Frugivorous Flies of the Drosophilidae, Lonchaeidae and Tephritidae and Their Parasitoids in Brazil
Carlos Henrique Marchiori1*
1Department of Biological Science, Institution Federal Goiano, Goiânia, Goiás, Brazil
*Correspondence to: Carlos Henrique Marchiori, PhD, Professor, Department of Biological Science, Institution Federal Goiano, Rua T-65 No.1050, Goiânia 74230120, Brazil; E-mail: chmarchiori@yahoo.com.br
Abstract
Objective: Brazil is one of the leaders in the production of fruit in temperate climates but is still plagued by pests, particularly frugivorous flies. The damage caused by these insects results in reduced yields and difficulties in exporting due to quarantine barriers imposed by importing countries. The objective of this study was to investigate the main species of frugivorous flies of the Drosophilidae, Lonchaeidae and Tephritidae and their parasitoids in Brazil.
Methods: The collected fruits (orange, star fruit, guava, mango, and pitanga) were deposited on a layer of fine sand, in plastic containers, cylindrical, transparent, and open at the top. Each week, the pupae were separated from the substrate by flotation. They were removed and placed in glass flasks with fine sand at room temperature until the emergence of dipterans and/or their parasitoids.
Results: In Goiás, Doryctobracon areolatus (Szepligeti, 1911) (Hymenoptera: Braconidae) was the most frequent with 65.0%, and in Minas Gerais, Trichopria anastrepha Costa Lima, 1940 (Hymenoptera: Diapriidae) accounted for 44.4%. In Goiás, Doryctobracon areolatus presented the highest percentage of parasitism with 4.3%, and in Minas Gerais the percentage of parasitism in Trichopria anastrepha was 5.7%. In both states, Zaprionus indianus Gupta, 1970 (Diptera: Drosophilidae) the largest species with the highest percentage of parasitism was Pachycrepoideus vindemmiae (Rondani, 1875) (Hymenoptera: Pteromalidae) with 96.7%. In Psidium guajava, L. (Myrtaceae) the percentage of natural parasitism was 16.7%, being 0.9% by Aganapis pelleranoi (Brèthes, 1924) 8.0%.
Conclusion: In Brazil, frugivorous flies are important pests of fruits and vegetables. Knowledge of the population fluctuation of these species in each biome is an important requirement for the adoption of pest control.
Keywords: insects, parasitoids, damage, flies, dipterans, hymenopterans
1 INTRODUCTION
The expansion of frontiers for fruit growing causes changes in population dynamics and modifies the spatial distribution of fruit fly species. The Brazilian fruit industry covers an area of 2.3 million hectares planted and an annual production of more than 38 million tons. Brazil is the third largest fruit producer in the world, surpassed only by China (133 million tons) and India (58 million tons), generating 6 million direct jobs, 27% of the total agricultural labor employed in the country, and a gross domestic product of $11 billion[1].
This sector demands intensive and qualified labor, keeping people in the countryside and allowing a life dignity for farmers and their families on both small farms and large projects. However, Brazil ranks 20th among exporting countries. Tropical climate zone has great potential for year-round fruit production. The variations of temperature fluctuate by only 2 to 3% between the monthly averages, without the extremes seen in the Southeast and South regions of the country[1].
Fruit flies are one of the major problems faced by these producers. Fruit flies (Diptera: Tephritidae, Lonchaeidae and Drosophilidae) are among the main pests of world agriculture and are of particular concern to tropical developing countries that have an important role in fruit production of its trade balance. Essentially, the negative economic impacts of these insect pests are associated with direct damage and quarantine restrictions imposed by importing countries[2-4].
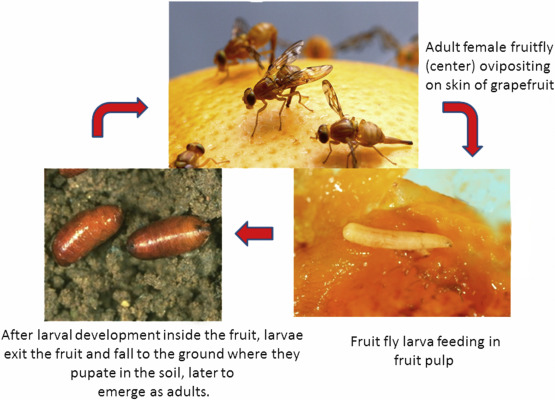
The Tephritidae family is one of the largest families of the Order Diptera. This family is one of the pests with a high economic impact in the world fruit industry because they attack the reproductive organs of plants, fruits with their pulp and flowers. These insects constitute an important group of pests in fruit production worldwide, as they have a life cycle in which their larval period develops inside the fruits, and generally feed on their pulp. Fruit flies are insects that cause high damage to fruit growers (Figure 1A)[5-7].
|
Figure 1. Specimens. A Specimen of Tephritidae family; B: Specimen of Lonchaeidae family; C: Specimen of Drosophilidae family. Sources: https://alchetron.com/Tephritidae, https://bugguide.net/node/view/1343473 and https://www.mapress.com/zt/article/view/zootaxa.5068.2.8
Some species of Lonchaeidae have been reported as pests of agricultural crops, causing economic damage by infesting fruits and/or flower buds. Therefore, interest in studying these insects has increased in recent years. Lonchaeidae from the Brazilian Amazon was developed to group and provide information on the diversity, distribution and hosts of Lonchaeidae species in the Legal Amazon (states of Acre, Amapá, Amazonas, Mato Grosso, Pará, Rondônia, Roraima, Tocantins and Maranhão) in a simple and accessible way (Figure 1B)[8-10].
The larvae of most species of the family Drosophilidae feed on microorganisms in spoiled fruits, slime fluxes, fungi, rotting cacti, or other decaying organic matter. The Drosophilidae family is represented by generally small flies and is distributed throughout the planet (it is cosmopolitan) (Figure 1C)[11-13].
In several countries, studies with a survey of fruit fly species are mainly based on trap collections and sporadic fruit sampling, and little is known about the hosts and infestation rates[14,15].
The survey with character traps allows for the analysis of the character quantitatively and qualitatively, while fruit collection allows assessment of infestation and population levels and determination of the association with hosts, as well as the abundance and diversity of natural enemies, which is not possible through the use of traps to capture the adults[16,17].
The objective of this study was to know the main species of frugivorous flies of the Drosophilidae, Lonchaeidae Tephritidae and their parasitoids in Brazil.
2 MATERIALS AND METHODS
The experiment was carried out in the municipalities of Itumbiara located in the State of Goiás and in Lavras in the State of Minas Gerais, Brazil. In Itumbiara, the collections were carried out on the farm of the Lutheran Institute of Higher Education in Itumbiara and in Lavras on the campus of the Federal University of Lavras. The collections were carried out from January to December 2001.
The collected fruits (orange, star fruit, guava, mango and pitanga) were deposited on a layer of fine sand, in plastic containers, cylindrical, transparent and open at the top. Each week, the pupae were separated from the substrate by flotation. They were removed and placed in glass flasks with fine sand at room temperature until the emergence of dipterans and/or their parasitoids. The experiments were carried out in 2001. Possible differences between the preference of parasitoids and flies for fruits were tested by Chi-square (Figure 2).
|
Figure 2. Fruit fly laboratory. A: Nipagim, sodium benzoate and citric acid dissolved in distilled water; B: Appearance of the artificial diet without the addition of sugar cane pomace; C: Increment from sugarcane bagasse to artificial diet; D: Mixture of cane cake to ingredients in the diet; E: Final aspect of artificial diet. Source: https://docplayer.com.br/56731187-Documentos-tecnicas-para-criacao-da-mosca-da-carambola-bactrocera-carambolae-drew-hancock-rio-para-pesquisa-cientifica.html
2.1 Experiment with Psidium guajava, L. (Myrtaceae)
The study was carried out in P. guajava crops located at Sítio Rio Grande in Divinopolis State of Minas Gerais. The experiment was performed from January to December 2002. The collected fruits were placed in plastic containers (five fruits for each container) 20cm high by 10cm in diameter, being deposited on a 5cm layer of fine autoclaved sand used in house construction, serving as a substrate for pupation. The opening of the containers was sealed with organza fabric tied with elastic to prevent the entry of other insects.
Weekly, the sand containing the fruit fly pupae was deposited in buckets of water, where the pupae were separated by the floating method. Then, they were removed from the water with the aid of a sieve and dried. After drying, they were counted and individualized in gelatin capsules (number 00) and kept in an acclimatized room at 25±2°C until the emergence of the fly adults and / or their parasitoids.
The percentage of parasitism was calculated: P = (parasitized pupae / total pupae) × 100.
3 RESULTS AND DISCUSSION
3.1 Fruit Flies (Tephritoidea) and Their Parasitoids
In the state of Goiás, the percentage of frequency of hosts was 64.0% for Anastrepha fraterculus (Wiedemann, 1830) (Diptera: Tephritidae) (917/1516), 30.5% for Ceratitis capitata (Wiedemann, 1830) (Diptera: Tephritidae) (463/1516), and 9.0% for Neosilba sp. (Diptera: Lonchaeidae) (136/1516). Anastrepha fraterculus presented the highest frequency, probably due to its polyphagia (Table 1 and Figure 3).
Table 1. Frugivorous Flies and Their Collected Parasitoids and Their Parasitoids in Goiás, Brazil
Taxonomic Group |
Number of Pupae |
Parasitoids |
Pupae Parasitized |
% Parasitism |
Anastrepha fraterculus |
917 |
Doryctobracon areolatus |
39 |
4.3 |
|
|
Pachycrepoideus vindemmiae |
02 |
0.2 |
|
|
Aganaspis pelleranoi |
26 |
2.8 |
Ceratitis capitata |
463 |
Doryctobracon areolatus |
02 |
0.4 |
Neosilba sp. |
136 |
Doryctobracon areolatus |
02 |
1.5 |
|
1516 |
|
71 |
|
|
Figure 3. Anastrepha fraterculus (Wiedemann, 1830) (Diptera: Tephritidae). Source: http://www.bio.ufpr.br/portal/pragasplantas/wp-content/uploads/sites/12/2013/11/frutiferas_resumida.pdf
Anastrepha fraterculus: This species of fruit fly is considered the main pest of many fruit species in Brazil. It is mostly found in avocado, plum, coffee, persimmon, citrus, fig, guava, apple, mango, passion fruit, quince, loquat, pear, peach, tomato, and grape. The adults lay their eggs on the fruits, and the larvae penetrate the fruit and feed from within, capable of destroying it completely.
Ceratitis capitata: Fruits attacked by flies show characteristic symptoms: around the place where the egg is laid, a halo appears approximately 2cm in diameter and dark in color. When the larvae hatch, this halo takes on a brownish color due to the rotting of the shell. Certain fungi develop on these destroyed tissues. The pest preferentially attacks fruits exposed to the sun. The pest predominates in coffee, where the eggs are deposited inside the ripe fruit and where the larva reaches its maturity, causing loss of coffee quality and great damage to the crop[18-20].
It is the species of the genus Neosilba, which has the widest geographic distribution and host diversity in Brazil, being considered an important pest of fruits grown in the Northeast and Southeast regions of the country. Therefore, studies focused on this family should be encouraged, especially regarding ecology and biology. It should be noted that a significant part of the insect species in the region is still unknown to science (Table 1 and Figure 4)[21-23].
|
Figure 4. Anastrepha fraterculus (Wiedemann, 1830) (Diptera: Tephritidae) life cycle. Source: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/anastrepha-fraterculus
The frequency of parasitoids was 65.0% for Doryctobracon areolatus (Szepligeti, 1911) (Hymenoptera: Braconidae) (46/71), 2.8% for (2/71), and 37.0% for (26/71). The high frequency of Doryctobracon areolatus may be attributed to its ability to search for hosts in the larval stage (Figure 5).
|
Figure 5. Doryctobracon areolatus (Szepligeti, 1911) (Hymenoptera: Braconidae). Source: https://entnemdept.ufl.edu/creatures/beneficial/wasps/doryctobracon_areolatus.htm
The results shown in Table 1 demonstrate the small diversity of Diptera. The results suggest that A. fraterculus can be considered the most important pest of fruit trees. Regarding parasitoids (Table 1), D. areolatus can be considered the most important parasitoid of frugivorous. Anastrepha fraterculus is the only species found in all the hosts sampled.
The total percentage of parasitism was (71/1516) 4.7%. The highest percentage of parasitism was in A. fraterculus by the parasitoid D. areolatus with (39/917) 4.3%.
In Brazil, the native parasitoid D. areolatus stands out for its constant presence, the greater number of specimens obtained in most surveys carried out in the country, and the aggressiveness in the parasitism of fruit fly larvae of different stages[24-26].
The Chi-square calculation showed that A. fraterculus showed a preference for pitanga and C. capitata for guava (χ²=27.39; GL=1; P<0.0001) with 5% significance Neosilba sp. (Diptera: Lonchaeidae) being more common in pitanga, accounting for 66.6% of individuals.
In the state of Minas Gerais, a total of 349 pupae of Anastrepha spp. 45 specimens were obtained from the following six parasitoids: Trichopria anastrepha Costa Lima, 1940 (Hymenoptera: Diapriidae) (20/45) 44.4%, Leptopilina boulardi Barbotin, Carton & Kelner-Pillault 1979 (Hymenoptera: Figitidae) (10/45) 22.2%, Spalangia endius Walker, 1839 (Hymenoptera: Pteromalidae) (6/45) 13.3%, D. areolatus (5/45) 11.1%, Odontosema anastrepha Borgameier, 1935 (Hymenoptera: Figitidae) (2/45) 4.4% and Pachycrepoideus vindemmiae (Rondani, 1875) (Hymenoptera: Pteromalidae) (2/45) 4.4%.
The genus Trichopria includes microhymenopterans that can be used for the biological control of flies, since they are parasitoids of immature stages of Diptera. Trichopria anastrephae is a species generalist, usually occurring in a single parasitoid by host puparium. (Table 2 and Figure 6)[27,28].
Table 2. Fruit Fly and Its Parasitoids Collected in Minas Gerais State, Brazil
Host / Puparium Number |
Parasitoids |
Frequency |
Parasitism (%) |
Anastrepha spp. / 349 |
Braconidae: |
|
|
|
Doryctobracon areolatus |
05 |
1.4 |
|
Diapriidae: |
|
|
|
Trichopria anastrepha |
20 |
5.7 |
|
Figitidae: |
|
|
|
Leptopilina boulardi |
10 |
2.9 |
|
Odontosema anastrepha |
02 |
0.6 |
|
Pteromalidae: |
|
|
|
Pachycrepoideus vindemmiae |
02 |
0.6 |
|
Spalangia endius |
06 |
1.7 |
Total |
|
45 |
|
|
Figure 6. Trichopria anastrepha Costa Lima, 1940 (Hymenoptera: Diapriidae). Source: https://www.sciproveg.com/?p=3370&lang=en
The total percentage of natural parasitism observed was 13.0%, which may be attributable to the density of the hosts, the number of collections carried out, the size of the sample area, and the characteristics of the place chosen for collection, with vegetation that guarantees basic conditions to maintain a diversified fauna of host insects. In the work carried out in 27 municipalities, the percentage of parasitism was found to range from 0.007 to 42.86%, and a percentage of parasitism of 6.2% was identified in Goiás[29,30].
These results demonstrate that T. anastrepha can be considered one of the most important parasitoids of fruit flies in this region. Probably, this fact may be influenced by the parasitoid's search capacity and its density[31,32].
3.2 Psidium guajava, L. (Myrtaceae)
Zaprionus indianus Gupta, 1970 (Diptera: Drosophilidae) is considered to be secondary to more than 70 fruit species, due to its ability to attack and feed on decaying or mechanically damaged fruit. The fig fly, Z. indianus is an invasive species with a high colonization potential and high occurrence in anthropic places and is considered one of the most abundant species among the drosophilid community in Brazil (Figure 7)[33-35].
|
Figure 7. Zaprionus indianus Gupta, 1970 (Diptera: Drosophilidae) on fig fruits. A: Oviposition of Z. indianus; B: Fig fruits susceptible to Z. indianus; C: Z. indianus (left) and (right) eggs. The arrow in Figure 2A points to eggs at the entrance of the ostiole. Source: https://www.researchgate.net/figure/Zaprionus-indianus-on-fig-fruits-A-Oviposition-of-Zaprionus-indianus-B-fig-fruits_fig2_321928658
A total of 1068 individuals of Z. indianus were collected, including 04 specimens of S. endius 03 specimens of L. boulardi and 285 specimens of P. vindemmiae (Table 3 and Figure 7C)
Table 3. Parasitoids of Zaprionus Indianus Gupta, 1970 (Diptera: Drosophilidae) Collected in Goiás and Minas Gerais, Brazil
Host/Puparium Number |
Parasitoids |
Frequency |
Parasitism (%) |
Zaprionus indianus / 1068 |
Spalangia endius |
04 |
0.4 |
|
Pachycrepoideus vindemmiae |
208 |
19.5 |
|
Leptopilina boulardi |
03 |
0.3 |
|
|
215 |
|
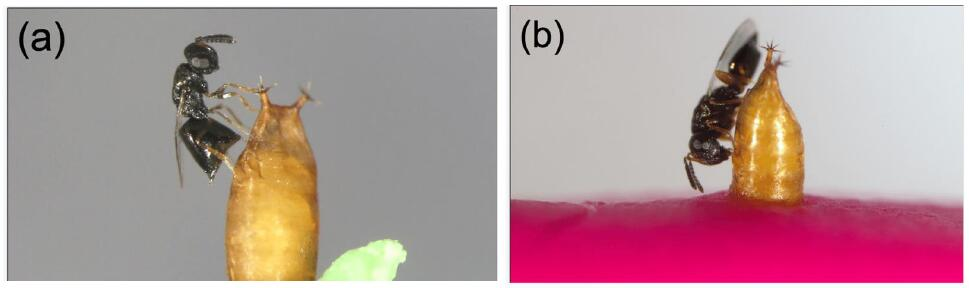
The frequency of parasitoids was 1.9% of S. endius (4/215) 1.9%, 96.7% of P. vindemmiae (208/215) and 1.4% of L. boulardi (3/215). Pachycrepoideus vindemmiae showed a higher frequency due to its ability to search for hosts (Table 3). A total percentage of 20.1% of P. vindemmiae showed a higher percentage of parasitism in Z. indianus, possibly due to the higher food supply (Figure 8).
|
Figure 8. Pachycrepoideus vindemmiae (Rondani, 1875). a: Female wasp inserting her ovipositor through the Drosophila suzukii (Matsumura, 1931) (Diptera: Drosophilidae) pupal case and performing evaluation of the host prior oviposition; b: Female wasp host-feeding on hemolymph of a D. suzuki pupa following ovipositor withdrawal from the pupal case. Source: https://www.researchgate.net/figure/Pachycrepoideus-vindemmiae-Hymenoptera-Pteromalidae-attacking-pupae-of-spotted-wing_fig1_334637885
Pachycrepoideus vindemmiae is a solitary parasitoid of numerous Diptera. It has a wide geographic distribution and is also found in North America, Latin America, and Africa[36,37].
3.3 Psidium guajava (Myrtaceae)
The guava tree P. guajava is widely distributed throughout the tropical and subtropical regions of the world. In addition to the economic importance, its fruits have a high nutritional value with high levels of sugars, iron, calcium, phosphorus and vitamins A, B and C[38,39].
Control of this pest in the guava crop has been carried out through the application of pesticides when the fruits are still small, with a moratorium 30 days before harvest. However, due to the strong requirements of the importing countries regarding the absence of pests and chemical residues, allied to the environmental awareness of the rural producer, other control alternatives have been sought, highlighting the bagging of fruit, the release of the sterile males, and the use of parasitoids[38,39].
Little knowledge is available to the action of fruit fly parasitoids in Brazil. The main Hymenoptera Parasitica groups of these flies belong to the Braconidae, Figitidae and Pteromalidae families. Among the parasitoids used in the biological control of fruit flies, those belonging to the Figitidae family, Eucoilinae subfamily, have received great academic attention due to their efficiency as a control agent, in addition to their wide geographic distribution and high potential for use in pest management programs[39,40].
One hundred and ninety pupae of Anastrepha sp. were collected, from which 38 parasitoids of 38 pupae belonging to the Figitidae family emerged, including 20 Aganapis pelleranoi (Brèthes, 1924) and 18 Dicerataspis grenadensis Ashmead, 1896, with a percentage of natural parasitism around 16.7%. The frequency of parasitoids was A. pelleranoi (20/38) 52.6% and (18/38) 47.4%.
The parasitic percentage of A. pelleranoi was 0.9% and D. grenadensis 8.0%. This percentage may be due to the density of the hosts, the number of collections carried out, the size of the sample area or the characteristics of the place chosen for collection, where vegetation exists that ensures the basic conditions for maintaining the diversity of host insects.
Aganapis pelleranoi is widely distributed in Brazil. In a survey carried out by this author on Eucoilinae species in Brazil, this species was the most abundant, representing 29.9% of all Eucoilinae associated with frugivorous fly larvae. Aganapis pelleranoi was released as a biological control agent for fruit flies in Tucumán province in Argentina (Figures 9-11)[38-42].
|
Figure 9. Aganapis pelleranoi (Brèthes, 1924). Source: http://www.waspweb.org/cynipoidea/figitidae/Eucoilinae/Aganaspis/Aganaspis_pelleranoi.htm
|
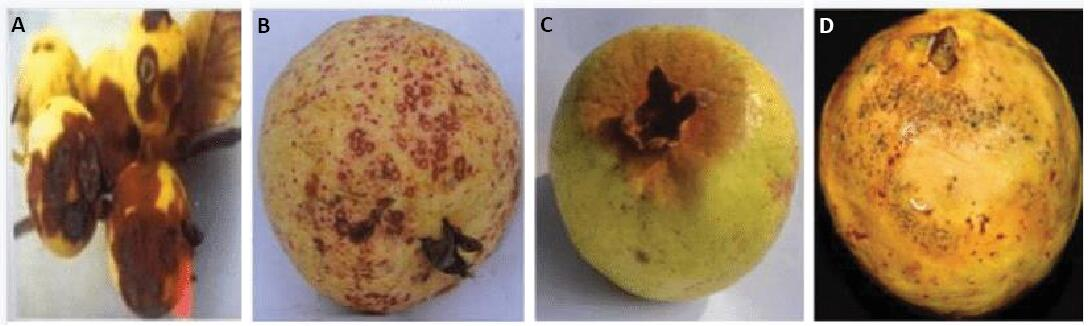
Figure 10. Fruits affected by guava diseases. A: Anthracnose; B: Algal spot; C: Styler end rot; D: Fruit fly. Source: https://www.researchgate.net/figure/Fruits-affected-by-guava-diseases-a-Anthracnose-b-Algal-spot-c-Styler-end-rot-d_fig1_353632026
|
Figure 11. Female fruit fly laying eggs on guava fruit. a: Ludhiana; b: Fruit fly-infested guava fruit on tree; c: Fruit fly-infested guava fruits in glass jar; d: Maggots of fruit fly developed from eggs in fruit; e: Collection of maggots from infested guava fruits; f: Rearing of maggots in artificial diet in laboratory; g: Maggots feeding on artificial diet; h: pupae of fruit fly developed from maggot. Source: https://ejbpc.springeropen.com/articles/10.1186/s41938-020-00345-7
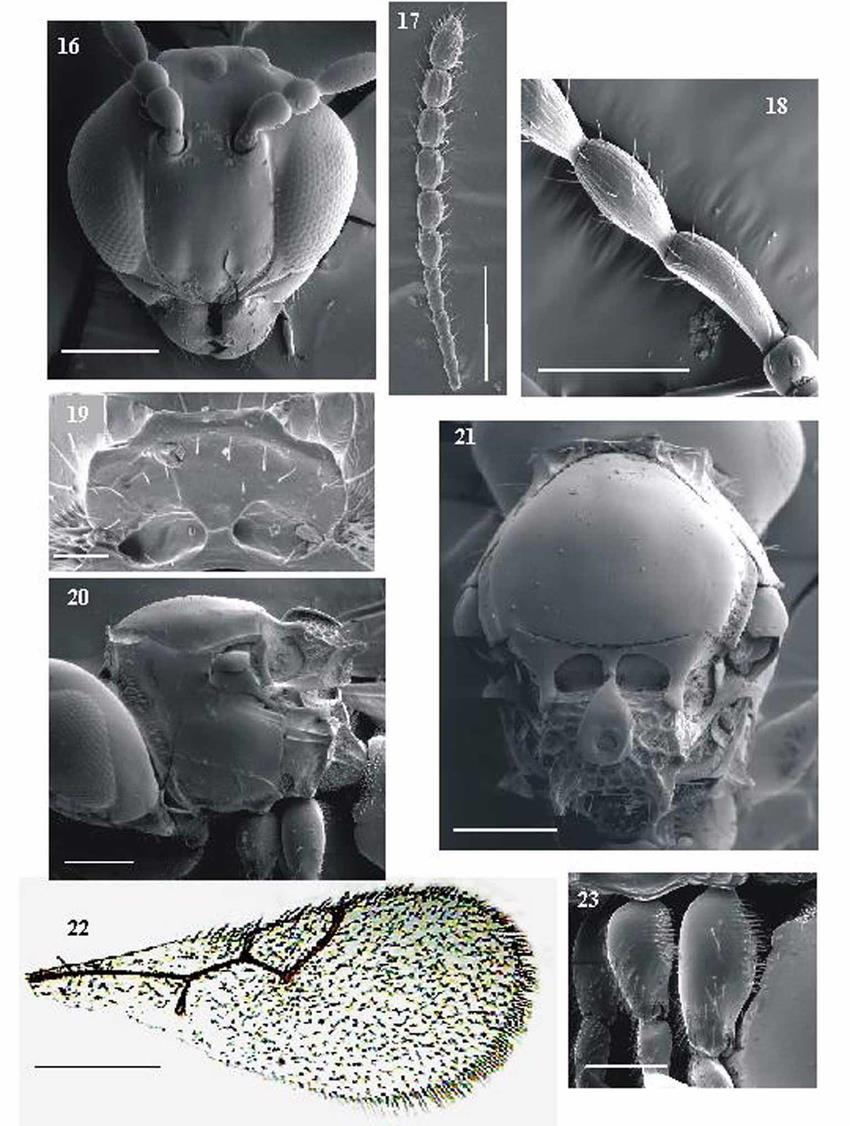
The genus Dicerataspis is currently represented by a single species, D. grenadensis. This species was recorded for the first time in Brazil in 1999 in the state of São Paulo, then in Pará, Goiás and Minas Gerais. Species of the Dicerataspis are potential parasitoids of Drosophilidae larvae, since they are much smaller than those associated with larvae of Tephritoidea. Species of the genera Dicerataspis and Leptopilina are the most commonly found Eucoilinae associated with drosophilid larvae-pupae, constituting important natural enemies of this family (Figure 12)[42-46].
|
Figure 12. Dicerataspis grenadensis Ashmead, 1896. 16: Head, anterior view; 17: Female antenna; 18: Flagellomeres 1 and 2 of male; 19: Pronotal plate; 20: Head, mesosoma and anterior part of metasoma, lateral view; 21: Mesosome, dorsal view; 22: Forewing; 23: Metacoxa. Source: https://www.researchgate.net/figure/FIGURES-16-23-Dicerataspis-grenadensis-16-Head-anterior-view-140x-100m-17_fig2_262413490
Knowledge about the diversity and distribution of these groups of natural enemies is essential, as these parasitoids contribute to regulating the frugivorous dipteran populations.
4 CONCLUSION
In Brazil, frugivorous flies are important pests of fruits and vegetables. Knowledge of the population fluctuation of these species in each biome is an important requirement for the adoption of pest control.
Acknowledgements
Not applicable.
Conflicts of Interest
The author declared no conflict of interest.
Author Contribution
Marchiori CH contributed to the manuscript and approved the final version.
References
[1] Seplag-State Secretariat for Planning, Management and Heritage. Fruticulture APL in Vale do Mundaú. Accessed 18 September, 2022. Available at: http://dados.al.gov.br/dataset/arranjos productive-locales/resource/bb0e2bd3-a53c-47e5-9ba4-2d477c1784c7
[2] Ferreira JTP, Ferreira EP, Pantaleão FS et al. Citriculture in the State of Alagoas a case study in the municipality of Santana do Mundaú-AL-Brazil. Rev ACSA-OJS, 2013; 9: 125-134.
[3] Uramoto K, Walder JMM, Zucchi RA. Biodiversity of fruit flies of the genus Anastrepha (Diptera, Tephritidae) at the ESALQ-USP Campus, Piracicaba, São Paulo. Rev Bras Entomol, 2004; 48: 409-414. DOI: 10.1590/S0085-56262004000300018
[4] Broglio SMF, Santos JM, Dias-Pini NDS et al. Frugivorous flies and their parasitoids associated with Surinam cherry fruits. Arq Inst Biol, 2016; 83: 1-5. DOI: 10.1590/1808-1657000112014
[5] Liu Y, Cui ZM, Kenis M et al. Antennal and behavioral responses of Drosophila suzukii to volatiles from a non-crop host, Osyris wightiana. Insects, 2021; 12: 166. DOI: 10.3390/insects12020166
[6] Aluja M, Mangan RL. Fruit fly (Diptera: Tephritidae) host status determination: Critical conceptual, methodological, and regulatory considerations. Annu Rev Entomol, 2008; 53: 473-502. DOI: 10.1146/annurev.ento.53.103106.093350
[7] Batista MS, Santos JM, Santos JRT et al. Frugivorous flies (Diptera: Tephritidae and Lonchaeidae) in the Mundaú Valley in the state of Alagoas, Brazil. Rev Ver, 2019; 14: 512-517. DOI: 10.18378/rvads.v14i4.6243
[8] Marchiori CH. Study of the ecology of the Tephritidae family (Insecta: Diptera). Access Res J Life Sci, 2021; 2: 047-080. DOI: 10.53022/oarjls.2021.2.1.0136
[9] Uchôa-Fernandes MA, Oliveira I, Molina RMS et al. Biodiversity of frugivorous flies (Diptera: Tephritoidea) captured in Citrus groves, Mato Grosso do Sul, Brazil. Neotrop Entomol, 2003; 32: 239-246. DOI: 10.1590/S1519-566X2003000200008
[10] Zucchi RA. Taxonomy, fruit flies of economic importance in Brazil: Basic and applied knowledge, 1st ed. Holos: Rio de Janeiro, Brazil, 2000.
[11] Marchiori CH. Check the biodiversity of the Lonchaeidae Family (Diptera). Access Res J Life Sci, 2022; 4: 27-43. DOI: 10.53022/oarjls.2022.4.1.0054
[12] MacGowan I, Rotheray G. British Lonchaeidae (Diptera, Cyclorrhapha, Acalyptratae), 1st ed. Royal Entomological Society of London: London, UK, 2008.
[13] Alvarenga CD, Canal NA, Zucchi RA et al. Fruit flies of economic importance in Brazil: Basic and applied knowledge, 1st ed. Holos: Rio de Janeiro, Brazil, 2015.
[14] Acurio AE, Rafael V. Taxonomic inventory of Drosophilidae (Diptera) in the Yasuni National Park, Ecuadorian Amazon. Acta Amazon, 2009; 39: 713-718. DOI: 10.1590/S0044-59672009000300028
[15] Bock IR. Current status of the Drosophila melanogaster species-group (Diptera). Sys Entomol, 1980; 5: 343-356. DOI: 10.1111/j.1365-3113.1980.tb00420.x
[16] Neelendra KJ, Biddinger DJ, Demchak K et al. First Report of Zaprionus indianus (Diptera: Drosophilidae) in commercial fruits and vegetables in Pennsylvania. J Insect Sci, 2014; 14: 1-4. DOI: 10.1093/jisesa/ieu121
[17] Marinho CF, Costa VA, Zucchi RA. Annotated checklist and illustrated key to braconid parasitoids (Hymenoptera, Braconidae) of economically important fruit flies (Diptera, Tephritidae) in Brazil. Zootaxa, 2018; 4527: 21-36. DOI: 10.11646/zootaxa.4527.1.2
[18] Souza AJB, Lima MGA, Guimarães JA et al. Fruit flies (Diptera: Tephritidae) associated to host plants from the orchard on the pici campus of the Federal University of Ceará, Brazil. Arq Inst Biol, 2009; 75: 21-27. DOI: 10.1590/1808-1657v75p0212008
[19] Alvarenga CD, Matrangolo CAR, Lopes GN et al. Fruit flies (Diptera: Tephritidae) and their parasitoids in host plants in three municipalities in the north of the state of Minas Gerais. Arq Inst Biol, 2009; 76: 21-17. DOI: 10.1590/1808-1657v76p1952009
[20] Leite GLD, Fialho A, Zanuncio JC et al. Bagging tomato fruits: A viable and economical method of preventing diseases and insect damage in organic production. Fla Entomol, 2014; 97: 50-60. DOI: 10.1653/024.097.0106
[21] Noyes JS. A study of five methods of sampling Hymenoptera (Insecta) in a tropical rainforest, with special reference to the Parasitica. J Nat Hist, 1989; 23: 285-298. DOI: 10.1080/00222938900770181
[22] Cruz PP, Neutzling AS, Garcia FRM. First record of Trichopria anastrephae, parasitoid of fruit flies, in the Rio Grande do Sul State, Brazil. Cienc Rural, 2011; 41: 1297-1299. DOI: 10.1590/S0103-84782011000800001
[23] Bragard C, Baptista P, Chatzivassiliou E et al. Pest categorisation of Zaprionus indianus. EFSA J, 2022; 20: e7144. DOI: 10.2903/j.efsa.2022.7144
[24] Marchiori CH, Silva CG. First occurrence of parasitoid Spalangia endius (Walker) (Hymenoptera: Pteromalidae) in pupae of Zaprionus indianus Gupta (Diptera: Drosophilidae) in Brazil. Braz J Biol, 2003; 63: 361-362. DOI: 10.1590/S1519-69842003000200022
[25] Bernardi D, Andreazza F, Botton M et al. Susceptibility and bioecology of Zaprionus indianus (Diptera: Drosophilidae) in strawberry culture interactions of Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae) in damaging strawberry. Neotrop Entomol, 2016; 7: 1-7. DOI: 10.1007/s13744-016-0423-9
[26] Cherre S, Silva B, Briana E et al. Factors affecting the biology of Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae), a parasitoid of spotted-wing drosophila (Drosophila suzukii). PLoS ONE, 2019; 14: e0218301. DOI: 10.1371/journal.pone.0218301
[27] Frank DL. Evaluation of fruit bagging as a pest management option for direct pests of apple. Insects, 2018; 9: 1-12. DOI: 10.3390/insects9040178
[28] Paranhos BJ, Nava DE, Malavasi DE. Biological control of fruit flies in Brazil. Pesqui Agropecu Bras, 2019; 54: e26037.
[29] Lachaise D, Tsacas L. Breeding sites in tropical african drosophilids. The genetics and biology of Drosophila, 1st ed. Academic Press London: London, UK, 1988.
[30] Vilelam CR, Teixeira EP, Stein CP. Mosca-africana-do figo, Zaprionus indianus (Diptera: Drosophilidae) Pragas introduzidas. Holos: Ribeirão Preto, Brazil, 2001.
[31] Gattelli T, Silva FF, Meirelles RN et al. Frugivorous flies associated with Myrtaceae and 'Ceu' orange in the region of Vale do Rio Cai, Rio Grande do Sul, Brazil. Cienc Rural, 2008; 38: 236-239. DOI: 10.1590/S0103-84782008000100038
[32] Lima MAC, Assis JS. Characterization of guava fruits and selection of cultivars in the Submédio São Francisco region. Rev Bras Frutic, 2002; 24: 273-276. DOI: 10.1590/S0100-29452002000100061
[33] Uramoto K, Walder JMM, Zucchi RA. Quantitative analysis and distribution of the population of species in the genus Anastrepha (Diptera: Tephritidae) on Luiz de Queiroz Campus, Piracicaba, SP, Brazil. Neotrop Entomol, 2005; 34: 33-39. DOI: 10.1590/S1519-566X2005000100005
[34] Alvarenga CD, Brito ES, Lopes EN. Introduction and recovery of the exotic parasitoid Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) in commercial guava orchards in northern Minas Gerais. Neotrop Entomol, 2005; 34: 133-136. DOI: 10.1590/S1519-566X2005000100020
[35] Bittencourt MAL, Silva ACM, Silva VES. Fruit flies (Diptera: Tephritidae) and their parasitoids (Hymenoptera: Braconidae) associated with host plants in southern Bahia. Neotrop Entomol, 2011; 40: 405-406. DOI: 10.1590/S1519-566X2011000300016
[36] Barclay HJ. Models for pest control: Complementary effects of periodic releases of sterile pests and parasitoids. Theor Popul Biol, 1987; 32: 76-89. DOI: 10.1016/0040-5809(87)90041-4
[37] Canal DNA, Zucchi RA, Silva NM et al. Faunistic analysis of parasitoids (Hymenoptera, Braconidae) of Anastrepha spp. (Diptera, Tephritidae) in Manaus and Iranduba, State of Amazonas. Acta Amazon, 1995; 25: 235-246. DOI: 10.1590/1809-43921995253246
[38] Deus EG, Pinheiro LS, Lima CR et al. Wild hosts of frugivorous dipterans (Tephritidae and Lonchaeidae) and associated parasitoids in the Brazilian Amazon. Fla Entomol, 2013; 96: 1621-1625. DOI: 10.1653/024.096.0453
[39] Dutra VS, Ronchi-Teles B, Garcia MVB. Native hosts and parasitoids associated with Anastrepha fractura and other Anastrepha species (Diptera: Tephritidae) in the Brazilian Amazon. Fla Entomol, 2013; 96: 270-273. DOI: 10.1653/024.096.0144
[40] Flores S, Campos S, Villaseñor A et al. Sterile males of Ceratitis capitata (Diptera: Tephritidae) as disseminators of Beauveria bassiana conidia for IPM strategies. Biocontrol Sci Technol, 2013; 23: 1186-1198. DOI: 10.1080/09583157.2013.822473
[41] Garcia FRM, Corseuil E. Native hymenopteran parasitoids associated with fruit flies (Diptera: Tephritidae) in Santa Catarina State, Brazil. Fla Entomol, 2004; 87: 517-521. DOI: 10.1653/0015-4040(2004)087[0517:NHPAWF]2.0.CO;2
[42] Garcia FRM, Ricalde MP. Augmentative biological control using parasitoids for fruit fly management in Brazil. Insects, 2013; 4: 55-77. DOI: 10.3390/insects4010055
[43] Leonel JR, Zucchi RA, Wharton RA. Distribution and tephritid hosts (Diptera) of braconid parasitoids (Hymenoptera) in Brazil. Int J Pest Manag, 1995; 4: 208-213. DOI: 10.1080/09670879509371951
[44] Marinho CF, Souza Filho MF, Raga A et al. Parasitoids (Hymenoptera: Braconidae) of fruit flies (Diptera: Tephritidae) in the State of São Paulo: Associated plants and parasitism. Neotrop Entomol, 2009; 38: 321-326. DOI: 10.1590/S1519-566X2009000300004
[45] Guimarães JA, Zucchi RA. Parasitism behavior of three species of Eucoilinae (Hymenoptera: Cynipoidea: Figitidae) fruit fly parasitoids (Diptera) in Brazil. Neotrop Entomol, 2004; 33: 217-224. DOI: 10.1590/S1519-566X2004000200012
[46] Gallardo FE, Diaz NB, Guimarães JA. Contribution to the systematics of Dicerataspis Ashmead, 1896 (Hymenoptera: Cynipoidea: Figitidae). Entomol News, 2010; 121: 23-30. DOI: 10.3157/021.121.0105
Copyright © 2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.













 Copyright ©
Copyright ©