Deciphering the Causal Links: A Mendelian Randomization Study of Circulating Inflammatory Proteins and Cardiovascular Diseases
Liantai Song1, Yujia Zhao1, Xinyang He1, Yibing Duan1, Minxuan Hu1, Suti Han1, Yifan Chi1, Qian Xu2*
1Basic Medical College of Chengde Medical University, Chengde, Hebei Province, China
2Department of Biochemistry, Chengde Medical University, Chengde, Hebei Province, China
*Correspondence to: Qian Xu, Department of Biochemistry, Chengde Medical University, Anyuan Road, Chengde, 067000, Hebei Province, China; e-mail: qianxu@cdmc.edu.cn
DOI: 10.53964/cme.2024011
Abstract
Objective: The main objective was to gain a deeper understanding of the underlying mechanisms of cardiovascular illnesses and to identify the key components and potential treatment targets using Mendelian randomization (MR).
Methods: This study utilizes a two-sample MR approach to examine the causal association between 91 circulating inflammatory proteins and four primary categories of cardiovascular disease.
Results: Ultimately This study identifies 28 significant causal links with key findings revealing the correlation between Macrophage colony-stimulating factor 1 and both coronary artery disease and heart failure.Additionally, the study highlights the diverse functions of various interleukins in different cardiovascular diseases.
Conclusions: Our analysis extends existing knowledge by uncovering potential therapeutic targets for peripheral artery disease, heart failure, and stroke, previously under-researched in the context of these inflammatory proteins. This research highlights the importance of inflammatory biomarkers in predicting cardiovascular events and opens new avenues for therapeutic intervention.
Keywords: cardiovascular diseases, circulating inflammatory proteins, Mendelian randomization
1 INTRODUCTION
Cardiovascular diseases (CVD), also known as circulatory system disorders, predominantly encompass coronary artery atherosclerosis, peripheral artery disease, heart failure, and stroke. It has been observed in recent years that cardiovascular disease incidence and mortality rates are on the rise. Currently, CVD has become the primary factor leading to death globally[1,2], presenting a substantial public health issue and posing a grave threat to human health and safety. According to a WHO report, an estimated 117.3 million people died from cardiovascular disease in 2008, accounting for 30% of global deaths; About 23.6 million people are expected to die from cardiovascular disease by 2030[3].
Hence, investigating the origin and potential treatment targets of these disorders carries significant clinical importance in terms of diagnosis and prevention. The prevention and treatment of cardiovascular disease helps to protect people’s health, improve the quality of life and reduce the socio-economic burden.
Inflammation is the body’s natural reaction to infection and injury. Nevertheless, abnormal inflammatory activities can result in harm to tissues and serve a pivotal function in the progression of various diseases. The inflammatory process plays a crucial role in the development of cardiovascular illnesses. Research indicates that inflammation plays a crucial role in the advancement of arteriosclerosis in both young and old individuals[3,4]. The inflammatory response is regulated by a highly coordinated network of cells and mediators[5,6], including cytokines and circulating proteins like soluble receptors. Recent data suggests a possible connection between inflammatory proteins and the onset of cardiovascular illnesses. Macrophages and T lymphocytes become increasingly activated with the development of atherosclerotic damage. The activated macrophages and vascular cells produce inflammatory proteins, potentially modulating the progression of atherosclerosis[7,8]. Endothelial dysfunction, a hallmark of CVD, has been linked to local and systemic inflammatory proteins[9,10]. Research in mice and humans indicates that inflammatory proteins exacerbate cardiac injury in CVD, promoting myocardial cell apoptosis and triggering vascular inflammation, arteriosclerosis, and vascular fibrosis[11-13]. Furthermore, studies have discovered elevated levels of the inflammatory protein lipid transport protein 2 in the blood and cardiac tissue of patients post-acute myocardial infarction and those with various CVD[14,15]. Although there is increasing evidence suggesting a connection between inflammatory proteins and cardiovascular illnesses, additional research is necessary to fully understand the precise cause-and-effect relationship between them.
Mendelian randomization (MR) utilizes genetic variants that are linked to an exposure as instrumental variables (IVs) to investigate the association between the exposure and outcomes[16]. As genetic variants are assigned randomly during conception, MR can offer valuable insights into causal linkages within observational data, effectively minimizing biases caused by confounding factors or reverse causation. This strategy is highly effective for examining causal links between exposures and outcomes[17]. In our investigation of the potential link between inflammatory proteins and CVD, we adopted the MR approach. This entailed utilizing inherent stochastic genetic variations to deduce the influence of inflammatory proteins on illness outcomes. Our research involved performing a comprehensive examination of 91 plasma proteins using genome-wide association analysis. The main objective was to gain a deeper understanding of the underlying mechanisms of cardiovascular illnesses and to identify the key components and potential treatment targets using MR. This work has the potential to provide new and valuable information about the connection between inflammatory proteins and diseases. As a result, it can contribute to the development of more effective strategies for preventing and treating CVD.
2 MATERIALS AND METHODS
2.1 Research Design
A two-sample MR study was undertaken to investigate the causal link between inflammatory circulating proteins and cardiovascular disease. This analysis has its foundation on three basic assumptions: (1) There is a substantial association among exposure and IVs. (2) An IV does not have any confounding factors that could affect the link between exposure and outcome. (3) IVs affect outcomes solely through exposure[18]. The studies included in our investigation had obtained approval from pertinent institutional review boards, with individuals providing informed consent.
2.2 Data Sources
The IVs utilized by this study originated from a recent genome-wide association study (GWAS) conducted by Zhao et al.[19] on 91 inflammatory circulating proteins, involving 14,734 European participants. GWAS data for each inflammatory protein is publicly accessible from the GWAS Catalog (accession numbers from GCST90274758 to GCST90274848). We used the most extensive Genome-Wide GWAS available for MR investigation into cardiovascular disorders. The meta-analysis undertaken by van der Harst et al. utilized data from CARDIoGRAMplusC4D and the UK Biobank to generate summary statistics for coronary artery disease (CAD). The analysis included 122,733 cases and 424,528 controls[20]. The information on peripheral artery disease were 11,924 cases and 288,638 controls from The FinnGen Consortium[21]. Data on heart failure were 47,309 cases and 930,014 controls sourced from the HERMES Consortium[22]. The summary statistics for stroke were 40,585 cases and 406,111 controls from MEGASTROKE Consortium[23] (Table 1).
Table 1. Detailed Information Regarding Studies and Datasets Used in The Present Study
Exposure or Outcome |
Source |
Ancestry |
Participants |
Circulating inflammatory proteins |
PMID: 37563310 |
European |
14,734 individuals |
Coronary artery disease |
PMID: 29212778 |
European |
122,733 cases and 424,528 controls |
Peripheral artery disease |
FinnGen consortium |
European |
11,924 cases and 288,638 controls |
Heart failure |
PMID: 31919418 |
European |
47,309 cases and 930,014 controls |
Stroke |
PMID: 29531354 |
European |
40,585 cases and 406,111 controls |
2.3 IV Selection
With the aim to find single nucleotide polymorphisms (SNPs) that correspond to the exposure factors and to verify the accuracy of the causal relationship between circulating inflammatory proteins and the likelihood of developing cardiovascular illnesses, the following steps were employed for SNP selection. In the GWAS data of inflammatory proteins, a threshold of P<5×10-8 yielded a limited number of IVs. As a result, we chose a threshold of P<1×10-5 for SNP selection in order to acquire a greater number of IVs and ensure trustworthy outcomes. For CVD, the significance level was adjusted to 5×10-8. A linkage disequilibrium r2<0.001 and a distance of 10,000kb were set to minimize bias from residual linkage disequilibrium[24]. The F-statistics, represented as F= beta2/se2, were computed to evaluate the correlation between SNPs and exposure. In this equation, beta denotes the allele’s effect size, while se indicates the standard error. SNPs with a F<10, demonstrating a weak connection with the exposure, were eliminated from the study[25]. SNPs with palindromic structures were automatically excluded.
2.4 Statistical Analysis
Four common MR methods were used, including inverse variance weighted (IVW), MR Egger, Weighted Median, and Weighted Mode. The IVW tests utilize the meta-analysis technique to combine the Wald estimates of each SNP and create a cumulative impact estimate of the exposure. This is done by employing the reciprocal of the result variance (se2)[26]. IVW is the primary analytical method, with other methods serving as supplementary tools.
In order to assess the strength and dependability of the results, sensitivity studies were conducted to identify any possible biases. The Cochran’s Q test was utilized to assess heterogeneity in the IVW model, adopting a fixed-effects IVW model when the p-value is greater than 0.05 and a random-effects IVW model otherwise[27]. The MR-Egger intercept test was run to assess pleiotropy among IVs[28]. The Mendelian Randomization Pleiotropy RESidual sum and Outlier (MR-PRESSO) test was employed to evaluate potential pleiotropic effects and identify outliers[29]. A “leave-one-out” sensitivity analysis was conducted to figure out whether the results were affected by a single SNP[30]. In order to quantify the possible cause-and-effect connection between inflammatory proteins and cardiovascular disease, the MR Steiger test was employed[31].
By employing SNPs related with cardiovascular disease as IVs. Subsequently, a reverse MR analysis was performed for all positive analytical outcomes. The procedures and parameters were similar to the forward MR, except for different association thresholds for the genetic variants.
Statistical significance was determined using the “TwoSampleMR” and “MR-PRESSO” packages in R software (version 4.2.3), with a threshold of P<0.05.
3 RESULTS
3.1 Selection of IVs for Circulating Inflammatory Proteins
A two-sample MR analysis was conducted on 91 circulating inflammatory proteins and four different CVD using GWAS data to evaluate their causal relationships. The analysis comprised 1,683 SNPs derived from 91 inflammatory proteins that are present in the circulation. The number of IVs for each protein varied between 3 and 30. The minimum F-statistics of these IVs varied from 20.84 to 1,477.14, as shown in Table S1. This range indicates that all 91 circulating inflammatory proteins had a satisfactory level of effectiveness for conducting MR analysis, with F-statistics above 10[32].
3.2 Causal Impact of Circulating Inflammatory Proteins on CVD
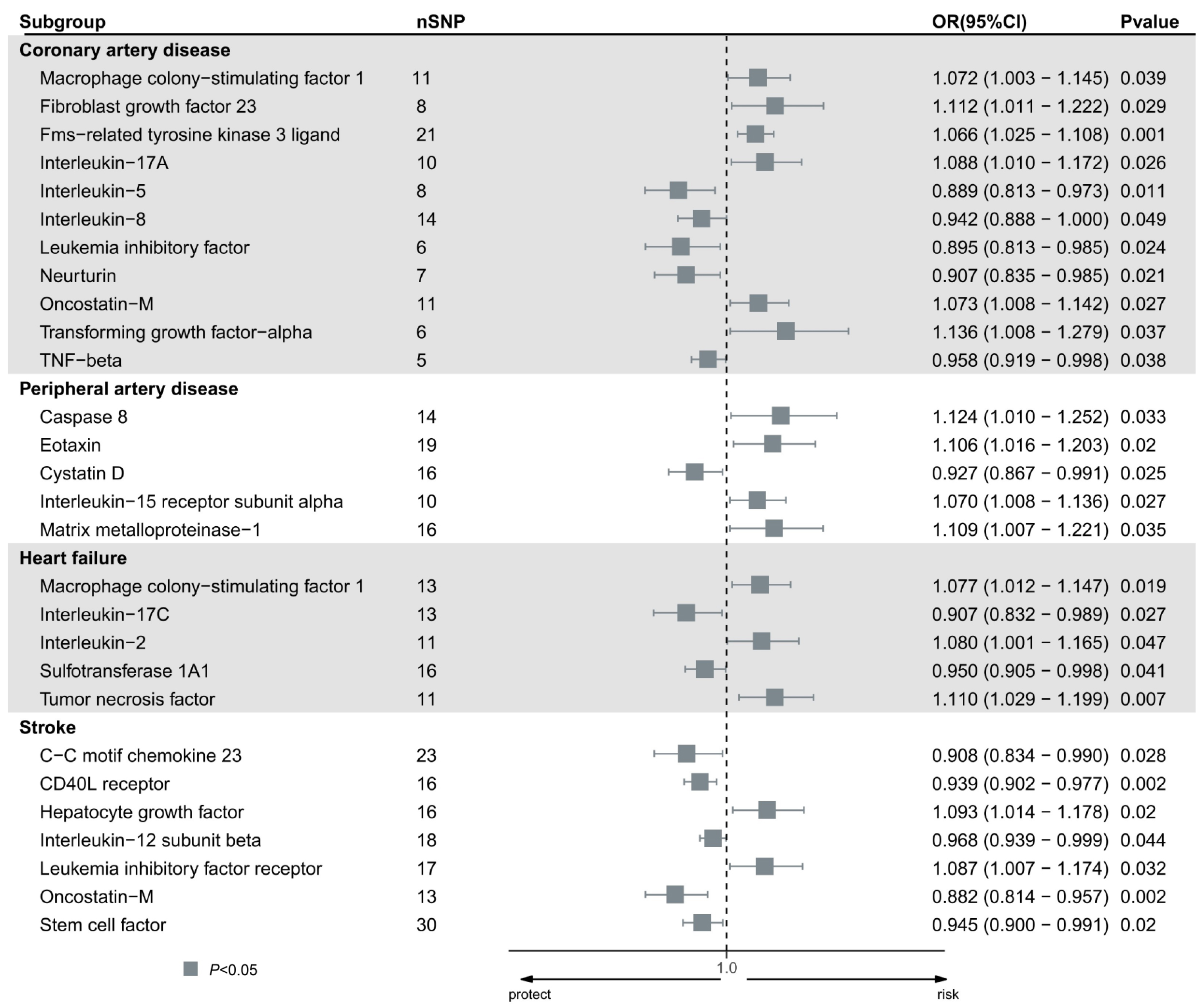
The results of the MR analysis of the 91 circulating inflammatory proteins and four CVD are detailed in Table S2. Using the IVW method, significant causal associations were observed for 28 proteins (Figure 1). Specifically, 11 inflammatory proteins showed nominal significance (IVW, P<0.05) for CAD in the IVW model. Six proteins increased the risk of CAD, including macrophage colony-stimulating factor 1 (MCSF1), fibroblast growth factor 23 (FGF23), Fms-related tyrosine kinase 3 (FLT3) ligand (FLT3L), interleukin (IL)-17A, Oncostatin-M, and Transforming growth factor-alpha (TGF-α). Conversely, five proteins decreased the risk, including IL-5, IL-8, leukemia inhibitory factor (LIF), neurturin, and tumor necrosis factor-beta (TNF-β).
Proteins linked to a higher likelihood of developing peripheral artery disease are Caspase 8, Eotaxin, IL-15 receptor subunit alpha, and matrix metalloproteinase-1 (MMP1). Conversely, Cystatin D reduces the risk. Three proteins have been connected to a heightened susceptibility to heart failure: MCSF1, IL-2, and TNF, whereas Sulfotransferase 1A1 and IL-17C decreased the risk.
In the analysis of stroke, Hepatocyte growth factor and LIF receptor increased the risk, while C-C motif chemokine 23, CD40L receptor, IL-12 subunit beta, Oncostatin-M, and Stem cell factor reduced the risk.
|
Figure 1. Forest plot of the causal effect of identified circulating inflammatory proteins on the risk of five types of cardiovascular disease (CAD, peripheral artery disease, heart failure, and stroke), derived from IVW analysis.
3.3 Sensitivity Analysis
Validation of the presumed causal connections between circulating proteins and cardiovascular disease outcomes was performed through sensitivity analysis (Table 2). The Cochran’s Q test indicated that there was no heterogeneity in the causal correlations pertaining to circulating inflammatory proteins. Additionally, the intercept test of the MR-Egger analysis did not find any indication of pleiotropy in these correlations. Complete results of Cochran’s Q test and MR-Egger intercept test for the 91 circulating inflammatory proteins are provided in Table S3. The MR-PRESSO test yielded no indications of potential outliers or horizontal pleiotropy, and the findings of the Steiger test showed that the observed relationships were unlikely to be caused by reverse causation (Table 2). The scatter plots (Figure S1) depict the evaluation of the causal connection between circulating proteins and the risk of cardiovascular disease. The “leave-one-out” test results indicated no large effect size SNP bias in estimates (Figure S2).
Table 2. Sensitivity Analysis for the Causal Association between Circulating Inflammatory Proteins and Cardiovascular Disease
Cardiovascular Disease |
Circulating Inflammatory Proteins |
IVW_Cochr an _Q |
IVW_Cochran _Q_pval |
Egger _inter cept |
Egger_intercept _pval |
Global test P-value |
MR-PRESSO_Glob al_test_pval |
Steiger test |
Coronary artery disease |
MCSF1 levels |
14.066 |
0.17 |
-0.014 |
0.246 |
0.188 |
0.35 |
TRUE |
Coronary artery disease |
FGF23 levels |
9.045 |
0.249 |
-0.001 |
0.908 |
0.291 |
0.291 |
TRUE |
Coronary artery disease |
FLT3L levels |
24.604 |
0.003 |
-0.003 |
0.464 |
0.382 |
0.327 |
TRUE |
Coronary artery disease |
IL-17A levels |
9.964 |
0.352 |
-0.008 |
0.866 |
0.377 |
0.327 |
TRUE |
Coronary artery disease |
IL-5 levels |
7.694 |
0.164 |
-0.008 |
0.569 |
0.225 |
0.225 |
TRUE |
Coronary artery disease |
LIF levels |
6.361 |
0.273 |
-0.012 |
0.375 |
0.328 |
0.328 |
TRUE |
Coronary artery disease |
Neurturin levels |
10.882 |
0.074 |
-0.011 |
0.214 |
0.359 |
0.359 |
TRUE |
Coronary artery disease |
Oncostatin-M levels |
7.195 |
0.408 |
-0.011 |
0.183 |
0.539 |
0.539 |
TRUE |
Coronary artery disease |
TGF-α levels |
17.859 |
0.106 |
-0.009 |
0.406 |
0.216 |
0.216 |
TRUE |
Coronary artery disease |
TNF-β levels |
7.63 |
0.106 |
-0.009 |
0.406 |
0.285 |
0.258 |
TRUE |
Peripheral artery disease |
Caspase 8 levels |
14.291 |
0.354 |
-0.029 |
0.405 |
0.371 |
0.371 |
TRUE |
Peripheral artery disease |
Eotaxin levels |
8.449 |
0.449 |
0.056 |
0.402 |
0.475 |
0.475 |
TRUE |
Peripheral artery disease |
Cystatin B levels |
6.917 |
0.75 |
0.046 |
0.358 |
0.54 |
0.54 |
TRUE |
Heart failure |
MCSF1 levels |
14.292 |
0.997 |
0.007 |
0.513 |
0.988 |
0.988 |
TRUE |
Heart failure |
IL-17C levels |
9.997 |
0.062 |
-0.003 |
0.651 |
0.098 |
0.098 |
TRUE |
Heart failure |
IL-2 levels |
9.062 |
0.926 |
0.006 |
0.451 |
0.93 |
0.93 |
TRUE |
Heart failure |
Sulfotransferase 1A1 levels |
4.402 |
0.347 |
0.034 |
0.48 |
0.743 |
0.743 |
TRUE |
Heart failure |
TNF levels |
16.358 |
0.764 |
0.042 |
0.847 |
0.43 |
0.43 |
TRUE |
Stroke |
C-C motif chemokine 23 levels |
32.853 |
0.003 |
0.006 |
0.979 |
0.274 |
0.274 |
TRUE |
Stroke |
CD40L receptor levels |
10.994 |
0.489 |
0.02 |
0.979 |
0.228 |
0.228 |
TRUE |
Stroke |
Hepatocyte growth factor levels |
16.952 |
0.094 |
0.007 |
0.464 |
0.503 |
0.503 |
TRUE |
Stroke |
IL-12 subunit beta levels |
11.694 |
0.074 |
0.014 |
0.233 |
0.502 |
0.502 |
TRUE |
Stroke |
LIF receptor levels |
11.487 |
0.586 |
0.014 |
0.339 |
0.53 |
0.53 |
TRUE |
Stroke |
Oncostatin-M levels |
7.194 |
0.474 |
-0.01 |
0.75 |
0.757 |
0.757 |
TRUE |
Stroke |
Stem cell factor levels |
35.942 |
0.175 |
0.974 |
0.207 |
0.926 |
0.926 |
TRUE |
3.4 Reverse MR Analysis
A reverse MR study was carried out to explore the causal relationship between CVD and circulating inflammatory proteins. This analysis utilized IVs that represented CAD, peripheral artery disease, heart failure, and stroke. IVs included for the four CVD are detailed in Table S4. The Steiger test confirmed that there was no reverse causation in the detected causative associations. However, the reverse MR analysis showed that there is a causal effect of stroke on the growth of LIF factor receptor. The odds ratio was 1.227, with a 95% confidence interval of 1.009 to 1.492, and a p-value of 0.041. Nevertheless, the reverse MR study did not reveal any inverse causal associations between the remaining cardiovascular illnesses and the levels of circulating inflammatory proteins. The sensitivity analysis did not find any indication of heterogeneity or horizontal pleiotropy (Table S5).
4 DISCUSSION
This research applied two-sample MR analysis in exploring the causal relationships between 91 circulating inflammatory proteins and four distinct CVD for the first time. The study uses genetic variants associated with the diseases to examine complex diseases that may involve multiple factors. Vascular inflammation is a crucial factor in the progression of cardiovascular disorders, and the levels of inflammatory markers in the bloodstream can accurately forecast cardiovascular events. In this MR analysis, we identified 28 significant causal relationships. Among these, the majority of inflammation proteins causally related to CAD have been confirmed by research, while fewer studies have confirmed the associations of inflammation proteins with peripheral artery disease, heart failure, and stroke. This provides new insights and directions for identifying therapeutic targets for various CVD.
Our analysis found that the levels of MCSF1 are highly associated with CAD and heart failure[33]. MCSF1 is an essential growth hormone that plays a crucial role in the differentiation, existence, proliferation, and renewal of macrophages and monocytes. The circulation of MCSF1 is mainly restricted by receptor-mediated endocytosis in liver and spleen macrophages, and understanding its receptor expression locations is crucial for interpreting MCSF1’s actions and disease-related targeting signals. A significant risk factor for CAD is atherosclerosis, where macrophages can induce lipid accumulation and typical foam cell formation, expressing a series of inflammatory factors to promote atherosclerosis[34]. Inflammation, hemodynamic disturbances, excessive renal tubular sodium reabsorption, and nephrotoxic drugs are risk factors for harmful cardiorenal interactions in patients with heart failure[35]. MCSF1 can promote phagocytosis, aiding in the clearance of apoptotic cells and the phenotypic transition from M1 to M2, thus playing a role in promoting the resolution of inflammation[36].This suggests that it may serve as a promising therapeutic target for heart failure and CAD.
Interleukins are cytokines that are involved in inflammation and have a crucial function in the development of arteriosclerosis, which leads to the creation and rupture of plaques[37]. They are also important in CAD. IL-17A, a regulator of inflammation, can enhance the production of inflammatory substances in the brain, leading to the activation of the sympathetic nervous system. Sympathetic nerve activation driven by inflammation can lead to heart dysfunction and remodeling, advancing heart failure, while atherosclerosis or coronary artery insufficiency can induce heart failure[38]. Studies using flow cytometry on ischemic heart failure mouse models have shown IL-17A’s relevance to the disease process[39]. Elevated levels of IL-17A in damaged hearts and circulation align with our analysis linking IL-17A levels to CAD. IL-5, a cytokine that reduces inflammation, has been demonstrated to have a safeguarding impact against arteriosclerosis by promoting the activity of natural immunoglobulin M antibodies[40,41]. IL-8 is a key chemokine in the development of coronary heart disease, massively released from neutrophils involved at various stages of the disease’s development[37]. Studies have shown IL-8’s reparative effects on cardiac tissues and its association with lower myocardial infarction rates in females, though the functional evidence remains controversial[42-44]. The analysis also indicated close associations of IL-15 receptor subunit alpha with peripheral artery disease, IL-12 subunit beta with stroke, and both IL-17C and IL-2 with heart failure.
Oncostatin-M, a pro-inflammatory cytokine belonging to the IL-6 cytokine family, is produced by immune cells in response to infection and tissue damage. It has been recognized as a potential therapeutic target for atherosclerosis by the European cardiovascular target discovery consortium, due to its significant involvement in inflammation and autoimmunity[45-49]. CAD is a condition that occurs due to the buildup of fatty substances and collagen fibers in the arteries, specifically caused by atherosclerosis. It is defined by the presence of persistent inflammation[46].
LIF is a versatile cytokine belonging to the IL-6 superfamily. It has the ability to promote the differentiation of myeloid leukemia cells, effectively preventing their excessive growth. LIF plays a vital role in regulating the immune system and can serve as a protective factor against various immunopathological diseases[50]. The signaling of the LIF receptor is facilitated by the LIF receptor complex, which consists of a heterodimer of LIF receptor and Gp130[51]. The investigation revealed a strong correlation between LIF and the development of CAD, whilst its receptor showed a significant relationship with stroke.
In addition to these circulating inflammatory proteins impacting various CVD, some are specifically associated with single cardiovascular conditions. FGF23, FLT3L, neurturin, TGF-α, and TNF-β have a strong association with the occurrence of CAD. Caspase 8, Eotaxin, Cystatin D, and MMP1 are closely associated with peripheral artery disease; CD40L receptor, Hepatocyte growth factor, C-C motif chemokine 23, and Stem cell factor are closely related to the occurrence of stroke.
FGF23 is a hormone that is released by osteocytes and has been discovered as a risk factor for higher mortality rates in several cardiovascular illnesses[52,53]. The REGARDS study focused on Americans aged 45 and above. AND it discovered a significant association between elevated levels of FGF23 and a heightened susceptibility to coronary heart disease[53]. Nevertheless, the study’s observational design does not show a definitive cause-and-effect association between FGF23 levels and coronary heart disease. The FLT3 is a receptor tyrosine kinase that is present in the hematopoietic compartments. FLT3-targeting tyrosine kinase inhibitors may have cardiovascular adverse effects. The heart expresses FLT3 and its ligand, which can cause the formation of dimers and activation of FLT3’s inherent tyrosine kinase activity[54,55]. Research on a mouse model of myocardial infarction found that intramyocardial administration of FLT3 ligand can confer cell-protective effects in the hearts of infarcted mice, suggesting that the protective role of FLT3L in human cardiac cells warrants further investigation[54,55].
Neurturin, a constituent of the glial cell line-derived neurotrophic factor family, is recognized for its involvement in nerve function[56]. However, the connection between TGF-α and CAD is still uncertain, even though TGF-α has a known impact on the movement of hepatocellular carcinoma cells[57]. TNFs, including TNF-α and TNF-β, are critical pro-inflammatory cytokines involved in systemic inflammation and are linked to diseases affecting the nervous, cardiovascular, pulmonary, autoimmune, and metabolic systems. TNF-β shares similarities with TNF-α but has unique characteristics, such as its expression being limited to immune cells and never being expressed on the cell surface[58,59]. Our research suggests a close association between Neurturin, TGF-α, TNF-β, and the incidence of CAD, a hypothesis that requires further investigation to confirm.
Caspase 8 is crucial in the regulation of inflammation[60]. Studies conducted in intensive care unit settings have demonstrated that patients who suffer from malignant cerebral artery stroke and have elevated levels of Caspase 8 experience a worse death rate compared to those with normal levels[61]. Pulmonary artery hypertension (PAH) involves progressive pulmonary arterial remodeling and perivascular inflammation, influenced by inflammation and autoimmunity, with studies indicating Caspase 8’s role in vascular remodeling in PAH[62]. This aligns with our study’s conclusion linking Caspase 8 to peripheral artery disease. Eosinophil chemotactic factor is highly expressed at vascular pathology sites, involved in vascular inflammation, and overexpressed in arteriosclerosis, providing a credible biological link to peripheral artery disease[63-65]. Research on the use of cystatin C as a marker for kidney function in the context of chronic renal disease and its progression, along with its association with lipid abnormalities and cardiovascular illnesses, is limited[66,67]. CVD entail complex pathophysiological changes, possibly driven by changes in enzyme activity[68]. The expression of MMP1 and MMP2 in pro-inflammatory macrophages within human carotid plaques is associated with increased HDAC9, impacting innate immunity positively.The lack of HDAC9 may have a beneficial effect on inflammation resolution, thus preventing arteriosclerosis from progressing[68]. Further investigation is required to establish the causal involvement of Cystatin D and MMP1 in peripheral artery disease.
Heart failure represents a state of inappropriate sustained inflammation related to the induction of cytokines and chemokines, potentially leading to adverse cardiac remodeling and dysfunction in contraction and relaxation, thereby inducing heart failure[69,70]. Sulfotransferase 1A1, previously associated with renal fibrosis, has been less studied in heart failure, indicating the need for further research into its mechanisms, highlighting the innovative nature of this analysis[71]. TNF is an effective pro-inflammatory cytokine mediating widespread inflammation in the body[72], with TNF-α closely related to ventricular remodeling following myocardial infarction[70]. This analysis first suggests a close link between these inflammatory proteins and heart failure.
Stroke, caused by thromboemboli or infections, results in varying degrees and types of brain damage, severely affecting mental and physical health[73,74]. The CD40-CD40L system is essential for controlling both humoral and cellular immunity and is commonly employed in transplantation[75]. Modified anti-CD40 antibodies can effectively address thromboembolic problems in the transplant region. Hepatocyte Growth Factor is a biomarker of cardiovascular disease risk, with studies showing its independent association with heart failure events, though its relation to stroke is less understood[76]. Research on C-C motif chemokine 23 and stem cell factor in stroke is limited and unclear.
Our study benefits from utilizing large-scale GWAS data on 91 inflammatory proteins and four CVD for a comprehensive and systematic analysis of their causal relationships. Additionally, employing multiple MR analysis methods to exclude potential polymorphism variabilities and assessing the potential causal relationships between the 91 circulating inflammatory proteins and four CVD yielded reliable conclusions. Nevertheless, it is important to exercise caution when generalizing the outcomes of our study to other ethnic groups, as the GWAS statistical data we utilized predominantly consists of individuals of European origin, hence highlighting the presence of racial disparities. It is not easy to demonstrate that the results are entirely independent of the horizontal pleiotropy effect; nevertheless, we performed many sensitivity analyses to demonstrate the stability of the results.
Acknowledgements
As part of the project, this work was supported by the Hebei Province Education Department (Project No. QN2016145), as well as the University-level Science Funds in CDMC (202118) and the Chengde Medical University’s Fundamental Research Funds (KY202220). Thanks for these help, we were also honoured to be rated as a national college student scientific research project (Project No.2023004).
Conflicts of Interest
The authors declare that there were no relationships or financial ties that may be seen as a potential conflict of interest throughout the course of the study.
Author Contribution
The study’s planning and manuscript writing were done by Song L and Zhao Y. The data was collected by He X, Duan Y, and Hu M. The final paper was examined and approved by Han S and Chi Y. Xu Q oversaw all aspects of the project, including data interpretation and article review. The final draft of the paper was approved by all authors after they had evaluated the findings.
Abbreviation List
CAD, Coronary artery disease
CVD, Cardiovascular diseases
FGF23, Fibroblast growth factor 23
FLT3, Fms-related tyrosine kinase 3
GWAS, Genome-wide association study
IL-17A, Interleukin-17A
IL-8, Interleukin-8
IVs, Instrumental variables
IVW, Inverse variance weighted
LIF, Leukemia inhibitory factor
MCSF1, Macrophage colony-stimulating factor 1
MMP1, Matrix metalloproteinase-1
MR, Mendelian randomization
MR-PRESSO, Mendelian randomization pleiotropy residual sum and outlier
PAH, Pulmonary artery hypertension
SNPs, Single nucleotide polymorphisms
TGF-α, Transforming growth factor-alpha
References
[1] Fuster V, Mearns BM. The CVD paradox: mortality vs prevalence. Nat Rev Cardiol, 2009; 6: 669.[DOI]
[2] Fornai F, Carrizzo A, Forte M et al. The inflammatory protein Pentraxin 3 in cardiovascular disease. Immun Ageing, 2016; 13: 1-9.[DOI]
[3] Ortega-Paz L, Capodanno D, Angiolillo DJ. Canakinumab for secondary prevention of coronary artery disease. Futur Cardiol, 2021; 17: 427-442.[DOI]
[4] Frostegård J. Immunity, atherosclerosis and cardiovascular disease. Bmc Med, 2013; 11: 1-13.[DOI]
[5] Dekens DW, Eisel ULM, Gouweleeuw L et al. Lipocalin 2 as a link between ageing, risk factor conditions and age-related brain diseases. Ageing Res Rev, 2021; 70: 101414.[DOI]
[6] Zhao J, Stacey D, Eriksson N et al. Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat Immunol, 2023; 24: 1540-1551.[DOI]
[7] Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010; 74: 213-220.[DOI]
[8] Carnevale D, Pallante F, Fardella V et al. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity, 2014; 41: 737-752.[DOI]
[9] Clapp BR, Hingorani AD, Kharbanda RK et al. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res, 2004; 64: 172-178.[DOI]
[10] Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA, 2017; 318: 1925-1926.[DOI]
[11] Buonafine M, Martínez-Martínez E, Amador C et al. Neutrophil Gelatinase-Associated Lipocalin from immune cells is mandatory for aldosterone-induced cardiac remodeling and inflammation. J Mol Cell Cardiol, 2018, 115: 32-38.[DOI]
[12] Shibata K, Sato K, Shirai R et al. Lipocalin-2 exerts pro-atherosclerotic effects as evidenced by in vitro and in vivo experiments. Heart Vessels, 2020; 35: 1012-1024.[DOI]
[13] Sung HK, Chan YK, Han M et al. Lipocalin‐2 (NGAL) attenuates autophagy to exacerbate cardiac apoptosis induced by myocardial ischemia. J Cell Physiol, 2017; 232: 2125-2134.[DOI]
[14] ouweleeuw L, Naudé PJW, Rots M et al. The role of neutrophil gelatinase associated lipocalin (NGAL) as biological constituent linking depression and cardiovascular disease. Brain Behav Immun, 2015; 46: 23-32.[DOI]
[15] Marques FZ, Prestes PR, Byars SG et al. Experimental and human evidence for lipocalin‐2 (neutrophil gelatinase‐associated lipocalin [NGAL]) in the development of cardiac hypertrophy and heart failure. J Am Heart Assoc, 2017; 6: e005971.[DOI]
[16] Skrivankova VW, Richmond RC, Woolf BAR et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA, 2021; 326: 1614-1621.[DOI]
[17] Burgess S, Butterworth A, Malarstig A et al. Use of Mendelian randomisation to assess potential benefit of clinical intervention. BMJ, 2012; 345: e7325.[DOI]
[18] Xiang S, Jia T, Xie C et al. Association between vmPFC gray matter volume and smoking initiation in adolescents. Nat Commun, 2023; 14: 4684.[DOI]
[19] Zhao JH, Stacey D, Eriksson N et al. Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat Immunol, 2023; 24: 1540-1551.[DOI]
[20] Van Der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res, 2018; 122: 433-443.[DOI]
[21] Kurki MI, Karjalainen J, Palta P et al. FinnGen: Unique genetic insights from combining isolated population and national health register data. MedRxiv, 2022; 2022: 03.[DOI]
[22] Shah S, Henry A, Roselli C et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun, 2020; 11: 163.[DOI]
[23] Malik R, Chauhan G, Traylor M et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet, 2018; 50: 524-537.[DOI]
[24] 1000 Genomes Project Consortium et al. “A map of human genome variation from population-scale sequencing”. Nature, 2010; 467: 1061-1073.[DOI]
[25] Burgess S, Thompson SG, Crp Chd Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol, 2011; 40: 755-764.[DOI]
[26] Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med, 2016; 35: 1880-1906.[DOI]
[27] Greco MFD, Minelli C, Sheehan NA et al. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med, 2015; 34: 2926-2940.[DOI]
[28] Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol, 2015; 44: 512-525.[DOI]
[29] Verbanck M, Chen CY, Neale B et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet, 2018; 50: 693-698.[DOI]
[30] Burgess S, Thompson S G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol, 2017; 32: 377-389.[DOI]
[31] Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. Plos Genet, 2017; 13: e1007081.[DOI]
[32] Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res, 2017; 26: 2333-2355.[DOI]
[33] Sehgal A, Irvine KM, Hume DA. Functions of macrophage colony-stimulating factor (CSF1) in development, homeostasis, and tissue repair. Semin Immunol, 2021; 54: 101509.[DOI]
[34] Taleb S. Inflammation in atherosclerosis. Arch Cardiovasc Dis, 2016; 109: 708-715.[DOI]
[35] Costanzo MR. The cardiorenal syndrome in heart failure. Heart Fail Clin, 2020; 16: 81-97.[DOI]
[36] Elliott MR, Koster KM, Murphy PS. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J Immunol, 2017; 198: 1387-1394.[DOI]
[37] Dechkhajorn W, Maneerat Y, Prasongsukarn K et al. Interleukin-8 in hyperlipidemia and coronary heart disease in Thai patients taking statin cholesterol-lowering medication while undergoing coronary artery bypass grafting treatment. Scientifica, 2020; 2020.[DOI]
[38] Yu Y, Weiss RM, Wei SG. Brain Interleukin-17A contributes to neuroinflammation and cardiac dysfunction in rats with myocardial infarction. Front Neurosci-Switz, 2022; 16: 1032434.[DOI]
[39] Chang SL, Hsiao YW, Tsai YN et al. Interleukin-17 enhances cardiac ventricular remodeling via activating MAPK pathway in ischemic heart failure. J Mol Cell Cardiol, 2018; 122: 69-79.[DOI]
[40] Ye D, Wang Z, Ye J et al. Interleukin-5 levels are decreased in the plasma of coronary artery disease patients and inhibit Th1 and Th17 differentiation in vitro. Revista Española de Cardiología, 2020; 73: 393-402.[DOI]
[41] Knutsson A, Björkbacka H, Dunér P et al. Associations of interleukin-5 with plaque development and cardiovascular events. Jacc-Basic Transl Sc, 2019; 4: 891-902.[DOI]
[42] Velásquez I M, Frumento P, Johansson K, et al. Association of interleukin 8 with myocardial infarction: results from the Stockholm Heart Epidemiology Program. Int J Cardiol, 2014; 172: 173-178.[DOI]
[43] Schömig K, Busch G, Steppich B et al. Interleukin-8 is associated with circulating CD133+ progenitor cells in acute myocardial infarction. Eur Heart J, 2006; 27: 1032-1037.[DOI]
[44] Zhao X, Zhang W, Xing D et al. Endothelial cells overexpressing IL-8 receptor reduce cardiac remodeling and dysfunction following myocardial infarction. Am J Physiol-Heart C, 2013; 305: H590-H598.[DOI]
[45] Stawski L, Trojanowska M. Oncostatin M and its role in fibrosis. Connect Tissue Res, 2019; 60: 40-49.[DOI]
[46] Carvalho VMF, Oliveira PSS, Albuquerque APB et al. Decreased serum levels of Soluble Oncostatin M receptor (sOSMR) and glycoprotein 130 (sgp130) in patients with coronary artery disease. Arq Bras Cardiol, 2023; 120: e20220326.[DOI]
[47] Masjedi A, Hajizadeh F, Dargani FB et al. Oncostatin M: A mysterious cytokine in cancers. Int Immunopharmacol, 2021; 90: 107158.[DOI]
[48] Rankouhi TR, Keulen D, Tempel D et al. Oncostatin M: risks and benefits of a novel therapeutic target for atherosclerosis. Curr Drug Targets, 2022; 23: 1345-1369.[DOI]
[49] Patel P, Rai V, Agrawal DK. Role of oncostatin-M in ECM remodeling and plaque vulnerability. Mol Cell Biochem, 2023; 478: 2451-2460.[DOI]
[50] Wang J, Chang CY, Yang X et al. Leukemia inhibitory factor, a double-edged sword with therapeutic implications in human diseases. Mol Ther, 2023; 31: 331-343.[DOI]
[51] Di Giorgio C, Bellini R, Lupia A, et al. Discovery of BAR502, as potent steroidal antagonist of leukemia inhibitory factor receptor for the treatment of pancreatic adenocarcinoma. Front Oncol, 2023; 13: 1140730.[DOI]
[52] Svensson EH, Söderholm M. Fibroblast growth factor 23 is associated with risk of intracerebral hemorrhage. Eur J Neurol, 2022; 29: 114-120.[DOI]
[53] Panwar B, Judd SE, Wadley VG et al. Association of fibroblast growth factor 23 with risk of incident coronary heart disease in community-living adults. JAMA Cardiol, 2018; 3: 318-325.[DOI]
[54] Della Verde G, Mochizuki M, Lorenz V et al. Fms-like tyrosine kinase 3 is a regulator of the cardiac side population in mice. Life Sci Alliance, 2022; 5.[DOI]
[55] Kazi JU, Rönnstrand L. FMS-like tyrosine kinase 3/FLT3: from basic science to clinical implications. Physiol Rev, 2019; 99: 1433-1466.[DOI]
[56] Matsushima-Nishiwaki R, Yamada N, Hattori Y et al. SERMs (selective estrogen receptor modulator), acting as estrogen receptor β agonists in hepatocellular carcinoma cells, inhibit the transforming growth factor-α-induced migration via specific inhibition of AKT signaling pathway. Plos one, 2022; 17: e0262485.[DOI]
[57] Rong W, Liu C, Li X et al. Caspase-8 promotes pulmonary hypertension by activating macrophage-associated inflammation and IL-1β (Interleukin 1β) production. Arterioscl Throm Vas, 2022; 42: 613-631.[DOI]
[58] Li K, Qiu H, Yan J et al. The involvement of TNF-α and TNF-β as proinflammatory cytokines in lymphocyte-mediated adaptive immunity of Nile tilapia by initiating apoptosis. Dev Comp Immunol, 2021; 115: 103884.[DOI]
[59] Trevaskis JL, Sacramento CB, Jouihan H et al. Neurturin and a GLP-1 analogue act synergistically to alleviate diabetes in Zucker diabetic fatty rats. Diabetes, 2017; 66: 2007-2018.[DOI]
[60] Lorente L, Martín MM, Pérez-Cejas A et al. Association between blood caspase-8 levels and mortality of patients with malignant middle cerebral artery infarction. Med Intensiva, 2022; 46: 305-311.[DOI]
[61] Satoh K. Caspase-8 Promotes the Development of Pulmonary Hypertension. Arteriosclerosis, thrombosis, and vascular biology, 2022; 42: 689-690.[DOI]
[62] McCarthy CP, Shrestha S, Ibrahim N et al. Performance of a clinical/proteomic panel to predict obstructive peripheral artery disease in patients with and without diabetes mellitus. Open Heart, 2019; 6: e000955.[DOI]
[63] Haley KJ, Lilly CM, Yang JH et al. Overexpression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation, 2000; 102: 2185-2189.[DOI]
[64] Emanuele E, Falcone C, D’Angelo A et al. Association of plasma eotaxin levels with the presence and extent of angiographic coronary artery disease. Atherosclerosis, 2006; 186: 140-145.[DOI]
[65] Chen DC, Potok OA, Rifkin D et al. Advantages, limitations, and clinical considerations in using cystatin C to estimate GFR. Kidney360, 2022; 3: 1807-1814.[DOI]
[66] Tapper M, McGrowder DA, Dilworth L et al. Cystatin C, vitamin D and thyroid function test profile in chronic kidney disease patients. Diseases-Basel, 2021; 9: 5.[DOI]
[67] Schiano C, Benincasa G, Franzese M et al. Epigenetic-sensitive pathways in personalized therapy of major cardiovascular diseases. Pharmacol Therapeut, 2020; 210: 107514.[DOI]
[68] Hanna A, Frangogiannis NG. Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc Drug Ther, 2020; 34: 849-863.[DOI]
[69] Bansal SS, Ismahil MA, Goel M et al. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation, 2019; 139: 206-221.[DOI]
[70] Hou H, Horikawa M, Narita Y et al. Suppression of Indoxyl Sulfate Accumulation Reduces Renal Fibrosis in Sulfotransferase 1a1-Deficient Mice. Int J Mol Sci, 2023; 24: 11329.[DOI]
[71] Chu CQ. Blocking tumor necrosis factor paved the way for targeted therapeutics in inflammatory diseases. Chinese Med J-Peking, 2021; 134: 2525-2528.[DOI]
[72] Zhao Y, Zhang X, Chen X et al. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment. Int J Mol Med, 2022; 49: 1-9.[DOI]
[73] Murala S, Nagarajan E, Bollu PC. Infectious causes of stroke. J Stroke Cerebrovasc, 2022; 31: 106274.[DOI]
[74] Singh AK, Goerlich CE, Zhang T et al. CD40-CD40L blockade: update on novel investigational therapeutics for transplantation. Transplantation, 2023; 107: 1472-1481.[DOI]
[75] Ferraro RA, Ogunmoroti O, Zhao D et al. Hepatocyte growth factor and incident heart failure subtypes: the multi-ethnic study of atherosclerosis (MESA). J Card Fail, 2021; 27: 981-990.[DOI]
[76] Di Giorgio C, Bellini R, Lupia A et al. Discovery of BAR502, as potent steroidal antagonist of leukemia inhibitory factor receptor for the treatment of pancreatic adenocarcinoma. Front Oncol, 2023; 13: 1140730.[DOI]
Copyright © 2024 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.




 Copyright ©
Copyright ©