Recent Advances in Bio-management of Plant Diseases
Danish Nigar1, Asfa Rizvi2, Bilal Ahmed3*, Mohammad Saghir Khan1*, Ees Ahmad4
1Department of Agricultural Microbiology, Faculty of Agricultural Sciences, Aligarh Muslim University, Aligarh, Uttar Pradesh, India
2Department of Botany, School of Chemical and Life Sciences, Jamia Hamdard University, New Delhi, India
3Department of Agricultural and Biological Engineering, College of Agriculture, Purdue University, West Lafayette, Indiana, USA
4National Agriculturally Important Microbial Culture Collection (NAIMCC), ICAR-National Bureau of Agriculturally Important Microorganisms, Kushmaur, Maunath Bhanjan, Uttar Pradesh, India
*Correspondence to: Mohammad Saghir Khan, PhD, Professor, Department of Agricultural Microbiology, Faculty of Agricultural Sciences, Aligarh Muslim University, Aligarh 202002, Uttar Pradesh, India; Email: khanms17@rediffmail.com;
Bilal Ahmed, PhD, Post Doc Research Associate, Department of Agricultural and Biological Engineering, College of Agriculture, Purdue University, 610 Purdue Mall, West Lafayette 47907, USA; Email: bilalahmed.amu@gmail.com
Abstract
Constantly increasing global human populations has put agricultural sector under astounding pressure to fulfil food demands growing worldwide. In order to optimize crop production, usage of agrochemicals in intensive agronomic practices have increased alarmingly. The negative impact of excessive application of agrichemicals on food production, human health and environment raises concerns about its long-term field application. Exploration of alternative means of crop protection and optimization, therefore, warrants inexpensive and environmentally friendly strategy. Use of microbes or microbes-based products among many options is one such important strategy that provides solution to the problems. In this regard, many biocontrol measures have been practiced over time, and some of them have shown exceptional potential and success. Realizing the importance of soil microbiota in crop optimization while reducing the chemical inputs, significance of plant beneficial bacteria in the amelioration of biotic stresses especially the phytopathogens employing microbiome management, microbial volatilomes, and nano-bioformulations is discussed. The relationship between the agriculturally useful soil microbiomes and food crops enables the development of microbes-based antagonist strategies for low-cost production of food crops in worrying open field environment.
Keywords: biotic stress, micro-biocontrol agents, nematophagous microbiome, nano-bioformulations, microbial volatilomes, hydrolytic enzymes
1 INTRODUCTION
The relationship between human populations and crop production has been very old but complex as well. World population is growing exponentially and therefore, food insecurity is increasing at greater pace worldwide[1]. Global human populations are likely to reach approximately eight billion in 2022 which will further increase gradually to 10.4 billion in 2100 (UN 2022). Due to this huge population size, there is tremendous pressure on agriculture sector to optimize crop production and to fulfil human food demands. The global food mandates that agriculture production must increase by 70% to circumvent human food hunger[2]. Despite all efforts, production of food crops faces massive challenges from biological/microbiological enemies which cause huge losses to yield and quality of eatable crops worldwide. According to some estimates, loss in crop production in India due to insect-pests and diseases is 30-35%[3]. Among phytopathogens, fungi cause 80%, viruses and phytoplasmas cause 9% and bacteria cause more than 7% yield losses[4]. Scientists are trying hard to find ways as to how to control such losses. In this regard, various methods like, use of agrichemicals, antibiotics, and host resistance[5,6] are applied to prevent the crop damage due to phytopathogens. These methods have been proved as a short-term option in disease management strategies. Moreover, the inappropriate application of agrochemicals leads to unintended toxic impacts on soil-plant environment, soil/rhizosphere microbiome[7] and human health via food chain[8]. The resilience of insect-pests or other microbial phytopathogens toward expensive toxic chemicals is yet another major concern. So, considering all factors, there is urgent need to find an eco-friendly and cost-effective long-term solution to solve the phytopathogens problem and to enhance crop production. Evidence reveals that among all applied methods, use of biological formulations to manage plant diseases, often called “biological control” has attracted greater attention as a sustainable and inexpensive disease management strategy[9].
Indeed, different kinds of biological control measures are available but among all options, application of rhizobacteria that occupy about 7-15% of rhizosphere, is most preferred and widely used method[10]. The biological control agents (BCAs) employ different mechanisms such as competition, lytic enzymes and iron chelating compounds “siderophore”, antagonism and induced systemic resistance (ISR) to suppress the severity and extent of diseases[11]. Attempts have been made herein to provide a broader and comprehensive view of new and emerging biocontrol strategies that focuses explicitly on microbial volatilome, plant microbiome engineering, and bio-nano-formulations for controlling phytopathogens.
2 CURRENT GLOBAL PERSPECTIVE OF CROP DISEASES
The loss in food crops due to insect-pests is increasing globally. Each year, plant diseases severely damage the crops resulting in losses in world economy which accounts for about $220 billion while pests alone cause $70 billion monetary losses. The plant diseases therefore, contribute significantly to crop yield losses which puts additional financial pressure on the agrarian community[12]. Like other countries, Indian economy which depends heavily on agriculture sector also suffers hugely from the menace of phytopathogens[13]. Among major food crops, the production of cereals especially maize, rice, and wheat[14] has been reported to decline by 30-70% due to huge biotic stresses[15], this is indeed an alarming situation regarding world food security. Savary et al.[16] projected yield loss at a global level for wheat, rice, maize, potatoes, and soybeans at 22, 30, 23, 17 and 21%, respectively. The maximum losses in the food production could be due to food-deficit areas where the population is growing rapidly and due to the emergence or re-emergence of similar or new plant diseases in the same agro-ecological regions. Put together, there is a pressing need for increasing crop production while minimizing/reducing chemical inputs in agricultural practices. Such ameliorating techniques employed for the management of crop diseases should however, be ecologically sustainable, highly reliable, greatly profitable, socially acceptable, and biologically/agronomically/medically and environmentally safe[17].
3 BIOCONTROL: CONCEPTS AND IMPLICATIONS IN DISEASE MANAGEMENT
Plant diseases caused generally by the phytopathogens belonging to different groups such as fungi, oomycetes, bacteria, nematodes, and viruses, primarily invade the plant root systems and gradually affects the other parts of the host plants[18]. The destruction of disease-causing ability of phytopathogens at any stage of infected plants therefore, becomes imminent. This can be achieved by employing one or simultaneous options like, biological approaches, chemical measures, plant genetic strategies or soil disinfection (fumigation, soil solarization, and anaerobic soil disinfestation) strategies[19]. Of all options, use of chemicals especially pesticides have been found rapid and effective. Ironically, the application of chemicals in the management of plant diseases pollute soil through drift or runoff, leaching and food via consumption of contaminated foods causing phytotoxicity to plants and human health[20]. Agrichemicals are also exorbitantly expensive and when applied injudiciously to control plant diseases, disrupt soil fertility, and crop productivity[21,22]. Considering this, interest in biological control via microbial agents has grown as an alternative to pesticides[23]. In order to limit the usage of synthetic agents, biological control measures involving microorganisms is considered an important component of integrated management strategies[23].
The term "biological control" or "biocontrol" refers to the use of living organisms to compete for resources or space, parasitise other organisms, or fight against certain plant diseases or pests[5]. Due to differences in understanding among scientists, the term biocontrol has been defined differently. For example, the use of biological agents, such as viruses, to combat pestilential organisms, such as pathogens, pests, and weeds, for a variety of objectives to benefit humans has been defined as biocontrol by some workers[24]. The beneficial organisms that are used to cope with the diseases are termed as BCAs by others. With the molecular advancement in biological control strategies, the term has been expanded further to include specialised metabolites, which can be effective for treating diseases and are often isolated from interactions or plant extracts. They are frequently referred to as "biopesticides" or "bioprotectants" and comprise compounds having signalling, antimicrobial or attractant properties (such as pheromones)[25]. Some of the widely used BCAs belongs to genera, Bacillus, Pseudomonas, Agrobacterium, Burkholderia, Azotobacter, Frankia, Azospirillum, Bradyrhizobium, Rhizobium, Serratia and Thiobacillus[26]. Apart from disease suppression, they also enhance plant growth by different direct/indirect mechanisms, biological nitrogen fixation, solubilizing essential elements such as P, Zn and K, secretion of iron chelating compounds (siderophores), phytohormones excretion, production of antibiotics, volatile organic compounds (VOCs), exopolysaccharide, hydrogen cyanide (HCN) and lytic enzymes[27]. Yan et al.[28] in a recent study, isolated Bacillus velezensis YYC strain from tomato rhizosphere and observed enhancement in tomato growth following inoculation by suppressing Ralstonia solanacearum through secondary metabolites such as plantazolicin, fengycin, difficidin and bacilysin and fengycin. Among all secondary metabolites, fengycin promoted plant disease resistance and reduced the growth of Sclerotinia sclerotiorum. Overall, BCAs are considered important in agriculture because they are inexpensive, environment friendly, simple to convey, easy to use, long shelf life and generates no toxic residues[29]. Also, BCAs can be used along with biofertilizers without contaminating soil as well as without causing any damage to the human health[30]. Considering all, there is need to scale up the BCAs production for aggressive application in real field situations for optimizing the crop yields[31,32].
4 PLANT MICROBIOME: AN EMERGING CONCEPT OF DISEASE SUPPRESSION
Plant microbiome, also known as the phytomicrobiome, is relatively a new concept that plays significant roles in disease suppression leading to crop optimization. The term microbiome has been defined as “a characteristic microbial community occupying a reasonably well-defined habitat which has distinct physio-chemical properties”. In simple terms, microbiome refers to both composition and functions of microbes thriving well in any given environment. Broadly, plant microbiome, can be divided into the rhizosphere, phyllosphere and endosphere microbiomes, all of which harbour antagonists that may inhibit various phytopathogens in a plant system[33]. Overall, the plant microbiome benefits plants through different mechanisms such as synthesis of a specialised antagonistic metabolite (rhizobitoxine) that causes resistance against severe infections, suppression of soil-borne disease, antibiosis, competition for nutrients in the rhizosphere and hormone regulation[34]. Plant microbiome is affected by many factors related to plant itself for example, genotype, species, organs and plant health and abiotic factors like, land use and climate, agriculture practices like crop rotation, fertilizers and microbial applications[35].
Rhizosphere microbiome, also considered as the “second genome of plants”[36], consists of bacteria, protozoa, fungi, archaea, oomycetes, algae, nematodes, and viruses[37,38]. Some of these microbial populations exhibit antagonistic effects, while others benefit plants by other mechanisms[39]. Like rhizosphere microbiome, phyllosphere microbiome consists of mostly non-pathogenic microbial community whose interaction with plants can be positive, negative, neutral, or commensal[40]. According to some estimates, the total aerial surface of phyllosphere is about 6.4×108km² worldwide that provides a common and vital place for terrestrial microbiota including bacterial cells[41]. Phyllosphere microbiome aid plants in maintaining health by reducing the overgrowth of phytopathogens. For instance, Brevibacillus brevis, isolated from Chinese cabbage phyllosphere, when used as a biocontrol agent against Botrytis cinerea produced the antibiotics gramicidin S and another cyclic antibiotic, non-ribosomal decapeptide, and some major component of tyrothricin[42]. In addition, phyllosphere microbiome enhances plant productivity and health by influencing seed weight, apical growth, blooming, and fruit development, as well as eliminating pollutants[43]. Phyllosphere microbial populations, such as Microbacterium, Stenotrophomonas, and Methylobacterium have been found to improve the growth and nutritional quality of edible crops by producing natural growth regulators (such as IAA) and fixing di-molecular atmospheric N[44]. The importance of plant associated microbiome in phytopathogen suppression and consequently crop optimization is discussed in the following section.
4.1 Microbial Biocontrol Agents: A Conventional Biocontrol Approach
4.1.1 Bacterial Biocontrol Agents
Soil contains a diverse group of microbial populations which may be deleterious or beneficial to the plants. Among beneficial microorganisms, plant growth-promoting rhizobacteria (PGPR) or plant beneficial bacteria (PBB), firstly described by Kloepper and co-workers were isolated from the rhizosphere which after seed inoculation, quickly colonized plant roots and enhanced crop yields[45]. Also, any soil has the capability to prevent the establishment of diseases in host plants, even in the presence of a pathogen with a substantial inoculum density[46]. However, the abundance, diversity, and composition of PGPR/PBB depends largely on plant species and soil properties[47]. Despite this, the rhizosphere is considered a hotspot for interactions between plants and soil inhabiting heterogenous microbial populations and offer several advantages to mutualistic and symbiotic microorganisms, for example, PGP bacteria, archaea, mycorrhizal fungi, endophytic fungi, and other groups of organisms[48]. The scientific evidence suggests that PBB have been/being used in agriculture as potential biocontrol agents in place of agro-chemicals[49]. Some of the notable PBB commonly applied as bacterial antagonists belongs to genera Alcaligenes, Azospirillum, Arthrobacter, Acinetobacter, Bradyrhizobium, Bacillus, Burkholderia, Enterobacter, Erwinia, Flavobacterium, Pseudomonas, Rhizobium, Frankia, Azoarcus, Exiguobacterium, Paenibacillus, and Pantoea[26,50,51]. These bacterial BCAs mitigate the phytopathogens populations both directly and/or indirectly[52] as presented in Figure 1. In order to inhibit the phytopathogens directly, PBB synthesize secondary metabolites, organic compounds, antimicrobial compounds, toxins and various hydrolytic enzymes like beta-xylosidase, chitinase, catalase, pectin methylesterase, β-1,3-glucanase etc. (Table 1). Of these, production of enzymes is however, one of the key mechanisms evolved within microbial populations that disintegrate the glycosidic linkages of pathogen cell wall leading consequently to the death of target pathogens[5].
|
Figure 1. A mechanistic model explaining the plant disease suppression adopted by plant beneficial microbiome (Adapted from Khan et al.[53])
Table 1. Hydrolytic Enzymes Associated with Disease Suppression Produced by Bacterial Biological Control Agents
Antagonists |
Biocontrol Agents |
Target Phytopathogens |
Disease Caused by Phytopathogens |
Enzymes Involved |
Mechanisms of Destruction |
References |
Pseudomonas spp. |
Bacterial |
Ralstonia solanacearum |
Bacterial wilt of tomato |
Lipase Amylase Protease
|
Cell wall degradation |
[54] |
Bacillus subtilis |
Bacterial |
R. solanacearum |
Bacterial wilt of potato |
Protease |
Hydrolyze proteins and peptides |
[55] |
Pseudomonas fluorescens |
Bacterial |
Fusarium oxysporum f.sp. cumini |
Cumin wilt |
Chitinase, β-1, 3 Glucanase, and Protease |
Inhibits mycelial growth, cell wall degradation |
[56] |
Bacillus velezensis |
Bacterial |
Coniella vitis |
Grape white rot |
Cellulase, Protease, Amylase and Lipase |
Inhibits mycelial growth and spore germination |
[57] |
Serratia sp. Enterobacter sp. |
Bacterial |
F. oxysporum f.sp. ciceris |
Chickpea wilt caused |
Amylase, Protease, Cellulase, Chitinase |
Cell wall degradation |
[58] |
Pseudomonas aeruginosa |
Bacterial |
A. alternata, R. solani, X. euvesicatoria C. michiganensis subsp. michianensis, P. infestans, P. colocasiae, and B. cinerea |
Black spot of vegetables, Root rot and damping off vegetables |
Proteases and Lipases |
Destruct cell membrane and cell wall |
[59] |
The antagonists also inhibit the growth of phytopathogens indirectly by other mechanisms, hyperbiotrophy, ISR and competition. They also promote the growth of plants through effector and elicitor molecules released from the BCAs without killing the targeted pathogens[60]. Effectors are substances that are secreted by or linked to an organism that change the physiology, composition, or function of another organisms. Effectors are pathogen specific compounds that can alter the function and structural constituents of host cells to facilitate infection and/or elicit immune reactions allowing access to nutrients, proliferation, and growth[61]. A very few studies however suggests that effectors/elicitors may aid in uplifting the BCAs' capacity to manage plant diseases. For example, some species of Pseudomonas synthesize and excrete different elicitors such as lipopolysaccharides, phenazines and siderophores (pyochelin and pseudobactin) that efficiently stimulates the defence responses in host plants whereas growth and ISR were induced by 2,4-diacetylphloroglucinol and phosphogluconate dehydratase. The antibiotic compounds produced by Pseudomonas may have superior effectiveness when they are excreted in the rhizosphere because ISR may be triggered in plants prior to pathogen attack. As a result, they protect host plants from the damaging impact of pathogens and enhances overall growth of infected plants[62].
ISR is other beneficial trait of plant associated microbiomes that are used to suppress pathogens present either in soil or aboveground. The purpose and pathways underpinning ISR triggered by different beneficial biocontrol agents such as Bacillus spp., and Pseudomomas spp. etc. in containment of plant diseases is, however, poorly researched even though they can suppress numerous crop diseases[63]. Thus, systemic acquired resistance (SAR) and ISR both cause systemic resistance in plants and offer long-lasting defence against different phytopathogens. Among phytocompounds, salicylic acid (SA) is a necessary signal molecule for SAR, whereas ethylene and jasmonic acid (JA) acts as ISR signal molecules[64]. For instance, the seed inoculation of B. pumilus INR7 was found effective against the bacterial spot disease of pepper caused by Xanthomonas axonopodis pv. Vesicatoria[65]. PBB also manage biotic stresses indirectly through competition which occur among microbes for nutrients, oxygen, and appropriate niches both at root surface and in rhizosphere regions. The root exudates or the nutritional photosynthates released from different plant genotypes attracts PBB and favours their colonisation on appropriate plant surfaces. Rhizosphere microbiome, however, have a competitive advantage over plant pathogens due to their superior nutrient absorption and metabolic capabilities[66].
4.1.2 Fungal Biocontrol Agents
Majority of plant growth-promoting fungi are considered one of the safest methods for ISR and growth promotion of crops due to their ability to activate the plant immune system against pathogenic attack[67]. As a BCA, fungi are preferred due to - (i) a very high reproductive rate, (ii) a quick generation time, and (iii) they are target specific. Also, they can survive by switching from parasitism to saprotrophism in the absence of the host, thus preserving the sustainability of the environment[68]. Among fungi, Trichoderma, a genus with 25 BCAs have been used largely as a potential candidate to suppress a range of fungal phytopathogens. In addition to Trichoderma, other fungal genera such as Alternaria, Aspergillus, Candida, Fusarium, Penicillium, Pichia, Pythium, Talaromyces, and Verticillium have also been found as promising fungal biocontrol bioagents (FBCAs)[68]. The fungi adopt different mechanisms including secretion of hydrolytic enzymes (Table 2) to suppress the pathogenicity of the diseases. Furthermore, FBCAs activate host defence responses by producing pathogen-associated molecular patterns (PAMPs) or microbe-associated molecular patterns (MAMPs). These receptors can easily be sensed by the plants and in return induce PAMP/MAMP-triggered immunity to plants, antibiosis, hyperparasitism, competition and production of secondary metabolites[69]. Hyperparasitism is a fungal phenomenon exhibited by many antagonists wherein hyperparasites penetrate and kill cells of bacterial pathogens as well as the mycelium, spores, and dormant fungal pathogens[70].
Overall, the mycoparasitism includes different stages: (i) close contact with the pathogens, (ii) mutual/specific recognition and interaction between the pathogen and antagonists, (iii) secretion of lytic enzymes by the antagonists, dissolution of cell wall and penetration inside the host, and (iv) proliferation of the antagonists inside the host, and exit to the exterior environment[71]. In this context, mycoparasites such as species of Trichoderma have traditionally been considered as necrotrophic hyperparasite and fast colonizer of the spermosphere (seed zone) and rhizosphere (root zone). Following colonization and successful establishment, release of various hydrolytic enzymes such as chitinases, proteases, glucanases and other secondary metabolites, like, polyketides, non-ribosomal peptides, terpenoids and pyrones have been reported to suppress the fungal pathogen diseases and hence relieving plants from biotic stresses[72]. It has now become possible to enhance fungal strains and introduce fungal genes into the host plants using biotechnologies, genetic modification, and DNA recombination[68]. Employing these techniques, the gene encoding for trichodermin (tri5-trichodiene synthase) was cloned into T. brevicompactum Tb41tri5 which increased the production of trichodermin with greater antifungal efficacy against Aspergillus fumigatus and Fusarium spp.[73]. This and other developments in biological approaches for disease management warrants further use of genome editing technology (such as CRISPR/Cas9)[74].
Table 2. Some Examples of Hydrolytic Enzymes Associated with Disease Suppression Secreted by Fungal Biological Control Agents
Antagonists |
Target Phytopathogens |
Disease Caused by Phytopathogens |
Enzymes Involved |
Mechanisms of Destruction |
References |
Trichoderma harzianum sensu lato |
Bipolaris sorokiniana |
Wheat spot blotch |
Chitinase |
Decompose cell wall chitin |
[75] |
Trichoderma asperellum, |
F. oxysporum, F. fujikuroi, F. tricinctu, F. cantenulatum |
Fusarium wilt of banana |
Chitinase and β-1,3, Glucanase |
Degradation of chitin and cell wall |
[76] |
Cladosporium omanense |
Pythium aphanidermatum |
Cucumber and radish damping-off disease |
Cellulase, β-1,3-Glucanases |
Cellular leakage mycelium and inhibited oospore production. |
[77] |
Trichoderma species |
F. solani and R. solani |
Root rot of pea and bean |
Chitinase, Peroxidase, and Polyphenol oxidase |
Hydrolysis of cell wall |
[78] |
4.1.3 Fungal and Bacterial BCAs Against Plant Parasitic Nematodes: An Overview
Plant-parasitic nematodes (PPNs) among soil dwelling nematodes pose a serious global challenge to food crops. The annual global economic losses due to PPNs has been reported as USD 173 billion[79]. When food crops are attacked by PPNs, no specific symptoms appear on plants and hence the damages are quite often erroneously confused with abiotic stresses. This mis concept, allows PPN to proliferate and increase in density beyond threshold limit in the field. Some of the agronomically destructive nematodes are root-knot nematodes (Meloidogyne spp.), cyst nematodes (Globodera spp. and Heterodera spp.), root-lesion nematodes (Pratylenchus spp.), the burrowing nematode (Radopholus similis) and the stem and bulb nematode (Ditylenchus dipsaci)[80,81]. So, considering the nematode threats, chemical control with synthetic nematicides has been attempted to offset PPN populations under real field conditions. The broad-spectrum activity, environmental pollution, emergence of resistance among PPNs and lack of proper regulatory and legislative guidelines regarding use of synthetic chemicals to contain and maintain PPNs within limit warrants alternatives strategies[82]. In this regard, BCAs especially nematophagous fungi (NF) and nematophagous bacteria (NB) offers a promising alternative to expensive chemical measures. In this section, the role of some fungal and bacterial BCAs in the management of PPNs are highlighted.
Plant beneficial microbiome inhabiting soil/rhizosphere or colonizing and penetrating endophytically plant surfaces (endophytes/spermophytes) also adopts different strategies to protect plants from the damaging impact of PPNs[83,84]. Generally, such nematophagous microbes (NMs) including fungal (Table 3) and bacterial BCAs (Table 4) target various life stages/processes including both motile and sedentary growth stages of PPNs and eventually kill them. Broadly, the NF controls the PPNs through bionematicides by parasitism, secretion of lytic enzymes and toxins and induce defence and resistance mechanisms in plants against PPNs[85-87]. Essentially, the NF traps (nematode-trapping fungi) and prevent PPNs from escaping through-(i) specialized structures, such as constrictor rings that capture nematodes in their mycelium (ii) three-dimensional hyphae networks and (iii) adhesive spores[88,89]. For example, the opportunistic saprotrophic fungi attack non-motile stages, like eggs, cysts, and Meloidogyne females[90]. On the other hand, the endoparasitic fungi do not produce specialized structures but infect PPNs by spores (conidia or zoospores). Such spores produced for example by Harposporium spp. are either ingested or adheres onto the cuticle of PPNs and later the whole NF content is injected into the nematode as reported in Drechmeria coniospora and Verticillium spp.[91,92]. As an example, the Arthrobotrys oligospora, a nematode-trapping fungus has been found to have a significant deleterious effect on Meloidogyne javanica infecting tomato by forming a specialized penetration tube to pierce the nematode cuticles[93]. The killing of PPNs by NF is also done through nematicidal or nematostatic compounds released into the soil[94,95]. Among toxic metabolites, mycotoxins are commonly used by toxin-positive NF to immobilize or kill PPNs[96,97].
Table 3. Some Examples of Potential Fungal BCAs Against Plant-parasitic Nematodes
Antagonists |
Major PPN |
Nematode Species |
Host Plants |
Major Findings |
References |
Pochonia chlamydosporia |
Root-knot nematodes
|
Meloidogyne incognita |
Tomato and Cucumber |
Reduced infection by 32-43% and female fecundity by 14.7-27.6%, induced the expression of salicylic acid (SA) pathway and upregulated jasmonate signalling pathway |
[98] |
Purpureocillium lilacinum |
Tomato |
Namaticidal effect on second stage juvenile's survival and egg hatching of nematode, reduced nematode populations, number of galls and egg masses in plant roots |
[99] |
||
Arthrobotrys oligospora (MRDS 300) |
Tomato |
Reduced the number of females, galls and nematodes in different developing stages |
[100] |
||
Pochonia chlamydosporia var. chlamydosporia |
Pistachio |
Reduced reproduction parameters; Number of galls were significantly reduced |
[101] |
||
Pochonia chlamydosporia |
Cyst nematodes
|
Globodera pallida |
Potato |
Integrative characterization offers novel perspectives on the biology and biocontrol potential of P. chlamydosporia |
[102] |
Hirsutella minnesotensis |
Heterodera glycines |
Soybean |
Microscopic examinations revealed soybean root surface colonization by H. minnesotensis, H. minnesotensis inoculation significantly improved the biomass, colonization efficiency, relationship between nematode parasitism and fungal density, and enhancement in soybean growth provide evidence that H. minnesotensis may be used as a potential BCA. |
[103] |
|
Arthrobotrys oligospora, Purpureocillium lilacinum and Pochonia chlamydosporia, Glomus fasciculatum |
Root lesion nematodes
|
Pratylenchus zeae |
Sugarcane |
Individual or combined inoculations of BCAs increased shoot and root growth, G. fasciculatum with A. oligospora showed maximum shoot weight, P. zeae population reduced in all BCA inoculated plants, nematode reductions in roots varied between 50 and 77%, mycorrhizal colonization increased in combined treatments of AMF and antagonistic fungi |
[104] |
Fusarium oxysporum f. sp. cepae (foc) |
Stem and bulb nematode
|
Ditylenchu dipsaci |
Garlic |
Interaction of D. dipsaci and Foc reduced the severity of disease in bulb and lowered the nematode populations |
[105] |
Fusarium inflexum, Thielavia terricola, Trichoderma brevicompactum, T. harzianum, T. longibrachiatum, Penicillium citrinum |
Reniform nematode
|
Rotylenchulus Reniformis |
Coriander and Cowpea |
The NF fungi caused nematode mortality, allowed only 5 to 20% of the juveniles to hatch, fungal filtrates significantly reduced the number of egg masses and the reproductive factor of R. reniformis. |
[106] |
Purpureocillium lilacinum |
White tip nematode
|
Aphelenchoides besseyi |
Rice |
Significantly reduced white tip symptoms and kernel numbers in panicles and panicle weight |
[107] |
Volutella citronella |
Cyst nematode |
Aphelenchoides besseyi, Bursap-helenchus xylophilus, and Ditylenchus destructor |
Potato |
The mortality rate was 100, 100, and 55.63%, respectively for each nematode |
[108] |
Monacrosporium thaumasium |
Beer mat nematode |
Panagrellus redivivus |
In vitro |
BCA produced chitinases of two distinct molecular weights, 27 and 30 kDa with nematocidal activity, enzymes significantly reduced number of P. redivivus larvae by 80%. |
[109] |
Bacterial mechanisms to antagonize PPNs may include the production of antibiotics, endospores, hydrolytic enzymes, VOCs, Cry proteins (pore-forming toxins) etc[110,111]. Apart from directly suppressing the PPNs, many soil microbiome including arbuscular mycorrhizal fungi (AMF), indirectly facilitate the growth and development of agriculturally important crops by inducing plant defense mechanisms against PPNs, for example, inducing signal substrate production, regulating gene expression, and enhancing protein production and maintaining plant hormone levels[112-114]. Acknowledging the importance of interaction between NMs and PPNs, the area looks interesting because nematophagous BCAs can be used to manufacture inexpensive and environmentally friendly namatocides as an alternative to synthetic chemicals used to manage PPNs. Some hydrolytic enzymes such as serine protease, chitinase and toxins released by NMs into soils can play important roles in destroying infection and thereby protecting crops from the PPNs attack.
Table 4. Some Examples of Potential Bacterial BCAs Against Plant-parasitic Nematodes
Bacterial BCAs
|
Major PPN |
Nematode Species |
Host Plants |
Major Findings |
References |
B. cereus, B. subtilis, B. thuringiensis, B. megaterium |
Root-knot nematodes |
Meloidogyne spp. |
Soybean |
The filtrate mixture of BCA caused approximately 85–90% immobility of second- stage juveniles (J2) of nematode after 96 h |
[115] |
Bacillus subtilis |
Sugarcane |
Effectively controlled the nematodes in all three cycles of sugarcane production |
[116] |
||
Pasteuria penetrans |
M. arenaria |
Peanut |
Local change in specificity on a yearly basis, exhibited ability to infect and suppress target pest |
[117] |
|
Bacillus cereus, B. pumilus, B. subtilis, B. flexus, B. megaterium |
Cyst nematodes
|
Globodera rostochiensis |
Potato |
BCAs were nonpathogenic and had protease and chitinase activity; could be used as potential BCA for golden cyst nematode |
[118] |
Ensifer fredii |
Heterodera glycines |
Soybean |
The mortality of J2 treated with BCA increased with exposure time, reduced egg hatching within cysts, H. glycines were repelled by the BCA |
[119] |
|
Pseudomonas donghuensis, Pseudomonas sp. |
Root lesion nematodes
|
Pratylenchus Penetrans |
Onion |
PGPR application decreased nematode populations on onion roots, enhanced root length and dry weight, PGPR strains showed nematicidal activity, produced chitinases and proteases, and formed biofilms |
[120] |
Combination of P. fluorescens and Purpureocillium lilacinum |
Burrowing nematode
|
Radopholus similis |
Banana |
P. fluorescens with P. lilacinus effectively reduced the burrowing nematode populations in soil and roots |
[121] |
Bacillus sp. (CBSAL02), Pseudomonas sp. (CBSAL05) |
Stem and bulb nematode
|
Ditylenchus spp. and M. javanica
|
Garlic |
Bacterial BCAs CBSAL02 and CBSAL05 significantly reduced the hatching of M. javanica eggs by 74% and 54.77%, respectively and motility of Ditylenchus spp. by 55.19% and 53.53%, respectively |
[122] |
Bacillus amyloliquefaciens (FR203A), B. megaterium (FB133M), B. thuringiensis (FS213P), B. thuringiensis (FB833T), B. weihenstephanensis (FB25M), B. frigoritolerans (FB37BR), and P. fluorescens (FP805PU) |
Fanleaf virus nematode
|
Xiphinema index |
Grapevine |
The three initial consortia showed effective control of parasite, significantly lowered the reproductive indices, damages caused by X. index were declined by all BCAs without any difference among BCA formulations. |
[123] |
Xenorhabdus bovienii |
White tip nematode
|
Aphelenchoides besseyi |
Rice |
The X. bovienii suppressed the A. beseyi populations |
[107] |
5 MICROBIAL VOLATILOMES: A RECENT APPROACH FOR CONTROLLING PHYTOPATHOGENS
Control agents also synthesize VOCs that mitigates the crop diseases by preventing root colonization of plant pathogens. The VOCs released by microbiome, varying in chemical composition is preferred in sustainable agriculture over synthetic fungicides due to- (i) long-range of action, (ii) easy decomposition, and (iii) higher biocontrol efficiency[124,125]. Approximately 2000 bacterial VOCs are known to be secreted by almost 1000 microbial species, the predominant VOCs are alkenes, alcohols, ketones, terpenes, benzenoids, pyrazines, acids, and esters[124,126]. The fungal volatiles are dominated by alcohols, benzenoids, aldehydes, alkenes, acids, esters, and ketones[127]. Bacterial species that produce VOCs belongs to genera Bacillus, Burkholderia, Collimonas, Pseudomonas, Serratia, Stenotrophomonas and Streptomyces while fungi include Aspergillus, Fusarium, Muscodor and Alternaria (Table 5). The co-inoculations of B. cereus Rs-MS53 and P. helmanticensis Sc-B94 have been found to effectively suppress the pathogenic fungus R. solani[128]. Similarly, R. solani, a soil-borne pathogen, secreted a variety of VOCs that facilitated plant growth, development, altered plant emissions, and decreased insect resistance[129]. The synthesis of HCN by some Pseudomonas spp., such as P. fluorescens CHA0, is a well-known example of volatile-mediated fungal inhibition against Thielaviospis induced tobacco root rot[130].
Table 5. Volatilomes Synthesized by Antagonist Microbiomes Against Phytopathogens
Antagonists |
Target Organisms |
Main VOCs |
Effects of VOCs |
References |
Lysobacter |
Pythium ultimum Rhizoctonia solani, and Sclerotinia minor. |
2,5-Dimethylpyrazine, 2-ethyl-3-methoxypyrazine and 2-isopropyl-3-methoxypyrazine |
Induce resistance against abiotic and biotic stresses and Inhibits spore germination and mycelial growth |
[131] |
Trichoderma gamsii |
Panax notoginseng
|
dimethyl disulfide, dibenzofuran, methanethiol, ketones, |
Controls plant pathogens, activate plant immunity, and enhance plant growth |
[132] |
Streptomyces sp. |
Rhizoctonia solani, Phoma medicaginis, Fusarium solani Fusarium oxysporum and Sclerotium rolfsii |
3-carene 2,5-dione, geosmin, beta-cubebene and Phenol, 2-(1,1-dimethylethyl)-6-methyl- |
Alter hyphal morphology and inhibits conidial germination |
[133] |
Pseudomonas sp. |
Verticillium dahlia |
1-undecene, (methyldisulfanyl) methane and 1-decene, tridecane, 1-decene |
Plant growth promotion and anti-fungal activity |
[134] |
Bacillus spp. |
Macrophomina phaseolina |
Benzene, 1, 3-diethyl- and Benzene, 1, 4-diethyl followed by naphthalene, m-ethylacetophenone and ethanone, 1-(4-ethylphenyl) |
Mycelial growth inhibition, deformity of mycelium, inhibition of sclerotia germination, and ultrastructural alterations of cell organelles |
[135] |
Bacillus spp. |
Fusarium oxysporum f. sp. niveum |
2-heptanone, 2-ethyl-1-hexano, and 2-nonanone |
Plant growth promotion and antifungal activities |
[136] |
Corallococcus sp. |
Fusarium oxysporum f. sp. Cucumerinum |
trans-1, 2-pentyl-1-heptene, 259 1H-cyclopenta-1,3-cyclopropa-1,2-benzene and 3-Undecanone. 2-hexyl-1-decanol and 2-octyl-1-dodecanol |
Damage cell wall and cell membranes, Apoptosis, accumulation of reactive oxygen species (ROS), inhibits mycelial growth, antifungal activity |
[137] |
Bacillus spp. |
Fusarium kuroshium |
ketones and pyrazine compounds, |
Inhibits mycelial growth, antifungal activity |
[138]
|
Stenotrophomonas sp. |
Bacillus pumilus |
dodecane, 2,6,10-trimethyl dodecane, 2,6,11-trimethyl |
Antibacterial activity, inhibits root attachment, chemotaxis and motility, damages the cells |
[139] |
Pseudomonas putida |
Ralstonia pseudosolanacearum |
2, 5-dimethyl pyrazine; 2-methyl pyrazine; dimethyl trisulphide; 2-ethyl 5-methyl pyrazine; and 2-ethyl 3, 6-dimethyl pyrazine |
Antibacterial, antifungal, completely inhibits oomycetes |
[140] |
6 NANO-BIOFORMULATIONS IN DISEASE MANAGEMENT
Nanotechnology, an exciting technology with broader applications in different disciplines including agriculture, biomedicine, food packaging and environment[141] has generated exceptional interests among global researchers due to their unique features[142-144] such as size (10 to 100nm), large surface area to volume ratio, high surface energy, quantum confinement and many other catalytic and magnetic activities of nanoparticles (NPs)[145,146]. The application of NPs or NPs based formulations are increasing in agriculture including plant disease management also[147,148] due to- (i) precise delivery of NPs to targeted sites (ii) capability of NPs to enhance nutrient use efficiency and (iii) its ability to reduce nutrient losses during application or leaching into water systems[149]. The NPs based formulation often called nanoformulations or nanobioformulations involves the use of biological resources such as microbes. The nanoformulations should contain all properties of NPs like shape, size, no eco-toxicity, easy transport delivery and disposal[150]. Due to these properties, nanoformulations are considered potential plant growth enhancer in crop production practices[151-153]. Realizing the phytopathogen inhibiting abilities of NPs, the nanomaterial-based formulations have been applied and found useful in up-regulating crop production by impeding the phytopathogens[154,155]. Due to many problems linked with the application of synthetic pesticides in disease management, metal oxide NPs (MONPs) have received greater attention as nanoformulations in plant disease management because they are- (i) much smaller than bulk molecule, (ii) required in small quantities, (iii) inexpensive relative to expensive conventional agrichemicals, and (iv) benign[156-158].
Nowadays, Bacillus based nanoformulations are preferred against phytopathogens since Bacillus spp. secretes a variety non-ribosomically antagonist substance, including iron chelating compounds, lipopeptides, antimicrobial peptides, and polyketide compounds[159]. The Bacillus species such as B. stearothermophilus, B. laterosporus, B. circulans, B. licheniformis, B. amyloliquefaciens, B. pabuli, B. magaterium, B. thuringiensis and B. subtilis produce chitinase which can break the cell wall chitin of the conidia, hyphae, sclerotia as well as chlamydospores and have shown antifungal activity against Aspergillus favus, A. niger and Penicillium chrysogenum[151]. Nanoformulations are also used as carrier materials for the controlled release of BCAs due to slow delivery of the active ingredient and improved solubility to have maximum inhibitory effect against phytopathogens[160]. Recently, bacterial based nanobioformulations prepared using carbon nanotubes and silicon oxide NPs was found effective against Phytophthora drechsleri, capable of causing pistachio gummosis, and increased the growth and crop yields[161]. Besides bacteria-based formulations, fungus-based formulations are also used against the phytopathogens. In a study, two types of nano-capsules, nanoemulsion, and powdered nanoformulations were prepared using fungus Talaromyces flavus and tested against the pathogenic fungus F. oxysporum f. sp. cucumerinum. The nanopowder was found as the most potent nanoformulation that maximally inhibited the pathogen compared to nanoemulsion based nanofungal formulations[162]. Similarly, the biocontrol capability of Serratia marcescens SU05's bovine serum albumin (BSA) NPs loaded with extra cellular chitinase had inhibitory effect against phytopathogenic fungus Alternaria alternata. Nano-enzyme conjugate at all concentrations significantly reduced the fungal biomass, with maximum damage to fungal hyphae fragments. It was suggested that the nanoformulations could be developed and applied in field environments for efficient control of phytopathogenic fungi[163]. The conventional and new emerging trends highlighting the importance of microbiomes in disease suppression are summarized in Figure 2.
Besides controlling the phytopathogens, NPs also play significant roles in plant growth and development beginning from seed germination to optimization of crop yields[164]. In a study, selenium NPs were found to exhibit positive effects on the length of shoots, roots, and the germination percentage of Hordeum vulgare[165]. Similarly, the silver and titanium oxide NPs revealed positive impacts on seed germination, seedling growth, chlorophyll content, antioxidant activity and carotenoid content of tomato plant[166]. In yet other reports, Zinc oxide nanoparticles, prepared from foliage of Coriandrum sativum, when used as nanofertilizer had stimulatory effects on growth of various pulses, such as Bengalgram, Turkishgram, and greengram. The effects were obvious on seed germination, chlorophyll, and protein content leading to overall improvement in plant performance[167].
|
Figure 2. Schematic overview of recent approaches involving plant microbiome, microbial volatilomes and nanobioformulations strategies that can be adopted and integrated to generate a more holistic picture of microbiome consortia and mechanisms involved in phytopathogen suppression leading to crop optimization. BCA, Biocontrol agents; JA, Jasmonic acid; SA, Salicylic acid; ROS, Reactive oxygen species; N, P and Fe indicates nitrogen, phosphorus and iron respectively; NPs, Nanoparticles.
7 CONCLUSION
Crops in general are susceptible to many biotic stresses including soil borne bacterial and fungal pathogens that distinctly diminish the food quality and crop yields. The reduction in production can be managed by adopting both traditional and advance approaches. The unreasonable cost, development of resistant pathogens and environmental hazards resulting from pesticidal applications are serious concerns. The intervention of microbial formulations and nanobioformulations in intensive crop production practices provide a safe and an effective solution to the problems of biotic stresses. They can also act as growth enhancer by supplying nutrition to plants and consequently enhances crop yields under stressed open field conditions. The microbiome endowed with many disease suppressing abilities could be developed and commercialized for upregulating the food production under real field conditions. The genetic manipulation of microbial antagonists especially genes associated with disease suppression/growth promotion into bacterial/fungal microbiome lacking such activity are needed.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
Rizvi A and Nigar D contributed to the literature search strategy, manuscript screening, data compilation and validation. Khan MS and Rizvi A contributed to original draft preparation. Ahmed B, Ahmed E and Rizvi A contributed to graphics design and reviewed the manuscript. Khan MS conceptualized, supervised, and edited the whole manuscript. The manuscript was read and approved by all the authors for publication.
Abbreviation List
AMF, Arbuscular mycorrhizal fungi
BCAs, Biological control agents
BSA, Bovine serum albumin
FBCAs, Fungal biocontrol bioagents
HCN, Hydrogen cyanide
ISR, Induced systemic resistance
JA, Jasmonic acid
MAMPs, Microbe-associated molecular patterns
MONPs, Metal oxide NPs
NB, Nematophagous bacteria
NF, Nematophagous fungi
NMs, Nematophagous microbes
NPs, Nanoparticles
PAMPs, Pathogen-associated molecular patterns
PBB, Plant beneficial bacteria
PGPR, Plant growth-promoting rhizobacteria
PPNs, Plant-parasitic nematodes
ROS, Reactive oxygen species
SA, Salicylic acid
SAR, Systemic acquired resistance
VOCs, Volatile organic compounds
References
[1] Aiyedogbon JO, Anyanwu SO, Isa GH et al. Population growth and food security: Evidence from Nigeria. Probl Perspect Manag, 2022; 20: 402-410. DOI: 10.21511/ppm.20(2).2022.33
[2] Carvajal-Yepes M, Cardwell K, Nelson A et al. A global surveillance system for crop diseases. Sci, 2019; 364: 1237-1239. DOI: 10.1126/science.aaw1572
[3] Prasad BK, Singh G, Sharma AK. Bio-management of diseases and insect pests in vegetable crops. Pharm Innovation J, 2022; 11: 153-155.
[4] Khakimov A, Salakhutdinov I, Omolikov A et al. Traditional and current-prospective methods of agricultural plant diseases detection: A review. IOP Conf Ser Earth Environ Sci, 2022; 951: 012002. DOI: 10.1088/1755-1315/951/1/012002
[5] Pandit MA, Kumar J, Gulati S et al. Major biological control strategies for plant pathogens. Pathogens, 2022; 11: 273. DOI: 10.3390/pathogens11020273
[6] Wang YP, Pan ZC, Yang LN et al. Optimizing plant disease management in agricultural ecosystems through rational in-crop diversification. Front Plant Sci, 2021; 12: 767209. DOI: 10.3389/fpls.2021.767209
[7] Lacava PT, Bogas AC, Cruz FPN. Plant growth promotion and biocontrol by endophytic and rhizospheric microorganisms from the tropics: A review and perspectives. Front Sustain Food Syst, 2022; 6: 796113. DOI: 10.3389/fsufs.2022.796113
[8] Adedayo AA, Babalola OO, Prigent-Combaret C et al. The application of plant growth-promoting rhizobacteria in Solanum lycopersicum production in the agricultural system: A review. Peer J, 2022; 10: e13405. DOI: 10.7717/peerj.13405
[9] An C, Ma S, Liu C et al. Burkholderia ambifaria XN08: A plant growth-promoting endophytic bacterium with biocontrol potential against sharp eyespot in wheat. Front Microbiol, 2022; 13: 906724. DOI: 10.3389/fmicb.2022.906724
[10] Elbouazaoui A, Sijilmassi B, Maafa I et al. Biocontrol activity of Bacillus, Paenibacillus and Pseudomonas against Fusarium wilt of chickpea in Morocco. Acta Agric Scand Section B-Soil & Plant Sci, 2022; 72: 847-859. DOI: 10.1080/09064710.2022.2100819
[11] Hyder S, Gondal AS, Rizvi ZF et al. Biological control of chili damping-off disease, caused by Pythium myriotylum. Front Microbiol, 2021; 12: 587431. DOI: 10.3389/fmicb.2021.587431
[12] Raza MM, Bebber DP. Climate change, biotic yield gaps and disease pressure in cereal crops. bioRxiv, 2022; 2022: 08. DOI: 10.1101/2022.08.12.503729
[13] Dhanyaa N, Kumar RS, Adhithya V et al. A Review of plant disease detection techniques using artificial intelligence. ICACCS, 2022; 1: 512-515. DOI: 10.1109/ICACCS54159.2022.9785224
[14] Neupane D, Adhikari P, Bhattarai D et al. Does climate change affect the yield of the top three cereals and food security in the world? Earth, 2022; 3: 45-71. DOI: 10.3390/earth3010004
[15] Dutilloy E, Oni FE, Esmaeel Q et al. Plant beneficial bacteria as bioprotectants against wheat and barley diseases. J Fungi, 2022; 8: 632. DOI: 10.3390/jof8060632
[16] Savary S, Willocquet L, Pethybridge SJ et al. The global burden of pathogens and pests on major food crops. Nat Ecol Evol, 2019; 3: 430-439. DOI: 10.1038/s41559-018-0793-y
[17] Ismaila AA, Ahmad K, Siddique Y et al. Fusarium wilt of banana: current update and sustainable disease control using classical and essential oils approaches. Hortic Plant J, 2022; 9: 1-28. DOI: 10.1016/j.hpj.2022.02.004
[18] De Medeiros EV, Lima NT, de Sousa Lima JR et al. Biochar as a strategy to manage plant diseases caused by pathogens inhabiting the soil: a critical review. Phytoparasitica, 2021; 49:713-726. DOI: 10.1007/s12600-021-00887-y
[19] Katan J. Diseases caused by soilborne pathogens: biology, management, and challenges. J Plant Pathol, 2017; 99: 305-315.
[20] El-Sheekh MM, Deyab MA, Hasan RSA et al. Biological control of Fusarium tomato-wilt disease by cyanobacteria Nostoc spp. Arch Microbiol, 2022; 204: 116. DOI: 10.1007/s00203-021-02673-0
[21] Pawaskar M, Kerkar S. Microbial biocontrol agents against chilli plant pathogens over synthetic pesticides: a review. Proc Indian Nat Sci Acad, 2021; 87: 578-594. DOI: 10.1007/s43538-021-00053-2
[22] Sood M, Kapoor D, Kumar V et al. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants, 2020; 9: 762. DOI: 10.3390/plants9060762
[23] Raymaekers K, Ponet L, Holtappels D et al. Screening for novel biocontrol agents applicable in plant disease management-a review. Biol Control, 2020; 144: 104240. DOI: 10.1016/j.biocontrol.2020.104240
[24] Stenberg JA, Sundh I, Becher PG et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J Pest Sci, 2021; 94: 665-676. DOI: 10.1007/s10340-021-01354-7
[25] Collinge DB, Jensen DF, Rabiey M et al. Biological control of plant diseases-what has been achieved and what is the direction? Plant Pathol, 2022; 71: 1024-1047. DOI: 10.1111/ppa.13555
[26] Azeem S, Agha SI, Jamil N et al. Characterization and survival of broad-spectrum biocontrol agents against phytopathogenic fungi. Rev Argent Microbiol, 2022; 54: 233-242. DOI: 10.1016/j.ram.2021.10.005
[27] Cochard B, Giroud B, Crovadore J et al. Endophytic PGPR from tomato roots: isolation, in vitro characterization and in vivo evaluation of treated tomatoes (Solanum lycopersicum L.). Microorganisms, 2022; 10: 765. DOI: 10.3390/microorganisms10040765
[28] Yan Y, Xu W, Chen W et al. Complete genome sequence of Bacillus velezensis YYC, a bacterium isolated from the tomato rhizosphere. Arch Microbiol, 2022; 204: 44. DOI: 10.1007/s00203-021-02709-5
[29] Tariq M, Khan A, Asif M et al. Biological control: a sustainable and practical approach for plant disease management. Acta Agric Scand Section B-Soil Plant Sci, 2020; 70: 507-524. DOI: 10.1080/09064710.2020.1784262
[30] Sharma A, Diwevidi VD, Singh S et al. Biological control and its important in agriculture. Int J Biotechnol Bioengg Res, 2013; 4: 175-180.
[31] Teixidó N, Usall J, Torres R. Insight into a Successful Development of Biocontrol Agents: Production, Formulation, Packaging, and Shelf Life as Key Aspects. Horticulturae, 2022; 8: 305. DOI: 10.3390/horticulturae8040305
[32] He DC, He MH, Amalin DM et al. Biological control of plant diseases: An evolutionary and eco-economic consideration. Pathogens, 2021; 10: 1311. DOI: 10.3390/pathogens10101311
[33] Gong T, Xin XF. Phyllosphere microbiota: community dynamics and its interaction with plant hosts. J Integr Plant Biol, 2021; 63: 297-304. DOI: 10.1111/jipb.13060
[34] Rodríguez M, Torres M, Blanco L et al. Plant growth-promoting activity and quorum quenching-mediated biocontrol of bacterial phytopathogens by Pseudomonas segetis strain P6. Sci Rep, 2020; 10: 4121. DOI: 10.1038/s41598-020-61084-1
[35] Zhang J, Cook J, Nearing JT et al. Harnessing the plant microbiome to promote the growth of agricultural crops. Microbiol Res, 2021; 245: 126690. DOI: 10.1016/j.micres.2020.126690
[36] Li J, Wang C, Liang W et al. Rhizosphere microbiome: The emerging barrier in plant pathogen interactions. Front Microbiol, 2021; 12: 772420. DOI: 10.3389/fmicb.2021.772420
[37] Mohanram S, Kumar P. Rhizosphere microbiome: revisiting the synergy of plant-microbe interactions. Ann Microbiol, 2019; 69: 307-320. DOI: 10.1007/s13213-019-01448-9
[38] Enespa, Chandra P. Tool and techniques study to plant microbiome current understanding and future needs: an overview. Communicative Integrative Biol, 2022; 15: 209-225. DOI: 10.1080/19420889.2022.2082736
[39] Rajput VD, Minkina T, Feizi M et al. Effects of silicon and silicon-based nanoparticles on rhizosphere microbiome, plant stress and growth. Biology, 2021; 10: 791. DOI: 10.3390/biology10080791
[40] Jia T, Yao Y, Wang R et al. Dynamics relationship of phyllosphere and rhizosphere bacterial communities during the development of Bothriochloa ischaemum in copper tailings. Front Microbiol, 2020; 11: 869. DOI: 10.3389/fmicb.2020.00869
[41] Li M, Hong L, Ye W et al. Phyllosphere bacterial and fungal communities vary with host species identity, plant traits and seasonality in a subtropical forest. Environ Microbiome, 2022; 17: 1-13. DOI: 10.1186/s40793-022-00423-3
[42] Legein M, Smets W, Vandenheuvel D et al. Modes of action of microbial biocontrol in the phyllosphere. Front Microbiol, 2020; 19: 1619. DOI: 10.3389/fmicb.2020.01619
[43] Liu H, Brettell LE, Singh B. Linking the phyllosphere microbiome to plant health. Trends Plant Sci, 2020; 25: 841-844. DOI: 10.1016/j.tplants.2020.06.003
[44] Abadi VAJM, Sepehri M, Rahmani HA et al. Role of dominant phyllosphere bacteria with plant growth-promoting characteristics on growth and nutrition of maize (Zea mays L.). J Soil Sci Plant Nutr, 2020; 20: 2348-2363. DOI: 10.1007/s42729-020-00302-1
[45] Kloepper JW, Schroth MN, Miller TD. Effects of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Phytopathology, 1980; 70: 1078-1082.
[46] Volpiano CG, Lisboa BB, São José JFB et al. Soil-plant-microbiota interactions to enhance plant growth. Revista Bras Ciênc do Solo, 2022; 46: e0210098. DOI: 10.36783/18069657rbcs20210098
[47] Ling N, Wang T, Kuzyakov Y. Rhizosphere bacteriome structure and functions. Nat Commun, 2022; 13: 836. DOI: 10.1038/s41467-022-28448-9
[48] Chamkhi I, El Omari N, Balahbib A et al. Is-the rhizosphere a source of applicable multi-beneficial microorganisms for plant enhancement? Saudi J Biol Sci, 2022; 29: 1246-1259. DOI: 10.1016/j.sjbs.2021.09.032
[49] Besset-Manzoni Y, Rieusset L, Joly P et al. Exploiting rhizosphere microbial cooperation for developing sustainable agriculture strategies. Env Sci Poll Res, 2018; 25: 29953-29970. DOI: 10.1007/s11356-017-1152-2
[50] Lahlali R, Ezrari S, Radouane N et al. Biological control of plant pathogens: A global perspective. Microorganisms, 2022; 10: 596. DOI: 10.3390/microorganisms10030596
[51] Ebrahimi-Zarandi M, Saberi Riseh R, Tarkka MT. Actinobacteria as effective biocontrol agents against plant pathogens, an overview on their role in eliciting plant defense. Microorganism, 2022; 10: 1739. DOI: 10.3390/microorganisms10091739
[52] Al-Ani LKT, Aguilar-Marcelino L, Fiorotti J et al. Biological control agents and their importance for the plant health. Microbial Services In Restoration Ecology, 2020; 13-36. DOI: 10.1016/B978-0-12-819978-7.00002-6
[53] Khan MS, Rizvi A, Ahmed B et al. Amelioration of pathogen induced biotic stress to vegetable crops by plant be-neficial bacteria: A Review. J Mod Agric Biotechnol, 2022; 1: 1. DOI: 10.53964/jmab.2022001
[54] Mohammed AF, Oloyede AR, Odeseye AO. Biological control of bacterial wilt of tomato caused by Ralstonia solanacearum using Pseudomonas species isolated from the rhizosphere of tomato plants. Arch Phytopathol Plant Protec, 2020; 53: 1-16. DOI: 10.1080/03235408.2020.1715756
[55] Prihatiningsih N, Asnani ARI, Djatmiko HADI. Extracellular protease from Bacillus subtilis B315 with antagonistic activity against bacterial wilt pathogen (Ralstonia solanacearum) of chili. Biodiversitas J Biol Diversity, 2021; 22: 1291-1295. DOI: 10.13057/biodiv/d220327
[56] Rathore R, Vakharia DN, Rathore DS. In vitro screening of different Pseudomonas fluorescens isolates to study lytic enzyme production and growth inhibition during antagonism of Fusarium oxysporum f. sp. cumini, wilt causing pathogen of cumin. Egy J Biol Pest Control, 2020; 30: 1-8. DOI: 10.1186/s41938-020-00259-4
[57] Yin X, Li T, Jiang X et al. Suppression of grape white rot caused by Coniella vitis using the potential biocontrol agent Bacillus velezensis GSBZ09. Pathogens, 2022; 11: 248. DOI: 10.3390/pathogens11020248
[58] Fatima I, Hakim S, Imran A et al. Exploring biocontrol and growth-promoting potential of multifaceted PGPR isolated from natural suppressive soil against the causal agent of chickpea wilt. Microbiol Res, 2022; 260: 127015. DOI: 10.1016/j.micres.2022.127015
[59] Ghadamgahi F, Tarighi S, Taheri P et al. Plant Growth-Promoting Activity of Pseudomonas aeruginosa FG106 and its ability to act as a biocontrol agent against potato, tomato, and taro pathogens. Biology, 2022; 11: 140. DOI: 10.3390/biology11010140
[60] Srivastava DA, Harris R, Breuer G et al. Secretion-based modes of action of biocontrol agents with a focus on Pseudozyma aphidis. Plants, 2021; 10: 210. DOI: 10.3390/plants10020210
[61] Dalio RJD, Magalhaes DM, Rodrigues CM et al. PAMPs, PRRs, effectors and R-genes associated with citrus-pathogen interactions. Annals Bot, 2017; 119: 749-774. DOI: 10.1093/aob/mcw238
[62] Guzmán-Guzmán P, Santoyo G. Action mechanisms, biodiversity, and omics approaches in biocontrol and plant growth-promoting Pseudomonas: an updated review. Biocontrol Sci Technol, 2022; 32: 527-550. DOI: 10.1080/09583157.2022.2066630
[63] Nguvo KJ, Gao X. Weapons hidden underneath: bio-control agents and their potentials to activate plant induced systemic resistance in controlling crop Fusarium diseases. J Plant Dis Prot, 2019; 126: 177-190. DOI: 10.1007/s41348-019-00222-y
[64] Niwas R, Gupta RN, Rani N. Systemic resistance: Plant responses to interaction with fungal bio-control agents. J Eco-Friendly Agriculture, 2022; 17: 363-368. DOI: 10.5958/2582-2683.2022.00070.3
[65] Bathke KJ, Jochum CC, Yuen GY. Biological control of bacterial leaf streak of corn using systemic resistance-inducing Bacillus strains. Crop Protec, 2022; 155: 105932. DOI: 10.1016/j.cropro.2022.105932
[66] Dimkić I, Janakiev T, Petrović M et al. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms-A review. Physiol Mol Plant Pathol, 2022; 117: 101754. DOI: 10.1016/j.pmpp.2021.101754
[67] Jogaiah S, Abdelrahman M, Tran LSP et al. Different mechanisms of Trichoderma virens‐mediated resistance in tomato against Fusarium wilt involve the jasmonic and salicylic acid pathways. Mol Plant Pathol, 2018; 19: 870-882. DOI: 10.1111/mpp.12571
[68] Thambugala KM, Daranagama DA, Phillips AJL et al. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front Cell Infec Microbiol, 2020; 10: 604923. DOI: 10.3389/fcimb.2020.604923
[69] Latz MAC, Jensen B, Collinge DB et al. Endophytic fungi as biocontrol agents: elucidating mechanisms in disease suppression. Plant Ecol, 2018; 11: 555-567. DOI: 10.1080/17550874.2018.1534146
[70] Köhl J, Kolnaar R, Ravensberg WJ. Mode of Action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front Plant Sci, 2019; 10: 845. DOI: 10.3389/fpls.2019.00845
[71] Alizadeh M, Vasebi Y, Safaie N. Microbial antagonists against plant pathogens in Iran: A review. Open Agric, 2020; 5: 404-440. DOI: 10.1515/opag-2020-0031
[72] Mukherjee PK, Mendoza-Mendoza A, Zeilinger S et al. Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol Rev, 2022; 39: 15-33. DOI: 10.1016/j.fbr.2021.11.004
[73] Sarethy IP, Saharan A. Genomics, proteomics and transcriptomics in the biological control of plant pathogens: A review. Ind Phytopathol, 2021; 74: 3-12. DOI: 10.1007/s42360-020-00302-2
[74] Leng J, Yu L, Dai Y et al. Recent advances in research on biocontrol of postharvest fungal decay in apples. Crit Rev Food Sci Nutr, 2022: 1-14. DOI: 10.1080/10408398.2022.2080638
[75] Villa-Rodriguez E, Lugo-Enríquez C, Ferguson S et al. Trichoderma harzianum sensu lato TSM39: a wheat microbiome fungus that mitigates spot blotch disease of wheat (Triticum turgidum L. subsp. durum) caused by Bipolaris sorokiniana. Biol Control, 2022; 175: 105055. DOI: 10.1016/j.biocontrol.2022.105055
[76] Win TT, Bo B, Malec P et al. Newly isolated strain of Trichoderma asperellum from disease suppressive soil is a potential bio-control agent to suppress Fusarium soil borne fungal phytopathogens. J Plant Pathol, 2021; 103: 549-561. DOI: 10.1007/s42161-021-00780-x
[77] Halo BA, Al-Yahyai RA, Al-Sadi AM. Biological control of Pythium aphanidermatum-induced cucumber and radish damping-off by an endophytic fungus, Cladosporium omanense isolate 31R. Biocontrol Sci Technol, 2021; 31: 235-251. DOI: 10.1080/09583157.2020.1844148
[78] Ketta HA, Hewedy OAER. Biological control of Phaseolus vulgaris and Pisum sativum root rot disease using Trichoderma species. Egypt J Biol Pest Control, 2021; 31: 1-9. DOI: 10.1186/s41938-021-00441-2
[79] Elling AA. Major Emerging Problems with Minor Meloidogyne Species. Phytopathology, 2013; 103: 1092-1102. DOI: 10.1094/PHYTO-01-13-0019-RVW
[80] Jones JT, Haegeman A, Danchin EGJ et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol, 2013; 14: 946-961. DOI: 10.1111/mpp.12057
[81] Kantor M, Handoo Z, Kantor C et al. Top Ten Most Important US-Regulated and Emerging Plant-Parasitic Nematodes. Horticulturae, 2022; 8: 208. DOI: 10.3390/horticulturae8030208
[82] Chitwood DJ. Nematicides. Encyclopedia of Agrochemicals, 2003; 3: 1104-1115. DOI: 10.1002/047126363X.agr171
[83] Al-Ani LKT, Soares F, Sharma A et al. Strategy of nematophagous fungi in determining the activity of plant parasitic nematodes and their prospective role in sustainable agriculture. Front Fungal Biol, 2022; 3: 863198. DOI: 10.3389/ffunb.2022.863198
[84] Pires D, Vicente CSL, Menéndez E et al. The fight against plant-parasitic nematodes: Current status of bacterial and fungal biocontrol agents. Pathogens, 2022; 11: 1178. DOI: 10.3390/pathogens11101178
[85] Girardi NS, Sosa AL, Etcheverry MG et al. In vitro characterization bioassays of the nematophagous fungus Purpureocillium lilacinum: Evaluation on growth, extracellular enzymes, mycotoxins and survival in the surrounding agroecosystem of tomato. Fungal Biology, 2022; 126: 300-307. DOI: 10.1016/j.funbio.2022.02.001
[86] Comans-Pérez RJ, Sánchez JE, Al-Ani LKT et al. Biological control of sheep nematode Haemonchus contortus using edible mushrooms. Biological Control, 2021; 152: 104420. DOI: 10.1016/j.biocontrol.2020.104420
[87] Sarker MS, Mohiuddin KM, Al-Ani LKT et al. Effect of bio-nematicide and bau-biofungicide against root-knot (Meloidogyne spp.) of soybean. Malays J Sustain Agric, 2020; 4: 44-48. DOI: 10.26480/mjsa.02.2020.44.48
[88] Al-Ani LKT, Soares F, Sharma A et al. Strategy of nematophagous fungi in determining the activity of plant parasitic nematodes and their prospective role in sustainable agriculture. Front Fungal Biol, 2022; 3: 863198. DOI: 10.3389/ffunb.2022.863198
[89] Jiang X, Xiang M, Liu X. Nematode-Trapping Fungi. Microbiol Spectr, 2017; 5: 5.1.10. DOI: 10.1128/microbiolspec.FUNK-0022-2016
[90] Liu X, Xiang M, Che Y. The living strategy of nematophagous fungi. Mycoscience, 2009; 50: 20-25. DOI: 10.1007/S10267-008-0451-3
[91] Morton O, Hirsch P, Kerry B. Infection of plant-parasitic nematodes by nematophagous fungi-a review of the application of molecular biology to understand infection processes and to improve biological control. Nematology, 2004; 6: 161-70. DOI: 10.1163/1568541041218004
[92] De Freitas Soares FE, Sufiate BL, De Queiroz JH. Nematophagous fungi: Far beyond the endoparasite, predator and ovicidal groups. Agric Nat Resour, 2018; 52: 1-8. DOI: 10.1016/j.anres.2018.05.010
[93] Mostafanezhad H, Sahebani N, Nourinejhad Zarghani S. Control of root-knot nematode (Meloidogyne javanica) with combination of Arthrobotrys oligospora and salicylic acid and study of some plant defense responses. Biocontrol Sci Technology, 2014; 24: 203-215. DOI: 10.1080/09583157.2013.855166
[94] Seong J, Shin J, Kim K et al. Microbial production of nematicidal agents for controlling plant-parasitic nematodes. Process Biochemistry, 2021; 108: 69-79. DOI: 10.1016/j.procbio.2021.06.006
[95] Li J, Zou C, Xu J et al. Molecular Mechanisms of Nematode-Nematophagous Microbe Interactions: Basis for Biological Control of Plant-Parasitic Nematodes. Annu Rev Phytopathol, 2015; 53: 67-95. DOI: 10.1146/annurev-phyto-080614-120336
[96] Degenkolb T, Vilcinskas A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as alternatives for biological control. Part II: Metabolites from nematophagous basidiomycetes and non-nematophagous fungi. Appl Microbiol Biotechnol, 2016; 100: 3813-3824. DOI: 10.1007/s00253-015-7234-5
[97] Degenkolb T, Vilcinskas A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: Metabolites from nematophagous ascomycetes. App Microbiol Biotechnol, 2016; 100: 3799-3812. DOI: 10.1007/s00253-015-7233-6
[98] Ghahremani Z, Escudero N, Saus E et al. Pochonia chlamydosporia Induces Plant-Dependent Systemic Resistance to Meloidogyne incognita. Front Plant Sci, 2019; 10: 945. DOI: 10.3389/fpls.2019.00945
[99] Isaac GS, El-Deriny MM, Taha RG. Efficacy of Purpureocillium lilacinum AUMC 10149 as biocontrol agent against root-knot nematode Meloidogyne incognita infecting tomato plant. Braz J Biol, 2021; 84: e253451. DOI: 10.1590/1519-6984.253451
[100] Soliman MS, El‐Deriny MM, Ibrahim DSS et al. Suppression of root‐knot nematode Meloidogyne incognita on tomato plants using the nematode trapping fungus Arthrobotrys oligospora Fresenius. J Appl Microbiol, 2021; 131: 2402-2415. DOI: 10.1111/jam.1510
[101] Ebadi M, Fatemy S, Riahi H. Biocontrol potential of Pochonia chlamydosporia var.chlamydosporia isolates against Meloidogyne javanica on pistachio. Egypt J Biol Pest Control, 2018; 28: 45. DOI: 10.1186/s41938-018-0047-y
[102] Vieira Dos Santos MC, Horta J, Moura L et al. An integrative approach for the selection of Pochonia chlamydosporia isolates for biocontrol of potato cyst and root knot nematodes. Phytopathol Mediterr, 2019; 58: 187-199. DOI: 10.14601/Phytopathol_Mediterr-23780
[103] Liu J, Zhu W, Hamid MI et al. Population dynamics and biocontrol efficacy of the nematophagous fungus Hirsutella minnesotensis in pot assay. Nematology, 2016; 18: 823-830. DOI: 10.1163/15685411-00002996
[104] Sankaranarayanan C, Hari K. Integration of Arbuscular Mycorrhizal and Nematode Antagonistic Fungi for the Biocontrol of Root Lesion Nematode Pratylenchus zeae Graham, 1951 on Sugarcane. Sugar Tech, 2021; 23: 194-200. DOI: 10.1007/s12355-020-00876-1
[105] McDonald MR, Ives L, Adusei-Fosu K et al. Ditylenchus dipsaci and Fusarium oxysporum on garlic: One plus one does not equal two. Can J Plant Pathol, 2021; 43: 749-759. DOI: 10.1080/07060661.2021.1910345
[106] Lira VL, Santos DV, Barbosa RN et al. Biocontrol Potential of Fungal Filtrates on the reniform nematode (Rotylenchulus reniformis) in coriander and cowpea. Nematropica, 2020; 50: 86-95.
[107] Tülek A, Kepenekçi I, Oksal E et al. Comparative Effects of Entomopathogenic Fungi and Nematodes and Bacterial Supernatants against Rice White Tip Nematode. Egypt J Biol Pest Control, 2018; 28: 1-6. DOI: 10.1186/s41938-017-0011-2
[108] Zhang X, Zhang H, Jiang Z et al. A new strain of Volutella citronella with nematode predation and nematicidal activity, isolated from the cysts of potato cyst nematodes in China. BMC Microbiol, 2021; 21: 323. DOI: 10.1186/s12866-021-02385-x
[109] Soares FE, de Queiroz JH, de Araújo JV et al. Nematicidal action of chitinases produced by the fungus Monacrosporium thaumasium under laboratorial conditions. Biocontrol Sci Technology, 2015; 25: 337-344. DOI: 10.1080/09583157.2014.979133
[110] Khan, A, Mfarrej MFB, Tariq M et al. Supplementing Pochonia chlamydosporia with botanicals for management of Meloidogyne incognita infesting chickpea. Acta Agric Scand Sect B Soil Plant Sci, 2021; 72: 164-175. DOI: 10.1080/09064710.2021.2003853
[111] Topalovi´c O, Hussain M, Heuer H. Plants and Associated Soil Microbiota Cooperatively Suppress Plant-Parasitic Nematodes. Front Microbiol, 2020; 11: 313. DOI: 10.3389/fmicb.2020.00313
[112] Weng W, Yan J, Zhou M et al. Roles of Arbuscular mycorrhizal Fungi as a Biocontrol Agent in the Control of Plant Diseases. Microorganisms, 2022; 10: 1266. DOI: 10.3390/microorganisms10071266
[113] Huang M, Bulut A, Shrestha B et al. Bacillus firmus I-1582 promotes plant growth and impairs infection and development of the cyst nematode Heterodera schachtii over two generations. Sci Rep, 2021; 11: 14114. DOI: 10.1038/s41598-021-93567-0
[114] Ghahremani Z, Escudero N, Beltrán-Anadón D et al. Bacillus firmus Strain I-1582, a Nematode Antagonist by Itself and Through the Plant. Front Plant Sci, 2020; 11: 796. DOI: 10.3389/fpls.2020.00796
[115] Engelbrecht G, Claassens S, Mienie CMS et al. Filtrates of mixed Bacillus spp inhibit second-stage juvenile motility of root-knot nematodes. Rhizosphere, 2022; 22: 100528. DOI: 10.1016/j.rhisph.2022.100528
[116] Mazzuchelli RCL, Mazzuchelli EHL, de Araujo FF. Efficiency of Bacillus subtilis for root-knot and lesion nematodes management in sugarcane. Biol Control, 2020; 143: 104185. DOI: 10.1016/j.biocontrol.2020.104185
[117] Liu C, Gibson AK, Timper P et al. Rapid change in host specificity in a field population of the biological control organism Pasteuria penetrans. Evol Appl, 2019; 12: 744-756. DOI: 10.1111/eva.12750
[118] Widianto D, Pramita AD, Kurniasari I et al. Bacillus is one of the most potential genus as a biocontrol agent of golden cyst nematode (Globodera rostochiensis). Arch Phytopathol Plant Prot, 2021; 54: 2191-2205. DOI: 10.1080/03235408.2021.1925501
[119] Wang YY, Sikandar A, Zhao YS et al. Effect of Culture Filtrate of Sinorhizobium fredii Sneb183 on the Activity and Behavior of Soybean Cyst Nematode (Heterodera glycines Ichinohe, 1952). Appl Ecol Environ Res, 2020; 18: 1129-1140. DOI: 10.15666/aeer/1801_11291140
[120] Marin-Bruzos M, Grayston SJ, Forge T et al. Isolation and characterization of Streptomycetes and Pseudomonas strains with antagonistic activity against the plant parasitic nematode Pratylenchus penetrans and fungi associated with replant disease. Biol Control, 2020; 158: 104599. DOI: 10.1016/j.biocontrol.2021.104599
[121] Thammaiah N, Shirol AM, Prakash P et al. Management of Burrowing Nematode, Radopholus similis in Banana by Using Biocontrol Agents. J Entomol Zool Stud, 2019; 7: 985-989.
[122] Turatto MF, Dourado FS, Zilli JE et al. Control potential of Meloidogyne javanica and Ditylenchus spp. Using fluorescent Pseudomonas and Bacillus spp. Braz J Microbiol, 2018; 49: 54-59. DOI: 10.1016/j.bjm.2017.03.015
[123] Aballay E, Prodan S, Correa P et al. Assessment of rhizobacterial consortia to manage plant parasitic nematodes of grapevine. Crop Prot, 2020; 131: 105103. DOI: 10.1016/j.cropro.2020.105103
[124] Tilocca B, Cao A, Migheli Q. Scent of a killer: Microbial volatilome and its role in the biological control of plant pathogens. Front Microbiol, 2020; 11: 41. DOI: 10.3389/fmicb.2020.00041
[125] Sdiri Y, Lopes T, Rodrigues N et al. Biocontrol ability and production of volatile organic compounds as a potential mechanism of action of olive endophytes against Colletotrichum acutatum. Microorganisms, 2022; 10: 571. DOI: 10.3390/microorganisms10030571
[126] Kumar V, Singh M, Devi A et al. Microbial Volatile Organic Compounds: Applications and Future Prospects. Asian J Biol Life Sci, 2021; 10: 533-538. DOI: 10.5530/ajbls.2021.10.71
[127] Poveda J. Beneficial effects of microbial volatile organic compounds (MVOCs) in plants. Appl Soil Ecol, 2021; 168: 104118. DOI: 10.1016/j.apsoil.2021.104118
[128] Schulz-Bohm K, Martín-Sánchez L, Garbeva P. Microbial volatiles: small molecules with an important role in intra-and inter-kingdom interactions. Front Microbiol, 2017; 8: 2484. DOI: 10.3389/fmicb.2017.02484
[129] Cordovez V, Mommer L, Moisan K et al. Plant phenotypic and transcriptional changes induced by volatiles from the fungal root pathogen Rhizoctonia solani. Front Plant Sci, 2017; 8: 1262. DOI: 10.3389/fpls.2017.01262
[130] Groenhagen U, Baumgartner R, Bailly A et al. Production of bioactive volatiles by different Burkholderia ambifaria strains. J Chem Ecol, 2013; 39: 892-906. DOI: 10.1007/s10886-013-0315-y
[131] Vlassi A, Nesler A, Perazzolli M et al. Volatile organic compounds from Lysobacter capsici AZ78 as potential candidates for biological control of soilborne plant pathogens. Front Microbiol, 2020; 11: 1748. DOI: 10.3389/fmicb.2020.01748
[132] Chen JL, Sun SZ, Miao CP et al. Endophytic Trichoderma gamsii YIM PH30019: a promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng. J Ginseng Res, 2016; 40: 315-324. DOI: 10.1016/j.jgr.2015.09.006
[133] Ayed A, Kalai-Grami L, Ben Slimene I et al. Antifungal activity of volatile organic compounds from Streptomyces sp. strain S97 against Botrytis cinerea. Biocontrol Sci Technol, 2021; 31: 1330-1348. DOI: 10.1080/09583157.2021.1947982
[134] Montes-Osuna N, Cernava T, Gómez-Lama Cabanás C et al. Identification of volatile organic compounds emitted by two beneficial endophytic Pseudomonas Strains from Olive Roots. Plants, 2022; 11: 318. DOI: 10.3390/plants11030318
[135] Nagrale DT, Gawande SP, Shah V et al. Biocontrol potential of volatile organic compounds (VOCs) produced by cotton endophytic rhizobacteria against Macrophomina phaseolina. Eur J Plant Pathol, 2022; 163: 467-482. DOI: 10.1007/s10658-022-02490-1
[136] Wu Y, Zhou J, Li C et al. Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. Microbiol Open, 2019; 8: e00813. DOI: 10.1002/mbo3.813
[137] Ye X, Chen Y, Ma S et al. Biocidal effects of volatile organic compounds produced by the myxobacterium Corrallococcus sp. EGB against fungal phytopathogens. Food Microbiol, 2020; 91: 103502. DOI: 10.1016/j.fm.2020.103502
[138] Guevara-Avendaño E, Bravo-Castillo KR, Monribot-Villanueva JL et al. Diffusible and volatile organic compounds produced by avocado rhizobacteria exhibit antifungal effects against Fusarium kuroshium. Braz J Microbiol, 2020; 51: 861-873. DOI: 10.1007/s42770-020-00249-6
[139] Safara S, Harighi B, Bahramnejad B et al. Antibacterial activity of endophytic bacteria against sugar beet root rot agent by volatile organic compound production and induction of systemic resistance. Front Microbiol, 2020; 13: 921762. DOI: 10.3389/fmicb.2022.921762
[140] Agisha VN, Kumar A, Eapen SJ et al. Broad-spectrum antimicrobial activity of volatile organic compounds from endophytic Pseudomonas putida BP25 against diverse plant pathogens. Biocontrol Sci Technol, 2019; 29: 1069-1089. DOI: 10.1080/09583157.2019.1657067
[141] Hazarika A, Yadav M, Yadav DK et al. An overview of the role of nanoparticles in sustainable agriculture. Biocat Agric Biotechnol, 2022; 43: 102399. DOI: 10.1016/j.bcab.2022.102399
[142] Huang D, Dang F, Huang Y et al. Uptake, translocation, and transformation of silver nanoparticles in plants. Environ Sci Nano, 2022; 9: 12-39. DOI: 10.1039/D1EN00870F
[143] Liu H, Di Valentin C. Shaping magnetite nanoparticles from first principles. Phys Rev Lett, 2019; 123: 186101. DOI: 10.1103/PhysRevLett.123.186101
[144] Saleem S, Ahmed B, Khan MS et al. Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb Pathog, 2017; 111: 375-387. DOI: 10.1016/j.micpath.2017.09.019
[145] Vallabani NVS, Singh S, Karakoti AS. Magnetic nanoparticles: current trends and future aspects in diagnostics and nanomedicine. Curr Drug Metabol, 2019; 20: 457-472. DOI: 10.2174/1389200220666181122124458
[146] Khan MN, Mobin M, Abbas ZK et al. Role of nanomaterials in plants under challenging environments. Plant Physiol Biochem, 2017; 110: 194-209. DOI: 10.1016/j.plaphy.2016.05.038
[147] Jakhar AM, Aziz I, Kaleri AR et al. Nano-fertilizers: A Sustainable Technology for Improving Crop Nutrition and Food Security. NanoImpact, 2022; 27: 100411. DOI: 10.1016/j.impact.2022.100411
[148] Verma KK, Song XP, Joshi A et al. Recent trends in nano-fertilizers for sustainable agriculture under climate change for global food security. Nanomaterials, 2022; 12: 173. DOI: 10.3390/nano12010173
[149] Balestra GM, Fortunati E. Nanotechnology-Based Sustainable Alternatives for the Management of Plant Diseases. Elsevier. 2021.
[150] Das A, Dutta P. Antifungal activity of biogenically synthesized silver and gold nanoparticles against sheath blight of rice. J Nanosci Nanotechnol, 2021; 21: 3547-3555. DOI: 10.1166/jnn.2021.18996
[151] Kumar P, Pandhi S, Mahato DK et al. Bacillus-based nano-bioformulations for phytopathogens and insect-pest management. Egy J Biol Pest Control, 2021; 31: 1-11. DOI: 10.1186/s41938-021-00475-6
[152] Saleem S, Khan MS. Phyto-interactive impact of green synthesized iron oxide nanoparticles and Rhizobium pusense on morpho-physiological and yield components of greengram. Plant Physiol Biochem, 2023; 194: 146-160. DOI: 10.1016/j.plaphy.2022.11.013
[153] Rui M, Ma C, Hao Y et al. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front Plant Sci, 2016; 7: 815. DOI: 10.3389/fpls.2016.00815
[154] Kumari B, Mallick MA, Solanki MK et al. Applying nanotechnology to bacteria: an emerging technology for sustainable agriculture. In: Role of plant growth promoting microorganisms in sustainable agriculture and nanotechnology. Woodhead Publishing: Duxford, UK, 2019; 121-143. DOI: 10.1016/B978-0-12-817004-5.00008-7
[155] Servin A, Elmer W, Mukherjee A et al. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J Nanopart Res, 2015; 17: 1-21. DOI: 10.1007/s11051-015-2907-7
[156] Poddar K, Sarkar D, Sarkar A. Nanoparticles on photosynthesis of plants: effects and role. Green Nanoparticles, 2020; 7: 273-287. DOI: 10.1007/978-3-030-39246-8_13
[157] Tawfik MM, Mohamed MH, Sadak MS et al. Iron oxide nanoparticles effect on growth, physiological traits and nutritional contents of Moringa oleifera grown in saline environment. Bull Natl Res Cent, 2021; 45: 1-9. DOI: 10.1186/s42269-021-00624-9
[158] Irum S, Jabeen N, Ahmad KS et al. Biogenic iron oxide nanoparticles enhance callogenesis and regeneration pattern of recalcitrant Cicer arietinum L. PLoS One, 2020; 15: e0242829. DOI: 10.1371/journal.pone.0242829
[159] Fira D, Dimkić I, Berić T et al. Biological control of plant pathogens by Bacillus species. J Biotechnol, 2018; 285: 44-55. DOI: 10.1016/j.jbiotec.2018.07.044
[160] Avila-Quezada GD, Golinska P, Rai M. Engineered nanomaterials in plant diseases: can we combat phytopathogens? Appl Microbiol Biotechnol, 2022; 106: 117-129. DOI: 10.1007/s00253-021-11725-w
[161] Moradi Pour M, Saberi Riseh R, Skorik YA. Sodium alginate-gelatin na noformulations for encapsulation of Bacillus velezensis and their use for biological control of Pistachio Gummosis. Mater, 2022; 15: 2114. DOI: 10.3390/ma15062114
[162] Naraghi L, Negahban M. Efficacy of Talaromyces Flavus coated with nanoparticles in the growht inhibitory of Fusarium Oxysporum F. SP. Cucumerinum. 3c Tecnología: glosas de innovación aplicadas a la Pyme, 2020; 9: 31-45. DOI: 10.17993/3ctecno/2020.v9n1e33.31-45
[163] Narendrakumar G, Namasivayam SKR, Manikanta M et al. Enhancement of biocontrol potential of biocompatible bovine serum albumin (BSA) based protein nanoparticles loaded bacterial chitinase against major plant pathogenic fungi Alternaria alternata. Biocat Agric Biotechnol, 2018; 15: 219-228. DOI: 10.1016/j.bcab.2018.05.015
[164] Khan M, Khan AU, Hasan MA et al. Agro-nanotechnology as an emerging field: a novel sustainable approach for improving plant growth by reducing biotic stress. Appl Sci, 2021; 11: 2282. DOI: 10.3390/app11052282
[165] Siddiqui SA, Blinov AV, Serov AV et al. Effect of selenium nanoparticles on germination of Hordéum vulgáre barley seeds. Coatings, 2021; 11: 862. DOI: 10.3390/coatings11070862
[166] Sonawane H, Arya S, Math S et al. Myco-synthesized silver and titanium oxide nanoparticles as seed priming agents to promote seed germination and seedling growth of Solanum lycopersicum: a comparative study. Int Nano Lett, 2021; 11: 371-379. DOI: 10.1007/s40089-021-00346-w
[167] Ukidave VV, Ingale LT. Green synthesis of zinc oxide nanoparticles from Coriandrum sativum and their use as fertilizer on bengal gram, turkish gram, and green gram plant growth. Int J Agron, 2022; 2022: 1-14. DOI: 10.1155/2022/8310038
Copyright © 2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.

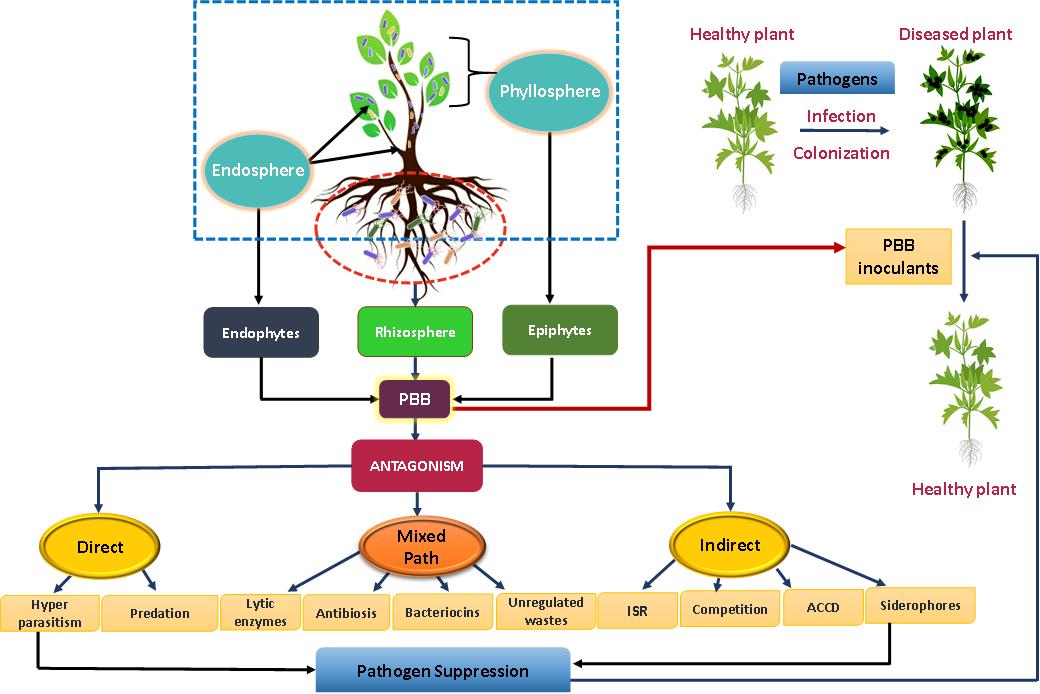
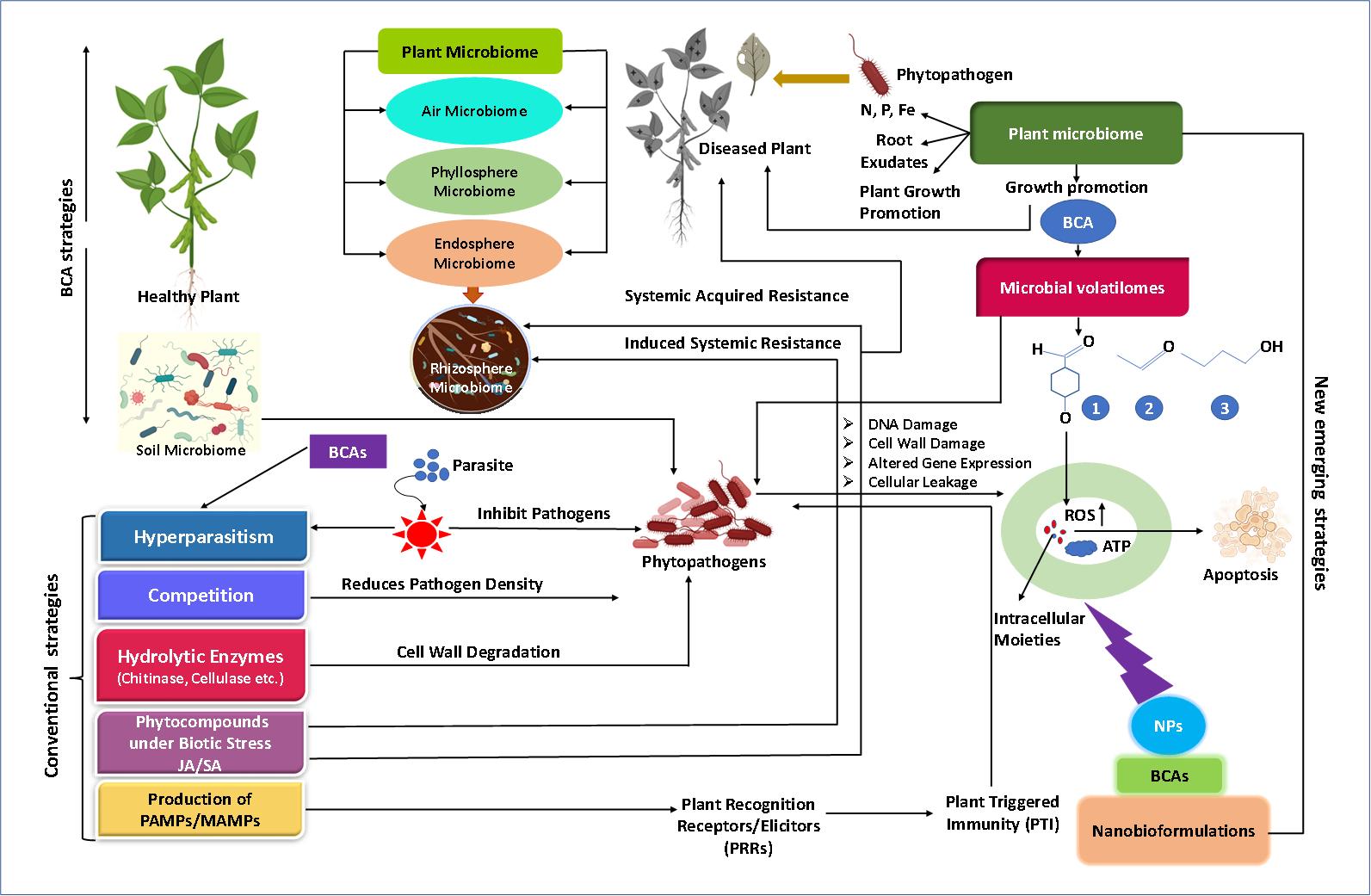




 Copyright ©
Copyright ©