Biochemical Properties of Crude Alkaline Protease Produced by Bacillus thuringiensis dendrolimus IP 4A/4B under Solid-State Fermentation Conditions
Amira M Roshdy1*, Hoda M Shata1, Safaa M Ali2, A M Youssef2, M S Foda1, T Kahil1
1Microbial Chemistry Department, Biotechnology Research Institution, National Research Centre, Cairo, Egypt
2Agricultural Biochemistry Department, Faculty of Agriculture, Mansoura University, Mansoura, Egypt
*Correspondence to: Amira M Roshdy, PhD, Associate Professor, Department of Microbial Chemistry, Biotechnology Research Institution, National Research Center, 33 El Buhouth St., Dokki, Cairo 12622, Egypt; Email: amiraroshdy82@yahoo.com
Abstract
Objective: This paper aims to, study the biochemical properties of the crude enzyme, extracted from the bacterial strain Bacillus thuringiensis dendrolimus IP 4A/4B, such as the concentration of the enzyme, the duration of the enzymatic reaction, the effect of changing the temperature of the enzymatic reaction and the effect of the pH of the enzymatic reaction medium.
Methods: Inoculum preparation, submerged and solid-state fermentation media, enzymes assays spectrophotometrically, protein determination were performed.
Results: The results indicated that 0.181mg protein/mL of enzyme produced maximum alkaline protease activity (269U/mL/min) followed by 0.227mg protein/mL enzyme (260U/mL/min). The slope of the first period (up to 5min reaction time) exhibited steeper slope indicating reactions velocity near the theoretical initial velocity (Vmax) that has become lesser in value as the reaction was extended up to 120min. The maximum enzyme activity was at 60°C (246U/mL/min), while decreased gradually to reach (188U/mL/min) at 90°C. It was clear that the optimum pH was 9.0 when glycine-NaOH and Tris-HCl buffers were used.
Conclusion: The optimum pH for the enzymatic reaction pH 9 indicates the potential for this crude enzyme to be used in many industries that require an alkaline pH, and at the same time, the optimum temperature for the enzymatic reaction of 60°C enhances this applicability; this temperature is widely available in many industries.
Keywords: biological control, Bacillus thuringiensis dendrolimus, crude alkaline protease, alkaline protease characterization
1 INTRODUCTION
Bacillus thuringiensis (B.t.) genus is considered one of the most important bio insecticides, because of distinguishing feature of its crystal protein toxin (prototoxin) that is selective to infect many pests without causing any harm to humans, vertebrates or plants, enabling it the forefront of applied bio pesticides. It represents about 90% of the bio pesticides already used globally for many insects such as Diptera, Lepidoptera, Hymenoptera, Hemiptera, Coleoptera, Mallophaga, Orthoptera and others, and it is also considered a biocidal for many other pests, as is the case in nematodes, snails and protozoa.
There are three scientific theories interpreting the mechanism of action of the crystal prototoxin protein. a) Classic pathway mechanism. The prototoxin is ingested by the insect, which dissolves in the alkaline environment of the insect's intestine and then hydrolyzed by alkaline protease enzymes in the insect's midgut, producing proteins of low molecular weight (about 60kDa). It in turn binds to the cellular receptors of the insect, forming polymeric structures with the ability to penetration of the lipid membrane of the insect cells, causing holes in the membrane, disturbing the cell pressure balance, causing dehydration and cell death. b) Signal pathway mechanism. Here, B.t. prototoxin is considered an activator of the signal transduction system dependent on Mg2+ and protein kinase, and a series of cellular phenomena such as membrane venting, cell swelling and the appearance of nuclear phantoms begin, then the cells decompose and die. c) Continuous binding pathway mechanism. After the B.t. prototoxin is converted into a low molecular weight protein, during its journey through the insect's intestine and its alkaline protease enzymes, it induces conformational changes that promote the introduction of the toxin into the cell membranes; Programmed cell death occurs and the insect dies[1].
Protease enzymes represent two-thirds of the enzymes of industrial application[2]. Proteases are considered one of the most important industrial enzymes because of their long applied history in wide sectors of different industrial fields, such as food, pharmaceutical, chemical, leather and many others industries. With the applied importance of proteases as a bio-catalyst factor, microbial protease is on the throne for its ease, abundance and economical production, so studying its biochemical properties such as pH, temperature, reaction time and enzyme concentration is of great importance to raise the efficiency of its application. In view of the role of B.t. in the biological resistance of insects through crystalline protein prototoxin and alkaline protease enzymes of the insect midgut itself, the strains of B.t. vector of alkaline protease in addition to the prototoxin will enhance its function as a biocidal[3].
Hence, the current study aims to study the biological properties of alkaline protease produced by B.t. subspecies dendrolimus IP-4A/4B.
2 MATERIALS AND METHODS
2.1 Bacterial Strains
The B.t. subspecies dendrolimus IP-4A/4B examined in the present studies was obtained from Microbial Chemistry Department, National Research Centre, Dokki, Cairo, Egypt culture collection (Institute Pasteur Collection, Paris, France).
2.2 Media Used for Growth and Production of Alkaline Protease (AP)
2.2.1 Standard Medium (Nutrient-broth Yeast-extract Salt Medium)
According to Foda et al.[4] these media were used for activation of the inoculum for 24h. Medium composition is as follows (g/L); peptone (5), beef extract (3), yeast extract (0.5), MnCl2 (0.01), CaCl2 (0.1), MgCl2 (0.2), (NH4)2 SO4 (8), K2HPO4 (5.6), KH2PO4 (2.4), MgSO4 (0.8), MnCl2 (0.3), FeCl2 (0.14), ZnSO4 (0.2), thiamin (0.2) and biotin (2µg/L).
2.2.2 Production of AP under Solid-State Fermentation
With some modifications of Prakasham et al.[5] method, five grams of coarse wheat bran was taken in a 500mL Erlenmeyer flask, a predetermined quantity of 50mM potassium phosphate buffer, pH 7.4, was added, mixed thoroughly and autoclaved at 121°C and 15lbs pressure for 15min. The flasks were cooled to room temperature, inoculated with 2mL of 24h grown bacterial culture under sterile conditions and incubated at 30°C temperature for 3d.
2.3 Assay of Alkaline Protease Activity (APA)
2.3.1 APA
The activity of AP was determined as described by Lowry et al.[6] with some modifications. The standard reaction mixture composition (unless otherwise stated) contained 0.5mL of enzyme source (culture supernatant or supernatant after purification steps), 1mL of glycine-sodium hydroxide buffer 0.1M, pH 9.6, in which 1% casein was dissolved as substrate. The reaction mixture was incubated at 40ºC for 20min after which the enzymatic reaction was stopped by addition of 1mL of 20% trichloroacetic acid with thoroughly mixing. The stopped reaction mixture was then centrifuged at 4000rpm for 10min without disturbing the precipitated proteins.
Series of clean tubes were used, and 0.5mL of the clear solution was pipetted in each clean tube and 5mL of Reagent C were added and mixed well. The mixture was let to stand at room temperature for 10min. Reagent D was added rapidly to the mixture and mixed immediately. The mixture was allowed to stand at room temperature for 10min and then the optical density of the developed color was read at 600nm. The readings of the optical densities of zero time cleared reaction mixtures were subtracted from those of the experimental tubes.
The optical densities were converted to the equivalent grams of tyrosine using standard curve of authentic tyrosine concentrations (10-200mg/mL) that were treated similarly to experimental enzyme-catalyzed reaction mixtures.
The enzyme activity was expressed in term of enzyme units. One AP unit was defined as the amount of enzyme that liberates one-microgram tyrosine per mL enzyme per minute under the specified reaction conditions.
|
Reagents:
Reagent A: 2% Na2CO3 in 0.1N NaOH
Reagent B: 0.5% CuSO4.5H2O in 1% sodium and potassium tartrate
Reagent C: 50mL of reagent A was mixed with 1mL of reagent B. Made fresh daily.
Reagent D: 1 part Folin Ciocalteau's phenol reagent + 2 parts water
2.3.2 Protein Determination
Protein was determined as described by Lowry et al[6]. To determine soluble protein concentrations series of clean tubes were used, 0.5mL of the enzyme solution was pipetted in each clean tube and 5mL of Reagent C were added and mixed well. The mixture was let to stand at room temperature for 10min. Reagent D was added rapidly to the mixture and mixed immediately. The mixture was allowed to stand at room temperature for 10min, then the optical density of the developed color was read at 600nm. The readings of the optical densities of enzyme solutions were observed and recorded.
The optical densities were converted to the equivalent grams of bovine serum albumin using standard curve of authentic bovine serum albumin concentrations (10-200mg/mL) that were treated similarly to experimental enzyme solutions.
3 RESULTS AND DISCUSSION
3.1 Effect of Enzyme Concentration on Reaction Rate of Crude APA Produced by B.t. (B.t). dendrolimus IP 4A/4B
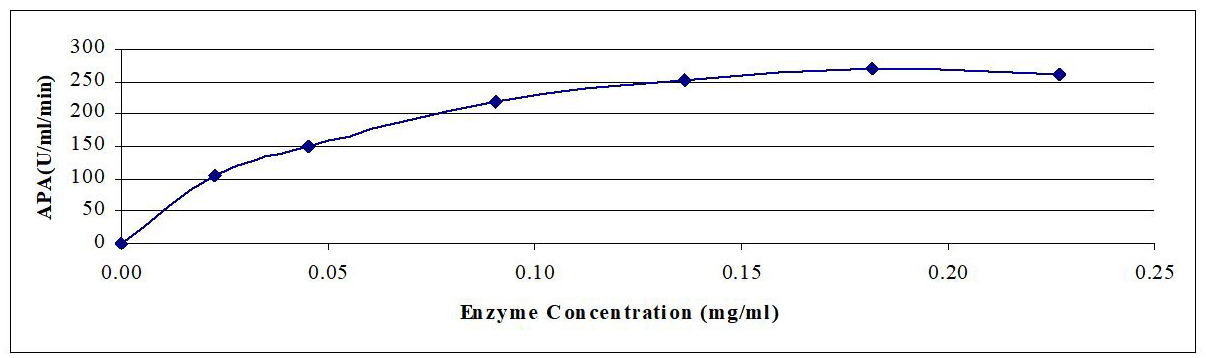
In this experiment, the enzyme concentrations in the reaction mixtures are varied between 0.023-0.227mg protein/mL of the reaction mixture. The enzyme activity was determined under standard conditions. Obtained results are graphically illustrated in Figure 1. The reaction rate exhibited approximately linear response with enzyme protein concentration at least up to 0.045mg/mL of reaction mixture followed by leveling off with no further increase in rate of reaction at higher enzyme concentrations.
|
Figure 1. Effect of enzyme concentration on reaction rate of crude AP produced by B.t. dendrolimus IP 4A/4B culture.
The results indicated that 0.181mg protein/mL of enzyme produced maximum protease activity (269 APA [U/mL/min]) followed by 0.227mg protein/mL enzyme (260 APA [U/mL/min]).
On the other hand, the lowest activity (106 APA) is obtained by using 0.023mg protein/mL enzyme. In this respect, Kaur et al.[7] stated that 0.023mg protein/mL enzyme produced the maximum enzyme activity. While Huang et al.[8] found that 0.34mg protein/mL of crude protease resulted in the maximum enzyme activity.
3.2 Effect of Reaction Time on Reaction Rate of Crude APA Produced by B.t. dendrolimus IP 4A/4B
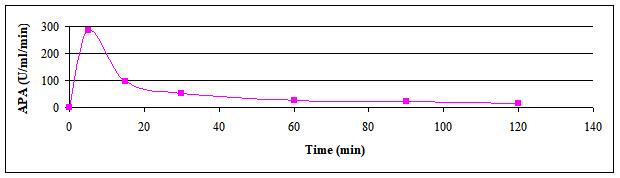
The effect of reaction time on the reaction rate of AP was done with periodic sampling for measuring the reaction progress with the reaction intervals. Figure 2 illustrated reaction progress with time. The slope of the first period (up to 5min reaction time) exhibited steeper slope indicating reactions velocity near the theoretical initial velocity (Vmax) that has become lesser in value as the reaction was extended up to 120min. Upon further longer reaction periods, more leveling off (gentler slope) of the reaction rate was evident.
|
Figure 2. Effect of time on reaction rate of crude AP produced by B.t. dendrolimus IP 4A/4B culture.
Aboul-Soud et al.[9] illustrated that a proportional increase in the enzymatic specific activity was obtained for the reaction times tested in the range of 0 to 30min, with a maximum activity of 145U/mL/min when the enzymatic reaction was allowed to proceed for 30min.
3.3 Effect of Temperature on Reaction Rate of Crude APA Produced by B.t. dendrolimus IP 4A/4B
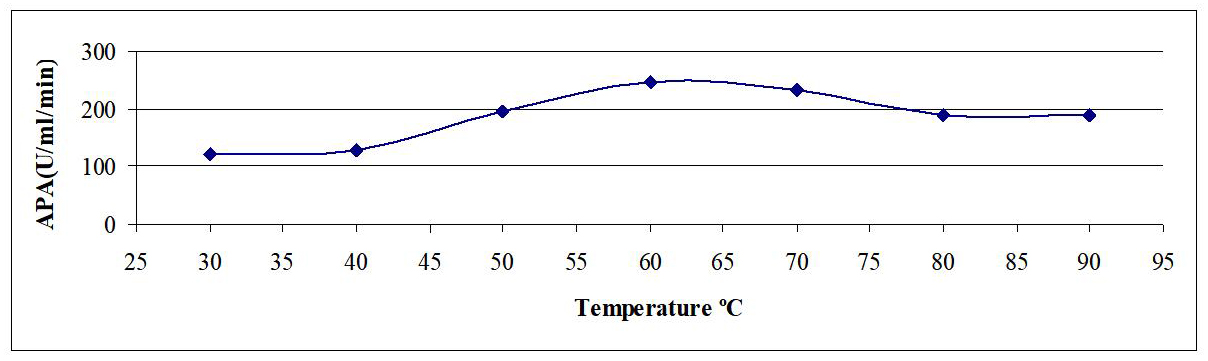
Temperature is a critical factor for maximum enzyme activity and it is a prerequisite for industrial enzymes to be active and stable at higher temperature. Assay mixture was incubated at different temperatures ranging from 30-90°C for 20min. From Figure 3, it could be observed that enzyme activity gradually peaked at 60°C, and then followed by decreasing enzyme activity until 90°C. The maximum enzyme activity was at 60°C (246U/mL/min), while decreased gradually to reach (188U/mL/min) at 90°C.
|
Figure 3. Effect of temperature on reaction rate of crude AP produced by B.t. dendrolimus IP 4A/4B culture.
Several researchers examined the effect of temperature on reaction rate of enzyme. For example, Li et al.[10] reported that alkaline protease isolated from Thermomyces lanuginose P134 had a broad temperature optimum of 50°C. While Samal et al.[11] reported that an alkaline protease from Tritirachium album Limber to be quite thermo-stable even up to 50°C. Additionally, the thermo-stability activity for alkaline protease enzyme produced by Aspergillus niger was stable up to 60min 40°C and lost activity gradually after 60min. The similar reports were observed for Thermomyces lanuginosus and in Aspergillus species by Nehra et al[12]. The results of Sumandeep et al.[13] showed that alkaline protease appeared to be more stable at alkaline pH. More than 40% of the initial activity was preserved after 1h reaction with the substrate at 80°C in Bacillus sp. Takami et al.[14] isolated a new AP from alkalophilic Bacillus sp. strain AH-101, and found that the optimum temperature was about 80°C in the presence of 5mM calcium ions.
3.4 Effect of pH on Reaction Rate of Crude APA Produced by B.t. dendrolimus IP 4A/4B
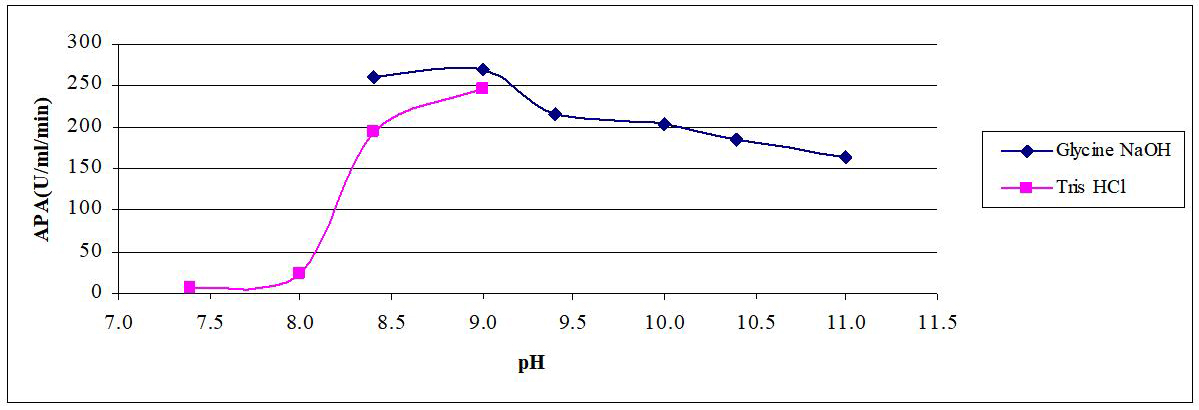
Effect of pH on activity of crude AP was studied by using casein as substrate and glycine-NaOH buffer (pH from 8.4 up to 11). In addition, Tris-HCl buffer of pH values (7.4-9.0) was used. Enzyme activity was determined and then plotted against pH values.
Data for the determination of optimum pH were found in (Figure 4). It was clear that the optimum pH 9.0 when glycine-NaOH and Tris-HCl buffers were used.
|
Figure 4. Effect of pH on reaction rate of crude AP produced by B.t. dendrolimus IP 4A/4B culture.
The effect of pH on the activity of alkaline protease has been previously explored. For instance, Durham et al.[15] mentioned that the pH optima for proteases produced by Bacillus species, Thermus aquaticus, Xanthomonas maltophila and Vibrio metscnikovii were ranged from 10-10.5. Similar observations were shown by Kalpana-Devi et al.[16], who determined the optimum pH as 10.
On the other hand, Takami et al.[14] isolated a new AP from alkalophilic Bacillus sp. strain AH-101; the enzyme was most active toward casein at pH 12-13. Also, Horikoshi[17] studied the production of an extra-cellular alkaline serine protease from alkaliphilic Bacillus strain 221. That strain, isolated from soil, produced large amounts of alkaline protease that differed from the subtilisin group. The optimum pH of the purified enzyme was 11.5 and 75% of the activity was maintained at pH 13.0.
4 CONCLUSION
The study showed that the crude enzyme produced from bacteria under solid fermentation conditions has the following biochemical characteristics: The optimum enzymatic concentration for the enzymatic reaction is 0.045mg/mL of reaction mixture, the best pH for the enzymatic reaction medium is 9, the best temperature for the enzymatic reaction is 60°C, and the best time for the enzymatic reaction is 5min. This calls for subsequent studies on appropriate industrial applications. The biochemical properties of the enzyme are promising, where it attains its best efficiency at a low enzymatic concentration (applied economic indicator) within a quick period of time (time is money in the language of industry and economics), the pH is 9 and the optimum temperature is 60°C. These qualities are impressive, including but not limited to industrial detergents and silver recovery.
Acknowledgements
A due thanks to the National Research Center, as this research was extracted from a Ph.D. thesis that was completed as work requirements under the support of the National Research Center.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
Roshdy AM studied, wrote, reviewed and corrected this article; Shata HM studied and wrote this article; Ali SM studied and wrote this article; Youssef AM studied and wrote this article; Foda MS studied and wrote this article and Kahil T studied and wrote this article.
Abbreviation List
AP, Alkaline protease
APA, Alkaline protease activity
B.t., Bacillus thuringiensis
References
[1] Li Y, Wang C, Ge L et al. Environmental behaviors of Bacillus thuringiensis (B.t.) insecticidal proteins and their effects on microbial ecology. Plants, 2022; 11: 1212. DOI: 10.3390/plants11091212
[2] Sedaghat S, Yazdi TF, Mortazavi A et al. Enhancement of alkaline protease production of Bacillus strains isolated from dairy sludge under cold, salt and ultrasound stress. Int Dairy J, 2022; 129: 105335. DOI: 10.1016/j.idairyj.2022.105335
[3] Asar RA. Biochemical studies on production, purification and characterization of chitinase and protease enzymes of Bacillus thuringiensis [PhD thesis]. Mansoura, Egypt: Mansoura University; 2013.
[4] Foda S, Safaa A, Youssef M et al. Production physiology of alkaline protease by Bacillus thuringiensis spp. under solid-state fermentation conditions. J Appl Sci Res, 2013; 9: 1975-1984.
[5] Prakasham RS, Subba-Rao CH, Sarma PN. Green gram husk - An inexpensive substrate for alkaline protease production by Bacillus sp. in solid-state fermentation. Bioresource Technol, 2006; 97: 1449-1454. DOI: 10.1016/j.biortech.2005.07.015
[6] Lowry OH, Rosebrough NJ, Farr A et al. Protein measurement with the Folin phenol reagent. J Biol Chem, 1951; 193: 265-275. DOI: 10.1016/S0021-9258(19)52451-6
[7] Kaur M, Dhillon S, Chaudhary K et al. Production, purification and characterization of thermostable alkaline protease from Bacillus polymyxa. Indian J Microbi, 1998; 38: 63-67.
[8] Huang Q, Peng Y, Li X et al. Purification and characterization of an extracellular alkaline serine protease with dehairing function from Bacillus pumilus. Curr Microbiol, 2003; 46: 169-173. DOI: 10.1007/s00284-002-3850-2
[9] Aboul-Soud MAM, Afify AEMMR, Foda MS et al. Purification and biochemical characterization of alkaline protease from an Egyptian biopesticide-producing Bacillus sphaericus strain. Afr J Microbiol Res, 2011; 5: 5076-5084. DOI: 10.5897/AJMR11.939
[10] Li DC, Yang YJ, Shem CY. Protease production by the thermophilic fungus Thermomyce lanuginosus. Myco Res, 1997; 101: 18-22. DOI: 10.1017/S0953756296002109
[11] Samal BB, Karan B, Parker C et al. Isolation and thermal stabilities of two novel serine proteinases from the fungus Tritirachium album Limber. Enzyme Microbiol Technol, 1991; 13: 66-70. DOI: 10.1016/0141-0229(91)90190-L
[12] Nehra KS, Singh A, Sharma J et al. Production and characterization of alkaline protease from Aspergillus species and its compatibility with commercial detergents. Asian J Microbiol Biotech Env Sc, 2004; 6: 67-72.
[13] Bhushan B, Beg QK, Hoondal GS. Partial purification and characterization of a thermostable alkaline protease of an alkalophilic Bacillus sp. NG 27. Ind J Microbiol, 1999; 39: 185-187.
[14] Takami H, Akiba T, Horikoshi K. Production of extremely thermostable alkaline protease from Bacillus sp. no. AH-101. Appl Microbiol Biot, 1989; 30: 120. DOI: 10.1007/BF00263997
[15] Durham DR, Stewart DB, Stellwag EJ. Novel alkaline- and heat-stable serine proteases from alkalophilic Bacillus sp. strain GX6638. J Bacteriol, 1987; 169: 2762. DOI: 10.1128/jb.169.6.2762-2768.1987
[16] Devi MK, Banu AR, Gnanaprabhal GR et al. Purification, characterization of alkaline protease enzyme from native isolate Aspergillus niger and its compatibility with commercial detergents. Indian J Sci Technol, 2008; 1: 1-6. DOI: 10.17485/ijst/2008/v1i7.8
[17] Horikoshi K. Alkaliphiles: Some applications of their products for biotechnology. Microbiol Mol Biol R, 1999; 63: 735. DOI: 10.1128/MMBR.63.4.735-750.1999
Copyright © 2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.





 Copyright ©
Copyright ©