Relevant MicroRNAs of MMPs and TIMPs with Certain Gut Microbiota Could Be Involved in the Invasiveness and Metastasis of Malignant Tumors
Moeka Nakashima1, Naoko Suga1, Yuka Ikeda1, Sayuri Yoshikawa1, Satoru Matsuda1*
1Department of Food Science and Nutrition, Nara Women's University, Kita-Uoya Nishimachi, Nara, Japan
*Correspondence to: Satoru Matsuda, MD, Professor, Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara, 630-8506, Osaka, Japan; Email: smatsuda@cc.nara-wu.ac.jp
DOI: 10.53964/id.2024010
Abstract
Malignant tumors are heterogeneous diseases characterized by uncontrolled cell proliferation, invasion, and/or metastasis. In particular, cancer stem cells within these tumors might be responsible for the invasiveness and the property of recurrence of malignancies. It has been reported that matrix metalloproteases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) could contribute to the development of tumor invasiveness. Remarkably, specific MMPs can drive the malignant phenotype of tumors counting the acquisition of stem cell feature, in which STATs signaling might play an important role. Furthermore, MMPs might be crucial for extra-cellular matrix remodeling in the pathological condition of cancer stem cells. Some immune responses against cancer stem cells in the connection to MMPs and/or TIMPs have been revealed, which might be an important issue in the treatment of aggressive cancers. MicroRNAs (miRNAs) have emerged as favorable biomarkers owing to their important roles in gene regulation and/or stability. Differential expression patterns of specific miRNA may be associated with different types of tumor. In addition, miRNAs can be detected in various body fluids, proposing easily available specimens for diagnostic purposes in tumor microenvironment. Remarkably, it has been shown that miRNA expression might be also related with the gut microbiota composition. Early detection and accurate monitoring of tumors are critical for effective treatment and/or improved patient outcomes. A better understanding of this mechanism of epigenetics could be of use to bring about innovative tactics for the treatment of malignant tumors.
Keywords: malignant tumors, invasion, metastasis, matrix metalloproteinases, tissue inhibitors of metalloproteinase, gut microbiota, microRNAs, cancer therapy
1 INTRODUCTION
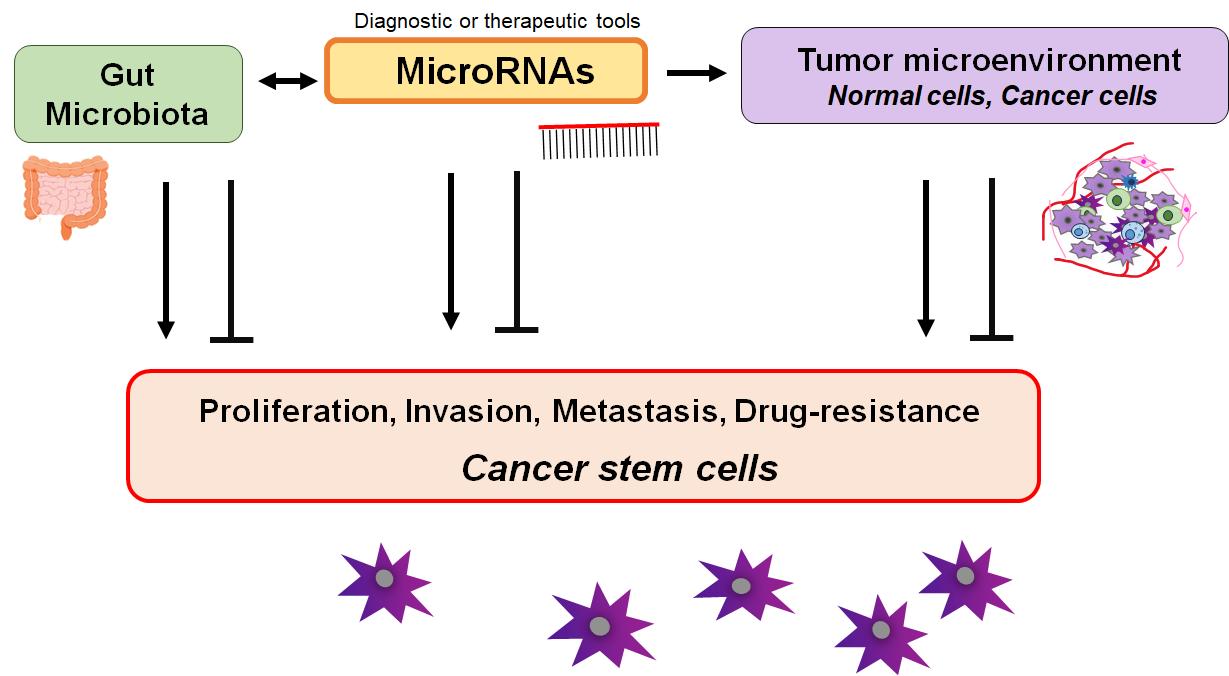
MicroRNAs (miRNAs) might characterize a broad group of post-transcriptional gene expression regulator in eukaryotes. These regulatory molecules usually consist of 20-24 nucleotides, which may exert their function over various cellular processes of RNAs[1]. The certain miRNA could play a crucial role in diverse cancer cells proliferation, invasion, metastasis, resistance to chemotherapy, and/or immune modulation[2]. Several miRNAs could also play an important role in regulating the development of normal cells, which may also reduce the development of cancers via the modulation of the surrounding microenvironment[3] (Figure 1). In addition, much evidence has shown that miRNAs might regulate intracellular transcription, posttranscriptional gene expression, and mRNA decay to manage features of cancer stem cell. In general, cancer cells may possess stem-like properties[4]. Therefore, some miRNAs could interrupt senescence, which might maintain stemness properties of cancer stem cells. In fact, it has been reported that several miRNAs could play important roles in regulating the maintenance and/or acquisition of stem cell features[5], which could also contribute to cancer metastasis and/or cancer recurrence after therapy[6]. Owing to a deep comprehension of cancer-relevant miRNAs, certain miRNA-based diagnosis and/or therapeutics have been feasible and safe in humans.
|
Figure 1. Illustration of the Relationship between miRNAs and Some Characters of Tumors or Cancer Stem Cells. Surrounding microenvironment with normal/cancer cells might be also involved in the connection via the regulation of maintenance and/or acquisition for the tumor cell features. Consequently, miRNAs could be diagnostic or therapeutic tools for various malignant tumors.
Cancer stem cells are a small population of malignant cells, which may profoundly contribute to tumor initiation, progression, therapy-resistance, and relapse, which might ultimately result in the mortality of host[7]. In particular, tumor recurrence from cancer stem cells may often occur within several cancer therapies, which could lead to the challenge of targeted cancer therapy. There has been growing interest in understanding the molecular mechanisms of cancer progression and/or therapy-resistance with cancer stem cells, as they are still associated with high mortality. Cellular strategies against specific characteristics of cancer stem cells might become to be encouraged. Interestingly, it has been reported that octamer-binding transcription factor 3/4 and matrix metalloproteinase-9 (MMP9) could be associated with the resistance of cancer radiotherapy[8]. In addition, it has been also shown a correlation between activated activator of transcription molecule-3 (STAT3) and the existence of cancer stem cells in chemoresistance-associated tumors in ovarian cancer[9,10]. Furthermore, STAT3 activation might be indispensable for keeping cancer stem cells in brain glioblastoma[11] as well as in mammary adeno-carcinoma[12]. Interestingly, some miRNAs such as miR-221 may contribute to cancer tumorigenicity by regulating stemness[13].
The invasiveness and/or metastasis of malignant tumors may be similarly related the function of matrix metalloproteinases (MMPs)[14,15]. In addition, the expression of tissue inhibitors of metalloproteinase-1 (TIMP1), TIMP2, and MMPs might be upregulated in response to various chemotherapeutic treatments in cancer stem cells[16]. Therefore, cancer stem cells might alter the expression of MMPs and/or TIMPs though the modulation of STAT-mediated signaling pathway[17]. Cancer stem cells seems to be considered as main reasons for invasion, metastasis, chemoresistance, the failure of treatment, and the concomitant poor prognosis. In addition, the identification of reliable and non-invasive biomarkers is crucial for early detection, accurate prognosis, and personalized therapeutic interventions. Here, we would like to discuss about the invasiveness brought by cancer stem cells on the basis of understanding the function of MMPs and/or STAT signaling with the alteration of several miRNAs. This review would also discuss miRNAs as biomarkers and/or promising cancer therapeutics, and presents a comprehensive summary of currently validated targets involved in specific signaling pathways. A better understanding of these mechanisms might be used to design more efficient cancer therapies[18]. Such knowledge could also be of use to engineer novel strategies for innovative cancer treatment.
2 MMPS, TIMPS, AND THEIR RELATED miRNAs COULD BE INVOLVED IN CANCER INVASION
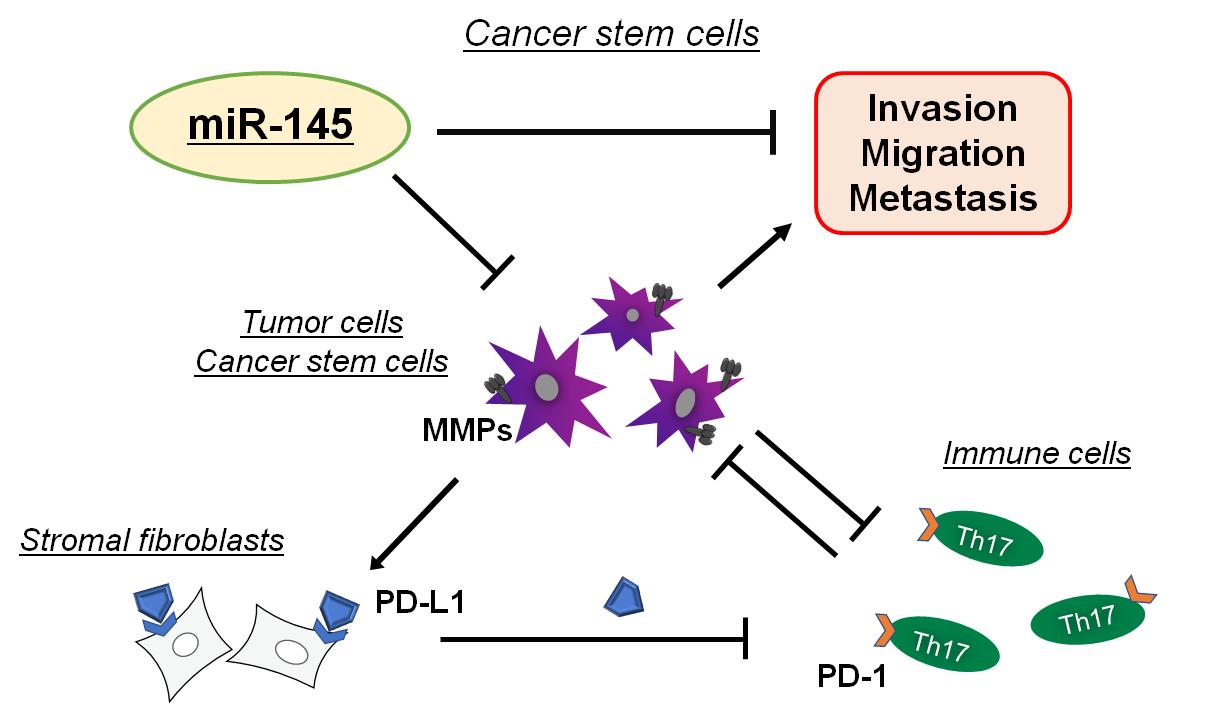
Human cells may express more than 23 different MMPs, which are essential for tissue development and/or homeostasis. In carcinogenesis, certain MMPs are deregulated to meet the homeostatic demand in the tumor development[19]. MMPs are a family of proteinases that could play a major role in remodeling of the extracellular matrix (ECM) as breakdown and/or rapid turnover of ECM molecules, which might be crucial for cancer progression and metastasis[20]. In fact, several MMPs can promote tumor angiogenesis, invasion and metastasis by facilitating ECM degradation[21]. Hence, there has been considerable interest in the posttranscriptional regulation of MMPs via miRNAs. Some microRNAs might ultimately influence the translation and expression of MMP genes[22]. Elucidating the function and regulation of MMP-related miRNAs in malignant tumors may reveal novel biomarkers that could enhance early detection and/or a complement existing therapeutics. MMPs are synthesized as pre-proMMPs, from which the signal peptide is removed during translation to produce mature proMMPs. MMP expression can be affected by several hormones, growth factors, and/or cytokines[23]. Higher expression of MMPs has been shown to be a potential marker of higher invasiveness and/or worse prognosis in various cancers[24]. In addition, ovarian hormones could affect the expression of several MMPs, which might participate in endometrial tissue remodeling during the menstrual cycles[25]. Therefore, MMP's might join in supporting physiological stability of the endometrium. Also, increases in estrogen and/or progesterone as well as vascular endothelial growth factor (VEGF) during pregnancy could promote the expression of several MMPs, which might be required for follicle rupture[26]. Interestingly, it has been shown that miR-145 could inhibit the proliferation and/or invasion of ovarian cells by regulating the expression of MMP2/MMP9[27] (Figure 2).
|
Figure 2. Hypothetical Schematic Image of the Relationship Among MMP Molecules (MMPs), Immune Check Point PD-1, PD-L1, and Invasion and/or Metastasis of Tumor Cells Has Been Presented. By inhibiting the function of MMPs, miR-145 could reduce the invasion and/or metastasis of malignant tumor cells. Indicated molecules and/or cell-types are for examples. Arrowhead means stimulation whereas hammerhead represents inhibition. Note that some critical pathways such as inflammation activation and/or cancer cells proliferation pathway have been omitted for clarity.
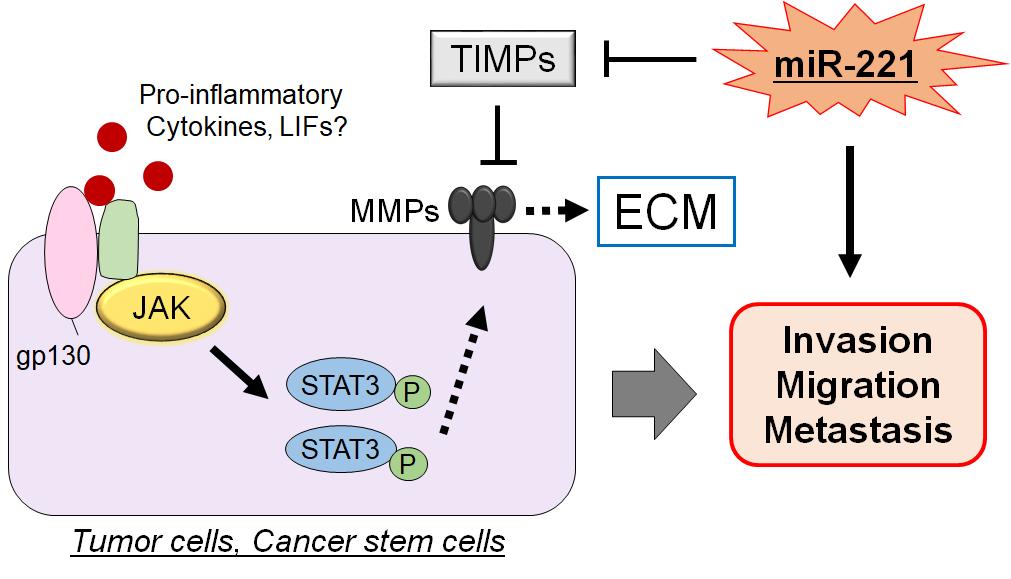
Activity of MMPs could be regulated by endogenous tissue inhibitors of matrix metalloproteinases (TIMPs) which are multifunctional proteins, belonging to a family of secreted and ECM bound proteins that naturally inhibit the proteolytic activity of MMPs[28]. The four TIMP family members share a substantial homology in their sequences[29], which could also regulate remodeling and turnover of the ECM[30]. The N-terminal domain of each TIMP proteins holds the inhibitory activity for the wasting potential of the MMPs[31]. In general, MMPs are regulated at multiple levels including mRNA expression, activation of the proenzyme to the active form, and the counteracting actions of those TIMPs. Therefore, MMPs such as MMP-9 is highly correlated with tumor progression including invasion, angiogenesis and metastasis in pancreatic cancer TIMPs[32,33]. It has been shown that miR-221 may be involved in the downregulation of TIMP3 gene during tumor development and that miR-221 may promote cell invasion through an up-regulation of MMP-9[34,35] (Figure 3). Increased MMPs activity and/or decreased TIMPs expression could lead to MMP/TIMP imbalance, which might result in various pathological conditions including cancer invasion and/or metastasis. For example, TIMP1 has been shown to interact with several MMPs, which could play a fundamental role in the serious spread of cancer[36].
|
Figure 3. In Cancer Stem Cells, the Activation and/or Phosphorylation of STAT3 Brought from the Extracellular Proinflammatory Cytokine Signals Could Eventually Trigger to Increase the Expression of MMP Family Proteins (MMPs), Which Might Lead to the Potentiation of Invasion, Migration, and/or Metastasis of Tumor Cells. MMPs could play a major role in remodeling of the extracellular matrix (ECM), which might be regulated by endogenous tissue inhibitors of matrix metalloproteinases (TIMPs). By inhibiting the function of TIMPs, miR221 could promote the invasion and/or metastasis of tumor cells. Arrowhead means stimulation, whereas hammerhead represents inhibition. Note that some critical pathways have been omitted for clarity.
3 MICROENVIRONMENT AND MMPS IN CANCER STEM CELLS
The microenvironment may contribute to regulating the behavior of cancer stem cells. The place surviving stem cells may consist of a beneficial microenvironment of adjacent cells and/or surrounding extracellular matrix, which might provide restricted signals that cold protect the stem cells from depletion, while also preventing uncontrolled proliferation[37]. Due to their ability to cleave, degrade, and rearrange ECM molecules, MMPs could modulate a variety of stem cell functions[38]. Interestingly, some stem cells could increase the expression of miR-101-3p for promoting the ECM remodeling[39]. In addition, MMPs could take an active role in determining the microenvironment of the stem cells with changing the availability of cytokines and/or chemokines that may affect the function of stem cells. For example, MMP9 could be associated with Kit ligand, which enables bone marrow populating cells to translocate to a permissive place that might permit the reconstitution after irradiation-therapy. In MMP9−/− mice, release of soluble Kit ligand may be impaired, resulting in the failure of hematopoietic recovery after bone marrow removal[40]. MMP14 could also regulate the gene transcription of chemokines and/or cytokines surrounded by stem cells[41]. Ovary with an assistance of MMP2 for the cleavage of their ECM component might promote the proliferation of intimate stem cells[42]. However, the ECM cleavage by MMPs may also lead to the devastation of interconnected structures. In the case of human epidermal stem cells, long-term survival may be sustained by inhibiting proteolysis through MMP2 and/or MMP14[43], which then would lead to proteolytic degradation of favorable place with stem cells.
There may be more complexity to the regulation of stem cells by MMPs rather than simple proteolytic degradation of ECM. For example, MMP3 has been suggested to possess a direct impact on the maintenance of epithelial stem cells in mammary glands[44]. Interestingly, the MMP3 could regulate stem cells in mammary glands independent from its proteolytic activity[44,45]. Hence, MMP3 could still work in the absence of proteolytic activity. Similarly, overexpression of inactive MMP3 may be enough to trigger a growth to hyperplasia in mammary glands[45]. In addition, MMP10 might be also involved in the proliferation of cancer stem cells in lung. It has been shown that MMP10 expression is associated with highly metastatic potential in human lung cancers[46]. These studies may provide interesting view for the susceptibility of signaling pathways from the activation of MMPs, which might stimulate stem cells leading to enlarged cancer possibility.
4 ACTIVATED STATS AND MMPS COULD CONTRIBUTE TO THE ENHANCED MIGRATION, IVASION, AND/OR METASTASIS OF CANCER STEM CELLS
STAT signaling pathway might also play an important role in various types of cancer stem cells. It has been shown that miR-1246 could regulate the activity of JAK/STAT and/or PI3K/AKT pathways, which can influence stemness and resistance of cancer cells in addition to affecting cell cycle progression and proliferation[47]. Activation of these pathways may lead to increased, chemoresistance, metastatic ability in cancer[48]. Nanog is found at high levels in different cancer stem cells, which could induce stemness, self-renewal, metastasis, invasiveness, and chemoresistance of cancer stem cells at least through the STAT signaling pathway[49]. The up and down-regulation of Nanog may be associated with several important signaling pathways including JAK/STAT and several miRNAs[50]. STAT3 may influence on the chemoresistance in ovarian cancer[51]. In addition, STAT3 could modulate the expression of several genes that are associated with cell proliferation, metastasis, angiogenesis, chemoresistance, and immune evasion in cancer cells[52] (Figure 3). STAT3 could be involved in the metastasis and chemoresistance with the cooperative action of MMP9[53].
Cancer metastasis and chemoresistance might be a complex process, during which various cells including cancer stem cells might communicate each other with the neighboring microenvironment. Possibly, MMPs may be involved in the remodeling of the extracellular matrix in the microenvironment to allow invasion and/or metastasis of cancer cells[54]. Therefore, several MMPs might be upregulated in various cancers. Among them, MMP2 and MMP9 are the most distinctive MMPs characterized by a robust proteolytic activity in the extracellular matrix[55], which may be linked to metastasis and/or poor prognosis with being overexpressed in tumor cells[56]. Mesenchymal stem cells could modulate the inflammatory reaction via the MMP2/STAT3 pathway[57], which could also improve the expression of MMPs and TIMPs via the modification of STAT-mediated signaling pathway[58]. On the contrary, inhibition of STAT3 could decrease the expression of MMP2 and/or MMP9, which might inhibit the proliferation of human glioblastoma stem cells[59].
Again, MMPs might be of crucial importance for invasiveness and/or chemoresistance of cancer cells[60]. For the implementation of invasion, cancer cells must adjust the activation rate of MMPs suitably corresponding to the solidity of surrounding ECM. STAT3 could contribute to the invasiveness of cancer cells via properly upregulating the expression of MMP2 and/or MMP9[61]. Accordingly, their expression might correlate with a significant prognostic marker. In this regard, it has been shown that miRNAs have the potential to be used as good biomarkers and therapeutic targets for the treatment of various cancers[62,63].
5 MMPS COULD ALSO MODULATE THE RESPONSES OF IMMUNE CELLS AGAINST CANCER CELLS VIA THE ACTIVATION OF STAT MOLECULES
Cancers must have eluded immune responses to proliferate and expand. In fact, cancer cells could often escape from immune surveillance which has been shown to be associated with various types of immune cells including T regulatory (Treg) and T helper-17 (Th17) cells[64]. Most tumor cells express antigens that can mediate recognition by host CD8+ T cells. STAT3 and STAT5 could oppose one another in regulation of the reciprocal development of Treg and Th17 cells[65]. Interestingly, high levels of MMP9 detected in laryngeal cancer could play a critical role in the development of Treg cells which have an ability to suppress the tumor-specific CD8+ T cells[66]. In addition, increased production of MMP7 might cause an upsurge in the suppressive function of Treg cells[67]. Likewise, the expression of MMP9 might be correlated with the markers of exhaustion in Th1 cells and/or T cells[68]. Furthermore, upregulated expressions of MMP2 and/or MMP9 could help the migration and/or invasiveness of esophageal cancer via the action of IL-17A which is a pro-inflammatory cytokine secreted from Th17 cells[69]. Similarly, MMPs inhibitors might regulate the expression of TGF-β, consequently reducing the number of Treg cells[70]. Remarkably, it has been shown that expression of MMP7 by H. pylori infection could contribute to inadequate adaptive immune responses characterized by insufficient Th1 and/or Th17 immune cells[71,72]. Increased production of MMPs could be also enhanced by the Treg cells[67,73]. Human chorionic gonadotropin (hCG), a hormone essential for pregnancy, is also ectopically expressed by a variety of cancers and is associated with poor prognosis, which could induce the synthesis of MMP2 and/or MMP9, thereby increased invasiveness in the MMPs-dependent manner. The hCG could also up-modulate the secretion of TGFβ and IL-10, thereby inhibiting T cell proliferation[74]. Interestingly, Th17 cells possess polyfunctional cytokine profile, and have stem cell-like features[75]. Moreover, by inhibiting the MMP9/AKT/NF-κB pathway, inflammation is relieved, as reflected in the restoration of the Th17/Treg balance[76]. Targeting the STAT3 pathway in cancer cells seems to control the tumor formation with an impact on immune cells shifting to antitumor Th17 population[77]. Adhesions could modulate the activity of STAT5, which is critical for the development of Th17 and Treg cells[78].
Constitutive activation of STAT3 in cancer cells and immune cells in the tumor microenvironment is a crucial contributor to the development of chemoresistance and immune evasion in most cancers, which may suggest that combined blockade of STAT3 and PD-1 signaling pathways may be an effective treatment option for overcoming poor therapeutic outcomes associated[79]. Consistently, inhibition of MMP2/MMP9 may improve the efficacy of PD-1 or cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade therapy in the treatment of aggressive metastatic cancers[80]. The PD-1 or CTLA4 checkpoint blockade are vivid therapies for several cancers by enhancing the activity of antitumor immune cells. Immune checkpoints are thoroughly related to tumor immune escape, which may be related to the poor prognosis of several tumors in the survival analysis[81]. ligand programmed death ligand 1 (PD-L1) is regulated through proteolytic cleavage by endogenous MMPs from stromal fibroblasts (Figure 2). For example, increased expression of MMP10 in stromal fibroblasts may be contributing to mucosal immune tolerance through the cleavage of cell surface PD-L1, which could suppress Th1 and/or Th17 immune cells response from activated T cells[82]. Supplementation of the MMP inhibitors could return the suppression of Th1/Th17 cells. PD-L1 could be also cleaved by MMP13, while ligand programmed death ligand 2 (PD-L2) is sensitive to wider activities of various MMPs. Accordingly, MMPs might have a significant role in the immune checkpoint responses in cancer therapy. The MMPs dependent cleavage of PD-L1 on stromal fibroblasts might regulate their immunosuppressive capacity[83]. Interestingly, combined treatment with MMP inhibitors and anti-CTLA-4 antibody could obstruct the tumor growth and reduce the metastases compared with the anti-CTLA-4 treatment alone in liver and/or lung cancers[84]. Similarly, MMP9 inhibition with an anti-MMP9 monoclonal antibody could elevate anti-tumor immunity[85]. Since PD-L1 expression has been shown to maintain stemness of cancer stem cells, targeting PD-L1 has shown promising anti-tumor effects[86]. Interestingly, it has been demonstrated that the delivery of aerosolized miRNAs targeting PD-L1 could be effective in preventing lung cancer development and progression in mice[87].
6 MMPS, TIMPS AND STAT3 MAY BE INVOLVED IN PROLIFERATION AND STEMNESS OF CANCER CELLS
TIMPs could stimulate cell growth, and exhibits an anti-apoptotic activity in addition to the MMP-inhibitory function[88,89]. Thus, TIMPs might regulate a whole range of signaling pathways including mitogen activated protein kinase (MAPK), cyclic adenosine monophosphate (cAMP)-protein kinase A, and activation of Ras pathways to promote cell growth[90]. An imbalance in the expression of MMP-9 and TIMP1 has been noted in gastric and laryngeal squamous cell carcinomas[91], while an imbalance in expression of MMP2 and TIMP2 has been reported for hepatocellular carcinoma[92]. In colorectal cancer, disproportionate expression of MMP8 and TIMP1 has been reported[93]. High mRNA expression of MMP2, MMP9, MMP14 and TIMP2 has been also reported in ovarian cancer, and correlated with low survival rate[94]. Abdominal ascites in ovarian cancer patients is enriched in MMP2, MMP9, and MMP14, which may play a key role in peritoneal dissemination[95]. The expression of TIMP2 is ubiquitous in most cell types, where it functions as an endogenous inhibitor of MMPs[96]. For example, TIMP2 could also regulate signalling pathways by direct interaction with the cell surface receptors[97]. In addition, TIMP2 might mediate anti-angiogenic effects by inhibiting endothelial cell migration and invasion[98]. The microenvironment of tumor may deliver paracrine signals, which could regulate these TIMP2 dependent actions in cancer cells[99]. Interestingly, the expression of TIMPs and MMPs as well as cancer stem cells might be upregulated in response to chemotherapy treatments in cancer cells[16,100]. In particular, TIMP2 could regulate proliferation, invasion and STAT3-mediated cancer stem cell-dependent chemoresistance in ovarian cancer[101].
While stem cell expansion is important during active phases of tissue regeneration, unregulated proliferation of cancer stem cells is a recognized feature of various cancer cells. Taking an active role in shaping the microenvironment, MMPs and TIMPS might be able to modulate a variety of stem cells. For example, it has been reported that MMP7 and TIMP7 might cooperate to support the the epithelial-to-mesenchymal transition and cancer stem cells[102]. MMP9 can cleave and mobilize Kit ligand[40,103]. MMP10 is involved in lung tumorigenesis based on stem cells expansion in the background of lung cancer[46,104]. MMP14 could regulate several genes transcription within the hematopoietic stem cells, suggesting that MMP14 could modify the microenvironment of the bone marrow stem cells by changing the bioavailability of cytokines and/or chemokines that influence the function of stem cells[41,105]. MMP14 also contributes to mesenchymal stem cell differentiation through promotion of the trafficking behavior of these cells into type I collagen-rich environments[106]. Decreased expression of MMP2 and/or MMP9 proteins might contribute to a decrease in the stemness of cancer cells[107]. TIMP1 has been shown to have a key role in glioblastoma stemness[108]. TIMP1 was also reported to participate in various cell functions including proliferation and survival, leading to reduced sensitivity to chemotherapy in colon cancer[109]. The activation of STAT3 has been also revealed to correlate with cancer stem cells markers that confer stem cell-like properties to tumor cells. For example, STAT3 activation has been shown to correlate with the self-renewing CD44-positive cells in hepatocellular carcinoma[110]. STAT3 could also play a role in promoting the invasive capacities of cancer cells by regulating the expression of MMPs, such as MMP2 and MMP9[111]. STAT3 pathway contributes to the maintenance of stemness, suggesting that inhibition of the STAT3 pathway provides a promising strategy for the treatment of cancer stem cells[112]. MMPs, TIMPs, and STAT3 might be potential new targets for intervention to overcome aggressive invasiveness or chemoresistance in cancer stem cells. Interestingly, IL-6 might induce miR-503-5p expression through STAT3 activation and promotes the survival of multiple myeloma cells, suggesting that miR-503-5p is a potential biomarker for tumor therapy[113]. However, there might be a lot of work that remains to be done for understanding the precise roles of MMPs/TIMPs/STAT3 axis in cancer stem cells.
7 PROSPECT
The miRNAs are potent regulators of tumorigenesis in various cancers. The abnormal expression of several miRNAs can be observed in tumor cells. The enrichment of various miRNAs can be also found in exosomes to further clarify their application as reliable diagnostic and prognostic modalities[114]. Evidence has shown that miRNAs enclosed in exosomes could be safe from immune attacks. In addition, the low expression level of several miRNAs has been well-documented based on the development of RNA sequencing and microarrays. Interestingly, it has been shown that miRNA expression might be bi-directionally related with the gut microbiota composition[115] (Figure 1). Several bacteria such as B. fragilis, F. nucleatum, and/or F. prausnitzii could modulate the level of miRNAs in cancer cells. In addition, commensal bacteria could also create extracellular vesicles carrying non coding RNAs with a potential regulation of gene expression for host epithelial cells changing the effectiveness of the cancer treatment and/or cancer progression[116]. In fact, some of strains in gut microbiome may extensively represented in the early metastatic stages of colorectal cancer, while other strains while are more prevalent in patients with non-invasive and intramucosal cancer[117,118]. Furthermore, gut microbiota and their metabolites could contribute to modulating the responses of the immune checkpoint cancer therapy[119]. Consequently, those miRNA interactions might facilitate cancer development, invasion, metastasis, and/or therapy resistance, which may exemplify the development of superior cancer therapeutic tactics. Now, investigators have instigated to explore the inhibitory effects of vitamin D on cancer stem cells[120]. Vitamin D might be useful in protecting normal stem cells from DNA damages via the oxidative stress, or inhibiting cancer stem cell growth with cell apoptosis by the action of autophagy[120]. In addition, vitamin D could increase the effectiveness of anticancer drugs as well as proton therapy[121,122]. Furthermore, an emerging role of vitamin D as an inverse agent against treatment resistance induced by various chemotherapies has been dispersed[123-126]. Cancer stem cells might be in charge for the treatment resistance of cancers. Interestingly, it has been shown that some miRNAs could be responsible for the anticancer activity of vitamin D in human leukemia and lymphoma[127].
Numerous studies have been shown that the crosstalk between cancer stem cells and the tumor microenvironment could support the survival, proliferation, invasion, and stemness of cancer stem cells[128]. In addition, cancer stem cells have been revealed to develop neovascularization by expressing various angiogenic factors, which in turn could also contribute to their own maintenance of cancer stem cells[129]. The progression of cancers may critically rely on the function of some components including cancer stem cells, tumor microenvironment and/or neovascularization with close relation each other. Further investigations are needed to fully understand the molecular mechanisms to improve possible treatment strategies and favorable clinical outcomes.
Acknowledgements
A special thanks to all the coauthors for sincerely assisting with the search of this meta-analysis and consultation in the drafting of this manuscript. The project was partially supported by Nara Women’s University of Japan.
Conflicts of Interest
The authors declared that they have no competing financial interests.
Data Availability
No additional data are available.
Copyright Permissions
Copyright © 2024 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Author Contribution
The conceptualization and visualization of the project was done by Nakashima M, Suga N, Ikeda Y, and Matsuda S. The original draft was prepared and edited by Nakashima M, Suga N, Ikeda Y, Yoshikawa S, and Matsuda S. Matsuda S also provided supervision for the project. Each author has participated sufficiently in this work of drafting the article and/or revising the article for the important rational content. Then, all authors gave final approval of the version to be submitted. Finally, all authors have read and agreed to the published version of the manuscript.
Abbreviation List
cAMP, Cyclic adenosine monophosphate
ECM, Extra-cellular matrix
hCG, Human chorionic gonadotropin
JAK, Janus kinase
LIFs, Leukemia inhibitory factors
MAPK, Mitogen activated protein kinase
miRNAs, MicroRNAs
MMPs, Matrix metalloproteinases
PD-L1, Programmed cell death ligand 1
PD-1, Programmed cell death protein 1
STAT3, Signal transducer and activator of transcription 3
TIMPs, Tissue inhibitors of metalloproteinases
VEGF, Vascular endothelial growth factor
VSMCs, Vascular smooth muscle cells
References
[1] Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet, 2010; 11: 597-610.[DOI]
[2] Qiu S, Xie L, Lu C et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res, 2022; 41: 296.[DOI]
[3] Nallasamy P, Nimmakayala RK, Parte S et al. Tumor microenvironment enriches the stemness features: the architectural event of therapy resistance and metastasis. Mol Cancer, 2022; 21: 225.[DOI]
[4] Burr ML, Sparbier CE, Chan KL et al. An Evolutionarily Conserved Function of Polycomb Silences the MHC Class I Antigen Presentation Pathway and Enables Immune Evasion in Cancer. Cancer Cell, 2019; 36: 385-401.[DOI]
[5] Fitriana M, Hwang WL, Chan PY et al. Roles of microRNAs in Regulating Cancer Stemness in Head and Neck Cancers. Cancers, 2021; 13: 1742.[DOI]
[6] Sato R, Semba T, Saya H et al. Concise Review: Stem Cells and Epithelial-Mesenchymal Transition in Cancer: Biological Implications and Therapeutic Targets. Stem Cells, 2016; 34: 1997-2007.[DOI]
[7] Zhang S, Balch C, Chan MW et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res, 2008; 68: 4311-4320.[DOI]
[8] Ko YS, Jung EJ, Go SI et al. Polyphenols Extracted from Artemisia annua L. Exhibit Anti-Cancer Effects on Radio-Resistant MDA-MB-231 Human Breast Cancer Cells by Suppressing Stem Cell Phenotype, β-Catenin, and MMP-9. Molecules, 2020; 25: 1916.[DOI]
[9] Abubaker K, Luwor RB, Zhu H et al. Inhibition of the JAK2/STAT3 pathway in ovarian cancer results in the loss of cancer stem cell-like characteristics and a reduced tumor burden. BMC Cancer, 2014; 14: 317.[DOI]
[10] Quintás-Cardama A, Verstovsek S. Molecular pathways: Jak/STAT pathway: mutations, inhibitors, and resistance. Clin Cancer Res, 2013; 19: 1933-1940.[DOI]
[11] Sherry MM, Reeves A, Wu JK et al. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells, 2009; 27: 2383-2392.[DOI]
[12] Staniszewska AD, Pensa S, Caffarel MM et al. Stat3 is required to maintain the full differentiation potential of mammary stem cells and the proliferative potential of mammary luminal progenitors. PLoS One, 2012; 7: e52608.[DOI]
[13] Roscigno G, Quintavalle C, Donnarumma E et al. MiR-221 promotes stemness of breast cancer cells by targeting DNMT3b. Oncotarget, 2016; 7: 580-592.[DOI]
[14] Zhou J, Liu T, Wang W. Prognostic significance of matrix metalloproteinase 9 expression in osteosarcoma: A meta-analysis of 16 studies. Medicine, 2018; 97: e13051.[DOI]
[15] Dofara SG, Chang SL, Diorio C. Gene Polymorphisms and Circulating Levels of MMP-2 and MMP-9: A Review of Their Role in Breast Cancer Risk. Anticancer Res, 2020; 40: 3619-3631.[DOI]
[16] Escalona RM, Kannourakis G, Findlay JK et al. Expression of TIMPs and MMPs in Ovarian Tumors, Ascites, Ascites-Derived Cells, and Cancer Cell Lines: Characteristic Modulatory Response Before and After Chemotherapy Treatment. Front Oncol, 2022; 11: 796588.[DOI]
[17] Deng W, Chen QW, Li XS et al. Bone marrow mesenchymal stromal cells with support of bispecific antibody and ultrasound-mediated microbubbles prevent myocardial fibrosis via the signal transducer and activators of transcription signaling pathway. Cytotherapy, 2011; 13: 431-440.[DOI]
[18] Thuault S, Ghossoub R, David G et al. A Journey on Extracellular Vesicles for Matrix Metalloproteinases: A Mechanistic Perspective. Front Cell Dev Biol, 2022; 10: 886381.[DOI]
[19] Piperigkou Z, Kyriakopoulou K, Koutsakis C et al. Key Matrix Remodeling Enzymes: Functions and Targeting in Cancer. Cancers, 2021; 13: 1441.[DOI]
[20] Moss NM, Barbolina MV, Liu Y et al. Ovarian cancer cell detachment and multicellular aggregate formation are regulated by membrane type 1 matrix metalloproteinase: a potential role in I.p. metastatic dissemination. Cancer Res, 2009; 69: 7121-7129.[DOI]
[21] Apte SS, Parks WC. Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix Biol, 2015; 44-46:1-6.[DOI]
[22] Li L, Li H. Role of microRNA-mediated MMP regulation in the treatment and diagnosis of malignant tumors. Cancer Biol Ther, 2013; 14: 796-805.[DOI]
[23] Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem, 2007; 15: 2223-2268.[DOI]
[24] Wang J, Ye C, Lu D et al. Matrix metalloproteinase-1 expression in breast carcinoma: a marker for unfavorable prognosis. Oncotarget, 2017; 8: 91379-91390.[DOI]
[25] Grzechocińska B, Dąbrowski F, Cyganek A et al. The role of metalloproteinases in endometrial remodelling during menstrual cycle. Ginekol Pol, 2017; 88: 337-342.[DOI]
[26] Duffy DM, Stouffer RL. Luteinizing hormone acts directly at granulosa cells to stimulate periovulatory processes: modulation of luteinizing hormone effects by prostaglandins. Endocrine, 2003; 22: 249-256.[DOI]
[27] Wang M, Zhang S. MiR-145 on the Proliferation of Ovarian Cancer Cells by Regulating the Expression of MMP-2/MMP-9. Cell Mol Biol, 2022; 67: 141-148.[DOI]
[28] Chirco R, Liu XW, Jung KK et al. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev, 2006; 25: 99-113.[DOI]
[29] Escalona RM, Chan E, Kannourakis G et al. The Many Facets of Metzincins and Their Endogenous Inhibitors: Perspectives on Ovarian Cancer Progression. Int J Mol Sci, 2018; 19: 450.[DOI]
[30] Gomis-Rüth FX, Maskos K, Betz M et al. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature, 1997; 389: 77-81.[DOI]
[31] Raeeszadeh-Sarmazdeh M, Greene KA, Sankaran B et al. Directed evolution of the metalloproteinase inhibitor TIMP-1 reveals that its N- and C-terminal domains cooperate in matrix metalloproteinase recognition. J Biol Chem, 2019; 294: 9476-9488.[DOI]
[32] Awasthi N, Mikels-Vigdal AJ, Stefanutti E et al. Therapeutic efficacy of anti-MMP9 antibody in combination with nab-paclitaxel-based chemotherapy in pre-clinical models of pancreatic cancer. J Cell Mol Med, 2019; 23: 3878-3887.[DOI]
[33] Adissu HA, McKerlie C, Di Grappa M, Waterhouse P, Xu Q, Fang H, et al. Timp3 loss accelerates tumour invasion and increases prostate inflammation in a mouse model of prostate cancer. Prostate, 2015; 75: 1831-1843.[DOI]
[34] Garofalo M, Di Leva G, Romano G et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell, 2009; 16: 498-509.[DOI]
[35] Xu Q, Li P, Chen X et al. miR-221/222 induces pancreatic cancer progression through the regulation of matrix metalloproteinases. Oncotarget, 2015; 6: 14153-14164.[DOI]
[36] Thaysen-Andersen M, Thøgersen IB, Lademann U et al. Investigating the biomarker potential of glycoproteins using comparative glycoprofiling - application to tissue inhibitor of metalloproteinases-1. Biochim Biophys Acta, 2008; 1784: 4554-63.[DOI]
[37] Scadden DT. The stem-cell niche as an entity of action. Nature, 2006; 441: 1075-1079.[DOI]
[38] Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature, 2014; 505: 327-334.[DOI]
[39] Wang Z, Ding X, Cao F et al. Bone Mesenchymal Stem Cells Promote Extracellular Matrix Remodeling of Degenerated Nucleus Pulposus Cells via the miR-101-3p/EIF4G2 Axis. Front Bioeng Biotechnol, 2021 ;9: 642502.[DOI]
[40] Heissig B, Hattori K, Dias S et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell, 2002; 109: 625-637.[DOI]
[41] Nishida C, Kusubata K, Tashiro Y et al. MT1-MMP plays a critical role in hematopoiesis by regulating HIF-mediated chemokine/cytokine gene transcription within niche cells. Blood, 2012; 119: 5405-5416.[DOI]
[42] Wang X, Page-McCaw A. A matrix metalloproteinase mediates long-distance attenuation of stem cell proliferation. J Cell Biol, 2014; 206: 923-936.[DOI]
[43] Muffler S, Stark HJ, Amoros M et al. A stable niche supports long-term maintenance of human epidermal stem cells in organotypic cultures. Stem Cells, 2008; 26: 2506-2515.[DOI]
[44] LaBarge MA. Breaking the canon: indirect regulation of Wnt signaling in mammary stem cells by MMP3. Cell Stem Cell, 2013; 13: 259-260.[DOI]
[45] Kessenbrock K, Dijkgraaf GJ, Lawson DA et al. A role for matrix metalloproteinases in regulating mammary stem cell function via the Wnt signaling pathway. Cell Stem Cell, 2013; 13: 300-313.[DOI]
[46] Regala RP, Justilien V, Walsh MP et al. Matrix metalloproteinase-10 promotes Kras-mediated bronchio-alveolar stem cell expansion and lung cancer formation. PLoS One, 2011; 6: e26439.[DOI]
[47] Ghafouri-Fard S, Khoshbakht T, Hussen BM et al. A Review on the Role of miR-1246 in the Pathoetiology of Different Cancers. Front Mol Biosci, 2022; 8: 771835.[DOI]
[48] Jin W. Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial-Mesenchymal Transition. Cells, 2020; 9: 217.[DOI]
[49] Vasefifar P, Motafakkerazad R, Maleki LA et al. Nanog, as a key cancer stem cell marker in tumor progression. Gene, 2022; 827: 146448.[DOI]
[50] Alemohammad H, Asadzadeh Z, Motafakker Azad R et al. Signaling pathways and microRNAs, the orchestrators of NANOG activity during cancer induction. Life Sci, 2020; 260: 118337.[DOI]
[51] Sheng H, Feng Q, Quan Q et al. Inhibition of STAT3 reverses Taxol-resistance in ovarian cancer by down-regulating G6PD expression in vitro. Biochem Biophys Res Commun, 2022; 617: 62-68.[DOI]
[52] Mohan CD, Kim C, Siveen KS et al. Crocetin imparts antiproliferative activity via inhibiting STAT3 signaling in hepatocellular carcinoma. IUBMB Life, 2021; 73: 1348-1362.[DOI]
[53] Lee JH, Chiang SY, Nam D et al. Capillarisin inhibits constitutive and inducible STAT3 activation through induction of SHP-1 and SHP-2 tyrosine phosphatases. Cancer Lett, 2014; 345: 140-148.[DOI]
[54] Liao WT, Ye YP, Deng YJ et al. Metastatic cancer stem cells: from the concept to therapeutics. Am J Stem Cells, 2014; 3: 46-62.
[55] Huang Q, Lan F, Wang X et al. IL-1β-induced activation of p38 promotes metastasis in gastric adenocarcinoma via upregulation of AP-1/c-fos, MMP2 and MMP9. Mol Cancer, 2014; 13: 18.[DOI]
[56] Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol, 2009; 27: 5287-5297.[DOI]
[57] Kim C, Kim HJ, Lee H et al. Mesenchymal Stem Cell Transplantation Promotes Functional Recovery through MMP2/STAT3 Related Astrogliosis after Spinal Cord Injury. Int J Stem Cells, 2019; 12: 331-339.[DOI]
[58] Deng W, Chen QW, Li XS et al. Bone marrow mesenchymal stromal cells with CD47 high expression via the signal transducer and activators of transcription signaling pathway preventing myocardial fibrosis. Int J Clin Exp Pathol, 2015; 8: 10555-10564.
[59] Shi L, Wan Y, Sun G et al. miR-125b inhibitor may enhance the invasion-prevention activity of temozolomide in glioblastoma stem cells by targeting PIAS3. BioDrugs, 2014; 28: 41-54.[DOI]
[60] Tune BXJ, Sim MS, Poh CL et al. Matrix Metalloproteinases in Chemoresistance: Regulatory Roles, Molecular Interactions, and Potential Inhibitors. J Oncol, 2022; 2022: 3249766.[DOI]
[61] Mirzaei S, Gholami MH, Mahabady MK et al. Pre-clinical investigation of STAT3 pathway in bladder cancer: Paving the way for clinical translation. Biomed Pharmacother, 2021; 133: 111077.[DOI]
[62] Kobayashi N, Uemura H, Nagahama K et al. Identification of miR-30d as a novel prognostic maker of prostate cancer. Oncotarget, 2012; 3: 1455-1471.[DOI]
[63] Yu R, Zhao R, Sun X et al. MicroRNA-588 regulates the invasive, migratory and vasculogenic mimicry-forming abilities of hypoxic glioma cells by targeting ROBO1. Mol Biol Rep, 2023; 50: 1333-1347.[DOI]
[64] Srivastava MK, Zhu L, Harris-White M et al. Targeting myeloid-derived suppressor cells augments antitumor activity against lung cancer. Immunotargets Ther, 2012; 2012: 7-12.[DOI]
[65] Wang W, Edington HD, Rao UN et al. Effects of high-dose IFNalpha2b on regional lymph node metastases of human melanoma: modulation of STAT5, FOXP3, and IL-17. Clin Cancer Res, 2008; 14: 8314-8320.[DOI]
[66] Wang BQ, Zhang CM, Gao W et al. Cancer-derived matrix metalloproteinase-9 contributes to tumor tolerance. J Cancer Res Clin Oncol, 2011; 137: 1525-1533.[DOI]
[67] Lee S, Yamamoto S, Srinivas B et al. Increased production of matrix metalloproteinase-7 (MMP-7) by asbestos exposure enhances tissue migration of human regulatory T-like cells. Toxicology, 2021; 452: 152717.[DOI]
[68] Zeng Y, Gao M, Lin D et al. Prognostic and Immunological Roles of MMP-9 in Pan-Cancer. Biomed Res Int, 2022; 2022: 2592962.[DOI]
[69] Liu D, Zhang R, Wu J et al. Interleukin-17A promotes esophageal adenocarcinoma cell invasiveness through ROS-dependent, NF-κB-mediated MMP-2/9 activation. Oncol Rep, 2017; 37: 1779-1785.[DOI]
[70] Krstic J, Santibanez JF. Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. ScientificWorldJournal, 2014; 2014: 521754.[DOI]
[71] Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology, 2007; 133: 288-308.[DOI]
[72] Ogden SR, Noto JM, Allen SS et al. Matrix metalloproteinase-7 and premalignant host responses in Helicobacter pylori-infected mice. Cancer Res, 2010; 70: 30-35.[DOI]
[73] Kim D, Lo E, Kim D et al. Regulatory T Cells Conditioned Media Stimulates Migration in HaCaT Keratinocytes: Involvement of Wound Healing. Clin Cosmet Investig Dermatol, 2020; 13: 443-453.[DOI]
[74] Khare P, Bose A, Singh P et al. Gonadotropin and tumorigenesis: Direct and indirect effects on inflammatory and immunosuppressive mediators and invasion. Mol Carcinog, 2017; 56: 359-370.[DOI]
[75] Wei S, Zhao E, Kryczek I et al. Th17 cells have stem cell-like features and promote long-term immunity. Oncoimmunology, 2012; 1: 516-519.[DOI]
[76] Zhang X, Gu J, Zhao C et al. Sweeteners Maintain Epithelial Barrier Function Through the miR-15b/RECK/MMP-9 Axis, Remodel Microbial Homeostasis, and Attenuate Dextran Sodium Sulfate-Induced Colitis in Mice. J Agric Food Chem, 2022; 70: 171-183.[DOI]
[77] Hajimoradi M, Rezalotfi A, Esmaeilnejad-Ahranjani P et al. STAT3 inactivation suppresses stemness properties in gastric cancer stem cells and promotes Th17 in Treg/Th17 balance. Int Immunopharmaco, 2022; 111: 109048.[DOI]
[78] Dong L, Zheng X, Wang G. Peritoneal adhesions induce Th17/Treg imbalance in mice. Int J Clin Exp Pathol, 2018; 11: 4352-62.
[79] Kim TW, Kim Y, Keum H et al. Combination of a STAT3 inhibitor with anti-PD-1 immunotherapy is an effective treatment regimen for a vemurafenib-resistant melanoma. Mol Ther Oncolytics, 2022; 26: 1-14.[DOI]
[80] Ye Y, Kuang X, Xie Z et al. Small-molecule MMP2/MMP9 inhibitor SB-3CT modulates tumor immune surveillance by regulating PD-L1. Genome Med. 2020; 12: 83.[DOI]
[81] Chen Z, Zhuo S, He G et al. Prognosis and Immunotherapy Significances of a Cancer-Associated Fibroblasts-Related Gene Signature in Gliomas. Front Cell Dev Biol, 2021; 9: 721897.[DOI]
[82] Aguirre JE, Beswick EJ, Grim C et al. Matrix metalloproteinases cleave membrane-bound PD-L1 on CD90+ (myo-) fibroblasts in Crohn’s disease and regulate Th1/Th17 cell responses. Int Immunol, 2020; 32: 57-68.[DOI]
[83] Dezutter-Dambuyant C, Durand I, Alberti L et al. A novel regulation of PD-1 ligands on mesenchymal stromal cells through MMP-mediated proteolytic cleavage. Oncoimmunology, 2015; 5: e1091146.[DOI]
[84] Li M, Xing S, Zhang H et al. A matrix metalloproteinase inhibitor enhances anti-cytotoxic T lymphocyte antigen-4 antibody immunotherapy in breast cancer by reprogramming the tumor microenvironment. Oncol Rep, 2016; 35: 1329-1339.[DOI]
[85] Juric V, O'Sullivan C, Stefanutti E et al. MMP-9 inhibition promotes anti-tumor immunity through disruption of biochemical and physical barriers to T-cell trafficking to tumors. PLoS One, 2018; 13: e0207255.[DOI]
[86] Banz-Jansen C, Helweg LP, Kaltschmidt B. Endometrial Cancer Stem Cells: Where Do We Stand and Where Should We Go? Int J Mol Sci, 2022; 23: 3412.[DOI]
[87] Zhang Q, Pan J, Xiong D et al. Aerosolized miR-138-5p and miR-200c targets PD-L1 for lung cancer prevention. Front Immunol, 2023; 14: 1166951.[DOI]
[88] Luparello C, Avanzato G, Carella C et al. Tissue inhibitor of metalloprotease (TIMP)-1 and proliferative behaviour of clonal breast cancer cells. Breast Cancer Res Treat, 1999; 54: 235-44.[DOI]
[89] Guedez L, Stetler-Stevenson WG, Wolff L et al. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J Clin Invest, 1998; 102: 2002-2010.[DOI]
[90] Yamashita K, Suzuki M, Iwata H et al. Tyrosine phosphorylation is crucial for growth signaling by tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2). FEBS Lett, 1996; 396: 103-107.[DOI]
[91] Matulka M, Konopka A, Mroczko B et al. Expression and Concentration of Matrix Metalloproteinase 9 and Tissue Inhibitor of Matrix Metalloproteinases 1 in Laryngeal Squamous Cell Carcinoma. Dis Markers, 2019; 2019: 3136792.[DOI]
[92] Giannelli G, Bergamini C, Marinosci F et al. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int J Cancer, 2002; 97: 425-31.[DOI]
[93] Böckelman C, Beilmann-Lehtonen I, Kaprio T et al. Serum MMP-8 and TIMP-1 predict prognosis in colorectal cancer. BMC Cancer, 2018; 18: 679.[DOI]
[94] Davidson B. Ovarian carcinoma and serous effusions. Changing views regarding tumor progression and review of current literature. Anal Cell Pathol, 2001; 23: 107-128.[DOI]
[95] Ghosh S, Wu Y, Stack MS. Ovarian cancer-associated proteinases. Cancer Treat Res, 2002; 107: 331-351.[DOI]
[96] Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta, 2010; 1803: 55-71.[DOI]
[97] Fernandez CA, Roy R, Lee S et al. The anti-angiogenic peptide, loop 6, binds insulin-like growth factor-1 receptor. J Biol Chem, 2010; 285: 41886-41895.[DOI]
[98] Seo DW, Li H, Guedez L et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell, 2003; 114: 171-180.[DOI]
[99] Sánchez-Pozo J, Baker-Williams AJ, Woodford MR, Bullard R, Wei B, Mollapour M, et al. Extracellular Phosphorylation of TIMP-2 by Secreted c-Src Tyrosine Kinase Controls MMP-2 Activity. iScience, 2018; 1: 87-96.[DOI]
[100] Drishya G, Nambiar J, Shaji SK et al. RECK and TIMP-2 mediate inhibition of MMP-2 and MMP-9 by Annona muricata. J Biosci, 2020; 45: 89.[DOI]
[101] Escalona RM, Bilandzic M, Western P et al. TIMP-2 regulates proliferation, invasion and STAT3-mediated cancer stem cell-dependent chemoresistance in ovarian cancer cells. BMC Cancer, 2020; 20: 960.[DOI]
[102] Xu T, Jing C, Shi Y et al. microRNA-20a enhances the epithelial-to-mesenchymal transition of colorectal cancer cells by modulating matrix metalloproteinases. Exp Ther Med. 2015; 10: 683-688.[DOI]
[103] Zhu B, Zhang J, Chen J et al. Molecular biological characteristics of the recruitment of hematopoietic stem cells from bone marrow niche in chronic myeloid leukemia. Int J Clin Exp Pathol, 2015; 8: 12595-12607.
[104] Justilien V, Regala RP, Tseng IC et al. Matrix metalloproteinase-10 is required for lung cancer stem cell maintenance, tumor initiation and metastatic potential. PLoS One, 2012; 7: e35040.[DOI]
[105] Shirvaikar N, Marquez-Curtis LA, Shaw AR et al. MT1-MMP association with membrane lipid rafts facilitates G-CSF-induced hematopoietic stem/progenitor cell mobilization. Exp Hematol, 2010; 38: 823-35.[DOI]
[106] Lu C, Li XY, Hu Y et al. MT1-MMP controls human mesenchymal stem cell trafficking and differentiation. Blood, 2010; 115: 221-229.[DOI]
[107] Chen Z, Yang H, Zhang Q et al. Chelerythrine Inhibits Stemness of Cancer Stem-Like Cells of Osteosarcoma and PI3K/AKT/mTOR Signal. J Oncol, 2022; 2022: 6435431.[DOI]
[108] Aaberg-Jessen C, Sørensen MD, Matos ALSA et al. Co-expression of TIMP-1 and its cell surface binding partner CD63 in glioblastomas. BMC Cancer, 2018; 18: 270.[DOI]
[109] Sørensen NM, Byström P, Christensen IJ et al. TIMP-1 is significantly associated with objective response and survival in metastatic colorectal cancer patients receiving combination of irinotecan, 5-fluorouracil, and folinic acid. Clin Cancer Res, 2007; 13: 4117-4122.[DOI]
[110] Toh TB, Lim JJ, Hooi L et al. Chow EK. Targeting Jak/Stat pathway as a therapeutic strategy against SP/CD44+ tumorigenic cells in Akt/β-catenin-driven hepatocellular carcinoma. J Hepatol, 2020; 72: 104-118.[DOI]
[111] Huang L, Jian Z, Gao Y et al. RPN2 promotes metastasis of hepatocellular carcinoma cell and inhibits autophagy via STAT3 and NF-κB pathways. Aging, 2019; 11: 6674-6690.[DOI]
[112] Sasaki N, Hirano K, Shichi Y et al. Gp130-Mediated STAT3 Activation Contributes to the Aggressiveness of Pancreatic Cancer through H19 Long Non-Coding RNA Expression. Cancers, 2022; 14: 2055.[DOI]
[113] Tan G, Jiang L, Li G et al. ESTAT3 Inhibitor AG-490 Inhibits the Growth of Prostate Cancer by miR-503-5p Both In Vivo and In Vitro. Technol Cancer Res Treat, 2020; 19: 1533033820948062.[DOI]
[114] Lin T, Pu X, Zhou S et al. Identification of exosomal miR-484 role in reprogramming mitochondrial metabolism in pancreatic cancer through Wnt/MAPK axis control. Pharmacol Res, 2023; 197: 106980.[DOI]
[115] Nikolaieva N, Sevcikova A, Omelka R et al. Gut Microbiota-MicroRNA Interactions in Intestinal Homeostasis and Cancer Development. Microorganisms, 2022; 11: 107.[DOI]
[116] Anfossi S, Calin GA. Gut Microbiota: A New Player in Regulating Immune- and Chemo-Therapy Efficacy. Cancer Drug Resist, 2020; 3: 356-370.[DOI]
[117] Yachida S, Mizutani S, Shiroma H et al. Metagenomic and Metabolomic Analyses Reveal Distinct Stage-Specific Phenotypes of the Gut Microbiota in Colorectal Cancer. Nat Med, 2019; 25: 968-976.[DOI]
[118] Luan C, Xie L, Yang X et al. Dysbiosis of Fungal Microbiota in the Intestinal Mucosa of Patients with Colorectal Adenomas. Sci Rep, 2015; 5: 7980.[DOI]
[119] Yoshikawa S, Taniguchi K, Sawamura H et al. Potential tactics with certain gut microbiota for the treatment of unresectable hepatocellular carcinoma. Explor Target Antitumor Ther, 2023; 4: 556-568.[DOI]
[120] Marigoudar JB, Sarkar D, Yuguda YM et al. Role of vitamin D in targeting cancer and cancer stem cell populations and its therapeutic implications. Med Oncol, 2023; 40: 2.[DOI]
[121] Ma Y, Yu WD, Trump DL, Johnson CS. 1,25d3 enhances antitumor activity of gemcitabine and cisplatin in human bladder cancer models. Cancer, 2010; 116: 3294-3303.[DOI]
[122] Podgorska E, Drzal A, Matuszak Z et al. Calcitriol and calcidiol can sensitize melanoma cells to low(-)let proton beam irradiation. Int J Mol Sci, 2018; 19: 2236.[DOI]
[123] Wei D, Wang L, Liu Y et al. Activation of Vitamin D/VDR Signaling Reverses Gemcitabine Resistance of Pancreatic Cancer Cells Through Inhibition of MUC1 Expression. Dig Dis Sci, 2023; 68: 3043-3058.[DOI]
[124] Jia Z, Zhang Y, Yan A et al. 1,25-dihydroxyvitamin D3 signaling-induced decreases in IRX4 inhibits NANOG-mediated cancer stem-like properties and gefitinib resistance in NSCLC cells. Cell Death Dis, 2020; 11: 670.[DOI]
[125] Huang Z, Zhang Y, Li H et al. Vitamin d promotes the cisplatin sensitivity of oral squamous cell carcinoma by inhibiting lcn2-modulated nf-kappab pathway activation through rps3. Cell Death Dis, 2019; 10: 936.[DOI]
[126] Provvisiero DP, Negri M, de Angelis C et al. Vitamin d reverts resistance to the mtor inhibitor everolimus in hepatocellular carcinoma through the activation of a mir-375/oncogenes circuit. Sci Rep, 2019; 9: 11695.[DOI]
[127] Gleba JJ, Kłopotowska D, Banach J et al. Micro-RNAs in Response to Active Forms of Vitamin D(3) in Human Leukemia and Lymphoma Cells. Int J Mol Sci, 2022; 23: 5019.[DOI]
[128] Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci, 2018; 25: 20.[DOI]
[129] Garza Treviño EN, González PD, Valencia Salgado CI et al. Effects of pericytes and colon cancer stem cells in the tumor microenvironment. Cancer Cell Int, 2019; 19: 173.[DOI]





 Copyright ©
Copyright ©