Effect of Caffeine in the Diffusion Behaviour of Analgesic Drugs in Aqueous Solutions
Filipa R.L. Fernandes1, Simão F.R.G. Soares2, Ana C.F. Ribeiro3*![]()
1Instituto Superior Técnico, Lisboa, Portugal
2Instituto Superior Miguel Torga, Coimbra, Portugal
3Institute of Molecular Sciences, Department of Chemistry, University of Coimbra, Coimbra, Portugal
*Correspondence to: Ana C.F. Ribeiro, PhD, Professor, Institute of Molecular Sciences, Department of Chemistry, University of Coimbra, Coimbra, 3004-535, Portugal. Email: anacfrib@ci.uc.pt
DOI: 10.53964/id.2024006
Abstract
The aim of the study was to examine the effect of caffeine on the diffusion of two pain-relieving drugs, namely paracetamol and sodium salicylate, in aqueous solutions at a temperature of 25.00ºC, using the Taylor dispersion method to determine the mutual diffusion coefficients for these ternary aqueous solutions. Ternary mutual diffusion coefficients are reported for aqueous solutions of paracetamol + caffeine, and sodium salicylate + caffeine, at 25.00ºC, and concentrations up to 0.050mol·dm-3. The data indicate that the presence of caffeine affects the diffusion of analgesic drugs. For example, a coupled diffusion of these drugs with caffeine was observed through the non-zero values of the cross-diffusion coefficients. Support for this came from the good agreement between our data and the predicted by a model of the diffusion that includes 1:1 complex, being obtained the association constants for systems [(caffeine and paracetamol) and (caffeine and salicylate sodium)], K=70mol-1·dm3 and K=80, respectively. These data provide us with a better understanding of the mechanisms of mass transport through diffusion in biological systems, as well as the structure of these systems.
Keywords: caffeine, diffusion, electrolytes, paracetamol, sodium salicylate
1 INTRODUCTION
Caffeine (Caff), also known as 1,3,7-trimethylxanthine, is a stimulant drug acting on the central nervous system having various pharmaceutical applications (e.g., it is used for a variety of purposes, including certain respiratory conditions of the premature newborn, pain relief, and to combat drowsiness[1,2]). It can be found in many beverages, including coffee, tea, and soft drinks[3,4]. Although there are advantages, harmful pharmacological effects on human health have been reported, including prolonged insomnia, elevated serum cholesterol, and peptic ulcers[2]. In addition, excessive consumption of caffeine can lead to adverse reactions[5]. For example, elevated serum cholesterol, and peptic ulcers[2] it can interfere with the effectiveness of certain medications by altering the metabolic pathways of those medications[5]. Furthermore, the ingestion of caffeine on a long-term basis has been linked to several clinical conditions such as osteoporosis, cardiovascular disease, and reproductive disorders[6].
Some studies, however, suggest that consuming caffeine can have several health benefits for humans, such as improving neuromuscular coordination, stimulating the central nervous system and cardiac muscle, and enhancing cognitive functioning. For instance, Renner et al.[7] and Weiser et al.[8] have demonstrated that caffeine can enhance the analgesic effect of certain analgesic drugs like sodium salicylate (NaSal). This means caffeine can act as an adjuvant analgesic, potentially boosting the effectiveness of these drugs. According to Goldstein et al.[9], caffeine can enhance the pain-relieving properties of these drugs and improve their effectiveness. The combination of aspirin, caffeine, and paracetamol (HPA) is believed to have a potent analgesic effect when compared to taking these drugs separately.
Several studies have examined the spectroscopic[10-13] and computational[14,15], transport[16-18], ultrasonic[19] and thermodynamic behaviour of individual drugs (including analgesics)[20-23], as well as their behaviour in combination with caffeine or other components (e.g., Ref.[24-29]). However, to the best of our knowledge, no data exists on the ternary mutual diffusion coefficients of analgesic drugs and caffeine in aqueous solutions. This study aims to fill this gap by providing experimental data on the diffusion coefficients measured by the Taylor dispersion method for two ternary systems (HPA/Caff/H2O and Sodium Salicylate/Caffeine/H2O) at carrier concentrations between 0.000mol·dm-3 and 0.010mol·dm-3 at 25.00ºC. The selection of these analgesics (Figure 1) is justified, having in mind that they are commonly used as first-line drugs for managing acute pain[30-33] and are included in the World Health Organization's (WHO) list of essential medicines[34].
|
Figure 1. Structures of Drugs. A: Caffeine[16]; B: Paracetamol[36]; C: Sodium salicylate (NaSal)[15].
2 MATERIALS AND METHODS
2.1 Materials
Table 1 shows all reagents used as received in the present work without further purification. Nevertheless, they were stored under low pressure in a desiccator over silica gel. All solutions were freshly prepared at 25.00ºC before each experiment.
Table 1. Reagents Description
Chemical Name |
Source |
CAS Number |
Mass Fraction Puritya |
Caff |
Sigma-Aldrich |
58-08-2 |
>0.985 |
HPA |
Sigma-Aldrich |
103–90-2 |
>0.99 |
NaSal |
Panreac |
54-21-7 |
>0.99 |
H2O |
Millipore-Q water (ρ = 1.82×105 Ω m at 25.0ºC) |
|
|
Notes: aThe mass fraction purity is on water-free basis; this information is provided by the suppliers.
2.2 Taylor Dispersion Technique
Taylor dispersion technique, used for measuring diffusion coefficients, is well described in the literature[37-41], and hence, only we summarize some relevant points regarding the equipment and the method. In this method, a 6-port Teflon injection valve (Rheodyne, model 5020) is used to generate dispersion profiles by injecting 0.063mL of solution into a laminar carrier stream of slightly different composition at the entrance to a Teflon capillary dispersion tube of length 3048.0±0.1cm, and internal radius 0.03220±0.00003cm. The radius of the tube was determined by weighing it when empty and when filled with distilled water of known density. The length of the tube was measured directly by stretching it out lengthwise in a large hall, using two high-quality theodolites and appropriate mirrors to focus the tube ends accurately. This tube and the injection valve were kept at 25.00±0.01℃ in an air thermostat. A Waters model 2,410 differential refractometer monitored the broadened distribution of the injected samples at the tube outlet. The refractometer output voltages, V(t), were measured at 5s intervals by a digital voltmeter (Agilent 34401A).
The dispersion profiles of ternary solutions containing HPA (1) or NaSal and Caff (2) were analysed by fitting equation (Equation 1) to pairs of dispersion peaks. These were created by injecting an excess of HPA (or Nasal) or an excess of caffeine into each carrier solution.
$$ V\left(t\right)={V}_{0}+{V}_{1}+{V}_{max}{({t}_{R}/t)}^{1/2}$$
$$ \left[{W}_{1}exp\left(-\frac{12{D}_{1}{\left(t-{t}_{R}\right)}^{2}}{{r}^{2}t}\right)+(1-{W}_{1})exp\left(-\frac{12{D}_{2}{(t-{t}_{R})}^{2}}{{r}^{2}}\right)\right]\left(1\right)$$
Equation 1 contains the eigenvalues of the matrix of ternary Dik coefficients as represented by D1 and D2. The equation also incorporates three other variables, which are baseline voltage V0, baseline slope V1, and peak high Vmax. Moreover, the equation includes two normalized pre-exponential factors, W1 and (1–W1).
The diffusion of mixed aqueous solutions containing HPA (or NaSal) and caffeine (Caff) can be described through the following ternary diffusion equations (Equations 2 and 3).
$$ {J}_{1}={D}_{11}{}_{{C}_{1}}{D}_{12}{}_{{C}_{2}}\left(2\right)$$
$$ {J}_{2}={-D}_{21}{}_{{C}_{1}}{D}_{22}{}_{{C}_{2}}\left(3\right)$$
J1 and J2 are the molar fluxes of HPA (or Nasal) and Caff driven by the concentration gradients c1 and c2 of each solute 1 and 2, respectively. Main diffusion coefficients D11 and D22 give the flux of each solute driven by its own concentration gradient. Cross-diffusion coefficients D12 and D21 give the coupled flux of each solute driven by a concentration gradient in the other solute. A negative Dik coefficient indicates counter-current coupled transport of solute i from regions of lower to higher concentration of solute k. A positive Dik cross-coefficient (i ≠ k) indicates co-current coupled transport of solute i from regions of higher to lower concentrations of solute k.
3 RESULTS
Tables 2 and 3 present the average Dik coefficients determined for each carrier-solution composition by fitting Equation 1 to five or six replicate pairs of dispersion profiles for two ternary aqueous systems, [HPA (1) plus Caff (2)] and [NaSal (1) plus Caff (2)]. Main diffusion coefficients D11 and D22 were generally reproducible within (±0.010×10-9m2·s-1). The cross-diffusion coefficients D12 and D21, which describe the coupled diffusion of HPA (or NaSal) and caffeine, were reproducible within (±0.020×10-9m2·s-1).
Table 2. Ternary Diffusion Coefficients (D11, D12, D21, D22) of Aqueous Paracetamol (HPA, C1) Caffeine (Caff, C2) Solutions (Predicted Dik Values in Parentheses)a
C1b |
C2b |
X1c |
D11±SDd |
D12±SDd |
D21±SDd |
D22±SDd |
HPA(C1) Caff (C2) solutions |
||||||
0.000 |
0.010 |
0.000 |
0.600±0.010 |
0.009±0.001 |
0.055±0.015 |
0.705±0.014 |
|
|
|
(0.615) |
(0) |
(0.093) |
(0.760) |
0.002 |
0.008 |
0.200 |
0.605±0.009 |
0.029±0.001 |
0.058±0.021 |
0.700±0.010 |
|
|
|
(0.628) |
(0.006) |
(0.069) |
(0.748) |
0.005 |
0.005 |
0.500 |
0.610±0.015 |
0.050±0.010 |
0.060±0.025 |
0.697±0.010 |
|
|
|
(0.656) |
(0.025) |
(0.016) |
(0.711) |
0.008 |
0.002 |
0.800 |
0.620±0.011 |
0.080±0.010 |
0.030±0.010 |
0.693±0.010 |
|
|
|
(0.651) |
(0.013) |
(0.023) |
(0.691) |
0.050 |
0.000 |
1.000 |
0.625±0.008 |
0.170±0.008 |
0.001±0.004 |
0.690±0.007 |
|
|
|
(0.664) |
(0.084) |
(0) |
(0.600) |
Notes: aPredicted values by using k=70mol·dm-3[18]. bCi in units of mol·dm-3. cX1 the solute fraction of HPA or NaSal. dDij ±SD in units 10-9m2·s-1 is the mean diffusion coefficient from 5 to 6 replicate measurements and SD is the standard deviation from the mean.
Table 3. Ternary Diffusion Coefficients (D11, D12, D21, D22) of aqueous Sodium Salicylate (NaSal, C1) Caffeine (Caff, C2) Solutions (predicted Dik values in parentheses)a
C1b |
C2b |
X1c |
D11±SDd |
D12±SDd |
D21±SDd |
D22±SDd |
NaSal(C1) Caff(C2) solutions |
||||||
0.000 |
0.010 |
0.000 |
0.918±0.010 |
0.005±0.008 |
0.050±0.014 |
0.704±0.009 |
|
|
|
(0.913) |
(0) |
(0.031) |
(0.747) |
0.002 |
0.008 |
0.200 |
0.940±0.020 |
0.006±0.030 |
0.015±0.010 |
0.701±0.005 |
|
|
|
(0.959) |
(0.020) |
(0.023) |
(0.756) |
0.005 |
0.005 |
0.500 |
0.975±0.010 |
0.005±0.008 |
0.030±0.014 |
0.704±0.009 |
|
|
|
(1.015) |
(0.070) |
(0.013) |
(0.747) |
0.008 |
0.002 |
0.800 |
0.989±0.011 |
0.085±0.018 |
0.010±0.014 |
0.700±0.009 |
|
|
|
(1.062) |
(0.126) |
(0.004) |
(0.737) |
0.010 |
0.000 |
1.000 |
0.995±0.007 |
0.100±0.014 |
0.009±0.010 |
0.699±0.011 |
|
|
|
(1.086) |
(0.163) |
(0) |
(0.731) |
Notes: aPredicted values by using k=80mol·dm-3[18]. bCi in units of mol·dm-3. cX1 the solute fraction of HPA or NaSal. dDij±SD in units 10-9m2·s-1 is the mean diffusion coefficient from 5 to 6 replicate measurements and SD is the standard deviation from the mean.
By looking these tables, it can be seen that at the limiting situations of X1=0 and X1=1 (where X1 represents the solute fraction of HPA or NaSal), the values of D11 correspond, respectively, to the tracer diffusion coefficient of paracetamol (or sodium salicylate) in caffeine and, the binary mutual diffusion coefficient of aqueous paracetamol (or sodium salicylate) at 0.050 and 0.010mol·dm-3, respectively. A good agreement is observed between these last values for D11 obtained for HPA and NaSal (D11 = 0.625×10-9m2·s-1 and D11 = 0.995×10-9m2·s-1), and the respectively binary diffusion coefficient values, previously obtained in other studies[15,17] (i.e., D(HPA)=0.6×10-9m2·s-1 and D(NaSal)=1.02×10-9m2·s-1[15]. The deviations less than 2.5% are acceptable and are within the uncertainties of the method (in general, <3%).
In the limit as X1 approaches zero, cross-coefficient values D12 are zero within experimental error, due to the inability of caffeine concentration gradients to drive coupled flows of NaSal in NaSal-free solutions (or HPA in HPA-free solutions). Similarly, at the other composition extreme, X1 → 1, the cross-coefficient values D21 are also close to zero. These values are not surprising since HPA or NaSal concentration gradients cannot drive coupled fluxes of caffeine in caffeine-free solutions.
At X2=0, the D22 values represent the tracer diffusion coefficients of caffeine in paracetamol and sodium salicylate, which are 0.690×10-9m2·s-1 and 0.699×10-9m2·s-1, respectively. On the other hand, when X2=1, the D22 values (0.705×10-9m2·s-1 and 0.704×10-9m2·s-1) are near the binary mutual diffusion coefficient of aqueous caffeine at 0.01mol·dm-3 (DCaff =0.703×10-9m2·s-1), as awaited.
4 DISCUSSION
Based on Tables 2 and 3, it is evident that the cross-diffusion coefficients D12 and D21 generally take negative values, but are lower for D12. In this scenario, D12<0 indicates that the concentration gradient of caffeine leads to counter-current coupled flows of paracetamol and NaSal. These observations can readily be interpreted by considering the binding interactions between these drugs and caffeine molecules, leading to formation of aggregates (e.g., complexes 1:1) in solution. This phenomenon leads to a decrease in free paracetamol or NaSal molecules. This loss results in a counterflow of paracetamol or NaSal.
Experimental results and computational simulations have provided evidence for the formation of complexes between caffeine and sodium salicylate in solution[10]. These complexes are believed to form initially as parallel stacking aromatic (π-π) complexes between the aromatic rings of caffeine and salicylate anions. Subsequently, salicylate molecules are released from the complex with caffeine, leading to hydration of caffeine and formation of caffeine-caffeine complexes through further π-π interactions. The observed increase in caffeine solubility in water upon addition of NaSal is attributed to these complex formation phenomena.
Thus, assuming the formation of 1:1 complexed species between paracetamol (or sodium salicylate) and caffeine (Equations 4 and 5), which details of this theoretical framework (including the respective limitations) are well described in the literature (e.g.,[17]), the binding constants equilibrium K (Equations 6 and 7) for these species have been determined to be 80 and 70 mol·dm-3, respectively,
$$ {H}{P}{A}\left({a}{q}\right)+{C}{a}{f}{f}\left({a}{q}\right){H}{P}{A}-{C}{a}{f}{f}\left({a}{q}\right)\left(4\right)$$
$$ {S}{a}{l}\left({a}{q}\right)+{C}{a}{f}{f}\left({a}{q}\right){S}{a}{l}-{C}{a}{f}{f}\left({a}{q}\right)\left(5\right)$$
$$ K=\frac{{C}_{\left(HPA-{C}{a}{f}{f}\right)}}{{C}_{HPA\times }{C}_{{C}{a}{f}{f}}}c({K}=80)\left(6\right)$$
$$ K=\frac{{C}_{\left(Sal-{C}{a}{f}{f}\right)}}{{C}_{{Sal}^{\times }}{C}_{{C}{a}{f}{f}}}({K}=70)\left(7\right)$$
where: HPA-Caff: the 1:1 complexed species between paracetamol and caffeine molecules;
Sal-Caff: the 1:1 complexed species between salicylate anion and caffeine molecules.
Table 4 shows the estimated values for the limiting diffusion coefficients of the free and complexed species, as well as the radii of the respective species.
Table 4. Limiting Diffusion Coefficients, Ds, of Species s at 25.00ºC
Species |
Ds/(10-9 m2·s-1) |
Radius/nm (RH) |
Caff |
0.760[16] |
0.323 |
HPA |
0.664[17] |
0.369 |
NaSal |
1.086[15] |
0.226 |
Paracetamol/Caffeine |
0.560a[18] |
0.440 |
Sal/Caffeine |
0.689b[18] |
0.356 |
Notes: aD = (DParacetamol-3 + DCaffeine-3)-1/3. bD = (DSal-3 + DCaffeine-3)-1/3.
The estimation of radii can be done using the Stokes-Einstein approximation[18], assuming the solvent behaves like a continuous medium. According to this relation, Ds is inversely proportional to the hydrodynamic radius of species s, and, therefore, inversely proportional to the cube root of the species’ hydrodynamic volume. Considering that the volume of complexed species [(HPA/Caff) or (NaSal/Caff)] is the sum of the volumes of the paracetamol molecules (or Sal ions) and caffeine molecules isolated, the diffusion coefficient of these complexes were estimated from the known values of DHPA (or DSal) using (DHPA-3 + DCaff-3)-1/3.
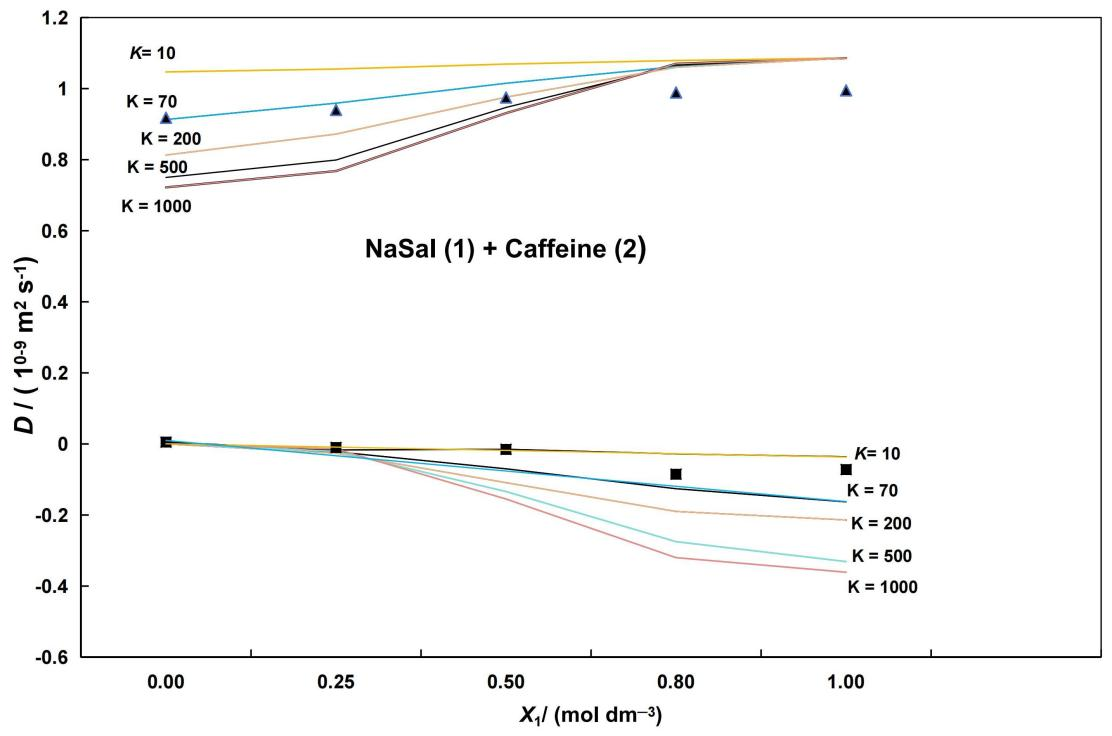
As shown in Tables 2 and 3, and in Figure 2, despite the limitations of this theoretical framework (e.g., only valid in dilute solutions[18]), a good agreement between these predicted values and the measured ones for equilibrium constants values 70mol-1·dm3 and 80mol-1·dm3 is found (e.g., for the cross diffusion D12, the deviations are generally ≤4%).
|
Figure 2. Ternary Mutual Diffusion Coefficients of Aqueous NaSal (1) + Caff (2) Solutions Plotted Against the Solute Fraction of NaSal, X1, and 25.00℃. Dij values predicted by a model indicated in[18] by for different values of K (Equation 7). Measured values: ▲ (D11); ■ (D12). The main diffusion coefficient, D11, represents the flux of HPA (or NaSal) driven by its concentration gradient. Meanwhile, the cross-diffusion coefficient, D12, indicates the coupled flux of each solute driven by a concentration gradient in the other solute.
However, as shown in Figure 2, there are significant differences between the predicted and experimental values for higher values of K (K≥200). That is, the predicted values of D11 and D12 are lower than the experimental values. Therefore, it can be concluded that the equilibrium constants mentioned above provide acceptable estimates for these parameters. These results suggest that the interaction between paracetamol (or NaSal) and caffeine is weak, but not negligible.
It is well-established that NaSal has the ability to act as a structure-maker[22], causing what is known as a salting-out effect. However, the explanation for the increase in caffeine solubility and the diffusion behaviour of these systems lies in the formation of aggregates due to potential π–π interactions between NaSal and caffeine. This phenomenon can be observed through the larger negative cross-diffusion values Dik. Support for these interactions as well as caffeine can act as an analgesic adjuvant and enhances the efficacy of paracetamol comes from the literature[10,24,42-44].
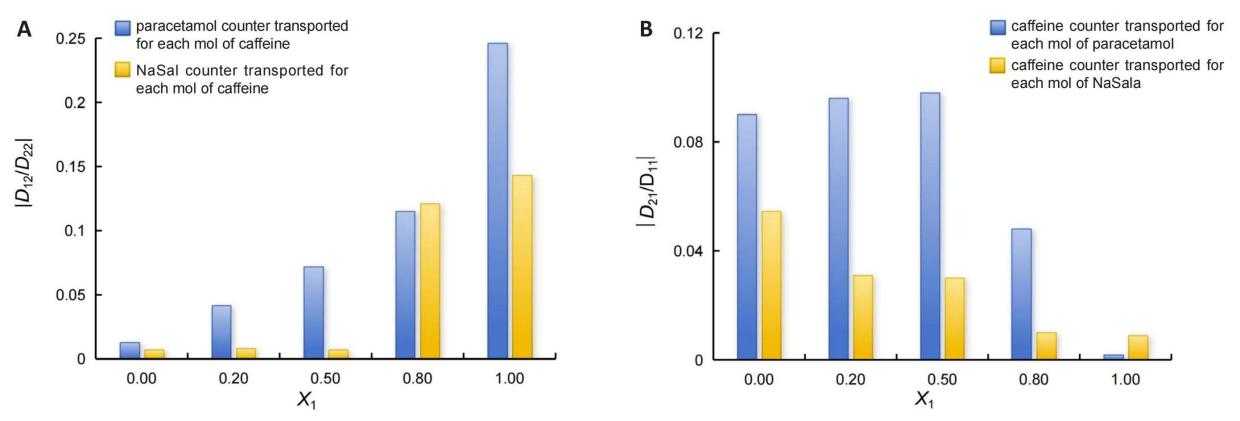
One can infer information about coupled diffusion from the calculated values of the ratio of D12/D22 (Figure 3A). To simplify the visualization of data, we are only considering the absolute values of these ratios. Upon analysis, it is observed that the HPA/Caff and NaSal/Caff systems show higher values for this relationship at X1 = 1. This indicates that during diffusion, one mole of caffeine can counter-transport up to 0.25 moles of HPA (or up to 0.14 moles of NaSal). Relative to the D21/D11 values (Figure 3B), it can be noticed these values are lower in comparison with the D12/D22 ones.
When considering the HPA/Caff system at the same concentrations, it can be expected that a mole of diffusing HPAcounter-transports at most 0.09mol of caffeine, whereas for the NaSal/Caff system, a mole of diffusing NaSal counter-transports at most only 0.05mol of caffeine. Since paracetamol is transported in the opposite direction of the caffeine concentration gradient, its drug concentration gradient will be higher in the target areas in the human body. This could explain why it may be more effective in treating pain. In fact, it is widely known in the scientific community that caffeine acts as an analgesic adjuvant, enhancing the efficacy of paracetamol[32].
|
Figure 3. Estimation of Moles of Drug Transported for Each mol of Caffeine for Different Values of Molar Fraction. A: X1. ![]() D12/D22 represents paracetamol counter transported for each mol of caffeine.
D12/D22 represents paracetamol counter transported for each mol of caffeine. ![]() D12/D22 represents NaSal counter transported for each mol of caffeine. B: X1.
D12/D22 represents NaSal counter transported for each mol of caffeine. B: X1. ![]() D21/D11 represents caffeine counter transported for each mol of paracetamol.
D21/D11 represents caffeine counter transported for each mol of paracetamol. ![]() D21/D11 represents caffeine counter transported for each mol of NaSala.
D21/D11 represents caffeine counter transported for each mol of NaSala.
5 CONCLUSION
Based on the measured ternary diffusion coefficients for the aqueous ternary systems studied, (that is, Paracetamol/Caffeine and Sodium Salicylate/Caffeine), it can be concluded that the presence of caffeine at low concentrations (C0.010mol·dm-3) affects the diffusion behavior of these drugs.
There is coupled diffusion of paracetamol (or NaSal) and caffeine, as indicated by negative cross-diffusion coefficients, D12 and D21, which suggests that there is interaction between the solutes. This behavior of these coefficients was interpreted on the basis of the formation of complexes between salicylate (or paracetamol) and caffeine molecules, whose K values obtained for these equilibria were 70mol·dm-3 and 80mol·dm-3, respectively.
Of course, further research is needed to identify all the species that contribute to the influence on this transport property, but it is out of the scope of the current study. In addition, it could be said that what is more important for some areas of interest (e.g., pharmaceutical applications) is the thermodynamic and transport behaviour of the involved species, not so much the complex question of the nature of their internal binding interactions. In this sense, it will be necessary to expand this work. For example, to evaluate the transport and thermodynamic behaviour of these drugs in different carriers, such as cyclodextrins and resorcinarenes, at different temperatures and chemical compositions. It is to learn how the properties related to both mobility (diffusion, conductivity, viscosity and transport number) and thermodynamics (optimum carrier concentration, drug solubility as a function of carrier concentration, binding constants, pH, temperature, and activity coefficients) can be used for the rational design of advanced drug-carrier systems. Characterizing these physical-chemical properties of systems containing analgesic drugs and caffeine will allow us to draw up guidelines for developing formulations for the safe and reliable delivery of these drugs.
Acknowledgements
This research was funded by the Coimbra Chemistry Centre, which is supported by the Fundação para a Ciência e a Tecnologia (FCT), Portuguese Agency for Scientific Research, through the projects UID/QUI/UI0313/2013 and COMPETE Programme (Operational Programme for Competitiveness).
Conflicts of Interest
The authors declare no conflict of interest.
Data Availability
All data generated or analyzed during this study are included in this published article.
Copyright Permissions
Copyright © 2024 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Author Contribution
Ribeiro A was responsible for concept, designing; Ribeiro A, Soares S and Fernandes F were responsible for data collection and processing, analysis, interpretation, literature search and writing. All authors contributed to the manuscript and approved the final version.
Abbreviation List
Caff, Caffeine
HPA, Paracetamol
NaSal, Sodium salicylate
Sal, Salicylate anion
References
[1] The United States Pharmacopeia. US Pharmacopeial Convention. Rockville: The National Formulary, 2009; 27; 2009; British Pharmacopoeia, 2016. London: The Stationary Office.
[2] George AJ. Central nervous system stimulants. Best Pract Res Clin Endocrinol Metab, 2000; 14: 79-88.[DOI]
[3] Murthy PS, Manonmani HK. Physico-chemical, antioxidant and antimicrobial properties of Indian monsooned coffee. Eur Food Res Technol, 2009; 229: 645-650.[DOI]
[4] Vuong QV, Roach PD. Caffeine in green tea: its removal and isolation. Sep Purif Rev, 2014; 43: 155-174.[DOI]
[5] Higdon JV, Frei B. Coffee and health: A review of recent human research. Crit Rev Food Sci Nutr, 2006; 46: 101-123.[DOI]
[6] Carrillo JA, Benitez J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin Pharmacokinet, 2000; 39: 127-153. [DOI]
[7] Renner B, Clarke G, Grattan T et al. Caffeine accelerates absorption and enhances the analgesic effect of acetaminophen. J Clin Pharmacol, 2007; 47: 715-726.[DOI]
[8] Weiser T, Weigmann H. Effect of caffeine on the bioavailability and pharmacokinetics of an acetylsalicylic acid-paracetamol combination: Results of a phase I study. Adv Ther, 2019; 36: 597-607.[DOI]
[9] Goldstein J, Silberstein SD, Saper JR et al. Acetaminophen, aspirin, and caffeine in combination versus ibuprofen for acute migraine: results from a multicenter, double-blind, randomized, parallel-group, single-dose, placebo-controlled study. Headache, 2006; 46: 444-453.[DOI]
[10] Baranovskii SF, Bolotin, PA. Association of riboflavin, caffeine, and sodium salicylate in aqueous solution. J Appl Spectrosc, 2007; 74: 211-218.[DOI]
[11] Singh DK, Sahu A. Spectrophotometric determination of caffeine and theophylline in pure alkaloids and its application in pharmaceutical formulations. Anal Biochem, 2006; 349: 176-180.[DOI]
[12] Riccardi C, Campanella A, Montesarchio D et al. Investigating the interaction of an anticancer Nucleolipidic Ru(III) complex with human serum proteins: A spectroscopic study. Molecules, 2023; 28: 2800.[DOI]
[13] Manea YK, Qashqoosh MTA, Rezakazemi M. In vitro hemoglobin binding and molecular docking of synthesized chitosan-based drug-carrying nanocomposite for ciprofloxacin-hcl drug delivery system. ACS Omega, 2024, 9: 6339-6354.[DOI]
[14] Constantinos D. Zeinalipour Yazdi, A DFT study of the interaction of aspirin, paracetamol and caffeine with one water molecule. J Mol Model, 2022; 28: 285.[DOI]
[15] Galindres DM, Espitia-Galindo N, Valente AJM et al. Interactions of sodium salicylate with β-cyclodextrin and an anionic resorcin[4]arene: Mutual diffusion coefficients and computational study. Int J Mol Sci, 2023; 24: 3921.[DOI]
[16] Ribeiro ACF, Lobo VMM, Santos CIAV et al. Diffusion of Caffeine in Different Aqueous Media at Physiological Temperature. In: Predy VR Ed. Caffeine: Chemistry, Analysis, Function and Effects. Royal Society of Chemistry Publishing, 2012; 6: 89-100.[DOI]
[17] Ribeiro ACF, Barros MCF, Verissimo LMP et al. Diffusion coefficients of paracetamol in aqueous solutions. J Chem Thermodyn, 2012; 54: 97-99.[DOI]
[18] Ribeiro ACF, Musilová L, Mrácek A et al. Host-guest paracetamol/cyclodextrin complex formation evaluated from coupled diffusion measurement. J Chem Thermodyn, 2021; 161: 106551.[DOI]
[19] Singh S, Talukdar M, Dash UN. Ultrasonic studies on paracetamol in aqueous solutions of sodium salicylate and nicotinamide. J Mol Liq, 2018; 249: 815-824.[DOI]
[20] Singh S, Talukdar M, Dash UN. Solute-solvent and solute-solute interactions of ibuprofen in aqueous and in aqueous solutions of urea, sodium salicylate and nicotinamide by volumetric and interferometric techniques. J Mol Liq, 2017; 241: 934-945.[DOI]
[21] Hosseinkhani H. Biomedical Engineering: Materials, Technology, and Applications, 1st edition, Wiley-VCH, 2022.[DOI]
[22] Vraneš M, Borovi TT, Drid P et al. Influence of sodium salicylate on self-aggregation and caffeine solubility in water-A new hypothesis from experimental and computational data. Pharmaceutics, 2022; 14: 2304.[DOI]
[23] Hosseinkhani H. Nanomaterials in Advanced Medicine, 1st edition, Wiley-VCH, 2019.[DOI]
[24] Dichi E, Sghaier M, Guiblin N. Pharmaceutical phase diagram: aspirin‑caffeine‑paracetamol. J Therm Anal Calorim, 2023; 148: 6107-6118.[DOI]
[25] Simões A, Veiga F. Vitorino C. Question-based review for pharmaceutical development: An enhanced quality approach. Eur J Pharm Biopharm, 2024; 195: 114174.[DOI]
[26] Banjare MK, Banjare BS. Study of the molecular interaction of a phosphonium-based ionic liquid within myo-inositol and non-steroidal anti-inflammatory drugs. RSC Adv, 2024.[DOI]
[27] Saha B, Barman S, Majumder S et al. Investigation of intermolecular interactions of l-Valine and l-Phenylalanine with muscle relaxant drug mephenesin molecule prevalent in aqueous solution by various physico-chemical methods at T=298.15K-313.15K. Heliyon, 2024, 10: e23562.[DOI]
[28] Pathania V, Garg A, Kaur N et al. Molecular interaction studies of antituberculosis drug Isoniazid in aq. β-cyclodextrin solution: A volumetric, spectroscopic and molecular docking approach. J Chem Thermodyn, 2024, 189: 107194.[DOI]
[29] Beri A, Kant R, Banipal TS. Interactional behaviour of analgesic drugs in aqueous solution of caffeine at different temperatures using multi-technique approach, Can. J Chem, 2024; 102: 1.[DOI]
[30] Sachs CJ. Oral analgesics for acute nonspecific pain. Am Fam Physician, 2005; 71: 913-918.
[31] Clissold P. Paracetamol and phenacetin. Drugs, 1986; 32: 46-59.[DOI]
[32] Freo U, Ruocco C, Valerio A et al. Paracetamol: A Review of Guideline Recommendations. J Clin Med, 2021; 10: 3420.[DOI]
[33] Brune K, Renner B, Tiegs G. Acetaminophen/paracetamol: A history of errors, failures and false decisions. Eur J Pain, 2015; 19: 953-965.[DOI]
[34] World Health Organisation. WHO model list of essential medicines, 2017. [Accessed 28 June 2018]. Available at:[Web]
[35] Tyrrell HJV, Harris KR. Diffusion in Liquids: A Theoretical and Experimental Study. Butterworths, 1984.
[36] Koletic ZM, Dosenovic S, Puljak L. Efficacy and safety of modified-release paracetamol for acute and chronic pain: a systematic review protocol. BMJ Open 2019; 9: e029728.[DOI]
[37] Erdey-Grúz T. Transport phenomena in aqueous solutions. Hilger (Adam), 1974.
[38] Robinson RA, Stokes RH. Electrolyte Solutions: Second Revised Edition. Dover Publications, Incorporated, 2012.
[39] Loh W. Taylor dispersion technique for investigation of diffusion in liquids and its applications. Quím Nova, 1997; 20: 541-545.[DOI]
[40] Callendar R, Leaist DG. Diffusion coefficients for binary, ternary, and polydisperse solutions from peak-width analysis of Taylor dispersion profiles. J Solut Chem, 2006; 35: 353-379.[DOI]
[41] Barthel J, Gores HJ, Lohr CM et al. Taylor dispersion measurements at low electrolyte concentrations. I. Tetraalkylammonium perchlorate aqueous solutions. J Solut Chem, 1996; 25: 921-935.[DOI]
[42] Ali Z, Burnett I, Eccles R et al. Efficacy of a paracetamol and caffeine combination in the treatment of the key symptoms of primary dysmenorrhoea. Curr Med Res Opin, 2007; 23: 841-851.[DOI]
[43] Derry CJ, Derry S, Moore RA. Caffeine as an analgesic adjuvant for acute pain in adults. The Cochrane Collaboration. Published by John Wiley & Sons, Ltd, 2019.[DOI]
[44] Owayda AM, Hajeer MY, Murad RMT et al. The efficacy of low-level laser therapy versus paracetamol-caffeine in controlling orthodontic separation pain and changes in the oral-health-related quality of life in Class I malocclusions: A 3-arm, randomize d, placebo-controlled clinical trial. J World Fed Orthod, 2022; 11: 75-82.[DOI]
Brief of Corresponding Author(s)
Ana Cristina Faria Ribeiro She currently serves as a Researcher and Professor at the Chemistry Department of University of Coimbra. She obtained a Ph.D in 1999 from Coimbra University. In 2010 she got the Habilitation in Chemistry. Her research interests include electrolytes and non-electrolytes, transport and thermodynamic properties of drugs and cyclodextrins, multicomponent diffusion, and supramolecular host-guest compounds. She has over 150 publications in ISI international journals, additionally, she has published 35 book chapters and is co-editor of 23 books, with an h-index of 31. |






 Copyright ©
Copyright ©