Direct-Fed Microbials for Dairy Calves: Gut Microbial Diversity and Host Health
Akbar Nikkhah1*, Masoud Alimirzaei2
1Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
2Behroozi Dairy Complex, Tehran, Iran
*Correspondence to: Akbar Nikkhah, PhD, Chief Highly Distinguished Professor, Faculty of Veterinary Medicine, University of Tehran, 16th Azar St., Enghelab Sq., Tehran 6619-14155, Iran; Email: anikkha@yahoo.com
Abstract
Direct-fed microbial (DFM) might provide young calves with healthy supplements to promote gut microbial diversity and host health if calves are appropriately managed. The possible usefulness of DFM, however, should not be overstated. The main objective of this review article was to investigate whether DFM is practically workable in preventing digestive tract disorders, namely diarrhea. Another objective was to delineate the possible effectiveness of such products in promoting calf health and performance. Protecting newborn calves against infectious diseases and improving their health and growth in early life is closely related to herd economics and sustainability. Diarrhea is the most prevalent contagious disease-causing calf morbidity and mortality worldwide. Supplementing dairy calf diets (milk or starter) with beneficial microorganisms such as bacteria and yeast has been an attempt to improve the gut microbial ecosystem and optimize nutrient assimilation. However, infectious diarrhea is still a problem in many dairy and beef farms. Since diarrhea has a multifactorial nature, it seems that the possible efficacy of DFM depends on the environmental and management conditions in which the calves are raised. The rearing system (individual vs. group housing), diet, environmental microbial load, and other management factors are involved in the incidence of diarrhea. Therefore, probiotic products may interact with such factors, thus influencing their effectiveness and likely causing inconsistencies concerning probiotics' usefulness in commercial dairy farms. Thus, animal responses to dietary DFM supplementation and their usefulness are practically dependent on management conditions.
Keywords: newborn calf, probiotics, diarrhea, growth, gut, microbial diversity, host health
1 INTRODUCTION
In modern dairy farming, raising healthy calves with optimal growth is crucial to herds' future reproductive and productive performance[1]. Different feeding and housing systems have been developed and recommended to improve calf health, welfare, and growth in the early stages of life. For instance, intensive milk feeding systems and new housing designs (pair- or group-housing) are being developed and refined worldwide[1]. In addition to these advancements in management practices, efforts have been made to modify and optimize the gut microbial ecosystems in calf diets by supplementing them with commercially available feed additives like colostrum supplements, plant secondary metabolites (such as phenolic and essential oils)[2], prebiotics, probiotics, and symbiotics.
Amongst the feed additives mentioned above, probiotics are well known on many farms. Based on Food and Agriculture Organization/World Health Organization definition, probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host[3]. Various beneficial microorganisms, such as yeast and bacteria, can be classified as probiotics. Saccharomyces cerevisiae, Lactobacillus spp., Enterococcus spp., and Bacillus spp. are the most common species in calf diets (milk and starter feed)[3]. Probiotics are primarily used in dairy calf diets to prevent diarrhea[4]. In addition, improved growth performance was claimed when calves were fed probiotics. However, the literature review shows an inconsistency regarding the effect of probiotics on calf growth[4]. Environmental and management conditions where experiments are conducted could influence the results.
Altering the intestinal microbiota in favor of beneficial bacteria, modulation of inflammatory responses, stimulating immune function, and intestinal barrier improvement seems to be important mechanisms by which probiotics impact calf health and possibly growth performance[5]. The colonization of the gastrointestinal tract in human and cattle infants is a key factor affecting their health and subsequent performance[6]. It is noteworthy that establishing a microbial population in the infants' intestine is a complex process with many interactions with environmental factors. Maternal microbiomes, diet type (colostrum, milk, and solid feed), and antibiotic use might affect the gut microbial colonization[6]. For instance, it has been demonstrated that Lactobacillus and Bifidobacteria populations were reduced by delaying colostrum feeding for about 12h after birth[7]. Heavy loads of pathogenic microbes in the calf environment can influence intestinal colonization of harmful microorganisms such as Escherichia coli (E. coli) K99[8].
Regardless of using a variety of probiotic products available commercially, the incidence of diarrhoea is still high in dairy farms all over the world, indicating that other factors besides feed additives could highly impact calf health and performance in early life. A better understanding of interrelationships between gut microbial colonization, management factors, and diarrhea may help producers use probiotics wisely and effectively.
2 PERSPECTIVES ON GUT MICROBIAL COLONIZATION
Immediately after birth, the newborn gastrointestinal tract is colonized by a wide range of microorganisms[9]. It has been reported that the host microbiome plays a substantial role in developing intestinal barrier function and mucosal immunity[9,10]. During the first weeks of life, the colonizing microbiota suffers from tremendous evolutionary events, rendering it susceptible to dietary or environmental changes[6]. Since neonatal calves are prone to enteric diseases in the early stages of life, considering the small intestine's microbial population dynamics may help work against harmful organisms more effectively and reduce the likelihood of diarrhea incidence[6,9].
Post-birth diversity of the microbial population is not very high but increases rapidly in a few hours. Individual variations amongst calves are believed to be considerable during the first days of life, implying the plasticity of the microbial community in the early stages of life[10]. Analyzing the calf meconium, the first excrement material following colostrum intake, revealed that Citrobacter, Lactobacillus, Leoconostoc, and Lactococcus are the first colonizers of the calf gut 6h to 12h after birth[11]. However, the abundance of colonizing bacteria can be changed over time as calves grow. Such revolutionary occurrences include the decrease in the relative abundance of the Lactobacillus, Bifidobacterium, and Faecalibacterium species with calf age[10]. E. coli also present in the calf gut within a few hours after birth and may become the predominant bacteria in the normal intestine[12]. Differences in feeding protocols, sampling, and methods used for analyzing the gut microbiota, the resulting bacterial community of newborn calves is relatively inconsistent. It has been reported that Firmicutes and Bacteroidetes genera were the most prevalent bacteria in pre-weaned calves[13]. As noted earlier, genetics and breed type, age, and diet may affect the microbial composition of pre-weaned calves. Thus, the calf gut microbiota can be manipulated by adopting different management decisions and systems.
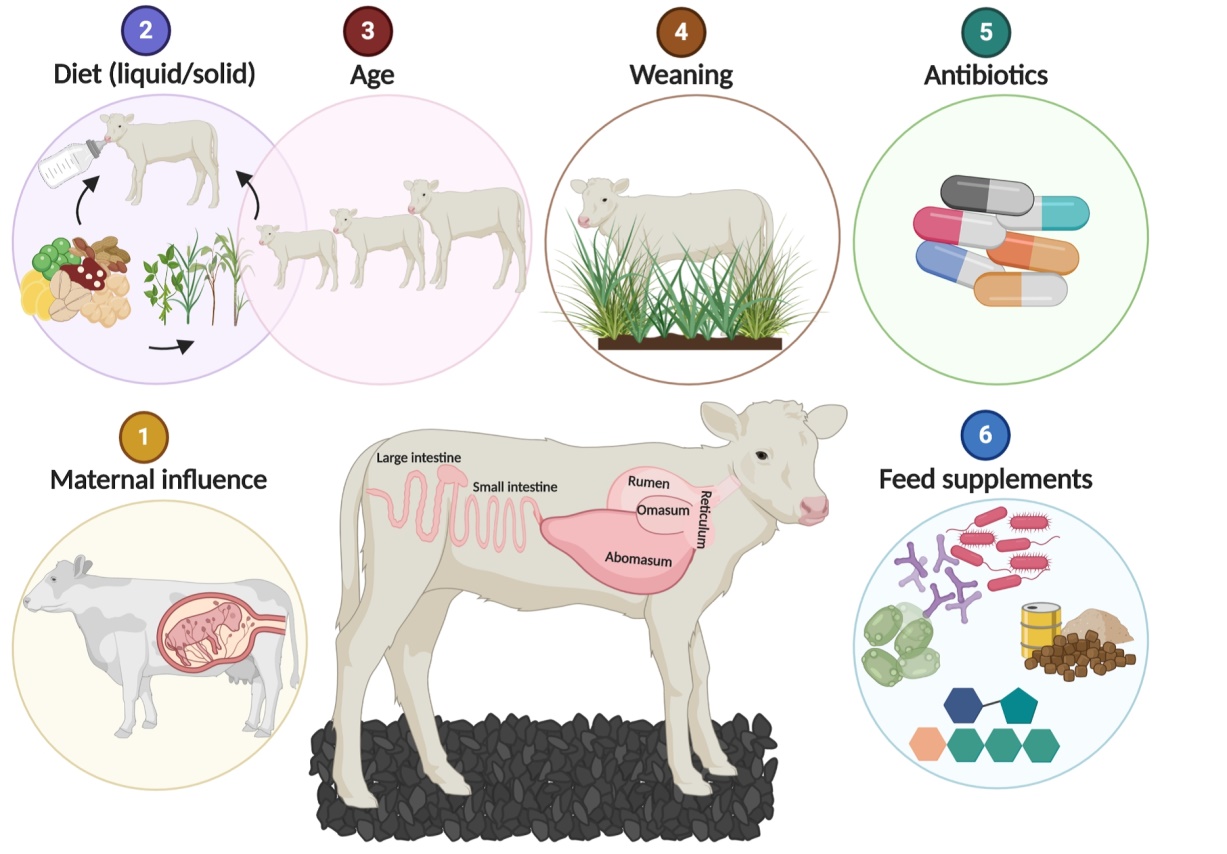
The factors affecting the calf gut microbiota establishment were simply shown in Figure 1[14]. Liquid feed type (milk replacer, whole milk, and waste milk) and starter feed intake may influence the gut microbial composition. It has recently been demonstrated that Lactobacillus genera abundance was higher in calves fed unpasteurized whole milk than those fed acidified whole milk[15]. In addition, the prevalence of Faecalibacterium spp. in whole milk-fed calves was associated with a lower incidence of diarrhea and increased weight gain during the first 4 weeks of life.
|
Figure 1. The factors influencing the establishment of the calf gut microbiota[14]. Source from: Amin N, Seifert J. Dynamic progression of the calf's microbiome and its influence on host health. Comput Struct Biotechnol J, 2021; 19: 989-1001.
Human studies have shown that the oligosaccharides present in milk may act as a substrate for beneficial intestinal bacteria such as Lactobacillus[15]. Such mechanisms may improve young calves' performance and health because bovine milk is an enriched source of prebiotic substances for the intestine microflora[16]. Comparing the fecal microbiota of calves fed milk replacer or pasteurized waste milk revealed a higher prevalence of Clostridial and Bacteroidales in the pasteurized waste milk fed calves[17]. This indicates that the gut microbial ecosystem may be affected by diet type in early life. In addition to milk ration, scientific evidence suggested that the provision of starter diets might influence intestinal barrier function and immune response by altering the genes involved in microbial identification and barrier function[18]. Most of the studies regarding starter intake in young calves have focused on possible roles in the rumen microbiota diversity and development, but less is known about the impact of starter feeds on the microbial establishment of the lower parts of the digestive tract. More studies are needed to determine starter feeds' effects on the intestinal microbiota in detail.
Dairy calf diets may be supplemented with a variety of antibiotics to prevent enteric and respiratory diseases[9]. However, it seems that practical uncertainty exists amongst producers or farm experts due to the effects of antibiotics in changing the gut microbial population and bacterial resistance. In this regard, studies have reported that administring bacitracin methylene disalicylate can potentially increase the number of pathogenic E. coli and shigella while reducing the abundance of the beneficial bacteria Faecalibacterium in fecal samples of calves[19]. Such alterations have been observed for other antibiotics, such as oxytetracycline. A broad-spectrum antibiotic, oxytetracycline, can significantly decrease the number of Lactobacillus species[20]. In contrast, medicated milk replacer fed calves containing tetracycline, ceftiofur, penicillin, and streptomycin showed a reduced abundance of E. coli in the ileum[21]. However, it has been demonstrated that antibiotic-resistant bacteria increase in the calf gut with increased residual antibiotics in whole milk[22]. Moreover, a higher prevalence of antimicrobial-resistant E. coli in the feces was observed in whole milk-fed calves containing antibiotic residuals versus milk replacer-fed calves[23]. Therefore, it can be concluded that unnecessary use of antibiotics during the early weeks of life should be avoided.
Probiotics are common feed additives supplemented in milk or starter feeds to reduce diarrhoea incidence in dairy calves. Altering the bacterial community of newborn calves towards beneficial bacteria and avoiding harmful bacteria attachment to calves' intestines are the most identified mechanisms describing the effects of the probiotic. In this case, decreased E. coli colonization was reported for probiotic strains fed calves in the pre-weaning period[24]. A similar study reported decreased fecal E. coli following direct-fed microbials (DFM) feeding[25]. As a result, because of the positive effects of probiotics use in controlling pathogenic E. coli, substituting antibiotics with probiotics can be a logical practice to reduce antibacterial resistance. Although positive effects of probiotics in controlling harmful bacteria populations exist in the literature, it should be underlined that environmental contaminations, herd-level sanitary protocols, and biosecurity indices and qualities have tremendous effects on E. coli and other pathogenic organisms shedding in practice[8,26]. Overall, efforts to optimize management conditions are vitally important when probiotics are used to control or prevent diarrhea.
3 PROBIOTICS AGAINST DIARRHOEA AND FOR HOST IMMUNITY AND HEALTH
Infectious diarrhoea is the most known disease of newborn calves. Despite the many advances in feeding programs, housing systems, and treatment protocols, the diarrhoea remains a major problem in dairy and beef herds worldwide. In addition to the economic cost of diarrhea treatment, calf health and performance in early life are associated with later performance and sustainability of dairy cattle[1]. Amongst many organisms (bacteria, viruses, and protozoa) involved in the diarrhea etiology, pathogenic E. coli is the most prevalent bacteria causing severe diarrhea and likely calf death on most farms. Vaccination of dry cows against E. coli, feeding antibiotics, and feed supplementation with probiotics and prebiotics have been conducted for years to reduce the likelihood of diarrhea occurrence[4,9]. However, Sadly, most calf mortality still results from diarrhea, despite the widespread availability of commercial probiotic supplements and other feed additives[8]. Therefore, as mentioned above, the establishment of gut microbiota depends on numerous environmental and management factors that can interact to determine the gut microbial community and diversity. It is important to note that the microbial population of newborns shows more plasticity to any changes in diet and management factors, making it feasible to be manipulated in the first weeks of life[4]. As such, promoting the growth of beneficial bacteria or preventing colonization of harmful bacteria in the first two weeks of life should be the main goal of any preventive programs against E. coli invasion.
It has been reported that the early microbial composition of calves has a vital role in their susceptibility to diarrhea[13]. Increased abundance of Bifidobacterium in calves fed heat-treated colostrum and concurrently decreased E. coli colonization might be good reasons for lower diarrhea occurrence in colostrum-fed calves[27]. Furthermore, feeding yeast cultures to pre-weaned calves has reduced diarrhea incidence and mortality rate within the 70d of life[28]. Similarly, supplementing calves with Lactobacillus and Bifidobacterium in the first 7d of age led to decreased diarrhea incidence and improved feed conversion ratio[29]. The occurrence of diarrhea with pathogenic E. coli seems to be negatively related to the abundance of Lactobacillus. In a study conducted to investigate pathogenic E. coli effects on the intestinal immunity of orally infected dairy calves, the higher E. coli abundance was related to a lower abundance of Lactobacillus[30]. On the other hand, reduced microbial diversity has been observed in calves with watery feces (diarrhea) vs. normal feces in healthy calves[31]. As such, isolated Lactobacillus strains inhibited the growth of pathogenic strains such as E. coli. The authors concluded that providing multiple potential probiotics can restrict E. coli colonization and prevent calf diarrhoea. A healthy gut with suitable microbial diversity is believed to prevent pathogenic bacterial colonization. Thus, enhanced microbial and intestinal integrity and mucosal immunity are essential for controlling pathogens invasion and diarrhea occurrence[31].
In addition to bacterial probiotics, live yeast and yeast culture are frequently used in calf diets. Beneficial effects were also observed for calves on yeast products[4]. Preventing pathogenic agents' attachment to the epithelium of the intestine and enhancing mucosal immunity are the most common mechanisms described for yeast products[32]. Improved intestinal barrier function could also result from supplementing calf diets with yeast culture[33]. More recently, enhancement in the release of secretory IgA in the ileum and colon of calves supplemented by Saccharomyces cerevisiae boulardii was reported[34]. Although most studies show positive effects of probiotics on improving gut health and reducing diarrhea, it is important to remember that diarrhea is a multifactorial disease, and numerous environmental and management factors are involved in its incidence. As a result, modifying the gut microbial ecosystem is just a single part of the diarrhea prevention equation. Thus, probiotics may be efficiently used if the other determining management factors are carefully and successfully controlled. For example, feeding adequate and timely colostrum, clean ambiance, optimal nutrition, and proper housing are necessary factors for any calf rearing program to make the best use of probiotics regarding modifying and optimizing the gut microbial diversity to prevent diarrhea.
A recent literature review regarding the effects of probiotics on calf growth showed no significant differences between treated and untreated calves[4]. Indeed, in normal rearing conditions, using probiotics may have no or little effects on calf growth. However, probiotics may show more usefulness under highly stressed conditions (e.g., thermal, transport, and nutritional transition stresses). Therefore, other principle management conditions (e.g., nutrition, behavior, health, comfort, environmental sanitation, etc.) should be kept normal or nearly optimized in any practical scenario[35-38]. This implies that the possible use of DFM should not be overstated.
4 CONCLUSION
Preventing diarrhoea is an important management practice affecting ruminant herd productivity, health, and economics. The optimal establishment of the gut microbial ecosystem is essential for the optimal functioning of the intestinal immune system. It seems that probiotics should be administered rather in the early weeks of life to catch better outcomes. Probiotics may reduce diarrhea incidence; however, basic management practices, including colostrum feeding, milk feeding, housing system, sanitation protocols, and biosecurity, must not be disregarded. Under stressful conditions, probiotics may be more efficient, especially concerning calf growth.
Acknowledgements
Nature for its inspiring nature.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
Nikkhah A and Alimirzaei M contributed equally to this work including idea conceptualization, review topic critical discussion and development, writing, and editing. Nikkhah A formed the idea and led the project.
Abbreviation List
DFM, Direct-fed microbials
E. coli, Escherichia coli
References
[1] Nikkhah A, Alimirzaei M. On pre-weaning calf weight gain differences: Opportunities to improve herd productivity, health and longevity. Biomed J Sci Tech Res, 2022; 42: 33420-33425. DOI: 10.26717/BJSTR.2022.42.006717
[2] Reddy PRK, Elghandour MMMY, Salem AZM et al. Plant secondary metabolites as feed additives in calves for antimicrobial stewardship. Anim Feed Sci Tech, 2020; 264: 114469. DOI: 10.1016/j.anifeedsci.2020.114469
[3] Hill C, Guarner F, Reid G et al. The international scientific association for probiotics and prebiotcs consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol, 2014; 11: 506-514. DOI: 10.1038/nrgastro.2014.66
[4] Cangiano LR, Yohe TT, Steele MA et al. Invited review: Strategic use of microbial-based probiotics and prebiotics in dairy calf raising. Appl Anim Sci, 2020; 36: 630-651. DOI: 10.15232/aas.2020-02049
[5] Halloran K, Underwood MA. Probiotic mechanism of action. Early Hum Dev, 2019; 135: 58-65. DOI: 10.1016/j.earlhumdev.2019.05.010
[6] Malmuthuge N, Griebel PJ, Guan L. The gut microbiome and its potential role in the development and function of newborn calf gastrointestinal tract. Front Vet Sci, 2015; 2: 36. DOI: 10.3389/fvets.2015.00036
[7] Fischer AJ, Song Y, He Z et al. Effect of delaying colostrum feeding on passive transfer and intestinal bacterial colonization in neonatal male Holstein calves. J Dairy Sci, 2018; 101: 3099-3109. DOI: 10.3168/jds.2017-13397
[8] Nikkhah A, Alimirzaei M. Preventing diarrhoea to reduce calf morbidity and mortality: A pragmatic outlook. Inter J Biomed Res, 2022; 2: 1-3.
[9] Lyons T, Hanne J, Bradly J et al. Integrated analyses of the microbiological. Immunological and ontological transitions in the calf ileum during early life. Sci Rep-UK, 2020; 10: 21264. DOI: 10.1038/s41598-020-77907-0
[10] Malmuthuge N, Guan LL. Understanding the gut microbiome of dairy calves: Opportunities to improve early-life gut health. J Dairy Sci, 2017; 100: 1-10. DOI: 10.3168/jds.2016-12239
[11] Mayer M, Abenthuma JM, Matthessa D et al. Development and genetics influence of neonatal bacterial flora of newborn calves. Vet Microbial, 2012; 161: 179-185. DOI: 10.1016/j.vetmic.2012.07.023
[12] Janke BH, Francis DH, Collins JE et al. Attaching and affecting Eschericia coli infections in calves, pigs, lambs, and dogs. J Vet Diagn Invest, 1989; 1: 6-11. DOI: 10.1177/104063878900100104
[13] Oikonomou G, Teixeira AG, Fodlitsch C et al. Fecal microbial diversity in pre-weaned dairy calves as escribed by pyrosequencng of metagenomic 16S rDNA. Associations of Fecalibacterium species with health and growth. PLos One, 2013; 8: e63157. DOI: 10.1371/journal.pone.0063157
[14] Amin N, Seifert J. Dynamic progression of the calf's microbiome and its influence on host health. Comput Struct Biotechnol J, 2021; 19: 989-1001. DOI: 10.1016/j.csbj.2021.01.035
[15] Virginio Junior GF, Coelho MG, de Toledo AF et al. The liquid diet composition affects the fecal community in pre-weaning dairy calves. Front Anim Sci, 2021; 2: 649468. DOI: 10.3389/fanim.2021.649468
[16] Pacheco AR, Barile D, Underwood MA et al. The impact of milk glycobiome on the neonate gut microbiota. Annu Rev Anim, 2015; 3: 419-445. DOI: 10.1146/annurev-animal-022114-111112
[17] Maynou G, Migura-Garcia L, Subirats J et al. Impact of milk feeding programs on fecal bacterial population and antimicrobial resistance genes in Escherichia Coli isolated from faeces in pre-weaned calves. J Anim Sci, 2016; 94: 593. DOI: 10.2527/jam2016-1232
[18] Malmuthuge N, Li M, Goonewardene LA et al. Effect of calf starter feeding on gut microbial diversity and expression of genes involved in host immune responses and tight junctions in dairy calves. J Dairy Sci, 2013; 96: 3189-3200. DOI: 10.3168/jds.2012-6200
[19] Xie G, Duff GC, Hall LW et al. Alteration of digestive tract microbiome in neonatal Holstein bull calves by bacitracin methylene disalicylate treatment and scours. J Anim Sci, 2013; 91: 4984-4990. DOI: 10.2527/jas.2013-6304
[20] Oultram J, Phipps E, Teixeira AG et al. Effects of antibiotics (oxytetracycline, florfenicol or tulathromycin) on neonatal calves faecal microbial diversity. Vet Rec, 2015; 177: 598. DOI: 10.2527/jas.2013-6304
[21] Yousif M, Li JH, Li ZQ et al. Low concentration of antibiotic modulates gut microbiota at different levels in pre-weaning dairy calves. Microorganisms, 2018; 6: 118. DOI: 10.3390/microorganisms6040118
[22] Langford FM, Weary DM, Fisher L. Antibiotic resistance in gut bacteria from dairy calves: a dose response to the level of antibiotics fed in milk. J Dairy Sci, 2003; 86: 3963-3966. DOI: 10.3168/jds.S0022-0302(03)74006-5
[23] Maynou G, Bach A, Terre M. Feeding of waste milk to Holstein calves affects antimicrobial resistance of Escherichia coli and Pasteurella multocida isolated from fecal and nasal swabs. J Dairy Sci, 2017; 100: 2682-2694. DOI: 10.3168/jds.2016-11891
[24] Zhao T, Doyle MP, Hammon BC et al. Reduction of carriage of enterohemorrhagic Eschericia coli O157: H7 in cattle by inoculation with probiotic bacteria. J Clin Microbial, 1998; 36: 641-647. DOI: 10.1128/JCM.36.3.641-647.1998
[25] Krehbiel C, Rust S, Zhang G et al. Bacteral direct-fed microbials in ruminant diets: performance response and mode of action. J Anim Sci, 2003; 81: E120-132.
[26] Cho YI, Yoon KJ. An overview of calf diarrhea-infectious etiology, iagnosism and intervention. J Vet Sci, 2014; 15: 1-17. DOI: 10.4142/jvs.2014.15.1.1
[27] Godden SM, Smonenski DJ, Donahue M et al. Heat-treated colostrum and reduced morbidity in pre-weaned dairy calves: Results of a randomize trial and examination of mechanisms of effectiveness. J Dairy Sci, 2012; 95: 4029-4040. DOI: 10.3168/jds.2011-5275
[28] Magalhaes VJ, Susca F, Lima FS et al. Effect of feeding yeast culture on performance, health, and immunocompetence of dairy calves. J Dairy Sci, 2008; 91: 1497-1509. DOI: 10.3168/jds.2007-0582
[29] Abe F, Ishibashi N, Shimamura S. Effect of administration of bifidobacteria and lactic acid bacteria to newborn calves and piglets. J Dairy Sci, 1995; 78: 2838-2846. DOI: 10.3168/jds.S0022-0302(95)76914-4
[30] He L, Wang C, Simujide H et al. Effect of early pathogenic Eschericia coli infection on the intestinal barrier and immune function in newborn calves. Front Cell Infect Microbial, 2022; 12: 818276. DOI: 10.3389/fcimb.2022.818276
[31] Fan P, Kim M, Liu G et al. The gut microbiota of newborn calves and influence on potential probiotics on reducing diarrheic disease by inhibition of pathogen colonization. Front Microb, 2021; 12: 772863. DOI: 10.3389/fmicb.2021.772863
[32] Wang Z, Guo Y, Yuan J et al. Effect of dietary b-1,3/1,6-glucan supplementation on growth performance, immune response and plasma prostaglandin E2, growth hormone and ghrelin in weaning piglets. Asian-Australas J Anim, 2008; 21: 707-714. DOI: 10.5713/ajas.2008.70559
[33] Kvidera SK, Dickson MJ, Abuajamieh M et al. Intentionally induced intestinal barrier dysfunction causes inflammation, affects metabolism and reduces productivity n lactating Holstein cows. J Dairy Sci, 2017; 100: 4127. DOI: 10.3168/jds.2016-12349
[34] Villot C, Chen Y, Pedgerachni K et al. Early supplementation of Saccharomyces cervisiae boularii CNCM 1-1070 in newborn dairy calves increases Ig A production in the intestine at 1 week of age. J Dairy Sci, 2020; 103: 8615-8628. DOI: 10.3168/jds.2020-18274
[35] Nikkhah A, Alimirzaei M. Splanchnic malfunction in a male holstein calf: A Problem beyond abomasal ulcers and bloat. Anim Husb Dairy Vet Sci, 2022; 6: 2-2.
[36] Nikkhah A, Alimirzaei M. Individual vs. group housing of dairy calves: An arbitrary or provisional rule? World J Agri Soil Sci, 2021; 7: 1-2.
[37] Nikkhah A, Alimirzaei M. Mini review: Bedding management for young calves: Health, welfare and growth perspectives. Open Access J Biomed Sci, 2022; 4: 1561-1562. DOI: 10.38125/OAJBS.000396
[38] Alimirzaei M, Nikkhah A. Hypothermia as a threat to young calves in cold ambient temperatures: Development of a workable therapeutic protocol. World J Agri Soil Sci, 2021; 7.
Copyright © 2022 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.





 Copyright ©
Copyright ©