Isolation and Identification of Lactic Acid Bacteria from Traditional Fermented Milk "Dahi" Towards Developing Probiotic Dahi in Bangladesh
Umme Salma Amin1, AKM Humayun Kober1*, Nasima Akter1, M.A. Hossain1, Eaftekhar Ahmed Rana2
1Department of Dairy and Poultry Science, Chattogram Veterinary and Animal Sciences University (CVASU), Chattogram, Bangladesh
2Department of Microbiology and Veterinary Public Health, Chattogram Veterinary and Animal Sciences University (CVASU), Chattogram, Bangladesh
*Corresponding to: AKM Humayun Kober, Department of Dairy and Poultry Science, Chattogram Veterinary and Animal Sciences University (CVASU), Chattogram, Bangladesh; E-mail: akmhumayun@cvasu.ac.bd
Abstract
Objective: An attempt was made herein to isolate lactic acid bacteria (Lactobacillus sp. and Streptococcus thermophilus) from local Dahi to develop Dahi with isolated culture.
Methods: These bacteria were identified by morphological characteristics, microscopic observation, catalase reaction, and finally examined by Polymerase Chain Reaction (PCR). Five Dahi samples (T1, T2, T3, T4, and T5) were prepared using the PCR culture. Dahi samples (T1, T2, and T3) were prepared using three Lactobacillus sp., T4 by Streptococcus thermophilus, and T5 by a combination of Lactobacillus sp. (T3) and Streptococcus thermophilus (T4) bacteria. Physicochemical, microbiological, and sensory properties were measured to assess the quality of the developed Dahi. The shelf life of the products was assessed through the determination of pH and titrable acidity of the samples measured on day 1 and 7, respectively.
Results: Ash and protein percentage of T3 sample were significantly (P<0.05) better than others. The viable count was well above the minimum recommended value (106cfu/ml) in all the Dahi samples. Results of sensory properties namely taste, aroma, and overall acceptability of T3 and T4 Dahi were significantly (P<0.05) found superior compared to other samples.
Conclusion: The lactic acid bacteria isolated and utilized in this study demonstrate great potential in the production of probiotic Dahi in future.
Keywords: isolation, lactic acid bacteria, Lactobacillus sp., Streptococcus thermophilus, Dahi
1 INTRODUCTION
Fermented dairy products have long been an essential component of a nutritional diet. Dahi is a popular fermented dairy product in the Indian subcontinent including Bangladesh, with a similar appearance to that of yoghurt[1]. It is prepared by fermenting milk from cows, buffalos, or goats with mesophilic lactic cultures, and its preparation process and physicochemical characteristics are well known. Yoghurt, a western counterpart of Dahi, in addition to its nutritive value and high palatability, is believed to be effective in preventing and treating various illnesses, viz., gastrointestinal disorders, heart diseases, and tumor development, both in humans and animals. Several health benefits of Dahi and yoghurt have been reported. Lactic acid bacteria (LAB) are known as “milk souring organisms”. With their extensive use in the development of fermented foods, the LAB have received considerable attention, which are characterized by hygienic safety, improved organoleptic properties, and potential probiotic qualities[2]. Some of them also constitute a natural component of the intestinal microflora[3]. Lactobacilli and Bifidobacteria are the most common bacteria considered as potential probiotics. Probiotics serve to supplement the host microbes and provide protection against several enteric pathogens. The yoghurt/Dahi is a good source of probiotics/immunoregulatory probiotics, as they feature various physiological functions which contribute to the health of the host environment regulating microflora and also to combating overweight and obesity[4]. Fermented milk products such as Dahi may possess various potential benefits for the human body, including as similation of cholesterol[5], destroying enteric pathogen, prevention of inflammatory bowel disease, and lowering blood pressure[6].
Dahi is easily digestible and canreduce the risk of cardiovascular problems and cancerous tumours, as it boosts body immunity.
In view of the above, fermented dairy foods, introduced to a balanced diet and healthy lifestyle, potentially contribute to human health and disease prevention, which, however, has been overlooked by people of Bangladesh to date. Furthermore, very little information is available on the characteristics of Lactobacillus microflora present in locally available Dahi in Bangladesh. In developed countries, milk and food companies have recently developed and sold many types of functional yoghurts using selected probiotics including beneficial bacteria such as Lactobacillus sp. and Bifidobacterium sp. The conventionally fermented dairy product could be considered a valuable resource for probiotic strain screening and starter culture[7]. However, very limited work has been done in Bangladesh regarding the specific strain Dahi production, which, with sufficient evidence, will facilitate the local production of functional fermented dairy products the promotion of the national Gross Domestic Product (GDP), and the improvement of national health. Hence, the present research was undertaken to isolate and identify Lactobacillus sp. and Streptococcus thermophilus from traditional fermented milk “Dahi” through phenotypic and genotypic characterization, to develop Dahi of specific strain culture and to assess the physicochemical, microbiological, and sensory properties of the prepared product as a primary step towards developing the probiotic Dahi in Bangladesh.
2 MATERIALS AND METHODS
2.1 Statement of the Experiment
The experiment was carried out in the Dairy Science laboratory of the Department of Dairy and Poultry Science (DDPS) and Poultry Research & Training Centre (PRTC) of Chattogram Veterinary and Animal Sciences University (CVASU), Bangladesh, from July to November 2019.
2.2 Sample Collection
The Dahi samples (Totally 8 types, both branded and non-branded Dahi) were purchased from the local markets of Chattogram metropolitan city, Chattogram, Bangladesh. After collection, the samples were stored in a sterile plastic container and then preserved aseptically at a temperature of 4 C for the isolation of bacteria (Lactobacillus sp. and Streptococcus thermophilus).
2.3 Isolation of Lactobacillus sp. and Streptococcus Thermophilus
The Lactobacillus sp. were isolated from Dahi using streak plate technique on De Mann Rogosa Sharpe (MRS) agar and were investigated by microscopic characteristics of Gram staining and catalase reaction.
Samples were directly streaked onto blood agar to isolate Streptococcus thermophilus, followed by a 24h incubation of the agar plates at 37°C. Characteristic S. thermophilus colonies were then subcultured to attain pure cultures, which were further investigated by microscopic characteristics of Gram staining and catalase reaction.
2.4 Catalase Test (Slide test)
A small amount of bacterial colony was transferred to a clean, dry glass slide surface using a loop, prior to the addition of a drop of 3% H2O2 and mixed well. A positive result is the rapid evolution of oxygen (within 5-10 sec.) as evidenced by bubbling. A negative result is no bubbles or only a few scattered bubbles. Lactobacillus sp. and S. thermophilus are both catalase negative and no O2 production (gas bubbles) was observed when 3% H2O2 solution is dropped on top of the colonies grown overnight on agar medium. Then the slide was discarded in the biohazard glass disposal container.
2.5 Preservation of Cultures
All presumptive isolates were cultured into brain heart infusion (BHI) (Oxoid Ltd., UK) broth and incubated overnight at 37°C. Investigation of each isolate was conducted after 700 μl BHI broth culture was mixed with 300 μl 50% glycerol in 2 ml sterile eppendorf tube and stored at -80°C.
2.6 Deoxyribonucleic Acid (DNA) Extraction
Chromosomal DNA was isolated by the conventional boiling method[8], and the bacterial DNAs were preserved at -20°C for further testing.
2.7 Polymerase Chain Reaction (PCR) of Lactobacillus sp.
The identification of Lactobacillus sp. was carried out by PCR using specific primer sets (Table 1) to amplify 16s rRNA gene of Lactobacillus sp[9]. PCR reactions were conducted with a 25 μl reaction volume which was prepared with 2.5 μl of DNA (20 picomole/μl), 11.75 μl of master mix (Thermo Scientific Dream Taq PCR Master Mix (2x) Ready to use), 5 μl of both primer sets (20 pmol), and 0.75 μl of nuclease-free water. The PCR program was carried out by the following program: Initial denaturation 94°C for 3 min, 29 cycles of denaturation at 94°C for 30s, annealing at 55°C for 30s, extension at 72°C for 3 min and a final extension of 72°C for 10 min[9].
Table 1. Primers Used for the Detection and Confirmation of Lactobacillus sp. and Streptococcus Thermophilus for this Study
Target Organisms |
Primer Set |
Primer Sequence (5’-3’) |
Ref |
Lactobacillus sp. |
LAC1F LAC2R |
AGCAGTAGGGAATCTTCCA ATTTCACCGCTACACATG |
(Gebreselassie et al. 2016) |
Streptococcus thermophilus |
ThI ThII |
ACGGAATGTACTTGAGTTTC TGGCCTTTCGACCTAAC |
(Vanatkova et al. 2009) |
Amplification (PCR) was performed in a thermal cycler (Applied Biosystem®, 2720).
2.8 Polymerase Chain Reaction(PCR) of Streptococcus Thermophilus
Primers used to identify S. thermophilus are enlisted in Table 1. The PCR reaction mixture (25 μl) was prepared from 4 μl of chromosomal DNA (20 picomole/μl), 12.5 μl of master mix (Thermo Scientific Dream Taq PCR Master Mix (2x) Ready to use), 2 μl of specific primer sets (20 pmol), and 4.5 μl of nuclease-free water. The PCR program used for S. thermophilus had 1 cycle of 95°C for 5 min, followed by 40 cycles of 95°C for 1 min, 52°C for 30s and 72°C for 1 min, then 1 cycle of 72°C for 5 min[10].
2.9 Visualization of the PCR Products by Agarose Gel Electrophoresis
PCR amplified products were separated by agarose gel electrophoresis in 1.5% agarose solution (Seakem® LE agarose, Lonza) and the gel was stained with ethidium bromide. DNA fragments were visualized by an ultra-violet (UV) illuminator (BDA digital, biometra GmbH, Germany). The specific size of the amplified product is 340 base pair (bp)[11] and 250bp[12] in the case of Lactobacillus sp. and S. thermophilus, respectively.
2.10 Development of Dahi
2.10.1 Preparation of Lactobacillus sp. Culture
Lactobacillus sp. cultures (eighteen isolates) were reconstituted from frozen stock cultures by plating out on MRS agar. The plates were incubated for 48-72 hours at 37°C. Thereafter, a loopful colony was inoculated in 10 ml MRS broth and was incubated at 37°C for 24 hours to get samples with a close concentration level of 106 colony forming units per ml(cfu/ml) susceptibility testing.
2.10.2 Preparation of Streptococcus Thermophilus Culture
S. thermophilus cultures (single isolate) were reconstituted from frozen stock cultures by plating out on blood agar. The plates were incubated for 24 hours at 37°C. Afterward, a loopful colony was inoculated in 10 ml BHI broth and was incubated at 37°C for 24 hours to get samples with a close concentration level of 106 cfu/ml susceptibility testing.
2.10.3 Preparation of Mother Culture
The Lactobacillus sp. and S. thermophilus cultures grown in MRS and BHI broth respectively were centrifuged at 4000 rotations per minute (rpm) for 5 minutes to get bacterial pellets which were rinsed twice with sterile phosphate buffered saline (PBS) and were then reconstituted in 2 ml of PBS. Subsequently, a 200 μl aliquot containing 5 x 108 cfu/ml was added to 10 ml of the milk that was cooled down to 35°C after a boil and then incubated for 18 hours at 37°C.
2.10.4 Preparation of Dahi
Whole fresh cow milk was used for the preparation of Dahi. After collection, milk was filtrated and boiled to inactivate viable microorganisms. The volume was reduced to 25% by boiling, followed by the addition of 5% of milk powder and 4% of sugar. The fat and protein content of the milk was found 3.8% and 3.6% respectively on testing after adding milk powder. The milk was stirred continuously to cool down to 30-35°C and was then seeded with 2% of prepared culture. In Dahi prepared using all the eighteen different cultural isolates of Lactobacillus sp., T1, T2, T3 presented the best results among the others. In addition, In the Dahi prepared with the combination of Lactobacillus sp. (T1, T2, T3) and S. thermophilus (T4) cultures together, the combination of T3 and T4 displayed the best results. Finally, five different types of Dahi such as T1, T2, T3 were prepared using three different cultural isolates of Lactobacillus sp., T4 was prepared by using S. thermophilus culture, and T5was prepared by adding the combination of Lactobacillus sp. (T3) and S. thermophilus (T4) cultures together. After filling in, the containers were then incubated at 37°C and 42°C for 6-8 hours for Lactobacillus sp. and S. thermophilus cultures, respectively. Then the prepared Dahi was stored at 4°C for further use.
2.11 Determination of Physicochemical and Microbiological Properties of Developed Dahi
The developed Dahi was analyzed in terms of pH, titrable acidity, moisture, ash, and protein. All the determinants were conducted in triplicates and the results were expressed as the average. The pH and titrable acidity were detected on day 1 and 7. Acidity percentage was determined followed by the procedure described by Aggarwala and Sharma[13]. The pH of the preparations was measured using a digital microprocessor pH meter (pHepÒ3, Hanna Instruments, USA). The Dahi samples were tested for proximate analysis to determine moisture %, crude protein (CP %), and ash content[14]. For microbiological analysis, total viable count[15] and coliform count were determined by the methods described in the "Standard Methods for the examination of Dairy Products" APHA[16]. The microbial counts were expressed in log cfu/ml.
2.12 Sensory Evaluation of Prepared Dahi
The sensory characters of the developed Dahi samples were evaluated by the panel expert following the method as described by Shekhar et al.[17] using “9-point hedonic scale” (1, 2, 3, 4, 5, 6, 7, 8, and 9 represent dislike extremely, dislike very much, dislike moderately, dislike slightly, neither like nor dislike, like slightly, like moderately, like very much, and like extremely, respectively).
2.13 Statistical Analysis
All the collected data were subjected to statistical analyses by using one-way analysis of variance (ANOVA) (Minitab version 16, 2000). The significance of differences between means was determined by Fisher’s least significant difference at P≤0.05.
3 RESULTS AND DISCUSSION
3.1 Isolation of Lactobacillus sp. and Streptococcus Thermophilus
3.1.1 Morphological Characteristics
In this study, the presumptive isolates of Lactobacillus sp. grew as cream-coloured, circular, convex, shiny, and moist with a smooth edge (Figure 1A). All the isolates were morphologically similar to Lactobacillus sp[18]. The isolates of presumptive Streptococcus thermophilus were selected on the basis of characteristic colony morphologies of the isolates; small, dew-drop like colony producing hemolysis on blood agar (Figure 1B).
|
Figure 1A. Growth of Lactobacillus sp. on MRS agar. The isolates were cream coloured, circular, convex, glossy and moist with smooth edge. B. Growth of Streptococcus thermophilus on blood agar. The isolates grew as small, dew-drop like colony producing hemolysis on blood agar.
3.1.2 Microscopic Observation and Catalase Test
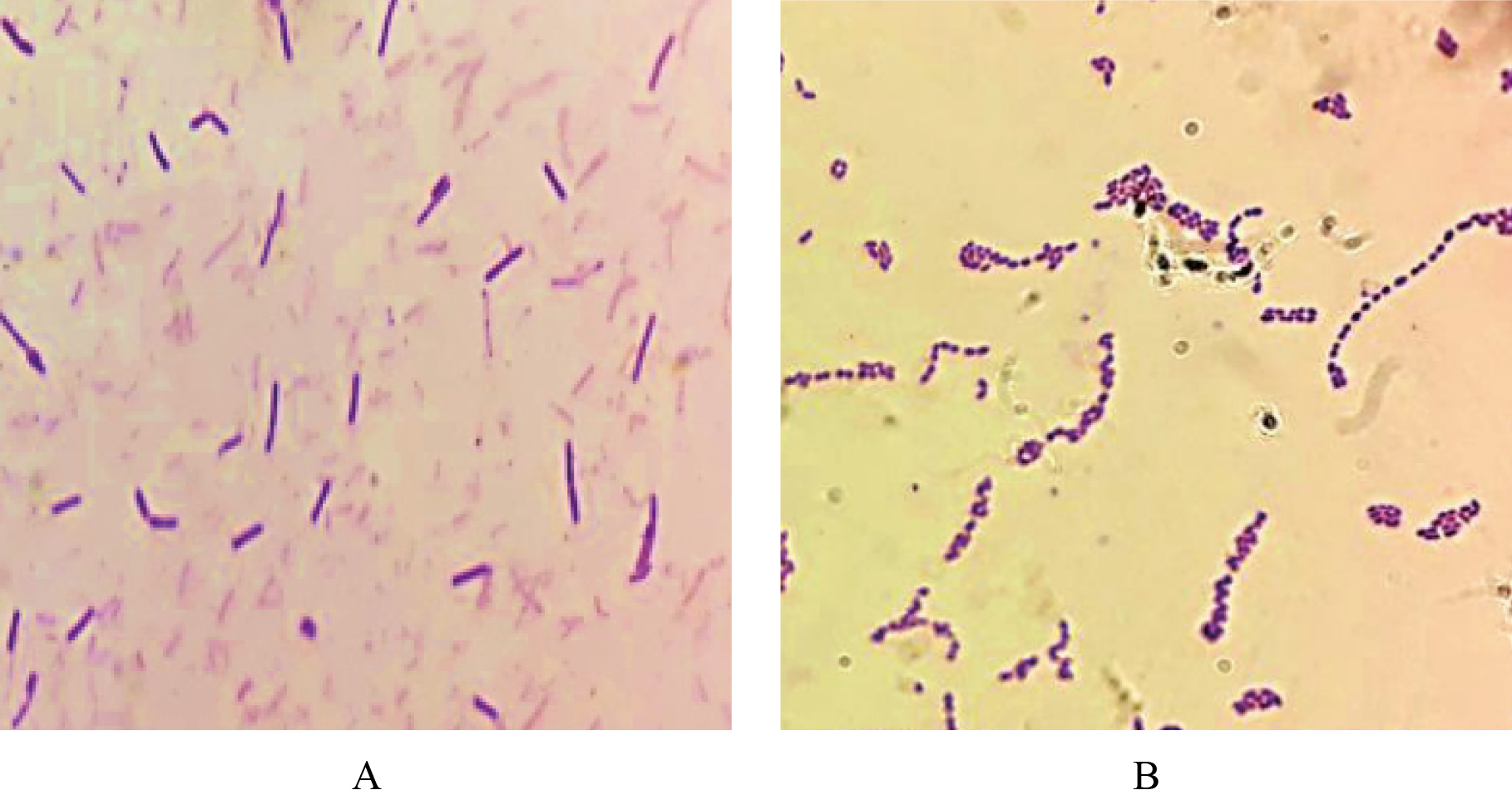
Gram stain morphology of Lactobacillus sp. varies, including as short, long, plump rods, slender rods, in chains or palisades. In this study, all the presumptive isolates of Lactobacillus sp. and S. thermophilus were of the purple coloured rod[18] (Figure 2A) and Gram positive ovoid-spherical cocci of 0.7 to 0.9 μm in diameter that were found in chains and in pairs[19] (Figure 2B), respectively under a microscope on Gram staining. During Gram staining, they took the color of primary stain crystal violet, as they possess a thick mesh-like cell wall which is composed of peptidoglycan layer (50–90% of cell envelope), and appeared as purple under a light microscope. No gas bubble was formed during the catalase reaction, indicating all the presumptive isolates as catalase negative. Catalase enzyme breaks down hydrogen peroxide into oxygen and water molecules (2H2O2→2H2O+O2) and oxygen production is observed by the generation of O2 bubbles. The generation of gas bubbles indicates the presence of the enzyme and hence the catalase positive nature of the bacterium.
|
Figure 2A. Gram stained Lactobacillus sp. 100X magnified. Microscopically they were rod-shaped, gram-positive bacilli. Scale bars indicate 20 μm. B. Gram stained Streptococcus thermophilus 100X magnified. The isolates were gram-positive chain-forming cocci. Scale bars indicate 50 μm.
3.2 Molecular Identification of Lactobacillus sp. and Streptococcus Thermophiles
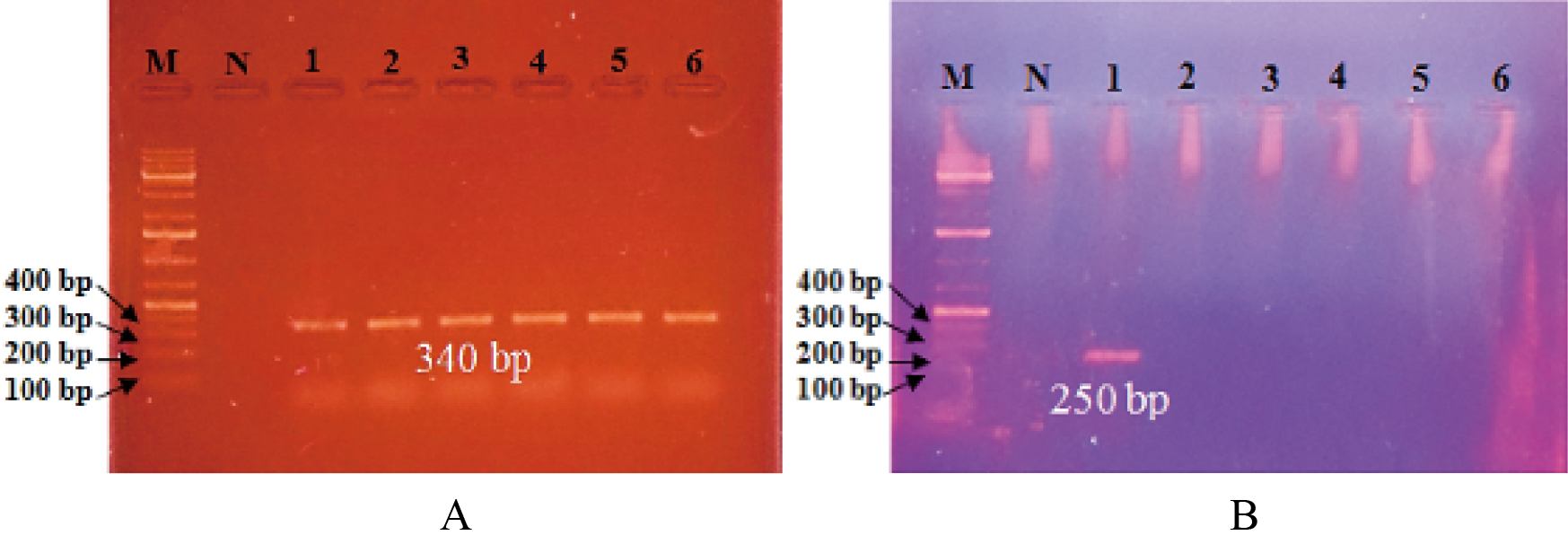
In this experiment, eighteen isolates of Lactobacillus sp. were identified by PCR using primer set LAC1F/LAC2R which amplified a 340bp fragment of the 16s rRNA gene (Figure 3A). Likewise, Gebreselassie et al.[9] confirmed the Lactobacillus sp. by the method PCR-DGGE using the primer set LAC1F/LAC2R. In their study, they obtained the Lactobacillus culture from naturally fermented buttermilk. Walter et al.[11] identified different Lactobacillus sp. by using the above-mentioned primers which produced a PCR product of the intended size of 340bp. S. thermophilus (single isolate) was confirmed by PCR using primer set ThI/ThII. Mentioned primers provided a PCR product with the expected size of 250bp (Figure 3B). The result is similar to that described by Brigidi et al.[12] and Vanatkova et al.[10] who identified the PCR product size of primer set ThI/ThII specificity to target species.
|
Figure 3A. Lactobacillus sp. specific PCR assay. This figure illustrates fragments specifically amplified by PCR by means of the primer set LAC1F/ LAC2R. Lane M: 1 kb plus DNA marker, Lane N: negative control. Lanes 1-6: PCR products of amplified chromosomal DNA of Lactobacillus sp. (340bp). B. Streptococcus thermophilus specific PCR assay.
3.3 Development of Dahi
3.3.1 Physicochemical Analysis
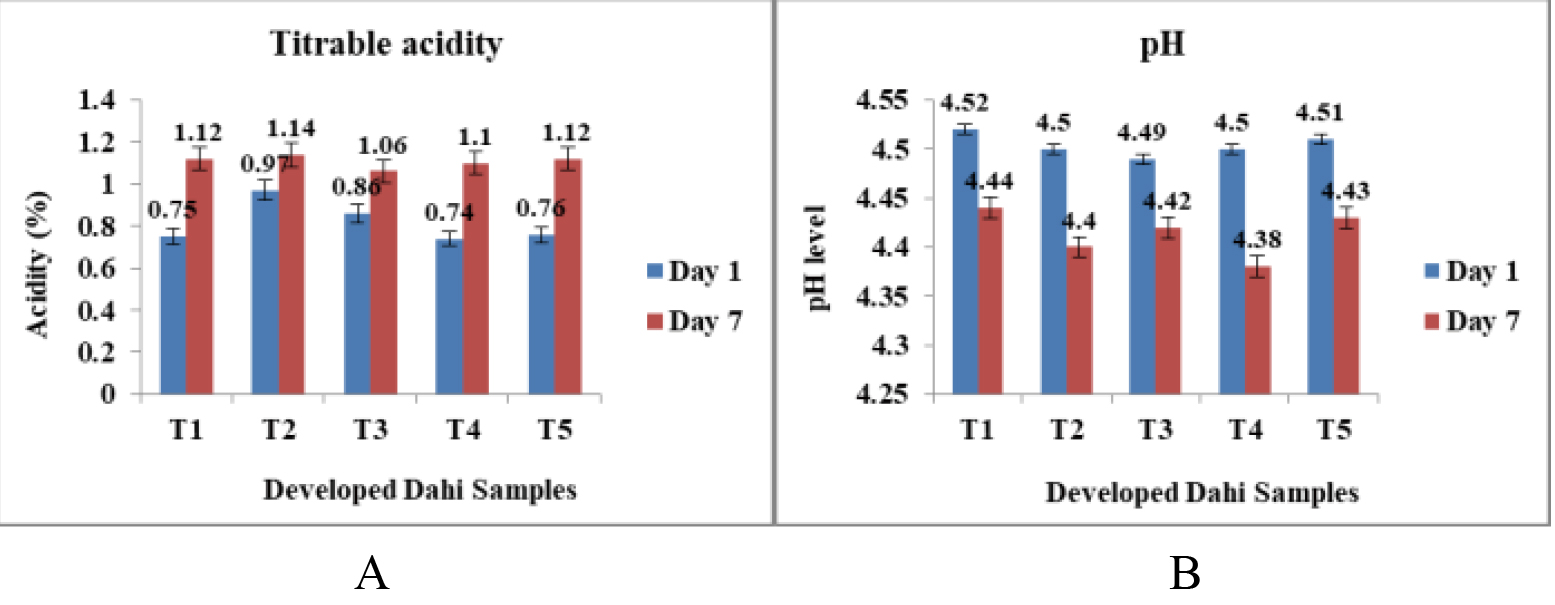
The development of Dahi using isolated Lactobacillus sp. and S. thermophilus was successful as the preparation yielded a complete process. There was an increase in acidity with the progress of the storage time (Figure 4A). However, the results of titrable acidity of all our experimental samples are in line with the results reported by Kober et al.[1] and Vijayendra and Gupta[20]. Aryana and Olson[21] reported that the most desirable yoghurt resulted in a titrable acidity of 0.74 to 0.83% when being placed into cold storage and acidity of 0.91 to 0.93% during cold storage. The pH values of different Dahi samples were decreased with time (Figure 4B). The drop in pH is attributed to the absorption of lactose by microbial culture, which ultimately leads to the production of lactic acid, formic acid, and small amounts of CO2[22].
|
Figure 4A. Titrable acidity of the Developed Dahi on day 1 and day 7. T1, T2, T3 treatments refer to Dahi samples made from three different cultural isolates of Lactobacillus sp., whereas T4 means Dahi sample from Streptococcus thermophilus culture and T5 denotes Dahi sample prepared by a combination of Lactobacillus sp. (T3) and S. thermophilus (T4) cultures together. (Data shown are of three independent experiments performed in triplicates). B. pH of the Developed Dahi on day 1 and day 7. (Data shown are of three independent experiments performed in triplicates).
The results of the chemical composition of the developed Dahi are shown in Table 2. Significant variation was observed in the values of moisture, protein, and ash contents of the different samples of developed Dahi. From Table 2, it is obvious that the ash and protein% of T3 sample were significantly higher in comparison with that of other samples. Low moisture content and ingredient composition might be the reasons behind the enhanced protein and ash contents of the T3 Dahi.
Table 2. Chemical Composition (Moisture, Protein and Ash) of Developed Dahi
Developed Dahi Samples |
Moisture% |
Protein% (Fresh Basis) |
Ash% (Fresh Basis) |
T1 |
77.63a |
5.15b |
1.31c |
T2 |
74.10b |
5.64ab |
1.34b |
T3 |
74.46b |
5.85a |
1.37a |
T4 |
79.33a |
4.31c |
1.09d |
T5 |
79.58a |
4.41c |
1.09d |
SEM |
0.200 |
0.050 |
0.001 |
P- value |
P<0.001 |
P<0.001 |
P<0.001 |
Data refer to mean values consisting of three replicates; a, b, c, d Means bearing uncommon superscripts within a column is significantly different at the level mentioned in the above Table; SEM=Standard error of the means; T1, T2, T3 treatments refer to Dahi samples made from three different cultural isolates of Lactobacillus sp., whereas T4 means Dahi sample from Streptococcus thermophilus culture and T5 denotes Dahi sample prepared by a combination of Lactobacillus sp. (T3) and S. thermophilus (T4) cultures together.
3.3.2 Microbiological analysis
The values of the lactic acid bacterial counts (cfu/ml) of the Dahi samples are presented in Table 3. To achieve the therapeutic benefit, a minimum number of 106–107cfu of viable cells of probiotic cultures is a must in the products until the time of consumption[23]. The viable count of Dahi cultures was well above the minimum recommended amount at their production. The total coliform count was nil in all Dahi samples. As reported in Indian standard 9617[24], the coliform count per gram is limited to 10 cfu for Dahi. Bakr et al[25]. prepared bio yoghurt and reported the nil of coliform counts in all fresh and stored treatments, which might be due to the effect of heat treatment and the role of yoghurt bacteria in coliform control by producing various antibacterial compounds.
Table 3. Viable Count in the Developed Dahi (Data Shown are of Three Independent Experiments Performed in Triplicates)
Developed Dahi Samples |
Total Viable Count (cfu/ml) |
T1 |
1.6×107 |
T2 |
1.89×107 |
T3 |
1.9×107 |
T4 |
1.96×107 |
T5 |
1.98×107 |
3.3.3 Sensory evaluation of Developed Dahi
In our experimental, Dahi, various sensorial characters such as colour, appearance, taste, aroma, body and texture, overall acceptability, etc. were evaluated by the panel expert following the method described by Shekhar et al[17]. The scores of aroma, taste, and overall acceptability showed a significant difference between treatments (Table 4). The colour and appearance, body, and texture scores obtained by different samples showed no significant difference between and within treatments. Dahi samples produced from Streptococcus thermophilus (T4) and Lactobacillus sp. (T3) were preferred by the panelists. The higher palatability, increased nutritive value, and the compatible action of using suitable organisms might serve to enrich these characters of the Dahi (T3, T4). Higher fat content of the sample might increase the palatability, taste, or flavour nature of the Dahi, and which in turn, could enhance the consumer acceptability to the products. We also found that the quality of T3, T4 Dahi was better than that of local Dahi available in the market.
Table 4. Sensory Evaluation Scores of Developed Dahi
Developed Dahi Samples |
Colour& Appearance |
Aroma |
Taste |
Body & Texture |
Overall Acceptability |
T1 |
8.66 |
8.66ab |
8.33ab |
8.66 |
8.00b |
T2 |
8.33 |
8.00b |
7.33b |
8.00 |
8.00b |
T3 |
9.00 |
9.00a |
9.00a |
8.66 |
8.66ab |
T4 |
9.00 |
9.00a |
8.33ab |
8.66 |
9.00a |
T5 |
8.33 |
8.33ab |
8.00ab |
8.33 |
8.33ab |
SEM |
0.115 |
0.094 |
0.115 |
0.133 |
0.094 |
P- value |
0.233 |
0.029 |
0.013 |
0.452 |
0.029 |
Data refer to mean values of three replicates; a, b Means bearing uncommon superscripts within a column is significantly different at the level mentioned made from in the Table; SEM=Standard error of the means; T1, T2, T3 treatments refer to Dahi samples made from three different cultural isolates of Lactobacillus sp., whereas T4 means Dahi sample from Streptococcus thermophilus culture and T5 denotes Dahi sample prepared by a combination of Lactobacillus sp. (T3) and S. thermophilus (T4) cultures together.
4 CONCLUSION
The developed Dahi with the specific starter organisms can be prepared successfully using cultures obtained from the locally produced Dahi available in the market of Bangladesh. The viable count of the Dahi denoted sufficient viability of starter bacteria to reach the standard limit (>106cfu/ml) in all the developed Dahi samples. Notwithstanding the variation in pH and acidity measured in this study, their levels were acceptable which approximate the normal level that contributes to prolonging the shelf life of the dairy food products. T3 (Lactobacillus sp.) and T4 (Streptococcus thermophilus) Dahi samples showed the best performance over others on chemical composition, sensory evaluation, and shelf life. The experiment can surmise that Dahi developed using prepared cultures will certainly appeal to health-conscious consumers given the future establishment of probiotic properties.
Acknowledgements
The authors would like to express their sincere gratitude and profound appreciation to the Ministry of Science and Technology, Bangladesh for providing National Science and Technology (NST) Fellowship 2018-2019 Award for this research.
Conflicts of Interest
The authors declared that there are no conflict of interest.
Author Contribution
Amin US performed this study and wrote the article. Kober AKMH and Hossain MA supervised the study and revised the papers for important intellectual content. Akter N and Rana EA supervised the methods of the study; all authors approved the final version.
Abbreviation List
bp, Base pair
cfu, Colony forming unit
LAB, Lactic acid bacteria
PBS, Phosphate buffer saline
PCR, Polymerase chain reaction
SEM, Standard errors of mean
sp., Species
References
[1] Kober AKMH, Mannan MA, Debnath GK et al. Quality evaluation of dahi/yogurts sold in Chittagong metropolitan area in Bangladesh. Int J Sustain Agr Technol, 2007; 3: 41-46.
[2] Aly S, AT OC, Alfred TS et al. Bacteriocins and lactic acid bacteria-A minireview. Afr J Biotechnol, 2006; 5: 678-683. DOI: 10.5897/AJB05.388
[3] Holzapfel WH, Haberer P, Geisen R et al. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am J Clin Nutr, 2001; 73: 365s-373s. DOI: 10.1093/ajcn/73.2.365s
[4] Tada A, Kober AH, Islam MA et al. Evaluation of fat accumulation and adipokine production during the long-term adipogenic differentiation of porcine intramuscular preadipocytes and study of the influence of immunobiotics. Cells, 2020; 9: 1715. DOI: 10.3390/cells9071715
[5] Noh DO, Kim SH, Gilliland SE. Incorporation of Cholesterol into the Cellular Membrane of Lactobacillus acidophilus ATCC 43121. J Dairy Sci, 1997; 80: 3107-3113. DOI: 10.3168/jds.S0022-0302(97)76281-7
[6] Alhaj OA, Kanekanian AD, Peters AC. Investigation on whey proteins profile of commercially available milk-based probiotics health drinks using fast protein liquid chromatography (FPLC). Brit Food J, 2007; 109: 469-480. DOI: 10.1108/0007 0700710753526
[7] Harun-ur-Rashid M, Togo K, Ueda M et al. Probiotic characteristics of lactic acid bacteria isolated from traditional fermented milk “Dahi” in Bangladesh. Pak J Nutr, 2007; 6: 647-652. DOI: 10.3923/pjn.2007.647.652
[8] Ahmed OB, Asghar AH, Elhassan MM. Comparison of three DNA extraction methods for polymerase chain reaction (PCR) analysis of bacterial genomic DNA. Afr J Microbiol Res, 2014; 8: 598-602. DOI: 10.5897/AJMR2013.6459
[9] Gebreselassie N, Abay F, Beyene F. Biochemical and molecular identification and characterization of lactic acid bacteria and yeasts isolated from Ethiopian naturally fermented buttermilk. J Food Sci Technol, 2016; 53: 184-196. DOI: 10.1007/s13197-015-2049-z
[10] Vanatkova Z, Okenkova E, Drab V et al. Molecular Diagnostic of Streptococcus thermophilus. Ecol Chem Eng A, 2009; 16: 1627-1635.
[11] Walter J, Hertel C, Tannock GW et al. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella Species in Human Feces by Using Group-Specific PCR Primers and Denaturing Gradient Gel Electrophoresis. Appl Environ Microb, 2001; 67: 2578-2585. DOI: 10.1128/AEM.67.6.2578-2585.2001
[12] Brigidi P, Swennen E, Vitali B et al. PCR detection of Bifidobacterium strains and Streptococcus thermophilus in feces of human subjects after oral bacteriotherapy and yogurt consumption. Int J Food Microbiol, 2003; 8: 203-209. DOI: 10.1016/S0168-1605(02)00245-3
[13] Aggarwala AC, Sharma RM. A laboratory manual of milk inspection, 4th ed. Asia Publishing House: New Deli, India, 1961.
[14] AOAC (Association of Official Analytical Chemists). AOAC: Official methods of analysis. 2005.
[15] Behrad S, Yusof MY, Goh KL et al. Manipulation of probiotics fermentation of yogurt by cinnamon and licorice: Effects on yogurt formation and inhibition of Helicobacter pylori growth in vitro. World Acad Sci Eng Technol, 2009; 60: 590-594. DOI: 10.5281/zenodo.1085691
[16] Marshall RT. Standard methods for the examination of dairy products. American Public Health Association: Washington, DC, USA, 1992.
[17] Shekhar S, Joe J, Kumar R et al. Effect of heat treatment of milk on the sensory and rheological quality of dahi prepared from cow milk. J Food Dairy Technol, 2012; 1: 9-15.
[18] Tharmaraj N, Shah NP. Selective enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus, and propionibacteria. J Dairy Sci, 2003; 8: 2288-2296. DOI: 10.3168/jds.S0022-0302(03)73821-1
[19] Hajira B, Naeem M, Nabi S et al. Review on molecular detection of Streptococcus thermophilus in dairy products. Indo Am J Pharm Sci, 2017; 4: 941-949.
[20] Vijayendra SVN, Gupta RC. Assessment of probiotic and sensory properties of dahi and yoghurt prepared using bulk freeze-dried cultures in buffalo mil. Ann Microbiol, 2012; 62: 939-947. DOI: 10.1007/s13213-011-0331-5
[21] Aryana KJ, Olson DW. A 100-Year Review: Yogurt and other cultured dairy products. J Dairy Sci, 2017; 100: 9987-10013. DOI: 10.3168/jds.2017-12981
[22] Panesar PS. Fermented Dairy Products: Starter Cultures and Potential Nutritional Benefits. Food Nutr Sci, 2011; 2: 47-51. DOI: 10.4236/fns. 2011.21006
[23] Boylston TD, Vinderola CG, Ghoddusi HB et al. Incorporation of bifidobacteria into cheeses: Challenges and rewards. Int Dairy J, 2004; 14: 375-387. DOI: 10.1016/j.idairyj.2003.08.008
[24] ISI. Specification for Dahi IS: No.9617-1980. Indian Standards Institution: New Delhi, India, 1980.
[25] Bakr IA, Mohamed TH, Tammam AA et al. Characteristics of bioyoghurt fortified with fennel honey. Int J Curr Microbiol App Sci, 2015; 4: 959-970.
Copyright © 2022 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.






 Copyright ©
Copyright ©