.9c |
27.1±1.4e |
14.0±0.7abc |
4.8±0.5b |
16.6±0.6abc |
6.9±0.5abc |
2054±54abc |
|
150s |
10.6±1.9c |
30.7±1.4cd |
14.7±0.7ab |
4.9±0.5b |
15.6±0.6c |
7.1±0.5ab |
1978±54c |
|
180s |
17.3±1.9a |
34.1±1.4a |
14.8±0.7a |
6.2±0.5a |
17.1±0.6ab |
7.3±0.5ab |
1825±54d |
|
|
C16:1 |
C17:0 |
C17:1 |
C18:0 |
C18:1n9c |
C18:1n9t |
C18:2n6c |
CP |
O |
18.4±0.5a |
13.6±0.2a |
6.2±0.1c |
203±19de |
693±13bc |
10.5±3.4e |
6510±147b |
Species |
He |
14.8±0.5b |
13.9±0.2a |
6.6±0.1b |
489±19a |
612±13d |
65.8±3.4a |
5191±147d |
CP |
0s |
19.1±0.9a |
13.5±0.4a |
5.5±0.3d |
180±36e |
726±24ab |
5.1±6.4e |
7273±275a |
time |
30s |
19.2±0.9a |
13.6±0.4a |
6.4±0.3bc |
257±36d |
737±24a |
24.1±6.4d |
6728±275ab |
|
60s |
17.9±0.9a |
13.8±0.4a |
6.2±0.3bc |
335±36c |
674±24c |
40.7±6.4c |
5895±275c |
|
90s |
18.2±0.9a |
14.1±0.4a |
6.7±0.3ab |
366±36c |
729±24ab |
42.5±6.4bc |
6299±275bc |
|
120s |
15.1±0.9b |
13.9±0.4a |
6.3±0.3bc |
393±36bc |
666±24c |
48.2±6.4bc |
5809±275c |
|
150s |
13.8±0.9bc |
13.9±0.4a |
7.1±0.3a |
447±36ab |
563±24e |
55.3±6.4b |
4845±275d |
|
180s |
12.7±0.9c |
13.3±0.4a |
6.7±0.3ab |
444±36ab |
473±24f |
51.2±6.4bc |
4104±275e |
Factors |
Levels |
C18:2n6t |
C18:3n3 |
C18:3n6 |
C20:0 |
C20:1 |
C20:2 |
C20:3n3 |
Species |
O |
6.4±0.7d |
401±10b |
14.6±0.4c |
12.1±0.6e |
75.9±1.7a |
7.4±0.5c |
11.2±0.9ab |
|
He |
16.9±0.7a |
309±10d |
16.3±0.4a |
19.1±0.6a |
58.2±1.7d |
6.5±0.5cd |
11.1±0.9ab |
CP |
0s |
2.3±1.4e |
465±19a |
11.2±0.8d |
10.2±1.1f |
78.3±3.3a |
17.6±1.0a |
9.7±1.7c |
time |
30s |
9.7±1.4c |
419±19b |
7.2±0.8e |
14.2±1.1d |
79.5±3.3a |
7.8±1.0c |
12.0±1.7ab |
|
60s |
14.3±1.4b |
355±19c |
15.6±0.8abc |
15.6±1.1cd |
69.5±3.3bc |
3.1±1.0ef |
11.0±1.7ab |
|
90s |
12.6±1.4b |
386±19bc |
14.6±0.8bc |
17.8±1.1abc |
75.7±3.3ab |
11.9±1.0b |
13.6±1.7a |
|
120s |
12.7±1.4b |
351±19c |
16.6±0.8a |
17.9±1.1ab |
68.3±3.3c |
5.1±1.0de |
9.9±1.7bc |
|
150s |
14.9±1.4ab |
278±19e |
16.9±0.8a |
17.8±1.1abc |
54.0±3.3d |
1.5±1.0fg |
10.2±1.7b |
|
180s |
15.4±1.4ab |
229±19f |
15.9±0.8ab |
15.7±1.1bcd |
44.4±3.3e |
0.8±1.0g |
11.5±1.7b |
Notes: C10: 0, Capric acid; C12: 0, Lauric acid; C14: 0, Myristic acid; C14: 1, Myristoleic acid; C15: 0, Pentadecanoic acid; C15: 1, cis-10-Pentadecenoic acid; C16: 0, Palmitic acid; C16: 1, Palmitoleic acid; C17: 0, Heptadecanoic acid; C17: 1, Ginkgolic acid; C18: 0, Stearic acid; C18: 1n9c, Oleic acid; C18: 1n9t, Elaidic acid; C18: 2n6c, Linoleic acid; C18: 2n6t, Linolelaidic acid; C18: 3n3, α-Linolenic acid; C18: 3n6, Methyl linolenate; C20: 0, Arachidic acid; C20: 1, cis-11-Eicosenoic acid; C20: 2, cis-11, 14-Eicosatrienoic acid; C20: 3n3, cis-11, 14, 17-Eicosatrienoic acid. Means with the different superscript letters in a column are different significantly (P<0.05) among different CP treatment times.
Table 5. Effect of Feed Gas and CP Treatments on the Contents of Fatty Acids in Wheat Shorts
|
|
|
|
Fatty |
Acids |
(μg/g) |
|
Factors |
Levels |
C20: 3n6 |
C20: 4n6 |
C20: 5n3 |
C21: 0 |
C22: 0 |
C22: 1n9 |
CP |
O |
24.9±2.6d |
6.7±0.2ab |
17.1±0.5bc |
16.7±1.4d |
11.3±1.3c |
2.2±0.3a |
Species |
He |
43.4±2.6a |
6.3±0.2bcd |
18.0±0.5ab |
24.2±1.4ab |
9.3±1.3cd |
0.3±0.3c |
CP |
0s |
33.4±4.8bc |
7.1±0.3a |
19.0±0.9a |
15.4±2.5d |
15.6±2.4ab |
1.5±0.6ab |
time |
30s |
38.0±4.8abc |
6.5±0.3abcd |
18.4±0.9ab |
23.9±2.5abc |
11.3±2.4bcd |
1.6±0.6ab |
|
60s |
30.6±4.8cd |
6.2±0.3bcd |
16.5±0.9c |
17.9±2.5d |
19.2±2.4a |
0.6±0.6bc |
|
90s |
40.4±4.8ab |
6.6±0.3abc |
17.9±0.9abc |
27.0±2.5a |
9.1±2.4cd |
0.7±0.6bc |
|
120s |
29.1±4.8cd |
5.9±0.3d |
17.9±0.9abc |
19.6±2.5cd |
2.4±2.4e |
1.8±0.6ab |
|
150s |
30.3±4.8cd |
7.1±0.3a |
17.2±0.9abc |
20.4±2.5bcd |
7.6±2.4cd |
0.9±0.6bc |
|
180s |
37.1±4.8abc |
6.1±0.3cd |
16.1±0.9c |
18.9±2.5cd |
6.9±2.4de |
1.8±0.6ab |
Factors |
Levels |
C22:2n6 |
C22:6n3 |
C23:0 |
C24:0 |
C24:1 |
|
CP |
O |
16.0±0.9de |
64.9±5.8b |
14.8±0.9d |
27.0±1.3c |
27.1±0.9b |
|
Species |
He |
21.1±0.9b |
29.9±5.8d |
21.8±0.9b |
32.3±1.3b |
23.2±0.9c |
|
CP |
CK |
13.6±1.7e |
4.6±10.8e |
11.0±1.6e |
19.3±2.4d |
14.4±1.8d |
|
time |
30s |
16.9±1.7cde |
30.3±10.8cd |
11.8±1.6e |
25.4±2.4c |
22.8±1.8c |
|
|
60s |
18.1±1.7cd |
46.9±10.8c |
16.0±1.6cd |
30.6±2.4bc |
25.7±1.8bc |
|
|
90s |
21.9±1.7b |
28.9±10.8cd |
17.4±1.6c |
38.1±2.4a |
24.8±1.8bc |
|
|
120s |
25.7±1.7a |
32.4±10.8cd |
18.6±1.6c |
32.6±2.4b |
27.8±1.8b |
|
|
150s |
14.6±1.7e |
86.0±10.8a |
22.6±1.6b |
32.6±2.4b |
32.1±1.8a |
|
|
180s |
19.1±1.7bc |
101.2±10.8a |
30.4±1.6a |
29.1±2.4bc |
28.1±1.8b |
|
Factors |
Levels |
Total |
Saturated |
Unsaturated |
Mono- unsaturated |
Poly- unsaturated |
Ratio |
Species |
O |
10346±186b |
2421±38cd |
7925±168b |
845±13b |
7079±155b |
3.26±0.06b |
|
He |
9134±186e |
2672±38a |
6461±168d |
793±13c |
5669±155d |
2.45±0.06d |
CP |
CK |
11070±348a |
2354±71d |
8716±314a |
859±25ab |
7856±291a |
3.70±0.11a |
time |
30s |
10722±348ab |
2516±71bc |
8205±314ab |
902±25a |
7804±291a |
3.27±0.11b |
|
60s |
9805±348cd |
2546±71bc |
7259±314c |
847±25b |
6412±291c |
2.87±0.11c |
|
90s |
10479±348abc |
2716±71a |
7763±314bc |
909±25a |
6854±291bc |
2.88±0.11c |
|
120s |
9777±348d |
2616±71ab |
7161±314c |
845±25b |
6316±291c |
2.79±0.11c |
|
150s |
8671±348e |
2611±71ab |
6060±314d |
738±25d |
5322±291d |
2.36±0.11d |
|
180s |
7654±348f |
2467±71cd |
5187±314e |
631±25e |
4556±291e |
2.13±0.11e |
Notes: C20: 3n6, cis-8, 11, 14-Eicosatrienoic acid; C20: 4n6, Arachidonic acid; C20: 5n3, cis-5, 8, 11, 14, 17-Eicosapentataenoic acid; C21: 0, n-Heneicosanoic acid; C22: 0, Behenic acid; C22: 1n9, cis-13-Docosenoic acid; C22: 2n6, cis-13,16-Docosadienoic acid; C22: 6n3, cis-4, 7, 10, 13, 16, 19-Docosahexaenoic acid; C23: 0, Tricosanoic acid; C24: 0, Lignoceric acid; C24: 1, Nervonic acid. Means with the different superscript letters in a column are different significantly (P<0.05) among different CP treatment times.
There were 14 species of FAs in the range of 10-20μg/g, including palmitoleic acid (C16: 1), cis-5, 8, 11, 14, 17-eicosapentataenoic acid (C20: 5n3), n-heneicosanoic acid (C21: 0), cis-13, 16-docosadienoic acid (C22: 2n6), pentadecanoic acid (C15: 0), tricosanoic acid (C23: 0), methyl linolenate (C18: 3n6), myristic acid (C14: 0), capric acid (C10: 0), heptadecanoic acid (C17: 0), arachidic acid (C20: 0), behenic acid (C22: 0), cis-11, 14, 17-eicosatrienoic acid (C20: 3n3), and elaidic acid (C18: 1n9t).
There were six species of FAs with contents ˂5μg/g, including arachidonic acid (C20: 4n6), cis-11, 14-eicosatrienoic acid (C20: 2), linolelaidic acid (C18: 2n6t), ginkgolic acid (C17: 1), cis-10-pentadecenoic acid (C15: 1), and myristoleic acid (C14: 1).
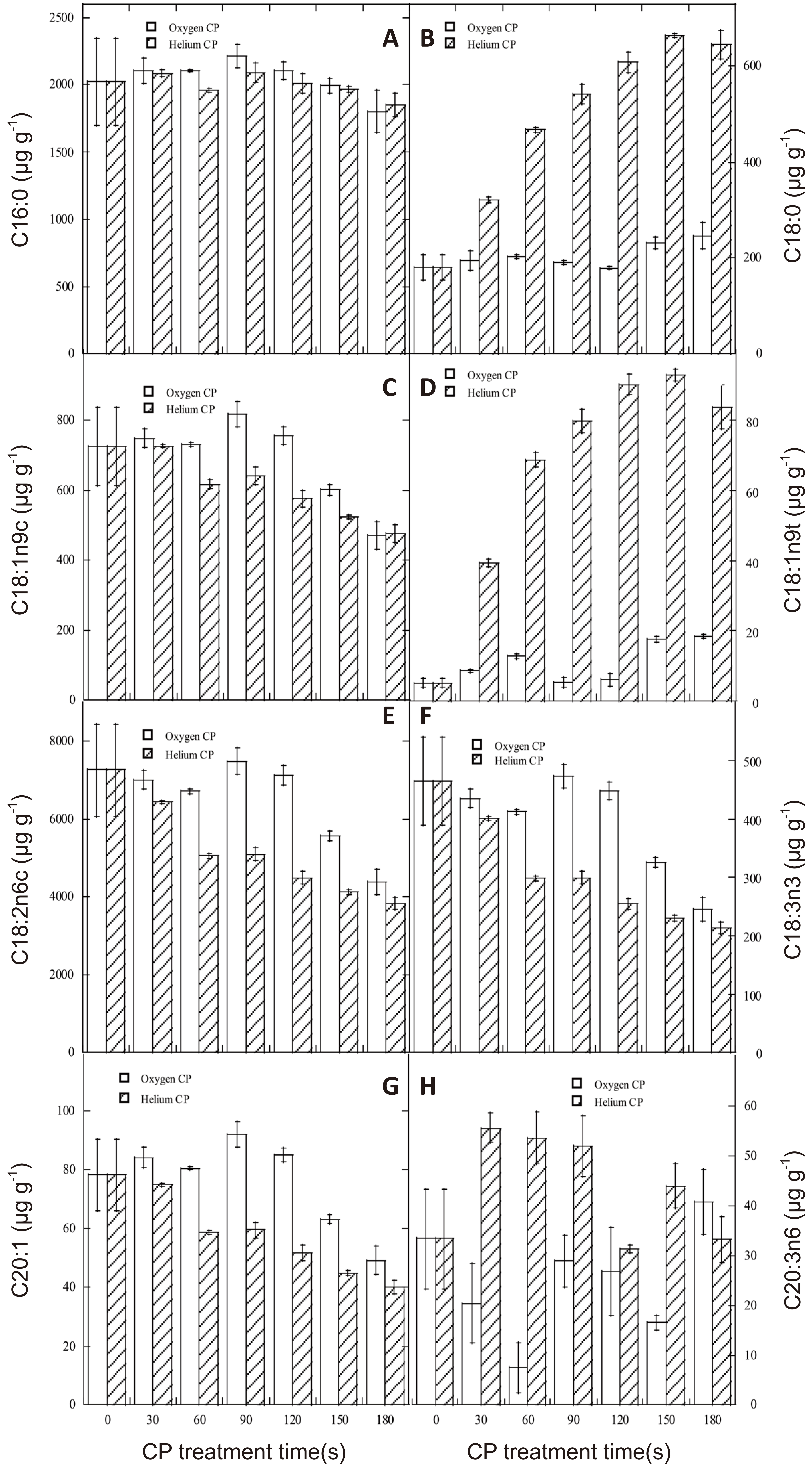
Figures 3 and 4 show the changes in the FA types in the wheat shorts samples during CP treatment. Among the eight important FA components, neither of the low-pressure CP treatments resulted in significant reductions in C16: 0 in wheat shorts (Figure 3A). Significant reductions were observed in C18: 1n9c (Figure 3C), C18: 2n6c (Figure 3E), C18: 3n3 (Figure 3F), and C20: 1 (Figure 3G) with increasing plasma treatment time, while significant increases were observed in C18: 0 (Figure 3B), C18: 1n9t (Figure 3D), and C20: 3n6 (Figure 3H). Helium plasma treatment had a greater effect on the levels of the FAs than oxygen plasma treatment.
|
Figure 3. Changes in the important components of fatty acids in wheat shorts with cold plasma treatment time.
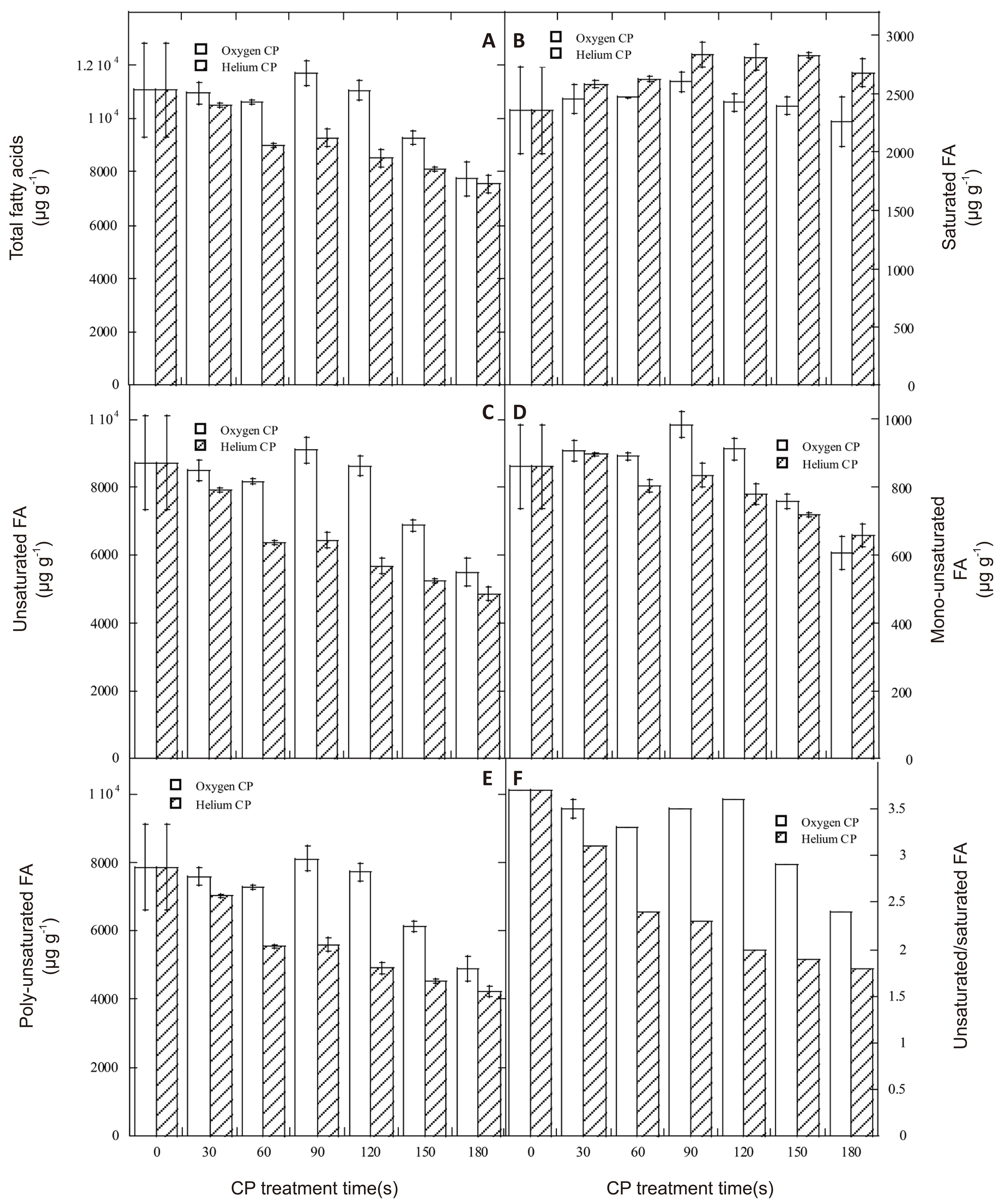
Compared with the untreated samples (0s CP treatment), both of the low-pressure CP treatments decreased the contents of total FAs (Figure 4A), unsaturated FAs (Figure 4C), mono-unsaturated FAs (Figure 4D), and poly-unsaturated FAs (Figure 4E), as well as the ratio of unsaturated to saturated FAs (Figure 4F), in wheat shorts samples with increasing treatment time. The degree of reduction induced by helium plasma was greater than that of oxygen plasma. Both CP treatments increased the contents of saturated FAs in wheat shorts, with helium plasma having a greater effect (Figure 4B).
|
Figure 4. Changes in FA types in wheat shorts with CP treatment time.
Further analysis using the GLM method showed that, for nine major FA components, compared to the untreated samples, both of the CP treatments did not change the C16: 0 content, increased the contents of C18: 0 and C22: 6n3, and decreased the contents of C18: 2n6c and C18: 3n3. Oxygen plasma did not change the contents of C18: 1n9t, C18: 1n9c, and C20: 1, while helium plasma increased the C18: 1n9t content and decreased the contents of C18: 1n9c and C20: 1. Oxygen plasma decreased the C20: 3n6 content, while helium plasma increased its content (Tables 4 and 5).
These findings indicate that oxygen plasma did not change the contents of palmitic acid (C16: 0), oleic acid (C18: 1n9c), trans-oleic acid (C18: 1n9t), and cis-11-eicosenoic acid (C20: 1) in the wheat shorts, but reduced the contents of linoleic acid (C18: 2n6c), α-linolenic acid (C18: 3n3), and cis-8, 11, 14-eicosatrienoic acid (C20: 3n6), and increased the contents of stearic acid (C18: 0) and cis-4, 7, 10, 13, 16, 19-docosahexaenoic acid (DHA, C22: 6n3). Helium plasma reduced the contents of oleic acid, linoleic acid, α-linolenic acid, and cis-11-eicosenoic acid, but increased the contents of stearic acid, trans-oleic acid, cis-8, 11, 14-eicosatrienoic acid, and DHA, and did not change the palmitic acid content.
Compared to the untreated samples, both the oxygen and helium CP treatments reduced the contents of total, unsaturated, and polyunsaturated FAs, and the ratio of unsaturated to saturated FAs, with helium plasma producing greater reductions. Oxygen plasma did not change the contents of saturated and monounsaturated FAs, while helium plasma significantly increased the content of saturated FAs but decreased the content of monounsaturated FAs. This suggests that oxygen plasma oxidized polyunsaturated FAs, leading to a decrease in the unsaturated FA content, while helium plasma caused the decomposition of monounsaturated and polyunsaturated FAs and partially converted some unsaturated FAs into saturated FAs (Table 5).
Compared with the untreated samples, when the 120W CP treatment time was 90s, the contents of total and monounsaturated FAs of wheat shorts did not significantly change, while 89.1% of unsaturated FAs and 87.2% of polyunsaturated FAs were maintained (Table 5).
4 DISCUSSION
In the present study, the wheat shorts initially contained 2.47μg/g of ERG. When they were treated by 120W low-pressure RF oxygen or helium CP, all CP treatments for 30-180s steadily degraded the ERG, and most treatments could convert ERG into VD2 or VD3-like compounds, with the 90-120s treatments showing the greatest effect. To our knowledge, this result is the first observed effect of cold plasma on cereals, maybe due to the meta-stable helium atoms and high-energy photons generated from low-pressure RF helium CP[31] and high concentration of oxygen ion from oxygen CP[32].
Two previous studies reported that CP-treated starches have lower gelatinization temperatures[33,34], but Bie et al.[35] observed an increase in gelatinization temperature and a decrease in the gelatinization enthalpy of starches after CP treatment. The cross-linking, depolymerization, and oxidization mechanisms of CP action greatly influence the gelatinization temperatures of starches; higher cross-linking starches show greater gelatinization temperatures than starches with lower cross-linking[36]. In this study, 120W low-pressure RF helium or oxygen CP significantly increased the gelatinization temperature of wheat shorts and tended to decrease the gelatinization enthalpy (though this was not statistically significant), possibly indicating a cross-linking mechanism of CP. The GLM further confirmed that helium plasma treatment induced a higher gelatinization temperature of wheat shorts, as compared to the samples treated with oxygen plasma and the untreated samples.
In the present study, the content (11,070μg/g) of fatty acids in wheat shorts was much higher than that of wheat flour (3,751μg/g[6]). CP treatment can increase lipid oxidation, leading to rancidity in lipid-rich foods[37]. Thus, it is necessary to optimize the CP processing parameters and CP generators. In this study, 32 types of FAs were identified in CP-treated wheat shorts. The main species were C16: 0, C18: 0, C18: 1n9c, C18: 1n9t, C18: 2n6c, C18: 3n3, C20: 1, C20: 3n6, and C22: 6n3. Compared with the untreated samples, both the oxygen and helium low-pressure RF CP treatments did not change the C16: 0 content, reduced the contents of C18: 2n6c and C18: 3n3, and increased the content of C22: 6n3. Oxygen CP did not change the contents of C18: 0, C18: 1n9c, C18: 1n9t, and C20: 1, and reduced the C20: 3n6 content, while helium CP increased the contents of C18: 0, C18: 1n9c, C18: 1n9t, and C20: 3n6, and reduced the content of C20: 1. The decreases in the contents of C18: 1n9c and C20: 5n3 in the oxygen and helium CP-treated wheat shorts are similar to the decreases in oleic acid (C18: 1, n-9) and eicosapentaenoic acid (20: 5, n-3) in mackerel fillets after CP treatment[38]. Oxygen CP did not increase the content of trans-oleic acid (C18: 1n9t) in wheat shorts. Our results suggest that the active substances of CP induced intra- and inter-molecular reactions in lipid molecules, including polymerization and hydrogenation reactions. Both CP treatments reduced the total, unsaturated, and polyunsaturated FAs and the ratio of unsaturated to saturated FAs, but helium CP increased the content of saturated FAs and decreased the mono-unsaturated FA content, while oxygen CP did not change the contents of saturated and mono-unsaturated FAs. These results further suggest that oxygen CP oxidized polyunsaturated FAs, leading to a decrease in the unsaturated FA content, while helium plasma caused the decomposition of monounsaturated and polyunsaturated FAs and partially converted some unsaturated FAs into saturated FAs.
A reduction in polyunsaturated FAs was noted by Yepez et al.[39], who used 99.8% hydrogen as the feed gas and high-voltage atmospheric pressure CP (HVACP) to treat soybean oil to achieve hardening of the oil without producing trans-FAs. In the present study, with increases in the low-pressure RF CP treatment time, the contents of C18: 1n9t and C18: 2n6t in wheat shorts increased, with helium CP inducing higher contents than oxygen CP. Therefore, low-pressure RF oxygen and helium CP treatments on wheat shorts can reduce fungal contaminants. The optimized process inevitably induces lipid molecule polymerization and hydrogenation reactions.
5 CONCLUSIONS
More powder sample like 70g wheat shorts was used to treat each time with low-pressure RF cold plasma. The 120W oxygen or helium RF CP treatments of wheat shorts for 90-120s reduced the ERG content by 46-64% producing 0.26μg/g VD2 and 1.52μg/g VD3-like compounds, did not change the content of monounsaturated FAs, maintained 88.3-95% of total FAs and 82.2-89.1% of unsaturated FAs and 80.4-87.2% of polyunsaturated FAs, but increased 11.1-15.4% of saturated FAs, and tended to decrease (non-significantly) the gelatinization enthalpy. Cold plasma treatment is useful to remove contaminants like ERG in wheat shorts.
Acknowledgments
The authors acknowledge the Operating Expenses of the Basic Scientific Research Project of the Central Public-Interest Scientific Institution, China (JY2007).
Conflicts of Interest
The authors declared that there are no conflict of interest in this study
Author Contribution
Zhao M was responsible for conceptualizing the study, developing methodologies, conducting validation, formal analysis, curating data, writing the oringinal draft preparation, investigation and resources. Wang M was responsible for conceptualizing the study, developing methodologies, conducting validation, formal analysis, curating data, writing the oringinal draft preparation, investigation and writing the review and editing. Gao G was responsible for developing methodology and curating data. Wu J was responsible for conducting validation, supervision, and writing the review and editing. Li X was responsible for conceptualizing the study, developing methodologies, conducting validation, formal analysis, investigation, conducting supervision, administrating the project, and writing the review and editing. Ren Y was responsible for conducting supervision and writing the review and editing. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data, models, and code generated or used during the study appear in the submitted article.
Abbreviation List
CP, Cold plasma
DON, Deoxynivalenol
DSC, Differential scanning calorimeter
ERG, Ergosterol
FA, Fatty acid
FAME, Fatty acid methyl esters
FAs, Free fatty acids
GLM, General Linear Model-Univariate method
HPLC, High-pressure liquid chromatography
RF, Radio frequency
RNS, Nitrogen species
ROS, Reactive oxygen species
Tc, The conclusion temperature of gelatinization
To, The onset temperature of gelatinization
Tp, The peak temperature of gelatinization
UV, Ultraviolet
VD, Vitamin D
VD2, Vitamin D2
VD3, Vitamin D3
ΔH, Enthalpy of gelatinization
References
[1] Misnal MFI, Redzuan N, Zainal MNF et al. Cold plasma: A potential alternative for rice grain postharvest treatment management in Malaysia. Rice Sci, 2022; 29: 1-15.[DOI]
[2] Gao GB, Wu JZ, Li YY et al. Advance in applied research of cold plasma in grains and oils industry. China Oil, 2022; 47: 133-140.[DOI]
[3] Pan Y, Cheng JH, Sun DW. Cold plasma-mediated treatments for shelf life extension of fresh produce: A review of recent research developments. Compr Rev Food Sci F, 2019; 18: 1312-1326.[DOI]
[4] Zahoranová A, Hoppanová L, Šimončicová J et al. Effect of cold atmospheric pressure plasma on maize seeds: Enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem Plasma P, 2018; 38: 969-988.[DOI]
[5] Puligundla P, Kim JW, Mok C. Effect of corona discharge plasma jet treatment on decontamination and sprouting of rapeseed (Brassica napus L.) seeds. Food Control, 2017; 71: 376-382.[DOI]
[6] Gao G, Wu J, Wei Z et al. Effects of low-pressure radio-frequency cold plasma on the biochemical parameters and fatty acid profile of wheat flours. Cereal Chem, 2023; 100: 393-413.[DOI]
[7] Basaran P, Basaran-Akgul N, Oksuz L. Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment. Food Microbiol, 2008; 25: 626-632.[DOI]
[8] Bermúdez-Aguirre D, Wemlinger E, Pedrow P et al. Effect of atmospheric pressure cold plasma (APCP) on the inactivation of Escherichia coli in fresh produce. Food Control, 2013; 34: 149-157.[DOI]
[9] Iqdiam BM, Abuagela MO, Boz Z et al. Effects of atmospheric pressure plasma jet treatment on aflatoxin level, physiochemical quality, and sensory attributes of peanuts. J Food Process Pres, 2020; 44: e14305.[DOI]
[10] Bahrami N, Bayliss D, Chope G et al. Cold plasma: A new technology to modify wheat flour functionality. Food Chem, 2016; 202: 247-253.[DOI]
[11] Sarangapani C, O’Toole G, Cullen PJ et al. Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innov Food Sci Emerg, 2017; 44: 235-241.[DOI]
[12] Usman I, Afzaal M, Imran A et al. Recent updates and perspectives of plasma in food processing: A review. Int J Food Prop, 2023; 26: 552-566.[DOI]
[13] Janić Hajnal E, Vukić M, Pezo L et al. Effect of Atmospheric Cold Plasma Treatments on Reduction of Alternaria Toxins Content in Wheat Flour. Toxins, 2019; 11: 704.[DOI]
[14] Chen X, Qiu Y, Zhang J et al. Degradation efficiency and products of deoxynivalenol treated by cold plasma and its application in wheat. Food Control, 2022; 136: 108874.[DOI]
[15] Xi DQ. Grain dictionary. China Goods Press: Beijing, China, 2009; 64-65.
[16] Posner ES, Hibbs AN. Wheat flour milling. American Association of Cereal Chemists: St. Paul, Minnesota, USA, 1999; 215-217.
[17] Liu J, Wang R, Chen Z et al. Effect of cold plasma treatment on cooking, thermomechanical and surface structural properties of Chinese milled rice. Food Bioprocess Tech, 2021; 14: 866-886.[DOI]
[18] Lee KH, Woo KS, Yong HI et al. Assessment of microbial safety and quality changes of brown and white cooked rice treated with atmospheric pressure plasma. Food Sci Biotechnol, 2018; 27: 661-667.[DOI]
[19] Lee JH, Woo KS, Jo C et al. Quality evaluation of rice treated by high hydrostatic pressure and atmospheric pressure plasma. J Food Quality, 2019; 9: 4253701.[DOI]
[20] Ng HE, Raj SSA, Wong SH et al. Estimation of fungal growth using the ergosterol assay: a rapid tool in assessing the microbiological status of grains and feeds. Lett Appl Microbiol, 2008; 46: 113-118.[DOI]
[21] Olsson J, Börjesson T, Lundstedt T et al. Detection and quantification of ochratoxin A and deoxynivalenol in barley grains by GC-MS and electronic nose. Int J Food Microbiol, 2002; 72: 203-214.[DOI]
[22] Pietri A, Bertuzzi T, Pallaroni L et al. Occurrence of mycotoxins and ergosterol in maize harvested over 5 years in northern Italy. Food Addit Contam, 2004; 21: 479-487.[DOI]
[23] Sun Y, Nzekoue FK, Vittori S et al. Conversion of ergosterol into vitamin D2 and other photoisomers in Agaricus bisporus mushrooms under UV-C irradiation. Food Biosci, 2022; 50: 102143.[DOI]
[24] Varga M, Bartók T, Mesterházy A. Determination of ergosterol in Fusarium-infected wheat by liquid chromatography-atomospheric pressure photoionization mass spectrometry. J Chromatogr A, 2006; 1103: 278-283.[DOI]
[25] Papoutsis K, Grasso S, Menon A et al. Recovery of ergosterol and vitamin D2 from mushroom waste-Potential valorization by food and pharmaceutical industries. Trends Food Sci Tech, 2020; 99: 351-366.[DOI]
[26] Chen Z, Wang R, Li X et al. Sorption equilibirum moisture and isosteric heat of cold plasma treated milled rice. Innov Food Sci Emerg, 2019; 55: 35-47.[DOI]
[27] China’s national standard. GB/T 25221-2010. Inspection of grain and oils-Determination of ergosterol in grain-normal phase high-performance liquid chromatography. Issued March 1, 2011.
[28] Tao LS, Qin W, Wei Z et al. Effects of small-scale storage on the cooking property and fatty acid profile of sea rice paddy. Appl Food Res, 2022; 2: 100175.[DOI]
[29] SPSS Inc. SPSS for Windows, Release 17.0.1. SPSS Inc: Chicago, USA, 2017.
[30] Li X, Han X, Gao G et al. Rice freshness determination during paddy storage based on solvent retention capacity. Cereal Chem, 2022; 99: 593-602.[DOI]
[31] Cao Z, Li X, Song H et al. Effect of intermittent low-pressure radiofrequency helium cold plasma treatments on rice gelatinization, fatty acid, and hygroscopicity. Foods, 2024; 13: 1056.[DOI]
[32] Zhao M, Li X, Wu J et al. Study on the characteristics of monolayer water and bound water of wheat flour after cold plasma treatment. Science and Technology of Cereals. Oils and Foods, 2024; 32: 55-64.[DOI]
[33] Zhang B, Chen L, Li X et al. Understanding the multi-scale structure and functional properties of starch modified by glow-plasma: A structure-functionality relationship. Food Hydrocolloid, 2015; 50: 228-236.[DOI]
[34] Thirumdas R, Trimukhe A, Deshmukh RR et al. Functional and rheological properties of cold plasma treated rice starch. Carbohyd Polym, 2017; 157: 1723-1731.[DOI]
[35] Bie P, Li X, Xie F et al. Supramolecular structure and thermal behaviour of cassava starch treated by oxygen and helium glow-plasmas. Innov Food Sci Emerg, 2016; 34: 336-343.[DOI]
[36] Thirumdas R, Kadam D, Annapure US. Cold plasma: an alternative technology for the starch modification. Food Biophys, 2017; 12: 129-139.[DOI]
[37] Ekezie FGC, Sun DW, Cheng JH. A review on recent advances in cold plasma technology for the food industry: current applications and future trends. Trends Food Sci Tech, 2017; 69: 46-58.[DOI]
[38] Albertos I, Martín-Diana AB, Cullen PJ et al. Effects of dielectric barrier discharge (DBD) generated plasma on microbial reduction and quality parameters of fresh mackerel (Scomber scombrus) fillets. Innov Food Sci Emerg, 2017; 44: 117-122.[DOI]
[39] Yepex XV, Baykara H, Xu L et al. Cold plasma treatment of soybean oil with hydrogen gas. J Am Oil Chem Soc, 2021; 98: 103-113.[DOI]
Copyright © 2024 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.




 Copyright ©
Copyright ©