Assessing the Potential of Irrigating Against Rainfed Manihot Esculenta Crantz Under Different Levels of NPK Fertilizers on Storage Root Yields in Tanzania Lake Victoria Zone
Deusdedit Peter Mlay1, Jacob Bulenga Lisuma2*
1Department of Research, Tanzania Agricultural Research Institute of Tanzania (TARI), Ukiriguru, Mwanza, Tanzania
2Department of Research, Tobacco Research Institute of Tanzania (TORITA), Tabora, Tanzania
*Correspondence to: Jacob Bulenga Lisuma, PhD, Research Fellow, Department of Research, Tobacco Research Institute of Tanzania (TORITA), Tumbi, Tabora 45120, Tanzania; Email: jbulenga@gmail.com
Abstract
Objective: To assess the response of new registered Kiroba cassava variety on different rates of fertilizer under rainfed and irrigated regimes on the low fertility land of Tanzania Agricultural Research Institute of Tanzania (TARI), Ukiriguru, Mwanza, Tanzania.
Methods: The experiment was laid in a randomized complete block design in a split-plot design with irrigation and rainfed regimes being the site-main plot and fertilizer-subplots replicated three times. The treatments were absolute control and three rates i.e., 150:40:180, 75:20:90 and 300:80:360 kg ha-1 of N, P, and K respectively, under the rainfed and irrigated regimes.
Results: Half rate of fertilizer blend N75P20K90 under irrigation regime had significantly high root storage yields ranging from 24.76 to 25.21t ha-1, to both cropping seasons. However, even though the root storage yields were slightly low (24.59 to 24.14t ha-1) in the same treatment, it did not differ with the rainfed treatment. The high root storage yields were attributed to the narrow gap between the small and large storage root size and adequate moisture levels.
Conclusion: Cassava crop requires less fertilizer nutrients, but with appropriate proportion on soil moisture for increased root storage yield. Therefore, the study recommends further research to determine the potential yields using fertilizer blend within N50P10K70 and N100P30K120.
Keywords: Kiroba variety, small and large storage roots, soil nutrients, soil health
1 INTRODUCTION
The agriculture sector is the back bone of the Tanzanian economy accounting for 30% of agricultural produce export[1]. The food crops in the country are growing at 3.5% per annum[2]. The most common food and cash crops in Tanzania are maize, cassava, sweet potatoes, bananas, sorghum, and sugar cane[3,4]. It is estimated that about 82% of Tanzanian farmers grow maize, and 24% grow cassava in an estimated 655,700 ha of land and annual production of 1,786,400 ton[3]. Recently, the cassava crop has become the most widely used food crop in Tanzania, with minimal post-harvest losses[5].
Cassava (Manihot esculenta Crantz) is a perennial crop growing up to 2.4m tall[6]; it is a starchy storage root crop that also serves as a local food crop that strengthens food security in developing countries[7,8]. About one billion smallholder households in sub-Saharan Africa depend on cassava as a staple food crop[9]. The preference of the crop to sub-Saharan Africa is a result of the crop's ability to grow on low fertility soils[5,10]. For example, in Tanzania, the cassava crop is grown to the soil fertility constraints surrounding Lake Victoria in Mara, Mwanza, Simiyu, Geita and Kagera regions[11,12].
Despite the cassava crop resilience and ability to grow on low soil fertility areas[5,7,8,10], it requires fertility as when cultivated continuously, its storage root yields may decrease[13]. Nitrogen (N), phosphorus (P) and potassium (K) are major nutrients required by cassava crops. Applying these nutrients in splitting has proven to be effective in increasing cassava root yields[14,15]. The crop is efficient in N, P and K uptake[16,17]. However, the crop removes a large quantity of K compared to other nutrients because of its involvement in the synthesis and accumulation of starch in storage roots[16,18]. The high uptake of K increases the concentration of N and S by 2-3-fold because K is involved in N metabolism[19]. Like other plants, cassava could naturally obtain nutrients such as N and P by forming beneficial relationships with microbes[20]. A recent study revealed the role of microbes such as arbuscular mycorrhizal fungi (AMF) in contributing to the P nutrients availability to the cassava rhizosphere for increasing storage root yield[21]. In fact, the crop is highly dependent on AMF for P uptake from the soils, which contributes to the increase in cassava storage root yield[17].
Although, cassava is a drought tolerant crop, recent persistence of drought for an extended period, has been observed to reduce the storage root yield[22,23]. Drought can hinder nutrient uptake where fertilizers are applied hence reducing cassava yield potential. Thus, irrigation could be an option for increasing cassava storage root yield under climate change in the sub-Saharan countries by increasing moisture availability and nutrient solubility uptake. Based on the importance of the cassava crop in Tanzania, a need to research on irrigating cassava applied with different NPK fertilizers throughout the year was initiated. Although, in Tanzania, only 1.8% of all cropped land is irrigated[24], research for irrigated cassava crops is rarely studied. Therefore, the current study aimed to assess cassava biomass yields under rainfed and irrigated regimes with different NPK fertilizer blends using Kiroba, a newly released improved cassava variety in Lake Victoria zone, Tanzania.
2 MATERIALS AND METHODS
2.1 Site Description and Experimentations
Field experiments were conducted at TARI Ukiriguru site during two cropping seasons (2019-20 and 2020-21). The site is located at 2o 42'S and 33o 01'E at an altitude of 1,198 meters above sea level. Rainfall and temperature collected during the two cropping seasons are indicated in Table 1.
Table 1. Rainfall and Temperature of the Study Site During the 2019-20 and 2020-21 Cropping Seasons
Month |
Cropping Season 2019-20 |
Cropping Season 2020-21 |
||
Rain (mm) |
Temp (°C) |
Rain (mm) |
Temp (°C) |
|
Oct |
105.1 |
29.8 |
101.4 |
30.5 |
Nov |
133.9 |
29.4 |
171.1 |
29.5 |
Dec |
226.7 |
27.8 |
178.5 |
28.3 |
Jan |
197.3 |
30.2 |
174.1 |
28.2 |
Feb |
30.6 |
31.2 |
91.8 |
30.2 |
Mar |
55.4 |
31.8 |
65.2 |
29.4 |
Apr |
146.8 |
30.6 |
133.3 |
28.9 |
May |
23.8 |
30.7 |
45.8 |
29.9 |
Jun |
31.8 |
29.7 |
1.8 |
30.2 |
Jul |
3.1 |
30.9 |
0.2 |
30.4 |
Aug |
71.6 |
30.7 |
12.4 |
31.5 |
Sep |
46.3 |
31.4 |
59.8 |
31.7 |
A randomized complete block design in split-plot arrangement with treatments being fertilizer rates-main plot and moisture regimes (irrigation vs rainfed)-subplot replicated three times was used in the experiment. The main plot treatments involved an improved cassava variety at three different application rates of NPK fertilizer; half rate (75:20:90), full rate (150:40:180), double rate (300:80:360) kg ha-1, checked against a control with no fertilizer application (Table 2).
Table 2. Treatment Description and Rates
Treatment |
Blend |
N |
P |
K |
Urea |
TSP |
MOP |
Urea |
TSP |
MOP |
kg ha-1 |
kg ha-1 |
g plot-1 |
||||||||
T1 |
CON |
0 |
0 |
0 |
0 |
0 |
0 |
0.0 |
0.0 |
0.0 |
T2 |
HALF |
75 |
20 |
90 |
163 |
100 |
180 |
1956.5 |
1194.8 |
2160.0 |
T3 |
FULL |
150 |
40 |
180 |
326 |
199 |
360 |
3913.0 |
2389.6 |
4320.0 |
T4 |
DBLE |
300 |
80 |
360 |
652 |
398 |
720 |
7826.1 |
4779.1 |
8640.0 |
Notes: Key: CON=Control; HALF=Half NPK fertilizer blend; FULL=Full fertilizer blend; DBLE=Double fertilizer blend.
The subplot treatments involved an improved cassava variety (fertilized and control) with rainfed versus irrigated regimes. A recommended cassava variety of Kiroba was used as a test crop and was obtained from the TARI of Ukiriguru, Mwanza. Crop cuttings were planted (one per hill) in December at a spacing of 1m between the cuttings as well as between ridges in a plot size of 8m x 8m. Inter-plot separation was 2m, while the inter-block separation was 4m. Cassava was irrigated (10L plant-1) twice a week during the dry season when the sum of rainfall for the three consecutive days was less than 10mm. The irrigation application was done either in the morning or evening to minimize evaporation.
The N, P and K were applied as Urea, triple superphosphate (TSP) and muriate of potash (MOP), respectively. Basal application (100%) of TSP was done at the time of planting. Urea and MOP were applied in 3 splits 30:35:35 at 4, 10 and 12 weeks after planting about 10-15cm away from each cutting. Weeding and earthing-up of ridges were observed throughout the experimental period, and the cassava crop was harvested after 12 months (in January) for each cropping season.
2.2 Physical and Chemical Properties of the Soil from the Experimental Site
Some physical and chemical properties of the soil from the experimental site were analyzed (Table 3). The physical analysis included soil constituents and textural class determination, while chemical analysis involved exchangeable Ca, K and Mg, available P, total N, OC and soil pH.
Table 3. Initial Physical and Chemical Characteristics of the Ukiriguru Experimental Soil
Soil Constituents |
Depth |
Rating* |
|
1-15cm |
15-30cm |
||
Sand (%) |
73 |
71 |
|
Silt (%) |
11 |
12 |
N/A |
Clay (%) |
16 |
17 |
N/A |
Textural class |
Sandy loam |
Sandy loam |
N/A |
pH (H2O) |
6.55 |
6.71 |
Slightly acidic to neutral |
Total N (%) |
0.04 |
0.03 |
Very low |
Organic C (%) |
0.46 |
0.32 |
Very low |
Bray I P (mg kg-1) |
8.00 |
9.00 |
Medium |
Exchangeable K (cmol kg-1) |
0.12 |
0.07 |
Very low |
Exchangeable Ca (cmol kg-1) |
1.84 |
1.54 |
Medium |
Exchangeable Mg (cmol kg-1) |
0.64 |
0.54 |
Medium |
Notes: * Source: Landon[25].
2.3 Soil Data Collection
Before starting the experiment, each composite soil sample from 0-15 and 15-30cm depth were collected from the experimental site for determining the soil texture, pH, OC, Total N, extractable P, exchangeable Ca, Mg and K. Soil texture was determined using the Bouyoucos hydrometer method as described by[26].
The pH measurements were done potentiometrically in water and in 1M KCl both at a ratio of 1:2.5, soil: water and soil: 1M KCl suspensions, respectively[27], and the pH meter was used to read the pH, soil samples were ground to pass through a 0.25mm sieve, then procedure by the Walkley and Black wet digestion method[28], was used to determine the organic carbon (OC). For the determination of total N, about 1g of soil samples was hydrolyzed with 15mL concentrated sulfuric acid (H2SO4)-hydrogen peroxide method for 2h. Then, total N was determined using the micro-Kjeldahl procedure[29]. Available P was determined by Bray and Kurtz method[30]. Exchangeable Ca and Mg in 1M neutral ammonium acetate (NH4OAc) filtrates were determined by atomic absorption spectrophotometer (AAS) and exchangeable K by flame photometer[31].
2.4 Plant Data Collection, Harvesting of Cassava Storage Roots
Five plants from each plot were selected randomly for plant data collection. Data on cassava parameters included plant height (PH) ‘measurement of the plant from the ground to the top-most portion of the plant at maturity’, stem diameter (SD) ‘measurement of stem base girth’, canopy length (CL) ‘measurement of the longest part of the canopy diameter’, canopy width (CW) ‘measurement of the shortest part of the canopy diameter’, canopy height (CH) ‘measurement from the lowest node of the stem or branch that had a green leaf to the highest leaf of the plant’, canopy volume (CV) ‘calculated from the CL, CW and CH parameters’, and weight of small and large roots. At cassava maturity (12 months after planting), CL, CH and CW were measured using a measuring tape, PH was measured from the soil surface (at the root collar of the plant) to the plant apex by a calibrated measuring tape. Stem diameter (SD) was measured using a vernier caliper. Finally, the weight of small and large storage roots was determined at 12.5% moisture content by weighing the roots on a digital scale after removing soil particles adhered to the roots.
2.5 Statistical Analyses
Data were analyzed using STATISTICA 8th Edition, StatSoft, Inc., Tulsa, OK, USA. Cassava storage root weights were evaluated based on the interactions among the treatments. One-way ANOVA statistical analyses were performed through the split-plot design with treatments being fertilizer rates, rainfed, or irrigated regime. A Fisher’s comparison test was used, and the significance threshold was set at P=0.05 and P=0.001 for high significance. The treatment means were compared to the standard error of the difference of the mean.
2.5.1 Regression and Correlation Assessment
Multiple linear regression was used to determine the parameter(s) that contribute significantly to root yields. Thus, total root yield as a response parameter (Y) was regressed against CH, CW, CL, CV, PH and SD in the following model:
Y = x1Wi + x2Wii+x3Wiii+x4Wiv+x5Wv+x6Wvi+x7Wvii + C (1)
Where Y is the total root yield, Wi to Wvii stand for parameters CH, CW, CL, CV, PH and SD, x1 to x7 represent coefficients of the parameters and C is the constant. A diagnostic test of coefficient of determination (R2) was used to predict the results perfectly.
Correlations of total root yield against CH, CW, CL, CV, PH and SD were carried out to determine the association impact of total root yield.
3 RESULTS
3.1 Weather Data, Physical and Chemical Properties of the Soil from the Experimental Site
Precipitation and atmospheric temperature data for the first season (2019-20) were an annual average of 89.37mm and 30.35oC respectively. During the cropping season, 2020-21 recorded average rainfall (86.28mm) and temperature (29.89oC) were slightly lower than in the previous season (Table 1).
The high rates of fertilizer (Table 2) were not in favour of significantly higher cassava root yields. Ukiriguru texture soil was categorized as a sandy loam (Table 3). Soil available P content ranged from 8 to 9mg kg-1, while exchangeable Ca ranged from 1.54 to 1.84cmol kg-1 and Mg from 0.54 to 0.64cmol kg-1, were considered as medium. Soil total N (0.03-0.04%), K (0.07-0.12cmol kg-1) and OC (0.32-0.46%) were very low. Soil pH was slightly acidic to neutral, ranging from 6.55 to 6.71 at the depth of 0-15 and 15-30cm respectively.
3.2 Effect of Fertilizer Treatments on Aerial Cassava Characteristics
The effects of growing cassava crop under fertilization to both rainfed and irrigated crop regimes on aerial cassava characteristics for 2019-20 and 2020-21 crop seasons are shown in Table 4 and Table 5. The absolute control (T1), which received no fertilizer, had significantly (P≤0.001) lowest SD (6.42-6.45cm), PH (137.50-137.70cm), CL (116.22-117.10cm), CW (85.12-85.86cm), CH (24.60-24.95cm), and CV (135.22-139.18cm3) than the rest of treatments to both cropping seasons.
With exception to the absolute control treatment (T1), application of different fertilizer rates, T2 (N150P40K180 i.e. 1,957, 1,195, 2,160g plot-1 for Urea, TSP and MOP, respectively), T5 (N300P80K360 i.e. 3,913, 2,390, 4,320g plot-1 for Urea, TSP and MOP, respectively) and T6 (N300P80K360 i.e. 7,826, 4,779, 8,640g plot-1 for Urea, TSP and MOP, respectively), did not significantly (P≤0.001) affect the SD, PH, CL, CW, CH, and CV during both cropping seasons (2019-20 and 2020-21). On the other hand, the application of half fertilizer rates N75P20K90 under rainfed (T4) had reduced stem diameter in both cropping seasons. However, the cassava plant height did not differ significantly (P≤0.001) to all fertilized treatments under the rainfed regime.
Table 4. Effect of Fertilizer on Cassava Above Ground Canopy for 2019-20 Cropping Season
Treatments |
Cropping Season 2019-20 |
|||||
Canopy Length (cm) |
Canopy Width (cm) |
Canopy Height (cm) |
Canopy Volume (cm3) |
Stem Diameter (mm) |
Plant Height (cm) |
|
1. Absolute control no fertilizer-Rainfed |
117.10±9.30c |
85.86±8.34b |
24.95±2.68b |
139.18±37.67b |
6.45±0.20c |
137.70±7.53b |
2. Standard fertilizer rate N150P40K180-Rainfed |
190.05±19.25ab |
147.30±18.41a |
32.80±2.71ab |
515.12±124.30a |
10.25±0.22a |
185.25±4.58a |
3. Half fertilizer rate N75P20K90-Irrigated |
163.55±9.90b |
124.30±9.33a |
36.30±3.61a |
396.15±66.05a |
10.45±4.57a |
174.75±8.03a |
4. Half fertilizer rate N75P20K90-Rainfed |
161.50±9.76b |
123.37±8.82a |
34.65±3.69a |
375.43±60.60a |
9.80±0.42a |
173.57±7.64a |
5. Double fertilizer rate N300P80K360-Irrigated |
188.75±11.77ab |
130.60±9.28a |
36.75±3.59a |
482.27±91.33a |
10.35±0.22a |
182.65±6.03a |
6. Double fertilizer rate N300P80K360-Rainfed |
214.95±17.62a |
138.45±2.55a |
36.45±2.34a |
569.08±63.59a |
9.90±0.52ab |
184.65 ±11.35a |
F-statistics |
6.15*** |
4.05** |
2.05ns |
3.74* |
15.19*** |
5.32** |
Notes: a, b, and c means in the same category of evaluated interface sharing similar letter(s) do not differ significantly based on their respective standard error (SE) at 5% error rate. Values presented are means ± SE (standard error of means); *P<0.05, **P<0.01, ***P<0.001; ns = non-significant (P≥0.05)
Table 5. Effect of Fertilizer on Cassava Above Ground Canopy for 2020-21 Cropping Season
Treatments |
Cropping Season 2020-21 |
|||||
Canopy Length (cm) |
Canopy Width (cm) |
Canopy Height (cm) |
Canopy Volume (cm3) |
Stem Diameter (mm) |
Plant Height (cm) |
|
1. Absolute control no fertilizer-Rainfed |
116.22±9.4c |
85.12±8.55b |
24.60±2.62b |
135.22±36.65b |
6.42±0.22c |
137.50±7.69b |
2. Standard fertilizer rate N150P40K180-Rainfed |
189.00±9.24ab |
145.85±18.42a |
32.30±2.56ab |
499.55±120.35a |
10.12±0.20a |
184.65±4.44a |
3. Half fertilizer rate N75P20K90-Irrigated |
162.40±10.06b |
122.75±9.48a |
35.77±3.69a |
382.44±63.88a |
10.37±0.64a |
174.47±8.01a |
4. Half fertilizer rate N75P20K90-Rainfed |
158.75±9.38b |
122.22±9.42a |
35.00±4.12a |
370.48±64.61a |
9.95±0.65a |
173.20±7.52a |
5. Double fertilizer rate N300P80K360-Irrigated |
187.72±11.79ab |
129.47±9.35a |
35.92±3.54a |
465.96±90.35a |
10.20±0.19a |
182.27±6.08a |
6. Double fertilizer rate N300P80K360-Rainfed |
212.37±17.01a |
137.40±2.69a |
36.07±2.34a |
552.21±61.45a |
9.75±0.42ab |
183.97 ±11.25a |
F-statistics |
6.17*** |
3.85** |
1.94ns |
3.62* |
11.80*** |
5.29** |
Notes: a, b, and c means in the same category of evaluated interface sharing similar letter(s) do not differ significantly based on their respective standard error (SE) at 5% error rate. Values presented are means ± SE (standard error of means); *P<0.05, **P<0.01, ***P<0.001; ns = non-significant (P≥0.05).
3.3 Effect of Fertilizer Treatments on Cassava Storage Root Yields
The effects of growing cassava crop under fertilization to both rainfed and irrigated crop regimes for 2019-20 and 2020-21 crop seasons on storage root yield are shown in Table 6. The lowest significant (P≤0.001) root storage yields were 11.20, and 11.09t ha-1 obtained in absolute control (T1) for 2019-20 and 2020-21 cropping seasons, respectively. Application of N150P40K180 under the rainfed regime (T2) increased root storage to 16.82 and 16.57t ha-1 in 2019-20 and 2020-21 respectively. The N75P20K90 treatment (T3) under the irrigated regime, had significantly highest (P≤0.001) yields with 25.21 and 24.76t ha-1 for the cropping seasons, respectively.
The T4 treatment (N75P20K90) had a decreased yield, though not significant, of 24.59 and 24.14t ha-1 in 2019-20 and 2020-21 cropping seasons, respectively. Doubling the fertilizer (N300P80K360) under irrigation regime (T5) produced 20.66 to 20.75t ha-1 storage yield for both cropping seasons, which did not differ significantly from those of T3 and T4. However, a similar fertilizer treatment but under a rainfed regime (T6), had a substantial increase in root storage yield (21.64-21.87t ha-1) in spite of insignificant difference with T3, T4 and T5.
Table 6. Effect of Fertilizer on Cassava Below Ground Root Yields for 2019-20 and 2020-21 Cropping Seasons
Treatments |
Crop Season 2019-20 |
Crop Season 2020-21 |
||||
Large storage roots yield (t ha-1) |
Small storage roots yield (t ha-1) |
Overall root storage yield (t ha-1) |
Large storage roots yield (t ha-1) |
Small storage roots yield (t ha-1) |
Overall root storage yield (t ha-1) |
|
1. Absolute control no fertilizer-Rainfed |
5.75±0.45c |
5.44±0.70c |
11.20±0.87c |
5.66±0.41c |
5.43±0.61c |
11.09±0.75c |
2. Standard fertilizer rate N150P40K180-Rainfed |
9.30±0.73abc |
7.52±1.04bc |
16.82±0.35bc |
9.22±0.74abc |
7.34±1.04bc |
16.57±0.34bc |
3. Half fertilizer rate N75P20K90-Irrigated |
13.12±2.17a |
12.08±1.77a |
25.21±3.24a |
12.91±2.16a |
11.84±1.76a |
24.76±3.19a |
4. Half fertilizer rate N75P20K90-Rainfed |
13.04±2.02a |
11.54±1.86a |
24.59±3.24a |
12.68±2.04a |
11.46±1.72a |
24.14±3.02a |
5. Double fertilizer rate N300P80K360-Irrigated |
11.67±1.15ab |
9.07±0.72abc |
20.75±1.31ab |
11.57±1.08ab |
9.08±0.64abc |
20.66±1.07ab |
6. Double fertilizer rate N300P80K360-Rainfed |
11.42±1.15ab |
10.45±0.38ab |
21.87±0.91ab |
11.30±1.09ab |
10.34±0.36ab |
21.64±0.97ab |
F-statistics |
3.86** |
4.35** |
6.85*** |
3.74** |
4.61*** |
7.23*** |
Notes: a, b, and c means in the same category of evaluated interface sharing similar letter(s) do not differ significantly based on their respective standard error (SE) at 5% error rate. Values presented are means ± SE (standard error of means);*P<0.05, **P<0.01, ***P<0.001; ns = non-significant (P≥0.05).
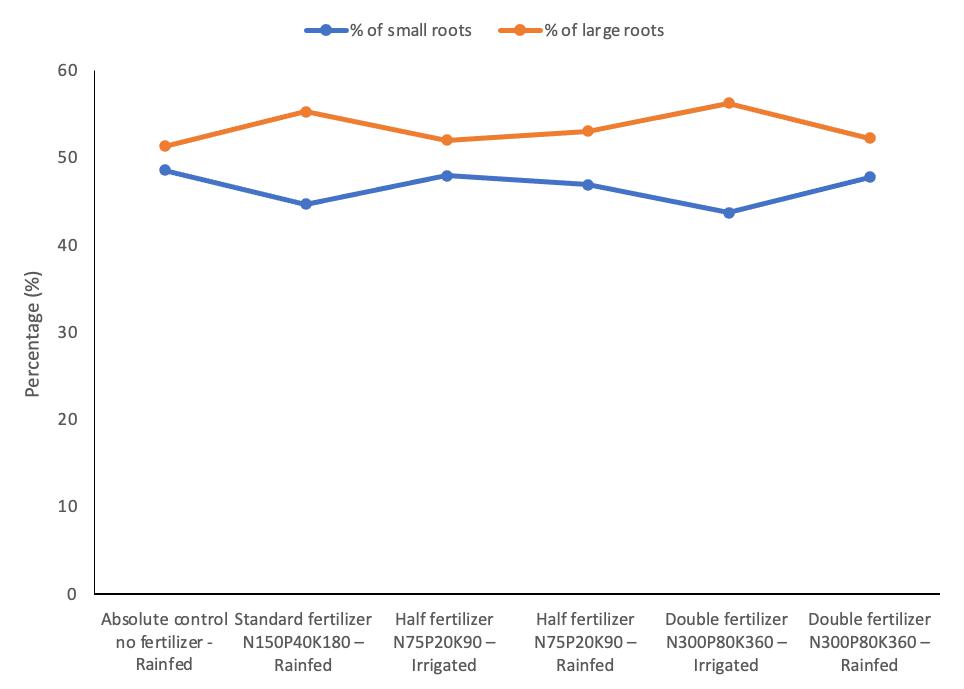
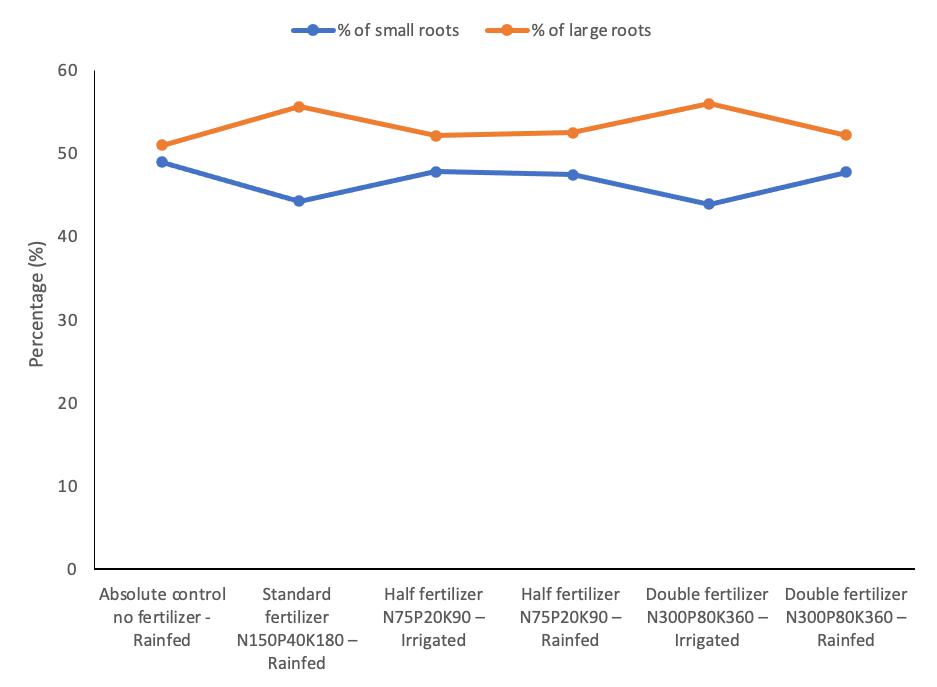
3.4 Effect of Fertilizer on the Root Size Distributions
The effect of fertilization on the distribution percentage of root size under both rainfed and irrigated crop regimes for the 2019-20 and 2020-21 crop seasons is shown in Figure 1 and Figure 2. The absolute control under the rainfed regime (T1) had small roots ranging from 48.57 to 48.96% and large roots ranging from 51.04 to 51.34%. Applying fertilizer at N150P40K180 (T2) resulted in a broader gap of root size due to a decrease in the small roots ranging from 44.29 to 44.70% and an increase in large roots by ranging from 55.29 to 55.64% in both cropping seasons. The N75P20K90 fertilizer application under the irrigated regime (T3) narrowed the gap between small roots, which ranged from 47.82 to 47.92%, and large roots that ranged from 52.04 to 52.14%. However, the same fertilizer treatment rate under the rainfed regime (T4) lowered slightly the percentage of small roots distribution ranging from 46.92 to 47.47%, but increased large roots distribution ranging from 52.53 to 53.03% in both cropping seasons compared to T3.
Applying N300P80K360 fertilizer under the irrigated regime (T5) significantly widened the distribution gap between small and large roots. This treatment had the lowest small roots distribution compared to the rest of the treatments by ranging from 43.71 to 43.94%, and the highest large roots distribution ranging from 56.00 to 56.24% for both cropping seasons. On the other hand, the treatment (T6), which had a similar application rate as T5 but under the rainfed regime, had an increased small roots distribution by 47% compared with T5, but decreased large roots distribution by 52.22%.
|
Figure 1. Small and large roots distribution size for 2019-20 cropping season.
|
Figure 2. Small and large roots distribution size for 2020-21 cropping season.
3.5 Association between Total Root Storage Yield and Leaf Canopy, Stem Diameter and Plant Height
Multiple linear regression results (Table 7) generated based on a regression model with total root storage yield as a response variable (Y) and the fitted constants CV, CL, CW, CH, PH and SD such that:
Y = 2.63SD+0.16PH+0.10CH-0.03CW-0.03CL-0.01CV-18.91 (2)
The coefficient of determination (R2) was 0.85. The model indicated that for every unit increase of SD, PH and CH, total root yield increased by 2.36, 0.16 and 0.1% respectively. But, with the same unit increase in CW, CL and CV the amount of total root yield would decrease by 0.03, 0.03 and 0.01% respectively.
Table 7. Multiple Linear Regression Analysis of Root Yield Versus Canopy, Stem Diameter and Plant Height
Parameter |
Estimate |
Standard Error |
T-statistics |
P-value |
Constant (C) |
-18.91 |
13.37 |
-1.41 |
0.17 |
Canopy width (CW) |
-0.03 |
0.09 |
-0.32 |
0.75 |
Canopy length (CL) |
-0.03 |
0.07 |
-0.40 |
0.69 |
Canopy height (CH) |
0.10 |
0.25 |
0.39 |
0.69 |
Canopy volume (CV) |
-0.01 |
0.02 |
-0.67 |
0.51 |
Stem diameter (SD) |
2.36 |
1.03 |
2.30 |
0.03 |
Plant height (PH) |
0.16 |
0.08 |
1.90 |
0.07 |
Model: Total Root Yield (Y)=2.36SD+0.16PH+0.10CH-0.03CW-0.03CL-0.01CV-18.91 |
||||
The correlation analysis (Table 8) indicates that SD (r=0.70), and PH (r=0.63) had a positive and significant relationship with total root yields. In addition, there was positive relationship between CV and CL (r=0.91), SD and PH (r=0.83).
Table 8. Correlations between Total Root Yields and Leaf Canopy, Stem Diameter and Plant Height
Measured Variables and Their Correlations |
||||||||
|
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
1 |
Total root yield |
1 |
|
|
|
|
|
|
2 |
Canopy width (CW) |
0.14 |
1 |
|
|
|
|
|
3 |
Canopy length (CL) |
0.16 |
0.85 |
1 |
|
|
|
|
4 |
Canopy height (CH) |
0.35 |
0.49 |
0.53 |
1 |
|
|
|
5 |
Canopy volume (CV) |
0.17 |
0.89 |
0.91 |
0.73 |
1 |
|
|
6 |
Stem diameter (SD) |
0.70 |
0.63 |
0.59 |
0.60 |
0.64 |
1 |
|
7 |
Plant height (PH) |
0.63 |
0.64 |
0.73 |
0.59 |
0.71 |
0.83 |
1 |
4 DISCUSSIONS
The soil of Ukiriguru site was slightly acidic to the depth of 0-15cm to neutral from 15 to 30cm and had sandy loam texture which is categorized as Arenosols[32,33]. The soil had the low OC (Table 3) reflecting low organic matter resulting from high temperature (Table 1) that enhances rapid decomposition of organic matter and hence leaching of minerals[32]. As a result, the N and K levels are low while P and Ca are at medium range.
With exception to absolute control treatment, Kiroba cassava variety, aerial vegetative parts did not differ significantly, and were better in 2019-20 cropping season compared to 2020-21 cropping season as a result of favourable precipitation and atmospheric temperature (Table 1). Higher fertilizer rates (Table 2) favoured but not significant the PH, CH and CW indicating that varying fertilizer did not influence significant change of these vegetative characteristics for Kiroba variety used in this study. These results suggest that aerial parts of Kiroba variety did not differ significantly upon fertilization. A research study by Mwamba et al.[34] also observed similar results for the aerial cassava varieties to have no significant changes upon application of fertilizer.
The lowest significant (P≤0.001) SD, PH, CL, CW, CH, CV and total root yields observed in absolute control treatment (Table 4, Table 5, and Table 6), was a result of very low inherent soil N and K nutrients. Application of N150P40K180 fertilizer influenced good results on cassava aerial vegetative parts and storage root yields due to good fertilizer response following inherent low soil N and K (Table 3). Doubling the fertilizer blending (N300P80K360) also increased root storage yields under the irrigated regime. However, the same treatment under the rainfed regime slightly improved the root storage yields, indicating that supplementation of N, P and K improved soil fertility and favoured cassava root yield increase.
The lowest N75P20K90 application under irrigated regime improved significantly (P≤0.001) the cassava root yields indicating that enhancing the N, P and K balance contributes to high root yields. However, the rainfed N75P20K90 treatment had a slightly lower but insignificant root yield than irrigated crop. This is due to the nature of the sandy loam textural class that may have an adverse effect on soil water retention and storage capacity which could lead to water stress. The increase of root storage yields for irrigated and rainfed N75P20K90 treatments could also be related to the CH as these treatments had significant CH. A research study by de Oliveira et al.[35] also observed that the vegetative canopy architecture has a direct influence on the growth and yield of cassava crops. Furthermore, it indicates that the cassava crop requires less nutrients for balancing required nutrients for high root storage yields, as these treatments were applied half blend of the fertilizer. However, the irrigated cassava crop was observed to have increased potential of root storage yields than rainfed crop. Other studies also supported that application of balanced N, P and K in the presence of adequate soil moisture is of paramount importance to the cassava root yield increase[14,15].
The cassava crop grown in unfertilized soil had the highest proportion of small roots reaching the maximum of 48.96% and accounted for the total low root yields. Application of NPK fertilizer under the irrigated regime lowered small storage root proportions and favoured an increase of large storage root proportions. However, as fertilizer rates and levels increased under the rainfed regime, it favoured more increase of small roots proportions and decreased the large root proportions (Figure 1 and Figure 2). The applied higher (double) fertilizer levels, could be associated with unbalancing of NPK nutrients, thus, did not influence proper roots distributions to enhance the increase of storage root yields. Adiele et al.[36] observed that the cassava root growth and storage yield, require a balanced supply of N and P and moderate K. On the other hand, cassava yield loss was mainly be caused by the low soil fertility and suboptimal management practices by farmers[37].
The significantly higher storage root yields to both cropping seasons were observed to the half blend (N75P20K90) treatments under the irrigated and rainfed regimes, respectively. This indicates that cassava crops require a balancing state of nutrients, and the irrigation has potential for the timely spreading and uptake of nutrients due to soil sufficient moisture as a major factor associated with root storage yield increase. The narrow gaps between the cassava small and large roots distribution were mainly observed in these treatments and hence associated with significantly high storage root yields.
Therefore, narrowing the gap between the small and large cassava roots proportion is a key determinant for the significantly higher root storage yield associated with balancing NPK nutrients and adequate soil moisture. Furthermore, these treatments had large stem diameter, canopy height and plant height (Table 4 and Table 5) that contributed significantly to the total root yields (Table 7). Thus, SD (r=0.70) and PH (r=0.63) correlated significantly to the total root yields (Table 8). Our results are almost similar to the results obtained by Yonis et al.[38], who observed that roots with a large size were generally heavier and reflected high root storage yields than small and roots and hence lighter in weight. The increase in cassava storage yields is influenced significantly by inappropriate nutrient balance[35]. Furthermore, water deficit affects the development of cassava roots and, consequently reduces root storage yields[39].
The current study reveals that the potential yields of cassava crops could be achieved using fertilizer blend within N50P10K70 and N100P30K120. Further research is required to review the fertilizer blends while balancing the N, P, K as the potential yields may increase beyond 25.21t ha-1. Our findings suggest that balancing soil moisture and nutrients is critical for high cassava root storage yields as it narrows the gap between the small and large roots proportions.
5 CONCLUSION
Cassava crops require a less fertile soil with balanced N, P and K nutrients to achieve significant yields. In addition, the nutrients supplied through fertilizer require adequate moisture as significant factor associated with root storage yield increase. These factors, contribute significantly to the narrow gap between the small and large roots that determine the significant higher root storage yield and increased cassava yield ranging from 24.14 to 25.21t ha-1. However, the current study pinpointed the potential for further research using fertilizer blends within N50P10K70 and N100P30K120 to identify the best fertilizer blend to increase storage root yield beyond 25.21t ha-1.
Acknowledgements
The authors acknowledge the Tanzania Agricultural Research Institute (TARI), Ukiriguru, Mwanza, Tanzania for funding the research trial, and the Tobacco Research Institute of Tanzania (TORITA), Tumbi, Tabora, Tanzania for the provision of researchers and technical staff to prepare this manuscript jointly with TARI.
Conflicts of Interest
The authors declared no conflict of interest regarding this paper publication.
Author Contribution
Mlay DP designed the study, supervised field officers, collected data, wrote the article, revised the article; Lisuma JB performed the statistical analysis, wrote the article, revised the article. All authors contributed equally to the manuscript and approved the final version.
Abbreviation List
CH, Canopy height
CL, Canopy length
cmol, Centimole
CV, Canopy volume
CW, Canopy width
ha, Hectare
K, Potassium
MOP, Muriate of potash
N, Nitrogen
OC, Organic carbon
PH, Plant height
P, Phosphorus
SD, Stem Diameter
t, tone
TARI: Tanzania Agricultural Research Institute
TSP, Triple superphosphate
[1] Chongela J. Contribution of agriculture sector to the Tanzanian economy. Am J Res Commun, 2015; 3: 57-70.
[3] Lazaro E, Lisasi H, Assenga A. Proceedings of the 13th ISTRC Symposium: Cassava in Tanzania: A famine or commercial reserve, Morogoro, Tanzania, 2007.
[4] Derksen-Schrock K. Anderson CL, Gugerty MK. Tanzania: agricultural sector overview. Gates Open Res, 2019; 3: 1376. DOI: 10.21955/gatesopenres.1116266.1
[5] Mtunguja MK, Beckles DM, Laswai HS et al. Opportunities to commercialize cassava production for poverty alleviation and improved food security in Tanzania. Afr J Food, Agr Nutr Dev, 2019; 19: 13928-13946. DOI: 10.18697/ajfand.84.BLFB1037
[6] Cock JH, Connor DJ. Cassava. In Crop Physiology Case Histories for Major Crops. Sadras VO, Calderini D ed. Academic Press: London, UK, 2021; 588-633. DOI: 10.1016/B978-0-12-819194-1.00019-0
[7] Reincke K, Vilvert E, Fasse A et al. Key factors influencing food security of smallholder farmers in Tanzania and the role of cassava as a strategic crop. Food Secur, 2018; 10: 911-924. DOI: 10.1007/s12571-018-0814-3
[8] Purnamasari RA, Ahamed T, Noguchi R. Land suitability assessment for cassava production in Indonesia using GIS, remote sensing and multi-criteria analysis. Asia-Pac J Region Sci, 2019; 3: 1-32. DOI: 10.1007/s41685-018-0079-z
[9] Jacobson A, Duffy S, Sseruwagi P. Whitefly-transmitted viruses threatening cassava production in Africa. Curr Opin Virol, 2018; 33: 167-176. DOI: 10.1016/j.coviro.2018.08.016
[10] Imakumbili ML, Semu E, Semoka JM et al. Soil nutrient adequacy for optimal cassava growth, implications on cyanogenic glucoside production: A case of konzo-affected Mtwara region, Tanzania. PLoS One, 2019; 14: 0216708. DOI: 10.1371/journal.pone.0216708
[11] Maggidi I. Cassava value chain: willingness to pay for improved cassava planting material in coastal and Lake Victoria areas of Tanzania [Masters Dissertation]. Morogoro, Tanzania: Sokoine University of Agriculture; 2019.
[12] Merumba MS, Msanya BM, Semu E et al. Pedological Characterization and Suitability Assessment for Cassava Production in Bukoba, Missenyi and Biharamulo Districts, Tanzania. Am J Agri Forest, 2020; 8: 144-166. DOI: 10.11648/j.ajaf.20200804.18
[13] Howeler RH. Sustainable soil and crop management of cassava in Asia: A Reference Manual. CIAT Publication: Cali, Colombia, 2014.
[14] Senkoro CJ, Tetteh FM, Kibunja CN et al. Cassava yield and economic response to fertilizer in Tanzania, Kenya and Ghana. Agron J, 2018; 110: 1600-1606. DOI: 10.2134/agronj2018.01.0019
[15] Byju G, Suja G. Mineral nutrition of cassava. Adv Agron, 2020; 159, 169-235. DOI: 10.1016/bs.agron.2019.08.005
[16] Chua MF, Youbee L, Oudthachit S et al. Potassium fertilisation is required to sustain cassava yield and soil fertility. Agron, 2020; 10: 1103. DOI: 10.3390/agronomy10081103
[17] Venegas RAP, Lee SJ, Thuita M et al. The phosphate inhibition paradigm: host and fungal genotypes determine arbuscular mycorrhizal fungal colonization and responsiveness to inoculation in cassava with increasing phosphorus supply. Front Plant Sci, 2021; 12: 693037. DOI: 10.3389/fpls.2021.693037
[18] Fernandes AM, Gazola B, Nunes JGDS et al. Yield and nutritional requirements of cassava in response to potassium fertilizer in the second cycle. J Plant Nutr, 2017; 40: 2785-2796. DOI: 10.1080/01904167.2017.1382520
[19] Xu G, Wolf S, Kafkafi U. Ammonium on potassium interaction in sweet pepper. J Plant Nutr, 2002; 25: 719-34. DOI: 10.1081/PLN-120002954
[20] Kimberly A, Lema B, Willis I et al. Corals Form Characteristic Association with Symbiotic Nitrogen Fixing Bacteria. Appl Environ Microb, 2012; 8: 3136-3144. DOI: 10.1128/AEM.07800-11
[21] Ceballos I, Mateus ID, Peña R et al. Using variation in arbuscular mycorrhizal fungi to drive the productivity of the food security crop cassava. BioRxiv, 2019; 830547. DOI: 10.1101/830547
[22] Marzouk N, Hassan N, Fawzy Z et al. Cassava cultivars response to different levels of potassium fertilization under drip irrigation and sandy soil conditions. Egypt J Soil Sci, 2020; 60: 317-334. DOI: 10.21608/ejss.2020.34054.1367
[23] Pushpalatha R, Gangadharan B. Assessing the influence of climate model biases in predicting yield and irrigation requirement of cassava. Model Earth Syst Environ, 2021; 7: 307-315. DOI: 10.1007/s40808-020-01038-8
[24] Derksen-Schrock K, Anderson CL, Gugerty MK. Tanzania: agricultural sector overview. Gates Open Res, 2019; 3: 1376.
[25] Landon JR. Booker Tropical Soil Manual: A hand book for soil survey and agricultural land evaluation in the tropics and subtropics. Longman Scientific & Technical: Michigan, USA, 1991.
[26] Gee GW, Bauder JW. Particle-size analysis. In: Methods of Soil Analysis, Part 1. Klute A ed. Agronomy Monograph 9, American Society of Agronomy, Madison Wisconsin: Madison, USA, 1986; 363-375.
[27] Thomas GW. Soil pH and soil acidity. In: Methods of Soil Analysis, Part 3: Chemical Methods. Sparks DL, Page AL, Helmke PA et al. ed. American Society of Agronomy: Madison, USA, 1996; 475-490.
[28] Nelson DW, Sommers EL. Total carbon, organic carbon, and organic matter. In: Methods of soil analysis Part 3: Chemical Methods. Sparks DL ed. American Society of Agronomy: Madison, USA, 1996; 961-1010.
[29] Bremner JM. Nitrogen-Total. In: Methods of Soil Analysis Part 3: Chemical Methods. Sparks DL, Page AL, Helmke PA et al. ed. American Society of Agronomy: Madison, USA, 1996; 1085-1122. DOI: 10.2136/sssabookser5.3.c37
[30] Kuo S. Phosphorous. In: Methods of Soil Analysis Part 3: Chemical Methods. Sparks DL, Page AL, Helmke PA et al. ed. American Society of Agronomy: Madison, USA, 1996; 869-920.
[31] Sumner ME, Miller WP. Cation exchange capacity and exchange coefficients. In: Methods of Soil Analysis Part 3: Chemical Methods. Sparks DL, Page AL, Helmke PA et al. ed. American Society of Agronomy: Madison, USA, 1996; 1201-1230.
[32] Ley G. Towards Integrated Soil Fertility Management in Tanzania: Developing Farmers' Options and Responsive Policies in the Context of Prevailing Agro-ecological, Socio-economic and Institutional Conditions. Royal Tropical Institute, KIT Publishers: Madison, USA, 2002.
[33] FAO. FAO-UNESCO Soil map of the world. Accessed 2022. Available at https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/faounesco-soil-map-of-the-world/en
[34] Mwamba S, Kaluba P, Moualeu-Ngangue D et al. Physiological and Morphological Responses of Cassava Genotypes to Fertilization Regimes in Chromi-Haplic Acrisols Soils. Agron, 2021; 11: 1757. DOI: 10.3390/agronomy11091757
[35] de Oliveira EC, de Almeida LHC, Zucareli C et al. Analysis of cassava growth at different harvest times and planting densities. Semin: Ciências Agrárias, 2019; 40: 113-126. DOI: 10.5433/1679-0359.2019v40n1p113
[36] Adiele JG, Schut AGT, Ezui KS et al. Dynamics of NPK demand and uptake in cassava. Agron Sustain Dev, 2021; 41: 1-14. DOI: 10.1007/s13593-020-00649-w
[37] Kintché K, Hauser S, Mahungu NM et al. Cassava yield loss in farmer fields was mainly caused by low soil fertility and suboptimal management practices in two provinces of the Democratic Republic of Congo. Eur J Agron, 2017; 89: 107-123. DOI: 10.1016/j.eja.2017.06.011
[38] Yonis BO, Del Carpio DP, Wolfe M et al. Improving root characterisation for genomic prediction in cassava. Sci Rep, 2020; 10: 1-12. DOI: 10.1038/s41598-020-64963-9
[39] Adu MO. Causal shoot and root system traits to variability and plasticity in juvenile cassava (Manihot esculenta Crantz) plants in response to reduced soil moisture. Physiol Mol Biol Pla, 2020; 26: 1799-1814. DOI: 10.1007/s12298-020-00865-4
Copyright © 2022 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.





 Copyright ©
Copyright ©