Induced Pluripotent Stem Cells: A New Dawn for the Treatment of Ischemic Cardiomyopathy
Tadahisa Sugiura1*, Sheeza Nawaz1,2, Brandon E. Ferrell1, Taizo Yoshida3
1Department of Cardiothoracic and Vascular Surgery, Montefiore Medical Center/Albert Einstein College of Medicine, New York, USA
2Department of Medicine, Fatima Jinnah Medical University, Lahore, Pakistan
3Department of Surgery, Montefiore Medical Center/Albert Einstein College of Medicine, New York, USA
*Correspondence to: Tadahisa Sugiura, MD, PhD, Assistant Professor, Department of Cardiothoracic and Vascular Surgery, Montefiore Medical Center/Albert Einstein College of Medicine, 3400 Bainbridge Avenue, Bronx, New York, 10467, USA; Email: tsugiura@montefiore.org
DOI: 10.53964/id.2024031
Abstract
Ischemic cardiomyopathy, a leading cause of heart failure globally, arises from the irreversible damage inflicted upon cardiac tissue following myocardial infarction. The limited regenerative capacity of the adult human heart necessitates the exploration of novel therapeutic strategies to restore cardiac function and improve outcomes. Induced pluripotent stem cells (iPSCs), with their remarkable ability to differentiate into virtually any cell type in the body, have emerged as a beacon of hope for regenerative medicine, including the treatment of ischemic cardiomyopathy. This review delves into the pathophysiology of ischemic cardiomyopathy, highlighting the cellular and molecular mechanisms underlying cardiac dysfunction. We provide a comprehensive overview of the current state of iPSC technology, focusing on its potential applications in disease modeling, drug discovery, and cell-based therapies for cardiac regeneration. Furthermore, we discuss the challenges and opportunities associated with translating iPSC-based therapies to the clinic, emphasizing the need for rigorous preclinical studies and well-designed clinical trials to ensure safety and efficacy. Finally, we offer perspectives on the future directions of iPSC research in the context of ischemic cardiomyopathy, highlighting the transformative potential of this technology to revolutionize cardiovascular medicine and usher in an era of personalized regenerative therapies for patients with heart failure.
Keywords: cardiac regenerative therapy, iPSCs, stem cell therapy, heart failure
1 INTRODUCTION
Cardiovascular diseases remain the leading cause of death worldwide, casting a long shadow over global health[1,2]. Among these, ischemic cardiomyopathy, a debilitating condition arising from reduced blood flow to the heart muscle, presents a significant challenge. This often occurs due to coronary artery disease, where plaque buildup narrows the arteries, depriving the heart of oxygen-rich blood. The resulting damage to the heart muscle weakens its ability to pump effectively, leading to heart failure and a cascade of debilitating symptoms.
Current treatment strategies for ischemic cardiomyopathy primarily focus on managing symptoms and slowing disease progression. Medications, lifestyle modifications, and surgical interventions such as angioplasty and bypass surgery aim to improve blood flow and alleviate symptoms (Table 1). However, these approaches do not address the fundamental issue: the irreversible loss of functional heart muscle[3]. Heart transplantation and left ventricular assist device (LVAD) are also treatment options, however, these treatments can lack effectiveness in the long term and are associated with risk and complications. These limitations underscore the urgent need for innovative therapies that can regenerate damaged heart tissue and restore cardiac function[4,5].
Table 1. Current Treatments for Ischemic Cardiomyopathy
Category |
Treatment |
Function |
Pharmacological |
ACE inhibitors |
Reduce afterload, prevent remodeling |
ARBs |
Alternative to ACE inhibitors |
|
Beta-blockers |
Decrease myocardial oxygen demand |
|
Aldosterone antagonists |
Prevent sodium retention, reduce fibrosis |
|
Diuretics |
Manage fluid overload |
|
Nitrates |
Relieve angina, reduce preload |
|
Antiplatelets |
Prevent coronary thrombus |
|
Interventional |
PCI |
Revascularize stenosed arteries |
CABG |
Surgical revascularization |
|
ICD |
Prevent sudden cardiac death |
|
CRT |
Improve ventricular function |
|
LVAD |
Mechanical support for heart failure |
|
Lifestyle & Other |
Lifestyle changes |
Reduce risk factors |
Cardiac rehab |
Improve cardiovascular function |
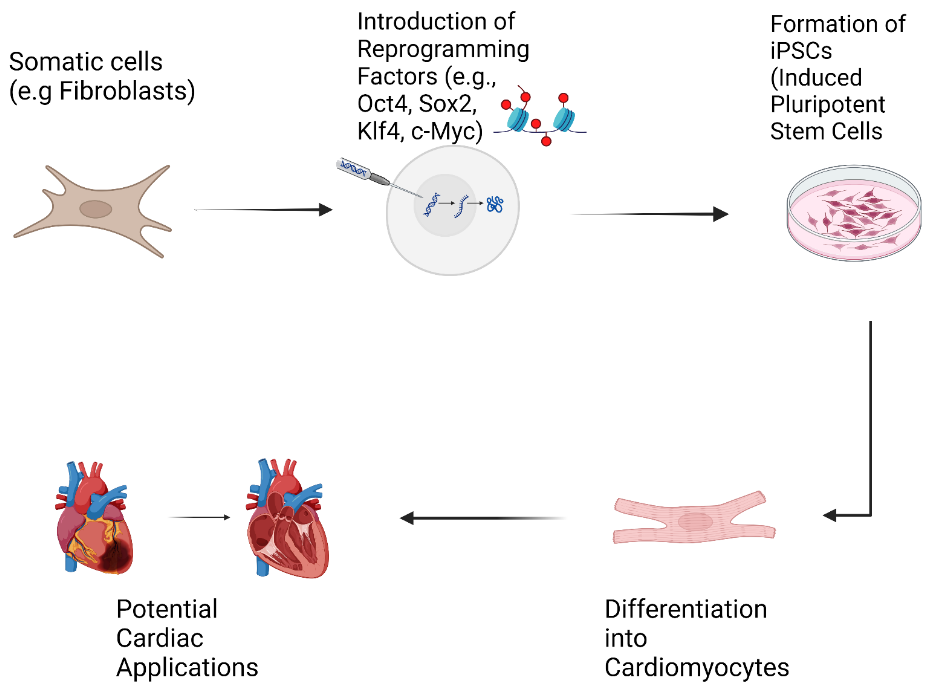
Regenerative medicine has emerged as a beacon of hope, offering the potential to repair or replace damaged tissues and organs. At the forefront of this revolution are induced pluripotent stem cells (iPSCs), a groundbreaking discovery that has transformed the landscape of biomedical research and therapeutic development[6]. iPSCs are adult cells that have been reprogrammed to an embryonic-like state, granting them the remarkable ability to differentiate into virtually any cell type in the body, including cardiomyocytes, the specialized cells responsible for the heart's pumping action (Figure 1)[7,8].
|
Figure 1. Flow Chart of Cardiac Regenerative Medicine Using iPS Cells.
This review delves into the transformative potential of iPSCs in the context of ischemic cardiomyopathy. We will explore how iPSCs are being harnessed to model the disease, accelerate drug discovery, and develop novel cell-based therapies. Furthermore, we will discuss the challenges that lie ahead and the future directions of this rapidly evolving field.
2 IPSCS
2.1 iPSCs for Disease Modeling and Understanding Pathophysiology
Unraveling the complex mechanisms underlying ischemic cardiomyopathy is crucial for developing effective treatments. Traditionally, researchers have relied on animal models and immortalized cell lines to study human diseases. However, these models often fail to fully recapitulate the intricacies of human pathophysiology. Animal models, while valuable, can exhibit significant differences in their physiology and response to treatments compared to humans[9]. Immortalized cell lines, on the other hand, often lack the genetic diversity and functional characteristics of primary cells, limiting their relevance to human disease[10].
iPSC-derived cardiomyocytes (iPSC-CMs) have emerged as a powerful tool for disease modeling, offering several advantages over traditional models. Firstly, iPSCs can be generated from patients with ischemic cardiomyopathy, allowing researchers to create patient-specific models that capture the unique genetic and environmental factors contributing to their disease. This personalized approach enables the study of disease mechanisms in a more relevant context, potentially leading to the identification of new therapeutic targets[11,12].
Secondly, iPSC-CMs provide a human-based platform to investigate the molecular and cellular events that drive ischemic injury and cardiac dysfunction. Researchers can use these cells to study how oxygen deprivation affects cardiomyocyte survival, metabolism, and contractility. They can also investigate the role of inflammation, oxidative stress, and other factors implicated in the progression of ischemic cardiomyopathy[12].
Thirdly, iPSC-CMs are amenable to high-throughput screening, allowing researchers to test the effects of thousands of compounds on cardiomyocyte function. This has opened up new avenues for drug discovery, enabling the identification of potential therapeutic agents that can protect cardiomyocytes from ischemic injury, promote cell survival, or enhance cardiac function[13,14].
Recent advances in 3D culture systems and organ-on-a-chip technology have further enhanced the utility of iPSC-CMs for disease modeling. 3D culture systems, such as engineered heart tissues and bioprinted cardiac constructs, allow for the creation of more physiologically relevant models that mimic the structure and function of the native heart. These models enable researchers to study cell-cell interactions, mechanical forces, and other factors that influence cardiac function in a more realistic setting[15,16]. Organ-on-a-chip technology takes this a step further by integrating microfluidic channels, sensors, and other components to create miniaturized, perfusable models of human organs, including the heart[17]. These “heart-on-a-chip” platforms allow researchers to study the effects of drugs, toxins, and other stimuli on cardiac function in a highly controlled and dynamic environment[18-20].
Numerous studies have demonstrated the power of iPSC-based disease modeling in ischemic cardiomyopathy. For instance, researchers have used iPSC-CMs to model familial forms of the disease caused by mutations in specific genes. These studies have provided valuable insights into the disease mechanisms and identified potential therapeutic targets[21]. In another study, iPSC-CMs were used to model the effects of diabetes on cardiac function, revealing new pathways that could be targeted to prevent or treat diabetic cardiomyopathy[22].
2.2 iPSCs for Drug Discovery and Cardiotoxicity Screening
The development of new drugs is a lengthy, costly and often inefficient process. Traditional drug discovery pipelines rely heavily on animal models and cell-based assays that often fail to accurately predict drug efficacy and safety in humans[2]. This is particularly true for cardiovascular drugs, where many promising candidates have failed in clinical trials due to unexpected toxicity or lack of efficacy. iPSC-CMs offer a transformative platform for drug discovery and cardiotoxicity screening, providing a human-relevant model system that can bridge the gap between preclinical studies and clinical trials[23].
One of the key advantages of iPSC-CMs for drug discovery is their amenability to high-throughput screening. Researchers can generate large numbers of iPSC-CMs from a single donor and use them to screen thousands of compounds in a rapid and cost-effective manner[19]. This allows for the identification of potential therapeutic agents that can protect cardiomyocytes from ischemic injury, promote cell survival, or enhance cardiac function. For example, iPSC-CMs have been used to identify novel compounds that activate pro-survival pathways, inhibit cell death, or stimulate angiogenesis in the context of ischemic injury[24].
Beyond identifying new drug candidates, iPSC-CMs are also invaluable for evaluating the efficacy of existing drugs in a more personalized manner. By generating iPSC-CMs from patients with different genetic backgrounds or disease subtypes, researchers can assess drug responses in a more patient-specific context. This is particularly relevant for ischemic cardiomyopathy, where individual responses to treatment can vary widely[25].
Furthermore, iPSC-CMs are emerging as a sensitive and predictive model for cardiotoxicity screening[26]. Many drugs, including some commonly used chemotherapeutic agents, can have adverse effects on the heart, leading to cardiotoxicity. Detecting cardiotoxic effects early in the drug development process is crucial to prevent patient harm and reduce costly late-stage drug failures. iPSC-CMs offer several advantages over traditional cardiotoxicity screening methods, such as animal models and human cell lines. They express the same ion channels and signaling pathways as human cardiomyocytes, making them more sensitive to drug-induced changes in cardiac electrophysiology and contractility[27].
Several studies have demonstrated the utility of iPSC-CMs for cardiotoxicity screening. For instance, researchers have used iPSC-CMs to identify drugs that prolong the QT interval, a measure of the heart's electrical recharging time[28]. Prolongation of the QT interval can lead to a life-threatening arrhythmia called torsades de pointes, and iPSC-CMs have proven to be a sensitive model for detecting this adverse effect[5]. In another study, iPSC-CMs were used to screen a library of FDA-approved drugs for cardiotoxicity, identifying several drugs that caused significant changes in cardiomyocyte function[7,13].
2.3 iPSC-Based Therapies for Ischemic Cardiomyopathy
While disease modeling and drug discovery are crucial steps towards improving treatment for ischemic cardiomyopathy, the ultimate goal is to develop therapies that can regenerate damaged heart tissue and restore cardiac function[29]. iPSCs hold immense promise for cell-based therapies offering the potential to replace lost or damaged cardiomyocytes and rebuild functional heart muscle[30,31]. Several cell therapy strategies using iPSCs are being explored:
1) Direct cell transplantation: This approach involves injecting iPSC-CMs directly into the damaged heart muscle. While conceptually straightforward, this method faces challenges in terms of cell delivery, survival, and engraftment[30]. Injected cells often struggle to survive in the harsh environment of the injured heart, and those that do survive may not integrate properly with the existing heart tissue[32].
2) Cell sheet engineering: This technique involves growing iPSC-CMs on specialized surfaces that allow for the detachment of intact sheets of cells. These cell sheets can then be transplanted onto the damaged heart muscle, potentially improving cell delivery and engraftment. This method is being actively explored in preclinical studies[33].
3) Biomaterial scaffolds: Another approach involves seeding iPSC-CMs onto biocompatible scaffolds that provide structural support and promote cell survival and integration. These scaffolds can be designed to mimic the architecture of the native heart tissue, providing a more natural environment for cell growth and function[34].
Preclinical studies in animal models have shown promising results using iPSC-based therapies for ischemic cardiomyopathy[35]. For example, transplantation of iPSC-CMs into mice or rats with myocardial infarction (heart attack) has been shown to improve cardiac function, reduce scar tissue formation, and enhance blood vessel growth[36]. While these results are encouraging, it's important to note that animal models do not fully recapitulate the complexities of human disease, and further research is needed to determine the safety and efficacy of these therapies in humans.
The use of iPSCs for myocardial regeneration therapy has been highlighted due to their capacity for unlimited proliferation and differentiation into cardiomyocytes[37]. Therefore, several clinical trials are currently underway to evaluate the safety and efficacy of iPSC-based therapies for ischemic cardiomyopathy. These trials are primarily focused on assessing the feasibility of cell delivery, cell survival, and the potential for adverse effects[38]. While it's still early days for iPSC-based therapies, the results of these trials are eagerly awaited and hold the potential to revolutionize the treatment of heart failure.
3 CHALLENGES AND FUTURE DIRECTIONS
In the realm of iPSC technology for treating ischemic cardiomyopathy, significant advancements have been made, showcasing its potential for therapeutic applications[13]. However, several challenges persist, hindering the widespread clinical adoption of iPSC-based therapies. One critical challenge lies in the maturation and functionality of iPSC-CMs[39]. Despite sharing some characteristics with mature cardiomyocytes, iPSC-CMs often exhibit functional immaturity, including weaker contractility, impaired calcium handling, and differences in electrophysiological properties compared to adult cardiomyocytes[40]. Enhancing the maturation and functionality of iPSC-CMs through techniques like 3D culturing, electrical stimulation, and mechanical conditioning is imperative for their successful transplantation and therapeutic efficacy[41].
Another significant challenge in iPSC-based therapies is the potential for immune rejection[42]. While iPSCs are derived from a patient's own cells, the reprogramming and differentiation processes can alter cell surface markers, increasing the risk of immune recognition and rejection. Strategies such as immunosuppression or the development of hypoallergenic iPSC lines are crucial for ensuring the long-term success of iPSC-based treatments[43]. Additionally, the risk of tumorigenicity poses a considerable concern in iPSC therapies, emphasizing the need for stringent quality control measures to eliminate undifferentiated iPSCs or cells with tumorigenic potential[44].
Scalability and cost-effectiveness are also significant hurdles in the widespread implementation of iPSC-based therapies for ischemic cardiomyopathy[45]. The complex and expensive process of generating, characterizing, and differentiating iPSCs into cardiomyocytes necessitates the development of cost-effective and scalable manufacturing processes to broaden the accessibility of these therapies to a larger patient population[46]. Despite these challenges, the future of iPSC-based therapies remains promising, with ongoing research endeavors aimed at addressing these limitations and exploring innovative avenues for improvement[47].
Researchers are actively investigating next-generation iPSC technologies to enhance the efficiency, safety, and tumorigenicity resistance of iPSCs[48]. Non-integrating reprogramming methods and small molecule-based reprogramming approaches are being explored to generate iPSCs with improved characteristics for therapeutic applications[49]. Moreover, the refinement of differentiation protocols to yield more mature and functional iPSC-CMs closely resembling adult cardiomyocytes is a focal point of current research efforts[50]. Techniques such as 3D culture systems, bioreactors, and specific growth factors are being employed to create an environment conducive to the maturation of iPSC-CMs[41].
Gene editing technologies like CRISPR-Cas9 hold immense potential for advancing iPSC-based therapies for ischemic cardiomyopathy[51]. These tools enable the correction of disease-causing mutations in patient-derived iPSCs, paving the way for the generation of healthy cells for transplantation[52]. Furthermore, gene editing can be utilized to create disease models by introducing specific mutations into iPSCs, facilitating the study of disease mechanisms and the development of novel therapeutics[53]. The combination of gene editing with other approaches such as biomaterial scaffolds, growth factors, or bioengineered cardiac patches presents a multi-faceted strategy to enhance cell survival, engraftment, and functional integration for comprehensive cardiac repair[54].
To summarize, while challenges persist in the maturation, immune rejection, tumorigenicity, scalability, and cost-effectiveness of iPSC-based therapies for ischemic cardiomyopathy, ongoing research efforts are actively addressing these limitations. The exploration of next-generation technologies, enhanced differentiation protocols, gene editing strategies, and combination therapies signifies a promising future for the advancement and widespread implementation of iPSC-based treatments in the field of cardiovascular regenerative medicine.
4 CONCLUSION
The discovery of iPSCs has significantly impacted regenerative medicine, offering extensive possibilities for disease modeling, drug discovery, and cell-based therapies[26]. iPSCs have demonstrated great potential in the realm of ischemic cardiomyopathy, with the capacity to create personalized treatments for regenerating damaged heart tissue and restoring cardiac function[55]. The rapid progress in research and development of iPSC-based therapies suggests a transformative role in addressing heart failure, potentially enhancing the quality of life for millions of patients globally[56].
Advancements in iPSC technology, along with gene editing and tissue engineering, have laid the foundation for personalized cardiac medicine, where a patient's cells could be reprogrammed to repair their damaged heart, potentially eliminating the necessity for organ transplantation and lifelong immunosuppression[57]. Despite existing challenges, the commitment of researchers worldwide instills hope for a future where heart failure can be managed effectively rather than being fatal[58].
The journey from laboratory research to clinical application of iPSC-based therapies for ischemic cardiomyopathy is ongoing, with these treatments still in early developmental stages[59]. Nevertheless, the potential advantages, combined with continuous research endeavors, offer promise for a future where iPSCs can revolutionize the landscape of heart disease treatment[25]. As our understanding of iPSC biology deepens and technological progress continues, the regenerative potential of iPSCs holds promise for transforming the lives of patients with heart disease[60].
In conclusion, iPSC technology represents a groundbreaking approach with significant implications for regenerative medicine, disease modeling, and drug discovery. The ongoing research and dedication of scientists worldwide suggest a future where iPSC-based therapies could revolutionize the treatment of heart failure, providing hope for enhanced patient outcomes and quality of life.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest.
Data Availability
No additional data are available.
Copyright Permissions
Copyright © 2024 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Author Contribution
The conceptualization was done by Sugiura T, while Nawaz S prepared the original draft; the writing was reviewed and edited by Sugiura T, Nawaz S, Ferrell BE, and Yoshida T.
Abbreviation List
ACE, Angiotensin-converting enzyme
ARB, Angiotensin receptor blocker
CABG, Coronary artery bypass grafting
CRT, Cardiac resynchronization therapy
iPSC, Induced pluripotent stem cell
iPSC-CMs, iPSC-derived cardiomyocytes
ICD, Implantable cardioverter-defibrillator
LVAD, Left ventricular assist device
PCI, Percutaneous coronary intervention
References
[1] Afzal MZ, Reiter M, Gastonguay C et al. Nicorandil, a Nitric Oxide Donor and ATP-Sensitive Potassium Channel Opener, Protects Against Dystrophin-Deficient Cardiomyopathy. J Cardiovasc Pharm T, 2016; 21: 549-562.[DOI]
[2] Del Buono MA-O, Moroni F, Montone RA et al. Ischemic Cardiomyopathy and Heart Failure After Acute Myocardial Infarction. Curr Cardiol Rep, 2022; 24: 1505-1515.[DOI]
[3] Cho HM, Cho JY. Cardiomyocyte Death and Genome-Edited Stem Cell Therapy for Ischemic Heart Disease. Stem Cell Rev Rep, 2021; 17: 1-16.[DOI]
[4] Biton Y, Baman JR, Polonsky B. Roles and indications for use of implantable defibrillator and resynchronization therapy in the prevention of sudden cardiac death in heart failure. Heart Fail Rev, 2016; 21: 433-446.[DOI]
[5] Chachques JC. Cellular cardiac regenerative therapy in which patients? Expert Rev Cardiovas, 2009; 7: 911-919.[DOI]
[6] Kim C, Wong J, Wen J et al. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature, 2013; 494: 105-110.[DOI]
[7] Contractor T, Beri A Fau - Gardiner J, Gardiner J Fau - Ardhanari S et al. Statins reduce appropriate implantable cardioverter-defibrillator shocks in ischemic cardiomyopathy with no benefit in nonischemic cardiomyopathy. Am J Ther, 2012; 19: 413-418.[DOI]
[8] Daiber A, Steven S, Euler G et al. Vascular and Cardiac Oxidative Stress and Inflammation as Targets for Cardioprotection. Curr Pharm Design, 2021; 27: 2112-2130.[DOI]
[9] Duerr GA-O, Dewald D, Schmitz EJ et al. Metallothioneins 1 and 2 Modulate Inflammation and Support Remodeling in Ischemic Cardiomyopathy in Mice. Mediat Inflamm, 2016; 2016: 7174127.[DOI]
[10] Diecke S, Jung SM, Lee J et al. Recent technological updates and clinical applications of induced pluripotent stem cells. Korean J Intern Med, 2014; 29: 547.[DOI]
[11] Theunissen TW, Friedli M, He Y et al. Molecular Criteria for Defining the Naive Human Pluripotent State. Cell Stem Cell, 2016; 19: 502-515.[DOI]
[12] Ramachandra CJA, Chua J, Cong S et al. Human-induced pluripotent stem cells for modelling metabolic perturbations and impaired bioenergetics underlying cardiomyopathies. Cardiovasc Res, 2021; 117: 694-711.[DOI]
[13] Hnatiuk AP, Briganti F, Staudt DW et al. Human iPSC modeling of heart disease for drug development. Cell Chem Biol, 2021; 28: 271-282.[DOI]
[14] Guthoff H, Hof A, Klinke A et al. Protective Effects of Therapeutic Neutrophil Depletion and Myeloperoxidase Inhibition on Left Ventricular Function and Remodeling in Myocardial Infarction. Antioxidants (Basel), 2022, 12: 33.[DOI]
[15] Wang YA-O, Elsherbiny A, Kessler L et al. Lamin A/C-dependent chromatin architecture safeguards naïve pluripotency to prevent aberrant cardiovascular cell fate and function. Nat Commun, 2022; 13: 6663.[DOI]
[16] Centeno EGZ, Cimarosti H, Bithell A. 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol Neurodegener, 2018; 13: 1-15.[DOI]
[17] Paloschi V, Sabater-Lleal M, Middelkamp H et al. Organ-on-a-chip technology: a novel approach to investigate cardiovascular diseases. Cardiovasc Res, 2021; 117: 2742-2754.[DOI]
[18] Wang G, McCain M, Yang L et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med, 2014; 20: 616-623.[DOI]
[19] Mathur A, Loskill P, Hong S et al. Human induced pluripotent stem cell-based microphysiological tissue models of myocardium and liver for drug development. Stem Cell Res Ther, 2013; 4: 1-5.[DOI]
[20] Gil JF, Moura CS, Silverio V et al. Cancer Models on Chip: Paving the Way to Large-Scale Trial Applications. Adv Mater, 2023; 35: 2300692.[DOI]
[21] Higo S. Disease modeling of desmosome-related cardiomyopathy using induced pluripotent stem cell-derived cardiomyocytes. World J Stem Cells, 2023; 15: 71.[DOI]
[22] Purnama U, Castro-Guarda M, Sahoo OS et al. Modelling Diabetic Cardiomyopathy: Using Human Stem Cell-Derived Cardiomyocytes to Complement Animal Models. Metabolites, 2022; 12: 832.[DOI]
[23] Kim JY, Nam Y, Rim YA et al. Review of the Current Trends in Clinical Trials Involving Induced Pluripotent Stem Cells.Stem Cell Rev Rep, 2022: 1-13.[DOI]
[24] Anghel L, Sascău R, Zota IM et al. Well-Known and Novel Serum Biomarkers for Risk Stratification of Patients with Non-ischemic Dilated Cardiomyopathy. Int J Mol Sci, 2021; 22: 5688. [DOI]
[25] Paik DT, Chandy M, Wu JC. Patient and Disease-Specific Induced Pluripotent Stem Cells for Discovery of Personalized Cardiovascular Drugs and Therapeutics. Pharmacol Rev, 2020; 72: 320-342.[DOI]
[26] Pang L. Toxicity testing in the era of induced pluripotent stem cells: A perspective regarding the use of patient-specific induced pluripotent stem cell–derived cardiomyocytes for cardiac safety evaluation. Curr Opin Toxicol, 2020; 23-24: 50-55.[DOI]
[27] Zeng H, Wang J, Clouse H et al. HiPSC-CMs from different sex and ethnic origin donors exhibit qualitatively different responses to several classes of pharmacological challenges. J Pharmacol Tox Met, 2019, 99: 106598.[DOI]
[28] Lu HR, Zeng H, Kettenhofen R et al. Assessing Drug-Induced Long QT and Proarrhythmic Risk Using Human Stem-Cell-Derived Cardiomyocytes in a Ca2+ Imaging Assay: Evaluation of 28 CiPA Compounds at Three Test Sites. Toxicol Sci, 2019; 170: 345-356.[DOI]
[29] Kim C. iPSC technology--Powerful hand for disease modeling and therapeutic screen. BMB Rep, 2015; 48: 256.[DOI]
[30] Hashimoto H, Olson EN, Bassel-Duby R. Therapeutic approaches for cardiac regeneration and repair. Nat Rev Cardiol, 2018; 15: 585-600.[DOI]
[31] Kadota S, Tanaka Y, Shiba Y. Heart regeneration using pluripotent stem cells. J Cardiol, 2020; 76: 459-463.[DOI]
[32] Hsiao ST, Dilley RJ, Dusting GJ et al. Ischemic preconditioning for cell-based therapy and tissue engineering. Pharmacol Therapeut, 2014, 142: 141-153.[DOI]
[33] Guo R, Morimatsu M, Feng T et al. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res Ther, 2020, 11: 1-13.[DOI]
[34] Silbernagel N, Körner A, Balitzki J et al. Shaping the heart: Structural and functional maturation of iPSC-cardiomyocytes in 3D-micro-scaffolds. Biomaterials, 2020, 227: 119551.[DOI]
[35] Higuchi T, Miyagawa S, Pearson JT et al. Functional and Electrical Integration of Induced Pluripotent Stem Cell-Derived Cardiomyocytes in a Myocardial Infarction Rat Heart. Cell Transplant, 2015; 24: 2479-2489.[DOI]
[36] Ong SG, Huber BC, Lee WH et al. Microfluidic Single-Cell Analysis of Transplanted Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes After Acute Myocardial Infarction. Circulation, 2015; 132: 762-771.[DOI]
[37] Sugiura T, Shahannaz DC, Ferrell BE. Current Status of Cardiac Regenerative Therapy Using Induced Pluripotent Stem Cells. Int J Mol Sci, 2024; 25: 5772.[DOI]
[38] Kawamura T, Ito Y, Ito E et al. Safety confirmation of induced pluripotent stem cell-derived cardiomyocyte patch transplantation for ischemic cardiomyopathy: first three case reports. Front Cardiovasc Med, 2023, 10: 1182209.[DOI]
[39] Sanchez-Freire V, Lee AS, Hu S et al. Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells. J Am Coll Cardiol, 2014; 64: 436-448.[DOI]
[40] Rapöhn M, Cyganek L, Voigt N et al. Noninvasive analysis of contractility during identical maturations revealed two phenotypes in ventricular but not in atrial iPSC-CM. Am J Physiol-Heart C, 2024; 326: H599-H611.[DOI]
[41] Jimenez-Vazquez EN, Jain A, Jones DK. Enhancing iPSC-CM Maturation Using a Matrigel-Coated Micropatterned PDMS Substrate. Curr Protoc, 2022; 2: e601.[DOI]
[42] Horikoshi Y, Yan Y, Terashvili M et al. Fatty Acid-Treated Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes Exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes. Cells, 2019, 8: 1095.[DOI]
[43] Lee J, Jung SM, Ebert AD et al. Generation of Functional Cardiomyocytes from the Synoviocytes of Patients with Rheumatoid Arthritis via Induced Pluripotent Stem Cells. Sci Rep, 2016, 6: 32669.[DOI]
[44] Peinkofer G, Maass M, Pfannkuche K et al. Persistence of intramyocardially transplanted murine induced pluripotent stem cell-derived cardiomyocytes from different developmental stages. Stem Cell Res Ther, 2021, 12: 1-13.[DOI]
[45] Shiba M, Higo S, Kondo T et al. Phenotypic recapitulation and correction of desmoglein-2-deficient cardiomyopathy using human-induced pluripotent stem cell-derived cardiomyocytes. Hum Mol Genet, 2021; 30: 1384-1397.[DOI]
[46] Kawamura T, Miyagawa S, Fukushima S et al. Structural Changes in N-Glycans on Induced Pluripotent Stem Cells Differentiating Toward Cardiomyocytes. Stem Cell Transl Med, 2015, 4: 1258-1264.[DOI]
[47] Wang Q, Chen KY, Yu F et al. Abnormal diastolic function underlies the different beneficial effects of cardiac resynchronization therapy on ischemic and non-ischemic cardiomyopathy. Clinics, 2017; 72: 432-437.[DOI]
[48] Kawamura T, Miyagawa S, Fukushima S et al. N-glycans: phenotypic homology and structural differences between myocardial cells and induced pluripotent stem cell-derived cardiomyocytes. PLoS One, 2014; 9: e111064.[DOI]
[49] Izadifar M, Berecz T, Li B et al. Speckle-Tracking Strain Analysis for Mapping Spatiotemporal Contractility of Induced Pluripotent Stem Cell (iPSC)-Derived Cardiomyocytes. Curr Protoc, 2023; 3: e889.[DOI]
[50] Lieu DK, Fu JD, Chiamvimonvat N et al. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ-Arrhythmia Elec, 2013; 6: 191-201.[DOI]
[51] Jeziorowska D, Fontaine V, Jouve C et al. Differential Sarcomere and Electrophysiological Maturation of Human iPSC-Derived Cardiac Myocytes in Monolayer vs. Aggregation-Based Differentiation Protocols. Int J Mol Sci, 2017; 18: 1173.[DOI]
[52] Barnes AA-O, Holmstoen TB, Bonham AA-O et al. Differentiating Human Pluripotent Stem Cells to Cardiomyocytes Using Purified Extracellular Matrix Proteins. Bioengineering, 2022; 9: 720.[DOI]
[53] Fang YH, Wang SPH, Gao ZH et al. Efficient Cardiac Differentiation of Human Amniotic Fluid-Derived Stem Cells into Induced Pluripotent Stem Cells and Their Potential Immune Privilege. Int J Mol Sci, 2020; 21: 2359.[DOI]
[54] Chiew MY, Wang E, Lan KC et al. Improving iPSC Differentiation Using a Nanodot Platform. ACS Appl Mater Inter, 2024; 16: 36030-36046.[DOI]
[55] Omole AE, Fakoya AA-O. Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ, 2018, 6: e4370., 2018, 6: e4370.[DOI]
[56] Silva M, Daheron L, Hurley H et al. Generating iPSCs: translating cell reprogramming science into scalable and robust biomanufacturing strategies. Cell Stem Cell, 2015; 16: 13-17.[DOI]
[57] Zhang Y, Xie X, Hu J et al. Prospects of Directly Reprogrammed Adult Human Neurons for Neurodegenerative Disease Modeling and Drug Discovery: iN vs. iPSCs Models. Front Cell Neurosci, 2020; 14: 546484.[DOI]
[58] Wattanapanitch MA-O. Recent Updates on Induced Pluripotent Stem Cells in Hematological Disorders. Stem Cells Int, 2019; 2019: 5171032.[DOI]
[59] Omar A, Zhou M, Berman A et al. Genomic-based diagnosis of arrhythmia disease in a personalized medicine era. Expert Rev Precis Med Drug Dev, 2016; 1: 497-504.[DOI]
[60] Ohnuki M, Takahashi K. Present and future challenges of induced pluripotent stem cells. Philos Trans R Soc Lond B Biol Sci, 2015, 370: 20140367.[DOI]




 Copyright ©
Copyright ©