Calliphoridae Family (Insecta: Diptera) of Importance in Forensic Entomology, Larval Therapy, Medical, Veterinary and Vector of Pathogens
Carlos Henrique Marchiori1*
1Department of Biological Science, Institution Federal Goiano, Goiânia, Goiás, Brazil
*Correspondence to: Carlos Henrique Marchiori, PhD, Professor, Department of Biological Science, Institution Federal Goiano, Rua T-65 No.1050, Goiânia 74230120, Brazil; E-mail: chmarchiori@yahoo.com.br
Abstract
Adults of the Calliphoridae family are attracted to the odor generated by the wounds of all types of vertebrates, but they can also be found in flowers and foliage. Eggs are laid in substrates such as feces, carrion, necrotic tissue or healthy open-wounded tissue from which there the larvae develop. Adults primarily feed on exudates from previous substrates. The objective of this work is to investigate the biology, ecology, habitat, geographic distribution, taxonomy, life cycle, phenology, biological control, and work done on the Calliphoridae family (Insecta: Calliphoridae). A literature search involving articles published between 1950 and 2022 was conducted in order to carry out research related to quantitative, taxonomic, and conceptual aspects. A mini-review was prepared in Goiânia, Goiás, between September and October 2021, by means of the Biological Abstracts, Periodicals CAPE S and Scielo.
Keywords: myiasis, decomposition, phenology, biology, ecology
1 INTRODUCTION
The family Calliphoridae has been extensively studied mainly from the forensic perspective due to its early presence in decomposing bodies. Some species can serve as mechanical vectors of bacteria, protozoa, and helminths. As a result of its large ecological diversity, this family is able to occupy a wide range of habitats (Figures 1 and 2)[1,2].
|
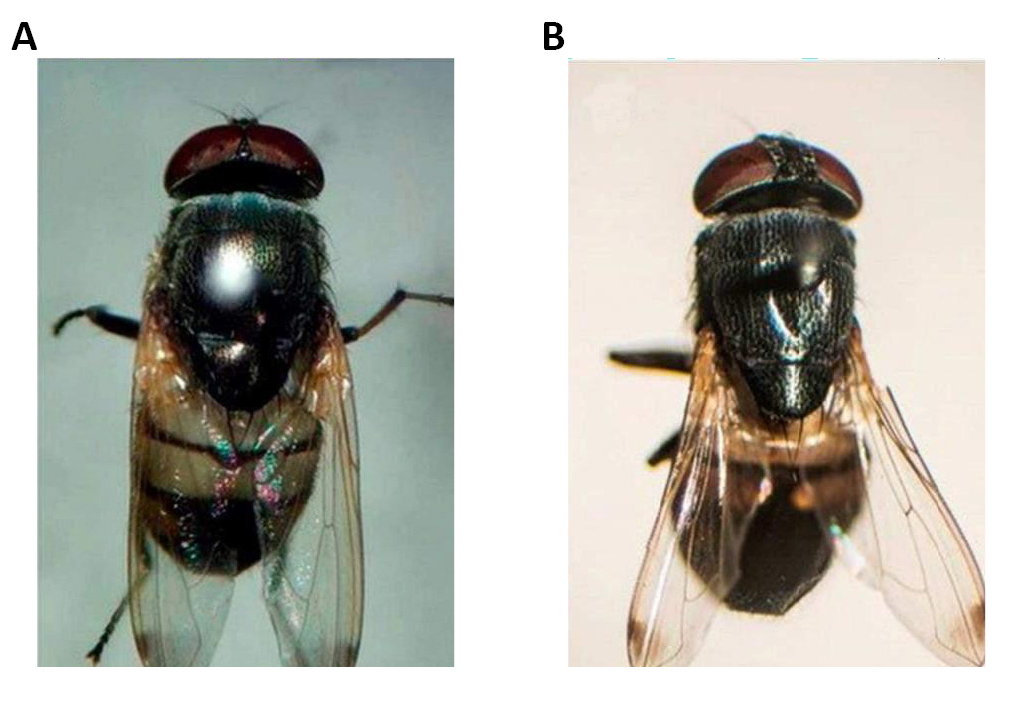
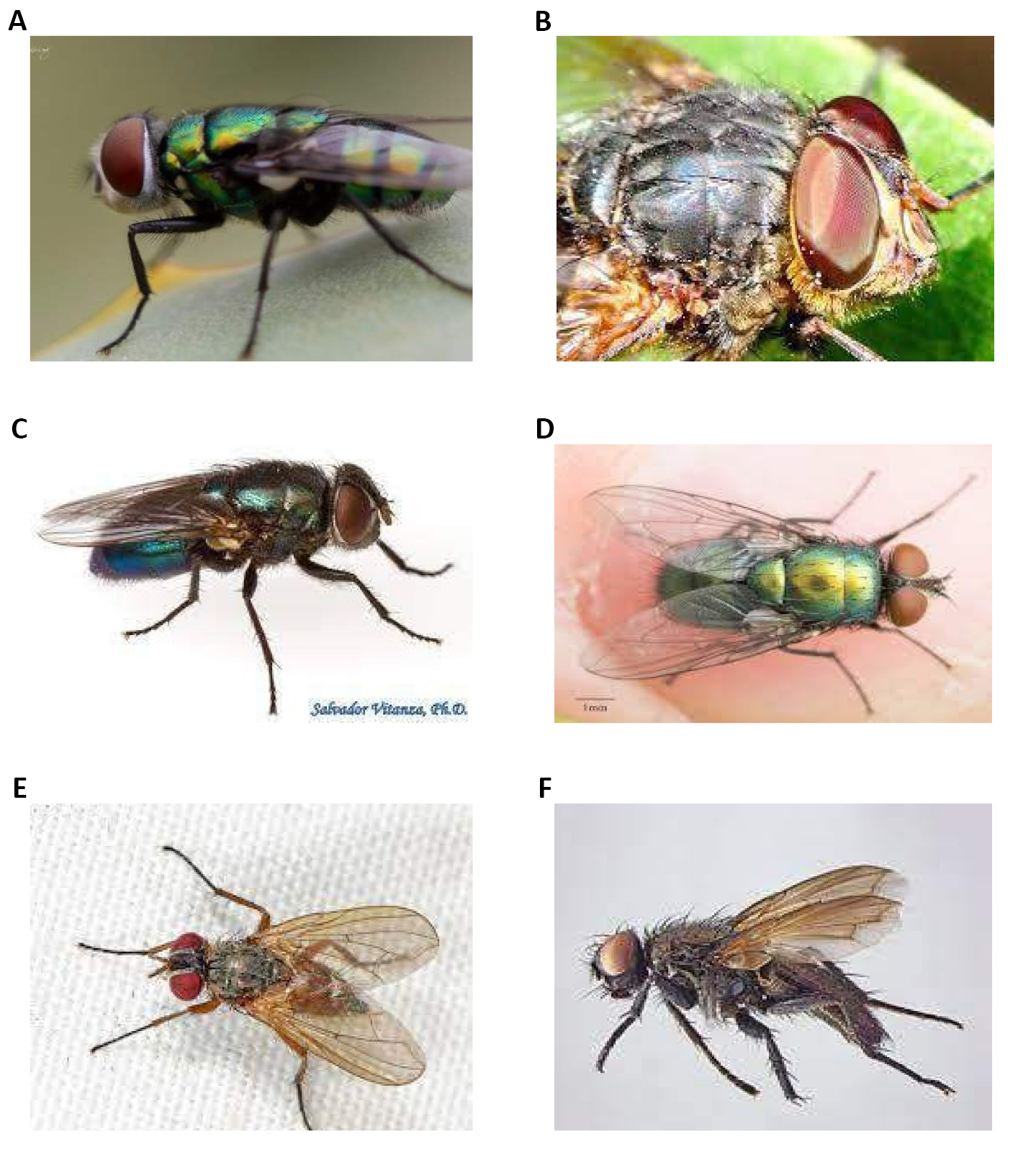
Figure 1. Specimens of Calliphoridae family. A: Side views; B: Side view and front view. Source: https://www.deviantart.com/bouzid27/art/Fly-6-730850616
|
Figure 2. Dorsal view of Stomorhina discolor (Fabricius, 1794). A: Male with holoptic eyes; B: Female with dichoptic eyes. Source: https://www.mdpi.com/2075-4450/8/1/11/htm
1.1 Description
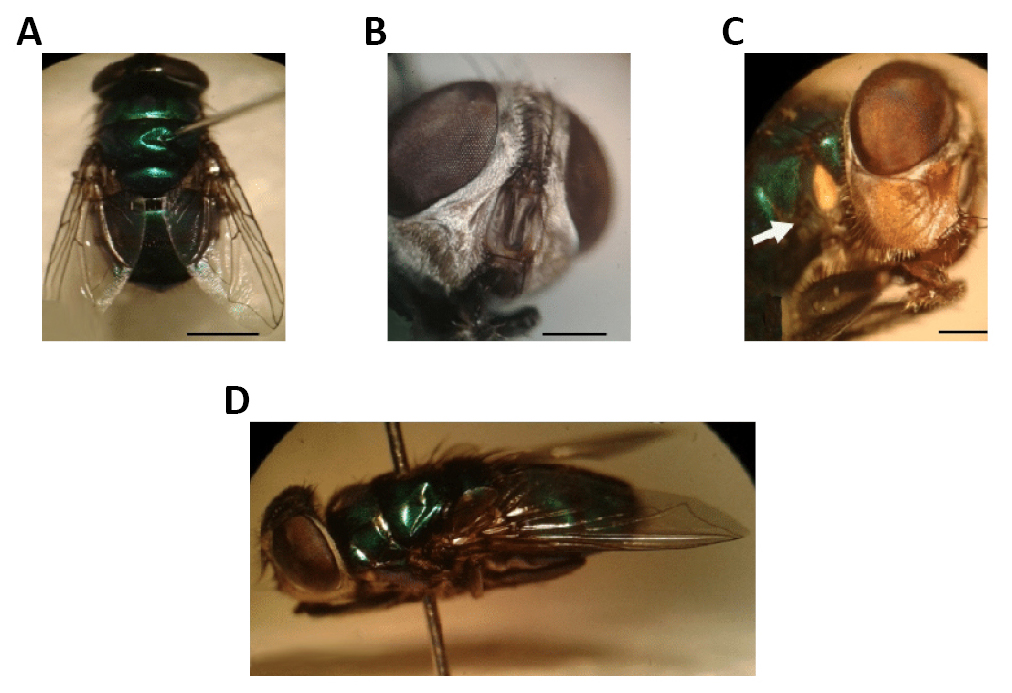
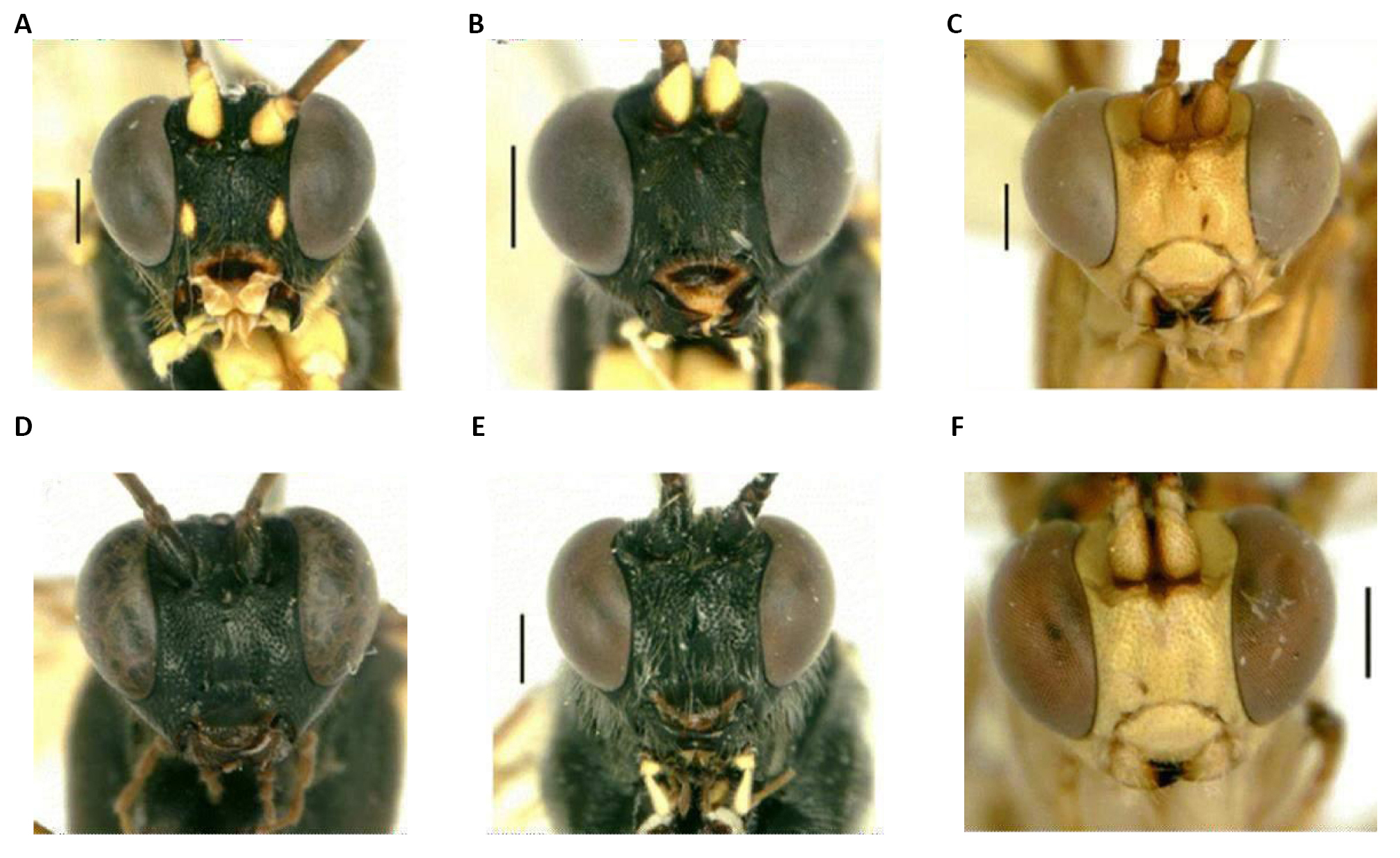
The awn is plumose, the bristles are typically long, reaching the apex, and there is no or a poorly developed post-scutellum on these Diptera. Mero and anepimere have well-developed bristles, katepisternum has three bristles, whilst notopleural has two bristles and sometimes an accessory bristle; the posterior posthumeral bristle is situated more laterally than the anterior presutural; the mesonotum may or may not have longitudinal black bands; the M1+2 rib is strongly curved forward distally, thereby making the apical cell (R4+5) narrower; it has well-developed thoracic calypters; it also segments the abdominals without distal bristles, or they will be poorly developed (Figure 3)[3-5].
|
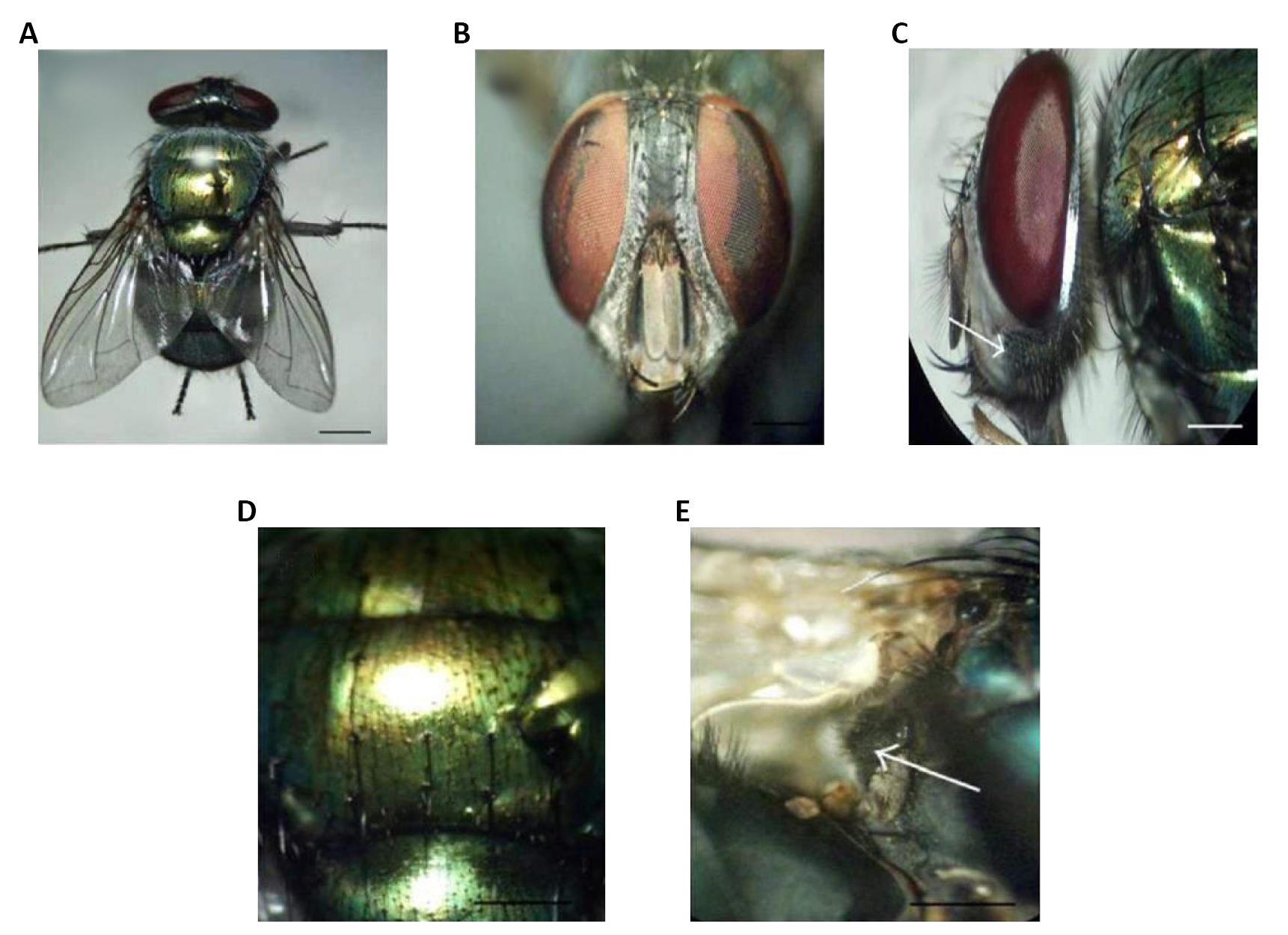
Figure 3. Diptera. A: Dorsal view of the female; B: Dorsal view of the male; C: Frons of the female showing dichoptic eyes; D: Frons of the male showing subholoptic eyes; E: Lateral view of the adult showing whitish squamae (arrow). Source: https://doi.org/10.1155/2012/371243
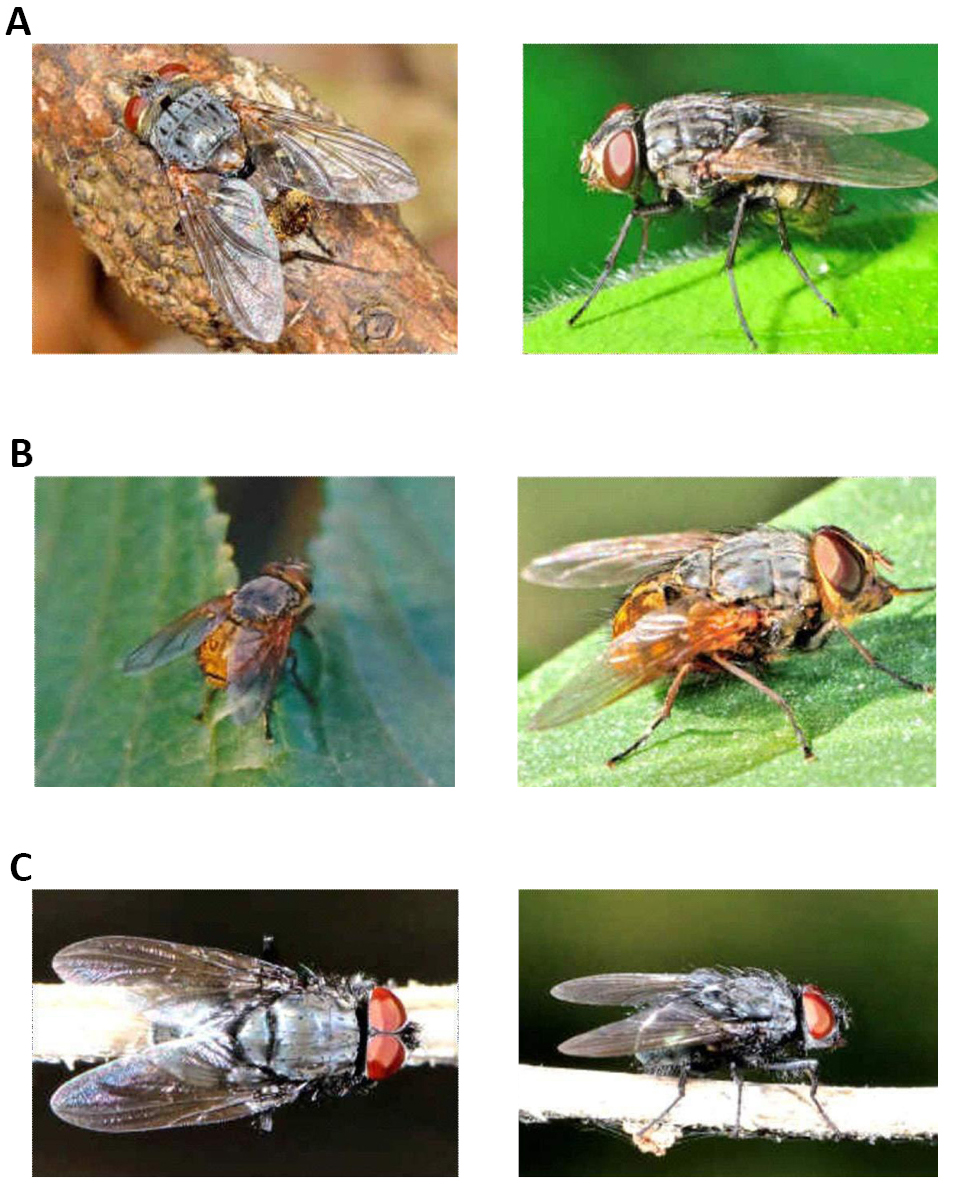
It has wings with hairy calyptera dorsally or ventrally and has a 5 part abdomen, with 3 or more segments forming the genitalia. Its coloration is partially or totally metallic green or blue, although it is sometimes opaque (Figures 4-6)[6-8].
|
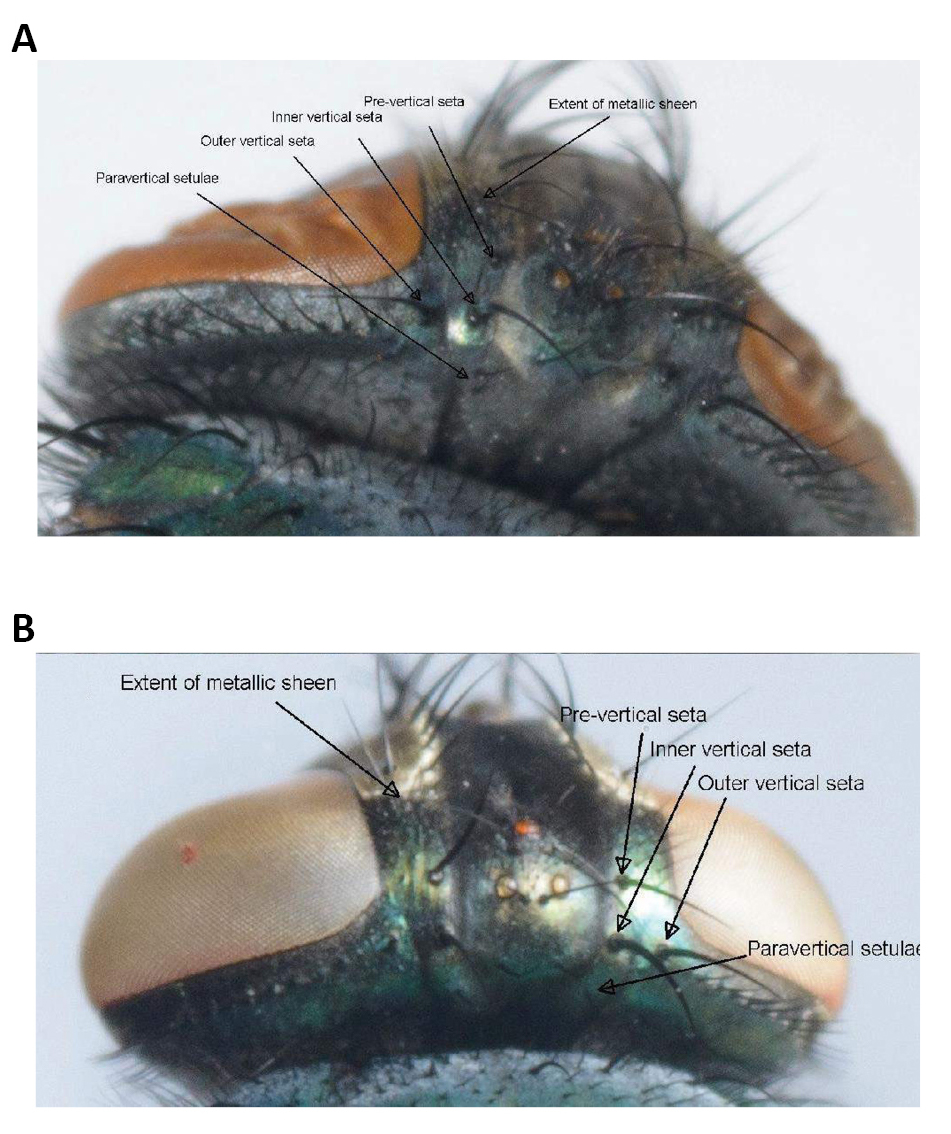
Figure 4. Paravertical setulae, distance between the outer and inner vertical setae, the size of the angle at the inner vertical triangle and extent of metallic sheen on parafrontal sclerites. A: Lucilia sericata (Meigen, 1826); B: Lucilia cuprina (Wiedemann, 1830). Source: https://www.semanticscholar.org/paper/Morphological-identification-of-Lucilia-sericata%2C-Williams-Villet/3f05085face0eb2e451f1b635a11d099df6ae699/figure/1
|
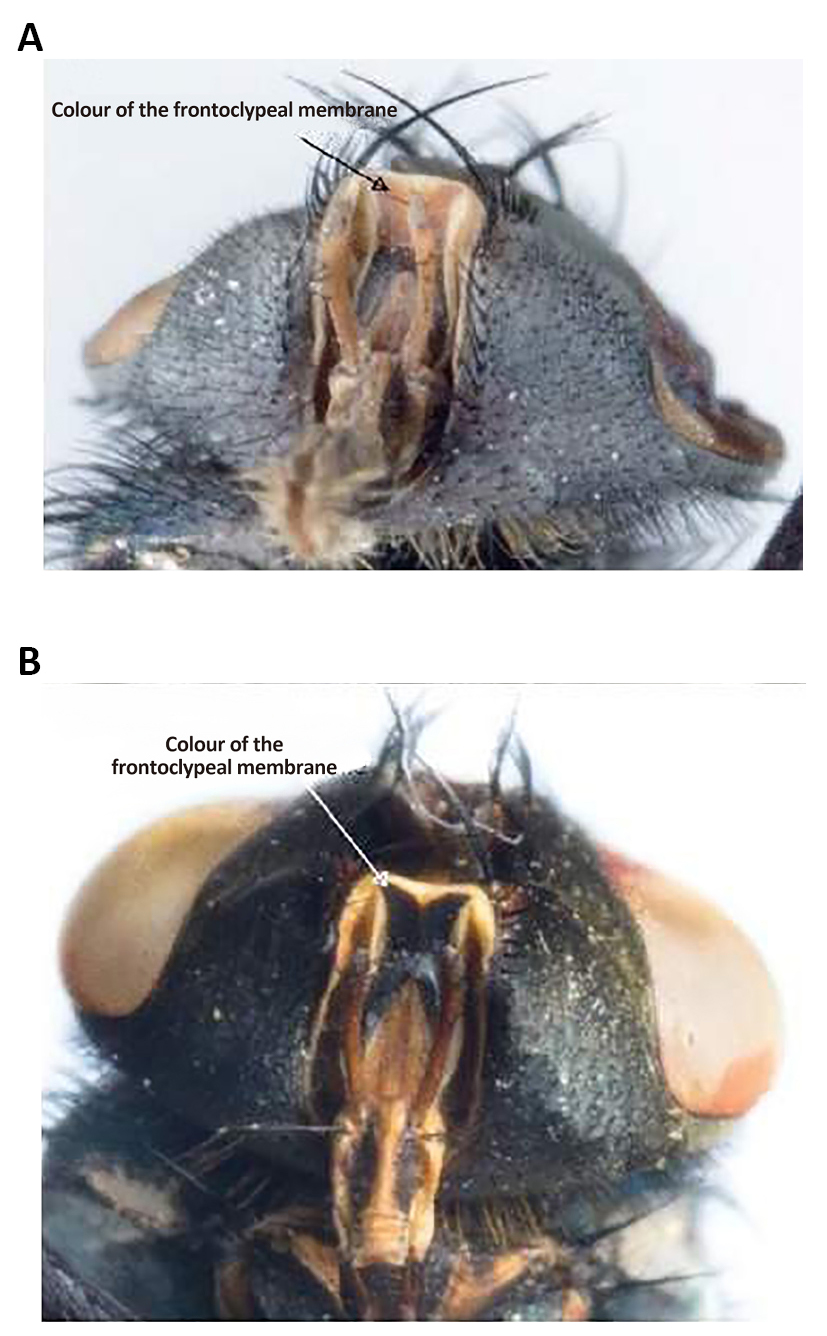
Figure 5. Colour of the fronto clypeal membrane. A: Lucilia sericata (Meigen, 1826); B: Lucilia cuprina (Wiedemann, 1830). Source: https://www.semanticscholar.org/paper/Morphological-identification-of-Lucilia-sericata%2C-Williams-Villet/3f05085face0eb2e451f1b635a11d099df6ae699/figure/2
|
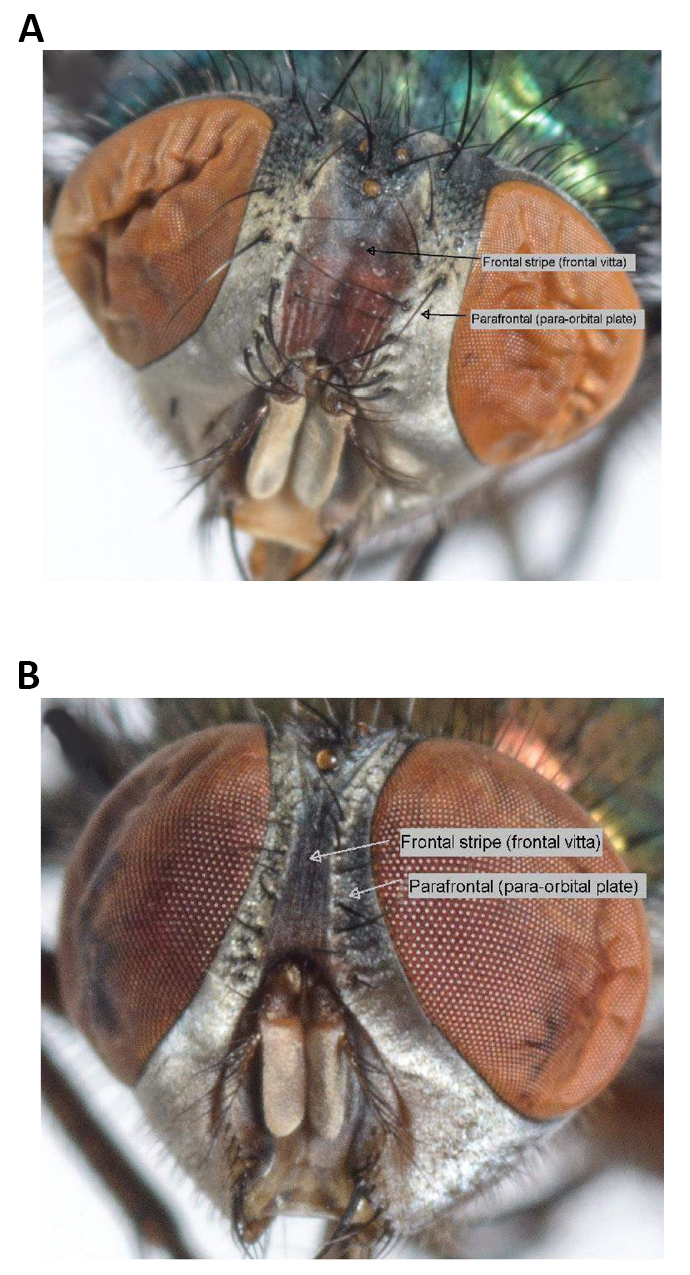
Figure 6. Lucilia sericata. A: Frontal stripe-Lucilia sericata (Meigen, 1826); B: Lucilia cuprina (Wiedemann, 1830). Source: https://www.researchgate.net/figure/Frontal-stripe-Lucilia-sericata-A-and-Lucilia-cuprina-B_fig2_263426596
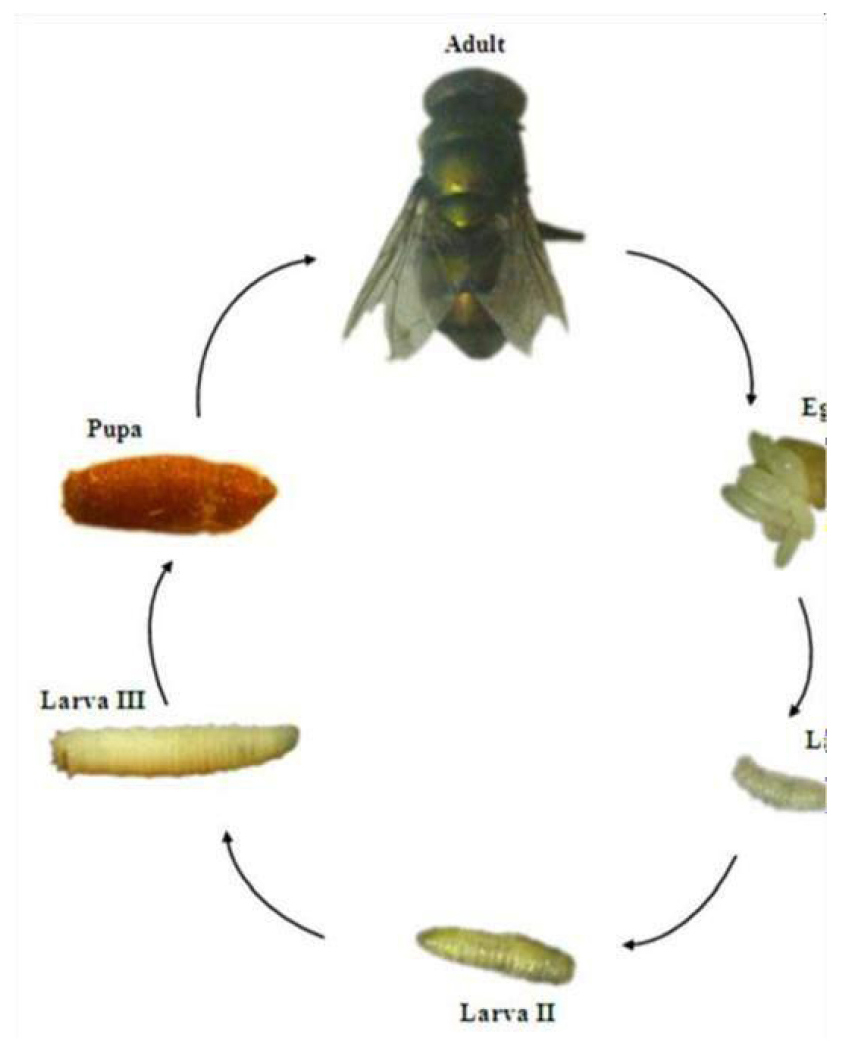
1.2 Life Cycle
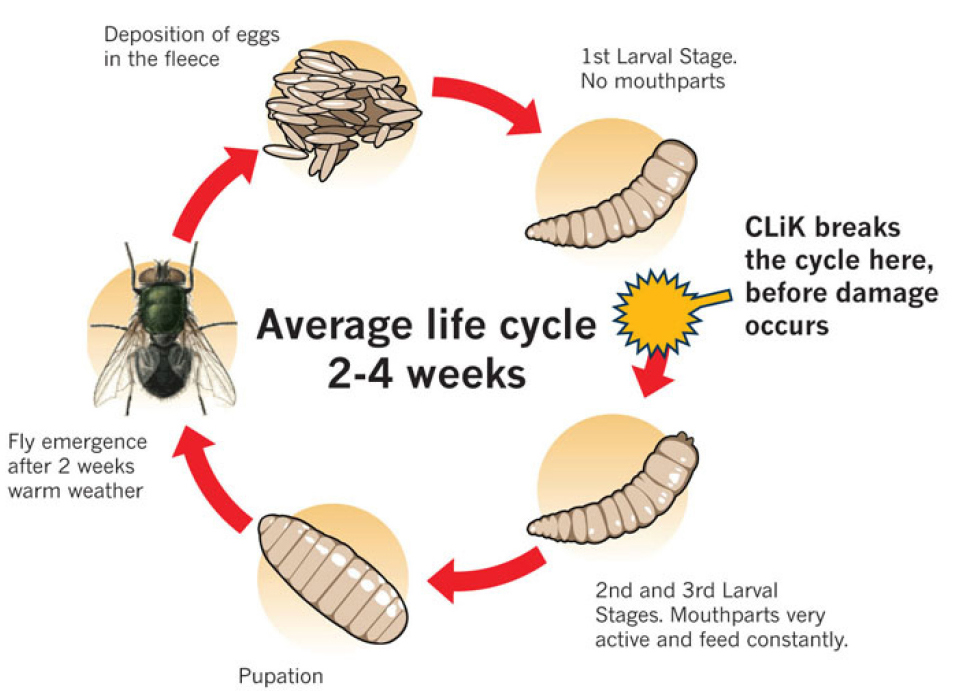
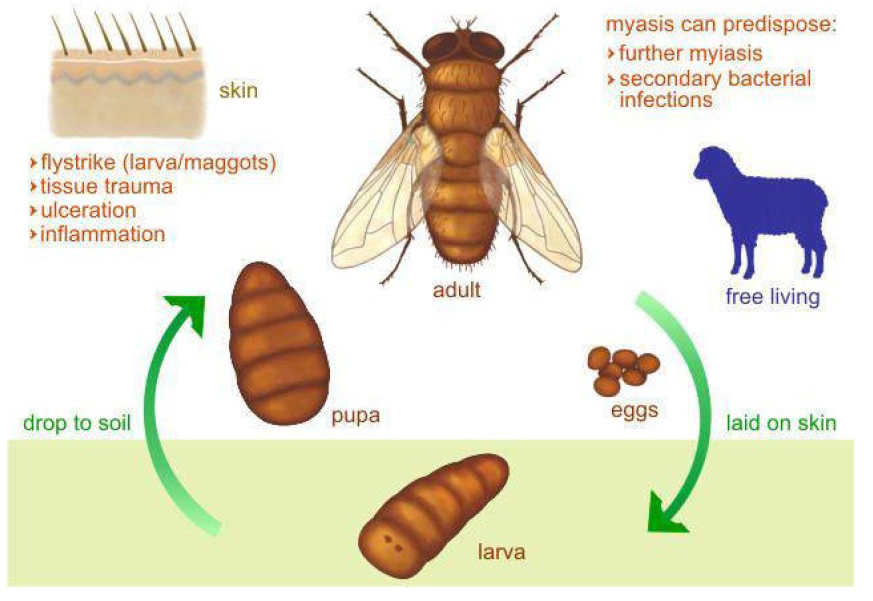
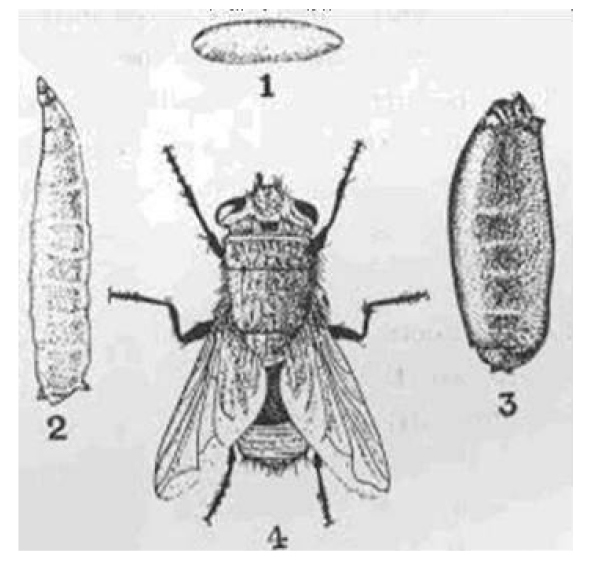
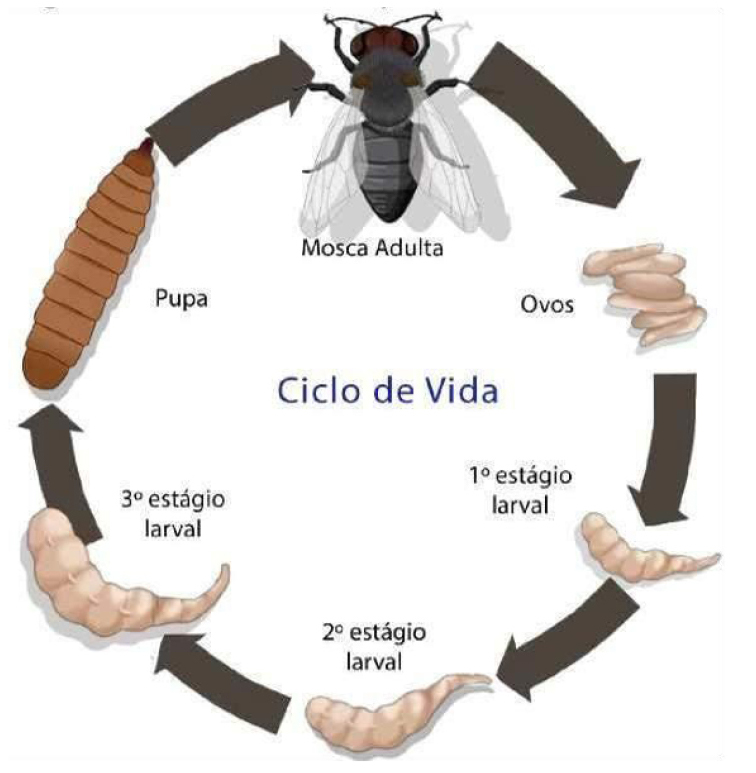
The life cycle comprises eggs, three larval instars, pupal instars and adult instars. During the pupal stage, a larva ceases to feed and leaves the substrate in search for a suitable place to pupate. At this stage the larva undergoes a post-feeding phase, commonly referred to as prepupa. The distance traveled during this migration is variable depending on the species (Figure 7)[9,10].
|
Figure 7. Life cycle of a blowfly Calliphoridae family. Source: https://www.crookwellvet.com.au/AnimalCare/Sheep/FlyStrike.aspx
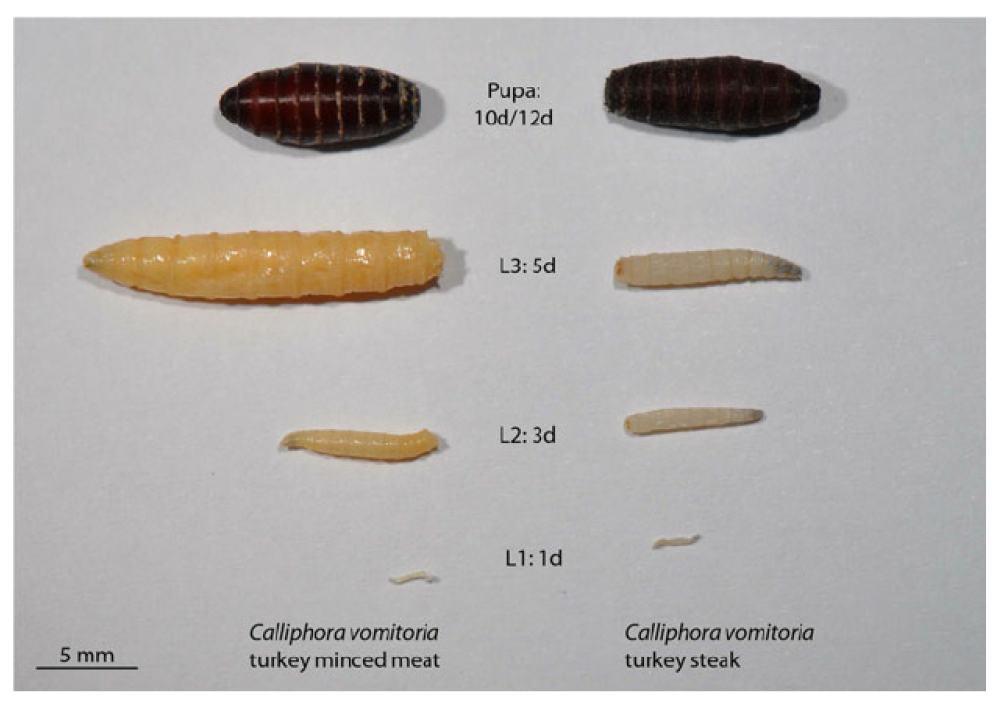
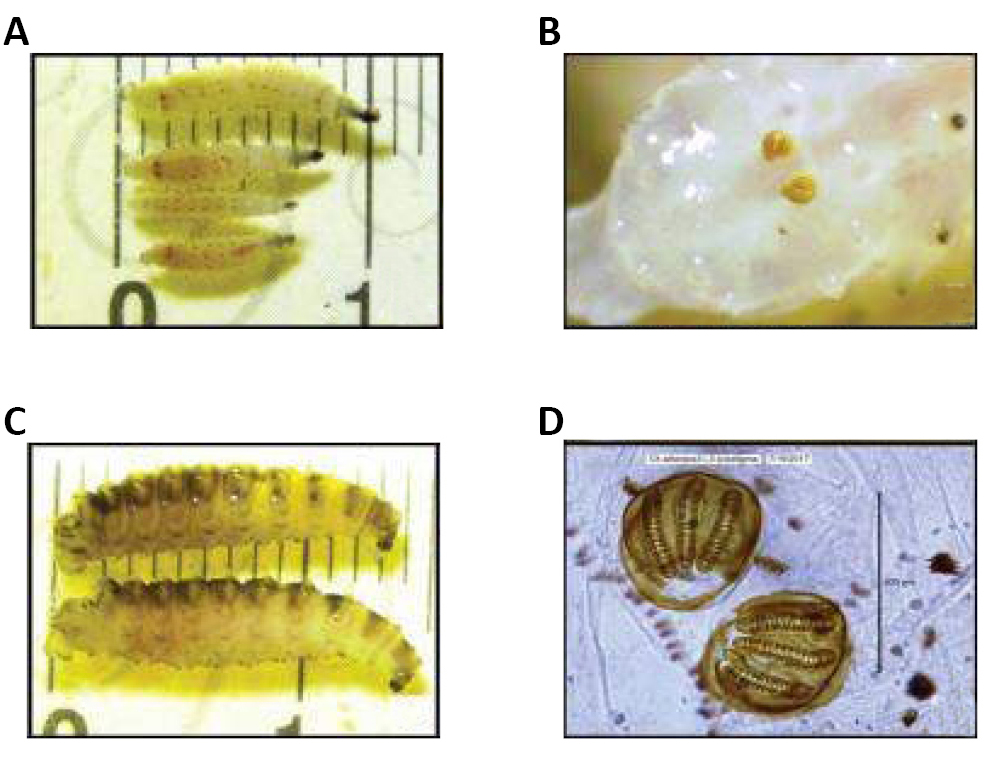
As larvae of Calliphoridae they are vermiform, acephalous, their anterior ends are sharpened with buccal structures called the cephalopharyngeal skeleton, whereas their posterior ends are truncated, formed by a disk more or less concave and surrounded by four to six pairs of conical tubercles (Figure 8)[11,12].
|
Figure 8. Comparison of development in Calliphora vomitoria (Linnaeus, 1758) on turkey minced meat (left) and turkey steak (right) from 1-day-old L1 larvae (bottom) to pupae (top) with an age of 10 days from turkey minced meat (left) and 12 days from turkey steak (right), respectively. Source: https://www.researchgate.net/figure/Comparison-of-development-in-C-vomitoria-on-turkey-minced-meat-left-and-turkey-steak_fig3_236915692
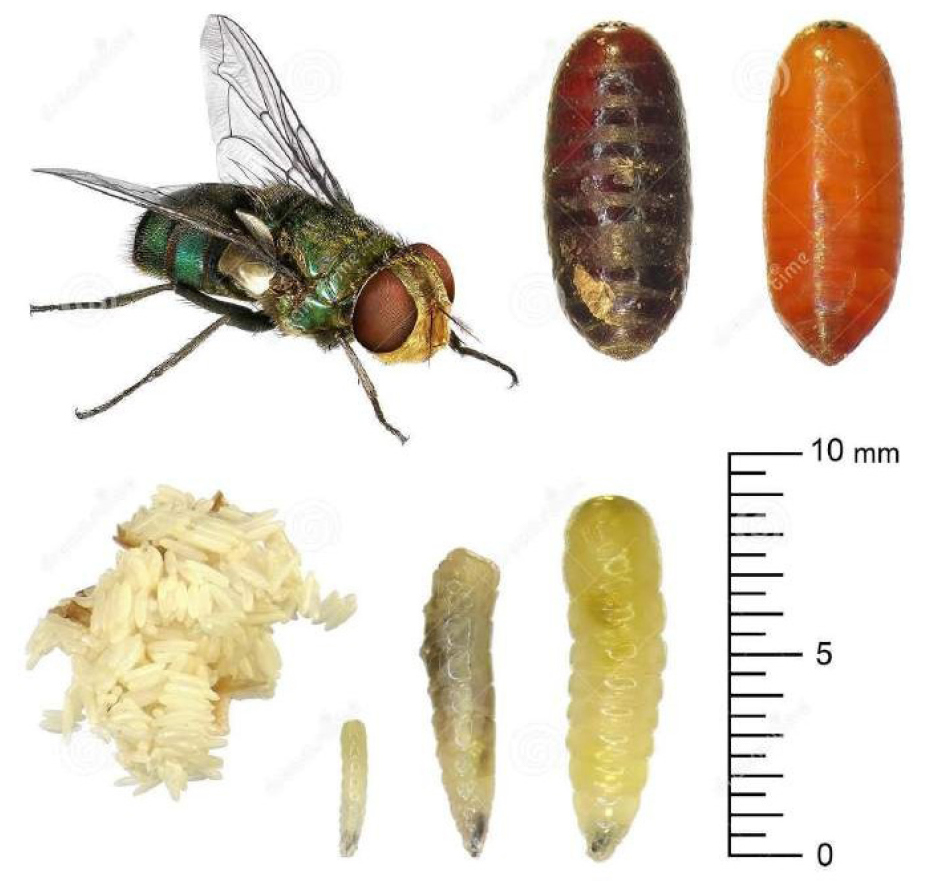
There are two posterior spiracles located in the center of this disk, each of which has a spiracular opening, that is usually used as an indicative character in the mature larvae (larva 3). There are 12 segments on the body, most of which are covered with parallel pigmented spines or patches, either covering the whole segment or only a portion of it (Figure 9)[13,14].
|
Figure 9. Egg, larva, pupa and adult Calliphoridae family. Source: https://www.dreamstime.com/stock-photo-housefly-development-stages-eggs-larva-imago-isolated-white-background-image63441861
1.3 Biology
In addition to being attracted by the odor generated in the wounds of vertebrates, adults are also found in flowers and foliage. The larvae of many species deposit their eggs in substrates such as feces, carrion, necrotic tissue or healthy open-wounded tissue where they develop. Adults of many species consume exudates from previous substrates[15,16].
In Brazil they are often referred to as blowflies because the larvae of this family can be biontophagous, scavengers or necrobiontophagous, which can cause obligatory and facultative myiasis, making them extremely important in the health of animals[15,16].
1.4 Habitat
Calliphorids are easily identifiable by their bright metal color that they flaunt: they are flies that we often find associated to the home environment and we recognize by its association with decomposition or rot (Figure 10)[17,18].
|
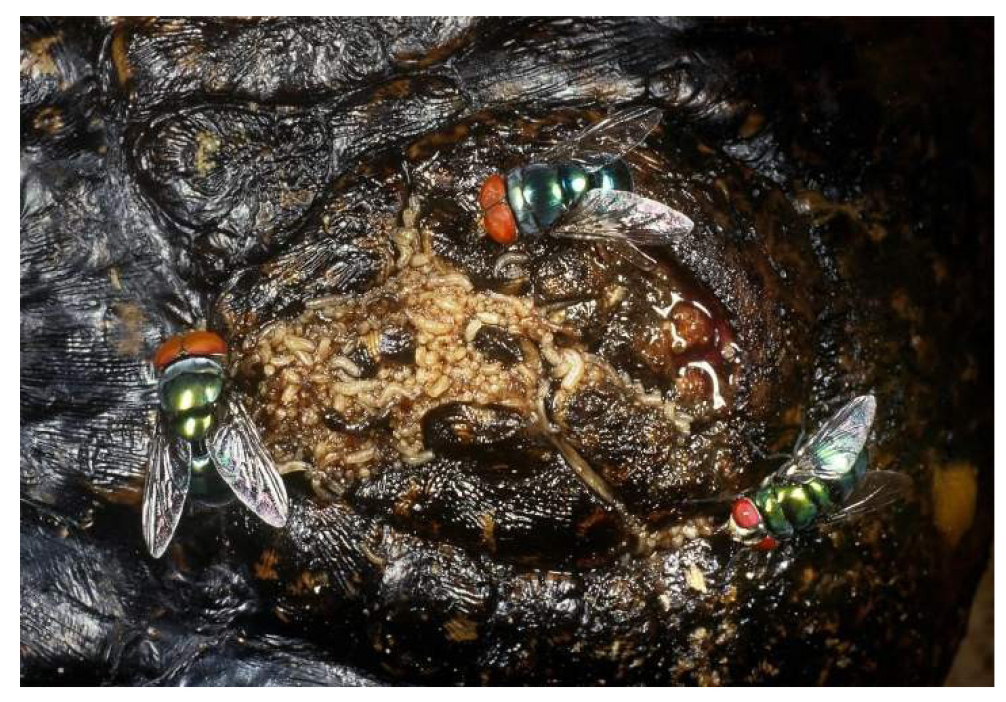
Figure 10. Decomposing remains of Eurasian jay bird seeing in a mass of Calliphoridae blow fly larvae, devouring dead body, carcass, Garrulus glandarius (Linnaeus, 1758) (Passeriformes: Corvidae: Garrulus). Source: https://www.alamy.com/decomposing-remains-of- eurasian-jay-bird-seething-in-a-mass-of-calliphoridae-blow-fly-larvae-devouring-dead-body-carcass-garrulus-glandarius-image358747404.html
1.5 Predators and Parasitoids
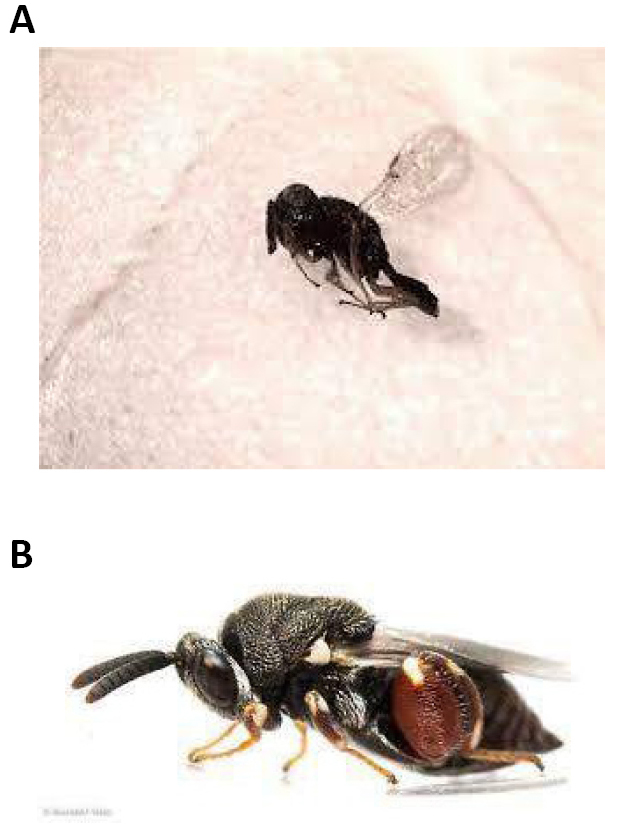
The predators of blow flies include spiders, beetles, frogs, birds, including chic (Figure 11), and mites of the Macrochelidae and Uropodidae families. Parasitoids: Pachycrepoideus vindemmiae (Rondani, 1875), Nasonia vitripennis (Walker, 1836) (Hymenoptera, Pteromalidae), Brachymeria podagrica (Fabricius, 1789) (Hymenoptera: Chalcididae) Spalangia spp. (Hymenoptera: Pteromalidae) and Trichopria sp. (Hymenoptera: Diapriidae) and Apanteles sp. (Hymenoptera: Braconidae) (Figure 11)[17,18].
|
Figure 11. Parasitoids. A: Pachycrepoideus vindemmiae (Rondani, 1875); Source: Carlos Henrique Marchiori; B: Brachymeria podagrica (Fabricius, 1789). Source: https://bugguide.net/node/view/1675698/bgpage
1.6 Taxonomy and Pylogeny
According to their taxonomy, the Calliphoridae family belongs to the Diptera order, Brachycera suborder, Muscomorpha Infraorder, Schizophora section, and Calyptratae group (due to them having a shell-shaped membranous expansion at the base of their wings)[19-21].
Subfamilies: Auchmeromyiinae, Calliphorinae, Chrysomyinae, Luciliinae, Melanomyinae and Polleniinae.
The species that cause myiasis in the Neotropics are of the genera: Cochliomyia Tonwsend, Compsomyiosps Townsend, Lucilia Robineau-Desvoidy (including Phaenicia Robineau-Desvoidy), Calliphora Robineau-Desvoidy and Chrysomya Robineau-Desvoidy. In the Americas, only Cochliomya hominivorax (Cocquerel 1858) causes obligate primary myiasis, and the other species mentioned are considered secondary wound invasive or facultative species (Figures 12-14)[22-26].
|
Figure 12. Subfamily. A: Subfamily Auchmeromyiinae. Source: https://gabrielpaladino.com/tag/auchmeromyiinae/; B: Subfamily Calliphorinae. Source: https://www.brisbaneinsects.com/brisbane_muscoid/Calliphorinae.htm; C: Subfamily Chrysomyinae. Source: https://elp.tamu.edu/ipm/bugs/order-diptera-flies/family-calliphoridae-blow-flies/diptera-calliphoridae-subfamily-chrysomyinae-blow-flies-b/; D: Subfamily Luciliinae. Source: https://www.inaturalist.org/taxa/202179-Luciliinae; E: Subfamily Melanomyinae. Source: https://inaturalist.nz/taxa/1115884-Melanomyinae; F: Subfamily Polleniinae. Source: https://zookeys.pensoft.net/article/51283/
|
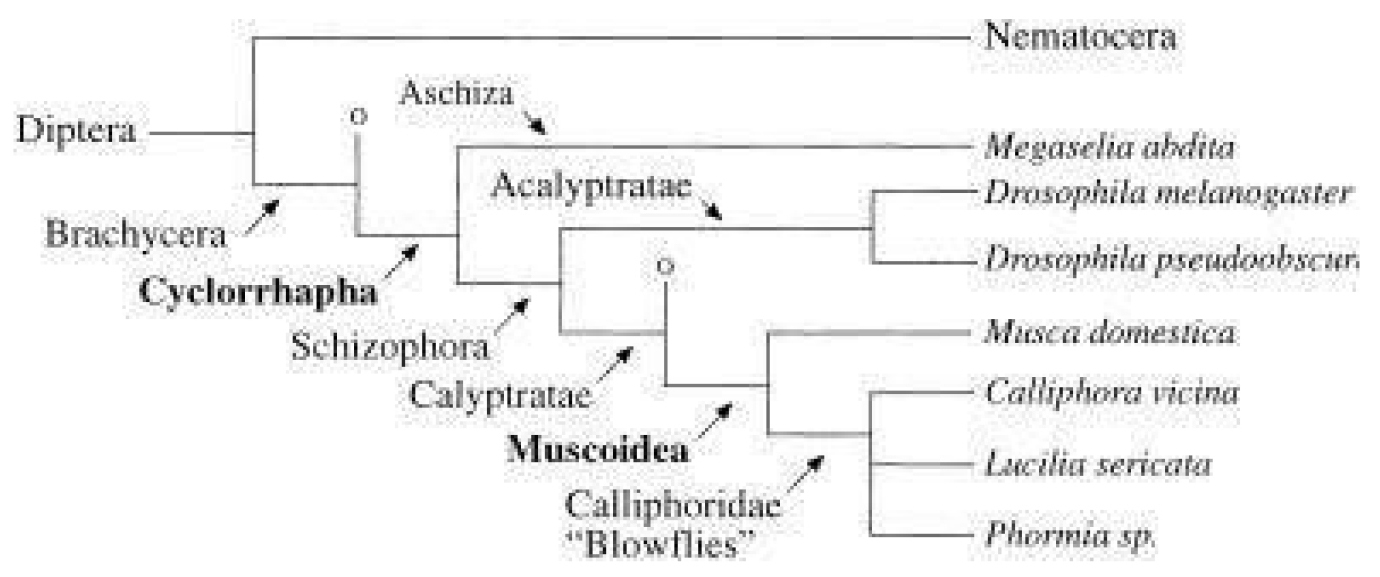
Figure 13. Phylogenetic tree of Diptera, adapted from Schmidt-Ott (2000). Species mentioned in the text are shown in italics. Other groups of Brachycera and Calyptratae are represented as “O” for simplification. Source: https://www.researchgate.net/figure/Phylogenetic-tree-of-Diptera-adapted-from-Schmidt-Ott-2000-Species-mentioned-in-the_fig5_11863473
|
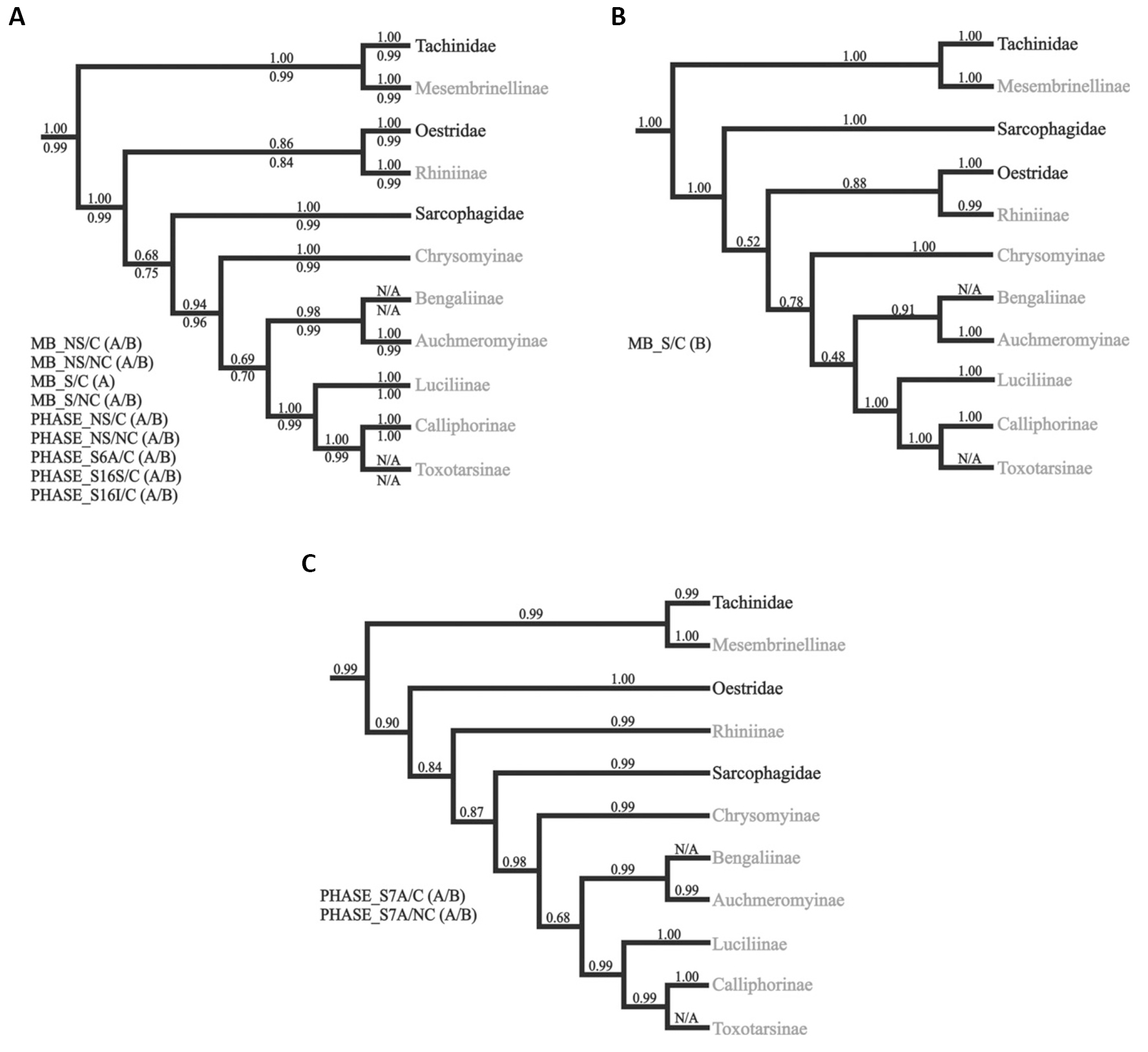
Figure 14. A Different hypothesis for interfamilial relationships in Oestroidea inferred by Bayesian analyses. A: Topology recovered by the majority of the partitioning strategies used (detailed to the left). Average posterior probabilities for MrBayes and PHASE conducted analyzes are shown above and below all branches, respectively; B: Relationships inferred in one of the two independent Bayesian inferences using the model/partition combination S/C; C: Topology recovered in the Bayesian analyzes using the S7A/C and S7A/NC model/partition combinations in PHASE. Names in gray are clades traditionally placed in Calliphoridae golden blowfly; Subfamily Calliphorinae (Calliphoridae). Source: https://www.sciencedirect.com/science/article/pii/S1055790312003168
1.6.1 Golden Blowfly-Subfamily Calliphorinae; Family Calliphoridae
The following pages contain pictures and information about Golden blowflies in the Calliphorinae subfamily that we found in the Brisbane area, Queensland, Australia[26-28].
1.6.1.1 Subgenus
Calliphora (Neocalliphora) ochracea Schiner, 1868.
Golden blowflies in this genus have the following characteristic:
1. Eyes may be densely haired, sparsely haired or bare.
2. There are some species with tessellated abdomens.
3. Legs are black with yellow colouring.
Reddish brown blowfly (Figure 15A).
|
Figure 15. Calliphora. A: (Neocalliphora) ochracea Schiner, 1868, body length 8mm. Source: https://www.brisbaneinsects.com/brisbane_muscoid/Calliphorinae.htm; B: Calliphora (Neocalliphora) stygia (Fabricius, 1781), body length 10mm. Source: https://www.brisbaneinsects.com/brisbane_muscoid/Calliphorinae.htm; C: Calliphora (Calliphora) vicina Robineau-Desvoidy, 1830, body length 8mm. Source: https://www.brisbaneinsects.com/brisbane_muscoid/Calliphorinae.htm; D: Calliphora (Neocalliphora) albifrontalis Malloch, 1932, body length 8mm. Source: https://www.brisbaneinsects.com/brisbane_muscoid/Calliphorinae.htm; E: Calliphora (Paracalliphora) augur (Fabricius, 1775), body length 8mm. Source: https://www.brisbaneinsects.com/brisbane_muscoid/Calliphorinae.htm; F: Calliphora sp. Source: https://www.brisbaneinsects.com/brisbane_muscoid/Calliphorinae.htm
1.6.1.2 Species
Calliphora canimicans Hardy, 1930 This genus has the following characteristics:
1. Bare eyes;
2. A submetallic bronzy abdomen with conspicuously tessellations;
3. Legs of some species are black, while those of others are brownish (Figure 16A).
|
Figure 16. Calliphora. A. Calliphora canimicans Hardy, 1930, body length 8mm. Source: https://www.brisbaneinsects.com/brisbane_muscoid/Calliphorinae.htm; B. Calliphora hilli Patton, 1925, (C.P. rufipes), body length 8mm. Source: https://www.brisbaneinsects.com/brisbane_muscoid/Calliphorinae.htm; C. Unknown specie, body length 6mm. Source: https://www.brisbaneinsects.com/brisbane_muscoid/Calliphorinae.htm
Black tessellated golden blowflies are dusty and metallic dark blue in color. Thorax, scutellum are metallic dark blue. Abdomen segments have the conspicuously tessellated markings. Legs with coax, femur and tibia in black.
Calliphora hilli, Patton, 1925 (C.P. rufipes) (Figure 16B).
Yellow tessellated golden blowflies are dusty metallic blue in colours. Thorax and scutellum are metallic blue. Abdomen segments have conspicuously tessellated markings and legs with coax, femur, and tibia in honey-yellow, while the tarsus is black in color.
Unknown specie (Figure 16C).
1.7 Objective
The objective of this work is to investigate the biology, ecology, habitat, geographic distribution, taxonomy, life cycle, phenology, biological control, and work carried out on the Calliphoridae family (Insecta: Hymenoptera).
2 METHODS
The method used to prepare this mini-review was Marchiori 2021 methodology[29].
3 STUDIES CONDUCTED AND SELECTED
3.1 Study 1
The present work will contribute to studies of the use of different species of flies as bioindicators of potentially toxic metals in anthropized environments (destined to be used as dumping gorunds), as well as, contribute new knowledge about the different biological bioindicators of contamination by potentially toxic metals in anthropized areas, with the aim of the final disposal of urban solid waste effectively (Figure 17)[30].
|
Figure 17. Diptera. Trap mounted based on Ferreira (1978). Source: http://tede2.uefs.br:8080/bitstream/tede/1030/2/Disserta%C3%A7%C3%A3o_Thayana%20Teles.pd.f
As shown by the concentrations of the metallic species investigated in the anthropized area (Dumping ground) in the municipality of Imperatriz-MA showed higher concentrations than that of the natural area. This, therefore, showcases evidence of the contribution of urban solid waste (MSW) improperly disposed of on the soil and organic matter degraded by the action of the leachate dissolving the solid species contained in the deposited material, which mainly contains iron, henceforth increasing its concentration and the other species that are associated with it (Figure 18)[30].
|
Figure 18. Chrysomya Robineau-Desvoidy, 1830. 19: Chrysomya megacephala (Fabricius, 1794), male eye with upper facets enlarged and sharp demarcation between upper and lower facets; 20: Chrysomya rufifacies (Macquart, 1844), proepisternal seta present (anterior spiracle in background). Source: https://zenodo.org/record/276284#.Y3PQ7XbMLIU
When comparing the contents of metallic species from the dumps of Imperatriz-MA and Senador La Rocque-MA, it was found that the dump of Imperatriz presented higher values for all metallic species surveyed.
The 2,593 specimens collected from the family Calliphoridae belong to the subfamily Chrysomyinae and are distributed in three genera and five species. In Imperatriz-MA, the most abundant species in the anthropized area were Chrysomya megacephala (Fabricius, 1794) (678 individuals), Chrysomya albiceps (Wiedemann, 1819) (250 individuals), and Cochliomyia macellaria (Fabricius, 1775), (133 individuals). In the natural area, the most abundant species were the C. albiceps with 98 individuals, followed by C. macellaria, with 56 individuals (Figure 19)[30].
|
Figure 19. Chrysomya albiceps (Wiedemann, 1819). A: Dorsal view; B: Parafrontal and antenna; C: Anterior spiracle (arrow); D: Side view of an adult. Source: https://www.researchgate.net/figure/Figura-3-Chrysomya-albiceps-Em-A-vista-dorsal-B-parafrontalia-e-antena-C_fig3_275657733
In the city of Senador La Rocque-MA, the most abundant species in the anthropized environment were C. megacephala (507 individuals) followed by C. albiceps (499 individuals). In the natural environment, the abundant species were C. albiceps (174 individuals) followed by C. megacephala (54 individuals) (Figure 20)[30].
|
Figure 20. Cochliomyia macellaria (Fabricius, 1775). Source: https://bugguide.net/node/view/1220239/bgimage
3.2 Study 2
This work sought to carry out an inventory of calliphorids of forensic interest occurring in Maringá/PR (Figure 21)[31].
|
Figure 21. Trap, mounted, based on Ferreira (1978) to capture calliphorid flies (Diptera) in the biological reserve of Tingua, RJ, Brazil. a: PvC pipe as base of the trap where the bait is sets inside its; b: Pvc pipe; c: transparent plastic container; d, inverted funnelmade 'with nylon screen; e: orifices of dipterous entrance; f: hooks to hang the traps. Source: https://www.scielo.br/j/isz/a/9QL6jcQkqvxTL5VSgjMxCVS/?lang=en#ModalFigfig01
The highest average temperature was recorded in February at 27.6°C, while the lowest was recorded in June at 20.1°C. In terms of relative air humidity, the mean values for each season were as follows: 64.42% in Spring, 68.92% in Summer, 76.05% in Autumn and 61.31% in Winter[31].
The months with the highest average relative humidity were April, May, and June with an average of 76%, on the other hand on the other hand, the month that presented the lowest average was August, with an average of 55%. In terms of rainfall in 24h measured in mm, in spring there was a total of 32319.7mm, in summer 611.8mm, in autumn 556.9mm, and in winter 64.5mm. The month with the highest rainfall was March (340mm), and the lowest was August (2.8mm) (Figure 22)[31].
|
Figure 22. Lucilia cuprina (Wiedemann, 1819). Source: https://www.researchgate.net/figure/Lucilia-cuprina-Wiedemann-1830-Male-Lucilia-sericata-Meigen-1826-Musca-sericata_fig4_332079297
A total of 264 adult insects were collected from the order Diptera, making a total of 224 (87.5%) representatives of the Calliphoridae family. Among the calliphorids collected, Chrysomya albiceps (Wiedemann 1819), Chrysomya megacephala (Fabricius1794), and Lucilia cuprina (Wiedemann 1819). The largest number of specimens found was C. megacephala, with a total of 126 specimen (56.25%), followed by C. albiceps with 87 (38.84%) specimen, and L. cuprina with 9 (4%) specimen[31].
3.3 Study 3
Originally from Africa, the Mediterranean, and the Middle East, Chrysomya albiceps (Wiedemann, 1819) is widely distributed in the Old World and in the Neotropics (Figures 23 and 24)[32].
|
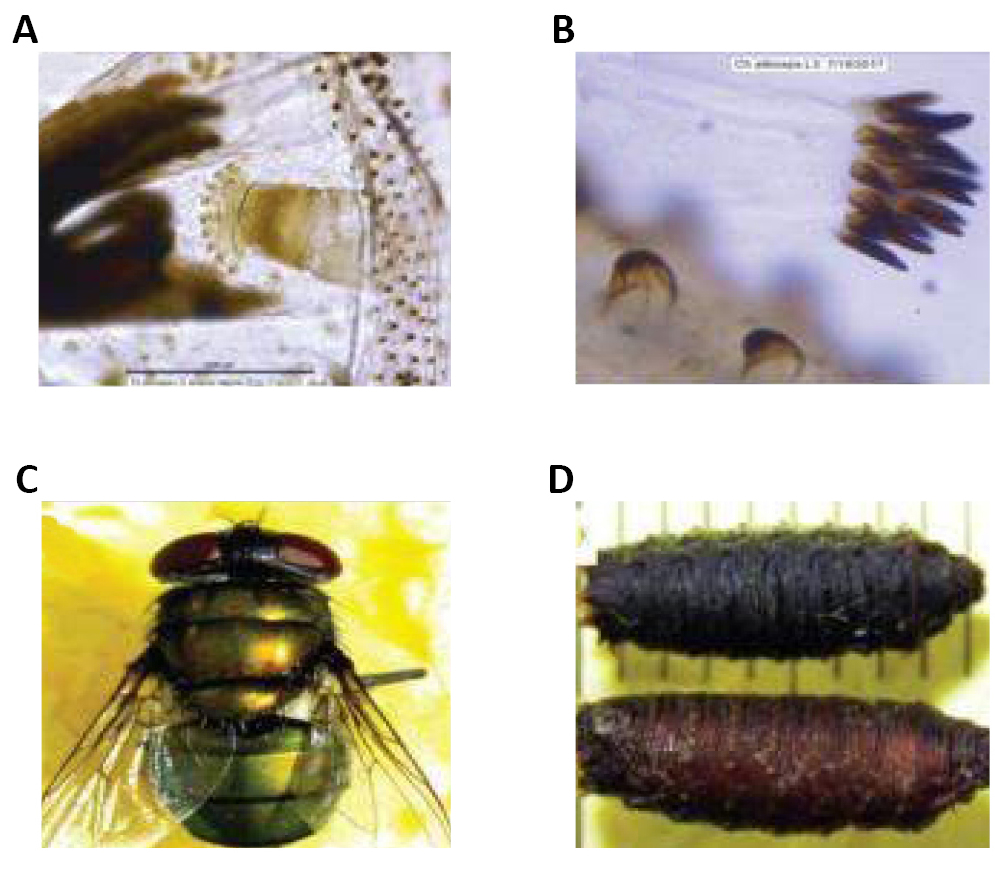
Figure 23. Second and third instars of Chrysomya albiceps. A: Second instars of Chrysomya albiceps (Wiedemann, 1819) is widely; B: Posterior stigma of second instar; C: Third instars of C. albiceps; D: Posterior stigma of third. Source: http://vetdergikafkas.org/uploads/pdf/pdf_KVFD_2517.pdf
|
Figure 24. Morphological characteristics of third instar, adult and pupa of Chrysomya albiceps (Wiedemann, 1819). A: Anterior stigma of third instar; B: Setiferous fleshy overhang on third instar; C: C. albiceps, adult; D: C. albiceps, pupa. Source: http://vetdergikafkas.org/uploads/pdf/pdf_KVFD_2517.pdf
Chrysomya albiceps is of great medical and sanitary importance, being associated with myiasis in Africa and in America, and plays a significant role as a predator of larvae of other dipterans. Its potential as a carrier of pathogenic microorganisms has been highlighted by several authors. Adults of this species arrive at carcasses hours after the death of the animal and feed mainly on flesh and blood from the bleeding cavities, thus making them capable of infecting vegetation and animals in the surrounding areas (Figure 25)[32].
|
Figure 25. Chrysomya albiceps (Wiedemann, 1819) decomposition of organic matter. Source: http://www.guiavisual-gorosti.org/galeria/details.php?image_id=10130&mode=search&sessionid=jgjdcd7dls1glm66qnm ehk5k01
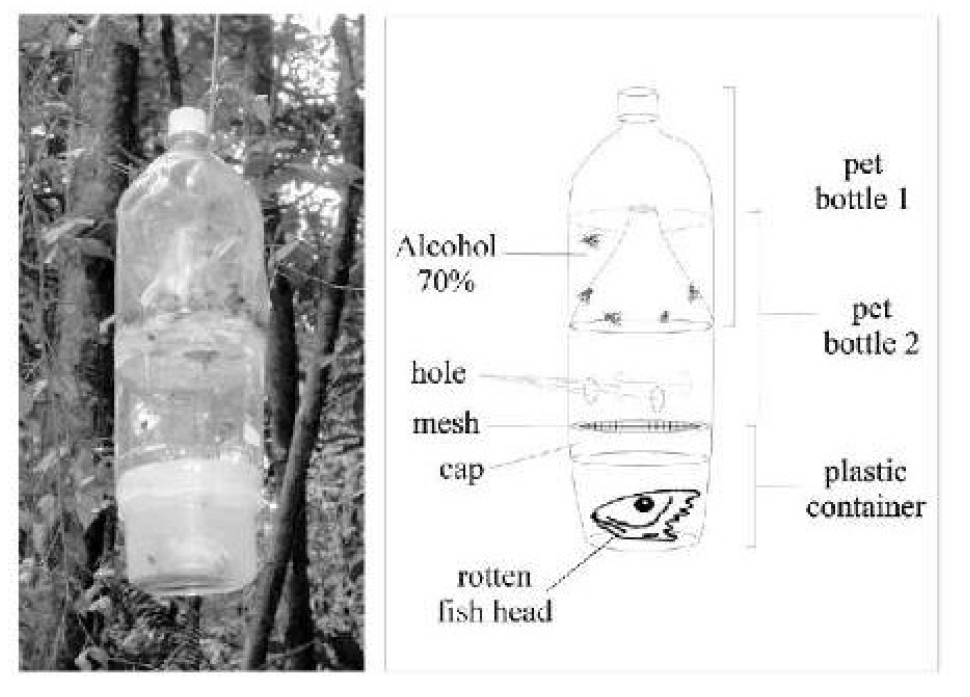
The aim of this study is to describe the occurrence of Tachinobia sp. (Hymenoptera: Eulophidae) in immature stages of C. albiceps in Brazil. Ferreira's trap (1978) was used for this experiment (Figure 26)[32].
|
Figure 26. The modified trap model proposed by Ferreira (1978) for fish carrion feeding flies. Source: https://www.researchgate.net/figure/The-modified-trap-model-proposed-by-Ferreira-1978-for-fish-carrion-feeding-flies_fig1_250021586
In September 2002, 43 pupae were obtained of C. albiceps, and from one of them emerged 30 specimens of Tachinobia sp. making it possibly one of the three described species of Tachinobia. The percentage of parasitism was 2.3% (1/43) (Figures 27 and 28)[32].
|
Figure 27. Tachinobia sp. (Hymenoptera: Eulophidae). Source: https://www.qeios.com/read/685488
|
Figure 28. First report of Tachinobia sp. location map of the Midwest Brazil experiment. Source: https://openaccesspub.org/ijen/article/1625
3.4 Study 4
Diptera of the Calliphoridae family are of great medical and sanitary importance due to the fact that the adults of many species are endophilic, they can frequently find food meant for human consumption after leaving contaminated environments, and the larval forms act as parasites, producing obligatory or facultative myiasis (Figure 29)[33].
|
Figure 29. Life cycle of Chrysomya putoria (Wiedemann, 1818). Source: https://www.semanticscholar.org/topic/Chrysomya-megacephala/181261
One species, Chrysomya putoria (Wiedemann, 1818), is of interest because it is a potential mechanical vector of Polyvirus types I and III, Coxsackie virus, Shilella sp., Salmonella sp., Escherichia coli (Enterobacteriales: Enterobacteriaceae) and Giardia lamblia Kunstler, 1882 (Diplomonadida: Hexamitidae), in addition to other enteric pathogens, and also act as irritating and exfoliating agents. They can invade necrotic tissues of vertebrates, as their larvae can produce secondary myiasis. In addition to this, larvae of the genus Chrysomya Robineau-Desvoidy (1830) actively act in the process of carcass degradation, thus making them useful in Forensic Entomology (Figure 30)[33].
|
Figure 30. Demonstrative scheme of the sterilization process sequence. A: Egg mass on chicken gizzard protein diet; B egg mass and separating for disassociation; C Bactor Sterile monitor filter cup and Whatman filter paper with sterilized egg mass; D: Sample of sterilized egg mass; E: Hatched larvae; F: Larvae with normal development. Source: https://www.researchgate.net/figure/Demonstrative-scheme-of-the-sterilization-process-sequence-a-egg-mass-on-chicken_fig1_268392125
3.5 Study 5
Individuals of Chrysomya megacephala Fabricius, 1794 are recorded in Afrotropical, Australian, and Eastern regions acting as pollinators in some places, such as vectors of helminth eggs, causing myiasis in humans and associated with decaying organic matter in some locations. In Brazil, in addition to being an important forensic indicator, C. megacephala also presents a potential threat to human health as it has been identified as a cause of myiasis in humans and a mechanical vector of agents causing acute diarrheal disease a fact that is aggravated by the fact that the species is well adapted to urban environments and places with a high population concentration (Figure 31)[34,35].
|
Figure 31. Life cycle of Chrysomya megacephala Fabricius, 1794. Source: https://www.semanticscholar.org/topic/Chrysomya-megacephala/181261
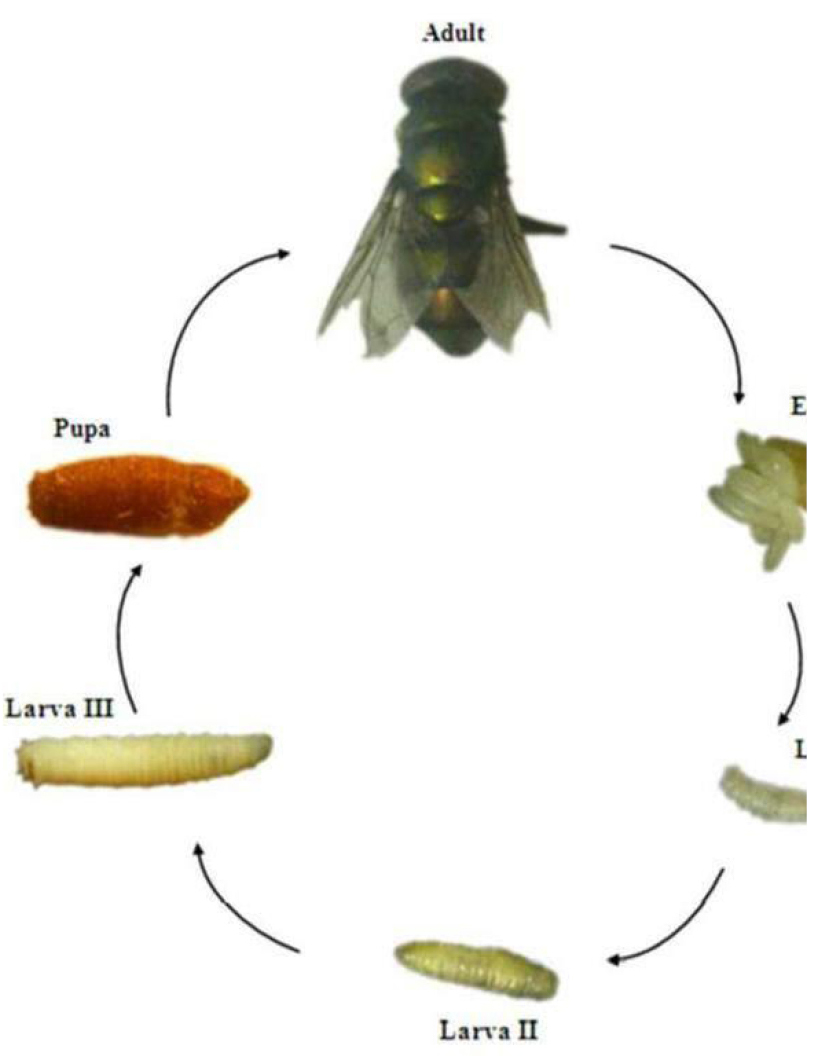
The species C. megacephala, has holometabolous development, which is a characteristic common to Diptera and, as well as the other species of Calliphoridae, its life cycle is made up of an egg stage, three larval stages, a pupal stage and the adult stage.
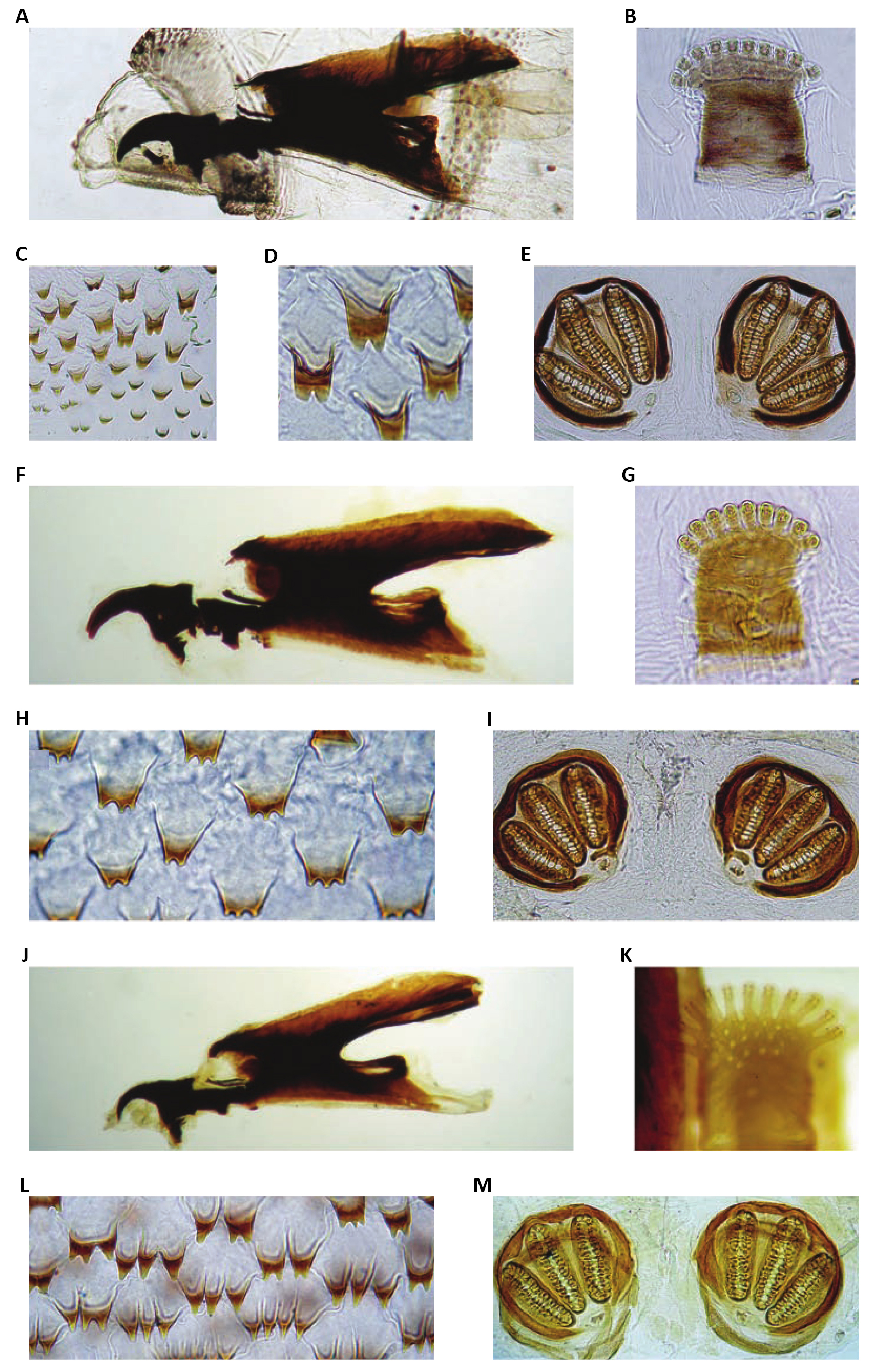
Nonetheless, some authors classify the larval development of calliphorids in six stages: 1st instar larva; farado 1st/2nd instar; 2nd instar larva, 2nd/3rd farado urge; 3rd instar larva; and pre-pupa. The classification into six larval stages is based on the fact that morphological and behavioral differences were observed between these stages of larval development. Entomology Forensics uses these differences to more precisely delimit possible the stage of development that an immature collected at the scene of crime is, that is, to more accurately estimate the age of this immature (egg, larva and pupa). (Figure 32)[34,35].
|
Figure 32. Chrysomya megacephala (Fabricius, 1794). A: Chrysomya megacephala, cephaloskeleton, lateral view; B: C. megacephala, anterior spiracle; C: C. megacephala, thoracic segment III, spines; D: C. megacephala, thoracic segment III, spines; E: C. megacephala, posterior spiracles; F: Phormia regina (Meigen, 1826), cephaloskeleton, lateral view; G: P. regina, anterior spiracle; H: P. regina, thoracic segment III, spines; I: P. regina, posterior spiracles; J: Protophormia terraenovae (Robineau-Desvoidy, 1830), cephaloskeleton, lateral view; K: P. terraenovae, anterior spiracle; L: P. terraenovae, thoracic segment III, spines; M: P. terraenovae, posterior spiracles. Source: https://www.researchgate.net/figure/Third-instars-of-Chrysomyinae-a-Chrysomya-megacephala-cephaloskeleton-lateral-view_fig5_226946269
Chrysomya megacephala eggs are small, translucent and are narrow at both ends. After the eggs hatch, the larval stages begin, which mainly differentiate by morphological characteristics of the anterior and posterior spiracles and by the arrangement, sclerotization, and pigmentation of the cephalopharyngeal skeleton. The larvae that leave the eggs soon after hatching correspond to 1st instar larvae, which in the first moments of life are called neo larvae (Figure 33)[34,35].
|
Figure 33. This image shows the larva and pupa of Chrysomya megacephala Fabricius, 1794. Source: https://www.scielo.br/j/ne/a/tw4Q76Jq3GVDLnmkb3jRRZQ/?format=pdf&lang=es
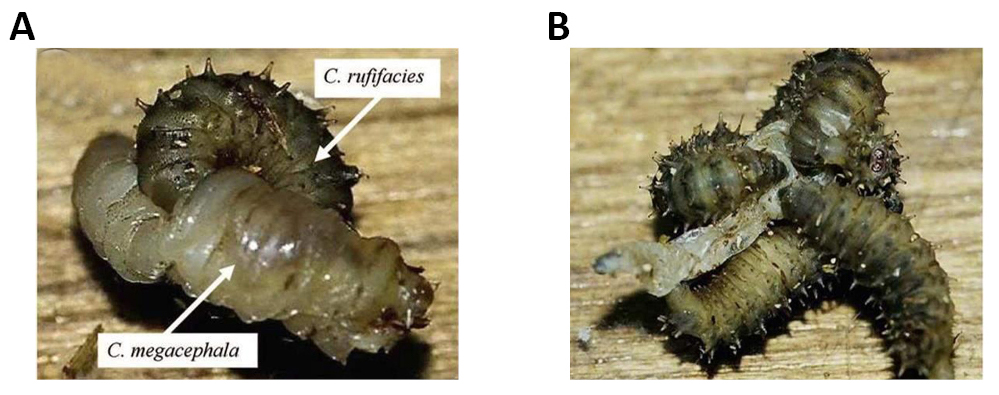
The second molting period of the larvae is known as farado 2nd/3rd larval instar, and is characterized by the simultaneous presence of a pair of 2nd and 3rd instar posterior spiracles, the latter of which has three spiracular openings, as well as the presence of 3rd instar anterior spiracles, which are larger and wider at the base that the first. 3rd instar larvae have a cephalopharyngeal skeleton fully formed from the anterior and posterior spiracles both of the 3rd instar (Figures 34 and 35).
|
Figure 34. Larval predation of thirdinstars of Chrysomya rufifacies (Macquart, 1844) on a third instar of Chrysomya megacephala Fabricius, 1794. A: single third instar of C. rufifacies trusses the prey with its curved body and sclerotized spines, and uses its mouthhook to penetrate the prey to extract buid; B: Several larvae usually work together until all of the body buid of the prey has been sucked out. Source: Shiao and Yeh: Larval competition of blow fly larvae.
|
Figure 35. Egg, larva, pupa and adult of the species Chrysomya megacephala Fabricius, 1794. Source: https://repositorio.ufrn.br/bitstream/123456789/23572/1/UsoTerapiaLarval_Pinheiro_2014.pdf
The pupal stage is characterized by the formation of the puparium, which corresponds to the hardening of the cuticle of the 3rd larval instar. The puparium is immobile and inside, the pupa undergoes major physiological and morphological transformations that will lead to the emergence of the adult. The puparium of C. megacephala has a reddish- brown color and is quite similar to that of other species of Calliphoridae[36,37].
3.6 Study 6
Lucilia cuprina (Wiedemann, 1830)
Currently, 17 species of the genus Lucilia Robineau-Desvoidy 1830 are known, existing in the Neotropical region. The species Lucilia cuprina (Wiedemann, 1830) feeds on dead tissue, however they can sometimes invade living tissue when there are few options, which makes them the cause of facultative myiasis (Figure 36).
|
Figure 36. Lucilia cuprina (Wiedemann, 1830). Source: Jesse Rorabaugh.
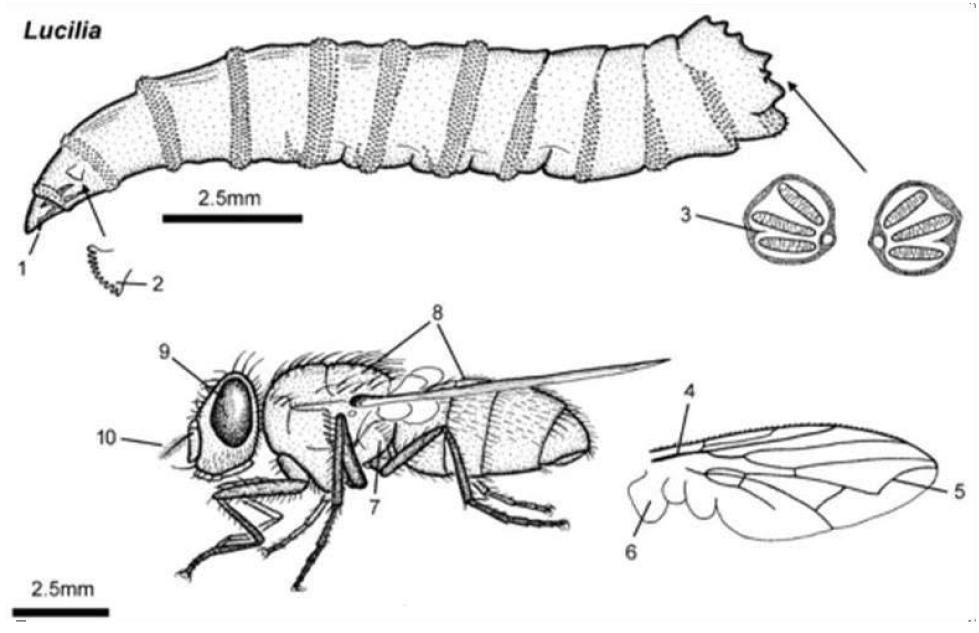
Some of the most striking features of this species include the metallic color with coppery reflections, the well-developed genitalia, the blackened frontalis, and the ocellar triangle, which has two pairs of ocellar bristles, which can be cited as a striking feature (Figure 37).
|
Figure 37. Comparison of the sizes of larvae of Lucilia cuprina (Wiedemann, 1830) after feeding on sera from control (three on left) and vaccinated (six on right) sheep. The small larvae (right) were fed on sera from sheep vaccinated with a semi-purified protein extract from the peritrophic membrane of L. cuprina. Source: https://www.researchgate.net/figure/Comparison-of-the-sizes-of-larvae-of-Lucilia-cuprina-after-feeding-on-sera-from-control_fig1_8251335
The average adult fly size can range from 8.0 to 10.0mm. It is an invasive species and is widely distributed worldwide, being found even in Brazil. In several regions, this species is primarily responsible for myiasis in animals, mainly affecting sheep. In Brazil, this fly can be found in urban waste, decomposing substrates, fallen fruits, flower nectar, and human feces, serving as a potential vector of entomopathogens for humans (Figure 38).
|
Figure 38. Life cycle of the species Lucilia cuprina (Wiedemann, 1830) (Insecta: Diptera). Source: http://parasite.org.au/para-site/lucilia/lucilia-clinical.html
This species has been found associated with human corpses, which makes it of forensic importance, demonstrating to be dominant during the active phase of decomposition, and directly participating in carcass removal[38-44].
3.7 Study 7
Lucilia sericata (Meigen, 1826) Blowfly, Greenfly, Green Blowfly. The common greenfly is one of the common blowflies in environments where there is meat, feces, and garbage. In fact, this species fills an important link in the trophic chain and is responsible for eliminating injured and dead animals. To that extent, it warrants particular attention in the context of animal health and food conservation (Figure 39)[45].
|
Figure 39. Lucilia sericata (Meigen, 1826). Source: https://www.biodiversity4all.org/taxa/128824-Lucilia-sericata
As well as being an important aid to forensic science, it also assists with determining the moment of death by determining the age of the larvae found in corpses. The imago (8mm to 10mm) is distinguished by its iridescent green body with a metallic sheen, covered with black hairs, as well as for its wine-red eyes, and its hyaline wings. Females can lay up to 3000 eggs in batches of 100 to 300 (Figure 40)[45].
|
Figure 40. Life cycle of Lucilia sericata (Meigen, 1826). Source: https://www.jstor.org/stable/24207545
A blowfly’s larvae are headless. These flies supplement the protein diet they find in the exudates of animal tissues with carbohydrates from the floral nectars. It is therefore common to find blowflies visiting flowers, so they participate in pollination, being considered as pollinators (Figures 41 and 42)[45].
|
Figure 41. Larva, pupa, decomposition and pollination of life cycle of Lucilia sericata (Meigen, 1826). Source: https://www.barewalls.com/art-print-poster/green-bottle-fly-lucilia-sericata_bwc8492126.html
|
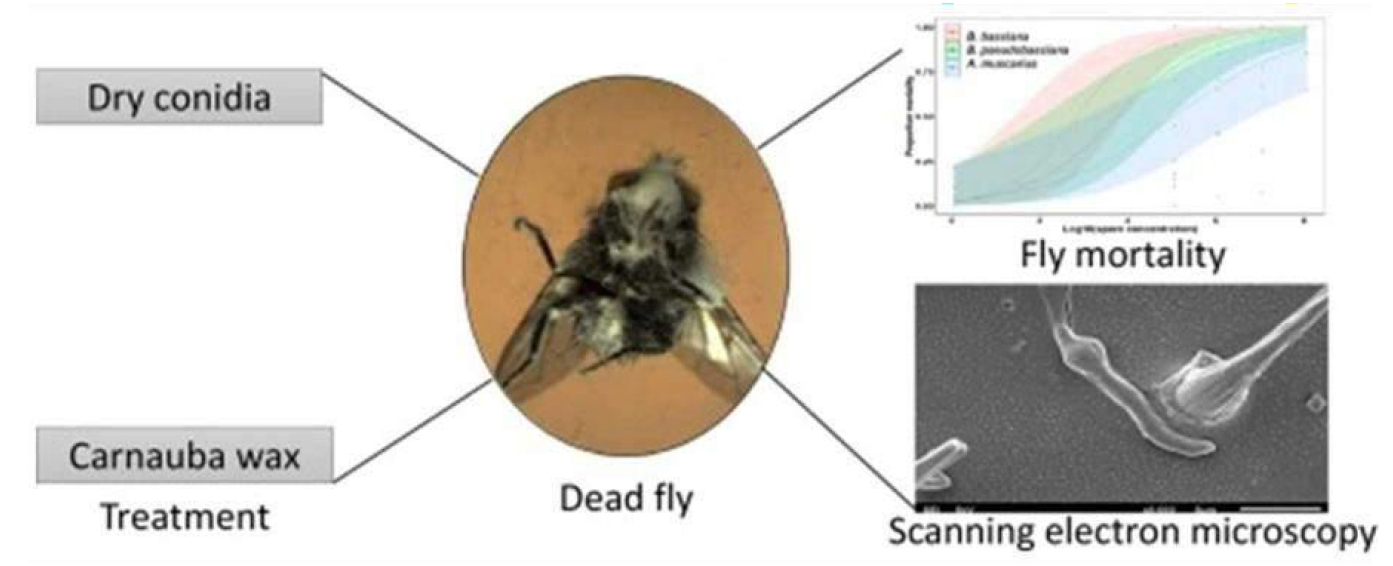
Figure 42. Lucilia sericata (Diptera: Calliphoridae), (Beauveria bassiana (Bals.), Beauveria pseudobassiana Rehner and Humber and Akanthomyces muscarius (Petch) (Fungi: Hypocreales: Cordycipitaceae) against) are considered eco-friendly tools, alternative to synthetic pesticides, for the control of arthropod pests. Source: https://www.sciencedirect.com/science/article/pii/S0022201120300975
3.8 Study 8
In the genus Lucilia, 27 species are described. Lucilia eximia (Wiedemann, 1819) whose larval habit is saprophagous, is a mechanical vector of pathogens of medical-veterinary importance (Figure 43).
|
Figure 43. Lucilia eximia (Wiedemann, 1819). Source: https://tede.ufrrj.br/jspui/bitstream/tede/210/18/2009%20-%20Jos%C3%A9%20Antonio%20Batista%20da%20Silva.pdf
Wide variety of substrates for food and reproduction, such as animal carcasses and human feces. The species is frequently found in forested and rural areas in Brazil, Peru, and Argentina. (Seems like there’s something missing at the beginning of the sentence.)
In Brazil, there are reports of myiasis by L. eximia in domestic animals such as cats and dogs, but none in rabbits. Regarding the domestic rabbit Oryctolagus cuniculus Linnaeus, 1758 (Lagomorpha: Leporidae) in the United States and Europe (Figure 44).
|
Figure 44. Larva, adult, lateral. 1: Scraping mouthparts of larva; 2: Previous spiracle has 7 to 10 openings; 3: Posterior spiracles have a thick periderm with one internal projection; 4: Wings have stem veins without a row of stout setae; 5: Vein 4 is angled sharply up toward the outermost edge; 6: Thoracic squama bears no setae on its upper surface; 7: Thorax has on its hypopleuron a row of stout setae; 8: Thorax and abdomen are metallic bright green; 9: Eyes are mid brown; 10: Antenna has a large arista with setae on both sides. Source: https://en.wikibooks.org/wiki/Parasitic_Insects,_Mites_and_Ticks:_Genera_of_Medical_and_Vete rinaryImportance/Blowflies
Lucilia eximia, normally found in animal carcasses, is one of the first colonizers of this substrate, even under the influence of predation and competition with other species, there is a reported probability of displacement of this species by flies of the genus Chrysomya in some areas of Brazil (Figures 45 and 46).
|
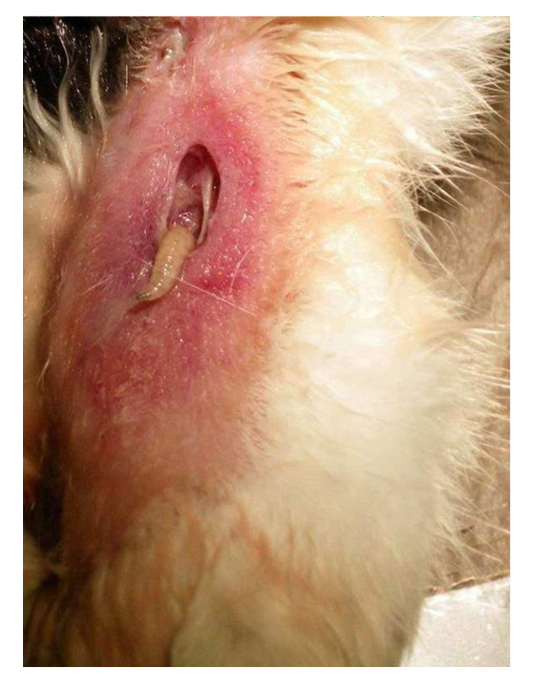
Figure 45. Myasis in a flesh wound on a cat leg. Source: https://pt.m.wikipedia.org/wiki/Ficheiro:Myiasis-cat.jpg
|
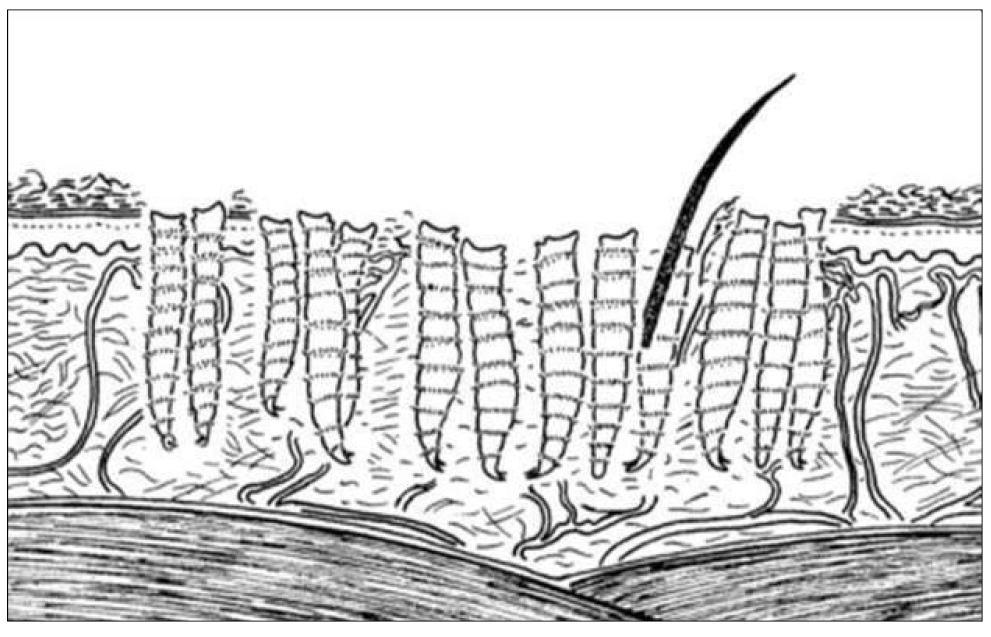
Figure 46. Diagram of feeding at skin represents a superficial infestation by immature larvae of fly. The proportions larvae to skin are not accurate. Source: https://en.wikibooks.org/wiki/Parasitic_Insects,_Mites_and_Ticks:_Genera_of_Medical_and_Vete rinaryImportance/
There are few reports of the species as a producer of primary myiasis, thus it is of to importance investigate its unusual behavior. Such changes suggest the occupation or creation of a new niche that may increase with the intensification of animal domestication. Interestingly, changes in the behavior of species causing myiasis, including an increase in the area of occupation, have been reported in the genera Lucilia and other genera[46-50].
3.9 Study 9
Larval therapy: The use of larvae in medicine
Larval therapy, therapy with medicinal larvae, biosurgery, biotherapy or biodebridement is the use of necrobiontophagous fly larvae (which feed exclusively on necrotic tissue) in the treatment of different types of wounds. These larvae act by removing necrotic tissue, stimulating granulation tissue, and promoting microbial decontamination (Figure 47).
|
Figure 47. Chrysomya megacephala Fabricius, 1794, female (left) and male (right), one of the existing species in Brazil with potential for use in larval therapy. Source: https://ledunirio.wordpress.com/2010/11/29/terapia-larval/
Larvae of different species have already been used in larval therapy, however, the most common is Lucilia sericata (Meigen, 1826), which is very common in northern hemisphere countries. In Brazil, species such as those of the genus Chrysomya (Robineau- Desvoidy, 1830) have potential for the same use, as they have biological and behavioral aspects similar to L. sericata. In Colombia, researchers are already developing studies using Lucilia eximia (Wiedemann, 1819) in patients1 (Figure 48).
|
Figure 48. Lucilia sericata (Meigen, 1826). Source: https://www.museubiodiversidade.uevora.pt/elenco-de-especies/biodiversidade-actual/animais/artropodes/insectos/lucilia-sericata/
Historic
Throughout the course of history, man has sought diversified and natural methods of treating wounds to reduce pain and speed up the healing time. The use of larvae of certain species of flies in cleaning wounds has been known for centuries. Ancient civilizations such as aboriginal tribes in Australia, inhabitants of Northern Myanmar, and the Mayans in Central America already used this technique to great advantage (Figure 49).
|
Figure 49. Larval debridement therapy is used clinically to treat ulcers that do not heal, especially in patients with underlying vascular problems. A promotes healing by inhibiting bacterial growth, removing necrotic tissue, decreasing the inflammatory response, and promoting healing through the formation of granular tissue. Source: https://maxscottlab.wordpress.ncsu.edu/sample-page/genetically-engineered-strains-of-lucilia-sericata-for-larval-debridement-therapy/
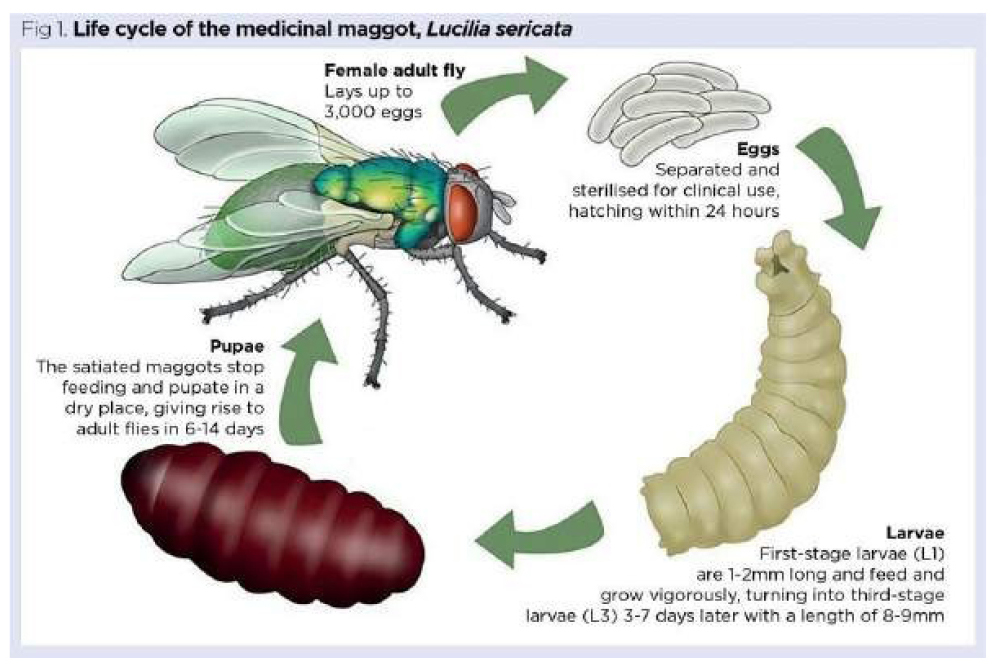
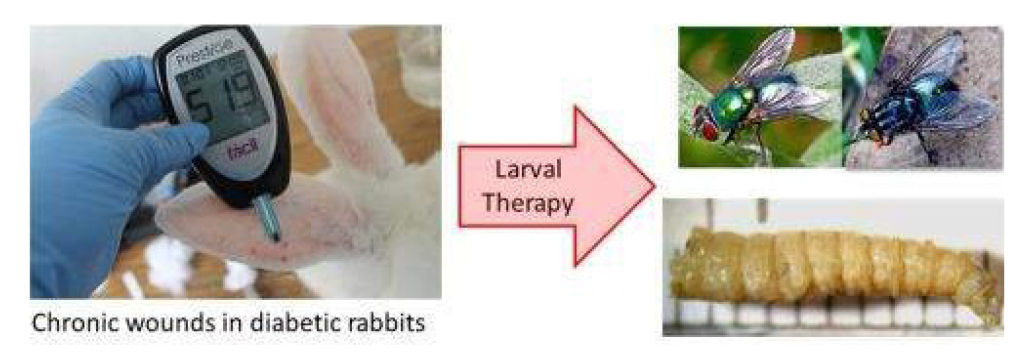
Larval therapy was used intentionally for the first time in the United States in 1931 by William Baer, an orthopedic surgeon at Johns Hopkins Hospital. During the first World War, Baer observed that wounds infested by fly larvae in soldiers collected from battlefields showed significant improvement compared to others, due to the debridement action (removal in necrotic tissue) carried out naturally by these larvae (Figure 50).
|
Figure 50. Maggot therapy, also known as larval therapy, is one option available for treating chronic, infected, necrotic and sloughy wounds. The maggots used are special clinical-grade, aseptically reared larvae of the common greenbottle fly Lucilia sericata (Meigen, 1826). For clinical use, disinfected eggs hatch under sterile conditions. Upon emerging, and just prior to being packaged for delivery, hungry first-stage (L1) larvae are fed once on a high-protein cereal-based diet, so they can survive for up to 24 hours in transit. Once placed on a wound, the larvae can feed and grow to their final third stage (L3); they usually remain on the wound for four days before being removed. Used larvae are treated as infectious clinical waste. Source: https://www.nursingtimes.net/clinical-archive/tissue-viability/the-principles-of-maggot-therapy-and-its-role-in-contemporary-wound-care-16-08-2021/
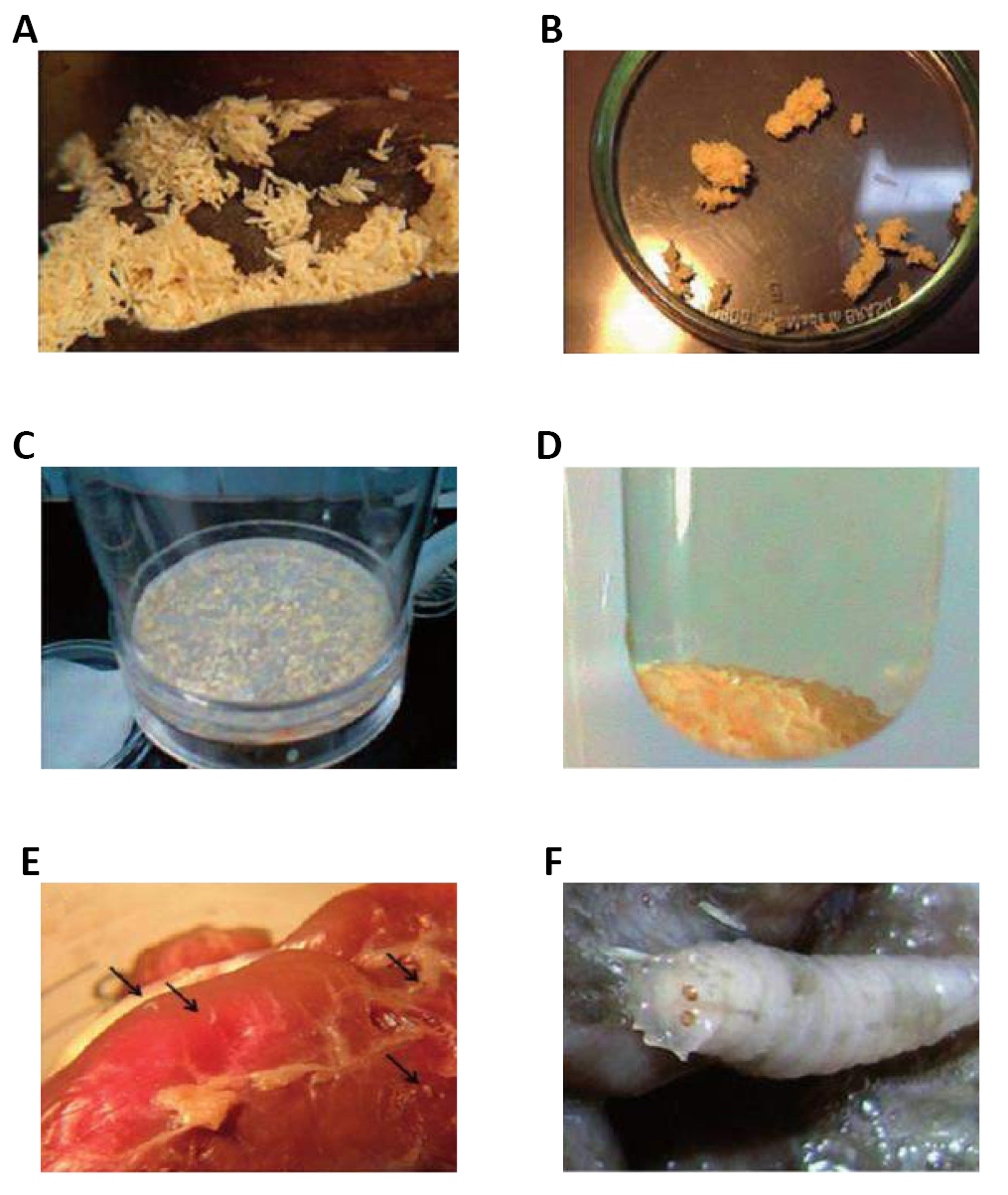
Based on these observations, 10 years later he repeated the same principle in the treatment of patients afflicted with osteomyelitis. Despite the success obtained (success in 90% of cases), the use of this technique had to be discontinued, as some patients had tetanus. It was then that research aimed at disinfecting larvae and sterilizing fly eggs began to be conducted (Figure 51)[51-55].
|
Figure 51. Evaluating Sarconesiopsis magellanica (Le Guillou 1842) (Diptera: Calliphoridae) blowfly-derived larval therapy and comparing it to Lucilia sericata-derived therapy in an animal model. Source: https://www.sciencedirect.com/science/article/abs/pii/S0001706X15301480
Therapy with medicinal larvae was routinely used until the mid-1940s in over 300 hospitals. However, with the advent of antibiotics and the improvement of surgical debridement techniques, larval therapy was considered obsolete until the early 1980s (Figure 52).
|
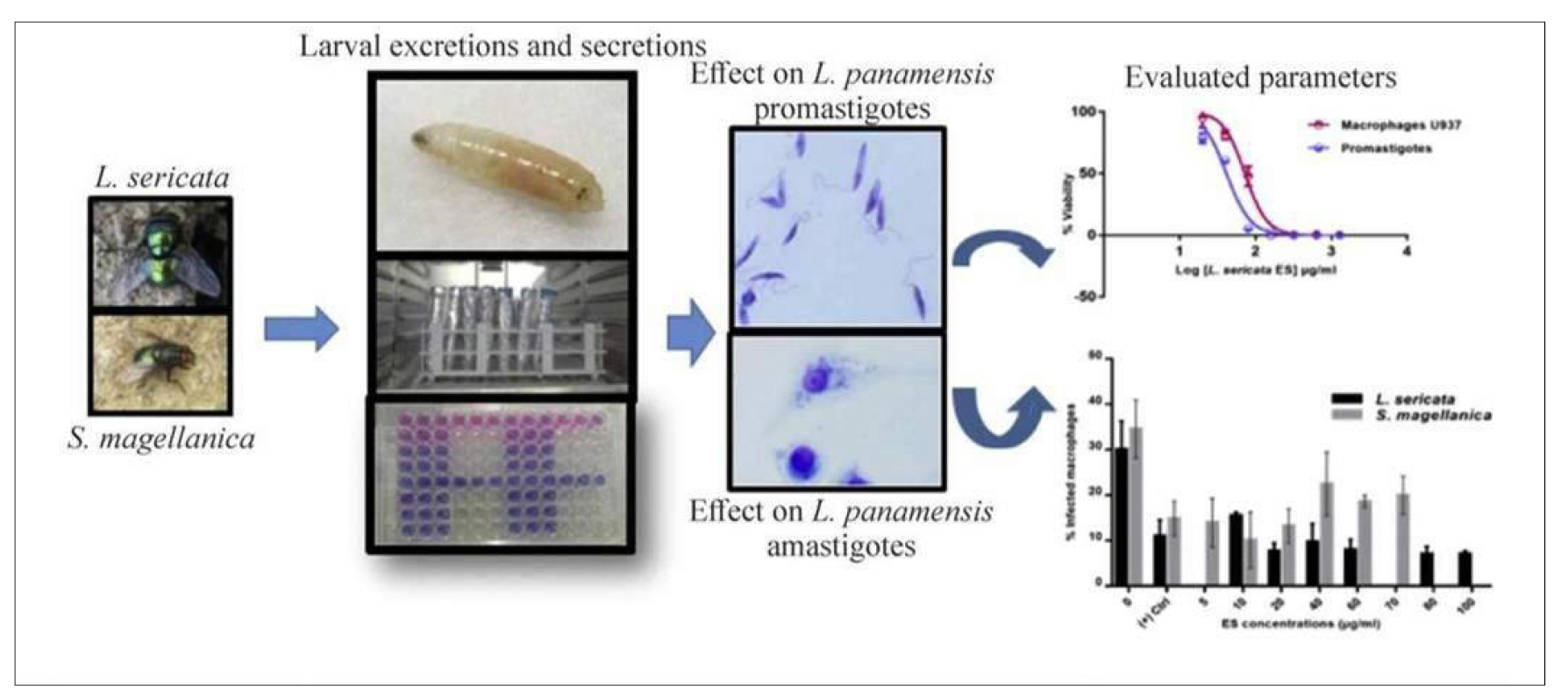
Figure 52. Evaluating Calliphoridae Lucilia sericata (Meigen, 1826) and Sarconesiopsis magellanica (Le Guillou 1842) derived larval excretions and secretions’ (ES) in vitro anti-leishmanial activity against Leishmania panamensis (Lainson & Shaw, 1972). Different larval- ES concentrations from both blowfly species were tested against either L. panamensis promastigotes or intracellular amastigotes using U937-macrophages as host cells. Source: https://www.sciencedirect.com/science/article/abs/pii/S0001706X17307155
During this period, due to the increase in patients with necrotic wounds, diabetic feet, and bacterial resistance to numerous antibiotics, larval therapy began to used again in the United States, United Kingdom and Israel. Currently, larval therapy is used in more than 30 countries around the world and around 24 laboratories provide sterile larvae for this type of treatment. In Brazil, mechanisms for sterilization of eggs and disinfection of larvae of different species have been researched, however, the procedures have not yet been applied in patients (Figure 53).
|
Figure 53. Anti-Leishmania activity of Lucilia sericata (Meigen, 1826) and Calliphora vicina Robineau-Desvoidy, 1830, maggots in laboratory models Use of sterile fly larvae (maggots) of blow flies for the treatment of many different types of skin and soft tissue wounds is called Maggot debridement therapy. The larvae of blow flies secrete a broad spectrum of compounds with diverse mechanisms of action in the gut and salivary glands called ES products which showed to have antimicrobial activities against Gram negative and positive bacteria. Cutaneous leishmaniasis which is the common form of leishmaniasis is difficult to treat. Source: https://www.udea.edu.co/wps/portal/udea/web/inicio/udea-noticias/udea-noticia/!ut/p/z0/!/
3.10 Study 10
Hemilucilia segmentaria (Fabricius, 1805) Type locality: “South America”. Distribution: Argentina, Bolivia, Brazil, Chile, Colombia, Costa Rica, El Salvador, Ecuador, Guatemala, Guyana, Mexico, Panama, Paraguay, Peru, and Trinidad and Tobago. The diagnostic characteristics of H. segmentaria are: posterior cream-yellow spire; dorsal surface of the naked upper calypter in the male, and long hair in the female (Figures 54-56)[56].
|
Figure 54. Thorax of Hemilucilia segmentaria (Fabricius, 1805) (Diptera: Calliphoridae). Source: EntomoBrasilis 7 (1): 12-15 (2014).
|
Figure 55. Larva Hemilucilia segmentaria (Fabricius, 1805) (Diptera: Calliphoridae). A: Last segment; B: Side view. Source: Oliveira-Costa, 2013.
|
Figure 56. Life cycle of Hemilucilia segmentaria (Fabricius, 1805) (Diptera: Calliphoridae). Source: EntomoBrasilis 7 (1): 12-15 (2014).
In a sinantropic study carried out in Rio de Janeiro, it indicated that this species had a preference for forest areas. Ichneumonidae are poorly known and only 10% of the species are well documented[56].
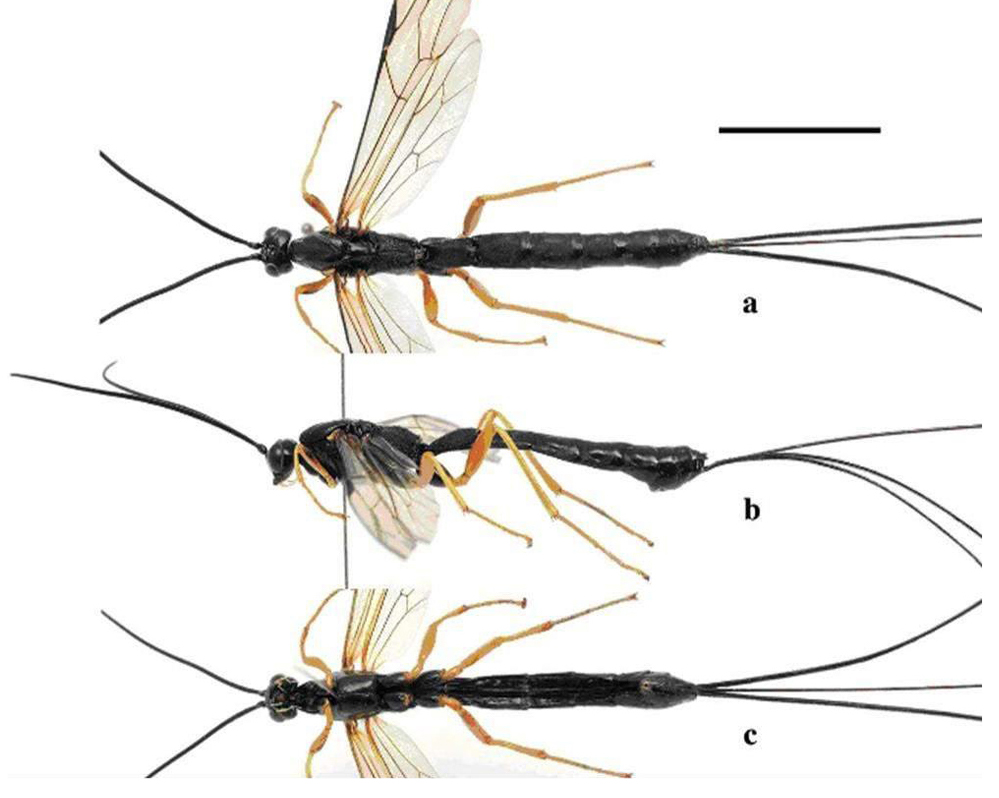
Ichneumonides are immature parasitoids (ecto or endoparasitoids) of other fully metamorphosed insects (holometabols) such as Lepidoptera, Coleoptera, Diptera, Neuroptera, Trichoptera and spiders (Figure 57)[56].
|
Figure 57. Holotype female of Dolichomitus flavicrus sp. a: dorWsal aspect; b: lateral aspect; c: ventral aspect. Source: Journal of Hymenoptera Research 62: 73-82. https://doi.org/10.3897/jhr.62.23559
The objective of this study was to verify Family Ichneumonidae specimens as Diptera Muscomorpha parasitoid in Brazil.
From November 2003 to January 2004, 50 species of H. segmentaria in pupae were collected, from which 10 emerged Pimpla sp. The percentage of parasitism was 20.0%. Hemilucilia segmentaria was created exclusively in the forest and the highest percentage of the specimens came from the liver[56].
Hemilucilia segmentaria was found carrying eggs of Dermatobia hominis (Linnaeus Jr., 1781) (Diptera: Oestridae), during a study of the diversity of Calliphoridae at Reserva Biológica do Tinguá, city of Nova Iguaçu, Rio de Janeiro. A female of H. segmentaria was captured in May 2001, carrying a mass of 20 eggs attached to the left side of its abdomen (Figure 58)[56].
|
Figure 58. Figure Pimpla sp. (Hymenoptera: Ichneumonidae). Source: http://www.waspweb.org/Ichneumonoidea/Ichneumonidae/Pimplinae/Itoplectis/images/Pimpla_castaniventris%20(1).JPG
The Pimplinae is a relatively large subfamily of the Ichneumonidae family. The subfamily includes idiobiont ecto and endoparasitoids, koinobiont ectoparasitoids, and egg predators. As one of the most species-rich genera in the Neotropical Pimplinae, it has 72 species currently known in this region and including 20 of these recorded from Brazil. Members of this genus are mainly idiobiont parasitoids of lepidopteran prepupae or pupae and hyperparasitoids of ichneumonids and tachinids parasitoids (Diptera: Tachinidae) (Figure 59)[56].
|
Figure 59. Pimpla Fabricius, 1804 (Hymenoptera: Ichneumonidae: Pimplinae). Source: Vietnam with descriptions of two new species. Nhi Thi Pham, Gavin R. Broad, Hoa Thi Dang, Wolfgang Böhme. Organisms Diversity & Evolution. 2013; 13(3): 397-407.
The diagnostic characters of Pimpla sp. are: it has a clippe normally separated from the forehead by a distinct groove, the first abdominal segment is often short and wide, with the spiracles located in the middle or slightly anterior to the middle of this segment and the tarsal claws of the females normally with the presence of a basal lobe (Figure 58)[56].
In Brazil, there is little knowledge of Diptera parasitoid species. To evaluate these species in the control of fly populations there is a need to conduct further research aiming to identify species found exclusively attacking these flies, for their use in biological control programs[56].
3.11 Study 11
Parasitoide de Chrysomya megacephala (Fabricius, 1794) associadas a carcaça de rato em área de vegetação secundária no município de Campinas, SP (Figure 60)[57].
|
Figure 60. Larva of Chrysomya megacephala (Fabricius, 1794). Source: https://pt.wikipedia.org/wiki/Chrysomya
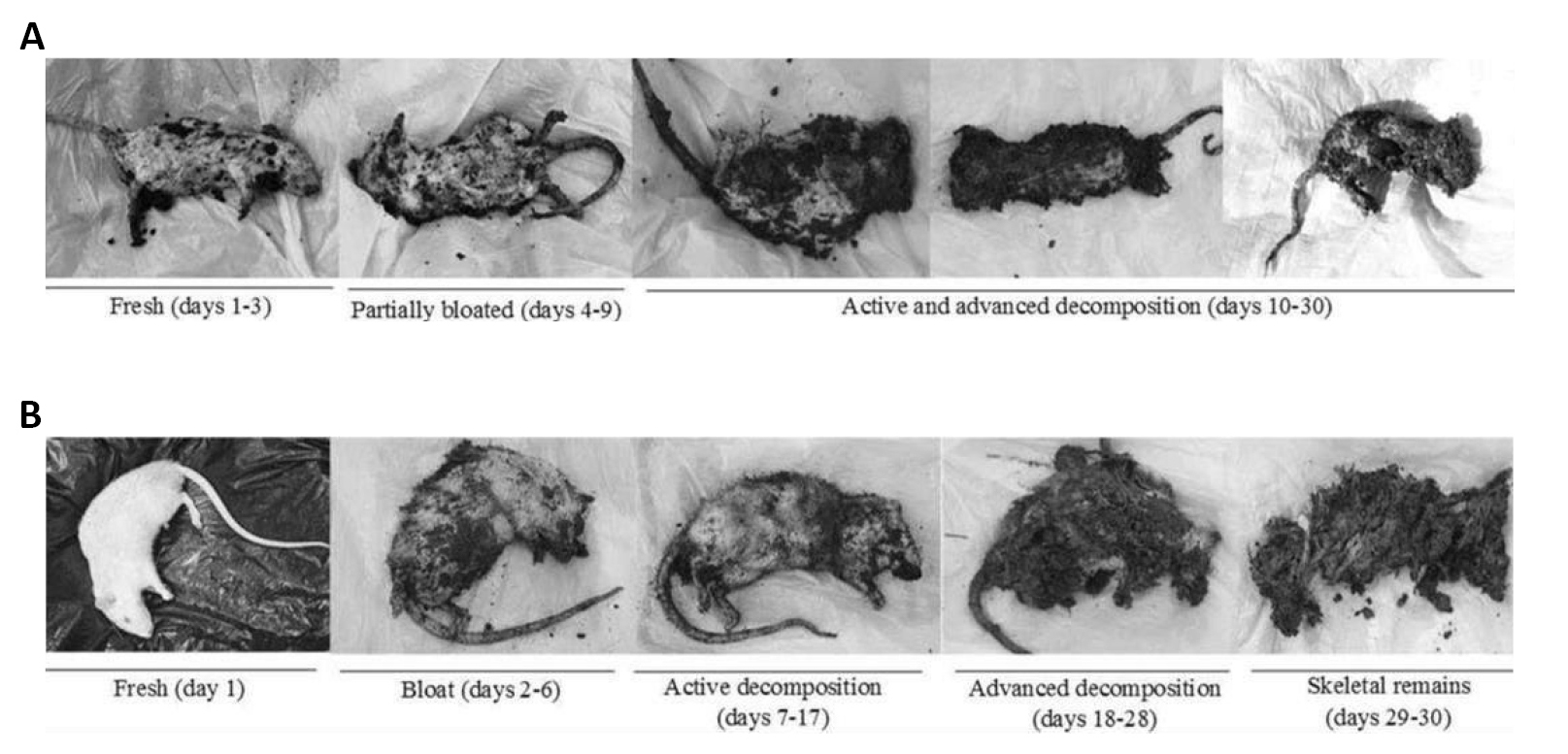
A 251.75g rat carcass (Rattus norvegicus L., 1758, Wistar strain) was exposed in a partially sunny location within the study area. After complete decomposition of the carcass, larvae and pupae were removed from the plastic packaging, then exposed to the environment and placed in a plastic pot containing vermiculite, a substrate for pupariation of the larvae. These pots were kept in the entomology laboratory at a temperature of 25±1ºC and a relative humidity of 60±10%, to monitor the emergence of adults (Figure 61)[57].
|
Figure 61. Decomposition stages of buried rat carcasses. Source: https://www.researchgate.net/figure/Decomposition-stages-of-buried-rat-carcasses-a-March-b-June_fig2_328389158
Five days after the transfer, 43 females and 24 males of the microhymenopteran identified as Tachinaephagus zealandicus Ashmead, 1904 (Hymenoptera: Encyrtidae) emerged. Sexual dimorphism was evident, mainly due to the antenna characteristics. The parasitism rate for this host was 91.8% (Figure 62)[57].
|
Figure 62. Tachinaephagus zealandicus Ashmead, 1904 (Hymenoptera: Encyrtidae). Source: https://www.inaturalist.org/taxa/397760-Tachinaephagus-zealandicus/browse_photos
4 CONCLUSION
Adults are attracted by the odor generated in the wounds of all kinds of vertebrates, but also visit flowers and can be found in foliage. They deposit their eggs in substrates such as feces, carrion, necrotic tissue or healthy open-wounded tissue, where there the larvae develop. Adults of many species feed on exudates from previous substrates. In Brazil they are popularly known as blowflies. The larvae of this family can be biontophagous, scavengers or necrobiontophagous, which can cause obligatory and facultative myiasis, which makes these larvae extremely important in animal health.
Acknowledgements
I thank the Editor and his working group for publishing my article in the Journal of Modern Agriculture and Biotechnology.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
Marchiori CH contributed to the manuscript and approved the final version.
Abbreviation List
ES, Excretion/secretion
References
[1] Amat E. Notes on necrophagous flies (Diptera: Calyptratae) associated to fish carrion in Colombian Amazon. Acta Amazon, 2010; 40: 397-400. DOI: 10.1590/s0044-59672010000200018
[2] Mariluis JC, Mulieri PR. Calliphoridae, caliphorids in updates in sanitary anthropology Argentina, 1st ed. Healthy World Foundation: Geneva, CH, 2005; 1-95.
[3] Greenberg B. Flies and disease: Biology and disease transmission, 2nd ed. Princeton University Press: Princeton, USA, 1973.
[4] Greenberg B, Szyska ML. Immature stages and biology of fifteen species of Peruvian Calliphoridae (Diptera). Ann Entomol Soc Am, 1984; 77: 488-517. DOI: 10.1093/AESA/77.5.488
[5] Laurence BR. Old world blowflies in the new world. Parasitol Today, 1986; 2: 77-79. DOI: 10.1016/0169-4758(86)90162-6
[6] Greenberg B. Behavior of postfeeding larvae of some Calliphoridae and a Muscid (Diptera). Ann Entomol Soc Am, 1990; 83: 1210-1214. DOI: 10.1093/aesa/83.6.1210
[7] Kosmann C, Macedo MP, Barbosa TAF et al. Chrysomya albiceps (Wiedemann) and Hemilucilia segmentaria (Fabricius) (Diptera, Calliphoridae) used to estimate the postmortem interval in a forensic case in Minas Gerais, Brazil. Rev Bras Entomol, 2001; 55: 621-623. DOI: 10.1590/S0085-56262011000400022
[8] Greenberg B, Kunich JC. Entomology and the law. Flies as forensic indicators, 1st ed. Cambridge University Press: Cambridge, UK, 2002.
[9] Byrd HJ, Castner LJ. Forensic Entomology: The utility of arthropods in legal investigation, 1st ed. Technical textbooks: Boca Raton, USA, 2010.
[10] Benecke M. A brief history of Forensic Entomology. Forensic Sci Int, 2001; 120: 2-14. DOI: 10.1016/s0379-0738(01)00409-1
[11] Dear JP. A revision of the new world Chrysomyini (Diptera: Calliphoridae). Rev Bras Zool, 1985; 3: 109-169. DOI: 10.1590/S0101-81751985000300001
[12] Stehr F. Immature Insect, 2nd ed. Iowa: Kendall/Aunt Publishing Company: Iowa, USA, 1991.
[13] Coast CS, Simonka CE. Insects immature: metamorphosis and identification, 1st ed. Hollos Editora: Rio de Janeiro, BR, 2006.
[14] Gagné RJ. Chrysomya spp., old world blow flies (Diptera: Calliphoridae), recently established in the Americas. Bull Entomol Soc Am, 1981; 27: 21-22. DOI: 10.1093/besa/27.1.21
[15] Guimarães JH, Papavero N, Prado AP. Myiasis in the Neotropics (Identification, biology, bibliography). Braz J Zool, 1983; 1: 239-416. DOI: 10.1590/S0101-81751982000400001
[16] Kosmann C, Mello RP, Harterreiten-Souza E et al. A list of current valid blow fly names (Diptera: Calliphoridae) in the Americas south of Mexico with key to Brazilian species. Entomol Brasilis, 2013; 6: 74-85. DOI: 10.12741/ebrasilis.v6i1.266
[17] Mañas-Jordá S, León-Cortés JL, García-García MD et al. Dipteran diversity and ecological succession on dead pigs in contrasting mountain habitats of Chiapas, Mexico. J Med Entomol, 2017; 55: 59-68. DOI: 10.1093/jme/tjx190
[18] Förster M, Klimpel S, Mehlhorn H et al. Pilot study on synanthropic flies (e.g., Musca, Sarcophaga, Calliphora, Fannia, Lucilia, Stomoxys) as vectors of pathogenic microorganisms. Parasitol Res, 2007; 101: 243-246. DOI: 10.1007/s00436-007-0522-y
[19] Florez E, Wolff M. Description and key of the immature stadiums of the main species of Calliphoridae (Diptera) of forensic importance in Colombia. Neotrop Entomol, 2009; 38: 418-429. DOI: 10.1590/S1519-566X2009000300019
[20] Förster M, Sievert K, Messler S et al. Comprehensive study on the occurrence and distribution of pathogenic microorganisms carried by synanthropic flies caught at different rural locations in Germany. J Med Entomol, 2009; 46: 1164-6. DOI: 10.1603/033.046.0526
[21] Toma R, Carvalho CJB. Phylogenetic study of Mesembrinellinae with emphasis on the genus Eumesembrinella Townsend (Diptera, Calliphoridae). Braz J Biol, 1995; 12: 127-144. DOI: 10.1590/S0101-81751995000100014
[22] Kutty SN, Pape T, Wiegmann BM et al. Molecular phylogeny of the Calyptratae (Diptera: Chyclorrhapha) with an emphasis on the superfamily Oestroidea and the position of Mystacinobiidae and McAlpine’s fly. Syst Entomol, 2010; 25: 614-635. DOI: 10.1111/j.1365-3113.2010.00536.x
[23] Mariluis JC, Schnack JA. Calliphoridae of Argentina. Systematic, ecology and sanitary importance (Diptera, Insect), 1st ed. Series diseases transmittables, monographic publication Healthy World Foundation: Geneva, CH, 2002; 3-23.
[24] McAlpine J. Phylogeny and classification of the Muscomorpha. Manual of Neartic Diptera. Agriculture Canada. Monogr, 1989; 32: 1397-1505.
[25] Gomes L, Zuben CJV, Sanches MR. Study of postfeeding larval radial dispersal in Chrysomya megacephala (Fabricius) (Diptera, Calliphoridae). Rev Bras Entomol, 2003; 47: 229-234. DOI: 10.1590/S0085-56262003000200012
[26] Marchiori CH. Biology and feeding behavior of ceratopogonid adult (Diptera: Ceratopogonidae). Int J Front Sci Tech Res, 2021; 1: 007-024. DOI: 10.53294/ijfstr.2021.1.2.0073
[27] Insects of Australia. CSIRO Division of Entomology. Accessed June 23, 2022. Available at https://www.brisbaneinsects.com/brisbane_muscoid/Calliphorinae.htm
[28] Tillyard RJ. Insects of Australia and New Zealand Angus. Accessed June 23, 2022. Available at https://www.brisbaneinsects.com/brisbane_muscoid/Calliphorinae.htm
[29] The interactive atlas and key to Australian fly families. Accessed June 23, 2022. Available at https://www.brisbaneinsects.com/brisbane_muscoid/Calliphorinae.htm
[30] Silva CPC, Santos Junior EGS, Silva VR. Blowflies (Diptera: Calliphoridae) as bioindicators and accumulators of potentially toxic metals in anthropic areas in the western region of Maranhão, Northeastern Brazil [in Portuguese]. Ibero-Am J Environ Sci, 2021; 12: 446-458. DOI: 10.6008/CBPC2179-6858.2021.008.0037
[31] Mello RP. Contribution to the study of the genus Phaenicia (R.D., 1830) (Diptera, Calliphoridae) [in Portuguese]. Mem Inst Oswaldo Cruz, 1961; 59: 259-278. DOI: 10.1590/s0074-02761961000300002
[32] Perez-Balam J, Quezada-Euan JJ, Alfaro-Bates R et al. The contribution of honey bees, flies and wasps to avocado (Persea americana) pollination in southern Mexico. J Pollination Ecol, 2012; 8: 42-47. DOI: 10.26786/1920-7603(2012)6
[33] Povolnỳ D. Synanthropy in Flies and diseases. Ecology, classification and biotic associations, 1st ed. Princeton University Press: New Jersey, USA, 1971; 17-54.
[34] Baumgartner DL. Spread of introduced Chrysomya megacephala blowflies (Diptera, Calliphoridae) in the Neotropics with records new to Venezuela. Biotropica, 1988; 20: 167-168. DOI: 10.2307/2388191
[35] Carvalho MH, Von Zuben CJ. Demographic aspects of Chrymosya megacephala (Diptera: Calliphoridae) adults maintained under experimental conditions: reproductive rate estimates. Braz Arch Biol Techn, 2006; 49: 457-461. DOI: 10.1590/S1516-89132006000400014
[36] Andrade HTA, Varela-Freire AA, Batista MJA et al. Calliphoridae (Diptera) collected from human cadavers in Rio Grande do Norte. Neotrop Entomol, 2005; 34: 855-856. DOI: 10.1590/S1519-566X2005000500021
[37] Castro P, Szpila C, Martínez-Sánchez L et al. The blowflies of the Madeira Archipelago: species diversity, distribution and identification (Diptera: Calliphoridae s.l.). Zookeys, 2016; 634: 101-123. DOI: 10.3897/zookeys.634.9262
[38] Norris KR. Evidence for the multiple exotic origin of Australian populations of the sheep blowfly, Lucilia-Cuprina (Wiedemann) (Diptera, Calliphoridae). Aust J Zool, 1990; 38: 635- 648. DOI: 10.1071/ZO9900635
[39] Biavati GM, Santana FHA, Pujol-Luz JR. A checklist of Calliphoridae blowflies (Insecta, Diptera) associated with a pig carrion in Central Brazil. J Foren Sci, 2010; 55: 1603-1606. DOI: 10.1111/j.1556-4029.2010.01502.x
[40] Borror DJ, Triplehporn CA, Johson JF. An introduction to the study of insects, 7th ed. Philadelphia: Saunders College Publishing: Philadelphia, USA, 2005.
[41] Carvalho CJB, Couri MS, Rafael JA et al. Insects from Brazil-Diversity and Taxonomy, 1st ed. Holos Editora: São Paulo, BR, 2012; 701-743.
[42] Costa C, Ide S, Simonka CE. Immature insects: metamorphosis and identification, 1st ed. Holos Editora: Ribeirão Preto: Brazil, BR, 2006.
[43] Mohamed HS, Fahmy MM, Attia MM et al. The insecticidal activity of two medicinal plants (Commiphora molmol) and (Balanites aegyptiaca) against the blowfly Lucilia sericata (Diptera: Calliphoridae). Int J Adv Res Biol Sci, 2016; 3: 144-158.
[44] Waterhouse DF, Paramonov SJ. The status of the two species of Lucilia (Diptera, Calliphoridae) attacking sheep in Australia. Aust J Sci Res, 1950; 3: 310-336. DOI: 10.1071/bi9500310
[45] Araújo J. Lucilia sericata (Meigen, 1826) blowfly, greenfly, greenfly. Virtual Museum of Biodiversity. Accessed June 23, 2022. Available at https://www.museubiodiversidade.uevora.pt/elenco-de-especies/biodiversidade-actual/animais/artropodes/insectos/lucilia-sericata
[46] Azeredo-Espin AML, Madeira NG, Madeira NG. Primary myiasis in dog caused by Phaenicia eximia (Diptera: Calliphoridae) and preliminary mitochondrial DNA analysis of the species in Brazil. J Med Entomol, 1996; 3: 839-843. DOI: 10.1093/jmedent/33.5.839
[47] Whitworth TL. Identification of Neotropical blow flies of the genus Calliphora Robineau-Desvoidy (Diptera: Calliphoridae) with the description of a new species. Zootaxa, 2012; 3209: 1-27. DOI: 10.11646/zootaxa.3209.1.1
[48] Whitworth TL. A revision of the Neotropical species of Lucilia Robineau- Desvoidy (Diptera: Calliphoridae). Zootaxa, 2014; 3810: 1-76. DOI: 10.11646/zootaxa.3810.1.1
[49] Sutherst RW, Spradbery JP, Maywald GF. The potential geographical distribution of the Old-World screwworm fly, Chrysomya bezziana. Med Vet Entomol, 1989; 3: 273-280. DOI: 10.1111/j.1365-2915.1989.tb00228.x
[50] Zumpt. Myiasis in man animals in the Old World, 1st ed. Butterworths: London, UK, 1965.
[51] Barros-Cordeiro KB, Pujol-Luz JR. Morphology and duration of post-embryonic development of Chrysomya megacephala (Diptera: Calliphoridae) under laboratory conditions [in Portuguese]. Pap Avulsos Zool, 2010; 50: 709-717. DOI: 10.1590/S0031-10492010004700001
[52] Thyssen PJ, Nassu MP, Costella AMU et al. Record of oral myiasis by Cochliomyia hominivorax (Diptera: Calliphoridae): case evidencing negligence in the treatment of incapable. Parasitol Res, 2012; 111: 957-959. DOI: 10.1016/S0002-9610(33)90461-4
[53] Weil GC, Simon RJ, Sweadner WR. A biological, bacteriological and clinical study of larval or maggot therapy in the treatment of acute and chronic pyogenic infections. Am J Surg, 1993; 19: 36-48. DOI: 10.1093/ecam/nel021
[54] Mumcuoglu KY, Ingber A, Gilead L et al. Maggot therapy for the treatment of intractable wounds. Int J Dermatol, 1999; 38: 623-627. DOI: 10.1046/j.1365-4362.1999.00770.x
[55] Ferraz ACP, Bento A, Gadelha B et al. Larval Therapy: The use of larvae in medicine. Accessed June 23, 2022. Available at https://ledunirio.wordpress.com/2010/11/29/terapia-larval/
[56] Marchiori CH. Ichneumonidae (Hymenoptera) as parasitoid of Diptera Muscomorpha in Brazil. Open J Biol Sci, 2020; 5: 001-003. DOI: 10.17352/ojbs.000014
[57] Moretti TC, Ribeiro OB. Occurrence of the parasitoid Tachinaephagus zealandicus (Ashmead) (Hymenoptera: Encyrtidae) in pupae of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) in rat carcass. Arq Bras Med Vet Zootec, 2006; 58: 137-140. DOI: 10.1590/S0102-09352006000100022
Copyright © 2022 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.



























 Copyright ©
Copyright ©