Copper Gluconate as an Ecofriendly Compound Protecting Apple and Orange Trees against the Most Common Pathogens Affecting Them during the Pre-harvest Stages
Natalia Muñoz Fambuena1*, Paula Vinaches Villarta1, Enrique Tinajero1
1Technical Department, Idai Nature, Valencia, Spain
*Correspondence to: Natalia Muñoz Fambuena, PhD, Crop Technology Coordinator, Technical Department, Idai Nature, 10 La Pobla de Vallbona, Valencia 46185, Spain; Email: natalia.munoz@idainature.rovensa.com
Abstract
Objective: The aim of this study was to investigate the efficacy of liquid copper (Cu) complexed with gluconic acid as an alternative to classical treatments based on large amounts of inorganic Cu, and to determine the efficacy of Cu gluconate in apple (Malus domestica) against bacterial and fungal pathogens, Erwinia amylovora, Venturia inaequalis, and Monilinia fructicola, as well as in orange (Citrus sinensis) against fungal pathogens, Alternaria alternata and Phytophthora citrophthora.
Methods: Applications of Cu gluconate were performed by foliar applications when the first symptoms of the diseases were detected. The doses tested of Cu gluconate were 3mL/L, 4mL/L, and 5mL/L compared to absolute control and a reference commercial product, namely Cu sulfate pentahydrate 25% at 3L/ha. Evaluations were conducted regarding the development of infection and the effectiveness of the treatment. After the first and second applications of this compound, the efficacy of the compound was evaluated.
Results: It was found that the higher dose of Cu gluconate (5mL/L) provided the best efficacy in controlling the infection, with no difference in efficacy between the reference commercial product and Cu gluconate.
Conclusion: It was concluded that Cu gluconate is an effective alternative for the control of bacterial and fungal diseases in apple trees and orange trees, with the advantage of reducing the levels of Cu in the environment as well.
Keywords: copper gluconate, fungi, vacteria, Erwinia amylovora, Venturia inaequalis, Monilinia fructicola, Alternaria alternata, Phytophthora citrophthora, orange, Citrus sinensis, apple, Malus domestica
1 INTRODUCTION
By developing new formulations of liquid copper (Cu) complexed with gluconic acid, it may be possible to protect plants with low concentrations of Cu, thereby reducing the level of Cu in the environment compared to classical treatments based on high concentrations of inorganic Cu.
Among the most significant damage caused by bacterial and fungal diseases is the destruction of the trees’ fruit. Furthermore, there is a reduction in yields due to the attack on flowers and a reduction in tree vigor as a result of the death of buds and branches during the sprouting and harvesting processes. In order to control them effectively and in an environmentally friendly manner, we need to find a more efficient and environmentally friendly solution.
Among these diseases, the most relevant and problematic in citrus are: Alternaria alternata (A. alternata) and Phytophthora citrophthora (P. citrophthora).
In the case of A. alternata, it is an opportunistic pathogenic fungus that can cause spots on the leaves, as well as rotting and discoloration in many parts of the plants[1]. More than 380 host plant species have been identified as being susceptible to such leaf spots and other diseases. The symptomatology begins in the basal leaves, gradually advancing towards the upper layer. Early plant symptoms begin with yellowing of the leaves at the tips and develop along the margins to the petiole. Later, the fungus produces spots on the leaves of variable size that can reach up to more than 1cm in diameter. The round or irregular spots, slightly depressed, have a well-marked purple border and a whitish or brownish center. If this occurs before flowering, there can be a total defoliation of the plants, which can lead to substantial harvest losses. There is also the possibility of the fruit being infected, which will show brown spots, causing it to be unappetizing and depreciating.
P. citrophthora, also known as citrus brown rot, is a soil-borne oomycete that infects several economically important citrus crops[2]. A diagnostic symptom of P. citrophthora is gummosis, in which sap is exuded from lesions around the base of the tree. The conditions of waterlogging of the soil, due to rain or excessive irrigation, favor the development of Phytophthora in the plot. The greatest parasitic activity of the pathogen occurs with average temperatures between 18 and 24ºC, although the optimum depends on the Phytophthora species. The symptoms of these diseases are only visible several months after infection. Affected trees usually show lack of vigor and general decline. In most cases, the leaves show a very marked chlorosis in the central nerve. First symptoms on the trunk the trunk and main branches are not visible externally because they are caused by darkening of the internal tissues of the phloem and the cambium. As the infection progresses, gummy exudations are released from the lesions, which vary in intensity depending on the health of the tree and the environmental conditions.
The following are some of the most problematic diseases that affect apple trees: Erwinia amylovora (E. amylovora), Venturia inaequalis (V. inaequalis) and Monilinia fructicola (M. fructicola).
E. amylovorais a bacterium of the Erwiniaceae family, a pathogen that causes "fire blight", a disease that affects various plant species of the Rosaceae family, especially pome fruit trees, as well as other ornamental and wild plants. Sensitivity to the disease is diverse according to species and varieties[3]. In some cases, the damage caused may lead to the death of the affected plant within a short period of time. Because of the disease's ease of spread and the lack of chemical treatment, its severity is increased. The life cycle of E. amylovora is in line with the seasonal development of the plant. The bacterium infects the plant generally in the spring, through the flowers or small buds in development. From that moment on, the bacterium begins to infect all the tissues of the plant, moving towards the base of the stem and causing the death of all the cells in its path. During the fall and winter the bacteria stop their activity. It remains dormant until spring on the edges of cankers formed at the end of the vegetative period.
V. Inaequalis is an ascomycete fungus that causes apple scab disease[4]. Symptoms may be found on leaves, petioles, sepals, fruits, pedicels, and less frequently on shoots. Leaves and fruits are the most evident in showing symptoms of the disease. As the leaves appear in spring, the first lesions appear on the underside. Later, symptoms also appear on the upper face. An infection of the petioles and pedicels results in the premature abscission of the leaves and fruits. There are symptoms of lesions on young fruits that are similar to those seen on leaves, but as the condition worsens, the lesions become larger, darker, and more corky. It is possible for early infections to kill the meristematic tissues near the surface of the fruit, and the fruit begins to deform due to the fact that the affected part stops growing while the healthy part continues to grow, or cracks begin to appear in the skin and pulp. The symptomatology is characterized by very small spots like the point of a pin, and the lesions are rough, black, circular from 0.1mm to 4mm in diameter.
The importance of M. fructicola lies in the attack on flowers, buds and fruits, causing their destruction[5]. It is a disease that is difficult to control when conditions favorable to its development occur. The first organ to be attacked is the flower, causing it to wilt. The stamens, pistils, petals, or sepals can be invaded by the fungus, producing small brown spots that spread to the entire flower and then turn blight. On these attacked flowers and in high humidity conditions, the sign of the fungus can be seen, consisting of mycelium and grayish chain conidia. The blighted flower may fall off or remain attached to the branch. If favorable conditions continue (high humidity and temperature), the fungus advances from the flower along the pedicel to the branch, producing cankers. These cankers are dark (brown) in color, somewhat depressed, and under high humidity conditions, the production of gummy exudates on them can be observed. In the ripening stage, the fruits are attacked, producing the symptom of brown rot that gives the disease its name. It consists of a firm rot, brown in color that progresses rapidly, taking over the entire fruit. On this fruit rot, the sporulation of the fungus with a powdery appearance and gray color can be seen.
The alternative compounds are not generalized or allowed in crop protection reducing the number of available compounds to mainly inorganic formulations of Cu, such as Cu hydroxide or Cu sulfate, or a mixture of Cu-based and ethylene bis-dithiocarbamates (EBDC). The generalized use of Cu-based compounds has led to the appearance of Cu-resistant species that dramatically reduce the effectiveness of classic treatments[6]. Moreover, use of combinations of Cu-based compounds with EBDC is restricted to the pre-harvest period because they can persist for up to 8 weeks in crops. There are also many environmental concerns about the accumulation of both Cu and EBDC in the soil. Extensive use of Cu-based fungicides is widespread in agriculture, with contamination reported not only in citrus or apple fields, but also in tomato fields, and, especially, vineyards[7-10]. Consequently, the development of organic compounds capable of protecting plants is becoming increasingly attractive as possible alternatives to chemicals in order to alleviate these environmental problems.
Research on new compounds based on organic formulations of copper and their effect as plant protectors provides farmers with a valuable alternative to the control of pathogens such as A. alternata, P. citrophthora, E. amylovora, V. inaequalis and M. fructicola. Cu gluconate is characterized by great Cu absorption and diffusion over the plant and is safer for the environment than other traditional inorganic Cu-based fungicides.
Therefore, the main purpose of this study was to determine whether the treatment had a direct effect on different pathogens. Field trials were conducted on orange (Citrus sinensis) and apple (Malus domestica) trees.
2 MATERIALS AND METHODS
The plant material used for the trials was orange tree (Citrus sinensis) var (variety). 'Valencia Late' for the control of A. alternata and P. citrophthora, located in San Rafael, Veracruz, Mexico, and apple tree (Malus domestica) var. 'Golden' for the control of E. amylovora, V. inaequalis and M. fructicola, located in Zacatlán, Puebla, Mexico. When the experiments were conducted, orange trees were in vegetative development, but the apple trees were in flowering period and fruiting.
For the experimental design, in both trials, randomised blocks were designed, each of these blocks consisting in three trees of about 8 years old arranged in a planting frame of 7×7m.There are four repetitions in each thesis, and a total of five theses were undertaken: i, Cu gluconate at 3mL/L; ii, Cu gluconate at 4mL/L; iii, Cu gluconate at 5mL/L; Commercial reference product with Cusulfate pentahydrate 25% at 3L/ha; and iv, negative control.
First treatments were started when the first symptoms of the diseases were detected. A total of two applications were performed at 5d intervals. According to the calibration performed in the field, the volume of water was 950L of ha-1. A 25L capacity motor sprinkler with hollow cone nozzle was used in this application. The sprinkler was pre-calibrated according to the volume of water required per hectare. In this case, the working pressure was 200PSI.
The evaluated parameter to know the efficacy of the different treatments was the percent of severity of the different diseases. To detect initial infections, before starting the applications, a preliminary evaluation was carried out using the scales of Tables 1-5 according to the disease. Subsequently, 5d after each application, disease severity assessments were carried out by sampling 50 leaves and fruits per experimental unit. In each experimental unit, three trees were randomly sampled.
Table 1. Scale Used to Assess Leaf Damage Caused by A. alternata on Orange Trees[11]
Index |
Description |
0 |
Without injuries |
1 |
1 to 2 injuries |
2 |
3 to 5 injuries |
3 |
6 to 10 injuries |
4 |
11 to 15 injuries |
5 |
>15 injuries |
Table 2. Scale Used to Assess Fruit Rot Damage Caused by P. citrophthora on Orange Fruit[12]
Index |
Description |
0 |
Healthy fruits |
1 |
0.1 to 3.125% of the area of damaged fruit |
2 |
3.2 a 6.25% of the area of damaged fruit |
3 |
6.3 a 12.5% of the area of damaged fruit |
4 |
12.6 a 25% of the area of damaged fruit |
5 |
25.1 a 50% of the area of damaged fruit |
6 |
50.1% or + of the area of damaged fruit |
Table 3. Scale Used to Determine the Severity of Apple Foliage Disease Caused by E. amylovora[13]
Index |
% of Damage |
0 |
Shoot without necrosis |
1 |
Initial necrosis on tender leaves |
2 |
Shoot with 10% damage, two-three tender leaves affected |
3 |
Shoot with 25% damage, damage in young leaves |
4 |
Up to 50% damage in shoots, young leaves and affected stems |
5 |
More than 50% damage, necrotic shoot |
Table 4. Scale Used to Determine Disease Severity in Apple Fruit Caused by V. inaequalis[14]
Index |
% of Damage |
0 |
Fruit without injuries |
1 |
Injuries causing up to 1% fruit damage |
2 |
Injuries causing 2-10% fruit damage |
3 |
Injuries causing 11-25% fruit damage |
4 |
Injuries causing 26-50% fruit damage |
5 |
More than 50% fruit damage |
Table 5. Scale Used to Determine Disease Severity in Apple Fruit Caused by M. fructicola[15]
Index |
% of Damage |
0 |
Fruit without injuries |
1 |
Injuries causing 1-5% fruit damage |
2 |
Injuries causing 6-15% fruit damage |
3 |
Injuries causing 16-25% fruit damage |
4 |
Injuries causing 26-50% fruit damage |
5 |
More than 50% fruit damage |
Phytotoxicity was evaluated according to the scale proposed by the European Weed Research Society (EWRS) used to assess phytotoxicity in crops.
The severity index was transformed to infection percentage using the formula of Townsend and Heuberger (1943). An analysis of variance and Tukey (α=0.05) test of means was carried out on the percentage of infection using the SAS statistical analysis package. In cases where the analysis of variance hypothetical case was not met, non-parametric tests were used, and the effectiveness of the treatments was calculated using Abbott's formula with.
Townsend and Heuberger formula. To transform the severity index to percentage of infection.
|
Where,
n: number of fruits or leaves in each scale
v: scale value
vmax: the highest scale value
N: observed total number of fruits.
Abbott formula. To calculate the biological effectiveness of products.
|
Orange trial started on 23 March 2019, pre-evaluation and the first application were carried out. The first evaluation took place on 28 March 2019 together with the second application. And the final assessment was conducted on 2 April 2019.
Apple trial started on 24 March 2019, pre-evaluation and the first application were carried out. The first evaluation took place on 29 March 2019 together with the second application. And the final assessment was conducted on 4 April 2019.
3 RESULTS
3.1 Study Carried Out on Orange Trees
3.1.1 A. alternata
Severity data and statistical analysis is shown in Table 6. At the beginning of the study, it was observed that the presence of the disease was homogeneous in all treatments, with an average of 6.95% infection in foliage. In the end it was 14.5% in the negative control.
Table 6. Percentage of Infection Caused by Brown Spot (A. alternata) on Foliage, in the First and Second Evaluation in Orange Trees
Treatments |
First Evaluation 5 DA1A |
Second Evaluation 5 DA2A |
||
% of Infection |
E (%) |
% of Infection |
E (%) |
|
1. Copper gluconate 3mL/L |
3.25 bz |
69.05 |
2.75 bz |
81.03 |
2. Copper gluconate 4mL/L |
2.75 b |
73.81 |
2.00 b |
86.21 |
3. Copper gluconate 5mL/L |
2.25 b |
78.57 |
1.50 b |
89.66 |
4. Commercial product 3L/ha |
2.50 b |
76.19 |
1.75 b |
87.93 |
5. Negative control |
10.50 a |
- |
14.50 a |
- |
Notes: zTreatments with the same letter are not significantly different (Tukey Test, α=0.05). DA1A: Days after first application; DA2A: Days after second application; E: Effectiveness.
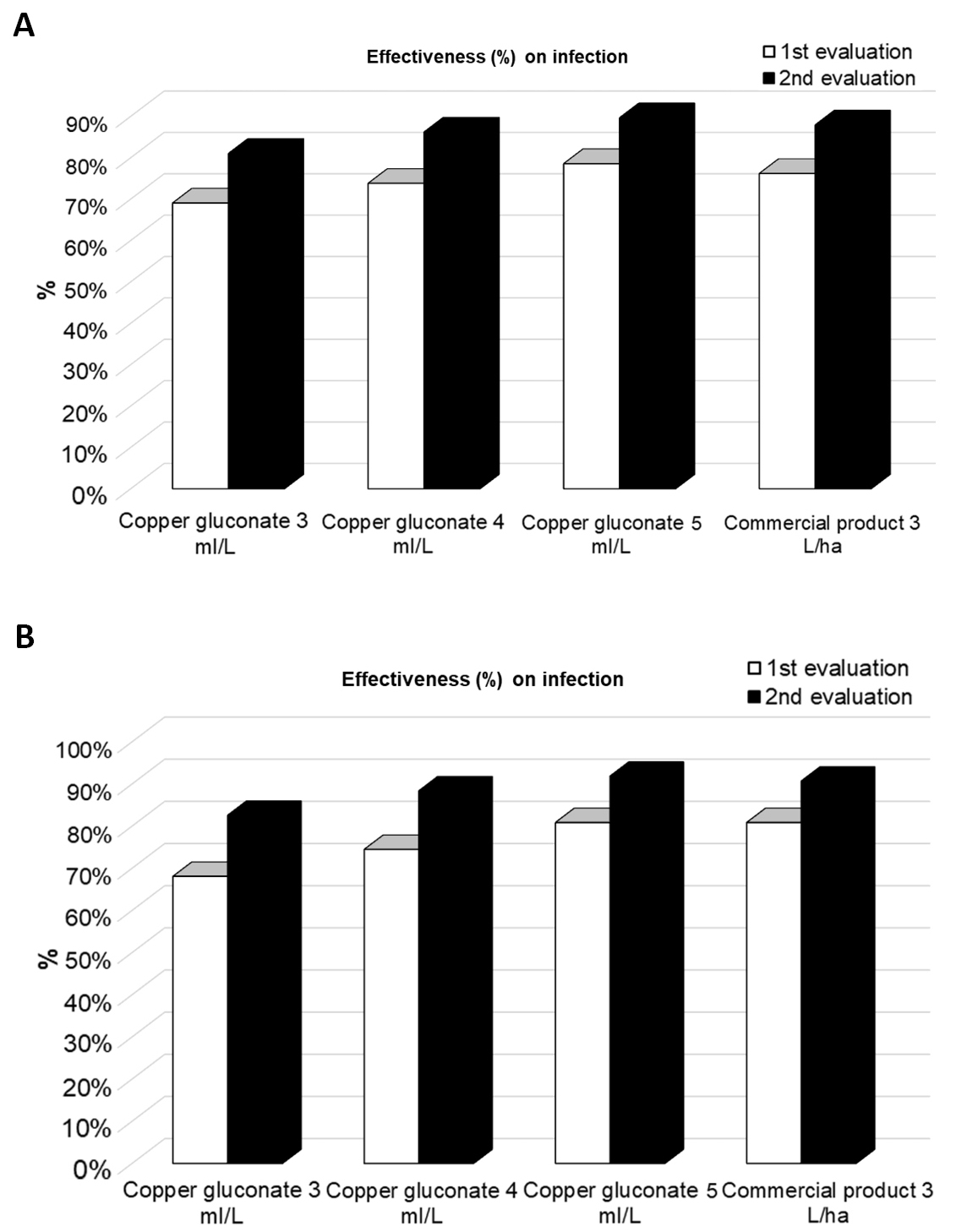
The results obtained in the first and second assessment, carried out 5 days after each application, are shown in Figure 1A. Cu gluconate at 5mL/L has the highest efficacy comparing with the other treatments, although it does not show significant differences with the lower doses or with the commercial reference product. All treatments improve their efficacy after the second application.
None of the treatments generated phytotoxicity at any of the evaluated doses.
|
Figure 1. Percentage of effectiveness of Cu gluconate against A. alternata (A) and P. citrophthora (B) in orange tree.
3.1.2 P. citrophthora
Severity data and statistical analysis is shown in Table 7. At the beginning of the study, the disease was homogeneous in all treatments, with an average of 4.95% infection in foliage.In the end it was 18% in the negative control.
Table 7. Percentage of Infection Caused by Citrus Brown Rot (P. citrophthora) on Fruit, in the First and Second Evaluation in Orange Trees
Treatments |
First Evaluation 5 DA1A |
Second Evaluation 5 DA2A |
||
% of Infection |
E (%) |
% of Infection |
E (%) |
|
1. Copper gluconate 3mL/L |
3.125 bz |
68.09 |
3.125 bz |
82.56 |
2. Copper gluconate 4mL/L |
2.500 b |
74.47 |
2.083 b |
88.37 |
3. Copper gluconate 5mL/L |
1.875 b |
80.85 |
1.458 b |
91.86 |
|
1.875 b |
80.85 |
1.667 b |
90.70 |
5. Negative control |
9.791 a |
- |
17.917 a |
- |
Notes: zTreatments with the same letter are not significantly different (Tukey Test, α=0.05). DA1A: Days after first application; DA2A: Days after second application; E: Effectiveness.
All treatments have a final efficacy of around 80-90% (Figure 1B).
In the study, the treatment that showed the greatest efficacy was Cu gluconate at the highest dose (5mL/L), although it did not show significant differences from the other doses or the commercial reference product. The effectiveness of all treatments increases with each subsequent application.
None of the treatments generated phytotoxicity at any of the evaluated doses.
3.2 Study Carried Out on Apple Trees
3.2.1 E. amylovora
Severity data and statistical analysis is shown in Table 8. In the pre-application assessment, it was observed that at the beginning of the study the disease presence was homogeneous, with an initial average of 7% infection in foliage. In the end it was 21.75% in the negative control.
Table 8. Percentage of Infection Caused by Fire Blight (E. amylovora) on Foliage, in the First and Second Evaluation in Apple Trees
Treatments |
First Evaluation 5 DA1A |
Second Evaluation 5 DA2A |
||
% of Infection |
E (%) |
% of Infection |
E (%) |
|
1. Copper gluconate 3mL/L |
4.00 bz |
70.91 |
3.75 bz |
82.76 |
2. Copper gluconate 4mL/L |
3.25 b |
76.36 |
2.50 b |
88.51 |
3. Copper gluconate 5mL/L |
2.75 b |
80.00 |
1.75 b |
91.95 |
4. Commercial product 3L/ha |
2.75 b |
80.00 |
2.00 b |
90.80 |
5. Negative control |
13.75 a |
- |
21.75 a |
- |
Notes: zTreatments with the same letter are not significantly different (Tukey Test, α=0.05). DA1A: Days after first application; DA2A: Days after second application; E: Effectiveness.
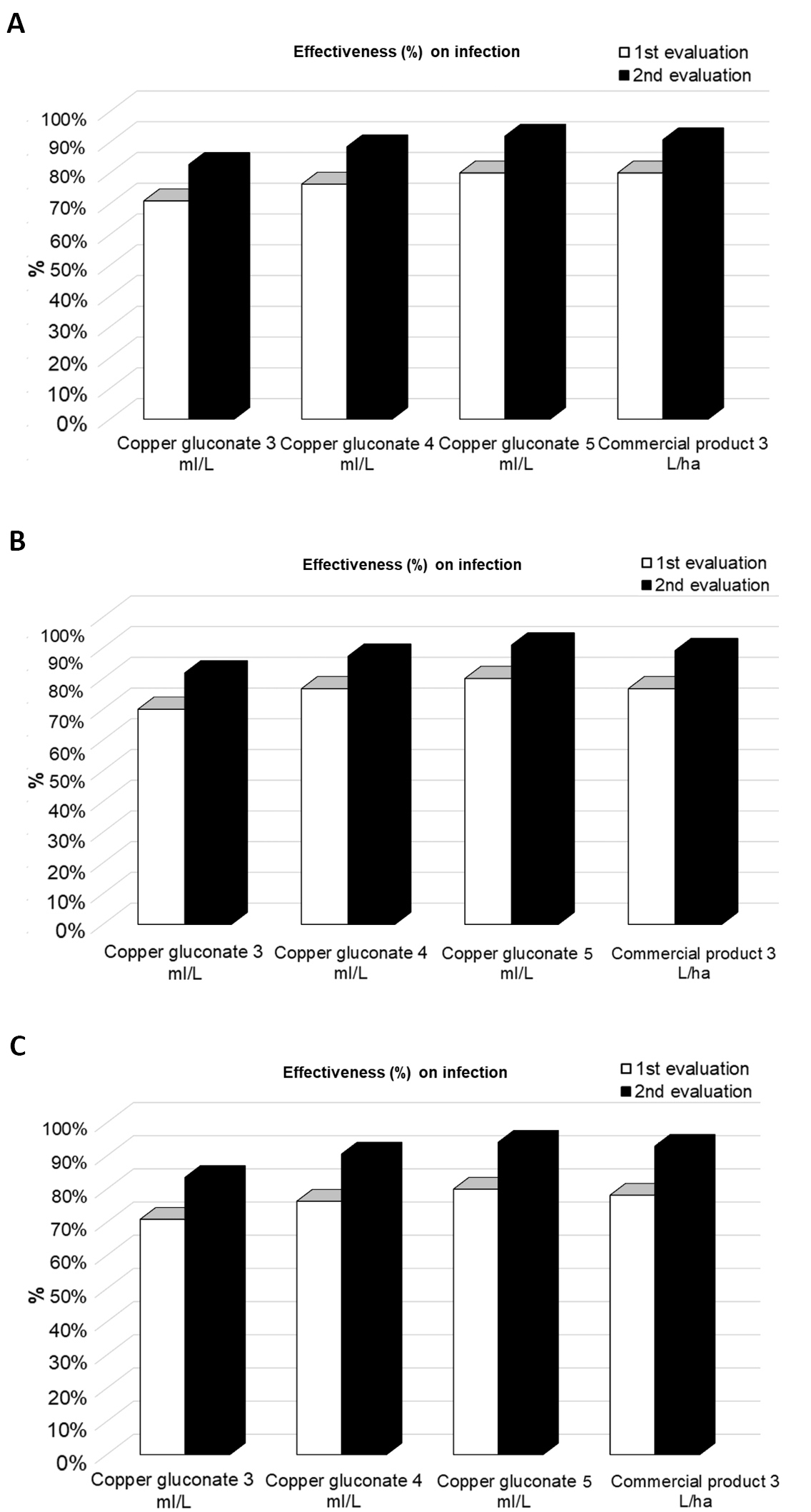
The results obtained in the first and second assessment, carried out 5 days after each application, are shown in Figure 2A.
|
Figure 2. Percentage of effectiveness of Cu gluconate against E. amylovora (A), V. inaequalis (B) and Monilinia fructicola (C) in apple tree.
In the first evaluation, the highest percentage of control was achieved by Cu gluconate at the highest dose (5mL/L), with an efficacy of 80% and no statistically significant differences with the reference product corresponding to the commercial reference product. In the final evaluation, 5 days after the second application, the highest dose of Cu gluconate (5mL/L) again achieved the highest percentage of disease control. The highest efficacies of the product Cu gluconate are obtained 5d after the second application at the three tested doses.
None of the treatments generated phytotoxicity at any of the evaluated doses.
3.2.2 V. inaequalis
Severity data and statistical analysis is shown in the Table 9. In the pre-application assessment, it was observed that the distribution was uniform with an average of 4 % infection in fruits. In the end it was 13.75% in the negative control. The results obtained are shown in Figure 2B.
Table 9. Percentage of Infection Caused by Apple Scab (V. inaequalis) on Fruit, in the First and Second Evaluation in Apple Trees
Treatments |
First Evaluation 5 DA1A |
Second Evaluation 5 DA2A |
||
% of Infection |
E (%) |
% of Infection |
E (%) |
|
1. Copper gluconate 3mL/L |
2.25 bz |
70.00 |
2.50 bz |
81.82 |
2. Copper gluconate 4mL/L |
1.75 b |
76.67 |
1.75 b |
87.27 |
3. Copper gluconate 5mL/L |
1.50 b |
80.00 |
1.25 b |
90.91 |
4. Commercial product 3L/ha |
1.75 b |
76.67 |
1.50 b |
89.09 |
5. Negative control |
7.50 a |
--- |
13.75 a |
--- |
Notes: zTreatments with the same letter are not significantly different (Tukey Test, α=0.05). DA1A: Days after first application; DA2A: Days after second application; E: Effectiveness.
In both, first and second evaluation, the highest dose of Cu gluconate (5mL/L) obtained the highest percentage of control of V. inaequalis. The effectiveness of all treatments increases with each subsequent application.
None of the treatments generated phytotoxicity at any of the evaluated doses.
Severity data and statistical analysis is shown in Table 10. During previous sampling, it was observed that the percentage of scab infections (Monilinia fructicola) in apple trees was statistically equal in all treatments, indicating a homogeneous presence of the disease before the study started. At the time of application of the treatments, there was an average infection rate of 6.25% in fruit. At the end of the study, the infection rate in the negative control was 21.25%.
Table 10. Percentage of Infection Caused by Monilinia fructicola on Fruit, in the First and Second Evaluation in Apple Trees
Treatments |
First Evaluation 5 DA1A |
Second Evaluation 5 DA2A |
||
% of Infection |
E (%) |
% of Infection |
E (%) |
|
1. Copper gluconate 3mL/L |
4.00 bz |
70.91 |
3.50 bz |
83.53 |
2. Copper gluconate 4mL/L |
3.25 b |
76.36 |
2.00 b |
90.59 |
3. Copper gluconate 5mL/L |
2.75 b |
80.00 |
1.25 b |
94.12 |
4. Commercial product 3L/ha |
3.00 b |
78.18 |
1.50 b |
92.94 |
5. Negative Control |
13.75 a |
- |
21.25 a |
- |
Notes: zTreatments with the same letter are not significantly different (Tukey Test, α=0.05). DA1A: Days after first application; DA2A: Days after second application; E: Effectiveness.
As can be seen in Figure 2C, Cu gluconate at 5mL/L obtains the highest efficacy against monilinia fructicola in apple trees with no significant differences with the commercial reference product and the other treatments with a lower dose of Cu gluconate.
None of the treatments generated phytotoxicity at any of the evaluated doses.
4 DISCUSSION
All treatments tested were effective for the control of A. alternata and P. citrophthora on orange trees and E. amylovora, V. inaequalis and M. fructicola on apple trees. Treatments with Cu gluconate controlled the infection, with the best efficacy observed at the higher dose of Cu gluconate (5mL/L), without differences compared to the reference commercial product. All the treatments improve their efficacy with the subsequent application. In other studies, carried out against Xanthomonas campestris pv. In both laboratory and field conditions indicated that Cu gluconate recorded significant control against the bacteria, with similar results as Cu hydroxide and mancozeb treatments[16]. Cu gluconate was able to inhibit the growth of the fungus Curvularia eragrostidis in in vitro assays[17].
For all diseases tested on both crops, the products work in a similar way, which shows reliability of the results obtained.
5 CONCLUSION
The results of this study indicated that Cu gluconate could be an effective alternative in apple and orange trees for the control of a wide variety of bacterial and fungal diseases, along with the advantage of reducing the level of Cu in the environment.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
All authors participated in conception and design, analysis and interpretation of the data, drafting the article or revising it critically for important intellectual content, and approval of the final version.
Abbreviation List
A. alternata, Alternaria alternata
Cu, Copper
E. amylovora, Erwinia amylovora
EBDC, Ethylene bis-dithiocarbamates
M. fructicola, Monilinia fructicola
P. citrophthora, Phytophthora citrophthora
V. inaequalis, Venturia inaequalis
Var, Variety
References
[1] Peever TL, Ibañez A, Akimitsu K et al. Worldwide phylogeography of the citrus brown spot pathogen, alternaria alternata. Phytopathology, 2002; 92: 794-802. DOI: 10.1094/PHYTO.2002.92.7.794
[2] AlvarezLA, VicentA, De la Roca E et al. Branch cankers on citrus trees in Spain caused by Phytophthora citrophthora. Plant Pathol, 2008; 57: 84-91. DOI: 10.1111/j.1365-3059.2007.01702.x
[3] Norelli JL, Aldwinckle HS, Beer SV. Differential host x pathogen interactions among cultivars of apple and strains of Erwinia amylovora. Phytopathology, 1984; 74: 136-139.
[4] Bowen JK, Mesarich CH, Bus VGM et al. Venturia inaequalis: The causal agent of apple scab. Mol Plant Pathol, 2011; 12: 105-122. DOI: 10.1111/j.1364-3703.2010.00656.x
[5] Grabke A, Hu MJ, Luo CX et al. First report of brown rot of apple caused by Monilinia fructicola in Germany. Plant Dis, 2011; 95: 772-772. DOI: 10.1094/PDIS-02-11-0113
[6] Griffin K, Gambley C, Brown P et al. Copper-tolerance in Pseudomonas syringae pv. tomato and Xanthomonas spp. and the control of diseases associated with these pathogens in tomato and pepper. A systematic literature review. Crop Prot, 2017; 96: 144-150. DOI: 10.1016/j.cropro.2017.02.008
[7] Adriano DC. Copper, trace elements in terrestrial environments. Springer: New York, USA, 2011.
[8] Komárek M, Čadková E, Chrastný V et al. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ Int, 2010; 36: 138-151. DOI: 10.1016/j.envint.2009.10.005
[9] Li W, Zhang M, Shu H. Distribution and fractionation of copper in soils of apple orchards. Environ Sci Pollut R, 2005; 12: 168-172. DOI: 10.1065/espr2005.04.243
[10] Schramel O, Michalke B, Kettrup A. Study of the copper distribution in contaminated soils of hop fields by single and sequential extraction procedures. Sci Total Environ, 2000; 263: 11-22. DOI: 10.1016/S0048-9697(00)00606-9
[11] Mondal SN, Vicent A, Reis RF et al. Efficacy of pre-and postinoculation application of fungicides to expanding young citrus leaves for control of melanose, scab, and Alternaria brown spot. Plant Dis, 2007; 91: 1600-1606. DOI: 10.1094/PDIS-91-12-1600
[12] Camele I, De Feo V, Altieri L et al. An attempt of postharvest orange fruit rot control using essential oils from Mediterranean plants. J Med Food, 2010; 13: 1515-1523. DOI: 10.1089/jmf.2009.0285
[13] Schroth MN, Thomson SV, Hildebrand DC et al. Epidemiology and control of fire blight. Annu Rev Phytopathol, 1974; 12: 389-412. DOI: 10.1146/annurev.py.12.090174.002133
[14] Köller W, Wilcox WF. Evaluation of tactics for managing resistance of Venturia inaequalis to sterol demethylation inhibitors. Plant Dis, 1999; 83: 857-863. DOI: 10.1094/PDIS.1999.83.9.857
[15] Lazar‐Baker EE, Hetherington SD, Ku VV et al. Evaluation of commercial essential oil samples on the growth of postharvest pathogen Moniliniafructicola (G. Winter) Honey. Lett Appl Microbiol, 2011; 52: 227-232. DOI: 10.1111/j.1472-765X.2010.02996.x
[16] Zhang Y, Mao R, Zheng J et al. Laboratory bioassay and field evaluation of copper gluconate as a new potential bactericide against Xanthomonas campestrispv. Citri (Hasse) dye. J Agr Sci-Cambridge, 2013; 5: 23. DOI: 10.5539/jas.v5n12p23
[17] García-Ordaz HA, Chan-Cupul W, Buenrostro-Nava MT et al. In vitro effectiveness of chemical fungicides against Curvularia eragrostidis (Henn) JA Mey, causal agent of pineapple leaf spot [in Spanish]. Scientia Agropecuaria, 2021; 12: 429-434. DOI: 10.17268/sci.agropecu.2021.047
Copyright © 2022 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.





 Copyright ©
Copyright ©