Test02
Amgad M. Rabie1,2*
1Dr. Amgad Rabie's Research Lab. for Drug Discovery (DARLD), Mansoura, Egypt
2Pharmaceutical Organic Chemistry Department, Faculty of Pharmacy, Mansoura University, Mansoura, Egypt
*Correspondence to: Amgad M. Rabie, PhD, Senior Drug Discovery Scientist & Clinical Research Associate, Mansoura University, 16 Magliss El-Madina Street, Dikernis City 35744, Dikernis, Dakahlia Governorate, Egypt; Email: amgadpharmacist1@yahoo.com
Abstract
Nucleic acid, nucleoside, nucleotide, DNA, and RNA analogs (i.e., nucleic acid therapeutics) are nowadays one of the most effective drug classes in medicinal chemistry against viral infections and tumors/cancers. Coronavirus disease 2019 (COVID-19) is still untreatable, with its causing virus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continuing to bring about losses everywhere. This difficult situation urged scientists to search for a potent anti-COVID-19 drug. Cordycepin (3'-deoxyadenosine) is a known natural adenosine analog of fungal origin. This bioactive phytochemical compound is characterized by several proven strong pharmacological actions that may effectively contribute to the comprehensive treatment of COVID-19, with the antiviral activities being the leading ones. Some new studies predicted the possible inhibitory affinities of cordycepin against the principal SARS-CoV-2 protein targets [e.g., SARS-CoV-2 spike (S) protein, main protease (Mpro) enzyme, and RNA-dependent RNA polymerase (RdRp) enzyme] based on the computational approach. Interestingly, the current research showed, for the first time, that cordycepin is able to potently inhibit the multiplication of the new resistant strains of SARS-CoV-2 with a very minute anti-SARS-CoV-2 half-maximal effective concentration (EC50) of about 2μM. Briefly, the current findings further support and suggest the repurposing possibility of cordycepin against COVID-19 and greatly encourage us to confidently and rapidly begin its preclinical/clinical evaluations for the comprehensive treatment of COVID-19.
Keywords: SARS-CoV-2, COVID-19, RNA-dependent RNA polymerase, cordycepin, adenosine, nucleic acid therapeutic, RNA therapeutic, drug design and discovery
1 INTRODUCTION
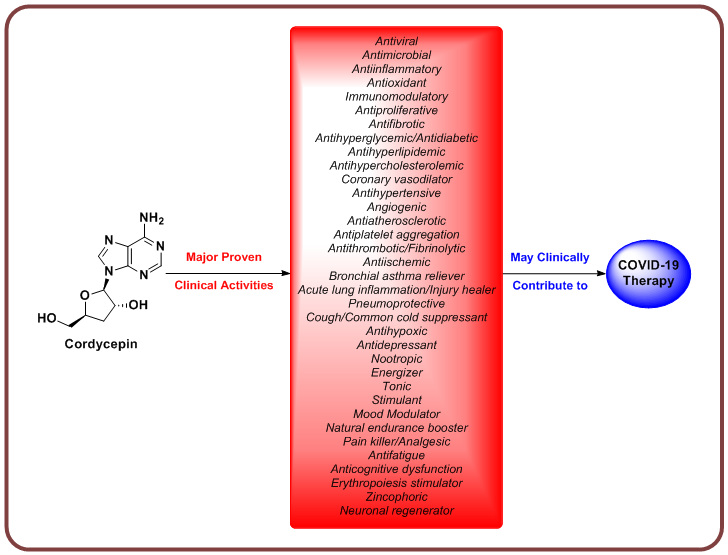
Coronavirus disease 2019 (COVID-19) is still untreatable, with its causing virus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continuing to give rise to losses everywhere. This complicated international situation urged all concerned scientists, including medicinal chemists and drug discoverers, to search for potent anti-COVID-19 drugs. Tens of new and repurposed promising compounds are nowadays under broad international investigations to be biologically evaluated as effective candidate anti-COVID-19 drugs, but none of them proved its satisfactory successful eventual broad-spectrum and comprehensive effectiveness to date[1-3]. Nucleoside analogs are certainly among the most successful bioactive classes of drug-like compounds in medicinal chemistry, as they are well-known agents for their numerous effective bioactivities in humans, specially as antivirals and anticancers[4,5]. Cordycepin (3'-deoxyadenosine) is a known natural adenosine nucleoside analog of fungal origin, which could be also synthetically produced[6]. This bioactive phytochemical compound is characterized by several proven strong pharmacological actions (e.g., it may act as antiviral, antimicrobial, antiinflammatory, antioxidant, immunomodulatory, antiproliferative, antifibrotic, antihyperglycemic/antidiabetic, antihyperlipidemic, antihypercholesterolemic, coronary vasodilator, antihypertensive, angiogenic, antiatherosclerotic, antiplatelet aggregation, antithrombotic/fibrinolytic, antiischemic, bronchial asthma reliever, acute lung inflammation/injury healer, pneumoprotective, cough/common cold suppressant, antihypoxic, antidepressant, nootropic, energizer, tonic, stimulant, mood modulator, natural endurance booster, pain killer/analgesic, antifatigue, anticognitive dysfunction, erythropoiesis stimulator, zincophoric, and neuronal regenerator) that may effectively contribute to the comprehensive treatment of COVID-19, with the antiviral and antiinflammatory activities being the leading ones[6,7] (Figure 1). Cordycepin is already being clinically investigated as a potential effective antileukemic/anticancer chemotherapeutic agent of the antimetabolites class since 1997 up till now in several clinical settings worldwide to pass the clinical phases 1 and 2 (e.g., clinical trials NCT00003005 and NCT00709215)[8,9]. Additionally, some new studies computationally predicted the possible inhibitory affinities of cordycepin against the principal SARS-CoV-2 protein targets [e.g., SARS-CoV-2 spike (S) protein, main protease (Mpro) enzyme, and RNA-dependent RNA polymerase (RdRp) enzyme] based on the in silico approach[10,11].
|
Figure 1. A chart enumerating the main pharmacological effects of cordycepin in humans which may efficiently alleviate most of the clinical sequelae of COVID-19 infection.
2 DISCUSSION
The currently-presented logic hypothesis, which suggests the potential strong anti-SARS-CoV-2/anti-COVID-19 properties of the cordycepin molecule, was actually tested and evaluated in this current work through a validated anti-SARS-CoV-2 bioassay (along with a full cytotoxicological bioevaluation), which was presupported by the promising results of in silico studies (see, e.g., Figures S1 and S2 in the Supplementary Data File). The complete procedures of the biological evaluation were carried out in a specialized biosafety level-3 laboratory against SARS-CoV-2 particles using Vero E6 cells (ATCC CRL-1586). For further details on the methodology, see the Materials and Methods section in the Supplementary Data File. Interestingly, in the current research we showed, for the first time, that cordycepin is able to potently inhibit the multiplication of the new resistant strains of SARS-CoV-2 [mainly against the new strain of SARS-CoV-2 virus, the first Variant of Concern from 2020, December (VOC-202012/01)] with a very minute in vitro anti-SARS-CoV-2 half-maximal effective concentration (EC50) of about 2μM, edging both remdesivir and its active metabolite GS-441524. Importantly, cordycepin was found to be about 10.5 and 7.8 times as effective as the two reference drugs remdesivir (EC50 = 21μM) and GS-441524 (EC50 = 15.60μM), respectively, in respect to the tested in vitro anti-VOC-202012/01/anti-SARS-CoV-2 bioactivity. According to the cytotoxicity assay, the in vitro half-maximal cytotoxic concentration (CC50) of cordycepin is significantly greater than 100μM, therefore this nucleoside analog is supposed to have very beneficial high clinical selectivity index (SI) (SIcordycepin>49.75; while remdesivir and GS-441524 have narrower SIs, SIremdesivir>4.76 and SIGS-441524>6.41), reflecting the specific/selective anti-RNA actions (RNA-disrupting activities) of the cordycepin molecule against the new coronaviral-2 genomes rather than the human genome. Cordycepin showed significantly small value of the concentration that results in 100% in vitro inhibition of the coronaviral-2 VOC-202012/01 cytopathic effects [full inhibitory concentration of cytopathic effects (CPEIC100) = 5.98μM], which is less than the corresponding values of remdesivir (CPEIC100 = 25.17μM) and GS-441524 (CPEIC100 = 17.40μM). In agreement with its potent antiviral-RNA activities, cordycepin also demonstrated very slight value of the concentration that is needed for 50% in vitro lowering in the number of RNA copies of the VOC-202012/01 strain of SARS-CoV-2 (2.35μM), which is clearly smaller than the corresponding values of both remdesivir and GS-441524 (22.92 and 16.04μM, respectively). Table 1 shows the detailed results of these in vitro assays[12].
Table 1. Anti-SARS-CoV-2/Anti-COVID-19 Activities (along with Cytotoxicity) of the Target Drug Cordycepin [Using Both Remdesivir and GS-441524 as the Positive Control/Reference Drugs, and Dimethylsulfoxide (DMSO) as the Negative Control/Placebo Drug] against SARS-CoV-2 (VOC-202012/01 Strain) in Vero E6 Cells[12]
Classification |
Compound Name |
CC50a (μM) |
Inhibition of SARS-CoV-2 Replication in vitro (Anti-VOC-202012/01 Bioactivities) (μM) |
||
100% CPE Inhibitory Concentration (CPEIC100)b |
50% Reduction in Infectious Virus (EC50)c |
50% Reduction in Viral RNA Copy (EC50)d |
|||
Target Compound |
Cordycepin |
>100 |
5.98±0.41 |
2.01±0.12 |
2.35±0.15 |
Reference Drugs |
Remdesivir |
>100 |
25.17±2.51 |
21.00±1.97 |
22.92±1.99 |
GS-441524 |
>100 |
17.40±1.83 |
15.60±0.76 |
16.04±0.81 |
|
Placebo Solvent |
DMSO |
>100 |
>100 |
>100 |
>100 |
Source from: https://pubs.acs.org/doi/10.1021/acsomega.1c05998. [Rabie AM. Potent Inhibitory Activities of the Adenosine Analogue Cordycepin on SARS-CoV-2 Replication. ACS Omega, 2022; 7 (3): 2960-2969. DOI: 10.1021/acsomega.1c05998]. Copyright © 2022 The Author. Published by American Chemical Society.
aCC50 or 50% cytotoxic concentration is the concentration of the tested compound that kills half the cells in an uninfected cell culture. CC50 was determined with serially-diluted compounds in Vero E6 cells at 48h postincubation using CellTiter-Glo Luminescent Cell Viability Assay (Promega).
bCPEIC100 or 100% CPE inhibitory concentration is the lowest concentration of the tested compound that causes 100% inhibition of the cytopathic effects (CPE) of SARS-CoV-2 VOC-202012/01 virus in Vero E6 cells under increasing concentrations of the tested compound at 48h postinfection. Compounds were serially diluted from 100μM concentration.
cEC50 or 50% effective concentration is the concentration of the tested compound that is required for 50% reduction in infectious SARS-CoV-2 VOC-202012/01 virus particles in vitro. EC50 is determined by infectious virus yield in culture supernatant at 48h postinfection (log10 TCID50/mL).
dEC50 or 50% effective concentration is the concentration of the tested compound that is required for 50% reduction in SARS-CoV-2 VOC-202012/01 viral RNA copies in vitro. EC50 is determined by viral RNA copies number in culture supernatant at 48h postinfection (log10 RNA copies/mL).
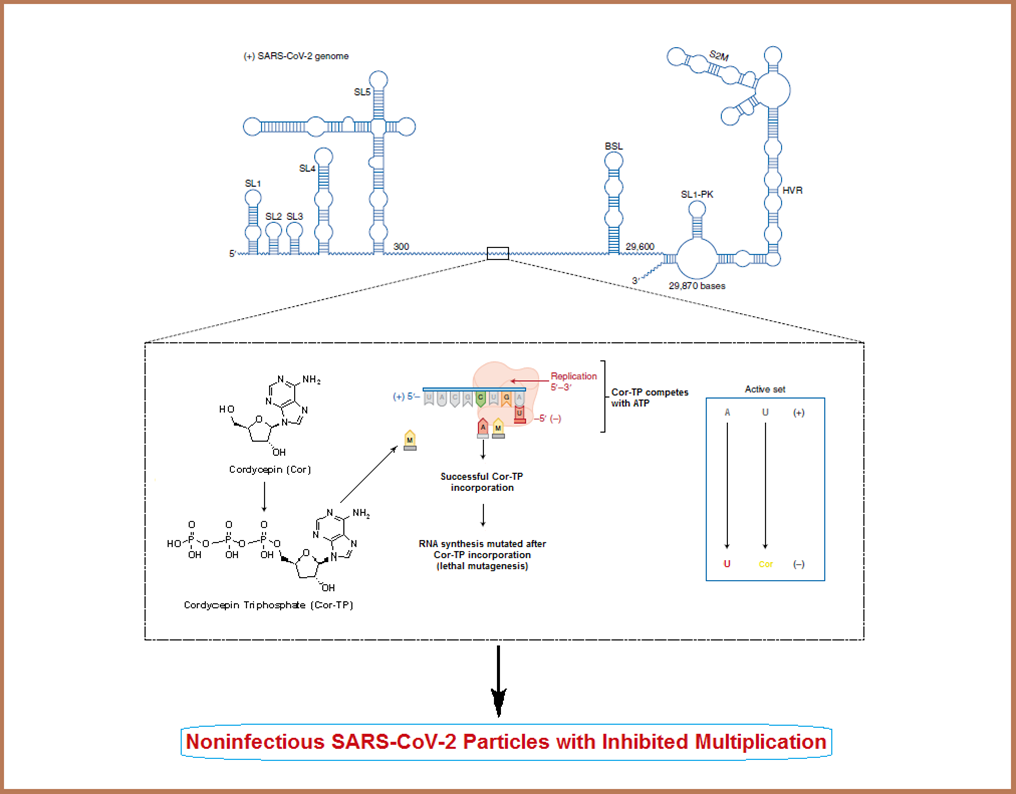
Herein, we suggest that cordycepin could effectively block the coronaviral replication (i.e., act as a SARS-CoV-2 replication inhibitor) through significantly reducing the number of replicated coronaviral copies, which is expected to be resulted from impairing mainly the genomic RNA synthesis occurred via the SARS-CoV-2 RdRp, using the nucleoside analogism strategy. In this effective mechanism of anti-RNA action, the nucleoside-like cordycepin molecule is first readily phosphorylated to its mono-, di-, and triphosphate forms (i.e., its nucleotide analogs) intracellularly, then the superactive nucleotide analog cordycepin triphosphate (Cor-TP) can be easily incorporated into RNA in place of the endogenous chemicosimilar ribonucleotide adenosine triphosphate (ATP), this inhibits and terminates transcription elongation and synthesis of viral RNA strands in all stages (i.e., acts as RNA elongation inhibitor due to the hydroxyl group absence at the 3' position of the molecule, this one-hydroxyl group deficiency significantly antagonizes the SARS-CoV-2 RdRp activity), giving incomplete disrupted premature RNAs in the developing mRNA strands and viral genomes, and finally this ambiguous coding leads to strong inhibition of SARS-CoV-2 copying/replication and generation of inactive, mutated, noninfectious, useless, nonpathogenic, and nonviral/non-SARS-CoV-2 particles instead of the active, parent, correct, original, infectious, pathogenic, and virulent SARS-CoV-2 particles (Figure 2).
|
Figure 2. Explanation of the newly-proposed mechanism of the anti-SARS-CoV-2 action of cordycepin.
It was surprisingly observed in the current research that cordycepin acts against the coronavirus in a time-dependent mode, since it reaches its maximal potency and effectiveness against the SARS-CoV-2 particles within 36-48h of treatment. This observation revealed the expected prolonged action (long-acting activities) against COVID-19 upon the clinical use. In spite of the specific expected high metabolic stability of cordycepin in the liver, a considerable portion of its molecules could be biologically transformed via metabolic phosphorylation inside the human cells to other active forms (e.g., its mono/di/triphosphate nucleotidic forms, with Cor-TP being the major metabolite among them)[6]. This type of intracellular metabolism, specifically, would not hinder the potential anti-COVID-19 activities of cordycepin and would not cause any clinical/therapeutic problems of cordycepin use, since it is an advantageous chemical transformation which modifies the nucleoside analog to the more biocompatible form the nucleotide analog (the more required form) without any metabolic hydroxylation of the 3' position of the molecule (as previously mentioned, the nucleotide analog Cor-TP could be successfully merged into the viral RNA, and strongly inhibits the transcription elongation and RNA generation because of the absence of a hydroxyl determinant at the 3' carbon of the ribose moiety of the molecule).
Promisingly, the total superiority, in almost all anti-COVID-19 parameters/properties/activities, of cordycepin over the potent antiviral agents remdesivir and GS-441524 supports cordycepin candidacy to be a leading potential comprehensive therapeutic agent against COVID-19. It is also worth mentioning that cordycepin molecule has more than ten sites that are considered as very active positions for chemical reactions, thus hundreds of possible derivatives (mainly potential RNA therapeutics) with required enhanced pharmacokinetic and/or pharmacodynamic properties can be designed and synthesized based on the biocompatible scaffold of this golden multitask molecule which belongs to the nucleoside analogs class of antiviral/antineoplastic therapeutic agents. Based on all the promising previous literature data together with the very interesting biological evaluation findings of the current research, cordycepin can be repurposed to in vivo evaluate its protective and inhibitory activities specifically against SARS-CoV-2 particles invasion (as an anti-RNA-virus agent) and to clinically evaluate its preventive and curative activities comprehensively against COVID-19 status as a whole (as an anti-COVD-19-condition agent) in COVID-19 patients. Please note that an extension of this short communication paper is now published as a full research article[12].
3 CONCLUDING HIGHLIGHTS
• COVID-19 polymerase (RdRp) inhibition via nucleoside analogism is the most successful tactic for COVID-19 infection therapy.
• Cordycepin (an adenosine analog) is a bioactive fungal metabolite with very important proven pharmacological actions in humans.
• Cordycepin inhibits SARS-CoV-2 replication and life cycle with interesting minute EC50 value of about 2μM.
• Cordycepin has relatively high values of SI (>49.75) and CC50 (>100μM).
• Cordycepin can be clinically tested, either alone or in combination with certain boosting agents like dipyridamole and/or pentostatin, against COVID-19.
Acknowledgments
I gratefully thank and deeply acknowledge anyone who gave a hand to make this new discovery and work coming out to light.
Conflicts of Interest
I hereby declare that I totally have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this new paper.
Supplementary Data and Availability of Data/Material
A complementary Supplementary Data File was provided to show the data that are not present herein in the Main Paper. Any data other than those present in the Main Paper and its complementary Supplementary Data File or any original data documents that are related to this current research work (a mixed Viewpoint/Short-communication article) are available from the Corresponding Author (Dr. Amgad M. Rabie) upon a reasonable request whenever possible.
Author Contribution
Not applicable (this article is a single-author research communication).
Abbreviation List
CC50, Half-maximal cytotoxic concentration
Cor-TP, Cordycepin triphosphate
COVID-19, Coronavirus disease 2019
CPEIC100, Full inhibitory concentration of cytopathic effects (CPE)
DMSO, Dimethylsulfoxide
EC50, Half-maximal effective concentration
RdRp, RNA-dependent RNA polymerase
SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
SI, Selectivity index
VOC-202012/01, First Variant of Concern from 2020, December
References
[1] Chitalia VC, Munawar AH. A painful lesson from the COVID-19 pandemic: the need for broad-spectrum, host-directed antivirals. J Transl Med, 2020; 18: 390. DOI: 10.1186/s12967-020-02476-9
[2] Rabie AM. Teriflunomide: A possible effective drug for the comprehensive treatment of COVID-19. Curr Res Pharmacol Drug Discovery, 2021; 2: 100055. DOI: 10.1016/j.crphar.2021.100055
[3] Rabie AM. Two antioxidant 2,5-disubstituted-1,3,4-oxadiazoles (CoViTris2020 and ChloViD2020): successful repurposing against COVID-19 as the first potent multitarget anti-SARS-CoV-2 drugs. New J Chem, 2021; 45: 761-771. DOI: 10.1039/D0NJ03708G
[4] Chien M, Anderson TK, Jockusch S et al. Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase, a key drug target for COVID-19. J Proteome Res, 2020; 19: 4690-4697. DOI: 10.1021/acs.jproteome.0c00392
[5] Rabie AM. Discovery of (E)-N-(4-cyanobenzylidene)-6-fluoro-3-hydroxypyrazine-2-carboxamide (cyanorona-20): the first potent and specific anti-COVID-19 drug. Chem Pap, 2021; 75: 4669-4685. DOI: 10.1007/s11696-021-01640-9
[6] PubChem. Cordycepin, PubChem CID: 6303. Accessed April 10, 2022. Available at https://pubchem.ncbi.nlm.nih.gov/compound/6303
[7] Tan L, Song X, Ren Y et al. Anti-inflammatory effects of cordycepin: A review. Phytother Res, 2021; 35: 1284-1297. DOI: 10.1002/ptr.6890
[8] ClinicalTrials.gov. Chemotherapy with cordycepin plus pentostatin in treating patients with refractory acute lymphocytic or chronic myelogenous leukemia, ClinicalTrials.gov Identifier: NCT00003005. Accessed December 16, 2021. Available at https://clinicaltrials.gov/ct2/show/NCT00003005
[9] ClinicalTrials.gov. Study of cordycepin plus pentostatin in patients with refractory TdT-positive leukemia, ClinicalTrials.gov Identifier: NCT00709215. Accessed December 16, 2021. Available at https://clinicaltrials.gov/ct2/show/NCT00709215
[10] Verma AK, Aggarwal R. Repurposing potential of FDA-approved and investigational drugs for COVID-19 targeting SARS-CoV-2 spike and main protease and validation by machine learning algorithm. Chem Biol Drug Des, 2021; 97: 836-853. DOI: 10.1111/cbdd.13812
[11] Bibi S, Hasan MM, Wang YB et al. Cordycepin as a promising inhibitor of SARS-CoV-2 RNA dependent RNA polymerase (RdRp). Curr Med Chem, 2022; 29: 152-162. DOI: 10.2174/0929867328666210820114025
[12] Rabie AM. Potent inhibitory activities of the adenosine analogue cordycepin on SARS-CoV-2 replication. ACS Omega, 2022; 7: 2960-2969. DOI: 10.1021/acsomega.1c05998
Copyright ©2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.




 Copyright ©
Copyright ©