Role of Microorganism in Agricultural Sustainability
Haleema Tariq1*
1Department of Plant Sciences, McGill University, Montreal, Quebec, Canada
*Correspondence to: Haleema Tariq, PhD, Department of Plant Science, McGill University, Macdonald Campus, 21111 Lakeshore Road, Sainte-Anne-de-Bellevue, Québec H9X 3V9, Canada; Email: haleema.tariq@mail.mcgill.ca
Abstract
Agriculture sustainability is the hot topic nowadays because of vigorous growing population of the world. In order to increase the yield of crops researcher work a lot on chemicals, to meet the nutrition demand of plants and cope the biotic stress, which exert pressure on economy and environmental health. Future food security need to be achieved without damaging environment, and this lead to the use of biological tools. Microbes play a vital role in achieving the food sustainability by increasing the yield of crops using their specific biochemical signaling and interaction with plants and increasing the overall yield of plants. Biological nitrogen fixation and nutrient solubilization and mobilization, phytohormone production are the most important functions of microorganism. The phytomicrobiome act as a biocontrol and save the plant from biotic stress, alter physiological and biochemical activity of plants in case of abiotic stress for its survival and growth. The holobiont that includes plants and its phytomicrobiome is taking part in agricultural sustainability and thriving to meet the demands of world in present environmental challenges using sustainable mechanisms in limited resources. The current review was therefore, carried out to document the current advances in the role of plant growth promoting rhizobacteria (PGPR) in biological nitrogen fixation, phytohormone production, solubilization of the insoluble nutrients and their role in mitigating the adverse effects of biotic and abioic stresses in plants.
Keywords: nitrogen fixation, phosphate solubilization, phytohormones, iron sequestration, potassium solubilization
1 INTRODUCTION
Food security is one of the most important challenges nowadays because of vigorous growth of world population. Agricultural sustainability is necessary to overcome hunger and poverty among the 7 billion people of the world[1]. Climate change is also the major factor which negatively impacts the agricultural yield. To overcome the global shortage of food, “Green Revolution” of 20th century increased the food production using chemical inputs, breeding and genetic manipulation of plants. This innovative idea puts a huge burden on the economy and also results in environmental damage[2] At the end of this century the world’s population will increase from 7 billion to 12 billion, so the agricultural yield must increase more than 50%[3]. Future food security is necessary but the environmental damages need to be avoided and pressure on the overall economy should need to be reduced. Climate changes cause the reduction in the yield of major crops (3.8% and 5.5% reduction in yield of maize and wheat), and the major climate changes include high temperature and other abiotic factors which are adversely affecting the agriculture system[4]. The idea of “Fresh Green Revolution” emerge out with Bio-revolution that involves the use of phytomicrobiome (use of microbial inoculants, use of microbial compounds etc.) and improved the agricultural yield through manipulation of phytomicrobial community[5]. The soil near the roots (rhizosphere), the root surface (rhizoplane) and the space between plant cells (endosphere) have specialized community of microbes that affect the plant growth, crop yield, phytoremediation, carbon sequesterization, help to resist the biotic and abiotic stress. Beneficial microbes including plant growth promoting rhizobacteria (PGPR) enhance plant growth through nutrient absorbtion, produce phytohormone, produce antibiotics and mitigate biotic stress. Since several decades there has been a lot of research on signal compounds that trigger and control the association between plants and microbes[6]. The plant host and its associated phytomicrobiome is known as “holobiont”[7]. Several studies have been taken place since many decades in order to achieve the signaling between phytomicribiomes and host plants in order to deploy these microbes in place of chemical fertilizers to achieve the agricultural sustainability. For instance, plants releases the flavonoids in response to this microbial strains releases the lipo-chitooligosaccharides (LCOs) which consists of receptors that have kinase activity and result in variation in phytohormone profile of roots and help in root development; this use of microbial strains will eliminate the use of chemical fertilizers.
The present study aims to elaborate plant-microbe interaction and role of PGPR as beneficial microbes and their role in agricultural sustainability in the present era of climate change.
2 BENEFICIAL MICROBES ASSIST PLANT HOLOBIONT ACQUIRE NUTRIENTS
Plants always heavily rely on beneficial microbes to assist in fulfilling their nutrient requirements. The most important tasks performed by microbes in nutrient acquisition of plants are 1) biological nitrogen fixation, 2) solubilization of insoluble nutrients, 3) increase of root surface area.
3 BIOLOGICAL NITROGEN FIXATION
Biological nitrogen fixation in agriculture systems ranges from 40 to 70Tg N y-1 which is approximately 50% of global production of N fertilizers[8]. Nitrogen is one of the most important nutrients of plants and an integral part of important physiological processes including photosynthesis and protein synthesis. Nitrogen has low reactivity because of triple bond between its two atoms and exists as dinitrogen in nature, which is unavailable to plants directly[9]. The most of the members of phytomicrobiome has ability to fix atmospheric nitrogen in plants through legume rhizobium interactions or assist the nitrogen fixers by secreting various components[10]. Nitrogen fixers are categorized as symbionts and free living nitrogen fixers. To develop the symbiosis between plants (legumes) and rhizobia, cascades of signal are involved which lead to the formation of nodules. The initial signal is from plant sides that involve the root exudates which include flavonoids, amino acids and organic acids. The flavonoids from plants trigger the nod gene induction, result in production of lipochitin-oligosaccharides which binds to the plant receptor kinases, and contribute to calcium spiking in root hair and root hair start curling and trap rhizobia[11]. Plant forms the tubular structure known as infection threads which mature into nodule tissue. The plants take up the bacteria through endocytosis and are surrounded by peribacteriod membrane (PBM), fill the plant cell cytoplasm and mature into bacteriod and metabolize the atmospheric nitrogen to ammonia[12,13]. Nitrogenase enzyme is highly sensitive to oxygen, O2 inhibit the activity of nitrogenase in aerobic conditions and prevent the fixation and assimilation of nitrogen[14]. For example, many diazotroph bacteria fix N2 only in aerobic and microaerophilic conditions. They have specialized structures to achieve the nitrogen fixation by producing cyst for nitrogen fixation and prevent the entry of O2 (Nostoc and Anabaena), while some have temporal separation between N2 production and O2 production (Non-hetrocyst cyanobacteria)[15]. The free living nitrogen fixers (Azospirillum, and Azotobacter) require the considerable amount of energy (16ATP) for reducing N2 to NH3, and this energy currency comes from the oxidation of photosynthetic sugars or non-photosynthetic bacteria that rely on other organism. To reduce the economy pressure and environmental damages, it is imperative to clarify the mechanism of nitrogen fixation through microorganism at commercial level and studies show that the deployment of nitrogen fixing bacteria plays a significant role in the future food security. BNF fixes 122 million tons of N per year among which 55 to 60 million tons is fixed in agricultural crops to achieve the sustainability[16].
4 PGPR PRODUCE PHYTOHORMONES
Plant hormones or phytohormones or PGR are chemical molecules produced by plants and have critical roles in regulating plant growth, serving as chemical messengers that modulate many cellular processes in plants[17]. Phytohormones develop root systems for better nutrient uptake, development of vascular tissue, shoot elongation and flowering. Microbe produces phytohormone auxins, and cytokinins regulate phytohormone levels of plants and promote the plant growth[18]. The plant respond to phytohormone produced by microorganism present in rhizosphere or supplement externally and affect the plant growth, cell enlargement, cell division and cell proliferation[19].
Indole acetic acid (IAA) controls many stages of plant growth including cell division, cell elongation, tissue differentiation and apical dominance[20]. IAA is produced by many plant growth-promoting microbes, develops the roots of plants and helps to take plant nutrients easily and promotes its growth[21]. IAA produced by phytomicroboime helps the plants to develop its adventitious root system, increases root weight and increases the surface area of roots for better uptake of nutrients from pool[22]. Cytokinin is another class of phytohormones produced by microorganism, and stimulates the cell division, enhances root development and enhances root formation, shoot initiation and inhibits root elongation in plants[23,24].
Ethylene is a gaseous phytohormone produced with plants in extremely low concentration in plants in biotic and abiotic stresses, and increases tolerance in plants and causes senescence[25]. PGPR produces ACC deaminase which lowers the ethylene level and promotes plant growth. Abscisic acid (ABA) is a stress hormone that helps the plant to promote adaptation against stress and regulate plant growth[26]. Various PGPR produce phytohormones, as listed in Table 1. Under drought stress ABA acts as anti-transpirant and stomatal closure. Gibberellins play an important role in seed dormancy and stimulate plant growth under abiotic stress. Plants root are colonized by microrganisms and take nutrients from root exudates and in turn produce phytohormones (auxins, cytokinins, gibberellins, and ABA) and assist growth and development of plants[27].
Table 1. PGPR Produces Phytohormones
Microbes |
Phytohormone |
Crop |
Ref |
Kocuria turfanensis |
IAA |
Suaeda fruticosa |
[19] |
Pseudomonas putida |
IAA |
Brassica nigra |
[28] |
Promicromonospora sp. |
GA |
Solanumlycopersicum |
[29] |
Azotobactersalinestris |
GA |
Sorghum |
[30] |
Bacillus subtilis |
Cytokinin |
Platycladus orientalis |
[31] |
Pseudomonas lini & Serratia plymuthica |
Affect ABA level |
Ziziphus jujuba |
[32] |
Glutamicibacter sp |
Regulate ACC activity & ethylene production |
Oryza sativa |
[33] |
5 SOLUBILIZATION OF INSOLUBLE NUTRIENTS
5.1 Phosphate Solubilization
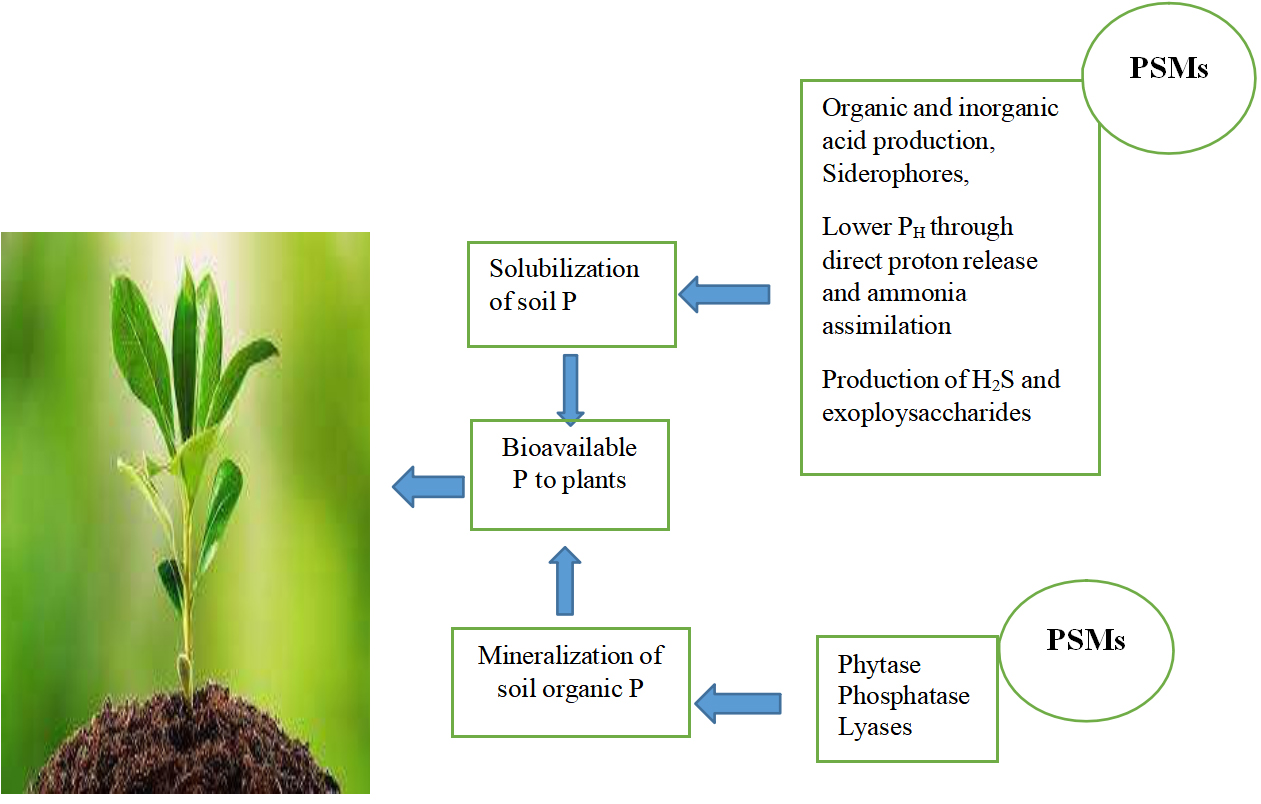
Phosphorus is the most important macronutrient after nitrogen in plants, plays an important role in growth, development and metabolism of plants[34]. The phosphorus is abundant in soil but it is insoluble and not available to plants because it forms complexes with metal ions in soil[35]. The use of chemical fertilizer to meet the phosphorus demands are not eco-friendly and immobilizes shortly after its application. Plant growth promoting microorganisms (PGPM) play an important role in biofortification of phosphorus by secreting organic acids, enzymes and produces siderophores to chelate the phosphorus with metal ion and make it available to plant[36]. It is reported that P. libanensis solubilize the phosphate in water-deficit conditions and improve the growth of cereal crops, hence used to overcome the challenges of sustainable agriculture in stressed environmental conditions. Pseudomonas stutzeri and Mesorhizobium sp can solubilize the phosphate in alleviated salt stress and various PH conditions in cress land plants (acacia, sugar beet, and wheat) and can be used as efficient inoculants in nutrient management in sustainable agriculture[37] (Figure 1).
|
Figure 1. Presentation of mechanism phosphate solubilization by phosphate solubilizing microorganism (PSMs).
5.2 Sequestration of Iron
Iron is a micronutrient and essential for the important physiological process in plants including photosynthesis, DNA synthesis, phytohormone synthesis but its homeostasis is necessary because it can cause program cell death in organisms[38]. Iron is abundantly present on earth but it is unavailable to plants because it is present in insoluble form, and microbes use the different approaches to assist the absorption of Fe in plants by producing low molecular weight iron binding compounds known as siderophores that are water soluble[39]. These iron chelators are classified by the ligands and are categorized as catecholates, hydroxamates, and carboxylates. At a neutral PH to alkaline PH, iron is present in the form of Fe+3[40,41]. Siderophores absorb the iron from the surrounding environment and make it unavailable to pathogens. Sideophore production for iron sequestrization binds with other heavy metals (Al, Cd, Cu, Ga, In, Cr, Pb, Zn and radionuclide U), in this way iron absorption and protection of plants from toxic effect of heavy metal is take place side by side[42] Microbes produce these siderophore and make the soluble form of Fe to plants as well protect the plants from phytopathogens and toxic effect of heavy metals. Iron is one the nutrient component in diet[43]. The co-inoculation of plant growth promoting microorganism Serratia marcescens, Microbacterium arborescens, and Enterobacter sp increases the iron content of wheat grains under pot, improves field condition, which benefits biofortifcation of wheat and control of iron deficiencies[44]. Pseudomonas fluorescens and Pseudomonas putida are siderophore producing microbes, facilitate the seed germination, root length of wheat and increase the iron content of grains[45]. Pseudomonas sp. produce siderophore and protect the plants from Fusarium oxysporum and increase biocontrol efficacy in oilseed crops and help to achieve the future food security[46].
5.3 Potassium Solubilization
Potassium is a macronutrient and plays an important role in primary metabolism of plants, stomatal opening, cell volume growth and plant movement[47]. Potassium is abundantly present on earth surface and only 1-2% is available to plants, and the remaining is bound to other minerals[48]. The potassium is the part of the most abundant minerals (feldspar and mica) of earth, and microorganism helps in decomposing the minerals and extracting the potassium and enhancing the soil fertility[49]. Microrganisms release the organic acids (oxalic acid, tartaric acids, gluconic acid, 2- ketogluconic acid, oxalic acid, citric acid, malic acid and succinic acid) for dissolving potassium from rocks or use the chelated silicon ion to dissolve potassium[50,51]. Potassium solubilizing bacteria (PSB) Enterobacter aerogenes, Pantoea agglomerans, Agrobacterium tumefaciens, Microbacterium foliorum, Myroides odoratimimus, and Burkholderia cepacia are the potassium solubilizing bacteria and can be used in place of potash fertilizers in order to achieve agricultural sustainability[52].
5.4 PGPM Controls Biotic and Abiotic Stress
Phytopathogens are threats to sustainable agriculture and ecosystem. Plant growth promoting microorganism has biocontrol properties and exerts beneficial effects on crops[53]. PGPM produces siderophores, β-1, 3-glucanase, chitinases, antibiotics, fluorescent pigment or by cyanide production. Phytopathogenics are devastating 15-30% crops daily, PGPM are eco-friendly and can be used as biocontrol agents in place of chemicals[54]. PGPR combats stress, as listed in Table 2.
The adverse environmental factors hinder growth and development of plants and reduce the overall yield of plants. The stress conditions affect the metabolism of plants by affecting enzyme activity, substrate scarcity, excess demands of various compounds or combination of all these mechanism[55,56]. The abiotic stress including high level of salinity and drought is directly proportional to osmotic stress, oxidative and ionic stress [29]. The biochemical and physiological functions of plants are affected accordingly, and PGPR maintains the homeostasis in plants by producing the various solutes including sugars, polyamines, betaines, glycine, polyhydric alcohol, trehalose in order to maintain the turgor pressure to mitigating the effects of high salinity and drought[57,58].
Table 2. PGPM Ameliorates Biotic and Abiotic Stress
PGPM |
Mechanism of Action |
Stress |
Crop |
Ref |
P. fluorescens |
Produce antibiotic 2, 4 Diacetylphloroglucinol |
Gaeumannomyc es graminis |
Wheat |
[59] |
Pseudomonas chlororaphis |
Production of antifungal metabolite (Phenazine-1-carboxamide) |
Colletotrichum lindemuthianum |
Bean |
[60] |
Burkholderia cepacia |
Gluconic acid, alpha, 2- ketogluconic acid, Benzoic acid, phenylacetic acid |
Phytopthora capsici |
Pepper |
[61] |
Paenibacillus polymyxa,Bacillus subtilis |
Antibiosis, ISR, production of volatiles |
Colletotrichum acutatum |
Pepper |
[62] |
Bradyrhizobium japonicum |
siderophore biogenesis |
Bacillus thuringiensis |
Soybean |
[63] |
Pseudomonas fluorescens |
Lower the activity of cellulase, polygalacturonase |
Aspergillus niger |
Groundnut |
[64] |
Bacillus cereus+ Bacillus megaterium+ Trichoderma simmonsii |
Act synergistically & increase seed germination & seedling growth by potassium uptake |
Salt and drought |
Soybean |
[65] |
Bacillus cereus |
Maintain photosynthesis efficacy & peroxidase activities |
Drought |
Tomato |
[66] |
Pseudomonas psychrotolerans |
Induced endogenous IAA, GA and improve growth |
Cu, Zn Cd |
Cucumber |
[67] |
Azospirillum Lipoferum and Azotobacter chroococcum |
Increase the activity of K+/Na+ ratio, and the activity of CAT, POD |
Salinity |
Maize |
[68] |
6 CONCLUSION
Environmental challenges are the biggest hurdles of crop production. Deployment of microbes and their application can overcome this challenge without harming the health of the ecosystem. Using of microorganism as Biofertilizers/Bioinoculants can help the plant to overcome the biotic and abiotic stress. PGPM are eco-friendly as well as cost-effective. Chemical fertilizers are proved to be detrimental to soil and environment health. Further research is needed for manipulation and usage of PGPM in increasing crop yield without greenhouse gas emission. This highlights the contribution of PGPM in the agricultural sector and environmental health. Microbial research based products need to be studied keenly to elicit robust results and gain the trust of the farmers, and the real stakeholders of agriculture. In addition, public awareness on use of biofertilizers and bioinoculants is needed for consumers and farmers for a better and greener future.
Acknowledgements
Not applicable.
Conflicts of Interest
The author declared no conflict of interest.
Author Contribution
Tariq H planned for the idea, collected the data required for writing draft of review article, revised the language and adjusted the article according to journal formatting.
Abbreviation List
GA, Gibberellic acid POD, Peroxidase
IAA, Indole acetic acid
PGPM, Plant growth promoting microrganism CAT, Catalase
PSB, Potassium solubilizing bacteria
PSMs, Phosphate solubilizing microorganisms ABA, Abscisic acid
References
[1] Pawlak K, Kołodziejczak M. The role of agriculture in ensuring food security in developing countries: Considerations in the context of the problem of sustainable food production. Sustain, 2020; 12: 5488. DOI: 10.3390/su12135488
[2] Backer R, Rokem JS, Ilangumaran G et al. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci, 2018: 1473. DOI: 10.3389/fpls.2018.01473
[3] Lal R. Anthropogenic influences on world soils and implications to global food security. Adv Agron, 2007; 93: 69-93. DOI: 10.1016/S0065-2113(06)93
[4] Shah A, Nazari M, Antar M et al. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front Sustain Food Syst, 2021; 5: 667546. DOI: 10.3389/fsufs.2021.667546
[5] Lyu D, Backer R, Subramanian S et al. Phytomicrobiome coordination signals hold potential for climate change-resilient agriculture. Front Plant Sci, 2020; 11: 634. DOI: 10.3389/fpls.2020.00634
[6] Antar M, Gopal P, Msimbira LA et al. Inter-organismal signaling in the rhizosphere[M]//Rhizosphere Biology: Interactions Between Microbes and Plants. Springer, Singapore, 2021: 255-293. DOI: 10.1007/978-981-15-6125-2_13
[7] Khan N, Zandi P, Ali S et al. Impact of salicylic acid and PGPR on the drought tolerance and phytoremediation potential of Helianthus annus. Front Microbiol, 2018; 9: 2507. DOI: 10.3389/fmicb.2018.02507
[8] Galloway JN, Townsend AR, Erisman JW et al. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Sci, 2008; 320: 889-892. DOI: 10.1126/science.1136674
[9] Foster SL, Bakovic SIP, Duda RD et al. Catalysts for nitrogen reduction to ammonia. Nat Catal, 2018; 1: 490-500. DOI: 10.1038/s41929-018-0092-7
[10] Malik G, Singh N, Hooda S. Nitrogen Stress in Plants and the Role of Phytomicrobiome. Springer, Singapore. DOI: 10.1007/978-981-15-2576-6_15
[11] Burghardt LT. Evolving together, evolving apart: measuring the fitness of rhizobial bacteria in and out of symbiosis with leguminous plants. New Phytol, 2020; 228: 28-34. DOI: 10.1111/nph.16045
[12] Kousar B, Bano A, Khan N. PGPR modulation of secondary metabolites in tomato infested with Spodoptera litura. Agronomy, 2020; 10: 778. DOI: 10.3390/agronomy10060778
[13] Roy S, Liu W, Nandety RS et al. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. The Plant Cell, 2020; 32: 15-41. DOI: 10.1105/tpc.19.00279
[14] Saha B, Saha S, Das A et al. Biological nitrogen fixation for sustainable agriculture. Agriculturally important microbes for sustainable agriculture. Springer, Singapore, 2017: 81-128. DOI: 10.1007/978-981-10-5343-6_4
[15] Issa AA, Abd-Alla MH, Ohyama T. Nitrogen fixing cyanobacteria: future prospect. Advances in biology and ecology of nitrogen fixation, 2014; 2: 24-48. DOI: 10.5772/56995
[16] Rao DLN, Balachandar D. Nitrogen inputs from biological nitrogen fixation in Indian agriculture. The Indian Nitrogen Assessment. Elsevier, 2017: 117-132. DOI: 10.1016/B978-0-12-811836-8.00008-2
[17] Backer R, Rokem JS, Ilangumaran G et al. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci, 2018; 9: 1473. DOI: 10.3389/fpls.2018.01473
[18] ALKahtani MDF, Attia KA, Hafez YM et al. Chlorophyll fluorescence parameters and antioxidant defense system can display salt tolerance of salt acclimated sweet pepper plants treated with chitosan and plant growth promoting rhizobacteria. Agronomy-Basel, 2020; 10: 1180. DOI: 10.3390/agronomy10081180
[19] Goswami D, Pithwa S, Dhandhukia P et al. Delineating Kocuria turfanensis 2M4 as a credible PGPR: a novel IAA-producing bacteria isolated from saline desert. J Plant Interact, 2014; 9: 566-576. DOI: 10.1080/17429145.2013.871650
[20] Amara U, Khalid R, Hayat R. Soil bacteria and phytohormones for sustainable crop production. Bacterial metabolites in sustainable agroecosystem. Springer, Cham, 2015; 15: 87-103. DOI: 10.1007/978-3-319-24654-3_5
[21] Mahmood Aulakh A, Qadir G, Hassan F U, et al. Desert Soil Microbes as a Mineral Nutrient Acquisition Tool for Chickpea (Cicer arietinum L.) Productivity at Different Moisture Regimes. Plants-Basel, 2020; 9: 1629. DOI: 10.3390/plants9121629
[22] Hakim S, Naqqash T, Nawaz MS et al. Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front Sustain Food S, 2021; 5: 16. DOI: 10.3389/fsufs.2021.617157
[23] Davies PJ. The plant hormones: their nature, occurrence, and functions. Plant hormones. Springer, Dordrecht, 2010: 1-15. DOI: 10.1007/978-1-4020-2686-7_1
[24] Verbon EH, Liberman LM. Beneficial microbes affect endogenous mechanisms controlling root development. Trends Plant Sci, 2016; 21; 218-229. DOI: 10.1016/j.tplants.2016.01.013
[25] Raza A, Mehmood SS, Tabassum J et al. Targeting plant hormones to develop abiotic stress resistance in wheat. Wheat production in changing environments. Springer, Singapore, 2019: 557-577. DOI: 10.1007/978-981-13-6883-7_22
[26] Ali S, Mehmood A, Khan N. Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. J Nanomater, 2021; 2021. DOI: 10.1155/2021/6677616
[27] Khan N, Bano A, Ali S et al. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul, 2020; 90: 189-203. DOI: 10.1007/s10725-020-00571-x
[28] Pal AK, Mandal S, Sengupta C. Exploitation of IAA producing PGPR on mustard (Brassica nigra L.) seedling growth under cadmium stress condition in comparison with exogenous IAA application. Plant Science Today, 2019; 6: 22-30. DOI: 10.14719/pst.2019.6.1.440
[29] Naseem H, Ahsan M, Shahid MA et al. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J Basic Microb, 2018; 58: 1009-1022. DOI: 10.1002/jobm.201800309
[30] Nakkeeran S, Saranya N, Senthilraja C et al. Mining the Genome of Bacillus velezensis VB7 (CP047587) for MAMP Genes and Non-Ribosomal Peptide Synthetase Gene Clusters Conferring Antiviral and Antifungal Activity. Microorganisms, 2021; 9: 2511. DOI: 10.3390/microorganisms9122511
[31] Liu F, Xing S, Ma H et al. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl Microbiol Biot, 2013; 97: 9155-9164. DOI: 10.1007/s00253-013-5193-2
[32] Zhang M, Yang L, Hao R et al. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil, 2020; 452: 423-440. DOI: 10.1007/s11104-020-04582-5
[33] Billah M, Khan M, Bano A et al. Rock phosphate-enriched compost in combination with Rhizobacteria; A cost-effective source for better soil health and wheat (Triticum aestivum) productivity. Agronomy-Basel, 2020; 10: 1390. DOI: 10.3390/agronomy10091390
[34] Rawat P, Das S, Shankhdhar D et al. Phosphate-solubilizing microorganisms: mechanism and their role in phosphate solubilization and uptake. J Soil Sci Plant Nut, 2021; 21: 49-68. DOI: 10.1007/s42729-020-00342-7
[35] Behera BC, Singdevsachan SK, Mishra RR et al. Diversity, mechanism and biotechnology of phosphate solubilising microorganism in mangrove-a review. Biocatal Agr Biotech, 2014; 3: 97-110. DOI: 10.1016/j.bcab.2013.09.008
[36] Kour D, Rana KL, Kaur T et al. Microbe-mediated alleviation of drought stress and acquisition of phosphorus in great millet (Sorghum bicolour L.) by drought-adaptive and phosphorus-solubilizing microbes. Biocatal Agr Biotech, 2020; 23: 101501. DOI: 10.1016/j.bcab.2020.101501
[37] Boroumand N, Behbahani M, Dini G . Combined effects of phosphate solubilizing bacteria and nanosilica on the growth of land cress plant. J Soil Sci Plant Nutr, 2020; 20: 232-243. DOI: 10.1007/s42729-019-00126-8
[38] Qarni A, Billah M, Hussain K et al. Isolation and chara-cterization of phosphate solubilizing microbes from rock phosphate mines and their potential effect for sustainable agriculture. Sustainability-Basel, 2021; 13: 2151. DOI: 10.3390/su13042151
[39] Sharma A, Singh RK, Singh P et al. Insights into the Bacterial and Nitric Oxide-Induced Salt Tolerance in Sugarcane and Their Growth-Promoting Abilities. Microorganisms, 2021; 9: 2203. DOI: 10.3390/microorganisms9112203
[40] Albelda-Berenguer M, Monachon M, Joseph E. Siderophores: From natural roles to potential applications.Adv Appl Microbiol, 2019; 106: 193-225. DOI: 10.1016/bs.aambs.2018.12.001
[41] Williamson AJ, Folens K, Matthijs S et al. Selective metal extraction by biologically produced siderophores during bioleaching from low-grade primary and secondary mineral resources. Miner Eng, 2021; 163: 106774. DOI: 10.1016/j.mineng.2021.106774
[42] Ma Y, Oliveira RS, Freitas H et al. Biochemical and molecular mechanisms of plant-microbe-metal interactions: relevance for phytoremediation. Front Plant Sci, 2016; 7: 918. DOI: 10.3389/fpls.2016.00918
[43] Ullah A, Heng S, Munis MFH et al. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot, 2015; 117: 28-40. DOI: 10.1016/j.envexpbot.2015.05.001
[44] Kusale SP, Attar YC, Sayyed RZ et al. Production of plant beneficial and antioxidants metabolites by Klebsiellavariicola under salinity stress. Molecules, 2021; 26: 1894. DOI: 10.3390/molecules26071894
[45] Ali S, Khan N. Delineation of mechanistic approaches employed by plant growth promoting microorganisms for improving drought stress tolerance in plants. Microbiol Res, 2021, 249: 126771. DOI: 10.1016/j.micres.2021.126771
[46] Subramanium N, Sundaram L. Siderophore producing Pseudomonas spp. isolated from rhizospheric soil and enhancing iron content in Arachis hypogaea L. plant. Int J Agric Technol, 2020; 16: 429-442.
[47] Hawkesford M, Horst W, Kichey T et al. Functions of macronutrients. Marschner’s mineral nutrition of higher plants. Academic Press, 2012: 135-189. DOI: 10.1016/B978-0-12-384905-2.00006-6
[48] Meena VS, Maurya BR, Verma JP et al. Potassium solubilizing microorganisms for sustainable agriculture. New Delhi: Springer, 2016.
[49] Sattar A, Naveed M, Ali M et al. Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Appl Soil Ecol, 2019; 133: 146-159. DOI: 10.1016/j.apsoil.2018.09.012
[50] Das I, Pradhan M. Potassium-solubilizing microorganisms and their role in enhancing soil fertility and health. Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, 2016: 281-291.
[51] Ullah A, Bano A, Khan N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front Sustain Food Syst, 2021; 161: 618092. DOI: 10.3389/fsufs.2021.618092
[52] Teotia P, Kumar V, Kumar M et al. Rhizosphere microbes: potassium solubilization and crop productivity–present and future aspects. Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, 2016: 315-325. DOI: 10.1007/978-81-322-2776-2_22
[53] Yadav AN, Verma P, Singh B et al. Plant growth promoting bacteria: biodiversity and multifunctional attributes for sustainable agriculture. Adv Biotechnol Microbiol, 2017; 5: 1-16.
[54] Purkayastha, GD. Biological control of important fungal pathogens of tea by antagonistic microorganisms isolated from tea rhizosphere (Doctoral dissertation, University of North Bengal), 2014.
[55] López-Gámez G, Elez-Martínez P, Martín-Belloso O et al. Pulsed electric fields affect endogenous enzyme activities, respiration and biosynthesis of phenolic compounds in carrots. Postharvest Biol Tec, 2020; 168: 111284. DOI: 10.1016/j.postharvbio.2020.111284
[56] Ali S, Waseem M, Hussain A et al. Combined Application of Citric Acid and Cr Resistant Microbes Improved Castor Bean Growth and Photosynthesis while it Alleviated Cr Toxicity by Reducing Cr+6 to Cr3+. Microorganisms, 2021; 9: 2499. DOI: 10.3390/microorganisms9122499
[57] Kaushal M. Microbes in cahoots with plants: MIST to hit the jackpot of agricultural productivity during drought. Int J Mol Sci, 2019; 20: 1769. DOI: 10.3390/ijms20071769.
[58] Jatav SS, Parihar M, Patra A et al. Soil Microbes in Plant Growth Promotion and for Mitigation of Abiotic Stress of Drought. Soil Microbiomes for Sustainable Agriculture. Springer, Cham, 2021; 27: 175-201. DOI: 10.1007/978-3-030-73507-4_7
[59] Kwak YS, Bonsall RF, Okubara PA, et al. Factors impacting the activity of 2, 4-diacetylphloroglucinol-producing Pseudomonas fluorescens against take-all of wheat. Soil Biol Biochem, 2012; 54: 48-56. DOI: 10.1016/j.soilbio.2012.05.012
[60] Pathak R, Shrestha A, Lamichhane J, Gauchan DP. PGPR in biocontrol: mechanisms and roles in disease suppression. Int J Agron Agric, 2017; 11, 69-80.
[61] Zhang C, Wang MY, Khan N et al. Potentials, utilization, and bioengineering of plant growth-Promoting Methylobacterium for sustainable agriculture. Sustainability-Basel, 2021; 13: 3941. DOI: 10.3390/su13073941
[62] Lamsal K, Kim S W, Kim Y S et al. Application of rhizobacteria for plant growth promotion effect and biocontrol of anthracnose caused by Colletotrichum acutatum on pepper. Mycobiology, 2012; 40: 244-251. DOI: 10.5941/MYCO.2012.40.4.244
[63] Le TTH, Jun SE, Kim GT. Current Perspectives on the Effects of Plant Growth-promoting Rhizobacteria. J Life Sci, 2019; 29: 1281-1293. DOI: 10.5352/JLS.2019.29.11.1281
[64] Deshmukh S, Bishi S, Vakharia D. Pseudomonas fluorescens modulate in-vitro lytic enzyme production and inhibit the growth of collar rot pathogen (Aspergillus niger) in groundnut (Arachis hypogaea L.). J Pure Appl Microbiol, 2015; 9: 1531-1538.
[65] Bakhshandeh E, Gholamhosseini M, Yaghoubian Y, et al. Plant growth promoting microorganisms can improve germination, seedling growth and potassium uptake of soybean under drought and salt stress. Plant Growth Regul, 2020; 90: 123-136.
[66] Chiappero J, del Rosario Cappellari L, Palermo TB et al. Antioxidant status of medicinal and aromatic plants under the influence of growth-promoting rhizobacteria and osmotic stress. Ind Crop Prod, 2021; 167: 113541. DOI: 10.1016/j.indcrop.2021.113541
[67] Khan N, Bano A. Role of plant growth promoting rhizobacteria and Ag-nano particle in the bioremediation of heavy metals and maize growth under municipal wastewater irrigation. Int J Phytoremediat, 2016; 18: 211-221. DOI: 10.1080/15226514.2015.1064352
[68] Abdel Latef AAH, Abu Alhmad MF, Kordrostami M et al. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum reinforces maize growth by improving physiological activities under saline conditions. J Plant Growth Regul, 2020; 39: 1293-1306. DOI: 10.1007/s00344-020-10065-9
Copyright © 2022 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.





 Copyright ©
Copyright ©