Biological Degradation of Nerve Agents
Vinita Chauhan Kushwah1, Ram Kumar Dhaked1*
1Biotechnology Division, Defence Research & Development Establishment, MP, India
*Correspondence to: Ram Kumar Dhaked, PhD, Biotechnology Division, Defence Research & Development Establishment, Jhansi Road, Gwalior, 474002, MP, India; Email: rkdhaked.drde@gov.in
Abstract
Objective: The aim of the present study was to determine biodegradation efficacy of nerve agents [methyl parathion, dimethyl methylphosphonate (DMMP), and sarin (GB)] by bacteria isolated from soil and their characterization.
Methods: Enrichment for bacteria that can use organophosphates (OPs) as a source of carbon from the soil, where the synthesis of organophosphorus (OP) compounds takes place. The enrichment of bacteria biodegrading OP compounds was carried out in mineral salt medium. Characterization of bacterial strains was done by sequencing, pyro GC, SDS-PAGE profiling and 16S rRNA. Degradation of OP compounds by bacterial isolates was determined by estimating inorganic phosphorus, gas chromatography and mass spectrometry analyses (GC/MS).
Results: After enrichment the bacteria were isolated. OPs were utilized as second preferred carbon source after glucose by these bacteria, whereas acetate and lactose exhibitedminimal effect on growth. These isolates were grouped together based on pyro GC and PAGE profiles and identified by 16S rRNA sequencing. Degradation of OP compounds was determined by the estimation of inorganic phosphorus after 12 and 24h of incubation. GC analysis revealed spp. of Pseudomonas and Acinetobactor effectively degraded the 200ppm concentration of OP compound up to 48.3%, and 59.8% respectively; after 24h of incubation.
Conclusion: Organophosphate compounds are extensively used as agricultural pesticides and some are listed chemical warfare agents by Organization for Prohibition of Chemical Weapons (OPCW); these compounds are highly toxic, show environmental persistence and contribute to numerous cases of poisoning. Therefore, the ability of microorganisms to reduce contaminants has been a preferred method to degrade these compounds. The biodegradation of methyl parathion, dimethyl methylphosphonate (DMMP), and GB proceeded rapidly with the formation of a series of intermediate products, which were analyzed using a combination GC/MS.
Keywords: organophosphorus, nerve agents, methyl parathion, enrichment, biodegradation, gas chromatography
1 INTRODUCTION
Organophosphates (OP) pesticides play an important role in controlling pests, especially in agriculture. It is estimated that OP pesticides cause around 3 million poisonings and 200,000 human deaths annually, mostly in developing countries[1,2]. OPs residues are also present in environment like crops/fruits, pond, water and many more[3]. In a recent study the environmental fate of the nerve agents was also elucidated by using quantitative structure-activity relationship (QSAR) models[4]. There are several manmade OP compounds like sarin (GB), soman (GD), tabun (GA), and venomous agent X (VX) are highly toxic nerve agents (NAs) that have been manufactured for use in warfare. The VX is more stable and has resistance to detoxification. Therefore, the environmental persistency of VX is higher than the other NAs[5]. It is also documented that VX is less volatile and more toxic when penetrating through skin. Hence, VX is labelled as a skin penetrant and lethal contact agent rather than an inhalation threat[6,7]. In cell metabolism, VX at 10μM is largely reduced within 2h[8]. However, the G agent absorption rate is much less rapid in percutaneous than in the inhalation form[9]. On the basis of animal studies, it was found that the G agents have a lethal inhaled dose of ~1.0mg in human beings. The overall relative lethality of NAs in animal studies is: VX>GD>GB>GA[10]. Use of these chemical warfare agents poses serious ecological and environmental problems[11].These NAs are mostly present in armed forces stockpiles of several nations, including the United States (http://www.opcw.org/chemical-weapons-convention).
NAs have mortal effects in the acute phase of toxicity and also have huge long-term complications due to irreversible inhibition of acetyl choline esterase (AChE)[12]. NAs have a very specific mechanism of action, which inhibit cholinesterase enzymes present in plasma, erythrocytes and at cholinergic nerve endings in tissues[13]. Once cholinesterase is inhibited by the OP compounds, the hydrolysis of phosphorylated AChE is extremely slow and results in the over-stimulation of the nervous system, which causes agitation, hyper salivation, confusion, convulsion, respiratory failure that ultimately leads to the death of insects in mammals[14]. OPs pose a great threat to the entire food chain, soil, water and the environment and require remedial measures[15]. Therefore, their degradation by biological processes has been intensively researched. In this sense, the ability of microorganisms to reduce contaminants has been a preferred method to degrade these compounds[16].
Biodegradation can offer an efficient and cost-effective option for disposal of OP compounds including NAs. The first microorganism identified as Flavobacterium sp. that could degrade OP compounds was isolated in 1973[17]. Since then, several bacterial and fungal species have been isolated which degrade a wide range of OP compounds in liquid cultures and soil systems. The main reactions involved in the degradation process are oxidation, hydrolysis, alkylation, and dealkylation[18]. Detoxification of chemical warfare agents and OP pesticides results in formation of stable alkyl-phosphonic acids[7]. The OP compounds form a covalent C-P bond, which is resistant to chemical hydrolysis[19]. This bond can be broken by an enzymatic system present in bacteria and utilizes the released phosphorus for biosynthesis of cellular components. The biochemistry of OP compound degradation by most bacteria seems to be identical, in which a structurally similar enzyme called organophosphate hydrolase or phosphotriesterase catalyzes the first step of the degradation. Organophosphate hydrolase encoding organophosphate degrading (opd) gene has been isolated from different taxonomic species isolated from different geographic regions[20]. The gene has been isolated, sequenced, cloned and manipulated for activity and stability improvement. Recently, genes encoding enzymes with similar functions but different sequences have also been isolated and characterized. Some engineered organisms have been checked for their ability to degrade different OP pollutants, including NAs[21]. A current study was carried out to isolate the bacterial strains from the contaminated soil of OP compound and characterization of these isolates was done using pyro GC, SDS-PAGE and 16S rRNA sequencing. These isolates capable of degrading OP compounds like methyl parathion, dimethyl methylphosphonate (DMMP), and GB effectively. Biodegradation of these compounds and the formation of a series of intermediate products is analyzed using gas chromatography equipped with mass spectroscopy (GC/MS).
2 MATERIALS AND METHODS
2.1 Culture Medium
Mineral salt (MS1) medium (pH 7.0) contained (g/L): NH4Cl, 2.0; MgSO4.7H2O, 0.2; K2SO4, 0.5; trace elements (mg/L); FeSO4·7H2O, 2.5; CaCl2·6H2O, 10.0; CuSO4·5H2O, 2.0; H3BO3, 0.06; ZnSO4·7H2O, 20.0; MnSO4·H2O, 1.0; NiCl2·6H2O, 0.05; Na2Mo4·2H2O, 0.3; sodium glutamate was used as the carbon source (20g/L). The same amino acid could serve as a nitrogen source, along with NH4Cl.
2.2 Enrichment and Isolation of OP Compounds Degrading Bacteria
The soil of the site where the synthesis of an OP compound being done place was collected and bacteria were isolated by an enrichment culture technique[22]. The enrichment of biodegrading bacteria for OP compounds was carried out in MS1 medium. It contains 100ppm of different OP compounds (methyl parathion, DMMP and GB). 1% weight/volume of soil sample was added to 300mL medium in a 1.0L flask for the enrichment at 30℃ in a shaker at 200rpm for a period of 7 days.
4 enrichment cycles were performed using the same experimental conditions and the addition of soil was replaced with the enriched culture of the previous cycle. 4 such transfers were made and every time the enrichment population was placed on MS1 medium plates containing 100ppm (methyl parathion, DMMP or GB) of OP compounds and as the sole carbon source and incubated at 30℃ for 24h. After the 4th transfer, a pure isolate capable of growth on a different OP compound was isolated. Bacterial colonies were picked up in the broth medium and bacteria were subjected to Gram staining. To check the degradation capability of bacterial isolates, all isolates were inoculated in MS1 medium using a different OP compound at 30℃ on a shaker at 200rpm for 24h. The medium with OP compounds and without bacterial inoculation was used as a control.
2.3 Identification of OP Degrading Gene
Total bacterial DNA was isolated by Qiagen tissue extraction kit (Germany) and used for amplification of 16s rRNA and opd genes. Based on the reported sequence information, primers were designed by Primer-BLAST (F1: ATG CAA ACG AGA AGG GTT GT, F2: TCA GTG AAT GAG GCC ATC CC, F3: TCA TGA CGC CCG GAA GGT CG, F4: TCT CGG CAG CTC GGC AGG ATT CT and F5: AGG GCT CAA GCT TCG GAC TCA AAA) from highly conserve region sequence to amplify opd genes. Polymerase chain reaction (PCR) was performed in thermal cycler (C1000TM, Bio-Rad, USA) in a sterile 0.2mL thin-walled tubes with the thermal profile; initial denaturation at 94℃/5min followed by 35 cycles of denaturation at 94℃/1min, annealing at 50℃/1min and extension at 72℃/1min and final extension at 72℃/10min. For optimization of annealing temperature for different primers, gradient PCR was carried out. PCR amplicon were purified by using gel extraction kit and used for sequencing. After sequence results were analyses by nucleotide BLAST.
2.4 Phosphate Determination
Concentration of inorganic phosphorus was determined spectrophotometrically by a procedure based on phosphomolibdate and malachite green complex formation in an acidic medium[23]. Reagent A was prepared by adding 1.75% ammonium heptamolibdate. 4H2O in H2SO4 (6.3N) and reagent B by 0.035% malachite green and 0.35% poly vinyl alcohol (PVA) dissolving in water at 80℃, both the reagents are stable at room temperature. To check the concentration of inorganic phosphate, 1.0mL of sample was mixed with 0.02mL of reagent A and incubated for 10min at RT minutes. After that, 0.2mL of reagent B was added. The solution was mixed again and further incubated at RT for 30min and absorbance was measured at 610nm with the help of a spectrophotometer. Potassium phosphate in the MS1 medium was used as the standard.
2.5 Characterization of Bacterial Isolates
2.5.1 SDS-PAGE Profiling
All bacterial isolates were grown overnight in MS1 media supplemented with either methyl parathion, DMMP or GB as a sole source of carbon. Cells were harvested by centrifugation at 4,000×g at 4℃ for 10min. The cell pellet was washed with PBS. Finally, the sample preparation involved denaturation of cell lysate by heating (85℃) with SDS sample lysis buffer for 10min. 4X sample lysis buffer used for supernatant and 1X lysis buffer used for pellet. Gel Electrophoresis was performed on amini protein II Gel Apparatus (BioRad Laboratories, USA) in 10% resolving gel at a constant current of 10mA per gel in the stacking gel and at 20mA per gel in the resolving gel. The gel was stained with 0.8% coomassie blue staining solution for overnight followed by destaining with successive bathes of destaining solution. The stained gels were scanned with a high-resolution densitometer (GS800, BioRad Laboratories, and USA).
2.5.2 Py-GC/MS of Bacterial Isolates
Pyrolysis-GC/MS analysis of bacterial isolates was performed on a Frontier Laboratories model PY-2020iD double-shot pyrolyser. The samples were pyrolyzed for 0.5min at 500℃. The pyrolyser was directly coupled to the inlet port of Agilent 6890N gas chromatograph interfaced with Agilent 5973 inert mass selective detector. The Agilent HP-5MS metal capillary column (30m×25mm ID) was used for analysis. 10µg of the cultivated bacteria from the pure samples were placed on the platinum foil of the pyrolyser using a sterile inoculating loop. The sample was then dried using a warm stream of air for 5min. The pyrolyser is based on a technique in which a small deactivated stainless steel cup is loaded with a sample into a small sized vertical micro-furnace by gravitational free-fall with a push button mechanism. This system provides precise temperature control and minimal condensation of pyrolysates in the system. Because the pyrolysation process works best with volatile compounds, the sample needs to be methylized in order to have the methyl esters of the fatty acids. The fatty acid methyl ester (FAME) is the same fatty acid with the proton in the carboxylic acid group -C-OOH changed by a methyl group -C-OOCH3. This makes the fatty acid more volatile and easier to measure in the chromatogram. To achieve this, around 1µL of tetra methyl ammonium hydroxide (TMAH) was applied to the platinum foil. The sample was dried again for 5min and placed in the pyrolyser chamber. In the pyrolyser chamber, the sample was decomposed under heat treatment at 500℃ in an inert atmosphere of helium gas. The helium gas was also used as a carrier gas for the pyrolysed sample throughout the chromatographic column and for the mass spectrometer. The program used begins at 50℃ and holds this temperature for 2min. The temperature was then increased from 10℃/min to 250℃. This temperature was held for 5min. The GC injector temperature was kept at 250℃ with the injector split ratio set to 50:1. Helium was used as a carrier gas with a constant flow rate of 1.0mL/min. The separated pyrolyzates from the GC were transferred to a mass spectrometer (MS) via a heated interface maintained at 280℃ temperature. The ion source and quadrupole mass analyzer temperatures were kept at 230℃ and 150℃, respectively. The total running time of the program is 27min. The mass spectrometer was tuned at 70eV in EI mode with the mass range of 50-550amu. Post-experimental data analysis (peak integration) was done by Chemstation software (Agilent Technologies, USA).
2.5.3 Identification of Bacterial Isolates by 16S rRNA Sequencing
Based on reported conserve 16S rRNA sequence forward primer (5-GAGTTTGATCCTGGCTCA-3) and reverse primer (5-CGGCTACCTTGTTACGACTT-3) were designed to amplify 16S rRNA gene from total genomic DNA of bacterial isolates. For amplification of 16S rRNA gene, thermal profile was; initial denaturation at 94℃/5min followed by 35 cycles of denaturation at 94℃/1min, annealing at 50℃/1min and extension at 72℃/1min and final extension at 72℃/10min. Taxonomic characterization of these strains was defined by direct sequencing of amplicons of 16S rRNA gene.
2.6 Characterization of Metabolites by GC-MS Analysis
The degradation products of methyl parathion, DMMP and GB were extracted degradation reaction after 24h of incubation with bacterial culture at 30℃. The samples were separated into 2 major fractions before analysis by GC/MS. The sample was first separated into solid and aqueous fractions by centrifugation (4000×g, 5min RT). The supernatant clear solution from centrifugation was extracted with equal volume of ethyl acetate with vortexing for 15min. An Agilent 7890A GC system coupled with 5976C inert mass detectors was used for analysis. The GC separation was performed on a C 18 column (30m2×50µm×0.25µm) with the helium carrier gas at the flow rate of 1.0mL/min. The injector temperature was kept at 250℃ and detector temperature at 300℃. The degradation product of methyl parathion, DMMP and GB was identified by comparing retention times and MS data those with known compounds.
3 RESULTS
3.1 Isolation of OP Compound Degrading Bacterial Strain
For isolation of OP compound degrading bacteria, the enrichment of bacterial culture was carried out in MS1 medium containing 100ppm of different OP compound (GB, DMMP and methyl parathion). After 4 enrichment cycles 10 isolates which capable of grown on different OP compound were isolated. These bacterial colonies were randomly picked up from different OP compound containing plate’s and were grown overnight. Total genomic DNA was isolated by using genomic DNA extraction kit from overnight grown culture. (Figure 1)
|
Figure 1. Genomic DNA of bacterial isolates by using total bacterial DNA Qiagen tissue extraction kit on 1.0% agarose gel.
3.1.1 Morphological and Biochemical Characterization of Isolates
To confirm the purity of isolates single colony of each isolates were picked and spread on organnophosphorus containing plates. After morphological and biochemical characterization it was found that bacterial colony has 2 types of isolates; a Gram-negative, non-motile, catalase-positive, oxidase-negative, bacilli and Gram-negative, catalase, and oxidase positive rod-shaped bacterium. These bacterial isolates were identified as A. baumannii and P. aeruginosa after 16S rRNA gene sequencing.
3.2 PCR Amplification of OP Degrading Gene
Genomic DNA of bacterial isolates was used for amplification of opd genes. On the basis of previous reports, 5 different sets of primers were designed to amplify the opd gene sequence. For that, thermal cyclers were used and optimization of the annealing temperature of prime sets was carried out by gradient PCR. In all primer sets, primer F1: ATG CAA ACG AGA AGG GTT GT, R2: TCA GTG AAT GAG GCC ATC CC, R3: TCA TGA CGC CCG GAA GGT CG were found to be optimally amplified opd genes. By the use of the F1 and R1 primer sets, the 1,100bp gene was amplified, while when the F1 and R3 sets of primer were used, a 900bp gene was amplified. Both PCR products were sequenced for confirmation of the opd gene. After sequencing, it was confirmed that in all bacterial isolate types of opd a gene is present that is responsible for biodegradation of methyl parathion, DMMP and GB (Figure 2).
|
Figure 2. Screening of the isolates for the presence of opd gene. A: amplicon with the use of F1 and R1 primer set, 1,100bp gene was amplified; B: F1 and R3 primers generated 900bp amplicon.
3.3 Inorganic Phosphate Estimation Assay
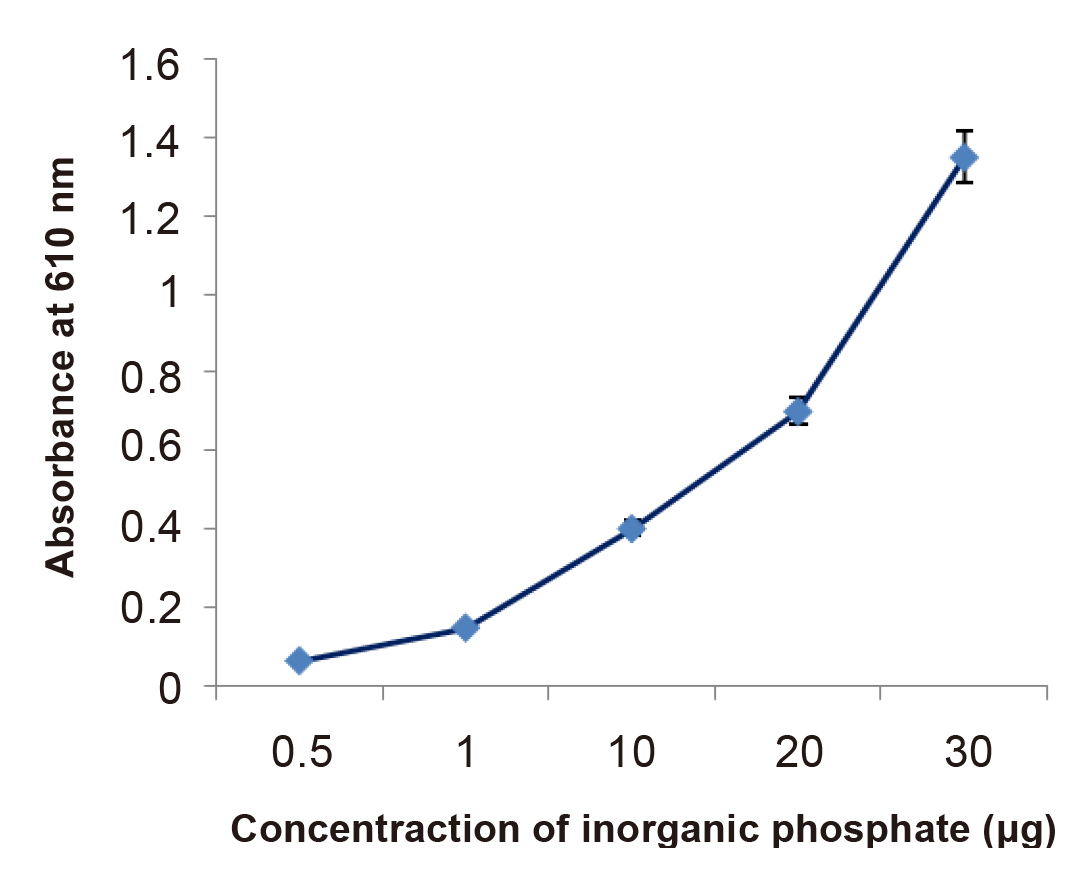
The concentration of inorganic phosphorus was determined at 610nm with the help of a spectrophotometer. In the presence of Pi, the colour of the solution becomes green, and remains stable for at least 24h. In the absence of Pi, the green colour of the dye disappears gradually and the solution becomes yellow. Colour development depends on the final acidity of the assay. For the determination of inorganic phosphate in a medium, a solution has OP compound in a medium used as a control. In the present studies, it was observed that the concentration of inorganic phosphate significantly increased in the presence of bacterial isolates. Figure 3 showed the standard graph plotted for the inorganic phosphate estimation, while Figure 4 showed the concentration of inorganic phosphorus after 12h of incubation in MS1 medium. It increases up to ~10 to 20μg in the presence of isolates, while in a control sample it is around 0.5μg after 12h. This showed a significant difference in the concentration of inorganic phosphorus as compared to their respective controls. These release of phosphorus indicate that degradation of OP compounds by bacterial isolates.
|
Figure 3. Standard curve used inorganic phosphate estimation.
|
Figure 4. Estimation of inorganic phosphate after degradation of OP compounds (methyl parathion, DMMP, GB) by spp. of Pseudomonas and Acinetobactor.
3.4 Characterization of OP Degrading Bacterial Isolates
3.4.1 SDS-PAGE Profiling
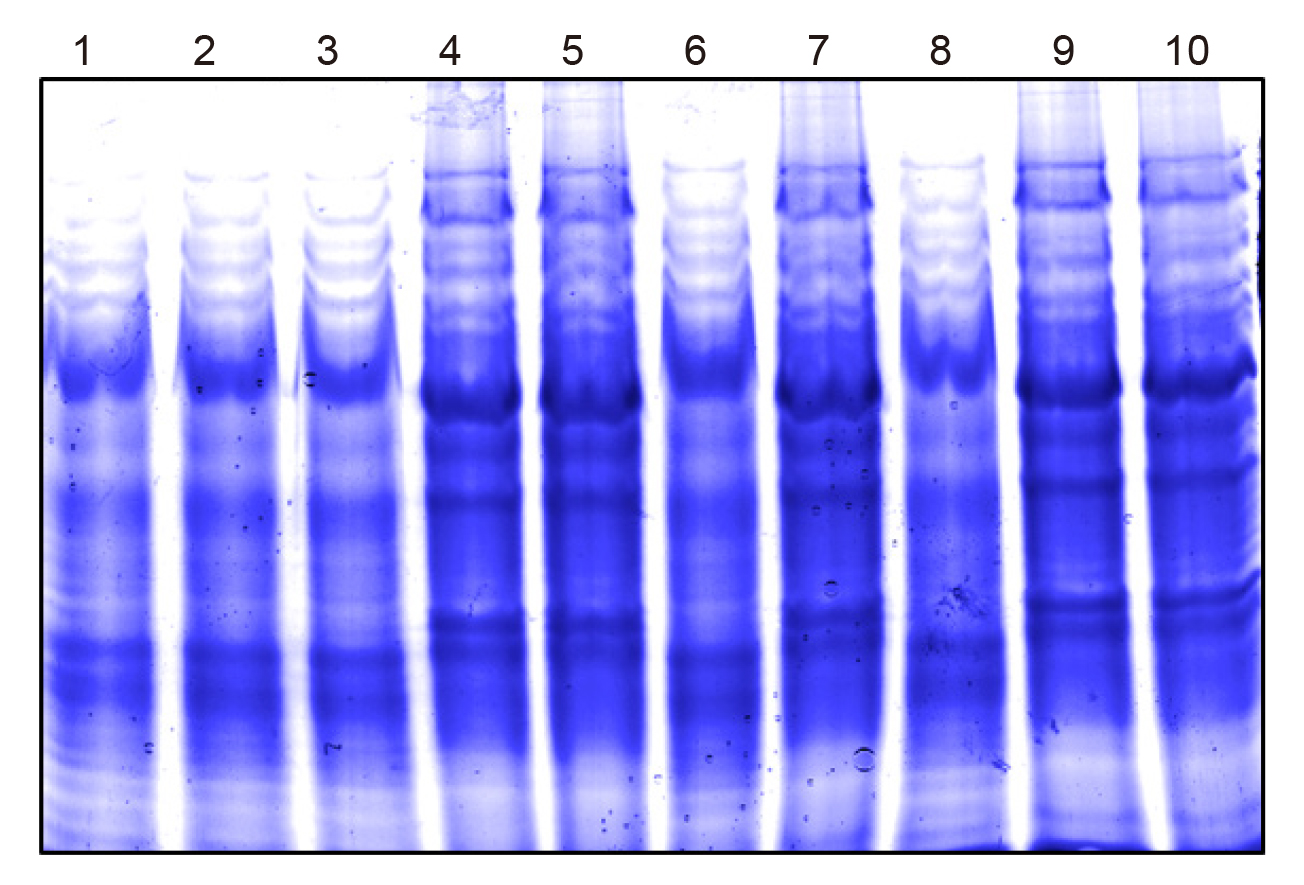
Protein profiling on SDS-PAGE is considered a tool to establish similarity between bacterial isolates. For characterization of bacterial isolates based on protein profiling, all the isolated are grown overnight (~16h) and harvested by centrifugation. The isolates 1, 2, 3, 6 and 8 on SDS-PAGE showed similar protein profiles. The protein profiling pattern of isolates 4, 5, 7, 9, and 10 was different from the first group and matched to each other. On the basis of protein profiling, it was concluded that bacterial isolates belong to 2 major bacterial types as shown in Figure 5.
|
Figure 5. Protein profile (10% SDS-PAGE) for the characterization of bacterial isolates.
3.4.2 Py-GC/MS of Bacterial Isolates
Pyrolysis-GC/MS analysis of all bacterial isolates was performed on grouping bacteria isolated from the OP biodegrading consortium. Total ion chromatograms (TIC) obtained by various isolates in pyrolysis at 600℃ temperatures are shown in Figure 6A and 6B. The post-experimental data analysis (peak integration) was done by Chemstation software (Agilent Technologies). The mass spectral data obtained from Py-GC/MS analysis suggests the formation of many simple aromatic compounds during pyrolysis of bacterial cell pellet at 600℃ temperatures (Table 1). After analysis, it was found that 2 types of peak integration pattern were obtained among all isolates. In all isolates, 1, 2, 3, 6 and 8 were showed similar patterns while another type of chromatographic pattern was recorded of 4, 5, 7, 9 and 10 bacterial isolates in post-experimental analysis as shown in Figure 6. When the tabulation of aromatic compounds was done on the basis of mass spectral data there were some products obtained that differed in both the isolates (Table 1). Hence, these results support the finding of SDS-PAGE analysis and we have considered these 10 bacterial isolates that are from 2 different bacterial species.
|
Figure 6. Py-GC/MS analyses of bacterial isolates. A: Bacterial isolates 1, 2, 3, 6 and 8 showed same pattern; B: Similar chromatographic pattern is recorded for bacterial isolates 4, 5, 7, 9 and 10 in post-experimental data analysis done by the Chemstation software.
Table 1. Identification of Pyrolysis Product of Pseudomonas and Acinetobactor spp.
Sr. |
Pseudomonas spp. Pyrolysis Products |
Acinetobactor spp. Pyrolysis Products |
1 |
Talubine |
Talubine |
2 |
3-methyl 1-H pyrole |
2-furonmethanol |
3 |
2-furon methanol |
Nonene |
4 |
Nonene |
Phenol |
5 |
Benzocyclobutene |
Pyrol 2-5, diol |
6 |
Phenol |
Octanol |
7 |
Pyrol 2-5, diol |
4-methyl Indole |
8 |
N-octaldehyde |
Indole |
9 |
Cresol |
Dodecanoic acid |
10 |
Indole |
Hexadecanoic acid |
11 |
Undecanol |
Octadecanoic acid |
12 |
3-methyl indole |
Hexadecanoic acid |
13 |
Dodecanoic acid |
Octadecanoic acid |
14 |
Hexadecanoic acid |
Farnesan |
15 |
Octadecanoic acid |
|
3.4.3 Identification of Bacterial Isolates 16S rRNA Gene Sequencing
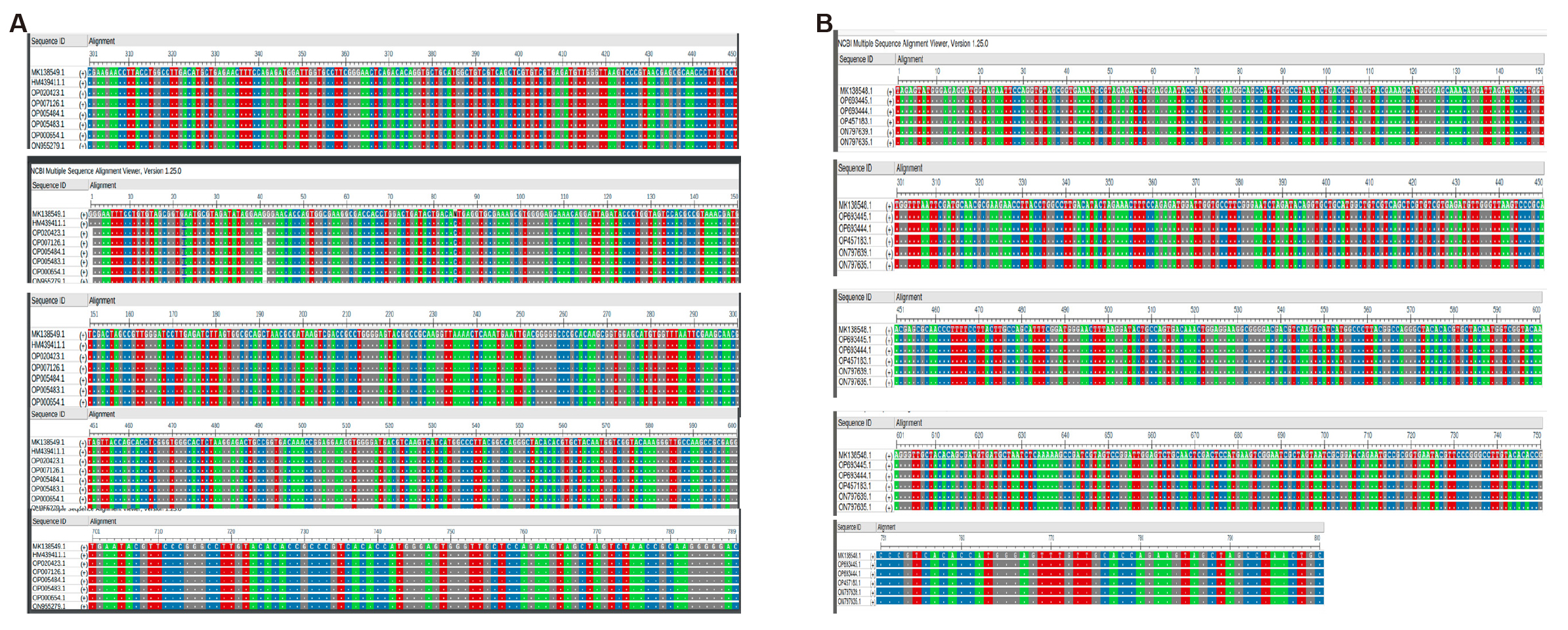
Further taxonomic characterization of all bacterial isolates was defined by 16S rRNA gene sequencing. Total bacterial genomic DNA was used for amplification of the 16S rRNA gene by using gene specific primers. After PCR amplification and sequencing, 2 spp. were identified as Acinetobactor and Pseudomonas. Sequence submitted to NCBI with accession numbers MK138548 and MK138549, respectively (Figure 7). Sequencing results also confirm that all these 10 bacterial isolates belong to 2 bacterial species as screening of the clones to check the presence of opd gene indicated by results of protein profiling on SDS-PAGE and Py-GC/MS.
|
Figure 7. The sequence alignment of bacterial isolates (NCBI multiple sequence alignment viewers 1.25.0). A: Sequence alignment of MK138548 isolate; B: Sequence alignment of MK138549 isolate.
3.5 GC/MS Analysis of Metabolic Intermediates of OP Compounds
Degradation of methyl parathion, DMMP, and GB was carried out in MS1 medium at 200ppm in a shaker incubator operating at 30℃ and 200rpm inoculated with 1% of O/N grown bacterial isolates. Supernatants collected after 12 and 24h of incubation were analyzed by GC/MS for degradation product of OP compounds. After degradation of these compounds by bacterial isolates, some intermediate products were found, like p-nitrophenol (PNP) in the case of methyl parathion, compared where GC/MS. Figure 8A and 8B were the GC/MS ion chromatograms of the degraded products of methyl parathion by bacteria, used to further identify the catabolic intermediates. Compound extracted from a degradation medium containing methyl parathion as a source of carbon showed the presence of PNP with a retention time of ~15.00min. The molecular ion (M+) at m/z 122.5 corresponding to the mass and major fragmentation ions was found to be identical to the mass spectral properties of authentic PNP. Similarly, a second characteristic peak with a retention time of ~19.00min and the molecular ion (M+) at m/z 199.8 showed identity with the mass of methyl parathion. These data support the conclusion that methyl parathion cannot be completely degraded by bacterial isolates. The presence of PNP shows degradation of the OP Compound (Figure 9B).
|
Figure 8. The GC/MS ion-chromatograms of the were and bacterial degraded products of methyl parathion. A: GC Chromatogram obtained from extracts of aqueous methyl parathion (200ppm) solution after incubation at 30℃ for 24h without the presence of bacterial isolates as the control sample; B: GC Chromatogram obtained from extracts of aqueous methyl parathion (200ppm) solution after incubation at 30℃ for 24h with the presence of bacterial isolates.
|
Figure 9. GC/MS Chromatogram. A: GC/MS Chromatogram showed presence of methyl parathion with retention time of 19.00min and molecular ion (M+) at m/z 199.8; B: GC/MS Chromatogram Showed degraded product of methyl parathion at retention time of 15.00min. The molecular ion (M+) at m/z 122.5 corresponding to the mass and major fragmentation ions was found to be identical to the mass spectral properties of authentic PNP.
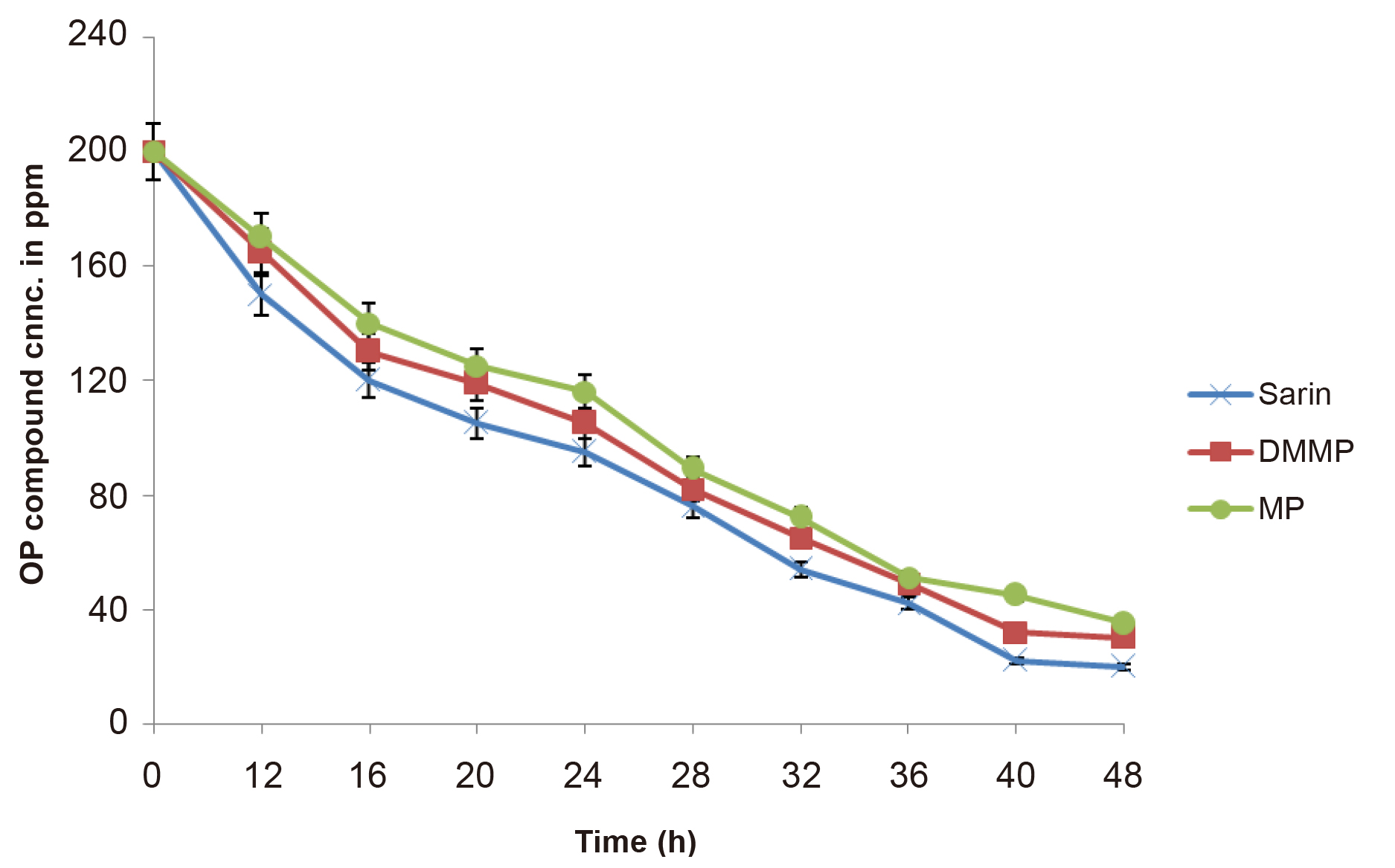
After GC analysis, it was found sp. of Pseudomonas and Acinetobactor effectively degraded the 200ppm concentration of OP compound up to 48.3%, and 59.8% respectively, after 24h of incubation (Figure 10).
|
Figure 10. Graph showed bacterial degradation of 200ppm concentration of methyl parathion, DMMP, GB after 24h at 30°C.
4 DISCUSSION
OP compounds, including pesticides and NAs, are extremely toxic compounds which exert toxicity by irreversibly inhibiting AChE activity[24]. The overuse of these compounds has resulted in contamination of the food chain, water, soil, and terrestrial ecosystems. Because of this, there are legitimate concerns over their exposure to humans and wildlife. As an estimate, unintentional exposures (water, soil, food and compound related accidents) cause 1 to 3 million poisonings and several thousand deaths annually worldwide[25]. This number does not reflect the full extent of the problem and, because of that, there is always the threat of chemical warfare exposure as witnessed in the recent past (Syria and other places). Therefore, there is an unmet need for an efficient bioremediation technology that can safely decontaminate these manmade toxic compounds. Some studies have been reported for biodegradation of OP compound in aerobic conditions[26-28].
In an effort we have isolated spp. of Pseudomonas and Acinetobactor that can use OPs as a source of carbon for their growth. These strains were isolated from the soil sample. These 2 strains were isolated from enriched culture obtained from OP polluted soil under liquid MS1 medium. The isolates grow well only in the liquid medium enriched with methyl parathion, DMMP and GB. After 4 enrichment cycles, the most active isolates that are active against compounds were selected. The strains were checked for the presence of opd genes by opd gene specific primers. These results were confirmed by sequencing. It is reported that Pseudomonas sp. is highly active in utilizing various chemical compounds, such as a hormone, fungicide, or insecticide like chlorpyrifos, methyl-parathion[29,30]. As concerned about Acinetobactor sp., it represents a new bacterium genus that plays a very efficient role in the degradation of OP compounds. It is widespread in nature and it is reported that Acinetobactor sp. can use various toxicants like biphenyl as carbon and energy source[31].
In our studies, the spp. of Pseudomonas and Acinetobactor efficiently degraded the methyl parathion, DMMP and GB. For that, an inorganic phosphate estimation assay was carried out. Results obtained by this assay revealed a significant increase in the concentration of inorganic phosphate (~10 to 20µg), after 12h of incubation in the presence of bacterial isolates as compared to controls. The increased concentration of inorganic phosphate is due to the OP compound used by the bacterial isolates as a carbon source for growth and multiplication. Some reports showed that the isolated bacterium can utilize OPs as a source of carbon or phosphorus[32]. A study on malathion biodegradation showed that physical and chemical characteristics of reaction also influence the rate of biodegradation, so optimization of all these factors will be required for an efficient degradation process[33].
After degradation study, bacterial isolates were characterized by SDS-PAGE, Pyro GC/MS, and by 16S rRNA sequencing. In the protein profiling experiment, all bacterial isolates showed 2 patterns. Similar results were obtained when all isolates were grouped on the basis of post-experimental data analysis (peak integration) done by the Chemstation software in Pyrolysis-GC/MS. These results were further confirmed by 16S rRNA gene sequencing. After sequencing, it was found that all 10 isolates have 2 bacterial spp., which are Acinetobactor and Pseudomonas. This sequence was submitted to NCBI with accession numbers MK138548 (Acinetobactor) and MK138549 (Pseudomonas).
To determine the metabolic intermediates of OP compounds, GC/MS base analysis was done. Degradation of methyl parathion, DMMP, and GB was undertaken in shake flask cultures and the percentage of degradation was determined by formation of intermediate products that were analyzed by GC/MS. After methyl parathion degradation by isolates, was the presence of PNP with a retention time of 15.00min was obtained with molecular ion (M+) at m/z 122.5. The degradation efficiency of individual isolates, a 200ppm concentration of methyl parathion, was found to be 48.3, and 59.8 % using Pseudomonas and Acinetobactor, respectively, within 24h in shake flask cultures. It has been reported that shaking accelerates the biodegradation process due to close contact between organisms and OP compounds. While in co-culture shown the degradation was achieved at 79.9% of DMMP. These isolates were also able to degrade GB up to 200ppm (data not shown). The findings from our study play an important role in the successful removal of various harmful chemical warfare agents.
5 CONCLUSION
The present study was performed with the aim of isolating bacteria which are capable of degrading OP chemical warfare agents. A total of 10 potential degrading bacteria were isolated from soil by the culture enrichment method. 2 unique bacterial isolates were identified using PAGE, pyro-GC and 16S rRNA analysis as spp. of Pseudomonas and Acinetobactor. Strains were able to grown maximum at 30℃ and at pH7.0. Organophosphate degrading gene (opd gene) was found to be present in both the bacterial isolates. Degradation of OP compounds by bacterial isolates was determined by the estimation of inorganic phosphorus after 12 and 24h. Degradation of methyl parathion, DMMP, and GB were undertaken in shake flask cultures and the percentage of degradation was determined by formation of intermediate products that were analyzed by estimation of inorganic phosphate and GC/MS. Current studies may play an important role in onsite biodegradation of OP pesticides industrial waste as well as decontamination of chemical warfare agents.
Acknowledgements
Authors are thankful Director, DRDE, for providing facilities and support required. Mr. Shiv Prakash Sharma and Mr. LNS Tomar were helped in GC/MS studies. Ms. Vinita Chauhan Kushwah is a women scientist fellow in DRDE.
Conflicts of Interest
The authors declare no conflicts of interest with respect to authorship for publication of this article.
Author Contribution
Ram Kumar Dhaked contributed to conceptualization, review and editing. Vinita Chauhan contributed to writing original draft and performing experiments. Both the authors read and approved the final version of manuscript.
Abbreviation List
AChE, Acetyl choline esterase
DMMP, Dimethyl methylphosphonate
GA, Tabun
GB, Sarin
GD, Soman
MS, Mass spectrometry
MS1, Mineral salt
NAs, Nerve agents
OP, Organophosphorus
OPs, organophosphates
PNP, p-nitrophenol
VX, venomous agent X
References
[1] WHO. Public health impact of pesticides used in agriculture. World Health Organization: Geneva, Switzerland, 1990.
[2] Karalliedde L, Senanayake N. Organophosphorus insecticide poisoning. Br J Anaesth, 1989; 63: 736-750.[DOI]
[3] Bose S, Kumar PS, Vo D-VN. A review on the microbial degradation of chlorpyrifos and its metabolite TCP. Chemosphere, 2021; 283: 131447.[DOI]
[4] Noga M, Michalska A, Jurowski K. The prediction of hydrolysis and biodegradation of organophosphorus-based chemical warfare agents (G-series and V-series) using toxicology in silico methods. Ecotoxicol Environ Saf, 2024; 272: 116018.[DOI]
[5] Groenewold, Gary S. Degradation kinetics of VX. Main Group Chem, 2010; 9: 221-244.[DOI]
[6] Munro N. Toxicity of the organophosphate chemical warfare agents GA, GB, and VX: implications for public protection. Environ Health Perspect, 1994; 102: 18-37.[DOI]
[7] Munro NB, Talmage SS, Griffin GD et al. The sources, fate, and toxicity of chemical warfare agent degradation products. Environ Health Perspect, 1999; 107: 933-973.[DOI]
[8] Cao CJ, Mioduszewski RJ, Menking DE et al. Cytotoxicity of organophosphate anticholinesterases. In Vitro Cell Dev Biol Anim, 1999; 35: 493-500.[DOI]
[9] Balali-Mood M, Balali-Mood K. Neurotoxic disorders of organophosphorus compounds and their managements. Arch Iran Med, 2008; 11: 65-89.
[10] Rickell DJ, Glenn JF, Houston WE. Medical defence against nerve agents: New direction. Milit Med, 1987; 152: 35-41.[DOI]
[11] Ganesan K, Raza SK, Vijayaraghavan R. Chemical warfare agents. J Pharm Bioallied Sci, 2010; 2: 166-178.[DOI]
[12] Balali-Mood M, Shariat M. Treatment of organophosphate poisoning. Experience of nerve agents and acute pesticide poisoning on the effects of oximes. J Physiol Paris, 1998; 92: 375-378.[DOI]
[13] Colović MB, Krstić DZ, Lazarević-Pašti TD et al. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol, 2013; 11: 315-335.[DOI]
[14] Ragnarsdottir KV. Environmental fate and toxicology of organophosphatepesticides. J Geological Soc, 2000; 157: 859-876.[DOI]
[15] Intisar A, Ramzan A, Sawaira T et al. Occurrence, toxic efects, and mitigation of pesticides as emerging environmental pollutants using robust nanomaterials—a review. Chemosphere, 2022; 293: 133538.[DOI]
[16] Kilonzi JM, Otieno S. Degradation kinetics and physiological studies of organophosphates degrading microorganisms for soil bioremediation. Stress Biol, 2024; 4: 11.[DOI]
[17] Mulbry WW, Karns JS. Purification and characterization of three parathion hydrolase from Gram negative bacterial strains. Appl Environ Microbiol, 1989; 55: 289-293.[DOI]
[18] Singh BK, Walker A. Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev, 2006; 30: 428-471.[DOI]
[19] Kononova SV, Nesmeyanova MA. Phosphonates and their degradation by microorganisms. Biochem (Mosc), 2002; 67: 184-195.[DOI]
[20] Singh BK. Organophosphorus-degrading bacteria: ecology and industrial applications. Nat Rev Microbiol, 2009; 7: 156-164.[DOI]
[21] Zhang R, Cui Z, Zhang X, et al. Cloning of the organophosphorus pesticide hydrolase gene clusters of seven degradative bacteria isolated from a methyl parathion contaminated site and evidence of their horizontal gene transfer. Biodegradation, 2006; 17: 465-472.[DOI]
[22] El-Fantroussi S. Enrichment and molecular characterization of a bacterial culture that degrades methoxy-methyl urea herbicides and their aniline derivatives. Appl Environ Microbiol. 2000; 66: 5110-5115.[DOI]
[23] Zhu S, Gan Z, Li Z, et al. The measurement of cyclic nucleotide phosphodiesterase 4 activities via the quantification of inorganic phosphate with malachite green. Anal Chim Acta, 2009; 636: 105-110.[DOI]
[24] Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem, 2004; 38: 151-216.[DOI]
[25] WHO: The World Health Report 2002: Reducing Risks, Promoting Healthy Life. World Health Organization: Geneva, Switzerland, 2002.
[26] Goda SK, Elsayed IE, Khodair TA et al. Screening for and isolation and identification of malathion degrading bacteria: cloning and sequencing a gene that potentially encodes the malathion-degrading enzyme, carboxylesterase in soil bacteria. Biodegradation, 2010; 21: 903-913.
[27] Kim YH, Ahn JY, Moon SH et al. Biodegradation and detoxification of organophosphate insecticide, malathion by Fusarium oxysporum f. sp. pisi cutinase. Chemosphere, 2005; 60: 1349-1355.[DOI]
[28] Mohamed ZK, Ahmed MA, Fetyan NA et al. Isolation and molecular characterisation of malathion-degrading bacterial strains from wastewater in Egypt. J Advanc Res, 2010; 2: 145-149.[DOI]
[29] Lakshmi CV, Kumar M, Khanna S. Biotransformation of chlorpyrifos and bioremediation of contaminated soil. Int Biodet Biodegrad, 2009; 62: 204-209.[DOI]
[30] Yasouri FN. Plasmid mediated degradation of diazinon by three bacterial strains Pseudomonassp., Flavobacterium sp. and Agrobacteriumsp. Asian J Chem, 2006; 18: 2437-2444.
[31] Sabit HH, Said OM et al. Molecular identification of Acinetobacter isolated from Egyptian dumpsite as potential bacteria to degrade malathion. Int J Acad, 2011; 3: 84-90.
[32] Singh DK. Utilization of monocrotophos as phosphorus source by Pseudomonas aeruginosa F10B and Clavibactermichiganense subsp. insidiosum SBL 11. Can J Microbiol, 2003; 49: 101-109.[DOI]
[33] Karpouzas DG, Walker A. Factors influencing the ability of Pseudomonas putida strains epI and II to degrade the organophosphate ethoprophos. J Appl Microbiol, 2000; 89: 40-48.[DOI]
Copyright © 2024 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.











 Copyright ©
Copyright ©