Advanced Techniques Using Ionic Liquids for Efficient Separation of Sugar Alcohols
Jyoti Bhattacharjee1#![]() , Subhasis Roy1#*
, Subhasis Roy1#*![]()
1Department of Chemical Engineering, University of Calcutta, Kolkata, India
#Both authors contributed equally to this manuscript.
*Correspondence to: Subhasis Roy, PhD, Assistant Professor, Department of Chemical Engineering, University of Calcutta, 92 A. P. C. Road, Kolkata, 700009, India; Email: srchemengg@caluniv.ac.in
DOI: 10.53964/id.2025003
Abstract
Recently, increasing interest has been reported in applying ionic liquids (ILs) in separating alcohols and proteins, an important process for several industrial and pharmaceutical applications. Traditional separation processes are poorly effective, with low selectivity, high energy consumption, and environmental problems. This review presents special properties of ILs concerning tunability and remarkable solvation ability that hold great promise for separation processes. The IL stationary phases modified with certain functional groups can enhance the interaction between IL and target molecules, increasing separation efficiency. Notably, the amino group-modified ILs were able to separate proteins by charge and size, a greener bioseparation technique owing to the low volatility and easy recyclability of ILs. AI's role in designing highly selective ILs for particular targets is discussed. Design of ILs, selection of solvent, and process optimization will be developed by applying techniques such as machine learning, molecular modeling, and data mining. Further, the review discusses the economic and environmental benefits of IL-based separations, and an attempt is made to discuss its cost-saving and resource-efficiency aspects. Separation of sugar alcohols using ILs, along with a discussion on advanced techniques, has been presented given their tunable solubility and low volatility being regarded as main issues in enhancing the separation processes. It also discusses the design of task-specific ILs for separating methanol from ethanol, which shows high selectivity toward methanol. Finally, the history of IL development, with current challenges in separating alcohols and proteins, gives a view of future research related to sustainable separation using ILs.
Keywords: alcohol, ionic liquids, molecular modeling, tailoring, sustainability, vitamins
1 INTRODUCTION
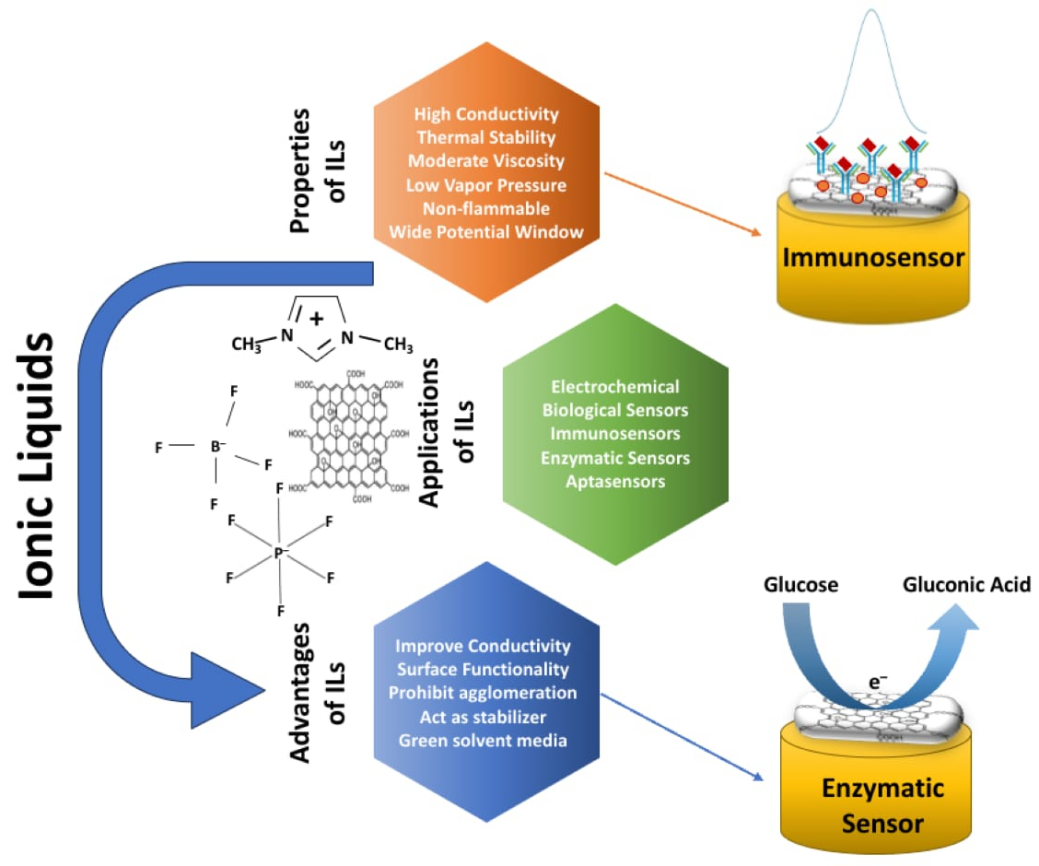
The quest for efficient and environmentally friendly separation technologies has become a critical endeavor in the dynamic landscape of modern chemistry[1]. The separation of the sugar alcohol family of chemicals necessary in many industries, including pharmaceuticals, food, and biofuels is one such field that has seen a boom in interest. Ionic liquids (ILs) have emerged as exceptional solvents among diverse separation technologies, promising increased efficiency and greener procedures[2]. This introduction explores the enthralling world of modern techniques aimed at maximizing the efficiency of ILs in separating sugar alcohols, providing light on the creative strategies that herald a watershed moment in chemical engineering. Due to their customized tunability, ILs have a significant advantage in selectively extracting and separating sugar alcohols from complex combinations[3]. Their ability to dissolve a wide range of compounds and their low vapor pressure make them an appealing alternative for efficient separation operations. However, researchers have looked into other ways to improve their efficacy in sugar-alcohol separation. In recent years, the need for sustainable and efficient operations in the chemical industry has fueled research into novel separation methods. ILs have attracted interest as attractive possibilities for chemical separation due to their low volatility, tunability, and unusual solvation properties[4,5]. Sugar alcohols are critical in industries ranging from pharmaceuticals to food and biofuels. Purer sugar alcohols mean better products, cleaner manufacturing, and a sweeter future. Modern methods to improving ILs for sugar alcohol separation center on adapting these solvents to specific requirements[5,6]. For instance, the functionalization of IL structures has yielded encouraging results. Functional groups that interact selectively with sugar alcohols have been successfully introduced by researchers, allowing for better separation efficiency, as shown in Figure 1.
|
Figure 1. Schematic Presentation of the Ionic Liquids, Their Properties, Advantages, and Applications.
Another very modern approach is the application of nanotechnology in IL-based separations. Introducing nanoparticles, such as magnetic or catalytic nanoparticles, into ILs could add another degree of selectivity, thus enabling sugar alcohols to be separated even more precisely. These new approaches reflect the constant change that characterizes research in this sector. The following review discusses the use of ILs as solvents for extracting and purifying bioactive compounds obtained by fermentation, synthetic pathways, or directly from biomass. The range of bioactive compounds includes small organic compounds like phenolic acids, alkaloids, fats, essential oils, carotenoids, vitamins, and amino acids to large biomolecules like nucleic acids, proteins, enzymes, and antibodies[6,7]. Different ILs have been widely explored in the context of various bioactive compounds and extraction techniques. The review is supported by a comprehensive table naming these ILs and abbreviating them, grouped according to their cation and anion constituents. IL-based techniques include liquid-liquid extractions using hydrophobic ILs, ionic-liquid-based aqueous biphasic systems, and aqueous micellar biphasic systems[8]. Other discussed solid-liquid extractions included techniques such as microwave-assisted and ultrasound-assisted extractions. Also discussed are techniques such as solid-phase extractions involving IL-modified materials and induced-precipitation techniques like three-phase partitioning and crystallization. Particular attention has been paid to IL-based techniques, which have shown scalability and have recovered significant amounts of target compounds at a moderate expense. Notably, this review excludes electrophoretic and chromatographic techniques since there have already been several reviews on these methods[9]. The paper underlines the most relevant challenges to IL-based extraction and separation processes, calling for further exploration and novelty. Properties and equilibrium conditions of systems, including extraction and solubility behavior using new solvents like ILs, are of paramount importance during the development and optimization of further processes. The present review also intends to provide an overall thermodynamic description based on simple but accurate tools represented by activity coefficient models and equations of state, which is a field that has been tackled very scantily so far. Therefore, 11 different calculation schemes based on the approaches above were applied to the largest databank ever investigated, including 13 solute-analyte systems, 21 ILs, and 653 data points. This is done regarding the performance analysis of such kinds of models[6-8].
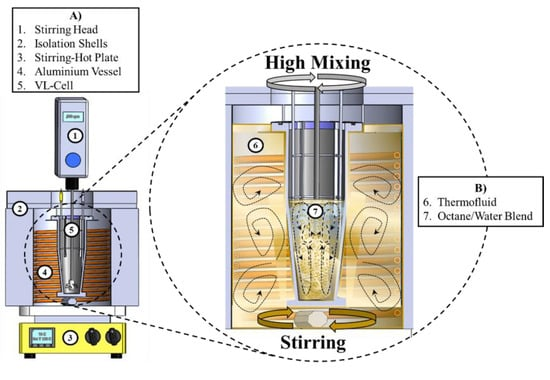
The models tested include some activity coefficient models (ACMs), namely NRTL, free-volume Flory-Huggins (FVFH), and Redlich-Kister (RK), using schemes other than athermal and ideal solution models. Three-parameter cubic Equations of state known for their good performance have also been tested and compared, including Peng-robinson (PR) and Valderrama-Patel-teja (VPT)[9]. The simple RK model and a three-parameter one provided the best results with a deviation of 0.82 % (2.77K) for the whole databank. Besides, the incorporation of the temperature-dependent interaction parameters into the FVFH ACM (3.55K, 1.05%), PR EoS (3.60K, 1.07%), and VPT EoS (3.66K, 1.08%) remarkably improved the accuracy of the estimations. The results show that the thermodynamic models based on the NRTL, FVFH, and RK ACMs are suitable for calculating the solid-liquid equilibrium conditions in the SA -IL systems because they provide a simple and reliable calculation method. Such models can be successfully applied within the simulation and optimization packages. An instance is given in Figure 2[10].
|
Figure 2. Thermodynamic Modeling of Sugar Alcohols Solubility in Ionic Liquids. This figure represents the overall modeling strategy for predicting the solubility of various sugar alcohols in ionic liquids. It also puts forward views on the efficacy and efficiency with which different thermodynamic models, such as activity coefficient models and equations of state, perform in reproducing solubility behavior. Reproduced from Ref.[10] with permission from MDPI.
1.1 The Role of ILs in Sugar Alcohol Separation
Considering their unique properties, ILs recently emerged as a promising alternative to traditional organic solvents in chemical processes. Usually, ILs are one class of salts in the liquid state, which consists of organic cations and inorganic or organic anions. They are characterized by low volatility, high thermal stability, and the ability to dissolve many substances, including sugars and sugar alcohols. These properties make ILs attractive in separation processes, especially where traditional solvents lack satisfactory performance.
The application of ILs to the separation of sugar alcohols has recently received much attention for some key advantages[11,12]:
High Selectivity and Solubility: ILs can be tailored by choice of cation and anion to selectively dissolve specific sugar alcohols, thus enabling efficient separation in complex mixtures. This selectivity reduces the need for multiple separation steps, thus lowering energy consumption.
Environmental Benefits: Because ILs are nonvolatile, they represent "green" solvents since these compounds’ VOC emissions into the atmosphere are negligible. This attribute is essential in many large-scale industrial applications where the loss of a given solvent may be rather high. A huge number of cation-anion combinations can be used in the design of task-specific ILs targeted to optimize separation processes. Researchers can modify ILs for improved selectivity, reduced viscosity, or other properties to better suit their intended use for separating certain sugar alcohols. Compatibility with Different Techniques: These ILs offer a wider versatility in applications with several industries because they can be combined with different separation methods such as liquid-liquid extraction, chromatography, and crystallization.
Despite these merits, the ILs involved in sugar alcohol separation pose several drawbacks, such as high cost, potential toxicity, and viscosity problems, which may prevent the industrial applications of ILs. More importantly, the molecular interactions between ILs and sugar alcohols have to be addressed to improve these processes, which demands highly developed thermodynamic models and simulations[13,14].
The review focuses on an overview of the present state of research concerning the application of ILs to sugar-alcohol separation. The scope of this review is as follows:
Summary of the Advantages and Disadvantages of IL Usage: The review will highlight such advantages of using ILs for sugar alcohol separation as high selectivity, low volatility, and the possibility to tune their properties, while challenges are high viscosity and possible toxicity.
In this perspective, the review discusses more contemporary thermodynamic modeling approaches that are applied in predicting and enhancing the efficiency of ILs in sugar alcohol separation, such as activity coefficient models, equations of state, COSMO-RS, and molecular dynamics simulations. The sustainability study of ILs compared with conventional solvents will be done based on their lifecycle impact, recoveries, and recycling possibilities[14-16].
2 SUGAR ALCOHOL SEPARATION
Separating sugar alcohols is indispensable in the pharmaceutical industry since a high degree of purity is required. Crystallization, distillation, and chromatography so far applied are generally non-efficient, non-selective, and often non-environmentally-friendly procedures. In separating sugar alcohols, ILs have become an ideal alternative. ILs are salts in the liquid state at room temperature or near it. Generally, they have low vapor pressure and high thermal stability, with adjustable polarity to enhance their solvation ability. To date, many strategies have been developed to improve the effectiveness of ILs in sugar-alcohol separation. For instance, task-specific ILs could be prepared by designing functional groups on the cation or anion to increase its affinity and selectivity for target sugar alcohols. Functionalized ILs, such as amino acid-based ILs or deep eutectic solvents (DESs), demonstrate possibilities of enhancing separation efficiency based on better sugar-alcohol interactions, for instance. Another strategy may be in advanced separation techniques, including membrane-based methods and IL-based liquid-liquid extraction. Membrane separation methods possess high selectivity, easy scale-up, and low energy consumption; incorporating ILs into the membrane systems will increase the transport efficiency of sugar alcohols[14,15]. New IL-based liquid-liquid extraction, for instance, may be considered a greener alternative route with the demonstration of less environmental impact, higher kinetics of extraction, and greater selectivity towards sugar alcohols. Another is novel process intensification, such as reactive distillation and hybrid separation processes; Figure 3[16] illustrates that it is within the purview of offering more efficiency in the separation of sugar alcohols.
|
Figure 3. Schematic Diagrams of Integrated Processes Using ILs. The figure presents schematic diagrams illustrating integrated processes that utilize ILs to extract and separate small organic compounds from biomass. It details the step-by-step procedures, including the extraction of target compounds, separation, and the subsequent recovery and reuse of the ILs. The diagrams emphasize the efficiency and sustainability of these processes in optimizing IL usage and minimizing waste. Reproduced from Ref.[16] with permission from America Chemistry Society.
2.1 Small and Extractable Bioactive Compounds from Biomass
The nutraceutical, cosmetic, and pharmaceutical product market is currently experiencing a trend of turning demand to natural compounds over synthetic ones. However, conventional extraction methods have several drawbacks: they are not selective and are lengthy procedures, have high energy consumption, and use large amounts of toxic organic solvents, which create environmental pollution and may put human health at risk. In this respect, the necessity of providing sustainable extraction techniques and safer solvents, also derived from renewable resources, must be investigated[17].
The area of IL-based extraction, separation, and purification of bioactive ingredients from biomass has been probed comprehensively. It is well-known that ILs and mixtures of ILs with water or organic solvents show a high ability for solid-liquid equilibria (SLE) of value-added compounds from biomass. Due to their ionic nature, ILs exhibit good interactions with electromagnetic fields; hence, IL-based microwave-assisted extraction (MAE) and ultrasound-assisted extraction (UAE) were quite efficient. On the other hand, IL-based Liquid -liquid extraction (LLE) is feasible for liquid matrices. Then, ILs confined to solid matrices (IL-modified sorbents for solid-phase extraction (SPE)) enhance the technique's performance by reducing IL consumption and improving its recycling[18,19].
Research in this area is focused on IL-based SLE, MAE, UAE, LLE, and SPE, with an emphasis on the extraction of alkaloids, terpenoids, flavonoids, phenolic compounds, saponins, and polycyclic aromatic compounds. The parameters that affect the efficiency of extraction, merits, and demerits of these approaches, and structure-property relationships between ILs and bioactive ingredients are considered, and this includes methods for back extraction and recovery of bioactive compounds from IL solutions and the reusability of ILs[20].
2.2 IL-Based Solid-Liquid Extractions
There are reports on IL-based SLE techniques using pure ILs, aqueous solutions, and mixtures with methanol or ethanol to extract natural compounds. The optimization parameters of simple SLE include temperature, extraction time, and solid-to-liquid ratio. Coupling with ultrasounds and microwave-assisted extractions could make the system further efficient in reducing time and minimizing the solvent used [21].
Numerous studies have been reported on the successful extraction of alkaloids using IL: glaucine, caffeine, piperine, and many others, with a high yield. For instance, ILs based on the imidazolium cations and various kinds of anions have indicated considerable extraction. On the other hand, MAE processes assisted by ILs gave enhanced yield and faster extraction of, e.g., trans-resveratrol and alkaloids[22,23]. IL cations and anions provide a definition that is important for functionalization and functionalization, which is a key process in developing properties for selective processes. Their phenomena were optimized from their properties with comprehensive characterization from spectroscopic, chromatographic, thermal, electrochemical, structural, rheological, and surface analyses[24].
3 EXTRACTION OF FLAVONOIDS USING ILS
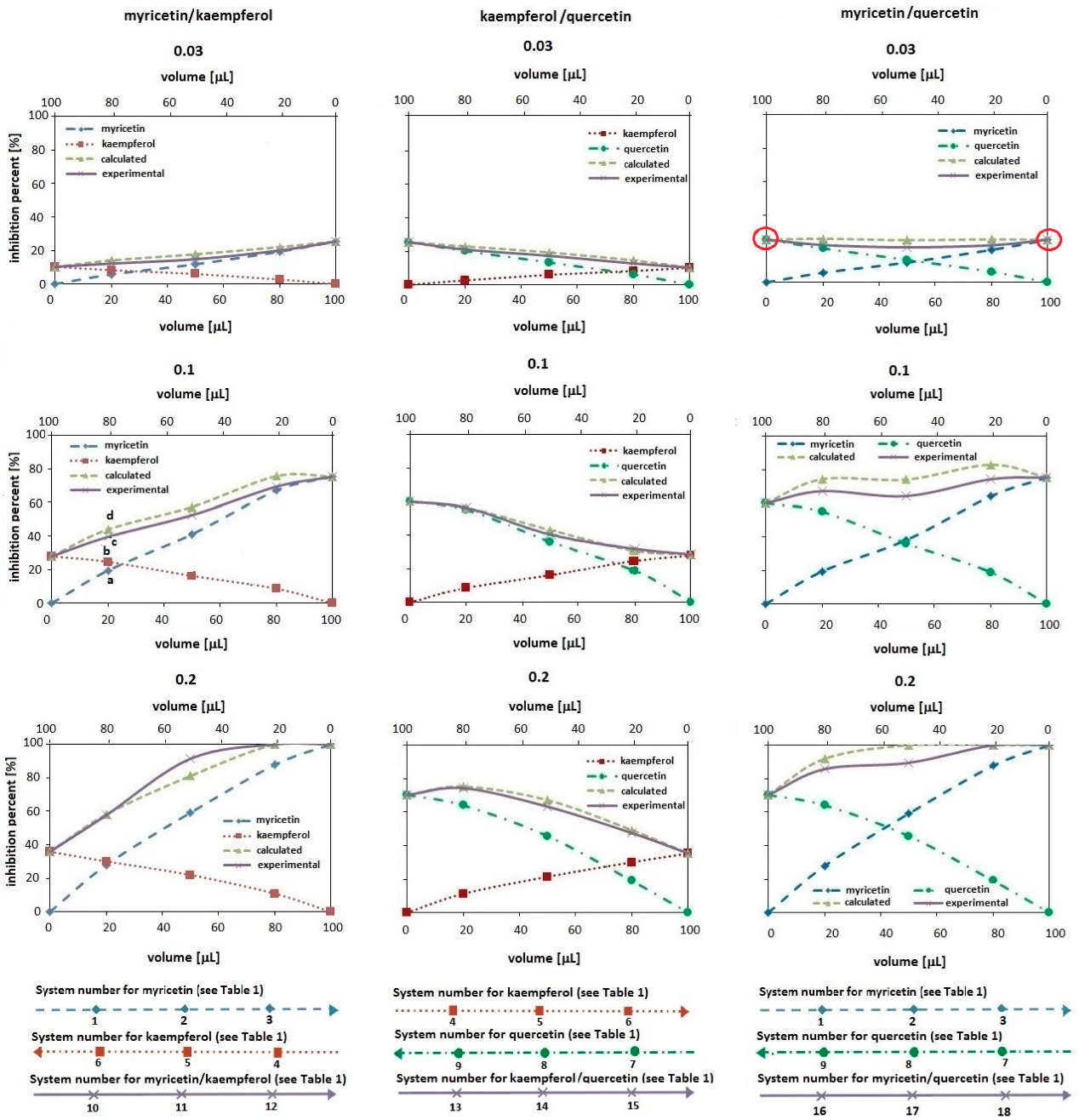
IL-based MAE has been proven effective for extracting flavonoids, including rutin, polyphenolic compounds, kaempferol, myricetin, and quercetin. The literature also showed that the efficiency of the extraction of flavonoids depends on the IL anion's hydrogen-bond-accepting abilities and the strength of π-π interactions from the IL cation. These, therefore, established that IL-based MAE is more efficient than traditional methods of yield and time of extraction[25,26] as shown in Figure 4.
|
Figure 4. The Variations of Antioxidant Activity were Measured using the DPPH (2,2-diphenyl-1-picrylhydrazyl) Radical Scavenging Method. The graphs show the effect of varying volumes of bioactive compound solutions on the activity. The studied systems are the myricetin solution (by a dashed line with diamonds), kaempferol solution (by a dotted line with squares), quercetin solution (by dashed-dotted line with circles), and their binary mixtures (by a solid line with 'x'). For comparison, the dashed line with triangles corresponds to the theoretically expected antioxidant activity values for the tested compound pairs. The experimental data represent mean values obtained from five replicates (n=5). Reproduced from Ref.[26] with permission from MDPI.
3.1 IL-based ABS
IL-based ABS containing hydrophilic ILs with inorganic salts can also be extended to separate the natural compounds. Two liquid phases exist in such systems, one rich in ILs and the other in salts. The IL-based ABS found successful applications for purifying alkaloids, phenolic compounds, and other natural products[27-31]. Even though IL-supported silica materials provided promising results, they face many limitations, such as high cost and limited pH stability. IL-modified polymers were developed to solve these limitations, and the performance in extracting natural compounds seems much more promising due to better pH stability. Recently, for instance, polyaminopropylimidazolium polymers, methylimidazolium-modified polymers, molecularly imprinted polymers, and alkyl pyridinium-modified polymers were developed, which show excellent selectivity and recovery[32-34].
3.2 Lipids and Other Hydrophobic Compounds
Lipids have nutritional and industrial value, so they get extracted and fractionated by the following LLE, SLE, ABS, and micro- to mini-SPE. Generally, ILs enhance these techniques, offering better effectiveness and selectivity.
3.3 Essential Oils
According to the application of ILs, MAE-based extraction has been considered effective in extracting essential oils while possessing many advantages like time reduction, energy consumption, maintaining quality oils, etc. Several have been referred to for the optimization of extraction parameters and have proved that IL-based MAE gives similar or better results compared to conventional methods[35,36].
3.4 Saponins
The surface-active nature of ILs has successfully been utilized to extract saponins. Different studies have also presented that the aqueous solutions of imidazolium and choline ILs extract saponins and polyphenols from other natural sources[37].
4 VITAMINS
4.1 Tocopherols
They are representatives of vitamin E; tocopherols present four types: α-, β-, γ-, and δ-tocopherol and are fat-soluble antioxidants. These homologs are structurally quite similar and have different biological activities, with α-tocopherol presenting the highest; therefore, fractionating these compounds has been difficult until now[38,39].
4.2 IL-Based Fractionation Techniques
A few researchers[40] introduced a new platform using ILs to separate tocopherol stereoisomers. Their works exploit the capability of ILs to interact with an organic molecule via hydrogen bonding. Examples of these studies are Hexane and IL/Methanol Mixtures: The 1:1.3 mixture of [C4C1im]Cl: methanol provided the highest selectivity ratio of 21.3 for δ- against α-tocopherol due to the possibility of interaction of an OH group interacting favorably with the chloride anion[40,41].
Hexane and IL-Cosolvent Mixtures: Other cosolvents were also used to study their effect on selectivity, e.g., acetonitrile and DMSO, which showed higher selectivity.
Anion Effect: The application of anion did not have any definite effect on the separation. Comparatively, Cl-, [BF4]-, and [CF3SO3]- have lower selectivity[42,43].
Vitamin D3 and Tachysterol: In different publications, few researchers investigated the LLE of the test compound using ILs with anions [NTf2]-, [CF3SO3]-, and functionalized alkyl chains. These were found to be highly effective. They believe that it is π···π stacking interactions that ensure a high selectivity of extraction. A conceptual continuous multistage extraction process allowed us to achieve purities of vitamin D3 higher than 98%. While some good progress has been made, the narrow range of applied IL structures, recovery, and recycling of ILs and solvents used in these extraction processes needs further research[44-46].
4.3 Applications of IL-based ABS in Enzyme Purification
4.3.1 Wheat Esterase Extraction
Efficient separation and purification of IL-based ABS for enzymes, including wheat esterase, that could be an alternative source instead of traditional AChE extracted from animal tissues have been performed. It was reported that wheat esterase partitions more into the IL-rich phase; under optimized conditions, the extraction yield recovered up to 88.93% with a PF of 4.23. According to other literature, these values can be because of the electrostatic interactions between the enzyme amino acids and IL cation[47,48].
4.3.2 Potential for Large-Scale Application
The IL-based ABS systems have the maximum potential for recycling and reusing the phase-forming constituents, resulting in the IL-based ABS having large-scale applications. However, a principal problem exists with the transference from batch to continuous processing. These microfluidic devices have the potential to handle processing from kilograms to tonnes down to the microquantity range, and they do offer a feasible solution for drastically improving yields and purities within very short times[49]. Besides, these microfluidic systems offer flexibility in operations, coupled with several economic and environmental benefits. However, the integration of ILs with microfluidic systems for extraction and purification remains underexplored. The most current applications have been for the synthesis of ILs or simply as solvent applications in biocatalysis, some of which are shown in Figure 5[50].
|
Figure 5. Distribution of Scientific Works on IL-Based Extraction Techniques. The next treemap describes the scientific literature about different IL-based techniques for extracting and separating small organic compounds from biomass. Circles are categorized by number, representing individual research studies on different families of natural compounds. Reproduced from Ref.[50] with permission from MDPI.
4.3.3 Recent Advances in Microfluidic Applications
In few relevant studies, probed the use of IL-based ABS and microfluidic devices towards the improvement of purification for bovine serum albumin using an ABS that consisted of [C4C1im] [BF4] and D-fructose working within a parallel flow microfluidic device. This method furnished partition coefficients of 14.6, which are 20 times higher than conventional ABS by polymers and salts. The predicted productivities of the IL-based microfluidic technique were 17.4kg/h·m³, much higher than the 1.48kg/h·m³ obtained with conventional systems. One key benefit of IL-based phases in microfluidic separations was their lower viscosity[51,52].
4.3.4 Hydrophobic ILs
The application of hydrophobic ILs in extraction processes is still limited due to the narrower range of available chemical structures compared to hydrophilic ILs. However, hydrophilic ILs offer a broader variety for customizing IL-based ABS, enhancing extraction and purification processes. Research typically falls into two categories: IL-based ABS for model proteins and enzymes and those for real, complex matrices. While model proteins provide preliminary insights, the real challenge lies in optimizing IL-based ABS for effective extraction from complex matrices[53,54].
4.3.5 ILs as Adjuvant in ABS
ILs used in traditional ABS systems show promise due to higher PFs and lower system costs. Green-biocompatible ILs are particularly promising for eliminating the need for additional buffers, though further optimization is necessary. Future research should focus on optimizing IL-based ABS for industrial downstream processes and exploring new strategies to tackle purification challenges, including pretreatment and sequential purification methods, such as liquid-liquid chromatography[55,56].
Regarding this approach, vitamins play an important role in separating sugar alcohols using ILs because they interfere with the processes of solubility and selectivity. In this respect, ILs can be regarded as a special class of solvent, defined by their ionic nature. Thus, they may enable special tuning of the extraction and purification processes. Water-soluble vitamins can greatly affect the behavior of sugar alcohols upon separation. These changes can enhance the selective separation of sugar alcohols like sorbitol, xylitol, and erythritol by tuning the solvation environment and the interaction energies between IL and sugar alcohol.
5 LIQUID-LIQUID EXTRACTIONS WITH HYDROPHOBIC ILS
5.1 Protein Extraction Challenges
Despite extensive research on hydrophobic IL-water biphasic systems for various extractions, their use in protein extraction is less common. The primary challenge is that proteins require "hydrated ILs" for effective dissolution. To address this, researchers have explored several methods:
Modification with Amphiphilic Polymers: Improves protein solubility and stability in hydrophobic phases.
Addition of Crown Ethers: Enhances extraction by coordinating with proteins.
Aqueous Microemulsion Droplets: Utilize hydrophobic ILs to form microemulsions for protein extraction[57-60].
5.2 Case Study
In 2006, Shimojo et al.[60] extracted heme proteins such as cytochrome c using ILs with dicyclohexano-18-crown-6. They hypothesized that the coordination interaction of cytochrome c with DCH18C6 would be a factor for its enhanced solubility in the hydrophobic IL phase and thus assist extraction. The current review updates the developing roles of ILs in extracting and purifying a wide array of bioactive compounds. ILs have been increasingly recognized for their versatility and effectiveness as primary solvents, cosurfactants, electrolytes, and functionalized materials to enhance silica and polymer adsorption capabilities. The application yields of their extracts and purification factors were considerably higher than the traditional methods, hence their potential use in research and industrial settings[60-62].
6 SOLID PHASE EXTRACTION OF ILS
SPE is a group of analytical techniques for sample pre-treatment based on the selective extraction of the analytes from the sample, usually aqueous, using a solid sorbent. ILs cannot be used for SPE in their pure form due to their aggregation state[63,64]. Incorporating ILs into novel hybrid materials as the sorbent coatings enables application in SPE. Among the preferred solid supports are nanomaterials such as carbon nanotubes, graphene, graphene oxide, and magnetic Fe3O4 nanoparticles, owing to their high surface-to-mass ratio, which can easily facilitate miniaturization of methodologies. Coupling this characteristic with the possibility offered by ILs for extraction gives rise to highly effective hybrid nanomaterials. Several methods involving SPE with hybrid materials and ILs have been reported in the recent literature. For instance, two SPE-HPLC methods for the determination of tea samples were developed, one of which determines pyrethroid-type insecticides using [C12mim]Br-modified attapulgite and the other detects benzoylurea insecticides using [C16mim]Br-modified alkalinized luffa sponge fibers[65]. Although these methods are precious alternatives to the existing techniques for insecticide determinations, they have some drawbacks: they require large volumes of HCl or NaOH for activation of the sorbent material and large volumes of organic solvents for chromatographic separation, producing too much waste and thus not corresponding to the green methods[66,67]. Vortex-assisted SPE has been applied to treat vegetable and fruit juice samples, with subsequent determination of PAHs by GC-FID. A metal-organic framework modified by the IL [C4mim]Cl has been used as a sorbent. While hydrophobic and π-π interactions made the analyte retention pretty efficient, methodology environmental friendliness was compromised due to oxidizing acids. Another SPE method for polyphenol extraction from wine samples was proposed, in which a homemade fiber coated with graphene oxide modified by the IL [APmim]NTf2 was used, and higher extraction efficiency in comparison with commercial fibers was achieved. Nevertheless, the process required methanol, thus affecting its greenness, as shown in Figure 6[68].
|
Figure 6. SEM and TEM Images of Three Nanoparticles. A and D: Fe3O4 nanoparticles; B and E: Fe3O4@SiO2 nanoparticles; C and F: Fe3O4@SiO2@VAN nanoparticles. Reproduced from Ref.[68] with permission from MDPI.
Various methods have combined magnetic NPs with ILs in MSPE. For instance, the NiFe2O4-based magnetic effervescent tablets incorporated with dicationic IL [C4(mim)2]Br2 were used to extract PAHs coupled with HPLC with fluorescence detection from meat samples. Thereafter, this method minimizes the consumption of organic solvents and does not take any unnecessarily complicated sample preparation steps. Another procedure utilized the hydrophilic magnetic ball of nano-Fe3O4, mesoporous silica, and the IL [C6mim]Cl to analyze parabens by GC-MS in cola and green tea samples. The developed method was rather selective, but the synthesis involved appreciable quantities of methylbenzene and hexanediol, compromising its greenness[69,70].
6.1 Magnetic IL-based Liquid-Liquid Extraction
Magnetic ILs were considered intrinsic magnetism with high extraction efficiency, which can avoid the disadvantages of IL-functionalized magnetic nanomaterials[71,72], such as IL leaching. Some literature has recently reported different MILdispersive liquid-liquid microextraction techniques for trace metal determination. For example, MILs of the phosphonium type, such as [P6,6,6,14]FeCl4, have been applied for Cd(II), As(III), and Cr(III) preconcentration in honey. Meanwhile, [P6,6,6,14]2MnCl4 was used to preconcentrate Pb(II) /. Though these methods reduce the consumption quantity of organic solvents, there were still defects concerning greenness while acid digestion and organic solvent were used in both sample pretreatment steps. In addition, the MIL-DLLME methods have been reported for the inorganic speciation of Se in rice and arsenic in vegetables using MIL [C4mim]FeCl4. Although these latter procedures still employed sample digestion with oxidizing acids, class-selective extraction was accomplished without chromatographic separation, and organic solvent use was dramatically reduced.
MIL-DLLME-HPLC-UV has also been applied to the preconcentration and determination of organic compounds. For example, [P6,6,6,14]2CoCl4 was applied to extract estrogens from milk. While [N1,8,8,8]FeCl4 was applied to extract parabens from beer and peach juice, realizing green extractions with very low consumption of organic solvent[73,74].
6.2 Polymeric IL-based Solid Phase Extraction
PILs provide better thermal and mechanical stability of the coating on micro-SPE fibers without leaching the extractant phase. Other recent applications are PIL-coated fibers for extracting VOCs in wines, which are more efficient than traditional coatings. A combination of PILs with nanomaterials showed their further increasing applicability, such as poly-VC6imPF6-coated graphene oxide@SiO2 for phenolic acid extraction in wolfberry yogurt. While such hybrids also exhibit stability and efficiency, using methanol in chromatographic separations diminishes the overall greenness of such hybrids[74,75]. Another stride toward this is magnetic nanomaterials coated with PILs. For instance, Fe3O4@SiO2 NPs functionalized with PILs have been applied to extract parabens from milk and pyrethroid residues from apples. These methodologies coupled the high extraction capacity of PILs with magnetic properties for phase separation; however, the sample preparation used trifluoroacetic acid and acetonitrile, which deteriorated their greenness[76,77].
Hybrid magnetic nanomaterials containing PILs and other selective components have also been developed. For example, magnetic NPs coated with poly(calixarene IL) have been described for flavonoid preconcentration from fruit juice and green tea, and magnetic NPs based on poly(β-cyclodextrin-IL) for extracting PAH in various food samples. Despite the low volume of organic solvents consumed by these methods for elution and sample preparation, the general greening of the process was still compromised by the consumption of methanol and acetonitrile. A new method was reported for determining sulfathiazole in milk and honey by using SPE with a membrane of copolymer [VC6im]Br and methylmethacrylate, coupled with fluorescence spectrometry, in which great findings were recorded. However, using trichloroacetic acid in the pretreatment of milk samples brought along an environmental concern. In contrast, honey samples were only required to be diluted with ultrapure water, which is much cleaner in its application.
6.3 Thermodynamic Modeling
Developing more efficient ILs for sugar alcohol separation is an important step in this process, drawing much interest in its applications in the food and pharmaceutical industries and biofuels. Sugar alcohols are a group of compounds produced from biomass, including sorbitol, xylitol, and mannitol, used as sweeteners and excipients in pharmacology. The challenge is essentially their efficient separation from complex mixtures with often similar molecular structures and physical properties. ILs hold much promise because of their unique capacity to dissolve various compounds. Present-day strategies for enhancing IL efficiency in the separation process are based on advanced thermodynamic modeling capable of predicting and optimizing IL-sugar alcohol interactions[78-80].
6.3.1 Role of Thermodynamic Modeling
Thermodynamic modeling enables the prediction of IL behavior in sugar alcohol mixtures and gives insight into phase behavior, solubility, and selectivity. Such models can assess the performance of different ILs concerning their interaction with the sugar alcohols at a molecular level, guiding the selection of adequate IL for specific means of separation. The idea is to find those ILs with the lowest cost and environmental impact possible that exhibit high selectivity and capacity toward sugar alcohols. Some current approaches to thermodynamic modeling include activity coefficient models such as the NRTL and UNIQUAC models, which are widely applied in modeling the non-ideal behavior of mixtures containing ILs and sugar alcohols. These models estimate the activity coefficient of components present in a mixture to enable the prediction of phase equilibrium data. These models have been parameterized using experimental data so that the performance of various ILs in separating sugar alcohols can be predicted and conditions optimized for maximum efficiency[80,81].
Equation of State Models: Equations of state, such as the Peng-Robinson EOS and the Soave-Redlich-Kwong EOS, have been adapted for ILs to predict high-pressure system phase behavior. Unlike the COSMO-RS model, EOS models account for the intermolecular forces between components; thus, they can be applied in systems containing ILs under different temperature and pressure conditions. In the case of sugar alcohol separations, EOS models will establish temperature and pressure changes in the solubility and selectivity of sugar alcohols in ILs to further process optimization.
COSMO-RS: Conductor-like Screening Model for Real Solvents The COSMO-RS model is a quantum chemical-based thermodynamic model used widely in IL applications. It enables the estimation of the chemical potential of the components in a mixture, making it possible to predict the phenomena of solubility and phase behavior of a considerable number of systems, even without extensive experimental data. Very useful for the prediction of sugar alcohol separations from just pure component data available in the literature is COSMO-RS with related capabilities[80,81]. COSMO-RS could be applied to predict the solubility of various sugar alcohols in different ILs, thus aiding the selection of the most appropriate ILs for specific separations. MD simulation provides a microscopic view of ILs and sugar alcohol interactions, which may allow an understanding of the solvation structure and dynamics. This may give insight into how specific cations and anions in ILs interact with various sugar alcohols by simulating the behavior of IL-sugar alcohol systems at a molecular level to guide the design of task-specific ILs, thus improving their separation efficiency[80-82].
6.3.2 Increasing Efficiency of ILs
Thermodynamic modeling is expected to improve IL efficiency in separating sugar alcohols by optimizing IL composition and predicting interactions between sugar alcohols and various ILs. This enables the selection or design of ILs with high selectivity toward target sugar alcohols, giving minimum energy input in separation and maximum yield. Table 1 Illustrates various immobilization techniques and the immobilization of different ILs[81-84].
Predict Phase Behavior and Solubility: Phase behavior and solubility can be accurately predicted, and thus, separation processes can be designed under optimal conditions of temperature and pressure, thereby reducing operational costs and leading to more efficient processes.
Minimize Environmental Impact: Thermodynamic modeling helps select ILs, among others, which will perform with the virtues of being environmentally benign in terms of properties like biodegradability and toxicity[81,82].
Table 1. Depicted Different Types of Immobilized Methods and Immobilization of Different ILs
Name |
Type of ILs Immobilized |
Method of IL Immobilization |
References |
N-methyl imidazole |
Stirring in the presence of acetonitrile and 3-chloropropyltriethoxysilane
|
Removal of 12 sulfonylurea herbicides |
[81] |
Tropine-type chiral ionic liquid with proline anion |
Chemical bonding method |
Separation of Cu2+, Fe3+, Ni2+ Separation of racemic amino acids
|
[82] |
Trihexyl (tetradecyl) phosphonium bis 2,4,4-trimethyl pentyl phosphinate—Cyphos IL-104 |
Sol-gel method |
Removal of Cr(III, VI) |
[83,84] |
7 FUTURE DEVELOPMENT
Over the last ten years, ILs and new alternatives to classical solvents have gained attention due to their unique properties workable in different chemical processes. They show a series of advantages, including low volatility, high thermal stability, and tunable physical and chemical properties through cationic-anionic combination. Most of them generally provide benefits in applications for ILs in catalysis, separation, and electrochemistry[85,86]. On the other hand, with these said, some disadvantages and challenges come with ILs, which need to be worked on to appreciate ILs in many applications.
7.1 Benefits of ILs
One of the cited utmost benefits of ILs is that they usually possess very low or negligible vapor pressure, giving them the least chance of volatilizing. This reduces the chances of causing volatile organic compound emissions, making them safer and friendlier to the environment than many common solvents. This feature is very useful in high-temperature applications or environments where solvent loss can be problematic. Besides, ILs represent thermal stability and can be involved in high-temperature processes without degradation. They can dissolve anything: organic compounds, metals, etc., which provides a lot of applications in all aspects of chemistry and engineering[85,86].
The other significant benefit is tunability. By switching cations and anions, researchers can develop ILs with desired properties suitable for their purposes. Such flexibility will hardly be obtained with any conventional solvent; this will be a specific peculiarity for the whole chemical processing chain. As an example, one strong conductor IL can be designed to be applied in batteries and fuel cells; separations, and especially liquid-liquid extractions, have constituted another field of application of the ILs, mainly because of the ability of the ILs to selectively dissolve some components from complex mixtures[87,88].
7.2 Disadvantages and Challenges of ILs
Even though all these properties of ILs render them quite fascinating from many points of view compared to conventional solvents, they still possess some drawbacks limiting their application. First, one of the critical issues is that they are relatively expensive in terms of synthesis and purification; that is, many ILs are elaborately synthesized and, therefore, expensive when compared with conventional solvents that require treatments. It is important to see this as an economic barrier to the better exploitation of the growing range of industrial applications for ILs. A more serious concern, however, is the issue of toxicity and environmental impact. Even though ILs have been considered "green" solvents due to their low volatility, some ILs are known to show aquatic toxicity and might even pose human health hazards. The potential for bioaccumulation and persistence in the environment is poorly understood; further research on their biodegradability and life-cycle impacts is essential of the hour[86,87].
The high viscosity of many ILs can also be a problem in chemical processes, mostly when mass transfer efficiency is required. High viscosities can impede the reactants and result in diffusion, causing low reaction rates and inefficiency of processes. In most cases, cosolvents and changes in process conditions must be adopted, which may obliterate the initial reason for IL exploitation.
Therefore, the following further areas of research would allow the ILs' real-world applicability: searching for cost-effective methods of IL synthesis, making them economically feasible, possibly by investigating new pathways of cation-anion synthesis or developing methods to utilize more abundant and inexpensive starting materials. It was prime that any improvement in the knowledge of IL toxicity and environmental impact had to be in front. The testing methodologies that would be applied for ILs on environment and health risks are to be identified and developed on standardized protocols required to design greener and more sustainable alternatives[85-87].
Also, the physical properties of ILs, such as viscosity and conductivity, need to be optimized to suit more well-defined applications. Besides, task-specific ILs designed for certain functions would also help overcome some of the present limitations. Such tuning would be very important, for example, in providing ILs that are less viscous and, hence, more capable of carrying out mass transfer-intensive processes. Thirdly, the more recyclable and reusable the ILs are, the lower their overall environmental impact and costs associated with their use will be. IL design materials that can be recovered and reused easily with no substantial degradation in properties are much more sustainable and interesting from an industrial point of view[88-90].
8 CONCLUSION
The range of bioactive compounds is of interest, from small organic molecules like alkaloids, flavonoids, terpenoids, antioxidants, and lipids to saponins, carotenoids, and vitamins. The paper highlights that the source of these compounds is also diverse, ranging from biomass, fermentation, plant and animal matrices to synthetic-derived broths. Biomass, within which are agricultural coproducts, underutilized native plants, and marine resources, presents opportunities and challenges. While this provides a rich source of value-added compounds, competition with established animal feed and human food impacts viability. In contrast, fermentation broths generally find applications mainly as sources of proteins or biopharmaceuticals and are usually expensive due to complicated and costly methodologies applied in their preparation.
Hydrophilic ILs offer many advantages, including diverse cations and anions, compared to hydrophobic ILs. They are more biocompatible and task-specific, hence, suitable for aqueous solutions. This preference is because they tend to minimize viscosity problems and overall solvent cost and improve the solubility in extraction processes. Hydrophilic ILs work excellently in extracting small-sized molecules and proteins due to their hydrated forms compared with hydrophobic ILs. Conclusion IL-based systems change the way bioactive compounds are extracted and purified. They stand a better chance at superior to conventional techniques with their increased efficiency, better biocompatibility, and lower associated costs. Optimizing IL formulation and scale-up processes are foreseen in future research that can deliver such benefits.
Acknowledgments
Roy S. would like to acknowledge ‘Scheme for Transformational and Advanced Research in Sciences (STARS)’ (MoE-STARS/STARS-2/2023-0175) by the Ministry of Education, Government of India for promoting translational India-centric research in sciences implemented and managed by Indian Institute of Science (IISc), Bangalore, for their support.
Conflicts of Interest
The authors declared no conflict of interest.
Data Availability
No additional data are available.
Copyright Permissions
Copyright © 2025 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Author Contribution
All authors have equal contributions to this manuscript.
Abbreviation List
ILs, Ionic liquids
ACMs, Activity coefficient models
FVFH, Free-volume flory-huggins
RK, Redlich-kister
References
[1] Vijayaraghavan R, Izgorodin A, Ganesh V et al. Long-term structural and chemical stability of DNA in hydrated ionic liquids. Angew Chem Int Edit, 2010; 49: 1631-1633.[DOI]
[2] Bhattacharjee J, Roy S. Density Functional Waltz: Molecular Theory of Spin Dynamics. J Phys Opt Sci, 2024; 242: 2-4.[DOI]
[3] Swatloski RP, Spear SK, Holbrey JD et al. Dissolution of Cellulose with Ionic Liquids. J Am Chem Soc, 2002; 124: 4974-4975.[DOI]
[4] Fort DA, Remsing RC, Swatloski RP et al. Can ionic liquids dissolve wood? Processing and analysis of lignocellulosic materials with 1-n-butyl-3-methylimidazolium chloride. Green Chem, 2007; 9: 63-69.[DOI]
[5] Bhattacharjee J, Roy S. A review on photocatalysis and nanocatalysts for advanced organic synthesis. Hybrid Adv, 2024; 100268.[DOI]
[6] Passos H, Freire MG, Coutinho JAP. Ionic Liquid Solutions as Extractive Solvents for Value-Added Compounds from Biomass. Green Chem. 2014; 16: 4786-4815.[DOI]
[7] Cláudio AFM, Neves MC, Shimizu K et al. The magic of aqueous solutions of ionic liquids: ionic liquids as a powerful class of catanionic hydrotropes. Green Chem, 2015; 17: 3948-3963.[DOI]
[8] Blesic M, Marques MH, Plechkova NV et al. Self-aggregation of Ionic Liquids: Micelle Formation in Aqueous Solution. Green Chem, 2007; 9: 481-490.[DOI]
[9] Ghoderao PNP, Narayan M, Dalvi V H et al. Patel-Teja cubic equation of state-A review of modifications and applications till 2022. Fluid Phase Equilibr, 2023, 567: 113707.[DOI]
[10] Lopez-Zamora S, Kong J, Escobedo S et al. Thermodynamics and Machine Learning Based Approaches for Vapor–Liquid–Liquid Phase Equilibria in n-Octane/Water, as a Naphtha–Water Surrogate in Water Blends. Processes, 2021; 9:413.[DOI]
[11] Pinkert A, Marsh KN, Pang S et al. Ionic liquids and their interaction with cellulose. Chem Rev, 2009; 109: 6712-6728.[DOI]
[12] Wang H, Gurau G, Rogers RD. Ionic liquid processing of cellulose. Chem Soc Rev, 2012; 41: 1519-1537.[DOI]
[13] Zhu S, Wu Y, Chen Q et al. Dissolution of cellulose with ionic liquids and its application: a mini-review. Green Chem, 2006; 8: 325-327.[DOI]
[14] Soares B, Passos H, Freire CSR et al. Ionic liquids in chromatographic and electrophoretic techniques: toward additional improvements in the separation of natural compounds. Green Chem, 2016; 18: 4582-4604.[DOI]
[15] Ho TD, Zhang C, Hantao LW et al. Ionic liquids in analytical chemistry: fundamentals, advances, and perspectives. Anal Chem, 2014; 86: 262-285.[DOI]
[16] Ventura SPM, e Silva FA, Quental MV et al. Ionic-liquid-mediated extraction and separation processes for bioactive compounds: past, present, and future trends. Chem Rev, 2017; 117: 6984-7052.[DOI]
[17] Koel M. Ionic Liquids in Chemical Analysis. Crit Rev Anal Chem, 2005; 35: 177-192.[DOI]
[18] Bhattacharjee J, Bhakta D, Roy S. Semiconducting Fiber-based Flexible Thermoelectrics. Semiconducting Fibers. CRC Press: Boca Raton, USA, 2024: 138-151.[DOI]
[19] Pokorný J. Are natural antioxidants better -and safer -than synthetic antioxidants?. Eur J Lipid Sci Technol, 2007; 109: 629-642.[DOI]
[20] Zhang L, Kujawinski DM, Federherr E et al. Caffeine in your drink: natural or synthetic?. Anal Chem, 2012; 84: 2805-2810.[DOI]
[21] Capello C, Fischer U, Hungerbühler K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem, 2007; 9: 927-934.[DOI]
[22] Tang B, Bi W, Tian M et al. Application of ionic liquid for extraction and separation of bioactive compounds from plants. J Chromatogr B, 2012; 904: 1-21.[DOI]
[23] Pereira MM, Coutinho JAP, Freire MG. In Ionic Liquids in the Biorefinery Concept: Challenges and Perspectives. The Royal Society of Chemistry: UK, 2016.
[24] Bhattacharjee J, Roy S. Review on green resources and AI for biogenic solar power. Energy Storage Convers, 2024; 2: 457.[DOI]
[25] De A, Bhattacharjee J, Chowdhury SR et al. A comprehensive review on third-generation photovoltaic technologies. J Chem Eng Res Updates, 2023; 10: 1-17.[DOI]
[26] Olszowy-Tomczyk M, Wianowska D. Antioxidant Properties of Selected Flavonoids in Binary Mixtures-Considerations on Myricetin, Kaempferol and Quercetin. Int J Mol Sci, 2023; 24: 10070.[DOI]
[27] Bhattacharjee J, Roy S. Utilizing a Variable Material Approach to Combat Climate Change. Mater Sci Res India, 2024; 20: 141-145.[DOI]
[28] Bogdanov MG, Svinyarov I. Ionic liquid-supported solid -liquid extraction of bioactive alkaloids. II. Kinetics, modeling and mechanism of glaucine extraction from Glaucium flavum Cr.(Papaveraceae). Sep Purif Technol, 2013; 103: 279-288.[DOI]
[29] Bogdanov MG, Svinyarov I, Keremedchieva R et al. Ionic liquid-supported solid -liquid extraction of bioactive alkaloids. I. New HPLC method for quantitative determination of glaucine in Glaucium flavum Cr.(Papaveraceae). Sep Purif Technol, 2012; 97: 221-227.[DOI]
[30] Cláudio AFM, Ferreira AM, Freire MG et al. Enhanced extraction of caffeine from guarana seeds using aqueous solutions of ionic liquids. Green Chem, 2013; 15: 2002-2010.[DOI]
[31] Ressmann AK, Zirbs R, Pressler M et al. Surface-active Ionic Liquids for Micellar Extraction of Piperine from Black Pepper. J Nat Res B, 2013; 68: 1129-1137.[DOI]
[32] Bogdanov MG, Keremedchieva R, Svinyarov I. Ionic liquid-supported solid -liquid extraction of bioactive alkaloids. III. Ionic liquid regeneration and glaucine recovery from ionic liquid-aqueous crude extract of Glaucium flavum Cr.(Papaveraceae). Sep Purif Technol, 2015; 155: 13-19.[DOI]
[33] Bhattacharjee J, Roy S. Green Remediation of Microplastics Using Bionanomaterials. Remediation of Plastic and Microplastic Waste. CRC Press: Boca Raton, USA, 2023: 240-260.[DOI]
[34] Chowdhury SA, Vijayaraghavan R, MacFarlane DR. Distillable Ionic Liquid Extraction of Tannins from Plant Materials. Green Chem, 2010; 12: 1023-1028.[DOI]
[35] Santos JHPM, e Silva FA, Coutinho JAP et al. Ionic liquids as a novel class of electrolytes in polymeric aqueous biphasic systems. Process Biochem, 2015, 50: 661-668.[DOI]
[36] Ressmann AK, Strassl K, Gaertner Pet al. New aspects for biomass processing with ionic liquids: towards the isolation of pharmaceutically active betulin. Green Chem, 2012; 14: 940-944.[DOI]
[37] Ressmann A K, Gaertner P, Bica K. From plant to drug: ionic liquids for the reactive dissolution of biomass. Green Chem, 2011; 13: 1442-1447.[DOI]
[38] Usuki T, Yasuda N, Yoshizawa-Fujita M et al. Extraction and isolation of shikimic acid from Ginkgo biloba leaves utilizing an ionic liquid that dissolves cellulose. Chem Commun, 2011; 47: 10560-10562.[DOI]
[39] Jin W, Yang Q, Huang B et al. Enhanced solubilization and extraction of hydrophobic bioactive compounds using water/ionic liquid mixtures. Green Chem, 2016; 18: 3549-3557.[DOI]
[40] Yang Q, Xing H, Su B et al. The essential role of hydrogen‐bonding interaction in the extractive separation of phenolic compounds by ionic liquid. Aiche J, 2013, 59: 1657-1667.[DOI]
[41] Ma W, Lu Y, Hu R et al. Application of ionic liquids based microwave-assisted extraction of three alkaloids N-nornuciferine, O-nornuciferine, and nuciferine from lotus leaf. Talanta, 2010; 80: 1292-1297.[DOI]
[42] Xu W, Chu K, Li H et al. Ionic liquid-based microwave-assisted extraction of flavonoids from Bauhinia championii (Benth.) Benth. Molecules, 2012; 17: 14323-14335.[DOI]
[43] Du FY, Xiao XH, Luo XJ et al. Application of ionic liquids in the microwave-assisted extraction of polyphenolic compounds from medicinal plants. Talanta, 2009; 78: 1177-1184.[DOI]
[44] Zeng H, Wang Y, Kong J, et al. Ionic liquid-based microwave-assisted extraction of rutin from Chinese medicinal plants. Talanta, 2010; 83: 582-590.[DOI]
[45] Gu H, Chen F, Zhang Q et al. Application of ionic liquids in vacuum microwave-assisted extraction followed by macroporous resin isolation of three flavonoids rutin, hyperoside and hesperidin from Sorbus tianschanica leaves. J Chromatogr B, 2016; 1014: 45-55.[DOI]
[46] Zhou Y, Wu D, Cai P et al. Special effect of ionic liquids on the extraction of flavonoid glycosides from Chrysanthemum morifolium Ramat by microwave assistance. Molecules, 2015; 20: 7683-7699.[DOI]
[47] Zhang Q, Zhao S, Chen J et al. Application of ionic liquid-based microwave-assisted extraction of flavonoids from Scutellaria baicalensis Georgi. J Chromatogr B, 2015; 1002: 411-417.[DOI]
[48] Liu T, Sui X, Zhang R et al. Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from Rosmarinus officinalis. J Chromatogr A, 2011; 1218: 8480-8489.[DOI]
[49] Yansheng C, Zhida Z, Changping L et al. Microwave-assisted extraction of lactones from Ligusticum chuanxiong Hort. using protic ionic liquids. Green Chem, 2011; 13: 666-670.[DOI]
[50] Bezelya A, Küçüktürkmen B, Bozkır A. Microfluidic Devices for Precision Nanoparticle Production. Micro. 2023; 3:822-866.[DOI]
[51] Ma C, Liu T, Yang L et al. Ionic liquid-based microwave-assisted extraction of essential oil and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. J Chromatogr A, 2011; 1218: 8573-8580.[DOI]
[52] Zirbs R, Strassl K, Gaertner P et al. Exploring ionic liquid -biomass interactions: towards the improved isolation of shikimic acid from star anise pods. RSC Adv, 2013; 3: 26010-26016.[DOI]
[53] Ma C, Wang S, Yang L et al. Ionic liquid-aqueous solution ultrasonic-assisted extraction of camptothecin and 10-hydroxycamptothecin from Camptotheca acuminata samara. Chem Eng Process, 2012; 57: 59-64.[DOI]
[54] Yang L, Wang H, Zu Y et al. Ultrasound-assisted extraction of the three terpenoid indole alkaloids vindoline, catharanthine and vinblastine from Catharanthus roseus using ionic liquid aqueous solutions. Chem Eng J, 2011; 172: 705-712.[DOI]
[55] Zhang L, Geng Y, Duan W et al. Ionic liquid‐based ultrasound‐assisted extraction of fangchinoline and tetrandrine from Stephaniae tetrandrae. J Sep Sci, 2009; 32: 3550-3554.[DOI]
[56] Wang W, Li Q, Liu Y et al. Ionic liquid-aqueous solution ultrasonic-assisted extraction of three kinds of alkaloids from Phellodendron amurense Rupr and optimize conditions use response surfac. Ultrason Sonochem, 2015; 24: 13-18.[DOI]
[57] Sun Y, Li W, Wang J. Ionic liquid based ultrasonic assisted extraction of isoflavones from Iris tectorum Maxim and subsequently separation and purification by high-speed counter-current chromatography. J Chromatogr B, 2011; 879: 975-980.[DOI]
[58] Chen F, Hou K, Li S et al. Extraction and Chromatographic Determination of Shikimic Acid in Chinese Conifer Needles with 1-benzyl-3-methylimidazolium Bromide Ionic Liquid Aqueous Solutions. J Anal Methods Chem, 2014; 2014: 1-12.[DOI]
[59] Tan T, Lai CJS, OuYang H, et al. Ionic Liquid-based Ultrasound-assisted Extraction and Aqueous Two-phase System for Analysis of Caffeoylquinic Acids from Flos Lonicerae Japonicae. J Pharm Biomed Anal, 2016; 120: 134-141.[DOI]
[60] Shimojo K, Kamiya N, Tani F et al. Extractive solubilization, structural change, and functional conversion of cytochrome c in ionic liquids via crown ether complexation. Anal Chem, 2006;78:7735-42.[DOI]
[61] Lin H, Zhang Y, Han M et al. Aqueous Ionic Liquid Based Ultrasonic Assisted Extraction of Eight Ginsenosides from Ginseng Root. Ultrason Sonochem, 2013; 20: 680-684.[DOI]
[62] Wu K, Zhang Q, Liu Q et al. Ionic Liquid Surfactant-mediated Ultrasonic-assisted Extraction Coupled to HPLC: Application to Analysis of Tanshinones in Salvia Miltiorrhiza Bunge. J Sep Sci, 2009; 32: 4220-4226.[DOI]
[63] Harde SM, Lonkar SL, Degani MS et al. Ionic Liquid Based Ultrasonic-assisted Extraction of Forskolin from Coleus Forskohlii Roots. Ind Crops Prod, 2014; 61: 258-264.[DOI]
[64] Yang L, Liu Y, Zu YG et al. Optimize the Process of Ionic Liquid-based Ultrasonic-assisted Extraction of Aesculin and Aesculetin from Cortex Fraxini by Response Surface Methodology. Chem Eng J, 2011; 175: 539-547.[DOI]
[65] Lu HT, Jiang Y, Chen F. Preparative High-speed Counter-current Chromatography for Purification of Shikonin from the Chinese Medicinal Plant Lithospermum Erythrorhizon. J Chromatogr A, 2004; 1023: 159-163.[DOI]
[66] Xiao Y, Wang Y, Gao S et al. Determination of the Active Constituents in Arnebia Euchroma (Royle) Johnst. by Ionic Liquid-based Ultrasonic-assisted Extraction High-performance Liquid Chromatography. J Chromatogr B, 2011; 879: 1833-1838.[DOI]
[67] Lu C, Wang H, Lv W et al. Ionic Liquid-based Ultrasonic/Microwave-assisted Extraction Combined with UPLC-MS-MS for the Determination of Tannins in Galla Chinensis. Nat Prod Res, 2012; 26: 1842-1847.[DOI]
[68] Tian Y, Xu Z, Liu Z et al. Fe3O4@SiO2@VAN Nanoadsorbent Followed by GC-MS for the Determination of Polycyclic Aromatic Hydrocarbons at Ultra-Trace Levels in Environmental Water Samples. Nanomaterials 2022, 12, 2921.[DOI]
[69] Lu C, Wang H, Lv W et al. Ionic Liquid-based Ultrasonic/Microwave-assisted Extraction combined with UPLC for the Determination of Anthraquinones in Rhubarb. Chromatographia, 2011; 74: 139-144.[DOI]
[70] Liu F, Wang D, Liu W et al. Ionic Liquid-based Ultrahigh Pressure Extraction of Five Tanshinones from Salvia Miltiorrhiza Bunge. Sep Purif Technol, 2013; 110: 86-92.[DOI]
[71] Dong LL, Fu YJ, Zu YG et al. Negative Pressure Cavitation Accelerated Processing for Extraction of Main Bioactive Flavonoids from Radix Scutellariae. Chem Eng Process, 2011; 50: 780-789.[DOI]
[72] Duan MH, Luo M, Zhao CJ et al. Ionic Liquid-based Negative Pressure Cavitation-assisted Extraction of Three Main Flavonoids from the Pigeonpea Roots and its Pilot-scale Application. Sep Purif Technol, 2013; 107: 26-36.[DOI]
[73] Wang Z, Hu J, Du H et al. Microwave-assisted Ionic Liquid Homogeneous Liquid -liquid Microextraction Coupled with High Performance Liquid Chromatography for the Determination of Anthraquinones in Rheum palmatum L. J Pharm Biomed Anal, 2016; 125: 178-185.[DOI]
[74] Ribeiro BD, Coelho MAZ, Rebelo LPN et al. Ionic Liquids as Additives for Extraction of Saponins and Polyphenols from Mate (Ilex paraguariensis) and Tea (Camellia sinensis). Ind Eng Chem Res, 2013; 52: 12146-12153.[DOI]
[75] Freire MG, Neves CMSS, Marrucho IM et al. Hydrolysis of Tetrafluoroborate and Hexafluorophosphate Counter Ions in Imidazolium-based Ionic Liquids. J Phys Chem A, 2010; 114: 3744-3749.[DOI]
[76] Shaikh AA, Bhattacharjee J, Datta P et al. A comprehensive review of the oxidation states of molybdenum oxides and their diverse applications. Sustain Chem Envir, 2024; 100125.[DOI]
[77] Tan Z, Li F, Xu X. Isolation and Purification of Aloe Anthraquinones based on an Ionic Liquid/Salt Aqueous Two-phase System. Sep Purif Technol, 2012; 98: 150-157.[DOI]
[78] Santos J, Martins M, Silvestre AJD et al. Fractionation of Phenolic Compounds from Lignin Depolymerisation using Polymeric Aqueous Biphasic Systems with Ionic Surfactants as Electrolytes. Green Chem, 2016; 18: 5569-5579.[DOI]
[79] Bhattacharjee J, Roy S. Exploration of Molybdenum Oxide Compounds-A Review. Catal Res, 2024; 4: 1-19.[DOI]
[80] Bhattacharjee J, Roy S. Synergistic insights: electro-organic photocatalysis and nanostructures. Chem Pap, 2024; 78: 8077-8105.[DOI]
[81] Liang P, Peng L. Ionic liquid-modified silica as sorbent for preconcentration of cadmium prior to its determination by flame atomic absorption spectrometry in water samples. Talanta, 2010; 81: 673-677.[DOI]
[82] Qian G, Song H, Yao S. Immobilized chiral tropine ionic liquid on silica gel as adsorbent for separation of metal ions and racemic amino acids. J Chromatogr A, 2016; 1429: 127-133.[DOI]
[83] Liu Y, Sun X, Luo F et al. The preparation of sol -gel materials doped with ionic liquids and trialkyl phosphine oxides for Yttrium(III) uptake. Anal Chim Acta, 2007; 604:107-113.[DOI]
[84] Bhakta D, Gayen D, Bhattacharjee J et al. Repurposing Industrial and Agricultural Wastes as Sustainable Alternatives in Photovoltaic Cells and Dye Degradation. ChemistrySelect, 2024, 9: e202403145.[DOI]
[85] Liu Y, Guo L, Zhu L et al. Removal of Cr(III, VI) by quaternary ammonium and quaternary phosphonium ionic liquids functionalized silica materials. Chem Eng J, 2010; 158: 108-114.[DOI]
[86] Bhattacharjee J, Roy S. Soft Computing Techniques in Wind Conversion Systems. Soft Comput Renew Energ Technol, 2024; 121-142.[DOI]
[87] Marwani HM, Alsafrani AE, Asiri AM et al. Silica-gel particles loaded with an ionic liquid for separation of Zr (IV) prior to its determination by ICP-OES. Sensors, 2016; 16: 1001.[DOI]
[88] Bhattacharjee J, Bhaskar S, Roy S. Green nanomaterials for antimicrobial and anticancer applications. Nano-Eng Funct Inter Multi-Dis App, 2025: 425-444.[DOI]
[89] Guibal E, Gavilan KC, Bunio P et al. Cyphos IL 101 (tetradecyl (trihexyl) phosphonium chloride) immobilized in biopolymer capsules for Hg (II) recovery from HCl solutions. Sep Sci Technol, 2008; 43: 2406-2433.[DOI]
[90] Bhattacharjee J, Roy S. A Comprehensive Review on Integrated Photo Rechargeable Batteries-Supercapacitors, and their Techno-Economic Feasibility. J Photochem Photobiol, 2024: 100257.[DOI]






 Copyright ©
Copyright ©