Investigation of the Characteristics of the Milichiidae Family (Arthropoda: Insecta: Diptera)
Carlos Henrique Marchiori1*
1Department of Biological Science, Instituto Federal Goiano, Goias, Brazil
*Correspondence to: Carlos Henrique Marchiori, PhD, Professor, Department of Biological Science, Instituto Federal Goiano, Rua T-65 No. 1050, Goiânia, Goiás 74230120, Brazil; E-mail: chmarchiori@yahoo.com.br
Abstract
Most Milichiidae species are very small and dark. Details of their biology have not been properly studied. It is known that the Milichiidae are associated with decomposing plant and animal matter, including animal and human corpses, a reason that has potential importance in their forensic importance; several species are myrmecophilous predators, pollinators and phoretic vectors. This journal aimed to investigate the bioecology, the bionomy, the biography and systematics of the Milichiidae family (Arthropoda: Insecta: Diptera). The research was carried out based on its systematic aspects and family characteristics. The review was inventoried from works published in national and international journals.
Keywords: decomposition, kleptoparasites, foresy, forensics entomology
1 INTRODUCTION
Milichiidae is a family of Diptera Milichiidae Meigen, 1830 is a family of acalipteran dipterans (Schizophora Becher, 1882; Muscomorpha Sharp, 1894) from small dimensions and by general coloring obscure distributed in all biogeographic regions (Figure 1)[1-3].
|
Figure 1. Specimens of Michillidae family. A: Desmometopa varipalpis Malloch, 1927; B: Genus Leptometopa; C: Genus Protophormia; D: Genus Milichiella. Source: https://www.biodiversity4all.org/taxa/329891-Milichiidae
1.1 Description
Most species are very small and dark in color, and their biological details have not yet been properly studied (Figures 2-6)[4-6].
|
Figure 2. Leptometopa veracildae sp. nov. A: Male sternite 5, ventral view; B: Synsternite 7/8, posterior view; C: Male genitalia, posterior; D: Male genitalia, lateral view. Notes: ce: cerci; ep: epandrium; hyp: hypandrium; ph: phallus; phap: phallapodeme; preg: pregonite; sur: surtylus. Source: https://www.scielo.br/j/paz/a/jDjG43B9g9jZyD99cf3Q5LF/?lang=en#
|
Figure 3. Leptometopa veracildae sp. nov. A: Female ovipositor, dorsal view; B: Female ovipositor, ventral; C: Wing. Notes: ce: cercil; rbs: ring of barbed spines; S: sternite; sba: supranal plates; T: tergite. Source: https://www.scielo.br/j/paz/a/jDjG43B9g9jZyD99cf3Q5LF/?lang=en#
|
Figure 4. Phyllomyza guangxiensis sp. n. (male). 1: Head, lateral view; 2: Wing; 3 Epandrium, cerci, and surstyli, posterior view; 4: Epandrium, cerci, and surstyli, lateral view. Source: https://zenodo.org/record/1278060#.Y1Df5XZBxPZ
|
Figure 5. Neophyllomyza lii Xi & Yang, 2014 (male). 25: Head, lateral view; 26: Wing; 27: Epandrium, cerci, and surstyli, posterior view; 28: Epandrium, cerci, and surstyli, lateral view; 29: Genitalia, posterior view (hypandrium; phallapodemic; subepandrial); 30: Genitalia, lateral view (hypandrium; phallapodemic; subepandrial). Source: https://zookeys.pensoft.net/article/36247/zoom/fig/15
|
Figure 6. Desmometopa leptometopoides Sabrosky, 1983 (Diptera: Milichiidae), Male. External genitalia. A: Ventral view; B: Lateral view; C: Dorso-lateral (5×); D: Hypandrial complex in ventral view (10×); E: Magnification of surstyle and cerci (10×), clarification: KOH 20%; F: Surstyle (South) magnification (40×), clarification: KOH 20%; G: Hypandrial complex in dorsal view; H: Magnification of basiphallus and distiphalus (40×). Yellow arrows show the basiphallus and red arrows the distiphalus. Notes: Ce: cerci; Ep: epandrium; Hyp: hypandrium; St: sternuml; Sur: surstylus. Source: https://www.researchgate.net/figure/Figura-3-Desmometopa-leptometopoides-Macho-Genitalia-externa-Vista-ventral-A-vista_fig2_325722467
1.2 Biology, Ecology, Habitat and Distribution Geographic
Diptera are attracted feed to the body fluids of the prey or predator and to them as commensals. It has been better to designate such behavior as kleptoparasitism if you know very little about the biology of most taxa that make up the Milichiidae; all groups are associated with decomposing plant and animal matter (saprophagy and coprophagy), including animal and human corpses, which is why those beans have potential importance in their forensic role; several species among them have very specialized ducts, predators are myrmecophiles or pollinators and a phoretic vector (Figure 7)[7-12].
|
Figure 7. Milichia patrizii Hennig, 1952. 1: A Crematogaster tricolor Gerstaecker, 1859 (Hymenoptera: Formicidae) trail on an Acacia (Fabaceae); 2: A Milichia sp. into the middle of the Crematogaster Lund, 1831 trail to pursue an individual ant. This attempt was unsuccessful, as the ant was able to move past the fly without stopping; 3: An ant in a successful attack by grabbing the ant’s antennal club between the paired basoflagellomeres of its own antennae; 4: An ant in a successful attack by grabbing the ant’s antennal club between the paired basoflagellomeres of its own antennae. The ant crouches down while the fly initiates the gurgitation process by extending its proboscis into the mouthparts of the ant. 5: General view; 6: An enlargement showing details of the food exchange interaction. The ant’s antenna is enfolded by the pilose clypeal membrane of the fly’s proboscis. The long hairs on the fly’s proboscis likely serve to capture liquids from the mouth of the ant; 7: The ant briefly sits motionless following the interaction; 8: Trophallaxis food-sharing behaviour between two Crematogaster tricolor Gerstaecker 1859 (Hymenoptera: Formicidae) nestmates. Sources: Wild & Brake, 2009 and https://milichiidae.myspecies.info/category/classification/milichia-patrizii
Kleptoparasitism or commensalism: It is a feeding form in which one animal deliberately takes food from another. This strategy is evolutionarily stable when theft is less costly than direct feeding, that can mean when food is scarce or when it is plentiful.
Phoretic vector: It captures a fly of another species during flight and lays its eggs in the captured fly's abdomen. Captured flies are true vectors for their eggs and are also called phoretics. The main vectors are the horn fly, stable fly and housefly.
Definition of forensic flies: The most common are those called scavengers, because in their larval stage they feed on the tissues of the corpse until they reach the moment when they take shelter somewhere to complete their metamorphosis.
Forensic importance: Entomology is a broad and diverse field of research that includes arthropods that interact with various aspects of life and human activities and products derived from them, such as food, housing and construction, health and the environment. Among the divisions of Entomology, it is in Forensic Entomology that the interaction between arthropodology and the judicial system takes place.
It can be said that Forensic Entomology is the science that applies the study of insects and other arthropods to legal proceedings. It is divided into three main areas focused on topics that are frequently the subject of litigation (Figure 8).
|
Figure 8. Milichiidae attending Rhinocoris bugs mating as the female feeds. A: Neophyllomyza sp. on bee killed by ambush bug; B: Freeloader flies (Milichiidae); C and D: Desmometopa; E, F and G: Milichiidae family. Sources: https://commons.wikimedia.org/wiki/File:Milichiidae_fg01.jpg; https://www.biodiversity4all.org/taxa/329891-Milichiidae; https://bugguide.net/node/view/1145632 and Photo 16193462, (c) Wynand Uys; Photo 16193462, (c) Wynand Uys; Photo 15693329, (c) Magriet b.
The larvae of these insects feed primarily on decaying plant matter, decaying wood or bark/rind and may be reared from manure or decaying plant materials (Figures 9-11)[13-15].
|
Figure 9. Family Milichiidae feeding on organic animal material. Sources: https://www.biodiversity4all.org/taxa/329891-Milichiidae
|
Figure 10. A crab spider (top left) that has killed a bullet ant queen and at least 2 species of small flies (Milichiidae & Phoridae) have arrived to feed from the carcass and/or lay their eggs. Source: https://www.antwiki.org/wiki/Spiders
|
Figure 11. Chemical structures of phytosterols identified in Momordica charantia L. (Cucurbitaceae) fruit and leaves. Numerous compounds that have been identified in the extracts of M. charantia, including phytosterols, terpenoids, fatty acids, phenolic compounds, phenolic acids and flavonoids. the biological effects of M. charantia extracts, including their antidiabetic, neuroprotective, anti-obesogenic, antimalarial, antioxidant, anti-inflammatory, antimicrobial and allelopathic activities. Source: https://www.semanticscholar.org/paper/Phytochemical-profile-and-biological-activities-of-Oliveira-Costa/c1f01cd9b99ad8bcb55149843ba94a28e259e0ce
The fly also feeds on other animals, but does not capture them, so it accompanies the bed bug in order to take advantage of the food it kills. The genus Desmometopa may be kleptoparasites of predatory insects such as Redbots (Heteroptera) and such some spiders such as Homises and Nephiles (Figure 12)[15,17-19].
|
Figure 12. Genus Nephila (spider). Source: https://en.wikipedia.org/wiki/Nephila
1.3 Taxonomy
The Michillidae family is composed of almost 250 species of small flies, distributed in 19 genera. Como for them classification schemes there are divergences among the expert’s group (Figure 13)[20,21].
|
Figure 13. Leptometopa latipes (Meigen, 1830). Source: https://www.diptera-info.nl/infusions/checklist/view_family.php?fam_id=158
1.3.1 Subfamily Madizinae
Aldrichiomyza Hendel, 1914, Desmometopa Loew, 1866, Enigmilichia Deeming, 1981 Leptometopa Becker, 1903, Madiza Fallén, 1810, Paramyia Williston, 1897 and Stomosis Melander, 1913 (Figure 14A)[22,23].
|
Figure 14. Subfamily Madizinae. Sources: https://bugguide.net/node/view/1210747 and https://en.wikipedia.org/wiki/Milichiidae
1.3.2 Subfamily Milichiinae
Eusiphona Coquillett, 1897, Milichia Meigen, 1830, Milichiella Giglio-Tos, 1895, Pholeomyia Bilimek, 1867 and Ulia Becker, 1907 (Figure 14B).
1.3.3 Subfamily Phyllomyzinae
Orneomyia Brake, 2004, Costalima Sabrosky, 1953, Microsimus Aldrich, 1926, Neophyllomyza Melander, 1913, Paramyioides Papp, 2001, Phyllomyza Fallén, 1810 and Xenophyllomyza Ozerov, 1992 (Figures 15 and 16)[24-26].
|
Figure 15. Subfamily Phyllomyzinae. Source: http://v3.boldsystems.org/index.php/Taxbrowser_Taxonpage?taxid=589086
|
Figure 16. Wings. A: Myolepta nigritarsis Coe 1957, wing; B: Myolepta dubia (Fabricius, 1805), wing; C: Hammerschmidtia ferruginea (Fallén, 1817), wing; D: Brachyopa pilosa Collin, 1939, wing; E: Melanogaster nuda (Macquart, 1829), wing; F: Dipteran phylogeny. Source: http://drawwing.org/insects/diptera/milichiidae
1.4 Objective
The objective of this work is to investigate the bioecology, bionomy, biography and systematics of the Milichiidae family (Arthropoda: Insecta: Diptera).
2 METHODS
The method used to prepare this mini review was Marchiori, 2021 methodology[27].
3 STUDIES CONDUCTED AND SELECTED
3.1 Study 1
3.1.1 Family Milichiidae
The Milichiidae are small, elongated, black or silver flies. The larvae live in decaying plant or animal material. Some are commensals or predators. Some species are ectoparasites of birds.
3.1.2 Subfamily Madizinae
Desmometopa (Platophrymyia) tarsalis Loew, 1866.
Desmometopa (Platophrymyia) tarsalis Loew, 1866.
Platophrymyia nigra Williston, 1896 [Desmometopa] (Figure 17).
|
Figure 17. Desmometopa. Source: https://species.wikimedia.org/wiki/Desmometopa
Distribution: Asia, Hawaii, USA, Cuba, St. Vincent, Grenada, Barbados, Sta. Lucía, Dominica, Dominican, Montserrat, Virgenes, Puerto Rico, Bahamas, Jamaica, Mexico, Guatemala, Belize, El Salvador, Honduras, Nicaragua, Costa Rica, Panama, Colombia, Tobago, Venezuela, Guyana and Ecuador.
Biology: Saprophagous.
3.1.3 Subfamily Milichiinae
Milichiella lacteipennis (Loew, 1866) (Figure 18).
|
Figure 18. Milichiella lacteipennis (Loew, 1866). Source: https://milichiidae.myspecies.info/category/classification/milichiella-lacteipennis
Distribution: Dominica, Cuba, Hispaniola, Jamaica, Puerto Rico, St. Vincent, Is. Virgins, St. Thomas, Mexico, Nicaragua (Managua), Costa Rica, Panama, Venezuela, Peru and Brazil.
Collected on: Poaceae: Zea.
3.1.4 Subfamily Phyllomyzinae
Pholeomyia longiseta (Becker, 1907).
Milichia longiseta Becker, 1907 [Paramilichia and Rhynchomilichia] (Figure 19).
|
Figure 19. Milichia longiseta Becker, 1907. Source: https://milichiidae.myspecies.info/category/classification/milichiella-longiseta
Distribution: Nicaragua, Paraguay and Argentina.
3.1.5 General Summary
1-Body symmetry: Bilaterally symmetrical.
2-Is eaten by Ictinogomphus rapax (Rambur 1842).
3-Diurnal behavioral circadian rhythm; holometabolous mode of development; eats Apis mellifera Linnaeus 1758.
4-First appearance 70.59999999999 million years ago.
5-Habitat: Field.
6-Number of fossil occurrences: 13.
7-Visits flowers of Lavandula stoechas L. (Lamiaceae).
8-Visual system ocelli wing[28-32].
3.2 Study 2
The aim of this study was to report the first record of D. leptometopoides for the Neotropical region and D. varipalpis for Venezuela (Figure 20).
|
Figure 20. Desmometopa leptometopoides Sabrosky, 1983 (Diptera: Milichiidae). Source: https://milichiidae.myspecies.info/category/classification/desmometopa-leptometopoides
The aim of this study was to report the first record of Desmometopa leptometopoides Sabrosky, 1983 (Diptera: Milichiidae) for the Neotropical region and Desmometopa varipalpis Malloch, 1927 for Venezuela.
Between May and July 2017, 10 daytime catches (N1=45, N2=210, N3=11, N4=55, N5=43, N6=25, N7=68, N8=54, N9=23, N10=91) with glass suction cups, small adult flies. The insects were transported to the Laboratory of Entomology, Parasitology and Tropical Medicine National Experimental University Francisco de Miranda (Figure 21).
|
Figure 21. Desmometopa varipalpis Malloch, 1927 for Venezuela. Source: https://bugguide.net/node/view/675592
Analysis of external and internal morphology of imagos, especially of 311 copies males, using taxonomic keys allowed the identification of the species D. leptometopoides (N=309♂; 5♀) and D. varipalpis (N=2♂).
Desmometopa leptometopoides has been reported on the African continent (Nigeria, South Africa, Liberia, Cameroon, Ghana and Tunisia) maintained from mud and debris from swimming pools, and in the Nearctic Region (Florida, the United State) obtained and bred to from the fruits of Momordica charantia L. (Cucurbitaceae) (Figure 22).
|
Figure 22. Momordica charantia L. fruit and leaves (Cucurbitaceae). Source: https://academicjournals.org/journal/AJB/article-abstract/14B9F2057674
Therefore, this appears as the first report of this species of Milichiidae for the Neotropical region and particularly for the country. In contrast, D. varipalpis has a wide distribution that covers practically all the zoogeographic zones.
In light of what has been discussed, detailed studies are needed to quantify the role of Desmometopa taxa as quality indicators in the dynamics of composting in the bioecological conditions given in Venezuela, especially in cities where urban agriculture can represent a viable alternative for many families.
It is important to highlight the findings of some species of Desmometopa (e.g. Desmometopa varipalpis) and others unidentified as part of the cadaverous fauna, both in humans and animals, so that they are considered as again that possess a potential to be used in legal medicine investigations, being able to be used for the estimation of the Intervals Post Morten (Forensic Entomology) (Figure 23).
|
Figure 23. Sources of inaccuracy in the estimation of the post-mortem interval based on insect evidence. Green circles represent the sources, their size represents importance of the sources. Sources: https://www.mdpi.com/2075-4450/12/4/314
The international trade of tropical fruits appears as the probable mechanism of expansion of the populations of D. leptomatotopoides and D. varipalpis, which seem to have adapted successfully in the Falconian urban semi-arid[33,34].
3.3 Study 3
Desmometopa varipalpis Malloch, 1927, an interesting species of Diptera common in urban areas. Species of this family are common in cities where they breed in culverts. They are also common in manure compost. Their appearance in large numbers in the bases of buildings is usually an indication that there is a blockage or leak in the sewage network (Figure 24).
|
Figure 24A. Desmometopa sordida (Fallén, 1820). Figure 24B. Desmometopa varipalpis Malloch. A: 1-Foresy exerted by the species a sordida Fallén; B: 2-Foresy exerted by the species D. varipalpis. Sources: https://milichiidae.myspecies.info/category/classification/desmometopa-sordida and https://bugguide.net/node/view/675592
In truth, in some areas it is more common and it can become a more captured species using an adhesive sheet of. This is a nice device from Sani trade in which Desmometopa varipalpis is or more abundant (Figure 25).
|
Figure 25. Detail of the adhesive sheet. Source: C. Pradera 08-2021
Description: 1-Wing venation with very visible hairs. 2-Head appears clearly between the eyes, black on a gray background. 3-Hind legs whose tarsomeres are light and also the gena are light (Figure 26).
|
Figure 26. Wing of Desmometopa varipalpis Malloch, 1927. Source: C. Pradera 08-2021.
Its presence in urban areas is due to the fact that the larva is coprophagous and carnivorous. Therefore, it can be found in wastewater and manure. It also appears to be saprophagous and can be found in other decaying plants. In fact, the device for capturing insects was located near a company dedicated to processing grain (Figure 27A and B).
|
Figure 27. Desmometopa varipalpis Malloch, 1927. A and B: Head of D. varipalpis; C: Back tarsi of D. varipalpis. Source: C. Pradera 08-2021
In adults of the Desmometopa genus, a common relationship with predators such as spiders has been observed. The spiders are slow at the time of ingesting their prey and they first have to detach their victims from the inside and suck them. These are used by flies to suck fluids from the prey that the spiders have captured. Adults measure between 2-3mm. The larva develops in 2-3 weeks (Figure 27C)[35].
3.4 Study 4
3.4.1 Genera and Species of Milichiidae in Venezuela.
In Venezuela, knowledge about the Milichiidae family is very limited, as far as it has been possible to investigate, only three genera have been reported Pholeomyia Bilimek, 1867, Milichiella Giglio-Tos, 1895, Desmometopa Loew, 1866 and six species, including Pholeomyia hurdi Sabrosky, 1959, Pholeomyia myopa Melander, 1913, Pholeomyia politifacies Sabrosky, 1959, Milichiella lacteipennis Loew, 1866 and Desmometopa tarsalis Loew, 1866 (Figures 28-30)[36].
|
Figure 28. Pholeomyia hurdi Sabrosky, 1959. Source: Hurd PD Collector.
|
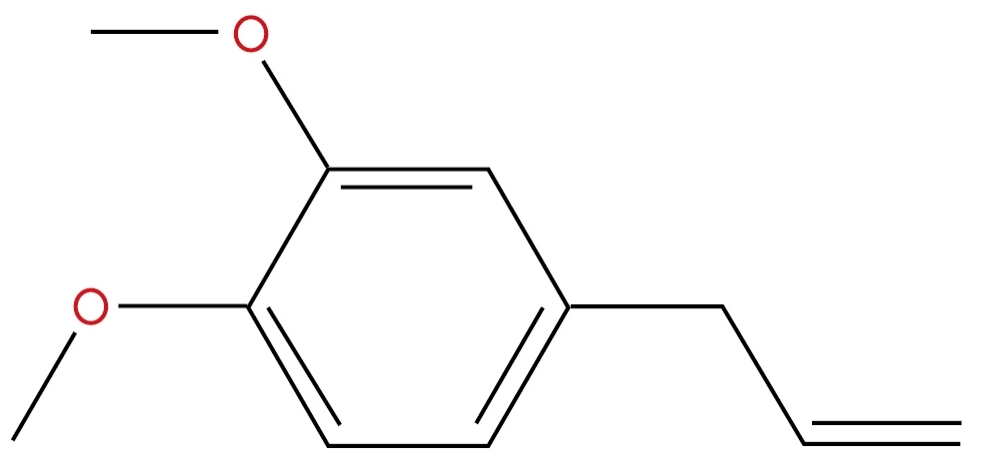
Figure 29. 1,2-Dimethoxy-4-(2-propenyl)-benzene Formula: C11H14O2. Semiochemical, Floral Compound um two components to attract floral agents. Source: https://www.pherobase.com/database/compound/compounds-index.php
|
Figure 30. Milichiella lacteipennis Loew, 1866. Source: Photo taken by I. Brake.
3.5 Study 5
The genus Eusiphona Coquillett, 1897, of the Milichiidae family, is characterized by its long and thin proboscis that, in addition, appears to be geniculate, it was known only from the Nearctic region. A key to aid your recognition, with notes on the bionomy of Eusiphona cooperi Sabrosky 1955 was published by Sabrosky in 1955 (Figure 31).
|
Figure 31. Genus Eusiphona Coquillett, 1897, of the Milichiidae family. Sources: Photo: P. Brake and https://bugguide.net/node/view/375129/bgpage
After the publication of the catalog, two specimens of an unidentified species of Eusiphona from Brazil, were collected in 1929 and 1937 and two others hunted in Costa Rica. These findings predate the introduction in Latin America, from the United State, of Megachile rotundata (Fabricius, 1793) (Hymenoptera: Megachilidae); in addition, the Brazilian specimens apparently correspond to the new species, which reinforces the opinion that we are in the presence of a Neotropical form. (Source: Sabtosky CW. A new species of Eusiphona from the Argentine Republic. (Diptera, Milichiidae). Rev Museo La Plata, 1982; 13: 1-128) (Figure 32)[37].
|
Figure 32. Megachile rotundata (Fabricius, 1793) (Hymenoptera: Megachilidae). Source: Pest and Diseases Image Library.
3.6 Study 6
In the late 1970s and early 1980s, the behavior of a species of Megachilidae, commonly called “cutting bees”, was investigated in the Canada de Gomez area, Santa Fe province, Argentina.
During observations in those years, it was found that many of the megachilid nests that were studied were parasitized with pupae that responded to the characteristics of some dipterans. They were found inside these nests. Once this cell is made, the bee carries pollen with its scopa which collects from different species of flowers, up to about half of the cell. He then lays an egg and closes the cell with circular cutouts (Figure 33).
|
Figure 33. Megachilidae family (Hymenoptera: Apoidea). Source: https://genent.cals.ncsu.edu/insect-identification/order-hymenoptera/family-megachilidae
In the first place, it was observed that these dipteran pupae, as mentioned above, were inside these cells, more precisely between the cuts that constitute them, leaving the interior empty, with remains of excrement and without the pollen and eggs that had been deposited by the bee. Then, in subsequent observations, the larvae were found inside the cells feeding on the pollen. There was no doubt that these pupae came from larvae that fed on pollen, leaving no food for the megachilid larva and, even more, unable to hatch the egg.
The type of interspecific relationship between the dipteran and the megachilid was thus determined: that of parasitism. Later, adult females could also be observed floating around megachilid nests to lay eggs. Possibly, egg laying occurred when the Megachilidae left the cell with pollen without completing it, for the next day, as night had already arrived. These bees do not stay in the nest at night or on rainy days or other inclement weather. Therefore, the nests are exposed to these dipterans and other natural enemies (Figure 34).
|
Figure 34. Costal breaks. hb: Humeral break; sb: Subcostal break. Longitudinal veins: C: Coast; Sc: Subcosta; R: Radius; M: Medium; Cu: Cubitus; A: Anal. Crossveins: h: Humeral; r-m: Radial-medial; bm-cu: Medial-ulnar basal; dm-cu: Medial-ulnar disc. Cells: br: 1st basal; bm: 2nd basal; dm: disc; cup: cell cup. Source: https://commons.wikimedia.org/wiki/File:Eusiphona_wing_veins.svg
In the first place, it was observed that these dipteran pupae, as mentioned above, were inside these cells, more precisely between the cuts that constitute them, leaving the interior empty, with remains of excrement and without the pollen and eggs that had been deposited by the bee. Then, in subsequent observations, the larvae were found inside the cells feeding on the pollen. There was no doubt that these pupae came from larvae that fed on pollen, leaving no food for the megachilid larva and, even more, unable to hatch the egg[38].
The type of interspecific relationship between the dipteran and the megachilid was thus determined: that of parasitism. Later, adult females could also be observed floating around megachilid nests to lay eggs. Possibly, egg laying occurred when the Megachilidae left the cell with pollen without completing it, for the next day, as night had already arrived. These bees do not stay in the nest at night or on rainy days or other inclement weather. Therefore, the nests are exposed to these dipterans and other natural enemies (Figure 35).
|
Figure 35. Pupae of genus Eusiphona. Source: https://milichiidae.myspecies.info/taxonomy/term/403/media
A systematic observation revealed a Diptera of the Milichiidae family, of the genus Eusiphona and of the cooperative species, first described by Sabrosky in 1955. Morphological characteristics for these species. Therefore, it turned out to be a new species of the genus Eusiphona which he called species Eusiphona vittata, Sabrosky 1982[38].
4 CONCLUSION
Most Milichiidae species are very small and dark in color and the details of their biology have not yet been properly studied. It is known that the Milichiidae are associated with decomposing plant and animal matter (saprophagy and coprophagy), including animal and human corpses, which is why it has potential importance in its forensic importance; several species have very specialized ducts, predators are myrmecophiles or pollinators and a phoretic vector.
Acknowledgements
I thank the Editor and his working group for publishing my article in the Journal of Modern Agriculture and Biotechnology.
Conflicts of Interest
The author declared no conflict of interest.
Author Contribution
Marchiori CH contributed to the manuscript and approved the final version.
References
[1] McGraw-hill dictionary of scientific & technical terms. Accessed August 18, 2022. Available at https://encyclopedia2.thefreedictionary.com/Milichiidae
[2] Dawah HA, Abdullah MA. New records of some filth flies species (Diptera: Milichiidae) in Southwest Saudi Arabia. Accessed August 18, 2022. Available at http://inis.iaea.org/search/search.aspx?orig_q=RN:40087325
[3] The Database of the Iberian Fauna. National Museum of Natural Sciences (CSIC). Accessed August 18, 2022. Available at https://www.gbif.es/en/instituciones/museo-nacional-de-ciencias-naturales-csic-madrid/
[4] Desiraju GR, Steiner T. The weak hydrogen bond in structural chemistry and biology, 2nd ed. Oxford University Press: Northampton shire, UK, 1999.
[5] Colless DH, McAlpine DK. Diptera (flies): The insects of Australia, 2nd ed. University Press: Melbourne, Australia, 1991.
[6] Richards OW, Davies RG. Imms general textbook of Entomology: Structure, physiology and development, classification and biology, 2nd ed. Springer: Berlin, Germany, 1977. DOI: 10.1007/978-94-017-0472-4
[7] Emily S, Steven T, Cassidy ST et al. Parasites of spiders: Their impacts on host behavior and ecology. J Arachnol, 2021; 49: 281-298. DOI: 10.1636/JoA-S-20-087
[8] Kahanpää J, Stuke JH. Checklist of the superfamilies Conopoidea, Diopsoidea and Nerioidea of Finland (Insecta, Diptera). Zookeys, 2014; 19: 251-2577. DOI: 10.3897/zookeys.441.7227
[9] Roskov Y, Ower G, Orrell T. Species 2000 & IT IS Catalog of life: Digital resource: Species 2000, 1st ed. Naturalis: Leiden, Netherlands, 2020.
[10] Dictionary of scientific & technical terms, 6E. Accessed August 18, 2022. Available at https://encyclopedia2.thefreedictionary.com/Milichiidae
[11] The Free Dictionary Milichiidae. McGraw-Hill dictionary of scientific & technical terms. Accessed August 18, 2022. Available at https://encyclopedia2.thefreedictionary.com/Milichiidae
[12] Amat E. Notes on necrophagous flies (Diptera: Calyptratae) associated to fish carrion in Colombian Amazon. Acta Amazon, 2010; 40: 397-400.
[13] Greenberg B. Flies and disease: Biology and disease transmission, 2nd ed. Princeton University Press: Princeton, USA, 1973.
[14] Greenberg B, Szyska ML. Immature stages and biology of fifteen species of Peruvian Calliphoridae (Diptera). Ann Entomol Soc Am, 1984; 77: 488-517. DOI: 10.1093/aesa/77.5.488
[15] Greenberg B, Kunich JC. Entomology and the law. Flies as forensic indicators, 1st ed. Cambridge University Press: Cambridge, UK, 2002.
[16] Byrd HJ, Castner LJ. Forensic Entomology: The utility of arthropods in legal investigation, 1st ed. Technical textbooks: Boca Raton, USA, 2010.
[17] Benecke M. A brief history of forensic entomology. Forensic Sci Int, 2001; 120: 2-14. DOI: 10.1016/S0379-0738(01)00409-1
[18] Stehr F. Immature insect, 2nd ed. Kendall/Aunt Publishing Company: Iowa, USA, 1991.
[19] Coast CS, Simonka CE. Insects immature: metamorphosis and identification. 1st ed. Hollos Editora: Rio de Janeiro, Brazil, 2006.
[20] Mañas-Jordá S, León-Cortés JL, García-García MD et al. Dipteran diversity and ecological succession on dead [pigs in contrasting mountain habitats of Chiapas, Mexico. J Med Entomol, 2017; 55: 59-68. DOI: 10.1093/jme/tjx190
[21] Carvalho CJB, Couri MS, Rafael JA et al. Insects from Brazil-diversity and taxonomy, 1st ed. Holos Publisher: São Paulo, Brazil, 2012.
[22] Förster M, Sievert K, Messler S et al. Comprehensive study on the occurrence and distribution of pathogenic microorganisms carried by synanthropic flies caught at different rural locations in Germany. J Med Entomol, 2009; 46: 1164-1166. DOI: 10.1603/033.046.0526
[23] Kutty SN, Pape T, Wiegmann BM et al. Molecular phylogeny of the Calyptratae (Diptera: Chyclorrhapha) with an emphasis on the superfamily Oestroidea and the position of Mystacinobiidae and McAlpine’s fly. Syst Entomol, 2010; 25: 614-635. DOI: 10.1111/j.1365-3113.2010.00536.x
[24] Torsten D. Natural history specimens collected and/or identified and deposited. Accessed August 11, 2022. Available at https://zenodo.org/record/6762844#.Yv6GGXbMLIU
[25] Sivinski J, Marshall S, Petersson E. Kleptoparasitism and phoresy in the Diptera. Fla Entomol, 1999; 82: 179. DOI: 10.2307/3496570
[26] Borror DJ, Triplehporn CA, Johson JF. An introduction to the study of insects, 7th ed. Saunders College Publishing: Philadelphia, USA, 2005.
[27] Marchiori CH. Biology and feeding behavior of ceratopogonid adult (Diptera: Ceratopogonidae). Int J Front Sci Tech Res, 2021; 1: 7-24. DOI: 10.53294/ijfstr.2021.1.2.0073
[28] BejBienko GY. Keys to the insects of the European, 1st ed. Brill Archive: New York, USA, 1989.
[29] Kumara TK, Hassan A, Salmah MR. A report on the pupae of Desmometopa sp. (Diptera: Milichiidae) recovered from a human corpse in Malaysia. Trop Biomed, 2010; 27: 131-133.
[30] Bugguide.net. Family Milichiidae-freeloader flies. Accessed August 18, 2022. Available at https://bugguide.net/node/view/23319
[31] Perez-Balam J, Quezada-Euan JJ, Alfaro-Bates R et al. The contribution of honey bees, flies and wasps to avocado (Persea americana) pollination in southern Mexico. J Pollination Ecol, 2012; 8: 42-47. DOI: 10.26786/1920-7603(2012)6
[32] Natural history specimens collected and/or identified and deposited. Accessed August 18, 2022. Available at https://clinicaltrials.gov/ct2/show/NCT00769704
[33] Mashaly A. Entomofaunal succession patterns on burnt and unburnt rabbit carrion. J Med Entomol, 2016; 53: 296-303. DOI: 10.1093/jme/tjv202
[34] Cazorla-Perfetti D, Morales-Moreno P. First record of Desmometopa leptometopoides Sabrosky, 1983 (Diptera: Milichiidae) for the Neotropical region and Desmometopa varipalpis Malloch, 1927 for Venezuela. Saber, 2018; 30: 126-137.
[35] Pradera C. On the fly Desmometopa varipalpis (Diptera, Milichiidae). Accessed August 19 2022. Available at https://desinsectador.com/2021/08/08/sobre-la-mosca-desmometopa-varipalpis-diptera-milichiidae/
[36] Insect pest control newsletter no. 89. Accessed August 18, 2022. Available at https://www.iaea.org/publications/12256/insect-pest-control-newsletter-no-89-july-2017
[37] Magazine of the Museum of La Plata. New Series. Section Zoology. Accessed August 18 2022. Available at https://publicaciones.fcnym.unlp.edu.ar/rmlp/article/view/1998
[38] Torresi NA. A new species of Diptera of the family Milichiidae (Entomology). Accessed August 18 2022. Available at http://biologiaengral.blogspot.com/p/una-nueva-especie-de.html
Copyright © 2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.







































 Copyright ©
Copyright ©