Assessment of Semi-autotrophic Hydroponics on in vitro propagated Jamaican Sweet Yam (Dioscorea alata) - Analysis of Variance and Principal Component Analysis
Kemar A. Rose1, Collin M. Scantlebury1*, Marsha C. Williams1, Ryan D. Francis1
1Biotechnology Department, The Scientific Research Council, Kingston, Jamaica
*Correspondence to: Collin M. Scantlebury, PhD, Team Leader, Biotechnology Department, The Scientific Research Council, Hope Gardens Complex, Kingston 6, Jamaica; Email: collins@src-jamaica.org
Abstract
Objective: Despite its benefits, plant tissue culture tends to incur a high production cost hence the need for cost-reduction strategies, especially with regards to acclimatization. One such strategy is to use semi-autotrophic hydroponics (SAH). SAH is a novel, low-cost technology that has been designed for the high-ratio and rapid propagation of clonal or vegetatively propagated crops. Sweet yam is a major export commodity of Jamaica and there is need to produce disease-free plants that require less pampering during hardening. The aim of this study is to analyze SAH in tissue culture of Jamaican sweet yams.
Methods: Rooted sweet yam (Dioscorea alata) in vitro cultures were transferred to a commercial sphagnum peat moss based potting substrate, with vermiculite and fine calcitic and dolomitic limestone and the SAH system was run in a growth chamber at a tissue culture facility. Six tissue culture yam plantlets were incubated in polypropylene vessels and placed on separate shelves with north facing rows and south facing rows. Samples were treated with commercial fertilizer and controls were treated with tap water.
Results: Fertilized plants showed significantly higher mean number of nodes (8.0±2.2 and 7.5±1.8) compared to non-fertilized plants (4.0±0.9 and 5.0±2.1) and significantly higher maximum leaf area index (41.42±15.49cm2 and 35.58±16.93cm2) versus non-fertilized plants (19.17±6.42cm2 and 23.58±6.55cm2). Fertilizer significantly reduced the mean number of mini tubers per plant (0.67±0.52). Non-fertilized (control) produced 2.50±1.05 and 1.83±0.41 mini tubers per plant. The mean mini tuber yields per plant were significantly reduced from 1.05±0.26g and 1.00±0.83g in non-fertilized (controls) to 0.28±0.30g and 0.34±0.40g in fertilized treatments. Principal Component Analysis (PCA) revealed three principal components accounting for 81.47% of the observed variance. PC1, 2 and 3 accounted for 39.28%, 30.49% and 11.64% of the variance respectively. PC1 described factors responsible for the vegetative growth, PC2 factors for mini tuberization and PC3 effects due to positioning of plants on shelves.
Conclusion: SAH is a very flexible and adaptable technique which was found to be very useful in the acclimatization of tissue culture for Jamaican sweet yam plants. Commercial fertilizer application was found to be an efficient means of nutrient addition to promote vegetative growth. Fertilizer application significantly increased the number of nodes and maximum leaf area while it significantly reduced tuber quantity and yield. The ANOVA and PCA showed that fertilizer application was positively correlated to vegetative production factors while it was negatively correlated to mini tuber yield.
Keywords: Dioscorea alata, fertilizer, mini tuber, semi-autotrophic hydroponics, sweet yam
1 INTRODUCTION
Plant tissue culture methods are conducted under controlled aseptic, nutritional and environmental conditions. These techniques produce plant tissues called explants which are indistinguishable replicas of the mother plant that are generated from the in vitro culture of cells, tissues, organs or the entire plant[1]. A single explant can be multiplied in a shorter time period compared to the traditional method and the demand for space is less; this is possible all throughout the year despite the season or weather[2]. Despite its benefits, plant tissue culture is capital intensive and comes with a high production cost. Many researchers have spoken to the issue of making plant tissue culture more cost-effective by addressing specific aspects of the vast range of activities in the in vitro process[3,4]. The focus herein is on acclimatization, the transition from in vitro to successfully weaned and hardened plant[5]. Semi-sterilized tissue culture (SSTC), photoautotrophic tissue culture system (PTCS) and semi-autotrophic hydroponics (SAH) systems have evolved from the need to get away from the aseptic constraints of in vitro culture. The resulting plants require less pampering during weaning and hardening.
A principal departure from conventional plant tissue culture involves ex vitro rooting of plantlets previously maintained in vitro. One such method that employs this is SSTC as described by Shan and Seaton[6]. Plantlets resulting from the cultivation of nodal cuttings or shoot tips are cultured in root pulsing media. Subsequently, they are transplanted to sterilized aerobic rooting substrate to induce root initiation and development. Afterwards, the rooted plantlets are then transferred to normal propagation beds in a greenhouse and potted on for acclimatization. SSTC is very advantageous as it can be performed under semi-sterilized conditions. Hence, degeneration is avoided and the occurrences of bacterial contamination are minimized in comparison to micropropagation techniques. By eliminating the time-consuming steps of the explant establishment, proliferation, and maintenance in vitro, the propagation process was simplified in contrast to typical sterile tissue culture techniques.
PTCS is a method that involves the application of sugar-free media in micropropagation. Once explants can photosynthesize, they can be micropropagated without the need for added sugar in the growth media. This capacity for autotrophic micropropagation is exploited to reduce in vitro production costs[7]. The PTCS reduces production costs because of large culture vessels, simple culture media formulations, and lower incidence of culture contamination. PTCS is also less affected by contamination and produces higher yields. Additionally, this system requires little or no hardening of plantlets[8,9].
SAH is similar in evolution to SSTC and PTCS. The technique was first developed for potato multiplication by a company in Argentina called SAHTecno LLC[10]. SAH is a novel, robust, more efficient and low-cost technology that has been designed for the high-ratio and rapid propagation of clonal or vegetatively propagated crops. This technique ensures the establishment of nodal cuttings from tissue culture plantlets that are true-to-type and disease-free. The plantlets are transplanted in boxes containing a mixture of substrate and growth nutrient medium[11,12]. It has been adapted for yams and cassava at the International Institute of Tropical Agriculture (IITA), Nigeria. It is very flexible and may be conducted in different facilities such as labs or screen houses[13,14]. It is a very easy and adaptable technology; additionally, it has been used in the successful acclimatization of pineapples[15]. As such, the technology has potential for a range of in vitro produced crops.
At the Scientific Research Council (SRC), Jamaica, as is common to tissue culture labs internationally, there is a constraint of high cost of inputs, notably labour and energy. Therefore, any reduction in tissue culture production costs is a welcomed innovation. Globally, Jamaica is a major exporter of yellow yam (Dioscorea cayenensis), negro yam (Dioscorea rotundata) and sweet yam[16]. Jamaica is currently second only to Ghana; however, a decade and a half earlier it was the leading yam exporter[17,18]. Therefore, sweet yam is an important crop to the agricultural sector in Jamaica and has been designated as a priority crop by the Jamaican government[19]. Hence, there is a need to produce disease-free sweet yam at a rapid rate to supply the needs of the industry. The aim of this present study is to analyze SAH in tissue culture Jamaican Dioscorea alata var. sweet yams, previously described by Riley et al.[20] and Riley et al.[21].
Principal component analysis (PCA) can be used to simplify similar multivariate plant data by transforming a number of potentially inter-correlated variables into a smaller number of variables known as principal components; this is performed with minimal information loss[22,23]. PCA was used in this present study to complement analysis of variance (ANOVA) and to identify the major underlying variables that accounted for the observed variances in the data.
2 MATERIALS AND METHODS
2.1 Plant Material, Experimental Design and Layout
In July 2023, rooted sweet yam (Dioscorea alata) in vitro cultures (Figure 1) were transferred to a commercial sphagnum peat moss based potting substrate, with vermiculite and fine calcitic and dolomitic limestone. The SAH system was run in a growth chamber at the SRC tissue culture facility, Kingston Jamaica. The facility is located at 18.0189° N latitude, 76.7497° W longitude and is 201m above sea level. Plants were incubated at 25±2°C with 16h photoperiod under LED light with photon flux of 75μmol∙m-2∙s-1.
|
Figure 1. Rooted sweet yam (Dioscorea alata) in vitro cultures.
For each treatment, six tissue culture yam plantlets were incubated in each of twelve 170mm×110mm×70mm polypropylene vessels (Figure 2A and 2B). Vessels were covered with lids to maintain a high relative humidity; each lid was perforated with four holes to allow for aeration. This was done for ten days to reduce transpiration. Each treatment was placed on a separate shelf. The layout on each shelf included two rows of six vessels each, north facing rows (samples 2 and 4) and south facing rows (samples 1 and 3). Samples 1 and 2 were fertilizer treatments, while samples 3 and 4 were controls.
|
Figure 2. SAH system. A: SAH system control (no fertilizer); B: SAH system using commercial fertilizer.
Plantlets were fertilized weekly (100mL/vessel) for 50 days in a modified SAH system with a commercial fertilizer comprising N:P2O5:K2O:S in the ratio (15:30:15:2.8) mixed at 1 tablespoon per gallon. Afterwards the fertilizer was substituted with another commercial fertilizer comprising N:P2O5:K2O:S in the ratio (28:6:14:5.6) plus micronutrients. The control was irrigated with tap water only.
2.2 Data Collection and Statistical Analysis
Plant growth was assessed after 120 days. At harvest 12 plants were randomly selected, one from each vessel. Due to the layout in double rows six plants were sampled from each row per shelf. Number of leaves, number of nodes, maximum leaf area, total fresh weight (fresh weight of the whole plant inclusive of the mini tubers if present), number of mini tubers, yield of mini tubers and shoot fresh weight were recorded. Two-way ANOVA and Tukey test were used to determine significant differences in each parameter according to Fowler et al[24]. Correlation analysis and Principal component analysis were computed using IBM SPSS Statistics for Windows, Version 29.0 (IBM Corp, Armonk, NY, USA) to account for the variance in the parameters.
3 RESULTS
Figure 3A and 3B show visible differences between fertilized and unfertilized plants. However, of the hypotheses tested - effects of fertilizer application, interference from layout position on shelves in the growth room, and interaction, two-way ANOVA showed that the F value was only significant for effects of fertilizer application. There were significant differences in means between samples due to fertilizer in four parameters. These were number of nodes per plant, maximum leaf area per plant, number of mini tubers per plant and total mini tuber yield per plant. All F values exceeded the critical F value 8.096 at df1,20, significant at P=0.01.
|
Figure 3. Differences between fertilized and unfertilized sweet yam. A: Sweet yam controls after 120 days; B: Sweet yam plants treated with commercial fertilizer after 120 days.
There were no significant differences in the number of leaves between samples (Tables 1 and 2). It was not possible to continue to the two-way ANOVA for total fresh weight or shoot fresh weight since the Fmax value for these exceeded the critical value for four samples and df=5 within samples. The Fmax values for total fresh weight and shoot fresh weight were 71.485 and 37.996 respectively, exceeding the critical value of 13.7.
Samples |
Number of Leaves |
Total Fresh Weight (g) |
Shoot Fresh Weight (g) |
1 |
9.67±1.97 |
3.6597±1.7507 |
3.3153±1.4766 |
2 |
8.17±1.83 |
2.7126±0.9543 |
2.4342±0.8612 |
3 |
6.67±1.86 |
2.6368±0.2390 |
1.5896±0.2399 |
4 |
7.67±3.44 |
2.9223±2.0255 |
1.9268±1.2052 |
Notes: Values are mean ±SD. Sample 1 - Fertilizer, south facing, Sample 2 - Fertilizer, north facing, Sample 3 - Control, south facing, Sample 4 - Control, north facing
Table 2. ANOVA Summary Table for Number of Leaves
Source of Variation |
Sum of squares |
df |
Variance |
F |
(Between samples) |
(0.09681) |
(3) |
|
|
Fertilizer |
0.07457 |
1 |
0.07457 |
4.14427 |
Position |
0.0018 |
1 |
0.0018 |
0.09987 |
Interaction |
0.02944 |
1 |
0.02944 |
1.13562 |
Within samples |
0.35989 |
20 |
0.01799 |
|
In addition to differences in parameter means between samples being due only to fertilizer application, the other major findings were that added fertilizer increased the number of nodes and the maximum leaf area possible. However, fertilizer application resulted in reduced yield in the number of mini tubers produced as well as the total yield of mini tubers per plant.
3.1 Number of Nodes
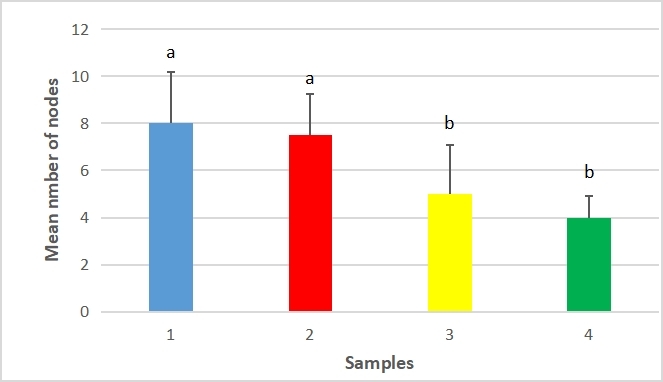
The mean number of nodes ranged from 5.0±2.1 and 4.0±0.9 for unfertilized plants to 8.0±2.2 and 7.5±1.8 for fertilized plants. Figure 4 showed the higher means for fertilized samples. According to Table 3, the ANOVA showed that regardless of position on shelf, fertilized samples produced significantly more nodes than unfertilized samples (F=24.247).
The Tukey’s statistic T=0.20 for 4 samples and 20df indicated that sample 1 (fertilized, south facing) was significantly different from both unfertilized samples. Sample 2 (fertilized, north facing) was significantly different from the unfertilized controls.
|
Figure 4. Mean number of nodes per plant between samples of fertilized and unfertilized sweet yam plants using SAH. Significant differences were determined by the Tukey’s test and are indicated by different letters (P≤0.01). Values are mean ±SD. Sample 1 - Fertilizer, south facing, Sample 2 - Fertilizer, north facing, Sample 3 - Control, south facing, Sample 4 - Control, north facing.
Table 3. ANOVA Summary Table for Number of Nodes
Source of Variation |
Sum of squares |
df |
Variance |
F |
(Between samples) |
(0.3816) |
(3) |
|
|
Fertilizer |
0.3605 |
1 |
0.3605 |
24.247** |
Position |
0.0168 |
1 |
0.0168 |
1.1317 |
Interaction |
0.0042 |
1 |
0.0042 |
0.2847 |
Within samples |
0.2974 |
20 |
0.0149 |
|
Notes: **P<0.01
3.2 Maximum Leaf Area
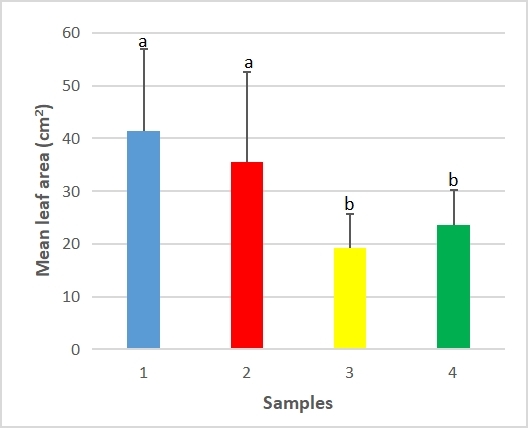
Figure 5 shows that the maximum leaf area ranged from 19.17±6.42cm2 and 23.58±6.55cm2 for the unfertilized plants to 41.42±15.49cm2 and 35.58±16.93cm2 for the fertilized plants. According to Table 4, shelf layout was not significant, nor interaction, but the effect of fertilizer application was significant (F=11.529).
The Tukey test showed that sample 1 (fertilized, south facing) mean maximum leaf area was significantly different from both unfertilized samples. Sample 2 (fertilized, north facing) was significantly different from the unfertilized controls. The critical value exceeded by this pairwise difference was T=19.97.
|
Figure 5. Maximum leaf area per plant between samples of fertilized and unfertilized sweet yam plants using SAH. Significant differences were determined by the Tukey’s test and are indicated by different letters (P≤0.01). Values are mean ±SD. Sample 1 - Fertilizer, south facing, Sample 2 - Fertilizer, north facing, Sample 3 - Control, south facing, Sample 4 - Control, north facing.
Table 4. ANOVA Summary Table for Maximum Leaf Area
Source of Variation |
Sum of squares |
df |
Variance |
F |
(Between samples) |
(1920.198) |
(3) |
|
|
Fertilizer |
1759.594 |
1 |
1759.594 |
11.529** |
Position |
3.010417 |
1 |
3.010417 |
0.0197 |
Interaction |
157.5938 |
1 |
157.5938 |
1.03256 |
Within samples |
3052.458 |
20 |
152.6229 |
|
Notes: **P<0.01
3.3 Yield Reduction from Added Fertilizer
Figure 6 shows mini tubers produced by sweet yam plants using SAH. In comparison to unfertilized controls, fertilizer application reduced the number of mini tubers produced and the total yield in mass per mini tuber per plant.
|
Figure 6. Sweet yam mini tubers produced after 120 days using SAH.
3.4 Number of Mini Tubers per Plant
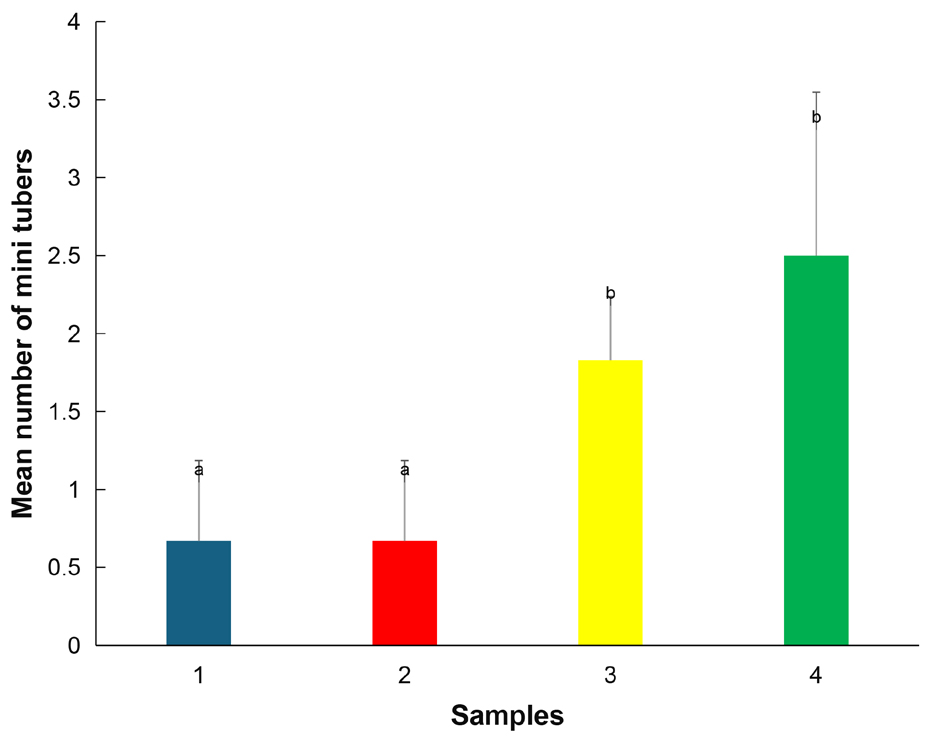
Table 5 shows that there was a significant difference in the number of mini tubers produced between treatments (F=27.013). The samples for fertilizer treatments produced 0.67±0.52 mini tubers per plant. The number of mini tubers produced for unfertilized control samples were 2.50±1.05 and 1.83±0.41 (Figure 7).
Table 5. ANOVA Summary Table for Number of Mini Tubers
Source of Variation |
Sum of squares |
df |
Variance |
F |
(Between samples) |
(0.510682) |
(3) |
|
|
Fertilizer |
0.492138 |
1 |
0.492138 |
27.013** |
Position |
0.009272 |
1 |
0.009272 |
0.50893 |
Interaction |
0.009272 |
1 |
0.009272 |
0.50893 |
Within samples |
0.364369 |
20 |
0.018218 |
|
Notes: ** P<0.01
|
Figure 7. Number of mini tubers per plant between samples of fertilized and unfertilized sweet yam plants using SAH. Significant differences were determined by the Tukey’s test and are indicated by different letters (P≤0.01). Values are mean ±SD. Sample 1 - Fertilizer, south facing, Sample 2 - Fertilizer, north facing, Sample 3 - Control, south facing, Sample 4 - Control, north facing.
The Tukey statistic T=0.98 indicated that both samples 1 and 2 were significantly different from samples 3 and 4. The unfertilized control samples produced more mini tubers than the fertilized samples.
3.5 Total Mini tuber Yield (g)
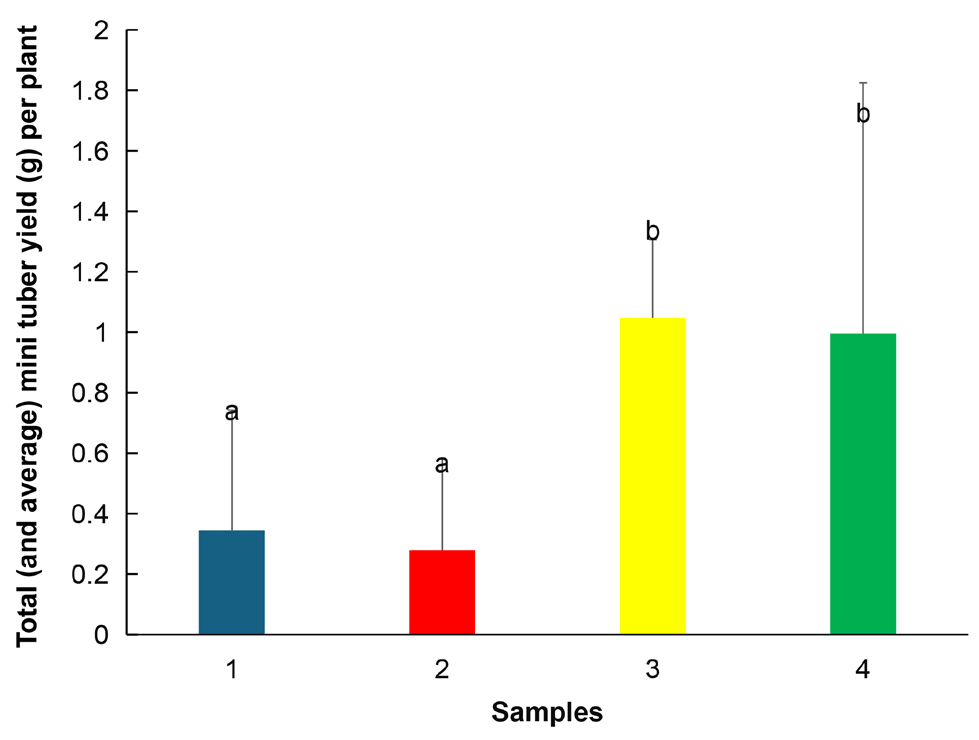
The computation of ANOVA (Table 6) and Tukey’s presented some interesting anomalies: while the mean yield between samples was found to be significantly different between samples (F=12.029), the Tukey statistic 3.63 was not exceeded by any pairwise differences. The largest pairwise difference was between sample 2 (fertilizer treatment north facing) and sample 3 (control south facing). The total yield ranged from 0.28±0.30g and 0.34±0.40g in fertilized treatments to 1.05±0.26g and 1.00±0.83g in non-fertilized controls (Figure 8).
Table 6. ANOVA Summary Table for Mini Tuber Yield
Source of Variation |
Sum of squares |
df |
Variance |
F |
(Between samples) |
(3.04563) |
(3) |
|
|
Fertilizer |
3.024529 |
1 |
3.024529 |
12.03948** |
Position |
0.020798 |
1 |
0.020798 |
0.0827 |
Interaction |
0.000309 |
1 |
0.000309 |
0.001229 |
Within samples |
5.028526 |
20 |
0.251426 |
|
Notes: **P<0.01
|
Figure 8. Total mini tuber yield per plant between samples of fertilized and unfertilized sweet yam plants using SAH. Significant differences were determined by the Tukey’s test and are indicated by different letters (P≤0.01). Values are mean±SD. Sample 1 - Fertilizer, south facing, Sample 2 - Fertilizer, north facing, Sample 3 - Control, south facing, Sample 4 - Control, north facing.
3.6 Correlation Analysis
Correlation analysis confirmed the obvious expected associations between the number of leaves, nodes and shoot fresh weight, as well as mini tubers and shoot fresh weight. It showed the expected strong positive association between fertilizer application and number of nodes (r=0.70). It also showed the unexpected strong negative association between fertilizer and mini tubers (r=-0.75) (Table 7).
|
Number of Nodes |
Total Fresh Weight |
Number of Mini Tubers |
Total Yield |
Shoot Fresh Weight |
Fertilizer |
0.70 |
|
-0.75 |
|
|
Number of leaves |
0.66 |
0.76 |
|
|
0.77 |
Total fresh weight |
|
|
|
|
0.74 |
Number of mini tubers |
|
|
|
0.87 |
0.66 |
Total yield |
|
|
|
|
0.87 |
3.7 Principal Component Analysis
Table 8 shows three principal components each with eigenvalues greater than 1.0 hence significant. As such, these principal components accounted for 81.42% of the variation seen in the yam data. Figure 9, the scree plot, confirms the three components based on the eigenvalues and all positioned along the vertical arm before the perpendicular intersection.
Table 8. Total Variance Explained for Principal Components
Component |
Total |
Initial Eigenvalues |
Extraction Sums of Squared Loadings |
|||
% of Variance |
Cumulative % |
Total |
% of Variance |
Cumulative % |
||
1 |
3.928 |
39.28 |
39.28 |
3.928 |
39.28 |
39.28 |
2 |
3.049 |
30.49 |
67.78 |
3.049 |
30.49 |
67.78 |
3 |
1.164 |
11.64 |
81.42 |
1.164 |
11.64 |
81.42 |
|
Figure 9. Scree plot for principal component analysis of sweet yam data.
Principal component (PC) 1 accounted for 39% of the variance while PC2 30%. PC3 was only 12%. From the component matrix (Table 9), PC1 correlated with six of the original variables, which all varied together. This component describes the vegetative production factors. It reinforces that increasing fertilizer increased leaves, nodes and associated total fresh weight and maximum leaf area.
Table 9. Component Matrixa for Principal Components of Sweet Yam Data
|
Component |
||
1 |
2 |
3 |
|
Fertilizer |
0.720 |
-0.616 |
|
Shelf position |
|
|
0.856 |
Number of leaves |
0.780 |
|
-0.321 |
Number of nodes |
0.877 |
|
|
Number of mini tubers |
-0.369 |
0.810 |
|
Total fresh weight |
0.751 |
0.614 |
|
Total mini tuber yield |
|
0.978 |
|
Shoot fresh weight |
0.917 |
|
|
Maximum leaf area |
0.658 |
|
|
Notes: a3 components extracted. Extraction Method: Principal Component Analysis.
PC2 correlated with three of the original variables. While the correlation was positive for mini tuber yield and associated variables, the correlation was negatively for fertilizer. It showed that decreasing the fertilizer meant increased mini tuber yield. PC2 therefore described the factors contributing to mini tuber production.
PC3 contributed the least, 12% to the 81% of the variation in the data. PC3 correlated strongly and positively with only one original variable, spatial or geographical layout. The loading with leaves was weak (-0.32). PC3 was therefore difficult to interpret but it indicated that shelf positioning may be important in the variance of the data.
4 DISCUSSION
The SAH technology for weaning and hardening of Jamaican sweet yam has been an ongoing demonstration at SRC Biotechnology facility. This illustrates how well adapted and easily adaptable this low cost technology is given the use of local substitutes. Additionally, it shows how useful SAH is for long-term growth, as well as, the production of mini tubers. The findings of major interest include identification of the requirements for tuberization analogous to observations in field production and the complementarity between the ANOVA analysis and the PCA. The recommendations for routine SAH culture are outlined.
Researchers at IITA have employed the use of formulated nutrient solutions to supply plantlets propagated by SAH with nutrients[13,25]. However, in this present study, locally sourced commercial fertilizer was used as a substitute to increase vegetative yield. Inorganic fertilizer components such as nitrogen (N), phosphorous (P) and potassium (K) are important nutrients for plant growth and the yield[26]. The combination treatment of NPK provides plants with the main plant nutrients needed to positively influence vegetative growth which includes leaves, stems and roots[27]. Therefore, fertilizer application, as expected, increased the number of nodes and leaf area. Total fresh weight and total shoot fresh weight were also expected to be affected by fertilizer application. Unfortunately, the ANOVA could not be computed and this was due to statistical procedure or protocol. This was because the sample variances were quite dissimilar. The homogeneity of variance test showed that the variances were significantly different hence ANOVA could not be applied. According to Fowler et al.[24], once the difference between the largest and smallest sample variance exceeds a certain limit one cannot proceed to the ANOVA. The simplest alternative to tell the differences between sample means was to perform a series of paired t-tests. For each pairwise test at df=10, the critical value was 2.228 at P=0.05. The matrix showed that there were no significant differences between sample means.
Initially, it may be surprising that leaf number or abundance was not affected by fertilizer application. From the observation of increased nodes with added fertilizer it may be inferred that yam leaves are not necessarily correlated to leaf number. This is an anatomical issue. Based on visual inspection, each node on the sweet yam vine does not necessarily translate to a leaf or pair of leaves because leaves are generally found more terminal on the vines and not restricted to nodes. In other crops, such as banana and elephant foot yam, leaf number was a poor index to differentiate between fertilizer and non-fertilizer treatments[28,29].
The findings were consistent with anecdotal and research data from in vivo production in yam. There are mixed reports of the usefulness of fertilizer. Generally, fertilizer application promoted increases in growth and yield in various Dioscorea species in nutrient deficient soil but the beneficial effects on yields were significantly lower in nutrient sufficient soil[30]. There are also varietal differences to different soil nutrient conditions[31].
In vivo, when yams are traditionally grown without fertilizer, added fertilizer is said to increase tuber yield but organoleptic quality is poor and taste is negatively affected[32,33]. Conversely, all mini tuber yield parameters were negatively affected. Our findings are similar to field studies where fertilizer increases aboveground biomass but was not reflected in tuber increase. This resulted in higher leaf area index and lower fresh tuber yield. This was possibly caused by a potential imbalance between source (leaves) and sink (mini tubers) in which fertilizer application has favoured top growth over the tubers[34].
The PCA and ANOVA are consistent in identifying that the conditions for foliage production are different from those favoring tuberization. In the context of reducing cost and maximizing production of propagation material, it may be more beneficial to opt for low fertilization systems. While there may be more nodes for propagation by cuttings, the production of mini tubers is an opportunity for enhanced multiplication rates. The merits of fertilizer use may be more visible in producing broad-leafed hardened plants for delivery to farmers.
5 CONCLUSION
SAH is a very flexible and adaptable technique which was found to be very useful in the acclimatization of tissue culture Jamaican sweet yam plants. Commercial fertilizer application was found to be an efficient means of nutrient addition to promote vegetative growth. Fertilizer application significantly increased the number of nodes and maximum leaf area while tuber quantity and yield were significantly decreased by the addition of fertilizer. SAH enabled the faster growth of sweet yam plantlets which will facilitate the increased availability of planting material for the field. The ANOVA and PCA showed that fertilizer application was positively correlated to vegetative production factors while it was negatively correlated to mini tuber yield. This research informs us that once mini tuber yield is the goal in SAH then it seems to be more beneficial to use low fertilizer systems. However, if foliage (leaf size, leaf area, number of nodes) is the goal then high fertilizer systems may be more beneficial.
Acknowledgements
Support for this investigation was given by the Scientific Research Council, Jamaica. The authors wish to thank all our colleagues who provided their expertise that greatly assisted this research project and enhanced the manuscript.
Conflicts of Interest
The authors declare that they have no conflict of interests.
Author Contribution
Rose KA carried out the experimental work of the manuscript, and contributed to the writing of the manuscript (including revisions). Scantlebury CM conceived the study, designed the study, generated the data, conducted the analysis and interpretation of the data, contributed to the writing of the manuscript and provided general supervision of the research. Williams MC carried out the experimental work of the manuscript. Francis RD provided general supervision of the research. All authors read and approved the final manuscript.
Abbreviation List
ANOVA: Analysis of variance
IITA, International Institute of Tropical Agriculture
PC, Principal component
PCA, Principal component analysis
PTCS, Photoautotrophic tissue culture system
SAH, Semi-autotrophic hydroponics
SRC, Scientific Research Council
SSTC, Semi-sterilized tissue culture
References
[1] Rodge RR, Patil P. Tissue Culture as a Plant Production Technique for Fruit Crops and Plant Part Used for Propagation. Int J Agric Sci, 2020; 12: 81-87.[DOI]
[2] Suresh Kumar C, Keshamma E. Perspectives on Plant Tissue Culture Technology: A Review. Int J All Res Educ Sci Methods, 2022; 10: 94-101.[DOI]
[3] George P, Manuel J. Low Cost Tissue Culture Technology for the Regeneration of Some Economically Important Plants for Developing Countries. Int J of Agric Environ Biotechnol, 2013; 6: 703-711.[DOI]
[4] Datta SK, Chakraborty D, Janakiram T. Low Cost Tissue Culture: An Overview. J Plant Sci Res, 2017; 33: 181-199.[DOI]
[5] Adam S. PennState Extension. Tissue Culture Finishing for Greenhouse Growers: Stage III-Stage IV. 2022. Accessed 27 December 2023. Available at:[Web]
[6] Shan F, Seaton K. Semi-sterilized Tissue Culture for Rapid Propagation of Grapevines (Vitis vinifera L.) Using Immature Cuttings. Hort Sci, 2014; 49: 949-954.[DOI]
[7] Ashrafzadeh S, Leung DWM. Photoautotrophic micropropagation system (PAM): a novel approach deserving increased uptake for commercial plant production. Vegetos, 2021; 34: 13-18.[DOI]
[8] Prakash S, Hoque MI, Brinks T. Culture Media and Containers: Low cost options for tissue culture technology in developing countries. Proceedings of a Technical Meeting organized by the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture. Vienna, Austria, 26-30 August 2002.[DOI]
[9] Aighewi BA, Asiedu R, Maroya N et al. Improved propagation methods to raise the productivity of yam (Dioscorea rotundata Poir.). Food Sec, 2015; 7: 823-834.[DOI]
[10] Maroya N, Balogun M, Aighewi B et al. Transforming Yam Seed Systems in West Africa. In: Root, Tuber and Banana Food System Innovations: Value Creation for Inclusive Outcomes. Springer: Cham, Switzerland, 2022; 421-451.[DOI]
[11] Darkwa K, Olasanmi B, Asiedu R et al. Review of empirical and emerging breeding methods and tools for yam (Dioscorea spp.) improvement: Status and prospects. Plant Breed, 2020; 139: 474-497.[DOI]
[12] Norman PE, Kawa MS, Karim KY. Effect of Pot Size and Transplanting Date on Tuber Production Using Micro-Tubers of White Yam. J Mod Agric Biotechnol, 2022; 1: 3.[DOI]
[13] Ogwuche TO, Adesanya TA, Diebiru-Ojo EM et al. Influence of growth nutrient and rooting hormone on survival and growth of Semi-Autotrophic Hydroponics (SAHTM) cassava plantlets: The GCP21 - IVth International Cassava Conference. Cotonou, Benin, 11-15 June 2018.[DOI]
[14] Pelemo O, Benjamin G, Adejumobi I et al. Semi-Autotrophic Hydroponics: A potential seed system technology for reduced breeding cycle and rapid quality seed delivery. Int I Trop Agr, 2019.[DOI]
[15] Olagunju YO, Aduloju AO, Akin-Idowu PE et al. Acclimatization of Tissue Culture Pineapple Plantlet Using Semi-Autotrophic Hydroponics Technique in Comparison with Other Conventional Substrates. J Exp Agric Int, 2021; 43: 61-67.[DOI]
[16] Davis G. Jamaica Information Service. Record Domestic Crop Production for Trelawny. 2023. Accessed 28 December 2023. Available at:[Web]
[17] Barker D, Beckford C. Innovation maintains yam production in Jamaica. Appropriate Technol, 2007; 34: 63-65.[DOI]
[18] Adingo T-M. B&FT Online. Yam exports reach record high. Accessed 27 December 2023. Available at:[Web]
[19] Ministry of Industry, Commerce, Agriculture & Fisheries. Ministry of Industry, Investment & Commerce. MICAF Annual Performance Report 2019-2020. Accessed 5 December 2023. Available at:[Web]
[20] Riley CK, Wheatley AO, Hassan I et al. In vitro Digestibility of Raw Starches Extracted from five Yam (Dioscorea spp.) Species Grown in Jamaica. Starch/Stärke, 2004; 56:69-73.[DOI]
[21] Riley CK, Wheatley AO, Asemota HN. Isolation and Characterization of Starches from eight Dioscorea alata cultivars grown in Jamaica. Afr J Biotechnol, 2006; 5:1528-1536.[DOI]
[22] Garcia DP, Caraschi JC, Ventorim G et al. Assessment of plant biomass for pellet production using multivariate statistics (PCA and HCA). Renew Energy, 2019; 139: 796-805.[DOI]
[23] Adilova SS, Qulmamatova DE, Baboev SK et al. Multivariate Cluster and Principle Component Analyses of Selected Yield Traits in Uzbek Bread Wheat Cultivars. Am J Plant Sci, 2020; 11: 903-912.[DOI]
[24] Fowler J, Cohen L, Jarvis P. Practical Statistics for Field Biology, 2nd ed. John Wiley & Sons: Chichester, UK, 1998.[DOI]
[25] Pelemo O, de Koeyer D, Matsumoto R et al. Semi-Autotrophic Hydroponics: A robust technique for accelerated basic seed yam production. Int I Trop Agr, 2019.[DOI]
[26] Handayati W, Sihombing D. Study of NPK fertilizer effect on sunflower growth and yield. AIP Conf Proc, 2019; 2120: 030031.[DOI]
[27] Hariyadi BW, Nizak F, Nurmalasari IR et al. Effect of Dose And Time of Npk Fertilizer Application on The Growth And Yield of Tomato Plants (Lycopersicum Esculentum Mill). Agric Sci, 2021; 2: 101-111.[DOI]
[28] Al-Harthi K, Al-Yahyai R. Effect of NPK fertilizer on growth and yield of banana in Northern Oman. J Hortic For, 2009; 1: 160-167.[DOI]
[29] Santosa E, Susila AD, Lontoh AP et al. NPK Fertilizers for Elephant Foot Yam (Amorphophallus paeoniifolius (Dennst.) Nicolson) Intercropped with Coffee Trees. J Agron Indonesia, 2016; 43: 257-263.[DOI]
[30] Diby LN, Tie BT, Girardin O et al. Growth and Nutrient Use Efficiencies of Yams (Dioscorea spp.) Grown in Two Contrasting Soils of West Africa. Int J Agron, 2011; 2011: 175958.[DOI]
[31] Matsumoto R, Ishikawa H, Asfaw A et al. Low Soil Nutrient Tolerance and Mineral Fertilizer Response in White Guinea Yam (Dioscorea rotundata) Genotypes. Front Plant Sci, 2021; 12: 629762.[DOI]
[32] Tiama D, Sawadogo N, Traore RE et al. Effect of Chemical Fertilisers on Production of Yams (Nyù) of Passore in Farmers’ Environment. Agronomie Africaine, 2018; 30: 99-105.[DOI]
[33] Afolabi F, Matsumoto R, Akinwande B et al. Effect of Mineral Fertilisation on Tuber Yield and Quality in Yams (Dioscorea alata and Dioscorea rotundata). Agriculture, 2023; 13: 2240.[DOI]
[34] Hgaza VK, Diby LN, Assa A et al. How fertilization affects yam (Dioscorea alata L.) growth and tuber yield across the years. Afr J Trop Agric, 2020; 8: 1-8.[DOI]
Copyright © 2024 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.










 Copyright ©
Copyright ©