Therapeutic Potential of Shatavari (Asparagus racemosus) for Psychological Stress-mediated Women's Reproductive Health Disorders
Ashutosh N. Pandey1, Pramod K. Yadav1, Karuppanan V. Premkumar1, Ajai K. Pandey2, Shail K. Chaube1*
1Cell Physiology Laboratory, Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi, 221005, Uttar Pradesh, ,India
2Department of Kayachikitsa, Faculty of Ayurveda, Institute of Medical Sciences, Banaras Hindu University, Varanasi, 221005, Uttar Pradesh, India
*Correspondence to: Shail K Chaube, PhD, Professor, Cell Physiology Laboratory, Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi, 221005, Uttar Pradesh, India, Email: shailchaubey@gmail.com
DOI: 10.53964/id.2025011
Abstract
In the contemporary era, large competition, limited resources and negative life events trigger generation of stress markers in women. Psychological stress modulates neuroendocrine network and lifestyle behaviors that generate oxidative stress (OS). The OS and excess level of cortisol induce various cell death pathways in the brain as well as ovary. Down regulation of hypothalmo-pituitary-gonadal axis, increased cortisol as well as OS negatively impacts ovarian functions by affecting follicular somatic cell functions, estradiol-β biosynthesis and oocyte quality. The impairment of ovarian function initiates the onset of several diseases including premature ovarian insufficiency, polycystic ovarian syndrome, anovulation and generates a salient threat to women’s ovary. To address these important health issues, search continues to find out a novel herbal medicine with a potential to mitigate psychological stress-mediated generation of OS and related various reproductive health disorders in women. Shatavari (Asparagus racemosus) with anti-stress, anti-anxiety, anti-depression, antioxidant properties have immense therapeutic potential as a new generation phytopharmaceutical drug for the management of psychological stress-mediated various women’s fertility issues. Nevertheless, the underlying mechanism(s) of psychological stress-mediated various reproductive disorders and therapeutic potential of various bioactive ingredients of Shatavari remains poorly understood. Therefore, this review focuses on the damage mechanism(s) of psychological stress and explores the therapeutic potential of bioactive ingredients of Shatavari as herbal medicine to improve women’s reproductive health.
Keywords: psychological stress, ovary, oocyte, women’s fertility, shatavari
1 INTRODUCTION
Stress is deeply rooted in modern society and considered as one of the major health concerns in the contemporary era globally. The number of cases is drastically increased due to large competition, limited resources, lifestyle changes, negative life events and post-coronavirus disease 2019 (COVID-19) stressors. This is supported by the data of Indian Psychiatric Society survey that the mental disorders have increased by 20% in India due to COVID-19 pandemic[1]. Mental disorders cases have drastically been increased from 80.8 million to 125.3 million globally[2]. The mental disorders particularly depression and anxiety are prevailing more frequently and affected nearly 264 million people globally[2]. The women are vulnerable and more likely to experience stress symptoms[3,4]. Anxiety and depression are more prevalent in women especially during reproductive age probably due to increased sensitivity of adrenal cortex and the influence of steroid hormones. As compared to men, women develop more frequently post-traumatic stress disorder, panic disorder and obsessive-compulsive disorder[5-7].
The psychological stress dysregulates hypothalamic-pituitary-gonadal (HPG) as well as hypothalamic-pituitary-adrenal (HPA) axes and initiates downstream signaling to generate excess levels of reactive oxygen species (ROS) that cause oxidative stress. In addition, psychological stress-mediated lifestyle changes such as alcohol consumption and cigarette smoking generate ROS that cause oxidative stress (OS) and infertility[8,9]. Depending on the level of insult, increased OS initiates various programmed cell death pathways in the ovary[10-12] that depletes ovarian reserve and oocyte quality[13-17]. Growing evidences suggest that elevated levels of anxiety and depression are associated with infertility whereas lowering the stress improves fertility in women[18,19]. Psychological stress is associated with the onset of various infertility issues and negatively affects the reproductive health of women[20,21]. The chronic psychological stress limits the in vitro fertilization (IVF) outcome by affecting ovarian functions, oocyte quality and fertility potential[22,23]. In addition, increased cortisol level inhibits estradiol-17β biosynthesis, affect ovarian functions and thereby women’s fertility potential[22,24].
In modern medicine, several drugs are frequently used to treat various mental disorders including stress, anxiety and depression as well as associated various fertility issues. Hence, there is a growing demand worldwide for alternative medicine that possess pleiotropic therapeutic potential to combat various mental disorders and their downstream adverse consequences on various fertility issues with minimum or no side effects. The herbal medicines possess the copious amount of polyphenols that impede OS-mediated downstream signaling pathways and exert anti-stress, anti-anxiety, anti-depression, anti-inflammatory and antioxidant abilities[25,26]. These herbal medicines act as adaptogens and tend to exhibit pleiotropic actions on the neuroendocrine-reproductive system. Hence in the contemporary era, the rationale use of herbal medicines is associated mainly to counter stress, anxiety, depression and associated impairment of various fertility issues[26,27].
In traditional system of medicines, bioactive ingredients of Shatavari, Asparagus racemosus (hereafter Shatavari) are well-known phytoadaptogens and their therapeutic potential have already been documented in Indian and British Pharmacopeias[28]. Several clinical and preclinical studies suggest that Shatavari not only possess therapeutic potential to treat various mental disorders including stress, anxiety, depression but also various reproductive health disorders with minimum or no side effects[27,29]. Although Shatavari has been used in ayurvedic system of medicine to treat various mental as well as associated reproductive disorders, the mechanism by which the bioactive ingredients of Shatavari prevent psychological stress-mediated downstream signaling and associated reproductive health disorders remains poorly understood. Therefore, this review explores the underlying mechanisms of psychological stress-mediated women’s reproductive health disorders and therapeutic potential of various bioactive ingredients of Shatavari.
2 IMPACT OF PSYCHOLOGICAL STRESS ON WOMEN’S HEALTH
Gender is an important determinant of overall health with a clear pattern for stress-mediated health disorders. Chronic pain, autoimmune diseases, anxiety and depression are more prevalent in women especially during reproductive age probably due to increased negative life events, sensitivity of adrenal cortex and the influence of steroid hormones such as estradiol as well as progesterone[30-32]. This notion is supported by the observations that the women are vulnerable towards various kinds of stressors and more likely to experience stress symptoms[3,4]. Women develop anxiety and depression disorder such as post-traumatic stress disorder, panic disorder and obsessive-compulsive disorder more frequently than men[5-7]. High stress level increases blood pressure, which generate negative impacts on the heart and thereby serious health issues[33]. In addition, long-lasting stress causes irritable bowel syndrome and obesity more frequently in women[34,35]. Further, high stress level affects estradiol sensitivity which increases the risk of obesity[35] and infertility in women[36]. Taken together, these studies suggest that the stress generates various health disorders and women are comparatively more vulnerable towards stress-mediated health disorders.
3 THERAPEUTIC POTENTIAL OF SHATAVARI IN INDIAN AYURVEDIC SYSTEM OF MEDICINES
In Indian ayurvedic system of medicine, Shatavari is renowned as “queen of herbs” due to its rejuvenating properties and counterbalancing day-to-day environmental stress. The various formulations of Shatavari are designed for the treatment of various women’s health disorders. The bioactive ingredients isolated from the root of Shatavari are considered to be effective as an anti-spasmodic, appetizer, stomach tonic, aphrodisiac, galactagogue, astringent, anti-diarhoeal, anti-dysenteric, laxative, anti-cancer, anti-inflammatory, blood purifier, anti-tubercular, anti-epileptic, kidney problems and in throat complaints as well as in night blindness[26,37]. The Shatavari is mentioned as medhya- the plants which increase intelligence and promote learning and memory, rasayana-the rejuvenator herbs which improve health by increasing immunity, vitality and resistance, imparting longevity as well as protection against stress and as balya- a strength promoter, stanya-a galactogogue and jeevaniya- an erythropoetic[38]. Although Shatavari is described for the management of variety of health disorders, special emphasis has been paid to the female reproductive health issues.
Charaka Samhita, Sushuruta Samhita and Ashtanga Hridaya are the three primary textbooks of ayurveda that are concerned with basic tenets of ayurvedic clinical practices. These texts have vividly described methods for the use of Shatavari, which helps in treating women’s health issues[39]. The root extracts of Shatavari are used to balance the hormonal levels, normalize the problems of sexual organs and improve women’s fertility[40]. It humidifies the dryness of the sexual organs of female, improves folliculogenesis and ovulation, strengthens the genital part for conceiving, prevents miscarriages, expands lactation by regularizing the hormone and possess therapeutic potential to treat premature ovarian insufficiency (POI), polycystic ovarian syndrome (PCOS), leucorrhea and menorrhagia[37-41].
4 MOLECULAR MCHANISMS UNDELYING PSYCOLOGICAL STESS
Excess levels of ROS in response to psychological stress disturb neuronal cell redox status in the hypothalamus and initiate programmed cell death pathways leading to brain tissue injury and neurological disorders[42]. Mitochondria is a major site of ROS production and excess levels of ROS alter structure and functions of mitochondria that further generate ROS. The increased ROS damage the brain tissue and impair neurological functions[43]. Further OS-mediated tissue damage causes inflammation that activates nuclear transcription factor-kappa B (NF-κB) signaling pathway in brain and further exaggerates neurological disorders[44].
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a redox sensitive transcription factor that negatively regulate the NF-κB signaling pathway and inhibit OS, inflammation, mitochondria dysfunction as well as neurological disorders[44]. In order to counter the OS, the redox sensitive the Keap1 (kelch-like ECH-associated protein 1)/ Nuclear factor erythroid 2-related factor 2 (Nrf2)/ antioxidant response element (ARE) signaling pathway is initiated in neuronal cells of the brain[44,45]. Under OS condition, Nrf2 and Keap1 are dissociated and transferred to the nucleus, wherein the activated Nrf2 and Keap1 combine with the corresponding sites of the ARE to improve the antioxidant capacity of the neuronal cells[44,45].
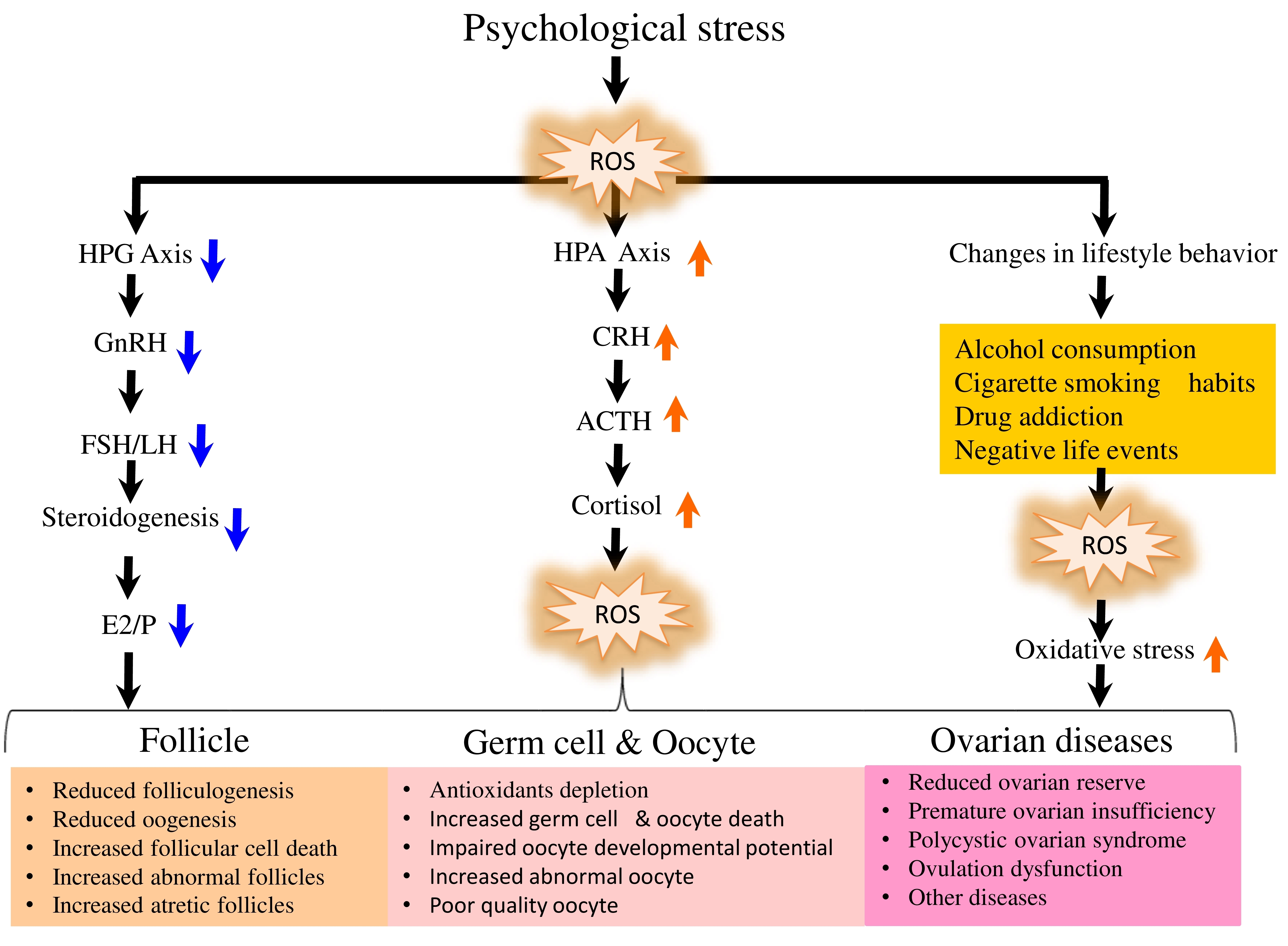
The psychological stress-mediated increased OS impair HPA as well as HPG axes and thereby fertility potential in reproductive-age women[22]. Psychological stress affects HPA axis by inducing the release of corticotropin-releasing hormone (CRH). The CRH binds to its receptor on the pituitary corticotropes and induce the secretion of adrenocorticotropin hormone that initiates downstream signaling to release cortisol[46]. The elevated cortisol affects functions of steroidogenic cells and inhibits estradiol-17β biosynthesis in the ovary (Figure 1). In addition, the psychological stress inhibits production of reproductive hormones such as gonadotropin releasing hormone (GnRH) from the hypothalamus, luteinizing hormone (LH) from pituitary and estradiol-17 β production from the ovary[47]. The subnormal level of estradiol-17β affects the ovarian reserve, follicular growth and development that results in the ovulation of poor quality oocyte and limits women’s fertility potential[24].
|
Figure 1. A Schematic Diagram Showing the Direct as well as Indirect Impact of Psychological Stress in Reducing Women’s Fertility Potential. The psychological stress modulates neuroendocrine network to directly activate hypothalmo-pituitary-adrenal (HPA) axis and down regulate hypothalmo-pituitary-gonadal (HPG) axis. The hyper activation of HPA axis stimulate overproduction of cortisol, while down regulation of HPG axis results in the production of subnormal levels of gonadotropic hormones. The subnormal levels of gonadotropic hormones and high cortisol that lead to impaired Estradiol-17β biosynthesis, folliculogenesis and oocyte quality in the ovary. The psychological stress indirectly generates oxidative stress by affecting life style behaviors that finally damage ovarian function and cause various diseases.
The psychological stress indirectly generates excess levels of ROS through lifestyle changes in infertile women[22,48,49]. For example, alcohol consumption and cigarette smoking generate excess levels of ROS that cause OS and limits women’s fertility[8,9]. Although antioxidant machinery is present in the ovary to maintain redox status, larger size (100-120 µm in diameter) of follicular oocyte makes it are more susceptible to OS insult. Hence, excess levels of ROS must be cleared as quick as possible in order to minimize OS insult and thereby impairment of oocyte quality and women’s fertility[12,17,50-52].
5 THERAPEUTIC POTENTIAL OF SHATAVARI IN PREVENTING MENTAL HEALTH DISORDERS
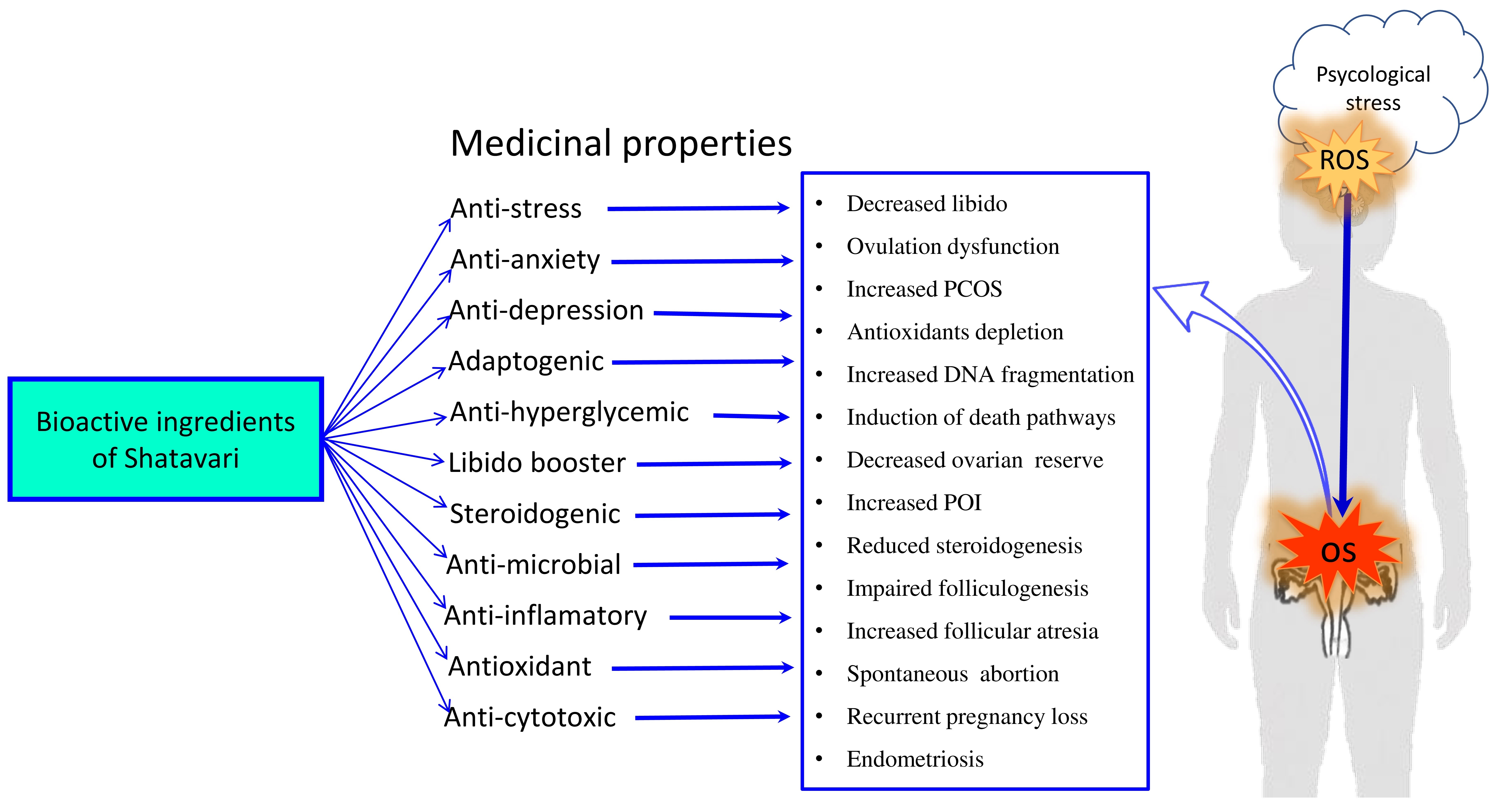
Shatavari has an immense therapeutic potential due to its anti-stress, anti-anxiety, anti-depressant, antioxidant, anti-cytotoxic properties (Figure 2). Shatavari activates monoaminergic system and reduces HPA axis-mediated downstream signaling. This is supported by the observations that the 100 and 200 mg/kg body weight of methanolic extract of Shatavari reduced OS generated by lipid peroxidation and increased enzymatic antioxidant activity in the hippocampus and striatum regions of rat brain[26]. These doses of methanolic extract of Shatavari decreased corticosterone as well as norepinephrine levels and showed anxiolytic activity by modulating GABA and serotonergic-mediated pathways further confirming its therapeutic potential for the treatment of anxiety disorders[53].
|
Figure 2. A Schematic Diagram Showing The Therapeutic Potential Of Bioactive Ingredients of Shatavari, Asparagus racemosus in Mitigating Psychological Stress-mediated Generation Of Reactive Oxygen Species (ROS) and Oxidative Stress-induced Ovarian Damage and the Onset of Various Reproductive Health Disorders in Women.
The saponin, a major bioactive ingredients of Shatavari, exhibit adaptogenic and neuroprotective properties, revitalizing monoaminergic neurotransmission and intra-neuronal calcium dynamic[54]. A glycoside flavonoid such as rutin exhibits an antidepressant activity[55]. The cholinergic dysfunction is identified as a biomarker of various neurological disorders. Studies demonstrated that the shatavarin IV significantly improves cholinergic transmission by increasing the synaptic acetylcholine level and nicotinic acetylcholine receptors (nAChR) further strengthening the beneficial impact of bioactive ingredients of Shatavari in preventing OS-mediated neuronal damage[26,56].
The enzyme-treated asparagus extract (50 mg and 250 mg/day) demonstrated anti-stress ability by managing daily psychological stress load and stabilized sleep quality[57,58]. The ethanolic extract of Shatavari alleviates stress level in mice by reducing the level of lipid peroxidation and oxidative damage in mice brain[59]. Without showing any remarkable side effects, Shatavari exhibits antioxidant, anti-inflammatory, immunomodulatory, neuroprotective, nootropic, antidepressant properties, restores the perturbed neurotransmitters, prevents oxidative neuronal damage, mitigate neurological disorders including stress, anxiety and depression[26,29]. Taken together, these findings suggest that Shatavari protects psychological stress-mediated OS damage and thereby mental health disorders.
6 THERAPEUTIC POTENTIAL OF SHATAVARI IN PREVENTING PSYCHOLOGICAL STRESS-MEDIATED OVARIAN DISORDERS
Normally pro- and antioxidant systems exist in a balanced state in mammalian cells. The ovary is a dynamic organ which generates comparatively excess levels of ROS during final stages of folliculogenesis, oocyte maturation and ovulation[48]. Although ROS are generated by various sub-cellular organelles, the major source of their production is mitochondria in mammalian cells[60]. The sustained high levels of ROS induce meiotic cell cycle arrest and apoptosis in follicular oocytes[15,61,62]. The high levels of ROS are associated with the decrease of catalase activity during folliculogenesis and oocyte maturation in rat[14,63]. Indeed, inhibition of enzymatic antioxidants and accumulation of excess levels of ROS generate OS in the ovary that directly affects oocyte quality and thereby reproductive outcome[10,14,64,65]. The increased OS not only induce germ cell depletion, deteriorates oocyte quality and ovarian aging but also limits reproductive outcome by affecting fertilization as well as pregnancy rates in women[17,20,66].
The increased OS initiates various death pathways, affects IVF outcome[67,68], embryo development[69,70] and triggers the onset of several ovarian diseases[71] including POI, PCOS[72,73], endometriosis, unexplained infertility, spontaneous abortion and recurrent pregnancy loss[71,74]. Although several antioxidants have been used to prevent OS-mediated oocyte damage[75] and improve fertility in stressed women[76-78], search continues to find out novel herbal medicine from natural origin that not only prevent psychological stress but also ROS-mediated damage at the level of ovary and oocytes (Figure 2).
Majority of bioactive ingredients of Shatavari exhibit strong antioxidant property[37]. For instance, the purified bioactive ingredients such as Racemoside (A,B,C), Sarasapogenin, Shatavarins, Asparanin A, Diosgenin, Asparagamine A, Racemofuran, Racemosol, Kaempferol, Quercetin, Rutin, Hyperoside etc. demonstrate strong antioxidant potential by preventing oxidative stress damage[37,79,80] Shatavari improve ROS scavenging ability by elevating enzymatic antioxidants such as catalase, superoxide dismutase and glutathione peroxidase activities that encounter depression[26]. This is supported by the observations that Shatavari root extract significantly reduced ROS levels and lipid peroxidation in human serum in vitro[81]. Taken together, these findings clearly suggest that the bioactive ingredients of Shatavari could be used as novel herbal medicine for the management both psychological as well as OS-mediated fertility disorders in women (Table 1)[82,83].
Table 1. Major Bioactive Ingredients of Shatavari, Asparagus racemosus and Their Biological Action on the Reproductive Health and Fertility Disorders
Chemical Nature |
Bioactive Ingredients |
Extracted From |
Biological Action |
References |
Steroids |
Sarsasapogenin Sapogenin Recemoside A,B,C Shatavaroside (A, B, C), Shatavarin I-X |
Root Fruit |
Anti-stress, Anti-anxiety, Antioxidant, Anti-inflammatory, Hormonal balance, Improve ovarian function, Folliculogenesis and oocyte maturation, Improve fertility and reproductive health |
[37-39,41,94] |
Sterols |
Racemofuran Racemosol Sistosterol |
Root |
Antioxidant, Adaptogenic, Improve fertility and reproductive health |
[37,40] |
Flavonoids |
Rutin, Quercetin, Quercetin-3-glucuronide |
Root Leaves Fruits |
Antioxidant, Anti-inflammatory, Improve fertility, Hormonal balance, Induce folliculogenesis and ovulation |
[26,40,91,95] |
Isoflavones |
4-Trihydro Isoflavine |
Root |
Anti-stress, Anti-amenorrhagic, Improve reproductive health |
[95] |
Alkaloids |
Asparagamine A |
Root |
Antioxidant, Anti-inflammatory, Hormonal balance, Correct menstrual disorders |
[37,38,41] |
Vitamins and Minerals |
Vitamins A, B1, B2, C, E,Zn,Co Mg, P, Ca, Fe, Folic Acid, Mn |
Root Leaves Flowers |
Improve general well-being and reproductive health |
[26,91] |
Essential Oils |
- |
Root Leaves Flowers |
Improve reproductive health and fertility |
[39,95] |
Lactones |
Lactones |
Root Leaves Flowers |
Improve overall health, vitality and reproductive health |
[95] |
Carbohydrates & Tannins |
- |
Root Leaves Fruits |
Strengthen immune system, improve overall health |
[91] |
7 THERAPEUTIC POTENTIAL OF SHATAVARI IN REJUVENATING REPRODUCTIVE HEALTH AND LIBIDO
The anti-stress, anti-inflammatory and anti-cytotoxic properties suggest that the bioactive ingredients of Shatavari could be used as herbal panacea for the treatment of stress-mediated fertility disorders in women[84,85]. This notion is strengthened by the observations that Shatavari reduces ROS as well as other stress markers[81,86] and prevent PCOS, POI, ovulation dysfunctions, impaired folliculogenesis and oogenesis, cell death in follicular somatic cells and oocytes, poor quality oocytes, spontaneous abortion, recurrent pregnancy loss, endometriosis etc. in women[40,85]. Shatavari enhances libido and rejuvenates female reproductive potential by promoting general well-being and/or support in counterbalancing day-to-day environmental stress[27,40]. The placebo-controlled randomized single-blind study using aged women between 40 to 60 years with menopausal syndrome suggests that Shatavari reduced hot flashes, night sweats, anxiety and insomnia[82]. Shatavari is well-reckoned as a female tonic in the Indian ayurvedic system of medicine due to its pleiotropic therapeutic potential on various women’s health issues[26].
In modern medicine, clomiphene citrate (CC) has widely been used to induce follicular growth, development and ovulation[61]. Although CC has good ovulation induction ability (>60%), the pregnancy rate is much lower (10%-20%)[87-89]. This discrepancy is due to anti-estrogenic effect of CC at the level of ovary. For instance, CC reduces estradiol 17β level in the ovary of rat[51,61] as well as monkey[90]. The CC-mediated hypoestrogenic conditions may lead to the generation of ROS[11]. For instance, CC treatment increases ROS levels and causes OS in rat ovary[51]. The increased OS induces apoptosis in follicular somatic cells as well as oocyte[11,51,64,66] and deteriorates oocyte quality[11,51]. Therefore, in order to counter the anti-estrogenic and ROS-mediated effects of CC, estradiol-17β or melatonin supplementation has been suggested[11,51].
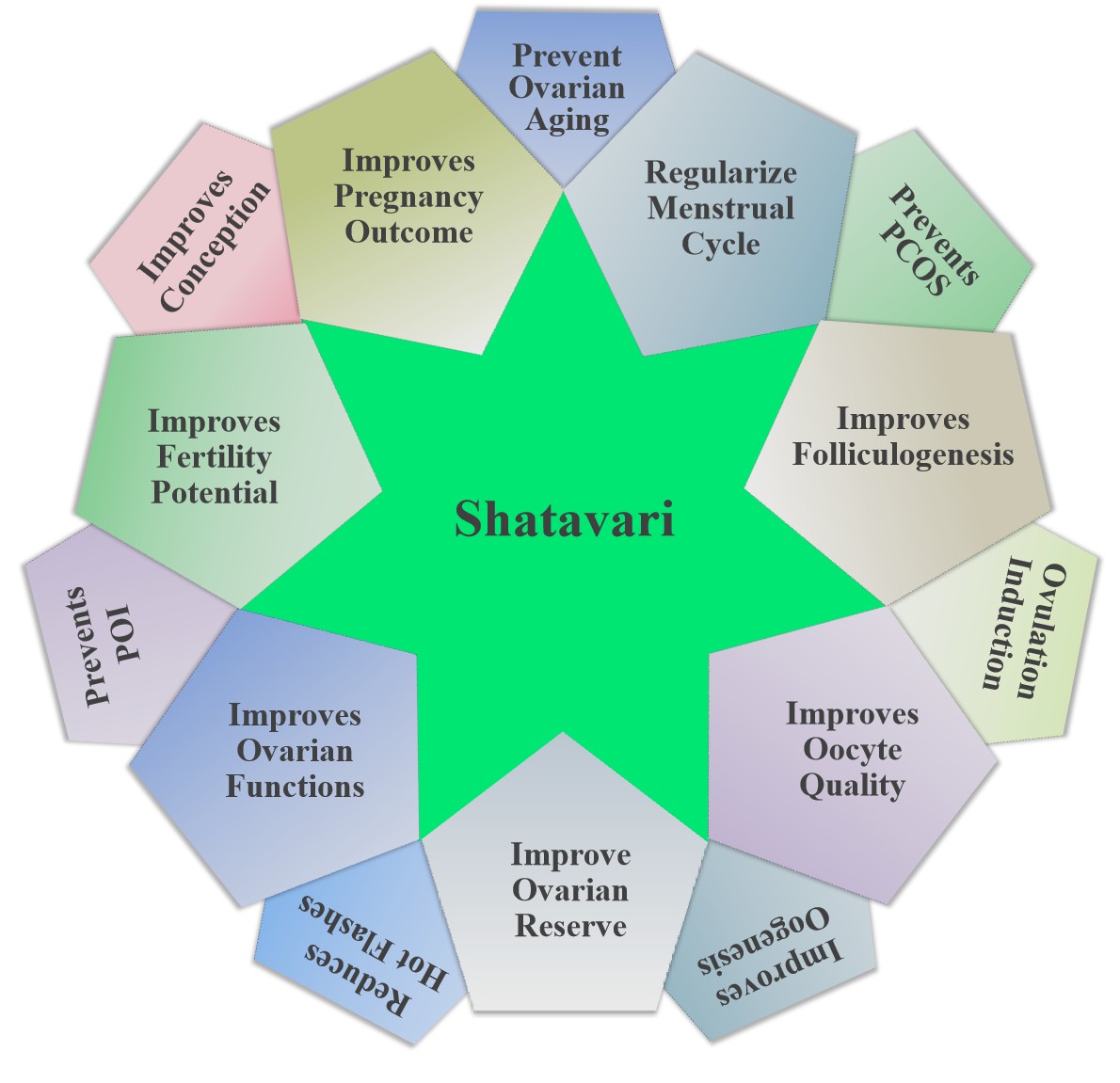
Shatavari is considered as potential candidate as an alternative medicine to replace CC for follicular growth and ovulation during assisted reproduction. In a randomized standard controlled study, when 40 infertile women in the age group of 18-40 years with menstrual irregularities, polycystic ovarian disease were fed either 50 mg of CC (once daily from day 2-6 of cycle) or 6 grams of Shatavari powder twice daily from day 1-14 of cycle) for 2 consecutive cycles, the follicular growth and ovulation inducing potential of Shatavari was comparable to CC[91]. These findings suggest that the bioactive ingredients of Shatavari could be used as a potential alternative medicine for ovulation induction in patients with anovulatory infertility experiencing significant side effects with modern medicine. To strengthen these findings, more detailed and long-term studies with large sample sizes would facilitate the development of an alternative medicine for ovulation induction in women. Taken together, these studies suggest that Shatavari increases libido, follicular growth and development, ovulation and rejuvenates women’s overall reproductive health with no/minimal side effects. These properties of Shatavari holds the promise as a potential alternative medicine for the management of psychological stress-mediated various fertility issues and rejuvenating various reproductive health issues in women experiencing significant side effects with the various modern medicines (Figure 3).
|
Figure 3. A Schematic Diagram Showing the Therapeutic potential of Shatavari, Asparagus racemosus in Rejuvenating Women’s Reproductive Health.
8 POTENTIAL SIDE EFFECTS OF SHATAVARI
Shatavari has extensively been used in ayurvedic system of medicine for a long time without any reported toxicity or side effects. Studies suggest that even high doses of Shatavari do not trigger any neuronal, behavioral, genotoxic changes and mortality in experimental animals[26,92]. However, a marked reduction in body weight and teratogenic effects were observed in experimental rats exposed to 100 mg/kg body weight/day of Shatavari root extract for 60 days[93]. Therefore, using modern biotechnological methods a detailed investigation into the safety profile of Shatavari is required to enhance the production of specific bioactive ingredients and their rigorous clinical trials to explore their targeted benefits. Further, isolation, purification of specific bioactive ingredient, molecular mechanism, interactions and potential herb-drug interactions are important to ensure the safe usage of Shatavari with minimal or no side effects.
9 CONCLUSION AND FUTURE DIRECTIONS
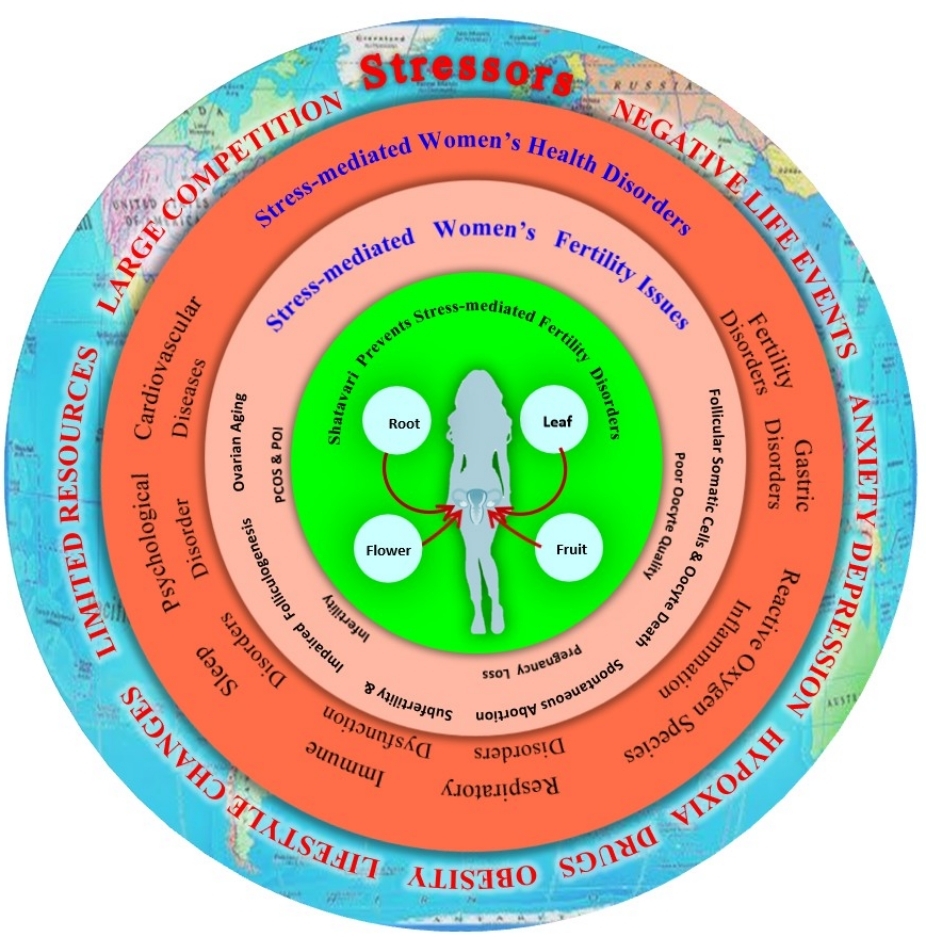
Psychological stress modulates neuroendocrine network, generates OS and play crucial roles in the pathophysiology of female infertility. The OS depletes ovarian reserve, affects ovarian function, causes ovarian diseases such as follicular atresia, PCOS, POI in stressed women. Various antioxidants are used to overcome the devastating effects of psychological stress-mediated OS, search continues to find out new generation phytopharmaceutical drugs that holds pleiotropic therapeutic potential for infertility treatment (Figure 4).
|
Figure 4. A Summarized Diagram Showing Psychological Stress-mediated Induction of Various General As Well As Reproductive Health Disorders in Women. Shatavari, Asparagus racemosus not only mitigate general and reproductive health disorders but also rejuvenates women’s reproductive health.
Although Shatavari has been used as an herbal boon for women’s reproductive health issues, future research warrants addressing following questions, 1) whether bioactive ingredients of Shatavari could be developed as alternative medicine for targeted reproductive health disorder? 2) whether specific bioactive ingredient of Shatavari could improve IVF outcomes in stressed women? 3) what are the long-term side-effects of Shatavari supplementation? To answer these questions, long-term detailed clinical studies with large sample sizes on the extraction procedure, dosage standardization, underlying mechanisms, herb-herb/herb-drug interactions would facilitate to development of a targeted alternative medicine for the holistic management of stress-mediated fertility disorders in women.
Acknowledgements
The financial support in part for the research reported herein has been provided to Shail K. Chaube from CAS, Department of Zoology, Institute of Science, BHU, Varanasi-221005, UP, India. The infrastructural facilities provided to Shail K. Chaube from the Department of Zoology, Institute of Science, BHU, Varanasi-221005, UP, India, are highly acknowledged. UGC Research fellowships provided to Ashutosh N. Pandey, and Karuppanan V. Premkumar from the Department of Zoology, Institute of Science, BHU are also acknowledged.
Conflicts of Interest
The authors declared no conflict of interest.
Data Availability
No data was used for the research described in the article.
Copyright Permissions
Copyright © 2025 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Author Contribution
Pandey AN and Yadav PK performed review/editing, image preparation, conceptualization and writing major parts of the original draft. Premkumar KV and Pandey AK performed further review/editing and finalize the images. Chaube SK performed final review/editing, visualization, project administration and the supervision.
Abbreviation List
ACTH, Adrenocorticotropin hormone
ARE, Antioxidant response element
ART, Assisted reproductive technology
CAT, Catalase
CC, Clomiphene citrate
COVID-19, Coronavirus disease 2019
CRH, Corticotropin-releasing hormone
GnRH, Gonadotropin releasing hormone
GPx, Glutathione peroxidase
H2O2, Hydrogen peroxide
HPA, Hypothalamic-pituitary-adrenal
HPG, Hypothalamic-pituitary-gonadal
IBS, Irritable bowel syndrome
IVF, In vitro fertilization
LH, Luteinizing hormone
MMP, Mitochondria membrane potential
nAChR, Nicotinic acetylcholine receptors
NF-κB, Nuclear transcription factor-kappa B
Nrf2, Nuclear factor erythroid 2-related factor 2
OS, Oxidative stress
PCOS, Polycystic ovarian syndrome
POI, Premature ovarian insufficiency
ROS, Reactive oxygen species
SOD, Superoxide dismutase
References
[1] India Today. 20% increase in patients with mental illness since coronavirus outbreak: Survey. Accessed April 10, 2025. Available at:[Web]
[2] GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry, 2022; 9: 137-150[DOI]
[3] Farooq K, Williams P. Headache and chronic facial pain. Contin Educ Anaesth Crit Care Pain, 2008; 8: 138-142.[DOI]
[4] Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci, 2001; 21: 6292-6297.[DOI]
[5] Bangasser DA. Sex differences in stress-related receptors: ″micro″ differences with ″macro″ implications for mood and anxiety disorders. Biol Sex Differ, 2013; 4: 2.[DOI]
[6] Hammen C, Kim EY, Eberhart NK et al. Chronic and acute stress and the prediction of major depression in women. Depress Anxiety, 2009; 26: 718-723.[DOI]
[7] Verma R, Balhara YP, Gupta CS. Gender differences in stress response: Role of developmental and biological determinants. Ind Psychiatry J, 2011; 20: 4-10.[DOI]
[8] Hakim RB, Gray RH, Zacur H. Alcohol and caffeine consumption and decreased fertility. Fertil Steril, 1998; 70: 632-637.[DOI]
[9] Howe G, Westhoff C, Vessey M et al. Effects of age, cigarette smoking, and other factors on fertility: findings in a large prospective study. Br Med J (Clin Res Ed), 1985; 290: 1697-1700.[DOI]
[10] Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertility - a clinician's perspective. Reprod Biomed Online, 2005; 11: 641-650.[DOI]
[11] Chaube SK, Prasad PV, Thakur SC et al. Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis, 2005; 10: 863-874.[DOI]
[12] Pandey AN, Yadav PK, Premkumar KV et al. Reactive oxygen species signalling in the deterioration of quality of mammalian oocytes cultured in vitro: Protective effect of antioxidants. Cell Signal, 2024; 117: 111103.[DOI]
[13] Goud AP, Goud PT, Diamond MP et al. Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic Biol Med, 2008; 44: 1295-1304.[DOI]
[14] Tiwari M, Prasad S, Tripathi A et al. Apoptosis in mammalian oocytes: a review. Apoptosis, 2015; 20: 1019-1025.[DOI]
[15] Tiwari M, Prasad S, Tripathi A et al. Involvement of reactive oxygen species in meiotic cell cycle regulation and apoptosis in mammalian oocytes. React Oxygen Spec, 2016; 1: 99-106.[DOI]
[16] Yadav AK, Yadav PK, Chaudhary GR et al. Autophagy in hypoxic ovary. Cell Mol Life Sci, 2019; 76: 3311-3322.[DOI]
[17] Yadav PK, Pandey AN, Premkumar KV et al. Follicular oocyte as a potential target for severe acute respiratory syndrome coronavirus 2 infection. Rev Med Virol, 2024; 34: e2568.[DOI]
[18] Palomba S, Daolio J, Romeo S et al. Lifestyle and fertility: the influence of stress and quality of life on female fertility. Reprod Biol Endocrinol, 2018; 16: 113.[DOI]
[19] Rooney KL, Domar AD. The relationship between stress and infertility. Dialogues Clin Neurosci, 2018; 20: 41-47.[DOI]
[20] Prasad S, Tiwari M, Pandey AN et al. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci, 2016; 23: 36.[DOI]
[21] Sharma R, Biedenharn KR, Fedor JM et al. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol, 2013; 11: 66.[DOI]
[22] Ebbesen SM, Zachariae R, Mehlsen MY et al. Stressful life events are associated with a poor in-vitro fertilization (IVF) outcome: a prospective study. Hum Reprod, 2009; 24: 2173-2182.[DOI]
[23] Lakatos E, Szabó G, Szigeti JF et al. [Relationships between psychological well-being, lifestyle factors and fertility]. Orv Hetil, 2015; 156: 483-492.[DOI]
[24] Kala M, Nivsarkar M. Role of cortisol and superoxide dismutase in psychological stress induced anovulation. Gen Comp Endocrinol, 2016; 225: 117-124.[DOI]
[25] Behl T, Rana T, Alotaibi GH et al. Polyphenols inhibiting MAPK signalling pathway mediated oxidative stress and inflammation in depression. Biomed Pharmacother, 2022; 146: 112545.[DOI]
[26] Singh N, Garg M, Prajapati P et al. Adaptogenic property of Asparagus racemosus: Future trends and prospects. Heliyon, 2023; 9: e14932.[DOI]
[27] Pandey AK, Gupta A, Tiwari M et al. Impact of stress on female reproductive health disorders: Possible beneficial effects of shatavari (Asparagus racemosus). Biomed Pharmacother, 2018; 103: 46-49.[DOI]
[28] Singh R. Asparagus racemosus: a review on its phytochemical and therapeutic potential. Nat Prod Res, 2016; 30: 1896-1908.[DOI]
[29] Majumdar S, Gupta S, Prajapati SK et al. Neuro-nutraceutical potential of Asparagus racemosus: A review. Neurochem Int, 2021; 145: 105013.[DOI]
[30] Kuehner C. Why is depression more common among women than among men? Lancet Psychiatry, 2017; 4: 146-158.[DOI]
[31] Li SH, Graham BM. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatry, 2017; 4: 73-82.[DOI]
[32] Oram S, Khalifeh H, Howard LM. Violence against women and mental health. Lancet Psychiatry, 2017; 4: 159-170.[DOI]
[33] Vaccarino V, Shah AJ, Rooks C et al. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med, 2014; 76: 171-180.[DOI]
[34] Grundmann O, Yoon SL. Irritable bowel syndrome: epidemiology, diagnosis and treatment: an update for health-care practitioners. J Gastroenterol Hepatol, 2010; 25: 691-699.[DOI]
[35] Michopoulos V. Stress-induced alterations in estradiol sensitivity increase risk for obesity in women. Physiol Behav, 2016; 166: 56-64.[DOI]
[36] Louis GM, Lum KJ, Sundaram R et al. Stress reduces conception probabilities across the fertile window: evidence in support of relaxation. Fertil Steril, 2011; 95: 2184-2189.[DOI]
[37] Akhtar S, Gupta AK, Naik B et al. Exploring pharmacological properties and food applications of Asparagus racemosus (Shatavari). Food Chem Adv, 2024; 4: 100689.[DOI]
[38] Sharma K, Bhatnagar M. Asparagus racemosus (Shatavari): A versatile female tonic. Int J Pharm Biol Arch, 2011; 2: 855-863.
[39] Thakur S, Kaurav H, Chaudhary G. Shatavari (Asparagus Racemosus) - The best female reproductive tonic. Int J Res Rev, 2021; 8: 73-84.[DOI]
[40] Patibandla S, Gallagher JJ, Patibandla L et al. Ayurvedic herbal medicines: a literature review of their applications in female reproductive health. Cureus, 2024; 16: e55240.[DOI]
[41] Meher D, Singh M, Meher B. An update on phytoconstituents and pharmacological importance of Asparagus racemosus. J Appl Pharm Res, 2024; 12: 11-20.[DOI]
[42] Malko P, Jiang LH. TRPM2 channel-mediated cell death: An important mechanism linking oxidative stress-inducing pathological factors to associated pathological conditions. Redox Biol, 2020; 37: 101755.[DOI]
[43] Qu Y, Zhang HL, Zhang XP et al. Arachidonic acid attenuates brain damage in a rat model of ischemia/reperfusion by inhibiting inflammatory response and oxidative stress. Hum Exp Toxicol, 2018; 37: 135-141.[DOI]
[44] Wang L, Zhang X, Xiong X et al. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants (Basel), 2022; 11: 2377.[DOI]
[45] Song Y, Wang LB, Bei Y et al. Carvacryl acetate, a semisynthetic monoterpenic ester obtained from essential oils, provides neuroprotection against cerebral ischemia reperfusion-induced oxidative stress injury via the Nrf2 signalling pathway. Food Funct, 2020; 11: 1754-1763.[DOI]
[46] Phumsatitpong C, Wagenmaker ER, Moenter SM. Neuroendocrine interactions of the stress and reproductive axes. Front Neuroendocrinol, 2021; 63: 100928.[DOI]
[47] Toufexis D, Rivarola MA, Lara H et al. Stress and the reproductive axis. J Neuroendocrinol, 2014; 26: 573-586.[DOI]
[48] Lázár L. The role of oxidative stress in female reproduction and pregnancy. In: Oxidative Stress and Diseases. IntechOpen; 2012.
[49] Ogden J, Stavrinaki M, Stubbs J. Understanding the role of life events in weight loss and weight gain. Psychol Health Med, 2009; 14: 239-249.[DOI]
[50] Combelles CM, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online, 2009; 18: 864-880.[DOI]
[51] Tripathi A, PremKumar KV, Pandey AN et al. Melatonin protects against clomiphene citrate-induced generation of hydrogen peroxide and morphological apoptotic changes in rat eggs. Eur J Pharmacol, 2011; 667: 419-424.[DOI]
[52] Yadav PK, Gupta A, Sharma A, et al. Fate of the germ cells in mammalian ovary: A review. J Reprod Health Med, 2020; 1: 3.[DOI]
[53] Garabadu D, Krishnamurthy S. Asparagus racemosus attenuates anxiety-like behavior in experimental animal models. Cell Mol Neurobiol, 2014; 34: 511-521.[DOI]
[54] Abbas G, Rauf K, Mahmood W. Saponins: the phytochemical with an emerging potential for curing clinical depression. Nat Prod Res, 2015; 29: 302-307.[DOI]
[55] Yusha'u Y, Muhammad UA, Nze M et al. Modulatory role of rutin supplement on open space forced swim test murine model of depression. Niger J Physiol Sci, 2017; 32: 201-205.
[56] Shuchi Smita S, Trivedi M, Tripathi D et al. Neuromodulatory potential of Asparagus racemosus and its bioactive molecule Shatavarin IV by enhancing synaptic acetylcholine level and nAChR activity. Neurosci Lett, 2021; 764: 136294.[DOI]
[57] Ito T, Ono T, Sato A et al. Toxicological assessment of enzyme-treated asparagus extract in rat acute and subchronic oral toxicity studies and genotoxicity tests. Regul Toxicol Pharmacol, 2014; 68: 240-249.[DOI]
[58] Takanari J, Nakahigashi J, Sato A et al. Effect of Enzyme-Treated Asparagus Extract (ETAS) on Psychological Stress in Healthy Individuals. J Nutr Sci Vitaminol (Tokyo), 2016; 62: 198-205.[DOI]
[59] Joshi T, Sah SP, Singh A. Antistress activity of ethanolic extract of Asparagus racemosus Willd roots in mice. Indian J Exp Biol, 2012; 50: 419-424.
[60] Keane JA, Ealy AD. An Overview of Reactive Oxygen Species Damage Occurring during In Vitro Bovine Oocyte and Embryo Development and the Efficacy of Antioxidant Use to Limit These Adverse Effects. Animals (Basel), 2024; 14: 330.[DOI]
[61] Chaube SK, Prasad PV, Thakur SC et al. Estradiol protects clomiphene citrate-induced apoptosis in ovarian follicular cells and ovulated cumulus-oocyte complexes. Fertil Steril, 2005; 84 Suppl 2: 1163-1172.[DOI]
[62] Tripathi A, Khatun S, Pandey AN, Mishra SK, Chaube R, Shrivastav TG, Chaube SK. Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic Res, 2009; 43: 287-294.[DOI]
[63] Pandey AN, Tripathi A, Premkumar KV, Shrivastav TG, Chaube SK. Reactive oxygen and nitrogen species during meiotic resumption from diplotene arrest in mammalian oocytes. J Cell Biochem, 2010; 111: 521-528.[DOI]
[64] Chaube SK, Prasad PV, Tripathi V, Shrivastav TG. Clomiphene citrate inhibits gonadotropin-induced ovulation by reducing cyclic adenosine 3',5'-cyclic monophosphate and prostaglandin E2 levels in rat ovary. Fertil Steril, 2006; 86: 1106-1111.[DOI]
[65] Pandey AN, Chaube SK. Reduction of nitric oxide level leads to spontaneous resumption of meiosis in diplotene-arrested rat oocytes cultured in vitro. Exp Biol Med (Maywood), 2015; 240: 15-25.[DOI]
[66] Chaube SK, Shrivastav TG, Prasad S, et al. Clomiphene Citrate Induces ROS-Mediated Apoptosis in Mammalian Oocytes. Open J Apoptosis, 2014; 3: 52-58.[DOI]
[67] Budani MC, Carletti E, Tiboni GM. Cigarette smoke is associated with altered expression of antioxidant enzymes in granulosa cells from women undergoing in vitro fertilization. Zygote, 2017; 25: 296-303.[DOI]
[68] Santini SJ, Cordone V, Falone S et al. Role of Mitochondria in the Oxidative Stress Induced by Electromagnetic Fields: Focus on Reproductive Systems. Oxid Med Cell Longev, 2018; 2018: 5076271.[DOI]
[69] Gardner DK, Reed L, Linck D et al. Quality control in human in vitro fertilization. Semin Reprod Med, 2005; 23: 319-324.[DOI]
[70] Gardner DK, Kelley RL. Impact of the IVF laboratory environment on human preimplantation embryo phenotype. J Dev Orig Health Dis, 2017; 8: 418-435.[DOI]
[71] Agarwal A, Aponte-Mellado A, Premkumar BJ et al. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol, 2012; 10: 49.[DOI]
[72] Pruett JE, Everman SJ, Hoang NH et al. Mitochondrial function and oxidative stress in white adipose tissue in a rat model of PCOS: effect of SGLT2 inhibition. Biol Sex Differ, 2022; 13: 45.[DOI]
[73] Uçkan K, Demir H, Turan K et al. Role of Oxidative Stress in Obese and Nonobese PCOS Patients. Int J Clin Pract, 2022; 2022: 4579831.[DOI]
[74] Chandra A, Surti N, Kesavan S et al. Significance of oxidative stress in human reproduction. Arch Med Sci, 2009; 5: S28-S42.
[75] Walczak-Jedrzejowska R, Wolski JK, Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol, 2013; 66: 60-67.[DOI]
[76] Aitken RJ. Impact of oxidative stress on male and female germ cells: implications for fertility. Reproduction, 2020; 159: R189-R201.[DOI]
[77] Lu J, Wang Z, Cao J et al. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol, 2018; 16: 80.[DOI]
[78] Xu Y, Nisenblat V, Lu C et al. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod Biol Endocrinol, 2018; 16: 29.[DOI]
[79] Kohli D, Champawat PS, Mudgal VD. Asparagus (Asparagus racemosus L.) roots: nutritional profile, medicinal profile, preservation, and value addition. J Sci Food Agric, 2023; 103: 2239-2250.[DOI]
[80] Singh AK, Srivastava A, Kumar V et al. Phytochemicals, Medicinal and Food Applications of Shatavari (Asparagus racemosus): An Updated Review. Nat Prod J, 2018; 8: 32-44.[DOI]
[81] Visavadiya NP, Soni B, Soni B et al. Suppression of reactive oxygen species and nitric oxide by Asparagus racemosus root extract using in vitro studies. Cell Mol Biol (Noisy-le-grand), 2009; 55 Suppl: OL1083-OL1095.
[82] Bhandary S, Sherly S, Kumari S et al. Acute and Subacute Toxicity Profile of Asparagus racemosus root extract, Isoprinosine and Shatvari Syrup in Swiss Albino Mice. J App Pharm Sci, 2017; 7: 129-135.
[83] Sushma, Yadava LP. Shatavari (Asparagus racemosus Willd): A herbal boon to women reproductive health and an overview of current research. World J Adv Res Rev, 2022; 16: 032-038.[DOI]
[84] Bhokardankar PS, Mane SG, Khairanar BP. An overview of shatavari (asparagus racemosus) an ayurvedic drug. Int J Ayurveda Pharma Res, 2019; 7: 60-65.[DOI]
[85] Bishoyi S, Tripathy U. Asparagus racemosus: Many problems, one solution on its phytochemical and pharmacological potential. J Med Plants Stud, 2023; 11: 03-07.[DOI]
[86] Visavadiya NP, Narasimhacharya AV. Ameliorative effects of herbal combinations in hyperlipidemia. Oxid Med Cell Longev, 2011; 2011: 160408.[DOI]
[87] Gupta S, Satwik R, Majumdar A et al. Clomiphene based ovarian stimulation in a commercial donor program. J Hum Reprod Sci, 2015; 8: 142-145.[DOI]
[88] Fisher SA, Reid RL, Van Vugt DA et al. A randomized double-blind comparison of the effects of clomiphene citrate and the aromatase inhibitor letrozole on ovulatory function in normal women. Fertil Steril, 2002; 78: 280-285.[DOI]
[89] Pourali L, Ayati S, Tavakolizadeh S et al. Clomiphene citrate versus letrozole with gonadotropins in intrauterine insemination cycles: A randomized trial. Int J Reprod Biomed, 2017; 15: 49-54.[DOI]
[90] Marut EL, Hodgen GD. Antiestrogenic action of high-dose clomiphene in primates pituitary augmentation but with ovarian attenuation. Fertil Steril, 1982; 38: 100-104.[DOI]
[91] Majeedi SF, Shameem I, Roqaiya M. Efficacy of Asparagus recemosus (Satavar) in stimulating follicular growth and ovulation in anovulatory infertility: a randomized controlled trial. Int J Reprod Contracep Obstet Gynecol, 2016; 5: 310-316.[DOI]
[92] Karuna DS, Dey P, Das S et al. In vitro antioxidant activities of root extract of Asparagus racemosus Linn. J Tradit Complement Med, 2018; 8: 60-65.[DOI]
[93] Goel RK, Prabha T, Kumar MM et al. Teratogenicity of Asparagus racemosus Willd. root, a herbal medicine. Indian J Exp Biol, 2006; 44: 570-573.
[94] Alok S, Jain SK, Verma A et al. Plant profile, phytochemistry and pharmacology of Asparagus racemosus (Shatavari): A review. Asian Pac J Trop Dis, 2013; 3:242-251.[DOI]
[95] Sinha B, Tare H. Asparagus racemosus: a holistic review of its traditional uses and modern research. Int J Pharm Qual Assur, 2024; 15:531-538.[DOI]





 Copyright ©
Copyright ©