Appetitive Lifestyles and Obesity with Risk of Senile Cataract: An Univariable and Multivariable Mendelian Randomization Study
Fan Cao1#, Yakun Wang2#, Baorui Chu1,3, Xianyang Liu2, Chao Wu1, Xinyuan Chen1, Shuhao Zeng2, Hui Feng1, Meng Tian4, Hong Wang4, Jing Ni5*, Shengping Hou1*
2The First Affiliated Hospital of Chongqing Medical University; Chongqing Key Laboratory of Ophthalmology; Chongqing Eye Institute, Chongqing, China
3Department of Ophthalmology, Qilu Hospital, Shandong University, Jinan, Shandong Province, China
4Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing, China
5Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University, Hefei, Anhui Province, China
#Both authors contributed equally to this manuscript.
*Correspondence to: Shengping Hou, MD, Professor, Beijing Institute of Ophthalmology, Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing Ophthalmology & Visual Sciences Key Laboratory, Beijing, 100005, China; Email: sphou828@163.com
Jing Ni, MD, Professor, Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University, 81 Meishan Road, Hefei, 230032, Anhui Province, China; Email: nijing@ahmu.edu.cn
DOI: 10.53964/cme.2024006
Abstract
Objective: To estimate the causal effects of appetitive lifestyles (cigarette smoking, alcohol use, coffee consumption) and obesity on risk of senile cataract (SC) using genetically based approaches.
Methods: Single nucleotide polymorphisms selected to be instrumental variables for exposures were identified at the level of genome-wide significance (P<5×10-8) from genome-wide association studies. Summary-level genetic statistics for SC was derived from the FinnGen consortium. Univariable and multivariable Mendelian randomization (MR) with inverse-variance weighted (IVW) method as main analysis were performed. Directional pleiotropy and heterogeneity were tested.
Results: For univariable MR, genetically determined predisposition to smoking initiation was associated with elevated risk of SC (IVW odds ratio (OR))=1.1241, 95% confidence interval (CI) (CI: 1.0240-1.2339, P=0.0140). Also, genetically predicted higher coffee consumption and body mass index (BMI) was associated with increased risk of SC (IVW OR=1.0050, 95% CI: 1.0012-1.0088, P=0.0101; IVW OR=1.1925, 95% CI: 1.0954-1.2982, P=4.8099×10-5, respectively). For multivariable MR, genetically predicted higher BMI still exerted a causal effect on SC risk (IVW OR=1.1712, 95% CI: 1.0786-1.2717, P<0.001). Nevertheless, the associations of smoking initiation and coffee consumption with SC risk disappeared. No directional pleiotropy was detected in both univariable and multivariable MR.
Conclusion: Our findings provide evidence for an adverse effect of higher BMI on SC risk, while no evidence supporting independent causal associations of appetitive lifestyles with SC risk. Improving the management of obesity may serve as a useful strategy protecting against SC.
Keywords: appetitive lifestyles, obesity, senile cataract, mendelian randomization
1 INTRODUCTION
Cataract, an opacification of the crystalline lens resulting in transparency loss, is one of the major causes of visual impairment accounting for approximately 51% of blindness worldwide, among which the senile cataract (SC) is the most common type[1,2]. The clouding or opacity of lens directly results from oxidative stress[3]. Accumulating evidence has revealed that the inherited genetic factors, environmental inducements, metabolic disturbance, and several chronic disorders collectively contribute to the initiation and progression of cataract genesis[1,4]. Over the last few decades, observational studies have suggested cigarette smoking[5-7] and obesity[8-10] as risk factors, coffee consumption as a protective factor[11,12], while alcohol use as a bidirectional factor for SC[13,14]. However, some other literature presented inconsistent results[15-20]. The overlap among obesity and appetitive lifestyles including cigarette smoking, alcohol use and coffee consumption may lead to confounding and make it difficult to clarify their independent associations with SC risk[21,22]. Therefore, the causal effects of obesity and appetitive lifestyles on the risk of SC remain elusive.
The Mendelian randomization (MR) utilizes genetic variants as instrumental variables (IVs) to make causal inference between exposure risk factors and disease outcomes, therefore overcomes several limitations of observational studies and traditional randomized controlled trials, including reverse causation, unmeasured confounding, strict inclusion and exclusion criteria, unethical design, etc[23]. Two-sample MR is one of the methods of MR with significant improved effectiveness, for that it identifies the associations between instrument-exposure and instrument-outcome based on two independent samples[24].
In the present study, we utilized genetic summary statistics from genome-wide association studies (GWAS) to explore the causal associations of appetitive lifestyles and obesity with the risk of SC, using a univariable and multivariable MR in two-sample framework.
2 MATERIALS AND METHODS
2.1 Study Design
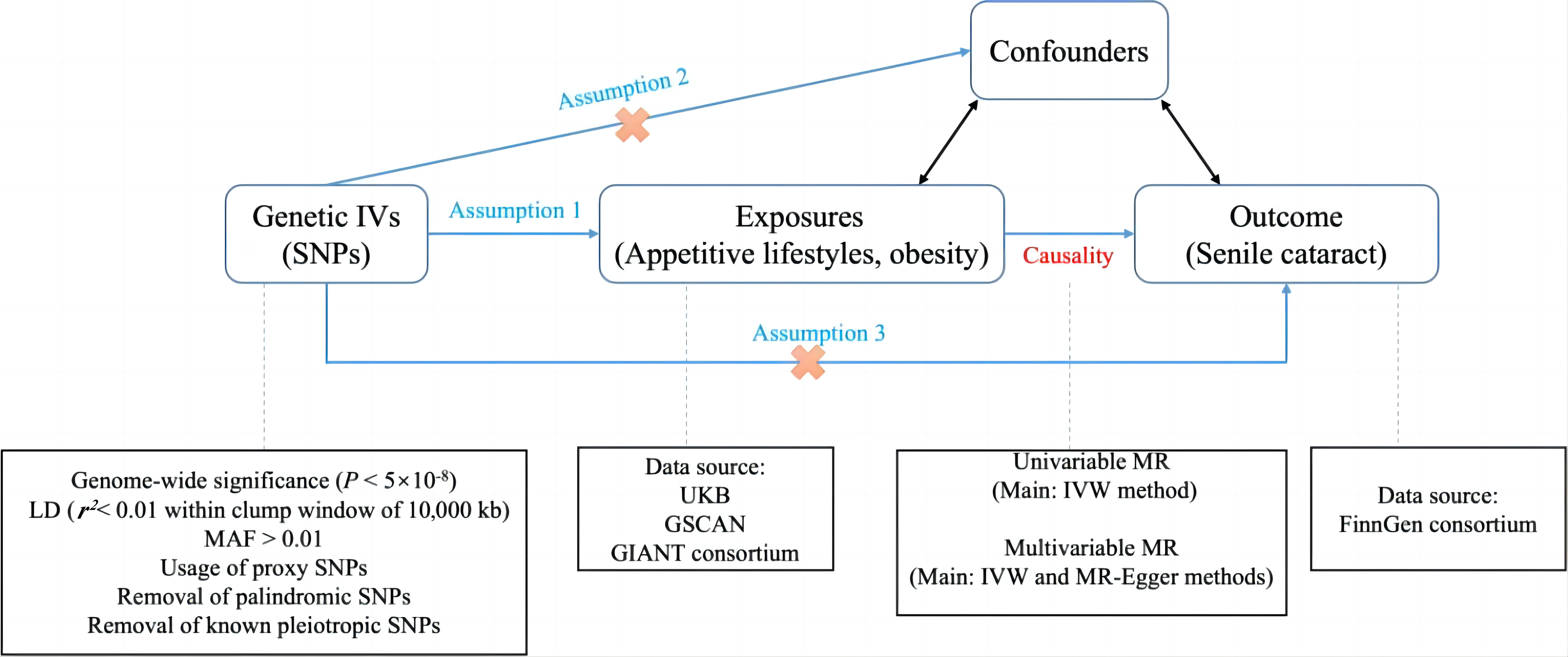
Study design of the present MR is illustrated in Figure 1. Single-nucleotide polymorphisms (SNPs) were selected to be IVs[23]. A univariable and multivariable MR in two-sample framework were carried out using the summary-level genetic data. Three major assumptions should be implemented to ensure the validity of MR[25,26]: Firstly, there should be robust IVs-exposure correlation, which is called relevance assumption and assessed by the F statistic in our study. The F statistic is calculated by R2(n-k-1)/[k(1-R2)]. In the formula, R2 is the exposure variance explained by included IVs, n represents the sample size and k refers to the number of selected IVs. If the F>10, the IVs-exposure correlation was thought to be powerful enough to avoid weak IVs-caused bias. Secondly, the included IVs should not be associated with any confounders of the exposure-outcome association, that is independence assumption. Thirdly, the IVs should impact the outcome only through the exposure rather than other pathways, which is called exclusion restriction assumption and essentially means a lack of pleiotropy of IVs-outcome. For more details, the MR Dictionary has provided comprehensive guidelines on definitions and descriptions for understanding and conducting MR[27].
|
Figure 1. Study design of the present MR. Three major assumptions should be implemented to ensure the validity of MR: Assumption 1, i.e., relevance assumption, which refers to robust IVs-exposure correlation; Assumption 2, i.e., independence assumption, which means that IVs should not be associated with any confounders of the exposure-outcome association; Assumption 3, i.e., exclusion restriction assumption, which indicates that IVs should impact the outcome only through the exposure rather than other pathways, essentially means a lack of pleiotropy of IVs-outcome.
Exposure datasets were derived from the UKB, GSCAN and Genetic Investigation of ANthropometric Traits (GIANT) consortium, and outcome datasets were obtained from the FinnGen consortium. SNPs selected to be IVs should meet the following criteria: (1) At the genome-wide significance (P<5×10-8). (2) LD meeting the condition of r2<0.01 within clump window of 10,000kb. (3) minor allele frequency (MAF)>0.01. (4) Usage of proxy SNPs at the condition that one certain SNP did not exist in the outcome GWAS. (5) Removal of palindromic SNPs. (6) Removal of known pleiotropic SNPs. Then, univariable and multivariable MR using the IVW and MR-Egger as main methods were conducted.
2.2 Data Source
The detailed information on the source of used data is demonstrated in Table 1. For the exposure datasets, summary-level genetic data for smoking initiation and alcohol use was derived from an international genetic association meta-analysis consortium named GWAS and Sequencing Consortium of Alcohol and Nicotine use (GSCAN)[28]. Summary-level genetic data for lifetime smoking index, which combined smoking initiation, duration, heaviness, and cessation, was obtained from a GWAS including individuals of current, former and never smokers in the UK Biobank (UKB)[29]. Genetic data for coffee consumption was retrieved from the hitherto largest GWAS investigating coffee consumption based on samples from the UKB[30]. Summary genetic statistics for body mass index (BMI) was derived from a meta-analysis in the UKB and GIANT consortium data[31]. For the outcome datasets, summary-level genetic statistics for SC was derived from the R5 release of FinnGen consortium[32]. The UKB is a very large UK collaborative research project in framework of population-based prospective study including over 500,000 individuals aged 40-69 years, with purpose of combining abundant and precise evaluation of exposures with longitudinal and comprehensive documentation of health-related outcomes[33]. The FinnGen is a public-private cooperation research project with growing individuals. It combines the genotype data in Finnish Biobank with the digital health records in Finnish health registries, therefore makes it possible to explore the genetic variation associated with diseases[32].
Table 1. Detailed Information on the Source of Used Data in the Present MR
Exposure/ Outcome |
Meaning |
Included Individuals |
Identified SNPs |
Ref |
Smoking initiation |
A binary phenotype indicating whether an individual had ever smoked regularly |
1,232,091 individuals from European ancestry |
378 |
[28] |
Lifetime smoking index |
Combination of smoking initiation, duration, heaviness, and cessation |
462,690 individuals in the UKB |
126 |
[29] |
Alcohol use |
Measurement of drinks per week |
941,280 individuals from European ancestry |
99 |
[28] |
Coffee consumption |
Measurement of drinks per day |
375,833 individuals in the UKB |
15 |
[30] |
BMI |
Weight/(Height)2 |
694,649 individuals from European ancestry |
543 |
[31] |
SC |
/ |
26,758 cases and 189,604 controls |
/ |
[32] |
2.3 Genetic Instrumental Variables Selection
SNPs selected to be IVs for smoking initiation[28], lifetime smoking index[29], alcohol use[28], coffee consumption[30], and BMI[31] were identified at the level of genome-wide significance (P<5×10-8) from corresponding GWAS. The PLINK clumping method was used to calculate linkage disequilibrium (LD) of selected significant SNPs associated with risk factors based on LD reference panel of European population. To avoid the strong LD-caused bias, SNPs with LD meeting the condition of r2>0.01 within clump window of 10,000kb were excluded. Then, the SNPs whose MAF<0.01 were further excluded. Moreover, if one SNP was not present in the outcome GWAS, another proxy SNP with high LD (r2≥0.80) would be used to replace it. To make the effect allele consistently relating to the same allele, the harmonization of exposure and outcome data was performed, with the action=3, that means all palindromic SNPs were dropped. Notably, 6 SNPs significantly associated with coffee consumption including rs4719497, rs1260326, rs574367, rs10865548, rs66723169, and rs34060476 were removed due to LD and known pleiotropic effects with several other potential risk factors especially BMI[34].
2.4 Univariable Mendelian Randomization
The inverse-variance weighted (IVW) method using multiplicative random effects model was used as the main analysis to estimate the causal effects of factors of interest on SC risk. Three other methods including MR-Egger regression, weighted median, and weighted mode, were utilized as the supplementary analyses to estimate the causal associations. The IVW method is in fact a meta-analysis model converting to a weighted regression of the outcome effects of IVs on the exposure effects, therefore provides an overall estimate of the effects of exposures on SC risk. The intercept of this weighted regression is 0, that means IVW could get an unbiased estimate if there was no horizontal pleiotropy[35]. Relatively, the MR-Egger regression method is easily influenced by outliers, nevertheless, it can make an unbiased estimate even with invalid IVs[36]. The weighted median model obtains consistent estimates on causal associations when at most 50% IVs are invalid[37]. On the condition that several IVs do not meet the criteria for causal inference of MR, the weighted mode method can still get valid estimates when most IVs are valid[35]. For directional pleiotropy among included SNPs of each exposure, it was detected and corrected by the MR-Egger regression, because its intercept is not limited to be 0. To strength the accuracy and power of pleiotropy test, the MR Pleiotropy Residual Sum and Outlier (MR-PRESSO) was also carried out. The MR-PRESSO can detect outliers, calculate estimates after removing outliers, as well as compare the estimates before and after outliers’ removal[38]. For heterogeneity among included SNPs of each exposure, it was assessed by the Cochran’s Q statistic.
2.5 Multivariable Mendelian Randomization
If a trait of interest demonstrated positive or negative causal effect on risk of SC in the univariable MR, multivariable MR would be performed to explore their independent associations with SC risk[39]. Multivariable IVW and MR-Egger methods using random-effect model were implemented to estimate the independent causal associations. The directional pleiotropy was detected and corrected by the MR-Egger and MR-PRESSO methods. The heterogeneity was evaluated by the Cochran’s Q statistic.
2.6 Statistical Analysis
All statistical analyses were conducted in R (version 4.1.0; R Foundation for Statistical Computing, Vienna, Austria) using the TwoSampleMR (version 0.5.6), MendelianRandomization (version 0.5.1), and MRPRESSO (version 1.0) packages. Statistical significance was set as two-tailed P<0.05.
3 RESULTS
3.1 Genetic Instrumental Variables
The detailed information of genetic IVs used in the univariable MR for smoking initiation, lifetime smoking index, alcohol use, coffee consumption and BMI, including effect allele, other allele, effect allele frequency, effect sizes (β), S.E. and P-value on exposure and outcome were demonstrated in Table S1. Likewise, the detailed information of genetic IVs used in the multivariable MR were shown in Table S2.
3.2 Univariable Mendelian Randomization
The results of univariable MR for smoking initiation, lifetime smoking index, alcohol use, coffee consumption and BMI on risk of SC were presented in Table 2. Genetically determined predisposition to smoking initiation was associated with elevated risk of SC in the data from GSCAN (IVW odds ratio (OR)=1.1241, 95% confidence interval (CI): 1.0240-1.2339, P=0.0140), GSCAN minus 23andMe (IVW OR=1.1159, CI: 1.0207-1.2200, P=0.0160), and GSCAN minus 23andMe and UKB (IVW OR=1.1115, CI: 1.0196-1.2117, P=0.0163). However, the positive association did not remain in the analyses between lifetime smoking index and SC. Genetically predicted increased coffee consumption was associated with elevated SC risk in the data from Joint-meta (IVW OR=1.0050, 95% CI: 1.0012-1.0088, P=0.0101) and Stage 1 (IVW OR=1.0051, 95% CI: 1.0012-1.0090, P=0.0096). Also, genetically predicted higher BMI was associated with increased SC risk (IVW OR=1.1925, 95% CI: 1.0954-1.2982, P=4.8099E-5). There was no genetic causal association of alcohol use with risk of SC in the present MR. No directional pleiotropy was found by the MR-Egger and MR-PRESSO methods. Heterogeneity was detected among the analyses of smoking initiation, alcohol use and BMI with SC risk. Additionally, the results of univariable MR using the MR-Egger regression, weighted median, and weighted mode methods were demonstrated in Table S3.
Table 2. Univariable MR of Appetitive Lifestyles and Obesity on SC Risk (IVW Method)
Exposure |
Exposure Dataset |
nSNP |
F statistic |
OR |
95% CI |
P |
Pleiotropy |
Heterogeneity |
|
PMR-Egger intercepta |
PMR-PRESSO distortion testb |
P Cochran’s Q testc |
|||||||
Smoking initiation |
GSCAN |
267 |
39.3118 |
1.1241 |
1.0240, 1.2339 |
0.0140 |
0.3268 |
0.7603 |
0.0005 |
GSCAN minus 23andMe |
266 |
22.5479 |
1.1159 |
1.0207, 1.2200 |
0.0160 |
0.3704 |
0.9948 |
0.0002 |
|
GSCAN minus 23andMe and UKB |
265 |
9.5344 |
1.1115 |
1.0196, 1.2117 |
0.0163 |
0.7584 |
0.8897 |
0.0001 |
|
Lifetime smoking index |
UKB |
107 |
38.4753 |
1.2463 |
0.9563, 1.6242 |
0.1032 |
0.3080 |
NA |
0.0647 |
Alcohol use |
GSCAN |
69 |
51.3976 |
0.8254 |
0.5918, 1.1512 |
0.2582 |
0.3578 |
NA |
0.0016 |
GSCAN minus 23andMe |
69 |
38.5517 |
0.8569 |
0.6467, 1.1353 |
0.2819 |
0.4268 |
NA |
0.0245 |
|
GSCAN minus 23andMe and UKB |
69 |
12.9532 |
0.7676 |
0.5566, 1.0585 |
0.1067 |
0.1620 |
NA |
0.0144 |
|
Coffee consumption |
UKB (Joint-meta data) |
6 |
239.8207 |
1.0050 |
1.0012, 1.0088 |
0.0101 |
0.5105 |
NA |
0.6856 |
UKB (Stage 1 data) |
6 |
209.9718 |
1.0051 |
1.0012, 1.0090 |
0.0096 |
0.5175 |
NA |
0.6994 |
|
BMI |
UKB plus GIANT consortium |
469 |
65.6347 |
1.1925 |
1.0954, 1.2982 |
4.8099E-05 |
0.1118 |
0.8944 |
1.9494E-05 |
Notes: a: PMR-Egger intercept<0.05 suggested a potential directional pleiotropy; b: PMR-PRESSO distortion test<0.05 suggested that estimates before and after outliers’ removal differed significantly. If no outlier was detected, PMR-PRESSO distortion test would present not available (NA); c: PCochran’s Q test<0.05 suggested a possible heterogeneity.
3.3 Multivariable Mendelian Randomization
The results of multivariable MR for smoking initiation, coffee consumption and BMI on risk of SC were presented in Table 3. Genetically predicted higher BMI still exerted a causal effect on SC risk after adjustment for smoking initiation and coffee consumption (IVW OR=1.1712, 95% CI: 1.0786-1.1217, P<0.001, MR-Egger OR=1.1607, 95% CI: 1.0690-1.2603, P<0.001; IVW OR=1.1712, 95% CI: 1.0786-1.1217, P<0.001, MR-Egger OR=1.1665, 95% CI: 1.0743-1.2666, P<0.001). However, smoking initiation and coffee consumption showed no causal associations with SC risk in the multivariable MR analyses. No directional pleiotropy was found by the MR-Egger and MR-PRESSO methods. Heterogeneity was detected in the present multivariable MR analyses.
Table 3. Multivariable MR of Appetitive Lifestyles and Obesity on SC Risk
Exposure |
Exposure Dataset |
Method |
OR |
95% CI |
P |
Pleiotropy |
Heterogeneity |
||
PMR-Egger intercepta |
PMR-PRESSO distortion testb |
P Cochran’s Q testc |
|||||||
Smoking initiation |
GSCAN minus 23andMe |
IVW |
1.0800 |
0.9947, 1.1727 |
0.0680 |
0.1490 |
NA |
<0.0001 |
|
Coffee consumption |
UKB (Stage 1 data) |
1.1085 |
0.8261, 1.4874 |
0.4920 |
|||||
BMI |
UKB plus GIANT consortium |
1.1712 |
1.0786, 1.2717 |
<0.001 |
|||||
Smoking initiation |
GSCAN minus 23andMe |
MR-Egger |
1.0030 |
0.8796, 1.1438 |
0.9610 |
<0.0001 |
|||
Coffee consumption |
UKB (Stage 1 data) |
1.1163 |
0.8319, 1.4978 |
0.4640 |
|||||
BMI |
UKB plus GIANT consortium |
1.1607 |
1.0690, 1.2603 |
<0.001 |
|||||
Smoking initiation |
GSCAN minus 23andMe and UKB |
IVW |
1.0747 |
0.9936, 1.1623 |
0.0710 |
0.4600 |
NA |
<0.0001 |
|
Coffee consumption |
UKB (Stage 1 data) |
1.1107 |
0.8278, 1.4903 |
0.4840 |
|||||
BMI |
UKB plus GIANT consortium |
1.1712 |
1.0786, 1.2717 |
<0.001 |
|||||
Smoking initiation |
GSCAN minus 23andMe and UKB |
MR-Egger |
1.0346 |
0.9108, 1.1752 |
0.6030 |
<0.0001 |
|||
Coffee consumption |
UKB (Stage 1 data) |
1.1129 |
0.8294, 1.4933 |
0.4760 |
|||||
BMI |
UKB plus GIANT consortium |
1.1665 |
1.0743, 1.2666 |
<0.001 |
|||||
Notes: a: PMR-Egger intercept<0.05 suggested a potential directional pleiotropy; b: PMR-PRESSO distortion test<0.05 suggested that estimates before and after outliers’ removal differed significantly. If no outlier was detected, PMR-PRESSO distortion test would present not available; c: PCochran’s Q test<0.05 suggested a possible heterogeneity.
4 DISCUSSION
Our present study indicated an independent and causal association of obesity with elevated risk of SC. There was no evidence supporting that appetitive lifestyles including cigarette smoking, alcohol use and coffee consumption were independently causally associated with SC risk.
Higher BMI reflecting overall obesity has been associated with increased risk of SC in meta-analysis of observational studies[8,9], which was corroborated by a previous MR[40] and our present MR in both univariable and multivariable design. Interestingly, an updated systematic review and meta-analysis revealed an association between obesity (defined by BMI) and increased risk of SC, posterior subcapsular cataract (PSC), and cortical cataract in adults. Nevertheless, such a positive association did not exist for nuclear cataract[41]. Previous studies have also investigated the genetic association of the obesity gene SNP with SC. It has been reported that the fat mass and obesity-related (FTO) gene was associated with obesity, and its SNP rs9939609 explaining between ∼1% and 0.24% of the variance of BMI was significantly associated with obesity in both adult and childhood[18]. Chandra et al. found that individuals with homozygous TT genotypes of the FTO SNP rs9939609 had higher risk of cataract than those with AT genotypes in a case-control study based on Indian sample[42]. Moreover, another population-based study in Malay revealed an association between the minor allele (A) of FTO SNP rs9939609 and elevated risk of nuclear cataract[17]. Although the difference in terms of risk allele of the FTO SNP rs9939609, these two studies both suggested a potential association between genetically determined obesity and cataract. Potential mechanisms responsible for the association may be that the proinflammatory meditators including interleukin-6, tumor necrosis factor-α and C reactive protein induced by adipose tissue serves as manifestations of oxidative stress[43]. Subsequently, the oxidative stress may directly contribute to the clouding or opacity of lens[44]. Experiments in Emory mouse have also indicated that moderate caloric restriction could delay cataract formation, which may be attributed to the enhanced proteolytic and antioxidative capacity of lens [45]. However, a previous study using MR method with FTO SNP rs9939609 as an IV for BMI-defined obesity revealed no causal association between obesity and SC in older Australian population[18]. Compared with the fact that FTO SNP rs9939609 could explain between ~1% and 0.24% of the variance of BMI, included SNPs in our present MR may explain ~6.86% of the variance of BMI. Additionally, the different population, and techniques applied into cataract ascertainment may also partly responsible for the distinct conclusion.
About cigarette smoking, despite the association between cigarette smoking and SC has been suggested in massive epidemiological studies, in which both current and past smokers appeared to have higher risk of developing cataract compared with never-smokers, even a dose-response effect between smoking and cataract was detected, especially for PSC and nuclear cataract[46-49], several other studies demonstrated inconsistent results that smoking was not associated with the higher risk for SC[15,16]. Interestingly, it is also reported that smoking had neither positive nor negative effects on the long-term incidence for early-onset cataract[50]. Of note, the latest umbrella review of systematic reviews and meta-analyses identified that smoking as a risk factor for cataract was the most robust association among various factors and major age-related eye disorders[51]. A previous MR study also indicated that genetic predisposition to smoking initiation was associated with SC[40]. Our present study found an association between genetically determined predisposition to smoking initiation and elevated risk of SC in univariable MR, nevertheless, this positive association disappeared in multivariable MR. Moreover, we utilized the genetic IVs of lifetime smoking index, which considered smoking status (i.e., ever and never smokers) and smoking duration, heaviness and cessation among ever smokers[29], to estimate the causal association of cigarette smoking with SC risk. Likewise, no causal association was observed. Therefore, our findings did not support the causal association between cigarette smoking and SC risk, previous observational studies reporting positive associations between smoking and SC may be biased due to several potential confounding factors such as BMI.
The data of previous observational studies investigating association between alcohol use and SC was inconsistent, with positive, negative, and null results reported. A meta-analysis revealed a nonlinear effect of alcohol use on cataract genesis varying by doses, as it suggested that moderate alcohol consumption seemed to protect against SC, whereas heavy consumption increased the risk of developing SC[13]. Moreover, a recent study using two large longitudinal cohorts found that the protective role of moderate alcohol intake on SC was particularly significant when consuming polyphenol-rich wine, which may be attributed to the strong antioxidant properties of polyphenol micronutrients[14]. Conversely, no association of heavy or moderate alcohol intake with susceptibility of SC were detected in another meta-analysis including seven prospective cohort studies[20]. Our present study supported no causal effect of alcohol use with risk of SC, which was consistent with a previous MR study[40]. As for coffee consumption, observational studies have found an association of higher amounts of coffee intake with lower risk or blindness incidence of cataract[11,12]. Nevertheless, a very early case-control study suggested no association between coffee or decaffeinated coffee intakes and cataract extraction[19]. A previous MR study indicated that higher genetically predicted coffee consumption was associated with an increased risk of SC in the FinnGen consortium[40]. However, our study only found an association in univariable MR, and did not support an independent causal association of genetically predicted increased coffee consumption with SC risk in multivariable MR.
This study comprehensively explored the independent and causal associations of appetitive lifestyles and obesity with the risk of SC, using univariable and multivariable MR design. The prominent strengths of our present study are the quality of used data and MR design including both univariable and multivariable. However, several limitations should be mentioned. First, assessment on the second and third assumptions may be not precise resulting from the weakness of MR method, therefore may cause potential bias. Second, the subgroup analysis could not be performed due to the lack of individual-level statistics such as their demographic information and clinical symptoms. Third, the generalization of conclusions of the present study should be given much caution due to the potential ethnic bias, which was caused by the selection of European ancestries in our study.
In conclusion, the present study using MR provides evidence for an adverse effect of higher BMI on the risk of SC, while no evidence supporting independent causal associations of appetitive lifestyles including cigarette smoking, alcohol use and coffee consumption with SC risk. Improving the management of obesity may serve as a useful strategy to protect against SC. Further studies using larger genetic statistics or longitudinal studies are warranted to verify the findings of our present study.
Acknowledgements
We want to acknowledge the participants and investigators of FinnGen study. This work was supported by the National Natural Science Foundation Project of China (No.82070951, No.82271078), Beijing Natural Science Foundation (No.7212016) and the Program for Youth Innovation in Future Medicine, Chongqing Medical University (w0047).
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
Cao F, Hou S, and Ni J conceptualized and designed the study. Cao F and Wan Y obtained the data, performed the analysis, and made the illustrations. Cao F wrote the manuscript. Hou S and Ni J revised the manuscript. Chu B, Liu X, Wu C and Chen X contributed to data collection and analysis. Zeng S, Hou S, Tian M, and Wang H participated in figures and tables preparation. Cao F, Hou S, and Ni J supervised the final version of manuscript. All authors reviewed the article, read the final manuscript and approved the submission.
BMI, Body mass index
CI, Confidence interval
FTO, Fat mass and obesity-related
GIANT, Genetic Investigation of ANthropometric Traits
GSCAN, Genome-wide association studies and Sequencing Consortium of Alcohol and Nicotine use
GWAS, Genome-wide association studies
IVs, Instrumental variables
IVW, Inverse-variance weighted.
LD, Linkage disequilibrium
MAF, Minor allele frequency
MR, Mendelian randomization
MR-PRESSO, MR Pleiotropy Residual Sum and Outlier
OR, Odds ratio
PSC, Posterior subcapsular cataract
SC, Senile cataract
SNPs, Single-nucleotide polymorphisms
UKB, UK Biobank
References
[1] Cicinelli MV, Buchan JC, Nicholson M et al. Cataracts. The Lancet, 2023; 401: 377-389.[DOI]
[2] Chen X, Xu J, Chen X et al. Cataract: advances in surgery and whether surgery remains the only treatment in future. Adv Ophth Pract Res, 2021; 1: 100008.[DOI]
[3] Lee B, Afshari NA, Shaw PX. Oxidative stress and antioxidants in cataract development. Curr Opin Ophthalmol, 2024; 35: 57-63.[DOI]
[4] Lim JC, Caballero Arredondo M, Braakhuis AJ et al. Vitamin C and the Lens: New Insights into Delaying the Onset of Cataract. Nutrients, 2020; 12: 3142.[DOI]
[5] Kelly SP, Thornton J, Edwards R et al. Smoking and cataract: review of causal association. J Cataract Refract Surg, 2005; 31: 2395-2404.[DOI]
[6] Ye J, He J, Wang C et al. Smoking and Risk of Age-Related Cataract: A Meta-Analysis. Invest Ophthalmol Vis Sci, 2012; 53: 3885-3895.[DOI]
[7] Lindblad BE, Håkansson N, Wolk A. Smoking Cessation and the Risk of Cataract: A Prospective Cohort Study of Cataract Extraction Among Men. JAMA Ophthalmol, 2014; 132: 253-257.[DOI]
[8] Ye J, Lou LX, He JJ et al. Body Mass Index and Risk of Age-Related Cataract: A Meta-Analysis of Prospective Cohort Studies. PLoS One, 2014; 9: e89923.[DOI]
[9] Pan CW, Lin Y. Overweight, Obesity, and Age-Related Cataract: A Meta-analysis. Optom Vis Sci, 2014; 91: 478-483.[DOI]
[10] Jacques PF, Moeller SM, Hankinson SE et al. Weight status, abdominal adiposity, diabetes, and early age-related lens opacities. Am J Clin Nutr, 2003; 78: 400-405.[DOI]
[11] Rautiainen S, Lindblad BE, Morgenstern R et al. Total Antioxidant Capacity of the Diet and Risk of Age-Related Cataract: A Population-Based Prospective Cohort of Women. JAMA Ophthalmol, 2014; 132: 247-252.[DOI]
[12] Varma SD. Effect of coffee (caffeine) against human cataract blindness. Clin Ophthalmol, 2016; 10: 213-220.[DOI]
[13] Gong Y, Feng K, Yan N et al. Different Amounts of Alcohol Consumption and Cataract: A Meta-analysis. Optom Vis Sci, 2015; 92: 471-479.[DOI]
[14] Chua SYL, Luben RN, Hayat S et al. Alcohol Consumption and Incident Cataract Surgery in Two Large UK Cohorts. Ophthalmology, 2021; 128: 837-847.[DOI]
[15] Han X, Wu C, Yan X et al. Are smoking intensity and cessation related to cataract surgical risk in diabetic patients? Findings from the 45 and Up Study. Eye, 2020; 34: 383-391.[DOI]
[16] Hugosson M, Ekström C. Prevalence and risk factors for age-related cataract in Sweden. Ups J Med Sci, 2020; 125: 311-315.[DOI]
[17] Lim LS, Tai ES, Aung T et al. Relation of age-related cataract with obesity and obesity genes in an Asian population. Am J Epidemiol, 2009; 169: 1267-1274.[DOI]
[18] Tan AG, Kifley A, Flood VM et al. Evaluating the associations between obesity and age-related cataract: a Mendelian randomization study. Am J Clin Nutr, 2019; 110: 969-976.[DOI]
[19] Tavani A, Negri E, Vecchia CL. Food and nutrient intake and risk of cataract. Ann Epidemiol, 1996; 6: 41-46.[DOI]
[20] Wang W, Zhang X. Alcohol intake and the risk of age-related cataracts: a meta-analysis of prospective cohort studies. PLoS One, 2014; 9: e107820.[DOI]
[21] Carmody TP, Brischetto CS, Matarazzo JD et al. Co-occurrent use of cigarettes, alcohol, and coffee in healthy, community-living men and women. Health Psychol, 1985; 4: 323-335.[DOI]
[22] Yuan S, Gill D, Giovannucci EL et al. Obesity, Type 2 Diabetes, Lifestyle Factors, and Risk of Gallstone Disease: A Mendelian Randomization Investigation. Clin Gastroenterol Hepatol, 2022; 20: 529-537.[DOI]
[23] Lawlor DA, Harbord RM, Sterne JA et al. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med, 2008; 27: 1133-1163.[DOI]
[24] Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol, 2013; 178: 1177-1184.[DOI]
[25] Gormley M, Dudding T, Sanderson E et al. A multivariable Mendelian randomization analysis investigating smoking and alcohol consumption in oral and oropharyngeal cancer. Nat Commun, 2020; 11: 6071.[DOI]
[26] Dan YL, Wang P, Cheng Z et al. Circulating adiponectin levels and systemic lupus erythematosus: a two-sample Mendelian randomization study. Rheumatology, 2021; 60: 940-946.[DOI]
[27] Lawlor DA, Wade K, Borges MC et al. A Mendelian Randomization dictionary: Useful definitions and descriptions for undertaking, understanding and interpreting Mendelian Randomization studies. OSF Preprints 2019 [Epub ahead of print].[DOI]
[28] Liu M, Jiang Y, Wedow R et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet, 2019; 51: 237-244.[DOI]
[29] Wootton RE, Richmond RC, Stuijfzand BG et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol Med, 2020; 50: 2435-2443.[DOI]
[30] Zhong VW, Kuang A, Danning RD et al. A genome-wide association study of bitter and sweet beverage consumption. Hum Mol Genet, 2019; 28: 2449-2457.[DOI]
[31] Pulit SL, Stoneman C, Morris AP et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet, 2019; 28: 166-174.[DOI]
[32] R5 release of genetic data from FinnGen consortium. Available at:[Web]
[33] Sudlow C, Gallacher J, Allen N et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med, 2015; 12: e1001779.[DOI]
[34] Yuan S, Larsson SC. No association between coffee consumption and risk of atrial fibrillation: A Mendelian randomization study. Nutr Metab Cardiovasc Dis, 2019; 29: 1185-1188.[DOI]
[35] Xiang K, Wang P, Xu Z et al. Causal Effects of Gut Microbiome on Systemic Lupus Erythematosus: A Two-Sample Mendelian Randomization Study. Front Immunol, 2021; 12: 667097.[DOI]
[36] Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol, 2015; 44: 512-525.[DOI]
[37] Bowden J, Davey Smith G, Haycock PC et al. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol, 2016; 40: 304-314.[DOI]
[38] Verbanck M, Chen CY, Neale B et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet, 2018; 50: 693-698.[DOI]
[39] Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol, 2015; 181: 251-260.[DOI]
[40] Yuan S, Wolk A, Larsson SC. Metabolic and lifestyle factors in relation to senile cataract: a Mendelian randomization study. Sci Rep, 2022; 12: 409.[DOI]
[41] Niazi S, Moshirfar M, Dastjerdi MH et al. Association between obesity and age-related cataract: an updated systematic review and dose-response meta-analysis of prospective cohort studies. Front Nutr, 2023; 10: 1215212.[DOI]
[42] Chandra A, Raza ST, Abbas S et al. Polymorphism of GST and FTO Genes in Risk Prediction of Cataract among a North Indian Population. Ophthalmic Genet, 2016; 37: 19-24.[DOI]
[43] Lindblad BE, Håkansson N, Philipson B et al. Metabolic syndrome components in relation to risk of cataract extraction: a prospective cohort study of women. Ophthalmology, 2008; 115: 1687-1692.[DOI]
[44] Vinson JA. Oxidative stress in cataracts. Pathophysiology, 2006; 13: 151-162.[DOI]
[45] Taylor A, Zuliani AM, Hopkins RE et al. Moderate caloric restriction delays cataract formation in the Emory mouse. Faseb j, 1989; 3: 1741-1746.[DOI]
[46] Foster PJ, Wong TY, Machin D et al. Risk factors for nuclear, cortical and posterior subcapsular cataracts in the Chinese population of Singapore: the Tanjong Pagar Survey. Br J Ophthalmol, 2003; 87: 1112-1120.[DOI]
[47] Tan JS, Wang JJ, Younan C et al. Smoking and the long-term incidence of cataract: the Blue Mountains Eye Study. Ophthalmic Epidemiol, 2008; 15: 155-161.[DOI]
[48] Wu R, Wang JJ, Mitchell P et al. Smoking, socioeconomic factors, and age-related cataract: The Singapore Malay Eye study. Arch Ophthalmol, 2010; 128: 1029-1035.[DOI]
[49] Kurt A, Ersekerci TK, Kiliç R et al. The Affects of Smoking on Cataract Formation in Turkish Patients. Ophthalmol Res: Int J, 2023; 18: 9-15.[DOI]
[50] Zhang J, Han X,Wang W et al. Findings from the 45 and Up Study: smoking is not associated with the risk of early-onset cataract. Ann Transl Med, 2021; 9: 1077.[DOI]
[51] Kai JY, Zhou M, Li DL et al. Smoking, dietary factors and major age-related eye disorders: an umbrella review of systematic reviews and meta-analyses. Br J Ophthalmol, 2023; 108: 51-57.[DOI]
Copyright © 2024 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.




 Copyright ©
Copyright ©