Survey of Recent Trends of IB-IVB Metals and Their Compounds in Cancer Treatment
Irena Kostova1*
1Department of Chemistry, Faculty of Pharmacy, Medical University-Sofia, Sofia, Bulgaria
*Correspondence to: Irena Kostova, PhD, Professor, Department of Chemistry, Faculty of Pharmacy, Medical University-Sofia, 2 Dunav St., Sofia 1000, Bulgaria; Email: irenakostova@yahoo.com
DOI: 10.53964/id.2024014
Abstract
Metals from IB to IVB groups of the periodic table, because of their flexible oxidation states, natures of coordinated bioligands, and geometries upon complexation, display various properties and efficiency of metal-based cytotoxic drugs. Recent decades have witnessed remarkable progress in the synthesis and therapeutic application of numerous inorganic compounds in cancer investigations. The main potential therapeutic agents, such as the complexes of platinum group metals possessing antineoplastic activity have been broadly described previously in the literature and are beyond the space of this analysis. The key objective of the present study is to analyze the possible activity of IB-IVB group metals their coordination compounds against the cancer cell types. The increasing number of anticancer drug candidates among these metals proves this field offers a remarkably variation of novel opportunities for the synthesis of advanced new pharmaceutics with diverse and specific modes of action. Furthermore, the review provides forthcoming insights into prospective clinical scenarios for approaching treatment applications. That is why this field of research deserves more attention. It is expected that this review can serve as a guiding framework for future investigations in cancer chemotherapy.
1 INTRODUCTION
The therapeutic status and application of metals and their complexes in medicine has been the subject of many investigations of Ru(II) and Ru(III) compounds[1-19], Rh(I), Rh(II), and Rh(III) complexes[20-22], palladium compounds[23-26], osmium compounds[27-30], iridium complexes[31-36] and Pt-based compounds[37-53]. Metals easily lose electrons thus forming cations which are soluble in biofluids Metals in their cationic forms play vital roles in numerous essential biological functions and processes. The electron deficient metal ions easily interact with electron rich biomolecules (proteins, DNA etc.). Additionally, metal cations have an affinity for various small biologically active molecules. In the last decades, quite a lot of groups of novel metal-based organometallic and coordination complexes have been thoroughly discovered as potential antitumor agents based on a varied range of metals, mainly from the d-block elements. Despite the overabundance of recently designed compounds, their precise mechanisms of antineoplastic action are often still unknown. The literature investigation exposes that plentiful reviews have been published on anticancer platinum group metals and their compounds, Table 1, but the delay in the therapeutic achievement of other metal-based compounds has hindered progress in this area of research. The biological role, therapeutical and diagnostic activity, and toxic effects of Pt group metals are briefly tabulated in Table 1 for comparison purposes.
Table 1. The Biological Role, Therapeutical and Diagnostic Activity, and Toxic Effects of Pt Group Metals
Metal |
Location and Role in the Body |
Medically–relevant Uses |
Toxic effects |
References |
Ru |
Retained strongly in bones; Ru complexes–antineoplastic agents with antimetastatic properties, selectivity and low total toxicity; Ru mimics Fe |
Ru immunosuppressant (Ru(III)-Cyclosporin A), antimicrobial agent (Ru(III)-Chloroquine), antibiotic agent (Ru(III)-Thiosemicarbazone), NO scavengers for therapy of septic shock (Ru(III)-ethylenediaminetetraacetic acid (EDTA) complexes); Ru complexes (KP1019, NKP-1339, IT-139, NAMI-A) - anticancer drugs; photodynamic agent |
CarcinogenicRuO4–highly toxic and volatile |
[3-10]
|
Rh |
Rh(II) complexes–anticancer, antiparasitic, antiviral agents and enzyme inhibitors |
Rh(I), Rh(II), and Rh(III) complexes–antitumor, antiparasitic, and antiviral agents; 105Rh (β−emitter), 105Rh-EDTMP - therapeutic agent for pain treatment in bone metastases |
Rh compounds - toxic and carcinogenic
|
[20-22]
|
Pd |
Pd–component of dental alloys; Pd complexes with low toxicity, closely related to Pt antitumor analogs; 103Pd - radiotherapy |
Pd in dental appliances and 103Pd needles–for prostate cancer and choroidal melanoma brachytherapy; PdCl2 for treatment of tuberculosis; Pd complexes for prostate and lung cancer; in radiotherapy 103Pd–in brachytherapy, for choroidal melanoma, prostate cancer |
Non-toxic; Pd compounds are relatively rare, highly toxic and carcinogenic |
[23-26] |
Os |
Ru and Os chemistry is typically comparable |
OsO4–in chronically inflamed arthritic joints, OsO4–SOD mimic, Os–containing sensors to check the blood glucose levels continuously; Os complexes–anticancer, redox activation, DNA targeting or inhibition of protein kinase; in photodynamic therapy (PDT) |
Os–nontoxic unreactive, all Os compounds are highly toxic |
[27-30] |
Ir |
Ir(III) organometallic complexes - anticancer and antimicrobial activities |
Ir and Ru–in dental alloys, Ir(III) complexes - inhibitors of protein kinase and protein-protein interactions; photoactive polypyridyl Ir complexes–for photodynamic therapy; organoiridium(III) cyclopentadienyl complexes - anticancer agents; 192Ir (β-emitter) for cancer brachytherapy and prostatic carcinoma; GAMMA-192Ir for coronary artery disease |
Metal has low toxicity, but all Ir compounds are highly toxic; 192Ir-acute radiation |
[31-36] |
Pt |
Pt accumulates in kidneys, excreted in urine; Pt complexes - the most used cancer chemotherapeutics |
Pt materials–pacemakers, urinary and cardiovascular catheters; Pt complexes - chemotherapeutic drugs used to treat breast, lung, ovarian, testicular cancers
|
Pt–biologically inert, almost all Pt compounds are highly toxic |
[41-44,50] |
There are many other metals with beneficial potential. The complexes of metals from groups IB, IIB, IIIB and IVB have been highlighted in this review with their distinct modes of anticancer action and different procedures for the design of their coordination compounds. Transition metals, belonging to groups IB, IIB, IIIB and IVB, are naturally found shiny metals, with lesser chemical reactivity than that of alkali and alkaline earth metals. Electrons of the outermost and the inner d-orbital can contribute in chemical bonds of their compounds. The elements of IB group (Cu, Ag, Au) and of IIB group (Zn, Cd, Hg) have filled d-orbitals and differ from the other transition metals, including group IIIB and IVB elements. In the last years, significant developments have been completed in the design of novel anticancer candidates containing biologically active ligands coordinated to the metals of IB, IIB, IIIB and IVB groups. Some of them could replace the most common platinum-based anticancer drugs based on their comparatively low toxicity, improved selectivity, and reduced resistance. The coordination of bioorganic molecules with metal ions can change the biological properties of both the metal ion and the ligand moieties that are known to have a broad variety of biological activities. In line with this, the current review focuses on recent discoveries regarding the anticancer action of these metal complexes and the influence of the ligands in modifying their pharmacological profiles. The analysis has shown that many candidates present most efficient antiproliferative and cytotoxic activity, with superior selectivity between malignant and normal cells, compared to traditional drugs. The number of published papers clearly shows the rising interest in this category of anticancer compounds with potent activities against numerous cancer cells and remarkable inhibition of cancer cells via multiple cells signaling pathways[51-53]. Some interesting review papers on these metal-based compounds, and the clinical investigations achieved, are discussed in detail in the further sections.
2 d-ELEMENTS of IB AND IIB GROUPS
The biological role, therapeutical activity, and toxic effects of d-elements of IB and IIB groups are briefly listed in Table 2.
Table 2. The Biological Role And Toxic Effects of D-elements of IB and IIB
Metal |
Location and Role in the Body |
Drugs |
Toxic Effect, Antidotes |
Cu |
Concentrates in the liver, kidney, and brain; processes of respiration, hematopoiesis, angiogenesis, and neuromodulation; Cu- based proteins and enzymes -1% of proteome |
CuSO4 and CuO–part of vitamin-mineral complexes; alloys of Au, Ag, and Cu-in dental practice for prosthetics; Cu(II) complexes–SOD-mimic, anti-Alzheimer, antioxidant, anti-inflammatory, antifungal
|
Excess of Cu–Wilson’s disease; Antidote-cysteine, D-penicillamine |
Ag |
Found in liver, kidney, endocrine glands, erythrocytes; bactericidal action |
Protargol (protein complex of silver) and colloid silver (colloidal Argentum); bactericidal, astringent, and anti-inflammatory activity; Ag nanoparticles-antiviral agents |
Interacts with proteins containing S, deactivates enzymes and proteins |
Au |
Gold forms stable complexes with sulphur and phosphorus, particularly with thiol (-SH) groups of blood proteins; inhibits ROS |
Ag(I) and Au(III) complexes in chrysotherapy or aurotherapy, effective in therapy of rheumatoid arthritis, reduce inflammation; Ag(I) and Au(III) complexes - anticancer candidates |
Cytotoxic potency |
Zn |
Found in the enzyme carbonic anhydrase, endocrine glands, reproduction processes. |
Astringent, anti-inflammatory activity; antibacterial |
Severe vomiting; antidote-D-penicillamine |
Hg |
Hg vapor absorbs in the lungs, dissolves in the blood, and then to the brain, where leads to irreversible damage to the CNS; accumulates mainly in the liver, kidney, and brain |
HgCl2-antiseptic; Hg2Cl2-laxative; HgO–in dermatology; Hg–in thermometers; amalgams in dentistry, composed of 52% mercury; Thimerosal (ethylmercury thiosalicylate)–for preserving vaccines |
Hg and HgCl2 affect the CNS; spilled Hg binds FeCl3, S, KMnO4; antidotes-dimercaprol, DMPS, DMSA, D-penicillamine |
2.1 Copper Complexes
Copper is an important trace element necessary for the normal functioning of organisms[54]. Copper(ΙΙ) complex compounds with biologically active ligands are involved in many metabolic processes, particularly in redox reactions and enzyme catalytic processes, thus being extensively used in medical practice as drugs.
There are many reports on the anticancer, anti-inflammatory, super oxide dismutase (SOD)-mimic, antioxidant, anti-Alzheimer, antifungal, etc. properties of copper(II) complexes[55]. The spectrum of activity varies among copper compounds and is strongly dependent on the type of bioligands in the complexes, such as N,N-diimine functional groups in thiosemicarbazone, Schiff base etc. ligands. Antineoplastic effects of Cu-containing complex compounds have been studied based on the postulation that endogenous Cu(II) can be less toxic to normal cells than to tumor cells as compared with the classical cytotoxic Pt(II), Au(III) and Ag(I) agents. Being an essential cellular element required for many biopathways, copper exists in two different oxidation states and can undergo redox activity and competitively bind to sites that could otherwise be occupied by other biometals. As mentioned above, Cu(II) complexes are identified to mimic SOD, which is a significant antioxidant enzyme that defends cells from superoxide radicals by its dismutation to nontoxic products[55]. The best mimics of SOD are the complexes of copper with low molecular weight. In addition, copper can act as an antioxidant and a prooxidant. As an antioxidant, it scavenges and neutralizes reactive oxygen species (ROS). As a prooxidant, copper accelerates the generation of toxic free radicals, helps ROS and reactive nitrogen species (RNS) damage and contributes to the progress of OS affecting immune functions[56]. Anomalies in Cu homeostasis are supposed to cause Parkinson’s, Alzheimer’s diseases and amyotrophic lateral sclerosis[57].
Cu(II) cations are ordinarily coordinated with N-donors or with a combination of N- and S-donor atoms in complexes with linear, octahedral and square-planar geometries[54]. The soft Cu(I) cation prefers to coordinate with S-based ligands, whereas the relatively hard Cu(II) cation prefers hard N-based functional groups. Normally, the Cu metalloenzymes, involved in redox reactions, include both kinds of ligands, so that the metal center can easily exist in both oxidation states.
It was previously presumed that Cu(II) complexes have an analogous mechanism of action to platinum(II) coordination compounds with a key target DNA. Copper coordination compounds are currently found to exhibit anticancer activity with mechanisms of action different from that of the clinically used platinum compound cisplatin and other Pt(II) complexes[58]. The principal mechanisms underlying the anticancer properties of copper(II) complexes are: insertion of the complex between the pairs of adjacent bases via intermolecular forces (Intercalation), inhibition of topoisomerases, proteasome inhibition, and some possible interactions with DNA nucleotides.
Most of the recently studied Cu(II) complexes contain N-donor heterocyclic ligand, for instance 2,2′-bipyridine and 1,10-phenanthroline (Figure 1). Cu(Sparfloxacinato)(2,2-bipyridin)Cl and Cu(Sparfloxacin)(1,10-phenanthroline)Cl complexes have displayed cytotoxic effects against peripheral blood human promyelocytic leukemia cell line HL-60[59]. The cytotoxic activity of Cu(II) chelated complexes with phenanthroline was mediated by the activation of proapoptotic processes in malignant cells.
|
Figure 1. Structures of 1,10-Phenanthroline and 2,2′-Bipyridine.
A series of Cu(II) complexes with tris(2-pyridyl)amine (tpa) and tris-(2-pyridylmethyl)amine (tmpa) (Figure 2), have been investigated against A431, HCT15, and A375 cell lines[60] and some of them have shown similar IC50 values to that of cisplatin. Cu(II) and other transition metal complexes of the type [M(CL)] with curcumin ligand (CL) have been synthesized and their in vitro cytotoxicity against MDA-MB-231, KCL-22, PBMC and HeLa cancerous cells has been screened out[61]. It has been observed that copper(II) complex with Curcumin was most effective against MDA-MB-231 and KCL-22 cells. New copper(II) complexes with different substituted multi-nitrogen heterocyclic ligands, such as 1,5-tetrazole-diacetic acid (atzpa), 5-(2-pyrazinyl)tetrazole-2(1-methyl)acetic acid (pytzipa) and 4-(4-hydroxyphenyl)-1,2,4-triazole (hphtz) (Figure 2), have been recently designed and tested against HeLa cell line[62]. The cytotoxicity of the complexes against HeLa cells has been evaluated by MTT assay. The complex with 4-(4-hydroxyphenyl)-1,2,4-triazole was found to be the most active against HeLa malignant cells in comparison with other Cu(II) complexes. Cu(II) and other transition metal complexes of 6-mercaptopurine (Figure 2) have been synthesized and their cytotoxic activity was measured against SK-MM-1 and Caco-2 cells[63]. The newly obtained complexes have shown better cytotoxicity than the organic ligand against tested cells. Between all transition metal complexes, the copper(II) complex was found to be most active towards the tested cancer cell lines.
|
Figure 2. Structures of a Series of Ligands of Cu(II) Complexes.
In vitro studies of Cu(I) complex of a scorpionate bis-pyrazolyl carboxylate ligand with auxiliary phosphine have detected its cytotoxic activity against growth of HepG2 cells, and this effect was comparable with that of Cisplatin[64]. Cu(II) complex with 2,2′-bipyridyl and 1-(4-(trifluoromethyl)benzyl)-1H-benzimidazole has exhibited antiproliferative effect against DU145 prostate cancer and SPC212 mesothelioma cell lines[65]. Copper-based coordination compounds with antitumor activity represent a cheaper alternative to classical platinum-containing chemotherapy with good selectivity.
2.2 Silver Complexes
The biological functions of silver have not been completely established. It is classified as a potentially toxic element with a supposed carcinogenicity. Many silver-containing complexes and alloys are known for their antimicrobial activities and are widely used in the treatment of infected wounds and burn cases as wound-care products, dressings, catheters and dental implants[66,67]. In fact, confusion exists over the benefits and hazards associated with Ag compounds and alloys. Silver nanoparticles, the most predominant nanomaterials, have become a widespread method of treating bacteria and viruses[68].
Earlier, Ag(I) complexes did not receive much consideration although they also demonstrated good cytotoxic effects against many cancer cells. Recently, they have received great attention as potential antineoplastic agents, with pronounced cytotoxic properties. Ag(I) complexes have been found to display more significant antiproliferation activity than cisplatin with comparatively low toxicity and higher selectivity. Based on recent reports, among various metal-based compounds, Ag(I) complexes are very efficient anticancer agents in treating different types of cancer, together with breast, colorectal, ovarian and lung cancer.
Ag(I) ions can affect the redox interactions of the thiol groups, which can cause obstruction of electron transfer, inactivation of vital enzymes and binding to DNA by forming disulphide bonds thus damaging the cancer cells. Functional groups with P, N, O and S donor atoms in the ligands are preferred for coordination with Ag(I) ions and exhibit excellent activity against the cancer cells. Most of known Ag(I) complexes contain phosphines, carboxylates, N-heterocyclic carbenes (NHCs), 5-fluorouracil, Non-steroidal anti-inflammatory drugs (NSAIDs), Schiff bases, and other biologically active ligands[69]. As typical σ-donors and π-acceptors phosphines possess lipophilic nature and can easily affect the mitochondria of cancer cells. Among silver(I) carboxylates with different chains, (AgO2CCH2OCH3) with the shortest chain was found to be more active on HeLa cell line. New Ag(I) complexes of camphor carboxylates and camphor carboxamides (Figure 3) have been tested against A2780 cancer cells and A2780 cisplatin-resistant cells and exhibited greater cytotoxic activity as compared with the normal cells.
|
Figure 3. Structures of Camphor Carboxylates and Camphor Carboxamides.
Due to the neutral nature, NHCs can interact with cations via σ-donation. Most studies have presented the imidazole-based nucleus (benzimidazole, xanthine derivatives like caffeine and theophylline, coumarin substituted benzimidazole-2-ylidenes, benzimidazolium acridine, etc.), presented in Figure 4. Ag(I) complexes with NHCs have exhibited a slow-release rate of Ag(I)[69].
|
Figure 4. Structures of Imidazole-Based Ligands.
The anticancer agent 5-fluorouracil is a good ligand which expresses synergistic activity in coordination with cations. It is generally used in treatment of gastrointestinal tract, breast and colorectal tumors. It prevents the enzyme thymidylate synthase, incorporated into DNA and RNA, which can destroy DNA. Ag(I) complex of 5-fluorouracil [Ag3(fu)(fu-H)] has been compared with cisplatin. Cisplatin interacts with DNA through the coordination of Pt(II) ions with nitrogen bases, while 5-fluorouracil acts by affecting the replication of DNA via inhibition of thymidylate synthase enzyme. Thus, the combination of 5-fluorouracil with Ag(I) ions overcomes the tumor resistance[70].
NSAIDs are analgesic, antipyretic and anti-inflammatory agents. It has been found that they possess antitumor activity associated with the inhibition of prostaglandin production by cyclooxygenase mediated pathways. The silver(I) complexes with mefenamic acid and tolfenamic acid (NSAIDs) (Figure 5) have been evaluated against different tumor cell lines. Mefenamic acid is used in the treatment of pain and inflammation in rheumatoid arthritis and osteoarthritis, migraine headache, acute pain including muscle and back pain, postoperative pain, toothache and menstrual pain. The Ag(I) complexes of mefenamic acid have been tested against colon HT-29, breast MCF-7, and hepatocarcinoma HepG2 tumor cells. The mode of action of these complexes is connected with the variation of the action of caspase-3 and p53 activated-Bax/Bcl-2 ratio and with the inhibition of aldo-keto reductase 1C activity[71]. Tolfenamic acid is used as a strong pain reliever for the treatment of acute migraine attacks, dysmenorrhea, rheumatoid, and osteoarthritis. The cytotoxicity of Ag(I) complexes with tolfenamic acid have been assessed against breast MCF-7 and MDA-MB-453 tumor cells and compared with normal 3T3L1 cells. The complexes exhibited greater antineoplastic potential compared to the classical drugs cisplatin and 5-fluorouracil. Their mode of action comprises mitochondrial membrane depolarization with NO and ROS generation, as well as activation of various caspases[72].
|
Figure 5. Structures of NSAIDs Mefenamic Acid and Tolfenamic Acid.
Silver complexes are promising candidates for antitumor therapy with different mechanisms of action involving DNA binding and cleavage, generation of ROS, topoisomerase inhibition, and induction of apoptosis. One of the most significant advantages of Ag(I) ions is their minor toxicity. The human body can tolerate low amounts of Ag(I) ions deprived of toxic side effects. This low toxicity made possible the usage of Ag(I)-based complexes in the design of drugs for the progress of new therapeutic low toxic agents.
2.3 Gold Complexes
For many centuries, it was supposed that gold possesses strong therapeutic values[73,74]. Gold can be found in oxidation states of +1 and +3 under biological conditions. Gold(I) with a completely filled outer electronic shell ([Xe]4f14d10), belongs to soft Lewis acids; therefore, its most stable coordination compounds contain heavier ligands (soft Lewis bases). Accordingly, P and S are more preferable than N and O donor atoms. The cation [Au(H2O)x]+ is not identified. Gold(I) complexes can be stabilized by π-acceptor ligands. In biosystems, the most favorite ligand for gold(I) is the S-donor atom of thiols, for instance Cys in proteins. The possible attraction of Au(I) to DNA is very small.
The rational use of gold compounds in medical practice has a long history, starting with K[Au(CN)2] against tuberculosis bacteria. Similar to various Au(I) complexes, the complex ion [Au(CN)2]– contains a linear fragment of two-coordinated Au(I) bound to C atoms of cyanide anions [NC–Au–CN]–. Complexes of three-coordinated and four-coordinated tetrahedral gold(I) complex compounds have been identified but less studied. Weak Au(I)– Au(I) interactions are frequently found in gold(I) complexes, usually perpendicular to the axis of linear geometry. These bonds are much shorter than the total sum of the van der Waals radii. This attraction of Au(I) cations to each other is named “aurophilicity”, which might be due to the impact of the relativistic effect in Au chemistry (the electrons from the inner shell cause the shell contract). Different from Cu(II), an analogous element of the IB group, Au(II) ions are not stable. Complexes of Au(III) with a square planar geometry can be obtained, but they are predisposed to reduction processes up to Au(I) or Au(0) in biological systems.
At the early XX century the use of the less toxic thiolate complexes of Au(I) began instead of K[Au(CN)2] in the tuberculosis treatment. The thiolate ligands RS− stabilize the complexes with Au(I)[74] and many gold(I) coordination compounds have been reported as good candidates for medical purposes. These Au(I) thiolate complexes show a composition with Au : thiolate ratio around 1:1, although the structures are more complicated in solutions. Most of the produced complexes are polymeric with -S-Au-S-Au-S- fragments in their linear and ring structures (Figure 6).
|
Figure 6. Structure of Polymeric Thiolate Au(I) Complexes.
Among the Au(I)-containing drugs, the oral Au(I) complex auranofin (tetraacetyl-β-D-thioglucose-gold(I)-thioethylphosphine), represented in Figure 7, has found extensive use[73,74]. Auranofin has been originally developed for the rheumatoid arthritis treatment, but now it is under investigation for oncological applications owing to its in vitro and in vivo antineoplastic activity on different tumor models and various types of cancer such as cisplatin-resistant gastrointestinal cancer, ovarian cancer, and chronic lymphocytic leukemia. Its exact mechanism of action is not clear, but it is supposed to work through immunological mechanisms. This drug induces cytotoxicity via ROS production in cancer cells inhibiting the activity of the selenocysteine-dependent enzyme thioredoxin reductase, which is important for maintaining the intracellular redox balance of the cytosol and mitochondria. Principally in cancer diseases, the inhibition of TrxR causes an increase in cellular OS and induces apoptosis.
Similar to other Au(I) coordination complexes, auranofin is a monomeric neutral complex with a linear structure. It has lipophilic properties appropriate for oral application. This stable complex has a low solubility in H2O but good solubility in organic solvents. Because of its stability, auranofin is safe for administration with low gold(I) content in the kidneys. The toxic and side effects of Au(I)-containing drugs are commonly associated with the possible formation of Au(III)[74].
Figure 7. Auranofin (Tetraacetyl-P-D-ThioglucoseAu(I) Triethylphosphine).
Au(I) and Au(III) complexes represent a good treatment option and alternative to antineoplastic platinum therapy overcoming adverse effects and cell resistance[75-82]. Au(III) compounds are isoelectronic and isostructural with Pt(II) complexes with a square-planar geometry. Square-planar geometries of Pt(II)-d8 systems are less typical in biosystems. They have been observed in the complexes of d8 transition cations, such as Au(III), Pt(II), Ni(II), Pd(II), and Ir(I) in strong ligand field. Since this geometry of Pt(II)complexes is typical and significant for the antitumor activity, Au(III) complexes can certainly be used in anticancer therapy with the supplementary advantage of their reduced toxicity.
The main features of gold(III) complexes are their strong oxidizing properties and easy reduction to gold(I) or gold(0). Gold(III) cations are more polarizing than platinum(II) ions. The binding of Au(III) complexes to nucleic acids is not as tight as the binding of Pt(II) drugs to the main target DNA, suggesting the existence of a different mechanism of action. Moreover, Au(III) complexes are less toxic, compared to classical platinum drugs. Gold(III) anticancer agents have a tendency to target thiol-based proteins and enzymes, predominantly thioredoxin reductases (TrxR). Research into the potential use of Au(III) derivatives with cytotoxic properties is still ongoing because of the similarity with Ru(III) and Ga(III) coordination complexes as alternatives of current platinum therapy.
Gold(III) complexes possessing antineoplastic activity have been widely studied during the last years[75-82]. The main representatives of Au(I) and Au(III) anticancer agents are shown in Figure 8. The most effective are the complexes with multidentate bioactive ligands, such as en (ethylenediamine), dien (diethylenediamine), and damp (N-benzyl-N,N-dimethylamine). Trichlorodiethylendiamine Au(III) [AuCl(dien)]Cl2, 1, and trichlorobisethylendiamine Au(III) [Au(en)2]Cl3, 2, Figure 8, have been estimated in vitro against A2780 human ovarian tumor cells. Gold(I) bis(diphosphine) complexes with tetrahedral geometry, for instance [Au(dppe)2]+, 3, have sown activity against several tumor cell lines by damaging mitochondrial function. The ligand 1,2-diphenylphosphinoethane (dppe) and its Au(III) complexes have also exposed antitumor activity in transplantable tumor models. Their clinical usage is not currently allowed due to their cardiotoxicity, which can be resolved by including phosphine substituents and by controlling the lipophilicity. Au(I) complex with mono- and diphosphine ligands, 4, has shown high cytotoxic effects with micromolar IC50 values.
|
Figure 8. Gold Compounds for the Treatment of Cancer.
Although attractive cytotoxic and antitumor effects of gold(III) coordination compounds, their progress has been run-down due to their low stability under biological conditions. This low stability is connected with the higher ligand exchange rates and the higher Au(III) reduction potentials[75]. Nevertheless, some metal-organic antitumor Au(III) complexes were found to be stable to reducers. Complexes of bis-pyridyls 5, 6, have shown activity with possible interaction with DNA components. The in vitro cytotoxicity of [Au(bipy)(OH)2]PF6, 5, and [Au(bipy-H)(OH)]PF6, 6, where bipy = 6-(1,1-dimethylbenzyl)-2,2'-bipyridine, has been established against different tumor cells. The cytotoxic effects of square-planar gold(III) complexes, such as trichloro(2-pyridylmethanol) Au(III) [AuCl3(Hpm)], 7 and dichloro(N-ethylsalicylaldiminato) Au(III) [AuCl2(esal)], 8, Figure 8, have been estimated against A2780 human ovarian tumor cell line in vitro. These Au(III) complexes have exhibited considerable cytotoxic activity, comparable to that of cisplatin. They have also overcome the cisplatin resistance[74,75].
Along with the complexes shown above, various gold complexes with cytotoxic activity, such as halo- and pseudohalo- gold(I) complexes with bioactive organic ligands, Figure 9, have been studied in last decades for their antineoplastic activity[76-78].
|
Figure 9. Recently Synthesized Gold Compounds[26-28].
It may be concluded that there are many results indicating that Au(I) and Au(III) complexes are an emerging class of compounds with potential anticancer properties alternative to cisplatin, but it would take time before their pharmacological potential could be explored and applied. Their cytotoxic effects, exhibited via non-cisplatin anticancer mechanism, low toxicity and good selectivity to thiol-based enzymes make them attractive probes in designing new candidates which can turn into clinically adequate drugs[76-82].
The radioactive isotope of gold 198Au is used to treat malignant tumors. It undergoes β-decay to stable 198Hg. The short half-life of 198Au (2.69 days) allows the drug to be injected into the body without its subsequent extraction, thus making it a preferred isotope with a relatively low complication rate[80]. Radioactive gold 198Au has therapeutic and radiochemical properties that make it an attractive opportunity for many types of cancer. It minimizes the radiation exposure to neighboring tissues.
2.4 Zinc Complexes
Coordination complexes of group IIB are of limited interest, since only Zn(II) is an important element in some metal-based enzymes, while Cd(II) and Hg(II) are toxic and hazardous pollutants. The search for antitumor metal-based drugs alternative to classical platinum complexes could not exclude zinc complexes due to the position of this essential microelement for the human physiology and the correct functioning of the human body. Zinc is a vital element with essential functions in many cellular processes, particularly protection against ROS, proliferation and differentiation of cells. Zinc functions in enzymes and proteins, including cellular signaling proteins, DNA repair enzymes, and transcription factors. Its lack of redox activity and its capability to support different coordination structures and to promote fast ligands exchange are very important. Analogously to other trace metals, the impairment of its homeostasis can cause various diseases and, in some cases, can be also related to cancer development.
Zinc, the second most abundant trace element in the human body, is active in regulating cell apoptosis, although its mechanism of action is not completely clarified. In some cell types, zinc activates apoptosis, while in others, zinc shows antiapoptotic properties. Because of its critical role in numerous biosystems, it is expected that different Zn levels are associated with some irregularities, including cancer incidences, depending on the type of tumor. Reduced zinc amounts have been observed in the cases of prostate, gallbladder, digestive tract, and liver cancer, while in the breast cancer cases zinc has exhibited higher zinc levels in malignant tissues[83]. Nevertheless, Zn(II) complexes generally exert lower toxicity in comparison to other metal-based compounds and many Zn(II) complexes have been proposed as antineoplastic agents.
Recently, design and pharmacological studies of Zn(II) complexes with N, O, S and P -donor ligands as anticancer agents have expanded considerably. Some of preferred ligands are depicted in Figure 10. Zn(II) complex of 2-aminimethylthiophenyl-4-bromosalicyaldehyde ligand (ATS)[84] has been obtained and tested against HCT116 and HEP2 tumor cells. Zn(II) complex has been found to be much active towards HCT116 and HEP2 cell lines than the ligand.
Khan et al.[85] have synthesized Zn(II) and other transition metal complexes of the ligand norharmane (9H-Pyrido[3,4-b]indole; Hnor) with bipyridine or phenanthroline as additional ligands. The complexes have been evaluated in vitro against A2780 and A549 cells. It has been observed that Zn(II) complexes with phenanthroline were more active than those with bipyridine ligands.
Novel Zn(II) and other transition metal complexes have been obtained by the condensation of 4-(4-amino-5-mercapto-4H-1,2,4-triazol-3-yl) phenol with salicyaldehyde derivatives[86]. The cytotoxicity of the complexes has been tested against HCT-116, DU145 and A549 cell lines. Zn(II) complex has displayed highest activity on the tested cancer cells, although being less potent than the standard drug Paclitaxel.
Newly obtained Zn(II) complexes of 1,1,3,3-tetrakis(3,5-dimethyl-1-pyrazolyl)propane[87] have been evaluated against Caco2 cells. The Zn(II) complexes exhibited good cytotoxicity at low concentrations.
Zn(II) and other transition metal complexes of Schiff base ethyl-2-(2-(4- chlorophenylcarbamothioyl)hydrazone)propanoate[88] have been obtained and their in vitro cytotoxicity has been tested against COLO-205 and K-562 human cancer cell lines.
Zinc(II) complexes possessed good anticancer activity against different tumor cells. Some of the complexes proved to be good antiproliferative agents in very low concentrations. Additionally, they are non-toxic. Hence, the discovery of new zinc complexes with antineoplastic activity proved to be beneficial.
Figure 10. Structures of the Ligands of Zn(II) Complexes.
3 d-ELEMENTS OF IIIB AND IVB GROUPS
Organometallic compounds with direct covalent metal-carbon bonds, especially those of group IIIB, have lately been found to show good anticancer properties[89].
Most of the biological properties of IIIB group metals are connected with their capability to substitute Ca(II) ions in biomolecules and their affinity for H2O molecules. Additionally, they can replace biogenic cations Mg(II), Mn(II), and Fe(III), which can alter the related enzymes functions.
It has been found that the complexes of IIIB and IVB group metals may possess distinct mode of action, resulting in an entirely different specificity to tumors thus changing the selectivity. The most probable mechanism of action of anticancer metals of IIIB group is associated with their probable inhibition of cations crucial for cells cycle regulation, and antitumor action is strongly improved by coordination with many bioligands.
The biological role, therapeutical activity, and toxic effects of d-elements of IIIB and IVB groups are briefly listed in Table 3.
Table 3. The Biological Role and Toxic Effects of D-elements of IIIB and IVB Groups
Metal |
Location and Role in the Body |
Drugs |
Toxic Effect, Antidotes |
Sc |
Sc is not biogenic; its radioactive isotopes are PET and SPECT agents |
43Sc and 44Sc–in PET imaging, while 47Sc is used in radiotherapy |
Sc is non-toxic, Sc compounds–cancerogenic |
Y |
Yttrium is not a vital element, but yttrium-based materials (90Y, 86Y) are used in medical lasers and biomedical implants |
Y–used in anticancer treatment as 90Y radionuclide (β-emitter): non–Hodgkin B-cell lymphoma radiotherapy and immunotherapy; 86Y–tracer for PET imaging |
Hazardous, causes lung embolisms, chances of lung cancer |
Ti |
Known to act as a stimulant; one of the most biocompatible metals |
Ti–in prosthetics, implants; Ti compounds–fodder additives; Ti(IV) complexes–for treatment of cancer |
Ti is not harmful or toxic; TiO2 is a carcinogen |
Zr |
Zr is not a vital element for living organisms |
Zr in dental, knee, and hip implants, reconstruction of ear ossicular chain, 89Zr in PET |
Zr exhibits little harmfulness |
Hf |
Unreactive metal, closely related to titanium and zirconium |
Hafnium–scavenger metal against oxygen and nitrogen, hafnium oxide nanoparticles in anticancer and radiation therapy |
The metal has no known toxicity |
3.1 Scandium Complexes
The group IIIB transition metals scandium and yttrium summarize the unique chemical properties of lanthanides, all together known as rare earths. Sc(III) ion is a strong Lewis acid. Its coordination chemistry has remained insufficiently studied until recently, though the utility of Sc(III) coordination complexes developed in biology and medicine. In contrast to other rare earth metals, Sc(III) has no d- or f-electrons, making their compounds difficult for spectral characterization[90]. Additionally, the smallest rare earth ion Sc(III) tends to form predominantly covalent bonds rather than ionic.
The literature data on biofunctions of non-radioactive scandium and its toxicity are quite scarce. The effect of Sc2O3 on human osteoblast-like cells TE85 HOS[91] has been described. Scandium exopolysaccharide complexes have been evaluated on different cancer cell lines (osteosarcoma, lung, glioblastoma, melanoma, breast)[92]. The new Sc(III), Y(III), and lanthanide(III) chlorido or triflate complex compounds with tridentate monoanionic quinolinephenoxyamine, quinolinephenoxyimine and ansa-monocyclopentadienyl-imino-pyridine ancillary ligands (Figure 11) have been synthesized and characterized. The cytotoxicity of the compounds has been estimated on murine fibroscarcoma WHEI-164, rat glioma C6 and human embryonic kidney HEK-293 cells[89]. The cytotoxic activity of Sc(III), Y(III), and lanthanide(III) complexes of N,N'-dicyclohexyl-2,2-bis-(3,5-dimethyl-pyrazol-1-yl)-acetamidinate (Figure 11) has been assessed on human epithelial lung adenocarcinoma A549, human epithelial cervix adenocarcinoma HeLa, human melanoma A375, human embryonic kidney HEK-293 and murine macrophage J774.A1 cells[93].
|
Figure 11. Structures of the Ligands of Sc(III) Complexes.
A number of unusual structures of Sc(III) calix[n]arenes (n = 4, 6, 8) with [Sc(OTf)3] (Tf = triflate) or [Sc(OiPr)3] (iPr = isopropyl) have been isolated and structurally characterized. Their cytotoxicity has been evaluated against cancerous cell lines HCT116 and HT-29[94].
The scandium(III) chemistry is of evolving interest for theranostic applications in nuclear medicine, because radioactive scandium isotopes are becoming more readily available as matched radionuclides. Scandium radioisotopes do not occur naturally. Relatively short half-lives on the order of hours to days are typical for them. Radioactive Sc isotopes have potential in positron emission tomography (PET) and SPECT imaging, and in cancer radiotherapy, specifically 44Sc and 47Sc, which display appropriate features for diagnostic or therapeutic purposes[95]. 44Sc and 47Sc may be used jointly for theragnostic purposes. The isotope 44Sc is an agent for PET imaging with radio-metalated peptides or some small targeting bioactive molecules, like 2,2′,2”,2”'-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid (DOTA)-functionalized ones. It is also a beneficial radioisotope for medical nuclear imaging and preclinical therapeutic dosimetry before treatment with the healing 177Lu labelled DOTA derivatives. The β-emitting isotope 47Sc is a therapeutic radioactive nuclide and in combination with 44Sc could permit the use of matching radiodrugs with similar pharmacokinetic properties. The application of positron (β+) emitting isotopes 43Sc (T1/2=3.9h) and 44Sc (T1/2=4.0h) for PET is beneficial regarding several aspects over longer periods compared to the commonly used 68Ga with T1/2=68min. The means of coordinating scandium(III) ions using bifunctional chelators for targeted isotope delivery constitute an important research area.
3.2 Yttrium Complexes
Yttrium is known to be an exceptional platform for the design of varied agents useful for multiple medical purposes. This element is considered as a rare earth because of its small chemical resemblance to the d-block elements. It forms predominantly metal-carbon bonds in organoyttrium compounds which is typical for lanthanides. The main reason for this behavior is the lanthanide contraction, therefore, yttrium like lanthanides does not exhibit the common characteristics of the transition metals. The main features of Y include greater reactivity, a wide range of coordination numbers, formation of labile complexes, almost constant oxidation state +III. Yttrium has a preference for highly electronegative donor atoms. Due to these chemical properties, Y(III) can easily form numerous complexes with many multidentate ligands, including EDTA, 1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid trisodium salt (DO3A), trans-1,2-diaminocyclohexane-N,N,N',N'-tetraacetic acid hydrate (CDTA), DOTA, diethylenetriaminepentaacetic acid (DTPA), presented in Figure 12[96]. These polyaminocarboxylic acids are multidentate which provides the Y(III) complexes with high thermodynamic stability and kinetical inertness. The complexes of octadentate ligands DTPA and DOTA exhibit very strong thermodynamic stability, that is why their compounds are extensively used for the preparation of radioactive pharmaceuticals. In addition, the presence of N donors, and the respective conformations displayed by these polyaminocarboxylates leads to formation of multiple isomers which can be readily interconverted and affect the NMR and magnetic resonance imaging (MRI) properties of the obtained complexes.
|
Figure 12. Structures of EDTA, DO3A, CDTA, DOTA, DTPA, OCTAPA Ligands.
The synthesis and characterization of a novel binuclear complex of Y(III) with anthranilic acid [Y2(HA)6(H2O)4] Cl6.2C2H5OH has been recently reported[97]. The bidentate binding of anthranilic acid (Figure 13) only via the oxygen atoms has been proven. The cytotoxic activity of the Y(III) complex against human prostate cancer PC-3, breast cancer MDA-MB-231, and bladder cancer T-24 cell lines has been tested. The Y(III) complex exhibited stronger cytotoxicity against the bladder cancer cell line.
The biological activity of Y(III) complex with 2,9-dimethyl-1,10-phenanthroline (Figure 13) has been studied for in vitro fish DNA (FS-DNA)/ bovine serum albumin (BSA) interactions, DNA-cleavage, antineoplastic and antibacterial activity[98].
Recently, Y(III) complex [Y(Daf)2Cl3.OH2] of 4,9 diazafluoren-9-one (Figure 13) has been synthesized and its interaction with DNA and BSA has been investigated. The antineoplastic activity of the yttrium complex has been tested on human breast MCF7 and human lung A549 cancer cells by means of MTT method. IC50 values obtained showed that the yttrium complex possesses anticancer activity[99].
|
Figure 13. Structures of the Ligands of Y(III) Complexes.
Because of the existence of numerous isotopes, yttrium and its complex compounds have been used in a wide variety of diagnostics and therapies in medicine. Many Y-based compounds are used in therapeutic lasers and medical implants. Yttrium-90 is the most biologically utilized radionuclide in immuno-radiotherapy against various types of cancers including lymphoma, leukemia, colorectal, pancreatic, ovarian, liver, and bone cancers[96]. 86Y tracers are used in PET imaging. Yttrium-90 (T1/2=2.67d) is a pure β-emitter lacking γ-photons, making it predisposed to various targeted radiotherapy applications including 90Y-labeled colloid, somatostatin-receptor targeting peptides, tumor-targeting antibodies, and resin/glass microspheres for catheter embolization of hepatic malignancies and metastases. It can be delivered to cells in the form of stable chelated complexes. 90Y radiotherapy is harmless and well-tolerated. It is also helpful in preserving normal tissues, as the radiation releases directly to the tumor, localizing β-emission in the area of cancer. Imaging techniques like PET can benefit from those radionuclides which exhibit β+ decay. 86Y (half-life 14.7h), that decays principally via β+ emission, has been studied as a potential PET imaging agent. The isotope yttrium-89 is naturally occurring and is quite stable. Yttrium(III) complexes can be used as multimodal agents where the same ligands can coordinate to 86Y for PET, to 90Y for radiotherapy, and to 89Y for NMR and MRI applications. The other isotopes yttrium-76 through 88 and yttrium-90 through 107 are artificially produced and are radioactive.
3.3 Titanium Complexes
The human body holds around 700mg Ti, although this metal does not play any substantial role in biofunctions. In contrast of Pt, Ti is non-toxic and the human body can tolerate comparatively high doses without accumulation. Titanium is used for the synthesis of anticancer complexes and nanomaterials, some of which, e.g., titanocene dichloride, TiCp2Cl2, have been approved by clinical trials[100]. Ti(IV) complexes were between the first metal compounds to enter clinical trials after platinum antitumor complex compounds with a distinct mode of action and range of activity from platinum(II) and platinum(IV) complexes as well as a good biocompatibility[101-110]. Titanocenes have been found rather effective against cisplatin resistant tumor cells in vitro and in vivo, which has proven that they acted through different mechanisms of action, hence, they have been recommended as potential agents in the treatment of cases of resistance to cisplatin. In contrast to the Pt(II) and Pt(IV) complexes, Ti(IV) compounds have not shown any sign of nephrotoxicity or myelotoxicity. The first antitumor metallocene was titanocene dichloride TiCp2Cl2. Budotitane (Figure 14A) and TiCp2Cl2 have exhibited antitumor activity with small toxicity in many cancer cell lines. Titanocene dichloride and Budotitane ( [Ti(IV)(bzac)2(OEt)2], where bzac = phenyl-butane-1,3-dione) have been the primary Ti(IV) compounds that reached clinical trials, but lastly failed due to their nonsufficient aqueous stability, leading to a rapid decomposition in the physiological aqueous environment and unspecified mode of action.
|
Figure 14. The Structures of Budotitane (A) and Titanocene Y (B).
The cis-structure of titanocene dichloride makes interesting matches with cisplatin, but this complex is too reactive and can hydrolyze to different Ti(IV) species and it is not known which of them is the active one. The Ti(IV) species formed have exhibited stronger cytotoxicity than freshly prepared titanocene dichloride and resulted in raised Ti uptake and accumulation into the cells. The toxicity effects detected for titanocene dichloride treatment have been comparable to those of budotitane, including hepatotoxicity, hypertension, anorexia, blurred vision, and insomnia. Comparison of cisplatin with titanocene dichloride shows that the [PtCl2] fraction is stable in aqueous solutions, while the analogous [TiCl2] moiety hydrolyzes forming hydrochloric acid, which causes vomiting and nausea.
The main weakness of titanium(IV) compounds is their hydrolytic instability in water solutions. Subsequently, new titanium(IV) complexes have been obtained to overcome this instability. Differently substituted titanocenes and Ti(IV) salan complexes have exposed potentials of better stability, solubility and cytotoxic activity[100,110,111]. Wide-ranging studies have been performed to substitute the Cl- groups and to modify the cyclopentadienyl ring of titanocene dichloride in order to expand solubility, stability, cytotoxic activity and alternative transport mechanistic routes of the titanocene compounds. Titanocene Y (Figure 14B), featuring alkylated derivatives of aromatic groups on both Cp rings and containing methoxyphenyl substituents, gives higher effectiveness. In addition, titanocene Y has been found to be a more hydrolytically stable complex. Titanocene Y is one of the most potent and promising second generation non-vectorized titanocenes. The titanium complexes Ti-Salan and titanocene-Y have displayed contrasting effects associated with their reactions with albumin and DNA, cellular uptake and circulation. Ti-Salan has demonstrated comparatively lower binding to biologically active molecules but increased serum-dependent cellular uptake whereas titanocene-Y has shown lower accumulation and higher binding to DNA and albumin. The biodistribution investigations have shown that for titanocene-Y the DNA interactions were critical while for Ti-Salan mitochondrial targeting was important. The inclusion of 2,6-dipicolinic acid as a second ligand to Ti-Salan has given novel heteroleptic complexes with better water stability and notable in vitro and in vivo cytotoxic activity[111], thus showing the higher adoptability to group IVB metals.
The success of cisplatin and titanocene dichloride has inspired numerous research groups to obtain similar coordination compounds comprising reactive Cl- ions in cis-location with vanadium, manganese, chromium and other analogs. These metallocenes MCp2X2, where Cp – cyclopentadienyl, (Figure 15) with different dihalides, represent a class of hydrophobic antineoplastic agents. They undergo quick hydrolysis in water[112]. The metallocenes MCp2X2 possess distorted tetrahedral structure where Cp ligands and the halide- or acido- ligands (X) are coordinated to M+4. The two cyclopentadienyl rings with delocalized negative charges are bonded to M+4 in a bent sandwich configuration in their structures. Metallocenes MCp2Cl2 have exhibited cytotoxicity against many cancer cell lines such as leukemias P388 and L1210, colon B adenocarcinoma, B16 melanoma and Lewis lung carcinoma, as well as solid and fluid Ehrlich ascites tumor cells (murine mammary cancer), and other carcinomas[100,111].
|
Figure 15. Structures of Antitumor Metallocene Dihalides.
The anticancer activity of MCp2X2 is dependent on the metal. In many studies, different approaches have been taken to functionalize the metallocene moiety to stabilize the complexes and improve their cellular uptake as well as to apply them to specific tumors. The complexes with Ti, Nb, V, Mo have shown strong activity, but Ta and W complexes have not revealed significant activity, and Zr and Hf complexes have been found inactive. Titanocene and vanadocene dichlorides have displayed good activity against breast, lung, and gastrointestinal cancers in vivo. Differences of halide and diacido anions have been extensively studied. It has been observed that halides did not affect the antineoplastic activity. Some studies have been made on different substituents in Cp ring, limited mainly to titanocenes[100,111].
The main disadvantages are the observed hepatotoxicity and gastrointestinal toxicity for TiCp2Cl2. Regarding the mode of action, it is still unclear whether DNA is the main target for titanium(IV) ions. The binding of Ti to N donor atoms in DNA appears to be weak at neutral pH values. It has been supposed that the phosphates are the favorite ligands for Ti(IV). Binding of titanium(IV) ions to iron(III)-transport protein serum transferrin might also play a substantial role[100,111]. Additionally, the hydrolysis studies, stability at different pH and the reactions with nucleic acids have shown that each of the metallocenes has its own mode of action specific for the metal cations.
The titanocene derivative of tamoxifen (Figure 16) with anticancer activity, revealed a higher proliferative action on the estrogen-dependent cancer cell line MCF7, derived from breast cancer cells holding estrogen receptor-positive[100], comparable to that detected with titanocene dichloride. Tamoxifen is a selective estrogen receptor modulator (SERM), thus the purpose was to observe whether the tamoxifen-corresponding modification of titanocene dichloride would improve its selectivity and activity. It has to be stated that the tamoxifen resistance, found in many breast cancer types, remains the most important problem.
|
Figure 16. The Structure of Titanocene Derivative of the Anticancer Drug Tamoxifen.
3.4 Zirconium Complexes
Zirconium has high coordination numbers and capability to form stable complex compounds. In spite of the success of titanium(IV) complexes, information for heavier Zr(IV) complexes with antineoplastic activity is rather scarce, which is owing to the insufficient water stability of zirconium(IV) complexes as established by previous reports on the activity of ZrCp2X2 and its amino derivatives[113] and zirconium(IV) 1,3-diketonates[114]. Among the many group 4 metal complexes obtained and evaluated for anticancer efficiency, the diaminobis-phenolato ligand namely Salan coordinated M(IV) complexes have achieved remarkable success. Zirconium(IV) bis-chelated Salan complexes have shown comparable anticancer activity to cisplatin and good water stability, but low solubility[115].
Metallocene-diacido complexes containing Ti, V, Nb, Zr, Mo, have been found to display antineoplastic activity in a wide range of tumors with low toxicity as compared to cisplatin. Zr(IV) proton-transfer complex compounds containing pyridine-2,6-dicarboxylic acid (Figure 17) with 2-methylimidazole and imidazole [2-mimH]2[Zr(pydc)3] and [imiH]2[Zr(pydc)3].4H2O] have been described. The pydc ligand has demonstrated relatively high adoptability to group 4 elements titanium and zirconium, leading them to a far broader research scope. The antiproliferative effects of these compounds has been assessed in vitro against human lymphocyte HL60, human breast cancer MCF7 and human colon adenocarcinoma HT29 cell lines. Significant cytotoxic effect has been observed on breast cancer MCF7 cell line (IC50=10μM)[116].
Zr(IV) complexes of pyrazoles, such as (4-[2-vinylthiophene]-3-methyl pyrozolin-5(4H)-one, 4-[4-chloro benzylidine]-3-methyl pyrozolin-5(4H)-one and 4-[4-dimethylnitro benzylidine]-3-methylpyrozolin-5(4H)-one have been reported. These Zr(IV) complexes have displayed considerable antineoplastic activity and cytostatic specificity against human HCT-116 colon carcinoma cell line[117].
Mixed ligand zirconium(IV) complexes of a primary ligand 8-hydroxyquinoline and secondary ligands amino acids (L-alanine, L-serine, glycine) (Figure 17) have been synthesized and characterized[118]. 8-Hydroxyquinoline stabilizes group 4 metal (Ti, Zr, Hf) complexes. Zr(IV) complexes have been tested for their antiproliferative properties on Ehrlich ascites and Daltonís lymphoma ascites cells. Novel stable zirconium(IV) complexes of 8-hydroxyquinoline with solid structures have been lately sinthesized[119]. These complexes have shown desirable stability and solubility in H2O and DMSO, which can elucidate their excellent inhibition effects against human derived hepatoma HepG2, human cervical tumor Hela S3 and human lung cancer PC9 cells through almost completely induced apoptotic pathway.
|
Figure 17. Structures of the Ligands of Zr(IV) Complexes.
Mononuclear oxy-V(IV) and oxy-Zr(IV) complexes (VO(ALz)2 and ZrO(ALz)2) of O,N-monobasic bidenate arylhydazone derivative 2-((2-(2,4-dinitrophenyl)hydrazineylidene)methyl)-4-nitrophenol, HALz (Figure 17) have been synthesized, characterized and their reactivity has been studied[120]. The binding action of the oxy-V(IV) and oxy-Zr(IV) complexes towards ctDNA has been investigated. Both the complexes have been found to be more active as antioxidants and anticancer agents than the free ligand. The V(IV) and Zr(IV) complexes have been assessed by MTT method against human breast adenocarcinoma MCF7, human colon carcinoma HCT-116 and human hepatocellular carcinoma HepG2 cells. It has been found that the antiproliferative activity of the complexes was attributable to the presence of the metal ions with high valency. In addition, the higher Lewis acidity of V(IV) cation compared to Zr(IV) cation resulted in better pharmacological activity.
Zirconium(IV) and other metal(IV) Schiff base complexes of the ligand (E)-1-((((1H-benzo[d]imidazol-2-yl)methyl)imino)methyl)naphthalen-2-ol (Figure 17) have been obtained[121]. The antineoplastic activity of the complexes has been evaluated in vitro against human hepatocellular carcinoma HepG2, human breast adenocarcinoma MCF-7 and human colon carcinoma HCT-116 cells. Zr(IV) complex has demonstrated substantial activity on HCT-116 colon cell line.
Although several isotopes of zirconium such as 86Zr (T1/2=17h, γ-emitter), 88Zr (T1/2=85d, γ-emitter), and 89Zr (T1/2=78.4h, β+-emitter) have been produced, zirconium-89 is the most popular for application as a radioactive pharmaceutical. The radionuclide 89Zr has found widespread use in PET imaging when it is coupled with antibodies, proteins and nanoparticles[122]. The availability of 89Zr radionuclide in the form of oxalate or chloride is vital to the progress of effective immuno-PET reagents. The research of the significant PET isotope 89Zr has advanced rapidly and was very important for understanding of Zr chemistry and for designing new ligands to efficiently chelate zirconium-89. Numerous ligands with hydroxamate, terepthalamide, hydroxyisopthalamide, hydroxypiridinoate, tetraazamacrocycle coordinating units have been studied for chelation of 89Zr isotope. Such ligands are predominantly effective in generating stable complexes because of their high chelate effects. These effects are principally evident for macrocyclic chelator ligands (Figure 12), although their rigid structure makes them more kinetically inert compared to their acyclic counterpart, thus high ligand amounts, extended reaction time, and heating are necessary for optimal radiochemical yield.
3.5 Hafnium Complexes
Hafnium is widely distributed in the Earth’s crust although it is considered a rare metal. It is a chemically active metal in the Ti-triad, closely related to Ti and Zr and is mostly used as an implant material for application in bone and soft tissues. Hafnium can be coordinated by ligands holding O, N and S donors in the oxidation state of +4 with a preference for soft bases containing N atoms. The ionic radius of hafnium(IV) is higher than the Ti(IV) and Zr(IV) radii, which may lead to some specific properties of the Hf(IV) complexes, for example, hafnium(IV) is a softer Lewis acid than Ti(IV) and Zr(IV) cations. Numerous Hf(IV) complexes have found interesting chemical, optical and biological applications, although, there are no many reports on their anticancer activity. Antitumor Hf(IV) complexes are primarily complexes of β-diketonates, but they are not stable and their mechanism of action is still undefined. The synthesis of new heptacoordinated hafnium(IV) complexes of Salan derivatives and 2,6-dipicolinic acid [SalanHf(IV)Dipic] with a quick cellular uptake have been reported[123]. The Hf(IV)complexes have been tested against human derived hepatoma HepG2 and human cervical carcinoma HelaS3 cell lines. However, these hafnium(IV) complexes were limited in their aqueous stability and activity, unclear hydrolytic performance and antitumor mechanism and suffered from complicated synthesis and purification. Hf(IV) alkoxyl Salan and bimetallic oxidobridged Hf(IV) complex compounds have been recently synthesized and characterized[124]. The Hf(IV) complexes have shown better hydrolytic stability and anticancer activity against HelaS3 cells (human cervical carcinoma) and HepG2 cells (human derived hepatoma). The Hf(IV) and Zr(IV) complexes are very similar with reference to their structures and coordination modes.
Hafnocene dichloride HfCp2X2 exhibited no anti-tumoral activity against several cell lines. β-Diketonate hafnium(IV) complexes such as Hf(IV)-bis-β-diketonates with unsatisfactory antitumor activity or Hf(IV)-tri-β-diketonate with comparable antineoplastic activity to cisplatin have been reported. Bis- or tris-β-diketonate Hf(IV) complexes had shown inhibitory activity against human breast cancer MCF-7 and human colon cancer HT-29 cells comparable to cisplatin. Actually, Hf(IV) ions exhibit biomedical advantages such as biological compatibility and low toxic effects[125]. Hafnocene oxide HfO2, possessing notable chemical inertness, has been applied as microneedles in transdermal drug delivery[126].
Additionally, Hf(IV) ions possess strong X-ray attenuation ability and can be used as a radio-sensitizer, including HfO2 nanocrystal assemblies[127], Hf(IV) based nanoscale metalloorganic frameworks and Hf carbon dots covering a photosensitizer have been used for combined radiotherapy and photodynamic therapy[128]. Numerous studies have explored the application of hafnium-based nanomaterials in tumor visualization and diagnosis, displaying promising safety profiles with controllable toxicity[129,130]. These nanomaterials not only hold potential for tumor imaging and diagnosis but also for cancer chemotherapy, due to their unique chemical structures and functionalities.
4 CONCLUSION AND PROSPECTIVE
Cancer diagnostics and treatment which include inorganic pharmaceuticals and metal complexes have been improved over the past years. In this review, the rapid development, recent research trends and application of metal-based anticancer compounds of IB, IIB, IIIB and IVB groups have been summarized. To date, many metal-based protective and therapeutic drugs have been reported to be strong candidates to serve in antitumor chemotherapy. Though earlier studies have been based on a concept of mechanistic resemblances of different metal-based drugs, it is currently acceptable that different antineoplastic non-Pt-based derivatives operate via different mechanisms. The different metallic centers of the non-Pt-group compounds confer their distinct coordination chemistry and reactions with biological molecules, which afterward results in different pharmacological effects. The mechanism of action of many metallodtugs is not analogous to that of cisplatin and does not include direct interaction with nucleic acids. Because of their specific modes of action and different pharmacological profiles, the classes of non-Pt-metal-based candidates provide new prospects for investigations and the development of anticancer drug design. Nevertheless, numerous challenges remain for the development and application of metal-based compounds in anticancer therapy. There is a great need for metal-based drugs that are biodegradable, eliminable, and non-toxic. Most of the heavy metals, discussed here, are relatively new discovered metals. All this necessitates systematic studies on their stability, biocompatibility, cytotoxicity, and safety and warrants further investigations required to clarify the cellular modes of action. The ongoing endeavors of researchers would certainly contribute to the progress and implementation of metal-based drugs in tumor diagnosis and treatment. Hopefully, this review article can serve as a suitable basis of information and motivation for new possible research directions for the development of new compounds as effective and reliable anticancer agents.
Acknowledgements
The administrative support received by the European Union-Next Generation EU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No. BG-RRP-2.004-0004-C01 is greatly acknowledged.
Conflicts of Interest
The author declared no conflict of interest.
Data Availability
No additional data are available.
Copyright Permissions
Copyright © 2024 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Author Contribution
Kostova I solely contributed to the manuscript and approved the final version.
Abbreviation List
BSA, Bovine serum albumin
CDTA, Trans-1,2-diaminocyclohexane-N,N,N',N'-tetraacetic acid hydrate
CL, Curcumin ligand
DO3A, 1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic acid trisodium salt
DOTA, 2,2′,2”,2”'-(1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid
DTPA, Diethylenetriaminepentaacetic acid
EDTA, Ethylenediaminetetraacetic acid
MRI, Magnetic resonance imaging
NHCs, N-heterocyclic carbenes
NSAIDs, Non-steroidal anti-inflammatory drugs
PET, Positron emission tomography
ROS, Reactive oxygen species
SOD, Super oxide dismutase
References
[1] Kenny RG, Marmion CJ. Toward multi-targeted platinum and ruthenium drugs—a new paradigm in cancer drug treatment regimens? Chem Rev, 2019; 119: 1058-1137.[DOI]
[2] Sundar H, Padmini S, Devi PB. Bioremediation of nuclear waste effluent using different communities of microbes. In Metagenomics to Bioremediation, Academic Press, 2023.[DOI]
[3] Zuba I, Zuba M, Piotrowski M et al. Ruthenium as an important element in nuclear energy and cancer treatment. Appl Rad Isot, 2020; 162: 109176.[DOI]
[4] Sahu AK, Dash DK, Mishra K et al. Properties and applications of ruthenium. In: Noble and Precious Metals-Properties, Nanoscale Effects and Applications. IntechOpen, 2018.[DOI]
[5] Coverdale JP, Laroiya-McCarron T, Romero-Canelón I. Designing ruthenium anticancer drugs: What have we learnt from the key drug candidates? Inorganics, 2019; 7: 31.[DOI]
[6] Liu J, Zhang C, Rees TW et al. Harnessing ruthenium (II) as photodynamic agents: Encouraging advances in cancer therapy. Coord Chem Rev, 2018; 363: 17-28.[DOI]
[7] Thangavel P, Viswanath B, Kim S. Recent developments in the nanostructured materials functionalized with ruthenium complexes for targeted drug delivery to tumors. Int J Nanomed, 2017; 12: 2749.[DOI]
[8] Lee SY, Kim CY, Nam TG. Ruthenium complexes as anticancer agents: A brief history and perspectives. Drug Des Devel Ther, 2020; 5375-5392.[DOI]
[9] Simović AR, Masnikosa R, Bratsos I et al. Chemistry and reactivity of ruthenium (II) complexes: DNA/protein binding mode and anticancer activity are related to the complex structure. Coord Chem Rev, 2019; 398: 113011.[DOI]
[10] Cole HD, Roque JA, Lifshits LM et al. Fine‐Feature Modifications to Strained Ruthenium Complexes Radically Alter Their Hypoxic Anticancer Activity. Photochem Photobiol, 2022; 98: 73-84.[DOI]
[11] Liang L, Wu X, Shi C et al. Synthesis and characterization of polypyridine ruthenium (II) complexes and anticancer efficacy studies in vivo and in vitro. J Inorg Biochem, 2022; 236: 111963.[DOI]
[12] Chen J, Tao Q, Wu J et al. A lysosome-targeted ruthenium (II) polypyridyl complex as photodynamic anticancer agent. J Inorg Biochem, 2020; 210: 111132.[DOI]
[13] Chen C, Xu C, Li T et al. Novel NHC-coordinated ruthenium (II) arene complexes achieve synergistic efficacy as safe and effective anticancer therapeutics. Eur J Med Chem, 2020; 203: 112605.[DOI]
[14] Elsayed SA, Harrypersad S, Sahyon HA et al. Ruthenium (II)/(III) DMSO-based complexes of 2-aminophenyl benzimidazole with in vitro and in vivo anticancer activity. Molecules, 2020; 25: 4284.[DOI]
[15] Janković N, Milović E, Jovanović JĐ et al. A new class of half-sandwich ruthenium complexes containing Biginelli hybrids: anticancer and anti-SARS-CoV-2 activities. Chem Biol Inter, 2022; 363: 110025.[DOI]
[16] Kar B, Das U, De S et al. GSH-resistant and highly cytoselective ruthenium (II)-p-cymene-(imidazo [4, 5-f][1, 10] phenanthrolin-2-yl) phenol complexes as potential anticancer agents. Dalton Trans, 2021; 50: 10369-10373.[DOI]
[17] Yufanyi DM, Abbo HS, Titinchi SJ et al. Platinum (II) and Ruthenium (II) complexes in medicine: Antimycobacterial and Anti-HIV activities. Coord Chem Rev, 2020; 414: 213285.[DOI]
[18] Rahul K, Shekhar S. Ruthenium based antifungal compounds and their activity. Res J Chem Environ, 2021; 25: 177-182.[DOI]
[19] Rospond-Kubiak I, Wróblewska-Zierhoffer M, Twardosz-Pawlik H et al. Ruthenium brachytherapy for uveal melanoma–single institution experience. J Contemp Brachyther, 2017; 9: 548-552.[DOI]
[20] Sohrabi M, Saeedi M, Larijani B et al. Recent advances in biological activities of rhodium complexes: Their applications in drug discovery research. Eur J Med Chem, 2021; 216: 113308.[DOI]
[21] Ohata J, Ball ZT. Rhodium at the chemistry–biology interface. Dalton Trans, 2018; 47: 14855-14860.[DOI]
[22] Loreto D, Merlino A. The interaction of rhodium compounds with proteins: A structural overview. Coord Chem Rev, 2021; 442: 213999.[DOI]
[23] Scattolin T, Voloshkin VA, Visentin F et al. A critical review of palladium organometallic anticancer agents. Cell Rep Phys Sci, 2021; 2: 100446.[DOI]
[24] Olesya S, Prosekov A. Antimicrobial activity of mono-and polynuclear platinum and palladium complexes. Foods Raw Mater, 2020; 8: 298-311.[DOI]
[25] Jahromi EZ, Divsalar A, Saboury AA et al. Palladium complexes: new candidates for anti-cancer drugs. J Iran Chem Soc, 2016; 13: 967-989.[DOI]
[26] Carneiro TJ, Martins AS, Marques MPM et al. Metabolic aspects of palladium (II) potential anti-cancer drugs. Front Oncol, 2020; 10: 590970.[DOI]
[27] Pelclova D. Chapter 25 - Osmium. In: Nordberg GF, Costa M (eds). Handbook on the Toxicology of Metals (Fifth Edition). Academic Press, 2022: 639-647. [DOI]
[28] Nkomo D, Mwamba A. Beneficiation opportunities for osmium: A review. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing, 2020, 839: 012014.[DOI]
[29] Hanif M, Babak MV, Hartinger CG. Development of anticancer agents: Wizardry with osmium. Drug Discov Today, 2014; 19: 1640–1648.[DOI]
[30] Quinson J. Osmium and OsOx nanoparticles: an overview of syntheses and applications. Open Res Eur, 2022; 2: 39.[DOI]
[31] Ohriner EK. Processing of iridium and iridium alloys. Platinum Metals Rev, 2008; 52: 186.[DOI]
[32] Sharma A, Sudhindra P, Roy N et al. Advances in novel iridium (III) based complexes for anticancer applications: A review. Inorg Chim Acta, 2020; 513: 119925.[DOI]
[33] Liang J, Sun D, Yang Y et al. Discovery of metal-based complexes as promising antimicrobial agents. Eur J Med Chem, 2021; 224: 113696.[DOI]
[34] Quinson J. Iridium and IrOx nanoparticles: an overview and review of syntheses and applications. Adv Colloid Interf Sci, 2022; 102643.[DOI]
[35] Singh SB. Iridium Chemistry and Its Catalytic Applications: a Brief. Green Chem Technol Lett, 2016; 2: 456-518.[DOI]
[36] Walker M, Smith JR. Iridium‐192: A literature review for further referencing the isotope, its activity units, and dosimetry techniques. Veterin Radiol, 1990; 31: 281-292.[DOI]
[37] Odularu AT, Ajibade PA, Mbese JZ et al. Developments in platinum-group metals as dual antibacterial and anticancer agents. J Chem, 2019; 2019: 5459461.[DOI]
[38] Abed A, Derakhshan M, Karimi M et al. Platinum nanoparticles in biomedicine: Preparation, anti-cancer activity, and drug delivery vehicles. Front Pharmacol, 2022; 13: 797804.[DOI]
[39] Vojtek M, Martins CB, Ramos R et al. Pd (II) and Pt (II) trinuclear chelates with spermidine: selective anticancer activity towards TNBC-sensitive and-resistant to cisplatin. Pharmaceutics, 2023; 15: 1205.[DOI]
[40] Marques MPM, Batista de Carvalho ALM, Mamede AP et al. A new look into the mode of action of metal-based anticancer drugs. Molecules, 2020; 25: 246.[DOI]
[41] Zhu Z, Wang Z, Zhang C et al. Mitochondrion-targeted platinum complexes suppressing lung cancer through multiple pathways involving energy metabolism. Chem Sci, 2019; 10: 3089-3095.[DOI]
[42] He C, Majd MH, Shiri F et al. Palladium and platinum complexes of folic acid as new drug delivery systems for treatment of breast cancer cells. J Mol Struct, 2021; 1229: 129806.[DOI]
[43] Adams M, Sullivan MP, Tong KK et al. Mustards-derived Terpyridine–platinum complexes as anticancer agents: DNA alkylation vs coordination. Inorg Chem, 2021; 60: 2414-2424.[DOI]
[44] Kutlu E, Emen FM, Kismali G et al. Pyridine derivative platinum complexes: Synthesis, molecular structure, DFT and initial anticancer activity studies. J Mol Struct, 2021; 1234: 130191.[DOI]
[45] Mbugua SN, Sibuyi NR, Njenga LW et al. New palladium (II) and platinum (II) complexes based on pyrrole Schiff bases: synthesis, characterization, X-ray structure, and anticancer activity. ACS Omega, 2020; 5: 14942-14954.[DOI]
[46] Lozada IB, Huang B, Stilgenbauer M et al. Monofunctional platinum (ii) anticancer complexes based on multidentate phenanthridine-containing ligand frameworks. Dalton Trans, 2020; 49: 6557-6560.[DOI]
[47] Islam MK, Baek A-R, Sung B et al. Synthesis, Characterization, and Anticancer Activity of Benzothiazole Aniline Derivatives and Their Platinum (II) Complexes as New Chemotherapy Agents. Pharmaceuticals, 2021; 14: 832.[DOI]
[48] Bai X, Ali A, Wang N et al. Inhibition of SREBP-mediated lipid biosynthesis and activation of multiple anticancer mechanisms by platinum complexes: Ascribe possibilities of new antitumor strategies. Eur J Med Chem, 2022; 227: 113920.[DOI]
[49] Zeynali H, Keypour H, Hosseinzadeh L et al. The non-templating synthesis of macro-cyclic Schiff base ligands containing pyrrole and homopiperazine and their binuclear nickel (II), cobalt (II) and mononuclear platinum (II) complexes: X-ray single crystal and anticancer studies. J Mol Struct, 2021; 1244: 130956.[DOI]
[50] Novohradsky V, Pracharova J, Kasparkova J et al. Induction of immunogenic cell death in cancer cells by a photoactivated platinum (IV) prodrug. Inorg Chem Front, 2020; 7: 4150-4159.[DOI]
[51] Kostova I. Biological and Medical Significance of Chemical Elements, Bentham Science Publishers, 2023.[DOI]
[52] Kostova I. The Role of Complexes of Biogenic Metals in Living Organisms. Inorganics, 2023; 11, 56.[DOI]
[53] Goswami AK, Kostova I. Medicinal and Biological Inorganic Chemistry. Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2022.[DOI]
[54] Nierengarten JF. In My Element: Copper. Chem Eur J, 2019; 25: 16-18.[DOI]
[55] Krasnovskaya O, Naumov A, Guk D et al. Copper coordination compounds as biologically active agents. Int J Mol Sci, 2020; 21: 3965.[DOI]
[56] Scheiber I, Dringen R, Mercer JF. Copper: effects of deficiency and overload. Met Ions Life Sci, 2013; 13: 359-387.[DOI]
[57] Gaggelli E, Kozlowski H, Valensin D et al. Copper homeostasis and neurodegenerative disorders (Alzheimer's, prion, and Parkinson's diseases and amyotrophic lateral sclerosis). Chem Rev, 2006; 106: 1995–2044.[DOI]
[58] Ge EJ, Bush AI, Casini A et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer, 2022; 22: 102-113.[DOI]
[59] Efthimiadou EK, Katsarou ME, Karaliota A et al. Copper(II) complexes with Sparfloxacin and nitrogen-donor heterocyclic ligands: structure–activity relationship. J Inorg Biochem, 2008; 102: 910-92.[DOI]
[60] Jopp M, Becker J, Becker S et al. Anticancer activity of a series of copper (II) complexes with tripodal ligands. Eur J Med Chem, 2017; 132: 274-281.[DOI]
[61] Kareem A, Khan MS, Nami SA et al. Curcumin derived Schiff base ligand and their transition metal complexes: Synthesis, spectral characterization, catalytic potential and biological activity. J Mol Struct, 2018; 1167: 261-273.[DOI]
[62] Zhang AL, Li XC, Min J et al. Synthesis and anticancer property of three new Cu (II) coordination polymers constructed by the bifunctional substituted-polynitrogen heterocyclic ligands. Inorg Chim Acta, 2021; 522: 120380.[DOI]
[63] Sharfalddin AA, Emwas AH, Jaremko M et al. Transition metal complexes of 6‐mercaptopurine: Characterization, Theoretical calculation, DNA‐Binding, molecular docking, and anticancer activity. Appl Organomet Chem, 2021; 35: e6041.[DOI]
[64] Khan RA, Usman M, Dhivya R et al. Heteroleptic copper(I) complexes of “scorpionate” bis-pyrazolyl carboxylate ligand with auxiliary phosphine as potential anticancer agents: an insight into cytotoxic mode. Sci Rep, 2017; 7: 45229.[DOI]
[65] Kacar S, Unver H, Sahinturk V. A mononuclear copper(II) complex containing benzimidazole and pyridyl ligands: synthesis, characterization, and antiproliferative activity against human cancer cells. Arab J Chem, 2020; 13: 4310-4323.[DOI]
[66] Eckhardt S, Brunetto PS, Gagnon J et al. Nanobio silver: its interactions with peptides and bacteria, and its uses in medicine. Chem Rev, 2013; 113: 4708-4754.[DOI]
[67] Gemmell CG, Edwards DI, Fraise AP et al. Guidelines for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J Antimicrob Chemother, 2006; 57: 589-608.[DOI]
[68] Galdiero S, Falanga A, Vitiello M et al. Silver nanoparticles as potential antiviral agents. Molecules, 2011; 16: 8894-8918.[DOI]
[69] Raju SK, Karunakaran A, Kumar S et al. Silver Complexes as Anticancer Agents: A Perspective Review. Germ J Pharmac Biomater, 2022; 1: 6-28.[DOI]
[70] Nunes JH, Bergamini FR, Lustri WR et al. Synthesis, characterization and in vitro biological assays of a silver (I) complex with 5-fluorouracil: A strategy to overcome multidrug resistant tumor cells. J Fluor Chem, 2017; 195: 93-101.[DOI]
[71] Altay A, Caglar S, Caglar B et al. Novel silver (I) complexes bearing mefenamic acid and pyridine derivatives: Synthesis, chemical characterization and in vitro anticancer evaluation. Inorg Chim Acta, 2019; 493: 61-71.[DOI]
[72] Harurluoglu B, Altay A, Caglar S et al. Binuclear silver (I) complexes with the non-steroidal anti-inflammatory drug tolfenamic acid: Synthesis, characterization, cytotoxic activity and evaluation of cellular mechanism of action. Polyhedron, 2021; 202: 115189.[DOI]
[73] Yuan Q, Zhao Y, Cai P et al. Dose-dependent efficacy of gold clusters on rheumatoid arthritis therapy. ACS Omega, 2019; 4: 14092-14099.[DOI]
[74] Raubenheimer HG, Schmidbaur H. The late start and amazing upswing in gold chemistry. J Chem Educ. 2014; 91: 2024-2036.[DOI]
[75] Yang Z, Jiang G, Xu, Z et al. Advances in alkynyl gold complexes for use as potential anticancer agents. Coord Chem Rev, 2020; 423: 213492.[DOI]
[76] Bian M, Fan R, Jiang G et al. Halo and pseudohalo gold (I)–NHC complexes derived from 4, 5-diarylimidazoles with excellent in vitro and in vivo anticancer activities against HCC. J Med Chem, 2020; 63: 9197-9211.[DOI]
[77] Walther W, Althagafi D, Curran D et al. In-vitro and in-vivo investigations into the carbene-gold anticancer drug candidates NHC*-Au-SCSNMe2 and NHC*-Au-S-GLUC against advanced prostate cancer PC3. Anti-Cancer Drugs, 2020; 31: 672-683.[DOI]
[78] Gulzar S, Ammara U, Abid Z et al. Synthesis, in vitro anticancer activity and reactions with biomolecule of gold (I)-NHC carbene complexes. J Mol Struct, 2022; 1255: 132482.[DOI]
[79] Alsaeedi MS, Babgi BA, Hussien MA et al. DNA-binding and anticancer activity of binuclear gold (I) alkynyl complexes with a phenanthrenyl bridging ligand. Molecules, 2020; 25: 1033.[DOI]
[80] Marmol I, Castellnou P, Alvarez R et al. Alkynyl Gold (I) complexes derived from 3-hydroxyflavones as multi-targeted drugs against colon cancer. Eur J Med Chem, 2019; 183: 111661.[DOI]
[81] Babgi BA, Alsayari J, Alenezi HM et al. Alteration of anticancer and protein-binding properties of gold (I) Alkynyl by phenolic Schiff bases moieties. Pharmaceutics, 2021; 13: 461.[DOI]
[82] Tabrizi L, Yang WS, Chintha C et al. Gold (I) Complexes with a Quinazoline Carboxamide Alkynyl Ligand: Synthesis, Cytotoxicity, and Mechanistic Studies. Eur J Inorg Chem, 2021; 2021: 1921-1928.[DOI]
[83] Frezza M, Hindo S, Chen D et al. Novel metals and metal complexes as platforms for cancer therapy. Curr Pharm Des, 2010; 16: 1813-1825.[DOI]
[84] El-Sherif AA, Eldebss TM. Synthesis, spectral characterization, solution equilibria, in vitro antibacterial and cytotoxic activities of Cu(II), Ni(II), Mn(II), Co(II) and Zn(II) complexes with Schiff base derived from 5- bromosalicylaldehyde and 2-aminomethylthiophene. Spectrochim Acta A Biomol Spectrosc, 2011; 79: 1803-1814.[DOI]
[85] Khan RA, De Almeida A, Al-Farhan K et al. Transition-metal norharmane compounds as possible cytotoxic agents: New insights based on a coordination chemistry perspective. J Inorg Biochem, 2016; 165: 128-135.[DOI]
[86] Deswal Y, Asija S, Kumar D et al. Transition metal complexes of triazole-based bioactive ligands: synthesis, spectral characterization, antimicrobial, anticancer and molecular docking studies. Res Chem Intermed, 2021; 1-27.[DOI]
[87] Beheshti A, Bahrani‐Pour M, Kolahi M et al. Synthesis, structural characterization, and density functional theory calculations of the two new Zn (II) complexes as antibacterial and anticancer agents with a neutral flexible tetradentate pyrazole‐based ligand. Appl Organomet Chem, 2021; 35: e6173.[DOI]
[88] Pathan AH, Ramesh AK, Bakale RP et al. Association of late transition metal complexes with ethyl 2-(2-(4-chlorophenylcarbamothioyl) hydrazono) propanoate: Design, synthesis and in vitro anticancer studies. Inorg Chim Acta, 2015; 430: 216-224.[DOI]
[89] Saturnino C, Napoli M, Paolucci G et al. Synthesis and cytotoxic activities of group 3 metal complexes having monoanionic tridentate ligands. Eur J Med Chem, 2010; 45: 4169-4174.[DOI]
[90] Vaughn BA, Koller AJ, Boros E. Aqueous chemistry of the smallest rare earth: Comprehensive characterization of radioactive and non-radioactive scandium complexes for biological applications. Meth Enzymol, 2021; 651: 343-371.[DOI]
[91] Herath HMTU, Silvio LD, Evans JRG. Scandia-A potential biomaterial? J Mater Sci Mater Electron, 2005; 16: 1061-1065.[DOI]
[92] Muñoz-Garcia J, Mazza M, Alliot C et al et al. Antiproliferative Properties of Scandium Exopolysaccharide Complexes on Several Cancer Cell Lines. Mar Drugs, 2021; 19: 174.[DOI]
[93] Saturnino C, Bortoluzzi M, Napoli M et al. New insights on cytotoxic activity of group 3 and lanthanide compounds: complexes with [N,N,N]-scorpionate ligands, J Pharm Pharmacol, 2013; 65: 1354-1359.[DOI]
[94] Alshamrani A, Mark RJ. Scandium calix [n] arenes (n=4, 6, 8): structural, cytotoxicity and ring opening polymerization studies. Dalton Trans, 2021; 50: 8302-8306.[DOI]
[95] Pniok M, Kubíèek V, Havlíèková J et al. Thermodynamic and kinetic study of scandium(III) complexes of DTPA and DOTA: a step toward scandium radiopharmaceuticals. Chem Eur J, 2014; 20: 7944-7955.[DOI]
[96] Tickner BJ, Stasiuk GJ, Duckett SB et al. The use of yttrium in medical imaging and therapy: Historical background and future perspectives. Chem Soc Rev, 2020; 49: 6169-6185.[DOI]
[97] Zidan ASA, Ibrahim ABM, Aly AAM et al. Synthesis, Solid State Structure, and Cytotoxic Activity of a Complex Dimer of Yttrium with Anthranilic Acid against Cancer Cells. Biol Trace Elem Res, 2023; 201: 4688-4696.[DOI]
[98] Jahani S, Aramesh-Boroujeni Z, Noroozifar M. In vitro anticancer and antibacterial activates of the yttrium (III) complex and its nano-carriers toward DNA cleavage and biological interactions with DNA and BSA; An experimental and computational studies. J Tr Elem Med Biol, 2021; 68: 126821.[DOI]
[99] Khorshidi M, Asadpour S, Aramesh-Boroujeni Z et al. Spectroscopic and molecular modeling studies of binding interaction between the new complex of yttrium and 1, 10-phenanthroline derivatives with DNA and BSA. Front Chem, 2023; 11: 1231504.[DOI]
[100] Wang X, Zhong X, Cheng L. Titanium-based nanomaterials for cancer theranostics. Coord Chem Rev, 2021; 430: 213662.[DOI]
[101] Cini M, Bradshaw TD, Woodward S. Using titanium complexes to defeat cancer: the view from the shoulders of titans. Chem Soc Rev, 2017; 46: 1040-1051.[DOI]
[102] Buettner KM, Valentine AM. Bioinorganic chemistry of titanium, Chem Rev, 2012; 112: 1863-1881.[DOI]
[103] Tshuva EY, Miller M. Metallo-drugs: Development and action of anti-cancer agents, in: A. Sigel, H. Sigel, E. Freisinger, R.K.O. Sigel (Eds.), Met. Dev. Action Anti-Cancer Agents, 18th ed, De Gruyter, Berlin, 2018, pp. 219-249.
[104] Loza-Rosas SA, Saxena M, Delgado Y et al. A ubiquitous metal, difficult to track: towards an understanding of the regulation of titanium(iv) in humans. Metallomics, 2017; 9: 346-356.[DOI]
[105] Shavit M, Peri D, Manna CM et al. Active cytotoxic reagents based on non-metallocene non-diketonato well-defined C2-symmetrical titanium complexes of tetradentate bis(phenolato) ligands. J Am Chem Soc, 2007; 129: 12098-12099.[DOI]
[106] Peri D, Meker S, Shavit M et al. Synthesis, characterization, cytotoxicity, and hydrolytic behavior of C2- and C1-symmetrical Ti(IV) complexes of tetradentate diamine bis(phenolato) ligands: a new class of antitumor agents. Chem Eur J, 2009; 15: 2403-2415.[DOI]
[107] Caruso F, Rossib M, Opazo C et al. Structural features of antitumor titanium agents and related compounds. Bioinorg Chem Appl, 2005; 3: 317-330.[DOI]
[108] Nahari G, Tshuva EY. Synthesis of asymmetrical diaminobis(alkoxo)-bisphenol compounds and their C 1-symmetrical mono-ligated titanium(iv) complexes as highly stable highly active antitumor compounds. Dalton Trans, 2021; 50: 6423-6426.[DOI]
[109] Pedko A, Rubanovich E, Tshuva EY et al. Hydrolytically stable and cytotoxic [ONON]2Ti(IV)-type octahedral complexes. Inorg Chem, 2022; 61: 17653-17661.[DOI]
[110] Miller M, Mellul A, Braun M et al. Titanium tackles the endoplasmic reticulum: a first genomic study on a titanium anticancer metallodrug. IScience, 2020; 23: 101262.[DOI]
[111] Skoupilova H, Hrstka R, Bartosik M. Titanocenes as anticancer agents: recent insights. Med Chem, 2017; 13: 334-344.[DOI]
[112] Zhao TK, Grützke M, Gotz KH et al. Synthesis and X-ray structure analysis of cytotoxic heptacoordinate sulfonamide salan titanium(IV)-bischelates. Dalton Trans, 2015; 44: 16475-16485.[DOI]
[113] Allen OR, Knox RJ, McGowan PC. Functionalised cyclopentadienyl zirconium compounds as potential anticancer drugs. Dalton Trans, 2008; 39: 5293-5295.[DOI]
[114] Lord RM, Mannion JJ, Hebden AJ et al. Mechanistic and Cytotoxicity Studies of Group IV β-Diketonate Complexes. ChemMedChem, 2014; 9: 1136-1139.[DOI]
[115] Schneider F, Zhao T, Huhn T. Cytotoxic heteroleptic heptacoordinate salan zirconium(IV)-bis-chelates – synthesis, aqueous stability and X-ray structure analysis. Chem Commun, 2016; 52: 10151-10154.[DOI]
[116] Abdolmaleki S, Ghadermazi M, Ashengroph M et al. Cobalt (II), zirconium (IV), calcium (II) complexes with dipicolinic acid and imidazole derivatives: X-ray studies, thermal analyses, evaluation as in vitro antibacterial and cytotoxic agents. Inorg Chim Acta, 2018; 480: 70-82.[DOI]
[117] El‐Shwiniy WH, Shehab WS, Mohamed SF et al. Synthesis and cytotoxic evaluation of some substituted pyrazole zirconium (IV) complexes and their biological assay. Appl Organomet Chem, 2018; 32: e4503.[DOI]
[118] Malghe YS, Prabhu RC, Raut RW. Synthesis, characterization and biological activities of mixed ligand Zr (IV) complexes. Acta Pol Pharm, 2009; 66: 45-50.[DOI]
[119] Yang M, Liu N, Wang P et al. Synthesis and cytotoxicity study of water soluble 8-hydroxyquinoline stabilized zirconium (IV) complexes. Inorg Chem Commun, 2023; 153: 110795.[DOI]
[120] Adam MSS, El-Hady OM, Makhlouf MM et al. Effect of oxy-vanadium (IV) and oxy-zirconium (IV) ions in O, N-bidentate arylhydrazone complexes on their catalytic and biological potentials that supported via computerized usages. J Taiwan Inst Chem Engin, 2022; 132: 104168.[DOI]
[121] Al-Hakimi AN, Alminderej F, Aroua L et al. Design, synthesis, characterization of zirconium (IV), cadmium (II) and iron (III) complexes derived from Schiff base 2- aminomethylbenzimidazole, 2-hydroxynaphtadehyde and evaluation of their biological activity. Arab J Chem, 2020; 13: 7378-7389.[DOI]
[122] Bhatt NB, Pandya DN, Wadas TJ. Recent Advances in Zirconium-89 Chelator Development. Molecules, 2018; 23: 638.[DOI]
[123] Zhao T, Wang P, Liu N et al. Synthesis and X-ray structure analysis of cytotoxic heptacoordinated Salan hafnium (IV) complexes stabilized with 2, 6-dipicolinic acid. J Inorg Biochem, 2023; 240: 112094.[DOI]
[124] Zhao W, Yuan P, Zhang Q et al. Novel bimetallic oxido-bridged phenolato hafniumIV complex with enhanced anti-tumor activity and aqueous stability. Res Chem, 2023; 6: 101161.[DOI]
[125] Jayaraman V, Bhavesh G, Chinnathambi S et al. Synthesis and characterization of hafnium oxide nanoparticles for bio-safety. Mater Express, 2014; 4: 375-383.[DOI]
[126] Zhang YH, Campbell SA, Karthikeyan S. Finite element analysis of hollow outof-plane HfO2 microneedles for transdermal drug delivery applications. Biomed Microdevices, 2018; 20: 19.1-19.7.[DOI]
[127] Li Y, Qi Y, Zhang H et al. Gram-scale synthesis of highly biocompatible and intravenous injectable hafnium oxide nanocrystal with enhanced radiotherapy efficacy for cancer theranostic. Biomaterials, 2020; 226: 119538.[DOI]
[128] Liu J, Yang Y, Zhu W et al. Nanoscale metal− organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials, 2016; 97: 1-9.[DOI]
[129] Marill J, Anesary NM, Zhang P et al. Hafnium oxide nanoparticles: toward an in vitro predictive biological effect? Radiat Oncol, 2014; 9: 150.[DOI]
[130] Pottier A, Borghi E, Levy L. New use of metals as nanosized radioenhancers. Anticancer Res, 2014; 34: 443-453.
Brief of Corresponding Author(s)
She graduated from Mendeleev University in Moscow, and is one of the World’s Top 1% of scientists according to Stanford Universit’s ranking of scientists with the greatest contribution to the development of modern science. She has been a lecturer at renowned universities in India, Austria, Italy, Romania, Spain, Slovakia etc. Her research interests include the developmental work in theoretical and coordination chemistry, vibrational spectroscopy, pharmacological investigations of biologically active compounds etc. Author of about 200 publications with high impact factor, several textbooks, education book materials and monographs with around 7000 citations in indexed journals with an h-index of 38. |

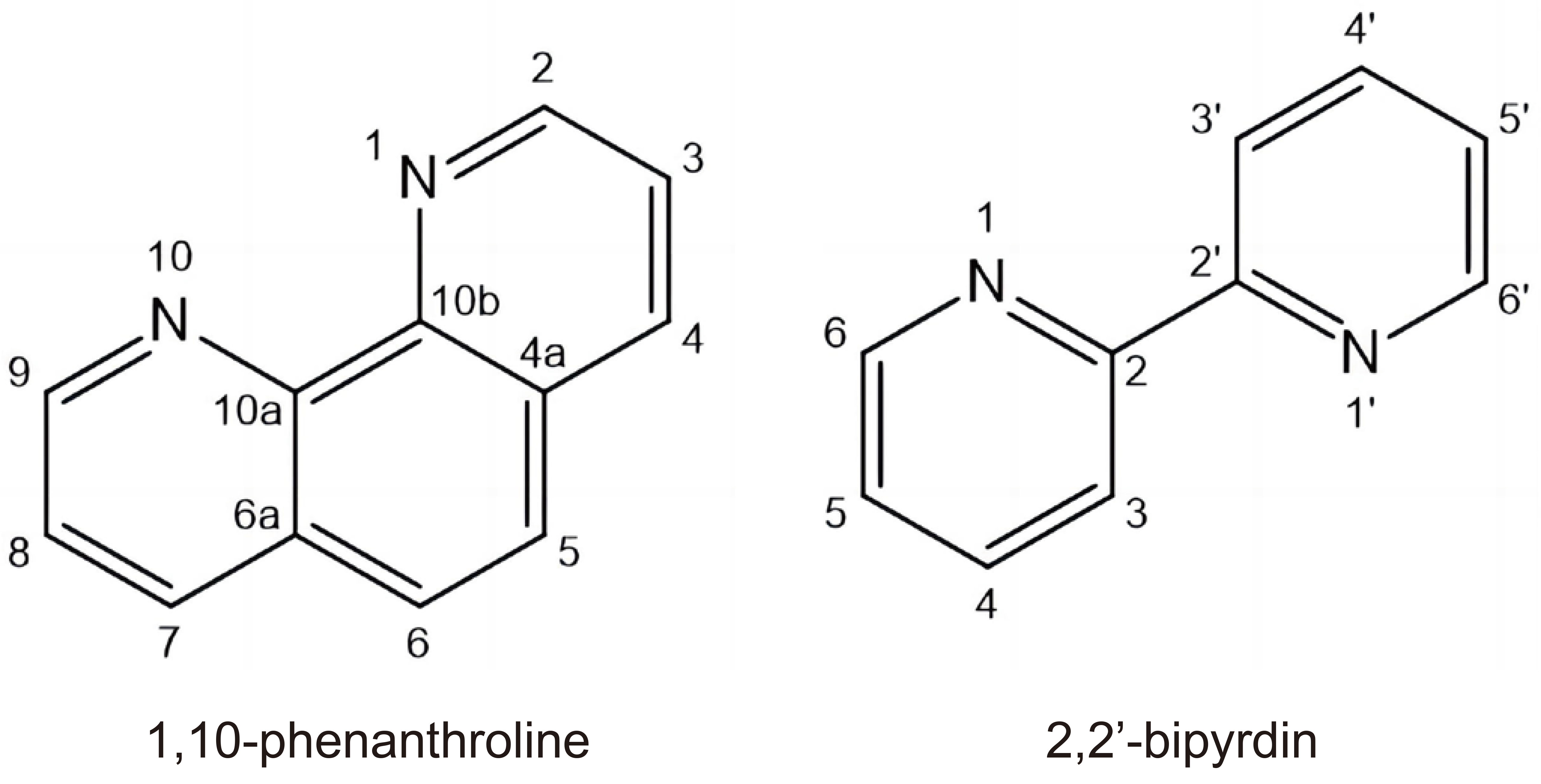
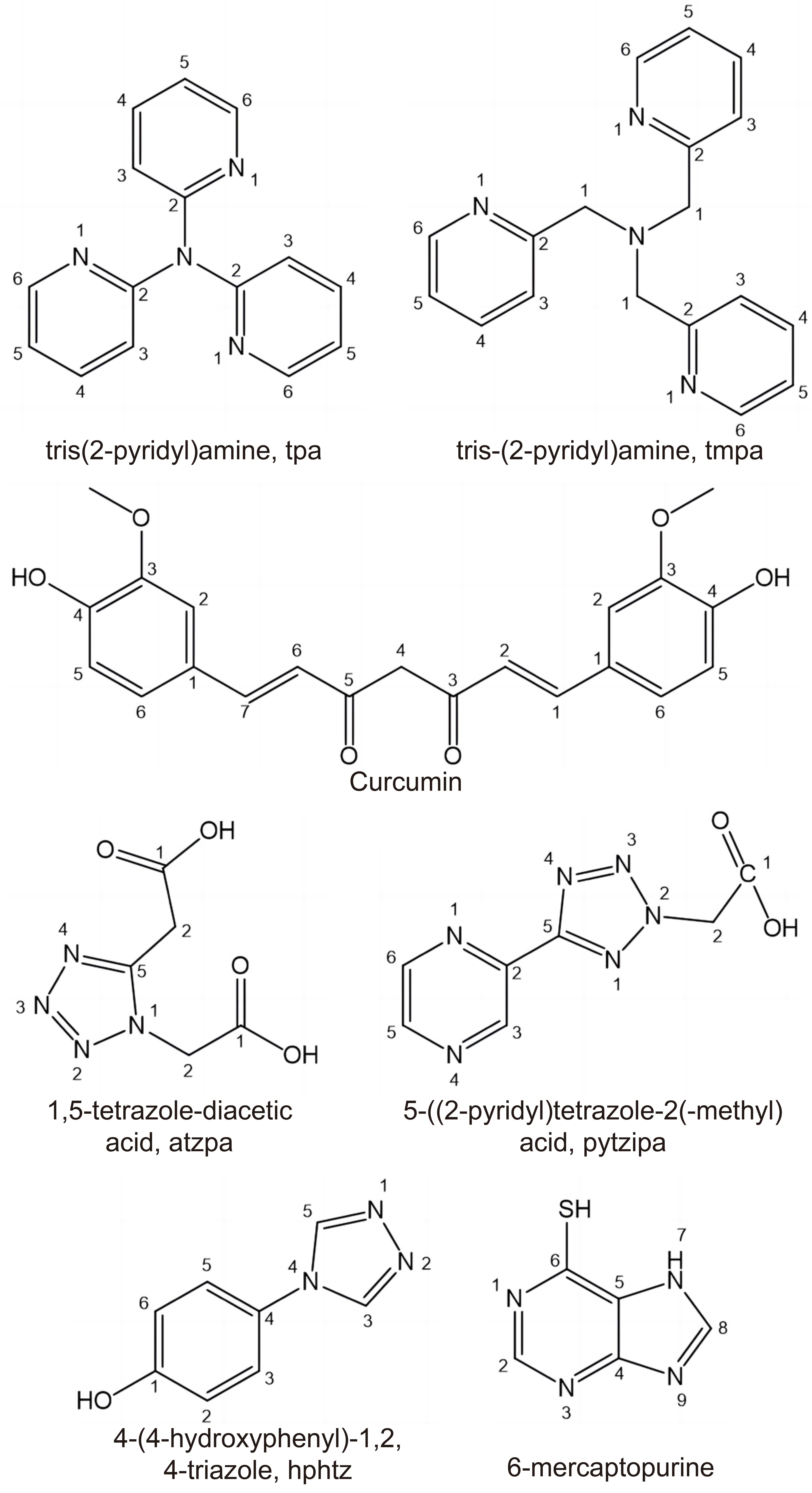
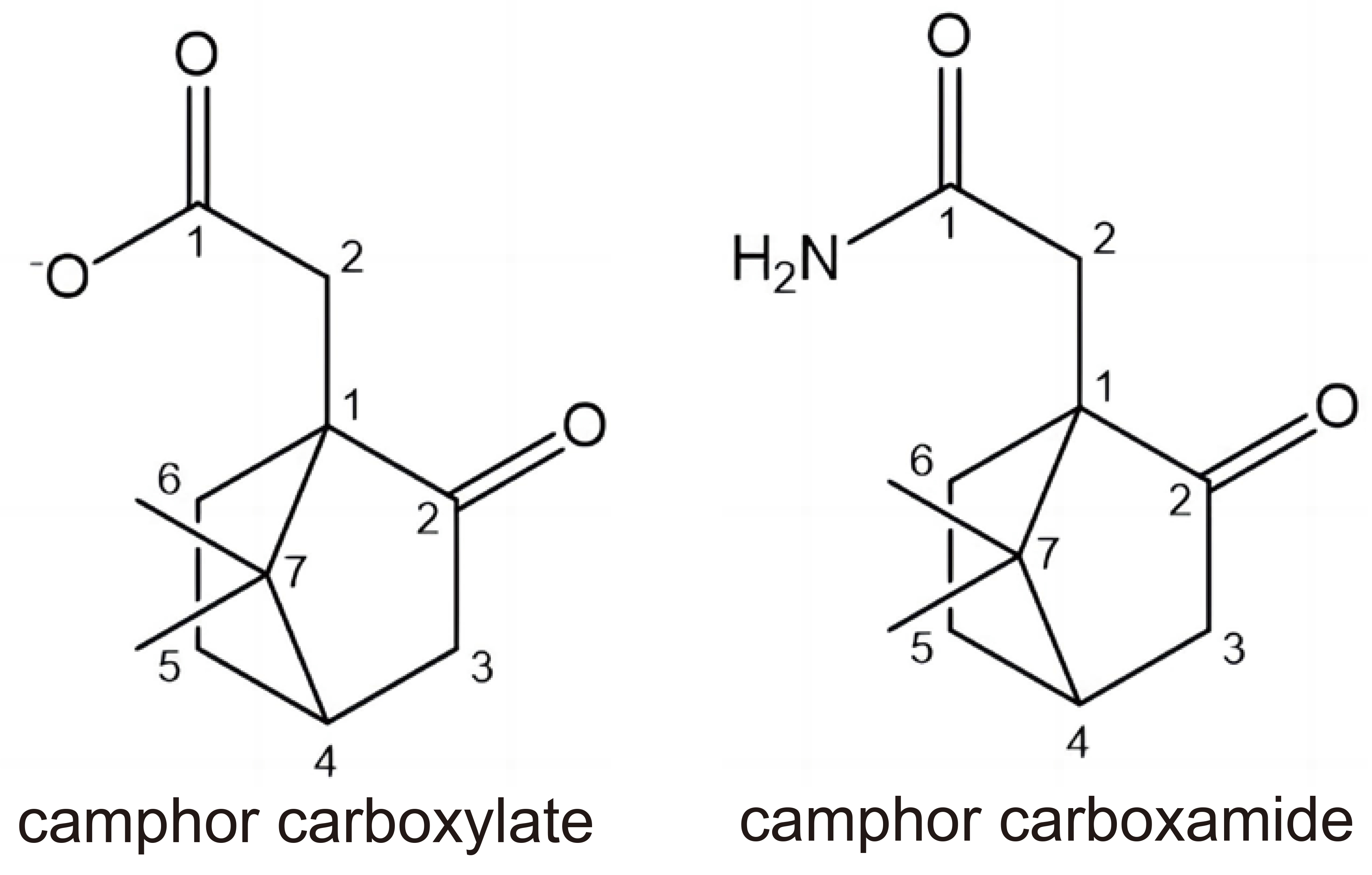
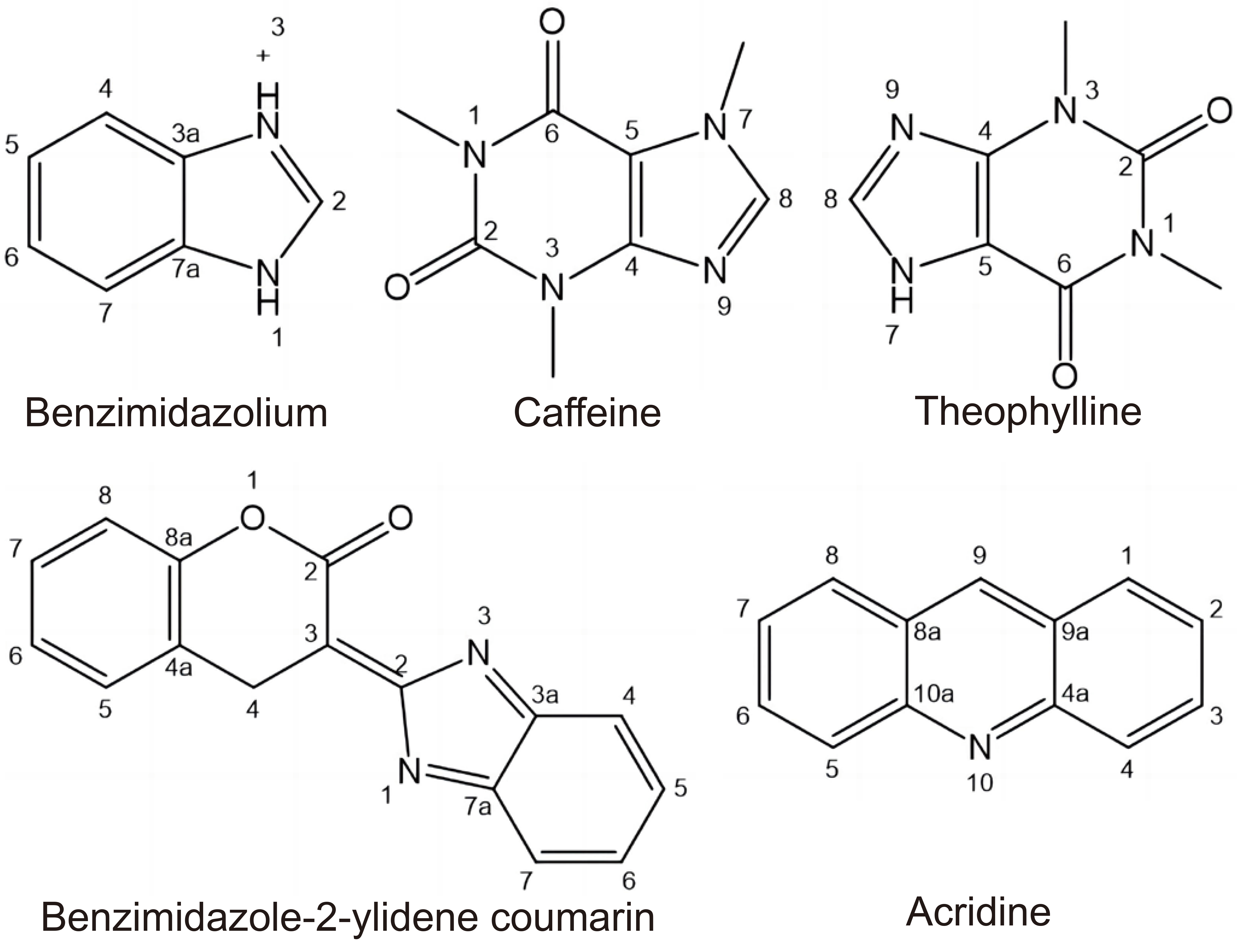
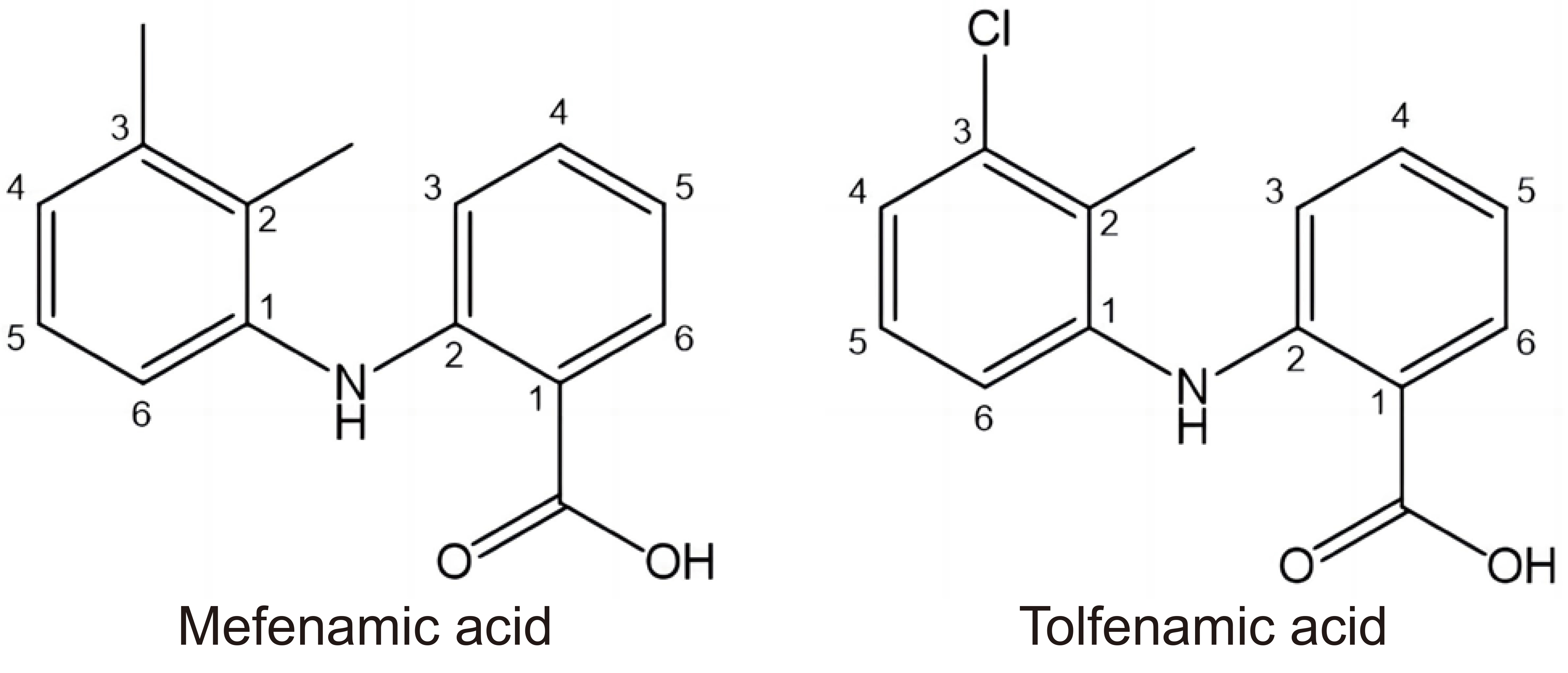
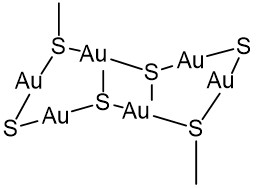
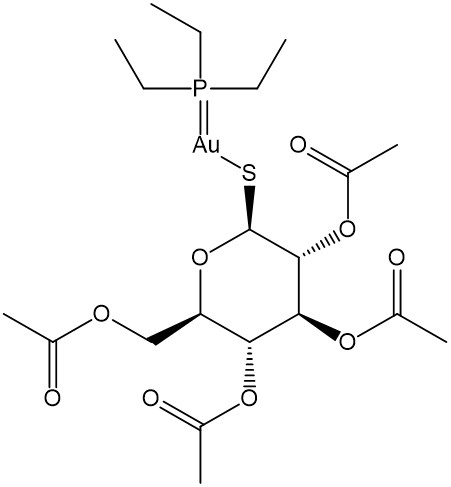
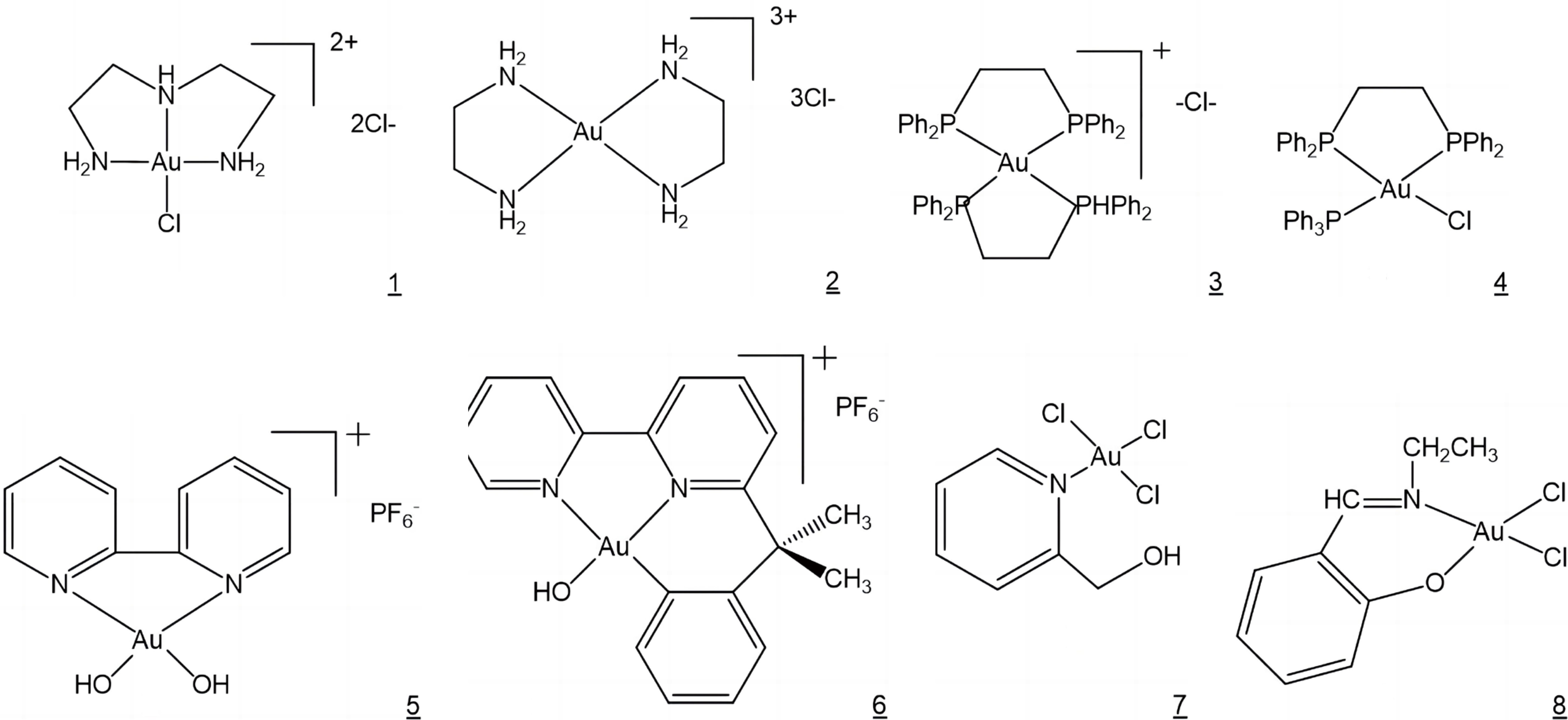
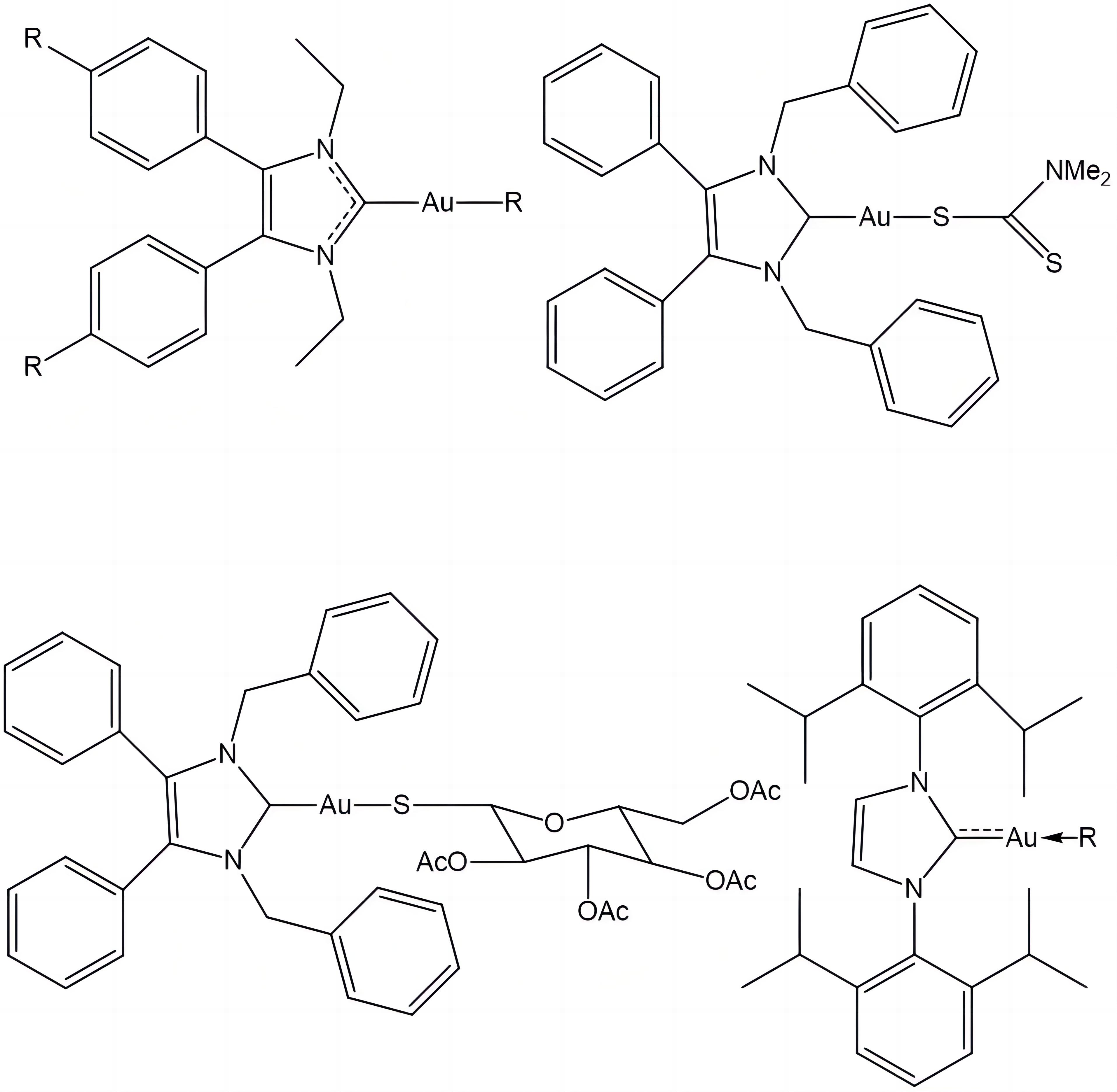
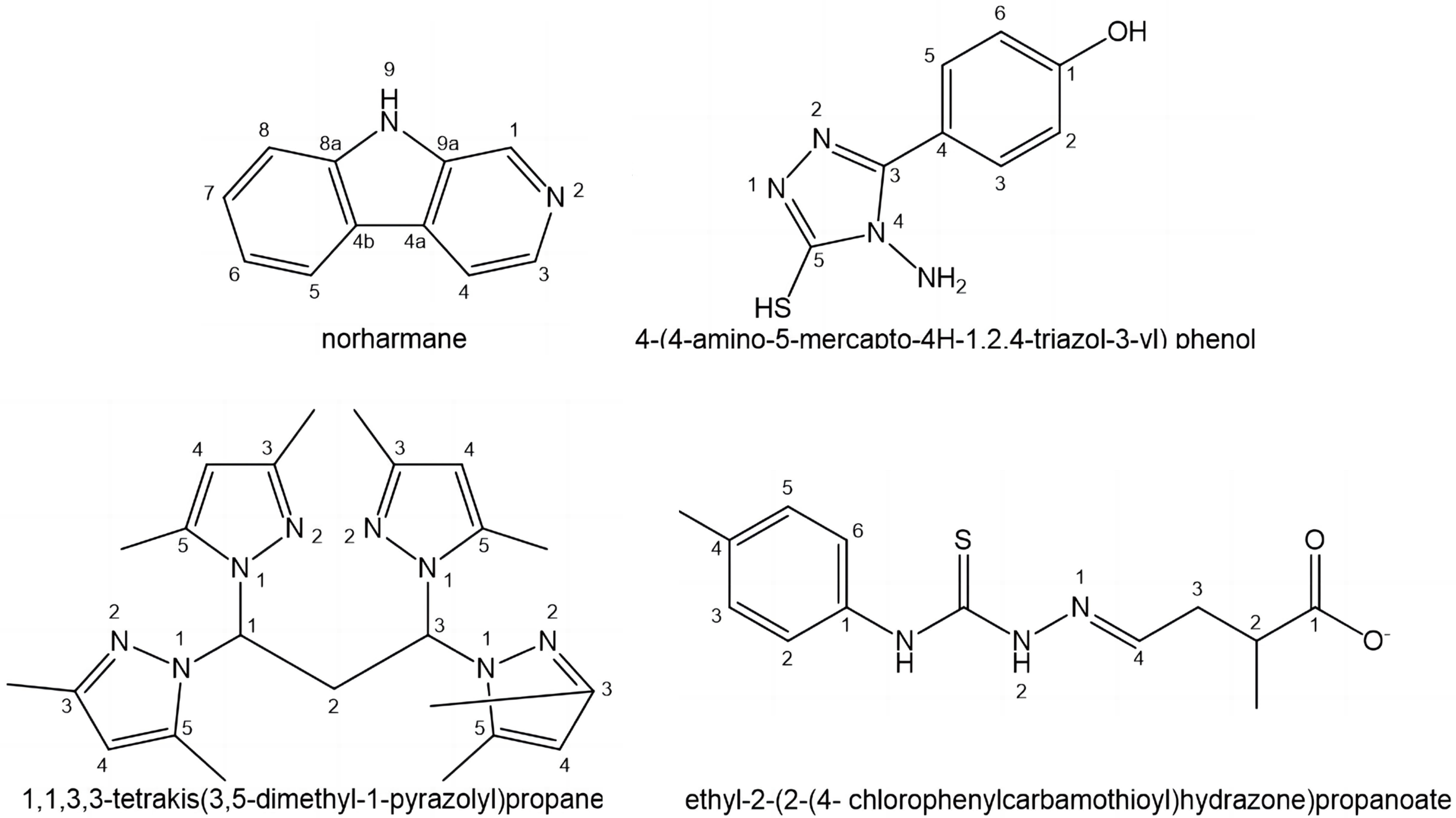
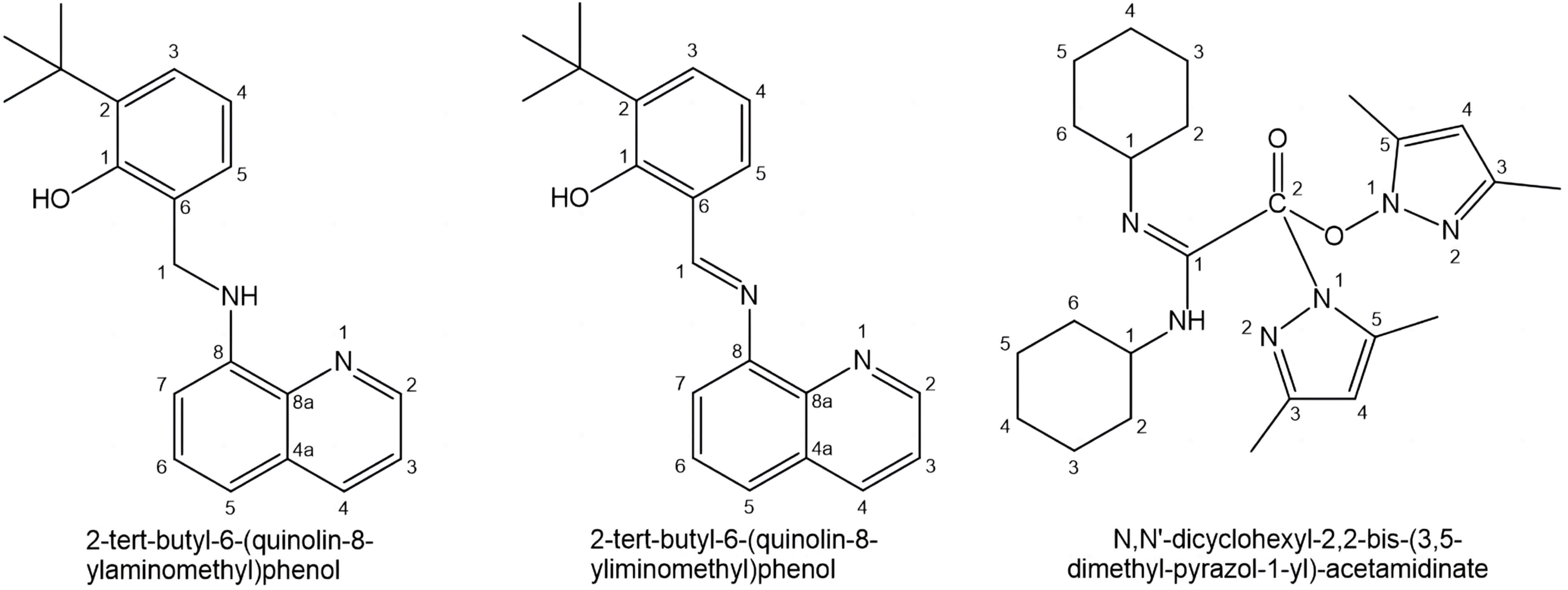

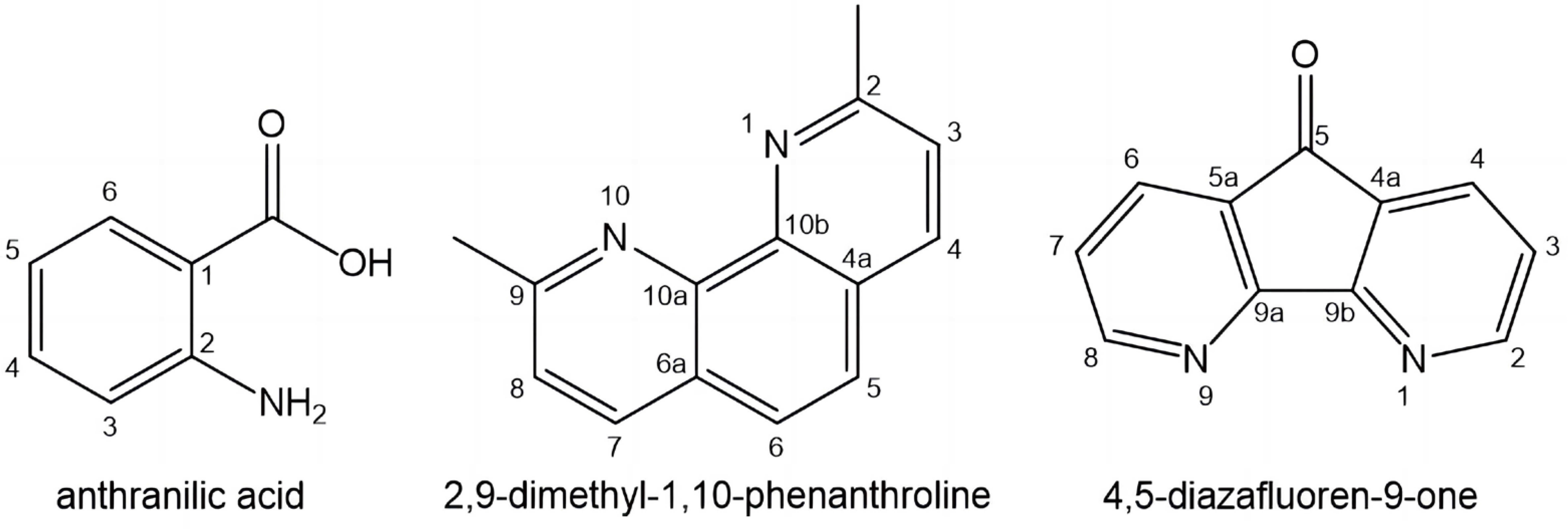
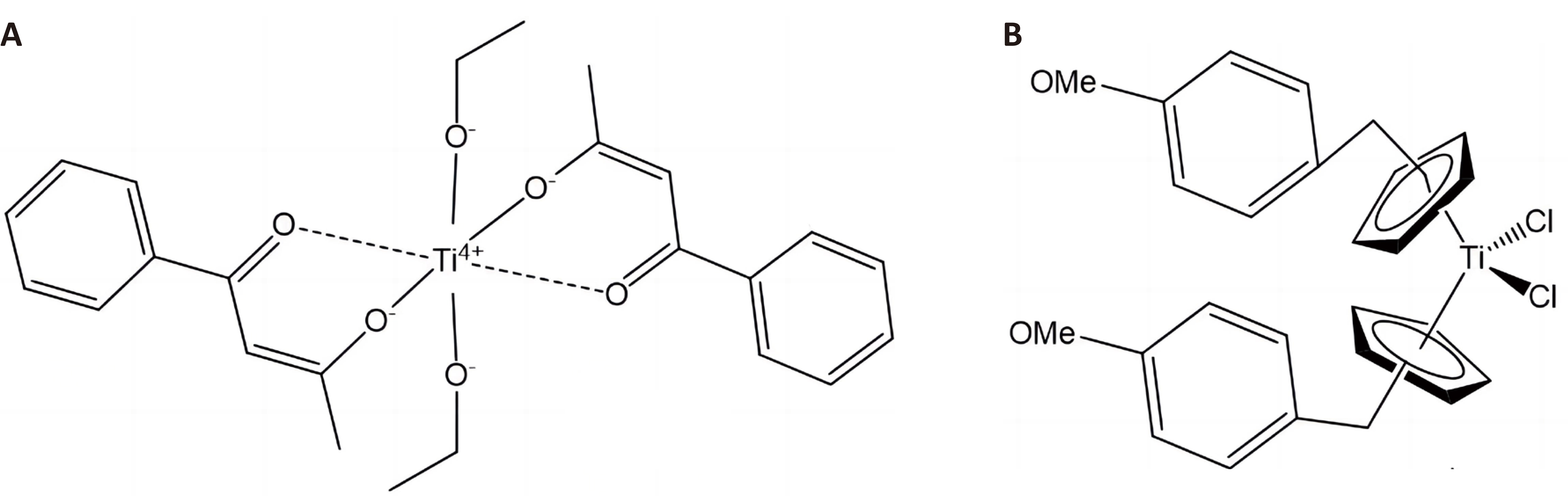

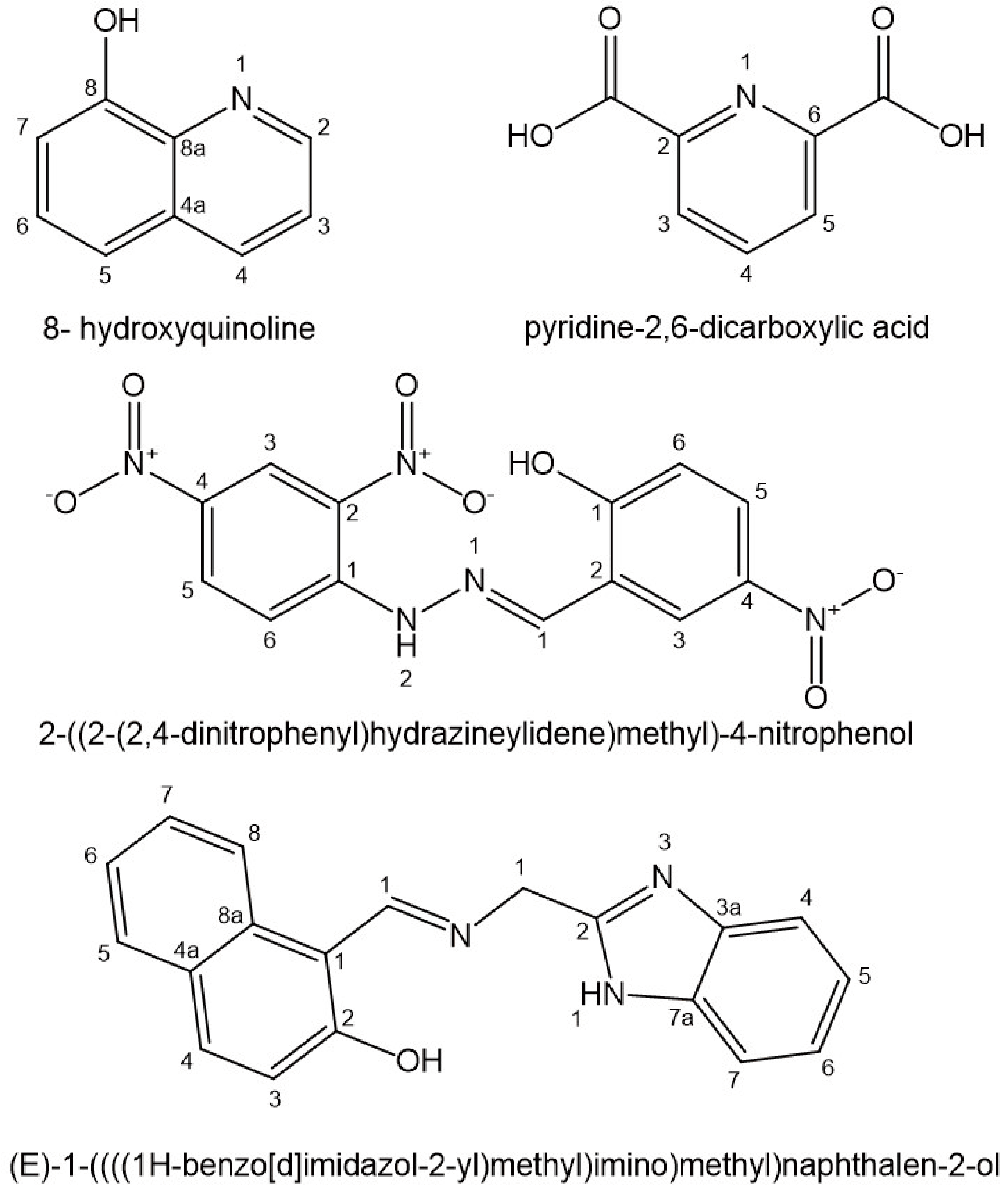





 Copyright ©
Copyright ©