Commercial Extraction of Applicable Alkaline Protease and Chitinase by Bacillus thuringiensis dendrolimus IP 4A/4B
Amira M Roshdy1*, Hoda M Shata1, Safaa M Ali2, A M Youssef2, M S Foda1,T Kahil1
1Microbial Chemistry Department, Biotechnology Research Institution, National Research Centre, Cairo, Egypt
2Agricultural Biochemistry Department, Faculty of Agriculture, Mansoura University, Mansoura, Egypt
*Correspondence to: Amira M Roshdy, PhD, Associate Professor, Department of Microbial Chemistry, Biotechnology Research Institution, National Research Center, 33 El Buhouth St., Dokki, Cairo 12622, Egypt; Email: amiraroshdy82@yahoo.com
Abstract
Objective: The study aims to produce alkaline protease and chitinase in the same fermentation process in the most economical way possible so as to reduce production costs and to investigate the application of alkaline protease in detergents compatibility.
Methods: Inoculums were prepared in nutrient yeast-extract standard medium, grown, and extracted from the solid medium, while the enzyme activities were measured using Lowry protein assay and high-performance liquid chromatography.
Results: The culture of Bacillus thuringiensis subspecies dendrolimus IP 4A/4B was performed under solid-state fermentation conditions. The efficiency and performance of extraction using tap water outperformed other solutions. The crude protease enzyme was compatible with some detergents available in the local market, such as Persil, Tide, and Ariel, where the crude enzyme retained its activity at rates that varied between 113%, 110% and 83%. No microbial growth and therefore no chitinase enzymatic activity was observed on the first and second days, while microbial growth and chitinase activity commenced on the third day and reached the peak of the curve on the fourth day and stabilized on the sixth day. The productivity of chitinase in solid-state was superior to submerged fermentation conditions, which were 2.050 and 0.693U (μmole/mL/min).
Conclusion: Production of alkaline protease by Bacillus thuringiensis dendrolimus IP/4B is efficient using tap water for extraction. The alkaline protease is compatible with (Arial, Tide and Persil) detergents, and the same bacterial strain also produced chitinase.
Keywords: biological control, Bacillus thuringiensis, alkaline protease, chitinase, detergents, protease characterization
1 Introduction
It is universally recognized that all species of the genus Bacillus thuringiensis are effective bio insecticidal materials for all orders of insects, as they secrete an inactive insect toxic protein, which is activated by proteolytic enzymes in the insect's mid-gut and presents insect toxin[1]. Khorramnejad et al.[2] showed that Bacillus thuringiensis produces proteins that are toxic to many insects, whether these toxic proteins accumulate in the stationary growth phase of the bacteria or are secreted in the vegetative phase.
In addition to this common use, Asar[3] outlines many other alkaline protease applications in the field of the food industry, leather industry, medical usage, management of industrial and household waste, photographic industry, peptide synthesis, silk de-gumming, and detergent industry. Horikoshi[4] stated that detergent enzymes represent 30% of enzyme sales worldwide, 89% of sales of protease enzymes are alkaline proteases, and genus Bacillus participates are involved in the production of alkaline protease enzymes in a noticeable way. Asar[3] reviewed multiple types of alkaline proteases applications in detergents, such as laundry detergent formulations, membranes cleaning and contact lenses and tears films cleansing. Pringgenies et al.[5] found that alkaline proteolytic enzymes extracted from Bacillus thuringiensis and B. ceres are effective in industrial detergents, given their features of low price with continuous productivity, environmental friendliness, easy production, and short production period.
Because of the role of Bacillus thuringiensis in the biological control of insects worldwide, research has shed light on the simultaneous applications of these bacterial strains. Studies have demonstrated the importance of Bacillus thuringiensis in the production of metabolites of various biological effects[6].
The present study was to maximize the efficiency of producing alkaline protease (which helps to convert protoxin to its active form in the insect gut), to apply the extract to industrial detergents as well as to produce chitinase (in the same fermentation process), which contributes to the economic production of both products from Bacillus thuringiensis strain IP 4A/4B.
2 MATERIALS AND METHODS
2.1 Bacterial Strain
The Bacillus thuringiensis subspecies dendrolimus IP-4A/4B strain was obtained from Microbial Chemistry Department, National Research Center, Dokki, Cairo, Egypt culture collection (Institute Pasteur Collection, Paris, France).
2.2 Media Used for Growth and Production of Alkaline Protease
2.2.1 Standard Medium (Nutrient-broth Yeast-extract Salt Medium)
According to Foda et al.[7], nutrient-broth yeast-extract salt medium was used to activate the inoculum for 24h at 30°C and 100rpm. The medium composition is as follows (g/L); peptone (5), beef extract (3), yeast extract (0.5), MnCl2 (0.01), CaCl2 (0.1), MgCl2 (0.2), (NH4)2SO4 (8), K2HPO4 (5.6), KH2PO4 (2.4), MgSO4 (0.8), MnCl2 (0.3), FeCl2 (0.14), ZnSO4 (0.2), thiamin (0.2) and biotin (2µg/L).
2.2.2 Production of Alkaline Protease under Solid State Fermentation
To reduce the economic cost of enzyme production to the maximum extent, the solid-state fermentation method was used according to Prakasham et al.[8] with modifications. 15mL of 50mM potassium phosphate buffer at a pH of 7.4 was added to 5g of wheat bran, mixed, and autoclaved at 121°C and 15lbs pressure for 15min. The flasks were cooled to room temperature, inoculated with 2mL of 24h grown bacterial culture under sterile static conditions, and incubated at 30°C temperature for 3d.
2.3 Effect of Extracting Solutions on Alkaline Protease Activity (APA) Produced by Bacillus thuringiensis dendrolimus IP 4A/4B Culture under Solid State Fermentation
According to our previous research[7], a known weight of fermented wheat bran was mixed with tap water, distilled water, NaOH, Triton X-100, Tween-80, Ethanol-glycerol, NaCl, glycine-NaOH pH (12.4, 9.4, 8.4, 7.4 and 6.6) (1:5, w/v) by stirring on a magnetic stirrer for 30min at room temperature. The slurry was squeezed through cheesecloth followed by centrifugation of the whole content at 4000rpm for 10min at 4°C to remove the insoluble matters. The clear supernatant was used for the protease assay.
2.4 Assay of APA
The activity of AP was determined as described by Lowry et al.[9] with modifications. The standard reaction mixture composition (unless otherwise stated) contained 0.5mL of enzyme source (culture supernatant or supernatant after purification steps), 1mL of glycine-sodium hydroxide buffer 0.1M, pH 9.6, in which 1% casein was dissolved as substrate. The reaction mixture was incubated at 40°C for 20min after which the enzymatic reaction was terminated by adding 1mL of 20% trichloroacetic acid. The terminated reaction mixture was then centrifuged at 4000rpm for 10min without disturbing the precipitated proteins. 0.5mL of the filtrate was added with 5mL of reagent C and mixed well. The mixture was rested at room temperature for 10min. Reagent D was quickly added to the mixture and mixed and rested at room temperature for 10min, and then the optical density of the developed color was measured spectrophotometrically at 600nm using JASCO V-730 Spectrophotometer. The readings of the optical densities of zero time cleared reaction mixtures were subtracted from those of the experimental tubes.
The optical densities were converted to the equivalent grams of tyrosine using a standard curve of authentic tyrosine concentrations (10-200µg/mL)[10] treated similarly to the experimental enzyme-catalyzed reaction mixture.
The enzyme activity was expressed in terms of enzyme units. One AP unit was defined as the amount of enzyme that releases one-microgram tyrosine per mL enzyme per minute under the specified reaction conditions.
Micrograms of tyrosine released X enzyme dilution
Enzyme units = -----------------------------------------------------------------------------------------------------------------------------------------------------
Reaction time (min)
= microgram tyrosine/min/mL culture supernatant
Reagents:
Reagent A: 2% Na2CO3 in 0.1N NaOH
Reagent B: 0.5% CuSO4.5H2O in 1% sodium and potassium tartrate
Reagent C: 50mL of reagent A was mixed with 1mL of reagent B. Made fresh daily.
Reagent D: 1 part Folin Ciocalteau's phenol reagent + 2 parts water
2.5 Application of Alkaline Protease as Washing Detergent Additive
The washing test with protease preparation was performed according to the method described by Kanmani et al[11]. Application of protease enzyme (100mg/mL) as a detergent additive was studied on white cotton cloth pieces (10×10cm) stained with human blood (0.075mL), chocolate (0.5mL of 60% chocolate solution) and egg yolk (0.25mL) as mentioned by Adinarayana et al[12]. The following steps were prepared and studied with modifications (the detergent solution was boiled in a water bath for 15min prior to the test). It was necessary to ensure no enzymatic activity in the laundry detergent before adding the studied enzymes.
1. Flask with 100mL distilled water + stained cloth (Cloth stained with blood, chocolate and egg yolk). Set as control.
2. [(Flask with 100mL distilled water + the stained cloth) + 1mL boiled detergent solution] (Arial, Tide or Persil) (10mg/mL).
3. [(Flask with 100mL distilled water + the stained cloth) + 1mL boiled detergent solution (10mg/mL)] + 2mL of enzyme solution.
4. (Flask with 100mL distilled water + the stained cloth) + 2mL of enzyme solution.
All treatments were incubated at 60°C for 15min. After incubation, cloth pieces were taken out, rinsed with water and dried. Visual examination of various pieces exhibited the effect of enzymes for removal of stains. Untreated cloth pieces stained with human blood (0.075mL), chocolate (0.5mL of 60% chocolate solution) and egg yolk (0.25mL) were used as the control. The result was confirmed spectrophotometrically by Lowrey's method, whereby the released amino acids in rinsing water, were measured as a result of the activity of the alkaline protease[9].
2.6 Media Used for Growth and Chitinase Production
2.6.1 Qualitative Medium for Chitinase
Overnight grown culture of Bt denrolimus IP 4A/4B were assayed on a chitinase-detection agar plate according to Usharani and Gowda[13]. The chitinase activity was detected by visualizing the clear zone formed around the bacterial growth.
Medium (1L) for chitinase-detection agar was prepared by mixing 10g of chitin powder and colloidal chitin individually and 20g of agar in M9 medium containing the following salts (g/L): Na2HPO4, 0.65; KH2PO4, 1.5; NaCl, 0.25; NH4Cl, 0.5; MgSO4, 0.12 and CaCl2, 0.005. The final pH was adjusted to 6.5.
2.6.2 Inoculum Preparation
Inoculum preparation was performed according to Vu et al.[14] with modifications. The pH of the medium used for inoculum preparation was adjusted to 7 before autoclaving at 121°C and 15lbs pressure for 15min. A loopful of Bacillus thuringiensis was used to inoculate 500mL Erlenmeyer flask containing 100mL of sterilized chitin broth medium (Nutrient Broth with 1% chitin powder). The flask was incubated on a rotary shaker at 100rpm and 30˚C for 24h. This media was used as submerged fermentation (SmF) media for chitinase production for 5d.
2.6.3 Chitinase Production on Solid State Fermentation
Commercially available wheat bran was used as the solid substrate. Solid media for chitinase production was prepared by mixing 2.5g with 25mg chitin powder (1% w: w) in 250mL Erlenmeyer flask[15]. The wheat bran supplemented with chitin powder was moistened with distilled water at a ratio of 1:1 (w/v) substrate. Media were autoclaved at 121°C for 1h, cooled to room temperature and inoculated with 2mL of the prepared inoculum suspension. The suspension was mixed carefully under strictly aseptic conditions to achieve uniform distribution of inoculum throughout the medium. The flasks were incubated at room temperature for 5d.
2.6.4 Chitinase Extraction
After completion of fermentation, the fermented wheat bran was transferred to 250mL Erlenmeyer flasks, and 5 volumes of distilled water were added (based on initial dry weight of the substrate) and the mixture was mixed at room temperature on a rotary shaker (100rpm) for 30min[15]. The slurry was squeezed through cheesecloth. After two extractions, the extracts were mixed and centrifugated at 4000rpm at 4°C for 15min, and the clear supernatant was used as a crude enzyme for assay through the determination of N-acetyl glucose amine (NAG) as a final product of chitin hydrolysis.
2.6.5 Chitinase Assay
Chitinase activity was assayed according to the method of Usharani and Gowda[13] with modifications as follows. The reaction mixture contained 0.125mL of chitin powder (17.5mg/mL; Alpha Chemika, Prod. No. AL 1161), 0.5mL enzyme (or 0.5mL of an inactive enzyme by boiling for 15min as a blank sample), and 0.375mL of 0.1M sodium acetate buffer, pH 5.6. The reaction mixture was incubated in a shaking water bath at 37°C for 2h and centrifuged at 11,000rpm for 10min to terminate the reaction. The supernatant was used for the determination of the resultant reaction product NAG by high-performance liquid chromatography (HPLC).
2.7 Determination of NAG by HPLC
The activity of chitinase was determined in the chitinase filtrate as NAG which is released by enzymatic hydrolysis of chitin. The filtrate was separated by HPLC under the following conditions[16]: mobile phase at a flow rate of 1mL/min; gradient prepared from 5% methanol in water (A) and 95% of 0.5% formic acid (B) at zero time. The composition of isocratic was as follows: 15min. 70 ACN agilent 1100 series (Waldborn, Germany), quaternary pump (G1311A), Degasser (G1322A), Thermostated autosamples (G1329A), variable wavelength detector (G1314A); and column: Zorbax 300SB C18 column (4.5×250mm) (Agilent Technologies, USA). The injection was carried out at wavelengths 210nm for separation.
NAG concentrations were quantified by measuring peak areas and then comparing those with those of standard samples with known concentrations.
HPLC analysis was carried out at Central Laboratory, National Research Center, Giza, Egypt.
3 Results and Discussion
3.1 Effect of Extracting Solutions
There are several factors with potential impacts on the quality and efficiency of extraction solutions. If the enzyme was extra-cellular or endo-cellular, the active groups of its activity center are hydrophilic or hydrophobic, and hydrogen bonds or Van der Waals forces were presented[17].
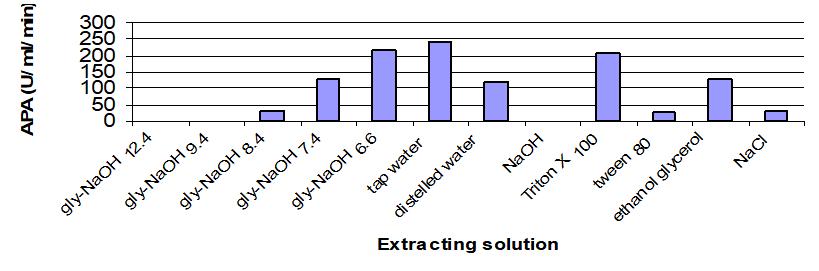
Results in Table 1 and Figure 1 showed the effect of various extracting solutions on APA produced by Bacillus thuringiensis dendrolimus IP 4A/4B culture. It demonstrated that tap water was considered a suitable extracting solution for efficient recovery of the enzyme (244U/mL/min), followed by glycine-NaOH buffer pH 6.6 (215U/mL/min) and Triton X-100 (206U/mL/min). The obtained results revealed that glycine-NaOH pH (12.4 to 8.4) was not suitable for APA extraction.
Contrary to the current results, it was found that the saline solution of NaCl at a concentration of 1% recovered the maximum percentage of extracted alkaline proteases from fermented bran by Mucor bacilliformis[17] and Rhizopus oligosporus[18]. 10% ethanol with 3% glycerol was the optimal extracting solution, for proteases extracted from the fermented bran of Rhizopus oryzae fungus[19].
Table 1. Effect of Extracting Solutions on Alkaline Protease Activity Produced by Bacillus thuringiensis dendrolimus IP 4A/4B Culture under Solid State Fermentation
Extracting Solution |
Glycine-NaOH Buffer pH |
Tap Water |
Distilled Water |
NaOH |
Triton X 100 |
Tween 80 |
Ethanol-glycerol |
NaCl |
||||
12.4 |
9.4 |
8.4 |
7.4 |
6.6 |
||||||||
Alkaline Protease Activity (U/mL/min) |
0 |
0 |
32 |
127 |
215 |
244 |
119 |
0 |
206 |
25 |
127 |
31 |
|
Figure 1. Effect of extracting solutions on alkaline protease activity produced by Bacillus thuringiensis dendrolimus IP 4A/4B culture.
Overall, industrial application of tap water as the optimal alkaline protease extraction solution in the present study contributes to the production of alkaline protease from Bacillus thuringiensis dendrolimus IP 4A/4B under conditions of solid-state fermentation of wheat bran.
3.2 Compatibility Application of Some Local Detergents with Crude Alkaline Proteases Produced by Bacillus thuringiensis dendrolimus IP 4A/4B
To study the feasibility of adding crude alkaline protease to industrial detergents, Ariel, Persil and Tide were used as industrial detergents available in the local market so as to study the chemical compatibility of enzymes with them and to identify the stability of the enzymatic activity in these detergents, without adding any stabilizers.
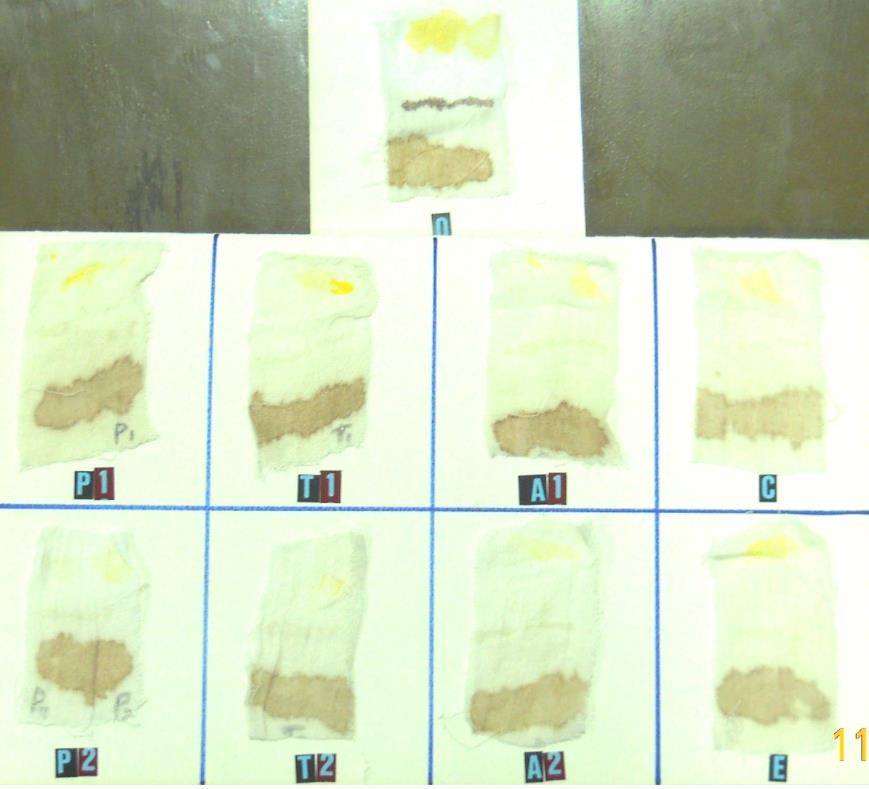
Results in Table 2 and Figure 2 showed the compatibility of crude alkaline protease with commercial detergents, whereas the alkaline protease showed the stability of its enzymatic activity with percentages of 113, 110 and 83% as a compatible activity with Persil, Tide and Ariel detergents, from the activity of this alkaline protease alone.
Table 2. Compatibility with Various Commercial Detergents of Alkaline Protease Produced by Bacillus thuringiensis dendrolimus IP 4A/4B Culture under Solid State Fermentation
Control (U/mL/min) (Tap Water and Crude Enzyme) (C) |
Enzymatic Treating without Detergent (U/mL/min) (E) |
Detergent |
Detergent without Enzyme (U/mL/min) |
Detergent with Enzyme (U/mL/min) |
Enzymatic Residual Activity (%) |
154 |
200 |
Arial (A) |
136 |
166 |
83 |
Persil (P) |
112 |
226 |
113 |
||
Tide (T) |
104 |
219 |
110 |
|
Figure 2. Compatibility of various commercial detergents with alkaline proteases produced by Bacillus thuringiensis dendrolimus IP 4A/4B culture. A1: Arial detergent without enzyme; A2: Arial detergent with enzyme; T1: Tide detergent without enzyme; T2: Tide detergent with enzyme; P1: Persil detergent without enzyme; P2: Persil detergent with enzyme; C: Control (tap water) and crude enzyme; E: Crude Enzyme; 0: tap water only.
Among the industrial applications of alkaline proteases from strains of Bacillus, there are three types of proteases from Bacillus produced by Novo Industry A.I.S. Company, Alcalase and Esperase from Bacillus licheniformis, and Savinase from Bacillus amyloliquefaciens. Gist Brocades produces and distributes Maxatase enzyme from Bacillus licheniformis. Temperatures from 10 to 65°C are suitable for the use of the enzymes Alcalase and Maxatase, with a pH ranging from 7 to 10.5. Savinase enzyme is used at a pH of up to 11 and Esperase is used at a pH of up to 12[20]. The pH of 10.5 is the optimum enzymes activities of the industrial detergents of the famous companies Carlsberg or Novo[21], which is highly similar to the results of the present study. The ideal alkaline protease quality meets the criteria for an industrial detergent additive with enzymatic activity that is stable over a wide range: pH, temperature, low enzyme concentration in the reaction medium (0.4 to 0.8%), long enzyme life, and compatibility of the enzyme action with many components of the different detergents in use, in addition to oxidation and sequestration factors[22].
A pH of 10.5 and a temperature of 65°C are the optimal conditions for the APA of Bacillus brevis when applied with several commercial detergents. Complete removal of blood stains was achieved with these detergents in the presence of glycine and Ca2+[21].
Among the alkaline proteases produced by a strain of Bacillus, alkaline protease is resistant to bleaching and heat, with stable activity in the presence of 10% (v/v) of the oxidizing agent H2O2 and 1% of the surfactant reducing agent sodium dodecyl sulfate, reaching optimal activity in the presence of ionic and nonionic detergents, at pH 10 and at temperatures of 60 to 70°C[23].
Phadatare et al.[24] explained that alkaline protease of Conidiobolus coranatus in the presence of 25mM CaCl2 at 50°C was compatible with several commercial detergents, retaining more than 80% of its activity. Some alkaline protease enzymes extracted from Tritirachium album retained about 90% of their activity up to one hour in the presence of some detergents such as ERA plus and Dyanamo, while some of them were considered inadequate given their loss of 96% of their activity after only 10min.
The ability of the alkaline protease enzymes of Bacillus polymyxa to maintain their activity varied from 20 to 84.5% with different industrial detergents[25]. The retained enzymatic activity varied between 16, 11.4 and 6.6% in the presence of the detergents Revel, Ariel and Wheel, respectively[12]. Kalpana-Devi et al.[26] mentioned that the enzyme retained its activity at rates from 80 to 92% with all the industrial detergents used in the study, save Ariel, with whom the enzyme retained only 23% of its enzymatic activity.
A study by Kanmani et al.[11] confirmed the ability of Bacillus cereus protease enzymes to remove blood, chocolate and egg yolk stains within 10min, and Nadeem et al.[27] demonstrated the ability of Bacillus licheniformis N-2 to remove blood stains from cotton fabrics. Similar conclusions have been drawn by Najafi et al.[28] that the enzymes of the Pseudomonas aeruginosa PD100 alkalophilic strain were able to remove blood stains from cotton fabrics.
Banerjee et al.[21] demonstrated that industrial detergents have a pH ranging from 9 to 12, requiring the production of enzymes with high activity in an alkaline environment to achieve the first conditions of compatibility with these industrial detergents.
The results of the current research showed that protease enzyme produced using Bacillus thuringiensi dendrolimus IP 4A/4B demonstrated good potential for extensive use in the detergent industry. Foda et al.[7] showed applicable silver recovery from used X-ray films by the same fermentation process, which enhance the economic productivity of alkaline protease from Bacillus thuringiensi dendrolimus IP 4A/4B.
3.3 Production of Chitinases by Bacillus thuringiensis dendrolimus IP 4A/4B
3.3.1 Qualitative Test for Chitinases
Bacillus thuringiensis dendrolimus IP 4A/4B was inoculated on chitinase-detective agar plates to evaluate chitinase activity based on visualizing the width of the translucent digestion haloes of chitin around the bacterium colony, and variation was also observed.
The obtained results in (Table 3) showed that on the 1st and 2nd days, there is no chitinase activity as no bacterial growth was observed. Vu et al.[14] found that the addition of chitin to the wastewater of the starch industry as a growth environment for Bacillus thuringiensis kurstaki inhibited the growth. Gooday[29] found that chitin was not a preferred nutrient source for bacterial growth, given its role as a carbon or nitrogen source[30]. Nonetheless, the growth and chitinase activity appeared on the 3rd day, increased on 4th and 5th day, and stabilized on the 6th day, and the bacterial growth stabilized from the 4th day.
Table 3. Hydrolysis of Chitin on Chitinase-detective Agar Plates by Bacillus thuringiensis. dendrolimus IP 4A/4B
Day |
1st |
2nd |
3rd |
4th |
5th |
6th |
Radius of clear zone (cm) |
- |
- |
0.5 |
1.0 |
1.5 |
1.5 |
There were few studies on chitinase production by Bacillus thuringiensis and these were focused only on its possible entomo-pathogenic effect[31]. Rojas-Avelizapa et al.[31] demonstrated the potential of Bacillus thuringiensis as a dual producer of insecticidal proteins and enzymes, because a highly proteolytic and chitinolytic strain was selected, showing moderate levels of insecticidal activity. This multiple effects of Bacillus thuringiensis as an enzyme producer, organic waste degrader and insecticidal microorganism showed a novel biotechnological approach for this bacterium.
The ability of microorganisms to produce titinase from strains that have produced protoxin is considered a biocide, which is considered a characteristic that supports the application of such strains as insecticides[26]. Usharani and Gowda[13] reported that the screening of Bacillus thuringiensis isolates for chitinolytic activity on chitinase-detective agar plates showed varied responses. They found that Bacillus thuringiensis cultures achieved medium to superior growth and also chitinolytic clear zone around their colonies. The hydrolysis of chitin reached a peak in the strains Bacillus thuringiensis 15.1, 14.2, 27B, 48E, 50B, 29 and 17.4.
Rojas-Avelizapa et al.[31] demonstrated that haloes were 0, 1, 2 and 3mm after 48h by Bacillus thuringiensis 53, 46, 24 and 29 strains respectively.
From the industrial point of view, studies on the enzymatic potential of Bacillus thuringiensis are significant for two reasons: (1) selection of strains with high enzymatic activity, combined with high insecticidal activity, could expand the diversity of wastes for biocide production; and (2) selection of strains with high enzymatic activity allows the expansion of the use of Bacillus thuringiensis as an enzyme producer or as a biomass producer of waste materials, avoiding safety problems such as those shown by other bacteria (i.e., Echerichia coli).
In conclusion, the greater the applied properties within one strain, the better it will result in the selection of one of the strains for field application. It also increases the chances of safe biological alternatives in the market, whether to produce pesticides or enzymes. Strain Bacillus thuringiensis dendrolimus IP 4A/4B showed proteolytic and chitinolytic levels, with some features that may indicated the uniqueness of this strain. Further selection may discover strains with higher proteolytic and chitinolytic activities.
3.3.2 Determination of NAG by HPLC
In solid-state fermentation experiments, wheat bran was used as a support material for fermentative growth in a solid growth environment, and chitin powder was added to induce bacteria to produce chitinase[32].
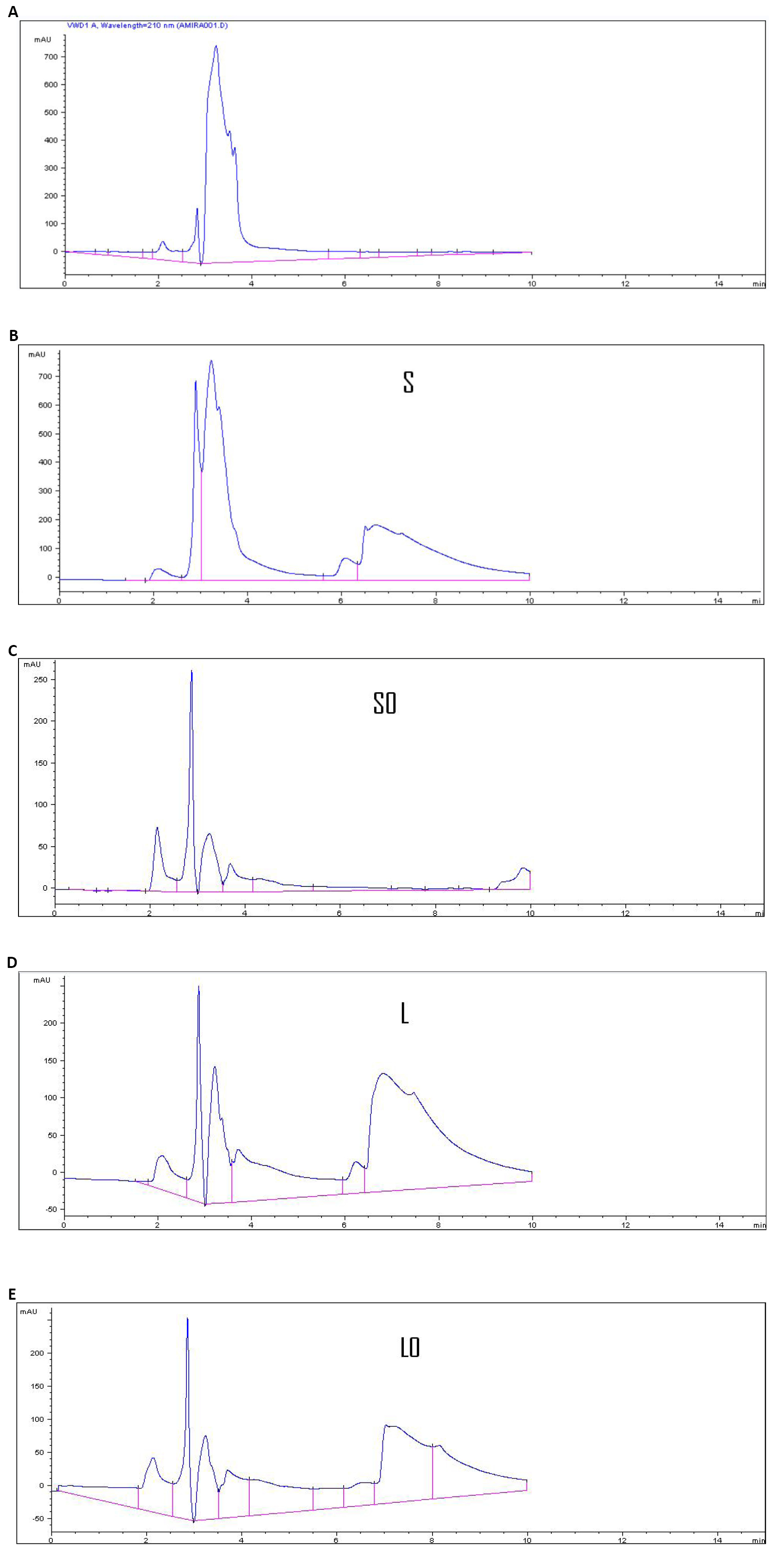
The selected strain Bacillus thuringiensis dendrolimus IP 4A/4B ability to produce chitinase activities were checked by HPLC to determine NAG as the final product of chitinase activity through hydrolyzing chitin after 5d. The results were illustrated in Table 4 and Figure 3. An increase in chitinase yield was observed under solid fermentation than under SmF, 2.050 and 0.693U (mole/mL/min) respectively.
Table 4. Determination of N-acetyl Glucose Amine by HPLC
Standard |
SSF |
SSF-0 |
SmF |
SmF-0 |
||
NAG (µmole/mL/min) |
18.836 |
2.163 |
0.113 |
2.314 |
1.621 |
|
Difference |
18.836 |
2.050 |
0.693 |
|||
|
Figure 3. Determination of Standard authentic peak of N-acetyl glucose amine by high performance liquid chromatography, concentration (50µg/20µL) (A), determination of N-acetyl glucose amine by high performance liquid chromatographyreleased by chitinase activity produced by Bacillus thuringiensis dendrolimus IP 4A/4B culture under solid state fermentation (B), and determination of N-acetyl glucose amine by high performance liquid chromatography released by chitinase activity produced by Bacillus thuringiensis dendrolimus IP 4A/4B culture under ubmerged fermentation conditions (SmF) (C, D and E). S: NAG produced under SSF conditions; S0: NAG produced under SSF conditions but by killed enzyme; L: NAG produced under SmF conditions; L0: NAG produced under SmF conditions but by killed enzyme.
The current results were consistent with those of Najafi et al.[28] who noted an abrupt and dramatic decrease in chitinase activity in Beauveria bassiana in liquid culture. In addition, it is in accordance with Tunga et al.[19] who stated that solid state fermentation is distinguished from SmF by superior productivity, better product recovery, less waste water, less capital, lower energy needs, simpler procedures, limited handling of raw materials and thus lower manufacturing costs and maximization of microorganism productivity.
Suresh and Chandrasekaran[15] reported that a total of 21 chitinolytic fungal cultures were isolated from marine sediments and screened for hyper-producing culture. Beauveria bassiana BTMF S10 was selected for its ability to produce chitinase at higher levels under solid state fermentation. Naturally, marine fungi were adapted to grow in nutrient diluted environments under submerged conditions[29], but this fungus grew in the solid-state fermentation and tolerated adverse conditions including the high carbohydrate concentration and the presence of other waste components.
A reduction in enzyme production under SmF conditions may be ascribed to a change in particle structure, reduction of gas volume, impaired oxygen transfer, and decreased diffusion[30].
4 ConclUsion
The alkaline proteases produced by Bacillus thuringensis dendrolimus IP/4B can be extracted using tap water with an efficiency that is superior to the rest of the extraction solutions under study. The same bacterial strain also produced chitinase in the same fermentation process. It has also been shown that alkaline proteases are chemically and stably compatible without adding stabilizers with some industrial detergents available in the local market (Arial, Tide and Persil). Therefore, we recommend the use of the bacterial strain Bacillus thuringiensis dendrolimus IP/4B in the production of alkaline protease and chitinase in addition to its leading role in biological resistance to pests.
Acknowledgements
A due thanks to the National Research Center, as this research was extracted from a Ph.D. thesis that was completed as work requirements under the support of the National Research Center.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
Roshdy AM studied, wrote, reviewed and corrected this article, and Shata HM, Ali SM, Youssef AM, Foda MS and Kahil T studied and wrote this article.
Abbreviation List
APA, Alkaline protease activity
HPLC, High performance liquid chromatography,
IP, Institute Pasteur
NAG, N-acetyl glucose amine
SmF, Submerged fermentation
References
[1] Talaei-Hassanloui R, Bakhshaei R, Hosseininaveh V et al. Effect of midgut proteolytic activity on susceptibility of lepidopteran larvae to Bacillus thuringiensis subsp. Kurstaki. Front Physiol, Invertebr Physiol, 2014; 4: 406. DOI: 10.3389/fphys.2013.00406
[2] Khorramnejad A, Bel Y, Talaei-Hassanloui R et al. Activation of Bacillus thuringiensis Cry1I to a 50 kDa stable core impairs its full toxicity to Ostrinia nubilalis. Appl Microbiol Biot, 2022; 106: 1745-1758. DOI: 10.1007/s00253-022-11808-2
[3] Asar RA. Biochemical studies on production, purification and characterization of chitinase and protease enzymes of Bacillus thuringiensis [PhD thesis]. Mansoura, Egypt: Mansoura University; 2013.
[4] Horikoshi K. Alkaliphiles-from an industrial point of view. Fems Microbiol Rev, 1996; 18: 259-270. DOI: 10.1111/j.1574-6976.1996.tb00242.x
[5] Pringgenies D, Setyati WA, Soenardjo N et al. Investigation of extra-cellular protease in indigenous bacteria of sea cucumbers as a candidate for bio-detergent material in bio-industry. Aims Environ Sci, 2020; 7: 335-349. DOI: 10.3934/environsci.2020022
[6] Martínez-Zavala SA, Barboza-Pérez UE, Hernández-Guzmán G et al. Chitinases of Bacillus thuringiensis: Phylogeny, modular structure, and applied potentials. Front Microbiol, 2020; 10: 3032. DOI: 10.3389/fmicb.2019.03032
[7] Foda S, Safaa A, Youssef M et al. Production Physiology of alkaline protease by bacillus thuringiensis spp. under solid-state fermentation conditions. J Appl Sci Res, 2013; 9: 1975-1984.
[8] Prakasham RS, Rao CS, Sarma PN. Green gram husk-An inexpensive substrate for alkaline protease production by Bacillus sp. in solid-state fermentation. Bioresource Technol, 2006; 97: 1449-1454. DOI: 10.1016/j.biortech.2005.07.015
[9] Lowry OH, Rosebrough NJ, Farr lA et al. Protein measurement with the Folin phenol reagent. J Biol Chem, 1951; 193: 265-275. DOI: 10.1016/S0021-9258(19)52451-6
[10] Chaykin S. Biochemistry laboratory techniques. John Wiley & Sons, Inc.: New York, USA, 1996.
[11] Kanmani R, Dhivya S, Jayalakshmi S et al. Studies on detergent additives of protease enzyme from an estuarine bacterium Bacillus cereus. Int Res J Biotechnol, 2011; 2: 157-163.
[12] Adinarayana KP, Ellaiah P, Prasad DS. Purification and partial characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. Pharm Sci Technol, 2003; 4: 56-64. DOI: 10.1208/pt040456
[13] Usharani TR, Gowda TKS. Cloning of chitinase gene from Bacillus thuringiensis. Indian J Biotechnol, 2010; 10: 264-269.
[14] Vu KD, Yan S, Tyagi RD et al. Induced production of chitinase to enhance entomotoxicity of Bacillus thuringiensis employing starch industry waste water as a substrate. Bioreactor Technol, 2009; 100: 5260-5269. DOI: 10.1016/j.biortech.2009.03.084
[15] Suresh PV, Chandrasekaran M. Impact of process parameters on chitinase production by an alkalophilic marine Beauveria bassiana in solid state fermentation. Process Biochem, 1999; 34: 257-267. DOI: 10.1016/S0032-9592(98)00092-2
[16] Krokeide IM, Synstad B, Gåseidnes S et al. Natural substrate assay for chitinases using high-performance liquid chromatography: A comparison with existing assays. Anal Biochem, 2007; 363: 128-134. DOI: 10.1016/j.ab.2006.12.044
[17] Fernendz-Lahore HM, Fraile ER, Cascone O. Acid protease recovery from a solid-state fermentation system. J Biotechnol, 1998; 62: 83-93. DOI: 10.1016/S0168-1656(98)00048-0
[18] Ikasari L, Mitchell DA. Leaching and characterization of Rhizopus oligosporus acid protease from solid state fermentation. Enzyme Microb Tech, 1996;19: 171-175. DOI: 10.1016/0141-0229(95)00227-8
[19] Tunga R, Banerjee R, Bhattacharya BC. Optimizing some factors affecting protease production under solid-state fermentation. Bioprocess Eng, 1998; 19: 187-190. DOI: 10.1007/s004490050504
[20] Chaplin M, Bucke C. The large-scale use of enzymes in solution. Cambridge University Press: Cambridge, UK, 1990.
[21] Banerjee UC, Sani RK, Azmi W et al. Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Process Biochem, 1999; 35: 213. DOI: 10.1016/S0032-9592(99)00053-9
[22] Kumar CG, Takagi H. Microbial alkaline proteases: from a bioindustrial viewpoint. Biotechnol Adv, 1999; 17: 561. DOI: 10.1016/S0734-9750(99)00027-0
[23] Gupta R, Gupta K, Saxena RK et al. Bleach-stable, alkaline protease from Bacillus sp. Biotechnol Lett, 1999; 21: 135. DOI: 10.1023/A:1005478117918
[24] Phadatare SU, Deshpande VV, Srinivasan MC. High activity alkaline protease from Conidiobolus coronatus (NCL 86-8.20) Enzyme production and compatibility with commercial detergents. Enzyme Microbiol Technol, 1993; 15: 72-76. DOI: 10.1016/0141-0229(93)90119-M
[25] Madan M, Dhillon S, Singh R. Production of alkaline protease by a UV mutant of Bacillus polymyxa. Ind J Microbiol, 2002; 42: 155-159.
[26] Kalpana-Devi M, Rasheedha-Banu AR, Gnanaprabhal GR et al. Purification, characterization of alkaline protease enzyme from native isolate Aspergillus niger and its compatibility with commercial detergents. Indian J Sci Technol, 2008; 1: 1-6. DOI: 10.17485/ijst/2008/v1i7.8
[27] Nadeem M, Qazi JI, Baig S et al. Effect of medium composition on commercially important alkaline protease production by Bacillus licheniformis N-2. Food Technol Biotech, 2008; 46: 388-394.
[28] Najafi MF, Deobagkar D, Deobagkar D. Potential application of protease isolated from Pseudomonas aeruginosa PD100. Electron J Biotechn, 2005; 8: 197-203. DOI: 10.2225/vol8-issue2-fulltext-5
[29] Gooday GW. The ecology of chitin decomposition. Adv Microb, 1990; 11. DOI: 10.1007/978-1-4684-7612-5_10
[30] Barboza-Corona JE, Contreras JC, Velázquez-Robledo R et al. Selection of chitinolytic strains of Bacillus thuringiensis. Biotechnol Lett, 1999; 21: 1125-1129. DOI: 10.1023/A:1005626208193
[31] Rojas-Avelizapa LI, Cruz-Camarillo R, Guerrero MI et al. Selection and characterization of proteo-chitinolytic strain of Bacillus thuringiensis, able to grow in shrimp waste media. World J Microb Biot, 1999; 15: 299-308. DOI: 10.1023/A:1008947029713
[32] Lonsane BK, Ghildyal NP, Budiatman S et al. Engineering aspects of solid-state fermentation. Enzyme Microb Technol, 1985; 7: 258-265. DOI: 10.1016/0141-0229(85)90083-3
Copyright © 2022 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.





 Copyright ©
Copyright ©