Water-responsive Contraction for Shape-adaptive Bioelectronics
Minyan Ge1, Shumao Xu1*![]() , Yurui Tang1, Yuchun Wang2, Yaqi Liao3, Jing Wang1*
, Yurui Tang1, Yuchun Wang2, Yaqi Liao3, Jing Wang1*
1Institute of Science and Technology for Brain-inspired Intelligence (ISTBI), Fudan University, Shanghai, China
2Huashan Hospital, Fudan University, Shanghai, China
3Shanghai University of Traditional Chinese Medicine, Shanghai, China
*Correspondence to: Shumao Xu, PhD, PI, Institute of Science and Technology for Brain-inspired Intelligence (ISTBI), Fudan University, Shanghai, 201203, China; Email: shumaoxu@psu.edu
Jing Wang, PhD, Associate Professor, Institute of Science and Technology for Brain-inspired Intelligence (ISTBI), Fudan University, Shanghai, 201203, China; Email: wangjing_@fudan.edu.cn
DOI: 10.53964/id.2025002
Abstract
Water-responsive adaptive contraction represents a pivotal advancement in bioelectronics, offering unparalleled advantages over traditional swollen hydrogels and actuator-based systems. This review explores mechanisms including phase transition, hydrogen bond disruption, and hierarchical hybridization to enable rapid and substantial shape adaptability upon moisture exposure without external control and highlights various component modulation and porous and heterostructure engineering strategies to provide directional control and multifunctionality. Additionally, recent advances in supercontractile films, achieved through cold-drawing processes that disrupt hydrogen bonds in aligned polymer chains are overviewed for wearable and implantable biomedical applications where seamless tissue interfacing is essential. These advances address long-standing challenges in device integration with biological tissues, such as rigidity and inflammation, by creating electrodes that naturally conform to tissue shapes. This review delves into water-responsive adaptive contraction in bioelectronics, emphasizing its potential to revolutionize medical implants, wearable health monitors, and soft robotics by naturally conforming to tissue shapes upon moisture exposure. They promise significant improvements in chronic electronic integration and patient care, paving the way for next-generation medical devices, soft robotics, and smart textiles that adapt dynamically to biological environments.
Keywords: water-responsive contraction, shape-adaptive bioelectronics, hydrogen bond disruption, supercontractile films, wearable biomedical devices
Seamlessly integrating electronic devices with biological tissues has long been a challenge in bioelectronics, a field that seeks to bridge the gap between rigid electronic materials and the soft, dynamic nature of biological tissues for applications in medical implants and wearable health monitors[1-11]. Early efforts focused on developing flexible electronics using PDMS and other elastomer materials[12-14], which offered improved mechanical compatibility but still required significant customization to match the complex geometries of tissues.
To enhance the integration of electronics with biological tissues, various adhesives, such as medical glues, sutures, and bioadhesive tapes have been developed and widely adopted[15-17]. However, tight adhesion can make removal difficult and often leads to potential tissue damage, inflammation, and limited durability under dynamic biological conditions. For example, cyanoacrylate-based glues provide strong adhesion but can cause cytotoxicity[18,19], while fibrin glues offer biocompatibility but limited mechanical strength[18,20].
To address these challenges, auxiliary adhesive methods have been developed, including the use of ultrasound to create microbubbles that enhance adhesive penetration[21-23], and light-activated adhesives that cure upon ultraviolet exposure or enable light-induced detachment[15,24,25]. These techniques offer more controlled adhesion and detachment, reducing tissue damage and inflammation while enhancing durability in dynamic biological environments. However, these methods can involve complex setups, external controls, and may not always provide consistent results, especially in the dynamic and moist environment of biological tissues.
To reduce reliance on adhesives and develop materials that can adapt to the complex, changing shapes and movements of biological tissues, shape-adaptive electrodes have been developed. However, these electrodes have typically relied on electric or other actuators[26-29], which often involve complex fabrication processes, various electrode integrations, and external controls for activation and positioning.
Water-responsive shape adaptive electrodes present a more efficient solution for wearable and implantable bioelectronics, which naturally and dynamically conform to tissue shapes upon exposure to moisture, simplifying the integration process. Traditional hydrogel swelling processes, while effective, often suffer from slow response times and limited control over shape changes[30].
Recent advances in shape-memory materials and hydrogels that respond to stimuli like temperature, light, and magnetism have enabled more adaptable, biocompatible, and conformal interfaces[1,31-40]. These advancements reduce the need for precise pre-shaping and extensive surgical manipulation. However, challenges remain in achieving rapid, large-scale conformal changes that are also biocompatible and mechanically stable under physiological conditions.
This review discusses the recent advances in water-responsive contraction for shape-adaptive bioelectronics. A comprehensive review of various water-responsive materials, including liquid crystal networks (LCNs), proteins, polypeptides, and shape memory polymers (Table 1), is provided to outline their different mechanisms and strategies for performance improvement, such as phase transition modulation, hydrogen bond disruption, and hierarchical hybridization. Furthermore, the potential applications of these materials in biomedical devices, soft robotics, and smart textiles are highlighted, emphasizing their significance in creating next-generation bioelectronics that seamlessly integrate with biological environments.
Table 1. Common Water-responsive Contraction Strategies and Materials
Types |
Materials |
Mechanism |
Parameters |
Performance |
Natural proteins |
Spider silk, Bombyx mori silk fibroin |
Disruption of hydrogen bonds in amorphous regions |
Crystallinity: 30%-50%; Hydration: 0-30s |
Contraction: Argiope trifasciata NS, 31%[45]; FS, 52%[46]. Nephila NS, 20%[47]; FS, 40%[46], 44%[48]. Tensile strength: 1-2GPa; Elasticity: ~30% elongation |
Shape-memory polymers |
PU[128], PLA[169], PEG-α-CD[139], et al. |
Phase transition or plasticization triggered by water |
Transition temperature: 20-40℃; Preset shape memory or stress |
Water response: <1min; Shape recovery ratio: >95%; Tensile strength: generally 10-50MPa |
Liquid crystal elastomers |
LCNs, LCPs |
Phase transition in liquid crystal domains; tuned by liquid crystal alignment |
Order parameter: 0.5-0.9; Transition temperature: 30-50℃ |
Reversible shape changes: >100% elongation; Water response: <1min |
Synthetic peptides and proteins |
ELPs, collagen hydrogels |
Folding/unfolding of peptide chains |
Peptide sequence; Folding: <10s |
Reversible contraction: >200% elongation; Biocompatibility; Tunable stiffness and elasticity |
Notes: PU: Polyurethane; PLA: Polylactic acid; PEG-α-CD: Poly(ethylene glycol)-α-cyclodextrin; LCNs: Liquid crystal networks; LCPs: Liquid crystal polymers; ELPs: Elastin-like polypeptides; NS, Naturally spun; FS, Forced silked.
2 NATURAL PROTEINS FOR SHAPE-ADAPTIVE BIOELECTRONICS
Natural proteins, like spider silk fibers, elastin, and collagen, exhibit remarkable mechanical strength and adaptability to environmental changes, which is attributed to their unique hierarchical structures[41-43]. Spider silk fibers, typically 1-5μm in diameter, exhibit a remarkable ability to undergo supercontraction—a reversible shortening by up to 60% upon exposure to water, owing to their unique hierarchical structure that enhances both strength and responsiveness to environmental changes. This structure combines organized crystalline β-sheet regions, which provide mechanical strength, with glycine-rich amorphous areas that offer elasticity (Figure 1A and B)[44-48]. The crystalline β-sheets in the structure act as rigid, load-bearing units, while the amorphous regions between these β-sheets allow flexibility. This arrangement enables rapid response to moisture while maintaining the fiber’s integrity under stress. The silk’s hierarchical structure is formed through the spider’s spinning process, where liquid–liquid phase separation and molecular alignment in the spinning duct led to poly(alanine) β-sheet crystallites linked by flexible glycine segments. These crystalline domains, composed of aligned nanocrystalline β-sheets, provide enhanced tensile strength by distributing and bearing loads effectively across the structure. They also act as structural anchors, ensuring that the fiber retains its shape even as the surrounding amorphous regions change due to moisture (Figure 1B[44]). The primary amino acids in spider silk are glycine, alanine, and serine, with the microstructure being semi-crystalline due to the specific polypeptide secondary structure. Upon wetting, the hydrogen bonds in the glycine-rich amorphous regions are disrupted, causing rapid contraction, while the crystalline β-sheets maintain the fiber’s overall structural integrity. This combination of strength and responsiveness in spider silk has inspired the design of water-responsive bioelectronic materials. By mimicking the hierarchical alignment of crystalline β-sheets and amorphous regions, materials can be fabricated to achieve controlled, rapid responses to moisture without sacrificing durability, which offers promising applications in bioelectronics, enabling flexible, shape-adaptive interfaces that naturally integrate with soft, moist tissues without requiring external controls.
|
Figure 1. Structural Insights into Spider Silk Under Varying Humidities. (A,B) Spider silk web, and β crystal sheets in silk thread. (C,D) Normalized orientation degree, deviation angle and stretch ratio as a function of relative humidity. Reproduced from Ref.[51] with permission from ACS. Dashed line indicates the glass transition relative humidity. (E) Simulations of the mechanical behavior of spider silk, correlating different stress-strain curve regions to conformational changes. Reproduced from Ref.[52] with permission from Nature. At low stress, amorphous strands stretch and lose hydrogen bonds, while at high stress, crystalline strands slide and hydrogen bonds break. (F) Experimental stress-strain curves for different silks. Reprinted from Ref.[54] with permission from Elsevier.
Mechanistically, as relative humidity (RH) rises, spider silk transitions from a densely packed glassy state to a more flexible, rubbery state[49,50]. In the glassy state, densely packed β-structures and hydrogen-bonded helices give the fiber its strength. As water acts as a plasticizer, these hydrogen bonds break, allowing minor expansion in the amorphous regions while the β-sheets continue to maintain structural stability. When RH reaches the glass transition point (ϕg), the silk’s molecular mobility increases, leading to contraction as bond dissociation becomes dominant. Figure 1C shows the relationship between the normalized orientation degree, α/α0 as a function of relative humidity (RH), ϕ, which could be reflected by the following equation[51]:
$$ {\alpha }\left({\varphi }\right)={{\alpha }}_{0}-\left({{\alpha }}_{0}-\frac{1}{3}\right)\frac{{\varphi }-{{\varphi }}_{{\xi }}}{1-{{\varphi }}_{{\xi }}}\left(1\right)$$
where ξ is the strength of a hydrogen bond. This equation describes the linear decrease in orientation as hydrogen bonds are disrupted, especially in amorphous regions. The angle, γm refers to that between a chain and the preferred direction in the glassy state. The normalized angle, γm/γ0, increases as RH increases, indicating a loss of orientation[51] (Figure 1C). As further moisture exposure causes swelling, the molecular alignment in the amorphous areas is lost, and the fiber transforms into a flexible, rubbery network maintained by β-sheet cross-links, which stabilize the structure (Figure 1D)[51]. The glassy state features dense packing and strong hydrogen bonds within the semi-amorphous matrix, characterized by β-structures, helices, and β-turns. Upon exposure to moisture, water molecules act as plasticizers, breaking hydrogen bonds and causing minor expansion. As ϕ approaches ϕg, the glass transition temperature (Tg) decreases, leading to increased molecular mobility. This results in fiber contraction due to the dominance of bond dissociation over swelling, reducing orientation. Beyond ϕg, swelling extends the polymer chains, increasing the deviation angle and losing alignment. In the fully supercontracted state, all hydrogen bonds are broken, and the network relies on β-sheet cross-links, behaving like a rubber network with high flexibility.
Atomistic simulations of spider silk under stress reveal how amorphous and crystalline strands respond to forces, reflecting conformational changes as hydrogen bonds are disrupted. This insight has informed the design of water-responsive bioelectronics that exploit stress-responsive behavior and the ability to dissipate energy for creating resilient, adaptable bioelectronic interfaces. At low stress, amorphous strands stretch and lose hydrogen bonds, while at high stress, crystalline strands slide and break hydrogen bonds. The mechanical behavior of spider silk under stress is detailed using atomistic simulations, highlighting regions of the stress-strain curve that correspond to specific conformational changes (Figure 1E)[52]. This behavior could be reflected by:
$$ {m}\left({\varphi }\right)={M}{exp}\left(\frac{{\varphi }-{{\varphi }}_{{\xi }}}{{\varphi }-1}\right)\left(2\right)$$
where m(ϕ) represents the number of hydrogen bonds remaining, M is the initial number of hydrogen bonds, and ξ is the strength of a hydrogen bond. This equation could explain how hydrogen bond dissociation leads to supercontraction. In the entropic unfolding phase, amorphous strands stretch, losing hydrogen bonds, contributing to elasticity. As stress increases, the stiffening region shows strain hardening, where crystalline β-sheets align and enhance load-bearing capacity. The stick-slip phenomenon at higher strains, marked by periodic stress changes, indicates the sliding and rupture of hydrogen bonds within crystalline regions. This behavior dissipates energy and contributes to the silk’s toughness. The area under the curve signifies the material's toughness, essential for bioelectronics and smart textile applications requiring elasticity, toughness, and environmental responsiveness[53].
Experimental findings also show that spider silk’s tensile properties, influenced by spinning conditions and humidity, can be engineered to meet the demands of bioelectronics and smart textiles, where toughness and environmental responsiveness are essential. Experimental stress-strain curves illustrate the differences between naturally spun and forcibly silked fibers (Figure 1F)[54]. Forcibly silked fibers show higher stiffness and lower deformation at breaking compared to naturally spun fibers. The tensile properties can be modulated by the silking stress, providing insights into the relationship between spinning conditions, microstructure, and mechanical behavior. The structure and mechanical properties of spider silk under varying humidities, supported by mathematical models and experimental data, offer a comprehensive understanding of how spider silk can be engineered for applications requiring dynamic mechanical responses to environmental changes, such as water-responsive contraction for shape-adaptive bioelectronics.
3 LCNs FOR SHAPE-ADAPTIVE BIOELECTRONICS
3.1 Chemical and Structural Modifications for Enhanced Water-responsiveness
LCNs have garnered significant interest for their unique ability to combine the fluidity of liquid crystals with the rigidity of polymer networks, allowing for substantial and controllable deformations in response to environmental stimuli, including water[55-57]. While traditional LCNs are typically non-hygroscopic, their water-responsiveness can be significantly enhanced by introducing chemical and structural modifications. Inherently hygroscopic molecules containing polar functional groups (e.g., C–O–C or C=O) facilitate water interaction without additional treatment[58,59]. In many non-hygroscopic LCNs, although these polar groups may be present, their effect is minimized by a composition rich in non-polar groups and limited network porosity, which restricts water diffusion and reduces responsiveness[60].
One approach to enhance water-responsiveness is creating thin LCN films, where reduced thickness facilitates rapid moisture diffusion across the material, allowing for quicker and more pronounced bending under humidity gradients (Figure 2A). Another effective strategy to enhance water-responsiveness in LCNs is to increase porosity by incorporating and subsequently removing particles, such as CaCO3[60], NaCl[61,62], or ZnO[63], from the LCN precursor. For instance, CaCO3 particles can be etched out to leave voids that significantly improve water diffusion throughout the material[57], while NaCl and ZnO offer distinct advantages. NaCl creates fine, evenly distributed pores for rapid hydration[62], and ZnO can form interconnected porous networks that enhance mechanical strength alongside water accessibility[63-65]. This increased porosity allows moisture to reach polar sites more readily, leading to quicker and more pronounced anisotropic swelling, which is beneficial for applications requiring rapid adaptability, such as aquatic soft robotics and bioelectronics.
|
Figure 2. Water-Responsiveness in Liquid Crystals, Proteins, Polypeptide and Shape-Memory Polymers. A: Assisted water diffusion in liquid crystal networks: Water molecules absorbed by reducing thickness or introducing pores disrupt molecular ordering, causing anisotropic deformation and bending. B: Hybridized liquid crystal films respond more in the hygroscopic layer than the single liquid crystal network layer when exposed to humidity, leading to bending or contraction. C and D: Shape change of bilayers in water based on liquid crystal elastomer (LCE) layer orientation, showing folding in different axes and bidirectional folding patterns. Reprinted from Ref.[82] with permission from RSC. E and F: Curvature change of alkalized-acrylic blue phase liquid crystal films with varying thickness under 70% relative humidity (RH) difference, and 70μm thickness exposed to different humidities. G: Deformation and recovery process of blue phase liquid crystal films in wet and dry conditions. E-G, reproduced from Ref.[95] with permission from RSC. H: Uversky plot of various proteins as a function of charge and hydrophobicity, showing elastin-like polypeptides (ELPs) ability to fold and unfold. Reprinted from Ref.[97] with permission from Elsevier. Inset, hydrated crosslinked elastin during stretching and folding, reprinted from Ref.[43] with permission from MDPI. ELP, elastin-like polypeptide. I: Contraction in a heated silicone shaped memory hybrid filled with CH3COONa∙3H2O (NaAc) upon immersing in water. Reprinted from Ref.[130] with permission from Expresspolymlett. J: Buckling of pre-stretched poly(methyl methacrylate) (PMMA) in ethanol. Reprinted from Ref.[135] with permission from AIP.
Bioelectronics, such as implantable sensors and neural interfaces, should dynamically conform to changing tissue conditions, including hydration, movement, and pressure while maintaining structural integrity and functionality[1,26,39,66-72]. The interconnected pore networks created through these porous reagents are advantageous for real-time bioelectronic applications, including devices for immediate physiological sensing or therapeutic response[73-76]. These materials can match the pace of physiological changes by adapting rapidly to hydration levels, making them ideal for applications that demand precise, quick adaptability in dynamic biological environments. Moisture-responsive LCN electrodes can adapt their shape to sustain close contact with biological tissues, ensuring reliable signal capture and effective therapeutic delivery[77-80]. This adaptability is crucial in wearable devices, where real-time adjustments to sweat or humidity levels maintain comfort, sensor accuracy, and device performance, making LCNs valuable in healthcare settings where continuous monitoring and adaptive responses provide timely feedback on health metrics like hydration, pH, and bioelectrical signals.
The rapid, reversible, and controlled responses enabled by porous LCNs are essential for bioelectronics in dynamically changing biological environments. Biological tissues experience constant fluctuations in hydration, temperature, and movement, which require bioelectronics to alter their configuration rapidly and precisely without relying on external power sources. The porosity within LCNs allows water to diffuse efficiently, enabling swift, anisotropic shape changes with controlled directionality. For instance, in neural interfaces, such rapid adaptability ensures electrodes maintain proximity to neurons, preserving signal fidelity despite tissue swelling. Similarly, in wearable sensors, the LCNs contract or expand in response to skin moisture changes, ensuring consistent sensor contact and accurate readings even during high-sweat activities. This real-time adaptability is crucial for creating reliable bioelectronics that can respond seamlessly to varied physiological and environmental conditions in real-world applications.
3.2 Hybridizing LCNs for Bioelectronics and Soft Robotics
In addition to porosity, hybridizing LCNs with hygroscopic layers can further increase their responsiveness to water[81,82]. In soft robotics, LCN-based actuators leverage water-responsiveness and molecular orientation to achieve biomimetic motion, simulating the contraction and expansion of biological tissues[78,83-85]. By fine-tuning the porosity and alignment, these actuators enable precise movements without complex mechanical systems, making them valuable in medical robotics. In applications like surgical tools or therapeutic devices, gentle, adaptive movement minimizes tissue damage and enhances patient safety[86]. Adaptive bioelectronics utilizing LCN-hydrogel bilayers are promising for smart sensors that adjust to varying moisture levels[87-90]. A bilayer of LCN and a hydrogel layer can act as a humidity-sensitive actuator, where the hydrogel expands rapidly in response to moisture, inducing bending or other deformations in the LCN layer (Figure 2B). Complex actuation modes can be programmed by varying the alignment in the LCN film, allowing for directional control and multifunctional responses. Additional advancements include spray-coating techniques to fabricate bilayer actuators where a cholesteric liquid crystal layer on a hydrophilic polyamide film results in twisting deformation upon humidity-induced polyamide swelling. Furthermore, fluorescent LCN-hydrogel bilayers can change luminescence color upon actuation. By incorporating an aggregation-induced emission (AIE) luminogen into a hydrophilic acrylate monomer mixture, researchers have created a hydrogel layer that emits humidity-dependent fluorescence, causing the bilayer to bend and shift fluorescence color[79,81,91].
LCNs are increasingly recognized for their potential in creating shape-changing materials responsive to environmental stimuli like water, positioning them as promising alternatives to traditional actuators, especially where power or size constraints apply[92]. LCN-based materials can be engineered to undergo tailored deformations-such as bending, twisting, and folding-by aligning the molecular orientation of LCEs within the structure[82,93,94]. This strategic alignment of the anisotropic modulus, which is approximately five times higher along the molecular orientation than perpendicular to it, allows for predictable and controlled shape responses to humidity (Figure 2C)[82]. For instance, a polymeric bilayer composed of a water-responsive hydrophilic polymer and an LCE was designed to change shape upon water exposure. When the hydrophilic layer absorbs moisture, it expands, and the bilayer bends along the direction determined by the LCE’s anisotropic stiffness. By adjusting the orientation of the stiffer axis in the LCE, the helical pitch of the bilayer can be controlled, ranging from 0.1 to 20mm, enabling the creation of complex 3D shapes through spatial patterning of the LCE’s alignment. This directional control is critical in bioelectronics, where devices should conform to tissue shapes and adapt seamlessly to biological changes, reducing mechanical stress and improving biocompatibility (Figure 2C)[82]. Patterning the director angle in LCEs further enables programming for intricate shape transformations. Introducing localized 90° patterns within a 0° oriented sample causes bending at specific points, which act as hinges, facilitating bidirectional folding or "mountain and valley" origami-inspired deformations. Each patterned section follows a helical curve proportional to the director angle, creating unique folding interactions where opposing helices meet (Figure 2D)[82].
Quantitative analysis of LCN actuation can be conducted using a custom-built humidity chamber to examine the effects of relative humidity differences, film thickness, and type on bending curvature and speed (Figure 2E)[95]. Acrylic blue-phase LCN films demonstrated thickness-dependent bending under a 70% RH difference, with a 70μm film reaching a steady-state curvature of 2.5cm−1 (Figure 2F)[95]. The behavior of these films in response to humidity can also be observed through color changes, as they curl upon exposure to moisture and revert as humidity decreases. This reversible curling, driven by directional expansion due to water absorption, highlights the potential of LCN films and their hybrid constructs for water-responsive actuation in adaptive bioelectronics (Figure 2G)[95].
LCNs’ ability to control mechanical and chemical properties in response to humidity shows promise for applications in adaptive bioelectronics. The dynamic capabilities of these materials-enabled by humidity-responsive shape programming, anisotropic stiffness, and advanced patterning techniques-position them as versatile tools in healthcare, wearable technology, and soft robotics. Their rapid, controlled, and reversible responses offer a flexible solution for real-time adaptability, crucial for maintaining reliable performance in dynamic biological environments.
4 WATER-RESPONSIVE SYNTHETIC PEPTIDES AND PROTEINS FOR SHAPE-ADAPTIVE BIOELECTRONICS
4.1 Structural Mechanics for Responsive Bioelectronics
Synthetic peptides and proteins, such as elastin-like polypeptides (ELPs), are renowned for their biocompatibility and ability to undergo significant conformational changes in response to environmental stimuli like water[43,96,97]. ELPs, derived from the hydrophobic domains of tropoelastin, are composed of a repeating VPGXG pentapeptide sequence, where X can be any amino acid except proline[98]. This sequence imparts ELPs with lower critical solution temperature phase behavior, enabling them to form an insoluble coacervate phase above their cloud point, similar to tropoelastin. In dry or low-humidity environments, ELPs exist in a more compact, disordered coil state. However, in the presence of water, they undergo a rapid conformational shift to form structured β-sheets. This phase behavior can be tuned to respond to various stimuli, such as temperature[99], salt concentration[100], pH[101], and light[102], making ELPs versatile for applications in drug delivery, tissue engineering, and adaptive bioelectronics.
The primary mechanism behind ELPs’ water-responsiveness lies in their ability to shift between random coil and β-sheet conformations. This transition enables ELPs to contract and expand reversibly, similar to elastin’s natural elasticity, and adapt to hydration changes in biological environments. The Uversky plot provides insight into these transitions by analyzing the folding and disorder tendencies of ELPs based on their hydrophobicity and net charge (Figure 2H)[43,97]. ELPs with highly hydrophilic or charged residues can exhibit thermal stability often associated with disordered proteins, allowing precise control of disorder propensity. Disorder, in addition to structure, may be encoded at the amino acid level.
The mechanics of ELPs mirror the structure of elastin, an essential extracellular matrix protein that provides elasticity to tissues like skin, ligaments, and blood vessels. Composed of cross-linked tropoelastin, elastin includes alternating hydrophobic and cross-linking domains that give it structural integrity and substantial elastic recoil. Hydration is crucial to elastin’s functionality; while dry elastin is hard and brittle, hydrated elastin retains flexibility. Elastin’s elasticity is driven by entropy changes in its disordered domains during stretching, where hydrophobic domains contract due to the hydrophobic effect, as modeled by the entropy Equation (3)[103]:
$${S}={c}{o}{n}{s}{t}-{{k}}_{{b}}{{N}}_{{c}}\left[{\rho}{\tau}\left({\rho }\right)+{ln}\left(\frac{{\tau }\left({\rho }\right)}{{sinh}\left({\tau }\left({\rho }\right)\right)}\right)\right]\left(3\right)$$
where const is a reference entropy value specific to the system, providing a baseline from which entropy changes are measured, kb is the Boltzmann constant, Nc is the number of chains, ρ is the ratio of the end-to-end distance to the contour length, and τ is a variable derived from the Langevin equation that describes the chain stretch. In elastin, the entropy changes during stretching and folding, driven by the hydrophobic effect and the structural disorder of the protein chains. This behavior underlies the extraordinary elasticity of these materials, making them suitable for applications in smart textiles, responsive coatings, and biomedical devices. The ability to precisely control the folding and unfolding processes is crucial for these applications, providing adaptability and functionality.
The potential of ELPs in bioelectronics lies in their capacity to function as smart, biocompatible interfaces. When integrated into devices, ELPs can dynamically respond to fluctuations in body fluids or sweat, adapting their shape and conforming closely to tissue surfaces[104,105]. This close interfacing reduces mechanical mismatch, minimizes inflammation, and enhances the longevity and functionality of implantable devices or wearable systems. In implantable devices, the ability of ELPs to dynamically conform to tissue movements, as they adjust to hydration levels, significantly improves the interface between the device and biological tissues. This adaptability minimizes mechanical mismatch and reduces the risk of inflammation, thus improving biocompatibility and device longevity. For instance, ELPs can be used in implantable sensors[106], neural interfaces[107,108], and cardiovascular stents[109,110] to maintain close and adaptable tissue contact. This adaptability reduces risks of dislodgement and improves device performance by naturally adjusting to the body’s movements and hydration levels over time. In wearable devices, ELPs’ water-responsive behavior allows for textiles that dynamically adjust to moisture, such as sweat, providing greater comfort and potentially serving as biofeedback mechanisms[104,111]. Smart garments incorporating ELPs could change their porosity or fit based on the wearer’s hydration levels, adjusting for comfort and potentially indicating dehydration[104,112]. This adaptability makes ELPs a compelling choice for personal health monitoring devices that aim to provide a more natural, comfortable user experience. ELPs can be used in soft robotics to create actuators that mimic the behavior of biological muscles[113-116]. Their water-responsive contraction and expansion enable these materials to move in a manner similar to muscle fibers, which contract or relax in response to stimuli. These actuators could be beneficial in medical robotics, where soft, tissue-like materials are crucial for safe interaction with human tissues. This capability to create natural, fluid motion in response to hydration changes makes ELPs valuable in surgical tools, rehabilitation devices, and other soft robotic systems requiring biomimetic movement.
To optimize ELP responsiveness, chemical modifications such as adding hydrophilic or charged residues (e.g., lysine or glutamate) can enhance water absorption, speeding up conformational changes and expanding applications in devices that require rapid shape adaptability[117-121]. This structural reinforcement ensures that ELP-based devices remain reliable and functional over extended periods.
4.2 Hybridization and Cross-linking for ELPs-based Bioelectronics
ELPs are highly adaptable and biocompatible, making them ideal candidates for bioelectronics and adaptive wearable integrations. Their functionality can be further expanded by combining them with other water-responsive materials, such as hydrogels[122]. Known for their high water content and swelling ability, hydrogels provide additional volume expansion and flexibility, which, when paired with ELPs, create a composite material that can respond dynamically to environmental humidity. Incorporating a hydrogel layer with ELPs enables rapid water absorption, triggering ELP contraction and facilitating more substantial or complex shape transformations. This synergy produces adaptable, layered responses that are especially useful in wearable devices or implants requiring advanced 3D adaptability[123,124].
Cross-linking methods further enhance ELPs’ durability and mechanical resilience, ensuring they withstand cyclic hydration and dehydration[119-121]. Cross-linking agents, including glutaraldehyde[125], genipin (a naturally derived cross-linking agent from Gardenia jasminoides)[126], and UV-induced bonds[24,127], provide additional stability by forming covalent bonds between polymer chains. Genipin, with its natural origin and biocompatibility, is advantageous for medical devices. By adjusting cross-linking density and selecting suitable cross-linkers, ELP properties can be finely tuned to achieve the right balance of flexibility and stability with the inherent water-responsive nature, adapting them to applications across bioelectronics, soft robotics, adaptive textiles, and medical coatings.
ELPs hold vast potential for shape-adaptive bioelectronics due to their water-responsive contraction, biocompatibility, and structural versatility. With advancements in chemical modification, hybridization with hydrogels, and cross-linking techniques, ELPs can be optimized to meet specific demands across biomedical applications, soft robotics, and adaptive wearables. ELP-based devices, able to interface seamlessly with biological tissues while providing flexibility and responsiveness, underscore their transformative role in next-generation bioelectronics.
5 SHAPE-MEMORY POLYMERS FOR SHAPE-ADAPTIVE BIOELECTRONICS
Shape-memory polymers (SMPs) are a versatile class of materials that can "remember" and return to their original shape upon exposure to specific triggers, such as water. This capability makes SMPs highly suitable for bioelectronic applications that require adaptive interfaces, including deployable medical devices, soft robotics, and smart sensors[128,129]. By incorporating hydrophilic components and applying shape-memory processing, SMPs achieve rapid, reversible deformations in response to moisture, enhancing their utility in various biomedical settings. The water-responsiveness of SMPs is achieved through their unique dual-phase structure: a temporary shape “locked” by stress-processing and a permanent shape “remembered” by the polymer matrix. Hydrophilic domains, such as polyethylene glycol (PEG) or polyvinyl alcohol (PVA), are incorporated to enable water absorption, which acts as a trigger to release internal stresses, returning the polymer to its original configuration. This moisture-driven shape recovery is advantageous in bioelectronics, where materials should interact dynamically with biological tissues in response to hydration. In deployable biomedical devices, SMPs enable minimally invasive insertion followed by water-induced expansion or shape recovery. Stents or vascular scaffolds can be introduced in a compact form and then expand upon exposure to body fluids, minimizing tissue damage while achieving a precise fit. This water-triggered adaptation is also beneficial in soft tissue support devices, where SMPs provide controlled shape recovery tailored to specific anatomical locations, reducing mechanical mismatch and enhancing biocompatibility.
5.1 Porosity and Microstructural Design for Rapid Water Responsiveness
The development of water-responsive SMPs introduces a highly adaptable class of materials with potential for bioelectronic applications, where precise and controlled shape changes are required. By combining porosity with tailored microstructural design, SMPs achieve enhanced water diffusion and rapid responsiveness, making them ideal for dynamic interactions in biological environments.
The development of a hybrid SMP ring made from silicone filled with 70 vol% sodium acetate trihydrate (NaAc) exemplifies an effective approach to creating water-responsive materials. This structure undergoes controlled contraction as NaAc dissolves, allowing the ring to recover its original shape when submerged in room-temperature water (Figure 2I)[130,131]. The initial star-shaped ring is pre-expanded at the melting temperature of NaAc and cooled to room temperature. Upon immersion in room temperature water, NaAc dissolves, and the silicone sponge recovers its original shape. This phenomenon can be extended to shape memory hybrids with different soluble inorganic salts where the transition component is designed to release in water, altering the device's configuration and enabling it to adapt its shape accordingly. This capability is useful for devices that need to change shape dynamically in response to environmental conditions, such as biomedical implants or soft robotics[132-134].
Pre-shape memory change or preset stress is essential for achieving effective and controlled water-responsive contraction. Materials like polymethyl methacrylate (PMMA) can be pre-stretched at high temperatures to induce a state of internal stress. When these materials are immersed in ethanol, they exhibit curving due to the penetration of the ethanol (Figure 2J)[135,136]. As the ethanol fully penetrates, the PMMA recovers its original shape.
In water-responsive systems, presetting the shape or stress within the material allows for precise control over the contraction and expansion processes. This is crucial for applications requiring exact and repeatable movements, such as in medical implants that need to adapt to the body's movements or in soft robotic components that should flex and contract reliably in response to environmental moisture[27,31,38,39,67,71,137, 38].
Inherently hygroscopic molecules typically contain polar functional groups (e.g., C–O–C or C=O) that interact with ambient humidity without additional treatment[58]. In many non-hygroscopic LCNs, although these polar groups may be present, their effect is minimized by a composition rich in non-polar groups and limited network porosity, which restricts water diffusion and reduces responsiveness. One approach to enhance water-responsiveness in these materials involves creating thin films. Due to reduced thickness, these films can deform quickly when exposed to a humidity gradient, as moisture can diffuse across the material more readily, facilitating bending and decreasing the alignment of the mesogens. Another effective method is to fabricate porous LCN actuators by incorporating and etching out particles, such as CaCO3, from the LCN precursor. The resulting porosity significantly improves water diffusion throughout the material, enabling access to polar sites that absorb moisture. This leads to quicker and more pronounced anisotropic swelling, resulting in complex deformation profiles that are beneficial for applications requiring rapid adaptability.
A recent study on poly(ethylene oxide) (PEO) and poly(ethylene glycol)-α-cyclodextrin (PEG-α-CD) inclusion complexes demonstrates the advantages of creating a porous, aligned microstructure to enhance water-responsive adaptability in bioelectronics (Figure 3A)[139,140]. By employing a repeated cold-drawing process, researchers created water-responsive adaptive polymer (WRAP) contractile electrodes that mimic the hierarchical structure of spider silk, featuring aligned microporous structures connected by fibrillar bridges (Figure 3B and C). This process aligns the polymer chains, forming a network of microscopic pores bridged by thin polymer fibers. The porosity enables rapid water diffusion, which, when combined with hydrogen bond disruption within the crystalline domains of the PEG-α-CD inclusion complex, causes the aligned chains to recoil quickly upon wetting, resulting in significant contraction. The crystalline domains maintain stability, preventing the film from dissolving in water, while the amorphous regions of PEO chains allow rapid contraction. WRAP wet films showed improved electrical properties with elongation (Figure 3D), ensuring that implanted recording electrodes achieved a high signal-to-noise ratio (SNR).
|
Figure 3. Water-Responsive Contracted Polymer Films. A: Schematic and photograph of a flexible water-responsive adaptive polymer (WRAP)-electrode array. SEBS, styrene-ethylene/butylene-styrene. B: Repeated cold drawing of IC/PEO to prepare WRAP films (drawn to 300% strain, then to a preset stress of 25MPa). IC/PEO, an inclusion complex where poly(ethylene oxide) (PEO) domains are crosslinked by poly(ethylene glycol) (PEG)-α-cyclodextrin (α-CD). C: SEM images of 700% elongated WRAP film with aligned and porous microstructures. D: Electrochemical impedance spectra (EIS) of wet WRAP films showing decreased impedance with increased elongation ratios. E: Optical microscope images of discontinuous Au domains on a swollen substrate (top) and continuous Au mesh on WRAP film (bottom). F: Hematoxylin and Eosin (H&E)-stained images of sciatic nerve cross-sections (top) and CD68 immunostaining (down) of sciatic nerves with Au-elastomer versus WRAP electrodes after two weeks of implantation. G: Recording setup with WRAP electrodes implanted on sciatic nerves through shape-adaptive contraction. H: Implantation of WRAP electrodes compared to conventional ones. I: Contraction of a WRAP electrode when wetted. J: WRAP electrode conformally wrapping around the peroneal nerve within 2min wetting. K: WRAP electrodes stimulate the rat sciatic nerve and record compound nerve action potential (CNAP) and compound muscle action potential (CMAP). I, Common peroneal nerve; II, tibial nerve; III, tibialis anterior muscle; IV, soleus muscle. L: Signal-to-Noise Ratio (SNR) recorded by WRAP and Au-elastomer electrodes implanted for 2 weeks, responding to mechanical stimuli. Reproduced from Ref[139] with permission from Springer Nature.
Unlike conventional hydrogels, which swell upon wetting and can tear Au films into discontinuous domains, WRAP films maintained a continuous Au mesh (Figure 3E). The shape-adaptive WRAP electrodes implanted on sciatic nerves showed superior biocompatibility (Figure 3F and G). The combination of porosity and flexibility allows WRAP electrodes to conformally wrap around nerves of different sizes, such as the rat sciatic and common peroneal nerves, expanding and contracting by over 50% within seconds of water exposure (Figure 3I and J). This rapid and reversible response supports precise applications in biomedical settings where adaptability is essential. Electrical stimulation induced leg and toe movements in rats, and simultaneous implantation on various nerves and muscles allowed for clear recording of compound nerve and muscle action potentials with lower baseline noise (Figure 3K)[141-146]. After two weeks of implantation, WRAP electrodes recorded clear electroneurography (ENG) signals, while Au-elastomer electrodes recorded almost no signals, demonstrating the superior recording performance of WRAP electrodes (Figure 3L). Unlike typical hydrogel swelling, which is often slow and uncontrolled, the adaptive contraction of WRAP films upon water exposure involves a rapid and controlled reduction in length due to the disruption of hydrogen bonds in the aligned polymer chains. This process transforms the inclusion complexes into a soft, stretchable hydrogel that quickly adapts to various shapes and sizes, enabling the creation of dynamic, biocompatible bioelectronic interfaces.
The rapid contraction and stretchability of these polymer films are attributed to their hierarchical microstructure, the hydrophilic nature of their components, and the stability provided by the polymer inclusion complex. Porosity and alignment achieved during the cold-drawing process allow PEO chains to contract rapidly when wetted. Moreover, the preset stress in the cold-drawing process determines the degree of chain alignment and the formation of a microporous, fibrillar microstructure, which can be fine-tuned by adjusting polymer chain length, crosslinking degree, ambient conditions, and polymer blend composition[147].
5.2 Molecular Composition and Processing for Targeted Responsiveness
Optimizing SMPs for bioelectronics requires adjustment of their molecular composition, hydrophilic component ratios, and stress processing conditions, as each of these factors enhances specific performance aspects crucial for bioelectronic applications[148]. The choice of base polymers and monomers in SMPs defines fundamental properties, such as flexibility, durability, and water-responsiveness. Incorporating hydrophilic monomers, like PEG or PVA, into the SMP matrix increases water affinity, enabling moisture absorption that activates shape recovery[148,149]. Adding hydrophobic segments, such as polycaprolactone or polylactic acid, enhances mechanical stability and controls water diffusion rates, creating a balanced design that ensures SMPs are both durable and sensitive to environmental changes-qualities that are essential for reliable bioelectronic interfaces[150,151].
The hydrophilic component ratio within an SMP directly impacts its water absorption capacity and responsiveness. A higher ratio of hydrophilic polymers increases water uptake, accelerating shape recovery in moist environments. Increasing PEG content within the polymer matrix improves water absorption, thereby speeding up shape change[152]. However, an excessive hydrophilic ratio can cause over-swelling, potentially compromising mechanical integrity. Optimizing this ratio is key to maximizing responsiveness while preserving stability, especially in implantable devices that should withstand varying hydration levels without structural deterioration.
The processing conditions of SMPs are crucial in defining their temporary shapes and ensuring efficient shape recovery upon water exposure. Stress-processing, which involves applying mechanical or thermal treatments, is used to “fix” a temporary shape within the polymer matrix. Thermal processing enables SMPs to be molded at elevated temperatures, setting a shape they can later revert to when exposed to water or heat. Mechanical treatments, such as stretching or compressing, instill a directional memory that governs how the material will contract or expand in response to hydration[139]. Key parameters-processing temperature, duration, and applied force-determine the strength and precision of the shape-memory effect, allowing SMPs to respond with tailored intensity and speed. This versatility makes SMPs adaptable for a range of bioelectronic applications, from rapid deployment in minimally invasive medical devices to gradual expansion in wearable sensors.
By adjusting SMPs’ molecular composition, hydrophilic component ratios, and stress-processing conditions, they can be fine-tuned to meet the diverse needs of bioelectronic applications. This customization enables SMPs to achieve precise, reliable shape recovery, making them versatile for adaptive bioelectronics, including neural interfaces and soft robotic actuators. SMP-based devices are thus well-suited for dynamic, hydration-sensitive biological environments.
The unique water-responsive properties of SMPs also position them as ideal materials for bioelectronic applications where adaptability and durability are essential. In soft robotics, SMP-based actuators mimic muscle-like movements through hydration-driven contraction and expansion, achieving biomimetic motion without motors or complex mechanical components[116]. This makes SMPs advantageous for robotic systems used in medical procedures, as SMP-based actuators can perform lifelike, adaptable movements, reducing tissue injury risks while allowing precise functionality during minimally invasive procedures.
Moreover, SMPs can leverage their shape-memory properties to maintain consistent contact with dynamic or irregular surfaces, such as the skin or internal[153] organs. Skin-mounted sensors can expand or contract in response to hydration changes, ensuring optimal contact despite fluctuations in sweating or environmental humidity. This real-time adaptability enhances the reliability and accuracy of physiological monitoring by maintaining continuous sensor contact. Additionally, SMPs can be engineered to respond to minimal amounts of moisture, making them highly sensitive for skin-based monitoring and wearable healthcare devices, where accurate data capture is essential[154,155]. Meanwhile, SMP-based polymeric bilayers, which combine hydrophilic polymers with LCEs, enable sophisticated 3D morphing controlled by the anisotropic modulus[156]. This bilayer structure allows devices to achieve complex, directional shape changes in response to water. The LCE layer provides structural rigidity along specific orientations, enabling anisotropic expansion or contraction. By adjusting the orientation and modulus of each layer, researchers can program complex shape changes that are particularly valuable in applications requiring precise spatial conformity, such as neural interfaces or biosensors for organs with irregular surfaces. These advanced morphing abilities of SMP-based bioelectronics enhance their versatility in soft robotics. SMPs serve as actuators that expand and contract with hydration changes, mimicking the movement of natural muscles. By integrating hydrophilic SMP actuators into robotic structures, soft robots achieve lifelike motion without relying on motors or electrical power, resulting in a more biomimetic and energy-efficient solution. This water-responsive actuation is particularly valuable in medical robotics, where safe, adaptable movement is crucial for preventing tissue injury and ensuring precise operation during minimally invasive procedures.
SMPs provide an exceptional platform for water-responsive, shape-adaptive bioelectronics due to their programmable shape-memory properties and hydrophilic responsiveness. With advances in polymer chemistry and stress-processing techniques, SMP-based bioelectronics can seamlessly integrate into real-world applications that demand dynamic interaction with biological tissues, such as deployable biomedical devices, smart sensors, adaptive interfaces, and soft robotics.
6 CONCLUSIONS AND OUTLOOK
Water-responsive adaptive contraction represents a significant advancement in bioelectronics, offering unique advantages over traditional swollen hydrogels and actuator-based systems. Unlike typical gels and hydrogels, which rely on swelling in response to light, temperature, or magnetic stimuli and often suffer from slow response times and limited shape control, water-responsive contraction offers rapid adaptability driven by structural changes at the molecular level. Actuator-based systems, while providing precise control, typically require external power sources and complex mechanisms, which can hinder their integration into soft, biocompatible applications.
Water contraction generally involves water-induced dissociation of intermolecular hydrogen bonds, and loss of chain orientation upon transitioning from a glassy to a rubbery state, different from swelling due to water molecule penetration. As the relative humidity increases, the distance between crystalline cross-linking domains decreases due to hydrogen bond dissociation, and highly oriented chains lose their orientation, leading to supercontraction.
Current findings in water-responsive materials suggest several optimization strategies to enhance their mechanical durability and biocompatibility, making them more suitable for biomedical applications. For instance, adding cross-linking agents can improve mechanical strength, while biocompatible surface coatings could reduce potential inflammatory responses upon implantation. Development of water-responsive materials that can achieve rapid and significant shape adaptability without the need for external control is crucial for wearable and implantable biomedical applications where seamless and efficient interfacing with the human body is essential[9-11,36,38,137,157-167]. These materials' intrinsic ability to respond to hydration changes allows for more natural and dynamic interactions within biological environments. Figure 4 illustrates various water-responsive materials, such as LCNs, proteins, polypeptides, and shape memory polymers, which offer exciting possibilities for adaptive bioelectronics through tailored chemical and structural modifications.
|
Figure 4. Summary of Various Water-responsive Contraction Mechanisms and Materials. The key mechanisms in water-induced contraction, involve hydrogen bond disruption, phase transition, and hierarchical structures. Materials that utilize these mechanisms include shape-memory polymers, liquid crystal elastomers, peptides, and proteins.
Natural proteins and ELPs, are notable for their ability to undergo significant conformational changes in response to water, shifting from random coils to β-sheet conformations. Such adaptability and biocompatibility make them ideal for smart textiles, responsive coatings, and biomedical devices.
LCNs are notable for combining the fluidity of liquid crystals with the rigidity of polymer networks, enabling significant and controllable deformations in response to water. Modifying the chemical components or microstructure of LCNs, such as by creating porous structures or hybridizing hygroscopic layers, can further enhance their water-responsiveness. Porous structures can be introduced into LCNs by incorporating materials like CaCO3 particles, which are subsequently etched out, leaving behind a network of voids. This improved porosity enhances moisture diffusion, allowing larger, faster, and more controlled deformations due to finely tuned water-induced swelling, which is beneficial for applications requiring rapid adaptability.
Hybridization with hygroscopic layers offers another way to increase water-responsiveness in LCNs[81,168]. Integrating a hydrogel layer within the LCN network can leverage the hydrogel’s rapid swelling properties to induce complex bending or other deformations upon water exposure. The liquid crystal elastomer layer within the LCNs provides directional control due to its anisotropic modulus, higher along the molecular orientation. By aligning the molecular orientation in specific patterns, this method enhances water responsiveness and allows for the creation of multi-functional materials capable of responding to multiple stimuli. Enhancing the water responsiveness through porosity and hybridization with hygroscopic layers results in materials that exhibit precise, controlled, and reversible shape changes, making them suitable for applications in soft robotics, adaptive bioelectronics, and smart sensors by providing flexibility, adaptability, and rapid responses.
Shape memory polymers can revert to their original shape upon water exposure by incorporating hydrophilic components and leveraging preset shape memory or stress processing, achieving rapid and reversible deformations. This makes them ideal for deployable structures, smart sensors, and adaptive bioelectronics, as demonstrated by polymeric bilayers combining hydrophilic polymers with liquid crystal elastomers, which allow for complex 3D morphing controlled by the anisotropic modulus.
Recent advances have introduced a precise cold-drawing process that aligns polymer chains and creates a microporous structure to fabricate a supercontractile soft, biocompatible water-responsive contractile films that facilitate adaptable chronic electronic integration, paving the way for next-generation biomedical devices that can seamlessly interface with the human body and simplify the implantation process, thereby improving patient care.
In conclusion, water-responsive adaptive contraction offers advantages over conventional swollen hydrogels and actuator-based cuff electrodes for biomedical applications, where rapid and significant shape adaptability without the need for external control is crucial. Its effectiveness lies in leveraging mechanisms like phase transition modulation, hydrogen bond disruption, and hierarchical hybridization. Ongoing research that focuses on optimizing material properties-such as mechanical durability, biocompatibility, and water-responsiveness-can enable these materials to become more effective tools in medical applications. Interdisciplinary efforts across material science, bioengineering, and medical sciences will be invaluable in advancing this field, facilitating the development of next-generation medical devices that seamlessly integrate with the human body, improve patient outcomes, and ultimately set new standards in biomedical engineering.
Acknowledgments
This work was supported from National Natural Science Foundation (No.22205254 and No.5230130320); Shanghai Science and Technology Innovation Sailing Special Project (No.23YF1401700) and the Initiative Postdoctor Supporting Program, China Postdoctoral Science Foundation (No.BX20200209). The authors thank editors' and reviewers' invaluable comments in improving this work.
Conflicts of Interest
The author declared no competing interests.
Data Availability
Data sharing is not applicable to this review as no datasets were generated or analyzed during the current study.
Copyright Permissions
Copyright © 2025 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Author Contribution
Xu S conceptualized the review topic, contributed to writing, and supervised the project. Ge M, Tang Y and Wang Y conducted literature research and drafted sections of the manuscript. Liao Y provided insights into ioelectronic applications. Wang J conceptualized the review topic, supervised the project, and finalized the manuscript.
Abbreviation List
ELP, Elastin-like polypeptides
FS, Forced silked
LCN, Liquid crystal network
LCE, Liquid crystal elastomer
LCP, Liquid crystal polymer
NS, Naturally spun
PDMS, Polydimethylsiloxane
PEO, Poly(ethylene oxide)
PEG, Poly(ethylene glycol)
PEG-α-CD, Poly(ethylene glycol)-α-cyclodextrin
PLA, Polylactic acid
PMMA, Polymethyl methacrylate
PU, Polyurethane
RH, Relative humidity
SMP, Shape memory polymer
WRAP, Water-responsive adaptive polymer
References
[1] Zhao X, Zhou Y, Song Y et al. Permanent fluidic magnets for liquid bioelectronics. Nat Mater, 2024; 23: 703-710.[DOI]
[2] Zhou W, Jiang Y, Xu Q et al. Soft and stretchable organic bioelectronics for continuous intraoperative neurophysiological monitoring during microsurgery. Nat Biomed Eng, 2023; 7: 1270-1281.[DOI]
[3] Zhang B, Li J, Zhou J et al. A three-dimensional liquid diode for soft, integrated permeable electronics. Nature, 2024; 628: 84-92.[DOI]
[4] Chen G, Xiao X, Zhao X et al. Electronic textiles for wearable point-of-care systems. Chem Rev, 2021; 122: 3259-3291.[DOI]
[5] Zhang N, Huang F, Zhao S et al. Photo-Rechargeable Fabrics as Sustainable and Robust Power Sources for Wearable Bioelectronics. Matter, 2020; 2: 1260-1269.[DOI]
[6] Xu S, Xiao X, Chen J. Stretchable fiber strain sensors for wearable biomonitoring. Nat Sci Rev, 2024; 11: nwae173.[DOI]
[7] Xu S, Yin J, Wan X, Chen J. Advances in luminescent fibers for interactive smart textiles. cMat, 2024.[DOI]
[8] Xiao X, Chen G, Libanori A, Chen J. Wearable triboelectric nanogenerators for therapeutics. Trends Chem, 2021; 3: 279-290.[DOI]
[9] Li M, Li W, Guan Q et al. Sweat-resistant bioelectronic skin sensor. Device, 2023; 1: 100006.[DOI]
[10] Tat T, Zhao X, Chen J. Leveraging fluidic programming for pneumatic haptic textiles. Device, 2023; 1: 100094.[DOI]
[11] Zhou H, Kim KN, Sung MJ et al. Intrinsically stretchable low-dimensional conductors for wearable organic light-emitting diodes. Device, 2023; 1: 100060.[DOI]
[12] Shim H, Sim K, Wang B et al. Elastic integrated electronics based on a stretchable n-type elastomer-semiconductor–elastomer stack. Nat Electron, 2023; 6: 349-359.[DOI]
[13] He Y, Cheng Y, Yang C et al. Creep-free polyelectrolyte elastomer for drift-free iontronic sensing. Nat Mater, 2024; 23: 1107-1114.[DOI]
[14] Gao M, Wu H, Plamthottam R et al. Skin temperature-triggered, debonding-on-demand sticker for a self-powered mechanosensitive communication system. Matter, 2021; 4: 1962-1974.[DOI]
[15] Yang Q, Wei T, Yin RT et al. Photocurable bioresorbable adhesives as functional interfaces between flexible bioelectronic devices and soft biological tissues. Nat Mater, 2021; 20: 1559-1570.[DOI]
[16] Yuk H, Wu J, Sarrafian TL et al. Rapid and coagulation-independent haemostatic sealing by a paste inspired by barnacle glue. Nat Biomed Eng, 2021; 5: 1131-1142.[DOI]
[17] Deng J, Yuk H, Wu J et al. Electrical bioadhesive interface for bioelectronics. Nat Mater, 2021; 20: 229-236.[DOI]
[18] Nam S, Mooney D. Polymeric tissue adhesives. Chem Rev, 2021; 121: 11336-11384.[DOI]
[19] Shagan A, Zhang W, Mehta M et al. Hot Glue Gun Releasing Biocompatible Tissue Adhesive. Adv Funct Mater, 2020; 30: 1900998.[DOI]
[20] Sun J, Su J, Ma C et al. Fabrication and Mechanical Properties of Engineered Protein-Based Adhesives and Fibers. Adv Mater, 2020; 32: 1906360.[DOI]
[21] Ma Z, Bourquard C, Gao Q et al. Controlled tough bioadhesion mediated by ultrasound. Science, 2022; 377: 751-755.[DOI]
[22] Lin M, Zhang Z, Gao X et al. A fully integrated wearable ultrasound system to monitor deep tissues in moving subjects. Nat Biotechnol, 2024; 42: 448-457.[DOI]
[23] Zhao X, Zhou Y, Li A et al. A self-filtering liquid acoustic sensor for voice recognition. Nat Electron, 2024; 7: 924-9321.[DOI]
[24] Xu S, Manshaii F, Chen G et al. Reversible metal–ligand coordination for photocontrolled metallopolymer adhesives. Chem, 2024; 10: 1638-1640.[DOI]
[25] Rong JZ, Cai TX, Bai YZ et al. A free-sealed high-voltage aqueous polymeric sodium battery enabling operation at -25°C. Cell Rep Phys Sci, 2022; 3: 100805.[DOI]
[26] Yuan M, Liu J, Qiu S et al. Mechanically adaptive supercontractile polymer for soft bioelectronics. Matter, 2024; 7: 745-749.[DOI]
[27] Xu S, Chen G, Scott K et al. Soft electrochemical actuators for intraoperative nerve activity monitoring, Matter, 2024; 7: 2795-2797.[DOI]
[28] Xu S, Han Z, Yuan K et al. Upcycling chlorinated waste plastics. Nat Rev Methods Primers, 2023; 3: 44.[DOI]
[29] Xu S, Ahmed S, Momin M et al. Unleashing the potential of 3D printing soft materials. Device, 2023; 1: 100067.[DOI]
[30] Li M, Guan Q, Li C, Saiz E. Self-powered hydrogel sensors. Device, 2023; 1: 100007.[DOI]
[31] Chen G, Zhou Y, Fang Y et al. Wearable Ultrahigh Current Power Source Based on Giant Magnetoelastic Effect in Soft Elastomer System. ACS Nano, 2021; 15: 20582-20589.[DOI]
[32] Xu S, Manshaii F, Xiao X et al. Artificial Intelligence Assisted Nanogenerator Applications. J Mater Chem A, 2024;[DOI]
[33] Xu J, Tat T, Zhao X et al. Spherical Magnetoelastic Generator for Multidirectional Vibration Energy Harvesting. ACS Nano, 2023; 17: 3865-3872.[DOI]
[34] Zhao X, Chen G, Zhou Y et al. Giant Magnetoelastic Effect Enabled Stretchable Sensor for Self-Powered Biomonitoring. ACS Nano, 2022; 16: 6013-6022.[DOI]
[35] Xu J, Tat T, Zhao X et al. A programmable magnetoelastic sensor array for self-powered human–machine interface. Appl Phys Rev, 2022; 9: 031404.[DOI]
[36] Xu J, Tat T, Yin J et al. A textile magnetoelastic patch for self-powered personalized muscle physiotherapy. Matter, 2023; 6: 2235-2247.[DOI]
[37] Ock IW, Zhao X, Wan X et al. Boost the voltage of a magnetoelastic generator via tuning the magnetic induction layer resistance. Nano Energy, 2023; 109: 108298.[DOI]
[38] Zhao X, Zhou Y, Xu J et al. Soft fibers with magnetoelasticity for wearable electronics. Nat Commun, 2021; 12: 6755.[DOI]
[39] Zhou Y, Zhao X, Xu J et al. Giant magnetoelastic effect in soft systems for bioelectronics. Nat Mater, 2021; 20: 1670-1676.[DOI]
[40] Zhou Y, Zhao X, Xu J et al. A multimodal magnetoelastic artificial skin for underwater haptic sensing. Sci Adv, 2024; 10: eadj8567.[DOI]
[41] Miranda-Nieves D, Chaikof EL. Collagen and Elastin Biomaterials for the Fabrication Of Engineered Living Tissues. ACS Biomater. Sci Eng, 2017; 3: 694-711.[DOI]
[42] Sionkowska A. Collagen blended with natural polymers: Recent advances and trends. Prog Polymer Sci, 2021; 122: 101452.[DOI]
[43] Trębacz H, Barzycka A. Mechanical Properties and Functions of Elastin: An Overview. Biomolecules, 2023; 13: 574.[DOI]
[44] Branković M, Zivic F, Grujovic N et al. Review of Spider Silk Applications in Biomedical and Tissue Engineering. Biomimetics, 2024; 9: 169.[DOI]
[45] Perez-Rigueiro J, Elices M, Guinea G. Controlled supercontraction tailors the tensile behaviour of spider silk. Polymer, 2003; 44: 3733-3736.[DOI]
[46] Elices M, Plaza GR, Arnedo MA et al. Mechanical Behavior of Silk During the Evolution of Orb-Web Spinning Spiders. Biomacromolecules, 2009; 10: 1904-1910.[DOI]
[47] Work RW. Viscoelastic Behaviour and wet supercontraction of Major Ampullate Silk Fibres of Certain Orb-Web-Building Spiders (Araneae). J Exp Biol, 1985; 118: 379-404.[DOI]
[48] Jelinski LW, Blye A, Liivak O et al. Orientation, structure, wet-spinning, and molecular basis for supercontraction of spider dragline silk. Int J Biolog. Macromol, 1999; 24: 197-201.[DOI]
[49] Hopfe C, Ospina-Jara B, Schulze T et al. Impact of environmental factors on spider silk properties. Curr Biolog, 2024; 34: 56-67.e55.[DOI]
[50] Plaza GR, Guinea GV, Pérez‐Rigueiro J et al. Thermo-hygro-mechanical behavior of spider dragline silk: Glassy and rubbery states. J Polymer Sci B, 2006; 44: 994-999.[DOI]
[51] Cohen N, Levin M, Eisenbach CD. On the Origin of Supercontraction in Spider Silk. Biomacromolecules, 2021; 22: 993-1000.[DOI]
[52] Yarger JL, Cherry BR, Van Der Vaart A. Uncovering the Structure-Function Relationship in Spider Silk. Nat Rev Mate., 2018; 3: 18008.[DOI]
[53] Xu S, Manshaii F, Chen J. Multiphasic Interfaces Enabled Aero-Elastic Capacitive Pressure Sensor. Matter, 2024; 7: 2351-2354.[DOI]
[54] Elices M, Plaza GR, Pérez-Rigueiro J et al. The Hidden Link Between Supercontraction and Mechanical Behavior of Spider Silks. J Mechan Behav Biomed Mater, 2011; 4: 658-669.[DOI]
[55] Zhang Z, Yang X, Zhao Y et al. Liquid Crystal Materials for Biomedical Applications. Adv Mater, 2023; 35: 2300220.[DOI]
[56] Pinchin NP, Guo H, Meteling H et al. Liquid Crystal Networks Meet Water: It's Complicated! Adv. Mater., 2023: 2303740.[DOI]
[57] Sommerdijk NA, With Gd. Biomimetic CaCO3 Mineralization Using Designer Molecules And Interfaces. Chem. Rev., 2008; 108: 4499-4550.[DOI]
[58] Wang X, Lin F, Wang X et al. Hydrovoltaic Technology: From Mechanism To Applications. Chem Soc Rev, 2022; 51: 4902-4927.[DOI]
[59] Zhao Y, Zhang S, Xu S et al. A π‐Conjugated Polyimide‐Based High‐Performance Aqueous Potassium‐Ion Asymmetric Supercapacitor. Macromol Rapid Commun, 2022; 43: 2200040.[DOI]
[60] Pinchin NP, Guo H, Meteling H et al. Liquid Crystal Networks Meet Water: It's Complicated! Adv Mater, 2024; 36: 2303740.[DOI]
[61] Sakamoto T, Ogawa T, Nada H et al. Development of Nanostructured Water Treatment Membranes Based on thermotropic Liquid Crystals: Molecular Design Of Sub‐Nanoporous Materials. Adv Sci, 2018; 5: 1700405.[DOI]
[62] Kim SK, Hanif A, Jang IY. Incorporating Liquid Crystal Display (Lcd) Glass Waste As Supplementary Cementing Material (SCM) in Cement Mortars-Rationale Based on Hydration, Durability, and Pore Characteristics. Materials, 2018; 11: 2538.[DOI]
[63] Zhu H, Luo W, Ciesielski PN et al. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem Rev, 2016; 116: 9305-9374.[DOI]
[64] Solhi L, Guccini V, Heise K et al. Understanding nanocellulose-water interactions: Turning a detriment into an asset. Chem Rev, 2023; 123: 1925-2015.[DOI]
[65] Xu S, Liang X, Ren ZC et al. Free‐standing air cathodes based on 3D hierarchically porous carbon membranes: Kinetic overpotential of continuous macropores in Li‐O2 batteries. Angew Chem Int Ed, 2018; 130: 6941-6945.[DOI]
[66] Lee S, Liang X, Kim JS et al. Permeable Bioelectronics toward Biointegrated Systems. Chem Rev, 2024; 124: 6543-6591.[DOI]
[67] Wang X, Xiao X, Feng Z et al. A Soft Bioelectronic Patch for Simultaneous Respiratory and Cardiovascular Monitoring. Adv Healthcar Mater, 2024; 13: 2303479.[DOI]
[68] Li J, Che Z, Wan X et al. Biomaterials and bioelectronics for self-powered neurostimulation. Biomaterials, 2023; 304: 122421.[DOI]
[69] Pang X, Lee H, Rong J et al. Self-Thermal Management in Filtered Selenium-Terminated MXene Films for Flexible Safe Batteries. Small, 2024; 20: 2309580.[DOI]
[70] Chen S, Manshaii F, Fan X et al. Creating breathable wearable bioelectronics using three-dimensional liquid diodes. Matter, 2024; 7: 2632-2635.[DOI]
[71] Yin J, Wang S, Tat T, Chen J. Motion artefact management for soft bioelectronics. Nat. Rev. Bioeng., 2024; 2: 541-558.[DOI]
[72] Wan X, Tat T, Zhou Y et al. Building bioelectronic fibres with a light touch. Nat. Electron., 2024; 7: 521-522.[DOI]
[73] Zhang Z, Zhu Z, Zhou P et al. Soft bioelectronics for therapeutics. ACS Nano, 2023; 17: 17634-17667.[DOI]
[74] Kim H, Rigo B, Wong G et al. Advances in wireless, batteryless, implantable electronics for real-time, continuous physiological monitoring. Nano-Micro Lett., 2024; 16: 52.[DOI]
[75] Yu Y, Nyein HYY, Gao W, Javey A. Flexible electrochemical bioelectronics: the rise of in situ bioanalysis. Adv Mater, 2020; 32: 1902083.[DOI]
[76] Won C, Kwon C, Park K et al. Electronic drugs: spatial and temporal medical treatment of human diseases. Adv Mater, 2021; 33: 2005930.[DOI]
[77] Hu L, Jiang J, Liu ZT et al. Fabricating 3D Moisture-and NIR Light-Responsive Actuators by a One-Step Gradient Stress-Relaxation Process. ACS Appl Mater Interfaces, 2023; 15: 40991-40999.[DOI]
[78] Yang S, Yang Y, Xia X et al. Biomimetic Stimulus Responsiveness: From Materials Design to Device Integration. Adv Funct Mater, 2024: 34, 2400500.[DOI]
[79] Das G, Shinde DB, Melepurakkal A et al. Synergistic humidity-responsive mechanical motion and proton conductivity in a cationic covalent organic framework. Chem, 2024: 10, 2500-2517.[DOI]
[80] Tabor J, Chatterjee K, Ghosh TK. Smart Textile-Based Personal Thermal Comfort Systems: Current Status and Potential Solutions. Adv Mater Technol, 2020; 5: 1901155.[DOI]
[81] Gu C, Yan Y, He J et al. Transparent and energy-efficient electrochromic AR display with minimum crosstalk using the pixel confinement effect. Device, 2023; 1: 100126.[DOI]
[82] Boothby J, Ware T. Dual-responsive, shape-switching bilayers enabled by liquid crystal elastomers. Soft Matter, 2017; 13: 4349-4356.[DOI]
[83] Wang Z, Chen Y, Ma Y et al. Bioinspired Stimuli-Responsive Materials for Soft Actuators. Biomimetics, 2024; 9: 128.[DOI]
[84] Lin S, Ma S, Chen K et al. A humidity-driven film with fast response and continuous rolling locomotion. Chem Eng J, 2024; 495: 153294.[DOI]
[85] Xue J, Ge Y, Liu Z et al. Photoprogrammable Moisture-Responsive Actuation of a Shape Memory Polymer Film. ACS Appl Mater Interfaces, 2022; 14: 10836-10843.[DOI]
[86] Yang Y, Wu Y, Li C et al. Flexible actuators for soft robotics. Adv Intellig System, 2020; 2: 1900077.[DOI]
[87] Zhang D, Ding J, Zhou Y, Ju J. Research Progress on Moisture-Sorption Actuators Materials. Nanomaterials, 2024; 14: 1544.[DOI]
[88] Yang M, Wang SQ, Liu Z et al. Fabrication of moisture-responsive crystalline smart materials for water harvesting and electricity transduction. J Am Chem Soc, 2021; 143: 7732-7739.[DOI]
[89] Montes‐García V, Samorì P. Humidity sensing with supramolecular nanostructures. Adv. Mater., 2024; 36: 2208766.[DOI]
[90] Zheng M, Liu M, Cheng Y et al. Stimuli-Responsive Fiber/Fabric Actuators for Intelligent Soft Robots: from Current Progress to Future Opportunities. Nano Energy, 2024; 129: 110050.[DOI]
[91] Wang A, Shi W, Huang J, Yan Y. Adaptive soft molecular self-assemblies. Soft Matter, 2016; 12: 337-357.[DOI]
[92] Chen M, Gao M, Bai L et al. Recent Advances in 4D Printing of Liquid Crystal Elastomers. Adv Mater, 2023; 35: 2209566.[DOI]
[93] Herbert KM, Fowler HE, McCracken JM et al. Synthesis and alignment of liquid crystalline elastomers. Nat Rev Mater, 2022; 7: 23-38.[DOI]
[94] Waters JT, Li S, Yao Y et al. Twist again: Dynamically and reversibly controllable chirality in liquid crystalline elastomer microposts. Sci Adv, 2020; 6: eaay5349.[DOI]
[95] Hu W, He W, Wang K et al. A blue phase liquid crystal film based on an interpenetrating network and its sensitive humidity response performance. Mater Adv, 2024; 5: 1930-1939.[DOI]
[96] Varanko AK, Su JC, Chilkoti A. Elastin-like polypeptides for biomedical applications. Annual Rev Biomed Eng, 2020; 22: 343-369.[DOI]
[97] Roberts S, Dzuricky M, Chilkoti A. Elastin-like polypeptides as models of intrinsically disordered proteins. FEBS Lett, 2015; 589: 2477-2486.[DOI]
[98] MacEwan SR, Chilkoti A. Applications of elastin-like polypeptides in drug delivery. J Control Rel, 2014; 190: 314-330.[DOI]
[99] Li NK, Quiroz FG, Hall CK et al. Molecular description of the LCST behavior of an elastin-like polypeptide. Biomacromolecules, 2014; 15: 3522-3530.[DOI]
[100] Prhashanna A, Taylor PA, Qin J et al. Effect of peptide sequence on the LCST-like transition of elastin-like peptides and elastin-like peptide–collagen-like peptide conjugates: Simulations and experiments. Biomacromolecules, 2019; 20: 1178-1189.[DOI]
[101] Wang B, Patkar SS, Kiick KL. Application of thermoresponsive intrinsically disordered protein polymers in nanostructured and microstructured materials. Macromol Biosci, 2021; 21: 2100129.[DOI]
[102] Duan T, Li H. In situ phase transition of elastin-like polypeptide chains regulates thermoresponsive properties of elastomeric protein-based hydrogels. Biomacromolecules, 2020; 21: 2258-2267.[DOI]
[103] Miserez A, Guerette PA. Phase transition-induced elasticity of α-helical bioelastomeric fibres and networks. Chem Soc Rev, 2013; 42: 1973-1995.[DOI]
[104] Hartzell EJ, Gonzalez Obeso C, Hasturk O et al. Exploration of Stimuli‐Responsive, Elastin‐Based Coatings for Textiles. Adv Funct Mater, 2024; 34: 2407882.[DOI]
[105] Bandiera A, Colomina-Alfaro L, Sist P et al. Physicochemical Characterization of a Biomimetic, Elastin-Inspired Polypeptide with Enhanced Thermoresponsive Properties and Improved Cell Adhesion. Biomacromolecules, 2023; 24: 5277-5289.[DOI]
[106] Lin R, Yan X, Hao H et al. Introducing Temperature-Controlled Phase Transition Elastin-like Polypeptides to Transient Electronics: Realization of Proactive Biotriggered Electronics with Local Transience. ACS Appl Mater Interfaces, 2019; 11: 46490-46496.[DOI]
[107] Nelson DW, Gilbert RJ. Extracellular Matrix-Mimetic Hydrogels for Treating Neural Tissue Injury: A Focus on Fibrin, Hyaluronic Acid, and Elastin-Like Polypeptide Hydrogels. Adv Healthcar Mater, 2021; 10: 2101329.[DOI]
[108] Suhar RA, Marquardt LM, Song S et al. Elastin-like Proteins to Support Peripheral Nerve Regeneration in Guidance Conduits. ACS Biomater Sci Eng, 2020; 7: 4209-4220.[DOI]
[109] Gonzalez de Torre I, Alonso M, Rodriguez-Cabello JC. Elastin-Based Materials: Promising Candidates for Cardiac Tissue Regeneration. Front Bioeng Biotechnol, 2020; 8: 657.[DOI]
[110] Venkatraman S, Boey F, Lao LL. Implanted cardiovascular polymers: Natural, synthetic and bio-inspired. Prog Polymer Sci, 2008; 33: 853-874.[DOI]
[111] El Maachi I, Loewen A, Acosta S et al. Protein‐Engineered Elastin Fibers as Building Blocks for The Textile-Based Assembly of Tissue Equivalents. Adv Funct Mater, 2024; 34: 2313204.[DOI]
[112] Wang Y, Li Z, Hu Q. Emerging self-regulated micro/nano drug delivery devices: a step forward towards intelligent diagnosis and therapy. Nano Today, 2021; 38: 101127.[DOI]
[113] Noh Y, Son E, Cha C. Exploring stimuli-responsive elastin-like polypeptide for biomedicine and beyond: potential application as programmable soft actuators. Front Bioeng Biotechnol, 2023; 11: 1284226.[DOI]
[114] Kim H, Ahn SK, Mackie DM et al. Shape Morphing Smart 3D Actuator Materials for Micro Soft Robot. Mater Today, 2020; 41: 243-269.[DOI]
[115] Yang Y, Liu Y, Shen Y. Plasmonic‐Assisted Graphene Oxide Films with Enhanced Photothermal Actuation for Soft Robots. Adv Funct Mater, 2020; 30: 1910172.[DOI]
[116] Wang Y, Huang W, Wang Y et al. Stimuli-responsive composite biopolymer actuators with selective spatial deformation behavior. PNAS, 2020; 117: 14602-14608.[DOI]
[117] Yeo GC, Aghaei‐Ghareh‐Bolagh B, Brackenreg EP et al. Fabricated elastin. Adv Healthcar Mater, 2015; 4: 2530-2556.[DOI]
[118] Varanko AK, Su JC, Chilkoti A. Elastin-like polypeptides for biomedical applications. Annual Rev Biomed Eng, 2020; 22: 343-369.[DOI]
[119] Zhao X, Chen X, Yuk H et al. Soft materials by design: unconventional polymer networks give extreme properties. Chem Rev, 2021; 121: 4309-4372.[DOI]
[120] Higuchi A, Ling QD, Hsu ST et al. Biomimetic Cell Culture Proteins as Extracellular Matrices for Stem Cell Differentiation. Chem Rev, 2012; 112: 4507-4540.[DOI]
[121] Ding X, Zhao H, Li Y et al. Synthetic Peptide Hydrogels as 3D Scaffolds for Tissue Engineering. Adv Drug Deliver Rev, 2020; 160: 78-104.[DOI]
[122] Chen M, Fu X, Chen Z et al. Protein-Engineered Functional Materials for Bioelectronics. Adv Funct Mater, 2021; 31: 2006744.[DOI]
[123] Herbert R, Lim HR, Park S et al. Recent Advances in Printing Technologies of Nanomaterials for Implantable Wireless Systems in Health Monitoring And Diagnosis. Adv Healthcar Mater, 2021; 10: 2100158.[DOI]
[124] Soliman BG, Nguyen AK, Gooding JJ et al. Advancing Synthetic Hydrogels through Nature-Inspired Materials Chemistry. Adv Mater, 2024; 36: 2404235.[DOI]
[125] Sallach RE, Cui W, Wen J et al. Elastin-Mimetic Protein Polymers Capable of Physical And Chemical Crosslinking. Biomaterials, 2009; 30: 409-422.[DOI]
[126] Zhao J, Yan Y, Shang Y et al. Thermosensitive Elastin-Derived Polypeptide Hydrogels Crosslinked by Genipin. Int J Polymer Mater Polymer Biomater, 2017; 66: 369-377.[DOI]
[127] Okesola BO, Lau HK, Derkus B et al. Covalent Co-Assembly Between Resilin-Like Polypeptide and Peptide Amphiphile into hydrogels with Controlled Nanostructure and Improved Mechanical Properties. Biomater Sci, 2020; 8: 846-857.[DOI]
[128] Liu W, Wang A, Yang R et al. Water-Triggered Stiffening of Shape-Memory Polyurethanes Composed of Hard Backbone Dangling PEG Soft Segments. Adv Mater, 2022; 34: 2201914.[DOI]
[129] Zhang Y, Zheng N, Cao Y et al. Climbing-Inspired Twining Electrodes Using Shape Memory for Peripheral Nerve Stimulation And Recording. Sci Adv, 2019; 5: eaaw1066.[DOI]
[130] Fan K, Huang W, Wang C et al. Water-responsive shape memory hybrid: Design concept and demonstration. Exp Polymer Lett, 2011; 5: 409-416.[DOI]
[131] Huang WM, Song C, Fu YQ et al. Shaping Tissue with Shape Memory Materials. Adv Drug Del Rev, 2013; 65: 515-535.[DOI]
[132] Ghanim R, Kaushik A, Park J, Abramson A. Communication Protocols Integrating Wearables, Ingestibles, and Implantables for Closed-Loop Therapies. Device, 2023; 1: 100092.[DOI]
[133] Yearley AG, Patel RV, Blitz SE et al. Implantable microdevices for Treating Brain Tumors. Device, 2023; 1: 100068.[DOI]
[134] Che Z, O'Donovan S, Xiao X et al. Implantable Triboelectric Nanogenerators for Self-Powered Cardiovascular Healthcare. Small, 2023; 19: 2207600.[DOI]
[135] Zhao Y, Wang C, Huang W et al. Buckling of poly (methyl methacrylate) in stimulus-responsive shape recovery. Appl Phys Lett, 2011; 99: 131911.[DOI]
[136] Wu X, Huang WM, Zhao Y et al. Mechanisms of the Shape Memory Effect in Polymeric Materials. Polymers, 2013; 5: 1169-1202.[DOI]
[137] Chen G, Zhao X, Andalib S et al. Discovering Giant Magnetoelasticity in Soft Matter For Electronic Textiles. Matter, 2021; 4: 3725-3740.[DOI]
[138] Damaševičius R. Explainable Artificial Intelligence Methods for Breast Cancer Recognition. Innov Discov, 2024; 1: 25.[DOI]
[139] Yi J, Zou G, Huang J et al. Water-Responsive Supercontractile Polymer Films for Bioelectronic Interfaces. Nature, 2023; 624: 295-302.[DOI]
[140] Yi J, Ren X, Li Y et al. Rapid‐Response Water‐Shrink Films with High Output Work Density Based on Polyethylene Oxide and α‐Cyclodextrin for Autonomous Wound Closure. Adv Mater, 2024; 36: 2403551.[DOI]
[141] Xu S, Scott K, Manshaii F et al. Heart-brain connection: How can heartbeats shape our minds? Matter, 2024; 7: 1684-1687.[DOI]
[142] Xu S, Manshaii F, Xiao X et al. Triboelectric Nanogenerators for Self-Powered Neurostimulation. Nano Res, 2024; 17: 8926-8941.[DOI]
[143] Xu S, Momin M, Ahmed S et al. Illuminating the Brain: Advances and Perspectives in Optoelectronics for Neural Activity Monitoring and Modulation. Adv Mater, 2023; 35: 2303267.[DOI]
[144] Xu S, Liu Y, Lee H et al. Neural interfaces: Bridging the brain to the world beyond healthcare. Exploration, 2024; 4: 20230146.[DOI]
[145] Xu S, Manshaii F, Chen J. Is Deep Brain Imaging on the Brink of Transformation with a Bioluminescence Molecule? BME Mat, 2024; 2: e12115.[DOI]
[146] Xu S, Xiao X, Manshaii F et al. Injectable fluorescent neural interfaces for Cell-Specific Stimulating And Imaging. Nano Lett, 2024; 24: 4703-4716.[DOI]
[147] De Keer L, Kilic KI, Van Steenberge PH et al. Computational Prediction of the Molecular Configuration Of Three-Dimensional Network Polymers. Nat Mater., 2021; 20: 1422-1430.[DOI]
[148] Chin SM, Synatschke CV, Liu S et al. Covalent-supramolecular hybrid polymers as muscle-inspired anisotropic actuators. Nat Commun, 2018; 9: 2395.[DOI]
[149] Zhao Q, Qi HJ, Xie T. Recent Progress in Shape Memory Polymer: New behavior, Enabling Materials, and Mechanistic Understanding. Prog Polymer Sci, 2015; 49: 79-120.[DOI]
[150] Ramaraju H, Akman RE, Safranski DL et al. Designing Biodegradable Shape Memory Polymers for Tissue Repair. Adv Funct Mater, 2020; 30: 2002014.[DOI]
[151] Hanif M, Zhang L, Shah AH et al. Mechanical Analysis and Biodegradation of Oxides-Based Magneto-Responsive Shape Memory Polymers for Material Extrusion 3D Printing of Biomedical Scaffolds. Addit Manuf, 2024; 86: 104174.[DOI]
[152] Xia Y, He Y, Zhang F et al. A Review of Shape Memory Polymers and Composites: Mechanisms, Materials, and Applications. Adv Mater, 2021; 33: 2000713.[DOI]
[153] Jeong YR, Lee G, Park H et al. Stretchable, Skin-Attachable Electronics with Integrated Energy Storage Devices for Biosignal Monitoring. ACC Chem Res, 2018; 52: 91-99.[DOI]
[154] Sessini V, Arrieta MP, Fernández-Torres A et al. Humidity-Activated Shape Memory Effect on Plasticized Starch-Based Biomaterials. Carboh Polymer, 2018; 179: 93-99.[DOI]
[155] Delaey J, Dubruel P, Van Vlierberghe S. Shape-Memory Polymers for Biomedical Applications. Adv Funct Mater, 2020; 30: 1909047.[DOI]
[156] Xu W, Kwok KS, Gracias DH. Ultrathin Shape Change Smart Materials. ACC Chem Res, 2018; 51: 436-444.[DOI]
[157] Brennan M. Wearable technology is a proving ground for device engineering and design. Device, 2023; 1: 100096.[DOI]
[158] Yin J, Kashyap V, Wang S et al. Self-powered eye-computer interaction via a triboelectric nanogenerator. Device, 2024; 2: 100252.[DOI]
[159] Deng W, Zhou Y, Libanori A et al. Piezoelectric nanogenerators for Personalized Healthcare. Chem Soc Rev, 2022; 51: 3380-3435.[DOI]
[160] Fang Y, Chen G, Bick M et al. Smart textiles for personalized thermoregulation. Chem Soc Rev, 2021; 50: 9357-9374.[DOI]
[161] Fang Y, Zhao X, Chen G et al. Smart polyethylene textiles for radiative and evaporative cooling. Joule, 2021; 5: 752-754.[DOI]
[162] Xiang S, Chen G, Wen Q et al. Fully addressable textile sensor array for self-powered haptic interfacing. Matter, 2024; 7: 82-94.[DOI]
[163] Libanori A, Chen G, Zhao X et al. Smart textiles for personalized healthcare. Nat Electron, 2022; 5: 142-156.[DOI]
[164] Pandiyan A, Veeramuthu L, Yan Z-L et al. A comprehensive review on perovskite and its functional composites in smart textiles: Progress, challenges, opportunities, and future directions. Prog Mater Sci, 2023; 140: 101206.[DOI]
[165] Che Z, Wan X, Xu J et al. Speaking without vocal folds using a machine-learning-assisted wearable sensing-actuation system. Nat Commun, 2024; 15: 1873.[DOI]
[166] Xu S, Scott K, Chen JA Bioinspired Three-Dimensionally Architected Electronic Skin. Device, 2024; 2: 100462.[DOI]
[167] Xu S, Wan X, Manshaii F et al. Advances in Piezoelectric Nanogenerators for Self-Powered Cardiac Care. Nano Trends, 2024; 6: 100042.[DOI]
[168] Xu SM, Liang X, Wu XY et al. Multistaged discharge constructing heterostructure with enhanced solid-solution behavior for long-life lithium-oxygen batteries. Nat Commun, 2019; 10: 5810.[DOI]
[169] Peng W, Yin J, Zhang X et al. 4D Printed Shape Memory Anastomosis Ring with Controllable Shape Transformation and Degradation. Adv Funct Mater, 2023; 33: 2214505.[DOI]

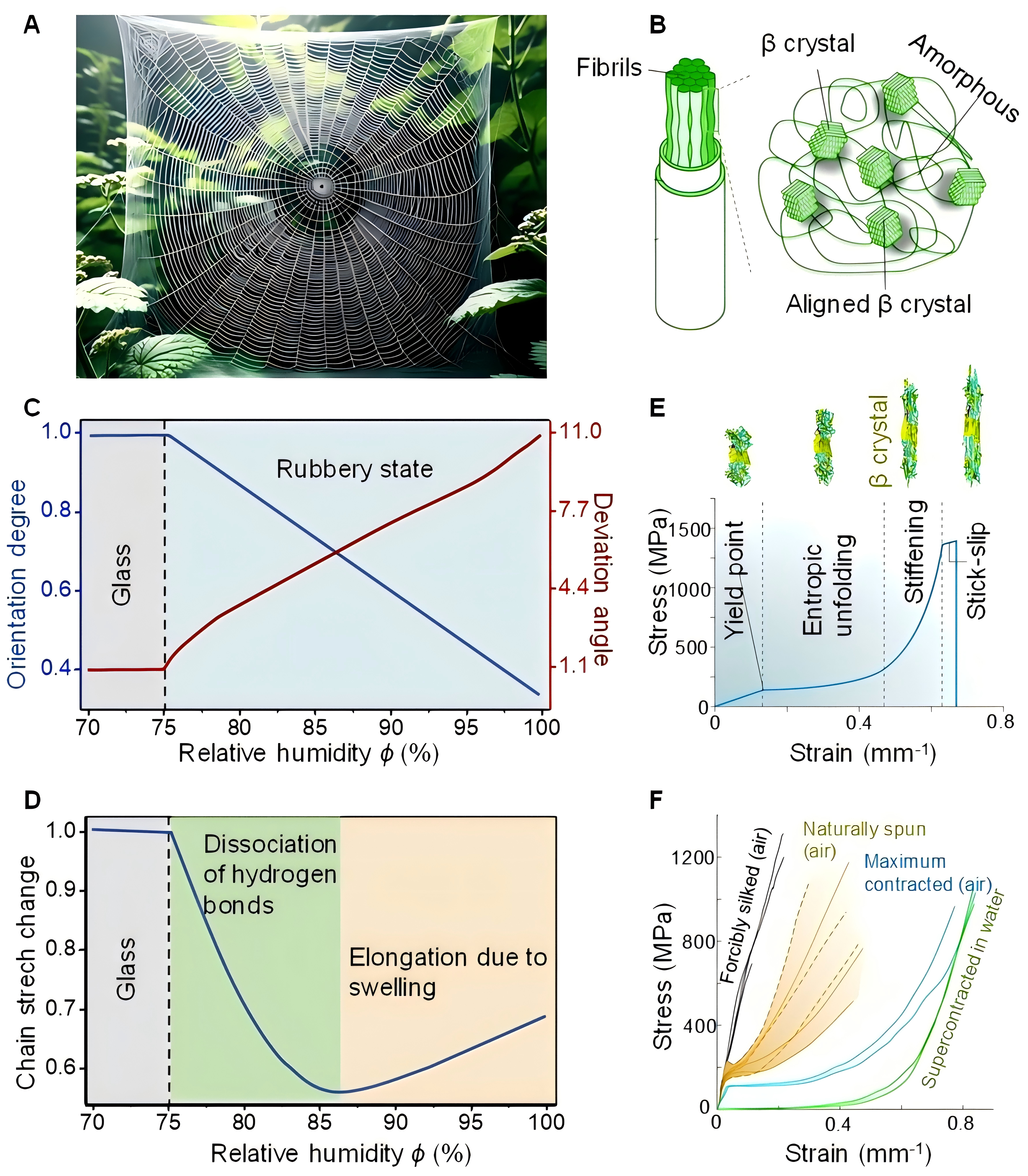
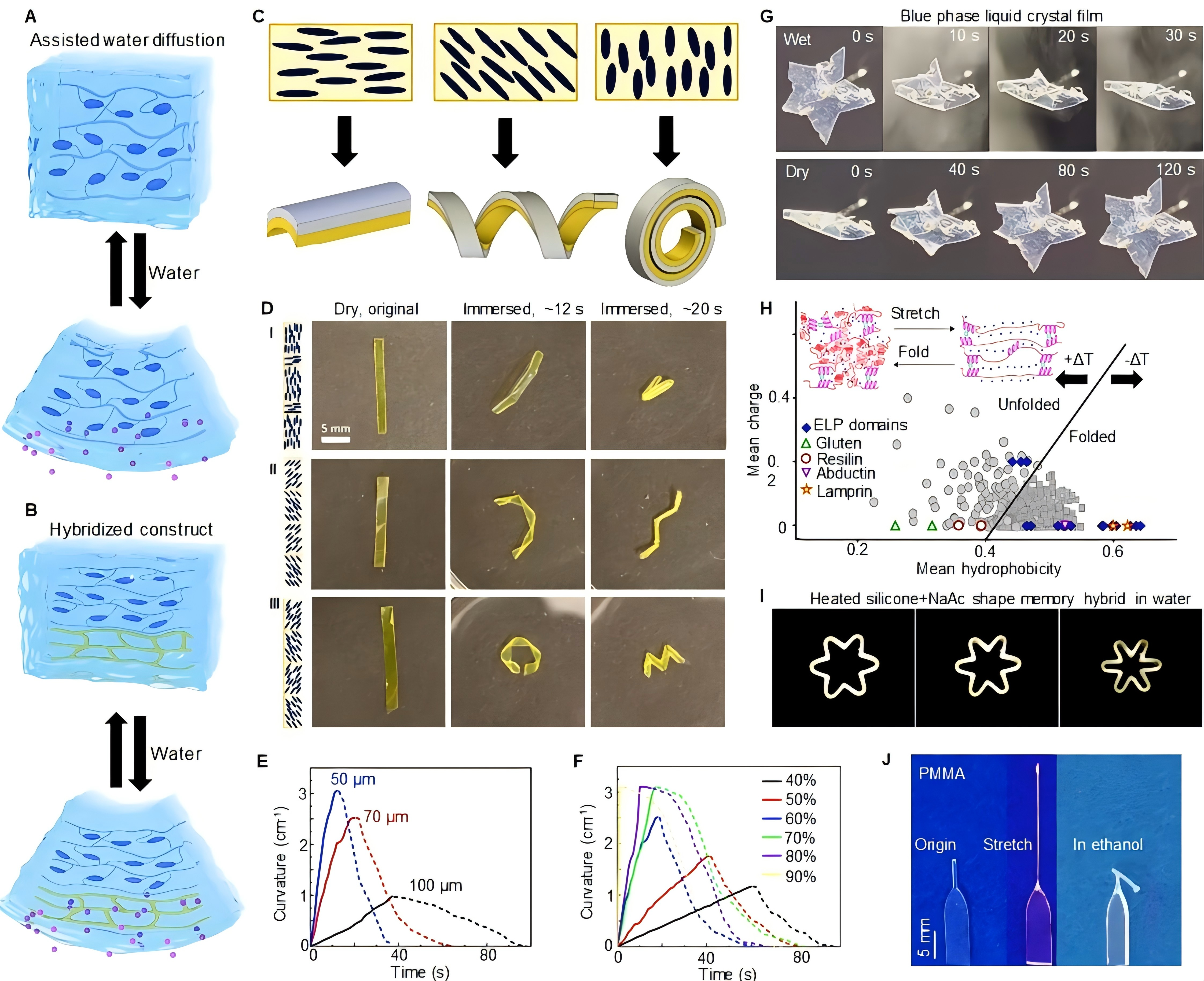
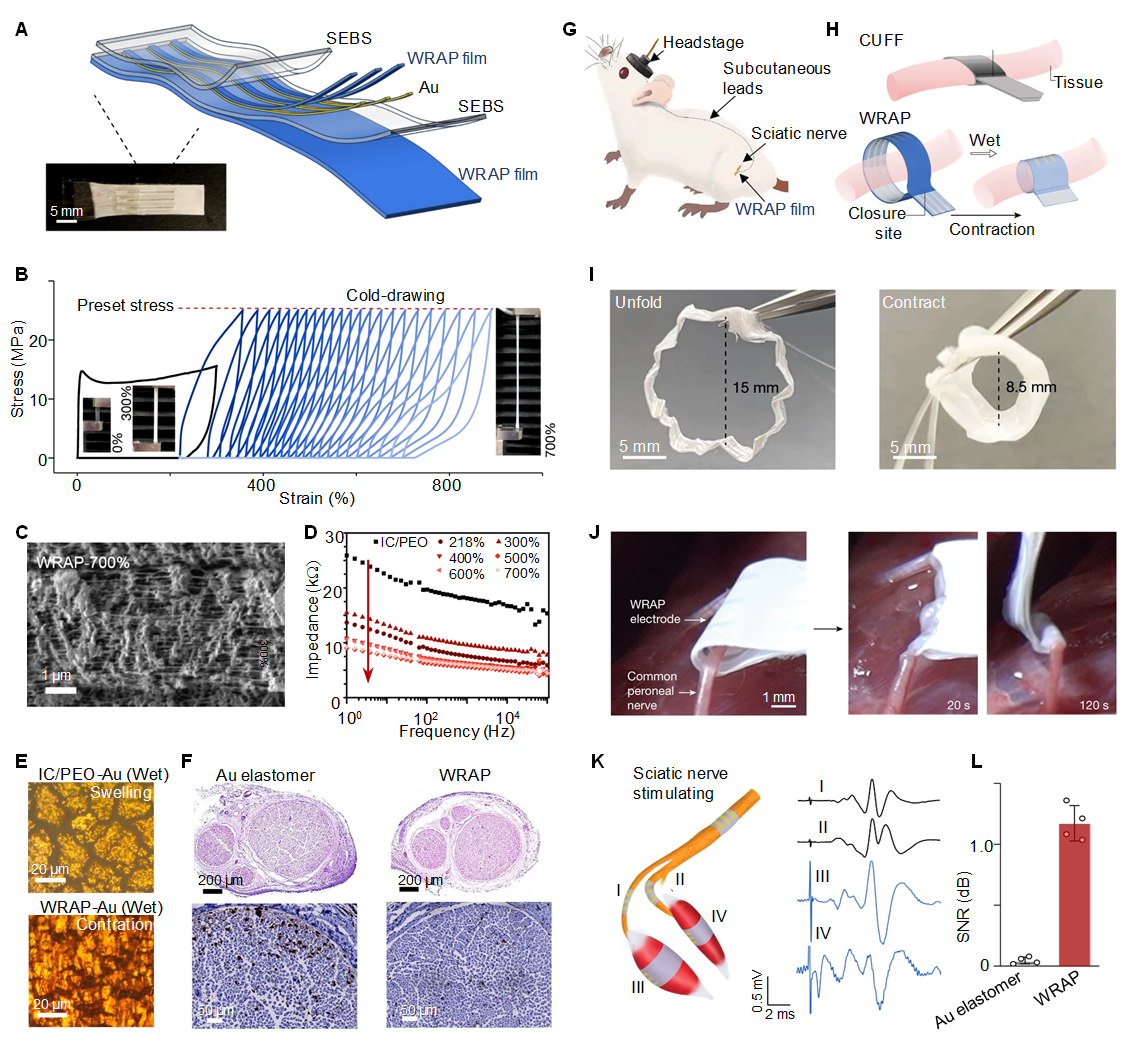
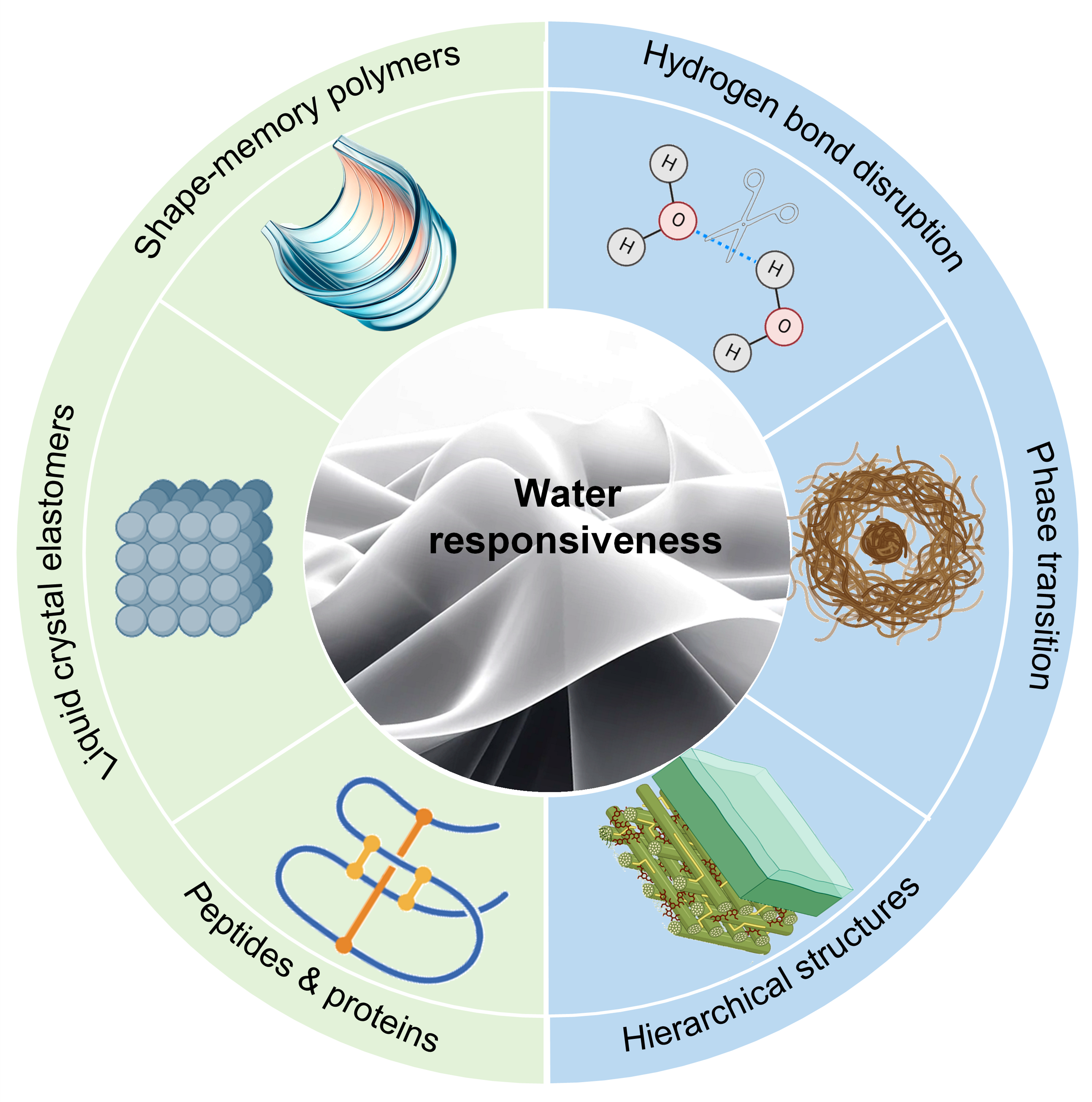



 Copyright ©
Copyright ©