Emerging Micromolecule-based Aqueous Two-phase Systems and Their Application in Biofuels
Shaoqu Xie1,2*, Zhenqi Sun1, Jialin Tan1, Wanli Zhang3, Xiaolan Cai1,2, Yanxiong Fang1,2
1School of Light Industry and Chemical Engineering, Guangdong University of Technology, Guangzhou, 510006, Guangdong Province, China
2Guangdong Provincial Laboratory of Chemistry and Fine Chemical Engineering, Jieyang Center, Jieyang, 515200, Guangdong Province, China
3R&D Center, Shanghai Shaanxi Coal Hi-tech Research Institute, Shanghai, 201613, China
*Correspondence to: Shaoqu Xie, PhD, Associate Professor, School of Light Industry and Chemical Engineering, Guangdong University of Technology, 100 Waihuan Xi Road, Panyu District, Guangzhou, 510006, Guangdong Province, China. Email: shaoqu.xie@gdut.edu.cn
DOI: 10.53964/id.2024030
Abstract
Aqueous two-phase system (ATPS) is a system composed of two incompatible solutions, which has excellent performance for extracting proteins, antibiotics, and other bioactive molecules, so it is a very simple, efficient, and green extraction and separation method. In this paper, we introduced three different emerging micromolecule-based ATPSs and introduced their characteristics, applications, advantages, and disadvantages respectively. This is of guiding significance for the synthesis of new ATPSs. One of the important prospects of this ATPS in industrial application is the separation and purification of fermentation-based biofuel. Adding a salt, sugar, or ionic liquid to a biofuel solution can remove most of the water, enabling efficient recovery and dehydration of biofuel. The two-step process can replace the traditional distillation and molecular sieve adsorption processes to obtain high-purity bioalcohols, thus introducing a new purification process for biofuel. An in-depth analysis of the separation performance of the emerging micromolecule-based ATPSs as well as an objective indication of their application provides valuable insights for further optimization and application of this kind of ATPSs in separation science.
Keywords: micromolecule-based aqueous two-phase system, salt, sugar, alcohols, ionic liquid, biofuel
1 INTRODUCTION
Since the industrial revolution, efforts have been made to optimize the use of energy, and numerous methods and equipment have been developed and optimized to reduce the amount of fuel consumed in separation and purification areas[1]. The chemical industry requires the separation and purification of chemical products from research to actual production, and there are a variety of traditional separation and purification techniques, such as distillation[2], membrane permeation[3], adsorption[4], and extraction[5]. However, all of these separation techniques consume a large amount of energy. Currently, the petroleum-based industrial system is facing a global crisis, including climate change and the depletion of fossil resources[6].
The conversion of biomass into clean fuels and high-quality chemicals through the action of microorganisms reduces dependence on fossil energy sources while lowering environmental pollution during the production process[7–9]. This approach promotes the recycling of resources and is an important approach to green and sustainable development[10]. Fermentation of biomass for the production of fuels and chemicals has significant advantages. Firstly, the method is environmentally sustainable and most of the waste generated during the production process can be disposed of through natural decomposition, reducing environmental pollution. Secondly, biomass fermentation utilizes renewable resources and reduces dependence on limited mineral resources. Furthermore, fermentation technology produces chemicals of high quality and purity, such as those used in the biopharmaceutical and cosmetic fields, which demonstrate excellent product performance. In addition, the technology has a high degree of controllability and safety in the production process and relatively low energy consumption. Thus, the production of fuels and chemicals by biomass fermentation is not only conducive to environmental protection but also promotes the sustainable utilization of resources and enhances product quality, which is an important direction for future development[11,12].
However, complications arise when azeotropic solutions are formed during low-concentration fermentation and separated for purification[13,14]. Therefore, it is critical to improve the competitiveness of products in terms of improved fermentation technology or purification methods[15]. The high energy content and portability of bio-derived fuels, as well as their remarkable compatibility with existing petroleum-based transportation infrastructures, can be considered attractive as a fuel source[16-19]. Aqueous two-phase extraction is a well-established method that does not require heat exchange[20]. Aqueous two-phase systems (ATPSs) have a wide range of applications in the isolation and purification of bioproducts from biologically active or low-molecule alcohols (e.g., isopropanol, carboxylic acids, and fermentation broths)[21,22]. The formation of a liquid-liquid equilibrium in these extraction systems can be driven by salting-out effects or sugaring-out effects[23,24]. The formation of liquid-liquid equilibria in these systems can be a key factor in the development of a new extraction method.
Traditional ATPSs can be formed when a hydrophilic polymer is added to a saline solution or another polymer at a certain concentration or a particular temperature[25]. The two phases formed usually include two polymers such as dextran and polyethylene glycol (PEG), polymers and salts (e.g., PEG and sodium sulfate, citrate, etc.), among other kinds of ATPSs. The characteristics of these systems, their basic concepts, and their implications for the partitioning behaviors of different substances have been studied in detail. It has been found that these ATPSs have good performance in the separation and recovery of proteins, monoclonal antibodies, DNA, and low molecular weight biologically active substances, but also maintain good activity and can be used to stabilize cell morphology by their biocompatibility and environmental friendliness[26]. In addition, ATPSs can be formed in a simple vessel and the low cost of the operating conditions gives the potential for industrial applications. Especially, the traditional ATPSs and their application have attracted a great deal of interest in academic and industrial areas, as ever reviewed by several published papers. For instance, Grilo et al.[27] extensively reviewed the theory of partitioning and the important factors affecting partitioning in the field of downstream processing of biomolecules by ATPSs and summarized the research trends in ATPS during 2008-2013. Hatti-Kaul summarized that systems formed by mixing two polymers or polymers and salt in water can be used to separate cells, membranes, viruses, proteins, nucleic acids, and other biomolecules, and have proven to have applications in the fields of biomolecule and cell surface analysis, separation of cell populations, and recycling of biotechnology products and are expected to be used for environmental remediation, with large-scale recycling of proteins receiving the most attention[26]. ATPSs have been successfully applied in other fields such as veterinary drug residue detection, precious metal separation, sewage treatment, etc[28]. New advances in the water-water interfacial dynamics, as well as advances in interface-assisted design of artificial cells, preparation of cytosolic and biocompatible particles, cell micropatterning, 3D bioprinting, and microfluidic separation of cells and biomolecules, were also reviewed, and challenges and prospects for expanding the applications utilizing the unique properties of ATPSs and their interfaces were discussed[29]. However, traditional ATPSs cannot be used for biofuel separation and purification.
ATPSs can also be formed when a hydrophilic small-molecule solvent is added to a saline solution at a certain concentration or at a particular temperature[25,30]. The two phases formed usually include alcohols and salts (e.g., ethanol and ammonium sulfate), alcohols and sugars (propanol and glucose, etc.), and ionic liquids (ILs) and salts[31–33]. It has been reported that these emerging ATPSs have been used in operations to isolate fermentation products[34]. Thus, this review can cover previous gaps that traditional ATPSs left in the biofuel separation from fermentation broth.
It is clear that the scientific community still sees great potential for the development of primary processes based on ATPSs due to the increasing number of reports on the use of new ATPSs for the recovery of different bioproducts. This review focuses on a detailed description of these ATPSs and summarizes the recent research progress of these systems and the advantages and disadvantages of the system. Their applications in biofuels are also included to highlight these emerging separation techniques.
2 EMERGING MICROMOLECULE-BASED AQUEOUS TWO-PHASE SYSTEMS
2.1.1 Introduction to the Salt/alcohol ATPSs
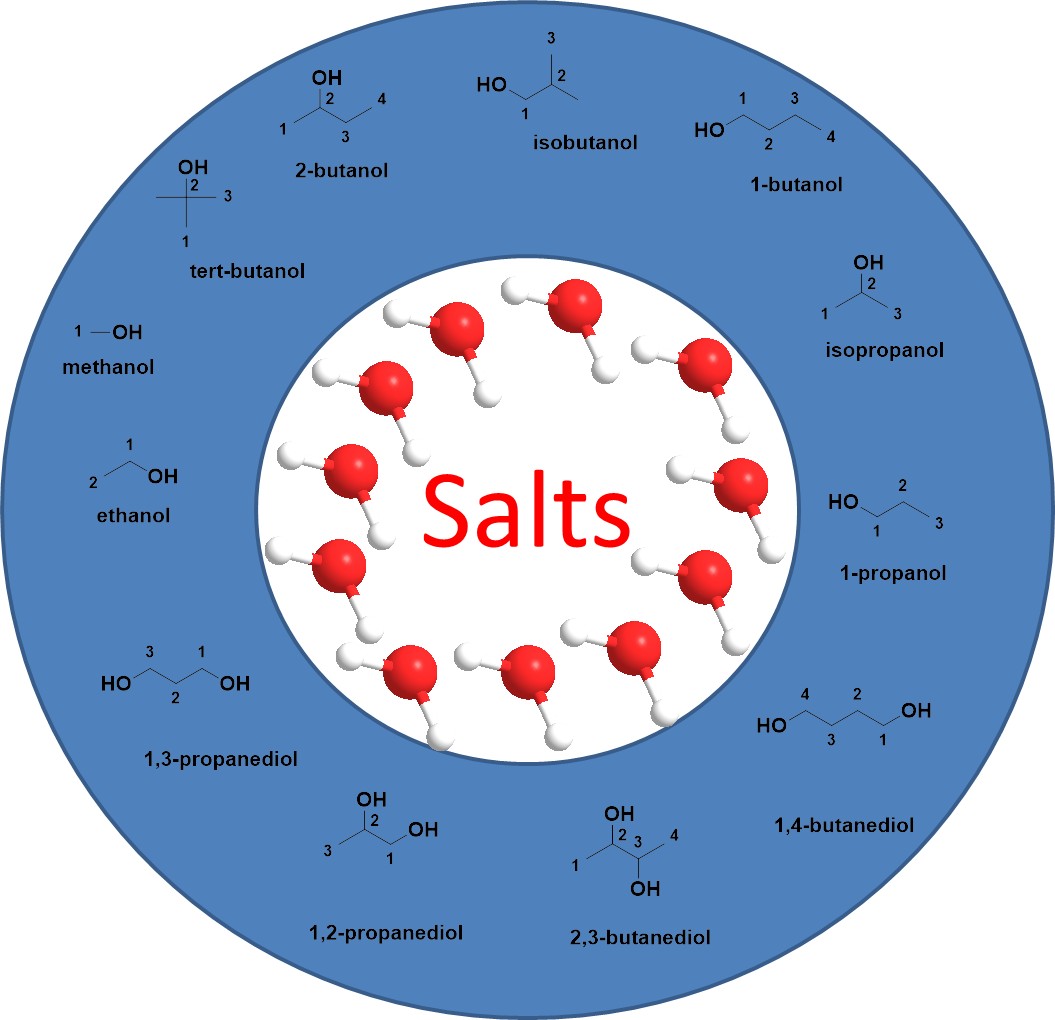
The addition of salt or other hydrophobic substances to an aqueous solution of alcohol induces a change in the interfacial properties of the solvent, its charge, and the formation of multiple forces such as hydrophobic, hydrogen, and ionic bonds, which results in the alcohol-rich phase becoming the upper phase, and the water+salt-rich phase becoming the lower phase, resulting in the formation of a salt/alcohol systems[8], as shown in Figure 1. The isolation and purification of the organic alcohols from the aqueous solution is achieved[35,36]. This liquid-liquid separation is usually used for the extraction of alcohols produced by fermentation[37]. In addition, the salting-out reagent can be mixed with a highly concentrated solution of the target product, and the target product can still be separated from the aqueous solution even without the addition of an extractant[38]. The formation of ATPS occurs spontaneously, so no additional organic solvents need to be added to form two phases. For example, if a conventional organic solvent is used as an extractant for highly polar alcohols (e.g., 2,3-butanediol), the water molecule is also highly polar and the two follow the solubility rule "like dissolve like", resulting in unsatisfactory extraction of butanediol from its aqueous solution[39]. When the salting-out effect of the salt separator is utilized, the repulsive effect on the butanediol molecules in the solution can be increased, thus improving the extraction efficiency[40].
|
Figure 1. Salt/alcohol ATPSs.
2.1.2 Advantages and Disadvantages of Salt/alcohol ATPSs
In the extraction of proteins in the water/acetonitrile system using dipotassium hydrogen phosphate as a separating agent, the pH of the solution can be stabilized to maintain a good extraction efficiency of the proteins because dipotassium hydrogen phosphate in the system is amphoteric[41]. The results of the study have shown that the efficiency of salting-out extraction of proteins can be improved by both the addition of salt and the cooling measures. The salt/alcohol system can also be used to improve the partitioning efficiency of the substance to be extracted in one of its phases by adding a small amount of adjuvant IL[42]. In addition to the influence of the choice of salt separator on the separation effect, the structure of the alcohol significantly affects the results, and alcohols with shorter carbon chains are usually chosen as the organic phase in the preparation of salt/alcohol ATPS, due to the fact that long-chained alcohols can be more unstable than short-chained and branched-chained alcohols in the extraction of certain biomolecules (e.g., proteins)[43]. The disadvantages of the salting-out effect are also evident in the fact that large volumes of salts are used in the salting-out process, which not only cause some corrosion of the equipment, but also the difficult-to-recovery salts may cause environmental problems and denaturation of the target proteins due to characteristics such as high ionic strength or high alkalinity. Certain salts (e.g., potassium phosphate) limit the application of their ATPSs due to their extreme water absorption, which leads to a reduced effect during salting-out[44].
2.2 Alcohol/sugar ATPSs
2.2.1 Introduction to the Sugar/alcohol ATPSs
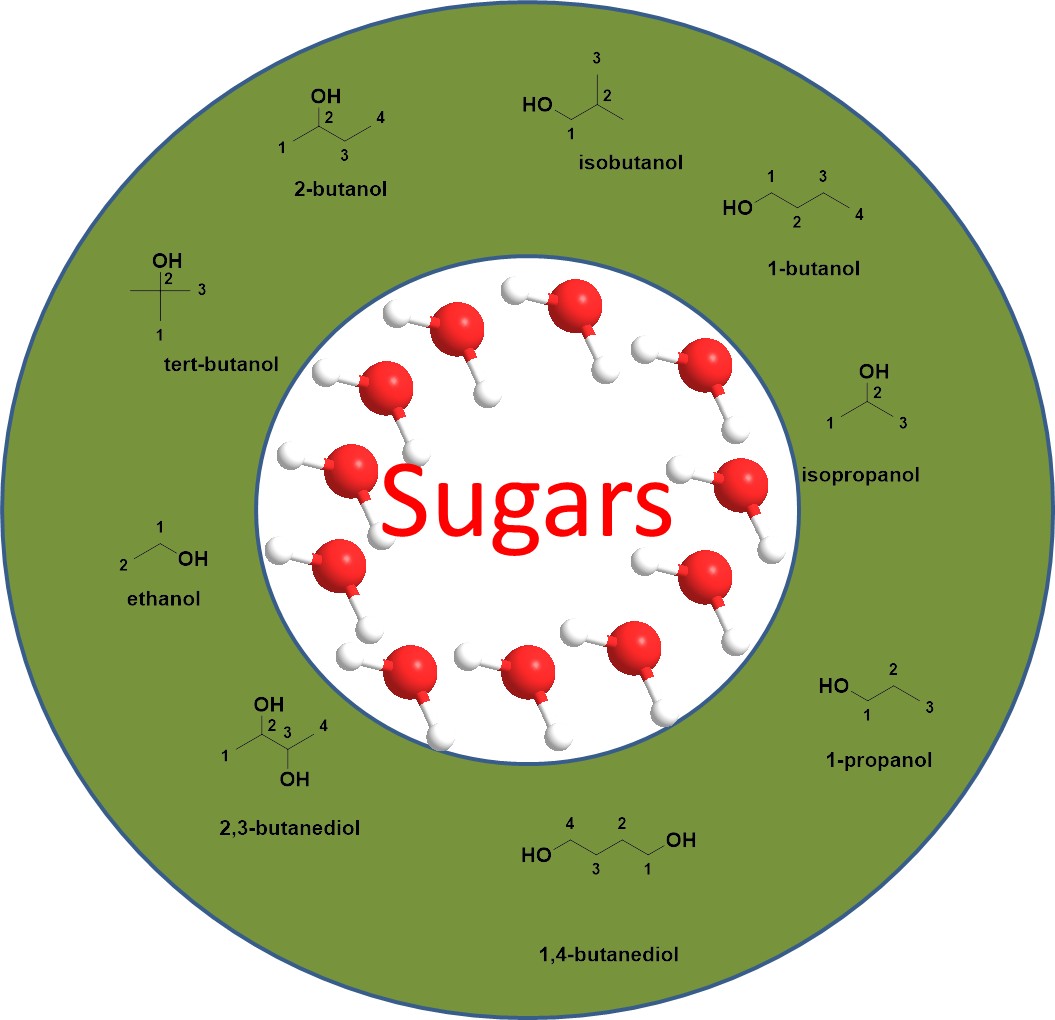
Sugar acts as a separator competing with the solute to attract water molecules, generating a new phase of small organic molecules of low polarity, which is dominated by small molecules, with the sugar mainly present in the aqueous phase[45], as shown in Figure 2. Thus, the sugaring-out system makes it possible to separate small molecules[46]. The carbohydrates are polyhydroxy aldehydes or ketones, which can be categorized into two main groups, i.e. monosaccharides and complex sugars. In terms of separation, sugar as an alternative to salt has many advantages such as the ability to create a milder separation environment that is more suitable for biomolecules, and the fact that alcohols are less viscous than polymers and IL, which endows the alcohol/sugar system with the ability to accomplish the phase separation process more rapidly and efficient mass transfer.
|
Figure 2. Sugar/alcohol ATPSs.
2.2.2 Advantages and Disadvantages of Sugar/alcohol ATPSs
The sugar/alcohol systems are greener, more environmentally friendly, and more efficient extraction and separation systems compared to the high cost of the traditional ATPS. The high viscosity characteristics of the traditional system tend to cause slow molecular mass transfer. The use of a large amount of salt in the salt/alcohol system may cause corrosion of the equipment. When utilizing sugar/alcohol ATPSs for ultrasound-assisted flotation of bioactive molecules is a green production mode with low energy consumption and pollution, and can be controlled ultrasound conditions can achieve higher yields than continuous ultrasound treatment[47,48]. The ultrasound-assisted separation process reduces heat consumption and vapor loss compared to conventional separation methods. The mild environment of the sugar/alcohol ATPSs also helps to maintain the activity of the biomolecules to be extracted and the molecular state of the separator in the system avoids corrosion of the equipment. Due to the disadvantage of the weak sugaring-out effect and its low extraction efficiency[33], the sugaring-out effect can be enhanced by the addition of some additives in the practical application process.
2.3.1 Introduction to IL-based ATPSs
ILs are generally organic salts with a melting point lower than or equal to room temperature and consist of cations and organic or inorganic anions[49]. They are friendlier and safer than conventional volatile molecular solvents due to their many excellent properties such as very low volatility, non-flammability, electrochemical stability, and excellent ionic conductivity even in the absence of water and recyclability. This allows them to be widely used as "green solvents" in the separation field[50]. In the past decades, many types of ILs have been reported and studied. Among them, the most representative one is imidazolium IL, which was used for the separation and identification of chloramphenicol[51]. Nowadays, ILs are considered alternatives to hazardous organic solvents and are widely used for the extraction and separation of various products[52].
2.3.2 Advantages and Disadvantages of IL/salt ATPSs
The greatest advantage of ILs is the ability to regulate their polarity and affinity by designing cation/anion combinations in aqueous two-phase systems[53]. ILs have a high recovery due to their low volatility, non-flammability, electrochemical stability, etc. After the recovery, the ILs can still maintain strong stability, and the separation efficiency is not affected by reuse[54]. However, the hydrophobic chiral magnetic ILs/salt system is greatly affected by temperature, and its phase separation ability decreases with increasing temperature under different temperature environments, which suggests that the interaction between the IL and water will be strengthened with increasing temperature, thus enhancing the mutual solubility between the ILs and water[55]. For the extraction of metal ions from solution using ILs-ATPS, the extraction efficiency also decreases with increasing temperature[44]. Therefore, it is recommended to separate and extract metal ions or chiral compounds at room temperature when extracting metal ions or chiral compounds based on the ILs/salt ATPSs. The ILs-based ATPSs also use a large amount of salt during the application process and therefore have the same disadvantage of the salting-out effect. The high cost of ILs also restricts the wide application of this system. Thus in the subsequent process, better methods should be developed for the recovery of salt and ILs as well as avoiding the phenomenon of equipment corrosion.
2.4 Mechanism of Micromolecule-based ATPSs
Salts are soluble in solvents such as water, organic mono-alcohols, diols, and certain organic substances. When the salt is mixed with a solvent, it will lead to changes in boiling point, liquid phase activity, mutual solubility, vapor-liquid phase equilibrium composition, etc. This phenomenon of relative change in the nature of the mixture due to the presence of salt is known as the salt effect[56].
The electrostatic mechanism focuses on the electrostatic interaction between salt ions and water molecules in ternary solutions, where molecules with different dielectric constants are considered to be redistributed under the action of the ionic electrostatic field, leading to changes in the free energy and changes in the activity coefficients of the components. For saturated non-electrical dielectrics, a rise in the activity coefficient reduces their solubility, leading to the phenomenon of supersaturation and precipitation of substances[57]. Whereas, when the dielectric constant of a non-electric dielectric is higher than that of water, the phenomenon of salting-in, i.e., an increase in solubility, may occur[58].
The process of structural changes induced by the addition of the salting-out agent to dilute amphiphilic aqueous solutions is described in detail[59]. Specifically, upon the addition of NaCl, approximately 1/3 to 1/2 of the t-butanol molecules, which originally relied on nonpolar headgroup interactions, formed anion-bridged polar contacts through rearrangement of their alcohol hydroxyl groups. In solutions without added salts, the pattern of polar-polar interactions between solutes was almost negligible. However, the introduction of NaCl significantly enhanced the polar-tail interactions between the solutes, which was mainly realized through the anion bridging effect. This anion bridging not only changed the arrangement of solute molecules but also increased the exposure of amphiphilic molecules to nonpolar surfaces, which, in turn, made aggregated solute molecules unfavorable in the aqueous environment.
The mechanism of sugaring-out mainly involves the interaction of sugar molecules with solute molecules and the resulting phase separation phenomenon[60]. The interaction between sugar molecules and water molecules is mainly the formation of hydrogen bonds. When sugar molecules (such as glucose, sucrose, etc.) dissolve in water, their hydroxyl or carbonyl group can form hydrogen bonds with water molecules. When sugar is added to a solution containing an organic substance that is miscible with water (e.g., acetonitrile), the sugar molecules will replace some of the organic molecules to form hydrogen bonds with water molecules[61]. Due to the formation of hydrogen bonds between sugar molecules and water molecules, the original balance of interaction between organic matter and water molecules is disrupted[62]. When the sugar concentration reaches a certain value, this change in interaction leads to phase separation of the solution, forming an organic-rich phase (upper phase) and a sugar-rich phase (lower phase).
The mechanism of IL extraction is primarily concerned with the interactions between the IL and the solute molecules, and how these interactions lead to the partitioning of the solute between the IL and the aqueous phase[63]. IL extraction involves not only mechanisms such as electrostatic interaction, hydrogen bonding, dispersion and hydrophobicity, but may also include complex processes such as ion exchange and solvation[64,65]. Due to the ionization properties of ILs, it incorporates similar mechanisms of salting-out and solvent extraction-based like-dissolves-like principle.
3 APPLICATION OF MICROMOLECULE-BASED ATPSS IN BIOFUELS
3.1 Overview of Bioethanol and Biobutanol
3.1.1 Bioethanol and Biobutanol Fermentation
The most common biofuel available today is ethanol, most of which is produced by brewer's yeast through fermentation of sucrose-rich crops or starch-rich crops[66]. Ethanol produced from these sources is known as a first-generation biofuel. Yeast fermentation can produce a range of additional valuable by-products that accumulate in the primary process of fermentation, such as protein concentrates, water-soluble metabolites, heterohydric alcohols, and industrial enzymes[67]. Current bacterial fermentation concentrations of bioethanol from molasses using high gravity technology by mutant S. cerevisiae can reach 12.0% (v/v)[68]. The potential of sweet sorghum for ethanol production was investigated and optimized to reach 87g/L ethanol concentration in sorghum seed and 72g/L ethanol concentration in sweet sorghum juice[69]. Ultra-high specific gravity ethanol fermentation technology is highly efficient. Using a central composite design to optimize the conditions, a concentration of 135.0g/L of ethanol was achieved after 30 hours of fermentation at 27.0°C in which a sugar concentration of 300.0g/L, and a cellular concentration of 15.0% was selected[70].
While first-generation fuel ethanol technology, which can use grain crops or sugars other than rice and wheat sugar/starch crops as feedstock, has contributed to the development of clean energy to some extent, its large-scale production poses a significant threat to the global food supply. This phenomenon of ‘competition for food and land’ not only exacerbates the risk of food shortages but may also lead to higher food prices and affect social stability. Therefore, the sustainable development of first-generation fuel ethanol faces serious challenges, and there is a need to explore alternative feedstocks and technological pathways that are more efficient, environmentally friendly, and do not affect food security.
Second-generation fuel ethanol production utilizes agricultural waste and lignocellulosic materials as feedstocks, a shift that not only broadens the pathway to energy production but also demonstrates the advantages of low feedstock costs, a wide range of sources, and relatively low investment costs[71]. Despite the potential of genetically modified algae for bioenergy production, difficulties in harvesting and high upfront costs have limited its large-scale application[72]. Chemical conversion of CO2 or fermentation of CO to produce ethanol has the advantage of cheap feedstock, but yields are generally quite low[73–75]. In contrast, second-generation fuel ethanol is more affordable and has a lower environmental impact, making it a more viable green energy option today. By further investigating ethanol fermentation, researchers can catalyze cellulose using catalysts, yielding higher concentrations of ethanol solutions[63]. In some processes, a second fermentation is carried out through a second fermentation with another fermenting organism. Such a two-stage fermentation can produce valuable compounds such as 1,3-propanediol, organic acids, and bacteriocins. The use of lactic acid bacteria also leads to the aggregation of precipitated proteins and the aggregation of proteins into concentrates[64].
Lignocellulosic biomass has great potential as a raw material for fuel ethanol and is an important direction for future ethanol fuel development. However, in the production process of cellulosic ethanol, the two key steps of pretreatment and enzymatic hydrolysis have large capital investment, which has become a major bottleneck restricting its large-scale application[76]. Choosing the right pretreatment method is the key to improving the efficiency of enzymatic hydrolysis. From a commercialization point of view, efficient and economical pretreatment needs to reduce water and energy consumption, suppress by-product generation, achieve low-cost catalyst recovery, and reduce waste. The importance of pretreatment is emphasized by the fact that the cost of enzymatic digestion accounts for about 25% of the total cost of a fuel ethanol biorefinery.
Butanol has significant advantages as a biofuel. Bio-butanol has a high energy density, which is closer to that of gasoline, and does not have the same hygroscopicity as ethanol, so there is no concern about engine corrosion from water[77]. However, butanol is currently facing technical problems such as low concentration of butanol in butanol fermentation broth and high separation costs, which makes butanol fermentation uncompetitive compared to the production of other biofuels[78]. Traditional n-butanol fermentation is carried out by Clostridium acetobutylicum to obtain the product 2% acetone-butanol-ethanol (ABE) solution, and the toxic effect of elevated butanol concentration on Clostridium acetobutylicum during the fermentation process is the main factor contributing to the low concentration of the fermentation broth[79]. Currently, there have been studies to increase the concentration of butanol fermentation broth by strain improvement and alternative substrates, which still can't well solve the problem of low concentration of butanol fermentation broth and high isolation cost[80]. Therefore, the development of a method that can increase the concentration of butanol fermentation can significantly improve the competitiveness of butanol in biofuels.
Similarly, the main feedstock for the production of biobutanol is biomass, specifically including, but not limited to, sugar-rich crops such as corn, cassava, molasses, sugar beets, and sugar cane, as well as woody fibrous materials such as woody stems, rice straw, agricultural residues, corn fibers, and husks. These raw materials can be converted into biobutanol through bioethanol-like processes.
The traditional ABE solvent titer is 2%[81], while acetobacter butyricus JB200, domesticated under long-term intermittent butanol addition, showed excellent butanol production capacity with butanol titer up to 20g/L, which made it the strain with the highest butanol yield under batch fermentation conditions[82]. This achievement provides strong support for the production of biobutanol and has important application value, as the ABE solvent titer reached 28g/L, the highest ever achieved in fermentation with sucrose.
3.1.2 Bioethanol and Biobutanol Separation
Microbial fermentation technology has demonstrated its unique advantages in the production of organic acids, alcohols, amino acids, and amines with a wide range of carbon chain lengths, providing a green and sustainable solution for the chemical and energy sectors[83]. The development of products such as bioethanol and butanol not only reduces dependence on fossil fuels but also promotes the recycling of resources, which is in line with the current trend of global sustainable development[17]. In particular, the promising application of isobutanol in the field of aviation fuels has injected new vitality into this field[84]. However, the azeotropic mixtures generated during the separation of low-concentration fermentation broth pose a great challenge to the subsequent separation and purification, which directly affects the purity and productivity of the product[13,14]. Therefore, the development of more efficient purification technologies has become the key to enhancing the competitiveness of products.
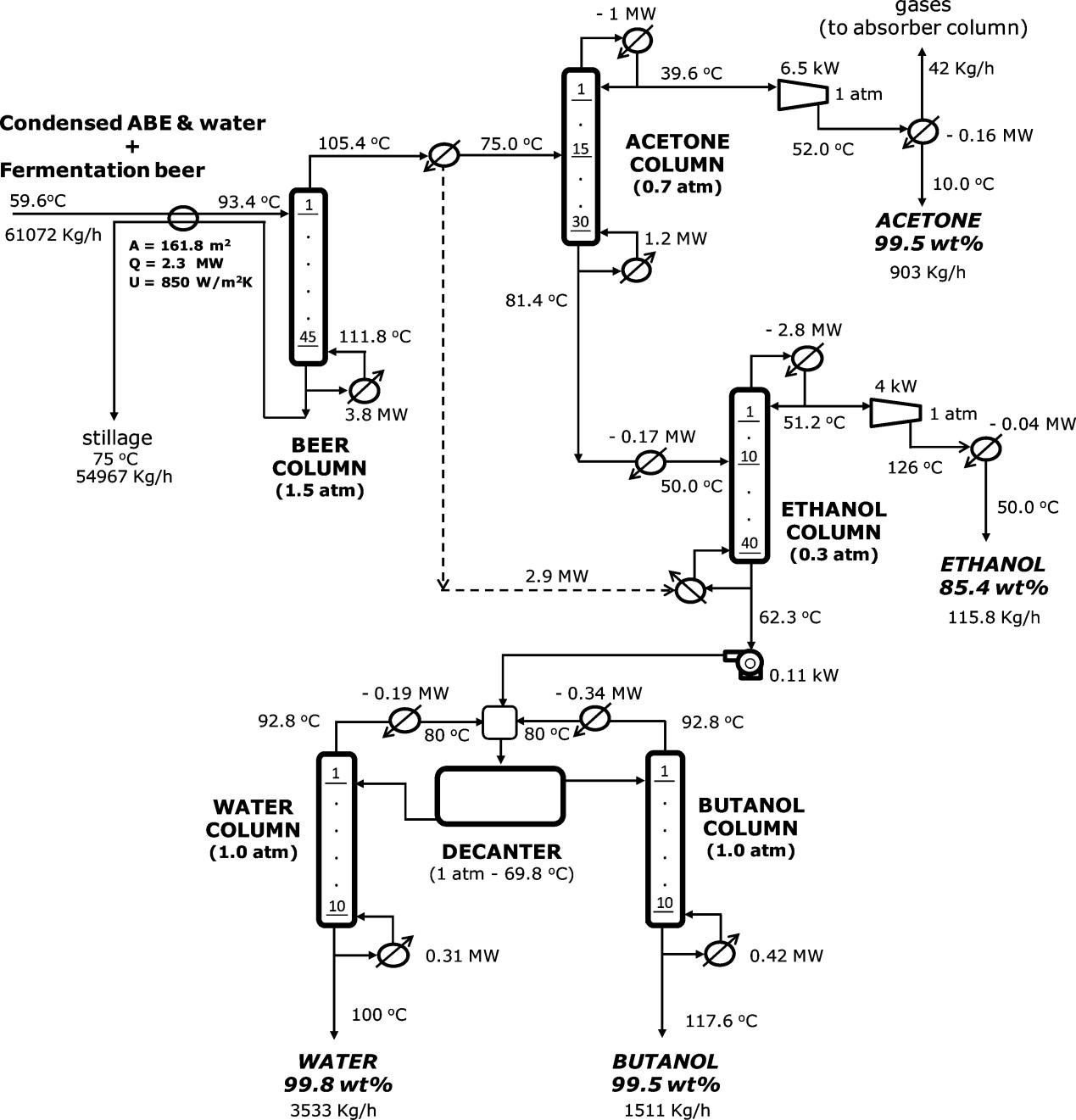
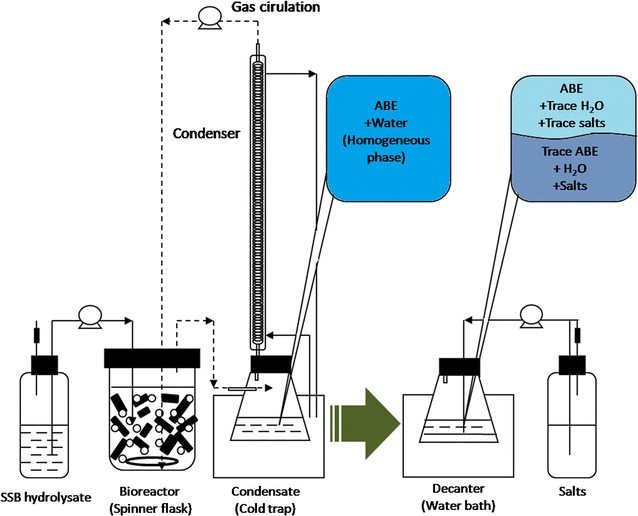
Distillation[85], membrane permeation[86], adsorption[87], and extraction[88,89] are effectively used for purification of bioalcohol fermentation broth. However, the separation of bioalcohols from azeotropes requires high reflux ratios and additional equipment, which is costly. Distillation also faces the challenges of high equipment investment and energy consumption, as shown in Figure 3[91]. Thus, more cost-effective purification solutions need to be explored.
|
Figure 3. Downstream Distillation Process for ABE Fermentation Broth. Reproduced from Ref.[91] with permission from the American Chemical Society.
As a well-established technique that does not require heat exchange[20,90], aqueous two-phase extraction shows significant advantages in the separation, purification, and retention of biological activity of bioproducts, and is particularly suitable for the treatment of low molecular alcohols and fermentation broths[21,91]. Salts, sugars, and ILs are not volatile while bioethanol and biobutanol are volatile. The micromolecule-based aqueous two-phase systems in the separation of bio-based alcohols not only improve the separation efficiency but also facilitate the purification process of bio-alcohols and separating agent recovery[23,24,90,92–95]. Therefore, ATPSs have a broad application prospect in the field of biofuels, which provides strong support for the realization of green and efficient preparation of biofuels.
3.1.3 Bioethanol and Biobutanol as Biofuels
Bioethanol and biobutanol have the potential to replace traditional fossil energy sources as new biofuels, which have the advantages of being sustainable and green compared to traditional fossil energy sources[96]. Bioethanol and biobutanol can be generated directly by fermentation using cellulose such as straw, and the gases produced during the fermentation process can be further converted by biochemical, physical, and thermochemical methods[97,98]. Although biofuels have partially replaced gasoline and diesel in the field of fossil fuels, they account for a relatively small proportion of the total. Bioethanol is not suitable for long-distance transportation and direct use in engines as a fuel due to its low energy density and water absorption. Biobutanol cannot be applied on a large scale for industrial production due to its low fermentation concentration and complex purification process, especially the azeotrope separation[99]. As an important biomass energy source, the potential and development of biofuels deserve further research and exploration, so finding an efficient separation and purification method is a major key to enhancing the competitiveness of biofuels.
3.2 Application of ATPSs in Bioethanol
Liquid-liquid separation of bioethanol is an important process in the purification of ethanol. The fermentation broth contains many components such as ethanol, water, raw materials, cells, and other impurities[100]. Separation of ethanol from the complex fermentation broth is an important technology. Currently, the industry separates and purifies ethanol from the ethanol fermentation broth by distillation, which can quickly separate ethanol from the fermentation broth, and then subsequently purify it further by distillation. However, when the ethanol concentration reaches 95%, ethanol and water form an azeotrope, and the difficulty of further purifying ethanol by distillation rises. To obtain anhydrous ethanol, water is usually removed by a molecular sieve[101]. Adsorption of the remaining water is the most difficult to reduce the energy consumption of the ethanol purification process[102].
Liquid-liquid phase equilibrium (LLE) separation can be achieved by a variety of methods, among which extraction and salting-out separations have been familiarly mastered. Extraction utilizes the difference in partitioning between two immiscible liquids to extract ethanol from the fermentation broth into the extractant. In the production of bioethanol, commonly used organic solvents include carbon tetrachloride and acetone[103]. These solvents can selectively bind to ethanol, thereby separating it from the aqueous phase. However, this will increase the process of ethanol-extractant separation for ethanol purification. Based on the high polarity of ethanol, most of the extractants still have part of ethanol remaining in the aqueous phase after the end of extraction, which increases the cost of ethanol separation. In contrast, the salting-out method does not require the recovery of the extractant, and the ethanol content in the aqueous phase is also in a very low state, which is an excellent means of separating and purifying ethanol[104].
3.2.1 Solvent Extraction of Bioethanol
Ethanol has a low activity coefficient in water, making extraction with conventional solvents difficult. Although ethanol is more volatile than water in dilute solution, it forms azeotropes with water at high concentrations, which makes the distillation process complex and potentially expensive[105]. However, solvent extraction is always unsatisfactory in terms of the water content of the extracted product when a high-polarity extractant is selected for the separation, which requires further separation of ethanol, extractant, and water. When a low-polarity extractant is selected for the extraction, based on the nature of ethanol itself, the low-polarity extractant tends to extract molecules with low polarity. Therefore some ethanol remains in the aqueous phase after the extraction, which increases the cost of ethanol separation[106]. Moreover, solvent extraction requires further solvent-extractant separation, which undoubtedly increases the complexity of ethanol separation.
3.2.2 Salting-out of Bioethanol
The salting-out extraction method for ethanol mainly utilizes different salts as salting-out agents to separate ethanol from aqueous solutions. The purification of different organic compounds by salting-out is a simple and efficient method, such as the addition of K2CO3 to an isopropanol-water azeotrope system to separate ethanol from the azeotrope mixture, which essentially changes the partition coefficient of isopropanol between the organic and aqueous phases[107]. So enhancing the salting-out effect of the salting-out agent is the key to improving the separation ability of the salting-out agent. Salt can also be regarded as a dehydrating agent, and the salting-out extraction with ethanol is also widely used for the separation of bio-based chemicals such as 2,3-butanediol and 1,3-propanediol from fermentation broths[108-110]. This method is simple, efficient, and suitable for the purification of hydrophilic products with low molecular weight[39].
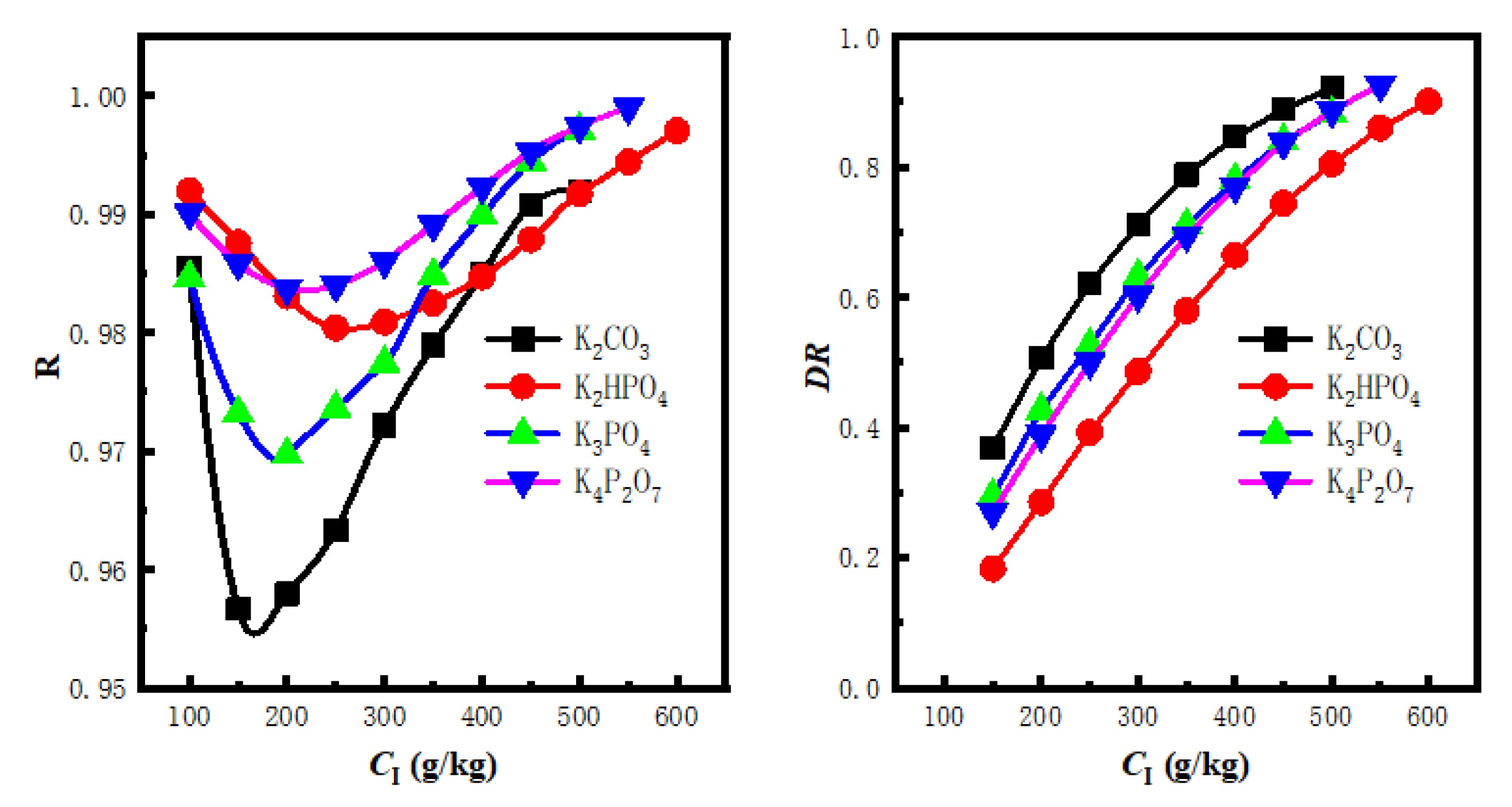
The effect of high-solubility inorganic electrolytes on the salting-out of ethanol-water systems was explored, as shown in Figure 4[111]. The experimental results show that the separation of ethanol from water can be effectively achieved with a carefully selected salting-out agent with little contamination of the organic phase (ethanol), while maintaining a high water content in the aqueous phase. In particular, it reveals significant differences in the dewatering efficiency of different dewatering agents in aqueous ethanol solutions, with K4P2O7 showing the best performance, followed by K3PO4, K2HPO4, and K2CO3, and this ranking provides a valuable reference for the optimization of dewatering processes in the industry. This finding not only enhances our understanding of the salting-out mechanism but also opens up new avenues for designing a more efficient and economical ethanol purification process in the future.
|
Figure 4. Recovery (left) and Dehydration (right) of Bioethanol. Reproduced from Ref.[111] with permission from Elsevier.
3.2.3 Ionic-liquid Extraction of Bioethanol
IL applications in bioethanol mainly include extractive distillation, liquid-liquid extraction, and support of liquid membranes[112]. ILs exhibit salt properties and reduce the formation of water-ethanol azeotropes. With regard to IL separation techniques, there is a rich literature on their wide application in the separation of butanol from water[113]. In contrast, the literature on the separation of ethanol from water is sparse. For ethanol-water azeotrope separation, the most promising IL anions are [Cl]- and [OAc]-, while bis-alkyl phosphate-based ILs have shown good results at low ethanol concentrations[52].
|
Figure 5. Ionic-liquid Extraction of Bioethanol. Reproduced from Ref.[114] with permission from the American Chemical Society.
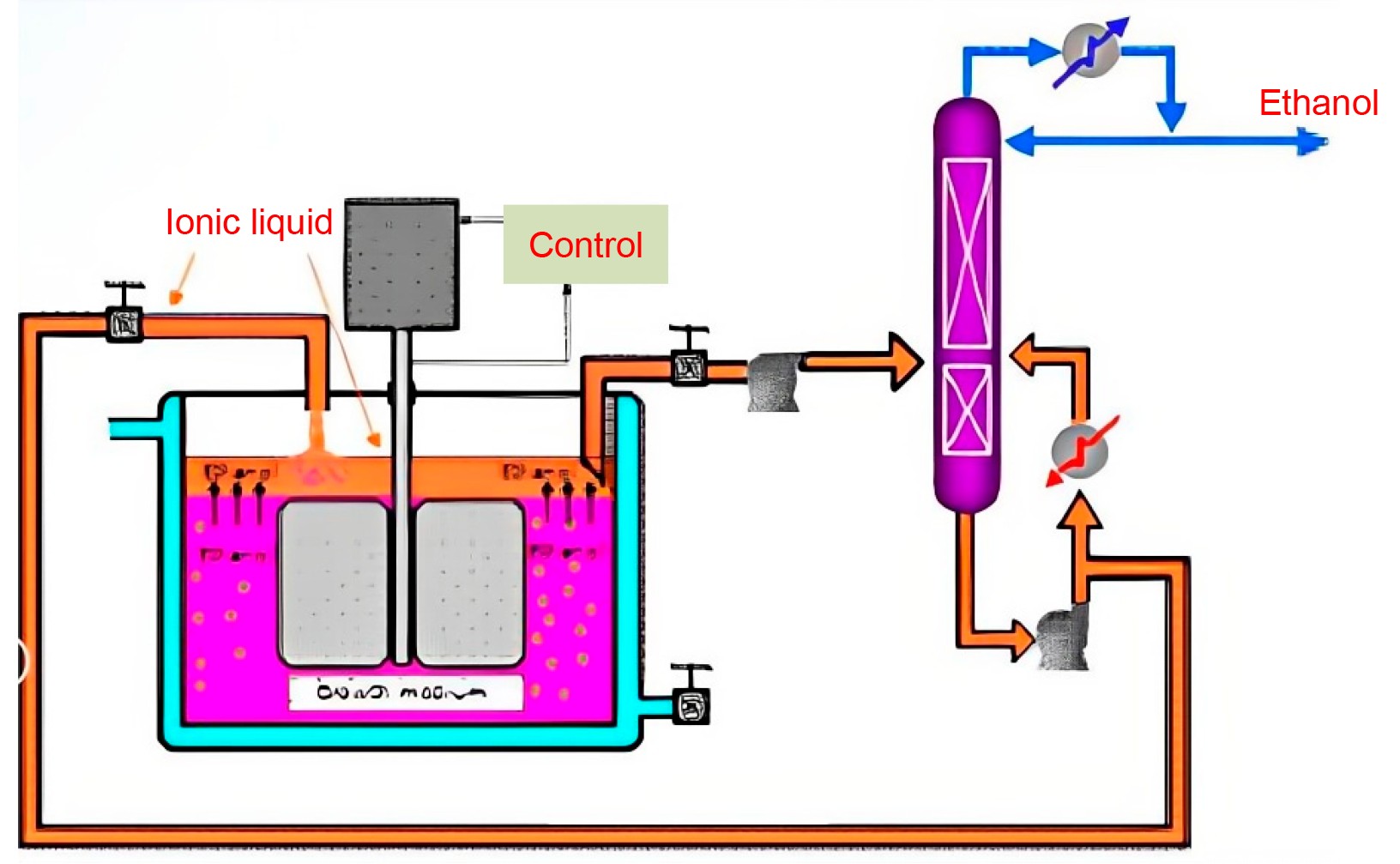
An integrated fermentation and separation process using ILs was developed[114], as shown in Figure 5. This study innovatively explored the application of ILs in the extraction-fermentation process for bioethanol production, which significantly enhanced the ethanol extraction efficiency up to 62%, demonstrating the great potential of ILs as efficient extractants. In particular, the applicability of two ILs, [MTOA+][NTf2-] and [Hex3TDP+][Cl-], provides a new strategy for ethanol production. This study not only verified the feasibility of the integration of IL extraction and fermentation but also predicted that this technology may open up a new era of microbial synthesis of polar compounds and bring revolutionary changes in the field of biofuels, which is highly prospective and promising for application.
3.3 Application of ATPSs in Biobutanol
There are many techniques for the separation and purification of biobutanol, including adsorption, gas stripping, liquid-liquid extraction, osmotic separation, reverse osmosis, and osmotic evaporation[115-117]. Among them, LLE separation can efficiently separate and purify butanol. However, liquid-liquid extraction suffers from solvent loss and toxicity to microorganisms and has some limitations in continuous fermentation production.
3.3.1 Traditional Solvent Extraction of Biobutanol
The main product of biobutanol fermentation is ABE solution or isobutanol solution, which is a multi-component complex fermentation broth. The selection of solvents for extraction usually needs to consider the subsequent separation of the extractant. Current studies on extractants include oleyl alcohol, polypropylene glycol, and glyceryl butyrate[118,119]. Current studies have also combined solvent extraction with gas stripping techniques to achieve higher separation efficiency. The stripping gas passes through the fermenter and the butanol vapors are left behind by adsorption through selective adsorbents[120]. However, traditional solvent extraction requires further butanol re-separation, which can make the biobutanol purification step more cumbersome.
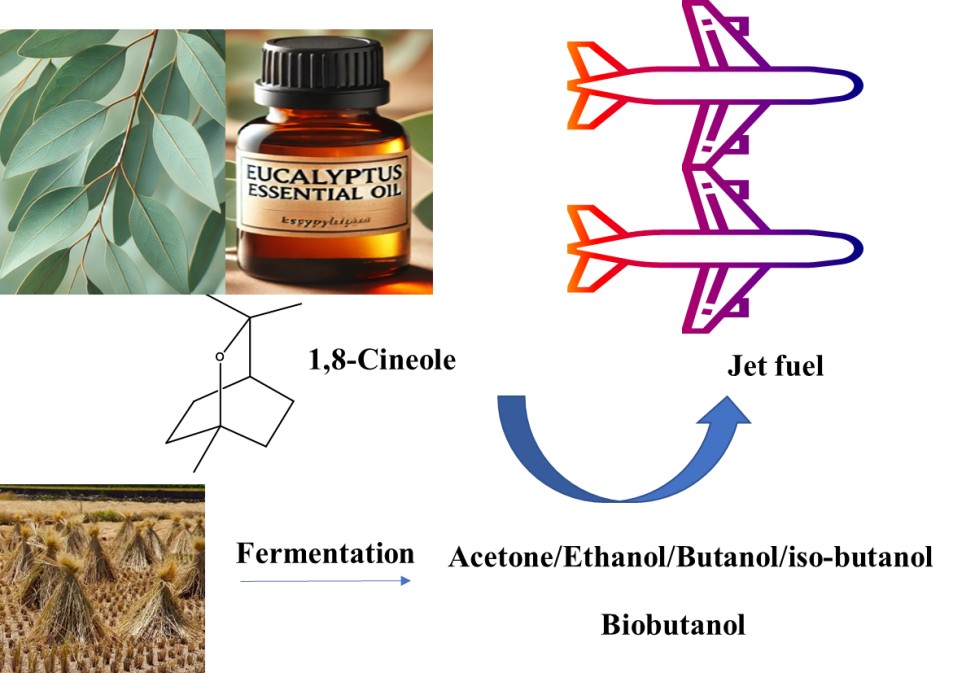
The extraction capacity and dehydration efficiency of 1,8-cineol for biobutanol in different solutions were investigated (Figure 6)[121]. Recoveries of 92% and 95% were achieved for n-butanol and isobutanol, respectively, while water removal exceeded 99% in a 2% butanol system. These results indicate that 1,8-cineol has a high selectivity for butanol, thus providing a theoretical basis for fermentation-based biofuel recovery and azeotropic system elimination. The introduction of high-density eucalyptus oil, similar to kerosene, into the low-energy-density biobutanol, will increase the energy density of the biofuel obtained from fermentation, yielding both linear and cyclic biofuel blends. Utilization of blended biofuel products has great potential to reduce human dependence on fossil fuels, and extraction of biofuels will yield significant economic benefits. Replacing gasoline/kerosene/diesel with blended fuels has significant advantages in reducing carbon emissions.
|
Figure 6. Eucalyptus Oil with Biobutanol for the Production of Linear and Cyclic Biofuel Mixtures.
3.3.2 Salting-out of Biobutanol
Salting-out in butanol separation is a commonly used separation technique. The separation of butanol is achieved by adding an appropriate amount of salt to the butanol solution, allowing the butanol solution to form a two-phase system. Adding NaCl to the butanol+water system makes a two-phase solution, but limited salting-out ability of NaCl cannot remove the water in the system efficiently[122]. (NH4)2SO4 is a commonly used salting-out agent, which can quickly separate the butanol from the aqueous solution[123]. However, recovery and dehydration of butanol with (NH4)2SO4 was still unsatisfactory.
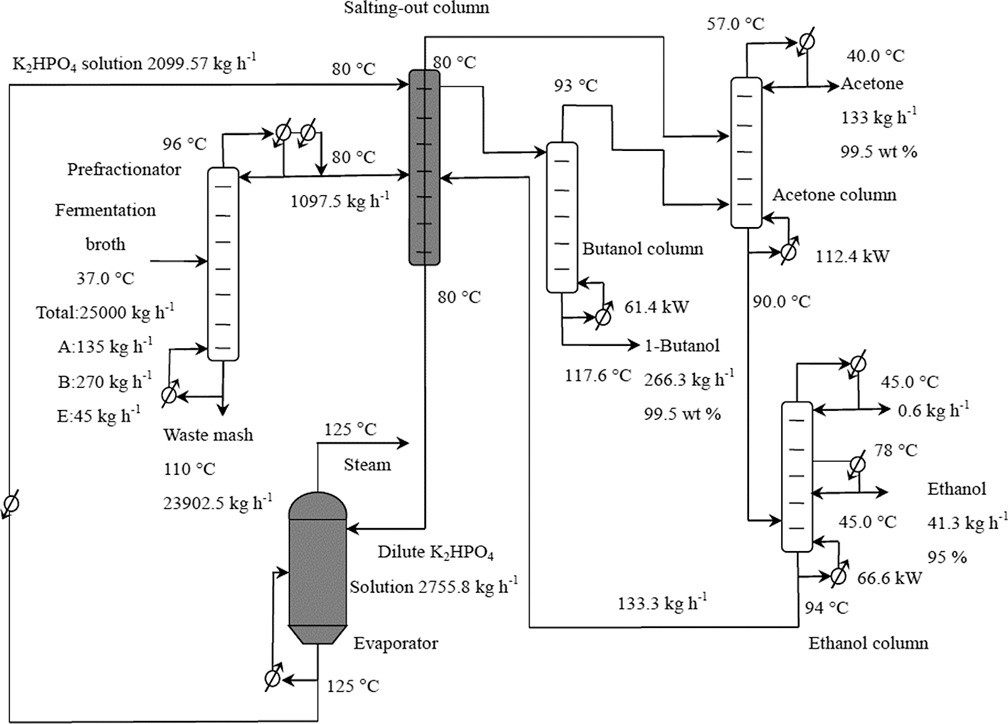
In the past 10 years, we have developed four excellent salting-out agents, namely K4P2O7, K3PO4, K2HPO4, and K2CO3. We have developed energy-efficient separation technologies for bio-based fuels and chemicals with these salts. We use salting-out technology to remove water from ABE fermentation broth[93] or fermentation broth concentrates[23,24,124,125] to reduce the amount of azeotropes or water in subsequent separations. Figure 7 shows the separation of ABE by the "distillation plus salting-out plus distillation" process, which can save more than 35% more energy than the conventional distillation-only process[126]. This separation strategy has also been applied to acetone[127], 1,3-propanediol[94], 2,3-butanediol[95], other fermentation products, and the separation of chemically catalyzed biomass-based products[128,129].
|
Figure 7. Separation of ABE by "Distillation plus Salting-ou plus Distillation" Process. Reproduced from Ref.[126] with permission from Wiley‐VCH.
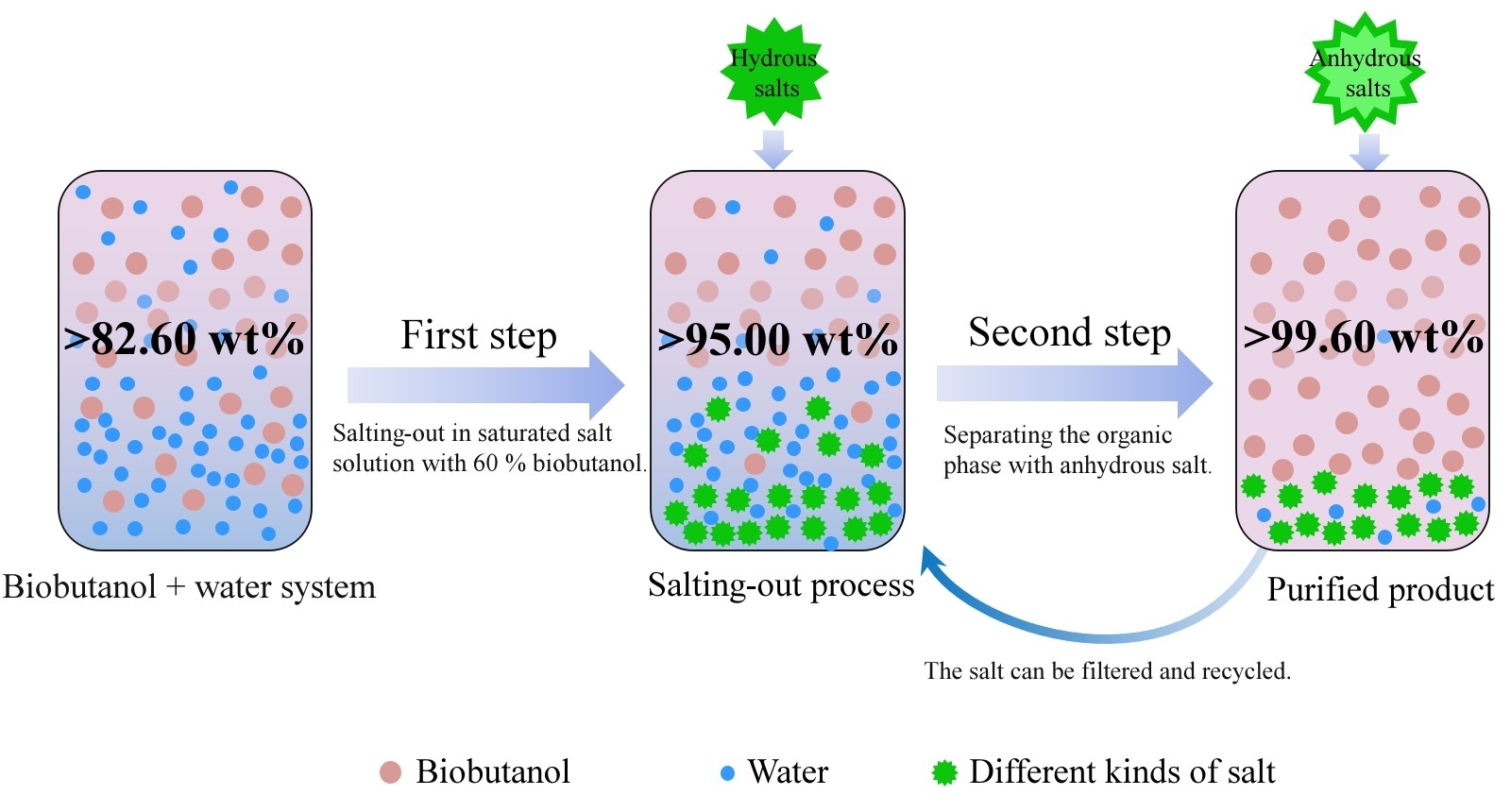
The purification of biobutanol is currently a hot area of research. Complete recovery of bioalcohols can be achieved at high salt concentrations (Figure 8)[130]. A novel separation and purification technology for biofuels was further developed, namely a two-step salting-out process, which can replace the traditional distillation processes to obtain high-purity butanol (>99.5%).
|
Figure 8. Isolation and Purification of Biobutanol with Salting-out Effect. Reproduced from Ref.[130] with permission from Elsevier.
The use of salt solutions or pure neutral, acidic, or basic salts as extractants significantly reduces the water content in butanol. Due to the low concentration of butanol during the salting-out process, the high energy consumption of 21.9MJ/kg-butanol[126] or 28.5MJ/kg-butanol[124] is required to evaporate the water from the salt solution and to remove the remaining water from the recovered organic solvent. In the first case, The flow rate of the ABE fermentation broth for separation and purification was 25,000kg∙h-1, producing 266.3kg butanol per hour. To reduce energy consumption and increase the solvent concentration, a novel two-stage gas stripping-salting-out system was developed to effectively separate ABE from fermentation broth using sweet sorghum straw as raw material[131]. The results showed that the concentration of ABE separated in the first stage after gas stripping was 143.6g/L, which was recovered and introduced into the second stage of the salting-out process (Figure 9). K4P2O7 and K2HPO4 were used for salting-out, respectively. The energy consumption of the downstream distillation process was 3.72MJ/kg ABE. The high concentration of cellulosic ABE was effectively separated from the fermentation broth by the novel two-stage gas stripping-salt precipitation process. Thus, the novel ATPSs can be integrated with another separation process to effectively and significantly reduce the energy consumption of the biofuel downstream process.
|
Figure 9. Integration of in situ Gas Stripping and Salting-out Process. Reproduced from Ref.[131] with permission from Springer Nature.
3.3.3 Sugaring-out of Biobutanol
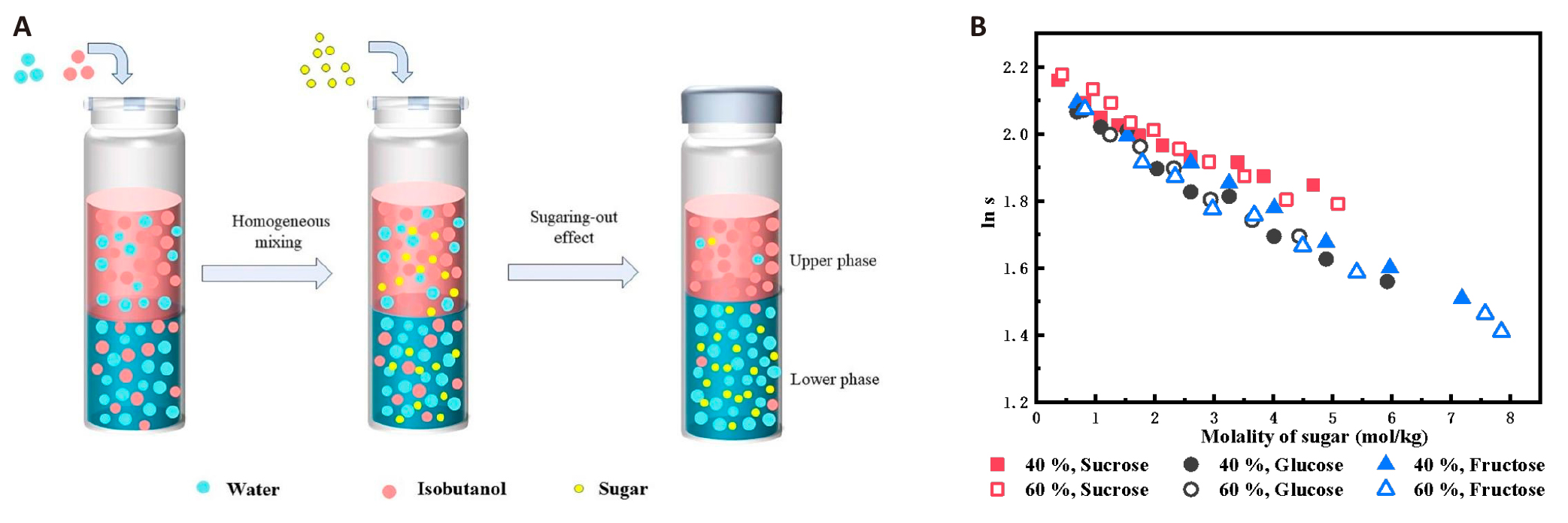
The sugaring-out system for butanol is generally created by glucose, fructose, sucrose, and other saccharides, and the recovery of butanol can reach more than 97%[33]. The strength of the sugaring-out effect was determined by quantifying the relationship between isobutanol content and salt/sugar concentration in the order glucose ≈ fructose > sucrose, as shown in Figure 10. Quantification of the separation effect was achieved during the sugaring-out of biobutanol.
|
Figure 10. Sugaring-out Process (A) and Quantification of the Separation Effect (B) during Sugaring-out of Biobutanol. Reproduced from Ref.[33] with permission from Elsevier.
Compared with traditional distillation, sugaring out of biobutanol has the advantages of lower energy consumption, simple and fast operation. Sugaring-out technology is also used to remove water from the fermentation concentrate, reducing the amount of n-butanol + water azeotrope and ethanol + water azeotrope[132]. The raffinate liquid is mainly a sugar solution, which can be used in the next step of ABE fermentation, reducing the process of raffinate liquid recycling, and saving energy significantly.
A novel separation and purification technology for biofuels was further developed, namely salting-out plus salting-out[33], which can replace traditional distillation to obtain high-purity biobutanol (>99.5%).
3.3.4 IL-based Extraction of Biobutanol
IL-based biphasic extraction is a new type of ATPS, which has been used in the extraction of antibiotics, amino acids, traditional Chinese medicine, and other fields because its simple and green composition can maintain the biological activity of proteins and so on. The partitioning of the substance to be extracted in ATPS mainly depends on ionic and hydrophobic interactions. Since ILs are charged in solution, the charged target product is separated into one of the phases due to ionic interactions and the entropy of the system changes during the process, which is typical of hydrophobic interactions[133].
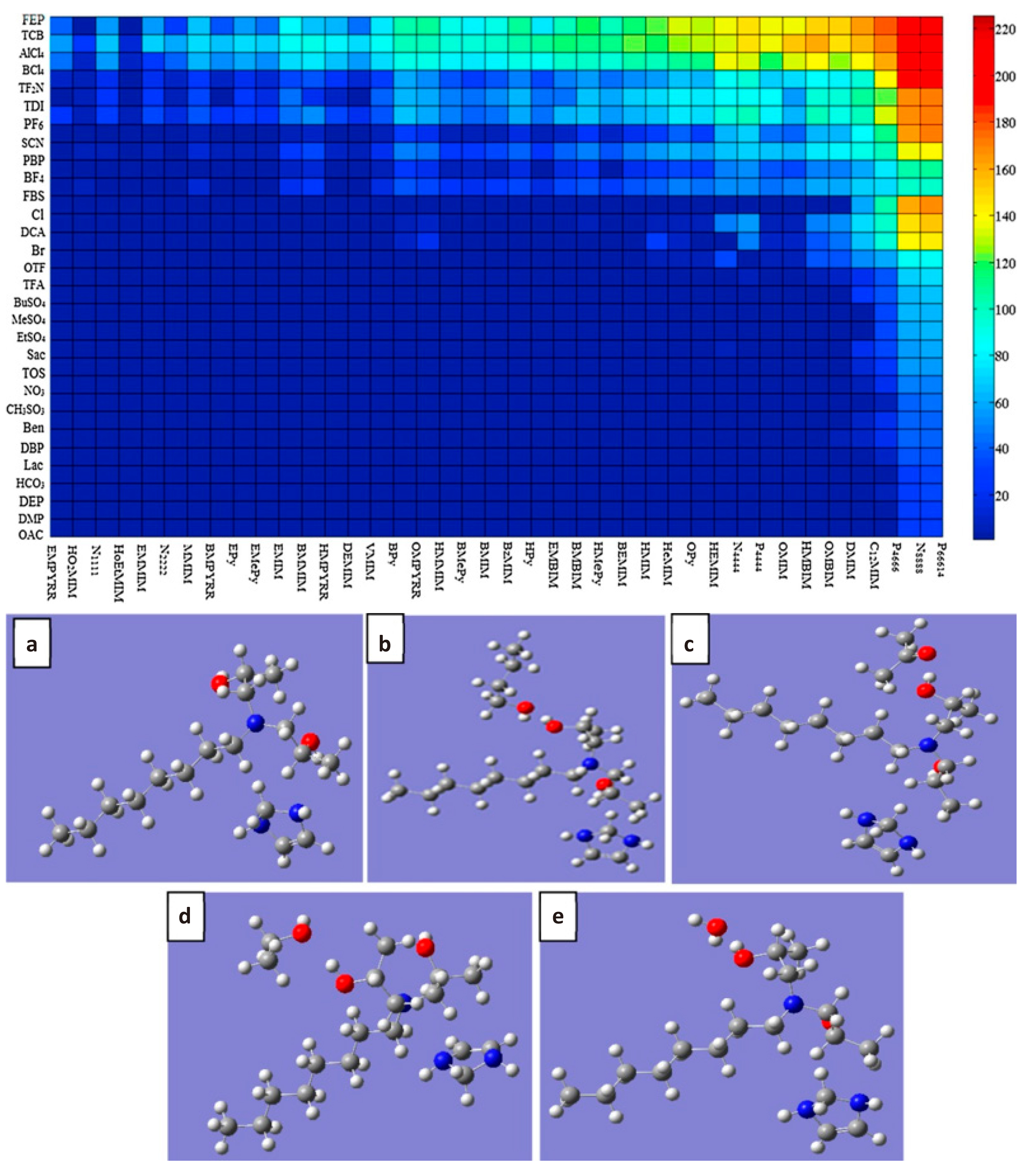
The principle of IL extraction of biobutanol is mainly to utilize the highly selective IL for biobutanol, and separate biobutanol from the fermentation broth. Then the biobutanol can be desorbed from the IL by means of depressurization or heating, to realize the separation and purification of the biobutanol. [C8DIPA][Im], [DPAIm][NTf2], [HeDBU][NTf2], [HMIM][FEP], etc. can be used to extract butanol from water[134,135]. The factors affecting the separation effect of ILs were investigated. It is pointed out that the partition coefficient and selectivity of imidazolium-based ILs increase with the increase of side-chain length and hydrophobic functional groups, and the same rule applies to other types of ILs (Figure 11). In addition, by comparing the separation effects of different types of ILs, it is found that quaternary ammonium-type and phosphonium-type ILs perform better when the number of carbons in the side-chain is larger, and especially phosphonium-type ILs have the advantages of density and viscosity, which make them potentially superior choices. However, each type of IL faces challenges in synthetic regulation and lack of stability, emphasizing the need to consider their separation efficacy and inherent defects in practical applications.
|
Figure 11. Partition coefficient of 1-butanol with different ILs (top) and geometries optimizations (bottom). Reproduced with permission from the American Chemical Society[134] and Elsevier[135].
ILs usually have high solubility and their selectivity for target products can be improved by adjusting different compositions[136]. However, ILs are costly and have complex separation mechanisms. Finding an IL with a good effect on substrate separation is challenging. ILs can cause corrosion of equipment in industrial applications, which is a major problem to be faced in practical production[137].
4 CONCLUSION AND OUTLOOK
The emerging micromolecule-based ATPSs have been recognized as an ideal alternative to conventional separation techniques in bioseparation technology due to their mild biocompatibility, high water content, low interfacial tension, and ease of process integration and scale-up. The emerging micromolecule-based ATPSs, including but not limited to those mentioned above, have been widely used for the extraction of biobutanol, acetone, organic acids, antibiotics, and other biomolecules. In particular, salt/alcohol systems, sugar/alcohol, and ILs-based systems have been widely investigated for biofuel separation due to their high-quality separation efficiency and yields, as well as their simplicity and cost-effectiveness. Adding a salt, sugar, or IL to a biofuel solution can remove most of the water, enabling efficient recovery and dehydration of biofuel. The two-step process can replace the traditional distillation and molecular sieve adsorption processes to obtain high-purity bioalcohols, thus introducing a new purification process for biofuel. An in-depth analysis of the separation performance of the emerging micromolecule-based ATPSs as well as an objective indication of their application provides valuable insights for further optimization and application of this kind of ATPSs in separation science.
However, ATPSs still have many challenges such as the difficulty of phase composition recovery, the more cumbersome process of ATPSs, and the unclear distribution model and mechanism of the target products[25]. All these drawbacks have hindered the development and promotion of ATPSs in the industry. However, ATPSs will certainly become reliable unit operations for the recovery and purification of biofuels, and it is expected that ATPSs will be implemented on a large scale shortly as a result of more robust systems developed on different scales.
One disadvantage of two-step salting-out experiments is the use of large amounts of salt. In general, a single separation unit cannot fulfill the energy-saving goal of the whole separation and purification process. If there are new energy-saving technologies, such as membrane distillation and heat recovery, they will greatly solve the energy consumption problem in the salt recovery process and reduce the cost of salt recovery. Recovery of high-value salt or enhanced research on low-cost and high-performance membranes may greatly improve process performance and cost-effectiveness. Therefore, for the dehydration of salt solution, it is necessary to develop new salt recovery methods.
Acknowledgments
This work was funded by the Start-up Fund of Guangdong University of Technology (No. 263113465), and the Guangdong Basic and Applied Basic Research Foundation, China (No. 2021A1515110329).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
No additional data are available.
Copyright Permissions
Copyright © 2024 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Author Contribution
Xie S was responsible for the conceptualization, data curation, formal analysis, writing - original draft preparation, funding acquisition, investigation, project administration, resources, supervision, validation, writing - review & editing. Tan J and Sun Z were responsible for data curation, methodology, software. Zhang W, Cai X, and Fang Y were responsible for the resources.
Abbreviation List
ABE, Acetone-butanol-ethanol
ATPS, Aqueous two-phase system
ILs, Ionic liquids
LLE, Liquid-liquid phase equilibrium
PEG, Polyethylene glycol
References
[1] Short W. Making energy value for money. Nature, 1974; 249: 715-717.[DOI]
[2] Lei Z, Li C, Chen B. Extractive Distillation: A Review. Sep Purif Rev, 2003; 32: 121-213. [DOI]
[3] Bolto B, Hoang M, Xie Z. A review of water recovery by vapour permeation through membranes. Water Res, 2012; 46: 259-266. [DOI]
[4] Da̧browski A. Adsorption - From theory to practice. Adv Colloid Interface Sci, 2001; 93: 135-224. [DOI]
[5] Raynie DE. Modern extraction techniques. Anal Chem, 2010; 82: 4911-4916. [DOI]
[6] Baumeister C, Kilian L. Forty Years of Oil Price Fluctuations: Why the Price of Oil May Still Surprise Us. J Econ Perspect, 2016; 30: 139-160.[DOI]
[7] Jang YS, Kim B, Shin JH et al. Bio-based production of C2-C6 platform chemicals. Biotechnol Bioeng, 2012; 109: 2437-2459.[DOI]
[8] Fu C, Li Z, Sun Z, Xie S. A review of salting-out effect and sugaring-out effect: Driving forces for novel liquid-liquid extraction of biofuels and biochemicals. Front Chem Sci Eng, 2020; 15: 854-871.[DOI]
[9] Xie S, Li Z, Zhu G, Yi C. One-pot reaction-separation process to produce jet fuel. Energy Convers Manag X, 2022; 13: 100155.[DOI]
[10] Taherzadeh MJ, Karimi K. Enzyme-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources, 2007.[DOI]
[11] Cheng KK, Zhao XB, Zeng J et al. Biotechnological production of succinic acid: Current state and perspectives. Biofuel Bioprod Bior, 2012; 6: 302-318.[DOI]
[12] Li Y, Tang W, Chen Y et al. Potential of acetone-butanol-ethanol (ABE) as a biofuel. Fuel, 2019; 242: 673-686.[DOI]
[13] Stockhardt JS, Hull CM. Vapor-Liquid Equilibria and Boiling-Point Composition Relations for Systems n-Butanol-Water and Isobutanol-Water1,2. Ind Eng Chem, 1931; 23: 1438-1440.[DOI]
[14] Higashide W, Li Y, Yang Y et al. Metabolic engineering of Clostridium cellulolyticum for production of isobutanol from cellulose. Appl Environ Microbiol, 2011; 77: 2727-2733.[DOI]
[15] Xie S, Li Z, Zhu G, et al. Cleaner production and downstream processing of bio-based 2,3-butanediol: A review. J Clean Prod, 2022; 343: 131033.[DOI]
[16] Bankar SB, Survase SA, Ojamo H et al. Biobutanol: The outlook of an academic and industrialist. RSC Adv, 2013; 3: 24734-24757.[DOI]
[17] Kumar S, Cho JH, Park J et al. Advances in diesel-alcohol blends and their effects on the performance and emissions of diesel engines. Renew Sustain Energy Rev, 2013; 22: 46-72.[DOI]
[18] Fenkl M, Pechout M, Vojtisek M. N-butanol and isobutanol as alternatives to gasoline: Comparison of port fuel injector characteristics. EPJ Web Conf, 2016; 114: 02021.[DOI]
[19] Li H, Cann AF, Liao JC. Biofuels: Biomolecular Engineering Fundamentals and Advances. Annu Rev Chem Biomol Eng, 2010; 1: 19-36.[DOI]
[20] Albertsson PÅ. Fractionation of particles and macromolecules in aqueous two-phase systems. Biochem Pharmacol, 1961; 5: 351-358.[DOI]
[21] Tang Z, Zhou R, Duan Z. Separation of isopropanol from aqueous solution by salting-out extraction. J Chem Technol Biotechnol, 2001; 76: 757-763.[DOI]
[22] Fu C, Li Z, Jia C et al. Recent advances on bio-based isobutanol separation. Energy Convers Manag X, 2021; 10: 100059.[DOI]
[23] Yi C, Xie S, Qiu X. Salting-out effect of dipotassium hydrogen phosphate on the recovery of acetone, butanol, and ethanol from a prefractionator. J Chem Eng Data, 2014; 59: 1507-1514.[DOI]
[24] Xie S, Yi C, Qiu X. Salting-out effect of potassium pyrophosphate (K4P2O7) on the separation of biobutanol from an aqueous solution. J Chem Technol Biotechnol, 2016; 91: 1860-1867.[DOI]
[25] Torres-Acosta MA, Mayolo-Deloisa K, González-Valdez J et al. Aqueous Two-Phase Systems at Large Scale: Challenges and Opportunities. Biotechnol J, 2019; 14: 1-12.[DOI]
[26] Hatti-Kaul R. Aqueous two-phase systems: A general overview. Mol Biotechnol, 2001; 19: 269-277.[DOI]
[27] Grilo AL, Aires-Barros MR, Azevedo AM. Partitioning in Aqueous Two-Phase Systems: Fundamentals, Applications and Trends. Sep Purif Rev, 2016; 45: 68-80.[DOI]
[28] Iqbal M, Tao Y, Xie S et al. Aqueous two-phase system (ATPS): an overview and advances in its applications. Biol Proced Online, 2016; 18: 1-18.[DOI]
[29] Chao Y, Shum HC. Emerging aqueous two-phase systems: from fundamentals of interfaces to biomedical applications. Chem Soc Rev, 2020; 49: 114-142.[DOI]
[30] Dhamole PB, Joshi N, Bhat V. A review of recent developments in sugars and polyol based soluting out separation processes. Sep Purif Technol, 2023; 312: 123394.[DOI]
[31] Ma B, Tan J, Liu S et al. Enhancing biofuel dewatering efficiency by ionizing food-additives-based salting-out agents. Sep Purif Technol, 2025; 354: 128906.[DOI]
[32] Zhou G, Tan J, Zhu M et al. Understanding the dewatering of fermentation-based 1,3-propanediol with acetone before cyclohexanone synthesis. J Ind Eng Chem, 2024; 138: 567-574.[DOI]
[33] Sun Z, Tan J, Zhou G et al. Sugaring-out plus salting-out: A novel separation and purification technique for biofuel. Fuel 2024; 357: 129787.[DOI]
[34] Xie S, Li Z, Zhang W. Techno-Economic Analysis of Upgrading Corn Stover-Based Acetone, n-Butanol, and Ethanol to Higher Ketones and Alcohols: Fuels or Fine Chemicals? ACS Sustain Chem Eng, 2023; 11: 3474-3485.[DOI]
[35] Fu C, Song W, Yi C et al. Creating efficient novel aqueous two-phase systems: Salting-out effect and high solubility of salt. Fluid Phase Equilib, 2019; 490:77-85.[DOI]
[36] Fu C, Xie S. Salts and 1-propanol induced aqueous two-phase systems: phase separation and application. J Chem Technol Biotechnol, 2019; 94: 2372-2381.[DOI]
[37] Xie S, Ji W, Zhang Y et al. Biobutanol recovery from model solutions/fermentation broth using tripotassium phosphate. Biochem Eng J, 2016;115:85-92.[DOI]
[38] Xie S, Fu C, Song W et al. Highly efficient synthesis and separation of fuel precursors from the concentrated ABE fermentation broth in a biphasic catalytic process. Fuel, 2019; 242: 41-49.[DOI]
[39] Dai JY, Sun YQ, Xiu ZL. Separation of bio-based chemicals from fermentation broths by salting-out extraction. Eng Life Sci, 2014; 14: 108-117.[DOI]
[40] Birajdar SD, Rajagopalan S, Sawant JS et al. Continuous countercurrent liquid-liquid extraction method for the separation of 2,3-butanediol from fermentation broth using n-butanol and phosphate salt. Process Biochem, 2015; 50: 1449-1458.[DOI]
[41] Gu Y, Shih PH. Salt-induced phase separation can effectively remove the acetonitrile from the protein sample after the preparative RP-HPLC. Enzyme Microb Technol, 2004; 35: 592-597.[DOI]
[42] Ran L, Yang C, Xu M et al. Enhanced aqueous two-phase extraction of proanthocyanidins from grape seeds by using ionic liquids as adjuvants. Sep Purif Technol, 2019; 226: 154-161.[DOI]
[43] Miyawaki O, Tatsuno M. Thermodynamic analysis of alcohol effect on thermal stability of proteins. J Biosci Bioeng, 2011; 111: 198-203.[DOI]
[44] Zhang Q, Sang Z, Li Q et al. Palladium(II) extraction from acidic chloride media using an ionic liquid-based aqueous two-phase system (IL-ATPS) in the presence of dipotassium hydrogen phosphate salting-out agent and reductive stripping with hydrazine hydrate to recover palladium meta. Hydrometallurgy, 2023; 216: 106017.[DOI]
[45] Dai JY, Ma LH, Wang ZF et al. Sugaring-out extraction of acetoin from fermentation broth by coupling with fermentation. Bioprocess Biosyst Eng, 2017; 40: 423-429.[DOI]
[46] Wang B, Ezejias T, Feng H et al. Sugaring-out: A novel phase separation and extraction system. Chem Eng Sci, 2008; 63: 2595-600.[DOI]
[47] Chia SR, Chew KW, Show PL et al. Isolation of protein from Chlorella sorokiniana CY1 using liquid biphasic flotation assisted with sonication through sugaring-out effect. J Oceanol Limnol, 2019; 37: 898-908.[DOI]
[48] Sankaran R, Manickam S, Yap YJ et al. Extraction of proteins from microalgae using integrated method of sugaring-out assisted liquid biphasic flotation (LBF) and ultrasound. Ultrason Sonochem, 2018; 48: 231-239.[DOI]
[49] Feng WQ, Lu YH, Chen Y et al. Thermal stability of imidazolium-based ionic liquids investigated by TG and FTIR techniques. J Therm Anal Calorim, 2016; 125: 143-154.[DOI]
[50] Tanimura K, Suga K, Okamoto Y et al. Enzymatic hydrolysis of cellulose recovered from ionic liquid-salt aqueous two-phase system. J Biosci Bioeng, 2020; 129: 624-631.[DOI]
[51] Han J, Wang Y, Yu C et al. Separation, concentration and determination of chloramphenicol in environment and food using an ionic liquid/salt aqueous two-phase flotation system coupled with high-performance liquid chromatography. Anal Chim Acta, 2011; 685: 138-145.[DOI]
[52] Pereiro AB, Araújo JMM, Esperança JMSS et al. Ionic liquids in separations of azeotropic systems - A review. J Chem Thermodyn, 2012; 46: 2-28.[DOI]
[53] Freire MG, Cláudio AFM, Araújo JMM et al. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem Soc Rev, 2012; 41: 4966-4995.[DOI]
[54] Li H, Bhadury PS, Song B et al. Immobilized functional ionic liquids: Efficient, green, and reusable catalysts. RSC Adv, 2012; 2: 12525-12551.[DOI]
[55] Yao T, Li Q, Li H et al. Extractive resolution of racemic phenylalanine and preparation of optically pure product by chiral magnetic ionic liquid aqueous two-phase system. Sep Purif Technol, 2021; 274: 119024.[DOI]
[56] Desai ML, Eisen EO. Salt Effects in Liquid-Liquid Equilibria. J Chem Eng Data, 1971; 16: 200-202.[DOI]
[57] Grover PK, Ryall RL. Critical appraisal of salting-out and its implications for chemical and biological sciences. Chem Rev, 2005; 105:1-110.[DOI]
[58] Ebrahimi N, Farahbod B, Sadeghi R. Salting-in and salting-out effects of organic and inorganic ammonium salts on the aqueous polymer solutions. J Chem Thermodyn, 2018; 123: 86-98.[DOI]
[59] Bowron DT, Finney JL. Structure of a salt–amphiphile–water solution and the mechanism of salting out. J Chem Phys, 2003; 118: 8357.[DOI]
[60] Sadeghi R, Coutinho JAP. Sugaring‐out assisted organic-aqueous biphasic systems: Characteristics, mechanisms and applications. Sep Purif Technol, 2024; 350: 127919.[DOI]
[61] Wang B, Feng H, Ezeji T et al. Sugaring-out separation of acetonitrile from its aqueous solution. Chem Eng Technol, 2008; 31: 1869-1874.[DOI]
[62] Saielli G, Bagno A. Preferential solvation of glucose and talose in water – acetonitrile mixtures: a molecular dynamics simulation study. Phys Chem Chem Phys, 2010; 12: 2981-2988.[DOI]
[63] Hou J, Lin S, Zhang M. Ionic-liquid-enhanced solvent extraction mechanism: A novel concept. J Environ Chem Eng, 2022; 10: 107899.[DOI]
[64] Papaiconomou N, Vite G, Goujon N et al. Efficient removal of gold complexes from water by precipitation or liquid–liquid extraction using ionic liquids. Green Chem, 2012; 14: 2050-2056.[DOI]
[65] Nakashima K, Kubota F, Maruyama T et al. Feasibility of Ionic Liquids as Alternative Separation Media for Industrial Solvent Extraction Processes. Ind Eng Chem Res, 2005; 44: 4368-4372.[DOI]
[66] Demirbas A. Progress and recent trends in biofuels. Prog Energy Combust Sci, 2007; 33: 1-18.[DOI]
[67] Tse TJ, Wiens DJ, Chicilo F et al. Value-added products from ethanol fermentation—A review. Fermentation, 2021; 7: 267.[DOI]
[68] Arshad M, Hussain T, Iqbal M et al. Enhanced ethanol production at commercial scale from molasses using high gravity technology by mutant S. cerevisiae. Brazilian J Microbiol, 2017; 48: 403-409.[DOI]
[69] Barcelos CA, Maeda RN, Santa Anna LMM et al. Sweet sorghum as a whole-crop feedstock for ethanol production. Biomass Bioenerg, 2016; 94: 46-56.[DOI]
[70] Cruz ML, de Resende MM, Ribeiro EJ. Improvement of ethanol production in fed-batch fermentation using a mixture of sugarcane juice and molasse under very high-gravity conditions. Bioprocess Biosyst Eng, 2021; 44: 617-625.[DOI]
[71] Jain S, Kumar S. A comprehensive review of bioethanol production from diverse feedstocks: Current advancements and economic perspectives. Energy, 2024; 296: 131130.[DOI]
[72] Choudhary P, Assemany PP, Naaz F et al. A review of biochemical and thermochemical energy conversion routes of wastewater grown algal biomass. Sci Total Environ, 2020; 726: 137961.[DOI]
[73] Wang L, Wang L, Zhang J et al. Selective Hydrogenation of CO2 to Ethanol over Cobalt Catalysts. Angew Chemie Int Ed, 2018; 57: 6104-6108.[DOI]
[74] Haas T, Krause R, Weber R et al. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat Catal, 2018; 1: 32-39.[DOI]
[75] Tang Y, Huang Y, Gan W et al. Ethanol production from gas fermentation: Rapid enrichment and domestication of bacterial community with continuous CO/CO2 gas. Renew Energy, 2021; 175: 337-344.[DOI]
[76] Devi A, Singh A, Bajar S et al. Ethanol from lignocellulosic biomass: An in-depth analysis of pre-treatment methods, fermentation approaches and detoxification processes. J Environ Chem Eng, 2021; 9: 105798.[DOI]
[77] Schiel-Bengelsdorf B, Montoya J, Linder S et al. Butanol fermentation. Environ Technol, 2013; 34: 1691-1710.[DOI]
[78] Fu J, Lu X, Savage PE. Hydrothermal decarboxylation and hydrogenation of fatty acids over Pt/C. ChemSusChem, 2011; 4: 481-486.[DOI]
[79] Zheng J, Tashiro Y, Wang Q et al. Recent advances to improve fermentative butanol production: Genetic engineering and fermentation technology. J Biosci Bioeng, 2015; 119: 1-9.[DOI]
[80] Ezeji TC, Qureshi N, Blaschek HP. Butanol fermentation research: Upstream and downstream manipulations. Chem Rec, 2004; 4: 305-314.[DOI]
[81] Chen T. Production Technology of Acetone and Butanol Fermentation. Beijing: Chemical Industry Press; 1991.
[82] Jiang W, Zhao J, Wang Z, Yang ST. Stable high-titer n-butanol production from sucrose and sugarcane juice by Clostridium acetobutylicum JB200 in repeated batch fermentations. Bioresour Technol, 2014; 163: 172-179.[DOI]
[83] Peter SC. Reduction of CO2 to Chemicals and Fuels: A Solution to Global Warming and Energy Crisis. ACS Energy Lett, 2018; 3: 1557-1561.[DOI]
[84] Service RF. CAN BIOFUELS REALLY FLY? Science, 2022; 376: 1394-1397. [DOI]
[85] Andre A, Nagy T, Toth AJ et al. Distillation contra pervaporation: Comprehensive investigation of isobutanol-water separation. J Clean Prod, 2018; 187: 804-818.[DOI]
[86] Hassankhan B, Raisi A. Separation of isobutanol/water mixtures by hybrid distillation-pervaporation process: Modeling, simulation and economic comparison. Chem Eng Process Process Intensif, 2020; 155: 108071.[DOI]
[87] Downarowicz D, Aleksandrzak T. Isobutanol Vapor Adsorption on Activated Carbons: Equilibrium and Kinetic Studies. J Chem Eng Data, 2017; 62: 3518-3524.[DOI]
[88] Yi C, Zhang Y, Xie S et al. Salting-out extraction of bio-based isobutanol from an aqueous solution. J Chem Technol Biotechnol 2018; 93: 372-384.[DOI]
[89] Li Q, Hu N, Zhang S et al. Energy-saving heat integrated extraction-azeotropic distillation for separating isobutanol-ethanol-water. Sep Purif Technol, 2021; 255: 117695.[DOI]
[90] Fu C, Li Z, Song W et al. A new process for separating biofuel based on the salt+1-butanol+water system. Fuel, 2020; 278: 118402.[DOI]
[91] Mariano AP, Keshtkar MJ, Atala DIP et al. Energy requirements for butanol recovery using the flash fermentation technology. Energ Fuel, 2011; 25: 2347-2355.[DOI]
[92] Xie S, Zhang Y, Yi C et al. Biobutanol recovery from model solutions using potassium pyrophosphate. J Chem Technol Biotechnol, 2017; 92: 1229-1235.[DOI]
[93] Xie S, Yi C, Qiu X. Salting-out of acetone, 1-butanol, and ethanol from dilute aqueous solutions. AIChE J, 2015; 61: 3470-3478.[DOI]
[94] Xie S, Qiu X, Ji W et al. Salting-out of 1,3-propanediol from aqueous solutions by inorganic electrolytes. J Chem Technol Biotechnol, 2016; 91: 2793-2801.[DOI]
[95] Xie S, Zhang Y, Zhou Y et al. Salting-out of bio-based 2,3-butanediol from aqueous solutions. J Chem Technol Biotechnol, 2017; 92: 122-132.[DOI]
[96] Rodionova MV, Poudyal RS, Tiwari I et al. Biofuel production: Challenges and opportunities. Int J Hydrogen Energy, 2017; 42: 8450-8461.[DOI]
[97] Kerry Yu KM, Tsang SC. A study of methyl formate production from carbon dioxide hydrogenation in methanol over a copper zinc oxide catalyst. Catal Letters, 2011; 141: 259-265.[DOI]
[98] Xie S, Li Z, Li H et al. Integration of carbon capture with heterogeneous catalysis toward methanol production: chemistry, challenges, and opportunities. Catal Rev, 2023; 1-40.[DOI]
[99] Cheng JJ, Timilsina GR. Status and barriers of advanced biofuel technologies: A review. Renew Energy, 2011; 36: 3541-3549.[DOI]
[100] Ho DP, Ngo HH, Guo W. A mini review on renewable sources for biofuel. Bioresour Technol, 2014; 169: 742-749.[DOI]
[101] Vane LM. Separation technologies for the recovery and dehydration of alcohols from fermentation broths. Biofuels, Bioprod Biorefining, 2008; 2: 553-588.[DOI]
[102] Shariati A, Azaribeni A, Hajighahramanzadeh P et al. Liquid –Liquid Equilibria of Systems Containingsunflower Oil, Ethanol and Water. APCBEE Procedia 2013; 5: 486-490.[DOI]
[103] Gyamerah M, Glover J. Production of ethanol by continuous fermentation and liquid-liquid extraction. J Chem Technol Biotechnol, 1996; 66: 145-152.[DOI]
[104] Neves CMSS, Granjo JFO, Freire MG et al. Separation of ethanol–water mixtures by liquid–liquid extraction using phosphonium-based ionic liquids. Green Chem, 2011; 13: 1517-1526.[DOI]
[105] Chiyoda C, Peixoto ECD, Meirelles AJA et al. Liquid-liquid equilibria for systems composed of refined soybean oil, free fatty acids, ethanol, and water at different temperatures. Fluid Phase Equilib, 2010; 299: 141-147.[DOI]
[106] Cacace JE, Mazza G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J Food Sci 2003; 68: 240-248.[DOI]
[107] Xie S, Li Z, Zhu G. Salting-out Effect on the Separation and Purification of Acetic Esters: Salting-out Agents, Theory, and Applications. Sep Purif Rev, 2022.[DOI]
[108] Fu H, Yang ST, Xiu Z. Phase separation in a salting-out extraction system of ethanol-ammonium sulfate. Sep Purif Technol, 2015; 148: 32-37.[DOI]
[109] Finkelde I, Newsome GA. Salting Out: A Simple and Reliable Method to Distinguish Between Common Fluid Preservatives and Estimate Alcohol Concentration. Collect Forum, 2020; 34: 11-31.[DOI]
[110] Liberato V, Ferreira T, Ribeiro BD et al. Salting-out as a fast and simple method for first- step separation of bio-based 1,3-propanediol. J Chem Eng Data, 2024: 1-25.[DOI]
[111] Xie S, Song W, Yi C et al. Salting-out extraction systems of ethanol and water induced by high-solubility inorganic electrolytes. J Ind Eng Chem, 2017; 56: 145-150.[DOI]
[112] Rainwater JC. Vapor-Liquid Equilibrium and the Modified Leung-Griffiths Model. In: Supercritical Fluid Technology. 2017: 57-162.
[113] Garcia-Chavez LY, Garsia CM, Schuur B et al. Biobutanol recovery using nonfluorinated task-specific ionic liquids. Ind Eng Chem Res, 2012; 51: 8293-8301.[DOI]
[114] Pérez De Los Ríos A, Hernández-Fernández FJ, Zapata Henríquez PA et al. Keys for Bioethanol Production Processes by Fermentation and Ionic Liquid Extraction. ACS Sustain Chem Eng, 2017; 5: 6986-6993.[DOI]
[115] Wang D, Wei F, Reyes-Labarta JA et al. Liquid-liquid equilibrium measurements and interaction explorations for separation of azeotrope n-butyl acetate and n-butanol using three ionic liquids. J Chem Thermodyn, 2021; 155: 106349.[DOI]
[116] Liang S, Li H, Zhang Y et al. Molecular mechanism, liquid–liquid equilibrium and process design of separating octane-n-butanol system by ionic liquids. J Mol Liq, 2022; 355: 118974.[DOI]
[117] Youqi Z, Qinqin Z, Hua X et al. COSMO-RS prediction, liquid-liquid equilibrium experiment and quantum chemistry calculation for the separation of n-butanol and n-heptane system using ionic liquids. J Chem Thermodyn, 2022; 167: 106719.[DOI]
[118] Xue C, Zhao JB, Chen LJ et al. Integrated butanol recovery for an advanced biofuel: Current state and prospects. Appl Microbiol Biotechnol, 2014; 98: 3463-3474.[DOI]
[119] Yu C, Wu S, Zhao Y et al. Liquid-Liquid Equilibrium Data of Water + Butyric Acid + {Butanal or n-Butanol} Ternary Systems at 293.15, 308.15, and 323.15 K. J Chem Eng Data, 2017; 62: 2244-2252.[DOI]
[120] Kamiński W, Górak A, Kubiczek A. Modeling of liquid-liquid equilibrium in the quinary system of water, acetone, n-butanol, ethanol, and ionic liquid. Fluid Phase Equilib, 2014; 384: 114-121.[DOI]
[121] Tan J, Huang H, Li H et al. Biobutanol extraction and dehydration with eucalyptus oil yielding linear and cyclic biofuel blends. AIChE J, 2024.[DOI]
[122] Kubiczek A, Kamiński W. Liquid-liquid extraction in systems containing butanol and ionic liquids-a review. Chem Process Eng, 2017; 38: 97-110.[DOI]
[123] Abdehagh N, Tezel FH, Thibault J. Separation techniques in butanol production: Challenges and developments. Biomass Bioenerg, 2014; 60: 222-246.[DOI]
[124] Xie S, Yi C, Qiu X. Energy-saving recovery of acetone, butanol, and ethanol from a prefractionator by the salting-out method. J Chem Eng Data, 2013; 58: 3297-3303.[DOI]
[125] Xie S, Qiu X, Yi C. Salting-out effect of tripotassium phosphate on the liquid-liquid equilibria of the (water+acetone+1-butanol+ethanol) system and the salting-out recovery. Fluid Phase Equilib, 2015; 386: 7-12.[DOI]
[126] Xie S, Qiu X, Yi C. Separation of a Biofuel: Recovery of Biobutanol by Salting-Out and Distillation. Chem Eng Technol, 2015;38:2181-2188.[DOI]
[127] Xie S, Song W, Fu C et al. Separation of acetone: From a water miscible system to an efficient aqueous two-phase system. Sep Purif Technol, 2018; 192: 55-61.[DOI]
[128] Xie S, Jia C, Go Ong SS et al. A Shortcut Route to Close Nitrogen Cycle: Bio-Based Amines Production via Selective Deoxygenation of Chitin Monomers over Ru/C in Acidic Solutions. iScience, 2020; 23: 101096.[DOI]
[129] Xie S, Jia C, Wang Z et al. Mechanistic Insight into Selective Deoxygenation of l -Lysine to Produce Biobased Amines. ACS Sustain Chem Eng, 2020; 8: 11805-11817.[DOI]
[130] Tan J, Sun Z, Huang H et al. Salting-out: A novel purification technique in biorefinery. Desalination, 2023; 564: 116790.[DOI]
[131] Wen H, Chen H, Cai D et al. Integrated in situ gas stripping-salting-out process for high-titer acetone-butanol-ethanol production from sweet sorghum bagasse. Biotechnol Biofuels, 2018; 11: 1-12.[DOI]
[132] Xie S, Zhang S, Qiu X et al. Sugaring-Out Effects of Sucrose and Glucose on the Liquid-Liquid Equilibria for the (Water + Acetone + 1-Butanol + Ethanol) System. J Chem Eng Data, 2015; 60: 2434-2441.[DOI]
[133] Pei Y, Wang J, Wu K et al. Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep Purif Technol, 2009; 64: 288-295.[DOI]
[134] Yu H, Cui K, Li T et al. Recovery of Butanol from ABE Fermentation Broth with Hydrophobic Functionalized Ionic Liquids as Extractants. ACS Sustain Chem Eng, 2019; 7: 9318-9329.[DOI]
[135] Dezhang S, Huisheng F, Feng X et al. Feasibility of ionic liquid as extractant for bio-butanol extraction: Experiment and simulation. Sep Purif Technol, 2019; 215: 287-298.[DOI]
[136] Ndaba B, Chiyanzu I, Marx S. n-Butanol derived from biochemical and chemical routes: A review. Biotechnol Rep, 2015; 8: 1-9.[DOI]
[137] Jin C, Yao M, Liu H et al. Progress in the production and application of n-butanol as a biofuel. Renew Sustain Energy Rev, 2011; 15: 4080-4106.[DOI]




 Copyright ©
Copyright ©