Protection by L-arginine Against Epinephrine-induced Arrhythmia and Cardiotoxicity
Omar M.E. Abdel-Salam1*![]() , Marawan Abd El Baset2, Enayat A Omara3, Amany A. Sleem2
, Marawan Abd El Baset2, Enayat A Omara3, Amany A. Sleem2
1Department of Toxicology and Narcotics, Medical Research and Clinical Studies Institute, National Research Centre, Cairo, Egypt
2Department of Pharmacology, Medical Research and Clinical Studies Institute, National Research Centre, Cairo, Egypt
3Department of Pathology, Medical Research and Clinical Studies Institute, National Research Centre, Cairo, Egypt
*Correspondence to: Omar M.E. Abdel-Salam, PhD, Professor, Department of Toxicology and Narcotics, Medical Research and Clinical Studies Institute, National Research Centre, Tahrir Street, Cairo, 11435, Egypt; Email: omasalam@hotmail.com
DOI: 10.53964/id.2024012
Abstract
Nitric oxide is important in control of coronary blood flow, cardiomyocyte contractility, cardiac rhythm, and thrombogenicity. We aimed to investigate the effect of the nitric oxide precursor L-arginine on cardiac arrhythmia and myocardial injury induced by epinephrine in male Sprague-Dawley rats. The possible modulation of L-arginine effects by methylene blue (MethyB), a nitric oxide synthase inhibitor was also examined. Cardiac arrhythmia was induced with 10μg/kg epinephrine given intravenously (i.v.). L-arginine (200mg/kg) or L-arginine and MethyB (100mg/kg) were intraperitoneally (i.p.) administered prior to i.v. epinephrine. Other groups received i.p. saline, only L-arginine or only MethyB (100mg/kg). Results showed that compared with the saline control, epinephrine caused a significant bradycardia (188.7±24.4 vs. 405.6±1.19beats/min), increased QRS duration (0.039±0.006 vs. 0.0185±0.0001s), decreased R wave amplitude (0.18±0.01 vs. 0.23±0.011mv), and ventricular extrasystoles. L-arginine alone showed no significant effect on heart rate (380.8±10.0 vs. 405.6±1.19beats/min) but increased QRS duration (0.029±0.0002 vs. 0.0185±0.0001s), and R wave amplitude (0.47±0.01 vs. 0.23±0.011mv). L-arginine given prior to epinephrine prevented bradycardia, shortened the duration of arrhythmia and decreased the number of extrasystoles. The administration of L-arginine and MethyB almost completely suppressed epinephrine-induced ventricular arrhythmias. Epinephrine caused severe degeneration of muscle fibres, widening of intercellular spaces, cellular infiltrations and interstitial haemorrhage. L-arginine markedly attenuated, while L-arginine/MethyB completely prevented these changes. It is concluded that L-arginine protects against cardiac arrhythmias and cardiac tissue damage caused by epinephrine.
Keywords: arrhythmia, epinephrine, L-arginine, methylene blue, myocardial injury, nitric oxide
1 INTRODUCTION
The free radical nitric oxide is an endogenous messenger molecule with important physiological effects on the vascular endothelium and cardiac function[1]. Nitric oxide is formed from the amino acid L-arginine via the activity of the enzyme, nitric oxide synthase. The three, nitric oxide synthase (NOS) enzymes which catalyze the reaction are expressed in endothelial cells, cardiomyocytes or cardiac nerves. These are the constitutively expressed endothelial (eNOS) and neuronal (nNOS) isoforms and the inducible NOS (iNOS) whose expression is induced by inflammatory cytokines. Nitric oxide produced by constitutive NOS is an endogenous vasodilator, which activates soluble guanylyl cyclase in vascular smooth muscle and causes vascular relaxation and vasodilatation[1,2]. In cardiac tissue, nitric oxide plays an important role in the control of vascular tone and coronary blood flow, cardiomyocyte contractility, and mitochondrial oxygen consumption[3]. In contrast to the low concentrations of nitric oxide produced by the constitutively expressed nitric oxide synthases, iNOS generates high nitric oxide fluxes for a relatively longer time, which can be damaging to the cell[4].
The amino acid L-arginine is the precursor for nitric oxide through the nitric oxide synthase pathway[5]. L-arginine administration improved endothelial function and induced endothelium-dependent vasodilatation in a number of vascular disease processes[6-8]. L-arginine reduced neutrophil adherence and consequent injury to coronary vascular endothelium dysfunction[9]. In patients subjected to coronary artery bypass grafting, L-arginine infusion, reduced blood levels of interleukins (IL)-2 receptor, IL-6 and tumour necrosis factor (TNF)-α[10]. The effects of exogenously administered L-arginine on the cardiovascular function are largely attributable to its ability to increase endothelial nitric oxide synthesis[11]. L-arginine is thus regarded as a pharmacologic intervention for increasing endothelial nitric oxide synthase activity[12].
The synthetic phenothiazine dye methylene blue (MethyB) is used to treat a variety of conditions such as methemoglobinemia, cyanide poisoning[13,14], and ifosfamid-induced neurotoxicity in cancer patients[15]. MethyB is an inhibitor of guanylate cyclase and nitric oxide synthase enzymes[16,17]. This effect of MethyB has been suggested to account for its usefulness in rescuing patients who developed vasoplegic shock during or after cardiopulmonary bypass, a condition characterized by hypotension and reduced systemic vascular resistance due to excessive release of nitric oxide[18].
The aim of the present study was therefore to examine whether the administration of L-arginine administration could protect against cardiac arrhythmia and myocardial injury induced by epinephrine in rats. We also examined the effect of the nitric oxide synthase inhibitor MethyB on the responses to L-arginine.
2 MATERIALS AND METHODS
2.1 Animals
Male Sprague-Dawley rats 8-10 weeks of age, weighing 140-150g were used in the study. Rats were obtained from the Animal House Colony of the NRC. Animals were kept under temperature- and light-controlled conditions (20-22℃ and a 12h light/dark cycle) and given free access to tap water and standard laboratory rodent chow. Animal procedures followed the guidelines of the Institute ethics committee for the use of animals in experimental studies and the Guide for Care and Use of Laboratory Animals by the U.S. National Institutes of Health (Publication No. 85-23, revised 1996).
2.2 Drugs and Chemicals
L-arginine, methylene blue (Sigma Chemical Co., St. Louis, MO, U.S.A) and epinephrine (Nile Co., Egypt) were used in the study and freshly dissolved in saline before the experiments to obtain the necessary doses.
2.3 Experimental Groups
Rats were randomly divided into six equal groups (n=8 per group) and treated as follows:
Group 1 was given i.p. saline (served as a negative control);
Group 2 was given i.p. saline prior to induction of cardiac arrhythmia by i.v. injection of epinephrine (10μg/kg) (served as a positive control);
Group 3 was given i.p. L-arginine (200mg/kg) only;
Group 4 was given i.p. MethyB (100mg/kg) only;
Group 5 was given L-arginine (200mg/kg, i.p.), 30min before the arrhythmia was induced by an i.v. injection of epinephrine;
Group 6 was given L-arginine (200mg/kg, i.p.) 30min before administering MethyB (100mg/kg, i.p.), and followed 30min later by an i.v. injection of epinephrine.
The dose of MethyB blue was selected based on previous studies[19].
2.4 Electrocardiography
After 30min of drug or saline administration, rats were anesthetized with 10% chloral hydrate (300mg/kg, i.p.). The electrocardiogram (ECG) was then recorded with the ECG Powerlab module. The latter consisted of Powerlab/8sp and Animal Bio-Amplifier (Australia), in addition to Lab Chart 7 software with an ECG analyzer. After the establishment of a steady state, arrhythmia was induced by the i.v. injection of 10µg/kg epinephrine. ECG recording was continued until the termination of the arrhythmia[20]. The average heart rate, RR interval, PR interval, QRS interval, QTc (corrected QT interval), R wave amplitude, ST height, number of ventricular extrasystoles, and duration of ventricular arrhythmia after different treatments were determined over a period of 15min. The mean value of three 5min of ECG measurements/recordings for each group obtained in the first 15min after the injection of epinephrine were used for the calculations. Arrhythmia was assessed by counting the number of premature ventricular beats, and runs of ventricular tachycardia during the first 15min after epinephrine. Arrhythmias were defined according to the Lambeth conventions[21]. Ventricular extrasystole is defined as a single premature ventricular complex, and ventricular tachycardia is defined as 4 or more consecutive ventricular premature beats[21].
2.5 Cardiac Histopathology
Cardiac specimens were immediately fixed in 10% formalin at room temperature, treated with a conventional grade of alcohol and xylol, embedded in paraffin, and sectioned at 5µm thicknesses. The sections were stained with haematoxylin and eosin in order to study the histopathological changes using a light microscope (Olympus CX41 with DP12 Olympous digital camera).
2.6 Statistical Analysis
Data are presented as mean±SE for measurement variables over a period of 15min. Comparison between groups was performed with a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. GraphPad Prism 6 for Windows (GraphPad Prism Software Inc., San Diego, CA, USA) was used, and differences were considered statistically significant when P values were less than 0.05.
3 RESULTS
3.1 Electrocardiographic Recordings
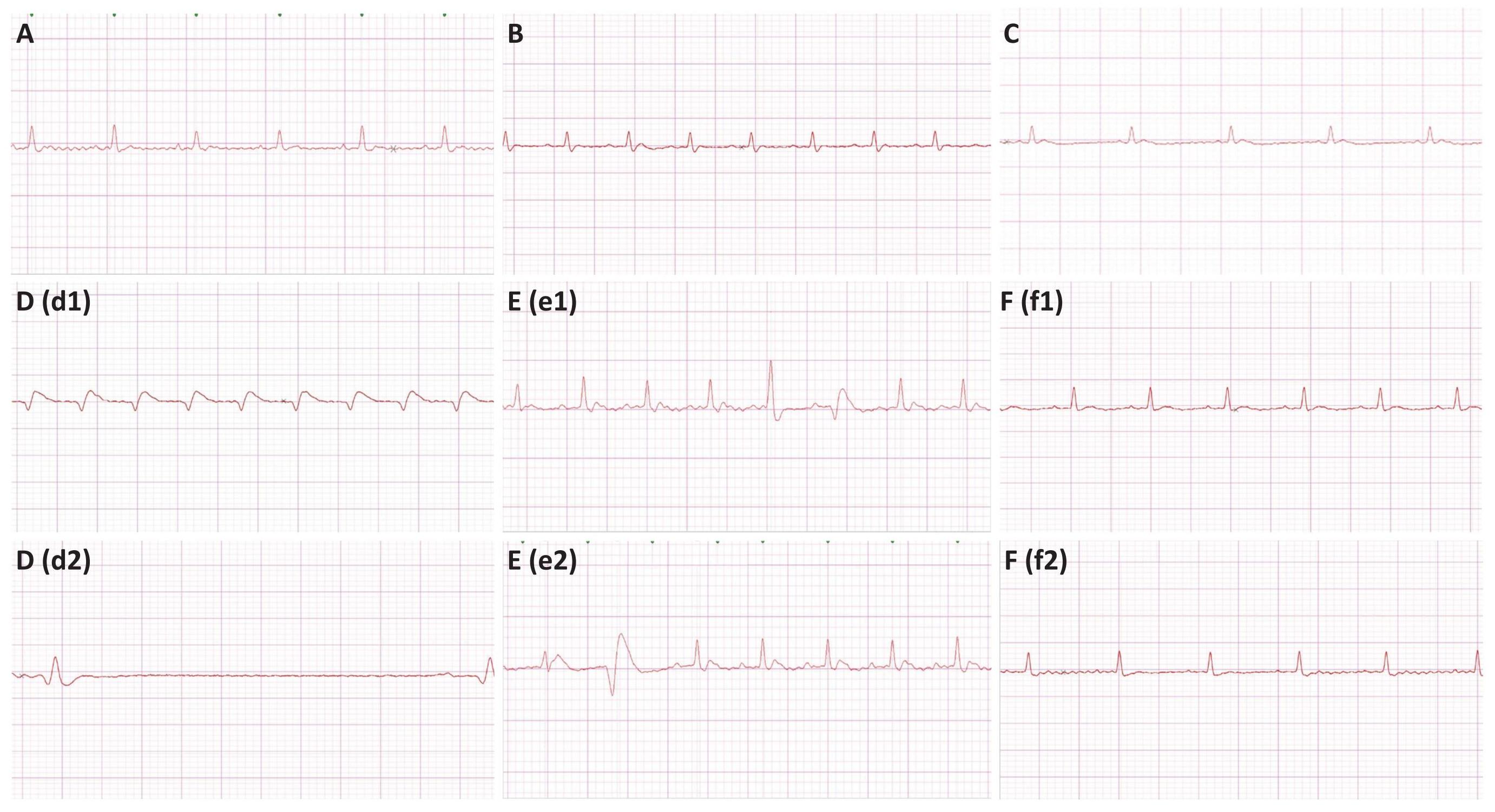
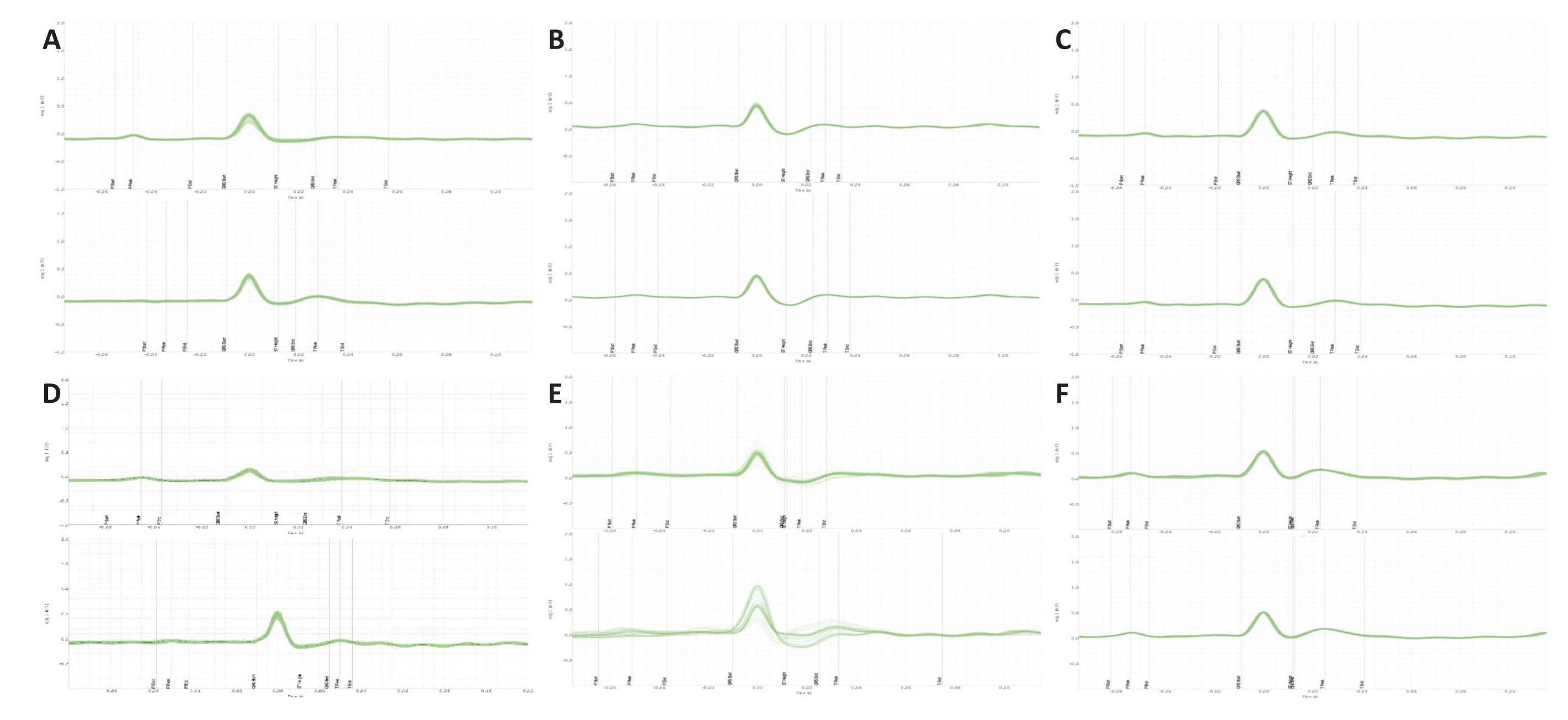
Representative ECG tracings of the different treatment groups are shown in Figure 1. ECG recordings in the epinephrine control group shows the presence of marked bradycardia, premature ventricular beats, and occasional ventricular tachycardia. The group treated with L-arginine plus epinephrine shows multiform ventricular premature beats. ECG changes in the L-arginine and epinephrine-treated group were prevented by MethyB. Representative ECG signals for different treatment groups are shown in Figure 2.
|
Figure 1. Representative ECG Tracings of Different Treatment. A:saline control-; B: L-arginine only-; C: MethyB only-; D: epinephrine only-, (d1) shows ventricular tachycardia, and (d2) shows bradycardia; E: epinephrine + L-arginine group, (e1) shows multiform premature ventricular beats (couplet), and (e2) shows a premature ventricular beat; F: epinephrine +L-arginine+MethyB, Both (f1) and (f2) tracings shows normalization of the ECG complexes after treatment.
|
Figure 2. Representative Successive Average ECG Wave Forms (1 Min Data) From Different Rats in the First 15 Min After Injection of Saline, Epinephrine, L-Arginine, MethyB Alone or in the First 15 Min After the I.V. Injection of Epinephrine in the Epinephrine + L-Arginine or Epinephrine + L-Arginine + MethyB Groups. A: ECG signals for saline control-; B: ECG signals for L-arginine only-; C: ECG signals for MethyB only-; D: ECG signals for epinephrine only-; E: ECG signals for epinephrine and L-arginine-; F: ECG signals for epinephrine and L-arginine and MethyB-treated groups. The green wave represents the individual ECG waves overlaid, while the black wave represents their collective average.
3.2 Electrocardiographic Parameters
3.2.1 Saline Control
The heart rate in the saline control group was 405.6±1.19beats/min. The ECG parameters of the saline group are shown in Tables 1, 2 and Figures 3, 4.
3.2.2 Effects of L-arginine
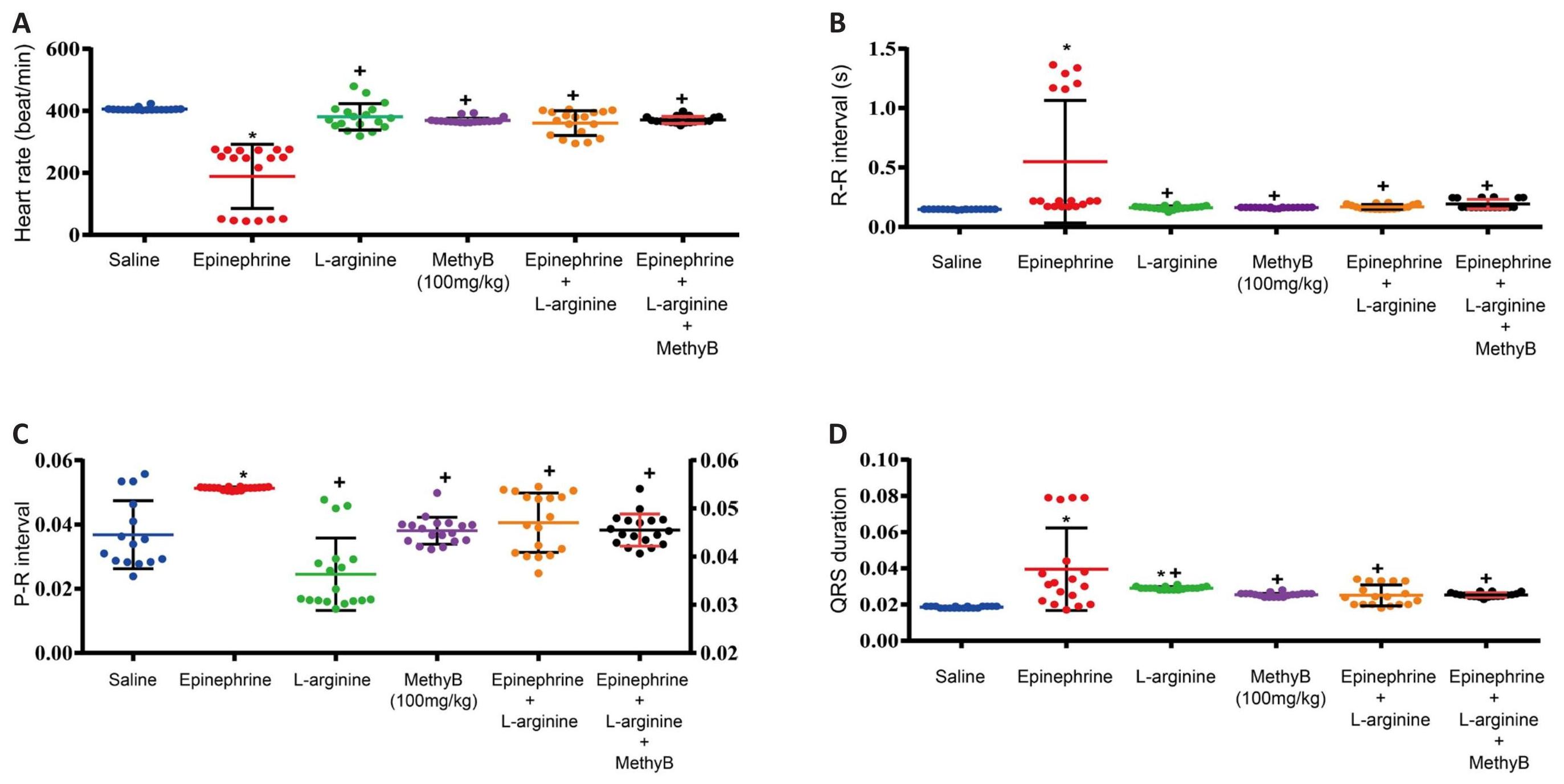
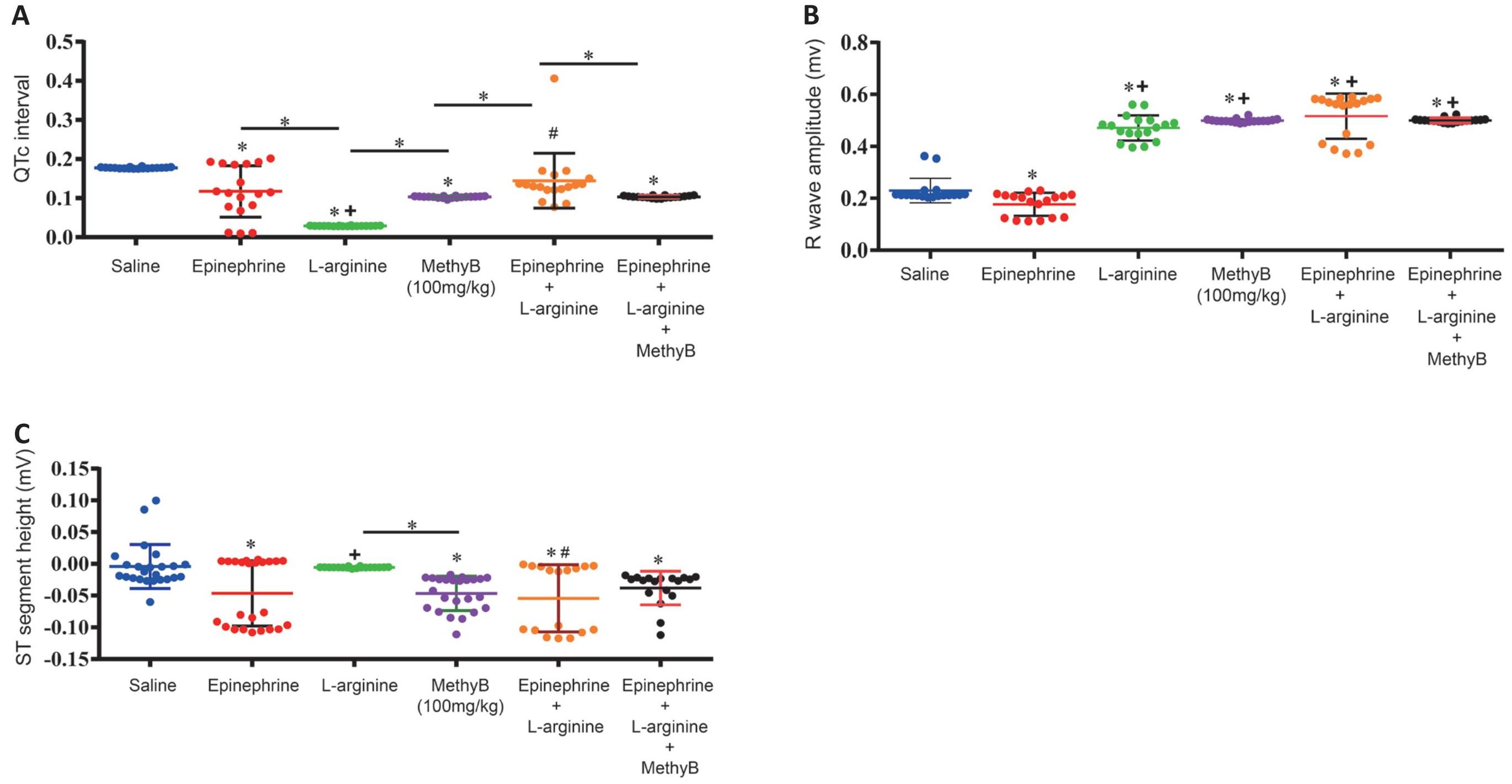
L-arginine alone had no significant effect on heart rate (380.8±10.0 vs. 405.6±1.19 beats/min) or PR interval, but caused a significant increase in QRS duration (0.029±0.0002 vs. 0.0185±0.0001s), shortened QTc interval (0.029±0.0002 vs. 0.177±0.0004s), and increased R wave amplitude (0.47±0.01 vs. 0.23±0.011mv) compared with the saline control group. L-arginine alone didn’t cause any arrhythmia (Tables 1, 2 and Figures 3-5).
3.2.3 Effects of Methylene Blue
The ECG changes induced by MethyB alone at 100mg/kg were those of a non-significant decrease in heart rate (369.5±2.18 vs. 405.6±1.19beats/min), significantly increased QRS duration (0.025±0.0002 vs. 0.0185±0.0001s), shortened QTc interval (0.103±0.0005 vs. 0.177±0.0004s), increased R-wave amplitude (0.498±0.002 vs. 0.23±0.011mv) and decreased ST segment (-0.046±0.005 vs. -0.004±007mv) relative to saline control values. MethyB didn’t cause any arrhythmia (Tables 1, 2 and Figures 3-5).
3.2.4 Effects of Epinephrine
The i.v. administration of epinephrine induced a significant bradycardia (188.7±24.37 vs. 405.6±1.19beats/min), increased PR interval (0.051±0.0001 vs. 0.037±0.003s), shortened QTc interval (0.117±0.021 vs. 0.177±0.0004s), increased QRS duration (0.0395 ±0.006 vs. 0.0185±0.0001s), decreased R-wave amplitude (0.18±0.01 vs. 0.23±0.011mv), ST segment height (-0.046±0.01 vs. -0.004±0.007mv) associated with bradycardia, ventricular extrasystoles and occasional ventricular tachycardia (Tables 1, 2 and Figures 3-5).
3.2.5 Effects of L-arginine and Epinephrine
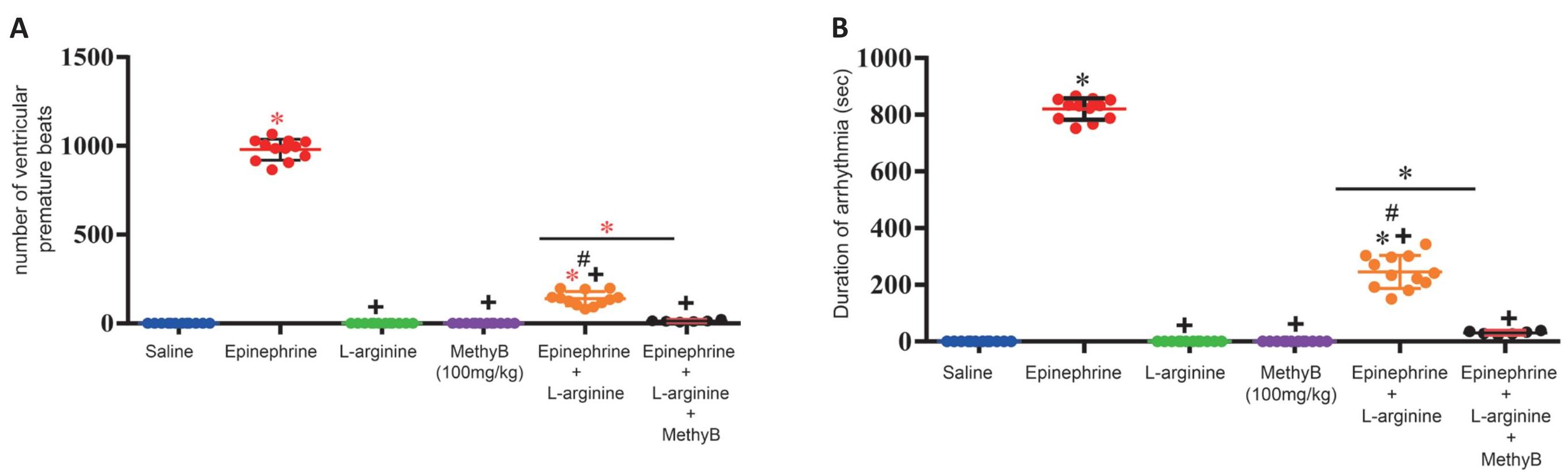
L-arginine given prior to i.v. epinephrine prevented the marked bradycardia associated with epinephrine (360.4±9.3 vs. 188.7±24.4beats/min), decreased QRS duration (0.025±0.001 vs. 0.0395±0.006s), and increased R-wave amplitude (0.516±0.02 vs. 0.18±0.01mv) compared with the group that received epinephrine alone. L-arginine given prior to i.v. epinephrine caused a significant and marked decrease in the duration of arrhythmia (245.2+16.9 vs. 820.0±10.9) and number of ventricular premature beats (139.5±11.5 vs. 979.1±17.2) caused by epinephrine. In addition, no ventricular tachycardia was observed (Tables 1, 2 and Figures 3-5).
3.2.6 Effects of L-arginine, Methylene Blue and Epinephrine
L-arginine and MethyB given prior to i.v. epinephrine prevented the epinephrine-associated bradycardiac response (370.6±2.6 vs. 188.7±24.3beats/min), normalized the PR interval, decreased QRS duration (0.025±0.0003 vs. 0.0395±0.006s), and increased R-wave amplitude (0.50±0.002 vs. 0.18±0.01mv) compared with the group that received epinephrine alone. The administration of L-arginine and MethyB almost completely suppressed epinephrine-induced ventricular arrhythmias (Tables 1, 2 and Figures 3-5).
Table 1. Effect of L-Arginine, Methylene Blue or L-Arginine Plus Methylene Blue on Epinephrine-Induced Electrocardiogram Changes and Ventricular Arrhythmia
Parameter/ Group |
Normal control |
Epinephrine |
L-arginine |
MethyB |
Epinephrine+ L-arginine |
Epinephrine+L-arginine+MethyB |
Heart rate/min |
405.6±1.19 |
188.7±24.3* |
380.8±10.0+ |
369.5±2.2+ |
360.4±9.3+ |
370.6±2.6+ |
RR interval (s) |
0.15±0.0004 |
0.55±0.12* |
0.16±0.003+ |
0.16±0.0009+ |
0.17±0.004+ |
0.19±0.001+ |
PR interval (s) |
0.037±0.003 |
0.051±0.0001* |
0.036±0.002+ |
0.038±0.001+ |
0.041±0.002+ |
0.038±0.00+ |
QRS duration (s) |
0.0185±0.0001 |
0.0395±0.006* |
0.029±0.0002*+ |
0.0254±0.0003+ |
0.0251±0.001+ |
0.0253±0.0003+ |
QTc interval (s) |
0.177±0.0004 |
0.117±0.02* |
0.029±0.0002*+ |
0.103±0.0005* |
0.145±0.02# |
0.103±0.0007* |
R wave amplitude (mv) |
0.23±0.011 |
0.18±0.01* |
0.47±0.011*+ |
0.50±0.002*+ |
0.52±0.02*+ |
0.50±0.002*+ |
ST segment height (mv) |
-0.004±0.007 |
-0.046±0.01* |
-0.006±0.002+ |
-0.047±0.005* |
-0.054±0.013*# |
-0.038±0.006* |
Notes: Data were expressed as mean ± SE (n=8). Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. *: P<0.05: significantly different from normal control group. +: P<0.05: significantly different from epinephrine control group. #: P<0.05: significantly different from the L-arginine group.
Table 2. Effect of L-arginine, Methylene Blue or L-Arginine Plus Methylene Blue on Epinephrine-Induced Ventricular Arrhythmia
Parameter/ Group |
Normal control |
Epinephrine |
L-arginine |
MethyB |
Epinephrine + L-arginine |
Epinephrine + L-arginine + MethyB |
Duration of arrhythmia (s) |
0.0 0.0±0.0 |
820.0±10.9* |
0.0±0.0+ |
0.0±0.0+ |
245.2±16.9*+# |
30.7±2.5+ |
Number of extrasystoles/15min |
0.0±0.0 |
979.1±17.2* |
0.0±0.0+ |
0.0±0.0+ |
139.5±11.5*+# |
13.0±2.2+ |
Notes: Data were expressed as mean±SE (n=8). Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. *: P<0.05: significantly different from normal control group. +: P<0.05: significantly different from epinephrine control group. #: P<0.05: significantly different from the L-arginine group.
|
Figure 3. Effects of Treatment With L-Arginine, L-Arginine Plus MethyB on the Epinephrine-Induced Changes in (A) Heart Rate, (B) RR Interval, (C) PR Interval, and (D) QRS Duration. *: P<0.05: significantly different from the normal control group. +: P<0.05: significantly different from the epinephrine control group.
|
Figure 4. Effects of Treatment With L-Arginine, L-Arginine Plus Methylene Blue (MethyB) on (A) QTc Interval, (B) R Wave Amplitude, and (C) ST Wave Height in Epinephrine-Treated Rats. *: P<0.05: significantly different from the normal control group and between different groups as shown in the figure. +: P<0.05: significantly different from the epinephrine control group. #: P<0.05: significantly different from the L-arginine group.
|
Figure 5. Effects of Treatment With L-Arginine, L-Arginine Plus Methylene Blue (MethyB) on the (A) Number, and (B) Duration of Ventricular Extrasystoles Induced by Epinephrine. *: P<0.05: significantly different from the normal control group and between different groups as shown in the figure. +: P<0.05: significantly different from the epinephrine control group. #: P<0.05: significantly different from the L-arginine group.
3.3 Histopathological Results
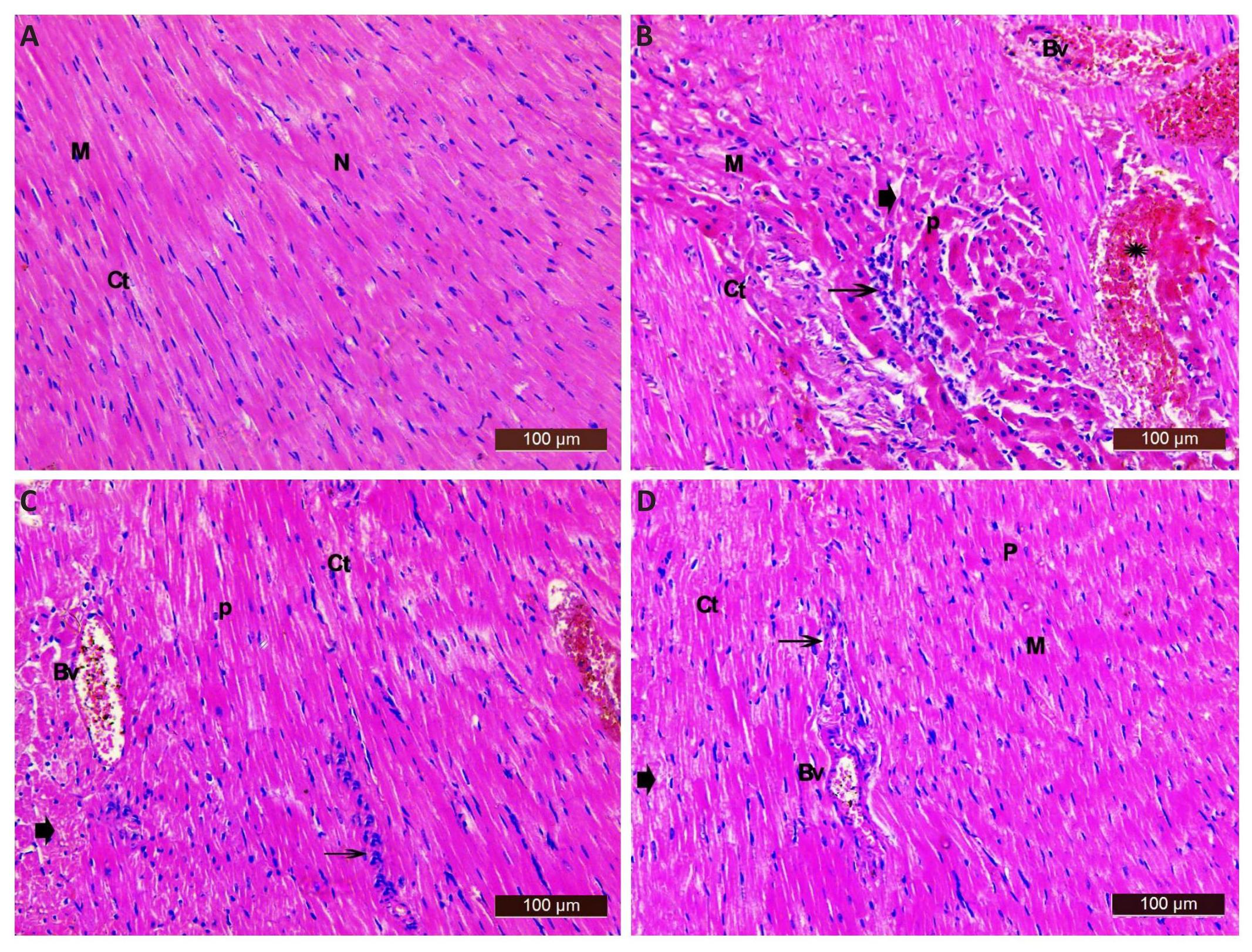
In cardiac sections from saline control rats, the myocardium appeared striated and arranged in a linear array. The cardiac muscle fibres were joined together by intercalated discs. They contained acidophilic cytoplasm with oval centrally located nuclei. The cardiac muscle fibres were separated by delicate layer of connective tissue containing fibroblasts that were identified by their flat nuclei with well evidenced myocardial blood capillaries (Figure 6A). The heart from the epinephrine control group showed a variety of histological changes including disarrangement with acidophilic cytoplasm of many cardiac myocytes. Also, degeneration of muscle fibres with widening of the intercellular spaces and pyknotic nuclei was seen. Marked congestion and dilatation of blood vessels with interstitial haemorrhage and cellular infiltrations were common in many sections (Figure 6B). Sections from the epinephrine and L-arginine treated group showed more or less normal architecture of cardiac myocytes with mild degeneration and normal vesicular nuclei. Some cellular infiltrate with mild interstitial haemorrhage and congestion blood vessels were seen. Most myofibers were intact, while others exhibited mild widening of the intercellular spaces and few pyknotic nuclei (Figure 6C). Sections from rats treated with epinephrine, L-arginine and MethyB showed obvious restoration of the normal architecture of the muscle fibres. Most myofibers were intact with mild degeneration. Other myocytes showed mild widening of the intercellular spaces and few pyknotic nuclei with minimal interstitial haemorrhage (star). Some cellular infiltrate and some congestion blood vessels were also seen (Figure 6D).
Histological damage was expressed using the following score system: ∅: absent; ⊥: few; +: mild; ++: moderate; +++: severe. The heart of 6 animals in every group was examined with 10 different microscopic fields (Table 3).
|
Figure 6. Representative Photomicrographs Showing Cardiac Tissue Stained With H&E. A: Saline control showing the typical histological architecture of cardiac myocytes (M). Most appear longitudinally with rounded vesicular centrally located nuclei (N), in-between the cardiac myocytes, there was a delicate layer of connective tissue (CT); B: Epinephrine control group showing disarrangement of cardiac myocyte, with acidophilic cytoplasm of many cardiac myocytes (M), degeneration of muscle fibres (arrowhead), with widening of the intercellular spaces (Ct), pyknotic nuclei (P), marked dilatation and congestion of blood vessels (BV), interstitial haemorrhage (star) marked cellular infiltrations (arrow); C: Epinephrine and L-arginine showing more or less normal histological appearance of cardiac myocytes (M) with mild degeneration (arrowhead), myofibers were intact while others had moderate widening of the intercellular spaces (Ct). Some cellular infiltrate is seen (arrow) and mild pyknotic nuclei (P). Notice congestion in blood vessels (Bv) and mild interstitial haemorrhage (star); D: Epinephrine and L-arginine and MethyB showing nearly normal myocardium with myofibers appeared intact (M) with mild degeneration (arrowhead); some had mild widening of the intercellular spaces (Ct) and few pyknotic nuclei (P). Some cellular infiltrate is seen (arrow). Notice some congested blood vessels (Bv).
Table 3. Histopathological Changes in the Heart of the Rats Treated With L-Arginine, L-Arginine Plus MethyB on Microscopic Observation
Treatment |
Degeneration of Muscle Fibres |
Inflammation with Fibrosis |
Interstitial Haemorrhage |
Widening of the Intercellular Spaces |
Dilatation and Congestion of Blood Vessels |
Pyknotic Nuclei |
Saline control |
∅ |
∅ |
∅ |
∅ |
∅ |
∅ |
Epinephrine |
+++ |
++ |
+++ |
+++ |
+++ |
+++ |
L-arginine + Epinephrine |
++ |
++ |
++ |
++ |
++ |
++ |
L-arginine + MethyB + Epinephrine |
+ |
+ |
∅ |
+ |
+ |
⊥ |
Notes: ∅: absent; ⊥: few; +: mild; ++: moderate; +++: severe (the heart of 6 animals in every group was examined).
4 DISCUSSION
In the present study, we examined the effect of L-arginine, the substrate for nitric oxide synthase, on the epinephrine-induced cardiac arrhythmia and myocardial injury in rats. The study provided evidence that exogenous administration of the nitric oxide precursor L-arginine exerted protective effects in the epinephrine model of cardiac arrhythmias. L-arginine suppressed cardiac arrhythmias and afforded protection against cardiac tissue injury (histological damage) caused by epinephrine. L-arginine itself caused a slight non-significant decrease in heart rate, shortened QTc interval, and increased both QRS duration and R wave amplitude. L-arginine given prior to i.v. epinephrine, however, counteracted the marked bradycardia observed in the group receiving only epinephrine. L-arginine also resulted in marked decrease in the number of ventricular extrasystoles and the duration of arrhythmia caused by epinephrine. These results are supported by the findings of a previous study where in the epinephrine model of cardiac arrhythmias, L-arginine, counteracted the bradycardiac response and suppressed the increase in the number of ventricular extrasystles and duration of arrhythmia that followed nitric oxide-blockade with L-NAME[22].
A number of preclinical and clinical studies indicated that provision of L-arginine was associated with beneficial effects on cardiac function[8,23,24]. In rats with ischaemia/reperfusion injury, i.p. administration of L-arginine (500mg/kg) conferred protection against myocardial cell injury as indicated by the decrease in serum cardiac troponin I concentration[25]. In pigs with ischaemia/reperfusion injury, Padilla et al.[26] showed that i.v. administration of L-arginine (100mg/kg) increased serum and myocardial cyclic guanosine monophosphate (cGMP) concentrations during early reperfusion and decreased cardiac infarct size. Moreover, provision of L-arginine attenuated left ventricular hypertrophy, collagen deposition, inflammatory cell infiltration, and decreased oxidative stress in response to hypertension induced in rats by deoxycorticosterone acetate-salt[23].
Cardiac arrhythmias and myocardial cell damage caused by epinephrine are attributed to direct stimulation of β1 adrenoceptors in cardiac myocytes, increasing cAMP levels and Ca2+ influx[27,28]. In support of this notion are studies that showed protection against cardiac cellular damage by β1-adrenoceptor antagonist atenolol[27]. There is also the effect of stimulating α-adrenoceptors with consequent vasoconstriction, decreased coronary perfusion, and thus profound effects on cardiac cellular functions. Moreover, a role for the local release of oxidation products of epinephrine e.g., adrenochrome, oxidant free radicals[29], and acetylcholine has been suggested[30].
Nitric oxide produced by the three NOS isoforms i.e., eNOS, nNOS, and iNOS is an important modulator of cardiac function being involved in the modulation of excitation-contraction coupling and in regulation of vascular tone and coronary perfusion[3]. Nitric oxide also is considered an endogenous antiarrhythmic agent[31[. In Pigs subjected to ischaemia/reperfusion cardiac injury, L-arginine supplementation increased serum and cardiac tissue levels of cGMP[26]. Tetrahydrobiopterin (BH4) is an essential co-factor for nitric oxide synthase[32]. In rats, dietary L-arginine supplementation increased plasma concentrations of arginine, BH4, and endothelial nitric oxide synthesis[7]. Healthy human subjects receiving L-arginine infusion showed urinary nitric oxide metabolites (NOx) and cGMP excretion, thereby, suggesting increased nitric oxide synthesis[33]. It is likely, therefore, that endothelial dysfunction which occurs under conditions of epinephrine infusion will result in decreased release and/or bioavailability of nitric oxide. L-arginine, thus, exerts its antiarrhythmic and cardiac protective effects by virtue of its ability to increase the bioavailability of nitric oxide and restoring cardiac nitric oxide concentrations, thereby, counteracting the epinephrine-induced endothelial dysfunction. The result is maintaining adequate tissue perfusion and cardiac cellular integrity and recovery of myocardial function. Increasing nitric oxide bioavailability will also inhibit platelet aggregation[34], neutrophil activation and infiltration[35], thereby, preventing neutrophil-endothelial interaction, thrombocyte aggregation and preserving endothelial integrity. Other researchers suggested that L-arginine prevents ischaemic/reperfusion myocardial injury via a direct effect against myocyte hypercontracture[26]. In healthy humans, L-arginine facilitated vagal control of heart rate, possibly through an increase in nitric oxide synthesis[33]. Moreover, in adult rat hearts, nitric oxide donors and nitric oxide derived from nNOS in intracardiac sympathetic neurons were reported to decrease norepinephrine release in response to electrical sympathetic nerve stimulation[36].
The present study also examined the possible modulation by MethyB of the cardioprotective effects of L-arginine in the epinephrine model of cardiac arrhythmia. Methylene blue targets both nitric oxide synthases and, guanylate cyclase. Methylene blue is a direct inhibitor of nitric oxide synthases, both constitutive and inducible. Nitric oxide stimulates soluble guanylate cyclase, which converts guanosine triphosphate into cGMP, that induces smooth muscle relaxation and vasodilation[16,37]. Methylene blue inhibits the enzyme guanylate cyclase and decreases the accumulation of cGMP, thereby, causing less vascular smooth muscle relaxation and vasodilation[37]. Mayer et al.[16] suggested that MethyB acts primarily as a direct inhibitor of nitric oxide synthase, but is only a poor inhibitor of soluble guanylyl cyclase. MethyB has been shown to inhibit the production of nitric oxide via the inhibition of nitric oxide synthases both in vitro and in vivo[16,17] and to decrease brain nitrite/nitrate[39] and serum nitric oxide concentrations[40].
Interestingly, in the present study, the antiarrhythmic and cardiac protective effects of L-arginine in rats treated with epinephrine were enhanced by MethyB. The anti-arrhythmogenic and cardioprotective actions of MethyB in this model have recently been reported[19], which explains the additive protective effect of MethyB in the present study.
The limitations in this study are that (i) the effects of MethyB on epinephrine-induced arrhythmia and cardiac muscle injury were not examined. This is because we used MethyB, which has been found to be an inhibitor of nitric oxide synthases[16,17] to investigate whether nitric oxide synthase inhibition would modulate the responses to L-arginine. Moreover, this action of MethyB in the epinephrine model has already been demonstrated in a previous study[19], and we aimed to avoid repeating the previous study; (ii) troponin I concentration as a measure of injury to cardiac muscle cells was not measured, which should be addressed in future studies.. However, in this work, we undertook a histopathological study to assess the extent of cardiac muscle damage.
5 CONCLUSION
Our data suggest that exogenous administration of L-arginine, the substrate for nitric oxide synthase suppressed cardiac arrhythmias and prevented histological cardiac tissue damage caused by epinephrine. It is suggested that these protective actions of L-arginine are mediated by increased nitric oxide bioavailability in cardiac tissue. However, a direct protective action for L-arginine on myocardial tissue may also be involved.
Acknowledgements
The authors declared that this study has received no financial support.
Ethics Statement
This research was approved by Medical Research and Clinical Studies Institute, National Research Centre, Cairo, Egypt. Animal procedures followed the guidelines of the Institute ethics committee for the use of animals in experimental studies and the Guide for Care and Use of Laboratory Animals by the U.S. National Institutes of Health (Publication No. 85-23, revised 1996).
Conflicts of Interest
The authors have no conflict of interest to declare.
Data Availability
All data generated or analyzed during this study are included in this published article.
Copyright Permissions
Copyright © 2024 The Author(s). Published by Innovation Forever Publishing Group Limited. This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Author Contribution
The conceptualization and design of the study were undertaken by Abdel-Salam OME and Baset MAE. Experimental studies conducted by Abd El Baset M. Data collection, analysis, and interpretation were carried out by Abdel-Salam OME, Abd El Baset M, and Sleem AA. Omara EA oversaw the histological studies and contributed to their interpretation. The composition of the written manuscript was predominantly the work of Abdel-Salam OME.
Abbreviation List
cGMP, Cyclic guanosine monophosphate
ECG, Electrocardiographic
eNOS, Endothelial nitric oxide synthase
IL, Interleukins
iNOS, Inducible NOS
MethyB, Methylene blue
NOS, Nitric oxide synthase
TNF, Tumour necrosis factor
References
[1] Forstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol, 2011; 164: 213-223.[DOI]
[2] Daff S. NO synthase: structures and mechanisms. Nitric Oxide 2010; 23: 1-11.[DOI]
[3] Massion PB, Feron O, Dessy C et al. Nitric oxide and cardiac function. Ten years after, and continuing. Circ Res, 2003; 93: 388-398.[DOI]
[4] Iova OM, Marin GE, Lazar I et al. Nitric oxide/nitric oxide synthase system in the pathogenesis of neurodegenerative disorders-An overview. Antioxidants (Basel). 2023; 12: 753.[DOI]
[5] Wu G, Meininger CJ, McNeal CJ et al. Role of L-Arginine in nitric oxide synthesis and health in humans. Adv Exp Med Biol, 2021; 1332: 167-187.[DOI]
[6] Gambardella J, Khondkar W, Morelli MB et al. Arginine and endothelial function. Biomedicines 2020; 8: 277.[DOI]
[7] Kohli R, Meininger CJ, Haynes TE et al. Dietary l-arginine supplementation enhances endothelial nitric oxide synthesis in streptozotocin-induced diabetic rats. J Nutr, 2004; 134: 600-608.[DOI]
[8] Sudar-Milovanovic E, Obradovic M, Jovanovic A et al. Benefits of L-Arginine on cardiovascular system. Mini Rev Med Chem, 2016; 16: 94-103.[DOI]
[9] Sato H, Zhao ZQ, Vinten-Johansen J. L-Arginine inhibits neutrophil adherence and coronary artery dysfunction. J Cardiovascular Res, 1996; 3; 63-72.[DOI]
[10] Colagrande L, Formica F, Porta F et al. L-arginine effects on myocardial stress in cardiac surgery: preliminary results. Ital Heart J, 2005; 6: 904-910.
[11] Böger RH, Bode-Böger SM. The clinical pharmacology of L-arginine. Ann Rev Pharmacol Toxicol, 2001; 41:79-99.[DOI]
[12] Cannon III RO. Oral L-arginine (and other active ingredients) for ischemic heart disease? JACC, 2002; 39: 46-48.[DOI]
[13] Clifton JI, Leikin JB. Methylene blue. Am J Ther, 2003; 10: 289‒291.[DOI]
[14] Bradberry SM. Occupational methaemoglobinaemia: mechanisms of production, features, diagnosis and management including the use of methylene blue. Toxicol Rev, 2003; 22: 13-27.[DOI]
[15] Zulian GB, Tullen E, Maton B. Methylene blue for ifosfamide-associated encephalopathy. N Engl J Med, 1995; 332:1239-1240.[DOI]
[16] Mayer B, Brunner F, Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem Pharmacol, 1993; 45: 367-374.[DOI]
[17] Volke V, Wegener G, Vasar E et al. Methylene blue inhibits hippocampal nitric oxide synthase activity in vivo. Brain Res, 1999; 826: 303-305.[DOI]
[18] Manghelli J, Brown L, Tadros HB et al. A reminder of methylene blue's effectiveness in treating vasoplegic syndrome after onpump cardiac surgery. Tex Heart Inst J, 2015; 42: 491-494.[DOI]
[19] Abdel-Salam OME, Sayed MABS, Omara EA et al. Cardioprotection by methylene blue against epinephrine-induced cardiac arrhythmias and myocardial injury. WSEAS Transactions on Biology and Biomedicine, 2023; 20; 64-72.[DOI]
[20] Rajani V, Hussain Y, Bolla BS et al. Attenuation of epinephrine-induced dysrhythmias by bradykinin: role of nitric oxide and prostaglandins. Am J Cardiol, 1997; 80: 153A-157A.[DOI]
[21] Walker MJ, Curtis MJ, Hearse DJ et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res, 1988; 22: 447-455.[DOI]
[22] Abdel-Salam OME, Sayed MABS, Omara EA et al. The effects of nitric oxide synthase inhibition on epinephrine-induced arrhythmia and myocardial damage. WSEAS Transactions on Biology and Biomedicine, 2023; 20: 145-154.[DOI]
[23] Fenning A, Harrison G, Rosemeyer R et al. L-Arginine attenuates cardiovascular impairment in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol, 2005; 289: H1408-H1416.[DOI]
[24] Lorin J, Zeller M, Guilland JC et al. Arginine and nitric oxide synthase: Regulatory mechanisms and cardiovascular aspects. Mol Nutr Food Res, 2014; 58: 101-116.[DOI]
[25] Yan Y, Davani S, Chocron S et al. Effects of L-arginine administration before cardioplegic arrest on ischemia-reperfusion injury. Ann Thorac Surg, 2001; 72: 1985-90.[DOI]
[26] Padilla F, Garcia-Dorado D, Agullo´ L et al. L-Arginine administration prevents reperfusion-induced cardiomyocyte hypercontracture and reduces infarct size in the pig. Cardiovascular Res, 2000; 46: 412-420.[DOI]
[27] Wheatley AM, Thandroyen FT, Opiea LH. Catecholamine-induced myocardial cell damage: Catecholamines or adrenochrome. J Mol Cell Cardiol, 1985; 17: 349-359.[DOI]
[28] Communal C, Singh K, Pimentel DR et al. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the β-adrenergic pathway. Circulation, 1998; 98: 1329-1334.[DOI]
[29] Dhalla NS, Adameova A, Kaur M. Role of catecholamine oxidation in sudden cardiac death. Fundam Clin Pharmacol, 2010; 24: 539-546.[DOI]
[30] Igić R. Mechanism of epinephrine-induced dysrhythmias in rat involves local cholinergic activation. Canad J Physiol Pharmacol, 1996; 74: 85-88. [DOI]
[31] Burger DE, Feng Q. Protective role of nitric oxide against cardiac arrhythmia-An update. Open Nitric Oxide J, 2011; 3: 38-47.[DOI]
[32] Schmidt K, Werner ER, Mayer B et al. Tetrahydrobiopterin-dependent formation of endothelium-derived relaxing factor (nitric oxide) in aortic endothelial cells. Biochem J, 1992; 281: 297-300.[DOI]
[33] Chowdhary S, Nuttall SL, Coote JH et al. L-arginine augments cardiac vagal control in healthy human subjects. Hypertension, 2002; 39: 51-56.[DOI]
[34] Radomski MW, Palmer RM, Moncada S. Modulation of platelet aggregation by an L-arginine-nitric oxide pathway. Trends Pharmacol Sci, 1991; 12: 87-88.[DOI]
[35] Wanikiat P, Woodward DF, Armstrong RA. Investigation of the role of nitric oxide and cyclic GMP in both the activation and inhibition of human neutrophils. Br J Pharmacol, 1997; 122: 1135-1145. [DOI]
[36] Schwarz P, Diem R, Dun NJ et al. Endogenous and exogenous nitric oxide inhibits norepinephrine release from rat heart sympathetic nerves. Circ Res, 1995; 77: 841-848. [DOI]
[37] Miclescu A, Wiklund L. Methylene blue, an old drug with new indications? J Rom Anest Terap Int, 2010; 17: 35-41.
[38] Booth AT, Melmer PD, Tribble JB et al. Methylene blue for vasoplegic syndrome. Heart Surg Forum, 2017; 20: E234-E238. [DOI]
[39] Wiklund L, Basu S, Miclescu A et al. Neuro- and cardioprotective effects of blockade of nitric oxide action by administration of methylene blue. Ann N Y Acad Sci, 2007; 1122: 231-244. [DOI]
[40] Abdel-Salam OME, SleemAA, Youness ER et al. Neuro- and hepatoprotective effects of methylene blue in rats treated with lipopolysaccharide endotoxin. React Oxygen Species, 2018; 6: 325-337. [DOI]
Brief of Corresponding Author(s)
Omar M.E. Abdel-Salam He has obtained his Bachelor of Medicine and Surgery (1985) and M.Sc. in Internal Medicine (1991) at Faculty of Medicine, Cairo University, Cairo, and his PhD in Medical Sciences at the Hungarian Academy of Sciences, Budapest, 1997. Became the head of the Department of Toxicology and Narcotics (2009-2017) & (2020-2022), Medical Research Institute, National Research Centre, Cairo. His research interests encompass pharmacology, pharmacy, neurosciences, neurology, physiology, life sciences, biomedicine, and other areas such as tropical medicine. He has authored or co-authored 296 research articles, 15 book chapters, 1 book, with an h-index of 29. |






 Copyright ©
Copyright ©