Catalytic Conversion of Complex Reactant Systems Makes the World Greener, More Sustainable and Hopeful: Where and How to Go?
Huiru Yang1, Ping Hu1, Zheng Li1, Changwei Hu1*
1Key Laboratory of Green Chemistry and Technology Ministry of Education, College of Chemistry, Sichuan University, Chengdu, Sichuan Province, China
*Correspondence to: Changwei Hu, PhD, Professor, Key Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064, Sichuan Province, China; Email: changweihu@scu.edu.cn
DOI: 10.53964/id.2024008
Abstract
Catalysis plays vital roles in chemical industries, which brings out significant convenience to human life in many aspects. With the development of both science and technology, various advanced and multivariate technologies have been cultivated in catalysis. Herein, a brief introduction in the frontiers of catalysis chemistry including single-atom catalysts, single crystal-based model catalysts, molecular switches catalysis and metal-organic frameworks / covalent-organic frameworks based catalysts has been given. Complex reactant systems (Biomass, vehicle exhausted gases, plastic wastes, and waste water) that featured with complex compositions, chemical-bonding and interactions endow researchers huge challenges to convert them to value-added chemicals or treat them to less-toxic or even non-toxic chemicals. Successes on catalytic conversion and degradation of these complex reactant systems sheds light on constructing a greener and more sustainable Earth via using both traditional and advanced catalysts combined with rational strategies. Progresses that achieved on the catalytic degradation of these complex reactant systems have been summarized here. However, the remaining issues concerned with these complex reactant systems drive us to think about how to further deal with them to achieve higher sustainability. Herein, four prospectives are proposed. We truly believe that with the continuous efforts on developing new catalysts with high efficiency and exploring reaction mechanisms under actual conditions towards these complex reactant systems, clearer understanding and better results could be achieved ultimately.
Keywords: catalytic conversion, complex reactant systems, biomass, vehicle exhausted gases, plastic wastes, waste water
1 INTRODUCTION
Since the concept of “catalysis” and “catalyst” was firstly proposed by Berzelius as early as 1835, considerable attentions have been paid to this field[1-3]. At present, the majority of the chemical processes and products closely related to human life is related to catalytic technologies, such as synthesis of ammonia[4], synthesis of sulfuric and nitric acid[5], Fischer-Tropsch process[6,7], petroleum refining including hydrocracking or hydro isomerization[8,9], and polymers production[10,11] etc. Therefore, the development of high-performance catalysts remains an ongoing and essential goal. Aiming to achieve sustainable progresses in catalysis, a multidisciplinary integrated discipline, the developed progress of various technologies and assistance from other fields are inevitably needed. Nowadays, with the rapid development of science and technology, the field of catalysis has evolved from the concept of simply facilitating reactions to proceed into precisely controlling of catalytic reactions or designing catalysts with fine structures or even at atomic level. These improvements and progresses benefit by the development of various reliable and advanced technologies, achieving transformation of the catalytic process from a “black box” reaction into touchable and regulable reaction. Various preparation methods have been developed subsequently. Here, we simply define these catalysts that prepared by simple methods or widely used in industries as traditional ones, and these prepared by more elaborate methods or with fine structure as advanced catalysts. For the preparation of traditional catalysts, methods that are rather easier to operate and more suitable for large production include impregnation, precipitation, comixing, thermal deposition, leaching, melting, electrolytic route, ion exchange and coat etc. have been developed well. Moreover, modifications on catalysts by defect engineering strategies[12] or salt-assisted gas-liquid interfacial element doping[13] etc. have been also explored. Besides, as for the synthesis of advanced catalysts, more elaborate operations and more stringent synthesis conditions are usually required. For example, sol-gel method, microwave ultrasonic method, supercritical technology, physical vapor deposition method, chemical vapor deposition method, solid-phase synthesis and template method, hydrothermal or solvothermal method, micro-emulsion method, electrochemical deposition method, self-assemble and micro-crystallization technologies etc[14,15]. These methods are beneficial for the synthesis of catalysts with fine structure, and the obtained catalysts are featured with specific structural characteristics, morphologies or active sites controllably.
Several new techniques or concepts that stepping into the vision of researchers are proposed here. Single-atom catalysts (SACs) are emerging as one of the research hot-pots recently. Figure 1 displays the number of publications in literature with the word “Single Atom Catalysts/Catalysis” contained in the title of articles since 2011[16], indicating its prosperous development trend. SACs are totally different from traditional nano-catalysts or subnano-catalysts. When metal dispersion reaches 100%, that is, single atoms are produced, various new characters are introduced accordingly, such as sharply increased surface free energy, quantum size effect, unsaturated coordination environment and support-metal interactions. These unique characteristics are significantly different from those of nano or sub-nano particles, vesting SACs superior catalytic performance[17].
|
Figure 1. Number of Publications Where "Single Atom Catalysts/Catalysis" Is in the Title Since 2011. The statistic data are from Web of Science. Reproduced from Ref.[16] with permission from American Chemical Society.
Meanwhile, it can change the adsorption/desorption selectivity to substrates, intermediates or products on the active sites of catalyst, thus affecting the reaction kinetics. Moreover, SACs are typically loaded with low amount of metal, which greatly improves the utilization efficiency of metal atoms. Zhang et al.[18] firstly reported the single-atom Pt1/FeOx catalyst, which showed both excellent stability and high activity for CO oxidation and preferential oxidation of CO in H2. Later, in 2012, Kyriakou et al.[19] achieved the high selectivity towards the hydrogenation of styrene and acetylene by using a SAC where isolated Pd atoms were loaded on the Cu (111) plane. By using means of desorption measurements combining with high-resolution scanning tunneling microscopy (STM), the SACs were characterized clearly, as shown in Figure 2. Meanwhile, theoretical calculation revealed that the isolated Pd atom was capable of lowering the energy barrier to both hydrogen uptake on and subsequent desorption from the Cu metal surface substantially[19]. Besides, employing Fe/SiO2 catalyst where single Fe atoms embedded in a silica matrix, enabled the direct conversion of methane to form ethylene and aromatics exclusively. The absence of adjacent Fe sites in the SACs prevented C-C coupling, further oligomerization, and hence, prohibited coke deposition[20]. The single-atom Pd1/TiO2 catalyst exhibited high catalytic activity in the hydrogenation of C=C bonds, exceeding that of commercial Pd catalysts by a factor of 9, reported by Liu et al[21]. Due to the outstanding and unique catalytic performance of SACs, continuous attentions and deeper researches have been paid to, thus more and more diversified monoatomic catalysts have been prepared, including Pt[22,23], Pd[24,25], Au[26,27], Ir[28], Ag[29], Rh[30], Fe[31,32], Co[33] etc. Nowadays, research interests over SACs are still expanding, and a variety of methods for synthesizing SACs are also being developed. Remarkable achievements have been also obtained by applying these SACs in multiple reactions[16,34-36]. Besides, the applications of MXene-based SACs for energy conversion have also attracted much attentions[37].
|
Figure 2. STM Images Showing Atomically Dispersed Pd Atoms in a Cu(111) Surface and Hydrogen Atoms That Have Dissociated and Spilled over onto the Cu Surface. Reproduced from Ref.[19] with permission from AAAS (Science).
Surface chemistry and catalysis based on single crystals have also gained wide research attentions, realizing the construction of another types of “model catalysts” that active sites are clearly built and demonstrated, thereby a clear activity-relationship can be also easily constructed. Different from nanocrystals, single crystal contributes to synthesize model catalysts with uniform and well-defined surface structures, which is an effective strategy to catalyze simple or precise reactions with deeper explorations on both reactivity and structure-activity relationship. Comparisons between single crystal-based catalysts and nano crystals-based catalysts have been clearly elaborated by Chen et al.[38], as illustrated in Figure 3. Moreover, single crystal-based model catalysts based on single metallic crystal phases have been also developed[39]. Noteworthy, in view of the well-developed and advanced spectroscopic and microscopic techniques, in-situ characterizations of surface structures and active species of single crystal-based catalysts have been easily accomplished[40].
|
Figure 3. Comparisons Between Single Crystal-Based Catalysts and Nano Crystal-Based Catalysts. Reproduced from Ref.[38] with permission from Elsevier.
In recent years, a novel concept “molecular switches” has entered the visions of researchers[41]. Molecular switches are defined as molecules in which bonds, electronic state, or structure change in response to environmental stimuli such as light, heat, electrical field, pH, atmosphere, or pressure[42,43]. They are widely used in photonics, bioscience, chiral synthesis, computer science for logical calculations, DNA sensors and chiral synthesis etc. Based on the ready availability, precise tunability and environment-adjustable properties, the applications of molecular switches in catalytic processes are gradually sieving attentions. Several typical molecular switches catalysis, such as “Redox switches”, “Photo switches”, “pH switches” and “Ligand switches” are introduced here. Hu et al.[44] proposed the Fe-based redox-switches to modulate the catalytic performance of ZSM-5-based catalyst towards the methanol-to-aromatics reaction, which enabled a para-xylene (PX) yield of up to 60% that was 3-6 times higher than previously reported values. Similarly, de Vries and Otten[45] applied this strategy to synthesis a formazanate-Zn catalyst with redox-switching properties, and accomplished the reversible on/off switching of lactide cyclopolymerization. A Ru-catalyzed redox-switchable catalytic system was reported to convert ketone into alcohol, and the catalytic activity of the complex was reversibly switched off and back on again over the course of the hydrogen transfer reaction[46].
Utilizing light to start or stop the chemical reactions has been studied widely, and molecular photo-switches are chromophores undergoing reversible isomerization between different states upon irradiation with light[47]. These molecular photo-switches find diverse applications as light-stimulated components in various research fields, where the ability to toggle the molecule between distinct states allows reversible control over the investigated systems. In 2011, Wang et al.[48] reported a light-driven molecular motor with integrated catalytic functions in which the stepwise change in configuration during a 360° unidirectional rotary cycle governed the catalyst performance with respect to both activity and absolute stereo-control in an asymmetric transformation. The schematic procedure of this system is proposed in Figure 4.
|
Figure 4. Schematic Illustration of This Integrated Unidirectional Light-Driven Molecular Motor and Bi-Functional Organocatalyst (top) and the Molecular Structure of (2R,2′R)-(P,P)-trans-1 (bottom). Reproduced from Ref.[48] with permission from AAAS (Science).
Neilson et al.[49] reported a photo-switchable organocatalysis system wheret light was used to modulate the catalytic activities of N-heterocyclic carbenes, that is, the rates of different reactions were successfully modelled between fast and slow states by alternating exposure to visible or UV light, respectively. The recent booms in the development of photo-switches catalysis indicate that they are valuable tools for the reversible activation/deactivation of different molecules. pH-responsive catalytic systems have also been developed well[50]. In 2008, Balof et al.[51], reported the first pH-responsive catalyst with high and adjustable activity to olefin metathesis which was decorated with dimethylamino groups that were ideal pH-responsive groups. Another switchable organo-catalyst based on a rotaxane architecture had been developed by Blanco et al.[52], which could be switched “on” or “off” by adding acids or bases, acting the role to move the rotaxane ring to either conceal or reveal the catalytic site, as shown in Figure 5. The system could effectively control the reaction rate of Michael addition of an aliphatic thiol to trans-cinnamaldehyde, either by adding the catalyst in its active form or by in situ switching.
|
Figure 5. Diagram of Acid-Base Switching of the Position of the Macrocycle in Rotaxane. Reproduced from Ref.[52] with permission from Wiley.
Besides, an elegant molecular switch for the reversible formation of emulsions by simple pH variation was reported by Walace et al.[53] Very recently, Zhang et al.[54] reported the pH-Triggered molecular switch toward texture-regulated Zn Anode. As reported, the periodical fluctuation of H+/OH- was feasible to trigger the pH-dependent molecular switch reaction to modulate Zn/electrolyte interface dynamically, achieving the reversible transformation between γ-butyrolactone and γ-hydroxybutyrate at the Zn/electrolyte interface, and enhancing the reversibility of Zn. Another type of molecular switches such as “Ligand switches” has also been applied to catalysis[55]. The weak-link approach, which builds upon the growing number of methods for preparing metal-containing macrocyclic complexes, allows us to synthesize fine structures through coordination chemistry and hemilabile ligands with transition-metal-based regulatory sites that can be modulated through ligand displacement reactions. In this manner, catalytic processes can be turned on or off depending upon the presence or absence of small molecules or elemental anion effectors, as called “Ligand switches”[56]. Foy et al.[57] developed coordination-coupled deprotonation (CCD) driven signaling and signal enhancement sequences by using the zinc(II)-initiated CCD of a hydrazone switch to instigate an acid catalyzed imine bond hydrolysis that separated a quencher from fluorophores, thus leading to emission amplification. Figure 6 disclosed the working procedure of this system. Similar hydrazone switches had been also proposed by Pramanik and Aprahamian[58], and later applied for sequestering zinc(II) from the environment.
|
Figure 6. The Imidazolyl-Containing Switch 1(E) Underwent CCD upon Addition of Zinc(II) Resulting in the Release of a Proton to the Environment. The Acidification of the Solution Can Be Used to Turn “On” pH-Sensitive Dyes (S/P), Leading to Fluorescence Output. Reproduced from Ref.[57] from the Royal Society of Chemistry.
In addition to the SACs, molecular-switch catalysis mentioned above, the applications of metal-organic frameworks (MOFs) and covalent-organic frameworks (COFs) materials in catalysis are also blooming, since they were firstly used as catalysts in 2000 and 2011, respectively[59]. MOF, a rapidly developing interdisciplinary novel material, attracts extensive interests in catalysis since it is a relatively new class of crystalline porous materials with high surface area, structural diversity, and tailorability[60]. In general, there were three parts of MOFs that could be utilized as active sites for catalytic process including metal nodes, organic linkers and pore space, which was clearly presented in Figure 7[61].
|
Mature and diverse approaches to synthesize MOFs, such as solution impregnation, double-solvent approach, chemical vapor deposition, solid grinding, thermal decomposition and so forth have been well developed[62]. Accurate control over the microenvironment of catalytic sites in MOFs that dominating the molecular/electron-transfer process and regulating the intrinsic activity of catalytic sites have got deeper and precise investigations[63]. Furthermore, these materials also exhibit superior catalytic performance in a variety of reactions such as electrochemical water splitting[64], treatments of waste water[65], electrocatalytic/photocatalytic CO2 reductions[66,67], hydrogen/oxygen evolution reactions[68], oxygen reduction reactions, nitrogen reduction reactions[69], CO2 selective hydrogenation[70], photocatalytic hydrogen peroxide production[71], and photocatalytic nitrogen fixation etc[72]. Meanwhile, graphene/MOFs composites are also widely used in energy and environmental protection fields, which have been reviewed by Nazir et al[73]. Furthermore, by means of decorations, MOFs derivatives have attracted widespread attentions due to their outstanding merits like large specific surface area, tunable porosity, unsaturated active sites inherited from MOFs precursors and higher water/thermal stability. Hence, they are further widely applied in catalyzing the removal of pollutants from water[74,75]. The advantages of using MOFs as catalysts, such as high metal-atom utilization efficiency, high reactivity, high selectivity and stable reusability etc. stimulate researchers to contribute more efforts in this field. The other kind of new material, COFs, is also acting as star in catalysis field due to adjustable porosities, high thermal and chemical stabilities, tunable functionalities and flexibility in installing catalytic active sites[76-79]. COFs are a class of crystalline porous polymers allowing for atomically precise integration of organic building units to create order skeletons and nanopores in the repetitive manner[77,80]. Based on the structure characteristics of COFs, three synthetic methodologies to construct COF-based catalysts have been systematically developed and further well elaborated, which can be simply summarized as in-situ synthesis, bottom-up synthesis and post-synthetic approaches[76]. Furthermore, COFs can be constructed into either two-dimensional or three-dimensional porous networks depending on their building block dimensions[79,81]. Currently, the post-synthetic modifications of COFs are introduced, such as (1) incorporation of a variety of active metal species by using metal complexation through coordination chemistry, (2) covalent bond formation between existing pendant groups and incoming constituents and (3) chemical conversion of linkages, so that both unique structure features and reactivity can be obtained[82]. Applications of COFs in the field of thermal catalysis, photocatalysis and electrocatalysis including organic coupling reactions, oxidation-reduction reactions, addition reactions, degradation/conversion reactions and so on have been all widely explored and investigated[83,84].
According to their structures and compositions, COF-based catalysts are classified as COFs with reactive skeletons, with reactive pendant groups and with reactive metals[76]. Compared with the well-developed MOFs and/or COFs-based catalysts, the development of MOF/COF composite-based catalysts merely starts[85]. These composites include MOF/COF hybrids, including MOF@COF and COF@MOF with core-shell structure, MOF + COF, C-MOF, and COF-in-MOF. Integration of MOFs and COFs is an effective strategy to construct new types of catalysts[86]. A detailed description for synthesizing a Pd doped MOF@COF core-shell material was presented in Figure 8, reported by Sun et al[87].
|
Figure 8. Schematic Scope of the Preparation of Pd Doped MOF@COF Core-Shell Material. Reproduced from Ref.[87] with permission from Wiley.
Despite model catalysts that with precisely controlled active sites we commented above, a great deal of model reactions, such as CO oxidation, NH3 synthesis/deposition, and H2O deposition etc. have been also studied extensively, and much dazzling achievements have been made. As for the CO oxidation reaction, various catalysts including noble-metal based catalysts such as Pt, Au and Pd[88,89], non-noble metal catalysts[90] have been synthesized successfully wherein efforts towards fulfilling high-catalytic performance via chemical-tuning strategies, in which the size, structure, shape and degree of alloys are controlled to alter the electronic structure, catalyst-oxide support interactions and resulting interactions between adsorbates and the catalyst etc[91]. Furthermore, detailed CO oxidation pathways over typical catalysts with different structural characteristics such as single atoms, nanoclusters or nanoparticles have been clearly elaborated[92,93] . Meanwhile, active species were also clarified[94,95]. Beniya et al.[91] had reviewed the progresses that achieved on CO oxidation reactions catalyzed by SACs, and then addressed the necessity of the development of low-cost, environment-friendly automotive catalysts, as shown in Figure 9.
|
Figure 9. Necessity of the Development of Low-Cost, Environment-Friendly Automotive Catalysts. Reproduced from Ref.[91] with permission from Springer.
Likewise, systemic investigations on the NH3 synthesis/deposition reactions[96,97] and H2O deposition reactions[98,99] have been conducted. In summary, in-depth systematic explorations on both model catalysts as well as model reactions are highly effective in establishing surface structure-activity relations and identifying active sites. To achieve these goals, a prerequisite is to fabricate a series of model catalysts with uniform and well-defined surface structures varying around specific structural parameters. Meanwhile, selecting model reactions that comprised of simple components as probe reactions provides possibilities to clarify real reaction mechanism.
To date, much efforts have been devoted to these field of catalysis, and remarkable achievements have been obtained accordingly, which is of great importance to improve the quality of human life. Therefore, lights have been shed on the conversion and utilization of complex reactant systems where compositions, chemical bonding and interactions are highly complicated due to the vigorous development of catalysis. Totally different from the simple systems that comprises of only single component, the complex reactant systems such as biomass (cellulose, hemicellulose, lignin, and chitin), vehicle exhausted gases, wasted plastics and waste water etc. have rather complexed compositions. Therefore, the catalytic conversion or degradation of these complex reactant systems not only faces the challenges that the applied catalytic systems should be effective for multiple components, but also encounters the obstacles that large quantities of pollutants or impurities included in these systems would lead to the poisoning or deactivation of catalysts. Accordingly, long-term sustainable developments would be highly restricted due to these reasons. Aiming to address these issues, using smart conversion strategies as well as rational protocols and powerful toolkits such as precisely designed catalysts with finely tuned active sites can devote to accomplish this goal. Finally, the full valorization or well disposal of these complexes could be achieved. Meanwhile, the newly developed analytic methods and advanced characterization techniques with high accuracy help to clarify the actual reaction mechanisms and guide the design of catalysts.
2 CATALYTIC CONVERSION OF COMPLEX REACTANT SYSTEMS
2.1 The Catalytic Conversion of Biomass
Biomass refers to a wide range of natural organic materials, either plant-based or animal-based, that is a potential resource for producing chemicals and fuels. Among various renewable resources (e.g., solar energy and wind, etc.), biomass is the only one containing C, H, N and O elements, which endows it unique merits in producing value-added chemicals (hydrocarbon, bulk and fine chemicals) directly, as shown in Figure 10[100-102]. Thereby, the exploitation of biomass conversions offers great potential for sustainable chemical supplies, as an alternative to current fossil fuel based chemical manufacturing industries[101,103]. A shift to renewable biomass would not only reduce the dependence on fossil fuels, but also alleviate the environment-pollution concerns about excessive green-house gases emissions, which are both beneficial for building a greener Earth[104]. Here, we mainly focus on the conversion of lignocellulose and chitin. The major components of lignocellulose include lignin, cellulose, and hemicellulose. All of them are comprised of numerous units and connected by complex chemical bonds. For instance, lignin, is a complex polymer consisting of various methoxylated phenylpropanoid units[105]. Selective scission of the crosslinked C-C/C-O bonds would produce aromatic products, such as benzaldehydes and phenols[106].
|
Figure 10. Utilization of Biomass for Production of Hydrocarbons, Bulk and Fine Chemicals. Chemical Structures of Major Components (Lignin, Cellulose and Hemicellulose) of Lignocellulosic Biomass. Reproduced from Ref.[102] with permission from The Royal Society of Chemistry.
As for cellulose, it is intrinsically recalcitrant owing to the abundant intra- and inter-molecular hydrogen bonds protecting the β-1,4-glycosidic bonds from attack by foreign molecule, which renders it difficult to be converted[107]. Furthermore, its insolubility in most solvents aggravates the difficulty for transformation. Only with concentrated mineral acids or supercritical liquids can cellulose be depolymerized to a substantial degree, and subsequence reactions can be proceeded. Hemicellulose with amorphous structure is composed of long chains with a variety of pentoses and hexoses, and is therefore easier to be degraded than cellulose, but is also with high difficulty[108]. Chitin is a linear polymer, and the second most abundant natural biopolymer on Earth (after cellulose), which is widely distributed in plankton and the exoskeletons of insects and crustaceans, and organisms. About 100 billion tons of chitin are generated per year[109]. Similar to other biomass mentioned above, the compositions and chemical-bonding in chitin are also highly complex, mainly comprised of N-acetyl glucosamine linking by β-(1→4) bonds. One point should be noted is that chitin contains natural nitrogen, which is totally different from lignocellulose biomass. Therefore, it is of great significance to make full use of the N element in chitin to prepare nitrogen-containing chemicals[110]. In order to achieve the catalytic conversion of biomass to produce value-added chemicals, the design of catalytic systems with high and tunable performance that can selectively cleave crosslinks in biomass and activate the target chemical bonds is highly pursued.
2.1.1 The Catalytic Conversion of Lignocellulose
2.1.1.1 The Catalytic Conversion of Cellulose into 5-Hydroxylmethylfurfural (5-HMF) and Levulinic Acid (LA)
Lignocellulosic biomass is consisted of cellulose (30-50 wt%), hemicellulose (20-40 wt%), lignin (10-20 wt%), extractives, and ash etc.[111], and its reserves in nature are abundant. Cellulose is a linear biopolymer of glucose units linked by β-1,4-glycosidic bond, where complicated inter/intramolecular hydrogen bonds (H-Bonds) network between hydroxyl group in cellulose was formed and robust crystal structures were produced accordingly[112]. 5-HMF as well as LA were both listed as important platform chemicals by U.S. energy department[113] since they can be converted into lots of bio-based fuels and value-added chemicals, which provides a desired alternative for fossil fuels-based products[114,115]. The conversion of cellulose into value-added chemicals is promising, and much works have been conducted. For the reaction networks from cellulose to 5-HMF/LA/FA, cellulose was firstly hydrolyzed into glucose, which was then isomerized into fructose, and 5-HMF was obtained from fructose via dehydration, and LA was produced via rehydration of 5-HMF[116,117]. Therefore, upgrading cellulose into 5-HMF/LA was significantly crucial for biorefinery of lignocellulosic biomass.
Recalcitrant structure of cellulose is one of the challenges for the conversion of cellulose to 5-HMF/LA due to its insolubility in most solvent, which leads to extremely limited accessibility for solvent molecules and catalysts[118]. Meanwhile, another problem for cellulose-to-5-HMF/LA conversion is repolymerization of reactive intermediates and products (i.e., 5-HMF and LA), giving much biomass-derived carbon loss and decreasing the selectivity and yield of 5-HMF/LA[119]. Therefore, the design and adjustment of catalytic systems rationally are of great concern for cascade reactions of cellulose to 5-HMF/LA. For tandem conversion of cellulose to 5-HMF/LA, both depolymerization of cellulose into glucose and isomerization of glucose to fructose are the determining step with activation energy of 113.0-180.0KJ·mol-1[120] and 59.0-103KJ·mol-1[121], respectively. Therefore, homogeneous, heterogeneous or phase-transition catalysts with appropriate Lewis acidity and/or Brønsted acidity could ensure catalytic performance well for converting cellulose to produce 5-HMF/LA.
2.1.1.1.1 Homogeneous Catalysts
Mineral acids such as HCl and H2SO4 were widely chosen as catalysts for cellulose-to-LA conversion, giving moderate yields of LA. Girisuta et al.[122] reported 1M H2SO4-catalyzed ‘one-pot’ conversion of cellulose with loading of 1.7 wt.% to LA with yield of 60.0mol% in water at 423K for 2.0h. While lowering the concentration of H2SO4 to 0.5M and 0.1M, the yield of LA was both decreased due to slow depolymerization of cellulose. In KCl-saturated aqueous solution, Qin et al.[123] obtained high LA yield of 67.3mol% from 10.0 wt.% cellulose catalyzed by H3PO4 at 453K for 1.0h. Furthermore, Hu et al.[124] reported an efficient catalytic system for the conversion of cellulose or lignocellulosic biomass-fractionated cellulose with high loading of 15.0 wt.% to high-yield LA of 60.8mol% and 80.1mol% catalyzed by benzenesulfonic acid in 2-methyltetrahydrofuran and water (VMTHF/VH2O = 2/1) biphasic solvent, respectively. Ionic liquids (ILs) bearing sulfonic groups or HSO4- anion show good dissolving capacity of cellulose via forming competing H-Bonds of ILs with hydroxyl group of cellulose[125] and behaved well for catalytic conversion of cellulose to LA. Due to the lack of Lewis acidity, Brønsted acid-catalyzed HMF formation from cellulose showed low catalytic activity[126,127]. Compared with Brønsted acids, metal salts or bifunctional acid-base organic catalysts exhibited excellent catalytic performance for HMF/LA formation from cellulose due to Lewis acid or Brønsted base-catalyzed rapid glucose-to fructose isomerization and dehydration of fructose. It has been reported that metal species such as [Cr(OH)2(H2O)4]+[128] and [Al(OH)2(H2O)4]+[129] could coordinate with glucose helping the isomerization of glucose to fructose via 1,2-H transfer[130] , followed by rapid dehydration of fructose to 5-HMF and rehydration of 5-HMF to LA catalyzed by Brønsted acid due to its lower activation energy. In γ-valerolactone (GVL) and water (VGVL/VH2O = 17/3) mixed solvent, a high LA yield of 88.5 mol% was obtained from 1.5 wt.% cellulose catalyzed by heteropolyacid H3PW12O40 with bifunctional Lewis and Brønsted acidity at 468K for 2.0h[131]. Compared with LA, 5-HMF was more unstable and was ready to repolymerization, especially at high reaction temperature. Hence, bifunctional Lewis and Brønsted catalysts combined with organic and water mixed/biphasic solvent was a better choice for the conversion of cellulose to 5-HMF. Gong et al.[132] used the prepared cellulose formate as the substrate for the synthesis of 5-HMF catalyzed by 0.1M AlCl3 and 0.05M HCl in dimethyl sulfoxide (DMSO) and water (VDMSO/VH2O = 4/1) mixed solvent at 423K for 0.5h, yielding 67.3 mol% 5-HMF. Shi et al.[133] reported Al2(SO4)3-catalyzed conversion of high-loading cellulose of 33.0 wt.% to 5-HMF with the yield of 71.2 mol% in tetrahydrofuran-cyclohexane (THF) and water biphasic solvent (VTHF-CHX/VH2O = 19/3) at 453K for 1.0h.
Homogeneous catalyst-catalyzed conversion of cellulose to 5-HMF/LA showed high catalytic activity due to easily controllable Lewis and Brønsted acidity with the help of solvent. However, stabilization of intermediate and products from cellulose deserve further considerations for its rapid repolymerization catalyzed by homogeneous catalyst.
2.1.1.1.2 Heterogeneous Catalysts
Lots of heterogeneous catalysts such as metal oxides, zeolites, metal phosphates, carbon materials, and organic materials was prepared for the conversion of cellulose to 5-HMF/LA due to its recyclability and adjustable Lewis/Brønsted acidic sites compared to homogeneous ones. Potvin et al.[134] utilized ion exchange resin as solid acid catalysts to catalyze the depolymerization of cellulose and further conversion of glucose to 72.0 mol% LA in NaCl-saturated aqueous solution at 463-473K for 5.0d. Ding et al.[135] reported the Al-modified NbOPO4 as catalyst for synthesis of LA from 5.0 wt.% cellulose in water at 453K for 24h, obtaining 52.9 mol% LA. Due to limited contact between cellulose and solid catalysts, the catalytic performance of heterogeneous catalysts behaved not well for depolymerization of cellulose. Yu et al.[136] prepared a hyperbranched poly(arylene oxindole)s organic material grafting by -Cl and -SO3H groups (5-Cl-SHPAO) with -Cl groups interacting with hydroxyl groups of cellulose, which catalyzed the conversion of cellulose to LA with the yield of 48.0 mol% in water at 438K for 5.0h.
For cellulose-to-5-HMF, metal oxides, metal phosphates, and zeolites bearing both Lewis and Brønsted acidic sites catch the fancy of 5-HMF synthesis from cellulose. Atanda et al.[137] modified TiO2 with phosphate group to get functionalized surface that with both Lewis and Brønsted acidity. Ball-milling cellulose was converted to 5-HMF with high yield of 86.2 mol% catalyzed by P-TiO2 in THF-NMP/NaCl-saturated aqueous biphasic solvent (VTHF-NMP/VH2O=4/1) at 453K for 105min. Cao et al.[138] designed the hafnium phosphate catalyst with various ratio of Hf/P, which could endow this catalyst with different Lewis/Brønsted acid densities, where 69.8 mol% 5-HMF was yielded from 2.0 wt.% cellulose catalyzed by (HfO)(PO4)2 in THF/NaCl-saturated aqueous biphasic solvent at 463K for 4.0h. To increase the contact between catalysts and cellulose, choline ion-functionalized HY zeolites was utilized to catalyze the conversion of cellulose to 5-HMF, giving 55.0 mol% 5-HMF in methyl isobutyl ketone and NaCl-saturated aqueous biphasic solvent (VMIBK/VH2O=3/1) at 453K for 3.0h[139].
Although heterogeneous catalysts showed excellent performance for cellulose-to-5-HMF/LA conversion, these catalytic systems still suffered from high reaction temperature and long reaction time, massive usage of organic solvent, and equipment-corrosive NaCl, due to limited solid-solid contact between catalysts and cellulose.
2.1.1.1.3 Phase-transition Catalysts
Phase-transition catalysts possess the advantages of both homogeneous and heterogeneous, overcoming the difficult recovery of liquid acid and restricted contact of solid acid. Zhang et al.[140,141] prepared choline-modified heteropoly-acid via facile ion-exchange, which was insoluble at room temperature and soluble at reaction temperature in water. High yield of 5-HMF and LA over 70.0 mol% could be obtained from cellulose at low reaction temperature below 413K. However, losses of catalysts appeared during the recovery of catalysts via recrystallization.
Compared to other types of catalysts, phase-transition catalysts are more desirable. In summary, these catalytic systems made great progresses for conversion of cellulose to 5-HMF/LA. Rational design of catalysts should be considered, including: 1) increasing the interaction of catalysts with cellulose via decorating the surface property of catalysts or solvent effects; 2) controlling the cascade reaction rate of depolymerization, isomerization, dehydration via functionalizing appropriate Lewis and/or Brønsted acidity; 3) decreasing the accessibility of products for catalysts; and 4) recycling of catalysts.
2.1.1.2 The Catalytic Conversion of Lignocellulose to GVL
GVL, which is accessible from renewable lignocellulosic biomass, has been identified as one of the most promising platforms for the sustainable production of fuels and value-added chemicals[142,143]. As shown in Figure 11, the reactions of lignocellulose to produce GVL are summarized, which can be performed through two paths. In the first path, cellulose is hydrolyzed to glucose and then isomerized to fructose, followed by further dehydration to form 5-HMF, which would be decomposed to form LA or levulinate. Finally, a hydrogenation-cyclization cascade reaction transforms both LA and levulinate into GVL[144,145]. In the other transforming path, hemicellulose, is firstly hydrolyzed to xylose. Later, it transforms to FAL, FAL alcohol and LA subsequently through continuous dehydration reactions. Finally, LA undergoes hydrogenation process to produce GVL. Therefore, it is very clear that the reaction networks for producing GVL from biomass is highly complicated, and LA is one of the indispensable intermediates. To accomplish the goal for producing GVL from biomass directly, demands on rational design of catalysts and precisely control of desired reactions should be meet.
Actually, researches focused on the formation of GVL from LA have been widely reported[146,147], which lays solid foundation for the conversion of biomass to produce GVL. However, compared to pure LA, direct utilization of raw biomass as feedstock for GVL production is more cost-effective, because of the elimination of biomass pretreatment and the accomplishment of minimal processing steps[148]. In General, an integrated approach, relative to one-pot multi-step process, is widely adopted when producing GVL from biomass. Wherein, an acidic catalyst is employed to catalyze several consecutive reactions to yield LA, which is further upgraded to GVL with the assistance of metal catalyst.
|
Figure 11. Reactions for Producing GVL from Hemicellulose and Cellulose in Lignocellulosic Biomass. Reproduced from Ref.[144] with permission from Elsevier.
Galletti et al.[149] reported the straight production of GVL directly from water slurries of giant reed by adopting bifunctional (acid and hydrogenating) catalytic systems based on Ru/C and NbO or NbOPO4. Mild reaction conditions (0.5MPa of hydrogen and 70℃) were possible for the hydrogenation step. GVL yield up to 16.6 wt.% calculated from the starting weight of dry biomass was reached, with an almost complete and selective conversion of the intermediate LA. Later, Ding et al.[135] realized the direct catalytic conversion of cellulose to GVL through sequential reactions that LA was firstly formed by using niobium-based solid acids with aluminum modified (Al-NbOPO4), and LA was further upgraded to GVL on a commercial Ru/C catalyst. It was found that the doping of Al enhanced the intensities of Lewis and Brønsted acids, especially the strong Lewis acid, thus resulting in the increase of LA yield from cellulose. Finally, a higher GVL yield from cellulose was achieved. Another integration that combined a highly cross-linked ionic liquid polymer (PDVB-IL) and Co/TiO2 to catalyze the degradation of cellulose was reported, and this catalytic system gave an overall GVL yield of 55%[150]. A combination of H3PW12O40 with Ru/TiO2 catalyst was also proved to be effective for GVL production from inulin with 70.5% yield. Cao and colleagues obtained a GVL yield in a range of 57% (from sucrose), 50% (from starch) and 33% (from cellulose) over H2SO4 and Au/ZrO2-VS catalyst[151]. A cheap Cu-based catalyst Cu/ZrO2-OG, cultivated by the same group also showed good catalytic performance for the conversion of giant reed through the one-pot process, and the yield of GVL decreased to 18.5% (based on the weight of dry biomass)[152]. A three-step method had been developed for the selective conversion of hemicellulose in pubescens to GVL by Pt/C catalysts without the addition of external hydrogen, reported by Luo et al[153]. A high yield of 20.0% GVL based on the weight of pubescens with a high selectivity of 90.5% was achieved. The reaction procedure was shown in Figure 12. By well characterizations, platinum species were found to interact with the carboxylic and lactonic groups of activated carbon, and the Pt (220) surface might exhibit high activity for the conversion of LA and formic acid (FA) to GVL. Besides, the Pt/C catalyst was effective for the in-situ generation of hydrogen due to the high selectivity towards the decomposition of FA.
|
Figure 12. The Origin of GVL from an Aqueous Mixture Derived from Hemicellulose in Pubescens. Reproduced from Ref.[153] with permission from the Royal Society of Chemistry.
Later, the same group developed a two-step integrated strategy for GVL production from FAL residue. Wherein, LA and FA were produced from furfural (FAL) residue using SnCl4 as a catalyst in the first step, and the obtained products were subsequently employed as feedstock for GVL production by a developed bimetallic Au-Ni/ZrO2 catalyst in the second step[154]. This work also gave a potential strategy for producing GVL directly from raw lignocellulosic biomass without external H2. Characterizations revealed that the loading of Ni promoted the reduction of Auδ+ to Au0, and facilitated Au0 dispersion with less aggregation, which improved the hydrogenation ability of this catalyst.
2.1.1.3 The Catalytic Conversion of Lignocellulose to FAL and Other Chemicals
Except the above-mentioned chemicals produced from biomass via catalytic degradation, other types of chemicals can be also obtained[155]. FAL, another type of important chemical, is mainly produced from hemicellulose. Both homogeneous and heterogeneous catalysts were adapted. For example, Zhang et al.[156] reported the preparation of FAL from pine wood with 33.6% yield by AlCl36H2O with the assistant of ionic liquid (1-butyl-3-methylimidazolium chloride). Besides, thiourea could also promote the yield of FAL from empty fruit bunch by using H2SO4 as catalysts, and the corresponding yields increased from 33. 8% to 61.0%[157]. Effective heterogeneous catalysts towards the catalytic degradation of hemicellulose to FAL had been also reported, such as WO3/SiO2 and Ga2O3/SiO2[158], zeolite SAPO-34[159], ZSM-5[160] etc., and the resultant FAL yields varied from 55-67%. By rationally designing a two-phase solvent system (GVL/water), the yields of FAL from corn stover and Eucalyptus sawdust catalyzed by SAPO-18[161] and H-SAPO-34[162] increased significantly to 95% and 99%, respectively. For the production of other value-added chemicals, Wang et al.[107] used 2%Ni-30%W2C/AC catalyst to convert cellulose to ethylene glycol (EG) via a hydrolytic hydrogenolysis method successfully at 503-523K and 6-10MPa H2, achieving 73.0% yield of EG . However, when applying this catalyst to convert birch wood, without any pretreatment, EG yield reduced sharply to 38.8% since it was found that the lignin component in the corn stalk significantly inhibited the conversion of cellulose to EG. Therefore, delignifying pretreatment must be conducted prior to the reaction. Besides, organic silicon especially tetraethyl orthosilicate (TEOS) could be also obtained from rice straw that contained a large amount of Si. Compared to the industrial production process of TEOS using SiCl4 or Si with multi-steps, where huge amount of HCl was used/produced or harsh conditions were needed, the direct production of TEOS from biomass represented a greener approach and showed greater potential. Fukaya et al.[163] used strong bases and a significant amount of dehydrating agent to promote the reaction of rice hull ash (The main component was SiO2) with ethanol to get TEOS. Sun et al.[164] used the real raw biomass (rice straw) to reacted with ethanol to obtain TEOS successfully. Meanwhile, it was found that reaction temperature influenced reactions remarkably, and the removal of lipids from rice straw facilitated the production of TEOS, giving the highest TEOS yield of 76.2%. In addition, silicon in different biomasses behaved differently in the production of TEOS, which might be related to the complex growth processes of the plants. Until now, there were few works focused on the direct catalytic conversion of biomass (such as rice straw) to TEOS, however, it can be predicted that more satisfied results can be obtained when proper catalysts have been designed and synthesized to promote this process.
In conclusion, at present, many studies that focused on the production of 5-HMF, LA, GVL, FAL, EG, TOES etc. from real biomass, cellulose, hemicellulose, and their derivatives have been reported. Scholars are continuing to explore multifunctional catalysts with high stability and efficiency. Meanwhile, the researches on exploring innovative catalytic systems to achieve high efficiency, safety, energy savings and environmental protection are also the focuses of them.
However, we should realize that the conversion of lignocellulose biomass is far more difficult than we imagine now. In fact, much works that focused on the conversion of biomass merely chose one single component such as cellulose or lignin that comes from the real biomass, which cannot represent the real biomass at all. The separation of these single components from real biomass is not simply a physical separation process. On the contrary, much chemical reactions occur, and interactions between different components simultaneously contribute to influence the separation process[165,166]. Meanwhile, the reaction networks for producing these chemicals are rather complicated than we think. Taken the conversion of biomass-derived hexose as example, due to the existence of multiple hydroxy groups in a hexose molecule, the protonation may occur at different positions leading to a variety of dehydrated intermediates, and then various products and side products form. Undoubtedly, these undesired parallel and consecutive side reactions lead to the low selectivity and higher difficulty in precise control. On account of the conformational complexities of the feedstock and the coexistence of multiple dehydration intermediates/products/side products, the reaction types involved in glucose dehydration are also diverse, including isomerization, dehydration, decarbonylation, decarboxylation, rehydration, retro-aldol condensation, and multiple polymerizations etc[167]. The diverse reaction types thereby constitute a complex reaction network, as illustrated in Figure 13[168]. One thing should be noted is that not only reactions can occur between initial substrates, but also between initial substrates and intermediates, intermediates and intermediates, products and intermediates or even products and initial substrates. The high complexity in reaction networks for converting model biomass molecular hexose have already significantly restricted the selective production of targeted products, not to say the conversion of real biomass. Proper or new degradation strategies are still urgently needed to be developed to achieve the full utilization of lignocellulose.
|
Figure 13. The Complex Reaction Network for Glucose-to-HMF Transformation Where G Value Indicates the Reaction Free Energy with a Unit of kJ·mol⁻¹. Reproduced from Ref.[168] with permission from Elsevier.
2.1.2 The Catalytic Conversion of Chitin
At the beginning, the valorization and utilization of shrimp shell or chitin were mainly conducted via bacterial or fungal fermentations and enzymatic degradation. For instance, Serratia marcescens B742 and Lactobacillus plantarum ATCC 8014 were effective in degrading chitin[169]. However, the high cost of enzymes and the low efficiency were some of the pitfalls of this strategy. Catalytic conversion of chitin gradually grabbed the attention of researchers. Under the guide of ‘shell biorefinery’ concept (as shown in Figure 14), distinctive protocols have been established to chemically transform chitin to amino sugars, amino alcohols, furanic amides, and N-heterocycles etc[110,170].
|
Figure 14. The Concept of Shell Biorefinery. Reproduced from Ref.[170] with permission from Wiley.
As for the overall conversion strategies towards chitin, firstly, the chitin polymer was decomposed into monomeric or oligomeric molecules with different functionalities on the side chains through selective depolymerization, deacetylation, and functionalization, whereas the glucosamine C6 backbone was maintained. This strategy was easy to carry out under rather milder conditions, but the obtained products were somehow simple with relatively low added-value. In the other category, the C6 backbone underwent breakage and rearrangement, leading to more diversified products. This method required high temperature to promote the cleavage of chemical bonds and more side reactions would occur accordingly.
The deacetylation of chitin led to the formation of chitosan, and its further depolymerization afforded low-molecular-weight chitosan (LMWC), which were both important compounds since they were featured with excellent antibacterial and antitumor properties. Chen et al.[171] established a one-step, solvent-free mechanochemical method to transform chitin and raw shrimp shell powders to LMWC. The base catalyst, NaOH, was found to not only facilitate chitin transformation into LMWC but also inhibited side reactions under ball milling conditions. Similarly, Yabushita et al.[172] showed that the depolymerization of chitin with a catalytic amount of H2SO4 rather than base could also give soluble short-chain oligomers. Subsequent hydrolysis of the ball-milled sample provided N-acetylglucosamine (NAG) with 53% yield, and methanolysis afforded 1-O-methyl-N-acetylglucosamine in yield of up to 70%. Despite strong base or acid could promote the depolymerization of chitin, effective solvent systems were indeed another ideal option. In 2020, Gözaydın et al.[173] established acidified lithium halide molten salt hydrate (AMSH) systems to convert native chitin into NAG effectively. 71.5% yield of NAG was achieved in LiBr AMSH containing only 40mM HCl at 120℃ after 30min. Likewise, several water-organic co-solvent systems had been studied and established for chitin depolymerization[174]. The effects of cosolvent types on conversion efficiency and product selectivity had been deeply explored. Water-free co-solvent systems, such as EG, FA etc. used for the conversion of chitin had been explored[175,176]. Finer nitrogen-containing chemicals were also obtained by catalytic conversion of chitin. For example, glucosaminic acid (GlcNA), an important amino acid applied in food, asymmetric synthesis, and medicines that could be generated by following a depolymerizaion-deacetylation-oxidation sequence[174]. Dai et al.[177] reported a two-step process by Amberlyst-15 and Au/MgO catalysts to produce GlcNA from chitosan (a typical chitin derivative) with an overall yield of 36%. Hydroxyethyl-2-amino-2-deoxyhexopyranoside (HADP) and hydroxyethyl-2-acetamido-2-deoxyhexopyranoside (HAADP) could be prepared under the catalysis of sulfuric acid in EG[175]. The conversion of NAG that originated from chitin to its corresponding amide/amino substituted sugar alcohols, smaller C2-C4 polyols and N-acetylmonoethanolamine had been reported over Ru/C catalysts in the presence of hydrogen in water[178]. A single compound, 5-(formyloxymethyl) furfural with 35% yield after a longer reaction time from chitin was reported by using formic acid as catalyst[176]. As for the straightforward method mentioned above, the core is to develop efficient solvents and catalysts to break the glycosidic bonds. Especially, developing catalysts with high selectivity is essential. Besides, pretreatments, such as ball milling, are also often involved to facilitate subsequent chemical reactions.
Further treatments of chitin that lead to the breakage and rearrangement of C6 backbone would help to get more diversified products. For example, 3-acetamido-5-acetylfuran (3A5AF), as a kind of versatile pharmaceutical precursor that produced mainly through the Haber process relying on fossil feedstocks[179]. Several works had reported the direct transformation of chitin into 3A5AF with a yield of 7% by using boric acid and alkaline chlorides as catalysts[180], and of 15.4% by using boric acid and HCl as catalysts in ionic liquid [BMIm]Cl[181]. Subsequently, it was further increased to 28.5% using a ball milling pretreatment technique[182]. Later, a range of organonitrogen chemicals derived from the upgrading of 3A5AF, such as 2-acetyl-4-aminofuran, 3-acetamido-5-(1-hydroxylethyl)furan and anticancer alkaloid proximicin had been also obtained, which further promoted the valorization of chitin[183,184]. A report focused on the conversion of chitin and waste shrimp shells into acetic acid and pyrrole by catalytic method using metal oxide (CuO, CeO2) and oxygen gas in basic water was shown. 38.1% and 47.9% yields of acetic acid were produced from chitin and crude shrimp shells, respectively[185]. Natural product syntheses (including Rhizochalinin, Pochonicine, Allosamizoline etc.) using chitin/chitosan represented another promising prospectives for the utilization of chitin[186,187]. Recently, a novel integrated biorefinery method for chitin upgrading had been established, which combined shrimp shell waste catalytic pretreatment and biological fermentation to transform shell waste to tyrosine and L-DOPA (a frontline drug treating Parkinson’s disease). These chemicals were previously unavailable from chitin by traditional chemical processes. This new protocol provides an alternative pathway to synthesize valuable aromatic amino acids from renewable chitin feedstock[188].
In conclusion, different strategies have been attempted to convert chitin to products with distinct structures. An effective dehydration catalyst with high catalytic performance with a proper solvent medium that can weaken the hydrogen bonding network in chitin is critical. However, there is still a long way for us to explore the utilization of chitin since its high complexity. In terms of these already achieved results, deeper explanations on reaction mechanisms when converted chitin to whatever products have not been reported, which is mainly ascribed to the high complexity of chitin. Therefore, advanced or even new convert strategies should be cultivated further.
2.2 The Catalytic Conversion of Plastic Wastes
Plastic products are used in almost all aspects of daily life because of their low cost, durability, and portability etc. Besides, the use of plastic products is rising per year. The mass production and the subsequent accumulation of waste-plastic products in Nature have caused serious environmental and management problems[189,190]. Therefore, proper and effective recycling methods should be developed. The diagram for upcycling plastics of its life circle is shown in Figure 15.
|
Figure 15. The Diagram for Upcycling in the Life Cycle of Plastics, Leading to Formation of New Polymers, Molecular or Materials. Reproduced from Ref.[189] with permission from Springer.
However, plastics, as a kind of typical polymer of large- or macro-molecule with highly repetitive subunits (or monomer units) that are linked to each other by specific types of chemical bonds, which brings out much difficulties in upcycling. Fortunately, significant progresses have been achieved. Indeed, there are many excellent reviews summarized the works focused on the conversion of plastic waste via bio-degradation[191-193], photocatalysis[194,195], electrocatalysis[196] and thermal catalysis etc[197,198]. Here, we briefly discussed the potential catalytic degradation strategies. Three strategies have been proposed based on whether a specific intermediate molecule is designed on the route of polymer transformation and the type of the intermediate molecule: (1) polymers are depolymerized to monomers, oligomers, or their derivatives, followed by the upcycling into high-value chemicals; (2) polymers are degraded into small platform molecules (e.g., CO2, CH4, FA, and methanol) and then upcycled into high-value chemicals; and (3) polymers are directly transformed into high-value chemicals[199]. There are advantages as well as disadvantages for each strategy. Usually, high yields of products can be achieved via the first strategy. However, it requires a high purity of substrate, and only polymers that comprised of single monomers can be totally converted, which is difficult to apply for practical use. The second one has advantages for producing various products but with rather lower atom utilization. The third one is beneficial for not requiring pretreatments or multi-conversion steps, leading to the reduced energy consumption and production costs. However, it is more demanding on the catalysts with superior catalytic performances.
López-Fonseca et al.[200] used different types of metal-salt such as zinc acetate, sodium carbonate, sodium bicarbonate, sodium sulphate and potassium sulphate as catalysts to convert polyethylene terephthalate (PET) into monomers. Comparable high yields (≈70%) of the monomer bis(2-hydroxyethyl terephthalate) were obtained with zinc acetate and sodium carbonate as depolymerization catalysts at 196℃. Moreover, other types of catalysts such as organic bases, solid acids, and metal oxides were also employed to degrade PET into monomers[201]. The second step for converting the formed monomers was more meaningful since it provided opportunity to synthesize different value-added chemicals. For instance, under thermo-catalytic conditions, the obtained monomers, dimethyl terephthalate, or bis(hydroxyalkyl) terephthalate from glycolysis in the presence of zinc(II) acetate catalyst, could be further hydrogenated or hydro-deoxygenated to dimethyl cyclohexane-1,4-dicarboxylate, 1,4-cyclohexanedime-thanol by Ni-based[202] and RuPtSn/Al2O3 catalysts[203] respectively, or gasoline and jet fuel range C7-C8 cycloalkanes and aromatics by using Pt/C catalysts[204]. In addition, under photo- or electro-catalytic conditions, the EG units of PET could be oxidized into glyoxal or formate in aqueous alkaline with H2 produced simultaneously over a carbon nitride/nickel phosphide catalyst[205]. Continuing with this guide line, other waste-plastics with ester or amide bonds, such as polyurethane (PU), polystyrene (PS), polycarbonate (PC), and polylactic acid (PLA), could be also upcycled by degrading into monomers.
Extensive progresses over the conversion of building-block molecules (such as CO2, CH4, CO and CH3OH etc.) into fuels and other high value-added chemicals have been obtained, therefore, the degradation of waste-plastic into these small platform molecules is also of great significance to chemical industries. As an example, Jiao et al.[206] accomplished the photodegradation of PE with 100% conversion into CO2 within 40 h by single-unit-cell thick Nb2O5 layers, while the produced CO2 could be further reduced to CH3COOH. This two-step waste-plastic-to-fuel conversion might help to solve the white pollution crisis and harvest highly valuable multi-carbon fuels in natural environments simultaneously. Meanwhile, the same group reported photo-catalyzing plastics to syngas by Co-Ga2O3 nanosheets[207]. In this process, H2O was photo-reduced into H2, while non-recyclable plastics including PE plastic bags, PP plastic boxes and PET plastic bottles were photodegraded into CO2, which was further selectively photo-reduced into CO. CH4 could also be obtained when transforming waste-plastics such as PE, PP and PS, reported by Lee et al[208]. They described that the Ru-modified zeolite exhibited excellent catalytic performance that grid-compatible methane (>97% purity) was obtained at 300-350℃ using near-stoichiometric amounts of H2, which might increase the intelligent use of plastic waste via energy recovery. A nickel-based catalyst involving Ni2Al3 phase enabled the direct transformation of mixed polyolefin plastics into natural gas, and the gas carbon yield reached up to 89.6%, presenting by Fang et al[209].
For the third strategy that polymers are directly transformed into high-value chemicals, it is the most difficult yet the most attractive. The core is to design catalytic systems rationally based on precise chemical bonds activation and proper chemical bonds cleavage, which is hopeful to achieve the disassembling of plastics by H2. Direct hydrogenolysis could lead to the cleavage of ester bonds, carbonate bonds, amide bonds, and urethane bonds etc. Lee et al.[208] reported the first example of converting PU to produce diol, diamine, and methanol in the presence of a ruthenium pincer catalyst at 150℃ and 70bar H2. Similarly, PU could be also transformed into diols, diamines, and methanol by using metal or metal oxide complexes under the presence of reducing agents (silanes)[210,211]. In 2020, Wang et al. reported the first example of the upgrading of various aromatic plastic wastes with C-O and/or C-C linkages to arenes (up to 75-85% yields) via catalytic hydrogenolysis over a Ru/Nb2O5 catalyst, which not only allowed the selective conversion of single-component aromatic plastic, and more importantly, enabled the simultaneous conversion of a mixture of aromatic plastic to arenes[212]. The work diagram of Ru/Nb2O5 catalyst to cleavage C-O and C@C bond of waste plastics was displayed in Figure 16, wherein the circular plastic economy could be achieved. Later, deeper work done by them showed a self-supported hydrogenation process of PET with H2-free hydrogenation system to generate benzene, toluene, and xylene (BTX)[213]. Ni2P catalysts were also proved to be effective towards the conversion of PET to BTX fraction[214]. The Co-Fe-Al catalyst containing CoFe alloy with tailored structural features could achieve xylene yields of >99.0% from PET[215]. Recently, Ma and coworkers achieved the one-pot direct amination of polylactic acid (PLA) to alanine catalyzed by Ru/TiO2 in ammonia solution, without the addition of external hydrogen. PLA was depolymerized to lactamide and ammonium lactate, and the following dehydrogenation of ammonium lactate on Ru nanoparticles initiated the amination, leading to the formation of alanine[216]. Besides, by combining a homogeneous catalyst (Hf(OTf)4) and a heterogeneous hydrogenation catalyst (Pd/C), PET were depolymerized into dicarboxylic acid and ethane under 1atm.
|
Figure 16. The Integration of C-O and C-C Bond Cleavage Catalyst (Ru/Nb₂O₅) into the Circular Plastic Economy. Reproduced from Ref.[212] with permission from Wiley.
H2, revealed by Kratish et al[217] . PET could also be completely converted into alkanes, dominated with cyclohexane and methane by Ru/TiO2 under the optimal conditions (200℃, 60bar H2, and small amount of H2O)[198]. Also, a CeO2-supported Ru nanoclusters catalyst showed good hydrogenolysis ability towards low-density PE, leading to the formation of alkane fuels[218]. All of these studies provide possibilities to selective degradation of plastic-wastes, which not only helps to alleviate the environment pollutions, but also contributes to achieve the full utilization of waste plastics.
Except the conversion of waste-plastics that only comprised of mono-components such as PET, PE, PP, PVC, PU, PS, and others, we should pay more attention to the conversion of mixed-plastic wastes since they are closer to the actual human life and recycle situations. For the chemical conversion of mixed-plastic wastes, there are two main strategies that have been proposed[219]: (1) transformation of mixed plastics into a product with a simple composition, and (2) stepwise transformation of the mixed plastics. The first strategy underscores the conversion of mixed polymers into simple products, such as CO, CH4 or H2, or a valuable hydrocarbon, like ethylene or fuels that can be used directly. It follows the same guideline for converting mono-composition plastic as we mentioned before. Once these mixed plastics are depolymerized into smaller molecules, we can further convert them into value-added chemicals through established catalytic methods. It should be noted that real-life plastic waste contains various toxic contaminants, which could potentially inhibit the transformation process. Thus, developing a robust catalyst that can tolerate contaminants is crucial for the success of this strategy. The second strategy involves separating and transforming mixed plastics into valuable chemicals in a step-by-step manner. Firstly, efficient extraction or separation methods allow us to separate these complicated plastics, and later, catalysts with high-catalytic performance allow us to harness the potential of each polymer component of the mixed plastic-wastes. Ultimately, to realize viable catalytic processes to deconstruct and upcycle waste plastics, the actual mechanisms of obtaining intermediates for upcycling via a depolymerization process or direct hydrogenolysis must be clear. Therefore, advanced monitoring and analyzing methods, characterization techniques and even in-situ reactions coupled with detection systems are highly pursued.
Towards the conversion of plastics, difficulties are mainly arisen from the complex chemical bonding in these polymers. However, it is far more complicated for recycling waste plastics since not only chemical bonding is complex, but also different types of polymers with different monomers are usually mixed as well as pollutants that can lead to the deactivation of catalysts. Thereby, more attentions are still needed to pay to achieve the efficient conversion of waste plastics.
2.3 The Catalytic Conversion of Vehicle Exhausted Gases
In recent decades, the environmental protection and long-term sustainability have become the focus of attention due to increasing pollution generated by the sharp increase of vehicles and intense industrialization[220]. It should be noted that there are some differences in the amount of toxic gas emissions released by different types of fuel vehicles, but harmful emissions with high composition complexity including NOx, COx, unburned alkanes, and solid particles etc. could be observed at all situations[221,222]. To overcome environmental issues caused by these vehicle exhausted gases, environmental catalysis has increasingly been used to solve the negative impacts of pollutant emissions on the global environment and human health. However, when the main pollutants in the tail gas COx, HC and NOx contact with catalysts, oxidation and reduction reactions occur on the catalyst at the same time with high complexity, and subsequently being converted into harmless CO2, H2O and N2. Actually, there are more than 400 reactions involved in this process, such as but not limited to these reactions: 2CO+O2→2CO2; CHx+(1+x/4)O2→CO2+x/2H2O; 2CO+2NO→2CO2+N2; CHx+2NO→CO2+x/2H2O+N2; 2H2+2NO→2H2O+N2; CO+H2O→CO2+H2; CHx+2H2O→CO2+(2+x/2)H2. Moreover, the real reaction mechanisms are still being explored. The above commented factors come to be one of the main reasons for its high conversion difficulty. Meanwhile, catalyst poisoning is also annoying, and high production costs and production discontinuity problems arise subsequently. Therefore, exploring robust catalysts with perfect catalytic performance towards the elimination of these exhausted gases simultaneously comes to be the focus in this field.
A worldwide effort to reduce vehicle emissions is advancing, which helps to build a greener, more sustainable Earth for mankind. The development of catalysts used for treating these exhausted gases has gone through three stages. The first generation of catalysts for vehicle exhausted gas purification was oxidation catalysts, with Pt and Pd as the main active components since Pt and Pd had high catalytic activity and stability towards the oxidation reaction of CO and CHx, which had experienced prosperous development between 1975-1980s[223,224]. Later, with the stricter requirements for controlling NOx pollutants, the developed oxidation catalysts could no longer meet the requirements. Accordingly, the second generation of catalysts was three-way catalysts (TWCs) that could deal with CO, CHx and NOx simultaneously. Further modifications on the earlier-developed TWCs that contained noble-metals or non-noble metals became the third generation of catalysts. A detailed description of each type of catalyst will discuss later.
Firstly, Pt and Pd-based catalysts were found to be highly active and stable for the oxidation of CO and CHx, and the reactions occurred according to the following equations:
$$ {C}{O}+1/2{{O}}_{2}\to {C}{{O}}_{2};{C}{{H}}_{{x}}+(1+{x}/4){{O}}_{2}\to {C}{{O}}_{2}+{x}/2{{H}}_{2}{O};$$
Considering the high cost of both Pt and Pd, and to maximize the catalyst performance further, attempts for new catalytic materials that comprised of non-noble metals had been tried, such as base metal oxides including Cr, Ni, Co, Cu and Mn etc. Unfortunately, all of them were unsuccessful at the early stage, which emphasized the essential importance of Pt and Pd[225]. However, decreasing or completely replacing precious metals into alternative cheap metals still continued to be a goal of some recent researches. Another strategy was loading Pt-group metals onto materials with high specific surface areas to increase the number of active sites. γ-Al2O3, CeO2-ZrO2[226,227] were the two main carriers used to disperse these active components (Pt, Pd, and later Rh). Heat treatment thermally fixed or anchored the active components to the carriers, resulting in a Pt-group metal-support catalytic system available for the reactants to chemisorb and convert with high efficiency. Strong interactions between metal and support induced by these operations contributed to anchor metal particles with higher stability, which achieved long-term durability under high temperature operation conditions. For the industrial production, the active components were commonly supported on extruded ceramic monolithic structures (cordierite; 2MgO-5SiO2-2Al2O3), which helped to improve catalytic effectiveness. These kinds of coated monoliths were sufficient with pores for coating a thin layer, and the reactants diffused from the bulk gas to the catalytic wash coat through its porous network in search of the catalytic sites, where they were chemisorbed and converted to products with high efficiency. Due to the recent advanced SACs catalysts preparation methods, applying SACs to eliminate gaseous pollutants had also attracted much attention and came to be another strategy to improve atom utilization efficiency[228,229].
Since 1980, with the increasing need for eliminating NOx from vehicles or industrial emissions, new catalytic systems have been established to convert CO, CHx and NOx simultaneously in concert with engine control and fuel composition change. With the discovery that Rh exhibited good catalytic performance towards NOx conversion[230], the active components of TWCs were mainly comprised of Pd/Rh or Pt/Rh[231-233]. Especially, Pd/Rh catalysts dominated the scene in recent years, aided by the spreading of low-sulfur fuels and cheaper Pd prices compared to Pt. Figure 17 showed the typical performance of a TWC under different air-to-fuel feed ratios, which remarkably influenced the gas composition after burning[232]. The advantages or uniqueness of the TWC was that it could operate primarily at stoichiometric air-to-fuel ratios that were compatible with the oxidation of the three pollutants. It can be seen clearly in Figure 17 that the operation window (in green column) for TWC to convert the pollutants at the same time was narrow. Therefore, an O2 sensor that could convert the partial pressure of O2 in the exhausted gases from the stoichiometric point (λ=1) into an electrical signal were required. Later, the generated voltage sent instructions to the electronic control computer, instructing the fuel supply system to increase or reduce the amount of fuel entering the engine in the feedback control loop. Based on this guideline, taking advantages of the reversible redox and oxygen storage capacities of CeO2-ZrO2 mixture, adding them to TWC help to buffer the disturbance, and ultimately achieved conversion goal. When there was a deficiency of O2 (that is, λ<1), the surface of CeO2 or ZrO2 was reduced while suppling the oxygen to convert CO. The reduced surface of Ce2O3/Zr2O3 was then oxidized back to CeO2/ZrO2 when the perturbation generated excess O2 (λ>1).
|
Figure 17. Typical Performance of a Three-Way Catalyst (Pd and Rh Stabilized on Alumina Plus CeO₂-ZrO₂/Ceramic Monolith) as a Function of Air-to-Fuel Ratio. Reproduced from Ref.[232] with permission from Springer.
Exploring other types of catalysts that can selectively catalyze the reduction (SCR) of NOx into N2, which can reduce or even replace Rh contents, and later couple them with Pt or Pd catalysts to construct TWCs has also obtained much attentions. Liu et al.[234] reported a novel WO3-doped Fe2O3 catalyst that exhibited high NH3-SCR activity in a wide range of operating temperatures and high resistance against H2O and SO2. The highly dispersed WO3 acted as both ‘‘chemical” and ‘‘structural” promoters, which led to the high surface area and more amounts of active sites. Similarly, V2O5-WO3/TiO2 catalysts[235], WO3/CeZrO2 catalysts[236], Fe or Fe2O3-based catalysts[237,238], Co-based catalysts[239,240] etc. had been also proved to be highly effective. More importantly, Cu-based catalysts including Cu/SSZ-13 that had already been commercially used[241,242]. Besides, Cu/SSZ-16[243], Cu/SSZ-39[244], modified Cu-SSZ-13@SiO2 catalysts with a core-shell monolithic structure[245] and coupled CeZrOx-Cu/SSZ-13 catalysts[246] had been also proposed successfully. The above catalysts provided opportunities to achieve the conversion of NOx and vehicle emissions by non-noble metals.
The adjustments or modifications of CeO2/ZrO2 based TWCs was an interesting and attractive strategies for converting these pollutants. On the one hand, modifications focused on the active metals brought out different catalytic performance. For example, alkali and alkaline earth metals were widely used to adjust the electronic structure of supported catalysts[247,248] due to their relatively small electronegativity, which were crucial for binding with active metals through surface oxygen linkages[249]. Constructions of catalysts with ternary or even quaternary metals also shed light on the conversion of vehicle emissions[250]. On the other hand, the modifications on CeO2/ZrO2, such as controlling the morphologies[251,252], crystal phases[253] and exposed lattice planes[254,255] etc. contributed to adjust the redox and oxygen storage properties, and accordingly improved the catalytic performance. Development of new synthetic processes for highly active CeO2-ZrO2 materials or even ternary oxide composites with high oxygen mobility was another vibrant research area[256,257].
In summary, the synthesis of three-way catalysts is the guide line for converting exhausted vehicle gases. At present, efforts have been continuously put to modify these catalysts with high catalytic performance but lower cost.
2.4 The Catalytic Conversion of Pollutants in Water
In the recent decades, the increasing serious water pollution from hazardous and toxic organic pollution agents is annoying worldwide. In the most prevalent examples of waste water, there are inorganic chemicals such as heavy metals, suspended solids, toxic organics and dyes, which are usually emanated from industrial sources[258]. By far, various techniques including chemical precipitation, adsorption, ion-exchange, membrane filtration and chemical catalytic conversion for remediation or purification of waste water samples have been reported. Here, we mainly pay attention to the chemical catalytic methods. As can be predicted, the complex compositions in waste water bring out high conversion difficulty, and multi-functional catalysts that can deal with different types of pollutants are the most ideal target. Indeed, it is difficult to achieve this goal as far as the technology that have been developed. At present, there are no efficient integrated methods that can deal with all types of pollutants in waste water simultaneously. The main strategy for converting waste water is transforming different kinds of pollutants stepwise. The essence for degrading different types of pollutants lies on the cleavage of different chemical bonds, such as COOH, -C-N, C=C, C-O, C-S, and N=N bonds, and halogen atoms. Therefore, the types of required catalysts with high catalytic performance differs, and the degradation paths of materials to different organic pollutants and heavy metals under different conditions are somewhat different.
Among the chemical catalytic methods, photocatalytic degradation dominated, which illustrated an effective and promising approach for the elimination and destruction of hazardous contaminants from waste water produced from industries. These photochemical degradation processes called “advanced oxidation processes” could completely degrade organic pollutants into harmless inorganic substances such as CO2 and H2O under moderate conditions. The fundamental mechanism of photocatalysis for the degradation of toxic pollutant was presented in Figure 18[259].
|
Figure 18. Photocatalytic Degradation of Toxic Pollutants. Reproduced from Ref.[259] with permission from Elsevier.
As for the photochemical materials, many effective photocatalysts had been developed well such as typical semi-conductor TiO2[260,261], other metal oxides and their composites[262], graphene-based materials (especially g-C3N4)[263,264], magnetic-MXene-based materials[265], and MOFs[75,266] etc. MOFs had emerged as promising and new photocatalysts to convert water pollutants, since they possessed an outstanding pore structure, adsorption capacity, and photocatalytic properties. The corresponding working mechanisms for the photocatalytic degradation of organic pollutants driven by MOF-based photocatalysts had been clearly demonstrated elsewhere[75]. Combining some reviews, we divided these complex pollutants into five main categories, that is, agrochemicals (including pesticides and herbicides), antibiotics, dyes, oil and grease, phenol and phenolic compounds and other pollutants.
2.4.1 Degradation of Agrochemicals
Nowadays, an interesting issue “Agrochemicals are our friends or our enemy” is debating. On the one hand, it helps to save the crop loss to a great extent by controlling pests and removing weeds. On the other hand, the overuse of agrochemicals including both pesticides and herbicides can badly impact the environment and human health as they can cause cancer and other harmful diseases. A CoOx/BiVO4 photocatalyst degraded propyl paraben (a typical herbicide) successfully, and the enhancement of the photocatalytic activity of the synthesized catalysts was described as the efficient electron-hole separation, achieving by the p-n junction formed between the p-type Co3O4 and the n-type BiVO4 semiconductors[267]. Unfortunately, this catalyst could only work in pure water system while the degradation was severely impeded in secondary treated waste water. Cao et al.[268] reported the efficient photocatalytic degradation of herbicide glyphosate (degradation rate reached 97%) by a magnetically separable and recyclable BiOBr/Fe3O4 nanocomposites under visible light irradiation in waste water. Another study successfully synthesized a ternary composite photocatalysts g-C3N4/BiOI/Bi2MoO6 via dispersion modification of BiOI and perovskite-like materials Bi2MoO6 on g-C3N4, which accomplished a high photodegradation rate of glyphosate (94%)[269]. Moreover, the degradation of another herbicide (paraquat) that used on large scale had been also conducted. Munshi et al.[270] reported that the high photodegradation activity of paraquat was obtained by using ZnO nano-catalyst, and the concentration of paraquat decreased from 100ppm to 35ppm in 12h. Adding WO3 to ZnO to modify the photocatalytic performance, a higher degradation rate of paraquat was achieved compared to single ZnO and WO3[271]. Moreover, the ZnO-WO3 composite was found to be stable for at least three cycles for reuse. Imidacloprid and profenofos etc., as typical pesticides, were widely used in agriculture field. The investigations on the conversion of these pesticides were also hot research topics. By using Ag3VO4/Ag2VO2PO4, an excellent degradation efficiency towards imidacloprid was achieved, as reported by Zhang et al[272]. The formation of Ag2VO2PO4/Ag3VO4 heterojunction suppressed the recombination of photoinduced charges and prolonged its lifetime. Therefore, enhanced catalytic performance was obtained. An optimized Bi2WO6/NH2‑MIL‑88B(Fe) composite derived MOFs materials was used to evaluate the degradation of imidacloprid under visible light, and about 84.5% of imidacloprid could be removed[273]. A hierarchical nanohybrid ZGO-MOF (comprised of zinc oxide, graphene oxide and MOFs) achieved excellent efficiency for converting profenofos[274]. Moreover, it showed excellent photo-stability over five repetitive runs for the removal of organic pesticides with minimal drop on catalytic activity, presenting a promising choice for applications in waste water management.
2.4.2 Degradation of Antibiotics
Antibiotics are substances that inhibit the reproductive growth of microorganisms such as bacteria, viruses, and fungi etc., and eventually eliminate them. However, humans and animals cannot completely absorb them, resulting in their discharge in the water environment, which causes environmental pollution. Typical antibiotics, for example, ofloxacin, norfloxacin, and ciprofloxacin, were mainly degraded by defluorination, carbon-nitrogen bond breaking, decarboxylation, and ring opening[75].
Yong et al.[275] had summarized the recent advances in photodegradation of antibiotic residues in water, as illustrated in Figure 19. In this review, we mainly list some typical cases. Heterogeneous TiO2-based catalysts had been widely examined for the photocatalytic degradation of ciprofloxacin under UV/visible light irradiation, which exhibited good degradation capability[276]. A BiOBr/Mn-Ti3C2Tx composite photocatalyst synthesized through in-situ ion modification method accomplished the nearly total degradation of ciprofloxacin with the assistant of peroxymonosulfate activation. This improved activity was ascribed to the superior light capture ability[277]. Besides, a novel γ-Fe2O3-MIL-53(Fe)-GO composite was found to display high photocatalytic activity towards norfloxacin, with a removal rate of 92.8% in 90min[278]. Another MOF (ZIF-67) etching-induced Co-doped hollow carbon nitride catalyst was reported to be efficient to remove antibiotic tetracycline, for achieving 99% degradation[279].
|
Figure 19. A Summary of Key Recent Advances in Photocatalytic Removal of Antibiotics in Water. Reproduced from Ref.[275] with permission from Elsevier.
2.4.3 Degradation of Dyes
Dyes play an important role in the production and life of human beings because they are widely used in various aspects of daily life. However, they also contribute to serious environmental problems even damages to human health once released into the environment[280,281]. Researches on degrading some typical dyes that were extensively utilized in the printing, textile, plastic, silk, food, leather, and textile industries, such as methylene blue (MB), methyl orange (MO), and rhodamine (Rh B) are presented here.
Ramezanalizadeh et al.[258] used a facile synthesis route to prepare a novel MOF-based composite with CuWO4 noted as MOF/CuWO4 successfully, and this photocatalyst could remove about 98% MB under LED light irradiation. The characterizations revealed that the formation of junction between MOF and CuWO4 effectively reduced the recombination of electron-hole pairs in the MOF/CuWO4 heterostructure. Furthermore, lower regeneration of electron-hole pairs in the MOF/CuWO4 was responsible for the higher photocatalytic activity. Degradation of MB as well as MO had been also conducted by Nguyen et al.[282] by using Pd-doped TiO2 photocatalysts that synthesized by sol-gel method. The degradation pathways of single MB and MO with this catalyst were also proposed. In addition, Kumar et al.[283] developed BiO-Ag(0)/C3N4@ZIF-67 for the degradation of MB and Congo red dyes, and the degradation rates of MB and Congo red dyes were 96.5% and 90%, respectively. A TiO2-based MOF (MIL-125-NH2) catalyst with decorations by introduction of both hierarchical pores and oxygen vacancies showed enhanced degradation rate of Rh B[284]. Hierarchical pores increased the active sites and oxygen vacancies increased the yield of active radicals, and therefore the synergistic effect of Ov and hierarchical pores was achieved, ultimately leading to a superior photocatalytic performance, which provided a new strategy to prepare modified catalysts for the treatment of waste water. Syed Shoaib and coworkers had also focused on the synthesis of advanced catalysts (SACs, MOFs-based catalysts etc.) and their applications for handling waste water. They synthesized tri-metallic layered double hydroxides (NiZnAl-LDH) nano-sheets with unique structure that comprised of continuous macrostructure skeleton with interconnected macropores, which showed excellent catalytic performance for the decontamination of Rh B and methyl orange[285]. Other types of catalysts including Chitosan/MnO2@MOF-801[286], ZIF-8@ZIF-67[287] were also prepared, which showed good performance towards the photocatalytic reduction or adsorption of Rh B.
2.4.4 Degradation of Oil and Grease
The oil and grease are not only used in cooking life daily, but also widely used as solvents and raw materials in the petroleum, petrochemical, pharmaceutical, and cosmetic industries[288]. Regardless of the domestic life effluent, industries effluent or oil spilling from the transportation on oceans, the negative effects such as disrupting the photosynthesis of aquatic plants and diminishing the amount of dissolved oxygen in the water bodies will be resulted, which further destroys the balance of ecosystem[289]. It is of great urgency to solve the problems of oil pollution of water worldwide. For the mechanism of photodegradation of oils: a whole list of redox reactions happened because of the electron and hole pairs migration to the photocatalyst surface when light irradiation was put[290]. Ultimately, oils and organic contaminants could be degraded into CO2 and H2O by reactive oxygen radicals. Saien and Shahrezaei[291] treated a real petroleum refinery waste water which contained a range of aliphatic and aromatic organic compounds by using nano-TiO2 particles as the photocatalyst under UV irradiation. It was reported that a degradation efficiency of more than 78% of these oil and grease pollutants was achieved under the optimum operating conditions. This work was expected to fulfill the primary required data on application of nano-photocatalyst particles for the treatment of petroleum refinery waste water. Later, Shivaraju et al.[292] fabricated a N-doped TiO2 photocatalytic catalysts with polyscale structural features to enhance the overall efficiency of oil and grease removal in waste water through sol-gel technique. Activity results clearly indicated the considerable removal level of the oil and grease from waste water, which was up to 85-90%±2% under natural sunlight, presenting a versatile, economical, and environmentally friendly technique due to the ease of handling and recovery, utilization of natural and renewable sunlight. Regardless of the modifications on photocatalysts, coupling photocatalytic and Fenton oxidation for oily waste water treatment was also adapted. Mokhbi et al.[293] reported a catalytic system (TiO2/UV/Fe2+/H2O2 (photocatalysis-Fenton’s reagent)), which was able to deal with oily organic pollutants with high efficiency. Solution pH was found to be the key factor governing the photodegradation performance.
2.4.5 Degradation of Phenol and Phenolic Compounds
Phenol and phenolic compounds such as p/m/o-nitrophenol, chlorophenols etc. are one type of volatile aromatic hydrocarbon with a white crystalline structure, which are highly soluble in water and widely utilized in the production of kinds of chemicals[294]. They are highly irritating to skin, eyes and mucous membrane of humans on acute inhalation or dermal exposure. Therefore, the residue of these phenol and phenolic compounds is annoying, and dealing with them is also essential. A novel p-LaFeO3/n-Ag3PO4 heterojunction photocatalyst for phenol degradation under visible light irradiation was reported by Yang et al.[295] and 95% of phenol was degraded. Compared with the individual Ag3PO4 and LaFeO3, the composite photocatalyst exhibited much higher photocatalytic performance and stability due to the promoted separation efficiency between photogenerated electron and hole pairs. Another photocatalyst (Bi2O3-Bi4V2O11) synthesized by a high-temperature calcination method was found to be effective for phenol degradation (40mg/L) under a visible light source (300W Xe lamp) for 30min[296]. The enhanced photocatalytic performance of Bi2O3-Bi4V2O11 compared to pure Bi2O3 and Bi4V2O11 was ascribed to the synergistic effect between Bi2O3 and Bi4V2O11, high interface quality and one-dimensionally ordered nanostructure. By constructing Cu2O/TiO2[297] and Ag3PO4/Bi2WO6[298] heterojunctions, they were capable of degrading 95.8% and 98.5% of phenol, respectively. Complete photocatalytic degradation of phenol was achieved on Ag2O/g-C3N4 heterostructure, synthesized by a simple chemical precipitation method at room temperature[299]. Similarly, TiO2/g-C3N4[300], synthesized by surface hybridization and dip-coating method was also able to degrade phenol completely in 1.5h under visible light illumination. Except the degradation of phenol, much works focusing on the conversion of phenol derivates had been reported. The photocatalytic property of Na2Ti6O13/TiO2 towards 2,4-dichlorophenol was evaluated, and it was found that the degradation rate reached 99.4%[301]. In addition, a trimetallic catalyst, FeCoCu-nitrogen-doped carbon (FeCoCu-NC) with core-shell structure was capable of degrading trichlorophenol efficiently with peroxymonosulfate activation[302]. In another study, Lam et al.[303] found that 95% of resorcinol was degraded by Ag2O/ZnO in 6h under visible light irradiation. Higher photocatalytic activity of Ag2O/ZnO than pure ZnO could be attributed to the high separation efficiency of the photogenerated electron-hole pairs based on the cooperative roles of Ag2O loading on ZnO nanorods. Recently, Zr6O8-porphyrinic MOFs had been reported as promising catalysts for boosting photocatalytic degradation of bisphenol A in high salinity waste water with degradation rate for nearly 100%[304]. This catalytic system could maintain consistent contaminant degradation efficiency over a wide pH range, with high concentrations of co-existing ions and in real water matrices, providing possibility for the treatment of highly saline waste water.
2.4.6 Degradation of Other Pollutants
In addition to the above-mentioned four categories of pollutants in waste water, various other pollutants are still presented in the waste water and atmospheric environment, such as heavy metal, pharmaceuticals, industrial compounds, volatile organic compounds (VOCs) and even bacteria. Reports for treating these contaminants had been also briefly presented. For example, both nanotubes and graphene-based photocatalysts had been proved to be effective towards the removal of heavy metals[305]. Besides, iron-based materials showed high removal performance towards heavy metals, reported by Zhang et al[306]. Cao et al.[307] used G-C3N4/ MIL-68(In)-NH2 to degrade ibuprofen (an anti-inflammatory and analgesic drug) under visible light. Fe-MOF derivative was successfully prepared by thermal treatment using MIL-100(Fe) as a precursor and the resulting M-300 catalyst displayed excellent performance in the degradation of VOCs and the bacteriostasis to Escherichia coli under visible light[308].
In summary, the photocatalytic degradation of all organic toxic pollutants was depending on their active groups, and the photocatalysts with high catalytic performance played vital roles on illuminating them. MOF-based materials that contained many active species were grabbing increased attentions on the degradation of waste water pollutants. However, as far as we know, there is no efficient catalytic system enables to catalyze or degrade these different types of pollutants in waste water simultaneously. The above commented works primarily displayed excellent catalytic performance towards one certain compound, rather than for several or even all the pollutants in the waste water. These catalysts were designed and synthesized particularly towards one certain compound. Moreover, for the waste water that really emitted from factories, a large quantity of sediments was mixed, which brought out converting difficulties further. The above issues would motivate researchers to think about how to convert the waste water with high complexity.
Even though a great deal of progresses has been achieved in the catalytic conversion of these complex reactant systems, deeper investigations on mechanism are still highly presumed since it guides us to fulfill the conversion of complex reactant systems with much higher efficiency and lower costs that follows the way we want. At present, there is merely rare scientific researches disclosing the real reaction mechanisms occurred in the complex reactant systems, and it is still a huge task for us to finish. Detailed mechanism explorations require not only reasonable and ingenious design of experimental processes, but also theoretical calculations, which are key steps to help realize this goal. To date, the complicated compositions of these complex reactant systems restrict or even imped the theory construction of models, so that accurate calculation data related to the real complex reactant systems cannot be obtained. Further efforts devoting to solve this problem should be put. As for the concrete examples for the catalytic conversion of complex reactant systems that we enumerated before, it can be clearly seen that traditional catalysis methods are widely adopted while those advanced catalysis we mentioned in the introduction section have not been used widely in this field even though they own prosperous application prospectives and huge development potentials. Hence, we should think about how to utilize these recent developed advanced catalysis approaches to promote the conversion of complex reactant systems? How can the advantages of these newly developed advanced catalytic methods be fully exploited in this application area? Whether new strategies should be developed from different viewpoints? Once the above questions have been well settled, it can be anticipated that the conversion of complex reactant systems would step into a new broader stage.
It should be noted that the poisoning phenomenon of traditional catalysts was obvious due to the complex compositions and high contents of toxic pollutants contained in these systems, not to say those advanced catalysts with precisely controlled active sites. Moreover, the interactions between components in a complex reactant system was highly complex, and the existence of other components might affect the conversion of a certain component, which might be positive or negative. Besides, the intercrossing or interactive effects of different reactions might also play an important role for such conversion. Therefore, new strategies that totally different from these presented or applied now should be cultivated since the majority of these methods currently developed are proposed based on the reaction systems that comprised of simple components rather than complicated ones.
3 CONCLUSIONS AND OUTLOOKS
In conclusion, there have been a lot of newly developed, advanced, and multivariate technologies related to catalysts emerging in recent decades. These technologies have been widely concerned with preparation, characterization and in-situ operation equipment. Based on both traditional and emerged technologies, and integrating with smart transformation strategies, the catalytic transformation or degradation of these complex reactant systems have also gained achievements. Researchers have fulfilled the conversion of biomass including lignocellulos and chitin into value-added chemicals, the recycle and re-utilization of waste-plastics accompanying solving white-pollution issues and the purification of both vehicle exhausted gases and water pollutants to less-toxic even non-toxic chemicals. All of these efforts contribute to build a more sustainable and greener Earth to live. However, due to the particularity of these complex reactant systems (not only limited to these we mentioned above), that is, the high complexity of their compositions, chemical-bonding and interactions, there is still huge development space and potential for their catalytic transformation, leaving researchers with a long way to explore. Based on this, we propose the following possible development strategies in the future to promote the catalytic transformation of complex reactant systems.
Firstly, take full advantages of “Artificial Intelligence” (AI) such as machine learning (ML) and deep learning (DL) that constructed and guided by huge database and naturally enables multi-task learning. AI has received widespread attention over the last few decades due to its potential to increase automation and accelerate productivity. The traditional trial-and-error method is inefficient and time-consuming to solve problems or synthesize new materials or chemicals. On the one hand, the development of new conversion methodologies towards both basic reactions or systems with complex networks is a tedious task demanding both time and resources. On the other hand, the vast amounts of chemical data generated during lab experiments are usually underutilized, which is indeed highly important for guiding further researches[309]. Therefore, the application of data-driven approaches for reaction discovery, optimization, and prediction can make a significant impact on efficient exploration of these complex reactant systems in multidimensional chemical spaces. Recently, the improvements in computing power that complemented by improved data availability have led to notable progresses in the use of ML and DL in many areas of chemistry, including the design and architecting of pharmaceuticals and other molecules[310,311], the discovery of novel multifunctional materials[312], the exploration and predication of kinetic pathways[313], the discovery of highly efficient catalysts and the understanding of the real reaction mechanisms etc[314]. Here, we primarily emphasize the goals and achievements using ML and DL on catalysis. By using ML and DL as tools, a deeper understanding of the relations between materials properties and activity, selectivity and stability, which are important merits of catalysts, can be established. Based on these insights, catalyst design principles can be established further, which hopefully guides us to discover highly efficient catalysts to solve pressing issues (such as the catalytic conversion of these complex reactant systems) to build a sustainable future and synthesis world[315-318]. In this research field, developing suitable and reliable machine learning models of ML or DL for designing and discovering superior catalysts in many aspects with relatively small and sparse labeled data, is also an important pursuit. To date, efforts have been devoted to these research fields, and some preliminary and fundamental results have been achieved. Cheng et al.[319] had summarized the works that focused on the design of SACs driven by AI, and the diagram of operation mechanism was shown in Figure 20. Firstly, an integrated design-synthesis-analysis approach could be established with the development of databases driven by AI, which helped to bridge the experimental science and computer-based models, and more and more parameters and effects involved in catalytic processes could be clarified, such as atomic confinement, local coordination, adsorption energy and kinetic and thermodynamic parameters etc. Secondly, AI could drive high throughput screening through various function-sensitive data-based descriptors of SACs towards structure-property relationship. Meanwhile, the theoretical calculations could be also promoted by AI since multi-parameters-involved as input descriptors during the calculation had been used to reveal the structure-activity relationships. These multiple-level descriptors obtained by machine learning were more reliable, which made the prediction of chemical reactions more accurate. As reported by Ramirez et al.[320], AI was also capable of accelerating exploration of heterogeneous CO2 hydrogenation catalysts by Bayesian-optimized high-throughput and automated experimentation.
|
Figure 20. The Proposed Diagram for AI-Driven Single-Atom Heterogeneous Catalysts Design and Discovery. Reproduced from Ref.[319] with permission from Wiley.
In addition to its positive impacts on catalyst design, AI is also important for clarifying the mechanism of catalytic reactions, where dynamic analysis is the core, which helps to directly test mechanism hypotheses from experimental data. Traditionally, kinetic analysis had relied on the use of methods such as initial rate, logarithmic graphs, and visual kinetic dynamics, combined with mathematical rate law derivations. However, the derivation of rate laws and their interpretation required many mathematical approximations, and as a result, they were prone to artificial errors and were limited to reaction networks with only a few steps at steady state. Burés and Larrosa[321] reported a deep neural network model could be trained to analyze ordinary kinetic data and automatically elucidated the corresponding mechanism class, without any additional user input. The model identified a wide variety of classes of mechanism with outstanding accuracy, including mechanisms out of steady states such as those involving catalyst activation and deactivation steps, and performed excellently even when the kinetic data contained substantial errors or only a few time points, which would lead to further advances in the development of fully automated organic reaction discovery and development. However, we should also realize even though AI is prospective, there are still some demerits needs to be considered. The required amounts of data in the database must be large enough, and the authenticity of the data must also be guaranteed. At the same time, the learning model adopted by AI must also be intelligent enough or suitable for complex reactant systems. Only when these two conditions are met simultaneously, the data filtered or simulated based on AI is effective. However, there are still tough ways to go to satisfy these two conditions.
Another pivotal approach is to shift our historic dependence on manual works to smart machines, that is, the recently hot “A mobile robotic chemist”[322,323]. Undoubtably, chemists spend a great deal of time tweaking the conditions of known reactions. Small changes to temperature, pressure, substrate concentration and catalyst amounts etc. have significant influences over product yields and selectivity in simple systems, not to say the complex reaction systems without well-developed methodologies. In this way, robots can assist in pre-experimental searches and materials researches, which have already applied in some simple synthesis process[324-327]. For example, very recently, Burger et al.[322] used a mobile robot to search for improved photocatalysts for hydrogen production from water, wherein the robot could operate autonomously over eight days, performing 688 experiments within a ten-variable experimental space, driven by a batched Bayesian search algorithm. This autonomous search identified photocatalyst mixtures that were six times more active than the initial formulations, selecting beneficial components and deselecting ones. This strategy used a dexterous free-roaming robot, automating with higher working-efficiency than human operation, which provided a new option for chemists to work with. Likewise, the similar robots or even with more sensitivity, flexibility and intelligence ability could promote the exploration and catalytic degradation of these complex reactant systems. Another prospect proposed here is an integration of AI and automatic robot workers towards autonomous discovery of highly efficient catalysts and subsequently reactions conduction, which will emerge within next decades, and provide a vision for converting complex reactant systems to desired products with higher yields as well as selectivity under much milder and greener conditions. Absolutely, the approach requires the integration of the following tools, which have already seen substantial development to date: high-throughput virtual screening, automated synthesis planning, automated laboratories, and machine learning algorithms etc., and each of them is indispensable. In spite of shortening the time to cultivate new catalyst materials by an order of magnitude, this integrated approach is also expected to lower the cost associated with the manpower resources, time costs and conditions optimizations etc. Consequently, the price of the final products as well as waste treatments will also decrease. This in turn will enable industries and governments to pay more attentions in terms of reducing toxic emissions, greenhouse gases emissions and white pollutants. In summary, bringing recent technological innovations in AI, automatic robotics and computer science together with current approaches in chemistry, catalysts synthesis and characterization will act as a catalyst for revolutionizing traditional research and development in both industry and academia[328]. However, we should realize that the research and building of smart robots are somehow difficult because they require the joint efforts of multiple disciplines including computer science, mechanical engineering, electronic engineering and control science etc., which brings a lot of uncertainties for the use of intelligent robots to transform complex reactant systems.
Thirdly, more efforts in both theoretical and practical levels should be contributed to implement the ‘precision chemistry’ concept. Developing more efficient and accurate theoretical methods and programs for theoretical calculations is essential. At present, various approximations are usually introduced in the actual calculations because the workload and difficulty of computation required augment exponentially with the increase of the sizes and amounts of molecules. These approximations bring errors in some extents because the complex reactant systems can not represent the real ones. Therefore, it is necessary to develop new methods to obtain the most accurate computational simulation results under the conditions of tolerable computational resources. It can be predicted that the emergence of quantum computers is anticipated to bring breakthrough progresses in this direction. Quantum computer can greatly improve the efficiency of electronic structure calculations. The other aspect is to develop accurate characterization methods for catalysts under real working conditions. To obtain experimentally accurate chemical details at the atomic scale, conditions of ultra-low temperature and ultra-high vacuum are usually required. However, the real chemical processes usually occur at normal temperature and pressure or even high temperature and pressure. Moreover, as for the treatments towards complex reactant systems, it is more intricated. To address these challenges, it will be necessary to gain a better understanding of the detailed reaction mechanism and reaction intermediates. It is a vital goal of precision chemistry to develop new characterization methods and obtain as much data as possible about the structures, properties and dynamic behaviors of the systems under working conditions, which will promote the establishing of structure-reactivity relationships and in turn guide the design of catalysts. In addition to this, the knowledge in precision chemistry will also provide insights into the deactivation phenomena that occurs during the conversion of these complex reactant systems. By unraveling the intricacies of the reaction mechanism and identifying key intermediates, researchers can develop strategies to mitigate catalyst deactivation and enhance catalyst stability, leading to more efficient and sustainable catalytic processes. Similarly, more precise identification of kinds of intermediates contributes to establish precision chemistry. In fact, there are still some obstacles for implementing the ‘precision chemistry’ concept in both theory and practical aspects. On the one hand, the development of precision chemistry theoretically needs the promotion of theoretical innovation. Also, when macromolecules are used to conduct the theoretical calculations to practice the concept of precise chemistry, not only will the calculation time be extended geometrically, the efficiency of the calculation will be reduced accordingly, but also the cost will be greatly increased.
Lastly, a newly conversion concept should be established. As for these complex reactant systems, researchers are widely adopting fractionation or categorized conversion strategies nowadays. An innovation concept that ‘Full components utilization and conversion’ could be a promising strategy, which exploits the full potential of all components in these complex reactant systems Besides, new strategies from different viewpoints, specifically for converting complex reactant systems, should be cultivated since these already developed methods are aiming for those systems with simple compositions, which is not suitable for the conversion of complex reactant systems. However, the time for developing new strategies from different viewpoints specifically suitable for converting complex reactant systems is unclear, which makes the process unpredictable.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21536007), the 111 Project (No. B17030), and the Fundamental Research Funds for the Central Universities.
Conflicts of Interest
The authors declare no competing financial interest.
Data Availability
No additional data are available.
Copyright Permissions
Copyright © 2024 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Author Contribution
Yang H and Hu P conducted the investigation and drafted the original manuscript. Li Z also contributed to the investigation. Hu C provided supervision, managed the project administration, acquired funding, and contributed to reviewing and editing the manuscript.
Abbreviation List
3A5AF, 3-acetamido-5-acetylfuran
5-HMF, 5-hydromethylfurfural
AI, Artificial intelligence
CCD, Coordination-coupled deprotonation
COFs, Covalent-organic frameworks
DL, Deep learning
DMSO, Dimethyl sulfoxide
EG, Ethylene glycol
FA, Formic acid
FAL, Furfural
GlcNA, Glucosaminic acid
GVL, γ-valerolactone
LA, Levulinic acid
LMWC, Low-molecular-weight chitosan
MB, Methylene blue
ML, Machine learning
MO, Methyl orange
MOFs, Metal-organic frameworks
NAG, N-acetyl glucosamine
PC, Polycarbonate
PET, Polyethylene terephthalate
PLA, Polylactic acid
PS, Polystyrene
PU, Polyurethane
PX, Para-xylene
Rh B, Rhodamine
SACs, Single-atom catalysts
SCR, Selectively catalyze the reduction
TEOS, Tetraethyl orthosilicate
THF, Tetrahydrofuran cyclohexane
TWCs, Three-way catalysts
VOCs, Volatile organic compounds
References
[1] Heinemann H, Davis BH, Mills GA. Comment on Heterogeneous catalysis since Berzelius: some personal reflections. Catal Lett, 2000; 69: 251-252.[DOI]
[2] Martín AJ, Mitchell S, Mondelli C et al. Unifying views on catalyst deactivation. Nat Catal, 2022; 5: 854-866.[DOI]
[3] Filippi E, Pizzolitto C. The past and the future of catalysis and technology in industry: a perspective from Casale SA point of view. Catal Today, 2022; 387: 9-11.[DOI]
[4] Liu H. Ammonia synthesis catalyst 100 years: Practice, enlightenment and challenge. Chin J Catal, 2014; 35: 1619-1640.[DOI]
[5] Lugovskoi AI, Linov NV. Sulfuric acid production: progressive technology. Chem Technol Fuels Oils, 2000; 36: 359-361.[DOI]
[6] Dieterich V, Buttler A, Hanel A et al. Power-to-liquidviasynthesis of methanol, DME or Fischer-Tropsch-fuels: a review. Energy Environ. Sci, 2020; 13: 3207-3252.[DOI]
[7] Ail SS, Dasappa S. Biomass to liquid transportation fuel via Fischer Tropsch synthesis-Technology review and current scenario. Renew Sust Energ Rev, 2016; 58: 267-286.[DOI]
[8] Wang X, Ma Y, Wu Q et al. Zeolite nanosheets for catalysis. Chem Soc Rev, 2022; 51: 2431-2443.[DOI]
[9] Chai Y, Dai W, Wu G et al. Confinement in a zeolite and zeolite catalysis. Acc Chem Res, 2021; 54: 2894-2904.[DOI]
[10] Zhu Y, Romain C, Williams CK et al. Sustainable polymers from renewable resources. Nature, 2016; 540: 354-362.[DOI]
[11] Ren J, McKenzie TG, Fu Q et al. Star polymers. Chem Rev, 2016; 12: 6743-6836.[DOI]
[12] Shah SSA, Sufyan JM, Najam T et al. Metal oxides for the electrocatalytic reduction of carbon dioxide: Mechanism of active sites, composites, interface and defect engineering strategies. Coord Chem Rev, 2022; 471: 214716.[DOI]
[13] Shah SSA, Najam T, Nazir MA et al. Salt-assisted gas-liquid interfacial fluorine doping: Metal-free defect-induced electrocatalyst for oxygen reduction reaction. Mol Catal, 2021; 514: 111878.[DOI]
[14] Debecker DP, Mutin PH. Non-hydrolytic sol-gel routes to heterogeneous catalysts. Chem Soc Rev, 2012; 41: 3624-3650.[DOI]
[15] Guan S, Li W, Ma J et al. A review of the preparation and applications of MnO2 composites in formaldehyde oxidation. J Ind Eng Chem, 2018; 66: 126-140.[DOI]
[16] Sarma BB, Maurer F, Doronkin DE et al. Design of single-atom catalysts and tracking their fate using operando and advanced x-ray spectroscopic tools. Chem Rev, 2022; 123: 379-444.[DOI]
[17] Liu L, Corma A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem Rev, 2018; 118: 4981-5079.[DOI]
[18] Qiao B, Wang A, Yang X et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat Chem, 2011; 3: 634-641.[DOI]
[19] Kyriakou G, Boucher MB, Jewell AD et al. Isolated Metal Atom Geometries as a Strategy for Selective Heterogeneous Hydrogenations. Science, 2012; 335: 1209-1212.[DOI]
[20] Guo X, Fang G, Li G et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science, 2014; 344: 616-619.[DOI]
[21] Liu P, Zhao Y, Qin R et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science, 2016; 352: 797-800.[DOI]
[22] Wei H, Liu X, Wang A et al. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat Commun, 2014; 5: 1-8.[DOI]
[23] Shi Y, Zhao C, Wei H et al. Single‐atom catalysis in mesoporous photovoltaics: the principle of utility maximization. Adv Mater, 2014; 26: 8147-8153.[DOI]
[24] Yan H, Cheng H, Yi H et al. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: remarkable performance in selective hydrogenation of 1,3-butadiene. J Am Chem Soc, 2015; 137: 10484-10487.[DOI]
[25] Pei G, Liu X, Wang A et al. Ag alloyed pd single-atom catalysts for efficient selective hydrogenation of acetylene to ethylene in excess ethylene. ACS Catal, 2015; 5: 3717-3725.[DOI]
[26] Li X, Yuan Z, He S. CO oxidation promoted by gold atoms supported on titanium oxide cluster anions. J Am Chem Soc, 2014; 136: 3617-3623.[DOI]
[27] Li Z, Yuan Z, Li X et al. CO oxidation catalyzed by single gold atoms supported on aluminum oxide clusters. J Am Chem Soc, 2014; 136: 14307-14313.[DOI]
[28] Lin J, Wang A, Qiao B et al. Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction. J Am Chem Soc, 2013; 135:15314-15317.[DOI]
[29] Hu P, Huang Z, Amghouz Z et al. Electronic metal-support interactions in single‐atom catalysts. Angew Chem Int Edit, 2014; 53: 3418-3421.[DOI]
[30] Wang L, Zhang S, Zhu Y et al. Catalysis and in situ studies of Rh1/Co3O4 nanorods in reduction of NO with H2. ACS Catal, 2013; 3: 1011-1019.[DOI]
[31] Deng D, Chen X, Yu L et al. A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature. Sci Adv, 2015; 1: e1500462.[DOI]
[32] Wu P, Du P, Zhang H et al. Graphyne-supported single Fe atom catalysts for CO oxidation. Phys Chem Chem Phys, 2015; 17: 1441-1449.[DOI]
[33] Fei H, Dong J, Arellano MJ et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat Commun, 2015; 6: 8668.[DOI]
[34] Li W, Guo Z, Yang J et al. Advanced strategies for stabilizing single-atom catalysts for energy storage and conversion. Electrochem Energy Rev, 2022; 9: 1-41.[DOI]
[35] Wang L, Wang D, Li Y. Single‐atom catalysis for carbon neutrality. Carbon Energy, 2022; 4: 1021-1079.[DOI]
[36] Liang X, Fu N, Yao S et al. The progress and outlook of metal single-atom-site catalysis. J Am Chem Soc, 2022; 144:18155-18174.[DOI]
[37] Din MAU, Shah SSA, Javed MS et al. Synthesis of MXene-based single-atom catalysts for energy conversion applications. Chem Eng J, 2023; 474: 145700.[DOI]
[38] Chen S, Xiong F, Huang W. Surface chemistry and catalysis of oxide model catalysts from single crystals to nanocrystals. Surf Sci Rep, 2019; 74: 100471.[DOI]
[39] Tao F, Dag S, Wang L et al. Break-up of stepped platinum catalyst surfaces by high CO coverage. Science, 2010; 327: 850-853.[DOI]
[40] Nørskov JK, Bligaard T, Rossmeisl J et al. Towards the computational design of solid catalysts. Nat Chem, 2009; 1: 37-46.[DOI]
[41] Klajn R. Spiropyran-based dynamic materials. Chem Soc Rev, 2014; 43: 148-184.[DOI]
[42] Wang Y, Tan X, Zhang Y et al. Dynamic behavior of molecular switches in crystal under pressure and its reflection on tactile sensing. J Am Chem Soc, 2015; 137: 931-939.[DOI]
[43] Niroui F, Wang AI, Sletten EM et al. Tunneling nanoelectromechanical switches based on compressible molecular thin films. ACS Nano, 2015; 9: 7886-7894.[DOI]
[44] Hu Q, Wang H, Cui C et al. “Redox switches” of Fe species on zeolite catalysts: Modulating the acidity and the para-xylene yield from methanol. Carbon Future, 2023; 1: 9200001.[DOI]
[45] De Vries F, Otten E. Reversible On/Off Switching of Lactide Cyclopolymerization with a Redox-Active Formazanate Ligand. ACS Catal, 2022; 12: 4125-4130.[DOI]
[46] Popp J, Caminade AM, Hey HE. Redox‐switchable transfer hydrogenations with P‐chiral dendritic ferrocenyl phosphine complexes. Eur J Inorg Chem, 2020; 2020: 1654-1669.[DOI]
[47] Zhang Z, Wang W, O'Hagan M et al. Stepping out of the blue: from visible to near‐ir triggered photoswitches. Angew Chem Int Edit, 2022; 61: e202205758 [DOI]
[48] Wang J, Feringa BL. Dynamic control of chiral space in a catalytic asymmetric reaction using a molecular motor. Science, 2011; 331: 1429-1432.[DOI]
[49] Neilson BM, Bielawski CW. Photoswitchable organocatalysis: using light to modulate the catalytic activities of N-heterocyclic carbenes. J Am Chem Soc, 2012; 134: 12693-12699.[DOI]
[50] Rabelo R, Toma L, Moliner N et al. pH-Switching of the luminescent, redox, and magnetic properties in a spin crossover cobalt(ii) molecular nanomagnet. Chem Sci, 2023; 14: 8850-8859.[DOI]
[51] Balof SL, Steven JP, Berger NJ et al. Olefin metathesis catalysts bearing a pH-responsive NHC ligand: a feasible approach to catalyst separation from RCM products. Dalton Trans, 2008; 42: 5791-5799.[DOI]
[52] Blanco V, Carlone A, Hänni KD et al. A rotaxane‐based switchable organocatalyst. Angew Chem Int Edit, 2012; 21: 5166-5169.[DOI]
[53] Do Pim WD, Oliveira WXC, Ribeiro MA et al. A pH-triggered bistable copper(ii) metallacycle as a reversible emulsion switch for biphasic processes. Chem Commun, 2013; 49: 10778-10780.[DOI]
[54] Zhang S, Hao J, Zhu Y et al. pH‐Triggered molecular switch toward texture‐regulated Zn anode. Angew Chem Int Edit, 2023; 62: e202301570.[DOI]
[55] Nuryyeva S. Structure-property analysis of a new family of hydrazone photoswitches [master’s thesis]. Hanover, New Hampshire: Dean of Graduate and Advanced Studies; 2017.
[56] Yoon HJ, Heo J, Mirkin CA. Allosteric regulation of phosphate diester transesterification based upon a dinuclear zinc catalyst assembled via the weak-link approach. J Am Chem Soc, 2007; 129: 14182-14183.[DOI]
[57] Foy JT, Ray D, Aprahamian I. Regulating signal enhancement with coordination-coupled deprotonation of a hydrazone switch. Chem Sci, 2015; 6: 209-213.[DOI]
[58] Pramanik S, Aprahamian I. Hydrazone switch-based negative feedback loop. J Am Chem Soc, 2016; 138: 15142-15145.[DOI]
[59] Rungtaweevoranit B, Diercks CS, Kalmutzki MJ et al. Spiers Memorial Lecture: Progress and prospects of reticular chemistry. Faraday Discuss, 2017; 201: 9-45.[DOI]
[60] Cai G, Yan P, Zhang L et al. Metal-organic framework-based hierarchically porous materials: Synthesis and applications. Chem Rev, 2021; 121: 12278-12326.[DOI]
[61] Jiao L, Jiang H. Metal-organic frameworks for catalysis: Fundamentals and future prospects. Chine J Catal, 2023; 45: 1-5.[DOI]
[62] Yang Q, Xu Q, Jiang H. Metal-organic frameworks meet metal nanoparticles: synergistic effect for enhanced catalysis. Chem Soc Rev, 2017; 46: 4774-4808.[DOI]
[63] Jiao L, Wang J, Jiang H. Microenvironment modulation in metal-organic framework-based catalysis. Acc Mater Res, 2021; 2: 327-339.[DOI]
[64] Zhang B, Zheng Y, Ma T et al. Designing MOF nanoarchitectures for electrochemical water splitting. Adv Mater, 2021; 33: 2006042.[DOI]
[65] Shah SSA, Sohail M, Murtza G et al. Recent trends in wastewater treatment by using metal-organic frameworks (MOFs) and their composites: A critical view-point. Chemosphere, 2024; 349: 140729.[DOI]
[66] Xue D, Xia H, Yan W et al. Defect Engineering on Carbon-Based Catalysts for Electrocatalytic CO2 Reduction. Nano-Micro Lett, 2020; 5: 1-23.[DOI]
[67] Shah SSA, Nazir MA, Khan K et al. Solar energy storage to chemical: Photocatalytic CO2 reduction over pristine metal-organic frameworks with mechanistic studies. J Energy Storage, 2024; 75: 109725.[DOI]
[68] Du J, Li F, Sun L. Metal-organic frameworks and their derivatives as electrocatalysts for the oxygen evolution reaction. Chem Soc Rev, 2021; 50: 2663-2695.[DOI]
[69] Peng Y, Sanati S, Morsali A et al. Metal-Organic frameworks as electrocatalysts. Angew Chem, Int Ed, 2023; 62: e202214707.[DOI]
[70] Liu J, Goetjen TA, Wang Q et al. MOF-enabled confinement and related effects for chemical catalyst presentation and utilization. Chem Soc Rev, 2022; 51: 1045-1097.[DOI]
[71] Kondo Y, Kuwahara Y, Mori K et al. Design of metal-organic framework catalysts for photocatalytic hydrogen peroxide production. Chem, 2022; 8: 2924-2938.[DOI]
[72] Li X, Li H, Jiang S et al. Constructing mimic-enzyme catalyst: polyoxometalates regulating carrier dynamics of metal-organic frameworks to promote photocatalytic nitrogen fixation. ACS Catal, 2023; 13: 7189-7198.[DOI]
[73] Nazir MA, Javed MS, Islam M et al. MOF@graphene nanocomposites for energy and environment applications. Composites Com, 2024; 45: 101783.[DOI]
[74] Wang F, Gao Y, Liu S et al. Fabrication strategies of metal–organic frameworks derivatives for catalytic aqueous pollutants elimination. Chem Eng J, 2023; 463: 142466.[DOI]
[75] Yue C, Chen L, Zhang H et al. Metal-organic framework-based materials: emerging high-efficiency catalysts for the heterogeneous photocatalytic degradation of pollutants in water. Environ Sci Water Res Technol, 2023; 9: 669-695.[DOI]
[76] Yusran Y, Li H, Guan X et al. Covalent organic frameworks for catalysis. Energy Chem, 2020; 2: 100035.[DOI]
[77] Feng X, Ding X, Jiang D. Covalent organic frameworks. Chem Soc Rev, 2012; 41: 6010-6022.[DOI]
[78] Diercks CS, Yaghi OM. The atom, the molecule, and the covalent organic framework. Science, 2017; 355: eaal1585.[DOI]
[79] Waller PJ, Gándara F, Yaghi OM. Chemistry of covalent organic frameworks. Acc Chem Res, 2015; 48: 3053-3063.[DOI]
[80] Ding S, Wang W. Covalent organic frameworks (COFs): from design to applications. Chem Soc Rev, 2013; 42: 548-568.[DOI]
[81] Guan X, Chen F, Qiu S et al. Three‐Dimensional Covalent Organic Frameworks: From Synthesis to Applications. Angew Chem Int Edit, 2022; 62: e202213203.[DOI]
[82] Segura JL, Royuela S, Mar RM. Post-synthetic modification of covalent organic frameworks. Chem Soc Rev, 2019; 48: 3903-3945.[DOI]
[83] Luo T, Gilmanova L, Kaskel S. Advances of MOFs and COFs for photocatalytic CO2 reduction, H2 evolution and organic redox transformations. Coord Chem Rev, 2023; 490: 215210.[DOI]
[84] Qi S, Guo R, Bi Z et al. Recent progress of covalent organic frameworks‐based materials in photocatalytic applications: A review. Small, 2023; 19: 2303632.[DOI]
[85] Li Y, Karimi M, Gong Y et al. Integration of metal-organic frameworks and covalent organic frameworks: Design, synthesis, and applications. Matter, 2021; 4: 2230-2265.[DOI]
[86] Zhang L, Liu Z, Deng Q et al. Nature‐inspired construction of MOF@COF nanozyme with active sites in tailored microenvironment and pseudopodia‐like surface for enhanced bacterial inhibition. Angew Chem Int Edit, 2020; 60: 3469-3474.[DOI]
[87] Sun D, Jang S, Yim SJ et al. Metal doped core–shell metal-organic frameworks@covalent organic frameworks (MOFs@COFs) hybrids as a novel photocatalytic platform. Adv Funct Mater, 2018; 28: 1707110-6.[DOI]
[88] Feng C, Liu X, Zhu T et al. Catalytic oxidation of CO on noble metal-based catalysts. Environ Sci Pollut Res, 2021; 28: 24847-24871.[DOI]
[89] Sankar M, He Q, Engel RV et al. Role of the support in gold-containing nanoparticles as heterogeneous catalysts. Chem Rev, 2020; 120: 3890-3938.[DOI]
[90] Wang L, Deo S, Dooley K et al. Influence of metal nuclearity and physicochemical properties of ceria on the oxidation of carbon monoxide. Chine J Catal, 2020; 41: 951-962.[DOI]
[91] Beniya A, Higashi S. Towards dense single-atom catalysts for future automotive applications. Nat Catal, 2019; 2: 590-602.[DOI]
[92] Wei Z, Zhu , Liu J et al. Recent advance in single-atom catalysis. Rare Metals, 2021; 40: 767-789.[DOI]
[93] Qing L, Sheng H. Oxidation of carbon monoxide on atomic clusters. Chem J Chine Universities, 2014; 35: 665-688.[DOI]
[94] Widmann D, Behm RJ. Activation of molecular oxygen and the nature of the active oxygen species for CO oxidation on oxide supported Au catalysts. Acc Chem Res, 2014; 47: 740-749.[DOI]
[95] Cheng X, Li F, Wang Ca. Density functional theory study of CO oxidation by O2 on Aun (n=11, 13 and 14) clusters as catalysis: From a comparative review. Comput Theor Chem, 2016; 1097: 1-7.[DOI]
[96] Shen J, Chen D, Bai M et al. Ammonia deposition in the neighbourhood of an intensive cattle feedlot in Victoria, Australia. Sci Rep, 2016; 6: 32793.[DOI]
[97] Shi L, Yin Y, Wang S et al. Rational Catalyst Design for N2 Reduction under Ambient Conditions: Strategies toward Enhanced Conversion Efficiency. ACS Catal, 2020; 10: 6870-6899.[DOI]
[98] Cao Y. Roadmap and Direction toward high-performance MoS2 hydrogen evolution catalysts. ACS Nano, 2021; 15: 11014-11039.[DOI]
[99] Chen F, Wu Z, Adler Z et al. Stability challenges of electrocatalytic oxygen evolution reaction: From mechanistic understanding to reactor design. Joule, 2021; 5: 1704-1731.[DOI]
[100] Huang Z, Luo N, Zhang C et al. Radical generation and fate control for photocatalytic biomass conversion. Nat Rev Chem, 2022; 6: 197-214.[DOI]
[101] Liao Y, Koelewijn S, Bossche G et al. A sustainable wood biorefinery for low-carbon footprint chemicals production. Science, 2020; 367: 1385-1390.[DOI]
[102] Wu X, Luo N, Xie S et al. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem Soc Rev, 2020; 49: 6198-6223.[DOI]
[103] Amidon TE, Liu S. Water-based woody biorefinery. Biotechnol Adv, 2009; 27: 542-550.[DOI]
[104] Deneyer A, Peeters E, Renders T et al. Direct upstream integration of biogasoline production into current light straight run naphtha petrorefinery processes. Nat Energy, 2018; 3: 969-977.[DOI]
[105] Li C, Zhao X, Wang A et al. Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev, 2015; 115: 11559-11624.[DOI]
[106] Zhang Z, Song J, Han B. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem Rev, 2016; 117: 6834-6880.[DOI]
[107] Wang A, Zhang T. One-Pot Conversion of Cellulose to Ethylene Glycol with Multifunctional Tungsten-Based Catalysts. Acc Chem Res, 2013; 46: 1377-1386.[DOI]
[108] Rao J, Lv Z, Chen G et al. Hemicellulose: Structure, chemical modification, and application. Prog Polym Sci, 2023; 140: 101675.[DOI]
[109] Yan N, Chen X. Sustainability: Don’t waste seafood waste. Nature, 2015; 524: 155-157.[DOI]
[110] Chen X, Song S, Li H et al. Expanding the boundary of biorefinery: organonitrogen chemicals from biomass. Acc Chem Res, 2021; 54: 1711-1722.[DOI]
[111] Mika LT, Cséfalvay E, Németh Á. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem Rev, 2017; 118: 505-613.[DOI]
[112] Chundawat SPS, Bellesia G, Uppugundla N et al. Restructuring the crystalline cellulose hydrogen bond network enhances its depolymerization rate. J Am Chem Soc, 2011; 133: 11163-11174.[DOI]
[113] Werpy T, Petersen G. Top value added chemicals from biomass: volume I--results of screening for potential candidates from sugars and synthesis gas. National Renewable Energy Lab: Golden, Colorado, USA, 2004.[DOI]
[114] Chen S, Wojcieszak R, Dumeignil F et al. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem Rev, 2018; 118: 11023-11117.[DOI]
[115] Sajid M, Farooq U, Bary G et al. Sustainable production of levulinic acid and its derivatives for fuel additives and chemicals: progress, challenges, and prospects. Green Chem, 2021; 23: 9198-9238.[DOI]
[116] Wu H, Zhang R, Zhai Y et al. Solvent effects enable efficient tandem conversion of cellulose and its monosaccharides towards 5‐hydroxymethylfurfural. Chem Sus Chem, 2022; 16: e202201809.[DOI]
[117] Daniele DB, Wang Y, Buvat JC et al. Production of levulinic acid and alkyl levulinates: a process insight. Green Chem, 2022; 24: 614-646.[DOI]
[118] Luo X, Wu H, Li C et al. Heteropoly acid-based catalysts for hydrolytic depolymerization of cellulosic biomass. Front Chem, 2020; 8: 1-29.[DOI]
[119] Badgujar KC, Wilson LD, Bhanage BM, Recent advances for sustainable production of levulinic acid in ionic liquids from biomass: Current scenario, opportunities and challenges. Renew Sust Energ Rev, 2019; 102: 266-284.[DOI]
[120] Questell YM, Galkin MV, Barta K et al. Stabilization strategies in biomass depolymerization using chemical functionalization. Nat Rev Chem, 2020; 4: 311-330.[DOI]
[121] Tang J, Zhu L, Fu X et al. Insights into the kinetics and reaction network of aluminum chloride-catalyzed conversion of glucose in NaCl-H2O/THF biphasic system. ACS Catal, 2017; 7: 256-266.[DOI]
[122] Girisuta B, Janssen LPBM, Heeres HJ, Kinetic study on the acid-catalyzed hydrolysis of cellulose to levulinic acid. Ind Eng Chem Res, 2007; 46: 1696-1708.[DOI]
[123] Qin K, Yan Y, Zhang Y et al. Direct production of levulinic acid in high yield from cellulose: joint effect of high ion strength and microwave field. RSC Adv, 2016; 6: 39131-39136.[DOI]
[124] Hu P, Hu Y, Li H et al. Orientated inhibition of humin formation in efficient production of levulinic acid from cellulose with high substrate loading: Synergistic role of additives. Carbohydr Polym, 2023; 309: 120692.[DOI]
[125] Swatloski RP, Spear SK, Holbrey JD et al. Dissolution of cellose with ionic liquids. J Am Chem Soc, 2002; 124: 4974-4975.[DOI]
[126] Weingarten R, Rodriguez-Beuerman A, Cao F et al. Selective conversion of cellulose to hydroxymethylfurfural in polar aprotic solvents. Chem Cat Chem, 2014; 6: 2229-2234.[DOI]
[127] He J, Liu M, Huang K et al. Production of levoglucosenone and 5-hydroxymethylfurfural from cellulose in polar aprotic solvent-water mixtures. Green Chem, 2017; 19: 3642-3653.[DOI]
[128] Choudhary V, Mushrif SH, Ho C et al. Insights into the interplay of Lewis and Brønsted acid catalysts in glucose and fructose conversion to 5-(hydroxymethyl)furfural and levulinic acid in aqueous media. J Am Chem Soc, 2013; 135: 3997-4006.[DOI]
[129] Tang J, Guo X, Zhu L et al. Mechanistic study of glucose-to-fructose isomerization in water catalyzed by [Al(OH)2(aq)]+. ACS Catal, 2015; 5: 5097-5103.[DOI]
[130] Zhao H, Holladay JE, Brown H et al. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science, 2007; 316: 1597-1600.[DOI]
[131] Li X, Lu X, Nie S et al. Efficient catalytic production of biomass-derived levulinic acid over phosphotungstic acid in deep eutectic solvent. Ind Crops Prod, 2020; 145: 112154.[DOI]
[132] Gong C, Meng X, Jin C et al. Green synthesis of cellulose formate and its efficient conversion into 5-hydroxymethylfurfural. Ind Crops Prod, 2023; 192: 115985.[DOI]
[133] Shi N, Zhu Y, Qin B et al. Conversion of cellulose into 5-hydroxymethylfurfural in a biphasic system catalyzed by aluminum sulfate and byproduct characterization. ACS Sustain Chem Eng, 2022; 10: 10444-10456.[DOI]
[134] Potvin J, Sorlien E, Hegner J et al. Effect of NaCl on the conversion of cellulose to glucose and levulinic acid via solid supported acid catalysis. Tetrahedron Lett, 2011; 52: 5891-5893.[DOI]
[135] Ding D, Wang J, Xi J et al. High-yield production of levulinic acid from cellulose and its upgrading to γ-valerolactone. Green Chem, 2014; 16C: 3846-3853.[DOI]
[136] Yu F, Thomas J, Smet M et al. Molecular design of sulfonated hyperbranched poly(arylene oxindole)s for efficient cellulose conversion to levulinic acid. Green Chem, 2016; 18: 1694-1705.[DOI]
[137] Atanda L, Shrotri A, Mukundan S et al. Direct production of 5‐hydroxymethylfurfural via catalytic conversion of simple and complex sugars over phosphated TiO2. Chem Sus Chem, 2015; 8: 2907-2916.[DOI]
[138] Cao Z, Fan Z, Chen Y et al. Efficient preparation of 5-hydroxymethylfurfural from cellulose in a biphasic system over hafnyl phosphates. Appl Catal B, 2019; 244: 170-177.[DOI]
[139] Peela NR, Yedla SK, Velaga B et al. Choline chloride functionalized zeolites for the conversion of biomass derivatives to 5-hydroxymethylfurfural. Appl Catal, A, 2019; 580: 59-70.[DOI]
[140] Zhang X, Zhang D, Sun Z et al. Highly efficient preparation of HMF from cellulose using temperature-responsive heteropolyacid catalysts in cascade reaction. Appl Catal B, 2016; 196: 50-56.[DOI]
[141] Zhang X, Zhang X, Sun N et al. High production of levulinic acid from cellulosic feedstocks being catalyzed by temperature-responsive transition metal substituted heteropolyacids. Renew Energ, 2019; 141: 802-813.[DOI]
[142] Santoro S, Ferlin F, Luciani L et al. Biomass-derived solvents as effective media for cross-coupling reactions and C-H functionalization processes. Green Chem, 2017; 19:1601-1612.[DOI]
[143] Kumar A, Jad Y, El-Faham A et al. Green solid-phase peptide synthesis 4. γ-Valerolactone and N -formylmorpholine as green solvents for solid phase peptide synthesis. Tetrahedron Lett, 2017; 58: 2986-2988.[DOI]
[144] Ye L, Han Y, Feng J et al. A review about GVL production from lignocellulose: Focusing on the full components utilization. Ind Crops Prod, 2020; 144: 112031.[DOI]
[145] Alonso DM, Wettstein SG, Dumesic JA. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem, 2013; 15: 584-595.[DOI]
[146] Xue Z, Liu Q, Wang J et al. Valorization of levulinic acid over non-noble metal catalysts: challenges and opportunities. Green Chem, 2018; 20: 4391-4408.[DOI]
[147] Liguori F, Moreno MC, Barbaro P. Environmentally friendly synthesis of γ-valerolactone by direct catalytic conversion of renewable sources. ACS Catal, 2015; 5: 1882-1894.[DOI]
[148] Yu Z, Lu X, Liu C et al. Synthesis of γ-valerolactone from different biomass-derived feedstocks: Recent advances on reaction mechanisms and catalytic systems. Renew Sust Energ Rev, 2019; 112: 140-157.[DOI]
[149] Galletti AMR, Antonetti C, Ribechini E et al. From giant reed to levulinic acid and gamma-valerolactone: A high yield catalytic route to valeric biofuels. Appl Energ, 2013; 102: 157-162.[DOI]
[150] Zhou H, Song J, Kang X et al. One-pot conversion of carbohydrates into gamma-valerolactone catalyzed by highly cross-linked ionic liquid polymer and Co/TiO2. RSC Adv, 2015; 5: 15267-15273.[DOI]
[151] Du X, He L, Zhao S et al. Hydrogen‐independent reductive transformation of carbohydrate biomass into γ‐valerolactone and pyrrolidone derivatives with supported gold catalysts. Angew Chem Int Edit, 2011; 50: 7815-7819.[DOI]
[152] Yuan J, Li S, Yu L et al. Copper-based catalysts for the efficient conversion of carbohydrate biomass into γ-valerolactone in the absence of externally added hydrogen. Energ Environ Sci, 2013; 6: 3308-3313.[DOI]
[153] Luo Y, Yi J, Tong D et al. Production of γ-valerolactone via selective catalytic conversion of hemicellulose in pubescens without addition of external hydrogen. Green Chem, 2016; 18: 848-857.[DOI]
[154] Zhou C, Xiao Y, Xu S et al. γ-valerolactone production from furfural residue with formic acid as the sole hydrogen resource via an integrated strategy on Au-Ni/ZrO2. Ind Eng Chem Res, 2020; 59: 17228-17238.[DOI]
[155] Sheldon RA. Green and sustainable manufacture of chemicals from biomass: state of the art. Green Chem, 2014; 16: 950-963.[DOI]
[156] Zhang L, Yu H, Wang P et al. Conversion of xylan, d-xylose and lignocellulosic biomass into furfural using AlCl3 as catalyst in ionic liquid. Bioresour Technol, 2013; 130: 110-116.[DOI]
[157] Xu W, Zhang S, Lu J et al. Furfural production from corncobs using thiourea as additive. Environ Prog Sustain, 2016; 36: 690-695.[DOI]
[158] Bhaumik P, Kane T, Dhepe PL. Silica and zirconia supported tungsten, molybdenum and gallium oxide catalysts for the synthesis of furfural. Catal Sci Technol, 2014; 4: 2904-2907.[DOI]
[159] Bhaumik P, Dhepe PL. Efficient, stable, and reusable silicoaluminophosphate for the one-pot production of furfural from hemicellulose. ACS Catal, 2013; 3: 2299-2303.[DOI]
[160] Gao H, Liu H, Pang B et al. Production of furfural from waste aqueous hemicellulose solution of hardwood over ZSM-5 zeolite. Bioresour Technol, 2014; 172: 453-456.[DOI]
[161] Li X, Xu R, Liu Q et al. Valorization of corn stover into furfural and levulinic acid over SAPO-18 zeolites: Effect of Brønsted to Lewis acid sites ratios. Ind Crops Prod, 2019; 141: 111759.[DOI]
[162] Li X, Yang J, Xu R et al. Kinetic study of furfural production from Eucalyptus sawdust using H-SAPO-34 as solid Brønsted acid and Lewis acid catalysts in biomass-derived solvents. Ind Crops Prod, 2019; 135: 196-205.[DOI]
[163] Fukaya N, Choi SJ, Horikoshi T et al. Direct synthesis of tetraalkoxysilanes from silica and alcohols. New J Chem, 2017; 41: 2224-2226.[DOI]
[164] Sun Q, Feng S, Li G et al. Influence of different treatments on the structure and conversion of silicon species in rice straw to tetraethyl orthosilicate (TEOS). ChemistryOpen, 2023; 12: e202300111.[DOI]
[165] Schutyser W, Renders T, Van S et al. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem Soc Rev, 2018; 47: 852-908.[DOI]
[166] Jing Y, Guo Y, Xia Q et al. Catalytic production of value-added chemicals and liquid fuels from lignocellulosic biomass. Chem, 2019; 5: 2520-2546.[DOI]
[167] Zhu L, Fu X, Hu Y et al. Controlling the reaction networks for efficient conversion of glucose into 5‐hydroxymethylfurfural. Chem Sus Chem, 2020; 13: 4812-4832.[DOI]
[168] Yang G, Pidko EA, Hensen EJM. Mechanism of Brønsted acid-catalyzed conversion of carbohydrates. J Catal, 2012; 295: 122-132.[DOI]
[169] Yan Q, Hong E, Fong S. Study of ChiR function in Serratia marcescens and its application for improving 2,3-butanediol from crystal chitin. Appl Microbiol Biotechnol, 2017; 101: 7567-7578.[DOI]
[170] Chen X, Yang H, Yan N. Shell biorefinery: Dream or reality? Chem Eur J, 2016; 22: 13402-13421.[DOI]
[171] Chen X, Yang H, Zhong Z et al. Base-catalysed, one-step mechanochemical conversion of chitin and shrimp shells into low molecular weight chitosan. Green Chem, 2017; 19: 2783-2792.[DOI]
[172] Yabushita M, Kobayashi H, Kuroki K et al. Catalytic depolymerization of chitin with retention of N‐acetyl group. Chem Sus Chem, 2015; 8: 3760-3763.[DOI]
[173] Gözaydın G, Song S, Yan N. Chitin hydrolysis in acidified molten salt hydrates. Green Chem, 2020; 22: 5096-5104.[DOI]
[174] Zhang J, Yan N. Production of glucosamine from chitin by co‐solvent promoted hydrolysis and deacetylation. Chem Cat Chem, 2017; 9: 2790-2796.[DOI]
[175] Pierson Y, Chen X, Bobbink FD et al. Acid-catalyzed chitin liquefaction in ethylene glycol. ACS Sustain Chem Eng, 2014; 2: 2081-2089.[DOI]
[176] Zhang J, Yan N. Formic acid-mediated liquefaction of chitin. Green Chem, 2016; 18: 5050-5058.[DOI]
[177] Dai J, Gözaydın G, Hu C et al. Catalytic conversion of chitosan to glucosaminic acid by tandem hydrolysis and oxidation. ACS Sustain Chem Eng, 2019; 7: 12399-12407.[DOI]
[178] Bobbink FD, Zhang J, Pierson Y et al. Conversion of chitin derived N-acetyl-d-glucosamine (NAG) into polyols over transition metal catalysts and hydrogen in water. Green Chem, 2015; 17: 1024-1031.[DOI]
[179] Ji X, Kou J, Gözaydın G et al. Boosting 3-acetamido-5-acetylfuran production from N-acetyl-D-glucosamine in γ-valerolactone by a dissolution-dehydration effect. Appl Catal, B, 2024; 342: 123379.[DOI]
[180] Chen X, Chew SL, Kerton FM et al. Direct conversion of chitin into a N-containing furan derivative. Green Chem, 2014; 16: 2204-2212.[DOI]
[181] Chen X, Liu Y, Kerton FM et al. Conversion of chitin and N-acetyl-d-glucosamine into a N-containing furan derivative in ionic liquids. RSC Adv, 2015; 5: 20073-20080.[DOI]
[182] Chen X, Gao Y, Wang L et al. Effect of treatment methods on chitin structure and its transformation into nitrogen‐containing chemicals. Chem Plus Chem, 2015; 80: 1565-1572.[DOI]
[183] Sadiq AD, Chen X, Yan N et al. Towards the shell biorefinery: sustainable synthesis of the anticancer alkaloid proximicin a from chitin. Chem Sus Chem, 2018; 11: 532-535.[DOI]
[184] Pham TT, Chen X, Söhnel T et al. Haber-independent, diversity-oriented synthesis of nitrogen compounds from biorenewable chitin. Green Chem, 2020; 22: 1978-1984.[DOI]
[185] Gao X, Chen X, Zhang J et al. Transformation of Chitin and Waste Shrimp Shells into Acetic Acid and Pyrrole. ACS Sustain Chem Eng, 2016; 4: 3912-3920.[DOI]
[186] Kühlborn J, Groß J, Opatz T. Making natural products from renewable feedstocks: back to the roots? Nat Prod Rep, 2020; 37: 380-424.[DOI]
[187] Tatyana N, Makarieva, Pavel S et al. Rhizochalins C and D from the sponge rhizochalina incrustata. A rare threo-sphingolipid and a facile method for determination of the carbonyl position in r,ω-bifunctionalized ketosphingolipids. J Nat Prod, 2007; 70: 1991-1998.[DOI]
[188] Ma X, Gözaydın G, Yang H et al. Upcycling chitin-containing waste into organonitrogen chemicals via an integrated process. Proc. Natl Acad Sci, 2020; 117: 7719-7728.[DOI]
[189] Jehanno C, Alty JW, Roosen M et al. Critical advances and future opportunities in upcycling commodity polymers. Nature, 2022; 603: 803-814.[DOI]
[190] Hou Q, Zhen M, Qian H et al. Upcycling and catalytic degradation of plastic wastes. Cell Rep Phys Sci, 2021; 2: 1-30.[DOI]
[191] Robles MA, Amigot SR, Fernandez LL et al. Sub-micro- and nano-sized polyethylene terephthalate deconstruction with engineered protein nanopores. Nat Catal, 2023; 6: 1174-1185.[DOI]
[192] Wei R, Tiso T, Bertling J et al. Possibilities and limitations of biotechnological plastic degradation and recycling. Nat Catal, 2020; 3: 867-871.[DOI]
[193] Shosuke Y, Kazumi H, Toshihiko T et al. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science, 2018; 351: 1196-1199.[DOI]
[194] Ouyang Z, Yang Y, Zhang C et al. Recent advances in photocatalytic degradation of plastics and plastic-derived chemicals. J Mater Chem A, 2021; 9: 13402-13441.[DOI]
[195] Chu S, Zhang B, Zhao X et al. Photocatalytic conversion of plastic waste: From photodegradation to photosynthesis. Adv Energy Mater, 2022; 12: 2200435.[DOI]
[196] Zhang S, Li M, Zuo Z et al. Recent advances in plastic recycling and upgrading under mild conditions. Green Chem, 2023; 25: 6949-6970.[DOI]
[197] Ellis LD, Rorrer NA, Sullivan KP et al. Chemical and biological catalysis for plastics recycling and upcycling. Nat Catal, 2021; 4: 539-556.[DOI]
[198] Li R, Zeng W, Zhao R et al. TiO2 nanoparticle supported Ru catalyst for chemical upcycling of polyethylene terephthalate to alkanes. Nano Res, 2023; 16: 12223-12229.[DOI]
[199] Cao R, Zhang M, Jiao Y et al. Co-upcycling of polyvinyl chloride and polyesters. Nat Sustain, 2023; 6: 1685-1692.[DOI]
[200] López-Fonseca R, Duque-Ingunza I, de Rivas B et al. Chemical recycling of post-consumer PET wastes by glycolysis in the presence of metal salts. Polym Degrad Stab, 2010; 95: 1022-1028.[DOI]
[201] Raheem AB, Noor ZZ, Hassan A et al. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J Clean Pro, 2019; 225: 1052-1064.[DOI]
[202] Hofmann M, Sundermeier J, Alberti C et al. Zinc(II) acetate catalyzed depolymerization of poly(ethylene terephthalate). Chemistry Select, 2020; 5: 10010-10014.[DOI]
[203] Hou D, Xin J, Lu X et al. Conversion of bis(2-hydroxyethylene terephthalate) into 1,4-cyclohexanedimethanol by selective hydrogenation using RuPtSn/Al2O3. RSC Adv, 2016; 6: 48737-48744.[DOI]
[204] Tang H, Li N, Li G et al. Synthesis of gasoline and jet fuel range cycloalkanes and aromatics from poly(ethylene terephthalate) waste. Green Chem, 2019; 21: 2709-2719.[DOI]
[205] Uekert T, Kasap H, Reisner E. Photoreforming of nonrecyclable plastic waste over a carbon nitride/nickel phosphide catalyst. J Am Chem Soc, 2019; 141: 15201-15210.[DOI]
[206] Jiao X, Zheng K, Chen Q et al. Photocatalytic Conversion of Waste Plastics into C2 Fuels under Simulated Natural Environment Conditions. Angew Chem Int Edit, 2020; 59: 15497-15501.[DOI]
[207] Xu J, Jiao X, Zheng K et al. Plastics-to-syngas photocatalysed by Co-Ga2O3 nanosheets. Natl Sci Rev, 2022; 9: nwac011.[DOI]
[208] Lee WT, Bobbink FD, van Muyden AP et al. Catalytic hydrocracking of synthetic polymers into grid-compatible gas streams. Cell Rep Phys Sci, 2021; 2:100342.[DOI]
[209] Si X, Chen J, Wang Z et al. Ni-catalyzed carbon-carbon bonds cleavage of mixed polyolefin plastics waste. J Energy Chem, 2023; 85: 562-569.[DOI]
[210] Nunes BFS, Oliveira MC, Fernandes AC. Dioxomolybdenum complex as an efficient and cheap catalyst for the reductive depolymerization of plastic waste into value-added compounds and fuels. Green Chem, 2020; 22: 2419-2425.[DOI]
[211] Monsigny L, Berthet JC, Cantat T. Depolymerization of waste plastics to monomers and chemicals using a hydrosilylation strategy facilitated by brookhart’s iridium(III) catalyst. ACS Sustain Chem Eng, 2018; 6: 10481-10488.[DOI]
[212] Jing Y, Wang Y, Furukawa S et al. Towards the circular economy: Converting aromatic plastic waste back to arenes over a Ru/Nb2O5 catalyst. Angew Chem Int Edit, 2021; 60: 5527-5535.[DOI]
[213] Lu S, Jing Y, Feng B et al. H2‐free plastic conversion: converting PET back to BTX by unlocking hidden hydrogen. Chem Sus Chem, 2021; 14: 4242-4250.[DOI]
[214] Golubeva M, Mukhtarova M, Sadovnikov A et al. PET waste recycling into BTX fraction using In situ obtained nickel phosphide. Polymers, 2023; 15: 2248.[DOI]
[215] Shao Y, Fan M, Sun K et al. The quantitative conversion of polyethylene terephthalate (PET) and Coca-Cola bottles to p-xylene over Co-based catalysts with tailored activities for deoxygenation and hydrogenation. Green Chem, 2023; 25: 10513-10529.[DOI]
[216] Tian S, Jiao Y, Gao Z et al. Catalytic amination of polylactic acid to alanine. J Am Chem Soc, 2021; 143: 16358-16363.[DOI]
[217] Kratish Y, Marks TJ. Efficient polyester hydrogenolytic deconstruction via tandem catalysis. Angew Chem, Int Ed, 2021; 61: e202112576.[DOI]
[218] Ji H, Wang X, Wei X et al. Boosting polyethylene hydrogenolysis performance of Ru‐CeO2 catalysts by finely regulating the Ru sizes. Small, 2023; 19: 2300903.[DOI]
[219] Ma D. Transforming end-of-life plastics for a better world. Nat Sustain, 2023; 6: 1142-1143.[DOI]
[220] Lee J, Theis JR, Kyriakidou EA. Vehicle emissions trapping materials: Successes, challenges, and the path forward. Appl Catal B, 2019; 243: 397-414.[DOI]
[221] Huang C, Shan W, Lian Z et al. Recent advances in three-way catalysts of natural gas vehicles. Catal Sci Technol, 2020; 10: 6407-6419.[DOI]
[222] Li T, Yang H, Xu L et al. Comprehensive treatment strategy for diesel truck exhaust. Environ Sci Pollut Res, 2023; 30: 54324-54332.[DOI]
[223] Jones J, Xiong H, Delariva AT et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science, 2016; 353: 150-154.[DOI]
[224] Wiebenga MH, Kim CH, Schmieg SJ et al. Deactivation mechanisms of Pt/Pd-based diesel oxidation catalysts. Catal Today, 2012; 184: 197-204.[DOI]
[225] Bennett GF, Catalytic air pollution control: Commercial technology. J Hazard Mater, 2003; 96: 99-100.[DOI]
[226] Jing Y, Cai Z, Liu C et al. Promotional effect of la in the three-way catalysis of la-loaded Al2O3-supported Pd catalysts (Pd/La/Al2O3). ACS Catal, 2020; 10: 1010-1023.[DOI]
[227] Li L, Zhang N, Wu R et al. Comparative study of moisture-treated Pd@CeO2/Al2O3 and Pd/CeO2/Al2O3 catalysts for automobile exhaust emission reactions: Effect of core-shell interface. ACS Appl Mater Inter, 2020; 12: 10350-10358.[DOI]
[228] Zhang N, Ye C, Yan H et al. Single-atom site catalysts for environmental catalysis. Nano Res, 2020; 13: 3165-3182.[DOI]
[229] Jeong H, Lee G, Kim BS et al. Fully dispersed Rh ensemble catalyst to enhance low-temperature activity. J Am Chem Soc, 2018; 140: 9558-9565.[DOI]
[230] Shelef M, Graham GW, Why rhodium in automotive three-way catalysts? Catal Rev, 1994; 36: 433-457.[DOI]
[231] Han L, Cai S, Gao M et al. Selective catalytic reduction of NOx with NH3 by using novel catalysts: state of the art and future prospects. Chem Rev, 2019; 119: 10916-10976.[DOI]
[232] Farrauto RJ, Deeba M, Alerasool S. Gasoline automobile catalysis and its historical journey to cleaner air. Nat Catal, 2019; 2: 603-613.[DOI]
[233] Hu Z, Allen FM, Wan CZ et al. Performance and structure of Pt–Rh three-way catalysts: mechanism for Pt/Rh Synergism. J Catal, 1998; 174: 13-21.[DOI]
[234] Liu Z, Su H, Chen B et al. Activity enhancement of WO3 modified Fe2O3 catalyst for the selective catalytic reduction of NO by NH3. Chem Eng J, 2016; 299: 255-262.[DOI]
[235] Zhu M, Lai JK, Tumuluri U et al. Nature of active sites and surface intermediates during SCR of NO with NH3 by supported V2O5-WO3/TiO2 catalysts. J Am Chem Soc, 2017; 139: 15624-15627.[DOI]
[236] Huang Y, Liu S, Pei M et al. Unveiling H2O2-optimized NOx adsorption-selective catalytic reduction (AdSCR) performance of WO3/CeZrO2 catalyst. Rare Metals, 2023; 42: 3755-3765.[DOI]
[237] Ye D, Gao S, Feng J et al. The development of Fe2O3-based catalysts used for the selective catalytic reduction of NO with NH3: A review. Mol Catal, 2023; 549: 113516.[DOI]
[238] Liu Q, Bian C, Ming S et al. The opportunities and challenges of iron-zeolite as NH3-SCR catalyst in purification of vehicle exhaust. Appl Catal A, 2020; 607: 117865.[DOI]
[239] Kang D, Shi Q, Zhang C et al. Synergistic removal of NOx and CB by Co-MnOx catalysts in a low-temperature window. Chem Eng J, 2023; 476: 146369.[DOI]
[240] Luo N, Gao F, Liu H et al. Hierarchical structured Ti-doped CeO2 stabilized CoMn2O4 for enhancing the low-temperature NH3-SCR performance within highly H2O and SO2 resistance. Appl Catal B, 2024; 343: 123442.[DOI]
[241] Peden CHF. Cu/Chabazite catalysts for ‘Lean-Burn’ vehicle emission control. J Catal, 2019; 373: 384-389.[DOI]
[242] Wu Y, Andana T, Wang Y et al. A comparative study between real-world and laboratory accelerated aging of Cu/SSZ-13 SCR catalysts. Appl Catal B, 2022; 318: 121807.[DOI]
[243] Li R, Jiang X, Lin J et al. Understanding the influence of hydrothermal treatment on NH3-SCR of NO activity over Cu -SSZ-16. Chem Eng J, 2022; 441: 136021.[DOI]
[244] Lin Q, Xu S, Zhao H et al. Highlights on Key Roles of Y on the Hydrothermal Stability at 900°C of Cu/SSZ-39 for NH3-SCR. ACS Catal, 2022; 12: 14026-14039.[DOI]
[245] Wei Y, Wang S, Chen M et al. Coaxial 3D printing of zeolite‐based core–shell monolithic Cu‐SSZ‐13@SiO2 Catalysts for Diesel Exhaust Treatment. Adv Mater, 2023: 2302912.[DOI]
[246] Xu S, Li J, Lin Q et al. Engineering CeZrOx-Cu/SSZ-13 coupled catalysts to synergistically enhance the low-temperature NH3-SCR activity. Chem Eng J, 2023; 476: 146767.[DOI]
[247] Li Y, Cai S, Wang P et al. Improved NOx reduction over phosphate-modified Fe2O3/TiO2 catalysts via tailoring reaction paths by In situ creating alkali-poisoning sites. Environ Sci Technol, 2021; 55: 9276-9284.[DOI]
[248] Zhang P, Wang P, Chen A et al. Alkali-resistant catalytic reduction of NOx by using Ce-O-B alkali-capture sites. Environ Sci Technol, 2021; 55: 11970-11978.[DOI]
[249] An H, McGinn PJ. Catalytic behavior of potassium containing compounds for diesel soot combustion. Appl Catal B, 2006; 62: 46-56.[DOI]
[250] Hirakawa T, Shimokawa Y, Miyahara Y et al. Activity-composition relationships of Fe-Ni-Cu ternary nanoparticles supported on Al2O3 as three-way catalysts for NO reduction. ACS Appl Nano Mater, 2021; 4: 10613-10622.[DOI]
[251] Tan Q, Shi Z, Wu D. CO2 Hydrogenation to methanol over a highly active Cu–Ni/CeO2-nanotube catalyst. Ind Eng Chem Res, 2018; 57: 10148-10158.[DOI]
[252] Ju T-J, Wang C-H, Lin SD. Insights into the CO2 deoxygenation to CO over oxygen vacancies of CeO2. Catal Sci Technol, 2019; 9: 2118-2124.[DOI]
[253] Zhou Y, Liu L, Li G et al. Insights into the Influence of ZrO2 Crystal Structures on Methyl Laurate Hydrogenation over Co/ZrO2 Catalysts. ACS Catal, 2021; 11: 7099-7113.[DOI]
[254] Zhu C, Wei X, Li W et al. Crystal-plane effects of CeO2{110} and CeO2{100} on photocatalytic CO2 reduction: synergistic interactions of oxygen defects and hydroxyl groups. ACS Sustain Chem Eng, 2020; 8: 14397-14406.[DOI]
[255] Xie L, Cheng J, Wang T et al. Mechanical wear behavior between CeO2(100), CeO2(110), CeO2(111), and silicon studied through atomic force microscopy. Tribol Int, 2021; 153: 106616.[DOI]
[256] Shen M, Wang J, Shang J et al. Modification ceria-zirconia mixed oxides by doping Sr using the reversed microemulsion for Improved Pd-only three-way catalytic performance. J Phys Chem C, 2009; 113: 1543-1551.[DOI]
[257] Eufinger J-P, Daniels M, Schmale K et al. The model case of an oxygen storage catalyst-non-stoichiometry, point defects and electrical conductivity of single crystalline CeO2-ZrO2-Y2O3 solid solutions. Phys Chem Chem Phys, 2014; 16: 25583-25600.[DOI]
[258] Ramezanalizadeh H, Manteghi F. Synthesis of a novel MOF/CuWO4 heterostructure for efficient photocatalytic degradation and removal of water pollutants. J Cleaner Prod, 2018; 172: 2655-2666.[DOI]
[259] Saravanan A, Kumar PS, Jeevanantham S et al. Degradation of toxic agrochemicals and pharmaceutical pollutants: Effective and alternative approaches toward photocatalysis. Environ Pollut, 2022; 298: 118844.[DOI]
[260] Park H, Park Y, Kim W et al. Surface modification of TiO2 photocatalyst for environmental applications. J Photochem Photobiol C, 2013; 15: 1-20.[DOI]
[261] Zeshan M, Bhatti IA, Mohsin M et al. Remediation of pesticides using TiO2 based photocatalytic strategies: A review. Chemosphere, 2022; 300: 134525.[DOI]
[262] Gusain R, Gupta K, Joshi P et al. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv Colloid Interface Sci, 2019; 272: 102009.[DOI]
[263] Zhang S, Li B, Wang X et al. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem Eng J, 2020; 390: 124642.[DOI]
[264] Balakrishnan A, Chinthala M, Polagani RK et al. Removal of tetracycline from wastewater using g-C3N4 based photocatalysts: A review. Environ Res, 2023; 216: 114660.[DOI]
[265] Hojjati-Najafabadi A, Mansoorianfar M, Liang T et al. Magnetic-MXene-based nanocomposites for water and wastewater treatment: A review. J Water Process Eng, 2022; 47: 102696.[DOI]
[266] Dias EM, Petit C. Towards the use of metal-organic frameworks for water reuse: a review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. J Mater Chem A, 2015; 3: 22484-22506.[DOI]
[267] Petala A, Noe A, Frontistis Z et al. Synthesis and characterization of CoOx/BiVO4 photocatalysts for the degradation of propyl paraben. J Hazard Mater, 2019; 372: 52-60.[DOI]
[268] Cao L, Ma D, Zhou Z et al. Efficient photocatalytic degradation of herbicide glyphosate in water by magnetically separable and recyclable BiOBr/Fe3O4 nanocomposites under visible light irradiation. Chem Eng J, 2019; 368: 212-222.[DOI]
[269] Sun H, Zou C, Liao Y et al. Modulating charge transport behavior across the interface via g-C3N4 surface discrete modified BiOI and Bi2MoO6 for efficient photodegradation of glyphosate. J Alloys Compd, 2023; 935: 168208.[DOI]
[270] Munshi GH, Ibrahim AM, Al-Harbi LM. Inspired preparation of zinc oxide nanocatalyst and the photocatalytic activity in the treatment of methyl orange dye and paraquat herbicide. Int J Photoenergy, 2018; 2018: 5094741.[DOI]
[271] Tariq SR, Chotana GA, Rashid A. Photocatalytic degradation of paraquat dichloride in the presence of ZnO.WO3 composite. Int J Environ Sci Technol, 2021; 19: 2583-2598.[DOI]
[272] Zhang T, Liu M, Meng Y et al. A novel method for the synthesis of Ag3VO4/Ag2VO2PO4 heterojunction photocatalysts with improved visible-light photocatalytic properties. Sep Purif Technol, 2018; 206: 149-157.[DOI]
[273] Chen M-L, Lu T-H, Li S-S et al. Photocatalytic degradation of imidacloprid by optimized Bi2WO6/NH2-MIL-88B(Fe) composite under visible light. Environ Sci Pollut Res, 2021; 29: 19583-19593.[DOI]
[274] Vigneshwaran S, Preethi J, Park CM et al. In-situ fabrication of ternary (3D/2D/2D) prism-like structures with dramatically enhancement on degradation of profenofos: A systemic study. J Water Process Eng, 2021; 39: 101720.[DOI]
[275] Yang X, Chen Z, Zhao W et al. Recent advances in photodegradation of antibiotic residues in water. Chem Eng J, 2021; 405: 126806.[DOI]
[276] Hu X, Hu X, Peng Q et al. Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2. Chem Eng J, 2020; 380: 122366.[DOI]
[277] Zuo S, Huang S, Wang Y et al. Surface modify BiOBr/Mn-Ti3C2Tx to enhance the carrier separation efficiency and weak electron utilization for photo-assisted peroxymonosulfate activation to degrade emerging contaminants. Chem Eng J, 2023; 475: 146230.[DOI]
[278] Wu Q, Liu Y, Jing H et al. Peculiar synergetic effect of γ-Fe2O3 nanoparticles and graphene oxide on MIL-53 (Fe) for boosting photocatalysis. Chem Eng J, 2020; 390: 124615.[DOI]
[279] Li X, Wang S, Xu B et al. MOF etching-induced Co-doped hollow carbon nitride catalyst for efficient removal of antibiotic contaminants by enhanced perxymonosulfate activation. Chem Eng J, 2022; 441: 136074.[DOI]
[280] Zinatloo-Ajabshir S, Morassaei MS, Salavati-Niasari M. Eco-friendly synthesis of Nd2Sn2O7-based nanostructure materials using grape juice as green fuel as photocatalyst for the degradation of erythrosine. Composites Part B, 2019; 167: 643-653.[DOI]
[281] Xie K, Fang J, Li L et al. Progress of graphite carbon nitride with different dimensions in the photocatalytic degradation of dyes: A review. J Alloys Compd, 2022; 901: 163589.[DOI]
[282] Nguyen CH, Fu C-C, Juang R-S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J Cleaner Prod, 2018; 202: 413-427.[DOI]
[283] Kumar OP, Ahmad M, Nazir MA et al. Strategic combination of metal–organic frameworks and C3N4 for expeditious photocatalytic degradation of dye pollutants. Environ Sci Pollut Res, 2022; 29: 35300-35313.[DOI]
[284] Chang Y, Huang H, Yang T et al. Simultaneous introduction of oxygen vacancies and hierarchical pores into titanium-based metal-organic framework for enhanced photocatalytic performance. J Colloid Interf Sci, 2021; 599: 785-794.[DOI]
[285] Nazir MA, Najam T, Jabeen S et al. Facile synthesis of Tri-metallic layered double hydroxides (NiZnAl-LDHs): Adsorption of Rhodamine-B and methyl orange from water. Inorg Chem Commun, 2022; 145: 110008.[DOI]
[286] Ishfaq M, Khan SA, Nazir MA. et al. The in situ synthesis of sunlight-driven Chitosan/MnO2@MOF-801 nanocomposites for photocatalytic reduction of Rhodamine-B. J Mol Struct, 2024; 1301: 137384.[DOI]
[287] Nazir MA, Najam T, Shahzad K et al. Heterointerface engineering of water stable ZIF-8@ZIF-67: Adsorption of rhodamine B from water. Surf Interfaces, 2022; 34: 102324.[DOI]
[288] Varjani SJ. Microbial degradation of petroleum hydrocarbons. Bioresour Technol, 2017; 223: 277-286.[DOI]
[289] Koe WS, Lee JW, Chong WC et al. An overview of photocatalytic degradation: photocatalysts, mechanisms, and development of photocatalytic membrane. Environ Sci Pollut Res, 2019; 27: 2522-2565.[DOI]
[290] Zheng W, Huang J, Li S et al. Advanced Materials with Special Wettability toward Intelligent Oily Wastewater Remediation. ACS Appl Mater Inter 2021; 13: 67-87.[DOI]
[291] Saien J, Shahrezaei F. Organic pollutants removal from petroleum refinery wastewater with nanotitania photocatalyst and UV light emission. Int J Photoenergy, 2012; 2012: 703074.[DOI]
[292] Shivaraju HP, Muzakkira N, Shahmoradi B. Photocatalytic treatment of oil and grease spills in wastewater using coated N-doped TiO2 polyscales under sunlight as an alternative driving energy. J Environ Sci Technol, 2016; 13: 2293-2302.[DOI]
[293] Mokhbi Y, Korichi M, Akchiche Z. Combined photocatalytic and Fenton oxidation for oily wastewater treatment. Appl Water Sci, 2019; 9: 35.[DOI]
[294] Parul, Kaur K, Badru R et al. Photodegradation of organic pollutants using heterojunctions: A review. J Environ Chem Eng, 2020; 8: 103666.[DOI]
[295] Yang J, Hu R, Meng W et al. A novel p-LaFeO3/n-Ag3PO4 heterojunction photocatalyst for phenol degradation under visible light irradiation. Chem Commun, 2016; 52: 2620-2623.[DOI]
[296] Liu T, Mao Y, Peng Y. Synthesis of Bi2O3-Bi4V2O11 heterojunctions with high interface quality for enhanced visible light photocatalysis in degradation of high-concentration phenol and MO dyes. Cryst Eng Comm, 2018; 20: 2553-2561.[DOI]
[297] Yang L, Luo S, Li Y et al. High efficient photocatalytic degradation of p-nitrophenol on a unique Cu2O/TiO2 p-n heterojunction network catalyst. Environ Sci Technol, 2010; 44: 7641-7646.[DOI]
[298] Fu G, Xu G, Chen S et al. Ag3PO4/Bi2WO6 hierarchical heterostructures with enhanced visible light photocatalytic activity for the degradation of phenol. Catal Commun, 2013; 40: 120-124.[DOI]
[299] Ren H-T, Jia S-Y, Wu Y et al. Improved photochemical reactivities of Ag2O/g-C3N4 in phenol degradation under UV and visible light. Ind Eng Chem Res, 2014; 53: 17645-17653.[DOI]
[300] Wei Z, Liang F, Liu Y et al. Photoelectrocatalytic degradation of phenol-containing wastewater by TiO2/g-C3N4 hybrid heterostructure thin film. Appl Catal B, 2017; 201: 600-606.[DOI]
[301] Jian Z, Huang S, Zhang Y, Photocatalytic degradation of 2,4-dichlorophenol using nanosized Na2Ti6O13/TiO2 heterostructure particles. Int J Photoenergy, 2013; 2013: 606291.[DOI]
[302] Gu A, Chen K, Zhou X et al. Trimetallic MOFs-derived Fe-Co-Cu oxycarbide toward peroxymonosulfate activation for efficient trichlorophenol degradation via high-valent metal-oxo species. Chem Eng J, 2023; 468: 143444.[DOI]
[303] Lam S-M, Sin J-C, Abdullah A et al. Efficient photodegradation of resorcinol with Ag2O/ZnO nanorods heterostructure under a compact fluorescent lamp irradiation. Chem Pap, 2013; 67: 1277-1284.[DOI]
[304] Wang Z, Liu Z, Huang J et al. Zr6O8-porphyrinic MOFs as promising catalysts for the boosting photocatalytic degradation of contaminants in high salinity wastewater. Chem Eng J, 2022; 440: 135883.[DOI]
[305] Tang W, Zeng G-M, Gong J-L et al. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci Total Environ, 2014; 468-469: 1014-1027.[DOI]
[306] Gong Y, Wang Y, Lin N et al. Iron-based materials for simultaneous removal of heavy metal(loid)s and emerging organic contaminants from the aquatic environment: Recent advances and perspectives. Environ Pollut, 2022; 299: 118871.[DOI]
[307] Cao W, Yuan Y, Yang C et al. In-situ fabrication of g-C3N4/MIL-68(In)-NH2 heterojunction composites with enhanced visible-light photocatalytic activity for degradation of ibuprofen. Chem Eng J, 2020; 391: 123608.[DOI]
[308] Qin J, Pei Y, Zheng Y et al. Fe-MOF derivative photocatalyst with advanced oxygen reduction capacity for indoor pollutants removal. Appl Catal B, 2023; 325: 122346.[DOI]
[309] Singh S, Sunoj RB, Molecular Machine Learning for Chemical Catalysis: Prospects and Challenges. Acc Chem Res, 2023; 56: 402-412.[DOI]
[310] Mayr A, Klambauer G, Unterthiner T et al. DeepTox: Toxicity Prediction using Deep Learning. Frontiers in Environ Sci, 2016; 3: 1-15.[DOI]
[311] Shen Y, Borowski JE, Hardy MA et al. Automation and computer-assisted planning for chemical synthesis. Nat Rev Methods Primers, 2021; 1: 23.[DOI]
[312] Merchant A, Batzner S, Schoenholz SS, Scaling deep learning for materials discovery. Nature, 2023; 624: 80-85.[DOI]
[313] Yoon J, Cao Z, Raju RK. et al. Deep reinforcement learning for predicting kinetic pathways to surface reconstruction in a ternary alloy. Machine Learning Sci Technol, 2021; 2: 045018.[DOI]
[314] Lamoureux PS, Winther KT, Torres JAG. et al. Machine Learning for Computational Heterogeneous Catalysis. Chem Cat Chem, 2019; 11: 3581-3601.[DOI]
[315] Wu S, Wang Z, Zhang H et al. Deep learning accelerates the discovery of two‐dimensional catalysts for hydrogen evolution reaction. Energy Environ Mater, 2022; 6: e12259.[DOI]
[316] Liu Y, Halder A, Seifert S et al. Probing active sites in CuxPdy cluster catalysts by machine-learning-assisted X-ray absorption spectroscopy. ACS Appl Mater Interf, 2021; 13: 53363-53374.[DOI]
[317] Zhou Y, Deng H, Huang X et al. Predicting the oxidation of carbon monoxide on nanoporous gold by a deep-learning method. Chem. Eng J, 2022; 427: 131747.[DOI]
[318] Yuan X, Cao Y, Li J et al. Recent advancements and challenges in emerging applications of biochar-based catalysts. Biotechnol Adv, 2023; 67: 108181.[DOI]
[319] Cheng L, Tang Y, Ostrikov K et al. Single‐atom heterogeneous catalysts: Human‐ and ai‐driven platform for augmented designs, analytics and reality‐enabled manufacturing. Angew Chem Int Edit, 2023; 63: e202313599.[DOI]
[320] Ramirez A, Lam E, Gutierrez DP et al. Accelerated exploration of heterogeneous CO2 hydrogenation catalysts by Bayesian-optimized high-throughput and automated experimentation. Chem Catal, 2024; 4: 100888.[DOI]
[321] Burés J, Larrosa I. Organic reaction mechanism classification using machine learning. Nature, 2023; 613: 689-695.[DOI]
[322] Burger B, Maffettone PM, Gusev VV et al. A mobile robotic chemist. Nature, 2020; 583: 237-241.[DOI]
[323] Sharma S, Sharma D. Intelligently applying artificial intelligence in chemoinformatics. Curr Top Med Chem, 2018; 18: 1804-1826.[DOI]
[324] King RD. Rise of the robo scientists. Sci Am, 2011; 304: 72-77.[DOI]
[325] Li J, Ballmer SG, Gillis EP et al. Synthesis of many different types of organic small molecules using one automated process. Science, 2015; 347: 1221-1226.[DOI]
[326] Dragone V, Sans V, Henson AB et al. An autonomous organic reaction search engine for chemical reactivity. Nat Commun, 2017; 8: 15733.[DOI]
[327] Bédard A-C, Adamo A, Aroh KC et al. Reconfigurable system for automated optimization of diverse chemical reactions. Science, 2018; 361: 1220-1225.[DOI]
[328] Tabor DP, Roch LM, Saikin SK et al. Accelerating the discovery of materials for clean energy in the era of smart automation. Nat Rev Chem, 2018; 3: 5-20.[DOI]
Brief of Corresponding Author(s)
Changwei Hu He is a Professor in the College of Chemistry, Sichuan University, China. He has got his Bachelor, Master, Ph.D. degree in Physical Chemistry at Sichuan University. His current research interests include the catalytic conversion of bio-based materials (bio-oil, sugars, raw algae, and lignocellulosic biomass) into fuels and useful chemicals; catalytic functionalization of C-H bonds from the viewpoint of atom economy; catalytic activation of green house gases; molecular modeling on catalytic systems; and other green chemistry interrelated researches. He has published more than 500 papers in peer-reviewed journals, with an h-index of 59. |

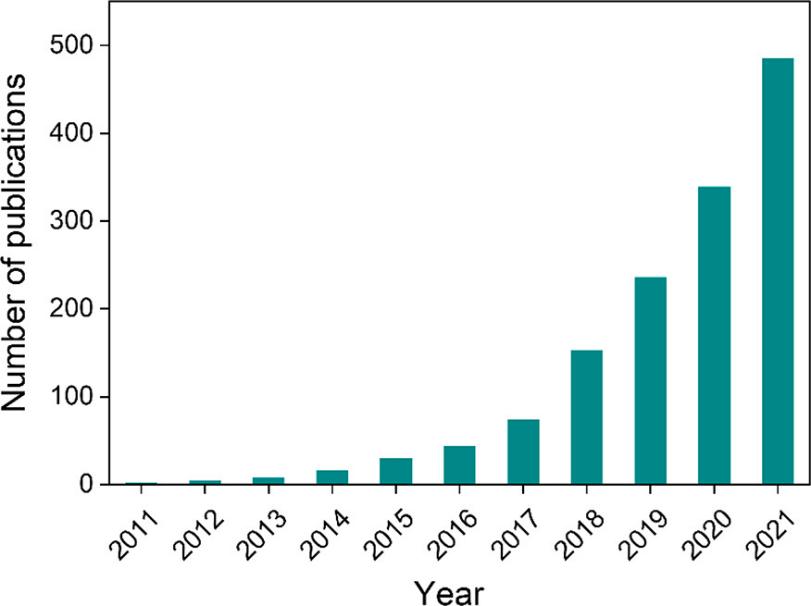
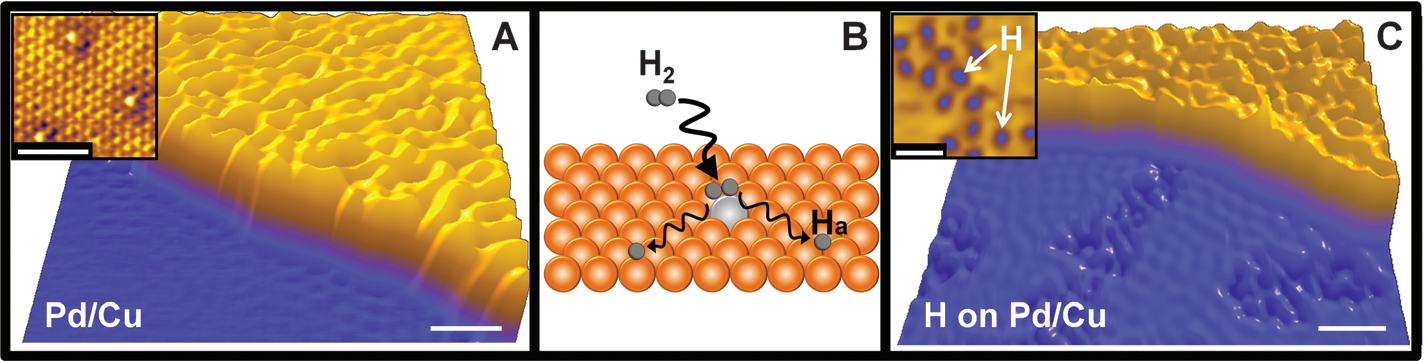
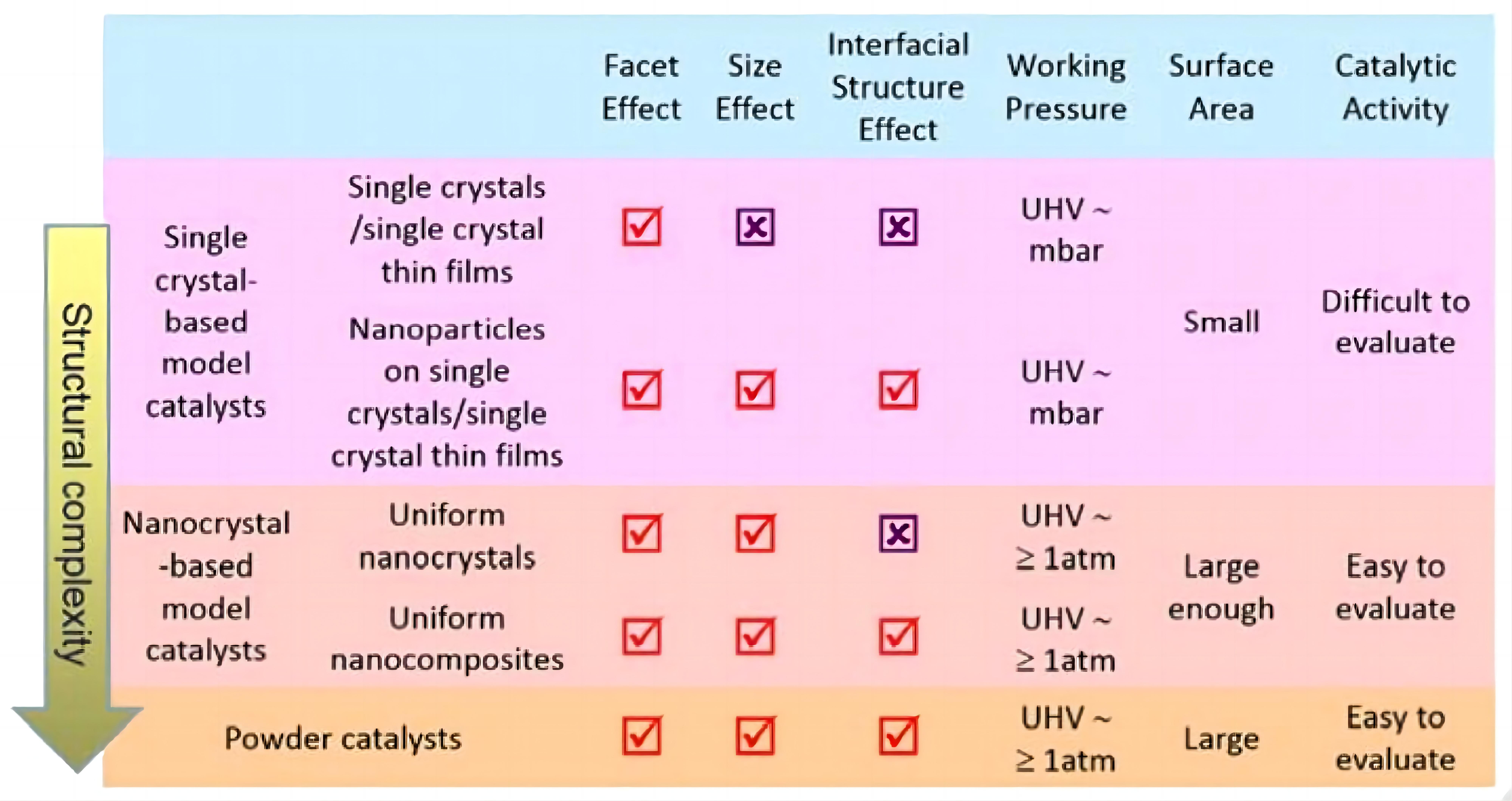
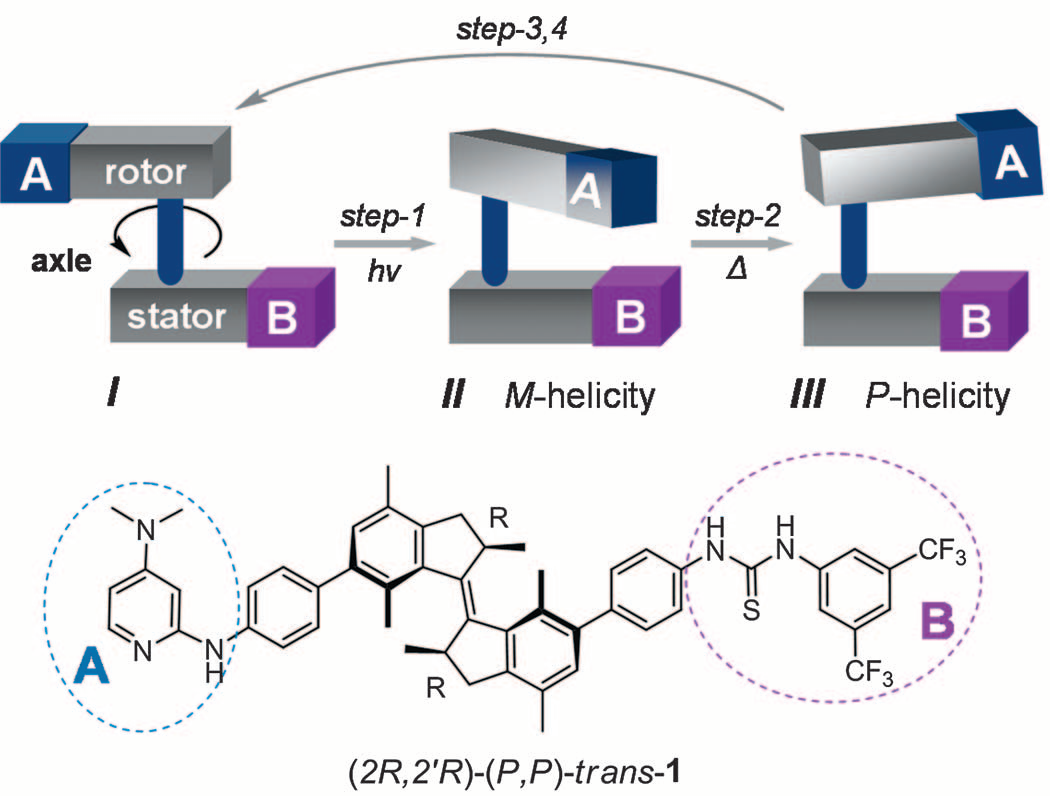
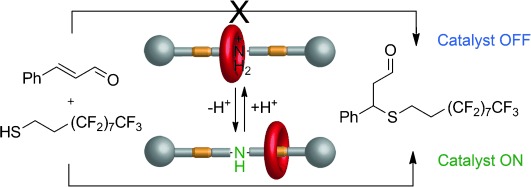
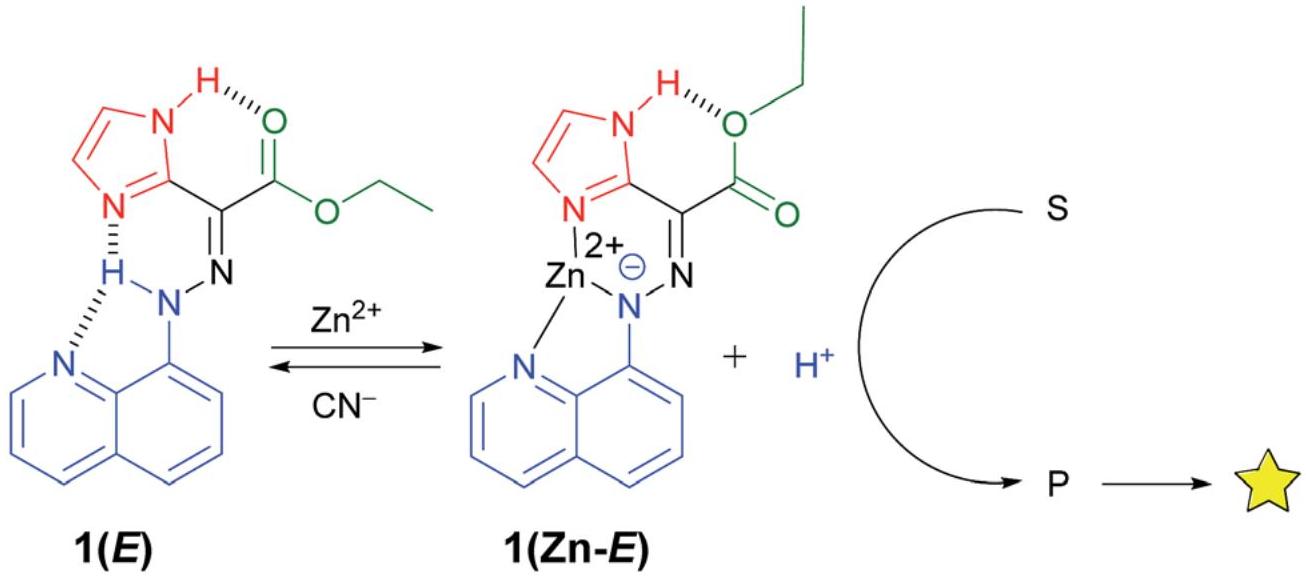
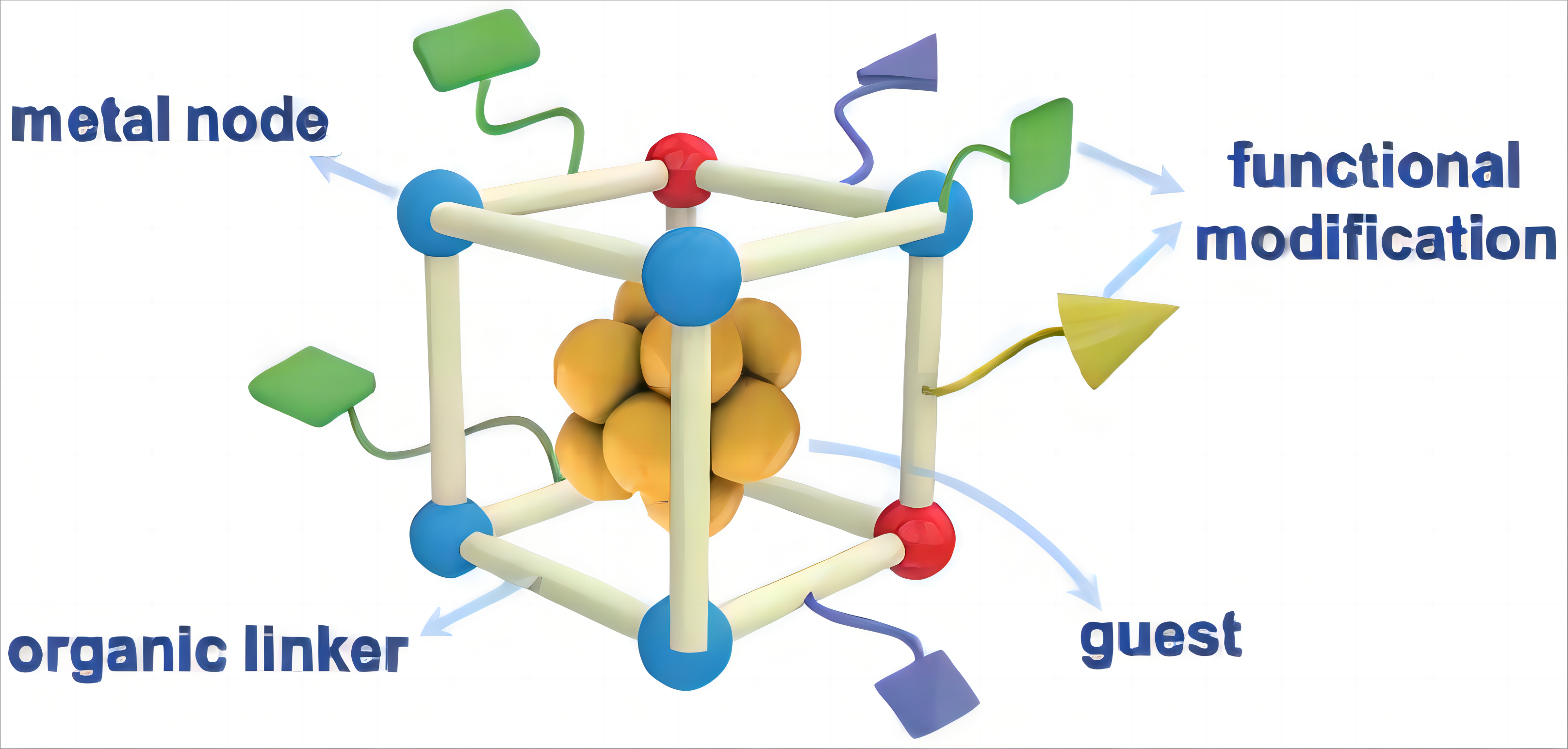
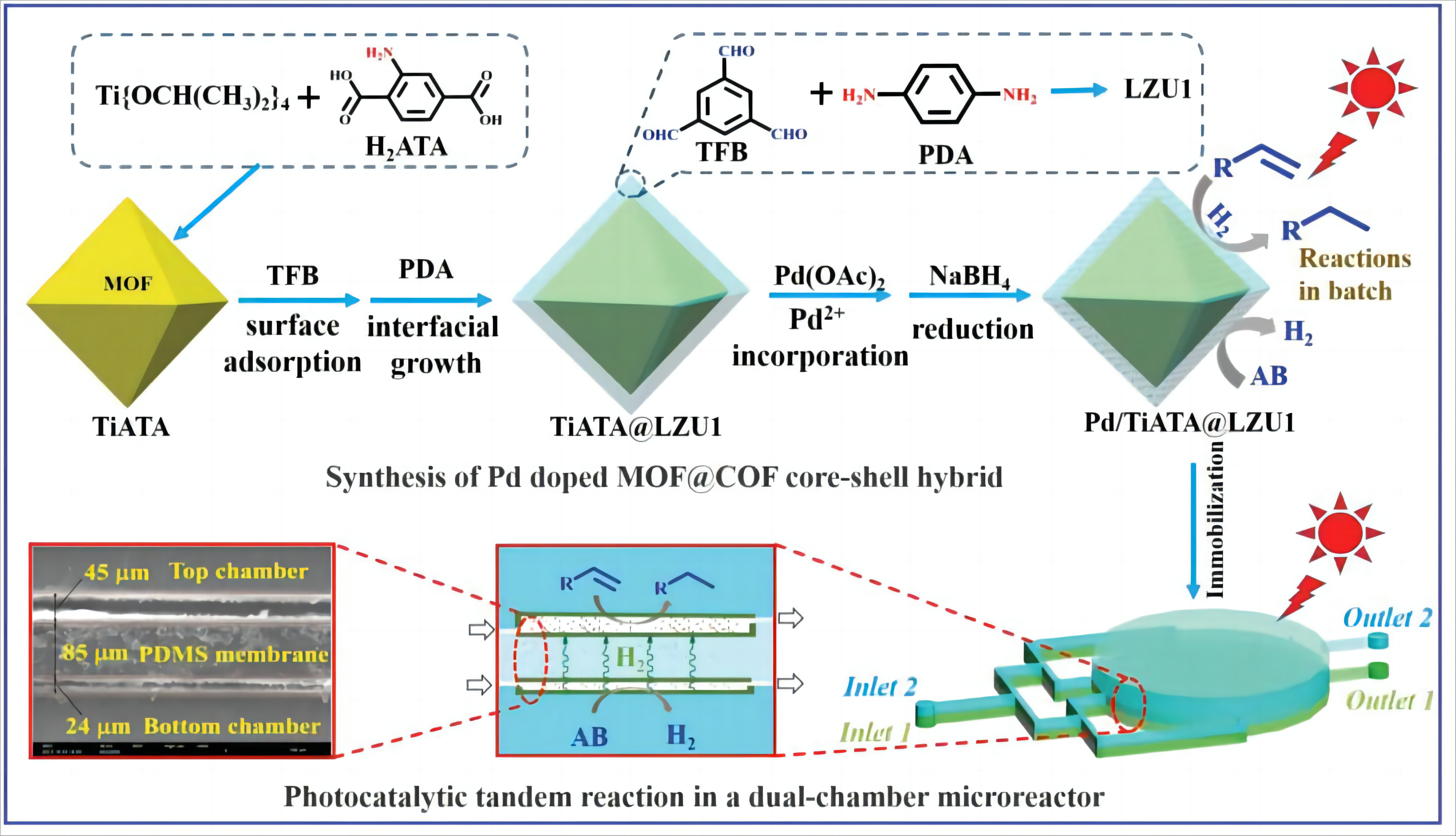
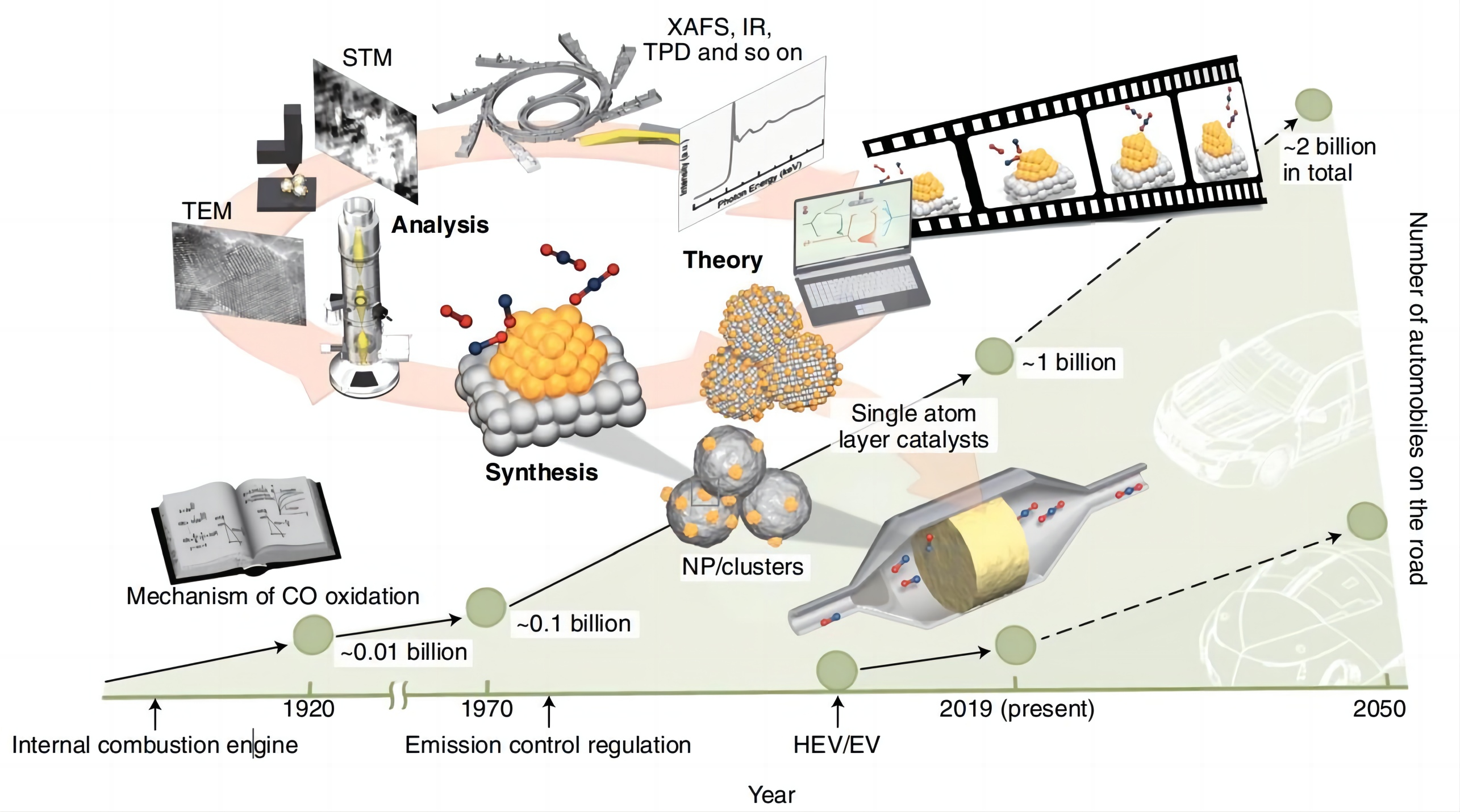
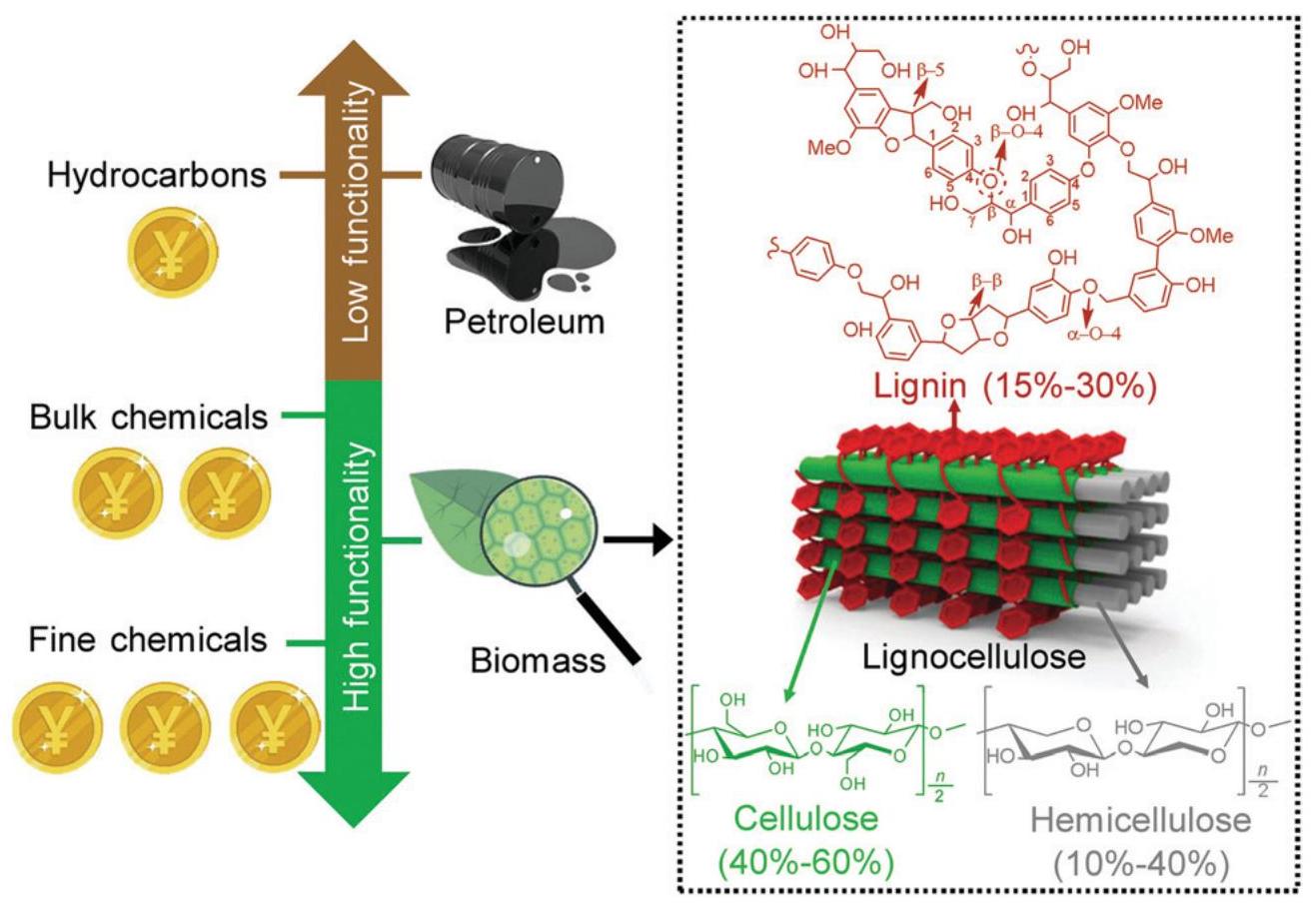
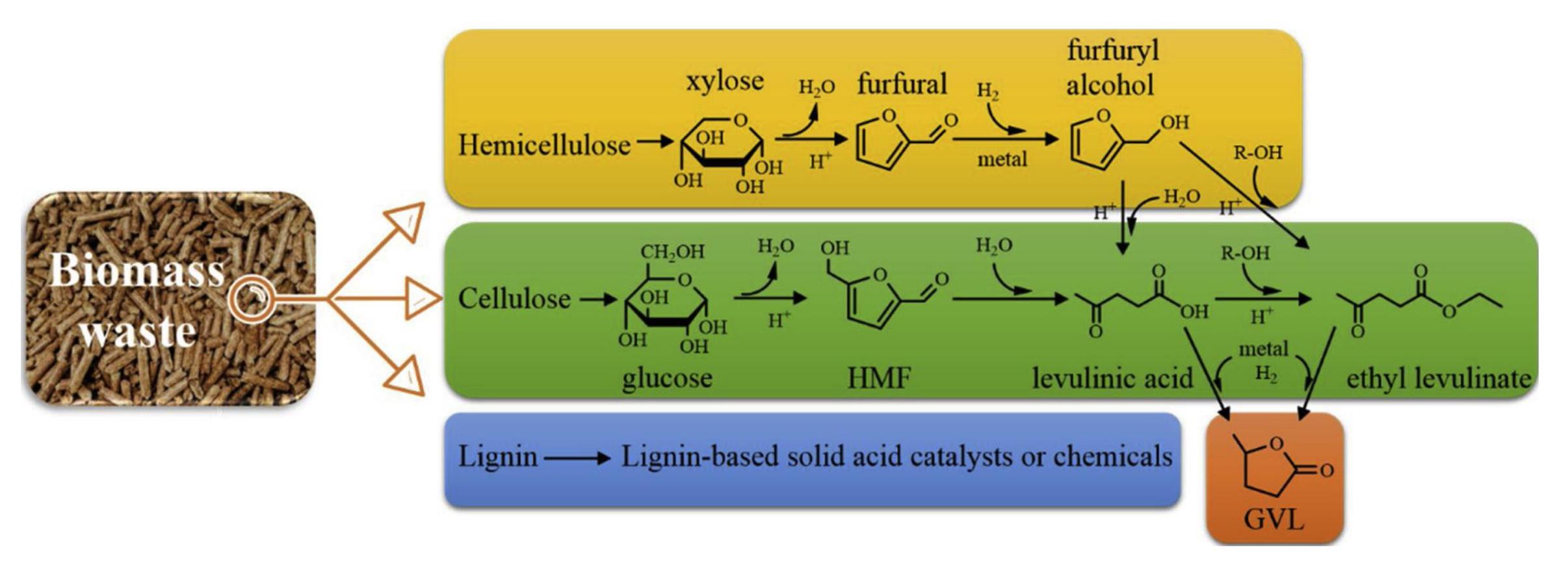
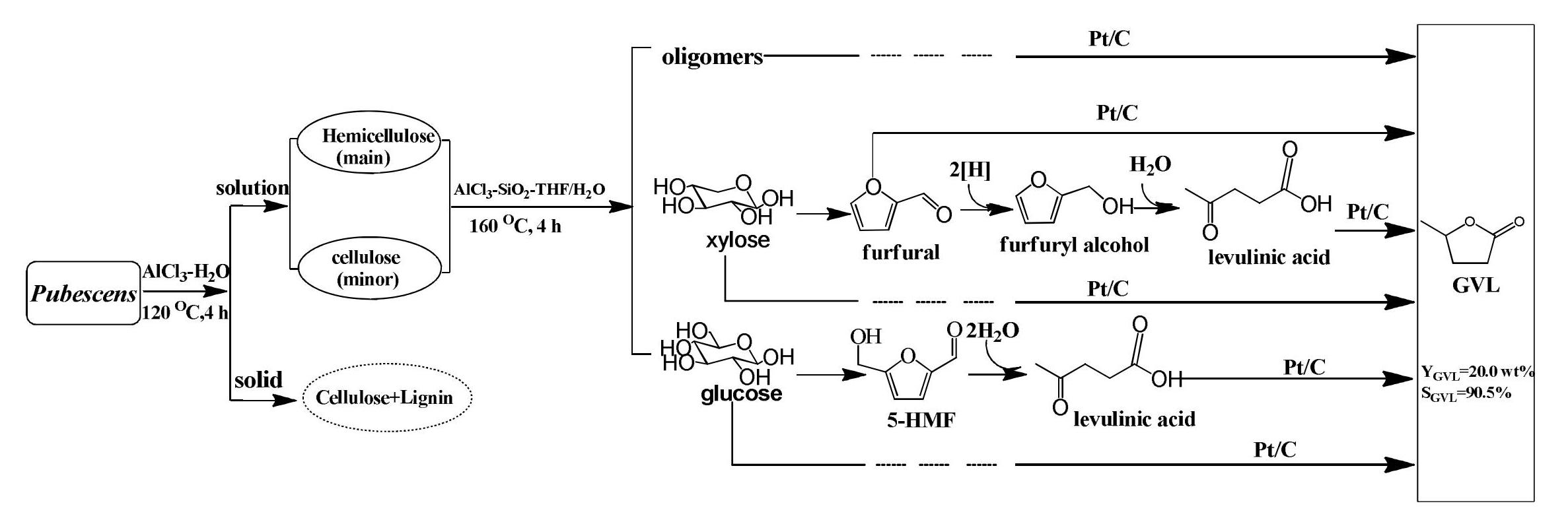
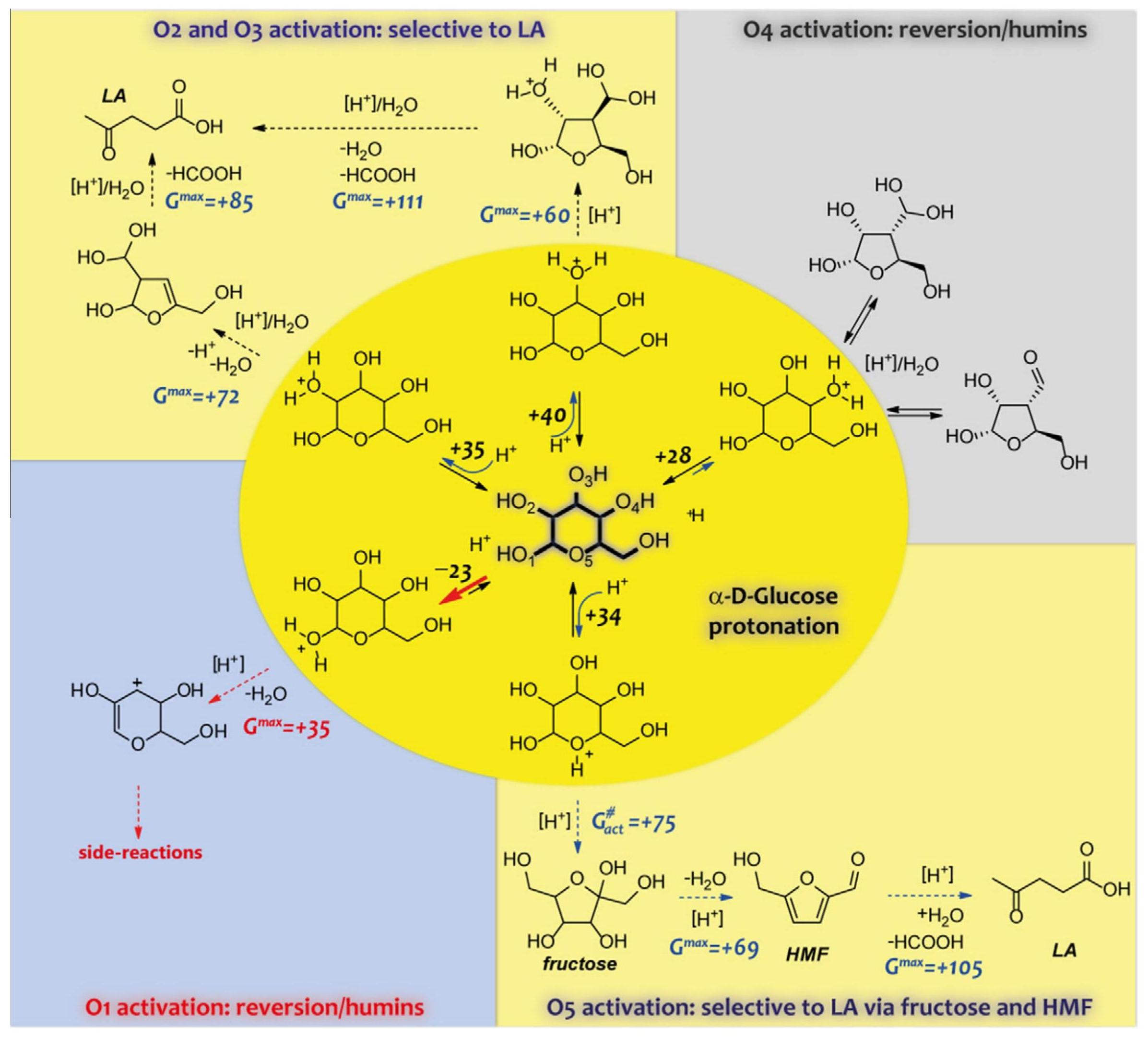
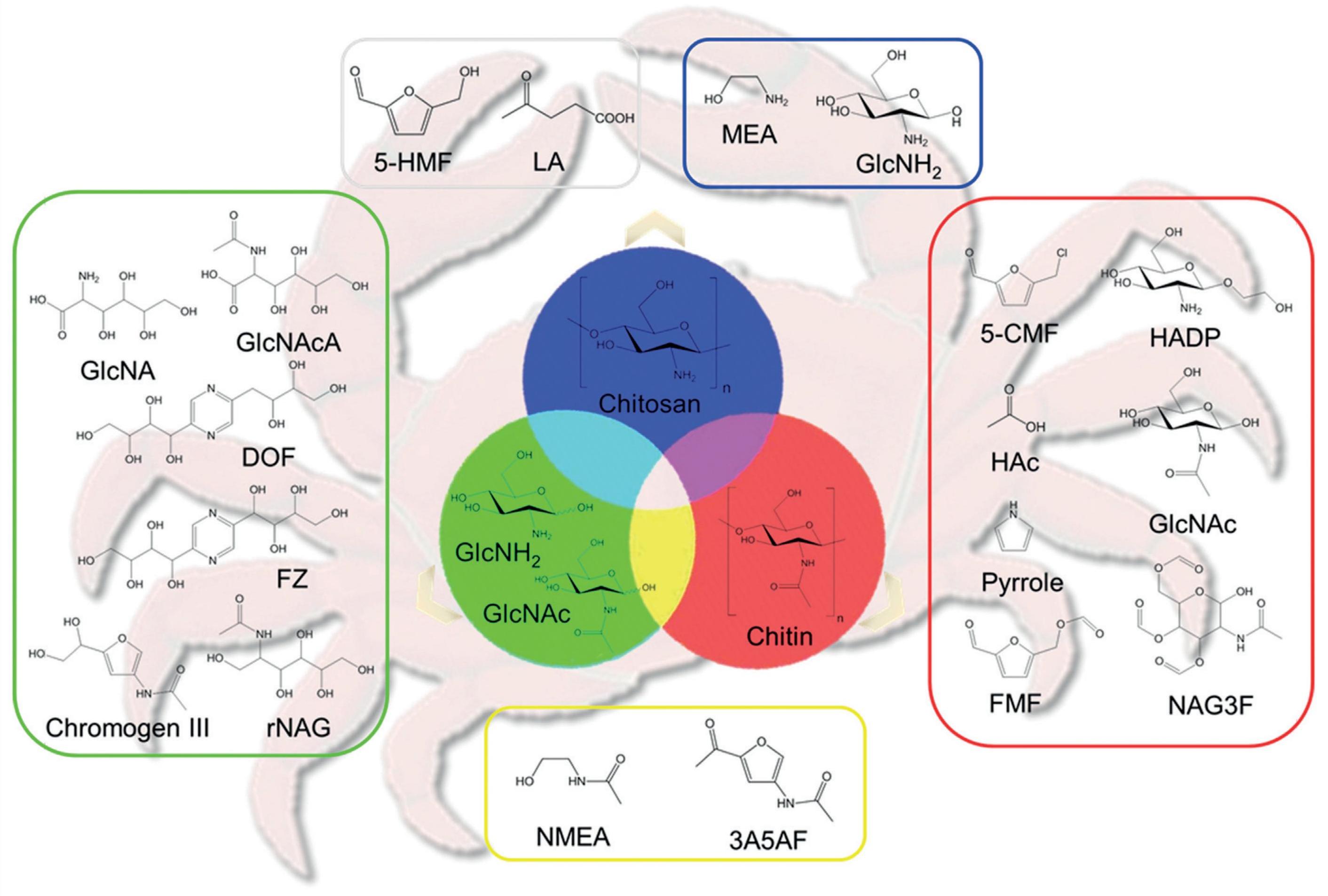
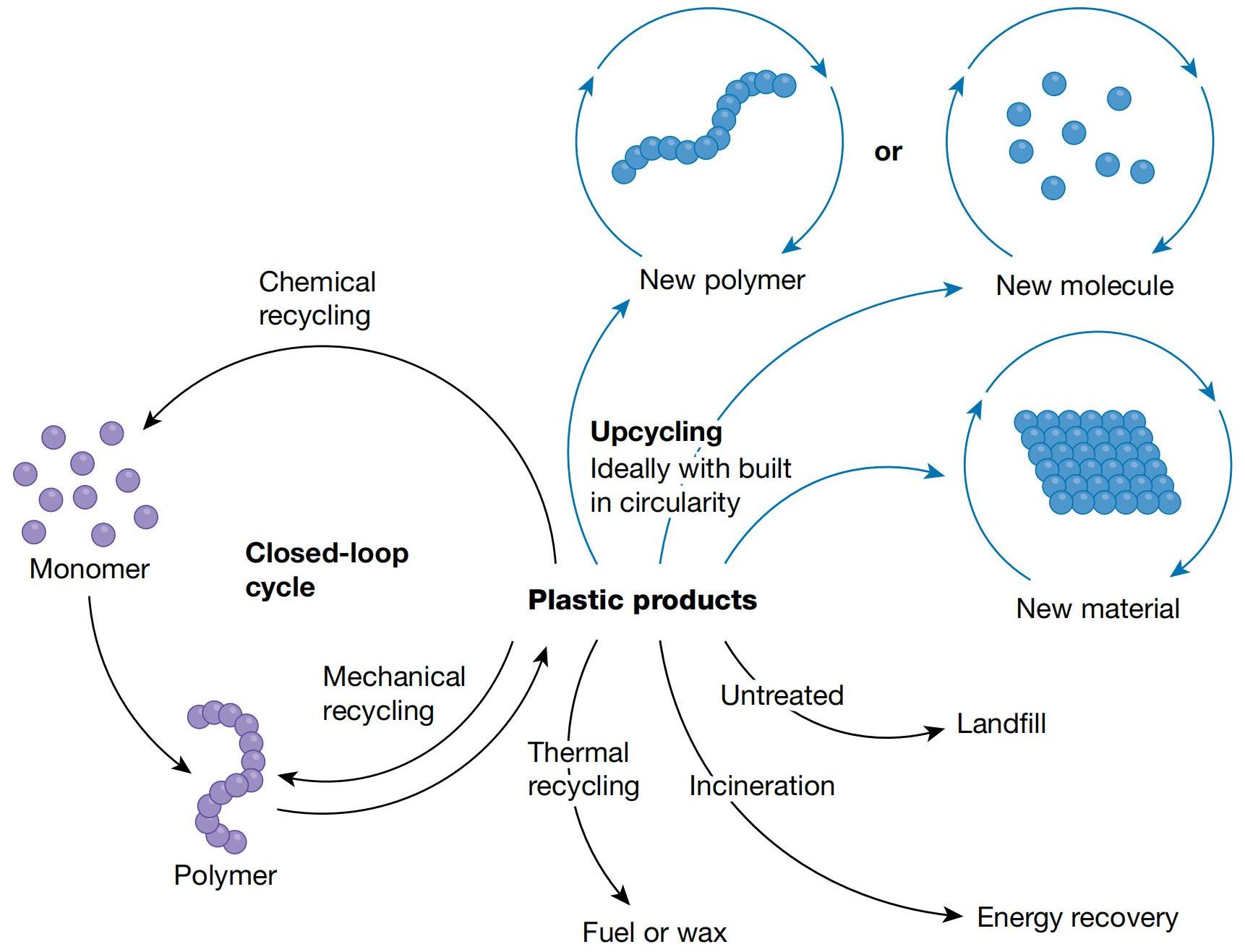
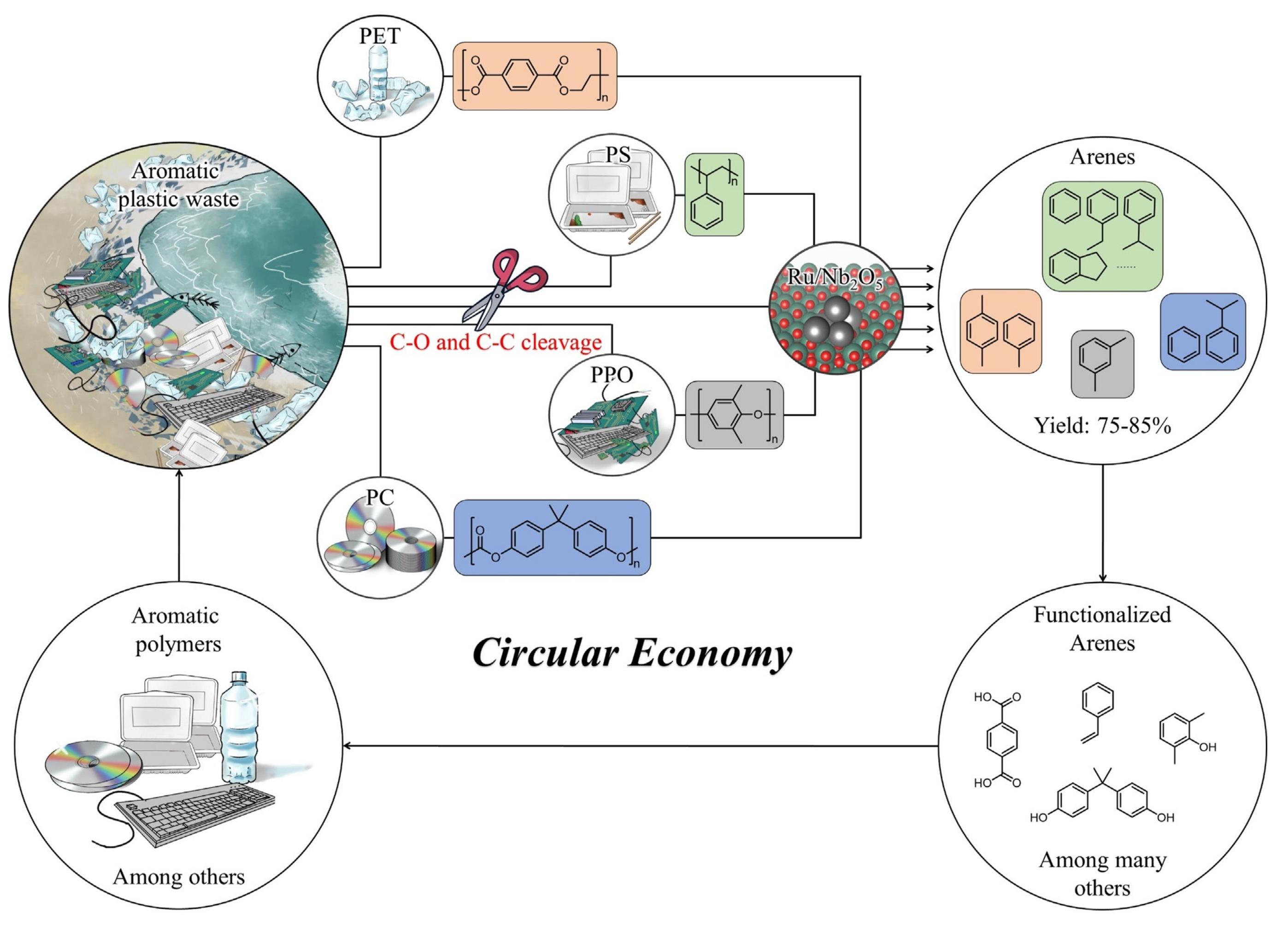
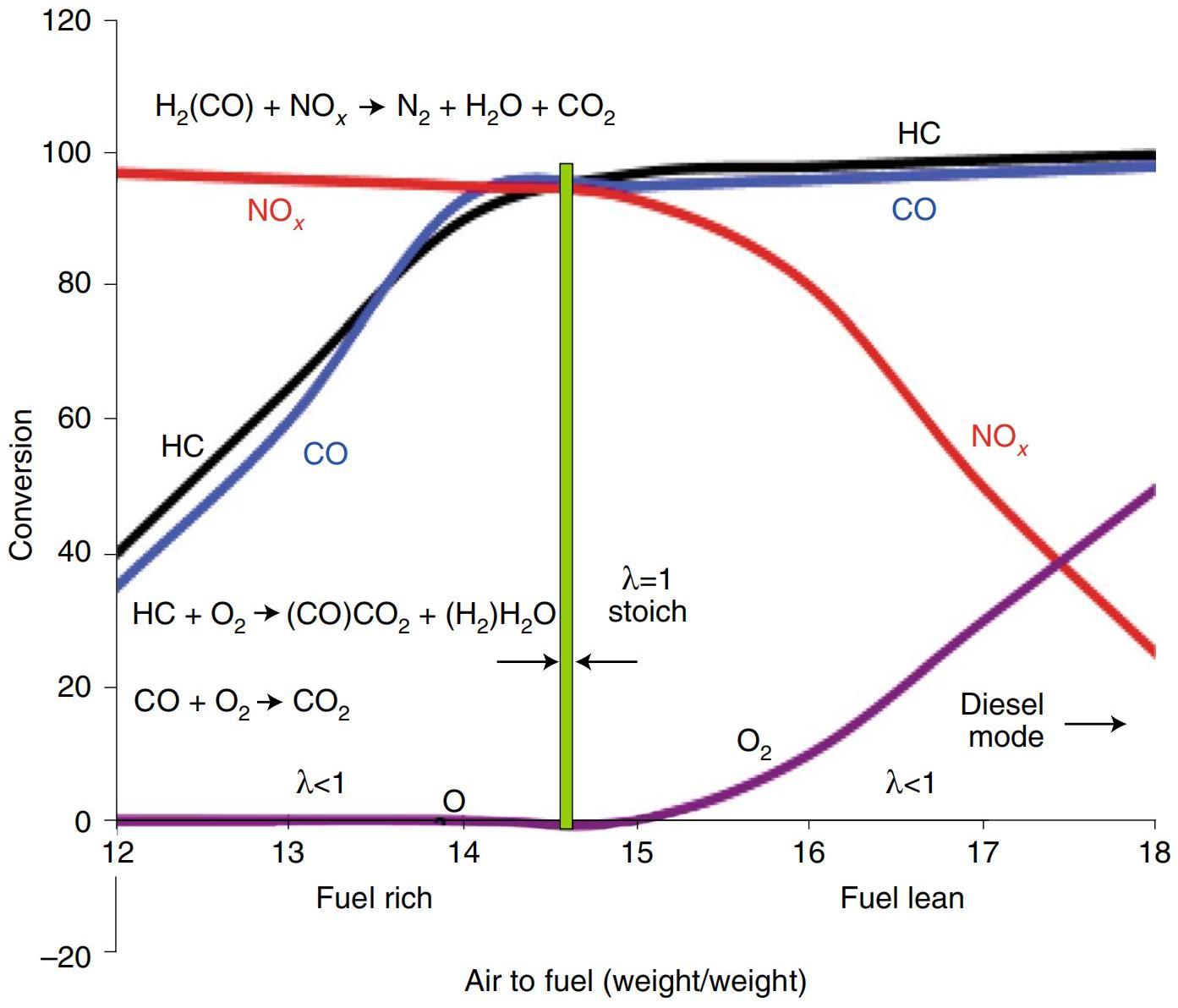
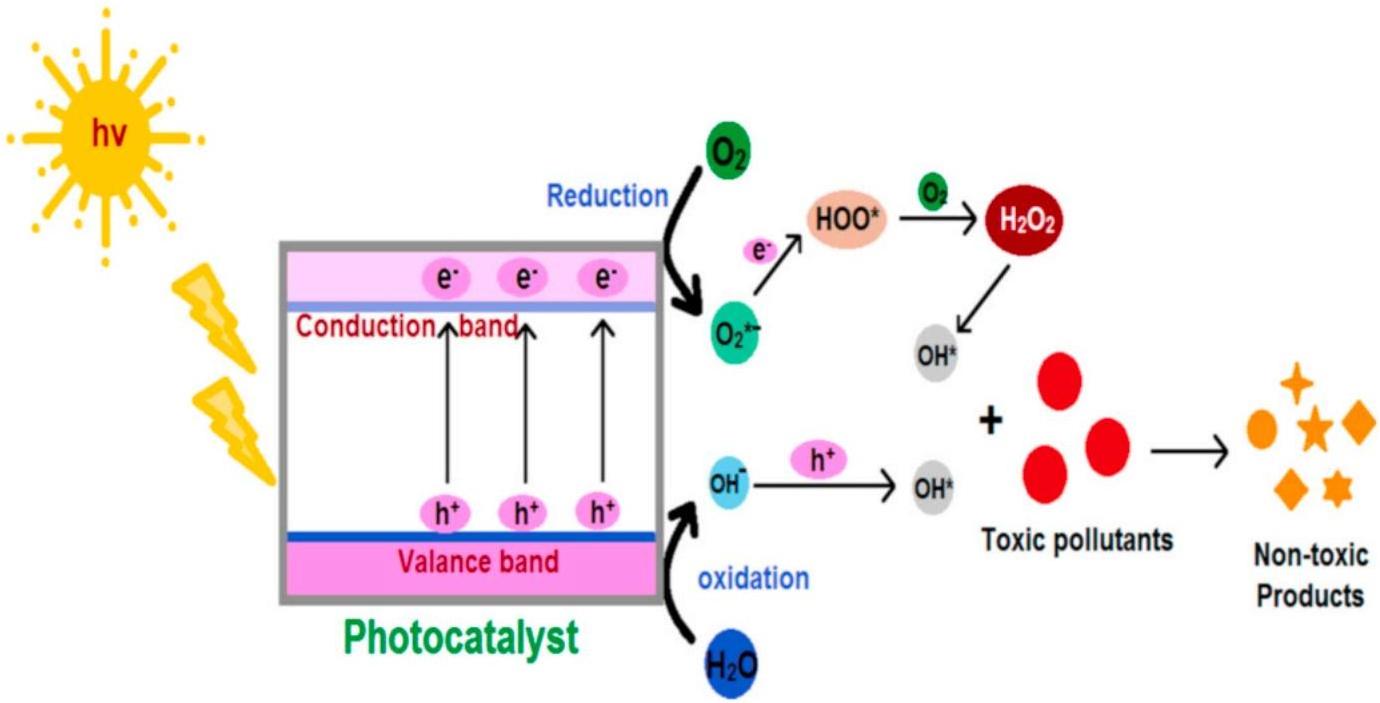




 Copyright ©
Copyright ©