Utilization of Biochar Alone and in Combination with Compost for Removal of Potentially Toxic Metals Accumulated in Soils Associated with Land-use Patterns
Emmanuel Hanyabui1*, Christian Adler Phares1, Emmanuel Botchway1, Atta Kena Sarpong1, Samuel Obeng Apori2, Patrick Ofori Manfo1
1Department of Soil Science, School of Agriculture, University of Cape Coast, Cape Coast, Ghana
2School of Food Science and Environmental Health, Technological University Dublin, Dublin, Ireland
*Correspondence to: Emmanuel Hanyabui, PhD Candidate, Researcher, Department of Soil Science, School of Agriculture, University of Cape Coast, Cape Coast, Ghana; Email: emmanuel.hanyabui@stu.ucc.edu.gh
Abstract
Background: Potentially toxic metals in soils are a threat to food security and human health because it enters the food chain through crop uptake. Hence, it is critical to understand the levels of potentially toxic metals in soils due to agricultural land use patterns and the approach to remove them from the soil.
Objective: This review discussed the effect of different land-use patterns on heavy metal accumulation and their removal using biochar.
Methods: A desktop review employing preferred reporting items for systematic review and meta-analysis was used to analyse information from peer-reviewed papers including journal articles, books, thesis, and reports.
Results: It was shown that potentially toxic metals mainly found in the soil include arsenic (As), copper (Cu), cadmium (Cd), zinc (Zn), chromium (Cr), cobalt (Co), nickel (Ni), antimony (Sb), mercury, thorium (Th), lead, silicon (Si), and selenium (Se). The sources of these potentially toxic metals accumulation in soils were the application of organic and inorganic fertilizers, irrigation, use of pesticides and weedicides, and atmospheric deposition. However, different land-use patterns (greenhouse field, vegetable field soils, forest field, and maize field soil) had a significant accumulation of heavy metals (Cr, Ni, Cu, As, Cd, and Zn) due to increasing crop yield after the use of fertilizers and pesticides. Biochar was found to be effective in the removal of 18 to 40% of these potentially toxic metals from the soil. The mechanisms of removal included precipitation, physical sorption, complexation, ion exchange, and electrostatic interaction.
Conclusion: Biochar applied alone or with compost is highly stable to remove heavy metals accumulated in soils due to land use patterns.
Keywords: land use, heavy metals, potentially toxic metals removal, biochar, compost, mechanisms
1 INTRODUCTION
Potentially toxic metals are the major principal pollutants found in the soil. Their presence in soils affect the quality and functions of soil and largely cause environmental hazard[1-3]. The soil is a non-renewable natural resource that provides various critical ecosystem functions, including supporting the growth of crops and the distribution of water and gases in the environment. It also serves as a buffer in the control of organic and inorganic substances[4,5]. Globally, heavy metal pollution of soil has attracted growing attention[6]. According to He et al.[7], about 10 million soil sites worldwide are polluted, with more than half of these pollution caused by heavy metal. The availability of heavy metals in soils is caused by multiple anthropogenic and natural factors. The toxicity of potentially toxic metals that naturally accumulate in agricultural soils in the environment is often insufficient to cause harm to human health[8]. The principal anthropogenic sources include waste disposal, fertilizer application, long-term wastewater application in agriculture, waste incineration and traffic emission[9,10]. The human use of land and management strategies have been implicated in the degradation of land including the introduction of heavy metals into soils[9]. Agriculture activities, deforestation, urban development, and other human activities have significantly altered the earth’s landscape[11-13]. In developing countries, heavy metal pollution in soils has become a major environmental concern due to the changes in land-use patterns over time[14]. Agriculture is one of the major land-use patterns leading to the contamination of heavy metals in soil, which is associated with the use of fertilizers, pesticides, and herbicides to increase crop yield[15]. Inconsistently, it has been documented that the application of organic and inorganic fertilizers continuously increases soil pollution of heavy metals[16], and these heavy metals contaminate the land from non-point sources when carried in runoff water[16].
Soil pollution due to heavy metal affects soil functions negatively, reduces plant diversity and the quality of crops such as rice, and impacts the evolution of soil microbes and their linked functional genes[17-19]. Accumulation of heavy metals in agricultural soils through various land-use patterns will cause disorder of functions of the soil, which in turn affects crop productivity. However, potentially toxic metals can be transferred to crops, thereby posing a risk to human health[20,21]. The concentration of arsenic (As), chromium (Cr), lead (Pb), cadmium (Cd), Nickel (Ni), zinc (Zn), and copper (Cu) above the threshold level is harmful to the health of humans[22]. To ensure safe and healthy food production, it’s critical to decrease the accessibility of heavy metals and phyto-availability to plants and to restore polluted soils[23]. According to Hayyat et al.[22], in situ method of remediation has been reported in studies about the remediation of soils polluted with potentially toxic metals. Biochar has been studied extensively as an in-situ soil amendment in recent years, and it contributes to removing potentially toxic metals in soils[24,25]. Biochar is a stable solid partially combusted (pyrolyzed) and a recalcitrant compound that improves soil's physical, chemical, and biological properties and carbon (C) stock in the soil[26,27]. Biochar is a carbonaceous sorbent usually prepared from biomass (crop residues), which is formed after specific thermochemical conversions (pyrolysis) under low oxygen (O) supply conditions[28]. Biochar sequesters large amounts of C and has high chemical stability in contaminated soils[22]. Biochar application has been observed to be an effective method for treating soils contaminated with potentially toxic metals, as heavy metals are absorbed through biochar application, bioavailability decreases and toxin-induced stress to plants and living organisms[23,29]. A few studies have looked at the accumulation of potentially heavy metals in soils, but few have done a systematic review of their removal from the soil[30-33]. Research on heavy metals accumulation and the variation in agriculture land use and other land use patterns is of theoretical and practical importance. It helps to optimize land use and prevents extreme accumulation of potentially toxic metals that leads to soil degradation and potentially entering the food chain[34-38]. The review focused on the effect of various land-use patterns on the accumulation of potentially toxic metals or heavy metals in soils and the use of sole application of biochar in a combination of compost for the removal of potentially toxic metals.
2 METHODS
A systematic review of the literature was conducted through the examination of scientific databases and grey literature to obtain scientific evidence relevant to the objective of the study. The results are reported according to Preferred Reporting Items for Systematic Review and Meta-analysis guidance[39].
2.1 Search Strategy
A literature search was conducted across a broad range of online databases, websites, and knowledge repositories such as Scopus, Web of Science, and Google Scholar, which allowed the identification of both peer-reviewed and grey literature. Boolean operators (AND, and OR) were used to broaden the search in combination with keywords and synonyms such as heavy metals, heavy metal accumulation, agricultural land use pattern, accumulation of heavy metals through land use pattern, biochar for heavy metal removal, a combination of biochar and compost, mechanisms for removal of potentially toxic metals through biochar and compost. The results were screened against inclusion criteria by two independent reviewers, with additional supervision by a third investigator. The search was restricted to peer-reviewed studies published in English. References were also cross-checked and screened to be included in the study. An initial check was performed to check repeated articles. The articles obtained were further screened for inclusion and exclusion.
2.2 Inclusion Criteria
All articles involving heavy metals and land-use patterns and written in English were included in the review. Studies focused on the heavy metal accumulation, heavy metals and their accumulation in soils as a result of land use patterns, use of biochar and compost for heavy metal removal, mechanism of heavy metals removal, types of biochars and the rate of application.
2.3 Exclusion Criteria
Articles that were excluded from the research review included studies that focused on mining, soil fertility management, water pollution, and waste management, biochar application for greenhouse gas mitigation. Selected articles were also limited to those that were written in English. Other reasons for exclusion were the impossibility to retrieve the full text and the type of article (systematic reviews or opinion/position papers, guidelines, editorials, or commentary were excluded).
2.4 Selection Process and Data Extraction
The titles and abstracts were read first, followed by reading the full texts in the screening process. The screening process was carried out by all authors (Hanyabui E, Phares CA, Botchway E, Sarpong AK, Apori SO, and Manfo OP) independently and data were extracted. Disagreement was resolved through thorough discussion among the authors until a consensus was reached, and in extreme cases, a colleague with the same or similar field of study was consulted. Data were collected and recorded into a spreadsheet after the final list of suitable full-text articles was defined. The results were synthesized using a narrative synthesis.
3 RESULTS AND DISCUSSION
The literature search yielded 170 articles on Scopus, 268 on Web of Science, and 184 on Google Scholar, among which 120 were removed before screening because of duplication, acceptability, and others, remaining 502 single citations. After title and abstract screening, 209 articles were rejected. Using inclusion and exclusion criteria, 42 articles were included in the qualitative synthesis (Figure 1).
|
Figure 1. Flow chart of article selection process. Source: https://www.bmj.com/content/372/bmj.n71
3.1 Land Uses
Climate change, socioeconomics (culture and population dynamics), and government policy are the key determinants of land use[40]. The use of the land would typically aim to promote social welfare, but its market value is far from being a reliable gauge of its social value, especially in developing nations where completely competitive land markets and rigorous legislative limitations of land use are infrequent[41]. Land use changed quickly in the second half of the 20th century as a result of the implementation of agricultural and economic policies[42]. Due to rural depopulation and the modernization of agriculture, significant changes occurred in human land use in the 20th century (second half). Plants, soil, nutrients, and water are all greatly affected by the way the land is used and managed[15].
The ramifications of changing agricultural land use described in the literature seem to vary by regions[43], which is supported by the fact that studies concentrate on various geographic locations[44,45]. Land-use change usually lead to cultivating management change, which might compromise the soil quality[46-48]. As heavy metals in soil have a direct impact on human health through food production, the level of heavy metals in soil is increasingly emerging as one of the key indicators of soil quality[49]. Currently, researchers have focused on the consequences of land use patterns on soil heavy metal accumulation, with an emphasis on agricultural land uses, including farmland, uncovered vegetable land, orchards and forest land[50-52]. The growing of vegetables in the greenhouse has evolved extremely rapidly in recent years and greatly aided in the supply of agricultural products, particularly vegetables, which is one of the significant land-use patterns in China. Some agricultural practices, such as the excessive and incorrect application of chemical and organic fertilizers, have caused the soil quality in greenhouse vegetable fields to deteriorate and decline[49].
3.2 Potentially Toxic Metals or Heavy Metals
There have been numerous debates over the definition of the term “heavy metals”[53]. Heavy metals were proposed by some researchers based on their high atomic weight, while others on the basis of density, chemical characteristics, toxicity, or density. However, the term "heavy metal" has recently been applied to metallic chemical substances and metalloids that impact the environment negatively and humans[54-56]. Heavy metals, according to Banfalvi[57], are naturally occurring elements with a high atomic weight and a density five times greater than that of water. Examples of heavy metals that are seen in our everyday life are titanium of atomic number 22, vanadium (atomic number 23), Cr (atomic number 24), manganese (atomic number 25), iron (atomic number 26), Co (atomic number 27), Ni (atomic number 28), Cu (atomic number 29) Zn (atomic number 30), As, molybdenum, silver, Cd, tin, platinum, gold, mercury (Hg) and Pb[54-56]. Potentially toxic metals, such as aluminium, Se, and As accumulate in the human body, cause damage to the soft tissues, and also become hazardous to the environment[58]. Metalloids also called trace elements such as Zn and molybdenum, are not hazardous or harmful to the environment, and they are referred to as microelements because of their limited activities in the soil[53].
3.3 Potentially Toxic Metal Sources in Soil
Sources of potentially toxic metals in the soil can be expressed as follows[59].
|
Where;
M is the potentially toxic metal; p is the parent material; a is the atmospheric deposition; f is the source of fertilizers; ag is the source of agrochemicals; ow are the sources of organic waste; ip are other inorganic pollutants; cr is crop removal; l is the losses by leaching, volatilization, etc.
Naturally, potentially toxic metals occur in the soil ecosystem from the weathering phase of the parent material and are regarded as trace (<1,000mg kg−1) and hardly toxic[60]. Cd has been reported to occur naturally in the soil at a concentration ranging between 0.1-1mg kg-1 and is mostly found in sedimentary rocks[61]. Enhanced concentrations of As are estimated from shales, clays and phosphates-bearing minerals, whiles Cr is found in all rocks but high concentrations are reported in mafic and ultramafic rocks[62]. Some of the natural sources of heavy metals are volcanoes, rock disintegration and soil erosion.
Anthropogenic sources of heavy metals in the soil are more mobile and thus more bioavailable than pedogenic heavy metals[63,64]. Potentially toxic metals linked to human activity, including leaded gasoline and Pb-based paints, fertilizer application, animal manure application, biosolids (sewage sludge), compost, pesticides, coal combustion residues, petrochemicals, and atmospheric deposition are partial sources of accumulation in the soil. Other sources include high metal waste disposal in unregulated landfills, leaded gasoline, Pb-based paints, and biosolids[65-67]. Heavy metals such as Cd, Cr, Hg, As, and Pb are associated with human activities. Anthrogenic activities that contribute to heavy metals include incomplete fossil burning, mineral extraction, landfilling, metal refining, electronic goods manufacturing, dyes, agricultural chemicals, military operations and vehicular emissions[68].
3.4 Distribution of Potentially Toxic Metals in Soil
Li et al.[69] studied the distribution and relationship with soil characteristics of the top 70 heavy metals under various land use and found that potentially toxic metals are distributed in soils depending on land use patterns, especially agriculture land use. Other related research has shown that potentially toxic metals in soil exhibit heterogeneous distribution in the vertical and horizontal directions in different ecosystems[70,71]. Ye et al.[72] suggested that the distribution of heavy metals in the soil near a river was high and decreased with the increase of distance from the river. Diane et al.[73] compared the distribution of elemental Pb in the riparian and agricultural land use in southern Canada and found that the concentrations of Pb in the soil of the riparian area were almost 12 times that of those in the agricultural area. Young et al.[74] carried out descriptive statistics for the heavy metal distribution in the agricultural soils of Taiyuan and concluded that out of seven metals, only Ni was normally distributed.
Potentially toxic metals are evenly distributed at the depth of 20cm of the soil profile and most of them extended down between a soil depth of 20 to 40cm, indicating highly varying characteristics nature of the soil profile[75]. Atafar et al.[76] stated that Pb distribution was in the range of 1.6-6.05mg kg-1 soil before fertilization and reached the range of 2.75-12.85 after harvesting, an increase by 2 folds. The distribution of the Mn, Zn, Cu, Ni, Co, Cr, Pb and Cd in soil profiles, and surface soils was investigated. It was shown that the soil-forming processes resulted in a separation of these elements between various soil components, causing differences in the distribution patterns. He concluded that the ionic radius is of major significance for such distribution[77].
3.4.1 Agricultural Land Use and Accumulation of Potentially Toxic Metals
Numerous studies have revealed that the massive accumulation of potentially toxic metals in agricultural soils has contaminated the food chain and become a serious problem for human and livestock consumption despite the wide variances in the distribution of heavy metals in soils[76-79]. The accumulation of heavy metals mostly occurs in surface soils and may result in deleterious effects on most plant species without any detectable effect on groundwater quality[80]. Cd and Pb are the two types of heavy metals that accumulate more readily in surface soils but significantly decrease in the lower horizons[81]. Recent studies in agricultural soils have indicated that potentially toxic metal accumulation has exceeded its thresholds[82-86]. Organic and inorganic fertilizer applications containing heavy metals are the main sources of potentially toxic metal accumulation in those soils. In addition, the application of pesticide-containing heavy metals, such as mancozeb, has been implicated to contribute substantially to heavy metal accumulation in the soils[49]. The variations in the concentration of the heavy metals could be attributed to the different rates of fertilizer application under different land-use patterns. Trace metals are essential nutrients but are required in small quantities. The deficiency of trace elements (Cu, Co, Fe, Mn, Mo, Ni and Zn) negatively affects the growth of plants[87]. The concentration of trace metals could be increased in deficient soils and improve crop yield using inorganic fertilizers[87]. Wuana et al.[88] posited that Cu deficient soils for cereal production are occasionally treated with Cu whilst Mn is supplied to root crops. Additionally, farmers apply large amounts of fertilizers to the soil in intensive farming systems to provide adequate N, P, and K for crop growth[88]. Heavy metals such as Cd and Pb are present in the compounds used to produce these elements at minor levels. Their levels in the soil may be much higher due to the continuous application of fertilizers[89]. Wuana et al.[88] reported that most weedicides used in agriculture productivity and horticulture contain substantial concentrations of heavy metals. The chemicals used to formulate the pesticides contain Cu, Hg, Mn, Pb, or Zn. Therefore, continuous application of pesticides, weedicides, fungicides, and insecticides in various fields results in the accumulation of heavy metals. Cu accumulation in the soil is mainly attributed to agricultural activities such as the continuous application of Cu-based fungicides and pesticides[90]. Application of biosolids such as manures, compost, and municipal sewage sludge to the soil inadvertently leads to heavy metal accumulation[88], though they serve as an organic amendment for soil fertility improvement.
Globally, twenty million hectares of cultivable land are irrigated with wastewater. According to studies, wastewater irrigation-based agriculture provides 50% of the urban areas' supply of vegetables in some Asian and African towns[91]. In general, farmers are more concerned with increasing their yields and earnings than with environmental advantages or risks. Despite the typically low levels of heavy metals in wastewater effluents, irrigation of the land for an extended period with wastewater can eventually lead to heavy metal buildup in the soil[88]. Different land-use patterns (greenhouse field, vegetable field soils, forest field, and maize field soil) have a significant difference in the accumulation of potentially toxic metals of As, Cr, Ni, Cu, Cd, and Zn but did not show any effect on the accumulation of Pb[49]. When heavy metal accumulation exceeds the standard, soil contamination occurs and negatively affects the sustainable development of the ecological environment and social economy[31,92]. Huang and Jin[93] concluded in their work that the accumulation of heavy metals in soils is significantly affected by land use, especially in agricultural lands.
Barman et al.[94] observed that the transfer of potentially toxic metals in the soil to part of a plant did not follow any trend and varied with the type of heavy metal, species and plant parts. Again, Barman et al.[94] observed in their studies that out of 32 plant samples analyzed, the percentage of the sample showing metal accumulation ratio (soil to other parts of the plant) ≥1 is as follows in descending order; Fe (84.0%)>Cu (81.3%)>Ni (59.3%)>Cr (46.9%)>Zn (31.3%)>Pb (17.4%)>Cd (9.4%). The ratio >1 indicates a very high accumulation in the plant tissues than in soil.
3.5 Types of Biochar
3.5.1 Agricultural and Forestry Waste Biochar
Waste generated from agricultural activities and forestry can be used to produce biochar. This type of biochar has gained popularity in the area of pollution control due to its low cost and ease of acquisition[95]. Biochar made from agricultural wastes such as seed shells[96], corn cob[97], corn straw[98], and potassium-iron rice straw[98] could effectively remove Cu2+ and Pb2+ ions from the soil, with removal capacities of 1.67, 2.08, and 0.41mmol/kg, respectively.
3.5.2 Wood Biochar
Studies have demonstrated that natural wood and waste wood are essential and common resources used to produce biochar. Some trees can survive all year round and can grow well even in hard situations, representing a plentiful source of the material. Recently, eucalyptus[99] and mulberry[100] have been used to prepare biochar, and biochar made from these materials are considered effective adsorbents for removing harmful chemicals from contaminated soil[101]. When utilizing biochar made from eucalyptus and pine, rates of Cu and Pb removal from the soil were 93% and 90%, respectively[102,103].
3.5.3 Industrial Waste Biochar
Industrial organic waste, such as sludge[104], and municipal solid trash comprise the majority of industrial waste[105]. From 2010 to 2019, research on biochar production from industrial waste increased by 70%. Biochar produced from industrial waste at a temperature of 750°C was observed as an efficient adsorbent for Cu2+ removal, with a maximum adsorption capacity of 18.5mg/g[106].
3.6 Characteristics of Biochar
Numerous studies have used the initial biomass feedstock and its biochar to illustrate the basic physicochemical properties of both raw and pyrolyzed content[107,108]. Zhang et al.[95] reported that biochar from feedstocks varies greatly in chemical characteristics due to the type of feedstock. The fundamental elements of biochar are C, hydrogen (H), O, S, and nitrogen (N). It is also known that biochar contains fixed C, which is used to estimate the amount of carbonaceous compounds present in the biochar solids. Van Krevelen's diagrams demonstrated the inconsistency of using the H/C and O/C ratios to calculate aromaticity and maturity degrees[108]. Basic O-H, O-C, and C-H rations have been established to determine the degree of pyrolysis and the amount of biochar oxidative modification in soil and solution systems[109,110]. However, it has also been shown that biochar contains functional groups such as carboxylic acids, lactic acids and phenols Due to the presence of tube fractures that were initially created by plant cells, biochar produced at various pyrolytic temperatures has a characteristic shape resembling honeycombs. Brunauer, Emmett, and Teller are widely distributed in biochar as a result of these well-developed pores[111,112]. Applied biochar produced at a low pyrolysis temperature in combination with inorganic fertilizer is considered suitable as it regulates nutrient release[111,112]. Additionally, biochar prepared at low temperatures is much more stable than biochar prepared at high temperatures. Once integrated into the soil, however, the porous structure becomes unstable and the fine fractions are abraded[108].
3.6.1 Biochar for Removing Heavy Metals Accumulated in the Soil
Heavy metals and their concentration can be removed from the soil following biochar application. Biochar is a stable material, mostly carbonaceous, produced from pyrolysis and gasification of biomass[113]. Due to the polar functional groups, large surface area and transition metals of biochar, it can absorb heavy metals through a variety of adsorption processes[95,113]. When it comes to heavy metal removal in soil, biochar is non-selective and is therefore beneficial for heavy metals accumulated in soils due to land use patterns[114]. The biomass used to produce the biochar, pyrolysis temperature and the method of production has a major impact on the biochar properties[113,114]. As, Cu, Cd, Zn, Cr, Co, Ni, Sb, Hg, Th, Pb, Si, and Se are the potentially toxic metals that can be found in the soil[113]. Their accumulation in the soil can be toxic to human and plant life. To produce biochar that can be used to remove heavy metals from the soil, the type of biomass and reactivity should be taken into consideration[115]. Also, the physical properties of biochar will change if the temperature during the pyrolysis is increased[113,116]. When the pyrolysis temperature is increased between 400°C to 900°C, the biochar surface area also increases from 0.1 to 3.2-100-500m2/g[117]. The unique characteristic of biochar (ion exchange and sieve-like nature) is the ability to absorb and trap heavy metals accumulated in soils[113].
The application of biochar for heavy metals removal can be done in three different ways, including functional group complexation, the release of cations, and physical adsorption or surface precipitation[116]. For functional group complexation, heavy metals react with the hydroxyl functionalities on the biochar surface[116]. A metal cation such as Ca2+ or Mg2+, which may be present in the structure of the biochar, exchanges heavy metals like Pb2+ for the process of ion exchange[116]. The amorphous nature of biochar traps heavy metals during the physical adsorption or surface precipitation process[116]. Biochar applied, which is pyrolyzed at a temperature of 900°C, can decrease Cu, Zn, As, Pb, Cd, and Cr from 91.65 to 9.44wt%, 98.82 to 63.34wt%, 97.91 to 52.11wt%, 55.91 to 4.87wt%, and 73.51 to 9.57wt%, respectively[116,118]. When the pyrolysis temperature of biochar increases, its adsorption capability gets better. This is because biochar pyrolyzed at higher temperatures has a higher concentration of functional groups that contain O on their surface[116]. Results from an experiment conducted by Liang et al.[119] using rice husk biochar produced at a temperature of 500°C to remove heavy metals accumulated in wetland surrounding soil showed that biochar could remove or reduce heavy metals. because the reason is that the concentration of heavy metals such as Cd, Cu and Zn reduced from 5.59 to 4.73mg kg-1, 53.9 to 51.57mg kg-1, and 210.82 to 194.59mg kg-1, respectively. Hu and Gholizadeh[116] reported that as a result of a large number of polar functional groups in biochar, such as carboxyl, hydroxyl, and carboxyl groups, the heavy metal ions, was adsorbate by physical sorption and complexation mechanisms. They added that the immobilization of heavy metals in the soil by biochar was also improved by ion exchange, precipitation, and the trapping of the potentially toxic metals in the nanopores of the material. Application of biochar can effectively reduce bio-availability of Pb, Zn, Ni, Cd, and Cu[120].
Different materials used to produce biochar cannot have the same efficiency to remove heavy metals from the soil, which may be attributable to the different structures and stability produced by biochar produced. This is consistent with the study conducted by Wang et al.[71] where biochar applied reduced heavy metals (Cd, Cr, Hg, and Pb) accumulated in the soil considerably. They used biochar produced from pig manure and corn straw, and it was noticed that the concentration of Hg (0.79mg/kg) was reduced by pig manure biochar to 0.34mg/kg and corn straw biochar to 0.59mg/kg. Biochar produced from pig manure was observed to remove more heavy metals from the soil systems due to its higher surface area (surface area for corn straw and pig manure biochar samples was 10.7 and 26.8m2/g, respectively)[116].
3.6.2 Mechanisms of Biochar for Heavy Metal Removal in Soil
Immobilization of heavy metal activities in the soil is achieved through the exchange adsorption of biochar surfaces. The higher the cation exchange, the heavier metals are retained[109,121]. Ion exchange occurs when positive modifications in the soil and negative charge groups on the biochar surface contact electrostatically[122]. This type of reaction falls under nonspecific adsorption and is reversible due to its lower adsorption energy. The cationic function is determined according to the way the biochar is aromatised[122]. The ability to lose electrons from functional groups increases and the impact of adsorption becomes more substantial as their conjugate aromatic structure is present to a larger extent[123]. According to Wang et al.[122], adsorption and dissolution-precipitation of mineral constituents in biochar could effectively reduce heavy metal activity. Soil pH can increase through biochar application and the reaction of heavy metal ions with -OH, PO43-, and CO32- can form hydroxide, carbonate or phosphate precipitation, effectively increasing heavy metal concentrations[121,122]. Biochar complexation is important for the fixing of heavy metal ions with high affinity[122]. Studies indicated that the reactions of oxygenic functional groups such as hydroxyl group (-OH), carboxyl group (-COOH) and amino group (-NH2) with heavy metals on the surface of biochar contribute significantly to the adsorption of heavy metal ions[123,124]. Biochar applied can absorb heavy metal contaminants more effectively and remove them from the soil because of their larger surface area and increased surface energy[122]. Many factors influence the effect of the adsorption of biochar on heavy metal ions, including biochar source materials, pyrolysis temperature, soil pH, physical and chemical properties of heavy metal ions, and the biochar application rate[122]. Under identical conditions, despite the biggest surface area, biochar produced from animal manure outperforms sludge biochar and plant biochar in terms of heavy metal ion adsorption. This is because P-rich biochar from animal feces can precipitate or coprecipitate with particular heavy metal ions, making the biggest contributions to the healing process.
3.7 Characteristics of Compost
One of the most efficient ways to benefit from organic waste is by converting it into useful amendments such as compost for soil fertility improvement[125]. Due to the growing demand for ecologically friendly methods of treating waste and organic agriculture products, interest in composting has recently surged[125]. Composting is a more effective technique of waste disposal that enables the recycling of organic matter, without mentioning that it is environmentally friendly to dispose of waste[126]. The utilization of compost prepared from manure has captured great attention recently. The management of the composting process depends on the succession of mesophilic and thermophilic microorganisms, both of which are active in the process[127]. The quality of composts made from various organic wastes varies, and depending on the composition of raw material and the composting process utilized[128]. The quality of compost is mostly dependent on its stability and maturity. Compost maturity and stability are related to phytotoxicity and the activities of microorganisms. Morel et al.[129] reported that the population of microorganisms, monitoring biochemical properties of microbial activity and biodegradable ingredients analysis are biological activities of compost, which can be used to determine the compost’s maturity.
3.7.1 Compost Application for Heavy Metal Removal
The presence of humic substances, mineral ions, and microorganisms in compost can decrease the risk of heavy metals' ecological and environmental effects and their immobilization in soils for agricultural productivity[130,131]. Composting may facilitate to lower the risk of agricultural failure, financial losses, and heavy metal exposure dangers to people. Compost is considered a fantastic waste management choice worldwide. Following the composting process, organic wastes lose most pathogens and parasites, lose weight and have their phytotoxicity from heavy metals and organic contaminants discharged[132,133]. In agriculture, compost has been used as an alternative to synthetic fertilizers. Evidence indicated that the use of compost improved the soil's physical characteristics and fertility, enhanced microbial activity and crop biomass, and enhanced crop development[134-136]. To reduce or eliminate heavy metals that built up in the soil through agricultural land use, composting is a cheap, extremely useful, and environmentally beneficial method.
The majority of research found that the addition of compost to soils can reduce or immobilize heavy metals in agricultural soil by altering the physical and chemical properties of the soil and reacting with the heavy metals[137,138]. The majority of research found that adding compost to soils can immobilize or reduce heavy metals in agricultural soils by altering the physical and chemical properties of the soil and interacting with the heavy metals[139,140]. However, the risk associated with applying compost in agriculture cannot be overlooked. Irfan et al.[141] found that the application of compost at the rate of 0.5, 1, 2, and 4% to artificially contaminated soil reduced the concentrations of Pb, Cd, and Cr, respectively. The level of lead decreased from 18.26 to 7.13mg k/g when 4% of compost was applied. Also, the concentration of Cd in soil was decreased from 9.33mg k/g to 5.36mg k/g at a 4% rate of compost application, and the Cr content was reduced at 4% compost application from 18.47mg k/g to 7.34mg k/g. The higher (amount) the compost application rate, the more the remediate or reduction of heavy metals[141]. The different methods used for removal of heavy metals accumulated in soil are presented in Figure 2.
|
Figure 2. A flowchart showing different methods used for heavy metal removal accumulated in the soil. Source: https://linkinghub.elsevier.com/retrieve/pii/S209549561830901X
3.7.2 Mechanism of Compost for Heavy Metal Removal in Soil
The application of compost serves as a bio sorbent to absorb potentially toxic metals[142,143], and the composting capacity of adsorption capacity can be assessed using kinetics of adsorption and experiment involved with adsorption isotherms[144-146]. Simantiraki and Gidarakos[147] reported that compost has a better removal capacity than zeolite and is reported to reduce or remediate the availability of heavy metals in water through chemical adsorption by 85-89%. The availability of potentially toxic metals in soil can be remediated using compost by altering the physicochemical characteristics (such as pH, oxidation-reduction potential, and organic matter content of soils. This will also help soil particles to more effectively bind with potentially toxic metals[148,149]. Predominantly, the application of compost immobilizes potentially toxic metals through the inorganic component, substance of humus, and microorganisms[150-152]. They further explained that the large number of organic functional groups (carboxyl, carbonyl, and phenols) is ascribed to the abundance of humus in the compost which binds metal ions through complexation. Furthermore, compost shows diverse affinities to different potentially toxic metals. According to Chien et al.[153], the linkage of humic substances with potentially toxic metals followed an increasing order: Pb>Cu>Cd>Zn. Because of the composition of humic (hydrophilic and hydrophobic compounds), it can act as surfactants[133].
Applied compost can immobilize potentially toxic metals through biosorption and biomineralization due to the availability of microorganisms in the compost[154,155]. Some isolated microbes (Penicillium chrysogenum, Graphiumputredinis, Fusarium solani) that are present during the composting process is able to absorb more than 90% of lead in the soil[156]. The presence of manganese, iron and aluminium in the compost can retain heavy metals regularly[157,158].
Removal of potentially toxic metals in soil with different biochar types and their mechanisms are presented in Table 1[159-174].
Table 1. Removal of Potentially Toxic Metals in Soil with Different Biochar Types and Their Mechanisms
Ref. |
Biochar Type |
Heavy Metals |
Matrix |
Adsorption Mechanism |
Lu et al.[159] |
Sludge biochar |
Pb2+ |
Soil |
Complex reaction with hydroxyl (-OH) and carboxyl (-COOH) Precipitation and complexation |
Cao and Harris[160] |
Dairy manure |
Pb2+ |
Soil |
Ion exchange, adsorption, and precipitation with PO43-, CO32- |
Liang et al.[161] |
Dairy manure, rice straw |
Pb2+ |
Soil |
Electrostatic adsorption, ion exchange |
Xie et al.[162] |
Walnut green husk |
Pb2+ |
Soil |
As a result of the aromatic structure, it reacts with the heavy metal and ion exchange with functional containing O groups |
Liu et al.[163] |
peanut shell, Chinese medicine residue |
Pb2+ |
Soil |
Complexation, ion exchange, electrostatic adsorption, and pH increase make the Pb2+ -carbonate bounded state to changed into Pb2+ insoluble phosphate and silicate state |
Kong et al.[164] |
Beanpoles |
Hg2+ |
Soil |
Precipitation, forming Hg (OH)2, HgCl2 |
Dong et al.[165] |
Brazilian pepper |
Hg2+ |
Soil |
A composite reaction with hydroxyl (-OH) and carboxyl (-COOH) reacts with an aromatic structure to form Hg-π |
Xu[166] |
Sugarcane, walnut wood chips |
Hg2+ |
Soil |
Composite reaction with hydroxyl (-OH) and carboxyl (-COOH), ion exchange, electrostatic adsorption |
Zhao[167] |
Rice husk and rice straws |
Hg2+ |
Soil |
Composite reaction with hydroxyl (-OH) and carboxyl (-COOH), ion exchange |
Ippolito et al. [168] |
Broiler litter |
Cu2+ |
Soil |
Complexation with functional groups to form Cu3(CO3)2 (OH)2, CuO |
Mohan et al.[169] |
Oak biochar |
Cr (VI) |
Soil |
Deoxidize Cr (VI) into Cr (VIII) complex reaction with hydroxyl (-OH) and carboxyl (-COOH) |
Yang et al.[74] |
Sugarcane leaves, tapioca stem, rice straw, silkworm excrement |
Cd2+ |
Soil |
Electrostatic adsorption precipitate with CO32-, OH- |
Zhang[146] |
Maize straw |
Cd2+ |
Soil |
Electrostatic adsorption, precipitation) |
Guan et al.[170] |
Pine needle, maize straw, dairy manure |
As5+ |
Soil |
Electrostatic adsorption |
Huang et al.[171] |
Maize straw |
As3+ |
Soil |
Non-electrostatic physical reversible adsorption and chemical irreversible adsorption with polar groups |
Wang et al.[172] |
Hardwood |
As3- |
Soil |
Increase the solubility of As3- |
Wu et al.[173] |
Almond Putamina, reed straw |
Ni2+ |
Soil |
Composite reaction with hydroxyl (-OH) and carboxyl (-COOH) |
Wang et al.[71] Chen et al.[174] |
Water hyacinth Hardwood |
Zn2+ |
Soil |
Electrostatic adsorption and ion exchange |
Xu[166] |
Dairy manure |
Pb2+, Cu2+, Zn2+, Cd2+ |
Soil |
Oxygenic functional groups and precipitate with PO43-, CO32- |
Xu[166] |
Rice husk |
Pb2+, Cu2+, Zn2+, Cd2+ |
Soil |
Phenolic hydroxy group with surface complex with |
The removal of potentially toxic metals in soils using different types of compost and their mechanisms are showed in Table 2[175-180].
Table 2. Removal of Potentially Toxic Metals in Soil With Different Types of Compost and Their Mechanisms
Feedstock type |
Heavy Metals |
Effect |
Effective Mechanisms |
Ref. |
Municipal organic waste (MOW) and Domestic organic waste |
Zn, Cu, Ni, Pb, Cd |
Compost produced from DOM and MOW can absorb heavy metals |
Process of adsorption |
Venegas et al.[175] |
Municipal solid waste |
Cu, Cd, Pb, Ni, Zn, Cr |
No significant influence on mobility factor of metals |
Both soluble and insoluble complexes were formed with organic compounds |
Achiba et al.[176] |
Green waste |
Cu, As Pb |
Mobility and solubility of As and other metals were increased |
Organic C and Fe in soil pore water were enhanced |
Clemente et al.[177] |
Animal manure and Green waste |
As, Cu |
Was able to reduce the mobility and leachability of Cu but not As |
Surface complexation |
Tsang et al.[178] |
Market waste |
As |
As levels in soil pore water were improved |
Availability of phosphorous improved and increase in water-soluble Fe and C in pore water |
Hartley et al.[179,180] |
The application of biochar and compost together for the removal of potentially toxic metals in soils and their mechanisms are presented in Table 3[181-186].
Table 3. Combined Application of Biochar and Compost for Potentially Toxic Metals Removal in Soil and Their Mechanisms
Combinations |
Heavy Metals |
Mechanisms |
Ref. |
Biochar + Compost |
Cu, Pb |
The high P and Fe content in the compost and biochar increased the soil organic matter content, soil pH |
Karami et al.[181] |
Biochar + Compost |
Zn, Cd, Cu, Pb |
Free ions form a complex with organic ligands and heavy metals exchange with Ca2+, Mg2+, and other cation associated |
Zeng et al.[182] |
Biochar + Compost |
Cu, Ni, Pb, Zn |
The pH of the soil increase and stable compounds between organic materials and heavy metals are formed. |
Rodríguez-Vila et al.[183,184] |
Biochar + Compost |
As, Zn, Cd, Cu |
Soil pH increases and dissolved organic C affects soluble phosphorous on As |
Beesley et al.[185] |
Biochar + Compost |
Pb, Cd, Cu, Zn, As |
Soil pH increases, dissolved organic C, and soluble phosphorous |
Beesley et al.[186] |
3.8 Biochar and Compost
The synergy between biochar and compost has the efficiency to remove heavy metals accumulated in soils[187]. An experiment conducted by Tang et al.[187] applied biochar (rice straw biochar at a pyrolysis temperature of 500°C) and compost (compost produced from rice straw, vegetable leaves, etc) at the same rate and showed that integration of biochar and compost, sole application of compost, and sole application of biochar reduced Cd by 87.1%, 69.6% and 65.8% respectively. Hu and Gholizadeh[116] reported that through electrostatic interactions and chelation processes, the high surface area and high amount of polar functional groups in biochar could immobilize the heavy metals. Due to the presence of humic substances, composting leads to improved stabilisation of heavy metal organometallic compounds.
4 CONCLUSION
The present review reveals that the accumulation of potentially toxic metals in agricultural soils is significant and has exceeded thresholds. This is associated with various agricultural land use patterns which are currently practiced. It was noticed that heavy metal accumulation through agricultural land uses is associated with the application of chemical and organic fertilizers, pesticides and herbicides by farmers to increase crop yield. Applying pesticides-containing heavy metals, such as mancozeb, is implicated to contribute substantially to heavy metal accumulation in the soils. Chemicals used to formulate the pesticides contain Mn, Cu, Hg, Pb, or Zn. Therefore, the continuous application of weedicides, pesticides, fungicides, and insecticides, in various fields leads to the accumulation of heavy metals in soils. As heavy metals are accumulated in the soil, biochar and compost application are available to remove the accumulated heavy metals. Biochar has a high surface area and includes abundant polar functional groups and transition metals, which can easily absorb potentially toxic metals through different adsorption mechanisms. The complexation, cationic and physical adsorption of biochar's functional groups allows for the potential removal of accumulated heavy metals. Considering the compost, its application can remediate potentially toxic metals in agricultural soils by changing the physical and chemical properties of soils and reacting with potentially toxic metals. Sometimes compost applications also introduce some potentially toxic metals into the soil. But the synergy between the biochar and compost helps to immobilize the heavy metals accumulated. The high levels of polar functional groups in biochar can immobilise potentially toxic metals through electrostatic interactions and chelation mechanisms, and the humus in the compost also enhances the stable organometallic complexes of potentially toxic metals.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
All the authors contributed to the conceptualization and methodology; Hanyabui E, Phares CA and Botchway E contributed to the definition of the search strategy, article screening, data extraction; all the authors contributed to original draft preparation, review and editing. The manuscript was read by all the authors and agreed to be published.
Abbreviation List
As, Arsenic
C, Carbon
Cd, Cadmium
Co, Cobalt
Cr, Chromium
Cu, Copper
H, Hydrogen
Hg, Mercury
N, Nitrogen
Ni, Nickel
O, Oxygen
Pb, Lead
Sb, Antimony
Se, Selenium
Si, Silicon
Th, Thorium
Zn, Zinc
References
[1] Zhang S, Lin H, Deng L et al. Cadmium tolerance and accumulation characteristics of Siegesbeckia orientalis L. Ecol Eng, 2013; 51: 133-139. DOI: 10.1016/j.ecoleng.2012.12.080
[2] Steffan JJ, Brevik EC, Burgess LC et al. The effect of soil on human health: An overview. Eur J Soil Sci, 2018; 69: 159-171. DOI: 10.1111/ejss.12451
[3] Feszterová M, Porubcová L, Tirpáková A. The monitoring of selected heavy metals content and bioavailability in the soil-plant system and its impact on sustainability in agribusiness food chains. Sustainability, 2021; 13: 7021. DOI: 10.3390/su13137021
[4] Brady NC, Weil RR. The nature and properties of soils. Pearson Education: New York, USA, 2008.
[5] Nuralykyzy B, Wang P, Deng X et al. Heavy metal contents and assessment of soil contamination in different land-use types in the qaidam basin. Sustainability, 2021; 13: 12020. DOI: 10.3390/su132112020
[6] Liu SH, Zeng GM, Niu QY et al. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour Technol, 2017; 224: 25-33. DOI: 10.1016/j.biortech.2016.11.095
[7] He Z, Shentu J, Yang X et al. Heavy metal contamination of soils: Sources, indicators, and assessment. J Environ Indic, 2015; 9: 17-18.
[8] Guo YB, Feng H, Chen C et al. Heavy metal concentrations in soil and agricultural products near an industrial district. Pol J Environ Stud, 2013; 22: 1357-1362.
[9] Kachenko AG, Singh B. Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Poll, 2006; 169: 101. DOI: 10.1007/s11270-006-2027-1
[10] Svendsen ML, Steinnes E, Blom HA. Vertical and horizontal distributions of Zn, Cd, Pb, Cu and Hg in uncultivated soil in the vicinity of a Zinc smelter at Odd, Norway. Soil Sediment Contam, 2007; 16: 585-603. DOI: 10.1080/15320380701623644
[11] Khormali F, Ajami M, Ayoubi S et al. Role of deforestation and hillslope position on soil quality attributes of loess-derived soils in Golestan province, Iran. Agr Ecosyst Environ, 2009; 134: 178-189. DOI: 10.1016/j.agee.2009.06.017
[12] Ayoubi S, Khormali F, Sahrawat KL et al. Assessing impacts of land uses change on soil quality indicators in a loessial soil in Golestan province, Iran. J Agr Sci Tech, 2011; 13: 727-742.
[13] Khormali F, Nabiollahi K. Degradation of mollisols in western iran as affected by land use change. J Agr Sci Tech, 2009; 11: 363-374.
[14] Adriano DC. Trace elements in terrestrial environments: Biogeochemistry, bioavailability and risks of metals. Springer: New York, USA, 2001. DOI: 10.1007/978-0-387-21510-5
[15] Ghorbani H, Hafezi Moghadas N, Kashi H. Effects of land use on the concentrations of some heavy metals in soils of Golestan province, Iran. J Agr Sci Tech Vol, 2018; 17: 1025-1040.
[16] Mendez A, Paz-Ferreiro J, Araujo F et al. Biochar from pyrolysis of drinking paper sludge and its use in the treatment of a nickel polluted soil. J Anal Appl Pyrolysis, 2014; 107: 46-52. DOI: 10.1016/j.jaap.2014.02.001
[17] Wang Y, Wang HS, Tang CS et al. Remediation of heavy-metal-contaminated soils by biochar: A review. Environ Geotech, 2019; 9: 135-148. DOI: 10.1680/jenge.18.00091
[18] Deng M, Zhu Y, Shao K et al. Metals source apportionment in farmland soil and the prediction of metal transfer in the soil-rice-human chain. J Environ Manag, 2020; 260: 110092. DOI: 10.1016/j.jenvman.2020.110092
[19] Huang J, Wang C, Qi L et al. Phosphorus is more effective than nitrogen in restoring plant communities of heavy metals polluted soils. Environ Pollut, 2020; 266: 115259. DOI: 10.1016/j.envpol.2020.115259
[20] Yadav RK, Goyal B, Sharma RK et al. Post-irrigation impact of domestic sewage effluent on composition of soils, crops and ground water-a case study. Environ Int, 2002; 28: 481-486. DOI: 10.1016/S0160-4120(02)00070-3
[21] Lee CS, Li X, Shi W et al. Metal contamination in urban, suburban, and country park soils of Hong Kong: A study based on GIS and multivariate statistics. Sci Total Environ, 2006; 356: 45-61. DOI: 10.1016/j.scitotenv.2005.03.024
[22] Hayyat A, Javed M, Rasheed I et al. Role of biochar in remediating heavy metals in soil. Springer: Cham, Switzerland, 2016. DOI: 10.1007/978-3-319-40148-5_14
[23] Al-Wabel MI, Usman ARA, El-Naggar AH et al. Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J Biol Sci, 2014; 22: 503-511. DOI: 10.1016/j.sjbs.2014.12.003
[24] Xu D, Zhao Y, Sun K et al. Cadmium adsorption on plant- and manure-derived biochar and biochar-amended sandy soils: Impact of bulk and surface properties. Chemosphere, 2014; 11: 320-326. DOI: 10.1016/j.chemosphere.2014.04.043
[25] Houben D, Evrard L, Sonnet P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere, 2013; 92: 1450-1457. DOI: 10.1016/j.chemosphere.2013.03.055
[26] Lehmann J, Skjemstad J, Sohi S et al. Australian climate-carbon cycle feedback reduced by soil black carbon. Nat Geosci, 2008; 1: 832-835. DOI: 10.1038/ngeo358
[27] Majumder S, Neogi S, Dutta T et al. The impact of biochar on soil carbon sequestration: Meta-analytical approach to evaluating environmental and economic advantages. J Environ Manag, 2019; 250: 109466. DOI: 10.1016/j.jenvman.2019.109466
[28] Jatav HS, Rajput VD, Minkina T et al. Sustainable approach and safe use of biochar and its possible consequences. Sustainability, 2021; 13: 10362. DOI: 10.3390/su131810362
[29] Mackie KA, Marhan S, Ditterich F et al. The effects of biochar and compost amendments on copper immobilization and soil microorganisms in a temperate vineyard. Agric Ecosyst Environ, 2015; 201: 58-69. DOI: 10.1016/j.agee.2014.12.001
[30] Li JY, Hou YL, Hua QX et al. Variation of soil nutrient and heavy metal contentrations in greenhouse soils. Soils, 2005; 37: 626-629.
[31] Wang L, Lu X, Ren C et al. Contamination assessment and health risk of heavy metals in dust from Changqing industrial park of Baoji, NW China. Environ Earth Sci, 2014; 71: 2095-2104. DOI: 10.1007/s12665-013-2613-7
[32] Duan W, Gu B, Whinston AB. Do online reviews matter?-An empirical investigation of panel data. Decis Support Syst, 2008; 45: 1007-1016. DOI: 10.1016/j.dss.2008.04.001
[33] Li S, Gu S, Liu W et al. Water quality in relation to land use and land cover in the upper Han River Basin, China. Catena, 2008; 75: 216-222. DOI: 10.1016/j.catena.2008.06.005
[34] Wen-qing L, Min Z, Hai-feng L. The study of soil nitrate status in fields under plastic house gardening. Acta Pedologica Sinica, 2002; 39: 283-287.
[35] Zhou JB, Zhai BN, Chen ZJ et al. Nutrient accumulations in soil profiles under canopy cultivation and their potential environmental impacts. J Agro-Environ Sci, 2004; 23: 332-335.
[36] Wang YQ, Peng ZP, Xue SC et al. Effect of excessive fertilization on soil ecological environment in the facility farm land. J Agro-Environ Sci, 2005; 24: 81-84.
[37] Liu YJ, Li Q, Liang WJ. Influence of installation cultivation on soil enviromental quality [in Chinese]. J Liaoning Tech Unviersity, 2006; 25: 468-470.
[38] Yu LI, Wang YB, Xin GOU et al. Risk assessment of heavy metals in soils and vegetables around non-ferrous metals mining and smelting sites, Baiyin, China. J Environ Sci, 2006; 18: 1124-1134. DOI: 10.1016/S1001-0742(06)60050-8
[39] Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev, 2021; 10: 1-11. DOI: 10.1136/bmj.n71
[40] Papazoglou IA, Bonanos G, Briassoulis H. Risk informed decision making in land use planning. J Risk Res, 2000; 3: 69-92. DOI: 10.1080/136698700376716
[41] Azqueta D. Land economic value and the environment: some ethical points. Wissenschaftsverlag Vauk Kiel KG: Kiel, Germany, 1995.
[42] Tanrivermis H. Agricultural land use change and sustainable use of land resources in the mediterranean region of Turkey. J Arid Environ, 2003; 54: 553-564. DOI: 10.1006/jare.2002.1078
[43] Tasser E, Tappeiner U. Impact of land use changes on mountain vegetation. Appl Veg Sci, 2002; 5: 173-184. DOI: 10.1111/j.1654-109X.2002.tb00547.x
[44] Lasanta T, Arnáez J, Oserín M et al. Marginal lands and erosion in terraced fields in the Mediterranean mountains. Mt Res Dev, 2001; 21: 69-76. DOI: 10.1659/0276-4741(2001)021[0069:MLAEIT]2.0.CO;2
[45] Krohmer J, Deil U. Dynamic and conservative landscapes? Present vegetation cover and land-use changes in the Serra de Monchique (Portugal). Phytocoenologia, 2003; 33: 767-799. DOI: 10.1127/0340-269X/2003/0033-0767
[46] Fu B, Chen L, Ma K et al. The relationship between land use and soil conditions in the hilly area of Loess Plateau in northern Shaanxi, China. Catena, 2000; 39: 69-78. DOI: 10.1016/S0341-8162(99)00084-3
[47] Guo XD, Fu BJ, Chen LD et al. Effects of land use on soil quality in a hilly area - A case study in Zunhua county of Hebei province. Acta Geographica Sinica, 2001; 56: 447-455.
[48] Hou PC, Xu XD, Pan GX. Variation of soil quality with different land use change in Tai Lake region, Jiangsu, China: A case study of soil quality survey of Wujiang municipality in 2003. Ecol Environ, 2007; 16: 152-157.
[49] Bai LY, Zeng X, Li LF et al. Effects of land use on heavy metal accumulation in soils and sources analysis. Agric Sci China, 2010; 9: 1650-1658. DOI: 10.1016/S1671-2927(09)60262-5
[50] Chen TB, Zheng YM, Chen H et al. Arsenic accumulation in soils for different land use patterns in Beijing [in Chinese]. Geogr Res, 2005; 24: 229-235.
[51] Zheng YM, Luo JF, Chen TB et al. Cadmium accumulation in soils for different land uses in Beijing. Geogr Res, 2005; 24: 542-548.
[52] Shen GX, Xie Z, Qian XY et al. Investigation and analysis of heavy metal accumulation in the soil of vegetable crop land in Shanghai. J Agro-Environ Sci, 2006; 25: 37-40.
[53] Briffa J. Heavy metals in Maltese agricultural soil [master's thesis]. University of Malta: Msida, Malta, 2020.
[54] Duffus JH. “Heavy metals” a meaningless term? Pure Appl Chem, 2002; 74: 793-807. DOI: 10.1351/pac200274050793
[55] Lenntech BV. Heavy metals. Accessed June, 2022. Available at https://www.lenntech.com/processes/heavy/heavy-metals/heavy-metals.htm
[56] Tchounwou PB, Yedjou CG, Patlolla AK et al. Heavy metal toxicity and the environment. Mol Clin Environ Toxicol, 2012; 101: 133-164. DOI: 10.1007/978-3-7643-8340-4_6
[57] Banfalvi G. Cellular effects of heavy metals. Springer: New York, USA, 2011. DOI: 10.1007/978-94-007-0428-2
[58] Masindi V, Muedi KL. Environmental contamination by heavy metals. Heavy Met, 2018; 10: 115-132. DOI: 10.5772/intechopen.76082
[59] Lombi E, Gerzabek MH. Determination of mobile heavy metal fraction in soil: results of a pot experiment with sewage sludge. Commun Soil Sci Plant Anal, 1998; 29: 2545-2556. DOI: 10.1080/00103629809370133
[60] Kabata-Pendias A, Pendias H. Trace metals in soils and plants, 2nd ed. CRC Press: Boca Raton, USA, 2001. DOI: 10.1201/9781420039900
[61] Traina SJ. The environmental chemistry of cadmium. Cadmium Soils Plants, 1999: 11-37. DOI: 10.1007/978-94-011-4473-5_2
[62] Kotas J, Stasicka Z. Chromium occurrence in the environment and methods of its speciation. Environ Pollut, 2000; 107: 263-283. DOI: 10.1016/S0269-7491(99)00168-2
[63] Kaasalainen M, Yli-Halla M. Use of sequential extraction to assess metal partitioning in soils. Environ Pollut, 2003; 126: 225-233. DOI: 10.1016/S0269-7491(03)00191-X
[64] Kuo S, Heilman PE, Baker AS. Distribution and forms of copper, zinc, cadmium, iron, and manganese in soils near a copper smelter. Soil Sci, 1983; 135: 101-109. DOI: 10.1097/00010694-198302000-00004
[65] Zhang MK, Liu ZY, Wang H. Use of single extraction methods to predict bioavailability of heavy metals in polluted soils to rice. Commun Soil Sci Plant Anal, 2010; 41: 820-831. DOI: 10.1080/00103621003592341
[66] Khan S, Cao Q, Zheng YM et al. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ Pollut, 2008; 152: 686-692. DOI: 10.1016/j.envpol.2007.06.056
[67] Basta NT, Ryan JA, Chaney RL. Trace element chemistry in residual-treated soil: key concepts and metal bioavailability. J Environ Qual, 2005; 34: 49-63. DOI: 10.2134/jeq2005.0049dup
[68] Bhat SA, Bashir O, Haq SAU et al. Phytoremediation of heavy metals in soil and water: An eco-friendly, sustainable and multidisciplinary approach. Chemosphere, 2022; 303: 134788. DOI: 10.1016/j.chemosphere.2022.134788
[69] Li F, Huang J, Zeng G et al. Toxic metals in topsoil under different land uses from Xiandao District, middle China: Distribution, relationship with soil characteristics, and health risk assessment. Environ Sci Pollut Res Int, 2015; 22: 12261-12275. DOI: 10.1007/s11356-015-4425-7
[70] Karbassi AR, Tajziehchi S, Khoshgalb H. Speciation of heavy metals in coastal water of Qeshm Island in the Persian Gulf. Global J Environ Sci Manage, 2018; 4: 91-98.
[71] Wang H, Xia W, Lu P et al. Adsorption characteristics of biochar on heavy metals of Pb and Zn in soil. Environ Sci, 2017; 38: 3944-3952. DOI: 10.13227/j.hjkx.201701171
[72] Ye C, Li S, Zhang Y et al. Assessing heavy metal pollution in the water level fluctuation zone of China’s Three Gorges Reservoir using geochemical and soil microbial approaches. Environ Monit Assess, 2013; 185: 231-240. DOI: 10.1007/s10661-012-2547-7
[73] Diane SL, Marlies H, Julien S. Comparative assessment of soil contamination by lead and heavy metals in riparian and agricultural areas (Southern Qu6bec, Canada). Int J Environ Res Public Health, 2010; 7: 3100-3114. DOI: 10.3390/ijerph7083100
[74] Young LIU, Huifeng W, Xiaoting LI et al. Heavy metal contamination of agricultural soils in Taiyuan, China. Pedosphere, 2015; 25: 901-909. DOI: 10.1016/S1002-0160(15)30070-9
[75] Zhou J, Ma D, Pan J et al. Application of multivariate statistical approach to identify heavy metal sources in sediment and waters: A case study in Yangzhong, China. Environ Geology, 2008; 54: 373-380. DOI: 10.1007/s00254-007-0824-5
[76] Atafar Z, Mesdaghinia A, Nouri J et al. Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess, 2010; 160: 83-89. DOI: 10.1007/s10661-008-0659-x
[77] Andersson A. The distribution of heavy metals in soils and soil material as influenced by the ionic radius. Swedish J Agric Res, 1977; 7: 79-83.
[78] Li Y, Shao J, Wang X et al. Characterization of modified biochars derived from bamboo pyrolysis and their utilization for target component (furfural) adsorption. Energy Fuels, 2014; 28: 5119-5127. DOI: 10.1021/ef500725c
[79] Xia X, Yang Z, Cui Y et al. Soil heavy metal concentrations and their typical input and output fluxes on the southern Song-nen plain, Heilongjiang province, China. J Geochem Explor, 2014; 139: 85-96. DOI: 10.1016/j.gexplo.2013.06.008
[80] Wu L, Pan X, Chen L et al. Occurrence and distribution of heavy metals and tetracyclines in agricultural soils after typical land use change in east China. Environ Sci Pollut Res Int, 2013; 20: 8342-8354. DOI: 10.1007/s11356-013-1532-1
[81] Gimeno-García E, Andreu V, Boluda R. Distribution of heavy metals in rice farming soils. Arch Environ Contam Toxicol, 1995; 29: 476-483. DOI: 10.1007/BF00208377
[82] Midrar-ul-Haq Puno HK, Khattak RA, Saif MS. Agricultural land contamination by industrial activities. Int J Agr Biol, 2003; 5: 150-153.
[83] Tariq SR, Shah MH, Shaheen N et al. Multivariate analysis of trace metal levels in tannery effluents in relation to soil and water: A case study from Peshawar, Pakistan. J Environ Manage, 2006; 79: 20-29. DOI: 10.1016/j.jenvman.2005.05.009
[84] Malik RN, Jadoon WA, Husain SZ. Metal contamination of surface soils of industrial city Sialkot, Pakistan: A multivariate and GIS approach. Environ Geochem Health, 2010; 32: 179-191. DOI: 10.1007/s10653-009-9274-1
[85] Mushtaq N, Khan KS. Heavy metals contamination of soils in response to wastewater irrigation in Rawalpindi region. Pak J Agric Sci, 2010; 47: 215-224.
[86] Ali AS, US SA, Ahmad R. Effect of different heavy metal pollution on fish. Res J Chem Environ Sci, 2014; 2: 74-79.
[87] Lasat MM. Phytoextraction of metals from contaminated soil: A review of plant/soil/metal interaction and assessment of pertinent agronomic issues. J Hazard Subst Res, 1999; 2: 5. DOI: 10.4148/1090-7025.1015
[88] Wuana RA, Okieimen FE. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol, 2011; 2011: 1-20. DOI: 10.5402/2011/402647
[89] Jones LHP, Jarvis SC. The fate of heavy metals. John Wiley & Sons: New York, USA, 1981.
[90] Apori OS, Hanyabui E, Asiamah YJ. Remediation technology for copper contaminated soil: A review. Asian Soil Res J, 2018; 8: 1-7. DOI: 10.9734/asrj/2018/v1i326338
[91] Bjuhr J. Trace metals in soils irrigated with waste water in a Periurban Area Downstream Hanoi city, Vietnam. Institutionen for markvetenskap, Sveriges lantbruk- suniversitet (SLU): Uppsala, Sweden, 2007.
[92] Zhao H, Li X. Risk assessment of metals in road-deposited sediment along an urban-rural gradient. Environ Pollut, 2013; 174: 297-304. DOI:10.1016/j.envpol.2012.12.009
[93] Huang SW, Jin JY. Status of heavy metals in agricultural soils as affected by different patterns of land use. Environ Monit Assess, 2008; 139: 317-327. DOI: 10.1007/s10661-007-9838-4
[94] Barman SC, Sahu RK, Bhargava SK et al. Distribution of heavy metals in wheat, mustard, and weed grown in field irrigated with industrial effluents. Bull Environ Contam Toxicol, 2000; 64: 489-496. DOI: 10.1007/s001280000030
[95] Zhang X, Wang H, He L et al. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ Sci Pollut Res Int, 2013; 20: 8472-8483. DOI: 10.1007/s11356-013-1659-0
[96] Liu H, Xu F, Xie Y et al. Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil. Sci Total Environ, 2018; 645: 702-709. DOI: 10.1016/j.scitotenv.2018.07.115
[97] Eduah JO, Nartey EK, Abekoe MK et al. Phosphorus retention and availability in three contrasting soils amended with rice husk and corn cob biochar at varying pyrolysis temperatures. Geoderma, 2019; 341: 10-17. DOI: 10.1016/j.geoderma.2019.01.016
[98] Guo J, Wen X, Yang J et al. Removal of benzo(a)pyrene in polluted aqueous solution and soil using persulfate activated by corn straw biochar. J Environ Manag, 2020; 272: 111058. DOI: 10.1016/j.jenvman.2020.111058
[99] Butphu S, Rasche F, Cadisch G et al. Eucalyptus biochar application enhances Ca uptake of upland rice, soil available P, exchangeable K, yield, and N use efficiency of sugarcane in a crop rotation system. J Plant Nutr Soil Sci, 2020; 183: 58-68. DOI: 10.1002/jpln.201900171
[100] Lu H, Li Z, Fu S et al. Effect of biochar in cadmium availability and soil biological activity in an anthrosol following acid rain deposition and aging. Water Air Soil Pollut, 2015; 226: 164. DOI: 10.1007/s11270-015-2401-y
[101] Ali A, Guo D, Jeyasundar PGSA et al. Application of wood biochar in polluted soils stabilized the toxic metals and enhanced wheat (Triticum aestivum) growth and soil enzymatic activity. Ecotoxicol Environ Saf, 2019; 184: 109635. DOI: 10.1016/j.ecoenv.2019.109635
[102] Debela F, Thring RW, Arocena JM. Immobilization of heavy metals by co-pyrolysis of contaminated soil with woody biomass. Water Air Soil Pollut, 2011; 223: 1161-1170. DOI: 10.1007/s11270-011-0934-2
[103] Lyu H, Zhang Q, Shen B. Application of biochar and its composites in catalysis. Chemosphere, 2020; 240: 124842. DOI: 10.1016/j.chemosphere.2019.124842
[104] Cea M, Gonzalez ME, Sangaletti N et al. Biochar derived from agricultural and forestry wastes: Characterization and potential application for enzyme immobilization. New Biotechnol, 2012; 29: S60. DOI: 10.1016/j.nbt.2012.08.167
[105] Karimi M, Diaz de Tuesta JL, dP Gonçalves CN et al. Compost from municipal solid wastes as a source of biochar for CO2 capture. Chem Eng Technol, 2020; 43: 1336-1349. DOI: 10.1002/ceat.201900108
[106] Wang Q, Lai Z, Mu J et al. Converting industrial waste cork to biochar as Cu (II) adsorbent via slow pyrolysis. Waste Manag, 2020; 105: 102-109. DOI: 10.1016/j.wasman.2020.01.041
[107] Yaashikaa PR, Kumar PS, Varjani S et al. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol Rep, 2020; 28: e00570. DOI: 10.1016/j.btre.2020.e00570
[108] Nartey OD, Zhao B. Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: An overview. Adv Mater Sci Eng, 2014; 2014: 715398. DOI: 10.1155/2014/715398
[109] Lehmann J, Gaunt J, Rondon M. Biochar sequestration in terrestrial ecosystems-a review. Miting Adapt Strat Glob Change, 2006; 11: 403-410. DOI: 10.1007/s11027-005-9006-5
[110] Yu Z, Zhou L, Huang Y et al. Effects of a manganese oxide-modified biochar composite on adsorption of arsenic in red soil. J Environ Manage, 2011; 163: 155-162. DOI: 10.1016/j.jenvman.2015.08.020
[111] Pan J, Gao B, Wang S et al. Waste-to-resources: Green preparation of magnetic biogas residues-based biochar for effective heavy metal removals. Sci Total Environ, 2020; 737: 140283. DOI: 10.1016/j.scitotenv.2020.140283
[112] Cantrell KB, Hunt PG, Uchimiya M et al. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol, 2012; 107: 419-428. DOI: 10.1016/j.biortech.2011.11.084
[113] Paz-Ferreiro J, Lu H, Fu S et al. Use of phytoremediation and biochar to remediate heavy metal polluted soils: A review. Solid Earth, 2014; 5: 65-75. DOI: 10.5194/se-5-65-2014
[114] Lyu H, Zhao H, Tang J et al. Immobilization of hexavalent chromium in contaminated soils using biochar supported nanoscale iron sulfide composite. Chemosphere, 2018; 194: 360-369. DOI: 10.1016/j.chemosphere.2017.11.182
[115] Gholizadeh M, Hu X. Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. J Environ Chem Eng, 2021; 9: 105830. DOI: 10.1016/j.jece.2021.105830
[116] Hu X, Gholizadeh M. Biomass pyrolysis: A review of the process development and challenges from initial researches up to the commercialisation stage. J Energy Chem, 2019; 39: 109-143. DOI: 10.1016/j.jechem.2019.01.024
[117] Zhao Z, Wang J, Han Y et al. Nutrients, heavy metals and microbial communities co-driven distribution of antibiotic resistance genes in adjacent environment of mariculture. Environ Pollut, 2017; 220: 909-918. DOI: 10.1016/j.envpol.2016.10.075
[118] Xing J, Li L, Li G et al. Feasibility of sludge-based biochar for soil remediation: Characteristics and safety performance of heavy metals influenced by pyrolysis temperatures. Ecotoxicol Environ Saf, 2019; 180: 457-465. DOI: 10.1016/j.ecoenv.2019.05.034
[119] Liang J, Yang Z, Tang L et al. Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere, 2017; 181: 281-288. DOI: 10.1016/j.chemosphere.2017.04.081
[120] Hassanzadeh Moghimi O, Daryabeigi Zand A, Nabi Bidhendi G. Assessing the immobilization of heavy metals in compost derived from organic fraction of municipal solid waste amended with forest-based biochar. Environ Energy Econ Res, 2022; 6: 1-12. DOI: 10.22097/EEER.2022.325249.1238
[121] Rees F, Simonnot MO, Morel JL. Short-term effects of biochar on soil heavy metal mobility are controlled by intra-particle diffusion and soil pH increase. Eur J Soil Sci, 2014; 65:149-161. DOI: 10.1111/ejss.12107
[122] Wang S, Xu Y, Norbu N et al. Remediation of biochar on heavy metal polluted soils. IOP Conf Ser Earth Environ Sci, 2018; 108: 042113. DOI: 10.1088/1755-1315/108/4/042113
[123] Li H, Dong X, da Silva EB et al. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere, 2017; 178: 466-478. DOI: 10.1016/j.chemosphere.2017.03.072
[124] Chao XU, Lin XB, Qi-Tang WU et al. Impacts of biochar on availability of heavy metals and nutrient content of contaminated soil under waterlogged conditions. J Soil Water Conserv, 2012; 26:194-198.
[125] Antil RS, Raj D, Abdalla N. Physical, chemical and biological parameters for compost maturity assessment. Springer International Publishing: New York, USA, 2014. DOI: 10.1007/978-3-319-08004-8_5
[126] Ko HJ, Kim KY, Kim HT et al. Evaluation of maturity parameters and heavy metal contents in composts made from animal manure. Waste Manage, 2008; 28: 813-820. DOI: 10.1016/j.wasman.2007.05.010
[127] Ishii K, Fukui M, Takii S. Microbial succession during a composting process as evaluated by denaturing gradient gel electrophoresis analysis. J Appl Microbiol, 2000; 89: 768-777. DOI: 10.1046/j.1365-2672.2000.01177.x
[128] Ranalli G, Botturea G, Taddei P et al. Composting of solid and sludge residues from agricultural and food industries: Bioindicators of monitoring and compost maturity. J Environ Sci Health, 2001; 36: 415-436. DOI: 10.1081/ESE-100103473
[129] Morel JL, Colin F, Germon JC et al. Methods for the evaluation of the maturity of municipal refuse compost. Composting Agric Other Wastes, 1985; 56-72.
[130] de la Fuente C, Clemente R, Martinez-Alcala I et al. Impact of fresh and composted solid olive husk and their water-soluble fractions on soil heavy metal fractionation; microbial biomass and plant uptake. J Hazard Mater, 2011; 186: 1283-1289. DOI: 10.1016/j.jhazmat.2010.12.004
[131] Udovic M, McBride MB. Influence of compost addition on lead and arsenic bioavailability in reclaimed orchard soil assessed using Porcellio scaber bioaccumulation test. J Hazard Mater, 2012; 205: 144-149. DOI: 10.1016/j.jhazmat.2011.12.049
[132] Kapanen A, Vikman M, Rajasarkka J et al. Biotests for environmental quality assessment of composted sewage sludge. Waste Manage, 2013; 33: 1451-1460. DOI: 10.1016/j.wasman.2013.02.022
[133] Kulikowska D, Gusiatin ZM. Sewage sludge composting in a two-stage system: carbon and nitrogen transformations and potential ecological risk assessment. Waste Manage, 2015; 38: 312-320. DOI: 10.1016/j.wasman.2014.12.019
[134] Calleja-Cervantes ME, Fernández-González AJ, Irigoyen I et al. Thirteen years of continued application of composted organic wastes in a vineyard modify soil quality characteristics. Soil Biol Biochem, 2015; 90: 241-254. DOI: 10.1016/j.soilbio.2015.07.002
[135] Proietti P, Federici E, Fidati L et al. Effects of amendment with oil mill waste and its derived-compost on soil chemical and microbiological characteristics and olive (Olea europaea L.) productivity. Agric Ecosyst Environ, 2015; 207: 51-60. DOI: 10.1016/j.agee.2015.03.028
[136] Tian W, Wang L, Li Y et al. Responses of microbial activity, abundance, and community in wheat soil after three years of heavy fertilization with manure-based compost and inorganic nitrogen. Agric Ecosyst Environ, 2015; 213: 219-227. DOI: 10.1016/j.agee.2015.08.009
[137] Liu L, Chen H, Cai P et al. Immobilization and phytotoxicity of Cd in contaminated soil amended with chicken manure compost. J Hazard Mater, 2009; 163: 563-567. DOI: 10.1016/j.jhazmat.2008.07.004
[138] Bolan N, Kunhikrishnan A, Thangarajan R et al. Remediation of heavy metalloids contaminated soils-to mobilize or to immobilize? J Hazard Mater, 2014; 266: 141-166. DOI: 10.1016/j.jhazmat.2013.12.018
[139] González V, García I, Del Moral F et al. Effectiveness of amendments on the spread and phytotoxicity of contaminants in metal-arsenic polluted soil. J Hazard Mater, 2012; 205: 72-80. DOI: 10.1016/j.jhazmat.2011.12.011
[140] Sharifi Z, Renella G. Assessment of a particle size fractionation as a technology for reducing heavy metal, salinity and impurities from compost produced by municipal solid waste. Waste Manage, 2015; 38: 95-101. DOI: 10.1016/j.wasman.2015.01.018
[141] Irfan M, Mudassir M, Khan MJ et al. Heavy metals immobilization and improvement in maize (Zea mays L.) growth amended with biochar and compost. Sci Rep, 2021; 11: 18416. DOI: 10.1038/s41598-021-97525-8
[142] Soares MAR, Marto S, Quina MJ et al. Evaluation of eggshell-rich compost as biosorbent for removal of Pb(II) from aqueous solutions. Water Air Soil Pollut, 2016; 227: 1-16. DOI: 10.1007/s11270-016-2843-x
[143] Anastopoulos I, Kyzas GZ. Composts as biosorbents for decontamination of various pollutants: A review. Water Air Soil Pollut, 2015; 226: 1-16. DOI: 10.1007/s11270-015-2345-2
[144] Paradelo R, Barral MT. Evaluation of the potential capacity as biosorbents of two MSW composts with different Cu, Pb and Zn concentrations. Bioresour Technol, 2012; 104: 810-813. DOI: 10.1016/j.biortech.2011.11.012
[145] Venegas A, Rigol A, Vidal M. Viability of organic wastes and biochars as amendments for the remediation of heavy metal-contaminated soils. Chemosphere, 2015; 119: 190-198. DOI: 10.1016/j.chemosphere.2014.06.009
[146] Zhang WM. Physical and chemical properties of biochar and its application in crop production. Shenyang, China: Shenyang Agricultural University; 2012.
[147] Simantiraki F, Gidarakos E. Comparative assessment of compost and zeolite utilisation for the simultaneous removal of BTEX, Cd and Zn from the aqueous phase: batch and continuous flow study. J Environ Manage, 2015; 159: 218-226. DOI: 10.1016/j.jenvman.2015.04.043
[148] Kargar M, Clark OG, Hendershot WH et al. Immobilization of trace metals in contaminated urban soil amended with compost and biochar. Water Air Soil Pollut, 2015; 226: 1-12. DOI: 10.1007/s11270-015-2450-2
[149] Vaca-Paulín R, Esteller-Alberich MV, Lugo-De La Fuente J et al. Effect of sewage sludge or compost on the sorption and distribution of copper and cadmium in soil. Waste Manage, 2006; 26: 71-81. DOI: 10.1016/j.wasman.2005.03.008
[150] Caporale AG, Pigna M, Sommella A et al. Influence of compost on the mobility of arsenic in soil and its uptake by bean plants (Phaseolus vulgaris L.) irrigated with arsenite-contaminated water. J Environ Manage, 2013; 128: 837-843. DOI: 10.1016/j.jenvman.2013.06.041
[151] Chang Chien SW, Wang MC, Huang CC et al. Characterization of humic substances derived from swine manure-based compost and correlation of their characteristics with reactivities with heavy metals. J Agric Food Chem, 2007; 55: 4820-4827. DOI: 10.1021/jf070021d
[152] Tsang DCW, Yip ACK, Olds WE et al. Arsenic and copper stabilisation in a contaminated soil by coal fly ash and green waste compost. Environ Sci Pollut Res Int, 2014; 21: 10194-10204. DOI: 10.1007/s11356-014-3032-3
[153] Chien SWC, Wang MC, Huang CC. Reactions of compost-derived humic substances with lead, copper, cadmium, and zinc. Chemosphere, 2006; 64: 1353-1361. DOI: 10.1016/j.chemosphere.2005.12.041
[154] Lloyd JR. Bioremediation of metals; the application of micro-organisms that make and break minerals. Interact, 2002; 2: M2.
[155] Bai RS, Abraham TE. Studies on chromium (VI) adsorption–desorption using immobilized fungal biomass. Bioresour Technol, 2003; 87: 17-26. DOI: 10.1016/S0960-8524(02)00222-5
[156] del Carmen Vargas-Garcia M, López MJ, Suárez-Estrella F et al. Compost as a source of microbial isolates for the bioremediation of heavy metals: in vitro selection. Sci Total Environ, 2012; 431: 62-67. DOI: 10.1016/j.scitotenv.2012.05.026
[157] Hettiarachchi GM, Ryan JA, Chaney RL et al. Sorption and desorption of cadmium by different fractions of biosolids‐amended soils. J Environ Qual, 2003; 32: 1684-1693. DOI: 10.2134/jeq2003.1684
[158] Tapia Y, Cala V, Eymar E et al. Chemical characterization and evaluation of composts as organic amendments for immobilizing cadmium. Bioresour Technol, 2010; 101: 5437-5443. DOI: 10.1016/j.biortech.2010.02.034
[159] Lu H, Zhang W, Yang Y. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res, 2012; 46: 854-862. DOI: 10.1016/j.watres.2011.11.058
[160] Cao X, Harris W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour Technol, 2010; 101: 5222-5228. DOI: 10.1016/j.biortech.2010.02.052
[161] Liang J, Yang Z, Tang L et al. Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere, 2017; 181: 281-288. DOI: 10.1016/j.chemosphere.2017.04.081
[162] Xie CR, Wang ZY, Zhu JM et al. Adsorption of lead and copper from aqueous solutions on biochar produced from walnut green husk [in Chinese]. Acta Scientiae Circumstantiae, 2016; 36: 1190-1198.
[163] Liu MH, Wang ZY, Chen L. Application of peanut shell and Chinese medication mixed biochar as soil amendment to lead contaminated soil [in Chinese]. Period Ocean Univ China, 2016; 46: 102-104.
[164] Kong H, He J, Gao Y et al. Cosorption of phenanthrene and mercury (II) from aqueous solution by soybean stalk-based biochar. J Agric Food Chem, 2011, 59: 1216-1213. DOI: 10.1021/jf202924a
[165] Dong X, Wang C, Li H et al. The sorption of heavy metals on thermally treated sediments with high organic matter content. Bioresour Technol, 2014; 160: 123-128. DOI: 10.1016/j.biortech.2014.01.006
[166] Xu XY. The Sorption and transformation of inorganic contaminants by biochars and the underlying mechanisms [in Chinese]. Shanghai Jiao Tong University: Shanghai, China, 2015.
[167] Zhao LY. Different sources of biochar on mercury removal performance and fixing mechanism research [in Chinese]. Nanjing Normal University: Jiangsu, China, 2015.
[168] Ippolito JA, Strawn DG, Scheckel KG et al. Macroscopic and molecular investigations of copper sorption by a steam-activated biochar. J Environ Qual, 2012; 41: 1150-1156. DOI: 10.2134/jeq2011.0113
[169] Mohan D, Pittman Jr CU, Bricka M et al. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J Colloid Interface Sci, 2007; 310: 57-73. DOI: 10.1016/j.jcis.2007.01.020
[170] Guan LZ, Zhou JJ, Zhang J et al. Effects of biochars produced from different sources on arsenic adsorption and desorption in soil. Chin J Appl Ecol, 2013, 24: 2941-2946.
[171] Huang DK, Li XQ, Dong ZQ et al. Soil environmental influence of biochar and its application in soil heavy metal restoration. Guizhou Agric Sci, 2014; 42: 159-165.
[172] Wang L, Lu X, Ren C et al. Contamination assessment and health risk of heavy metals in dust from Changqing industrial park of Baoji, NW China. Environ Earth Sci, 2015; 71: 2095-2104. DOI: 10.1007/s12665-013-2613-7
[173] Wu QW, Meng L, Zhang ZH et al. Adsorption behaviors of Ni2+onto reed straw biochar in the aquatic solutions. Environ Chem, 2015; 9: 1703-1709.
[174] Chen B, Chen Z, Lv S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour Technol, 2011; 102: 716-723. DOI: 10.1016/j.biortech.2010.08.067
[175] Venegas A, Rigol A, Vidal M. Viability of organic wastes and biochars as amendments for the remediation of heavy metal-contaminated soils. Chemosphere, 2015; 119: 190-198. DOI: 10.1016/j.chemosphere.2014.06.009
[176] Achiba WB, Gabteni N, Lakhdar A et al. Effects of 5-year application of municipal solid waste compost on the distribution and mobility of heavy metals in a Tunisian calcareous soil. Agric Ecosyst Environ, 2009; 130: 156-163. DOI: 10.1016/j.agee.2009.01.001
[177] Clemente R, Hartley W, Riby P et al. Trace element mobility in a contaminated soil two years after field-amendment with a greenwaste compost mulch. Environ Pollut, 2010; 158: 1644-1651. DOI: 10.1016/j.envpol.2009.12.006
[178] Tsang DCW, Olds WE, Weber PA et al. Soil stabilisation using AMD sludge, compost and lignite: TCLP leachability and continuous acid leaching. Chemosphere, 2013; 93: 2839-2847. DOI: 10.1016/j.chemosphere.2013.09.097
[179] Hartley W, Dickinson NM, Riby P et al. Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ Pollut, 2009; 157: 2654-2662. DOI: 10.1016/j.envpol.2009.05.011
[180] Hartley W, Dickinson NM, Riby P et al. Arsenic mobility and speciation in a contaminated urban soil are affected by different methods of green waste compost application. Environ Pollut, 2010; 158: 3560-3570. DOI: 10.1016/j.envpol.2010.08.015
[181] Karami N, Clemente R, Moreno-Jiménez E et al. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J Hazard Mater, 2011; 191: 41-48. DOI: 10.1016/j.jhazmat.2011.04.025
[182] Zeng G, Wu H, Liang J et al. Efficiency of biochar and compost (or composting) combined amendments for reducing Cd, Cu, Zn and Pb bioavailability, mobility and ecological risk in wetland soil. RSC Adv, 2015; 5: 34541-34548. DOI: 10.1039/C5RA04834F
[183] Rodríguez-Vila A, Covelo EF, Forján R et al. Phytoremediating a copper mine soil with Brassica juncea L., compost and biochar. Environ Sci Pollut Res Int, 2014; 21: 11293-11304. DOI: 10.1007/s11356-014-2993-6
[184] Rodríguez-Vila A, Asensio V, Forján R et al. Chemical fractionation of Cu, Ni, Pb and Zn in a mine soil amended with compost and biochar and vegetated with Brassica juncea L. J Geochem Explor, 2015; 158: 74-81. DOI: 10.1016/j.gexplo.2015.07.005
[185] Beesley L, Moreno-Jiménez E, Gomez-Eyles JL. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ Pollut, 2010; 158: 2282-2287. DOI: 10.1016/j.envpol.2010.02.003
[186] Beesley L, Inneh OS, Norton GJ et al. Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ Pollut, 2014; 186: 195-202. DOI: 10.1016/j.envpol.2013.11.026
[187] Tang J, Zhang L, Zhang J et al. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci Total Environ, 2020; 701: 134751. DOI: 10.1016/j.scitotenv.2019.134751
Copyright © 2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.

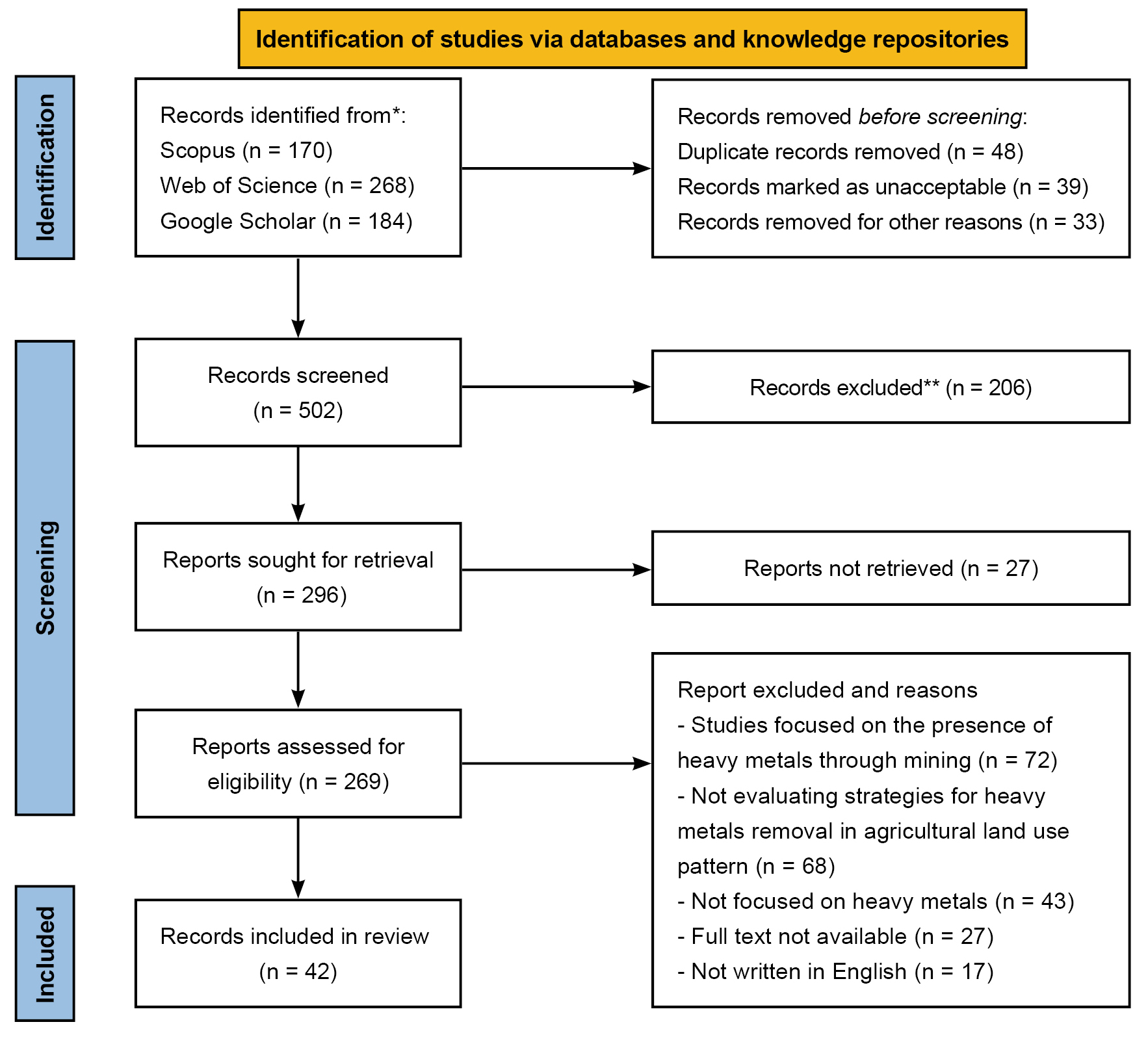
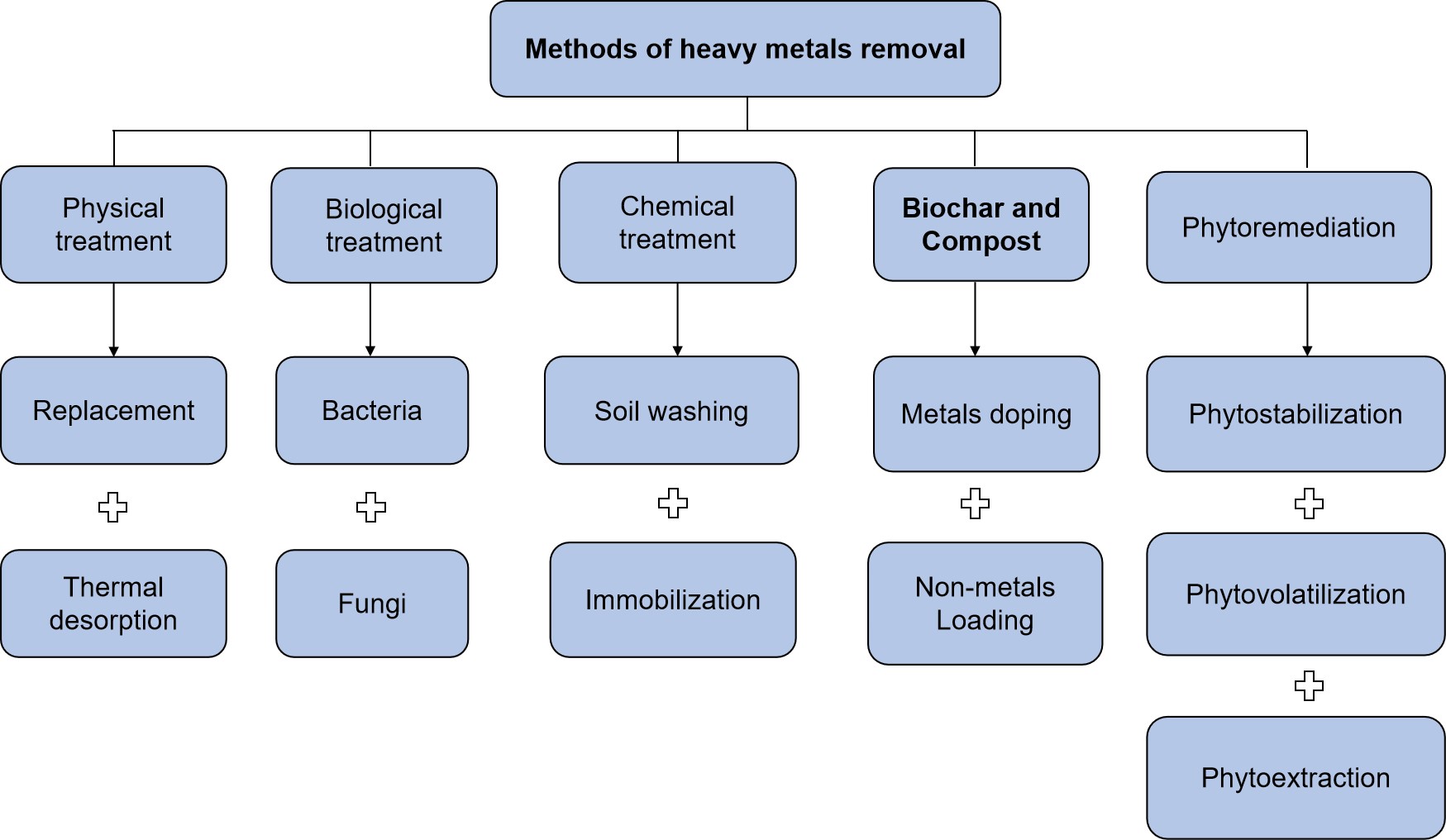



 Copyright ©
Copyright ©