Comparison of Three Cytokinins on In vitro Multiplication of Orbea semota, a Conical, Stout-Teethed Succulent
Kemar A Rose1*, Collin M Scantlebury1, Marsha C Williams1, Ryan D Francis1
1Biotechnology Department, The Scientific Research Council, Kingston, Jamaica
*Correspondence to: Kemar A Rose, MBA, BSc, Research Officer, Biotechnology Department, The Scientific Research Council, Hope Gardens, Kingston 6, Jamaica; Email: kemar.rose22@gmail.com
Abstract
Objective: Orbea semota (N.E. Br.) L.C. Leach is an erect or procumbent succulent with numerous branches. The stems of this plant are grey-green to dark green in color and possess flowers that are dark maroon or dark brown with yellow marks at the tips. It is native to Kenya, Tanzania and Rwanda and grows in rocky places. There is great potential for its use as a medicinal plant, also, there are immense prospects for commercial trade and cultivation of the succulent as an ornamental in Jamaica. The purpose of the study is to select the most effective cytokinin for the shoot multiplication of Orbea semota using stem cuttings.
Methods: Young shoots were initiated for 6-8 weeks on a modified Murashige and Skoog (MS) medium. Initiated shoots were then cultivated on Media A, B, C and D for 8 weeks to promote shoot multiplication. These were modified MS media supplemented with three different cytokinins [6-γ, γ-Dimethylallylamino purine (2iP), 6-Benzylaminopurine (BA) and Kinetin] and a control without any cytokinins.
Results: Medium A (containing 2iP) produced the best results at shoot multiplication than all other media used. The mean lengths of the shoots produced by Medium A were 19.0±11.4 mm and 10.5±3.8 mm. The mean number of shoots generated using Medium A were 3.4±1.3 and 4.0±1.9. These results were significantly greater than those of the shoots produced by all other media.
Conclusion: The present study details an efficient and reproducible protocol for the in vitro shoot multiplication of Orbea semota. The results obtained showed that Medium A was the most optimal medium for promoting shoot multiplication. Therefore, of the three cytokinins investigated 2iP produced the best results at stimulating shoot multiplication and growth of Orbea semota.
Keywords: 2iP, Apocynaceae, Asclepiadoideae, in vitro shoot multiplication, Orbea semota
1 INTRODUCTION
Orbea semota (N.E. Br.) L.C. Leach, also called Stapelia semota, belongs to the subfamily Asclepiadoideae of the family Apocynaceae[1]. It is native to Kenya, Tanzania and Rwanda growing in rocky places between 800-2000m above sea level[2]. This plant is a much-branched succulent growing up to 10cm tall with erect or procumbent stems forming compact cushions. These 4-angled stems are 10-18mm across excluding their stout, conical teeth, which can ascend or spread to 12mm. The stems are typically grey-green or dark green, occasionally blotched with dark reddish-brown. The flowers are transversely wrinkled and can appear as solitary or several together borne from any region of the stem with a diameter of 3.5-5cm across; they are dark maroon or dark brown with yellow marks at the tips or throughout the lobes[3,4]. Due to its colorful flowers, this plant could be utilized as an ornamental in Jamaica. It also produces a foul-smelling odor, which is primarily caused by butanoic acid. There is potential for Orbea semota to be used as a medicinal plant as the sap from the pounded stems is used to treat wounds and ulcers in Tanzania[5].
Orbea semota can be propagated by using stem cuttings and seed sowing only in the spring[6]. However, there are challenges with traditional propagation techniques as rotting often occurs rather than rooting whenever Asclepiadoideae stem cuttings are planted upright in rooting substrate[7]. In light of the issues with the traditional methods of propagation and the immense potential for medical use, as well as commercial trade and cultivation as an ornamental, tissue culture propagation can be employed as a means to rectify both issues.
Plant tissue culture methods are conducted under controlled aseptic, nutritional and environmental conditions. These techniques produce plant tissues called explants which are indistinguishable replicas of the mother plant that are generated from the in vitro culture of cells, tissues, organs or the entire plant[8]. A single explant can be multiplied in a shorter time period compared to the traditional method and the demand for space is less; this is possible all throughout the year despite the season or weather[9].
In vitro morphogenesis is greatly impacted by plant growth regulators; cytokinins have been known as plant growth regulators since 1950 when their ability to stimulate cell division was discovered[10,11]. Therefore, cytokinins are generally used on micropropagation media to promote in vitro shoot multiplication[12].
To the best of our knowledge on propagation, no published study on the in vitro propagation of Orbea semota exists. Due to the medicinal and ornamental benefits of this plant, the Biotechnology Department of the Scientific Research Council is conducting research on this plant. Therefore, the objective of this study was to select the most effective cytokinin for the shoot growth and multiplication of Orbea semota stem cuttings.
2 MATERIAL AND METHODS
2.1 Plant Material and Initiation of Culture
Orbea semota plants were obtained locally and maintained in a shade-house under 75% shade at the Scientific Research Council, Kingston, Jamaica (18.0189° N latitude and 76.7497°W longitude) in November 2022. Young explant shoots 3-6mm long were washed with tap water and 1% detergent. The shoots were then treated in a fungicide and bactericide mixture of 2.5g/L 70% 1,2-di-(3-methoxycarbonyl-2-thioureido) benzene (thiophanate methyl) (Topsin-M 70% WP), 3g/L 4% Methyl N-(2,6-dimethylphenyl)-N-(methoxy acetyl) alaninate +64% Manganese ethylene bisdithiocarbamate (Ridomil 68 WP), 3mL/L 21.36% Copper Sulfate Pentahydrate (Phyton-27 SC) for one hour by using an automatic stirrer to ensure continuous stirring.
Explants were surface sterilized by submerging shoots in a 70% ethanol solution for 1 minute, followed by 5.25% sodium hypochlorite plus Polyethylene glycol sorbitan monolaurate (Tween 80) for 15min. Disinfected explant shoots were rinsed in sterile distilled water 5-6 times.
The shoots were then cultured in Medium A since its protocol was used in the efficient in vitro propagation of another Asclepiadoideae species, Edithcolea grandis[13]. Medium A comprises of Murashige and Skoog (MS) basal salts[14], vitamins [100mg/L Myo-inositol (PhytoTechnology Laboratories, Shawnee Mission, Kansas, USA), 30mg/L Thiamine hydrochloride (HCl) (Sigma-Aldrich, St. Louis, Missouri, USA), 1mg/L Pyridoxine HCl (PhytoTech Labs, Lenexa, Kansas, USA), 10mg/L Nicotinic acid (PhytoTechnology Laboratories, Shawnee Mission, Kansas, USA)], 10mg/L L. Arginine (PhytoTech Labs, Lenexa, Kansas, USA), 80mg/L Adenine sulfate (PhytoTech Labs, Lenexa, Kansas, USA), 10mg/L 6-γ, γ-Dimethylallylamino purine (2iP) (Sigma Chemical Co., St. Louis, Missouri, USA), 0.5mg/L Indole-3-acetic acid (IAA) (Acros Organics, New Jersey, USA), 1 mg/L Indole-3-butyric acid (IBA) (PhytoTech Labs, Lenexa, Kansas, USA), 45g/L sucrose and 2.5g/L PhytagelTM (Sigma-Aldrich, St. Louis, Missouri, USA). The pH was adjusted to 5.3 and the medium was autoclaved at 121°C and 15 psi for 20min. Cultures were maintained for 6-8 weeks at 25±2°C with 16-h photoperiod under light emitting diode (LED) light with photon flux of 50 μmol m-2s-1.
2.2 Shoot Multiplication
Shoots were cultivated on the same modified MS media supplemented with three different cytokinins. Medium A contained 10mg/L 2iP, medium B comprised 10mg/L 6-Benzylaminopurine (BA) (Beantown Chemical, Hudson, New Hampshire, USA) and medium C constituted 10mg/L Kinetin (Sigma Aldrich, St. Louis, Missouri, USA). There was no added cytokinin in Medium D (the control). In each treatment, 15 explants were used and after an 8-week incubation period, the mean shoot number generated per explant and the shoot lengths were determined. Each experiment was repeated.
2.3 Statistical Analysis
Analysis of variance (ANOVA) was used to estimate the effects of cytokinins and repetition of the experiment on shoot multiplication rate (number of shoots generated per explant) and shoot length. This followed two-way ANOVA methodology by Fowler et al.[15] where the total sum of squares (SST) was partitioned into sum of squares for replications (SSa), sums of squares of cytokinins (SSb), sum of squares for interaction (SSi) and within sum of squares (SSwithin) (Equation (1)).
|
The Tukey’s Test in two-way ANOVA was used to determine significant differences between means (Equation (2)).
|
3 RESULTS
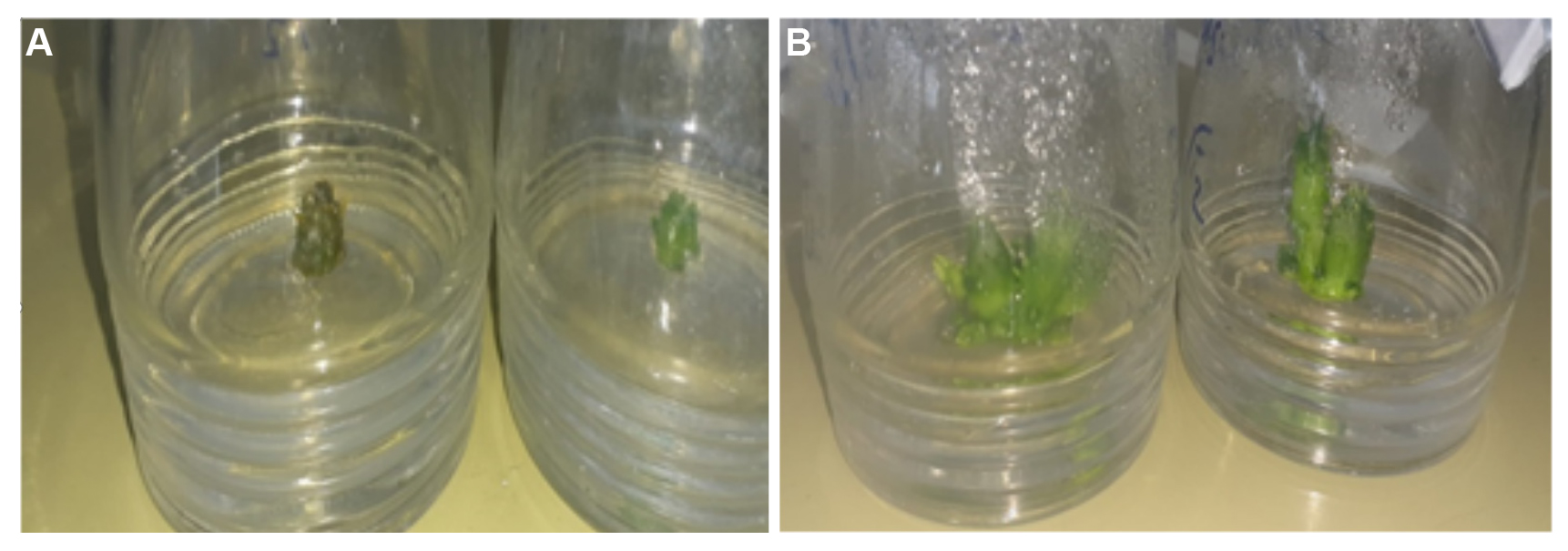
Figure 1A and B shows the in vitro propagation of Orbea semota explants. There were significant differences in explant shoot length between the four test media. There were also significant differences in shoot multiplication rates.
|
Figure 1. In vitro propagation of Orbea semota shoots - (A) Initiation of young shoots of 3-6mm in length on initiation media (B) Shoot multiplication on multiplication media (Medium A) after 8 weeks in culture.
3.1 Shoot Length
Table 1 shows the differences in shoot length among media containing different cytokinins. For both subcultures, 2iP produced the greatest shoot length. The two-way ANOVA summary table (Table 2) indicated that while there was no difference in shoot length between subcultures, nor interaction, the difference in shoot length was significant at P=0.01, F=419.079 for df3,112. The Tukey statistic indicated that the shoot length in Medium A (2iP supplemented medium) was greater than Medium B (BA supplemented medium); Medium B (BA supplemented medium) was greater than Medium C (kinetin supplemented medium) and the control.
Table 1. Effect of Media Containing Different Cytokinins on Shoot Length During Multiplication of Orbea semota After 8 Weeks of Culture
Media (Cytokinin) |
Shoot Length (mm) Subculture 1 |
Shoot Length (mm) Subculture 2 |
A (2iP) |
19.0±11.4a |
10.5±3.8a |
B (BA) |
6.9±4.1b |
6.4±3.4b |
C (Kinetin) |
5.5±2.0c |
6.1±2.1c |
D/control (no cytokinin) |
6.2±3.3c |
6.4±3.0c |
Notes: Significant differences were determined by the Tukey’s test and are indicated by superscripts with different letters (P≤0.01). Values are mean±SD. n=15.
Table 2. ANOVA Summary Table for Shoot Length
Source of Variation |
Sum of Squares |
df |
Variance |
F |
(Between samples) |
(83.756) |
(7) |
|
|
Subcultures |
0.004 |
1 |
0.004 |
0.055 |
Media (Cytokinins) |
83.469 |
3 |
27.823 |
419.079** |
Interaction |
0.324 |
3 |
0.108 |
1.625 |
Within samples |
7.436 |
112 |
0.066 |
|
Notes: ** Indicates significance at P=0.01.
3.2 Shoot Number Per Explant
Table 3 shows the differences in shoot number generated per explant among media containing different cytokinins. For both subcultures, 2iP generated the largest quantity of shoots per explant. The two-way ANOVA summary table (Table 4) indicated that while there was no difference in shoot number per explant between subcultures, nor interaction, the difference in number per explant was significant at P=0.01, F=65.645 for df3,112. The Tukey statistic indicated that the quantity of shoots produced in Medium A (2iP supplemented medium) was greater than Medium B (BA supplemented medium); Medium B (BA supplemented medium) was greater than Medium C (kinetin supplemented medium) and the control.
Table 3. Effect of Media Containing Different Cytokinins on Number of Shoots Generated per Explant During Multiplication of Orbea Semota After 8 Weeks of Culture
Media (Cytokinin) |
Number of Shoots Subculture 1 |
Number of Shoots Subculture 2 |
A (2iP) |
3.4±1.3a |
4.0±1.9a |
B (BA) |
2.7±1.4b |
2.3±1.6b |
C (Kinetin) |
1.5±0.7c |
1.9±1.2c |
D/control (no cytokinin) |
1.4±0.8c |
1.8±1.3c |
Notes: Significant differences were determined by the Tukey’s test and are indicated by superscripts with different letters (P≤0.01). Values are mean±SD. n=15.
Table 4. ANOVA Summary Table for Shoot Number per Explant Data
Source of Variation |
Sum of Squares |
df |
Variance |
F |
(Between samples) |
(11.927) |
(7) |
|
|
Subcultures |
0.002 |
1 |
0.002 |
0.028 |
Media (Cytokinins) |
11.725 |
3 |
3.908 |
64.645** |
Interaction |
0.201 |
3 |
0.067 |
1.106 |
Within samples |
6.771 |
112 |
0.060 |
|
Notes: ** Indicates significance at P=0.01.
4 DISCUSSION
There is great potential for Orbea semota as a medicinal plant as the stems of the Orbea genus have been used for a variety of medicinal purposes including the treatment of burns, eczema, wounds, diabetes, constipation, stomach ulcers and as an antidote for food poisoning[16]. The stems and roots of these plants have also been utilized in the suppression or curbing of appetite and the treatment or prevention of obesity[17]. Al-Fatimi et al.[18] and Mubarak et al.[19] have also reported on the antibacterial and cytotoxic effects of the Orbea genus. Orbea semota is a succulent that also has considerable prospects as an ornamental. The Orbea genus has high horticultural value and is in high demand by succulent collectors; plants belonging to this genus are grown and exchanged globally. The flowers are beautiful and their purple-blotchy stems against different shades of green backgrounds tend to be extremely attractive[20]. The plants can be grown in large pots, in gardens or in rockeries[21-23]. The practical applications of our findings can be of particular interest to stakeholders within the agricultural and horticultural sectors. The utilization of plant tissue culture allows for optimization in growth conditions as Orbea semota plantlets will have an avenue in which they can be cultivated all year round, even when weather and climate conditions become unfavorable. Additionally, yield can be maximized using the plant tissue culture technique as this method of propagation has a higher multiplication rate than traditional methods.
Environmental factors such as light conditions and temperature can significantly affect in vitro cultures. Artificial light, in combination with other factors such as medium composition, gas exchange in the culture vessel and temperature, plays a critical role in successful in vitro plant production. Maintaining the appropriate photon flux and photoperiod enable the production of plants with desired traits[29]. If the photon flux is too low, this may negatively affect the synthesis and activity of photosynthetic pigments and electron carriers. A high photon flux may result in the accelerated degeneration of chlorophylls and carotenoids[30]. Photoperiod impacts the vegetative growth, as well as the physiological and biochemical characteristics of a plant. Small changes in light quality can be sensed by plants through photoreceptors that regulate the growth and development of plants through exciting signaling pathways[31]. A photon flux of 50μmol m-2s-1 and a 16-h photoperiod were used in this study. Temperature is another crucial factor for plant growth in in vitro propagation since it may play a regulatory role in plant growth and in morphogenesis, additionally, growth room temperatures can directly or indirectly influence metabolic activities of growing tissues[32,33]. Photosynthesis is impacted by temperature with varying optimal levels depending on the species type, as well as the environmental conditions under which the plant is propagated. In vitro maintenance for many plant cultures occurs at 25°C, which is the optimum temperature for photosynthesis for many plants. At lower temperatures, shoot multiplication rates decrease because of slow growth and less differentiation of shoot meristems[34]. A temperature of 25±2°C was used in this study. Similar light conditions and temperature were used by other researchers of Asclepiadoideae species, Caralluma bhupenderiana Sarkaria and Ceropegia elegans, to induce in vitro shoot multiplication[35,36].
In plants, cytokinins are a category of phytohormones derived from adenine that stimulate cell differentiation. Cytokinin signaling is initiated by membrane-associated histidine kinase receptors and transduced through a phosphorelay system[37]. The physiological function of cytokinins is to activate RNA, protein synthesis, and enzyme activity. Different cytokinins can also cause multiple shoot proliferation by interfering with shoot apical dominance[38]. The multiplication of shoots is dependent on the accompanying of two iterative processes: the induction and formation of phytomer, which includes lateral meristem (axillary bud) formation from the apical meristem and the ensuing outgrowth of the axillary buds into newly developed shoots[29]. Cytokinins are associated with the maintenance of shoot meristem function since they are positive regulators of stem meristem activity. Therefore, they play a crucial role in shoot development, especially, in the shoot apical meristem[39]. There is an antagonistic relationship between cytokinins and auxins as cytokinin signaling is inhibited by auxin action in most parts of the plant[40]. However, Ganesan and Jayabalan[41] reported that high concentrations of cytokinins, in combination with low concentrations of auxins have been utilized for direct induction and elongation of multiple shoots in many plant species. This corresponds with the protocol used in this study as high concentrations of 2iP or BA (10mg/L), in combination with low concentrations of the auxins, IAA (0.5mg/L) and IBA (1mg/L) were able to induce shoot multiplication in Orbea semota. However, shoot multiplication produced by the cytokinin kinetin was not significantly different from the control. This is possibly due to kinetin being a weak cytokinin (especially when compared to BA) with low effectiveness in generating high shoot multiplication in some plant species[42]. BA is the most commonly used cytokinin in commercial micropropagation, due to its efficacy and affordability[43]. BA has a distinct impact on promoting the growth of auxiliary and adventitious buds and foliar development of shoot tip cultures[44]. This possibly explains why shoot multiplication produced by BA was significantly greater than kinetin. 2iP produced significantly greater results than BA, this could be due to the fact that 2iP is generally considered to be the second most potent cytokinin behind zeatin[45].
Cytokinins fall into two main categories: naturally occurring or synthetic. Common naturally occurring cytokinins include zeatin and 2iP, while synthetic cytokinins include BA, kinetin and thidiazuron[46]. Other researchers of Apocynaceae species had optimal success in shoot multiplication with the use of kinetin, 2iP or BA[35,36,47,48]. In addition, kinetin, 2iP and BA have been previously noted as some of the cytokinins that produced significant shooting response[49]. Therefore, these three cytokinins were selected for this present study. The cytokinins present in Media A, B and C were 2iP, BA and Kinetin respectively while Medium D had no cytokinins present.
Medium A, containing 2iP, produced the greatest number of shoots (3.4±1.3 and 4.0±1.9) of all the media investigated, as well as generating the shoots with the highest mean shoot length (19.0±11.4mm and 10.5±3.8mm). Similar results were reported in Grewia tenax (Forssk.) Fiori and another member of the Apocynaceae family, Decalepis hamiltonii Wight & Arn. in which 2iP induced the greatest shoot multiplication of all three cytokinins[47,50]. Kozak et al.[51] also reported that 2iP produced better shoot multiplication than BA in Mandevilla sanderi (Hemsl.) Woodson, another Apocynaceae species. Contrary to the present findings, it was reported that in other members of the Apocynaceae family, Rauvolfia serpentina Benth. ex Kurz and Decalepis arayalpathra BA generated the best results for optimal shoot multiplication[52,53]. Krishnareddy and Pullaiah[36] and Muthukrishnan et al.[54] reported that kinetin stimulated the greatest shoot multiplication in Ceropegia elegans and Ceropegia thwaitesii Hook. According to Enkhbileg et al.[12], the difference in results could be due to the effects of cytokinins differing depending on the plant species.
There has been very little study on the micropropagation of the Orbea genus, so little is known about how cytokinins affect these plants. This present investigation provides a foundation for the in vitro propagation of plants within the Orbea genus. Since this protocol was also used in the efficient in vitro propagation of Edithcolea grandis[13], it may also provide a framework for the micropropagation of other species within the Asclepiadoideae subfamily that possess similar morphological structures.
5 CONCLUSION
In conclusion, the present study details an efficient and reproducible protocol for the in vitro shoot multiplication of Orbea semota. This current investigation assured effective establishment, mass multiplication and could offer an in vitro strategy for the ex situ conservation of this succulent that has great potential as a medicinal plant, as well as, commercial cultivation and trade as an ornamental in Jamaica. The results obtained showed that Media A was the most optimal medium at promoting shoot multiplication. Therefore, of the three cytokinins investigated 2iP produced the best results at stimulating shoot multiplication and growth of Orbea semota.
Acknowledgements
Support for this investigation was given by the Scientific Research Council, Jamaica. The authors wish to thank all our colleagues who provided their expertise that greatly assisted this research project and enhanced the manuscript.
Conflicts of Interest
The authors declared that they have no conflicts of interest.
Author Contribution
Rose KA conceived the study, designed the study, carried out the experimental work of the manuscript, generated the data, participated in the analysis and interpretation of the data and wrote the manuscript (including revisions). Scantlebury CM participated in the analysis and interpretation of the data and provided general supervision of the research. Williams MC shared in the research proposal and design of the study. Francis RD shared in the research proposal and provided general supervision of the research. All authors read and approved the final manuscript.
Abbreviation List
2iP, 6-γ, γ-Dimethylallylamino purine
ANOVA, Analysis of variance
BA, 6-Benzylaminopurine
HCl, Hydrochloride
IAA, Indole-3-acetic acid
IBA, Indole-3-butyric acid
LED, Light emitting diode
MS, Murashige and Skoog
References
[1] Jürgens A, Dötterl S, Liede-Schumann S et al. Floral scent composition in early diverging taxa of Asclepiadoideae, and Secamonoideae (Apocynaceae). S Afr J Bot, 2010; 76: 749-761.[DOI]
[2] Goyder D, Harris T, Masinde S et al. Flora of Tropical East Africa: Apocynaceae (Part 2). Royal Botanic Gardens: London, UK, 2012.
[3] Albers F, Meve U. Illustrated Handbook of Succulent Plants: Asclepiadaceae. Springer-Verlag Berlin Heidelberg: New York, USA, 2004.
[4] Cullen J, Knees SG, Cubey HS et al. The European Garden Flora Flowering Plants: A manual for the identification of plants cultivated in Europe, both out-of-doors and under glass. Cambridge University Press: New York, USA, 2011.
[5] Schmelzer GH, Gurib-Fakim A. Plant Resources of Tropical Africa 11(2): Medicinal Plants 2. PROTA Foundation: Wageningen, Netherlands, 2013; 384.
[6] Anonymous. Llifle. Orbea semota (N.E. Br.) L.C. Leach. Accessed 11 November 2023. Available at:[Web]
[7] Hodgkiss RJ. The Succulent Plant Page. Cultivation of Asclepiads: milkweeds & carrion flowers. Accessed 25 November 2023. Available at:[Web]
[8] Rodge RR, Patil P. Tissue Culture as a Plant Production Technique for Fruit Crops and Plant Part Used for Propagation. Int J Agric Sci, 2020; 12: 81-87.[DOI]
[9] Suresh Kumar C, Keshamma E. Perspectives on Plant Tissue Culture Technology: A Review. Int J All Res Educ Sci Methods, 2022; 10: 94-101.
[10] Mok MC. Cytokinins and Plant Development — An Overview. In: Cytokinins: Chemistry, Activity, and Function. CRC Press: Boca Raton, USA, 2019; 155-166.
[11] Nowakowska K, Pińkowska A, Siedlecka E et al. The effect of cytokinins on shoot proliferation, biochemical changes and genetic stability of Rhododendron ‘Kazimierz Odnowiciel’ in the in vitro cultures. Plant Cell Tiss Org, 2022; 149: 675-684.[DOI]
[12] Enkhbileg E, Fári MG, Kurucz E. In vitro effect of different cytokinin types (BAP, TDZ) on two different Ocimum basilicum cultivars explants. Int J Hortic Sci, 2019; 25: 15-20.[DOI]
[13] Rose K, Williams M, Francis R et al. In Vitro Propagation of Edithcolea grandis. P Natl A Sci India B, 2023; 93: 589-594.[DOI]
[14] Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco cultures. Physiol Plant, 1962; 15: 473-479.[DOI]
[15] Fowler J, Cohen L, Jarvis P. Practical Statistics for Field Biology, 2nd ed. John Wiley and Sons: Chichester, UK, 1998.
[16] Al-Fatimi M. Ethnobotanical survey of medicinal plants in central Abyan governorate, Yemen. J Ethnopharmacol, 2019; 241: 111973.[DOI]
[17] Corley DG, Miller J. Plant derived or derivable material with appetite suppressing activity. United States Patent US 2006/0024389 A1, 2006.
[18] Al-Fatimi M, Wurster M, Schröder G et al. Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J Ethnopharmacol, 2007; 111: 657-666.[DOI]
[19] Mubarak AY, al-attab BM, Almaqtar MA. Evaluation of the Total Content of Phenols and Flavonoids in Two Different Extracts of Orbea wissmannii O. Schwart and their Toxic Effect on WI38 and HepG2 Cell Lines. J Amr Uni, 2023; 3: 101.[DOI]
[20] Bester SP. South African National Biodiversity Institute. Orbea Haw. Accessed 12 December 2023. Available at:[Web]
[21] Lenz TI, Facelli JM. Shade facilitates an invasive stem succulent in a chenopod shrubland in South Australia. Austral Ecol, 2003; 28: 480-490.[DOI]
[22] Honan I. A succulent escape in the arid lands of southern Australia — Carrion flower (Orbea variegata): 15th Australian Weeds Conference, Papers and Proceedings: Managing weeds in a changing climate. Adelaide, South Australia, 24-28 September 2006.
[23] Bester SP. South African National Biodiversity Institute. Orbea lutea (N.E.Br.) Bruyns subsp. Lutea. Accessed 12 December 2023. Available at:[Web]
[24] Mng’omba SA, Sileshi G, du Toit ES et al. Efficacy and Utilization of Fungicides and Other Antibiotics for Aseptic Plant Cultures. In: Fungicides for Plant and Animal Diseases. IntechOpen: London, UK, 2012; 245-254.[DOI]
[25] Dinçer D, Dündar H. The Use of Fungicides and Antibiotics in In Vitro Sterilization of Ornamental Plants. In: New Frontiers in Architecture, Planning and Design. Duvar Yayınları: Kemeraltı-Konak/İzmir, Turkey, 2023; 31-44.
[26] Torres GRC, Houllou LM, de Souza RA. Control of contaminants during introduction and establishment of Bambusa vulgaris in vitro. Res Biotechnol, 2016; 7: 58-67.[DOI]
[27] Jiménez VM, Castillo J, Tavares E et al. In vitro propagation of the neotropical giant bamboo, Guadua angustifolia Kunth, through axillary shoot proliferation. Plant Cell Tiss Org, 2006; 86: 389-395.[DOI]
[28] Ndakidemi CF, Mneney E, Ndakidemi PA. Development of Sanitation Protocol for Leaf Explants of B. huillensis for in Vitro Culture. Am J Plant Sci, 2013; 4: 41291.[DOI]
[29] Cavallaro V, Pellegrino A, Muleo R et al. Light and Plant Growth Regulators on In Vitro Proliferation. Plants, 2022; 11: 844.[DOI]
[30] Jo EA, Tewari RK, Hahn EJ et al. Effect of photoperiod and light intensity on in vitro propagation of Alocasia amazonica. Plant Biotechnol Rep, 2008; 2: 207-212.[DOI]
[31] Xu Y, Yang M, Cheng F et al. Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolata. BMC Plant Biol, 2020; 20: 269.[DOI]
[32] Sengar K, Sengar RS, Garg SK. The effect of in-vitro environmental conditions on some sugarcane varieties for micropropagation. Afr J Biotechnol, 2011; 10: 17122-17126.[DOI]
[33] Alexopoulos AA, Mavrommati E, Kartsonas E et al. Effect of Temperature and Sucrose on In Vitro Seed Germination and Bulblet Production of Pancratium maritimum L. Agronomy, 2022; 12: 2786.[DOI]
[34] Amoo SO, Finnie JF, Van Staden J. Effects of temperature, photoperiod and culture vessel size on adventitious shoot production of in vitro propagated Huernia hystrix. Plant Cell Tiss Org, 2009; 99: 233-238.[DOI]
[35] Ugraiah A, Sreelatha VR, Reddy PVK et al. In vitro shoot multiplication and conservation of Caralluma bhupenderiana Sarkaria — An endangered medicinal plant from South India. Afr J Biotechnol, 2011; 10: 9328-9336.[DOI]
[36] Krishnareddy PV, Pullaiah T. In vitro conservation of Ceropegia elegans, an endemic plant of South India. Afr J Biotechnol, 2012; 11: 12443-12449.[DOI]
[37] Yang W, Cortijo S, Korsbo N et al. Molecular mechanism of cytokinin-activated cell division in Arabidopsis. Science, 2021; 371: 1350-1355.[DOI]
[38] Ahmed MEAE. In vitro propagation for conservation and genetic fidelity of the near threatened Dimocarpus longan plant. J Genet Eng Biotechnol, 2022; 20: 130.[DOI]
[39] Li SM, Zheng HX, Zhang XS et al. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep, 2021; 40: 271-282.[DOI]
[40] Kurepa J, Shull TE, Smalle JA. Antagonistic activity of auxin and cytokinin in shoot and root organs. Plant Direct, 2019; 3: e00121.[DOI]
[41] Ganesan M, Jayabalan N. Influence of cytokinins, auxins and polyamines on in vitro mass multiplication of cotton (Gossypium hirsutum L. cv. SVPR2). Indian J Exp Biol, 2006; 44: 506-513.
[42] Amoo SO, Finnie JF, Van Staden J. The role of meta-topolins in alleviating micropropagation problems. Plant Growth Regul, 2011; 63: 197-206.[DOI]
[43] Werbrouck SPO. Merits and Drawbacks of New Aromatic Cytokinins in Plant Tissue Culture: ISHS Acta Horticulturae 865: IV International Symposium on Acclimatization and Establishment of Micropropagated Plants. Bangalore, India, 8 December 2008; 103-107.[DOI]
[44] Reddy DRD, Suvarna D, Rao DM. Effects of 6-Benzyl amino purine (6-bap) on in vitro Shoot Multiplication of Grand Naine (Musa sp.). Int J Adv Biotechnol Res, 2014; 5: 36-42.
[45] Kasem MM, Helaly AAE. Response of Syngonium podophyllum Plant to Some Synthetic Cytokinin Types and Concentrations as a Foliar Application. Sci J Flowers Ornam Plants, 2021; 8: 321-334.[DOI]
[46] Jana S, Sivanesan I, Jeong BR. Effect of cytokinins on in vitro multiplication of Sophora tonkinensis. Asian Pac J Trop Bio, 2013; 3: 549-553.[DOI]
[47] Giridhar P, Gururaj HB, Ravishankar GA. In vitro shoot multiplication through shoot tip cultures of Decalepis hamiltonii Wight & Arn., a threatened plant endemic to Southern India. In Vitro Cell Dev-Pl, 2005; 41: 77-80.[DOI]
[48] Karuppusamy S, Kiranmai C, Aruna V et al. In vitro conservation of Ceropegia intermedia- an endemic plant of south India. Afr J Biotechnol, 2009; 8: 4052-4057.[DOI]
[49] Goda MS, Ahmed SA, Sherif FE et al. In Vitro Micropropagation of Endangered Achillea fragrantissima Forssk. Combined with Enhancement of Its Antihyperglycemic Activity. Agronomy, 2023; 13: 278.[DOI]
[50] Daffalla HM, Elsheikh AM, Ali HA et al. Micropropagation of Grewia tenax (Forssk.) Fiori - an important ethnomedicinal plant. BioTechnologia, 2019; 100: 289-300.[DOI]
[51] Kozak D, Parzymies M, Świstowska A et al. The influence of growth regulators and explant position on the growth and development of Mandevilla sanderi (Hemsl.) Woodson in vitro. Acta Sci Pol-Hortoru, 2021; 20: 127-138.[DOI]
[52] Dey A, Nandy S, Nongdam P et al. Methyl jasmonate and salicylic acid elicit indole alkaloid production and modulate antioxidant defence and biocidal properties in Rauvolfia serpentina Benth. ex Kurz. in vitro cultures. S Afr J Bot, 2020; 135: 1-17.[DOI]
[53] Ahmad Z, Shahzad A, Sharma S. Enhanced multiplication and improved ex vitro acclimatization of Decalepis arayalpathra. Biol Plantarum, 2018; 62: 1-10.[DOI]
[54] Muthukrishnan S, Franklin Benjamin JH , Muthukumar M et al. In vitro propagation of Ceropegia thwaitesii Hook- an endemic species of Western Ghats of Tamil Nadu, India. Afr J Biotechnol, 2012; 11: 12277-12285.[DOI]
Copyright © 2024 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.





 Copyright ©
Copyright ©