Interface-engineering of the Catalysts for Efficient Electrocatalytic Upcycling of Polyethylene Terephthalate Waste
1Hubei Key Laboratory of Plasma Chemistry and Advanced Materials, School of Materials Science and Engineering, Wuhan Institute of Technology, Wuhan, Hubei Province, China
*Correspondence to: Xiang Peng, PhD, Professor, Hubei Key Laboratory of Plasma Chemistry and Advanced Materials, School of Materials Science and Engineering, Wuhan Institute of Technology, Wuhan, 430205, Hubei Province, China; Email: xpeng@wit.edu.cn
DOI: 10.53964/id.2024001
Abstract
Plastics have become an essential component of our daily lives, but their widespread use has resulted in significant environmental and ecological pollution. Conventional methods for plastic waste treatment are both environmentally unfriendly and energy-intensive. In recent years, the emergence of electrocatalytic degradation technology for plastic waste has opened up new possibilities for converting waste plastics into valuable chemicals, thus promoting the realization of a circular economy for plastics. This review aims to provide an in-depth overview of the fundamental principles and characteristics of interfaces, focusing on the electrocatalytic degradation of ethylene glycol (EG) derived from polyethylene terephthalate (PET). This review primarily examines the two primary methods of electrocatalytic oxidation reactions of PET-derived EG, along with the advancements in interface engineering technology. It delves into the research progress made in interface catalysts for the electrocatalysis of PET-derived EG, while also discussing future research directions. The objective is to delve deeper into the development of effective electrocatalytic degradation catalysts, to address the pressing challenges posed by the ecological environmental crisis and the imperative need for transforming and upgrading PET plastic waste.
Keywords: electrochemical upcycling, electrocatalysis, polyethylene terephthalate (PET), ethylene glycol, interface engineering
1 INTRODUCTION
The rapid growth of the modern economy and society has resulted in an unprecedented increase in fossil fuel consumption, causing severe damage to our ecological environment[1]. Simultaneously, the fast-paced nature of our lives has led to the ubiquitous presence of disposable plastic products[2,3]. Each plastic container is furnished with a distinctive identification sign resembling an “ID card”, typically positioned at the base. Enclosed within a triangular shape, a number ranging from 1 to 7 is displayed, with each number denoting a particular type of plastic material. The numbers are as follows: #1: polyethylene terephthalate (PET/PETE), #2: high-density polyethylene (HDPE), #3: polyvinyl chloride (PVC), #4: low-density polyethylene (LDPE), #5: polypropylene (PP), #6: polystyrene (PS), and #7: others such as acrylonitrile butadiene styrene (ABS)[4-8]. Plastic pollution, also known as white pollution[9,10], refers to the solid waste formed when high molecular compound plastics like PP and PET are carelessly discarded after use, resulting in adverse visual effects and substantial ecological damage and may even endanger human health[11,12]. This further exacerbates the challenges associated with subsequent disposal[13]. Consequently, the imperative to ban and restrict plastic usage has become increasingly urgent[14].
PET plastic, as a main type of plastic that is frequently contacted and used in daily life, undoubtedly brings great convenience to people. However, at the same time, the massive use of PET has also produced serious pollution problems for the environment[15]. To address the environmental pollution caused by PET, researchers have focused on developing efficient degradation methods. Currently, the primary methods for PET waste disposal involve chemical recycling and physical recycling[16]. Physical recycling entails crushing, cleaning, and drying PET waste to produce raw materials. However, the quality of the recycled products often falls short of the original raw material standards[17], and the treatment process generates wastewater and gas, significantly impacting the environment. In contrast, chemical recycling methods convert PET waste into monomers or intermediates, offering the advantage of obtaining high-purity monomers[18,19]. Establishing a catalytic recycling system using chemical means can help combat white pollution, reduce greenhouse gas emissions, and yield valuable chemicals, thereby offering economic benefits[20]. Among these chemical recovery methods, electrocatalysis stands out as an electrochemical technique that has been widely used in energy and environment applications[21-26]. Its application in PET plastic degradation enables an efficient and environmentally friendly process, transforming PET degradation products into high-value chemicals and fuels[27].
Consequently, electrocatalysis holds tremendous potential, facilitating efficient PET waste recycling, reducing environmental pollution, lowering greenhouse gas emissions, and generating economic benefits[28]. This technology plays a crucial role in achieving a sustainable circular economy for plastics. In recent decades, the demand for efficient catalysts has surged[29], leading to rapid advancements in interface engineering catalysts for the electrochemical oxidation of ethylene glycol (EGOR) derived from PET. However, comprehensive reviews on heterogeneous interface engineering for this process are currently lacking.
This review aims to present the PET depolymerization method and explore the fundamental principles of electrochemical conversion using PET-derived ethylene glycol. Additionally, the mechanisms and applications of interface engineering in the electrochemical conversion of PET-derived ethylene glycol will also be summarized. The primary goal is to provide a valuable reference for the development of new electrocatalysts that exhibit high efficiency, good selectivity, cost-effectiveness, and value addition. By adhering to principles of green technology, environmental protection, and sustainable development, we hope to contribute to the early realization of the “plastic reduction and recycling” goal.
2 FUNDAMENTALS OF PET DEPOLYMERIZATION
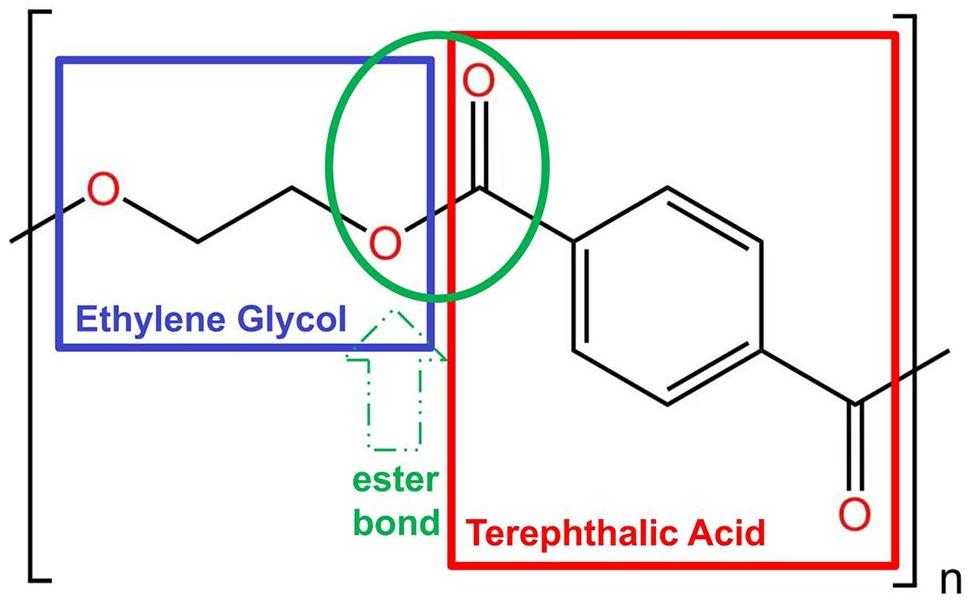
PET is a commonly used polymer, which has the chemical formula of (C10H8O4)n. When PET is spun into fibers for fabrics, it is commonly referred to as “polyester”. However, in container and packaging applications, it is known as “PET” or “PETE”[30]. The molecular structure of PET consists of a linear macromolecule with hydroxyl and carboxyl end groups connected by ester groups, with a benzene ring and an ethyl group positioned between them. With its regular molecular chain and symmetry, PET forms a linear macromolecule, as illustrated in Figure 1[31].
|
Figure 1. Molecular Structure of PET.
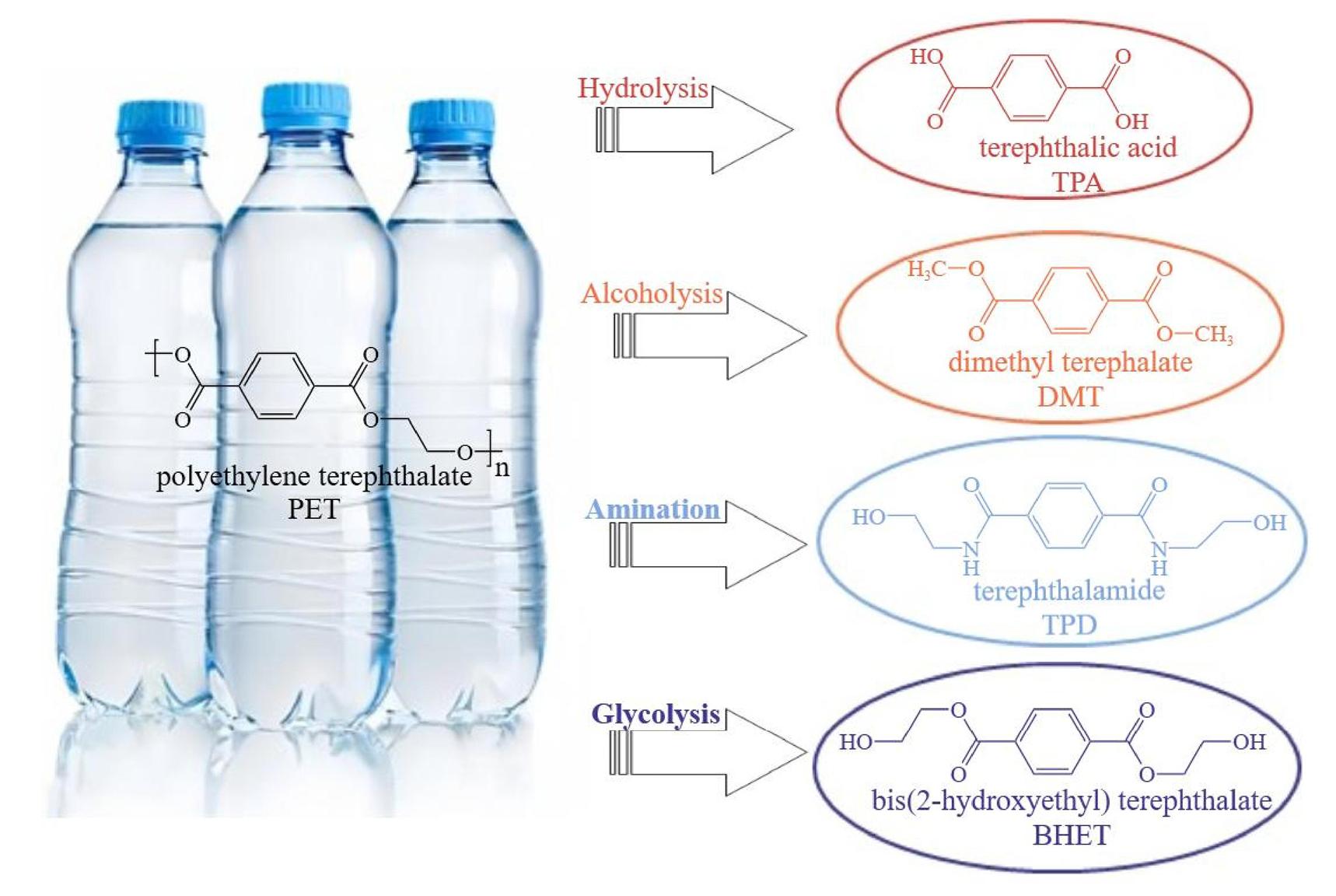
The PET molecule’s C-O bond typically possesses lower chemical energy compared to the C-C bond[32]. Various external factors, including water, heat, light, and oxygen, can cause the fracture of chemical bonds in PET, leading to its degradation. PET depolymerization methods are classified based on the chosen degradants, which include alcoholysis, glycolysis, hydrolysis, and aminolysis. Figure 2 illustrates the categorization of these depolymerization methods[33]. The primary depolymerized products include terephthalic acid (TPA/PTA) and EG, as well as combinations such as terephthalamide and EG, dimethyl terephthalate and EG, and bis(2-hydroxyehtyl) terephthalate. These products can be obtained through hydrolysis[34], ammonolysis[35], methanol alcoholysis[36], and EG alcoholysis (also known as glycolysis)[37].
|
Figure 2. Methods for PET Depolymerization.
PET is an abundant polyester plastic that can be hydrolyzed into TPA and EG in aqueous solutions of different media[38]. These media include acidic[39], neutral[40], and alkaline solutions[41]. However, acid hydrolysis is highly corrosive to the reaction system and generates waste liquid containing inorganic salts and acidic waste. Neutral hydrolysis requires a large amount of water, resulting in low EG content in the product and making separation challenging. Alkaline hydrolysis, on the other hand, allows for the precipitation of EG and TPA by acidifying the product solution and adjusting the pH to 2-3[42]. Thus, compared to acidic and neutral hydrolysis methods, alkaline hydrolysis offers advantages in terms of purification.
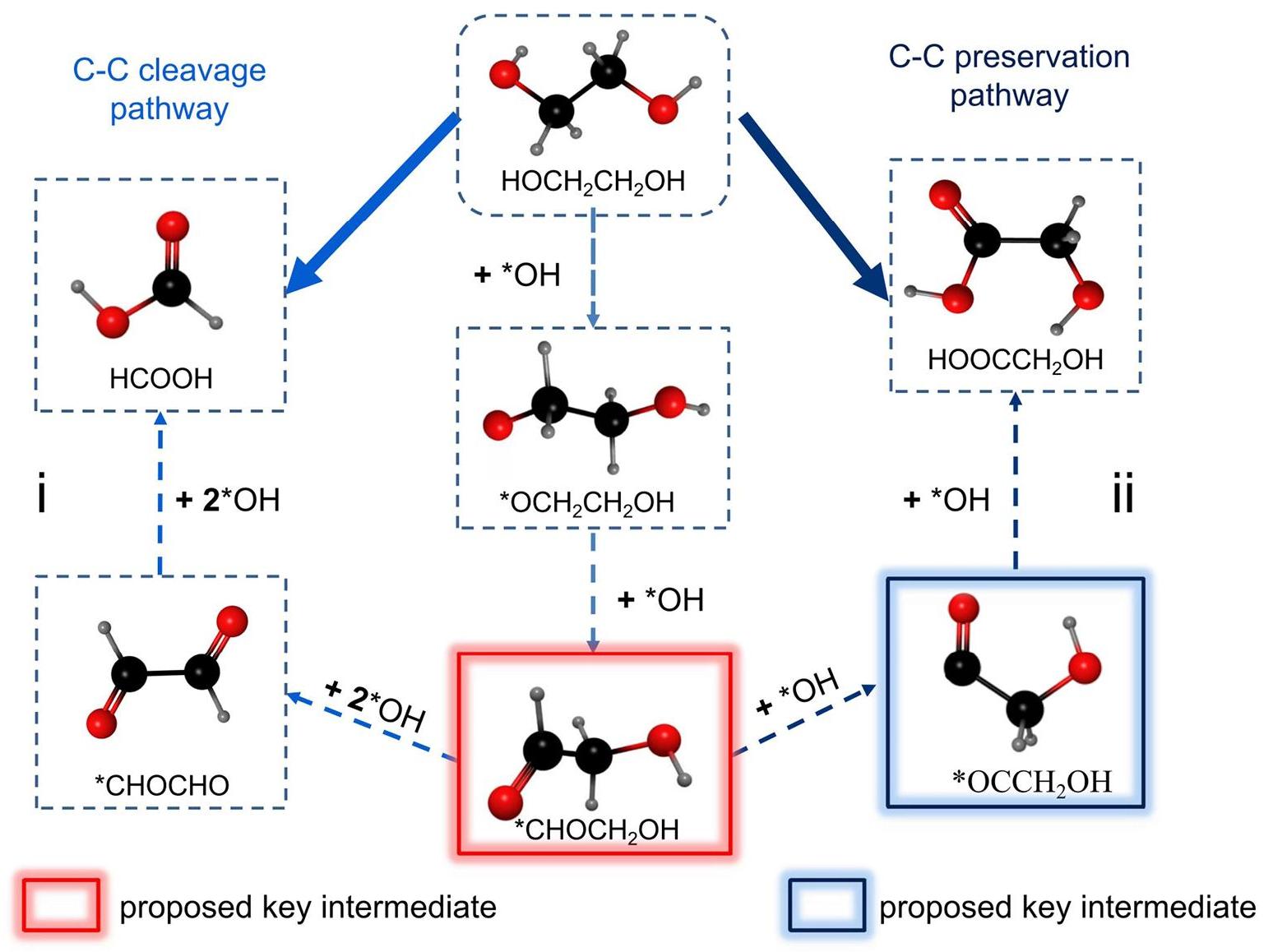
Electrocatalysis provides a promising approach for converting PET-derived EG into high-value products and recycling TPA[19]. In the electrocatalytic process of PET depolymerization, the oxidation current is attributed to the oxidation of EG in PET rather than TPA[43]. During electrocatalysis, there are typically two reaction paths for EGOR, as shown in Figure 3[44]. The C-C cleavage pathway leads to the formation of formic acid (C1 product), while the C-C preservation pathway results in the production of glycolic acid (C2 product). By modulating the adsorption strength of carbonyl intermediates on the catalyst surface, the C1/C2 reaction path can be controlled, enabling high selectivity of the EGOR products[45,46]. This means that the surface/interface structure of the catalysts can be tailored to facilitate the electrocatalytic transformation of PET-derived EG, enhancing product selectivity and yield.
|
Figure 3. Two Reaction Paths of EGOR: (i) C-C Cleavage Pathways and (ii) C-C Preservation Pathways.
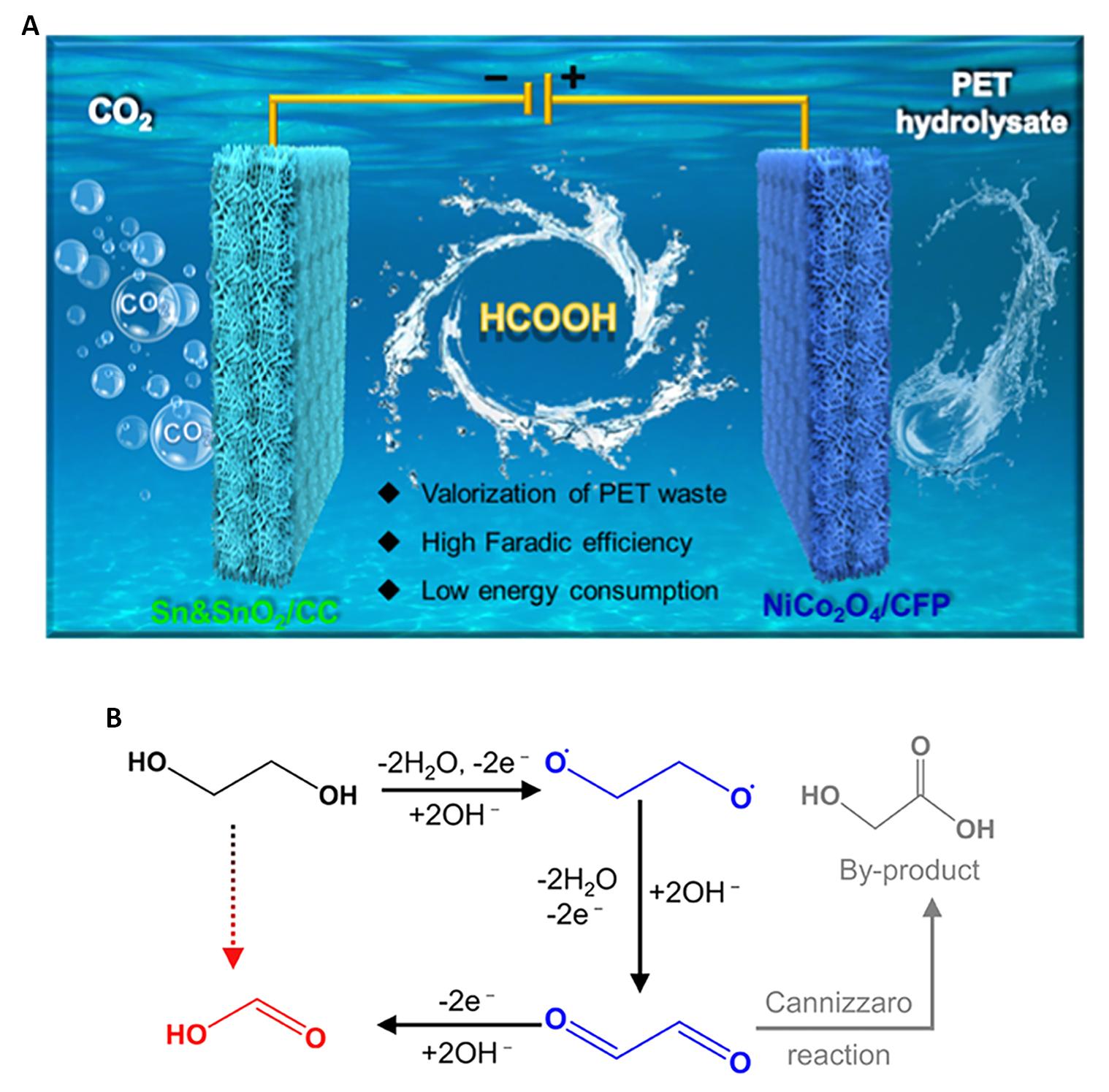
In a study conducted by Zhao and Wang[47], earth-abundant Ni and Co-based materials were employed as anodic catalysts for the electrocatalytic oxidation of PET-derived EG to produce formic acid. Simultaneously, cathodic coupling electrolysis of CO2 was employed to produce formic acid, as depicted in Figure 4A. High-performance liquid chromatography analysis revealed that the Faraday efficiency of formic acid remained consistently above 90% when the anode potential was maintained at 1.45V vs. the reversible hydrogen electrode (RHE). These results demonstrated that the NiCo2O4/carbon fiber paper catalyst exhibited remarkable stability and high selectivity for the electrochemical transformation of PET hydrolysate into formic acid.
To further investigate the reaction mechanism, an additional experiment was conducted to study the oxidation of glycolic acid (GA)[47]. It was observed that the main product of the glycolic acid oxidation reaction was oxalic acid, whereas the EGOR experiment primarily yielded formic acid. This finding suggested that glycolic acid was not a crucial intermediate in the EGOR catalyzed by the prepared NiCo2O4 electrocatalyst. Therefore, the proposed reaction mechanism for the EGOR on the NiCo2O4 electrocatalyst can be described as follows: Initially, the −OH deprotonation step occurs, leading to the formation of the reactive alkoxide intermediate OCH2−H2CO. Subsequently, the alkoxide intermediate undergoes oxidation to form the glyoxal intermediate OCH−HCO, which then undergoes C-C bond cleavage to yield formic acid. The by-product, glycolic acid, is a result of the transformation of the Cannizzaro reaction of glyoxal, and the reaction pathway is illustrated in Figure 4B.
|
Figure 4. Reaction Path of EGOR. A: Synchronous electrochemical conversion of anode PET hydrolysate with cathode CO2 to formic acid; B: Reaction path of the oxidation of ethylene glycol to formic acid. Reproduced from Ref.[47] with permission from American Chemical Society.
The selective oxidation of the hydroxymethyl groups in EG to produce GA is highly desirable in EGOR as GA is economically valuable and finds extensive applications in various industries such as industrial applications, food additives, pharmaceuticals, and agriculture. The market size of glycolic acid was $310.4 million in 2020 and is expected to reach $531.5 million by 2027[42].
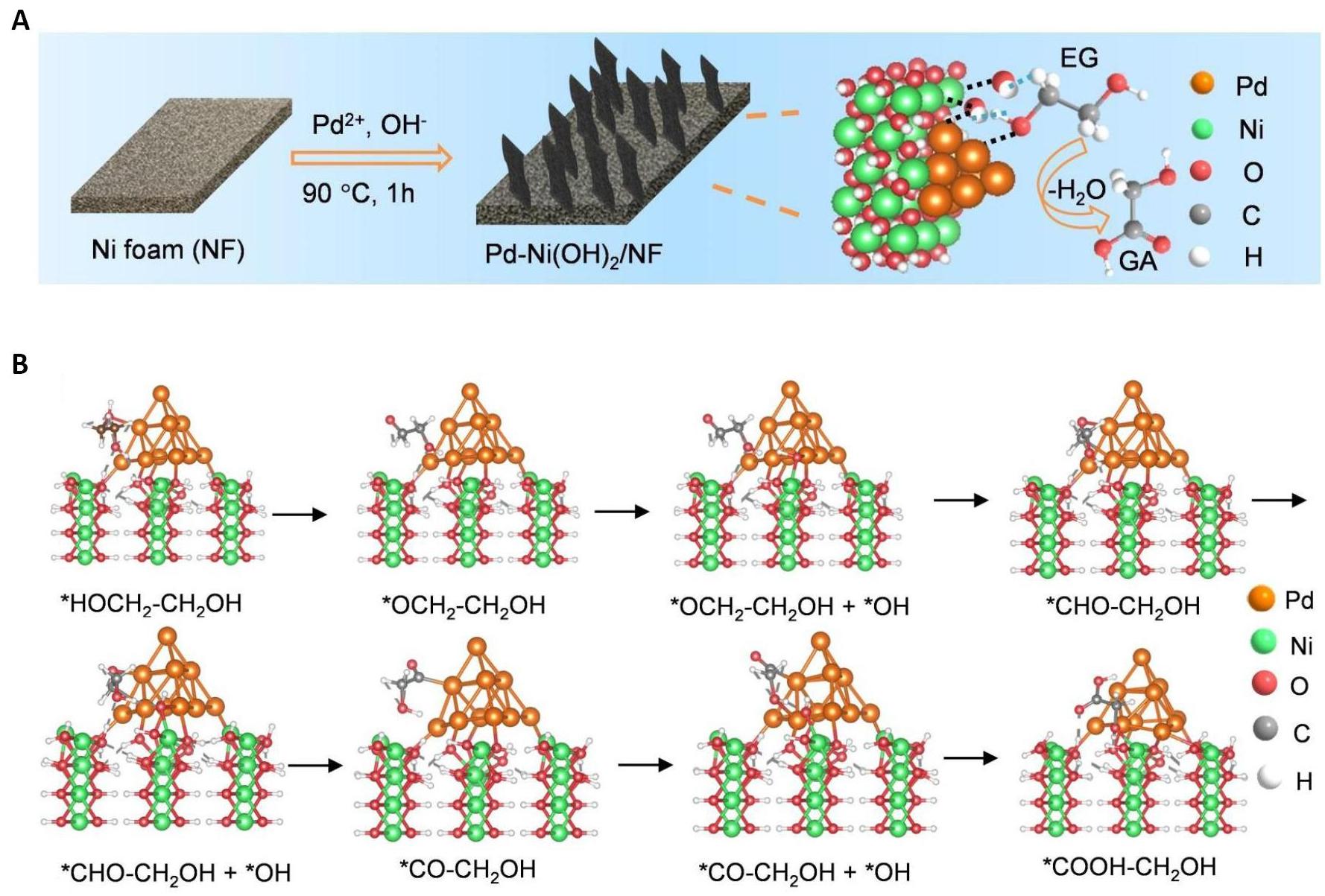
To achieve the selective oxidation of EG to GA, Chen et al.[48] designed and synthesized a Pd-Ni(OH)2 composite electrocatalyst, as depicted in Figure 5A. Through the synergistic interaction of Pd and Ni sites, ethylene glycol can be highly selectively oxidized to glycolic acid while generating hydrogen at the cathode. The catalyst exhibited a high selectivity of 91.6% for glycolic acid with an ethylene glycol conversion rate of 93.2%. Even at an industrial-scale current density of 600mA·cm-2, the catalyst maintained a high Faraday efficiency and selectivity of approximately 85%. In-situ Fourier Transform Infrared Spectroscopy during electrochemical experiments revealed that OH species rapidly convert the OC-CH2OH intermediate to glycolic acid products instead of C1 products through the C-C cleavage pathway. The electronic interaction between Pd and Ni(OH)2 leads to a decrease in the d-band center shift of Pd, reducing carbonyl adsorption and facilitating the transfer of the OC-CH2OH intermediate from the active Pd site to the adjacent Ni site. This interaction prevents excessive cleavage or oxidation of the C-C bond. Based on experimental results, the conversion pathway from EG to GA can be summarized as follows: HOCH2-CH2OH → HOCH2-CH2OH → HOCH2-CH2O (RO) → HOCH2-CHO* → HOCH2-CO* → HOCH2COOH* → HOCH2-COOH(GA), as illustrated in Figure 5B. Their work not only provides a promising approach for upgrading PET to high-value glycolic acids at an industrial level but also offers valuable insights into intermediate conversion pathways and catalyst design with synergistic active sites.
|
Figure 5. The Pd-Ni(OH)2 Catalyst for EGOR. A: Schematic diagram of the synthesis of Pd-Ni(OH)2 on nickel foam; B: Optimal configuration of EG oxidation process on Pd-Ni(OH)2. Reproduced from Ref.[48] with permission from Wiley.
3 CATALYSTS’ INTERFACE ENGINEERING FOR ELECTROCATALYTIC UPCYCLING OF PET-DERIVED EG
3.1 Electrocatalytic Conversion of EG to C1 Products
The surface and interface of catalysts encompass the atomic layers on the surface and the junction between phases, which do not have a well-defined boundary[49]. In the context of electrochemical reactions, the catalyst’s interface provides active centers for electrocatalytic reactions and plays a crucial role in achieving high efficiency[50-52]. The interface can modify the equilibrium relationship between reactants and products, thereby facilitating the reaction process. The overall electrocatalytic reaction can be described in three consecutive steps: Firstly, the chemical adsorption of reactants occurs on the surface or interface of the electrocatalyst. Secondly, the reactants undergo activation to generate intermediates. Lastly, the final product is desorbed from the electrocatalyst into the electrolyte[53,54]. The optimal performance of the entire electrocatalytic process relies on the optimization of the adsorption and desorption steps.
The limited catalytic performance of non-precious metal-based electrocatalysts hinders their widespread industrial application[55]. To overcome this limitation, various strategies, such as interface engineering[56], topography engineering[57], defect engineering[58], and crystal plane engineering[59], have been employed to enhance their electrocatalytic capability. Typically, the active sites responsible for electrocatalysis are concentrated at the edge sites; however, their number is relatively limited, posing a significant obstacle to achieving high efficiency. The atomic and molecular structure at the catalyst's interface plays a crucial role in determining the catalytic activity of these active sites[60]. Atoms located at the interface exhibit a coordination unsaturated state compared to bulk atoms, making them highly susceptible to physisorption, chemisorption, or direct chemical reactions with other species, leading to the formation of new species. Additionally, interface engineering holds great potential for improving electrochemical performance by optimizing the chemisorption of reaction intermediates, enhancing electron/mass transport, and preventing the aggregation of active components[61]. Therefore, a promising strategy to enhance the mass and energy conversion of electrocatalytic reactions is to increase the exposure of edge active sites and enhance the intrinsic activity of these sites through interface engineering of the catalysts. This approach has emerged as a research hotspot in the field of electrocatalysis[62].
Indeed, the electrocatalytic reforming of EG encounters challenges such as a complex reaction process, poor product selectivity, and catalyst deactivation. To achieve the electrochemical reforming of EG derived from PET in line with green and sustainable development, the design of high-performance catalysts is crucial. In recent years, interface engineering strategies have emerged as effective approaches to enhance the electrocatalytic oxidation performance of PET-derived ethylene glycols, making it a prominent research topic. By manipulating the atomic and molecular structure at the interface of catalysts, interface engineering can optimize the adsorption of reaction intermediates, facilitate electron/mass transport, and prevent the aggregation of active components.
In a significant discovery from a previous study, it was observed that secondary alcohols featuring a -C(OH)-C- structure can undergo oxidation, resulting in the cleavage of the C-C bond when cobalt hydroxyl oxide is employed as an anode catalyst in an alkaline electrolyte. This intriguing process leads to the formation of carboxylic acid compounds. Building upon this breakthrough, a team of researchers, under the leadership of Duan et al.[63] has successfully developed a highly efficient bifunctional CoNi0.25P electrocatalyst. This novel electrocatalyst serves as a pivotal component in their proposed electrocatalytic strategy, which aims to convert the hydrolyzed products of PET waste into potassium dicarboxylate and terephthalic acid within a KOH electrolyte.
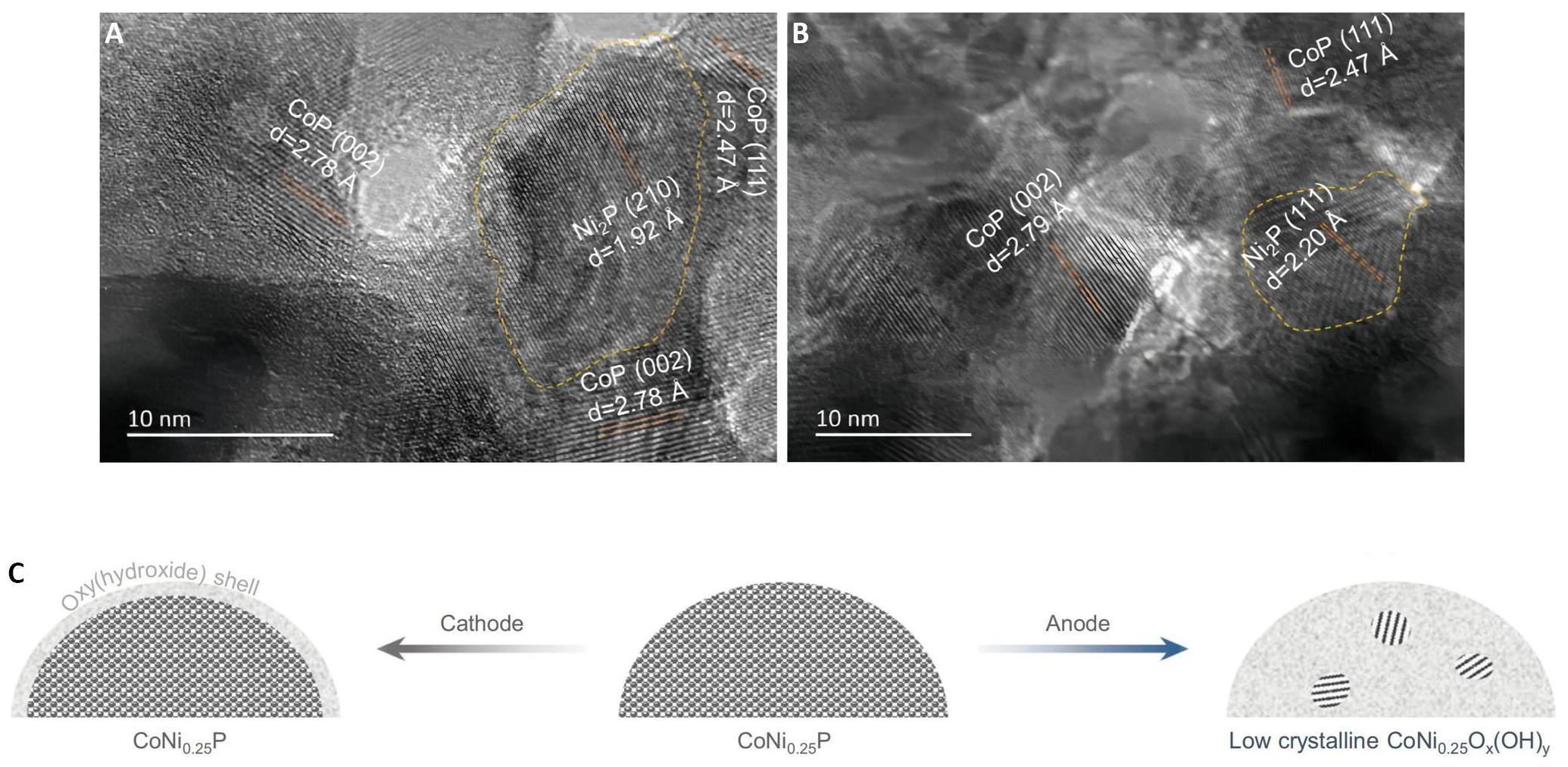
In this electrochemical process, the CoNi0.25P catalyst catalyzes the conversion of EG at the anode of the electrolytic cell, resulting in the formation of formate. Remarkably, the selectivity of this reaction exceeds 90%. Simultaneously, hydrogen is generated at the cathode. In the absence of EG, all electrocatalysts exhibit relative activity towards the oxygen evolution reaction (OER) with an initial potential of approximately 1.51V vs. RHE. However, with the introduction of 0.3M EG into the electrolyte, the initial potential of all electrocatalysts shifts to lower values (ranging from 1.22 to 1.33V vs. RHE). This shift indicates that, thermodynamically, the oxidation of EG is more favorable compared to the OER. High-resolution transmission electron microscopy (HR-TEM) observations revealed that the CoNi0.25P material consists of small Ni2P nanoparticles interconnected with CoP particles, forming a CoP-Ni2P heterostructure, as depicted in Figure 6A-B. When utilized in an H-type electrolyzer, the CoNi0.25P electrocatalyst demonstrates its capability to generate a high current density of approximately 350mA·cm-2 at a potential of 1.7V vs. RHE. Moreover, when applied to a zero-gap membrane electrode assembly with minimal energy loss, the catalyst achieves a significant current density of up to 500mA·cm-2 at a 1.8V. These impressive results are accompanied by a Faraday efficiency exceeding 80% and a formate selectivity of more than 80%.
A comprehensive investigation was undertaken to study the changes occurring in the CoNi0.25P catalyst during electrochemical reactions. The findings revealed that the catalyst retained the stability of its primary structure during the hydrogen evolution reaction. However, advanced characterization techniques including HR-TEM and X-Ray Diffraction provided evidence that the catalyst's structure underwent reconstruction during the anodic oxidation reaction. This reconstruction resulted in the formation of a low crystalline metal oxy(hydroxide) analogue, as illustrated in Figure 6C.
|
Figure 6. Structure of the CoNi0.25P Catalyst. (A-B): HR-TEM images of CoNi0.25P nanosheet; C: Schematic diagram of the structural evolution of the CoNi0.25P catalyst under reaction conditions. Reproduced from Ref.[63] with permission from Springer Nature.
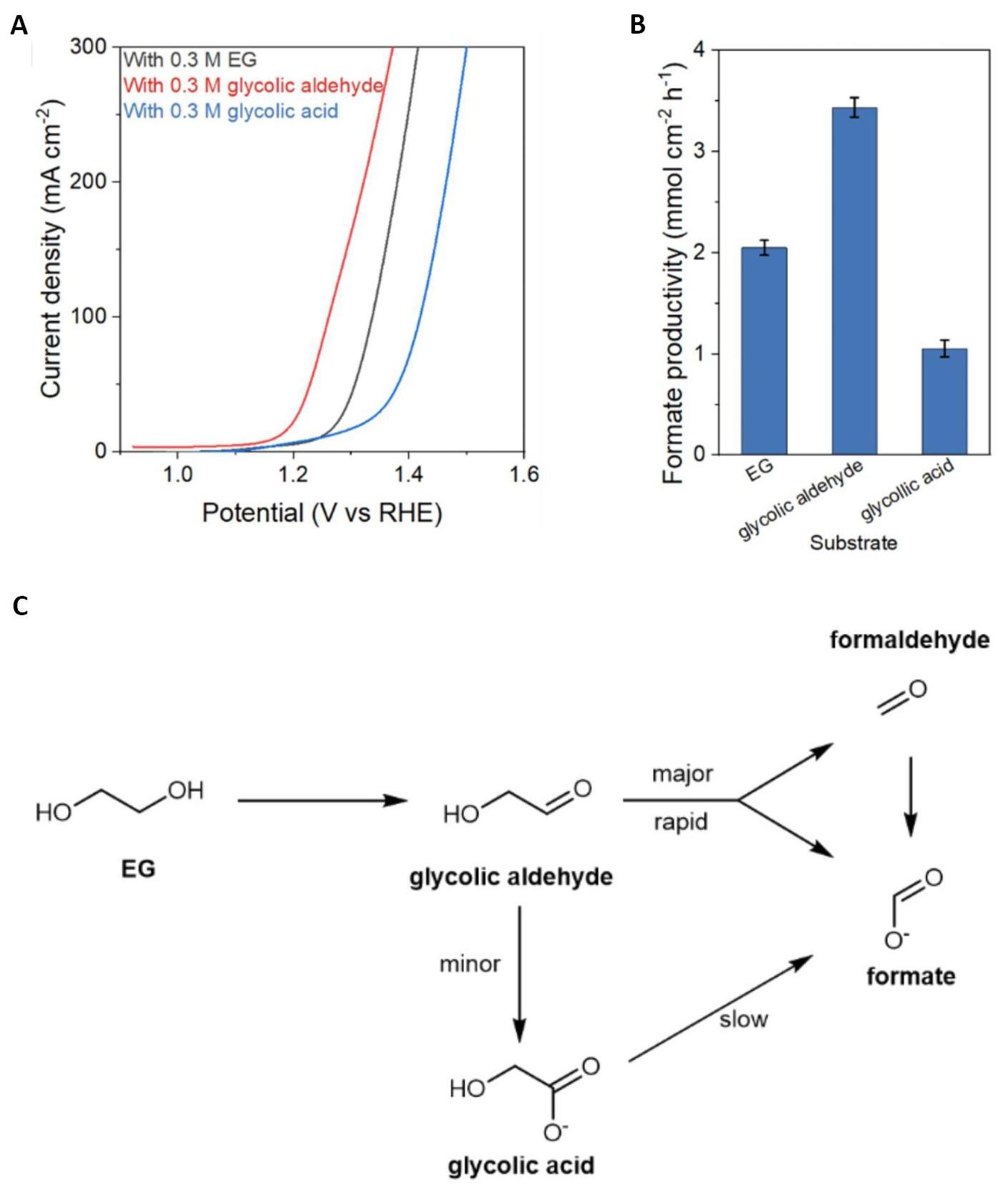
Moreover, the research team conducted an extensive analysis of the dynamic transformation of intermediate products during the oxidation reaction of EG. To gain insights into the electrochemical and kinetic aspects, three different starting substrates were evaluated: ethylene glycol, GA, and glycolic aldehyde The results unveiled that glycolaldehyde exhibited a significantly faster reaction rate in the formation of formic acid compared to ethylene glycol and glycolic acid, as illustrated in Figure 7A-B. This finding highlights the crucial role of glycolaldehyde as an intermediate in the formic acid formation process.
Based on the experimental findings, the researchers proposed an oxidation mechanism for EG, which is illustrated in Figure 7C. According to the proposed mechanism, EG is initially oxidized to glycolaldehyde, which undergoes rapid C-C cleavage to form formate ions. Additionally, a minor portion of glycolaldehyde is oxidized to glycolic acid, which then undergoes a slower C-C cleavage process, ultimately leading to the production of formic acid. This elucidation of the oxidation mechanism provides valuable insights that contribute to a deeper understanding of the EG oxidation reaction and its associated pathways.
|
Figure 7. The CoNi0.25P Catalyst for EGOR. A: Polarization curves with 85% iR correction for organic oxidation in 1 M KOH with 0.3 M substrate; B: Formate productivity at constant potential (1.5V vs. RHE) for different substrates; C: Proposed reaction route for electrocatalytic EG oxidation. Reproduced from Ref.[63] with permission from Springer Nature.
3.2 Electrocatalytic Conversion of EG to C2 Products
In a study conducted by Qiu et al.[42], the Pt/γ-NiOOH/NF electrocatalyst was prepared through corrosion engineering in the presence of K2PtCl4, NaCl, and nickel foam, as depicted in Figure 8A[42]. X-ray photoelectron spectroscopy (XPS) was employed to investigate the coupling effect between Pt and NiOOH in the catalyst, as shown in Figure 8B-D. The researchers discovered that the binding energy of the Ni3+ signal in the Pt/γ-NiOOH/NF catalyst was negatively shifted by 0.5eV compared to that of γ-NiOOH/NF (Figure 8B). Additionally, the binding energy of Pt-4f in Pt/γ-NiOOH/NF was positively shifted by 0.3eV compared to Pt/NF (Figure 8C). These shifts in the binding energy peaks of Ni and Pt indicate the presence of electronic interaction between Pt and the γ-NiOOH phases. The O-1s spectrum of Pt/γ-NiOOH/NF in Figure 8D demonstrates the existence of Pt-O-Ni bonds, which are not observed in the γ-NiOOH/NF catalyst. This finding serves as key evidence for the interaction between Pt and γ-NiOOH. Furthermore, calculations of the charge difference in the catalyst suggested that a significant amount of charge had accumulated at the Pt/γ-NiOOH interface, as illustrated in Figure 8E. Moreover, the calculated results indicated that the shortest oxygen-surface distance between EG and Pt/γ-NiOOH was 1.8Å. This distance suggests that the interfacial region between EG and Pt/γ-NiOOH is highly active for the adsorption of EG reactants.
|
Figure 8. The Pt/γ-NiOOH/NF Catalyst for EGOR. A: Schematic diagram of the preparation of Pt/γ-NiOOH/NF catalyst by corrosion engineering; High-resolution XPS spectra of (B) Ni-2p, (C) Pt-4f, and (D) O-1s for the Pt/γ-NiOOH/NF, γ-NiOOH/NF, and Pt/NF catalysts; E: Calculated charge difference isosurfaces at the Pt and γ-NiOOH interface; F: the Faraday efficiency, selectivity, and formation rate of glycolic acid at a fixed potential of 0.55V of the Pt/γ-NiOOH/NF (Pt mass loading of 0.18mg·cm-2), Pt/NF, and Pt/C catalysts. Reproduced from Ref.[42] with permission from Wiley.
The Pt/γ-NiOOH/NF catalyst successfully achieved the selective oxidation of EG from PET plastics to glycolic acid at a low potential of 0.55V vs. RHE. It achieved a high Faraday efficiency of 97.2% and a selectivity of glycolate production of 98.2%. Additionally, the catalyst demonstrated a production rate of 0.14mmol·cm-1·h-1, as depicted in Figure 8F.
Both experimental and theoretical findings suggest that the formation of glycolic acid involves three deprotonation steps. The first step involves the formation of adsorbed 1,2-dihydroxy ethyl or 2-hydroxy ethoxy species. In the second step, the adsorbed 2-hydroxyacetyl group is generated. Finally, in the third step, the adsorbed glycolic acid is formed. Theoretical calculations indicate that the production of the adsorbed 2-hydroxyacetyl species plays a crucial role in the formation of glycolic acid. The calculations reveal a free energy change of ΔG=0.33eV for the protonation process at the Pt/γ-NiOOH interface, which is smaller than the ΔG=1.14eV observed on the Pt surface. This suggests that the Pt/γ-NiOOH interface is favorable for the production of glycolic acid.
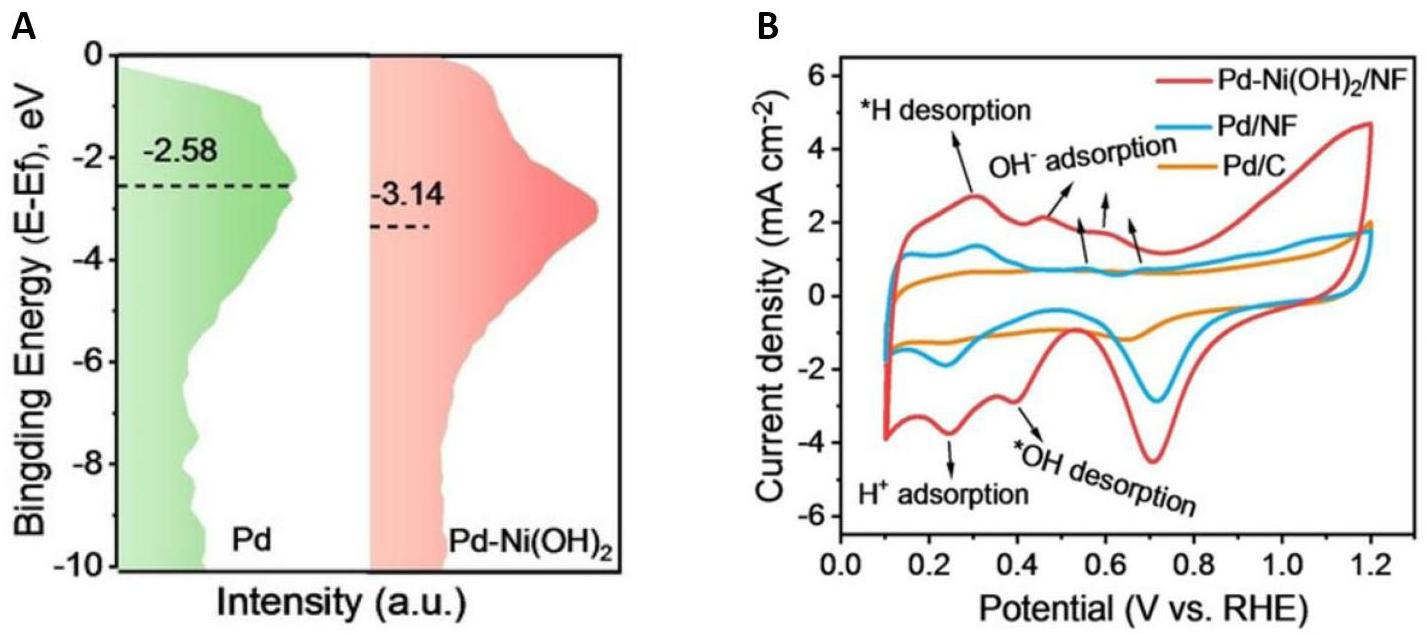
Chen et al.[48] designed a synergistic catalyst of Pd-Ni(OH)2/Ni foam (Pd-Ni(OH)2/NF) for the electrocatalytic oxidation reaction of EG to produce GA. Through charge density difference and Bader charge calculations, they found 1.71 electrons were transferred from Pd to Ni(OH)2, indicating a strong electronic interaction between the two components. The reduced electron density of Pd weakens the feedback effect of the Pd 3d electrons on the CO 2π* orbitals, diminishing the bond strength of the Pd-*CO species. To further investigate the interfacial interactions, they employed surface valence band photoelectron spectroscopy to evaluate the d-band centers of Pd and Pd-Ni(OH)2. Compared to the d-band center of the Pd at -2.58eV, the Pd-Ni(OH)2 exhibited a downshifted d-band center at -3.14eV. This downshift effectively weakens the binding strength of the carbonyl intermediate at the Pd site, as shown in Figure 9A.
|
Figure 9. Comparison of Pd and Pd-Ni(OH)2 Catalysts for EGOR. A: Surface valence band photoemission spectra of Pd and Pd-Ni(OH)2; B: CV curves of Pd-Ni(OH)2/NF, Pd/NF and Pd/C in 1 M KOH. Reproduced from Ref.[48] with permission from Wiley.
Furthermore, they conducted in-depth studies on the adsorption and activation of OH species through cyclic voltammetry (CV) tests. The experimental results revealed that Pd-Ni(OH)2 had a lower onset potential for OH adsorption/desorption peaks (0.4 vs. RHE) compared to Pd/NF (0.5 vs. RHE), while Pd/C demonstrated almost no peaks, as shown in Figure 9B. This suggests that the oxygenophilic nature of Ni(OH)2 assists in efficiently oxidizing OH- on the Pd surface to form *OH adsorbed species. By combining the downshift of the d-band center of the Pd and the enhanced oxyphilicity of Ni, the interfacial interactions synergistically facilitate the rapid desorption and transfer of GA from the active Pd site to the inactive Ni site, thereby preventing its over-oxidation.
Comparing different preparation processes for glycolic acid, the electrochemical method utilizing EG derived from PET plastic exhibits several advantages. These include a high yield of glycolic acid, low reaction voltage, and low precious metal mass loading. These advantages contribute to the economic viability of electrochemical reforming of PET waste plastics for glycolic acid production.
4 CONCLUSIONS AND PROSPECTS
With the escalating accumulation and environmental impact of plastic waste, research on plastic degradation and recycling has gained significant importance. In the context of electrochemical reforming and upgrading of PET waste, modification of catalyst surface interfaces plays a crucial role. The catalyst surface interface, as the region where gas, solid, and liquid phases interact, directly influences catalytic activity and reaction selectivity. This review discusses the fundamentals of PET depolymerization including the structure of PET, the decomposition mechanism as well as the decomposition path and products of the PET. In addition, the research progress of the catalysts’ interface engineering for electrocatalytic upcycling of PET-derived EG has been discussed detailedly from the perspectives of the type of electrocatalytic products, including C1 and C2 products. The aim is to inspire further research ideas to tackle the urgent challenges of the ecological environment crisis and the transformation and upgrading of PET plastic waste. Additionally, the review also analyzes and provides prospects for the problems and solutions in this field.
(i) Catalyst design and optimization remain an important area for future exploration. Researchers can continue to investigate novel catalysts for the electrocatalytic PET-derived ethylene glycol process, utilizing advanced computational methods and experimental techniques to study the active centers, surface structures, and lattice defects of catalysts, thereby enhancing their activity and selectivity.
(ii) Interface engineering and regulation of electrocatalytic performance are crucial factors in electrocatalytic processes. Future studies can focus on exploring the effects of different interface materials and structures on electrocatalytic performance. Suitable methods can be developed to regulate electron transport, proton transport, and mass transfer at the interface, thereby improving the yield and purity of ethylene glycol.
(iii) Application development for electrocatalytic PET-derived ethylene glycol holds substantial potential in areas such as the synthesis of high-value-added compounds and energy conversion. Future research can integrate electrocatalytic PET-derived ethylene glycol technology with other related fields, such as combining it with renewable energy sources like solar or wind power to drive the process using green energy. This integration can lead to the development of additional applications and products, promoting sustainable development and a circular economy.
(iv) Electrocatalysis holds great promise as an alternative technology to conventional thermal catalysis or traditional water electrolysis for the oxidation of alcohols into carbonyl compounds. Nevertheless, current research in this field still faces certain limitations. Firstly, there is a lack of comprehensive investigation into the relationships between working electrode materials, alcohol substrates, and electrogenerating intermediates. This knowledge gap hampers our understanding of reaction mechanisms and catalytic activities. Secondly, in electrocatalytic reactions, electrodes often struggle to differentiate between various reactant molecules. This can result in excessive oxidation/reduction of products and redox reactions of solvent molecules, thereby compromising reaction selectivity and product purity. To overcome these challenges associated with single-electron reactions, a viable approach is to combine photocatalysis with electrocatalysis, forming a synergistic photocatalytic system. By leveraging the potential of photocatalytic technology, it becomes possible to achieve higher reaction selectivity and activity in both homogeneous and heterogeneous catalytic reactions, thus opening up new prospects in this field.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 52002294) and Knowledge Innovation Program of Wuhan-Shuguang Project (No. 2022010801020364).
Conflicts of Interest
The authors declared no conflict of interest.
Data Availability
No additional data are available.
Copyright Permissions
Copyright © 2024 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Author Contribution
Liu J reviewed the literature and wrote the original manuscript. Peng X provided ideas and revised the final manuscript.
Abbreviation List
C1 product, Formic acid
C2 product, Glycolic acid
CV, Cyclic voltammetry
EG, Ethylene glycol
EGOR, Oxidation reaction of ethylene glycol
GA, Glycolic acid
HR-TEM, High-resolution transmission electron microscopy
LSV, Linear sweep voltammetry
OER, Oxygen evolution reaction
PP, Polypropylene
RHE, Reversible hydrogen electrode
TPA/PTA, Terephthalic acid
PET/PETE, Polyethylene terephthalate
References
[1] Zhang X, Song X, Lu J et al. How financial development and digital trade affect ecological sustainability: The role of renewable energy using an advanced panel in G-7 Countries. Renew Energ, 2022; 199: 1005-1015.[DOI]
[2] Kwon D, Jung S, Moon DH et al. Strategic management of harmful chemicals produced from pyrolysis of plastic cup waste using CO2 as a reaction medium. Chem Eng J, 2022; 437: 135524.[DOI]
[3] Arsalan M, Awais A, Chen T et al. Development of PANI/BN-based absorbents for water remediation. Water Qual Res J, 2019; 54: 290-298.[DOI]
[4] Okoffo ED, Ribeiro F, O'Brien JW et al. Identification and quantification of selected plastics in biosolids by pressurized liquid extraction combined with double-shot pyrolysis gas chromatography-mass spectrometry. Sci Total Environ, 2020; 715: 136924.[DOI]
[5] Liu Z, Liu X, Bai Y et al. Spatiotemporal distribution and potential sources of atmospheric microplastic deposition in a semiarid urban environment of Northwest China. Environ Sci Pollut R, 2023; 30: 74372-74385.[DOI]
[6] Enyoh CE, Wang Q, Ovuoraye PE et al. Toxicity evaluation of microplastics to aquatic organisms through molecular simulations and fractional factorial designs. Chemosphere, 2022; 308: 136342.[DOI]
[7] Wu S, Montalvo L. Repurposing waste plastics into cleaner asphalt pavement materials: A critical literature review. J Clean Prod, 2021; 280: 124355.[DOI]
[8] Ali M F, Siddiqui M N. Thermal and catalytic decomposition behavior of PVC mixed plastic waste with petroleum residue. J Anal Appl Pyrolysis, 2005; 74: 282-289.[DOI]
[9] Shen M, Song B, Zeng G et al. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ Pollut, 2020; 263: 114469.[DOI]
[10] Hameed M, Bhat RA, Singh DV et al. White pollution: A hazard to environment and sustainable approach to its management. Innovative Waste Management Technologies for Sustainable Development. IGI Global, 2020: 52-81.[DOI]
[11] Shafi M, Lodh A, Khajuria M et al. Are we underestimating stormwater? Stormwater as a significant source of microplastics in surface waters. J Hazard Mater, 2024; 465: 133445.[DOI]
[12] Allouzi MMA, Tang DYY, Chew KW et al. Micro (nano) plastic pollution: The ecological influence on soil-plant system and human health. Sci Total Environ, 2021; 788: 147815.[DOI]
[13] Fu Z, Zhang YS, Ji G et al. The interactions between mixed waste from discarded surgical masks and face shields during the degradation in supercritical water. J Hazard Mater, 2023; 459: 132338.[DOI]
[14] Woźniak-Budych M, Staszak K, Wieszczycka K et al. Microplastic label in microencapsulation field-consequence of shell material selection. J Hazard Mater, 2024; 465: 133000.[DOI]
[15] Jin Y, Qiu J, Zhang L et al. Biodegradation of polyethylene terephthalate: A review. Chinese J Biotechnol, 2023; 39: 4445-4462.
[16] Lerna M, Foti D, Petrella A et al. Effect of the chemical and mechanical recycling of PET on the thermal and mechanical response of mortars and premixed screeds. Materials, 2023; 16: 3155.[DOI]
[17] Zhang Q, Zhao J, Wang G et al. Mechanically strong and heat-resistant waste poly (ethylene terephthalate) derived by carbon dioxide treatment at ambient temperature. J Mater Res Technol, 2023; 25: 3298-3313.[DOI]
[18] Lalhmangaihzuala S, Laldinpuii ZT, Khiangte V et al. Orange peel ash coated Fe3O4 nanoparticles as a magnetically retrievable catalyst for glycolysis and methanolysis of PET waste. Adv Powder Technol, 2023; 34: 104076.[DOI]
[19] Kim HT, Kim JK, Cha HG et al. Biological valorization of poly(ethylene terephthalate) monomers for upcycling waste PET. ACS Sustain Chem Eng, 2019; 7: 19396-19406.[DOI]
[20] Shen M, Huang W, Chen M et al. (Micro)plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J Clean Prod, 2020; 254: 120138.[DOI]
[21] Yazdani A, Botte GG. Perspectives of electrocatalysis in the chemical industry: a platform for energy storage. Curr Opin Chem Eng, 2020; 29: 89-95.[DOI]
[22] Arsalan M, Ewis D, Mahmud N et al. Enhanced electrochemical conversion of CO2 into formic acid using PbSO4 electrode: Catalyst synthesis and process optimization. J Environ Chem Eng, 2023; 11: 111352.[DOI]
[23] Awais A, Arsalan M, Sheng Q et al. Rational design of highly efficient one-pot synthesis of ternary PtNiCo/FTO nanocatalyst for hydroquinone and catechol sensing. Electroanalysis, 2021; 33: 170-180.[DOI]
[24] Arsalan M, Saddique I, Baoji M et al. Novel synthesis of sensitive cu-zno nanorod–based sensor for hydrogen peroxide sensing. Front Chem, 2022; 10: 932985.[DOI]
[25] Miao B, Arsalan M, BaQais A et al. Highly efficient tetrametallic PtNiCuCo alloy nanoparticles for sensitive detection of hydrogen peroxide. Adv Compos Hybrid Ma, 2023; 6: 110.[DOI]
[26] Awais A, Arsalan M, Sheng Q et al. A non-enzymatic hydrogen peroxide sensor with enhanced sensitivity based on pt nanoparticles. Anal Sci, 2021; 37: 1419-1426.[DOI]
[27] Cao F, Wang L, Zheng R et al. Research and progress of chemical depolymerization of waste PET and high-value application of its depolymerization products. RSC Adv, 2022; 12: 31564-31576.[DOI]
[28] Sheer A, Fahad Sardar M, Younas F et al. Trends and social aspects in the management and conversion of agricultural residues into valuable resources: A comprehensive approach to counter environmental degradation, food security, and climate change. Bioresour Technol, 2024; 394: 130258.[DOI]
[29] Singh B, Indra A. Surface and interface engineering in transition metal–based catalysts for electrochemical water oxidation. Mater Today Chem, 2020; 16: 100239.[DOI]
[30] Oreski G, Ottersböck B, Barretta C et al. Degradation of PET-quantitative estimation of changes in molar mass using mechanical and thermal characterization methods. Polym Test, 2023; 125: 108130.[DOI]
[31] Tan Z, Liu S, Cui X et al. Application of macromolecular chain extender and contribution to the toughening of poly(ethylene terephthalate). J Thermoplast Compos Mater, 2014; 29: 833-849.[DOI]
[32] Cornwall W. The plastic eaters. Science, 2021; 373: 36-39.[DOI]
[33] Abedsoltan H. A focused review on recycling and hydrolysis techniques of polyethylene terephthalate. Polym Eng Sci, 2023; 63: 2651-2674.[DOI]
[34] Yang W, Liu R, Li C et al. Hydrolysis of waste polyethylene terephthalate catalyzed by easily recyclable terephthalic acid. Waste Manage, 2021; 135: 267-274.[DOI]
[35] Bäckström E, Odelius K, Hakkarainen M. Ultrafast microwave assisted recycling of PET to a family of functional precursors and materials. Eur Polym J, 2021; 151: 110441.[DOI]
[36] Liu J, Yin J. Carbon dioxide synergistic enhancement of supercritical methanol on PET depolymerization for chemical recovery. Ind Eng Chem Res, 2022; 61: 6813-6819.[DOI]
[37] Li M, Chen W, Chen W et al. Degradation of Poly(ethylene terephthalate) Using metal-free 1,8-diazabicyclo[5.4.0]undec-7-ene-based deep eutectic solvents as efficient catalysts. Ind Eng Chem Res, 2023; 62: 10040-10050.[DOI]
[38] Rosmaninho M G, Jardim E, Moura F C C et al. Surface hydrolysis of postconsumer polyethylene terephthalate to produce adsorbents for cationic contaminants. J Appl Polym Sci, 2006; 102: 5284-5291.[DOI]
[39] Barnard E, Rubio Arias J J, Thielemans W. Chemolytic depolymerisation of PET: A review. Green Chem, 2021; 23: 3765-3789.[DOI]
[40] Rahimi A, García J M. Chemical recycling of waste plastics for new materials production. Nat Rev Chem, 2017; 1: 0046.[DOI]
[41] Corak I, Tarbuk A, Dordevic D et al. Sustainable alkaline hydrolysis of polyester fabric at low temperature. Materials, 2022; 15: 1530[DOI]
[42] Du M, Zhang Y, Kang S et al. Electrochemical production of glycolate fuelled by polyethylene terephthalate plastics with improved techno-economics. Small, 2023; 19: e2303693.[DOI]
[43] Wang J, Li X, Zhang T et al. Electro-reforming polyethylene terephthalate plastic to Co-produce valued chemicals and green hydrogen. J Phys Chem Lett, 2022; 13: 622-627.[DOI]
[44] Chen J, Zhang L, Wang L et al. Toward carbon neutrality: Selective conversion of waste plastics into value-added chemicals. Matter, 2023; 6: 3322-3347.[DOI]
[45] Fu X, Wan C, Huang Y et al. Noble metal based electrocatalysts for alcohol oxidation reactions in alkaline media. Adv Funct Mater, 2022; 32: 2106401.[DOI]
[46] Gao F, Zhang Y, You H et al. Solvent-mediated shell dimension reconstruction of core@Shell PdAu@Pd nanocrystals for robust c1 and c2 alcohol electrocatalysis. Small, 2021; 17: e2101428.[DOI]
[47] Wang J, Li X, Wang M et al. Electrocatalytic valorization of poly(ethylene terephthalate) plastic and CO2 for simultaneous production of formic acid. ACS Catal, 2022; 12: 6722-6728.[DOI]
[48] Liu F, Gao X, Shi R et al. Concerted and selective electrooxidation of polyethylene-terephthalate-derived alcohol to glycolic acid at an industry-level current density over a Pd-Ni(OH)2 catalyst. Angew Chem Int Ed, 2023; 62: e202300094.[DOI]
[49] Erbil H Y. The debate on the dependence of apparent contact angles on drop contact area or three-phase contact line: A review. Surf Sci Rep, 2014; 69: 325-365.[DOI]
[50] Chen P, Xu K, Tao S et al. Phase-transformation engineering in cobalt diselenide realizing enhanced catalytic activity for hydrogen evolution in an alkaline medium. Adv Mater, 2016; 28: 7527-7532.[DOI]
[51] Majee R, Parvin S, Arif Islam Q et al. The perfect imperfections in electrocatalysts. Chem Rec, 2022; 22: e202200070.[DOI]
[52] Gao W, Hood ZD, Chi M. Interfaces in heterogeneous catalysts: advancing mechanistic understanding through atomic-scale measurements. Acc Chem Res, 2017; 50: 787-795.[DOI]
[53] Startsev A N. The crucial role of catalysts in the reaction of low temperature decomposition of hydrogen sulfide: Non-equilibrium thermodynamics of the irreversible process in an open system. Mol Catal, 2020; 497: 111240.[DOI]
[54] Zhang Y, Liu H, Ge R et al. Mo-induced in-situ architecture of NixCoyP/Co2P heterostructure nano-networks on nickel foam as bifunctional electrocatalysts for overall water splitting. Sustain Mater Techno, 2022; 33: e00461.[DOI]
[55] Turner J, Sverdrup G, Mann M K et al. Renewable hydrogen production. Int J Energy Res, 2008; 32: 379-407.[DOI]
[56] Sun K, Wu X, Zhuang Z et al. Interfacial water engineering boosts neutral water reduction. Nat Commun, 2022; 13: 6260.[DOI]
[57] Bharadwaj N, Das S, Pathak B. Role of morphology of platinum-based nanoclusters in ORR/OER activity for nonaqueous li-air battery applications. ACS Appl Energy Mater, 2022; 5: 12561-12570.[DOI]
[58] Zhang Y, Zhi X, Harmer J R et al. Facet-specific active surface regulation of BixMOy (M=Mo, V, W) nanosheets for boosted photocatalytic CO2 reduction. Angew Chem Int Ed, 2022; 61: e202212355.[DOI]
[59] Wang S, Jiang Q, Ju S et al. Identifying the geometric catalytic active sites of crystalline cobalt oxyhydroxides for oxygen evolution reaction. Nat Commun, 2022; 13: 6650.[DOI]
[60] Yuan W, Fang K, You R et al. Toward in situ atomistic design of catalytic active sites via controlled atmosphere transmission electron microscopy. Acc Mater Res, 2023; 4: 275-286.[DOI]
[61] Zhao R, Li Q, Jiang X et al. Interface engineering in transition metal-based heterostructures for oxygen electrocatalysis. Mater Chem Front, 2021; 5: 1033-1059.[DOI]
[62] Janani G, Surendran S, Lee DK et al. Aggregation induced edge sites actuation of 3D MoSe2/rGO electrocatalyst for high-performing water splitting system. Aggregate, 2023.[DOI]
[63] Zhou H, Ren Y, Li Z et al. Electrocatalytic upcycling of polyethylene terephthalate to commodity chemicals and H2 fuel. Nat Commun, 2021; 12: 4679.[DOI]
Brief of Corresponding Author(s)
Xiang Peng He is a professor and PhD supervisor of Materials Science and Engineering at the Wuhan Institute of Technology (WIT). He received his PhD in Physics and Materials Science from the City University of Hong Kong in 2017, where he laid the foundation for his future contributions to the field. After completing his doctoral studies, he continued to expand his expertise as a postdoctoral fellow at the City University of Hong Kong from 2017 to 2018. His research focuses on the design of functional nanomaterials and their application in energy storage and conversion. He has authored over 80 peer-reviewed papers, which have garnered significant recognition within the scientific community, accumulating more than 6200 citations with an h index of 45. He currently serves as the associate editor of Innovation Discovery as well as guest editor-in-chief and editorial board member of approximately 10 journals. |







 Copyright ©
Copyright ©