Evaluating the Influence of Ecological Diversity on Glomalin Production and Its Implications for Multifunctionality in Ecosystem Services
Sarita Mishra1, Ajay Kumar Mishra2,3*, Rahul Arya4, Vikash Chandra Mishra5
1Faculty of Science, Sri Sri University, Cuttack, Odisha, India
2Terrestrial Ecosystem Laboratory, Kyoto University, Kyoto, Japan
3International Rice Research Institute, South Asia Regional Centre, Varanasi, Uttar Pradesh, India
4Institute of Environmental Studies, Kurukshetra University, Kurukshetra, Haryana, India
5Department of Horticulture, Bihar Agriculture University, Sabour, Bihar, India
*Correspondence to: Ajay Kumar Mishra, PhD, Senior Associate Scientist, International Rice Research Institute, South Asia Regional Centre, Varanasi, 221001, India; Email: a.k.mishra@irri.org
DOI: 10.53964/id.2024020
Abstract
This review examines the role of Glomalin, a glycoprotein produced by arbuscular mycorrhizal fungi, in soil ecosystems. It covers methods for extracting Glomalin, its molecular characterization, and its function in soil health, including nutrient cycling, heavy metal sequestration, and organic contaminant degradation. The review emphasizes the importance of ecological diversity, highlighting how plant diversity, mycorrhizal associations, and root exudates influence Glomalin production and microbial community structure. These factors contribute to Glomalin's key role in soil stability, carbon sequestration, and overall ecosystem health. The study also explores Glomalin's implications in various systems such as forestry, agroforestry, and agriculture. Glomalin contributes to soil structure, nutrient cycling, and carbon storage in forestry systems, with tree species diversity affecting its accumulation. Agroforestry practices enhance Glomalin levels, promoting soil health and carbon sequestration. In agricultural systems, farming practices like monoculture vs. polyculture and crop rotation strategies impact Glomalin production, influencing soil fertility and health. Despite the progress, the review identifies methodological challenges in Glomalin research and suggests future directions, including the need for standardized protocols, interdisciplinary collaboration, and integration of emerging technologies. Understanding Glomalin's role in ecosystem services and managing its production can lead to sustainable soil management and environmental conservation.
Keywords: arbuscular mycorrhizal fungi, soil ecosystems, ecological processes, nutrient cycling, carbon sequestration, bioremediation
1 INTRODUCTION
The intricate association between plants and the phylum Glomeromycota, particularly the arbuscular mycorrhizal fungi (AMF), unfolds a captivating narrative within the soil ecosystem. Initially discovered by Sara and Wright in 1996, Glomalin is a glycoprotein secreted by AMF that forms symbiotic relationships with the roots of most plants[1]. This glue-like material binds soil particles into aggregates, improving soil structure[2]. Homologous to heat shock protein 60, Glomalin constitutes a critical component in the spores of arbuscular mycorrhizal fungi, germinating in the soil and establishing connections with host plant root epidermal cells[3]. Glomalin's molecular composition and characteristics make it a key player in soil health and functioning.
Liu et al.[4] comprehensive review of over 600 papers over the years concluded that Glomalin had received significant traction from the scientific community after 2015 and only focused on glomalin for remediation and other environmental concerns. Thus, the study highlights the need for further research on the scope of glomalin for carbon sequestration and this symbiotic relationship extends its influence to the intricate web of plant community structure, succession, and composition modulation[5]. The fungal hyphae residing on the surface of plant roots play a vital role in nutrient and water absorption, a process pivotal for the well-being of plants[6]. Positive impacts on plant growth, stress tolerance, nutrient absorption, and soil structure have been well-documented in the scientific literature[7].
Zooming into the rhizospheric zone, often called the heart of the soil, reveals a common platform where soil-inhabiting microorganisms converge with plant roots[8,9]. The exchange of photosynthates from plants to rhizospheric microorganisms forms a reciprocal relationship, profoundly influencing the growth and development of plants. Despite the challenges in distinguishing rhizospheric soils from others based on various characteristics, the symbiotic association of AMF and plants stands out as a vital force shaping the soil structure[10-12].
Unraveling the significance of Glomalin, an N-linked glycoprotein, raises intriguing questions[13]. Dubbed glomalin related soil protein (GRSP) due to its unclear connections with other protein fractions, GRSP plays a pivotal role in natural organic matter (NOM)[14]. The extraction methods involving high-temperature sodium citrate extraction, hydrochloric acid (HCl), or trichloroacetic acid (TCA) precipitation reveal the chemical features of GRSP in diverse soils. Astonishingly, GRSP constitutes a substantial portion of total carbon in mineral and organic soils.
Characterization studies utilizing nuclear magnetic resonance (NMR), infrared spectroscopy, and Bradford assay shed light on the unique features of GRSP[15-18]. While its resemblance to a typical glycoprotein remains questionable, GRSP showcases distinctive traits such as carbohydrate-type carbon content, carboxyl carbon content, aromatic richness, and aliphatic scarcity. The NMR spectra, albeit slightly resembling humic acid, maintain consistency across extraction methods and soil types.
The soil represents a dynamic and intricate ecosystem where various biological, chemical, and physical processes interact to sustain life. Within this complex matrix, Glomalin, a glycoprotein produced by arbuscular mycorrhizal (AM) fungi, is crucial in shaping soil structure and fertility. This section will provide a comprehensive introduction to the subject matter, emphasizing the fundamental concepts that set the stage for a deeper exploration of the influence of ecological diversity on glomalin production.
The significance of Glomalin extends beyond its structural role in soil. Research has revealed its involvement in carbon sequestration, nutrient cycling, and water retention, making it a central component in various ecosystem services[19,20]. Glomalin acts as a reservoir for carbon, aiding in mitigating climate change and facilitating the transfer of nutrients from the soil to plants, impacting overall ecosystem productivity[21,22]. The concentration of glomalin Glomalin was higher in conservation agriculture systems and shows amelioration of soil salinity[23,24].
In this review, we aim to synthesize existing knowledge on Glomalin, focusing on its extraction and characterization methods, and subsequently explore the intricate relationship between ecological diversity and glomalin production.
To ensure a thorough understanding and reproducibility of our literature review on Glomalin's role in soil ecosystems, we embarked on a detailed methodology that involved an extensive exploration of three major databases: Web of Science, Google Scholar, and PubMed. These platforms were chosen for their comprehensive coverage across various scientific disciplines, including environmental and agricultural sciences, life sciences, and biomedical literature.
Our search strategy was meticulously designed using specific keywords such as “Glomalin”, “arbuscular mycorrhizal fungi”, “soil health”, “ecosystem”, services, “nutrient cycling”, and “carbon sequestration”. We combined these keywords with Boolean operators “AND” and “OR” to formulate tailored queries for each database, aiming to capture the broadest relevant literature spectrum.
The inclusion criteria for our review focused on peer-reviewed articles published within the last 20 years to ensure the relevance and timeliness of the findings. We sought studies that provided empirical data on Glomalin’s impact on soil ecosystems, emphasizing articles written in English for uniformity in analysis. Conversely, we excluded duplicates, articles not directly addressing Glomalin’s impact on soil health, or those lacking empirical evidence.
Upon identifying pertinent articles, we engaged in a rigorous data extraction process. This involved cataloging each article’s title, authors, publication year, objectives, methodology, key findings, and conclusions regarding Glomalin’s environmental role. This structured approach facilitated a comprehensive literature synthesis. It allowed us to draw out significant themes such as Glomalin extraction methods, their influence on soil health, and implications for ecosystem services while identifying consensus points and literature gaps.
A critical aspect of our methodology was the quality assessment of the included studies. We evaluated the clarity of research objectives, the robustness of methodologies, and the significance of findings to ensure our review was grounded in high-quality, reliable evidence.
Our literature review, which analyzed 100 articles, uncovered profound insights into Glomalin’s multifaceted role within soil ecosystems, underscoring its importance in nutrient cycling, carbon sequestration, and overall ecosystem services. This comprehensive methodological approach not only ensured the reproducibility of our study but also significantly contributed to the broader understanding of Glomalin’s environmental significance, laying the groundwork for future research directions in this vital area of study. The specific objectives are as follows:
(1) To comprehensively review the methodologies employed in extracting and characterizing Glomalin, emphasizing recent advancements in molecular techniques.
(2) To examine the influence of ecological diversity, particularly plant diversity and microbial communities, on the production of Glomalin in soil ecosystems.
(3) To evaluate the bioremediation potential of Glomalin, specifically in nutrient cycling, heavy metal sequestration, and organic contaminant degradation.
(4) To assess the role of Glomalin in forestry systems, agroforestry systems, and agricultural systems, exploring its implications for sustainable land management and ecosystem services.
Understanding the factors influencing glomalin production is crucial for unlocking its full potential in enhancing ecosystem services. Ecological diversity, encompassing plant species richness and microbial community composition, has been identified as a critical determinant of glomalin dynamics[25,26]. This section will explore how plant-mycorrhizal interactions and microbial diversity contribute to variations in glomalin production across diverse ecosystems.
2 GLOMALIN: EXTRACTION AND CHARACTERIZATION
The extraction and characterization of Glomalin, a glycoprotein produced by AMF, have evolved significantly since Wright and Upadhyaya discovered it in the mid-1990s. Initially identified as a substance contributing to soil aggregation, the methods to isolate and study Glomalin have advanced, providing deeper insights into its structure, function, and role in ecosystem services.
2.1 Historical Context and Core Technologies
The journey to understand Glomalin began with the development of the Bradford Protein Assay, a colorimetric protein quantification method. This method was among the first techniques to estimate Glomalin in soil samples. However, while useful for general protein quantification, it offered limited specificity for Glomalin.
To address this, Wright and Upadhyaya introduced a more tailored approach using a hot water and sodium citrate extraction method. This method, published in 1996, leveraged the heat-stable nature of Glomalin to separate it from other soil components, marking a significant advancement in Glomalin research.
Understanding its extraction, molecular characterization, and functional roles in soil ecosystems is crucial for comprehending soil health and fertility[27].
2.1.1 Methodologies for Glomalin Extraction
2.1.1.1 Soil Sampling Techniques
Efficient glomalin extraction begins with strategic soil sampling. Representative sampling involves collecting soil cores from various locations within a site. The goal is to capture spatial heterogeneity, reflecting the diversity of soil microenvironments. Sampling at different depths, particularly in the root zone, provides a comprehensive understanding of Glomalin distribution.
Recently, soil techniques have undergone changes incorporating spatially explicit sampling, grid-based sampling, and transect designs to cover the samples’ spatial heterogeneity. Once collected, soil samples undergo processing to isolate the glomalin-containing fractions. A commonly employed technique is the wet-sieving method, where the soil is mixed with water and sieved through a series of meshes to separate glomalin-bound aggregates. Further refinement involves using density gradient centrifugation to isolate glomalin-rich fractions.
2.1.1.2 Laboratory Methods
Various methods are employed to extract Glomalin from soil in the laboratory. The modified Walker and Adams procedure, using citrate solution and ethanol precipitation, is widely used. Additionally, the sequential extraction method (SEM) proposed by Chen et al.[28] incorporates citrate, SDS buffers, and phenol extraction, making soil protein samples eligible for immunoblotting, SDS-PAGE, and 2-DE, thus contributing to soil metaproteomic.
Five different dilution and calibration methods were used for the samples collected in the French forests. The study strongly recommended pH-dependent coloring before applying the dilution of soil extracts and subtracting the pH-dependent coloring before applying the Bradford assay[29].
Infrared spectrometry has developed into a genuinely effective and quick way to quantify and determine. Near-infrared spectrometry has developed into a genuinely effective and quick way to quantify and determine Glomalin levels[30].
2.1.2 Molecular Characterization of Glomalin
2.1.2.1 DNA Sequencing
DNA sequencing is employed to delve into the genetic makeup of Glomalin. PCR amplification of glomalin genes, followed by sequencing, allows researchers to identify and study genetic variations. Metagenomic approaches enable the analysis of glomalin genes within complex soil microbial communities.
The study by Purin and Rillig[31] also proposed the hypothesis that Glomalin would be found in the cytoplasm, spore, and hyphal walls. Immune-electron microscopy, utilizing the monoclonal antibody MAb32B11, revealed more gold beads bound to spore walls and hyphae than in the cytoplasm, indicating potential interactions with other soil biota[31].
Proteomic analysis studying studies Glomalin’s protein composition. Techniques such as two-dimensional gel electrophoresis and mass spectrometry are employed to identify and characterize Glomalin proteins. The SEM method[28] introduced enhances the efficiency of extracting purified protein for proteomic analysis.
Bolliger et al.[32] also studied glomalin purity in fractions of glomalin-related soil protein using soil labeling, immunochemical, and lectin-affinity methods. Results showed that the protein part of the glomalin-related soil protein pool is chiefly composed of a single, glycosylated protein reactive to the glomalin-describing antibody, MAb32B11[32].
Over the years, the extraction methods have been refined to improve yield and purity. The introduction of the Modified Bradford Assay, which incorporates adjustments for soil’s complex matrix, represents one such development. This method has enhanced the specificity and reliability of Glomalin quantification (Table 1)[28,31-35].
Table 1. Different Methods of Extraction and Characterization and Its Significance
Extraction and Characterization |
Significance |
References |
Soil labeling, Immunochemical, and Lectin |
Glomalin purity in fractions of GRSP |
[32] |
Immune-electron microscopy by utilizing the monoclonal antibody MAb32B11 |
To detect the presence of Glomalin in the cytoplasm, spore, and hyphal walls |
[31] |
Soil extract dilution |
To gain more accuracy in the estimation of protein |
[33] |
SEM includes Citrate, SDS buffer, and phenol extraction. |
Made protein eligible for proteomic analysis |
[28] |
A Pressure cooker method |
A low-cost alternative for an autoclave for extraction of Glomalin from soil |
[34] |
Sodium pyrophosphate and Sodium citrate |
Extraction of SPRG (Soil Protein Related to Glomalin) |
[35] |
2.1.2.3 Characterization Techniques
Substantial progress has also been made in the molecular characterization of Glomalin. Initially relying on protein gel electrophoresis for size and purity assessments, recent advancements have moved towards mass spectrometry and NMR spectroscopy. These techniques offer detailed insights into Glomalin’s amino acid sequence and structure, providing a more comprehensive understanding of its role in soil ecosystems.
2.1.2.4 Latest Developments in Characterization
Recent years have witnessed the integration of genomic and proteomic technologies, enabling the exploration of Glomalin's genetic blueprint and its interaction with plant and soil microbiomes. Metagenomic sequencing has been utilized to study the AMF communities producing Glomalin, shedding light on its biosynthesis pathways and regulatory mechanisms.
2.1.2.5 Specific Examples
(1) Use of Mass Spectrometry in Glomalin Research: A landmark study by Gonzalez-Chavez et al.[36] utilized mass spectrometry to reveal the presence of hydrophobins, proteins known for their role in fungal structures, within Glomalin. This finding was pivotal in understanding Glomalin's contribution to soil structure and stability.
(2) NMR Spectroscopy Application: Singh et al.[37] employed NMR spectroscopy to characterize the chemical nature of Glomalin, identifying its unique carbohydrate and amino acid composition. This study provided essential clues about Glomalin's ability to bind heavy metals and its function in soil aggregation.
(3) Metagenomic Sequencing for AMF Community Analysis: In a recent study by Jie et al.[38], metagenomic sequencing was used to analyze the soil AMF community in different agricultural systems. The study highlighted the diversity of Glomalin-producing fungi and its correlation with soil health indicators, offering insights into managing soil ecosystems for enhanced Glomalin production.
These examples underscore the evolution of methodologies from elemental protein quantification to sophisticated genomic and proteomic analyses, marking significant progress in our understanding of Glomalin and its environmental implications. As research continues, integrating emerging technologies promises to unveil further details of Glomalin’s role in soil health and ecosystem services, guiding future conservation and management strategies.
2.1.3 Functional Roles of Glomalin in Soil Ecosystems
Glomalin, a glycoprotein produced by AMF, is pivotal in enhancing soil structure, facilitating nutrient cycling, and sequestering carbon, thereby significantly contributing to soil health and ecosystem services. This protein’s ability to bind soil particles together results in improved soil aggregation, which is crucial for maintaining soil porosity, water retention, and resistance to erosion[39]. Moreover, GRSP has been identified as a significant contributor to the soil organic carbon pool, highlighting its role in carbon sequestration and potential implications for climate change mitigation strategies[40].
The distribution of glomalin (both easily extractable glomalin (EEG) and total glomalin (TG)) and its contribution to soil organic carbon (SOC) across different soil depths (Figure 1). As observed, the amount of glomalin decreases linearly with soil depth, while the ratio of glomalin to SOC, indicating its contribution to carbon sequestration, increases, especially in deeper soil layers. This highlights glomalin's significant role in carbon storage and nutrient dynamics within the soil, underlining its importance in maintaining soil health and ecosystem sustainability across varying depths.
|
Figure 1. Glomalin Distribution and Contribution to SOC Across the Soil Depths[41].
Nutrient Cycling: Glomalin facilitates nutrient cycling, particularly phosphorus, by enhancing the mycorrhizal hyphal networks that transport nutrients from the soil matrix to the plant roots. This process is vital for plant health and agricultural productivity, underscoring the symbiotic relationship between plants and AMF[42].
Heavy Metal Sequestration: The role of Glomalin in heavy metal sequestration is another area of significant interest. Glomalin binds heavy metals, reducing their bioavailability and toxicity in the soil environment. This function is particularly relevant in polluted or contaminated soils, where Glomalin can contribute to bioremediation efforts and reduce heavy metal uptake by plants, thus protecting ecosystems and human health[43].
Ecosystem Services: Beyond its soil-related benefits, Glomalin impacts broader ecosystem services, including enhancing biodiversity, supporting water filtration, and contributing to the resilience and stability of ecosystems. By improving soil health, Glomalin indirectly supports the diverse microbial and plant communities essential for ecosystem functioning[44].
To further explore the connections between Glomalin's roles and ecosystem services, see sections IV and V of this review, where we discuss its implications for agricultural systems and its potential in forestry and agroforestry practices for sustainable land management. The following sections deal with each of the mentioned topics in detail.
Understanding these functional roles enhances our ability to manage and sustain soil ecosystems. Future research may explore the potential applications of Glomalin in soil conservation, agriculture, and climate change mitigation.
3 ECOLOGICAL DIVERSITY AND GLOMALIN PRODUCTION
Ecological diversity significantly shapes glomalin production, a key determinant of soil health and nutrient cycling. In this section, we delve deeper into the impact of plant diversity on Glomalin, considering mycorrhizal associations and root exudates, and explore the contributions of microbial communities, specifically bacteria and fungi, to synthesizing this crucial soil protein.
3.1 Impact of Plant Diversity on Glomalin
3.1.1 Mycorrhizal Associations
Mycorrhizal associations are central to glomalin production, with different plant species forming unique partnerships with AMF. The study by Johnson et al.[45] emphasizes that the variety of plant species present influences mycorrhizal diversity. Diverse plant communities foster a rich mycorrhizal network, increasing glomalin production and enhancing soil stability.
Furthermore, Klironomos’s work[46] suggests that mycorrhizal diversity is crucial for ecosystem resilience, especially under changing environmental conditions. This resilience is closely tied to the diversity of plant-mycorrhizal associations and their impact on glomalin synthesis.
3.1.2 Root Exudates and Glomalin Production
Root exudates, a diverse mix of organic compounds released by plant roots, profoundly impact glomalin synthesis. Badri and Vivanco’s study[47] underscores that the composition of root exudates varies among plant species, influencing the growth and activity of AMF. Certain exudates act as stimulants, promoting glomalin production as part of the mycorrhizal symbiosis.
Additionally, the research by Kuzyakov and Domanski[48] highlights the role of root exudates in regulating microbial processes in the rhizosphere. This includes stimulating glomalin-producing fungi and linking plant diversity to the intricate dynamics of glomalin synthesis.
3.2 Microbial Communities and Glomalin Synthesis
3.2.1 Bacterial and Fungal Contributions
Glomalin synthesis is a collaborative effort involving both bacterial and fungal communities in the soil. The study by Zhang et al.[49] demonstrates that bacteria contribute to the decomposition of organic matter, providing substrates that enhance fungal growth and, consequently, glomalin synthesis. The intricate balance between bacterial and fungal contributions is vital for maintaining glomalin levels in diverse ecosystems.
Moreover, the research by Treseder et al.[50] suggests that specific bacterial-fungal interactions influence nutrient cycling and carbon dynamics, further impacting glomalin synthesis. Understanding these complex relationships is essential for unraveling the ecological intricacies of glomalin production.
3.2.2 Soil Microorganism Interactions
Interactions among soil microorganisms are pivotal in regulating glomalin synthesis. The study by Philippot et al.[51] explores the competitive and mutualistic interactions among bacteria, fungi, and other microorganisms. These interactions influence nutrient availability, substrate utilization, and, consequently, the synthesis of Glomalin.
Additionally, Hartmann et al.[52] emphasize the importance of soil microorganism diversity in maintaining glomalin stability over time. Diverse microbial communities contribute to the resilience of Glomalin, ensuring its sustained presence in the soil ecosystem.
Understanding the intricate relationships between plant diversity, root exudates, and microbial communities provides a comprehensive view of the factors influencing glomalin production. This knowledge is essential for sustainable soil management practices that promote ecosystem health[52].
4 BIOREMEDIATION POTENTIAL OF GLOMALIN
The bioremediation potential of Glomalin, a glycoprotein produced by AMF, is an emerging area of research with promising implications for environmental sustainability. This section delves into Glomalin's multifaceted roles in nutrient cycling, heavy metal sequestration, and organic contaminant degradation, elucidating its capacity to contribute to soil remediation. The degraded contaminant soils in the coal mining reclaimed areas showed a significant increase in SOC levels and GRSP content over time[53]. In a study conducted in the undisturbed forests of the Atlantic region of Amazon forests in Brazil for the period of 2007-2020[54].
4.1 Glomalin-Mediated Nutrient Cycling
Glomalin plays a pivotal role in nutrient cycling within soil ecosystems. The symbiotic relationship between AMF and plants, facilitated by Glomalin, enhances nutrient uptake efficiency. Mycorrhizal hyphae extend into the soil, increasing the volume of soil explored for nutrients and transporting them to plant roots[55]. This nutrient-acquiring ability is particularly crucial in phosphorus-limited soils, where Glomalin helps pea plants mobilize and efficiently utilize phosphorus.
The nut hyphal network created by glomalin contributes to forming stable soil aggregates, protecting nutrients from leaching and erosion[56]. Glomalin's impact on nutrient cycling links back to the discussion on plant diversity, as the diverse mycorrhizal associations fostered by different plant species influence the types and quantities of nutrients cycled within the soil[45]. glomalin-mediated nutrient cycling is a fundamental ecological service with implications for soil fertility.
The discussion on nutrient cycling extends the understanding of glomalin's ecological functions. The nutrient cycling facilitated by glomalin builds upon its role in mycorrhizal associations discussed in the previous section, reinforcing the interconnectedness of glomalin dynamics in soil ecosystems.
4.2 Glomalin’s Role in Heavy Metal Sequestration
One of the remarkable aspects of Glomalin's bioremediation potential lies in its ability to sequester heavy metals in soil. The glycoprotein's structure, rich in functional groups such as carboxyl and hydroxyl, enables it to bind with metal ions effectively[20]. This sequestration mechanism is particularly relevant in environments impacted by anthropogenic activities such as mining and industrial discharges, where heavy metal contamination poses significant ecological threats.
Research by Rillig and Mummey[17] suggests that Glomalin is a critical player in the immobilization of heavy metals in soil, preventing plant mobility and uptake. The formation of stable aggregates by Glomalin contributes to the physical entrapment of heavy metals, reducing their bioavailability. This has implications for mitigating the toxic effects of heavy metals on both soil ecosystems and human health.
Furthermore, the specific interactions between glomalin-producing fungi and heavy metals are an area of ongoing investigation. The work by Jansa et al[57]. suggests that different AMF species exhibit varying abilities to accumulate and tolerate heavy metals, influencing the overall effectiveness of glomalin-mediated sequestration.
The discussion on heavy metal sequestration builds upon understanding Glomalin's role in nutrient cycling. The stable aggregates formed during nutrient cycling also contribute to the immobilization of heavy metals, showcasing Glomalin's multifunctionality in soil ecosystems (Table 2)[39,58-68].
Table 2. The Role of Glomalin in Soil Restoration and Improvement
Function |
Quantitative Data |
References |
Source of nutrients for plants and microbes |
Contains 30-40% C, 3-5% N, 3-4% P, 1-9% Fe, and 1-2% other nutrients (S, K, Ca, Mg, Zn, Cu) |
[58,59] |
Improves soil physical properties |
Acts as a binding agent for soil aggregates, improving porosity, moisture content, and water-holding capacity |
[60] |
Improves microbial activity |
Increases extracellular enzyme activities (e.g., β-glucosidase, phosphatase) and contributes to 25% total soil microbial respiration |
[61] |
Soil carbon storage |
Contributes up to 25% total SOC pool and has a persistence time up to 42 years or more in soil |
[39,62] |
Biostabilization of soil contaminants and pollutants |
Metal chelation capability allows it to act as a potential heavy metal biostabilizer (Cu, Al, Pb, Zn, Cd, Cr) and assists in the degradation of organic pollutants (e.g., PAHs) |
[63,64] |
Ecological restoration |
Helps in recovery of degraded land by improving soil physical, chemical, and biological properties across various conditions (e.g., long-term cropped land, coal mined land) |
[65-68] |
4.3 Glomalin and Organic Contaminant Degradation
In addition to its role in nutrient cycling and heavy metal sequestration, Glomalin demonstrates the potential for soil degradation by organic contaminants. The glycoprotein's interaction with the soil matrix, microbial communities, and the rhizosphere creates a microenvironment conducive to the breakdown of organic pollutants.
Studies by Purin and Rillig[31] propose that Glomalin might interact with other soil biota, indicating a potential role in the degradation of organic contaminants. The presence of Glomalin in spore walls, as observed under immune-electron microscopy, suggests interactions with the biochemistry of the soil matrix. This implies that Glomalin, in conjunction with soil microorganisms, may contribute to the degradation of organic compounds.
Moreover, the stable aggregates of Glomalin protects soil microorganisms engaged in decomposing organic matter. The physical structure provided by Glomalin may shield these microorganisms from environmental stresses, enhancing their resilience and capacity for organic contaminant degradation[56].
Exploring Glomalin's role in organic contaminant degradation complements the discussion on heavy metal sequestration. The protective microenvironment created by Glomalin, crucial for the stability of microbial communities in the presence of heavy metals, is also likely to contribute to the resilience of microorganisms involved in organic contaminant degradation.
4.3.1 Concurrence in Forestry, Agroforestry and Agriculture Systems
While existing research has made significant strides in unraveling the roles of Glomalin in diverse land use systems, several gaps still need to be addressed. Current research addresses these gaps to deepen our understanding of Glomalin's interplay in forestry, agroforestry, and agriculture. The response of Glomalin to varied agroecosystems, including monoculture and polyculture systems, still needs to be explored. Investigating glomalin dynamics in different agricultural contexts is crucial for tailoring management practices to optimize its benefits. Furthermore, the long-term implications of Glomalin on soil health and fertility in forestry, agroforestry, and agriculture need further elucidation (Figure 2). Longitudinal studies tracking glomalin levels and their correlation with soil parameters can provide valuable insights into its sustained impact. Also, the influence of changing climatic conditions on glomalin production and its effectiveness in soil processes is an emerging area. Understanding how climate change may alter the interplay of Glomalin in diverse systems is essential for anticipating and adapting to future challenges.
|
Figure 2. Representation of Glomalin Contribution in Different Land Uses.
While micro-scale studies have provided valuable insights, scaling up the impact of Glomalin to the macro level in agroforestry and forestry systems is an area requiring attention. Bridging this gap is crucial for developing holistic strategies for large-scale land management. The interplay of Glomalin in forestry, agroforestry, and agriculture systems holds immense significance for sustainable land use practices. Harnessing its bioremediation potential can improve soil fertility, reduce heavy metal toxicity, and enhance resilience to environmental stressors. As research continues to fill existing gaps, integrating glomalin-centric strategies into land management frameworks emerges as a promising avenue for fostering resilient and sustainable agroecosystems.
5 GLOMALIN IN FORESTRY SYSTEMS
Forests, vital components of terrestrial ecosystems, harbor intricate relationships between plants, microbes, and soil. Glomalin, a glycoprotein produced by AMF, plays a pivotal role in shaping the dynamics of forest ecosystems. This section explores the dynamics of Glomalin in forest ecosystems, the influence of tree species diversity on glomalin accumulation, and the broader implications for sustainable forest management.
5.1 Glomalin Dynamics in Forest Ecosystems
The dynamics of Glomalin in forest ecosystems are central to understanding nutrient cycling, soil structure, and overall ecosystem health. Research by Hossain[69] has demonstrated that Glomalin contributes to forming stable soil aggregates in forests, enhancing soil structure and water retention. The mycorrhizal associations facilitated by Glomalin play a crucial role in nutrient uptake by trees, influencing forest ecosystems' overall productivity and health[70].
The seasonal variations in glomalin production are noteworthy. Studies by Prasad et al., 2018 [71] indicate that glomalin levels fluctuate with changes in temperature, precipitation, and plant phenology; during periods of active root growth, glomalin production increases, highlighting the intricate linkages between plant-mycorrhizal interactions and glomalin dynamics in forested environments.
Furthermore, Glomalin contributes to carbon sequestration in forest soils. The stable aggregates formed by Glomalin protect organic matter from decomposition, accumulating soil carbon[25]. This has implications for climate change mitigation, emphasizing the role of Glomalin in the global carbon cycle within forest ecosystems.
Understanding the dynamics of Glomalin in forest ecosystems provides a foundation for assessing the health and functioning of these critical ecosystems.
5.2 Tree Species Diversity and Glomalin Accumulation
Tree species diversity is a crucial factor influencing glomalin accumulation in forest ecosystems. Different tree species establish unique mycorrhizal associations, influencing the soil's quantity and composition of Glomalin. Research by Chen et al.[72] reveals that diverse forests with various tree species exhibit higher glomalin levels than monoculture stands. This diversity-driven increase in Glomalin is attributed to the varied mycorrhizal associations fostered by different tree species.
Cissé et al.[73] conducted a long-term study in the broadleaf and conifers forests in the temperate forests of France, using 109 sites from the network of forest ecosystems known as RENECOFOR. It was identified that conifer forests have higher GRSP production than broadleaf forests.
GRSP composition can vary among tree species. Studies by Liang et al.[74] suggest that certain tree species promote the production of specific glomalin proteins, influencing the functional diversity of the soil microbial community. This tree species-driven variation in glomalin composition contributes to forest ecosystems' overall resilience and adaptability.
Moreover, the interplay between tree species diversity, Glomalin, and nutrient cycling is a topic of ongoing research. Jones et al.[75] demonstrate that diverse forests enhance nutrient cycling through the collaborative efforts of different mycorrhizal associations, highlighting the intricate relationship between tree diversity and glomalin-mediated ecosystem functions.
Understanding how tree species diversity influences glomalin accumulation provides insights into the mechanisms driving forest ecosystem resilience and sustainability.
5.3 Implications for Sustainable Forest Management
The dynamics of Glomalin in forest ecosystems have far-reaching implications for sustainable forest management practices. Recognizing the role of Glomalin in maintaining soil structure, enhancing nutrient cycling, and contributing to carbon sequestration is fundamental for developing management strategies that prioritize ecosystem health.
(1) Soil Health and Productivity: Sustainable forest management practices aim to preserve and enhance soil glomalin levels. Maintaining or promoting mycorrhizal associations through reduced disturbance during harvesting or selective logging can contribute to sustained glomalin production[76]. This, in turn, fosters soil health and supports the long-term productivity of forest ecosystems.
(2) Biodiversity Conservation: The link between tree species diversity, Glomalin, and overall ecosystem health underscores the importance of conserving forest biodiversity. Sustainable forest management should prioritize maintaining or restoring diverse forest stands to leverage the positive impacts of different mycorrhizal associations on glomalin dynamics[77,78].
(3) Climate Change Mitigation: Recognizing the role of Glomalin in carbon sequestration highlights its potential in climate change mitigation. Sustainable forest management practices prioritizing the preservation of glomalin-rich soils contribute to the global effort to sequester carbon in forest ecosystems.
(4) Adaptive Management: Adaptive management strategies are crucial given the potential influence of climate variability on glomalin dynamics. Monitoring glomalin levels and understanding the response of forest ecosystems to changing climatic conditions can inform adaptive management practices that enhance resilience and adaptive capacity[69].
The interplay of Glomalin in forestry systems is a complex yet crucial aspect of ecosystem dynamics. The dynamics of Glomalin contribute to soil health, nutrient cycling, and carbon sequestration in forest ecosystems. Recognizing the influence of tree species diversity on glomalin accumulation provides insights into the mechanisms that drive ecosystem resilience and sustainability. Sustainable forest management practices prioritize prioritizing the preservation and enhancement of glomalin levels that contribute to forest ecosystems' overall health and productivity.
6 GLOMALIN IN AGROFORESTRY SYSTEMS
Agroforestry, an integrated land-use management approach, combines the benefits of agriculture and forestry systems to promote sustainability and enhance ecosystem services. This section explores the role of Glomalin in agroforestry systems, focusing on practices that contribute to glomalin enhancement, the relationship between agroforestry systems and soil carbon sequestration, and the multifaceted benefits Glomalin has in agroecosystems.
6.1 Integrating Agroforestry Practices for Glomalin Enhancement
Agroforestry practices encompass a range of land-use systems that integrate trees, crops, and livestock, providing ecological, economic, and social benefits. Bhardwaj et al.[79] and Elevitch et al.[80] emphasize that physiographic, climatic, and socioeconomic variations influence categories of agroforestry practices and their yield. These variations underscore the need for context-specific agroforestry strategies to optimize glomalin-related benefits.
One significant contribution of agroforestry to glomalin enhancement is its positive impact on soil chemical indicators. The presence of trees in agroforestry systems influences soil nutrient content, leading to increased levels of essential elements such as calcium (Ca), magnesium (Mg), and potassium (K). Additionally, agroforestry systems contribute to pH regulation and reduce aluminum saturation, fostering favorable conditions for glomalin production[81].
Agroforestry systems also play a crucial role in maintaining enzymatic activity in the soil. During the rainy season, agroforestry systems support the activity of enzymes such as acid phosphatase, protease, and beta-glucosidase, akin to observations in natural forests. The production of glomalin-related soil protein is another notable outcome associated with agroforestry, contributing to the preservation of soil biological and chemical properties[81].
6.2 Agroforestry Systems and Soil Carbon Sequestration
Soil carbon sequestration is an essential ecosystem service provided by agroforestry systems[82], and Glomalin emerges as a crucial mediator in this process. Glomalin, a recalcitrant glycoprotein with a half-life of around 50 years, actively contributes to carbon sequestration in terrestrial ecosystems[83,84].
The intricate relationship between agroforestry systems and soil carbon sequestration is highlighted by Prasad et al.[84], who emphasize the regulatory role of Glomalin in nitrogen storage, carbon sequestration, and soil aggregation. Plant health is intimately connected to soil health, and Glomalin benefits from its protective role in mycorrhizal hyphae during nutrient transportation. The enhancement of soil aggregation, facilitated by Glomalin, improves nutrient and water-holding capacity[84].
Matos et al.[85] found a strong correlation between Glomalin, SOC, and soil quality in five areas, including three agroforestry settings, unmanaged secondary forests, and pasture lands in the Amazon forests. Silva et al.[86] concluded that glomalin production in both Agroforestry systems and secondary forests is similar and higher during the rainy season than during the dry season.
Perez-Sanz et al.[87] analyzed the four different agroforestry practices: home garden-based agroforestry, crop-based agroforestry, woodlot-based agroforestry, and Trees on soil and water-based agroforestry. The results varied for different AF practices, with the Home garden-based agroforestry practices having the highest SOC and Glomalin content in the soils, implying the importance of agroforestry practices on soil biodiversity and glomalin production and influencing various other parameters.
Curaqueo et al.[88] explored the impact of different tillage systems (no-tillage and conventional tillage) on arbuscular mycorrhizal fungal propagules, soil physical properties, and glomalin content. The study revealed that no-tillage systems enhance the beneficial role of GRSP and arbuscular mycorrhizal fungi, positively influencing soil aggregation. This underscores the potential of agroforestry practices, particularly those minimizing soil disturbance, to enhance glomalin-related benefits and contribute to sustainable soil management.
6.3 Glomalin-Mediated Benefits in Agroecosystems
The benefits mediated by Glomalin in agroecosystems extend beyond soil health to impact plant growth, seed germination, and the distribution of arbuscular mycorrhizal fungi. Govindu et al.[89] investigated the impact of GRSP from coal mine soils on three plant species (Acacia pinnata and Albizia lebbeck). The study revealed varying root colonization in different plant species, with Prongamia pinnata and Albizia lebbeck displaying good distribution of arbuscular mycorrhizal fungi.
The study by Govindu et al.[89] further highlighted the positive correlation between glomalin and spore density, indicating the influence of Glomalin on the abundance of arbuscular mycorrhizal fungi. The impact of glomalin-related extracts on seed germination varied among plant species, emphasizing the context-specific nature of these interactions.
Glomalin in agroforestry systems is multifaceted in enhancing soil health, promoting carbon sequestration, and influencing plant-microbe interactions. Enabling trees into agroecosystems positively influences soil chemical indicators, enzymatic activity, and glomalin-related benefits. Understanding these dynamics provides a foundation for developing sustainable agroforestry practices that optimize the contributions of Glomalin to ecosystem services.
7 GLOMALIN IN AGRICULTURAL SYSTEMS
Agricultural systems play a pivotal role in shaping the dynamics of Glomalin, a glycoprotein secreted by AMF that influences soil structure, nutrient cycling, and overall soil health. This section explores the effects of monoculture and polyculture on Glomalin, the impact of crop-rotation strategies, and how Glomalin contributes to enhancing soil health and fertility in diverse agricultural contexts.
7.1 Monoculture vs. Multicultural Effects on Glomalin
Lee and Eom[90] conducted experiments comparing organic and conventional farms to understand the impact of agricultural practices on AMF community structure and glomalin content. Soil samples revealed the presence of various Glomus species, Acaulospora longula, Paraglomus occultum, and Gigaspora margarita. Glomalin content was significantly higher in organic farm soil, indicating a positive correlation between organic farming practices and glomalin production.
The abundance and diversity of AMF species were also found to be higher in organic farm soil, emphasizing the role of organic farming in promoting a conducive environment for glomalin-producing fungi[90]. This underscores the potential of sustainable agricultural practices to foster a robust symbiotic relationship between plants and AMF, leading to enhanced glomalin production[91]. The study used cotton cultivation and intercropping with legumes, grass plants, and other border crops. It showed higher glomalin production with the intercrops and lower Urea (N) application. The study highlights the availability of glomalin in sandy soils.
Rajtor and Piotrowska-Seget[92] extended this understanding by suggesting that arbuscular mycorrhiza fungi, including glomalin-producing species, can contribute to phytoremediation, particularly in hydrocarbon-contaminated soils. The synergistic action of plant roots, AMF, and associated microorganisms facilitates the extraction and degradation of hydrocarbon pollutants, showcasing the multifaceted roles of Glomalin in agricultural ecosystems.
A mathematical model to analyze the soil data under various land management practices to analyze the microbial activity, GRSP and Banegas et al.[93] developed a mathematical model to analyze soil data under various land management practices, focusing on microbial activity, GRSP, and GRSP-C. The model showed a significant increase in pasture lands’ biomass, Organic Carbon, and GRSP.
7.2 Crop-Rotation Strategies and Glomalin Production
Nautiyal et al.[94] delved into the role of Glomalin in maintaining soil aggregation in temperate Himalayan soils under different cropping systems. Soil aggregation is crucial for preventing erosion and elevates the carbon pool, highlighting the correlation between aggregate formation and soil organic carbon.
The study observed varying concentrations of Glomalin in different land uses, with the highest value in undisturbed forest ecosystems and the lowest in wheat fields. Leguminous cropping in agricultural systems exhibited intermediate glomalin values, emphasizing the influence of land use and soil management practices on glomalin content[94].
Galazka et al.[95] expanded on this understanding by investigating the impact of agricultural management practices on Glomalin. The study revealed reduced tillage and direct sowing produced higher glomalin content than full tillage and crop rotation systems. Additionally, elevated enzyme activities and microbial biomass were observed in reduced tillage systems, highlighting the potential of specific agricultural practices to enhance glomalin-related benefits.
7.3 Enhancing Soil Health and Fertility through Glomalin
Different land uses act as Glomalin drivers, governing the soil carbon sequestration (Table 3)[30,41,86,94,95]. Singh et al.[96] examined disturbed (cultivated field) and undisturbed soils in the Indo-Gangetic plain, emphasizing the relationship between SOC, GRSP, and various soil properties. Disturbed lands exhibited lower concentrations of SOC, GRSP, and other components than undisturbed lands. The study demonstrated a linear correlation between SOC and GRSP, indicating the interdependence of these factors. Soil properties such as pH, total phosphorus, bulk density, available phosphorus, porosity, organic nitrogen, arbuscular mycorrhizal abundance, microbial activity, and cations showed similar relationships with both SOC and GRSP[96,97]. Bertagnoli et al.[98] focused on applying poultry litter and liquid swine slurry in agricultural lands, highlighting their impact on soil structure through Glomalin and extraarticular mycelium production. The study showcased that poultry litter, in particular, induced the production of total and active extraarticular mycelium and Glomalin, enhancing soil aggregation. Different manure types were found to influence soil aggregation and chemical fertility.
Table 3. Global Depth-Wise Distribution of Glomalin in Different Landuse
Location |
Soil Type |
Systems |
Soil Depth (cm) |
Glomalin (mg/g) |
References |
Forestry/Plantation systems |
|||||
Uttarakhand |
Silty loam |
Forest |
0-15 |
4.79 |
[94] |
Uttarakhand |
Silty loam |
Forest |
15-20 |
4.24 |
[94] |
China |
Black Soil |
Forest |
0-20 |
5.80 |
[41] |
|
|
|
20-40 |
4.17 |
|
|
|
|
40-60 |
3.50 |
|
|
|
|
60-80 |
3.07 |
|
|
|
|
80-100 |
2.40 |
|
France |
|
Conifer Forest Broad Leaves |
0-10 |
6.6 |
|
Czech Republic |
|
Forest |
0-10 |
7.35 |
[30] |
Brazil |
|
Secondary Forest |
0-10 |
|
[86] |
Agriculture systems |
|||||
Uttarakhand |
Silty loam |
Eleusine coracana |
0-15 |
2.32 |
[94] |
|
|
Amaranthus spp. |
0-15 |
2.57 |
|
|
|
Glycine max |
0-15 |
2.38 |
|
Uttarakhand |
Silty loam |
Eleusine coracana |
15-30 |
2.12 |
[94] |
|
|
Amaranthus spp. |
15-30 |
2.47 |
|
|
|
Glycine max |
15-30 |
2.18 |
|
Uttarakhand |
Silty loam |
Malus domestica |
0-15 |
3.14 |
[94] |
|
|
Prunus domestica |
0-15 |
3.95 |
|
|
|
Prunus persica |
0-15 |
3.16 |
|
Uttarakhand |
Silty loam |
Malus domestica |
15-30 |
3.01 |
[94] |
|
|
Prunus domestica |
15-30 |
3.63 |
|
|
|
Prunus persica |
15-30 |
3.02 |
|
Poland |
Brown podzolic |
Before sowing |
0-15 |
2.07 |
[95] |
Brazil |
|
Six leaves |
0-15 |
2.80 |
[86] |
|
|
12 leaves |
0-15 |
2.86 |
|
|
|
Flowering |
0-15 |
2.91 |
|
|
|
After harvest |
0-15 |
2.79 |
|
0-10 |
4.80 |
||||
The study analyzed the SOC and the influence of GRSP under different agricultural practices, such as zero tillage and minimum tillage, with the rhizosphere and mycorrhizal crops such as soybean, maize, fenugreek, sorghum, and marigold. It found a significant correlation between GRSP and microbial activity, with r=0.78 and 0.83, respectively[99]. The study also found that under a zero-tillage system, there is a strong correlation between SOC and GRP content.
Glomalin emerges as a key player in promoting soil health and fertility in agricultural systems. The choice of farming practices, land use, and crop rotation strategies significantly influences glomalin production, underscoring the importance of sustainable approaches to maximizing the benefits of this glycoprotein in agricultural ecosystems.
8 CHALLENGES AND FUTURE DIRECTIONS
Understanding and harnessing the potential of Glomalin in soil ecosystems presents many challenges and opportunities. This section explores the methodological challenges in glomalin research and the integration of glomalin studies into ecosystem management, and it outlines future research avenues and emerging technologies in the field.
8.1 Methodological Challenges in Glomalin Research
(1) Variability in Glomalin Extraction Techniques: Glomalin extraction methods vary widely, leading to inconsistencies in results. The sequential extraction method (SEM) introduced by Chen et al.[28] has improved protein extraction efficiency, but variations in soil types and compositions can still be challenging. Standardizing extraction protocols is crucial for the reliability of results.
(2) Complexities in Molecular Characterization: DNA sequencing and proteomic analysis provide valuable insights into glomalin structure. However, due to the diversity of soil microbial communities, challenges arise in identifying specific glomalin genes. Overcoming these challenges requires advancements in metagenomic approaches and high-throughput technologies[31].
(3) Functional Understanding of Glomalin: Despite progress, gaps persist in comprehending the functional roles of Glomalin in soil ecosystems. Investigating its interactions with other soil biota, as proposed by Purin and Rillig[31], requires more sophisticated methodologies such as immune-electron microscopy and biochemical analyses.
8.2 Integrating Glomalin Studies into Ecosystem Management
(1) Utilizing Glomalin for Sustainable Agriculture: Integrating glomalin research into agricultural practices holds significant potential. Understanding its role in nutrient cycling, soil structure, and plant health can lead to the development of sustainable farm strategies. This involves promoting mycorrhizal associations and optimizing glomalin-mediated benefits[33,100].
(2) Glomalin as an Indicator of Soil Health: Glomalin has been proposed to indicate soil health due to its correlation with soil organic carbon and aggregation. Implementing glomalin assessments in soil health monitoring programs can provide valuable information for land managers and policymakers. Such integrations require collaborative efforts between researchers and land management authorities[94].
(3) Mitigating Environmental Challenges: Glomalin's potential in mitigating environmental challenges, such as heavy metal sequestration and organic contaminant degradation, can be harnessed in ecological management strategies. Integrating glomalin-focused approaches into environmental remediation practices requires interdisciplinary collaboration and the development of targeted methodologies[101,102].
8.3 Future Research Avenues and Emerging Technologies
(1) Advanced Molecular Techniques: Future research should leverage advanced molecular techniques, such as metagenomics and transcriptomics, to unravel the diversity and functions of glomalin-producing organisms. Integrating these techniques can enhance our understanding of the genetic basis of glomalin synthesis and its regulation[31].
(2) Application of Imaging Technologies: Incorporating imaging technologies, including advanced microscopy and spectroscopy, can provide real-time visualization of Glomalin in soil matrices. This can contribute to a better understanding of glomalin distribution, dynamics, and its role in soil microenvironments[59,103].
(3) Remote Sensing for Large-Scale Monitoring: Developing remote sensing techniques for large-scale glomalin monitoring can revolutionize soil health assessments. Satellite-based or drone-assisted remote sensing technologies can offer insights into glomalin dynamics over extensive landscapes, aiding in precision agriculture and ecosystem management[41].
(4) Integrated Data Modelling: Future research should focus on integrating glomalin data into comprehensive soil health models. This includes developing predictive models considering glomalin dynamics in response to changing environmental conditions, land use, and management practices[94].
Addressing methodological challenges, integrating glomalin studies into ecosystem management, and embracing emerging technologies are crucial steps for unlocking the full potential of Glomalin in soil ecosystems. The interdisciplinary nature of glomalin research necessitates collaborative efforts to overcome challenges and pave the way for sustainable soil management practices.
9 CONCLUSION
The comprehensive review on the impact of ecological diversity on glomalin production within soil ecosystems underscores its pivotal role in bolstering soil health, facilitating nutrient cycling, sequestering heavy metals, and degrading organic contaminants. It highlights how ecological diversity, especially in plant diversity and microbial community composition, significantly influences glomalin levels, impacting soil stability, carbon storage, and overall ecosystem vitality. The interplay between glomalin and various land management practices, such as forestry, agroforestry, and agriculture, provides a promising avenue for sustainable land management and conservation efforts.
However, the review also points out several methodological challenges and gaps in current glomalin research, such as the need for standardized extraction and quantification protocols and a deeper understanding of glomalin's multifaceted roles in different ecosystems. Moving forward, addressing these challenges through interdisciplinary research, developing advanced molecular and imaging techniques, and integrating findings into ecosystem management practices will be crucial. Emphasizing collaborative efforts and leveraging emerging technologies will enhance our comprehension and utilization of glomalin, paving the way for innovative strategies to mitigate environmental challenges and promote sustainable ecosystem services.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest.
Data Availability
No additional data are available.
Copyright Permissions
Copyright © 2024 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Author Contribution
Mishra S led the development of the study’s concept and design and was primarily responsible for the initial drafting of the manuscript. Mishra AK supervised the review and contributed to the critical review and editing of the final manuscript. Arya R did the secondary data collection, tabulation and analysed the data, contributed to interpreting the results. Mishra VC was responsible for reviewing, scientific editing ,data visualization and preparing figures that aided in the interpretation of the data.
Abbreviation List
AMF, Arbuscular mycorrhizal fungi
GRSP, Glomalin related soil protein
NMR, Nuclear magnetic resonance
SEM, Sequential extraction method
EEG, easily-extracted GRSP
TG, Total GRSP
SOC, Soil Organic Carbon
References
[1] Wright SF, Upadhyaya A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci, 1996; 161: 575-586.[DOI]
[2] Rillig MC, Wright SF, Eviner VT. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: comparing effects of five plant species. Plant soil, 2002; 238: 325-333.[DOI]
[3] Yao Q, Zhu HH. Arbuscular mycorrhizal fungi: a belowground regulator of plant diversity in grasslands and the hidden mechanisms. In: Runas J, Dahlgren T. Grassland Biodiversity: Habitat Types, Ecological Processes and Environmental Impacts. New York: Nova Science Publisher, 2010.
[4] Liu S, Wang Q, Qian L et al . Mapping the scientific knowledge of glomalin-related soil protein with implications for carbon sequestration. Ecosyst Health Sustain, 2022; 8: 2085185.[DOI]
[5] Chen S, Sheng X, Qin C et al. Glomalin-related soil protein enhances the sorption of polycyclic aromatic hydrocarbons on cation-modified montmorillonite. Environ Int, 2019; 132: 105093.[DOI]
[6] Gao, WQ, Wang P, Wu QS. Functions and application of glomalin-related soil proteins: A review. Sains Malays, 2019; 48: 111-119.[DOI]
[7] Oyewole BO, Olawuyi OJ, Odebode AC et al. Influence of Arbuscular mycorrhiza fungi (AMF) on drought tolerance and charcoal rot disease of cowpea. Biotechnol Rep, 2017; 14, 8-15.[DOI]
[8] Napoli C, Mello A, Bonfante P. Dissecting the rhizosphere complexity: the truffle-ground study case. Rend Lincei, 2008; 19: 241-259.[DOI]
[9] Mishra AK, Mahinda AJ, Shinjo H et al. Role of conservation agriculture in mitigating soil salinity in indo-gangetic plains of India, 1st ed. In: Gupta SK, Goyal MR, Singh A (eds). Engineering Practices for Management of Soil Salinity. Apple Academic Press: Palm Bay, USA, 2018.[DOI]
[10] Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil, 2003; 255: 571-586.[DOI]
[11] Barea JM, Pozo MJ, Azcón R et al. Microbial co-operation in the rhizosphere. J Exp Bot, 2005; 56: 1761-1778.[DOI]
[12] Hinsinger P, Gobran GR, Gregory PJ et al. Rhizosphere geometry and heterogeneity arising from root‐mediated physical and chemical processes. New Phytol, 2005; 168: 293-303.[DOI]
[13] Schindler FV, Mercer EJ, Rice JA. Chemical characteristics of glomalin-related soil protein (GRSP) extracted from soils of varying organic matter content. Soil Biol Biochem, 2007; 39: 320-329.[DOI]
[14] Koide RT, Mosse B. A history of research on arbuscular mycorrhiza. Mycorrhiza, 2004; 14, 145-163.[DOI]
[15] Rillig MC, Steinberg PD. Glomalin production by an arbuscular mycorrhizal fungus: a mechanism of habitat modification? Soil Biol Biochem, 2002; 34: 1371-1374.[DOI]
[16] Rillig MC. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can J Soil Sci, 2004; 84: 355-363.[DOI]
[17] Rillig MC, Mummey DL. Mycorrhizas and soil structure. New Phytol, 2006; 171: 41-53.[DOI]
[18] Hallett PD, Feeney DS, Bengough AG et al. Disentangling the impact of AM fungi versus roots on soil structure and water transport. Plant Soil, 2009; 314, 183-196.[DOI]
[19] Wright SF, Upadhyaya A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant soil, 1998; 198: 97-107.[DOI]
[20] Rillig MC, Aguilar-Trigueros CA, Camenzind T et al. Why farmers should manage the arbuscular mycorrhizal symbiosis. The New Phytologist, 2019; 222: 1171-1175.[DOI]
[21] Wright SF, Nichols Ka. Glomalin: hiding place for a third of the world’s stored soil carbon. Agr Res, 2002; 50: 4.
[22] Lehmann A, Rillig MC. Arbuscular mycorrhizal contribution to crops’ copper, manganese and iron nutrient concentrations - A meta-analysis. Soil Biol Biochem, 2015; 81: 147-158.[DOI]
[23] Mishra AK, Das R, George KR et al. Promising management strategies to improve crop sustainability and to amend soil salinity. Front Environ Sci, 2023; 10: 962581.[DOI]
[24] Mishra AK, Shinjo H, Jat HS et al. Farmers’ perspectives as determinants for adopting conservation agriculture practices in Indo-Gangetic Plains of India. Resour Conserv Recycl Adv, 2022; 15: 200105.[DOI]
[25] Rillig MC, Treseder KK, Allen MF. Global change and mycorrhizal fungi. Mycorrhiza, 2010; 20: 343-351.[DOI]
[26] Verbruggen E, Kiers ET. Evolutionary ecology of mycorrhizal functional diversity in agricultural systems. Evol Appl, 2010; 3: 547-560.[DOI]
[27] Irving TB, Alptekin B, Kleven B et al. A critical review of 25 years of glomalin research requires a better mechanical understanding and robust quantification techniques. New Phytol, 2021; 232: 1572-1581.[DOI]
[28] Chen S, Rillig MC, Wang W. Improving soil protein extraction for metaproteome analysis and glomalin‐related soil protein detection. Proteomics, 2009; 9: 4970-4973.[DOI]
[29] Cissé G, Essi M, Nicolas M et al. Bradford quantification of Glomalin-Related Soil Protein in coloured extracts of forest soils. Geoderma, 2020; 372: 114394.[DOI]
[30] Zbíral J, Čižmár D, Malý S et al. Determination of glomalin in agriculture and forest soils by near-infrared spectroscopy. Plant Soil Environ, 2017; 63: 226-230.[DOI]
[31] Gadkar V, Rillig MC. The arbuscular mycorrhizal fungal protein glomalin is a putative homolog of heat shock protein 60. FEMS microbiology letters, 2006; 263: 93-101.[DOI]
[32] Bolliger A, Nalla A, Magid J et al. Re-examining the glomalin-purity of glomalin-related soil protein fractions through immunochemical, lectin-affinity and soil labelling experiments. Soil Biol Biochem, 2008; 40: 887-893.[DOI]
[33] Redmile-Gordon MA, Armenise E, White RP et al. A comparison of two colorimetric assays, based upon Lowry and Bradford techniques, to estimate total protein in soil extracts. Soil Biology Biochem, 2013; 67: 166-173.[DOI]
[34] Wright SF, Jawson L. A pressure cooker method to extract glomalin from soils. Soil Sci Soc Am J, 2001; 65: 1734-1735.[DOI]
[35] Pérez AB, Etchevers JD, Chávez MDCG et al. Extraction of glomalin and associated compounds with two chemical solutions in cultivated tepetates of mexico. Commun Soil Sci Plan, 2012; 43: 28-35.[DOI]
[36] Gonzalez-Chavez MC, Carrillo-Gonzalez R, Wright SF et al. The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ pollut, 2004; 130: 317-323.[DOI]
[37] Olk DC. Organic forms of soil nitrogen. Nitrogen in Agr Syst, 2008; 49: 57-100.[DOI]
[38] Eun Kang J, Ciampi A, Hijri M. SeSaMe PS Function: Functional analysis of the whole metagenome sequencing data of the arbuscular mycorrhizal fungi. Genom Proteom Bioinf, 2020; 18: 613-623.[DOI]
[39] Rillig MC, Wright SF, Nichols KA et al. Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil, 2001; 233: 167-177.[DOI]
[40] Wilson GWT, Rice CW, Rillig MC et al. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecol Lett, 2009; 12: 452-461.[DOI]
[41] Wang W, Zhong Z, Wang Q et al. Glomalin contributed more to carbon, nutrients in deeper soils, and differently associated with climates and soil properties in vertical profiles. Sci Rep, 2017; 7: 13003.[DOI]
[42] Jones MD, Smith SE. Exploring functional definitions of mycorrhizas: are mycorrhizas always mutualisms? Can J Bot, 2004; 82: 1089-1109.[DOI]
[43] Gonzalez-Chavez MC, Carrillo-Gonzalez R, Wright SF et al. The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ Pollut, 2004; 130: 317-323.[DOI]
[44] Pal A, Pandey S. Role of glomalin in improving soil fertility: a review. Int J Plant Soil Sci, 2014; 3: 1112-1129.[DOI]
[45] Johnson NC, Wilson GWT, Bowker MA et al. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. P Natl Acad Sci, 2010; 107: 2093-2098.[DOI]
[46] Klironomos JN. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology, 2003; 84: 2292-2301.[DOI]
[47] Badri DV, Vivanco JM. Regulation and function of root exudates. Plant Cell Environ, 2009; 32: 666-681.[DOI]
[48] Kuzyakov Y, Domanski G. Carbon input by plants into the soil. Review. J Plant Nutr Soil Sc, 2000; 163: 421-431.[DOI]
[49] Zhou F, Ding J, Li T et al. Plant communities are more sensitive than soil microbial communities to multiple environmental changes in the Eurasian steppe. Glob Ecol Conserv, 2020; 21, e00779.[DOI]
[50] Treseder KK, Balser TC, Bradford MA et al. Integrating microbial ecology into ecosystem models: challenges and priorities. Biogeochemistry, 2014; 109: 7-18.[DOI]
[51] Philippot L, Andersson SGE, Battin TJ et al. The ecological coherence of high bacterial taxonomic ranks. Nat Rev Microbiol, 2010; 8: 523-529.[DOI]
[52] Bonfante P, Anca IA. Plants, mycorrhizal fungi, and bacteria: a network of interactions. Annu Rev Microbiol, 2009; 63: 363-383.[DOI]
[53] Kumar S, Singh AK, Ghosh P. Distribution of soil organic carbon and glomalin-related soil protein in reclaimed coal mine-land chronosequence under tropical conditions. Sci Total Environ, 2018; 625, 1341-1350.[DOI]
[54] Yelikbayev B, Correa E, Duarte N et al. Sustainable restoration of degraded landscapes improves soil glomalin content. 2023 [Epub ahead of print].[DOI]
[55] Smith SE, Read DJ. Mycorrhizal Symbiosis, 3rd ed. Academic Press, 2008.[DOI]
[56] Sikes BA, Maherali H, Klironomos JN. Mycorrhizal fungal growth responds to soil characteristics, but not host plant identity, during a primary lacustrine dune succession. Mycorrhiza, 2014; 24: 219-226.[DOI]
[57] Jansa J, Bukovská P, Gryndler M. Mycorrhizal hyphae as ecological niche for highly specialized hypersymbionts–or just soil free-riders? Front Plant Sci, 2013; 4: 50022.[DOI]
[58] Wang W, Zhong Z, Wang Q et al. Glomalin contributed more to carbon, nutrients in deeper soils, and differently associated with climates and soil properties in vertical profiles. Sci Rep, 2017; 7: 13003.[DOI]
[59] Matos PS, Silva CFD, Damian JM et al. Beneficial services of Glomalin and Arbuscular Mycorrhizal fungi in degraded soils in Brazil. Sci Agr, 2021; 79: e20210064.[DOI]
[60] Li Y, Xu J, Hu J et al. Arbuscular mycorrhizal fungi and glomalin play a crucial role in soil aggregate stability in Pb-contaminated soil. Int J Env Res Pub He, 2022; 19: 5029.[DOI]
[61] Qiao L, Li Y, Song Y et al. Effects of vegetation restoration on the distribution of nutrients, glomalin-related soil protein, and enzyme activity in soil aggregates on the Loess Plateau, China. Forests, 2019; 10: 796.[DOI]
[62] Singh A K, Rai A, Singh N. Effect of long term land use systems on fractions of glomalin and soil organic carbon in the Indo-Gangetic plain. Geoderma, 2016; 277, 41-50.[DOI]
[63] Gujre N, Agnihotri R, Rangan L et al. Deciphering the dynamics of glomalin and heavy metals in soils contaminated with hazardous municipal solid wastes. J Hazard Mater, 2021; 416: 125869.[DOI]
[64] Khan AG. Promises and potential of in situ nano-phytoremediation strategy to mycorrhizo-remediate heavy metal contaminated soils using non-food bioenergy crops (Vetiver zizinoides & Cannabis sativa). Int J Phytoremediat, 2020; 22: 900-915.[DOI]
[65] Das S, Ganguly D, Chakraborty S et al. The first report of Glomalin from the Sundarban Mangrove Biosphere Reserve, India, a long-term sediment Carbon storage. Reg Stud Ma Sci, 2020; 39: 101398.[DOI]
[66] Fokom R, Adamou S, Teugwa MC et al, Glomalin related soil protein, carbon, nitrogen and soil aggregate stability as affected by land use variation in the humid forest zone of south Cameroon. Soil Till Res, 2012; 120, 69-75.[DOI]
[67] Holatko J, Prichystalova J, Hammerschmiedt T et al. Glomalin: A key indicator for soil carbon stabilization. Soil Carbon Stabilization to Mitigate Clim Change, 2024; 47-81.[DOI]
[68] Singh AK, Zhu X, Chen C et al. The role of glomalin in mitigation of multiple soil degradation problems. Crit Rev Env Sci Tec, 2020; 52: 1604-1638.[DOI]
[69] Hossain MB. Glomalin and contribution of glomalin to carbon sequestration in soil: a review. Turkish J Agr-Food Sci Tech, 2021; 9: 191-196.[DOI]
[70] Storer K, Coggan A, Ineson P et al. Arbuscular mycorrhizal fungi reduce nitrous oxide emissions from N2O hotspots. New Phytol, 2018; 220: 1285-1295.[DOI]
[71] Prasad M, Chaudhary M, Ramakrishnan S et al. Glomalin: a miracle protein for soil sustainability. Indian Farmer, 2018; 5: 1092-100.[DOI]
[72] De-la-Peña C, Loyola-Vargas VM. Biotic interactions in the rhizosphere: a diverse cooperative enterprise for plant productivity. Plant physiol, 2014; 166: 701-719.[DOI]
[73] G. Cissé, Essi M, Kedi B et al. Accumulation and vertical distribution of glomalin-related soil protein in French temperate forest soils as a function of tree type, climate, and soil properties. Catena, 2023; 220: 106635.[DOI]
[74] Huang L, Fu Y, Liu Y et al. Global insights into endophytic bacterial communities of terrestrial plants: Exploring the potential applications of endophytic microbiota in sustainable agriculture. Sci Total Environ, 2024; 927: 172231.[DOI]
[75] Isbell F, Craven D, Connolly J et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature, 2015; 526: 574-577.[DOI]
[76] Malobane ME, Madzivhandila MR.Chapter: Enhancement of Soil Arbuscular Mycorrhizal Fungi: A Step Towards Restoring Marginal Soils. In: The Marginal Soils of Africa. 2024; Springer Nature Switzerland.[DOI]
[77] Van der Heijden MGA, Martin FM, Selosse MA et al. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol, 2015; 205: 1406-1423.[DOI]
[78] Mishra AK, Singh K, Singh K et al. Carbon sequestered through biomass and soil organic carbon dynamics in Jatropha curcas L. Ecology, Environ Conserv, 2014; 20: 561-565, 2014.
[79] Fahad S, Chavan SB, Chichaghare AR, et al. Agroforestry systems for soil health improvement and maintenance. Sustainability, 2022; 14: 14877.[DOI]
[80] Elevitch CR, Wilkinson KM. (2000). Agroforestry guides for Pacific islands.
[81] Silva AMM, Araújo, VLVPD, Cardoso EJBN. Revisiting the past to understand the present and future of soil health in Brazil. Front Soil Sci, 2023; 3: 1172436.[DOI]
[82] Giri A, Kumar G, Arya R et al. Carbon sequestration in Populus deltoides based agroforestry system in Northern India. Int J Chem Stud, 2019; 7: 2184-2188.
[83] Jenkinson D, Andrew S, Lynch J et al. The turnover of organic matter in soil. Philos T R Soc B, 1990; 329: 361-368.
[84] Prasad M, Chaudhary M, Ramakrishnan S et al. Glomalin: a miracle protein for soil sustainability. Indian Farmer, 2018; 5: 1092-1100.
[85] Matos PS, Figueira da Silva C, Pereira MG et al. Short-term modifications of mycorrhizal fungi, glomalin, and soil attributes in tropical agroforestry. Acta Oecol, 2022; 114: 103815.[DOI]
[86] Silva CF, Pereira MG, Gomes JHG. Enzyme activity, glomalin, and soil organic carbon in agroforestry systems. Floresta Ambiente, 2020; 27: e20170716.[DOI]
[87] Perez-Sanz A, Yunta F, Lucena JJ et al. The effects of different agroforestry practices on glomalin related soil protein, soil aggregate stability and organic carbon-association with soil aggregates in southern Ethiopia. EGU General Assembly. Vienna, Austria, 24-28 April 2023.[DOI]
[88] Curaqueo G, Barea JM, Acevedo E et al. Effects of different tillage system on arbuscular mycorrhizal fungal propagules and physical properties in a Mediterranean agroecosystem in central Chile. Soil Till Res, 2011; 113: 11-18.[DOI]
[89] Govindu D, Duvva A, Shaikh AH et al. The impact of Arbuscular Mycorrhizal fungi on glomalin-related soil protein distribution and their relationship with soil properties of agroforestry plants in coal mine region of NorthTelangana. Indian Phytopathol, 2020; 73: 737-740.[DOI]
[90] Lee JE, Eom AH. Effect of organic farming on spore diversity of arbuscular mycorrhizal fungi and glomalin in soil. Mycobiology, 2009; 37: 272-276.[DOI]
[91] Cordeiro CFDS, Rodrigues DR, Rocha CH et al. Glomalin and microbial activity affected by cover crops and nitrogen management in sandy soil with cotton cultivation. Appl Soil Ecol, 2021; 167: 104026.[DOI]
[92] Rajtor M, Piotrowska-Seget Z. Prospects for arbuscular mycorrhizal fungi (AMF) to assist in phytoremediation of soil hydrocarbon contaminants. Chemosphere, 2016; 162: 105-116.[DOI]
[93] Banegas ND, Santos DAD, Molina FG et al. Glomalin contribution to soil organic carbon under different pasture management in a saline soil environment. Arch Agron Soil Sci, 2022; 68: 340-354.[DOI]
[94] Nautiyal P, Rajput R, Pandey D et al. Role of glomalin in soil carbon storage and its variation across land uses in temperate Himalayan regime. Biocatal Agr Biotech, 2019; 21, 101311.[DOI]
[95] Gałązka A, Gawryjołek K, Grządziel J et al. Effect of different agricultural management practices on soil biological parameters including glomalin fraction. Plant Soil Environ, 2017; 63: 300-306.[DOI]
[96] Aliasgharzad N, Malekzadeh E. Chapter: Glomalin and Carbon Sequestration in Terrestrial Ecosystems. In: Arbuscular Mycorrhizal Fungi and Higher Plants: Fundamentals and Applications. 2024; Springer Nature Singapore.[DOI]
[97] Arya R, Arora P, Rawal G et al. Impact of different agroforestry systems on depth-wise distribution of physico-chemical properties and soil carbon stock in north-west India. Indian J Ecol, 2021; 48: 1026-1031.
[98] Bertagnoli BG, Oliveira JF, Barbosa GM et al. Poultry litter and liquid swine slurry applications stimulate glomalin, extraradicular mycelium production, and aggregation in soils. Soil Till Res, 2020; 202: 104657.[DOI]
[99] Agnihotri R, Bharti A, Ramesh A et al. Glomalin-related protein and C16:1ω5 PLFA associated with AM fungi as potential signatures for assessing the soil C sequestration under contrasting soil management practices. Eur J Soil Biol, 2021; 103: 103286.[DOI]
[100] Suzuki K, Takemura M, Miki T et al. Differences in soil bacterial community compositions in paddy fields under organic and conventional farming conditions. Microbes Environ, 2019; 34: 108-111.[DOI]
[101] Chen S, Sheng X, Qin C et al. Glomalin-related soil protein enhances the sorption of polycyclic aromatic hydrocarbons on cation-modified montmorillonite. Environ Int, 2019; 132: 105093.[DOI]
[102] Chauhan S, Mahawar S, Jain D et al. Boosting sustainable agriculture by arbuscular mycorrhiza under stress condition: mechanism and future prospective. Bio Med Res Int, 2022; 2022.[DOI]
[103] Morris EK, Morris DJP, Vogt S et al. Visualizing the dynamics of soil aggregation as affected by arbuscular mycorrhizal fungi. The ISME J, 2019; 13: 1639-1646.[DOI]

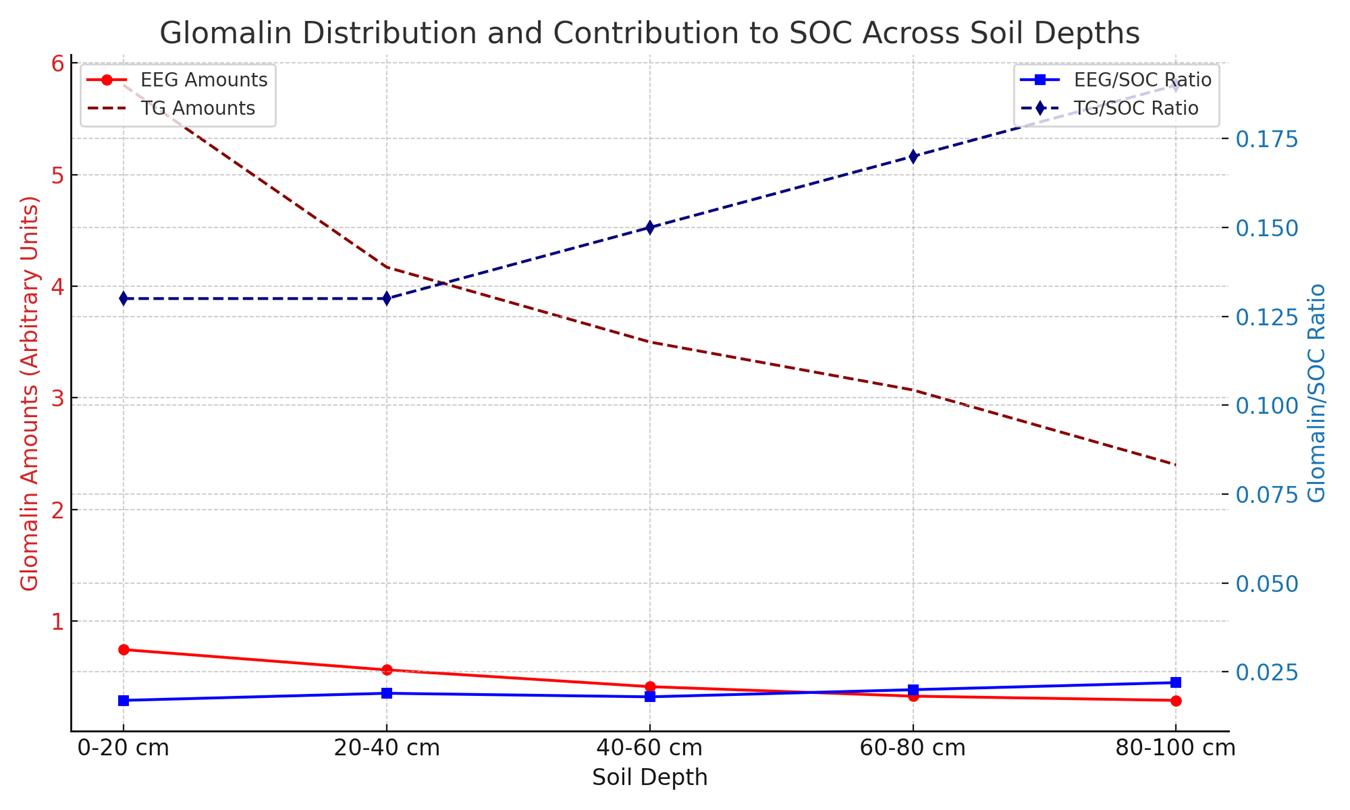
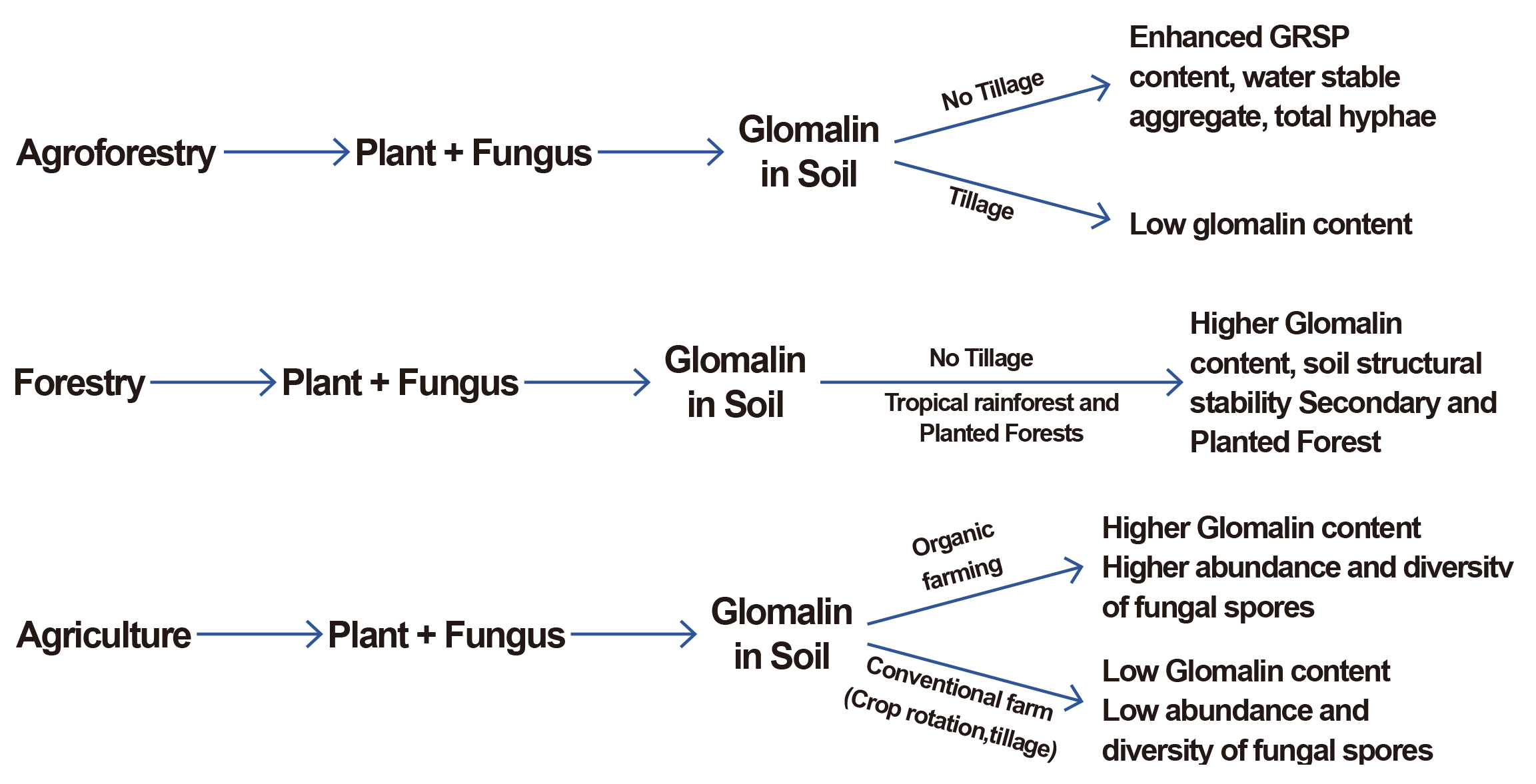



 Copyright ©
Copyright ©