Non-precious Electrocatalysts for the Hydrogen Evolution Reaction
Song Xie1, Hao Dong1, Xiang Peng1*![]() , Paul K. Chu2*
, Paul K. Chu2*![]()
1Hubei Key Laboratory of Plasma Chemistry and Advanced Materials, Engineering Research Center of Phosphorus Resources Development and Utilization of Ministry of Education, School of Materials Science and Engineering, Wuhan Institute of Technology, Wuhan, Hubei Province, China
2Department of Physics, Department of Materials Science and Engineering, and Department of Biomedical Engineering, City University of Hong Kong, Hong Kong, China
*Correspondence to: Xiang Peng, PhD, Professor, Hubei Key Laboratory of Plasma Chemistry and Advanced Materials, School of Materials Science and Engineering, Wuhan Institute of Technology, 206 Guanggu 1st Road, Wuhan, 430205, Hubei Province, China; Email: xpeng@wit.edu.cn
Paul K. Chu, PhD, Professor, Department of Physics, Department of Materials Science and Engineering, and Department of Biomedical Engineering, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong, 999077, China; Email: paul.chu@cityu.edu.hk
DOI: 10.53964/id.2024011
Abstract
Keywords: hydrogen evolution reaction, non-precious metal electrocatalyst, water splitting, hydrogen adsorption behavior, electronic structure
1 INTRODUCTION
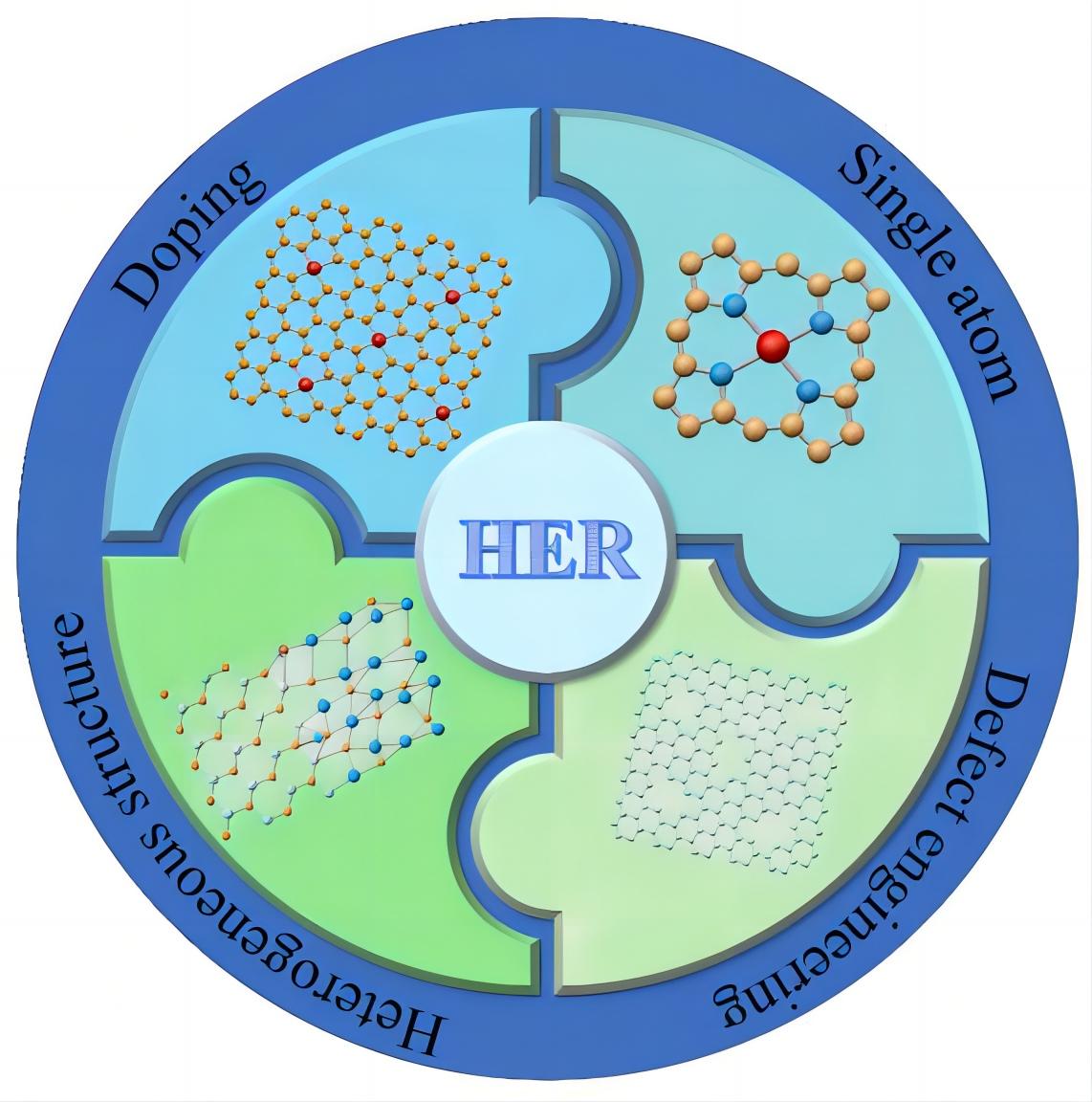
The development of efficient and cost-effective catalysts for the hydrogen evolution reaction (HER) is important to advanced water electrolysis and the widespread adoption of hydrogen as a clean and sustainable energy source[1,2]. While noble metals such as Pt and Pd exhibit exceptional HER activity, their limited natural reserve and high cost pose significant challenges for large-scale implementation[3,4]. As a result, there is a growing interest in exploring non-precious metal-based catalysts as alternatives. Non-precious transition metals, including Fe, Co, Ni, Cr, W, and Mo, are promising due to their earth abundance and lower cost compared to noble metals[5-7]. However, these metals often have unsatisfactory hydrogen adsorption properties and poor catalytic activity. To address these limitations, researchers have proposed and investigated various strategies to optimize the electronic structures of non-precious metal-based catalysts. These strategies include doping, defect engineering, construction of single-atomic catalysts, and engineering heterogeneous structures, as illustrated in Figure 1[8,9].
Figure 1. Scheme of the Regulation Strategies of Non-Precious Transition Metal-Based Catalysts for HER.
2 CATALYTIC MECHANISM OF HER
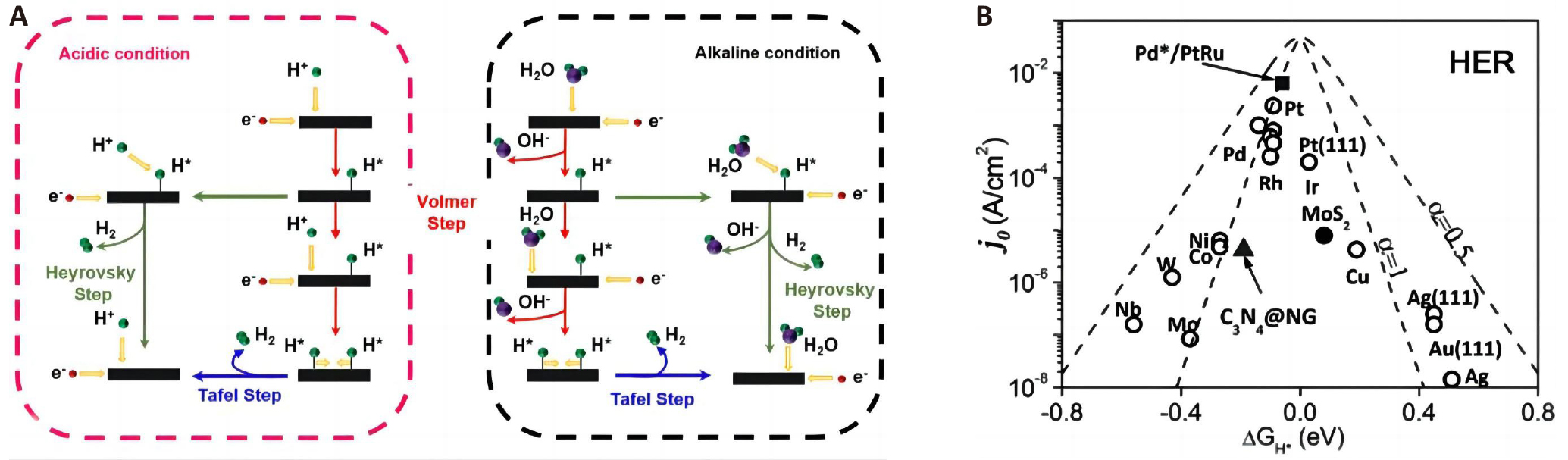
HER involves a two-electron transfer process in water electrolysis occurring on the cathode. During HER, H+ (under acidic conditions) or H2O (under alkaline and neutral conditions) accepts electrons on the catalyst surface to produce high-purity hydrogen gas[10]. Figure 2A illustrates the HER process in both acidic and alkaline media. In acidic conditions, the presence of H+ ions allow the Volmer step to occur on the catalyst’s surface, producing adsorbed hydrogen species (H*). However, in alkaline solutions where H+ ions are scarce, an additional water dissociation step becomes necessary within the Volmer process. Following the Volmer step, the catalyst proceeds to the subsequent steps, namely the Tafel or Heyrovsky steps, to generate H2. These steps contribute to the overall hydrogen evolution process. Thus, the catalyst’s proficiency in water dissociation and activation significantly influences the catalytic activity of alkaline HER. The Gibbs free energy of hydrogen adsorption (ΔGH) on the catalyst plays a crucial role in both the Volmer-Heyrovsky and Volmer-Tafel processes[11]. Generally, a small ΔGH makes it challenging for the hydrogen product to desorb, while a large ΔGH results in weak adsorption of H species[12]. Therefore, the desirable catalysts should possess a thermally neutral ΔGH of zero. The volcano plot, which compares different materials, reveals that noble metals like Pt and Pd exhibit excellent HER activity due to the suitable ΔGH, as shown in Figure 2B[13]. However, their natural scarcity and high cost are hampering widespread industrial applications.
|
Figure 2. HER Catalytic Mechanism. A: Schematic pathways of HER under acidic and alkaline conditions. B: Volcano plot for the HER for various metals. Reproduced from Ref.[13] with permission from Wiley-VCH.
3 REGULATION STRATEGIES OF NON-PRECIOUS METAL-BASED ELECTROCATALYSTS
Recently, extensive research has been conducted on non-precious metals such as Fe-group elements (Fe, Co, and Ni), Cr-group elements (Cr, Mo, and W), and so on to identify suitable alternatives with high natural abundance for HER. However, non-precious metals often have unsatisfactory ΔGH, which is closely related to the electronic structure of the active centers. To address this issue, various strategies have been proposed, for instance, doping, defect engineering, construction of single-atomic catalysts, and heterogeneous structure engineering. These approaches aim to modify the coordination environment of the active sites in non-precious metal-based catalysts, optimize hydrogen adsorption, and enhance the catalytic activity[11,14]. These strategies are important to the development of low-cost and efficient non-precious metal-based catalysts for hydrogen production.
Fe-group elements, including Fe, Co, and Ni are located in group VIII of the periodic table. The volcano plots reveal that these elements such as Co and Ni have high binding energies with hydrogen intermediates (Had), thus showing inferior activity compared to precious metals. However, these elements have good affinity to oxygen consequently facilitating the dissociation of water in alkaline electrolysis[15]. Non-precious transition metal based compounds such as NiSe2[16] and CoS2[17] have shown high potential as substitutes for Pt. Hence, researchers are focusing on developing effective strategies to optimize their performance, for example, by adjusting the phase, electronic, and geometric structures of the active centers.
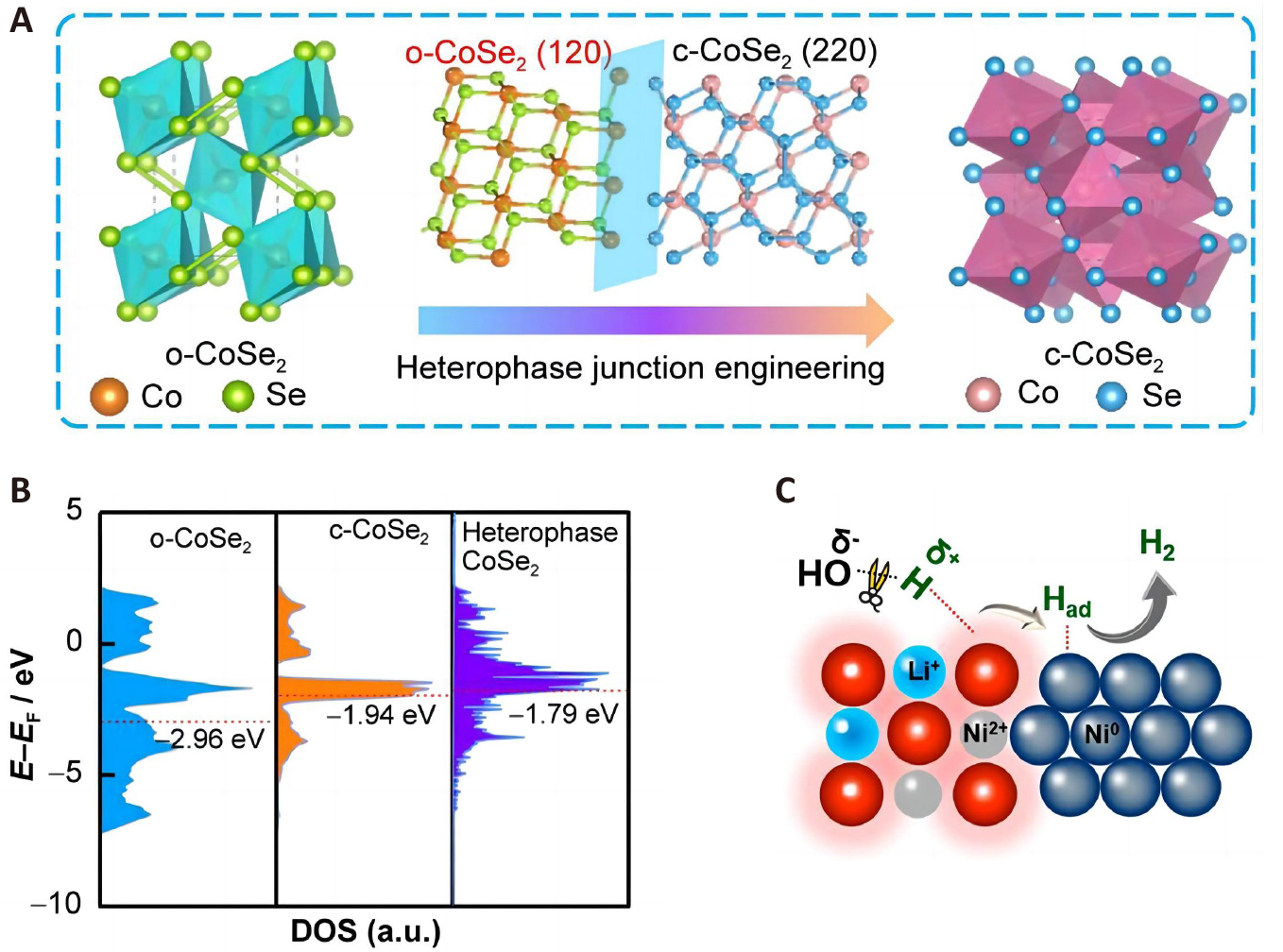
The catalytic activity depends on the surface phase and structure. Therefore, surface optimization can enhance the catalytic activity, increase the number of active sites, and improve the electron transfer efficiency. For instance, Xu et al.[18] have studied the phase transition of cubic CoSe2 (c-CoSe2) to orthorhombic CoSe2 (o-CoSe2) by a heat treatment, which produces a heterophase structure denoted as (n-c-CoSe2), where “n” represents the percent of c-CoSe2 in the composite. The transformation is illustrated in Figure 3A. Density functional theory (DFT) calculations disclose that the d-band center in the heterophase structure (–1.79eV) is higher than those of the pure o-CoSe2 (–2.96eV) and c-CoSe2 (–1.94eV) phases (Figure 3B). This upward shift in the d-band center modifies hydrogen adsorption and hydrogen evolution. Among the different compositions, the structure of c-CoSe2 30% (30-c-CoSe2) exhibits the highest HER activity, requiring an overpotential of only 240mV to achieve a current density of 1,000mA·cm−2. This innovative catalyst design and superior performance highlight the potential of non-precious metal-based catalysts for HER.
|
Figure 3. Regulation Strategies for Co(Ni)-Based Electrocatalysts. A: Schematic illustration showing phase conversion from o-CoSe2 to c-CoSe2 via heterophase junction engineering. B: d-band center diagrams of the o-CoSe2, c-CoSe2, and CoSe2 heterophases. Reproduced from Ref.[18] with permission from Springer Nature. C: Schematic of nanoscale LixNiO/Ni heterostructures. Reproduced from Ref.[19] with permission from American Chemical Society.
As another example, Lu et al.[19] constructed a heterogeneous structure composed of LixNiO nanoclusters and polycrystalline Ni nanocrystals, as shown in Figure 3C. The interface between LixNiO and Ni is closely connected, optimizing the electronic structure of the local sites and facilitating hydrogenation coupling and hydrogen desorption. The catalyst exhibits excellent HER activity over a wide pH range, with overpotentials of only 20, 50, and 36mV to achieve current densities of 10mA·cm–2 in acidic, neutral, and alkaline electrolytes, respectively.
In another study by Xiong et al.[20], the electronic structure of metallic Ni is optimized by constructing a heterostructure with Ni3N. The Ni sites in the heterostructure show optimal hydrogen adsorption attributed to the optimized electronic structure resulting from the electronic interactions at the heterointerface of the two components. Compared to pure Ni and Ni3N, the Ni/Ni3N heterostructure requires an overpotential of only 144mV for a current density of 10mA·cm–2.
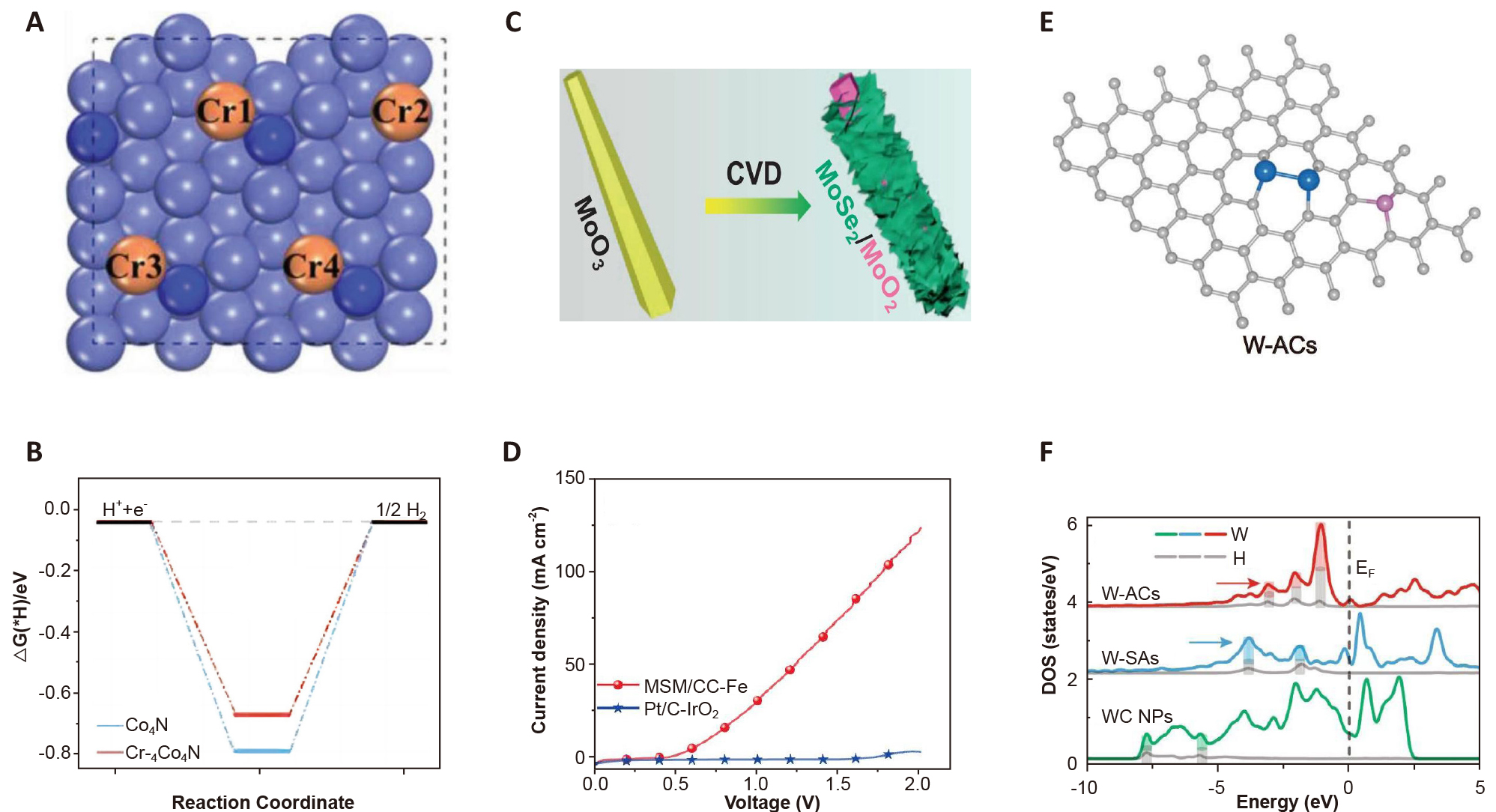
The Cr-group elements, including Cr, Mo, and W, are located in the 6th period of the periodic table and exhibit a wide range of oxidation states. Doping with high-valence transition metals can further enhance the adsorption and dissociation ability of H2O, which is beneficial to the alkaline Volmer and Heyrovsky steps and alkaline HER. Yao et al.[21] have constructed Cr-doped Co4N nanorods as shown in Figure 4A. DFT calculations (Figure 4B) indicate significantly weakened hydrogen adsorption on the Co4N surface after Cr doping, and the Cr-Co4N catalyst requires an overpotential of only 21mV for a current density of 10mA·cm–2. The excellent properties that arise from the chromium dopants modulate the electronic structure of Co4N to endow Co atoms with better hydrogen binding giving rise to accelerated HER.
|
Figure 4. Regulation Strategies for Cr(Mo, W)-Based Electrocatalysts. A: Surface atomic model of Cr-Co4N. B: ΔGH on Co4N and Cr-Co4N. Reproduced from Ref.[21] with permission from Wiley-VCH. C: Schematic representation of the heterogeneous structure of MSM. D: LSV curves of MSM/CC||Fe and Pt/C||IrO2 couples. Reproduced from Ref.[24] with permission from Elsevier. E: Atomic models of W-ACs, W (blue), C (gray), and P (pink). F: Local density-of-states of W and H atoms on the surface of W-SAs, W-ACs, and WC NPs. Reproduced from Ref.[25] with permission from Springer Nature.
Moreover, through defect engineering, by introducing an appropriate quantity of defects, it is possible to modify the electronic structure, surface active site density, and charge transfer performance of catalysts. This approach enhances both the activity and stability of the catalyst. Xu et al.[22] successfully induced phosphorus defects in nickel phosphide using bromine, effectively optimizing the hydrogen adsorption energy by adjusting the vacancy concentration. The resulting catalyst demonstrated exceptional catalytic activity for the HER in alkaline media, achieving an overpotential of only 18mV at 10mA·cm–2. Kumar et al.[23] developed defect-rich catalysts (Ni/MoS2) by integrating uniform nickel nanoparticles into molybdenum disulfide. Ni/MoS2 exhibited remarkable HER performance, with a current density of 10mA·cm−2 at an impressively low overpotential of only 89mV, along with a low Tafel slope of 59mV·dec−1.
Mo-based materials, including carbides, sulfides, selenides, and nitrides, are commonly used in HER[26-28]. However, their activity is typically lower than that of Pt-group metals due to the strong adsorption of hydrogen. To overcome this limitation, an effective strategy is to tune the coordination environment of Mo atoms to optimize hydrogen adsorption. For example, Peng et al.[24] have synthesized the MoSe2/MoO2 heterostructure (MSM) in situ from MoO3 by chemical vapor deposition, as shown in Figure 4C. Owing to electronic transfer and synergistic effects at the heterointerface, the electronic states of the atoms at the interface are optimized. MSM has excellent HER catalytic activity over a wide pH range. As a cathode in a neutral electrolyte together with a Fe anode, the hydrogen production system shows a current density of 10mA·cm–2 at only 0.68V, which is much lower than that the of Pt/C||IrO2 configuration (Figure 4D).
In another work, Feng et al.[29] have designed MoO2-MoN (MoON) heterostructured nanowire arrays with different Mo coordination environments by precisely controlling the Mo-O and Mo-N configurations by programmed nitridation. DFT calculations demonstrate that ΔGH of the Mo site in MoON is closer to 0 than the pure MoO2 and MoN. As a result, the MoON catalyst requires an overpotential of only 335mV to achieve a current density of 1A·cm–2. The optimal ratio of Mo-N and Mo-O in MoON is key to the coordination environment of Mo sites and ΔGH on the Mo sites for enhanced catalytic activity.
W and its compounds share similar properties as Mo for HER. In addition, single-atom catalysts and atomic clusters have outstanding catalytic activity compared to their bulk counterparts[30]. Chen et al.[25] have employed a thermal migration strategy to prepare W atomic clusters (W-ACs), as shown in Figure 4E. W-ACs consist of typical W-W bonds anchored by carbon atoms on the carbon substrate. The single-atom W (W-SAs) coordinates with three carbon atoms, while larger tungsten carbide nanoparticles (WC NPs) have a hexagonal packing structure. DFT calculations show that ΔGH of W-ACs (–0.31eV) is closer to 0 than that of W-SAs (–0.46eV) and WC NPs (–0.61eV). Furthermore, the local density-of-states shown in Figure 4F indicate that W-ACs have favorable interactions with W-H bonds due to the three hybridized peaks near the Fermi level (approximately at –3.1, –2.1, and –1.1eV), resulting in substantial weakening of the W-H interactions and outstanding HER activity exemplified by an overpotential of only 53mV for a current density of 10mA·cm–2.
These regulation strategies are interconnected and interactive. When designing and optimizing catalysts, it is crucial to consider the interplay between these factors comprehensively. By achieving precise control over the electronic structure, catalysts can be enhanced in terms of activity, selectivity, and stability. Table 1 provides an overview of the performance of non-precious transition metal HER electrocatalysts, showcasing recent advancements in this research field.
Table 1. Performance of Non-precious Transition Metal-Based Electrocatalysts for HER
Catalysts |
Electrolyte |
Overpotential (mV) at 10mA·cm–2 |
Tafel slope (mV·dec–1) |
Ref. |
|
Single atom |
Co@CCNS |
1.0M KOH |
70 |
70.6 |
[31] |
FeMo@CoNi-OH/Ni3S2 |
1.0M KOH |
89 |
92.2 |
[32] |
|
Ni-MSACs |
0.5M H2SO4 |
270 |
83.5 |
[33] |
|
CoN3-CSG |
0.5M H2SO4 |
82 |
59 |
[34] |
|
SAP-Mo2C-CS |
0.5M H2SO4 |
36 |
38.1 |
[35] |
|
Heterogeneous structures |
P-CoN/CMO/Co3O4/NF |
1.0M KOH |
109 |
89.1 |
[36] |
Fe-Co0.85Se/FeCo LDH |
1.0M KOH |
37 |
43.9 |
[37] |
|
Mo2N/Ni0.2Mo0.8N |
1.0M KOH |
26 |
31 |
[38] |
|
Cu3P/Ni2P@CF |
1.0M KOH |
88.1 |
94 |
[22] |
|
Ni3N/Mo2N |
1.0M KOH |
20 |
33.8 |
[39] |
|
FeP@CoP |
0.5M H2SO4 |
40 |
67 |
[40] |
|
Co2P-MoNiP/NF |
1.0M KOH |
46 |
49.3 |
[41] |
|
Doping |
Ni0.35Mo0.65O2 |
0.5M H2SO4 |
43 |
37 |
[42] |
C-Ni1−xO |
1.0M KOH |
27 |
36 |
[43] |
|
0.02Ni-MoP |
0.5M H2SO4 |
102 |
58.1 |
[44] |
|
Ni-WP2 NS/CC |
0.5M H2SO4 |
110 |
65 |
[45] |
|
N-NiMoS |
1.0M KOH |
50 |
86 |
[46] |
|
N-doped-CoxS/CC-14 |
1.0M KOH |
89 |
98 |
[47] |
|
W10%-MoxC/C |
1.0M KOH |
178 |
54.3 |
[48] |
|
|
Co-Ni2P |
0.5M H2SO4 |
31 |
47 |
[49] |
Defect engineering |
Ni-Sv-MoS2 |
1.0M KOH |
101 |
66 |
[50] |
Vs-Co3S4@NF |
1.0M KOH |
45 |
66 |
[51] |
|
S-NiFe2O4 |
1.0M KOH |
61 |
80 |
[52] |
|
Mo0.7V0.3Se2 |
0.5M H2SO4 |
114 |
43 |
[53] |
|
SV-Co9S8 |
0.5M H2SO4 |
217 |
97 |
[54] |
|
Cr-Co4N-Nv/NF |
1.0M KOH |
33 |
37 |
[55] |
4 CONCLUSION AND PROSPECTIVES
The development of non-precious metal-based catalysts for HER is crucial to advanced water electrolysis and the widespread adoption of hydrogen as a clean energy source. This commentary highlights the significance of efficient HER catalysts composed of non-precious metals and their compounds. Researchers have proposed various strategies to optimize the electronic structure of the active centers. Future research and development activities are expected to focus on optimizing the catalytic properties by various means, exploration of novel materials and synthetic techniques, and enriching our understanding of the effects on the fundamental processes in HER. As a result, non-precious metal-based catalysts will continue to attract research attention in the effort to continuously improve the catalytic activity, stability, and cost-effectiveness. Moreover, the integration of these catalysts into practical water electrolysis systems and their scalability for large-scale hydrogen production are the key research areas. Advancements in computation, modelling, as well as high-throughput screening techniques, are poised to play a crucial role in accelerating catalyst discovery and optimization. These innovative approaches are expected to empower researchers with the tools to improve the catalyst composition, structure, and configurations, in their pursuit to ultimately identify the ideal catalysts for commercial water splitting and foster a green environment.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No.52002294), the Knowledge Innovation Program of Wuhan-Shuguang Project (No.2022010801020364), City University of Hong Kong Donation Research Grants (No.DON-RMG 9229021 and 9220061), as well as City University of Hong Kong Strategic Research Grant (SRG) (No.7005505).
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
The authors contributed to the manuscript and approved the final version.
Data Availability
No additional data are available.
Copyright Permissions
Copyright © 2024 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Abbreviation List
HER, Hydrogen evolution reaction
DFT, Density functional theory
ΔGH, Gibbs free energy of hydrogen adsorption
References
[1] Mahmood N, Yao Y, Zhang JW et al. Electrocatalysts for hydrogen evolution in alkaline electrolytes: mechanisms, challenges, and prospective solutions. Adv Sci, 2018; 5: 1700464.[DOI]
[2] Feidenhans’l AA, Regmi YN, Wei C et al. Precious metal free hydrogen evolution catalyst design and application. Chem Rev, 2024; 124: 5617-5667.[DOI]
[3] Peng X, Pi C, Zhang X et al. Recent progress of transition metal nitrides for efficient electrocatalytic water splitting. Sustain Energ Fuels, 2019; 3: 366-381.[DOI]
[4] Chatenet M, Pollet BG, Dekel DR et al. Water electrolysis: from textbook knowledge to the latest scientific strategies and industrial developments. Chem Soc Rev, 2022; 51: 4583-4762.[DOI]
[5] Bhunia K, Chandra M, Sharma SK et al. A critical review on transition metal phosphide based catalyst for electrochemical hydrogen evolution reaction: Gibbs free energy, composition, stability, and true identity of active site. Coordin Chem Rev, 2023; 478: 214956.[DOI]
[6] Peng X, Yan Y, Jin X, et al. Recent advance and prospectives of electrocatalysts based on transition metal selenides for efficient water splitting. Nano Energy, 2020; 78: 105234.[DOI]
[7] Maduraiveeran G, Sasidharan M, Jin W. Earth-abundant transition metal and metal oxide nanomaterials: Synthesis and electrochemical applications. Prog Mater Sci, 2019; 106: 100574.[DOI]
[8] Liang W, Zhou M, Li X et al. Oxygen-vacancy-rich MoO2 supported nickel as electrocatalysts to promote alkaline hydrogen evolution and oxidation reactions. Chem Eng J, 2023; 464: 142671.[DOI]
[9] Li T, Ren S, Zhang C, et al. Cobalt single atom anchored on N-doped carbon nanoboxes as typical single-atom catalysts (SACs) for boosting the overall water splitting. Chem Eng J, 2023; 458: 141435.[DOI]
[10] Zhou Z, Pei Z, Wei L, et al. Electrocatalytic hydrogen evolution under neutral pH conditions: current understandings, recent advances, and future prospects. Energ Environ Sci, 2020; 13: 3185-3206.[DOI]
[11] Xiong L, Qiu Y, Peng X et al. Electronic structural engineering of transition metal-based electrocatalysts for the hydrogen evolution reaction. Nano Energy, 2022; 104: 107882.[DOI]
[12] Su H, Pan X, Li S et al. Defect‐engineered two‐dimensional transition metal dichalcogenides towards electrocatalytic hydrogen evolution reaction. Carbon Energy, 2023; 5: e296.[DOI]
[13] Zheng Y, Jiao Y, Jaroniec M et al. Advancing the electrochemistry of the hydrogen‐evolution reaction through combining experiment and theory. Angew Chem Int Edit, 2015; 54: 52-65.[DOI]
[14] Liu Y, Ding J, Li F et al. Modulating hydrogen adsorption via charge transfer at the semiconductor–metal heterointerface for highly efficient hydrogen evolution catalysis. Adv Mater, 2023; 35: 2207114.[DOI]
[15] Anantharaj S, Noda S, Jothi VR et al. Strategies and perspectives to catch the missing pieces in energy‐efficient hydrogen evolution reaction in alkaline media. Angew Chem Int Edit, 2021; 60: 18981-19006.[DOI]
[16] Peng X, Yan Y, Xiong S et al. Se-NiSe2 hybrid nanosheet arrays with self-regulated elemental Se for efficient alkaline water splitting. J Mater Sci Technol, 2022; 118: 136-143.[DOI]
[17] Zhang J, Xiao W, Xi P et al. Activating and optimizing activity of CoS2 for hydrogen evolution reaction through the synergic effect of N dopants and S vacancies. ACS Energy Lett, 2017; 2: 1022-1028.[DOI]
[18] Xu BC, Miao YP, Mao MQ et al. Heterophase junction engineering-induced Co spin-state modulation of CoSe2 for large-current hydrogen evolution reaction. Rare Metals, 2024; 43: 1-11.[DOI]
[19] Lu K, Liu Y, Lin F, et al. LixNiO/Ni heterostructure with strong basic lattice oxygen enables electrocatalytic hydrogen evolution with Pt-like activity. J Am Chem Soc, 2020; 142: 12613-12619.[DOI]
[20] Xiong L, Qiu Y, Dong H et al. Metallic Ni3N/Ni heterostructure for efficient hydrogen evolution reaction. Int J Hydrogen Energ, 2024; 59: 400-407.[DOI]
[21] Yao N, Li P, Zhou Z et al. Synergistically tuning water and hydrogen binding abilities over Co4N by Cr doping for exceptional alkaline hydrogen evolution electrocatalysis. Adv Energy Mater, 2019; 9: 1902449.[DOI]
[22] Xu T, Jiao D, Zhang L et al. Br-induced P-poor defective nickel phosphide for highly efficient overall water splitting. Appl Catal B-Environ, 2022; 316: 121686.[DOI]
[23] Kumar M, Ramulu B, Yu JS. Nanoarchitectonic Ni-doped edge dislocation defect-rich MoS2 boosting catalytic activity in electrochemical hydrogen production. J Clean Prod, 2023; 414: 137589.[DOI]
[24] Peng X, Xie S, Xiong S et al. Ultralow-voltage hydrogen production and simultaneous Rhodamine B beneficiation in neutral wastewater. J Energy Chem, 2023; 81: 574-582.[DOI]
[25] Chen Z, Xu Y, Ding D et al. Thermal migration towards constructing WW dual-sites for boosted alkaline hydrogen evolution reaction. Nat Commun, 2022; 13: 763.[DOI]
[26] Liu W, Wang X, Wang F et al. A durable and pH-universal self-standing MoC–Mo2C heterojunction electrode for efficient hydrogen evolution reaction. Nat Commun, 2021; 12: 6776.[DOI]
[27] Li R, Xie S, Zeng Y et al. Synergistic dual-regulating the electronic structure of NiMo selenides composite for highly efficient hydrogen evolution reaction. Fuel, 2024; 358: 130203.[DOI]
[28] Anjum MAR, Jeong HY, Lee MH et al. Efficient hydrogen evolution reaction catalysis in alkaline media by all‐in‐one MoS2 with multifunctional active sites. Adv Mater, 2018; 30: 1707105.[DOI]
[29] Feng S, Li D, Dong H et al. Tailoring the Mo-N/Mo-O configuration in MoO2/Mo2N heterostructure for ampere-level current density hydrogen production. Appl Catal B-Environ, 2024; 342: 123451.[DOI]
[30] Wang B, Zhu X, Pei X et al. Room-temperature laser planting of high-loading single-atom catalysts for high-efficiency electrocatalytic hydrogen evolution. J Am Chem Soc, 2023; 145: 13788-13795.[DOI]
[31] Huang Y, Hu Z, Huang L et al. Phosphorus-modified cobalt single-atom catalysts loaded on crosslinked carbon nanosheets for efficient alkaline hydrogen evolution reaction. Nanoscale, 2023; 15: 3550-3559.[DOI]
[32] Fang W, Wu Y, Xin S et al. Fe and Mo dual-site single-atom catalysts for high-efficiency wide-pH hydrogen evolution and alkaline overall water splitting. Chem Eng J, 2023; 468: 143605.[DOI]
[33] Park JW, Park G, Kim M et al. Ni-single atom decorated mesoporous carbon electrocatalysts for hydrogen evolution reaction. Chem Eng J, 2023; 468: 143733.[DOI]
[34] Huang K, Wei Z, Liu J, et al. Engineering the morphology and microenvironment of a graphene‐supported Co‐N‐C single‐atom electrocatalyst for enhanced hydrogen evolution. Small, 2022; 18: 2201139.[DOI]
[35] Fu W, Wang Y, Tian W et al. Non‐metal single‐phosphorus‐atom catalysis of hydrogen evolution. Angew Chem, 2020; 132: 23999-24007.[DOI]
[36] Ran J, Zhang Z, Feng H et al. P-doped Co-based nanoarray heterojunction with multi-interfaces for complementary HER/OER electrocatalysts towards high-efficiency overall water splitting in alkaline. Int J Hydrogen Energ, 2024; 64: 935-946.[DOI]
[37] Yu H, Zhu S, Hao Y et al. Modulating local interfacial bonding environment of heterostructures for energy‐saving hydrogen production at high current densities. Adv Funct Mater, 2023; 33: 2212811.[DOI]
[38] Wang X, Zhang X, Xu Y et al. Heterojunction Mo-based binary and ternary nitride catalysts with Pt-like activity for the hydrogen evolution reactio. Chem Eng J, 2023; 470: 144370.[DOI]
[39] Wang T, Miao L, Zheng S et al. Interfacial engineering of Ni3N/Mo2N heterojunctions for urea-assisted hydrogen evolution reaction. ACS Catal, 2023; 13: 4091-4100.[DOI]
[40] Zhang K, Jia J, Yang E et al. Work-function-induced electron rearrangement of in-plane FeP@CoP heterojunction enhances all pH range and alkaline seawater hydrogen evolution reaction. Nano Energy, 2023; 114: 108601.[DOI]
[41] Zhao Y, Zhang H, Chen J et al. Interfacial electronic engineering of heterostructured Co2P-MoNiP/NF nano-sea-urchin catalysts for efficient and stable hydrogen evolution via water/seawater splitting. Chem Eng J, 2023; 477: 147092.[DOI]
[42] Zeng H, Chen S, Jin YQ et al. Electron density modulation of metallic MoO2 by Ni doping to produce excellent hydrogen evolution and oxidation activities in acid. ACS Energy Lett, 2020; 5: 1908-1915.[DOI]
[43] Kou T, Chen M, Wu F et al. Carbon doping switching on the hydrogen adsorption activity of NiO for hydrogen evolution reaction. Nat Commun, 2020; 11: 590.[DOI]
[44] Xiao W, Zhang L, Bukhvalov D et al. Hierarchical ultrathin carbon encapsulating transition metal doped MoP electrocatalysts for efficient and pH-universal hydrogen evolution reaction. Nano Energy, 2020; 70: 104445.[DOI]
[45] Liu W, Geng P, Li S et al. Tuning electronic configuration of WP2 nanosheet arrays via nickel doping for high-efficiency hydrogen evolution reaction. J Energy Chem, 2021; 55: 17-24.[DOI]
[46] Huang C, Yu L, Zhang W et al. N-doped Ni-Mo based sulfides for high-efficiency and stable hydrogen evolution reaction. Appl Catal B-Environ, 2020; 276: 119137.[DOI]
[47] Zeng P, Meng Y, Liu Z et al. N‐doping coupled with Co‐Vacancies activating sulfur atoms and narrowing bandgap for CoS toward synergistically accelerating hydrogen evolution. Small, 2023; 19: 2301279.[DOI]
[48] Wang Q, Mi F, Li J et al. Tungsten doping generated Mo2C-MoC heterostructure to improve HER performance in alkaline solution. Electrochim Acta, 2021; 370: 137796.[DOI]
[49] Xiong L, Wang B, Cai H et al. Understanding the doping effect on hydrogen evolution activity of transition-metal phosphides: Modeled with Ni2P. Appl Catal B-Environ, 2021; 295: 120283.[DOI]
[50] Ma Y, Leng D, Zhang X et al. Enhanced activities in alkaline hydrogen and oxygen evolution reactions on MoS2 Electrocatalysts by in‐plane sulfur defects coupled with transition metal doping. Small, 2022; 18: 2203173.[DOI]
[51] Wang Q, Xu H, Qian X et al. Sulfur vacancies engineered self-supported Co3S4 nanoflowers as an efficient bifunctional catalyst for electrochemical water splitting. Appl Catal B-Environ, 2023; 322: 122104.[DOI]
[52] Jin J, Yin J, Liu H et al. Atomic sulfur filling oxygen vacancies optimizes H absorption and boosts the hydrogen evolution reaction in alkaline media. Angew Chem Int Edit, 2021; 133: 14236-14242.[DOI]
[53] Kwon IS, Kwak IH, Debela TT et al. Phase-transition Mo1–xVxSe2 alloy nanosheets with rich V–Se vacancies and their enhanced catalytic performance of hydrogen evolution reaction. ACS Nano, 2021; 15: 14672-14682.[DOI]
[54] Wu F, Yang R, Lu S et al. Unveiling partial transformation and activity origin of sulfur vacancies for hydrogen evolution. ACS Energy Lett, 2022; 7: 4198-4203.[DOI]
[55] Zhang Y, Ma Y, Yuan W et al. Symmetry or asymmetry: which one is the platform of nitrogen vacancies for alkaline hydrogen evolution. Mater Horiz, 2023; 10: 4480-4487.[DOI]
Brief of Corresponding Author(s)
Paul K. Chu He is the Chair Professor of Materials Engineering in the Department of Physics, Department of Materials Science & Engineering, and Department of Biomedical Engineering at City University of Hong Kong. He received his BS in mathematics from The Ohio State University and MS/PhD in chemistry from Cornell University. His research interests are quite diverse spanning plasma science as well as materials science and engineering. He has received more than 30 research and technical awards and is a highly cited researcher (8 consecutive years since 2026) with an h-index of 145. Besides being a fellow and council member of the Hong Kong Academy of Engineering Sciences, he is a fellow of the American Physical Society (APS), American Vacuum Society (AVS), Institute of Electrical and Electronics Engineers (IEEE), Materials Research Society (MRS), and Hong Kong Institution of Engineers (HKIE). |
Xiang Peng He is a Professor and PhD supervisor of Materials Science and Engineering at the Wuhan Institute of Technology (WIT). He received his PhD in Physics and Materials Science from the City University of Hong Kong in 2017. After completing his doctoral studies, he continued to expand his expertise as a Postdoctoral Fellow at the City University of Hong Kong from 2017 to 2018. His research focuses on the design of functional nanomaterials and their application in energy storage and conversion. He has authored over 80 peer-reviewed papers, accumulating more than 6,500 citations with an h-index of 46. He currently serves as the Associate Editor of Innovation Discovery as well as guest editor-in-chief and editorial board member of approximately 10 journals. |







 Copyright ©
Copyright ©