Efficacy of Different Solvents on the Extraction Process of Antioxidant and Potential Compounds in Alpinia Galanga L. from Indonesia
Yusuf Andriana1, Iwansyah Chandra Ade1, Quang Trung Nguyen3, Quang Minh Bui3, Minh Duc Nguyen2,4, Thi Tinh Phung3, Viet Anh Le3, Hoang Khanh Nguyen3, Ngoc Minh Truong2,3*
1Research Division for Natural Product Technology, Indonesian Institute of Sciences, National Research and Innovation Agency (Indonesia), Yogyakarta, Indonesia
2Graduate University of Science and Technology (GUST), Vietnam Academy of Science and Technology (VAST), Hanoi, Vietnam
3Center for Research and Technology Transfer (CRETECH), Vietnam Academy of Science and Technology (VAST), Hanoi, Vietnam
4Institute of Genome Research (IGR), Vietnam Academy of Science and Technology (VAST), Hanoi, Vietnam
*Correspondence to: Ngoc Minh Truong, Graduate University of Science and Technology (GUST), Vietnam Academy of Science and Technology (VAST), Hanoi, Vietnam; Center for Research and Technology Transfer (CRETECH), Vietnam Academy of Science and Technology (VAST), Hanoi, Vietnam; Email: anphuminh1011@gmail.com
Abstract
Objectives: To determine the most effective solvent for extraction of antioxidant and potential compounds in Alpinia galanga, expressed via the percentage of components in the sample.
Methods: A comparison test of 4 extractants, including water, hexane, chloroform and ethyl acetate, was conducted for the extraction procedure. In addition, 1, 1-diphenyl-2-picrylhydrazyl (DPPH) and 2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were used to perform antioxidant experiments. HPLC and GC-MS were then employed to determine quercetin content and scan the chemical compositions of the extract, respectively.
Results: Ethyl acetate extract showed the strongest antioxidant activity (DPPH IC50=127.67ppm, ABTS IC50=54.82ppm), containing the highest level of quercetin (39.61mg/g DW of extract) and 2 major components of methyl oleate (15.36%) and methyl palmitate (12.01%). In the correlation analysis, the antioxidant activity of Alpinia galanga extracts was proportional to its total phenolic, flavonoid, and quercetin contents.
Conclusion: Ethyl acetate produces the best performance in extracting anti-oxidative agents and quercetin in Alpinia galanga. The presence of quercetin and FAMEs in the extract highlighted the potentials of antioxidant, antihypertensive, anti-inflammatory, antibacterial, antiviral, and antidiabetic properties of Alpinia galanga.
Keywords: antioxidant activity, Alpinia galanga, methyl oleate, methyl palmitate, quercetin
1 INTRODUCTION
Medicinal plants are considered the most approachable pharmacological alternative for most people globally, and traditional herbs remain an integral part of the health system[1]. Alpinia galanga is a fragrant, herbaceous plant and widely applied for spice and folk medicines in Asian countries. It is commonly so-called greater galangal, galangal, Java galangal, lengkuwas (Indonesian) or Siamese ginger (English). This plant is a native of Indonesia, but its precise origin remains unknown. This herb is broadly distributed in South-East Asian countries such as Bangladesh, India, Suriname, and China[2]. The rhizome of Alpinia galanga is not only commonly utilized as spices for food such as Thai soups (Tom yam and Tom Khaa) or Indonesian/Malaysian curries, but in Southeast Asia, it also has been extensively applied as a traditional and pharmacological plant for several diseases such as asthma, fever, cough, hiccups, heart diseases, diabetes, rheumatism, chronic enteritis, renal calculus, kidney disorders, bronchitis, diarrhea, scurf, rheumatism, dysentery, and skin diseases[3].
Haraguchi et al.[4] reported antimicrobial activity interpinen-4-ol, the dominant compound of essential oil extracted from dried and fresh galangal rhizome. Diterpene (E)-8β, 17-epoxylabd-12-ene-15, 16-dial 1, isolated from galangal rhizome extract, can be resistant to Candida albicans since it can improve the antifungal mechanism of both chalcone and quercetin. Other active compounds of Alpinia galanga include galango flavonoid, 1′S-1′-acetoxychavicol acetate (ACE), phenylpropanoids and phydroxybenzaldehyde (1′S-1′-acetoxychavicol acetate and 1′S-1′-acetoxyeuginol acetate), acetoxycineoles (trans and cis)-2- and 3-acetoxy-1, 1, 8-cineoles, 1′-acetoxychavicol acetate (galangal acetate), β-Sitosterol diglucoside (AG-7) and β-sitsteryl Arabinoside (AG-8), hydroxy-1,8-cineole glucopyranosides, (1R, 2R, 4S)-and (1S, 2S, 4R)-trans-2-hydroxy-1,8-cineole β-D-glucopyranoside, and (1R, 3S, 4S)-trans-3-hydroxy-1, 8-cineole β-D-glucopyranoside that have been demonstrated to have various therapeutic effects[5-7].
Some active components from Alpinia galanga have been identified and reported to exhibit pharmaceutical effects against several diseases[8]. For example, quercetin, a flavonol belongs to the flavonoid group and is broadly detected in several kinds of vegetables. They have demonstrated abilities to reduce blood pressure, lower risk of the heart disease and cancer, and prevent infectious and neurological diseases[9]. Besides, fatty acid methyl esters from this plant have been reported for potential therapeutic potentials[10].
However, studies on the efficiency of extraction process of anti-free radical compounds and flavonoids in Alpinia galanga by using different solvents are still limited. Therefore, in this research, we used various types of solvents, due to their different polarities to assess anti-radical activity of Alpinia galanga as well as to determine its quercetin content.
2 MATERIALS AND METHODS
2.1 Chemicals and Plant Material
Ethanol, methanol, ethyl acetate, hydrochloric acid, sodium hydroxide, chloroform, sodium carbonate, Folin-Ciocalteu, gallic acid, aluminium(III) chloride, DPPH, and quercetin were purchased from Merck, Ltd., Singapore, Singapore. The rhizomes of Alpinia galanga were purchased commercially at a traditional market in Subang city, West Java, Indonesia. The sample was identified in Herbarium Bogoriense, Indonesian Institute of Sciences.
2.2 Plant Extraction and Fractionation
An amount of 15kg rhizomes of Alpinia galanga was sliced by a kitchen slicer to produce chips with a thickness of 3mm. The chips were dried using a cabinet dryer at 45°C for 3 days and then pulverized by a disc mill to obtain dried rhizome power that passed through the 40 mesh of a sieve. After that, an amount of 300g Alpinia galanga rhizome powder was immersed in 1.5L ethanol (80%) for 3 days at ambient conditions and the obtained solution was filtered out for the impurities. After filtration, ethanol was dried out using a rotary evaporator (QR 2005-S, Shimadzu, Tokyo Japan) at 40°C in a vacuum condition. Subsequently, the ethanol extract was fractionated using solvents of different polarities to produce 0.52, 0.84, 0.31 and 2.28g of hexane, chloroform, ethyl acetate, and water extracts respectively. These fractions were subjected to further analysis for anti-free radical activity and quercetin content.
2.3 Total Phenolic Contents
Total phenol was determined by Folin Ciocalteu-colorimetric method[11]. In brief, a volume of 0.125mL of either sample (2000ppm) or gallic acid standard solution (0-200ppm) was pipetted into a test tube and mixed with 0.125mL of FC reagent (10%v/v). After 6mins, the mixture was added with 1.25mL volume of Na2CO3 (7.5%). Finally, 1mL of aquades was transferred to 3mL mixture. The mixtures were then incubated at ambient conditions for 90mins. The absorbances of reactions were scanned at wavelength of 760nm using a spectrophotometer (Merk Company, Kota, Negara). The value of total phenol was presented as GAE per g extract (R2=0.998).
2.4 Total Flavonoid Contents
Referencing to Chompoo et al.[12], total flavonoid contents (TFC) were determined by a spectrophotometric technique. In brief, the reaction between 2mL aluminium (III) chloride hexahydrate (2%w/v in methanol) and2mL sample (2000ppm)/quercetin standard (0-200ppm) occurred. After 15mins of incubation, a spectrophotometer (Shimadzu 1800 Uv-Vis 115 VAC, Tokyo, Japan) was utilized to measure the absorbance of the mixtures at 430nm. Unit to interpret TFC is quercetin equivalent per gram of extract (mg QE/g) (R2=0.999).
2.5 DPPH Radical Scavenging Activity
A minor modification method from Minh et al.[13] was employed to measure the anti-free radical activities of Alpinia galanga sample, applying the DPPH reagent. The mixture of 1mL DPPH solution (0.5mM), 2mL sample (dissolved in MeOH) and 2mL acetate buffer (0.1M-pH5.5) was transferred in a test tube and kept at room temperature in shadow for half hour. The absorbance at 517nm was recorded by a spectrophotometer (Shimadzu 1800 Uv-Vis 115 VAC, Tokyo, Japan). Quercetin was used as a positive control. In addition, the IC50 value, a concentration required to inhibit 50% DPPH free radical, was also presented. The percentages of free-radical scavenging activity against DPPH were interpreted as the following formula:
|
Where:
Abs control represents the absorbance of the control
Abs sample represents the absorbance of the test sample
2.6 ABTS Radical Scavenging Activity
To evaluate the radical scavenging activity, a modified method of Andriana et al.[14] was applied, using the 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) radical cation (ABTS) solution as a reagent[13]. Soon after being stored at room temperature, isolated from any light source for 16h, ABTS solution was prepared by mixing 7mM ABTS with 2.45mM potassium persulfate solution. Next, dissolved 0.240mL sample in MeOH, then added 1.20mL the prepared ABTS into the solution. This mixture was kept away from any light source at room temperature for half an hour before the absorbance was measured by a spectrophotometer (Shimadzu 1800Uv-Vis 115VAC, Tokyo, Japan) at the wavelength of 734nm. BHT standard (5-125µg/mL) was used as a positive control. The ABTS radical scavenging activity was described as the same formula of DPPH calculation (1).
2.7 Quercetin Identification and Quantification
Referencing a method of Yue-ling et al.[15], a HPLC system (Agilent 1200HPLC system, Karlsruhe, Germany) integrated with a quaternary pump, an auto-sampler and a VWD detector was used to identify and quantify Quercetin content[14]. The stationary phase used for HPLC operation was C18 column (4.6x250mm, 5µm) (Agilent Eclips XDB-C18, Agilent, Karlsruhe, Germany), and the mobile phase included methanol and 0.40% phosphoric acid (49: 51, v/v) with the temperature of 25℃. Mobile phase flow rate and injection volume were installed at 1.0ml/min and 20µl, respectively. Identification of Quercetin was performed by comparing the retention time between quercetin standard and tested sample. While quantification of quercetin was conducted by establishing a standard curve of several concentrations of quercetin (R2=0.999).
2.8 Identification of Chemical Constituents by Gas Chromatography-Mass Spectrometry (GC-MS)
The Agilent GC-MS system (Agilent 7890B/MSD 5977A, Agilent Technology, Inc., Santa Clara, CA, USA) was employed to detect chemical constituents of ethyl acetate fraction of Alpinia galanga. 1µL was the volume for sample injection. The Agilent HP-5MS (30m, 250μm, 0.25μm) (19091S-433: 93.92873) (Agilent Technology, Inc., J&W Scientific Products, Santa Clara, CA, USA) was a column for MS operation. Helium was chosen as the carrier gas. The mode of helium inlet was splitless with a heater temperature of 250°C, a pressure of 7.0699psi, and a total flow of 104mL/min. The parameter for GC oven temperature was set up as follows: The starting temperature=40°C with hold time of 1min, the programmed rate=10°C/min up to 325°C with hold time of 4min. The scanning mass ranged from 122-1021amu. The Agilent ChemStation software (Agilent Technology, Inc., J&W Scientific Products, Santa Clara, CA, USA) was integrated for controlling the GC-MS instrument as well as processing the data peak.
2.9 Statistical Analysis
One-way ANOVA-Minitab® 16.2.3 (copyright© 2012 Minitab Inc., Philadelphia, PA, USA) was used as the statistical tool for data analysis; the result then was interpreted in the value of means ± standard deviation (SD). The Tukey’s test (confidence level of 95%; P<0.05) was applied to identify the significant difference among treatment and control. Pearson correlation analysis was also carried out to describe the correlation between TPC, TFC, quercetin contents, and anti-free radical activity of Alpinia galanga extracts.
3 RESULTS
3.1 Total Phenolic and Flavonoid Contents
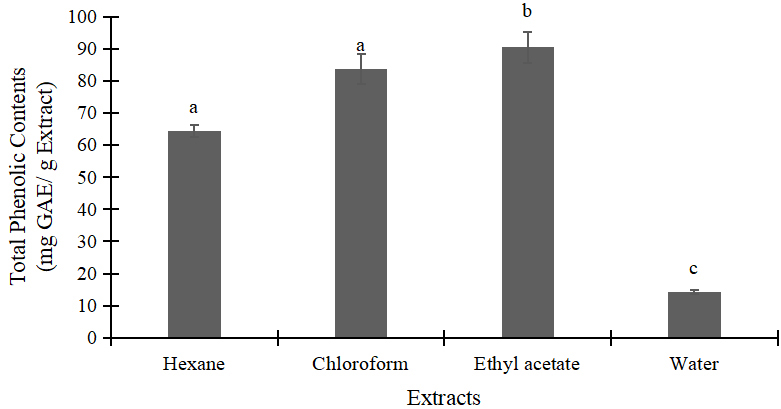
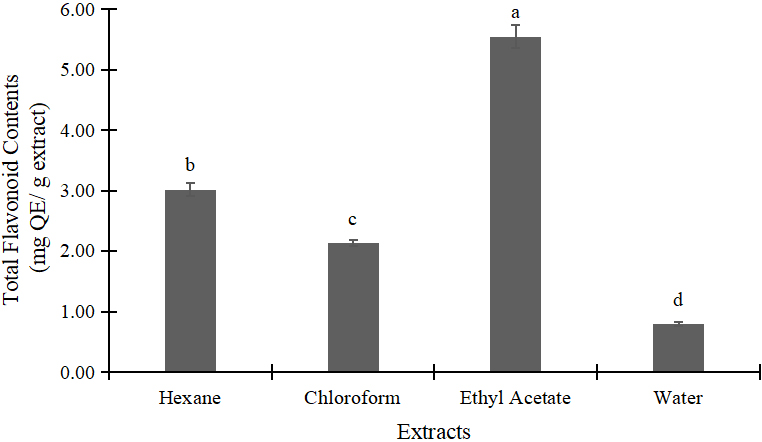
Figures 1 and 2 highlighted the total phenol and flavonoid of Alpinia galanga samples. Effects of different solvents on the content of total phenol and flavonoid of the Alpinia galanga can be explained as follows. The highest level of phenol was observed when ethyl acetate was used as extracting solvents. In contrast, water extract showed the lowest results of total phenol and flavonoid.
|
Figure 1. Total phenolic contents of A. galanga extracts. Data were presented as mean ± standard deviation (SD). Means with different small letter on the bars indicted significantly different by tukey’s test (P<0.05). GAE=gallic acid equivalent.
|
Figure 2. Total flavonoid contents of A. galanga extracts. Data represented mean±standard deviation (SD). Means with different small letter on the bars indicted significantly different by tukey’s test (P<0.05). GAE=gallic acid equivalent.
3.2 Identification and Quantification of Quercetin
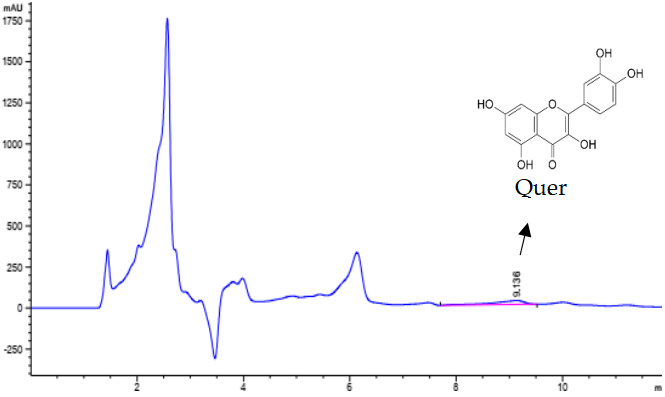
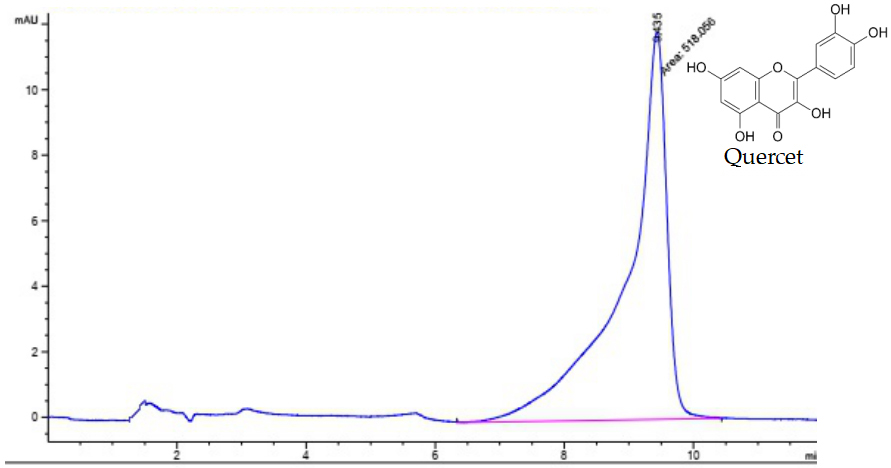
The HPLC chromatograms of ethyl acetate extract and quercetin standard are illustrated in Figure 3 and Figure 4. The results showed that quercetin was only detected in ethyl acetate extract and not detected in hexane, chloroform, and water extracts. Retention time of Quercetin was observed at the minute of 9.136 for both standard and sample. With the results of quantification analysis, the concentration of quercetin in ethyl acetate extract was 39.61±0.61mg/g DW. Thus, it can be concluded that the highest efficiency of quercetin separation for Alpinia galanga matrices was observed in the presence of ethyl acetate.
|
Figure 3. HPLC chromatogram of ethyl acetate extract of A. galanga.
|
Figure 4. HPLC chromatogram of quercetin standard.
3.3 Identification of Chemical Composition by Gas Chromatography-Mass Spectrometry (GC-MS)
Table 1 shows chemical compositions of ethyl acetate extracts of Alpinia galanga via GC-MS system. There were nine compounds, which are different classes found in ethyl acetate fraction. Of which, methyl oleate was counted as the major component followed by methyl palmitate, Cyclotrisiloxane, hexamethyl-(11.40%) and other components, accounting for 12.01%, 11.40% and 10.00%, respectively. Fatty acids and their derivates were the major class detected from the ethyl acetate extract.
Table 1. Chemical Composition of Ethyl Acetate Extract of A. Galanga
RT (min) |
Area (%) |
Compounds |
MW (g/mol) |
Class |
4.313 |
8.33 |
Dimethyl malate |
144.13 |
Hydroxyacid and derivates |
6.419 |
6.34 |
Decane, 2,6,7-trimethyl- |
184.36 |
Alkane |
18.177 |
12.01 |
Methyl palmitate |
270.5 |
Fatty acid ester |
18.606 |
2.35 |
Phthalic acid, butyl tetradecyl ester |
418.6 |
Carboxilyc ester |
19.828 |
15.36 |
Methyl oleate |
328.5 |
Lipid Peroxides |
20.055 |
1.64 |
Methyl stearate |
298.5 |
Fatty acid ester |
25.336 |
1.67 |
5-Acetamido-4,7-dioxo-4,7-dihydrobenzofurazan |
207.14 |
- |
27.151 |
11.40 |
Cyclotrisiloxane, hexamethyl- |
222.46 |
Siloxanes |
27.478 |
5.77 |
Tetrasiloxane, decamethyl- |
310.68 |
- |
Notes: RT=retention time. MW=molecular weight
3.4 DPPH and ABTS Free Radical Scavenging Activities
The antioxidant potentials of Alpinia galanga extracts of different solvents against DPPH and ABTS free radicals are illustrated in Table 2. The outcome revealed that ethyl acetate extract was found to have strongest of antioxidant activity (DPPH IC50=127.67, ABTS IC50=54.82ppm, respectively) followed by chloroform, water, and hexane extracts. However, compared with quercetin as positive control (IC50=7.53ppm for DPPH and IC50=4.65ppm for ABTS), ethyl acetate extract still presented a lower activity in this assay.
Table 2. Anti-free Radical Activity (IC50) of Extracts from A. Galanga.
Extracts |
IC50 (ppm) |
|
DPPH |
ABTS |
|
Hexane |
2187.85±14.07a |
1139.05±9.34a |
Chloroform |
130.69±2.83c |
115.46±1.11c |
Ethyl Acetate |
127.67±1.41c |
54.82±1.18d |
Water |
404.27±2.83b |
312.37±3.83b |
Quercetin* |
7.53±0.09d |
4.65±0.16e |
Notes: Data were presented as means ± standard deviations (SD). Means followed by different small letters. Different small letters indicate significantly different Tukey’s test (P<0.05). *=positive control.
3.5 Correlation of TPC, TFC, Quercetin, and Anti-Free Radical Activity(IC50)
Table 3 illustrates the correlation among TPC, TFC, quercetin, and anti-free radical activity of Alpinia galanga extracts (P<0.05). Significant and positive correlations were found between TPC and TFC (r=0.770), TFC and quercetin content (r=0.889), DPPH and ABTS (r=0.995). Simultaneously, a positive correlation was witnessed between TPC and quercetin (r=0.525), but it was not significant. In addition, negative correlations were observed between the TPC, TFC, and Quercetin to ABTS and DPPH IC50 (P<0.05).
Table 3. The Correlation Among TPC, TFC, Quercetin Content, and Antioxidant Activity
Parameters |
TPC |
TFC |
Quercetin |
IC50 (DPPH) |
IC50 (ABTS) |
TPC |
1 |
0.770* |
0.525 |
-0.106 |
-0.193 |
TFC |
|
1 |
0.889* |
-0.047 |
-0.141 |
Quercetin |
|
|
1 |
-0.393 |
-0.466 |
IC50 (DPPH) |
|
|
|
1 |
0.995* |
IC50 (ABTS) |
|
|
|
|
1 |
Notes: *=significantly correlation (P<0.05).
3.6 Therapeutic Potentials of Quercetin, Methyl Oleate, and Methyl Palmitate
Table 4 shows the origins of quercetin, methyl oleate, and methyl palmitate and their therapeutic potentials. The data indicate that quercetin is found in many plants and distributed widely in various families. Three species from the Rosaceae family, namely, Prunus domestica, Malus domestica, and Prunus aviumare highlighted quercetin sources. For therapeutic potentials, quercetin is reported to be a cure for many diseases due to its biochemical characteristic such as antioxidant, anti-inflammatory, anti-hypertensive, anti-bacterial, analgesic, anti-diabetic, anti-ulcer, and antitussive. Methyl oleate and methyl palmitate were found in vegetable oils extracted from soybean, corn and sun flower oils. They showed potent therapeutic properties against oxidative agents, fungi, and some bacteria.
Table 4. Quercetin, Methyl Oleate, and Methyl Palmitate Sources and Their Therapeutic Potentials
Plant Source |
Family |
Theurapeutic Effects |
References |
Quercetin |
|||
Apium graveolens |
Apiaceae |
Anti-inflammatory, antibacterial |
[16,17] |
Allium cepa (red onions) |
Amaryllidaceae |
Cardioprotective, immunostimulant, antioxidant |
[16,17] |
Centella asiatica |
Apiaceae |
Wound healing |
[16,17] |
Hypericum perforatum |
Hypericaceae |
Neurological effects, major depressive disorders |
[16,17] |
Moringa oleifera |
Moringaceae |
Antihypertensive, anti-inflammatory, antibacterial |
[16,17] |
Hypericum hircinum |
Hypericaceae |
Antioxidant |
[16,17] |
Brassica oleracea var. sabellica (Kale) |
Brassicaceae |
Reduces blood glucose, neuropathy, reduce the risk of stroke, |
[16,17] |
Brassica oleracea var. italica (broccoli) |
Brassicaceae |
Prevents cancer and fluid retention |
[16,17] |
Solanum lycopersicum |
Solanaceae |
Food supplement and salads |
[16,17] |
Coriandrum Sativum |
Apiaceae |
Cholesterol, dyspepsia, reduce blood pressure, |
[16,17] |
Morua alba |
Moraceae |
Diet |
[16,17] |
Nasturtium officinale |
Brassicaceae |
Reduces the risk of cancers |
[16,17] |
Asparagus officinalis |
Asparagaceae |
Antiulcer, antitussive, antineoplastic, |
[16,17] |
Lactuca sativa |
Asteraceae |
Osteoporosis, iron deficiency anemia |
[16,17] |
Malus domestica |
Rosaceae |
Decrease the risk of cardiovascular disease and cancer |
[16,17] |
Capparis spinosa |
Capparaceae |
Disinfectants, antiatherosclerotic agent, vermifuges, |
[16,17] |
Prunus domestica |
Rosaceae |
Laxative |
[16,17] |
Vaccinium oxycoccus |
Ericaceae |
Urinary tract infections |
[16,17] |
Prunus avium |
Rosaceae |
Diuretic, tonic, astringent, |
[16,17] |
Camellia sinensis |
Theaceae |
Antispasmodic, analgesic, antidiabetic, bronchodilator, antiviral |
[16,17] |
Methyl-oleate |
|||
Glycine max |
Fabaceae |
Antioxidant, antifungal |
[10] |
Helianthus annuus |
Asteraceae |
Antioxidant, antifungal |
[10] |
Zea mays |
Poaceae |
Antioxidant, antifungal |
[10] |
Ipomoea purpurea |
Convolvulaceae |
Acaricidal activity |
[18] |
- |
- |
Antibacterial activity |
[19] |
Methyl-palmitate |
|||
Zea mays |
Poaceae |
Anti-inflammatory activity |
[20] |
Glycine max |
Fabaceae |
Antioxidant, antifungal activity |
[11] |
Helianthus annuus |
Asteraceae |
Antioxidant, antifungal activity |
[11] |
Zea mays |
Poaceae |
Antioxidant, antifungalactivity |
[11] |
Withania somnifera |
Solanaceae |
Citotoxic activity, hepatoprotective agent |
[21] |
4 DISCUSSION
Alpinia galanga is a member of Alpinia genus of Zingiberaceae family. Many reports have emphasized its various bioactive components and therapeutic effects[22]. One of the most important therapeutic potentials of Alpinia galanga is antioxidant property[23]. Therefore, it is among the frequent indicators to evaluate the function and quality of bioactive compounds in foods and medicines. The DPPH radical scavenging assay is a famous spectrophotometric procedure that is widely used to evaluate the anti-free radical potentials in plants. The basic theory for this method is the reductions of DPPH in methanol solution, under the activity of hydrogen-donating antioxidants[24]. This therapeutic characteristic has been found in rhizomes of Alpinia galanga[25] and other Alpinia species such as A. zerumbet[12], A. officinarum, A. rafflesiana, and A. flabelata[26].
This study investigates the efficiency of different solvents to extract antioxidant substance of Alpinia galanga. According to the results, ethyl acetate showed the most effective solvent in antioxidant extraction with IC50 value of 127.67 ppm followed by chloroform (130.69ppm), water (404.27ppm), and hexane (2187.85ppm) (Table 2). As reported previously, ethyl acetate was the most effective extractant for antioxidants separation[13,20]. Its polarity index was considered moderate, 4.4, which is lower than that of water (9.0) and higher than chloroform (4.1) and hexane (0)[21]. In general, most of antioxidant compounds e.g. phenol and flavonoids are soluble in ethyl acetate[27]. In the analysis of total phenolic and flavonoid, ethyl acetate extract yielded the highest of 90.43mg GAE/g extract followed by chloroform (83.63mg GAE/g extract), hexane (64.43mg GAE/g extract), and water (14.30mg GAE/g extract) (Figure 1). This result is comparable with Jatropha podagrica as the efficiency of ethyl acetate was high, yielding the highest value of both total phenol and flavonoids[11].
Alpinia galanga has been reported to contain quercetin compound[22]. However, the information on suitable solvent for extraction is still limited. Quercetin is one of most important active compounds found in Alpinia galanga. It is classified as a flavonol, a subclass of flavonoid compounds. It has some hydroxyl (OH) groups attached at carbon bound positions of 3, 5, 7, 3′, and 4′ and according to IUPAC system, it is named 3, 3′, 4′, 5, 7-pentahydroxyflvanone[7]. Based on the result of this study, the most useful solvent to be addressed for quercetin extraction was ethyl acetate. Compared to the other test of different solvents, ethyl acetate remained the best solution. This might be because of quercetin belongs to flavonoid group that generally dissolved in ethyl acetate solvent.
Quercetin showed a broad range of therapeutic potentials such as antioxidant, anti-diabetic, anti-hypertensive, anti-inflammatory, diuretic, and laxative properties (Table 3). The wide range of beneficial effects from quercetin might be assosiated with the presence of elevated C-reactive protein (CRP) levels which linked with numerous diseases as reported by García-Mediavilla et al[28]. In preclinical in vitro studies, quercetin exhibited a significant reduction in the levels of inflammatory mediators such as NO synthase, COX-2, and CRP in human hepatocyte-derived cell line[29].
Quercetin is also found in many plant species from different families. Quercetin with flavonol type (quercetin glycosides) is the most abundant of the flavonoid and found in various types of foods, including apples, brassica vegetables, grapes, berries, capers, shallots, tea, onions, and tomatoes, as well as many nuts, flowers, seeds, leaves, and barks[17]. In medicinal plant quercetin has also been found in Hypericum perforatum, Ginkgo biloba, and Sambucus canadensis[8]. Quercetin is a potential compound that has a wide ranges of therapeutic potentials and easily found in many fruits, vegetables, and medicinal plants[22]. This compound is a promising material for drug development to treat various diseases.
Beside quercetin, some potent active compounds were detected in ethyl acetate extract of A. galanga, for example, fatty acid methyl ester (methyl palmitate and oleate) (Table 1). These compound might also be related to antioxidant activity of A. galanga as ethyl acetate fration showed the strongest activity compared to the other fractions (Table 2). This results was in line with previously report that stated that methyl oleate and methyl palmitate possessed antioxidant, antifungal, and antibacterial activities[7].
5 CONCLUSION
This study investigated the effects of different solvents on the extraction of antioxidant and potent chemical constituents of Alpinia galanga. The results showed that the solvents (hexane, chloroform, ethyl acetate, and water) played an important role in extracting anti-oxidative agent, quercetin and fatty acid methyl ester (FAMEs) contents of Alpinia galanga. Compared to other solvents, ethyl acetate presented as the most effective solvent to extract antioxidant agent and quercetin, a compound belonging to flavonoid group from Alpinia galanga rhizomes. Besides, GC-MS analysis showed methyl oleate (12.01, and methyl palmitate (15.36%) were the major component of ethyl acetate extract. This solvent also showed the most powerful effect in total phenol and total flavonoid contents. The correlation analysis showed that TPC, TFC, and Quercetin contents were proportional to the antioxidant activity (DPPH and ABTS) of Alpinia galanga. The presence of quercetin, methyl oleate and methyl palmitate in the ethyl acetate extract of Alpinia galanga underlined the promising anti-inflammatory, anti-hypertensive, anti-bacterial, anti-viral, antioxidant, and anti-diabetic properties of Alpinia galanga.
Acknowledgements
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.01-2019.316. The authors express their gratitude towards Center for Research and Technology Transfer, Vietnam for partly supporting this research.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
Ade IC and Andriana Y conceptualized, implemented the research, and wrote the manuscript. Nguyen QT, Nguyen MD, Bui QM, and Le VA wrote the first draft. Truong NM and Nguyen QT revised and gave advice to improve the research. Truong NM, Nguyen HK and Phung TT conceptualized and revised the paper.
Abbreviation List
ABTS, 2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)
ANOVA, Analysis of variance
BHT, 3, 5-Di-tert-4-butylhydroxytoluene
DPPH, 1, 1-diphenyl-2-picryhydrazyl
DW, Dry weight
FAMEs, Fatty acid methyl esters
FC, Folin Ciocalteu
GAE, gallic acid equivalent
GC-MS, Gas chromatography - Mass spectrometry
HPLC, High performance liquid chromatomgraphy
IC50, Half maximal Inhibitory Concentration
IUPAC, International Union of Pure and Applied Chemistry
QE, Quercetin equivalent
RSA, Radical scavenging activity
TFC, Total flavonoid content
TPC, Total phenolic content
VWD, Variable wavelength detector
References
[1] Ngarivhume T, Van’t Klooster CIEA, de Jong JTVM et al. Medicinal plants used by traditional healers for the treatment of malaria in the chipinge district in Zimbabwe. J Ethnopharmacol, 2015; 159: 224-237. DOI: 10.1016/j.jep.2014.11.011
[2] Ravindran PN, Balachandran I. Galangal. In: Handbook of Herbs and Spices: Volume 3. Peter KV ed. Woodhead Publishing Limited: Cambridge, England, 2006; 357-364. DOI: 10.1533/9781845691717.3.357
[3] Lim TK. Alpinia galanga. In: Edible Medicinal and Non-Medicinal Plant. Springer International Publishing: Cham, Germany, 2016; 133-168. DOI: 10.1007/978-3-319-26065-5_7
[4] Haraguchi H, Kuwata Y, Inada K et al. Antifungal activity from Alpinia galanga and the competition for incorporation of unsaturated fatty acids in cell growth. Planta Med, 1996; 62: 308-313. DOI: 10.1055/s-2006-957890
[5] Bendjeddou D, Lalaoui K, Satta D. Immunostimulating activity of the hot water-soluble polysaccharide extracts of anacyclus pyrethrum, Alpinia galanga and citrullus colocynthis. J Ethnopharmacol, 2003; 88: 155-160. DOI: 10.1016/S0378-8741(03)00226-5
[6] Morikawa T, Ando S, Matsuda H et al. Inhibitors of nitric oxide production from the rhizomes of Alpinia galanga: structures of new 8-9′ linked neolignans and sesquineolignan. Chem Pharm Bull, 2005; 53: 625-630. DOI: 10.1248/cpb.53.625
[7] Li Y, Yao J, Han C et al. Quercetin, inflammation and immunity. Nutrients, 2016, 8: 167. DOI: 10.3390/nu8030167
[8] Chouni A, Paul S. A Review on Phytochemical and Pharmacological Potential of Alpinia Galanga. Pharmacogn J, 2018; 10: 09-15. DOI: 10.5530/pj.2018.1.2
[9] Vicente-Vicente L, González-Calle D, Casanova AG et al. Quercetin, a promising clinical candidate for the prevention of contrast-induced nephropathy. Int J Mol Sci, 2019; 20: 4961. DOI: 10.3390/ijms20194961
[10] Pinto MEA, Araújo SG, Morais MI et al. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. An Acad Bras Cienc, 2017; 89: 1671-1681. DOI: 10.1590/0001-3765201720160908
[11] Andriana Y, Xuan TD, Quy TN et al. Biological Activities and Chemical Constituents of Essential Oils from Piper cubeba Bojer and Piper nigrum L. Molecules, 2019; 24: 1876. DOI: 10.3390/molecules24101876
[12] Chompoo J, Upadhyay A, Fukuta M et al. Effect of Alpinia zerumbet components on antioxidant and skin diseases-related enzymes. BMC Complement Altern Med, 2012; 12: 106. DOI: 10.1186/1472-6882-12-106
[13] Minh TN, Xuan TD, Tran H-D et al. Isolation and Purification of Bioactive Compounds from the Stem Bark of Jatropha podagrica. Molecules, 2019; 24: 889. DOI: 10.3390/molecules24050889
[14] Andriana Y, Xuan TD, Quy TN et al. Antihyperuricemia, Antioxidant, and Antibacterial Activities of Tridax procumbens L. Foods, 2019; 8: 21. DOI: 10.3390/foods8010021
[15] Yue-ling M, Yu-jie C, Ding-rong W et al. HPLC determination of quercetin in three plant drugs from genus sedum and conjecture of the best harvest time. Pharmacogn J, 2017; 9: 725-728. DOI: 10.5530/pj.2017.6.114
[16] Batiha GE, Beshbishy AM, Ikram M et al. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods, 2020; 9: 374. DOI: 10.3390/foods9030374
[17] Anand David AV, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev, 2016; 10: 84-89. DOI: 10.4103/0973-7847.194044
[18] Du J, Zhao L, Ma L et al. Acaricidal Activity of the Oleic Acid Methyl Ester from Pharbitis purpurea Seeds against Tetranychus Cinnabarinus. In: Information Technology and Agricultural Engineering. Zhu E, Sambath S ed. Springer: Berlin, Germany, 2012; 621-628. DOI: 10.1007/978-3-642-27537-1_76
[19] Padmini N, Rashiya N, Sivakumar N et al. In vitro and in vivo efficacy of methyl oleate and palmitic acid against ESBL producing MDR Escherichia coli and Klebsiella pneumoniae. Microb Pathog, 2020; 148: 104446. DOI: 10.1016/j.micpath.2020.104446
[20] Saeed NM, El-Demerdash E, Abdel-Rahman HM et al. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol Appl Pharmacol, 2012; 264: 84-93. DOI: 10.1016/j.taap.2012.07.020
[21] Saxena M, Faridi U, Srivastava SK et al. A Cytotoxic and Hepatoprotective Agent from Withania somnifera and Biological Evaluation of its Ester Derivatives. Nat Prod Commun, 2007; 2: 775-778. DOI: 10.1177/1934578X0700200714
[22] Chansriniyom C, Bunwatcharaphansakun P, Eaknai W et al. A synergistic combination of Phyllanthus emblica and Alpinia galanga against H2O2-induced oxidative stress and lipid peroxidation in human ECV304 cells. J Funct Foods, 2018; 43: 44-54. DOI: 10.1016/j.jff.2018.01.016
[23] Cheah PB, Hasim NHA. Natural Antioxidant Extract from Galangal (Alpinia galanga) for Minced Beef. J Sci Food Agr, 2000; 80: 1565-1571. DOI: 10.1002/1097-0010(200008)80:10<1565::AID-JSFA677>3.0.CO;2-7
[24] Tuyen PT, Xuan TD, Khang DT et al. Phenolic compositions and antioxidant properties in bark, flower, inner skin, kernel and leaf extracts of castanea crenata sieb. et zucc. Antioxidants-Basel, 2017; 6: 31. DOI: 10.3390/antiox6020031
[25] Pillai MK, Ismail R, Sasidharan S et al. Antioxidant and Antimicrobial Activities of Rhizome Extracts from Malaysian Species of Alpinia galanga and Alpinia officinarum. Pharmacol Online, 2019; 1: 366-375.
[26] Ma XN, Xie CL, Miao Z et al. An overview of chemical constituents from Alpinia species in the last six decades. RSC Adv, 2017; 7: 14114-14144. DOI: 10.1039/C6RA27830B
[27] Van TM, Xuan TD, Minh TN et al. Isolation and purification of potent growth inhibitors from piper methysticum root. Molecules, 2018; 23: 1907. DOI: 10.3390/molecules23081907
[28] García-Mediavilla V, Crespo I, Collado PS et al. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur J Pharmacol, 2007; 557: 221-229. DOI: 10.1016/j.ejphar.2006.11.014
[29] Yang D, Wang T, Long M et al. Quercetin: its main pharmacological activity and potential application in clinical medicine. Oxid Med Cell Longev, 2020; 2020: 8825387. DOI: 10.1155/2020/8825387
Copyright © 2022 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.





 Copyright ©
Copyright ©