A Comprehensive Review of Chemical Looping Ammonia Synthesis
Ziheng Han1, Qiuyan Xue1, Xiude Hu1, Tuo Guo1,2*, Jingjing Ma1*, Qingjie Guo1,2*![]()
1State Key Laboratory of High-efficiency Utiliztion of Coal and Green Chemical Engineering, College of Chemistry and Chemical Engineering, Ningxia University, Yinchuan, Ningxia Hui Autonomous Region, China
2Key Laboratory of Clean Chemical Engineering in Universities of Shandong, College of Chemical Engineering, Qingdao University of Science & Technology, Qingdao, Shandong Province, China
*Correspondence to: Tuo Guo, PhD, Professor, State Key Laboratory of High-efficiency Utiliztion of Coal and Green Chemical Engineering, College of Chemistry and Chemical Engineering, Ningxia University, Yinchuant, 750000, Ningxia Hui Autonomous Region, China; E-mail: tuo.guo.20@alumni.ucl.ac.uk
Jingjing Ma, PhD, Professor, State Key Laboratory of High-efficiency Utiliztion of Coal and Green Chemical Engineering, College of Chemistry and Chemical Engineering, Ningxia University, Yinchuan, 750000, Ningxia Hui Autonomous Region, China; E-mail: mjj_1022@163.com
Qingjie Guo, PhD, Professor, Key Laboratory of Clean Chemical Engineering in Universities of Shandong, College of Chemical Engineering, Qingdao University of Science & Technology, Qingdao, 266000, Shandong Province, China; E-mail: Chinaqingjie_guo@vip.sina.com
DOI: 10.53964/id.2024009
Abstract
Ammonia, being a vital chemical compound, has made substantial contributions to the progress of society. It addresses not only the issue of nitrogen fixation from the atmosphere but also helps alleviate the food crisis. Nevertheless, the conventional Haber-Bosch process for synthesizing ammonia is frequently associated with substantial greenhouse gas emissions and the consumption of fossil fuels. Consequently, in response to significant environmental challenges, the prospective trajectory for the synthetic ammonia sector involves the adoption of strategies such as carbon reduction, energy-efficient conversions, and the exploration of environmentally friendly and sustainable approaches to ammonia synthesis. Among the various methods for green ammonia synthesis, chemical looping ammonia synthesis (CLAS) technology is regarded as one of the most promising alternatives to conventional ammonia synthesis methods in the upcoming years. This can be attributed to the relatively mild operating conditions and the flexible and distributed characteristics of its processes. In this paper, the thermodynamic and kinetic properties of nitridation and ammoniation reactions were examined through the lens of CLAS. The design and regulation strategies of nitrogen carriers (NCs) are summarized based on various types of NCs. The process of CLAS was summarized in relation to various NCs. This paper serves as a foundational resource for the prospective advancement of the CLAS framework.
Keywords: chemical looping ammonia synthesis, nitrogen carriers, nitridation, ammoniation
1 INTRODUCTION
Ammonia is not only an important chemical raw material for the production of nitrogen fertilizer, but also 80% of nitrogen-containing chemicals are produced from ammonia[1]. However, the synthetic ammonia industry is a high-energy and high-emission industry. It is important for the synthetic ammonia industry to improve its technology. Furthermore, we are confronted with a series of environmental problems caused by the increase in greenhouse gases. The development of carbon-free fuels, the use of renewable energy instead of fossil fuels, and the development of low-cost, high-efficiency energy storage methods are the promising ways to achieve sustainable social development[2]. With the vigorous development of renewable technology, we have gradually realized that flexible storage and transportation of renewable energy can effectively address the instability and discontinuity of renewable energy, and alleviate energy pressure, especially in recent years. Ammonia has the advantages of high energy density and hydrogen content, easy storage and transportation, making it one of the most effective energy carriers[3,4]. The utilization rate of renewable energy can be effectively improved by storing energy in ammonia and reusing it after decomposition, which can ensure energy security. Ammonia as a carrier of hydrogen, can reduce the risks associated with hydrogen storage and transportation. It also addresses the challenge of storing and transferring hydrogen efficiently[5,6]. Compared with hydrogen, the liquefaction pressure of ammonia is only one-twentieth of that of hydrogen at room temperature, and the volume of hydrogen is 4.65 times that of ammonia when storing the same amount of energy. Furthermore, the pipeline and navigation transportation of ammonia are more mature[7]. Under atmospheric conditions, ammonia can be liquefied by increasing the pressure to 10 bar or cooling it to -33℃. This process enhances the properties of ammonia as an energy carrier and a carbon-free fuel. The excellent storage and transportation properties of ammonia make it highly competitive among many carbon-free fuels. The ammonia economy has unlimited potential in the future.
At present, the industrial synthesis of ammonia generally adopts the Haber-Bosch (H-B) method. The fossil fuel (coal or natural gas) is used as the feed to produce syngas. And then a certain amount of air is introduced into the syngas, where oxygen reacts with CO to form CO2, releasing heat to provide energy for the reaction[8]. The remaining N2 and H2 are used to produce ammonia. The reaction conditions of the H-B method are quite intense (pressure ranges from 10-30MPa and temperature ranges from 350-550℃), leading to a serious conflict between thermodynamics and kinetics. The reaction is difficult to occur at high temperatures. However, the low reaction temperature leads to a slow formation rate of ammonia, which results in a relatively low overall yield of ammonia[9]. A lot of research has been devoted to the development of efficient catalysts for ammonia synthesis. At present, the catalysts commonly used for ammonia synthesis by the Haber-Bosch method include Fe3O4 catalyst, Ru catalyst and Fe1-xO catalyst[10-12]. The synthesis of ammonia from N2 and H2 under mild conditions is one of the most challenging topics in the field of the Haber-Bosch method for ammonia synthesis, particularly for the traditional ammonia synthesis industry. In addition, the harsh reaction conditions not only impose higher demands on for the reactor but also necessitate the use of a significant amount of fossil fuels for hydrogen production and energy supply, leading to CO2 emissions. Statistics show that the synthetic ammonia industry emits 500 million tons of CO2 per year, accounting for 1.8% of global CO2 emissions[13]. It is important to realize green and low energy consumption synthesis of ammonia, implement energy-saving and carbon reduction transformation and develop green and low carbon energy production technologies for the future development of the ammonia industry.
At present, the green ammonia synthesis process can be achieved through electrocatalysis, photocatalysis and chemical looping technology. On the one hand, green ammonia synthesis technology is characterized by ability to obtain the hydrogen source through environmentally friendly methods such as electrolyzing water or photolyzing water, or by using water instead of hydrogen to react with N2 to produce ammonia[14-16]. However, the ammonia yield is still low. On the other hand, it has the characteristic of zero carbon emissions in its energy acquisition process. Solar energy and other renewable energy sources can be used to supply energy for the entire system instead of fossil fuels. Due to the unstable and discontinuous characteristics of renewable energy, there are still many challenges in the practical application of ammonia synthesis driven by electricity or light. For example, the efficiency of ammonia production and catalyst stability are still low[17]. Chemical looping ammonia synthesis (CLAS) can decouple the synthesis of ammonia into multi-step reactions and optimize the sub-reactions individually, which is enable to achieve the synthesis of ammonia under mild conditions[18,19]. Furthermore, the CLAS process can operate in atmospheric conditions. Due to the flexibility of the chemical looping technology process, CLAS can be integrated with various processes, offering the potential for low energy consumption and high efficiency in ammonia synthesis. Based on the reaction mechanism of CLAS, this paper systematically discusses various processes involved in CLAS. The selection criteria for nitrogen carriers (NCs) are also discussed from the perspectives of thermodynamics and kinetics. The structure and reaction mechanisms of various types of NCs are reviewed, and the advantages and disadvantages of different NCs are dialectically analyzed. The design principles and regulatory strategies of NCs are also reviewed to provide guidance for the design and development of NC, optimize the reaction process, and accelerate the application of CLAS process. Finally, the existing CLAS process is summarized, and future development directions for the CLAS process are proposed.
2 CLAS
The chemical looping technology refers to the process of decoupling the overall reaction into multiple step-by-step reactions and optimizing each individual reaction sequentially to achieve the goal of optimizing the overall reaction. Each step reaction can occur in different spatial and temporal conditions[20-22]. The consumption and regeneration of the carrier are utilized to link the step reactions in series to accomplish a comprehensive reaction process. Among them, due to the different properties of the materials, they can be classified as NCs, oxygen carrier, sulfur carrier, carbon carrier and so on[23-25]. Recently, CLAS technology has become a research hotspot. It can achieve efficient synthesis of ammonia under relatively mild reaction conditions. CLAS technology utilizes NCs to circulate between the nitridation and ammonification reactors, enabling low-energy and efficient synthesis of ammonia[26]. The basic principle is shown in Figure 1. Compared with H-B method, CLAS technology decouples the original ammonia synthesis reaction into two reactions of nitridation and ammonification reactions. This decoupling allows for precise control of each sub-reaction. CLAS technology can also prevent the competitive adsorption of H2 and N2, addressing the conflict between thermodynamics and kinetics[27]. Due to the characteristics of CLAS technology, the entire ammonia production process becomes milder, leading to significantly improved selectivity and yield of ammonia. Furthermore, efficient synthesis of ammonia can be achieved under atmospheric pressure by utilizing the mass transfer properties of the NCs. And the NCs can utilize the heat transfer relationship between the nitriding reactor and the ammoniation reactor to achieve the self-heating operation of the system. CLAS technology can eliminate the reliance of the ammonia synthesis industry on fossil fuels and enable the realization of a truly green ammonia synthesis process[28-30]. The chemical looping technology is more diverse in terms of obtaining hydrogen and nitrogen sources because of its flexible process. In the realm of hydrogen source acquisition, apart from utilizing green hydrogen production methods like photolyzed water and electrolytic water production, it can also be integrated with biomass chemical looping gasification and biomass chemical looping hydrogen production process[31,32]. The use of a high concentration of N2 at the outlet of the air reactor, instead of the traditional air separation process, can significantly reduce energy consumption and cost[33-35]. Therefore, the CLAS comprehensively understands the ammonia energy industry chain, from green energy to synthetic ammonia to terminal utilization scenes, and develops a green synthetic ammonia process route.
|
Figure 1. Schematic diagram of CLAS. (Alkali metal (AM); metal elements (Me); two-step water (H2O-2step); three-step water (H2O-3step)).
The performance of NCs in CLAS will determine the efficiency of ammonia synthesis in the entire system. NCs can be categorized into covalent NCs, ionic NCs, transition metal (TM) nitride NCs, and other types of NCs (such as composite NCs and perovskite NCs) based on their composition and properties. Different types of NCs have distinct reaction processes, and their regulation and optimization strategies vary accordingly. In order to achieve precise regulation of NCs, it is essential to comprehend the thermodynamic and kinetic properties of NCs. Based on the thermodynamic and kinetic properties of NCs screen out potential NCs materials can be identified and the reaction mechanism can be determined. Finally, developing the complete CLAS process to expedite the implementation of CLAS in the industry.
3 CLASSIFICATION OF NCs
3.1 NCs for Chemical Looping Synthesis Ammonia Mediated by H2
The CLAS process using H2 and N2 for production ammonia can be divided into the reaction dominated by TM NC (H2-TM CLAS), the reaction dominated by alkali metal hydrides (H2-AM CLAS), and the process of CLAS dominated by some special structure compound for example anti-perovskite (A3BN) and two-component composite (TCC) NC (H2-A3BN/TCC CLAS). These three reaction processes all use H2 as the hydrogen source, but there are obvious differences in reaction mechanism, reaction conditions and preparation methods.
3.1.1 The NCs for H2-TM CLAS
After a hundred years of development, the synthesis of ammonia using the Haber-Bosch method remains an energy-intensive industrial process. There is a significant conflict between thermodynamics and kinetics in the reaction. The synthesis of ammonia is thermodynamically favorable at low temperatures; however, the low temperature hinders the reaction rate. The synthesis of ammonia typically occurs under severe reaction conditions. In order to enhance the yield of ammonia and create milder reaction conditions, extensive research has been dedicated to developing ammonia synthesis catalysts with outstanding performance[36]. The catalyst composed of TM is considered an ideal material for ammonia synthesis[37,38]. At present, the ammonia synthesis catalyst has gradually evolved from the initial commercial Fe-based catalyst to the current second-generation Ru-based catalyst and Fe1-xO type metal oxide catalyst[39,40]. With the gradual maturity of advanced characterization techniques and theoretical calculation methods, researchers have conducted in-depth research on TM catalysts. They have proposed design principles for TM catalysts, such as Fe, Ru, and Co-Mo, based on the Sabatier principle[41,42]. It is found that the activation energy of the TM catalyst for N2 is proportional to the adsorption energy of N2 on the catalyst surface, which conforms to the Brønsted−Evans−Polanyi relationship, as shown in Figure 2A. It can be seen from Figure 2B that the ammonia synthesis activity of TM exhibits a typical volcanic curve[43,44]. he former TM is located on the left side of the volcanic curve, making it easy to break N≡N bonds, while the latter TM is generally difficult to nitride due to the weak adsorption energy of N2. The material located in the center of the volcanic curve is considered to have excellent NC and ammonia production properties. During the CLAS process, N2 and H2 are alternately introduced into the reactor. This method fundamentally prevents the competitive adsorption of N2 and H2, thereby enhancing the interaction between nitrogen activation energy and adsorption energy of the TM catalyst. The valence states of TM are diverse, resulting in a variety of nitrogen-containing species and the ability to accommodate different contents of lattice nitrogen[45,46]. The transformation between the nitrides facilitates the migration of lattice nitrogen. Therefore, TM NCs are also considered to be the most promising materials at present.
|
Figure 2. Activity of transition metal catalysts for ammonia synthesis. A: The relationship between the activation energy of N2 and the dissociation energy of N2 on the catalytic surface. Reproduced from Ref.[44] with permission from American Chemical Society. B: The relationship between the conversion frequency and the adsorption energy of nitrogen. Reproduced from Ref.[43] with permission from Elsevier.
TM NCs can be categorized into TM nitride NCs and TM oxide NCs. TM nitrides typically use hydrogen as a hydrogen source to synthesize ammonia, whereas TM oxide NCs use steam instead of hydrogen for synthesizing ammonia. This section mainly introduces the research progress of TM nitride NC. Compared with other NCs, TM nitride NCs can easily convert between nitrogen-rich and nitrogen-poor compositions. The poor NCs is nitrided in the nitridation reactor, and then the rich NCs are reduced by hydrogen in the ammonia production reaction to achieve a complete process of CLAS. The reaction process is illustrated by Equations 1 and 2.
TMaNb+3x/2H2→TMaNb-x+xNH3 (1)
TMaNb-x+x/2N2→TMaNb (2)
At present, there are three preparation methods for TM nitride NCs. (1) The ammoniation method involves the calcination and reduction of metal oxides or metal salt precursors in NH3. The NCs prepared by this method have a single crystal phase and high crystal purity. However, ammonia is the target product. When ammonia is added to the reaction medium, it will decrease the reaction efficiency of the process. (2) The pyrolysis method refers to the use of nitrogen-containing organic compounds and metal precursors for co-pyrolysis. Nitrogen-containing organic compounds mainly included melamine, urea and urotropine. Compared with ammonia hydrolysis, this method uses nitrogen-containing precursors instead of NH3, making the process more economical. (3) Mechanical mixing-pyrolysis is a method used to produce metal nitrides by pyrolyzing metal source materials and nitrogen-containing molecules after mechanical grinding and stirring. Its advantage is that the operation process is convenient and the steps are simple. However, solid-state mixing is evidently not as uniform and dispersed as the system produced by liquid/liquid mixing or liquid/solid mixing. This disparity will further impact the morphology and application performance of the product.
3.1.1.1 Binary TM Nitride NCs
Binary TM nitride NCs are that the NCs contains only one transition Me. For this type of NC, transition Me like Mn, Fe, and Mo show moderate adsorption strength for N2, aligning with the Sabatier principle and attracting considerable attention[47,48]. In the process of heterogeneous catalytic synthesis of ammonia, the Sabatier principle is usually considered an important criterion for screening catalysts for ammonia synthesis. This principle can also be used as a reference to screen appropriate NC. According to the thermodynamic calculations, the nitridation and hydrogenation reactions of certain TM nitrides. As shown in Table 1 it is found that the thermodynamic properties of the TMs are different. Among them, the nitridation reaction of Mn is a thermodynamically favorable reaction, but its ammonia production reaction is a thermodynamic climbing reaction. The late TM exhibits diametrically opposite properties, while Fe exhibits diametrically opposite properties as NC. However, molybdenum as a pre-TM is thermodynamically favorable for the nitridation and reduction reactions at low temperatures, as shown in Figure 3.
Table 1. TM NCs Nitridation and Ammonia Production Reaction
Nitridation |
Amination |
Fe2N+3H2(g)=2Fe4N+2NH3(g) (E9) |
|
2Fe4N+N2(g)=4Fe2N (E2) |
2Fe4N+3H2(g)=8Fe+2NH3(g) (E10) |
8Mn+N2(g)=2Mn4N (E3) |
5Mn3N2+6H2(g)=3Mn5N2+4NH3(g) (E11) |
10Mn4N+3N2(g)=8Mn5N2 (E4) |
8Mn5N2+9H2(g)=10Mn4N+6NH3(g) (E12) |
3Mn5N2+2N2(g)=5Mn3N2 (E5) |
2Mn4N+3H2(g)=8Mn+2NH3(g) (E13) |
4Mo+N2(g)=2Mo2N (E6) |
4MoN+3H2(g)=2Mo2N+2NH3(g) (E14) |
2Mo2N+N2(g)=4MoN (E7) |
2Mo2N+3H2(g)=4Mo+2NH3(g) (E15) |
6Co+N2(g)=2CoN (E8) |
2Co3N+3H2(g)=6Co+2NH3(g) (E16) |
|
Figure 3. The change trend of Gibbs free energy of (A) Fe, (B) Mn, (C) Mo, (D) Co nitridation reaction and H2 reduction reaction with temperature.
Studies have shown that manganese can form nitrides such as Mn4N, Mn5N2, Mn2N, Mn2N0.86, Mn3N2 and MnN under a nitrogen atmosphere. This property gives manganese a high nitrogen capacity, making it suitable for CLAS[49]. Laassiri et al.[50] found that although manganese has a good nitrogen holding capacity, its reaction performance is limited. Only 3.1% of lattice nitrogen in Mn3N2 can be converted into ammonia, and most of the lattice nitrogen is released in the form of N2. On the one hand, this is because the reaction temperature is too high to cause NH3 to decompose into N2. On the other hand, due to the weak interaction between Mn-N, the lattice nitrogen is released in the form of N2. However, the use of metal doping can promote more lattice nitrogen to NH3. When Li+ is doped, the low-temperature ammonia production characteristics of manganese-based NC are enhanced. Ammonia can be generated at 400℃, and the conversion ratio of lattice nitrogen to NH3 is increased to 15%. The process of atom doping can effectively inhibit the regeneration step of N2 and enhance the conversion rate of lattice nitrogen.
However, when Fe, K and other atoms are doped into the manganese-based NC, the yield of NH3 does not increase significantly. In addition, the electronic structure of manganese-based NC can be adjusted through doping or alloying. By doing so, the stability of lattice nitrogen in NC which in turn can affect the performance of ammonia production. Although the heteroatom deposition method is expected to enhance the reaction performance of manganese-based NC, further exploration of this method is still necessary. The role of TM alloying has been verified in the field of catalysis. Two types of TM alloying can create numerous reactive sites at metal interfaces, facilitating the nitriding process and ammonia production through interface interactions. The methods of alloying and heteroatom doping have a significant influence on the electronic structure and properties of TM. This influence is expected to enhance the reaction performance of TM nitrides. According to DFT calculation results, 3d TM can decrease the stability of lattice nitrogen. Moreover, the higher the proportion of 3d electrons in TM, the lower the formation energy of lattice nitrogen[51].
At present, on the one hand, it is believed that Fe-doped manganese-based NC can alter the electronic structure of manganese and decrease the diffusion energy barrier of lattice nitrogen. However, the experimental results show that the ammonia production performance of Fe-doped manganese-based NC is not as good as that of single manganese-based NC. Wang et al.[52] found that nitridation at 800℃ and reduction at 600℃ will cause the sintering of the NC and affect its cyclic ammonia production performance. When 50wt% Al2O3 is used as the carrier, it can effectively disperse the active component and prevent the sintering of the NC. The addition of Al2O3 can promote the conversion of lattice nitrogen to NH3 and increase the content of effective lattice nitrogen in NC. As shown in Figure 4, the addition of Al2O3 to the Me-Fe NC resulted in a significant increase in ammonia production. In addition to using Al2O3 as an inert carrier, other materials with good thermal stability and the ability to promote H2/N2 dissociation are utilized as inert carriers to disperse the active phase. While inhibiting the sintering of NC, the ammonia yield increases, and the cycle stability of NC is enhanced.
|
Figure 4. NH3 yield of Mn-based, Fe-based and MneFe-based N-carriers with 2% and 50% Al2O3 per gram of Mn at 600℃. Reproduced from Ref.[52] with permission from Elsevier.
The high temperature of the H2 reduction reaction of manganese-based NC leads to the significant decomposition of NH3. How to decrease the temperature of the reduction reaction while maintaining the ammonia yield unaffected is also a challenge to be addressed in the future. Manganese is positioned on the right side of the volcanic curve, making it prone to reacting with N2 to produce corresponding nitrides. However, its low reactivity in reduction reactions hinders the advancement of manganese-based NC. Fu et al.[53] reduced the stability of the Mn-N bond through Ni doping, which effectively enhanced the cyclic ammonia production performance of manganese-based NC. From Figure 5, it is not difficult to find that the low concentration Ni-doped manganese-based NC exhibits high hydrogenation activity and selectivity. At 750℃, the effective conversion rate of lattice nitrogen in 80Mn20Ni-N reaches 36.1%, which is 15.7% higher than that of undoped manganese-based NC. The introduction of Ni can enhance the bulk migration and interfacial reaction of lattice nitrogen by increasing the d-orbital electrons. This can achieve the best kinetic matching between the two, thereby improving the activity and selectivity of converting lattice nitrogen to NH3.
|
Figure 5. N mass balances of Ni-Mn-N NCs with different Ni/Mn ratios at different temperatures. A: 550℃; B: 600℃; C: 650℃; D: 700℃; E: 750℃; F: Effective conversion of lattice nitrogen for composite NCs at different temperatures. Reproduced from Ref.[53] with permission from Elsevier.
This method of using TM doping to improve the reaction performance of NC has also been proven in CrN. Wang et al.[54] modified the different contents of Co and Ni on the CrN NC prepared by ammonolysis. The synergistic effect between this TM and CrN greatly improved the lattice nitrogen utilization of the NC. The single CrN reaction is extremely inefficient, with only 4.5% of the lattice nitrogen able to react with H2. As shown in Figure 6, after loading cobalt onto CrN, the lattice nitrogen conversion of the composite NC reached 50.7%, and the ammonia selectivity reached 98.1%. In addition, Co-CrN exhibits excellent cycling stability, and the ammonia production rate of NC can still reach 466.1μmol·g−1·h−1 in 12 cycles, which is ten times higher than that of single CrN.
|
Figure 6. CLAS performance of Cr-based NCs. A: The average NH3 production rate of the pristine CrN and TM-CrN under an alternative flow of N2 and H2 700℃, WHSV=60,000ml·g−1·h−1; B: The average NH3 synthesis rate of TM-CrN as a function of temperature in CLAS; C: The temperature dependent activity of Co-CrN with different Co loading. Error bars denote the standard deviation of the recorded rates in at least three separated tests; D: Cyclic CLAS performance of 10wt% Co-CrN at 700℃, 1 bar. Reproduced from Ref.[54] with permission from Elsevier.
In addition, another widely concerning issue is molybdenum-based NC. The reaction conditions for molybdenum-based NC are milder than those for manganese-based NC. The ammonia yield of molybdenum-based NC prepared by pyrolysis is 4576μmol·g−1·h−1 at 450℃, which is three times higher than that of Ru-based catalysts under the same conditions[55], as illustrated in Figure 7A and B. As shown in Figure 8, there is no obvious deactivation of Mo2N in 8 cycles, and the ammonia yield remains stable, indicating promising application prospects for molybdenum-based NC. However, the nitriding process of Mo2N is relatively slow, which can facilitate the nitriding of NC after reduction under the action of H2. According to the DFT calculation results, hydrogen can reduce the reaction energy barrier of re-Mo2N and promote the nitridation of NC. However, the specific nitridation mechanism still requires further study.
|
Figure 7. Reaction characteristics of Mo based NCs for ammonia synthesis. A: The relationship between ammonia yield and time of Mo2N carrier at different reduction temperatures; B: Ammonia yield of Mo2N at different temperatures. Reproduced from Ref.[55] with permission from Elsevier.
|
Figure 8. Cyclic ammonia production performance of Mo2N. Reproduced from Ref.[55] with permission from Elsevier.
3.1.1.2 Ternary TM Nitride NCs
The ternary transition nitride NC can compensate for the performance deficiencies of single metal NC. For example, the combination of Mn which is not easily nitrided, and Fe which is not easily converted into ammonia, the construction of Fe-Mn bimetallic NC can help improve the characteristics of single Fe-based NC, which is not easily reduced, while promoting the nitriding of Mn. In addition, it is not difficult to find from the volcanic curve that CoMo has a moderate bond energy for ammonia synthesis, which is closer to the center of the curve. At present, it has been successfully proven that Co6Mo6N can produce Co3Mo3N in N2 atmosphere, and Co3Mo3N can be reduced by H2 to generate NH3[56,57]. According to isotope tracer experiments, it was found that Co6Mo6N can convert nitrogen in N2 into active lattice N, and the active lattice nitrogen generated is transformed into NH3 in the presence of H2. However, there is no unified theory for the preparation of ternary TM nitrides. At present, only Co3Mo3N has been reported as a ternary TM material that can be used for CLAS. Through the study of Fe-Mn and Co-Mo composite NC, it has been found that combining front TM with back TM is a common strategy for constructing composite TM NC. In terms of reactivity, the former TM is not easily nitrided but tends to produce ammonia at low temperatures, while the nitriding and reduction properties of the latter TM are completely opposite. Therefore, the combination of front and back TM can compensate for each shortcoming.
3.1.2 Discussion for H2-TM CLAS
TM NC are considered to be the most promising NC materials due to their moderate reaction temperature and variable valence. However, improving the yield of ammonia remains an important issue that needs to be urgently addressed. For a single-component TM NC, a reasonable design strategy is needed to enhance reactivity and cycle stability. On the one hand, it is necessary to reduce the formation energy of nitrogen vacancies and promote nitrogen fixation in NC. On the other hand, it is necessary to weaken the metal-nitrogen bond strength and promote the extraction of lattice nitrogen by H2. As the number of cycles increases, a single TM NC usually sinter due to agglomeration and structural collapse, which affects the ammonia production performance of the NC. This is due to the conflict between reaction kinetics and thermodynamics. The lower reaction temperature NC has a slower ammonia production rate, necessitating an increase in temperature to enhance the kinetic properties. Selecting the appropriate inert component to disperse the active phase can not only prevent the sintering of the NC, but also improve the reaction kinetic properties. Therefore, exploring the specific mechanism of the inert component in the active phase can guide the design of high-stability NC.
Secondly, due to the poor thermal stability of single component NC without inert carrier loading, the active components agglomerate at high temperatures. The composite TM NC is still in its infancy. Further optimization of the preparation method of NC and exploration of the nitridation mechanism and ammonia production of NC are areas of concern. Currently, the conversion rate of lattice nitrogen in NC is generally low. How to improve the effective conversion rate of lattice nitrogen is also a challenge that needs to be addressed in the future design and regulation of NC.
3.1.3 The NCs for H2-Alkali Metal CLAS (H2-AM)
Ionic NC can be divided into AM/alkaline earth metal hydride NC and AM/alkaline earth metal nitride NC. The process of CLSA dominated by ionic NC is usually thermodynamically feasible and has good kinetic performance[58]. Compared with TM and covalent NC, ionic NC have milder reaction conditions, but their stability is poor and the preparation conditions are harsh. At present, the hydrogenation of AM is typically carried out using high-pressure H2 or reducing agent reduction methods, which can make the preparation process hazardous[59,60].
The AM/alkaline earth metal hydride NC generates an AM/alkaline earth metal imino compound under the action of N2, and then produces ammonia and AM/alkaline earth metal hydride under the action of H2 to complete the cycle, as illustrated in Equations 3 and 4. TM NC use TM as electron donors to activate nitrogen, while AM/alkaline earth metal hydride NC use negative valence H as electron donors to activate N2. Guan et al.[61] found that a hydrogen vacancy in BaH2 is key to activating N2. The presence of a hydrogen vacancy enhances the efficiency of ammonia production in NC compared to that of the thermal catalytic process under similar conditions. As shown in Figure 9, the ammonia production rate of BaH2 can reach 3500μmol·g−1·h−1, which is ten times higher than that of the catalytic process.
4AHx+xN2=2xA2/xNH+xH2 (3)
xA2/xNH+2xH2=2AHx+xNH3 (4)
|
Figure 9. N2 fixation and ammonia synthesis over BaH2. A: Comparison of NH3 production rates of BaH2 at 723K and 1 bar via thermocatalysis chemical looping processes. The inserted figure is the enlarged view of the catalytic activity of BaH2. Reaction conditions: sample loading, 30mg; flow rate, 30mL·min-1; B: TG measurement of BaH2 in a flow of N2/H2 syngas (N2, 99%, H2%) or pure N2 with a ramping rate of 5K·min-1; C: XRD patterns for a self-made BaH2, b BaH2 sample collected after nitridation in a flow of N2/H2 syngas (N2 99%, H2%) at 723K for 3h, and c Ba sample collected after nitridation in a pure flow at 723K for 3h; D: Temperature dependence of N production rates of BaH2 via a chemical looping or catalytic (N2/H2=5/2) process. Reaction conditions: sample loading, 30mg; flow rate, 30mL·min-1; total pressure, 1 bar. Reproduced from Ref.[61] with permission from Wiley.
Although this process has excellent reactivity, H2 must be used as the hydrogen source. The formation of ammonia requires the interaction between lattice nitrogen and H2. In addition, the AM/alkaline earth metal hydride NC can facilitate the synthesis of ammonia under mild reaction conditions[62]. LiH and BaH2 can be nitrided at 400℃ and 250℃, respectively. Li2NH and BaNH can be hydrogenated to produce ammonia at 300℃ and 200℃, making the reaction process very mild. Gao et al.[63] found that doping TM atoms into LiH and BaH2 can enhance the nitridation of NC and increase the rate of ammonia production through hydrogenation. Among them, Ni+ has the most obvious promoting effect on NC, and Ni+ is considered to be a NC with weak catalytic activity in the process of thermocatalytic ammonia synthesis. This is because H2 and N2 enter the reactor alternately to prevent the competitive adsorption of H2 and N2 on the surface of the NC. This reduces the adsorption energy barrier of N2 on the surface of the NC, allowing Ni+ to facilitate the nitridation of the NC. Compared with typical ammonia synthesis catalysts like Cs-Ru/Mg in Figure 10, the reaction conditions of the AM-dominated process of CLAS are very mild and can be carried out at atmospheric pressure.
|
Figure 10. Comparison of NH3 production rates of Ni-catalysed AH-CL (1 bar) and the conventional thermo-catalytic process (10 bar). The activities of Co3Mo3N and Fe-K2O-Al2O3 were taken from Ref.[63] with permission from AIP publishing.
Compared with other NCs, the reaction conditions of CLAS dominated by Li2NH and BaNH are relatively mild and can be carried out at 100℃ and atmospheric pressure. Although the AM/alkaline earth metal hydride NC has excellent reaction performance and low-temperature ammonia production, the AM hydride has poor thermal stability and high preparation cost, which is not conducive to large-scale production. The preparation process of AM/alkaline earth metal hydrides is usually carried out in a glove box, and the corresponding hydrides are obtained by using H2 to reduce AM/alkaline earth Me. Although the AM/alkaline earth metal hydride NC reaction conditions are mild and the ammonia production performance is excellent, the preparation process requires the use of hydrogen, which significantly diminishes the process economy. Therefore, the development of AM dominated CLAS technology by green hydrogen can improve the process economy.
3.1.4 Discussion for H2-AM CLAS
The ion-type NC has the advantages of mild reaction conditions, normal temperature, and pressure. It can not only synthesize ammonia but also achieve a high ammonia yield, reaching the thermodynamic limit of the original reaction when other energy forms are introduced. However, the instability of the ionic NC itself and the high preparation costs limit their application. Therefore, the development of low-cost preparation methods and the improvement of the thermal stability of ionic NC need further consideration in the future.
3.1.5 The NCs for H2-A3BN/TCC (H2-A3BN/TCC) CLAS
In addition to the three types of NC mentioned above, composite NC with dual active components and perovskite NC with special structures have gradually attracted attention. Feng et al.[64] Prepared Li2NH-Mn2N and BaNH-Mn2N NCs by combining ionic NC with TM nitride NC. The two NC promote each other in the reaction process. As a NC, Mn2N can reduce the activation energy of the reaction involving Li2NH, BaNH and H2, thereby promoting the formation of ammonia. Li2NH and BaNH can also facilitate the nitridation and hydrogenation of Mn2N. This enables the composite NC to efficiently synthesize ammonia at 0.1MPa and 300℃. The efficiency of ammonia production is superior to that of the Cs-Ru/MgO catalyst under identical conditions, as illustrated in Figure 11. However, the reaction mechanism of the composite NC material, consisting of ionic and TM nitrides, still requires further exploration through advanced characterization methods and theoretical calculations. In addition, the alloying of Me can also adjust the nitrogen loading and ammonia production properties of NC. The alloying of Me can alter the characteristics of a single Me, modify its original electronic properties and structure, and create a new reactive center for reactions to occur. Yamaguchi et al.[65] successfully synthesized ammonia under mild conditions using a Li-Sn alloying strategy. Ammonia signal was successfully detected in mass spectrometry, as shown in Figure 12. Li17Sn4 can be nitrided at 500℃ and 0.1MPa, resulting in the production of ammonia at 300℃ in atmospheric H2. Compared with Li as a NC alone, the preparation strategy of alloying makes the reaction conditions of Li-Sn alloy NC milder, and the process does not require additional energy input to overcome the thermodynamic energy barrier. However, the stability of the cycle and the reaction mechanism of the NC after alloying require further exploration. A corresponding alloying strategy is proposed to guide the development and design of the NC.
|
Figure 11. Chemical looping ammonia production (CLAP) rates of different N carriers. A: Temperature-dependent ammonia production rates of LiH, BaH2, Mn4N and Mn4N-AH samples via chemical looping. Reaction conditions: WHSV ¼ 60000ml·g-1·h-1, P ¼ 1 bar; B: Comparison of ammonia production rates of Mn4N-AH via chemical looping and thermo-catalytic processes catalyzed by Mn4N-AH. The catalytic activity of Cs-Ru/MgO at 10 bar of syngas (N2:H2 ¼ 1:3) was also shown as a reference catalyst. Reproduced from Ref.[64] with permission from Royal Society of Chemistry.
|
Figure 12. TG-MS profiles of Li17Sn4 under 0.1MPa of (a)N2, (b)H2, and (c)Ar flow conditions. Reproduced from Ref.[65] with permission from Elsevier.
CLAS decomposes the total reaction of ammonia synthesis, as shown in Equation 5, into multiple independent step-by-step reactions. The inclusion of reaction intermediates enhances the energy efficiency of the reaction process and reduces the thermodynamic energy barrier for the reaction. Therefore, determining the appropriate transfer medium and screening the suitable nitrogen-loaded and ammonia-producing materials can not only optimize the reaction conditions but also enhance the equilibrium yield of ammonia. At present, under an operating pressure of 150 bar, the equilibrium yield of ammonia in a commercial ammonia synthesis catalytic device reaches 28%. This implies that the equilibrium yield of CLAS should exceed 38% under the same energy consumption. At the same cost, the ammonia equilibrium yield of the CLAS process exceeds 26%, making it competitive[66]. Among them, the ammonia yield of 26% is only a conservative estimate, and the actual cost of the reactor cannot be calculated due to insufficient data on the expenses associated with the chemical looping synthesis of ammonia reactor. Based on the above criteria, the Gibbs free energy of each distribution reaction can be calculated to determine the equilibrium yield of the NCs at different reaction temperatures and pressures and identify the appropriate operating conditions.
N2+3H2=2NH3 (5)
Recently, the thermodynamic properties of the process of CLAS mediated by AM and TM NC have been reported. In the thermodynamic evaluation of LiH/Li2NH, MgH2/MgNH, CaH2/CaNH, SrH2/SrNH, BaH2/BaNH and other AM NCs oxidation-reduction pairs, only LiH/Li2NH meets the requirement of NH3 equilibrium yield greater than 26%. As shown in Figure 13 under the reaction temperature of 250-450℃ and a reaction pressure of 150 bar, NCs located below the line exhibit a higher ammonia yield. The LiH/Li2NH compound demonstrates excellent thermodynamic properties with lower solid reaction heat and higher reaction entropy. Laron Burrows conducted a thermodynamic evaluation of 14 CLAS processes[67]. Compared with other NCs, the thermodynamic properties of Ca3N2/CaH2, SrH2/Sr3N2, MnO2/Mn5N2 and MoO2/Mo2N are superior. By analyzing the nitridation and reduction reactions of Ca3N2/CaH2, SrH2/Sr3N2, MnO2/Mn5N2 and MoO2/Mo2N, it is found that the nitridation and reduction processes of Ca-based NC and Sr-based NC are typical exothermic and endothermic reactions, respectively. In contrast, the heat relationship of nitridation and reduction reactions dominated by MnO2 and MoO2 is opposite. The heat relationship of 14 CLAS reactions is summarized. This is due to the different reaction processes dominated by the two types of NC. Through thermodynamic analysis of the CLAS reaction to determine the heat involved in each step of the reaction, and subsequently using the kinetic method to establish the reaction rate of each step, is a common approach in studying the NCs.
|
Figure 13. Minimum ΔHsolid and ΔSsolid are represented by the plotted lines. Pairings situated below or to the right of these lines have properties enabling them to have comparable or better performance than the conventional process. Three temperatures are considered for the benchmark at 150 bar. Reproduced from Ref.[67] with permission from Elsevier.
At present, there are few detailed studies on the kinetics of the nitridation reaction of NCs. The nitridation reaction is typically an exothermic process. The high reaction temperature will affect the degree of nitridation of NC, while a low reaction temperature will impact the rate of nitridation. CLAS is typically conducted under atmospheric pressure, and the reaction pressure is generally not taken into account. In addition to the influence of reaction conditions, the particle size, composition, and nitrogen fixation method of NC will affect the kinetics of the nitridation reaction.
The nitrogen fixation methods of NCs include reduction gas and N2 synergistic nitrogen fixation, solid reducing agent and N2 synergistic nitrogen fixation, and nitrogen fixation process using only N2. Due to the competition between N2 and other gases, the specific reaction kinetic mechanism needs further determination. Secondly, the particle size of NCs affects the diffusion and mass transfer processes of gas, thereby influencing the rate of nitrogen fixation rate[68,69]. At present, it is generally believed that as the particle size increases, the nitriding reaction becomes increasingly dependent on pressure. The nitriding reaction is typically conducted at atmospheric pressure, indicating that the adsorption on the surface of larger particles decreases. Conversely, smaller particles tend to agglomerate at higher reaction temperatures, impacting the nitrogen fixation of t NCs[70]. Therefore, choosing the appropriate size of the NCs particles is expected to improve the nitridation reaction kinetics. In addition, it is also expected to enhance the nitridation and reduction reaction kinetics of NCs by selecting suitable metal oxide carriers and loading appropriate additives. When the Mn4N-LiH/BaH2 composite NCs were prepared, it was found that the addition of LiH and BaH2 could significantly reduce the activation energy of Mn2N nitridation and reduction reactions, and promote the reactions of nitrogen fixation and ammonia production. As shown in Figure 14, when LiH and BaH2 participate in the reaction, the activation energy of the ammonia production reaction decreases by half compared to the original value.
|
Figure 14. Activation energy of N2 fixation over Mn4N-LiH and Mn4N-BaH2. (A) and (CV) The temperature-dependence of the N2 fixation rate based on TG results. (B) and (D) Arrhenius plots over LiH, BaH2, Mn4N-LiH and Mn4N-BaH2 samples. Reaction conditions: PN=1bar. Reproduced from Ref.[64] with permission from Royal Society of Chemistry.
The research on the kinetics of the reaction involving the reduction of ammonia production should be explored across various reaction routes. For the process of CLAS with H2 as the hydrogen source and water vapor as the reactant, the gas diffusion and mass transfer modes on the particle surface should be determined initially. Secondly, the various rate-limiting steps of the hydrogen source and the surface should be determined. Subsequently, the kinetic model of the reduction reaction should be established, providing a foundation for the design of more advanced NC materials.
In addition to the improvement strategy of alloying, the preparation of NC with a special structure can also enhance the reactivity of NC. Goto et al.[71] prepared a NC with an A3BN. The NC precursor can be aminolyzed in NH3 to obtain a NC with an A3BN structure. Four NCs, Co3ZnN, Ni3ZnN, Co3InN, and Ni3InN were prepared using this method. At 400℃, the ammonia yield was 3069, 2925, 289, and 1029μmol NH3·g-1, respectively. This indicates that the A3BN NC was not only easily reduced by H2 but also had a good ammonia yield. However, during the cyclic regeneration experiment conducted on TG analysis, the author discovered that only Ni3ZnN and Ni3InN were renewable. The specific reaction mechanism was not further verified.
3.1.6 Discussion for H2-AB3N/TCC CLAS
The development of composite NC is one of the most effective methods to enhance the reactivity of NC, and the composite mechanism should be determined. First of all, the NC with the same thermodynamic change trend can be combined with each other. Materials with similar thermodynamic properties exhibit comparable reaction temperature ranges and reaction conditions. Secondly, the kinetic properties are similar. The reaction rate of composite NC is similar under the same reaction conditions. Finally, while the composite NC exhibits good reaction performance, ensuring its stability remains a challenge that requires further consideration. In addition, the design of NC with special structures, such as the A3BN structure, not only ensures structural stability but also enhances reaction efficiency. This design can effectively address the issues of low ammonia production efficiency and poor cycle stability associated with NC. However, the NC with an A3BN structure is still in its early stages of development, and the selection of A-site and B-site Me, as well as the corresponding reaction mechanism, still need optimization.
3.2 NCs for Chemical Looping Synthesis Ammonia Mediated by H2O
In addition to the process of CLAS with H2 as the hydrogen source, the process of CLAS can also use H2O as the raw material for ammonia synthesis. This process can be divided into a H2O-2step CLAS process (H2O-2step CLAS) mediated by a covalent NC and a H2O-3step-based CLAS process (H2O-3step CLAS) mediated by a TM oxide and alkali oxide.
3.2.1 The NCs for H2O-2step CLAS
Among many covalent NC, Al2O3/AlN has received extensive attention due to its good thermal stability. Steinfeld et al.[72-74] proposed that the use of Al2O3 as a NC is expected to replace the H-B method for ammonia synthesis. It has been found that AlN can be produced through carbothermal reduction of Al2O3 at high temperatures in N2 atmosphere. The hydrolysis of AlN can produce ammonia and Al2O3, which can then be utilized in subsequent reaction processes to enable cyclic ammonia production. Compared with the H-B method, utilizing the carbothermal reduction process of Al2O3 to produce ammonia can significantly lower the reaction pressure, ensuring production safety. Secondly, using steam instead of H2 can reduce energy consumption and CO2 emissions. The by-product CO can be utilized as a raw material for methanol synthesis intermediates or Fischer-Tropsch products[75,76]. In the H2O-2step CLAS process, the carbothermal reduction reaction of Al2O3 is a strongly endothermic reaction that needs to occur at a higher reaction temperature (>1500℃), as shown in Equation 6. This process can utilize solar energy as a heat source to substitute fossil fuels for heating and decrease energy consumption. Additionally, the hydrolysis process of AlN is an exothermic reaction, as demonstrated in Equation 7. Other chemical looping technologies, such as the chemical looping combustion or gasification processes, utilize oxygen carriers that serve not only for oxygen release and catalysis but also as heat carriers to heat the system and sustain the self-heating equilibrium of the system[23]. Therefore, in the H2O-2step CLAS process, exploring how Al2O3 can utilize the heat relationship between the two-step reactions to sustain the self-heating operation of the system is a future research challenge.
Al2O3+3C+N2=2AlN+3CO (6)
2AlN+3H2O=Al2O3+2NH3 (7)
In order to further optimize the reaction process and improve the ammonia yield, a large number of studies have been devoted to exploring the influencing factors of Al2O3/AlN in the process of CLAS. These studies focus on optimizing the reaction conditions, regulating the composition and structure of NCs, and exploring the influence mechanism of the reducing medium on NC. By exploring the influence of different configurations of Al2O3 on the reaction, it was found that γ-Al2O3 had the best reaction efficiency among γ-Al2O3, Al(OH)3 and α-Al2O3[77]. Under the influence of graphite, the conversion rate from γ-Al2O3 to AlN was the highest. At the same time, the addition of additives such as CaF2 and Y2O3 can form aluminosilicate with Al2O3 to reduce the temperature of the carbothermal reaction and make the reaction conditions more moderate[78]. In addition to exploring the effect of Al2O3 structure and additives on the nitriding reaction. Zhang et al.[79] found that carbon black has better adsorption performance for N2 than graphene when investigating the carbothermal reduction process of Al2O3 using different reducing agents. At 1200℃, when the molar ratio of Al2O3 to C is three, the reaction efficiency is optimal.
The greater the disorder of carbon in the reducing agent, the more effective the reaction. Both coal char and biomass char can replace carbon black and graphite as the reducing agent. Among these, the relatively low price of biomass char has greater application potential. Biomass char can be converted into CO to enhance the utilization rate of biomass. However, with the increase in reaction temperature, the porosity of NC is significantly reduced. The collapse of the NC structure is the primary reason for the decrease in the cycling performance of aluminum-based NC. Therefore, by modifying the NC, the structural stability of the NC can be improved while maintaining the pore structure and specific surface area of the material. Xiong et al.[80] prepared silicon modified aluminum-based NC using the co-precipitation method. When the molar ratio of Si to Al is 1: 20, the NC has good thermal stability, which can inhibit the sintering of the NC and stabilize its structure. It is not difficult to find from Figure 15 that the modified NC exhibits a significant sintering phenomenon after the initial reaction. It is necessary to enhance the stability of the NC in the cyclic adsorption/desorption process by strengthening the pore structure and preventing sintering. When Si is added to the NC, there is no significant difference in the surface morphology of the fresh NC compared to the surface morphology of the NC after the cyclic reaction. The addition of Si increases the stability of the NC structure. In addition to the deterioration of the NC cycle performance caused by structural changes, promoting the directional conversion of other configurations of Al2O3 to γ-Al2O3 can also increase ammonia yield.
|
Figure 15. The structure of NC in cyclic experiment. A: SEM micrographs at different stages (N-sorption reaction:1200℃, 1h; N-desorption reaction: 1000℃, 1h); B: EM micrographs at different stages (N-sorption reaction:1200℃, 1h; N-desorptionreaction: 1000℃, 1h). Reproduced from Ref.[80] with permission from Elsevier.
At present, there are few studies on the process of hydrolysis and ammonia production. Most studies focus on enhancing the ammonia yield by incorporating additives. In general, the hydrolysis reaction rate of AlN is slow, and the addition of additives can reduce the activation energy of the reaction to increase the ammonia yield. Wu et al.[81] first proposed that the activation energy of the AlN hydrolysis reaction can be reduced by adding 5wt% Fe2O3, resulting in a 30% reduction in the activation energy of the reaction. When ZrO2 is used as a carrier, the generated NH3 molecules can be adsorbed by ZrO2, thereby preventing the decomposition of NH3 to N2 at high temperatures[82]. When ZrO2 was added to Al2O3 the yield and selectivity of NH3 increased by 85.6% and 53.6 %, respectively. It can be inferred from Equation 7 that promoting the decomposition of steam to generate hydroxyl radicals can increase the ammonia yield. During the photocatalytic hydrolysis process for hydrogen production, TiO2 can facilitate the generation of hydroxyl radicals from water. When TiO2 is used as the carrier for aluminum-based NC, the conversion rate of AlN and the yield of NH3 increase with the rise in TiO2 content and water vapor concentration. At present, the properties of NH3, which easily decomposes into H2 and N2 at high temperatures, will affect the yield of ammonia. Usually, the hydrolysis temperature of AlN is 900℃. Reducing the hydrolysis reaction temperature and inhibiting the decomposition of NH3 can also increase the yield of ammonia. To reduce the reaction temperature, increase the yield of ammonia, and inhibit ammonia decomposition while ensuring the reaction rate, further consideration is required.
3.2.2 Discussion for H2O-2step CLAS
When Al2O3 is used as the NC, the H2O-2step CLAS process, it can not only be operated under atmospheric pressure, but the NC can also be recycled. Compared with the traditional H-B method, this approach avoids the potential safety hazards caused by high-pressure reactions and reduces the requirements for the reactor. In addition, Al2O3 can replace some metal catalysts, such as ruthenium, making the process more efficient. The focus of future research will be on how to design the NC reasonably and reduce the reaction temperature. Decreasing the reaction temperature, designing the reactor rationally to utilize renewable energy as the heat source, and optimizing the energy supply method contribute to the industrialization process of the procedure. Secondly, the poor cycling stability of aluminum-based NC will affect their scale-up process. Therefore, ensuring the continuous and stable production of ammonia can be achieved by enhancing the stability of the NC structure and maintaining the crystal phase of γ-Al2O3 through reasonable regulation strategies. At present, the oxygen carrier with a core-shell structure has been proven to enhance cycle stability and oxygen release stability. Through rational design methods, an aluminum-based NC with a special structure is developed. It is expected to improve the problem of poor cycle stability of Al-based NC and better control the conversion of AlN to γ-Al2O3. Finally, the development of low-cost reduction media, such as biomass char as a reducing agent, can reduce production costs to a certain extent.
3.2.3 The NCs for H2O-3step CLAS
The ammonia production of TM oxide NC is shown in the reaction Equations 8-10. Firstly, the TM oxides are reduced to the corresponding transition Me under the action of a reducing gas. Secondly, the transition Me are nitrided in a N2 atmosphere to form corresponding metal nitrides[83,84]. Finally, the hydrolysis of metal nitrides can produce hydrogen and ammonia. This process is very similar to the reaction process of the covalent NC. Both of them use water as the hydrogen source, and the reduction process of the NC is a highly endothermic reaction, necessitating a higher reaction temperature to sustain the reaction. The nitridation reaction of Me and the hydrolysis of TM nitrides to produce ammonia are both exothermic reactions. In the actual operation process, the heat generated within the steps can be utilized to heat the reaction system and decrease the need for external energy supply. Cr/Cr2N/Cr2O3 and Mo/Mo2N/MoO2 have been proven to exhibit good reactivity in TM oxide NC. At the reduction temperature of 1000℃, Cr2O3 can be reduced to Cr under the action of H2. When N2 is introduced into the reactor, Cr is nitrided to form Cr2N. Cr2N can convert 53% of N in its lattice into NH3 under the action of water vapor. Adding a small amount of Ca2+ to chromium powder can slightly increase the yield of NH3[85,86]. In this process, carbon-based gas reducing agents such as CO and CH4 can be used instead of H2 to reduce metal oxides, thereby improving the efficiency of the process. When carbon-based reducing agents are used to reduce TM oxides, carbon deposition may occur, impacting the cycle stability of the NC. Since the Equation 8 reaction requires a higher temperature to reduce the TM oxide, utilizing solar collectors to provide energy for the reaction is a viable option to lower production costs.
MeO+R→Me+RO (8)
2Me+N2→2MeN (9)
2MeN+4H2O→2MeO+2NH3+H2 (10)
The reaction process dominated by alkali/alkaline earth metal nitride NC usually requires additional energy to intervene in the reaction process. Jaramillo et al.[87] proposed a process of CLAS based on LiOH, which is illustrated in Equations 11-13. The first step is the electrolysis of LiOH to produce lithium. The second step involves exposing the lithium to flowing nitrogen while maintaining the temperature at 22-100℃ to form Li3N. The third step involves the hydrolysis of Li3N to produce ammonia, enabling the synthesis of ammonia at room temperature and atmospheric pressure. It is also found that other forms of energy injection help to overcome the limitations of the original reaction thermodynamics and make the reaction conditions milder.
6LiOH→6Li+3H2O+3/2O2(g) (11)
6LiN2(g)→2Li3N(s) (12)
2Li3N+6H2O→6LiOH+2NH3 (13)
3.2.4 Discussion for H2O-3step CLAS
Although the TM oxide NC has a good ammonia yield, the reaction steps are complex and the reaction temperature is high. The use of solar collector reactors is a good choice. At the same time, combining other chemical looping technologies with the TM oxide-dominated CLAS can improve system efficiency. For example, synthesis gas can be produced through biomass chemical looping gasification to reduce metal oxides. It not only eliminates the dependence on fossil energy but also enhances the utilization rate of renewable energy. Furthermore, a more in-depth analysis of the economic, energy and environmental aspects of complex interconnected systems is still required.
3.3 Summary
At present, the high activity NC is still an urgent problem to be solved in CLAS. On the basis of understanding the thermodynamic and kinetic properties of NC, it is crucial for the development of CLAS to identify NCs suitable for various reaction processes, optimize the preparation methods, implement appropriate control strategies, and enhance the utilization rate of effective lattice nitrogen. The properties of various types NCs are shown in Table 2. The reduction and nitridation reaction conditions of various NC, the utilization rate of lattice nitrogen and the number of cycles are also summarized, as shown in Table 3.
Table 2. The Summary of NCs for CLAS
Classification |
Features |
Advantage |
Challenges |
Development |
TM NCs |
The reaction conditions are moderate (450-800℃, 0.1MPa) Nitrogen vacancy activates N2 |
Easy fabrication Excellent cycle stability Easy to scale application Low cost |
Low utilization rate of lattice nitrogen Few types of NCs Harsh kinetics |
Develop a variety of NCs Optimize regulation strategy to improve the NCs lattice |
Ionic NCs |
Hydrogen vacancy activates N2 Occur at atmospheric pressure and room temperature |
High ammonia yield rate Fast kinetics |
Preparation difficult Unstable property
|
Optimize the preparation method Enhanced stability |
Covalent NCs |
No need hydrogen Metal as electron donor activates N2 |
High utilization rate of lattice nitrogen By-product other gases A wide variety of materials |
High temperature Low rate of ammonia Harsh kinetics The reaction process is tedious |
Optimizing reaction kinetics Lowering the temperature Improve the cycle stability of NCs |
Other NCs |
Special structure Composed of more than two materials |
It has the advantages of two NCs Mild reaction conditions Good kinetics |
Poor cycle performance Composite strategy is unknown |
Develop more materials Determine the compounding and regulation strategy |
Table 3. Summary of NCs Performance
NCs |
Nitriding conditions (℃) |
Ammonia production conditions (℃) |
Effective lattice nitrogen conversion rate (%) |
Ammonia production rate (μmol·g-1·h-1) |
Mn |
/ |
/ |
23 |
5.2 |
Fe-Mn |
800 |
600 |
/ |
166.3 |
Ni-Mn |
750 |
750 |
36.5 |
130 |
Li-Mn4N |
/ |
500 |
24 |
/ |
Mo2N |
600 |
450 |
40 |
4576 |
Ni-CrN |
750 |
750 |
50.7 |
466.1 |
Co3Mo3N |
700 |
400 |
/ |
/ |
BaH2 |
300 |
500 |
/ |
3500 |
Ni-BaH2 |
300 |
250 |
/ |
5800 |
Al2O3/TiO2 |
1000 |
1000 |
/ |
/ |
Si-Al2O3 |
1200 |
1200 |
53.6 |
/ |
Mn4N-BaH2 |
250 |
250 |
/ |
1300 |
Ni3ZnN |
/ |
500 |
97.5 |
3461 |
4 THE PROCESS OF CLAS
The ammonia production process using the H-B method is characterized by high energy consumption and carbon emissions resulting from the harsh reaction conditions, making it an energy-intensive industrial process. At present, this process is the most commonly used method for ammonia synthesis, and it is challenging to replace it quickly. By developing efficient catalysts and implementing green hydrogen production methods, energy consumption and carbon emissions can be reduced to a certain extent. The second-generation ruthenium-based catalyst used in the KAAP process can reduce the reaction pressure to below 10MPa and the energy consumption of ammonia synthesis to 27GJ/t NH3[88,89]. However, due to the conflict between kinetics and thermodynamics and the competitive adsorption of H2 and N2, the reaction conditions for ammonia synthesis remain challenging. Nowadays, energy conservation, emission reduction and sustainable development are the primary tasks. It is necessary to explore and develop an environmentally friendly and sustainable N2 fixation method. Based on the aforementioned issues, photoelectrocatalytic ammonia synthesis technology and CLAS technology have been proposed.
However, the photocatalytic and electrocatalytic synthesis of ammonia is still in the laboratory stage, and there is still a certain distance from the industrial scale application[2]. The CLAS technology has strong potential applications due to its flexibility, adaptability, energy efficiency and carbon footprint reduction capabilities. The process of CLAS using covalent NC and TM oxide NC can utilize water vapor instead of hydrogen to produce ammonia. This approach can circumvent the laborious hydrogen production process, leading to a significant reduction in production costs and energy consumption. When the process of CLAS uses TM and ionic NCs, it can utilize green hydrogen production technology instead of traditional fossil energy hydrogen production methods, such as electrolytic water hydrogen production, photolysis water hydrogen production, and biomass hydrogen production[90,91]. The reaction temperature of the CLAS dominated by the ionic NC is more moderate. At present, the process of ammonia synthesis based on chemical looping technology can be divided into two types. One system is based on the H-B principle, while the other system using NC as a medium.
The process of CLAS use NCs as a reaction medium has the characteristics of operating at atmospheric pressure, uniform distribution, and flexibility. Compared with the traditional H-B ammonia synthesis method, the CLAS production process is economical and safe because it does not involve high-pressure reactions. In addition, the decentralized nature of the process can eliminate the reliance of ammonia synthesis on energy-intensive industries, allowing for the establishment of small-scale ammonia plants tailored to local requirements. In the future, the properties of ammonia as a carbon-free fuel will be valued. In this case, the significance of CLAS will be amplified. As the most promising alternative to H-B synthetic ammonia technology, CLAS technology is of great significance for energy conservation, emission reduction, and the green transformation of the synthetic ammonia industry.
4.1 CLAS Based on Haber-Bosch
The system based on the Haber-Bosch principle is typically combined with other chemical looping processes to produce ammonia. In our previous studies, a dual chemical looping system for hydrogen and ammonia co-production was proposed[92]. The process is shown in Figure 16A, where the chemical looping hydrogen production device is connected downstream of the chemical gasification device in series. As an additional component of the 100MW·h coal chemical looping gasification device, it serves the purpose of supporting combustion to generate hydrogen. The results show that when the gasification temperature is 900℃, the water carbon ratio is 0.84, and the oxygen carbon ratio is 1.5, the hydrogen production rate of the system is the largest. Through the heat exchange analysis of the logistics system, it was discovered that the system can generate 8.010×104kg·h-1 high pressure steam and 1.101×104kg·h-1 of medium-pressure steam. Additionally, the overall cost of the system is reduced by 48.69%. In addition to using coal as a raw material to synthesize ammonia, coke oven gas can also be utilized to co-produce NH3 while generating H2[93]. The exergy efficiency of the system is 78.7% and the energy efficiency is 70.1%.
|
Figure 16. The schematic diagram of the process. A: Energy and economic analysis of a hydrogen and ammonia co-generation system based on double chemical looping. Reproduced from Ref.[92] with permission from Elsevier. B: Design and Optimization of a Clean Ammonia Synthesis System Based on Biomass Gasification Coupled with a Ca−Cu Chemical Loop. Reproduced from Ref.[94] with permission from Elsevier
Tang et al.[95] constructed a CLAS system using biomass as the raw material. The process is divided into three parts. The syngas produced by biomass chemical looping gasification is sent to the carbon capture unit to obtain a higher concentration of H2 and N2 for the subsequent ammonia synthesis reaction. In addition, the process utilizes biomass, steam, and air as raw materials. This not only guarantees a high CO2 capture efficiency (96.4%) and ammonia synthesis capacity (0.281kg NH3/kg wet biomass) but also largely fulfills the self-heating requirements of the system. Economic analysis reveals that the investment cost of the system constitutes over 90% of the total annual cost, which is approximately 10 times the operating cost. The price of ammonia is affected by the price of biomass. The chemical looping synthesis process, based on the Haber-Bosch principle, offers a wider variety of raw materials compared to the traditional ammonia synthesis process. The utilization of renewable resources, such as biomass, as raw materials for ammonia synthesis can eliminate the reliance on fossil energy sources. However, there are still problems such as high reaction pressure and competitive adsorption in the ammonia production unit. The higher reaction pressure not only imposes greater demands on the reactor but also hinders safe production and raises production costs.
4.2 CLAS Based on NCs
The carbon thermochemical CLAS technology employs covalent metal oxides such as aluminum oxide, utilizing coal char, petroleum coke, and biomass char as reduction agents. During the production of ammonia, it has the capability to generate CO, exhibiting traits such as reduced carbon emissions and minimal energy consumption. Fang et al.[96] conducted a comprehensive energy and environmental assessment of the carbon CLAS co-production urea system. The process is illustrated in Figure 17A. The process comprises three stages: air separation, ammonia synthesis, and urea synthesis. The findings indicate that the energy consumption for ammonia production is merely 6.88GJ/t NH3, with a CO2 emission rate of 2.05kg/kg NH3. Additionally, the system has the capability to generate CO and high temperature steam. The operating conditions under atmospheric pressure alleviate the demands on the reactor. Sarafraz et al.[97] developted a three-stage CLAS system utilizing metal oxides such as chromium (Cr), magnesium (Mg), aluminum (Al), calcium (Ca) and manganese (Mn) as NC. The system configuration is illustrated in Figure 17B, and a comprehensive analysis of the heat transfer between reactors was conducted. The results indicate that the system exhibits the highest level of competitiveness when Cr2O3 is utilized as the NC. The self-heating factor of the system is 0.333, indicating that the heat generated by the reactions can contribute one-third of the total heat required by the system, while the remaining two-thirds of the heat must be externally supplied. To enhance the energy efficiency of the system, Weng et al.[98] introduced a biomass CLAS system that integrates solar and wind energy sources. As illustrated in Figure 18, the system comprises six units: i) biomass gasification, ii) syngas oxidation, iii) chemical looping air separation, iv) CLAP, v) electricity production, and vi) water electrolysis. At a biomass feed rate of 1kg/s, the ammonia selectivity reached 79.36%, with an ammonia production rate of 34.1kmol/h and an ammonia concentration of 65.65 vol%. During the ammonia synthesis process at ambient pressure conditions, biomass is utilized as a substitute for natural gas and coal in the production of ammonia. The NC alternates between the nitriding reactor and the ammonia production reactor. This method facilitates the ammonia synthesis process to occur under ambient pressure conditions, thereby preventing the competitive adsorption of N2 and H2. This process utilizes water vapor as an alternative to H2 for synthesizing ammonia, eliminating the requirement for supplementary hydrogen production facilities.
|
Figure 17. Schematics of the 3CLAP process for ammonia production. A: Utilization of carbon-based energy as raw material instead of fuel with low CO2 emissions: Energy analyses and process integration of chemical looping ammonia generation, Reproduced from Ref.[96] with permission from Elsevier. B: Sustainable 3CLAP process. Reproduced from Ref.[97] with permission from Elsevier.
|
Figure 18. Ammonia production from biomass via a chemical loopingebased hybrid system. Reproduced from Ref.[98] with permission from Elsevier.
4.3 Discussion for Chemical Looping System Using NC
In comparison to the process based on the H-B method, the chemical looping system that utilizes NC for ammonia production presents clear advantages. Currently, there have been no reports on the CLAS system utilizing TM nitrides and AM/alkaline earth metal hydrides as NCs has not been reported. From an energy supply perspective, it is essential to conduct heat balance calculations for both the ammonia production reactor and the nitriding reactor to ascertain the heat dynamics within the reactions, highlighting the significance of this analysis. Moreover, the reaction kinetic models of various types of NCs are established, providing guidance for reactor design and process optimization. It is crucial to conduct an analysis of the energy and environmental aspects of various processes and choose the most appropriate one for implementation. The analysis of energy, economic factors, and environmental aspects of the ammonia production system consisting of these two NC is beneficial for the utilization of NC.
5 CHALLENGES AND PROSPECTED
The pursuit of achieving green synthesis of ammonia under mild reaction conditions has consistently been a focal point of research endeavors. In recent years, there is an expectation that CLAS technology will enable the green synthesis of ammonia due to its ability to circumvent the competitive adsorption of H2 and N2, along with its operation under mild reaction conditions. Researchers employed machine learning techniques to conduct high-throughput screening of NCs suitable for the process of CLAS, and carried out an extensive thermodynamic analysis. Among these, AM, alkaline earth metals, and TM are potential candidates for this approach. Nevertheless, the current challenges in the field of nitrogen fixation catalysis include low efficiency in ammonia production and inadequate cycle stability, which continue to hinder the advancement of NC. The advancement of high-performance NCs has consistently been a primary concern in the field of CLAS technology.
In the field of CLAS dominated by TM, there is a focus on the development of composite TM NCs consisting of multiple metals, enhancing the efficient utilization of lattice nitrogen, and investigating the mechanisms involved in the transfer and phase transition of lattice nitrogen during the ammonia production process. Moreover, employing advanced characterization techniques can assist researchers in comprehending the reaction mechanism of NCs and enhancing their reactivity. Furthermore, it is imperative to elucidate the reaction mechanism of single component TM NCs and improve the cycle stability of the NCs. Based on this premise, the gradual transition to the composite NCs system is suggested, along with the design strategy for composite TM NC is proposed. Alkaline earth metal compound NC represent a novel class of materials with moderate thermodynamics conducive to nitrogen fixation and ammonia production through hydrogenation processes. Under the catalysis of TM, this material exhibits favorable low-temperature kinetic performance and represents the NC material with the highest reported rate of ammonia production in the literature. Enhancing its thermal stability and minimizing the production cost are crucial factors that need to be addressed in future research. An optimal solution has not yet been found for reducing the reduction temperature and separating the reduction medium from the covalent NC. In future research, it will be essential to optimize the preparation methods of NC. The investigation delves into the correlation between structure and composition when employing various preparation methods. Distinct preparation methods are developed for various types of NC.
The integration of renewable energy with CLAS technology has the potential to enhance the utilization rate of renewable energy sources and establish an industrial process characterized by negative carbon emissions. This integration can ultimately bolster the competitiveness of CLAS. The utilization of chemical simulation software in the design process, economic analysis, and energy balance assessment of the CLAS process is beneficial for advancing green ammonia synthesis technology. The application of CLAS technology represents a viable approach to realizing environmentally friendly ammonia synthesis. The utilization of CLAS technology relies on the interdisciplinary nature of catalytic chemistry, material science, chemical engineering, computational chemistry, and related fields. The growing interest of researchers in CLAS suggests its potential significance in facilitating the green synthesis of ammonia and the storage and conversion of renewable energy.
6 CONCLUSION
In the context of reducing carbon emissions, the utilization of ammonia as a novel carbon-neutral fuel holds significant importance in enhancing environmental conditions and mitigating CO2 emissions. The CLAS technology is considered the most promising method for ammonia synthesis under mild conditions. It is anticipated to facilitate the reduction of carbon emissions and energy consumption in the traditional ammonia synthesis industry, thereby enhancing the application potential of ammonia. Secondly, Controlled CLAS has the capability to achieve distinct regulation of the overall ammonia synthesis reaction, thereby enhancing the efficiency of ammonia production through the optimization of individual sub-reactions. The CLAS process facilitates the integration of the ammonia production process with technical processes, addressing the existing gap in small-scale and distributed ammonia synthesis. Nevertheless, there remain unresolved issues in the implementation of the CLAS process:
(1) Currently, the variety of NCs are still insufficient. It is imperative to enhance the diversity of NC and to design novel NC with improved reaction efficiency and milder reaction conditions.
(2) Various NCs large-scale preparation of NCs. These theories serve as guidelines for the practical application of NC.
(3) The elucidation of the relationship between the structure of NC and its reaction is facilitated by the reaction mechanism. This leads to enhanced kinetic and thermodynamic analyses of various NC types.
(4) Through the utilization of process simulation software, the CLAS process undergoes optimization, and comprehensive analyses including energy, economic, and environmental assessments are conducted for the CLAS process. The process of the CLAS package is designed to provide guidance for the industrial application process of the CLAS system.
Acknowledgements
The study was financially supported by Natural Science Foundation of China (No.U20A20124 and No.22379079), and Ningxia Natural Science Foundation (No.2022AAC01001).
Conflicts of Interest
The authors declared there is no conflict.
Author Contribution
Han Z was responsible for writing, editing, methodology, and data collection; Xue Q contributed methodology and data collection; Hu X responsible for reviewing and editing, methodology; Guo T was responsible for writing, reviewing editing, methodology, investigation, supervision, interpretation of results, and data management; Ma J responsible for the methods and data collection; Guo Q contributed to the investigation, grain warehouse management and data.
Abbreviation List
A3BN, Anti-perovskite
AM, Alkali metal
CLAP, Chemical looping ammonia production
CLAS, Chemical looping ammonia synthesis
H2O-2step, Two-step water
H2O-3step, Three-step water
Me, Metal elements
NCs, Nitrogen carriers
TCC, Two-component composite
TM, Transition metal
References
[1] Wang L, Xia M, Wang H et al. Greening ammonia toward the solar ammonia refinery. Joule, 2018; 2: 1055-1074.[DOI]
[2] Jiao F, Xu B. Electrochemical ammonia synthesis and ammonia fuel cells. Adv Mater, 2019; 31: 1805173.[DOI]
[3] Valera-Medina A, Xiao H, Owen-Jones M et al. Ammonia for power. Prog Energ Combust, 2018; 69: 63-102.[DOI]
[4] Ghavam S, Vahdati M, Wilson IAG et al. Sustainable ammonia production processes. Front Energy Res, 2021; 9: 34.[DOI]
[5] Cremonese L, Mbungu GK, Quitzow R. The sustainability of green hydrogen: An uncertain proposition. Int J Hydrogen Energ, 2023; 48: 19422-19436.[DOI]
[6] Farrell N. Policy design for green hydrogen. Renew Sust Energ Rev, 2023; 178: 113216.[DOI]
[7] Giddey S, Badwal SPS, Munnings C et al. Ammonia as a renewable energy transportation media. ACS Sustain Chem Eng, 2017; 5: 10231-10239.[DOI]
[8] Lin B, Wiesner T, Malmali M. Performance of a small-scale Haber process: A techno-economic analysis. ACS Sustain Chem Eng, 2020; 8: 15517-15531.[DOI]
[9] Huberty MS, Wagner AL, McCormick A et al. Ammonia absorption at haber process conditions. Aiche J, 2012; 58: 3526-3532.[DOI]
[10] Fang L, Yan Z, Wu J et al. Highly selective Ru/HBEA catalyst for the direct amination of fatty alcohols with ammonia. Appl Catal B Environ, 2021; 286: 119942.[DOI]
[11] Peng X, Chen X, Zhou Y et al. Size-dependent activity of supported Ru catalysts for ammonia synthesis at mild conditions. J Cata, 2022; 408: 98-108.[DOI]
[12] Wang Y, Wildfire C, Khan TS et al. Effects of support and promoter on Ru catalyst activity in microwave-assisted ammonia synthesis. Chem Eng J, 2021; 425: 130546.[DOI]
[13] Findlay A. Snowballing carbon emissions. Nat Clim Change, 2023; 13.[DOI]
[14] Qianru W, Jianping G, Ping C. The impact of alkali and alkaline earth metals on green ammonia synthesis. Chem, 2021; 3203-3220.[DOI]
[15] Rouwenhorst KHR, Engelmann Y, Veer K et al. Plasma-driven catalysis: green ammonia synthesis with intermittent electricity. Green Chem, 2020.[DOI]
[16] Liu X, Shen Z, Peng X et al. A photo-assisted electrochemical-based demonstrator for green ammonia synthesis. J Energ Chem, 2021; 68: 826-834.[DOI]
[17] Yang B, Ding W, Zhang H et al. Recent progress in electrochemical synthesis of ammonia from nitrogen: strategies to improve the catalytic activity and selectivity. Energ Environ Sci, 2020.[DOI]
[18] Brown S, Hu J. Review of chemical looping ammonia synthesis materials. Chem Eng Sci, 2023; 280: 119063.[DOI]
[19] Wang B, Shen L. Recent Advances in NH3 Synthesis with Chemical Looping Technology. Ind Eng Chem Res, 2022; 61: 18215-18231.[DOI]
[20] Adánez J, Abad A, Mendiara T et al. Chemical looping combustion of solid fuels. Prog Energ Combust, 2018; 65: 6-66.[DOI]
[21] Zhu X, Imtiaz Q, Donat F et al. Chemical looping beyond combustion - a perspective. Energ Environ Sci, 2020; 13: 772-804.[DOI]
[22] An M, Guo Q, Ma J et al. Chemical‐looping gasification of coal with CuFe2O4 oxygen carriers: The reaction characteristics and structural evolution. Canadian J Chem Eng, 2020; 98: 1512-1524.[DOI]
[23] Fu E, Gong F, Wang S et al. Chemical Looping Technology in Mild-Condition Ammonia Production: A Comprehensive Review and Analysis. Small, 2023; 20: 2305095.[DOI]
[24] Fang L, Chen S, Dan Z et al. Attrition and attrition-resistance of oxygen carrier in chemical looping process - A comprehensive review. Fuel, 2022.
[25] Tian H, Yuan F, Xu J et al. Performance Evaluation of a Gypsum-Based Desulfurizer for Sulfur Recovery from the Smelter Off-Gas: Experimental Analysis and Thermodynamic Performance. Energ Fuels, 2018; 32.[DOI]
[26] Zhao S, Ke L, Sam T et al. Ammonia synthesis via chromium-based nitrogen carrier looping. Chem Eng J, 2023.[DOI]
[27] Lai Q, Cai T, Tsang SCE et al. Chemical looping based ammonia production-A promising pathway for production of the noncarbon fuel. Sci Bull, 2022; 67: 2124-2138.[DOI]
[28] Wei Y, Cheng L, Leckner B et al. Design of an industrial chemical looping gasification system. Fuel, 2022; 330: 125541.[DOI]
[29] Yuan P, Guo T, Pan X et al. Process optimization and thermodynamic analysis of autothermal coal chemical looping gasification industrial demonstration system. Fuel, 2023; 334: 126667.[DOI]
[30] Cui Z, Tian W, Zhang H et al. Multi-scale modeling and control of chemical looping gasification coupled coal pyrolysis system for cleaner production of synthesis gas. J Clean Prod, 2021; 299.[DOI]
[31] Karina A, Olufemi OA, Amit K. Investigating the techno-economic and environmental performance of chemical looping technology for hydrogen production. Sustain Energy Tech, 2023.[DOI]
[32] Oruc O, Dincer I. Evaluation of hydrogen production with iron-based chemical looping fed by different biomass. Int J Hydrogen Energ, 2020; 45: 34557-34565.[DOI]
[33] Dou J, Krzystowczyk E, Mishra A et al. Perovskite Promoted Mixed Cobalt-Iron Oxides for Enhanced Chemical Looping Air Separation. ACS Sustain Chem Eng, 2018; 6: 15528-15540.[DOI]
[34] Dou J, Krzystowczyk E, Wang X et al. Sr1-xCaxFe1-yCoyO3-δ as facile and tunable oxygen sorbents for chemical looping air separation. J Phys Energ, 2020.[DOI]
[35] Syed S, Lizhong Y, Alessandro R et al. Coupling chemical looping air separation with the Allam cycle-A thermodynamic analysis. J Clean Prod, 2023.[DOI]
[36] Imamura K, Miyahara SI, Kawano Y et al. Kinetics of ammonia synthesis over Ru/Pr2O3. J Taiwan Inst Chem E, 2019; 105: 50-56.[DOI]
[37] Ellingsson V, Iqbal A, Skúlason E et al. Nitrogen Reduction Reaction to Ammonia on Transition Metal Carbide Catalysts. Chem Sus Chem, 2023.[DOI]
[38] Rostamikia G, Maheshwari S, Janik MJ. Elementary kinetics of nitrogen electroreduction to ammonia on late transition metals. Catal Sci Technol, 2018; 150: 041708.[DOI]
[39] Liu H, Liu C, Li X et al. Effect of an Iron Oxide Precursor on the H2 Desorption Performance for an Ammonia Synthesis Catalyst. Ind Eng Chem Res, 2003; 42: 1347-1349.[DOI]
[40] Guan S, Liu H. Effect of an Iron Oxide Precursor on the N2 Desorption Performance for an Ammonia Synthesis Catalyst. Ind Eng Chem Res, 2000.[DOI]
[41] Yang L, Fan J, Xiao B et al. Unveiling “Sabatier principle” for electrocatalytic nitric oxide reduction on single cluster catalysts: A DFT and machine learning guideline. Chem Eng J, 2023; 468: 143823.[DOI]
[42] Xu G, Cai C, Wang T. Toward Sabatier optimal for ammonia synthesis with paramagnetic phase of ferromagnetic transition metal catalysts. J Am Chem Soc, 2022; 144: 23089-23095.[DOI]
[43] Jacobsen CJH, Dahl S, Clausen BS et al. Catalyst design by interpolation in the periodic table: bimetallic ammonia synthesis catalysts. J Am Chem Soc, 2001; 123: 8404-8405.[DOI]
[44] Logadottir A, Rod TH, Nørskov JK et al. The Brønsted-Evans-Polanyi Relation and the Volcano Plot for Ammonia Synthesis over Transition Metal Catalysts. J Catal, 2001; 197: 229-231.[DOI]
[45] Guo J, Chen P. Interplay of Alkali, Transition Metals, Nitrogen, and Hydrogen in Ammonia Synthesis and Decomposition Reactions. Accounts Chem Res, 2021; 54: 2434-2444.[DOI]
[46] Tanaka H, Nishibayashi Y, Yoshizawa K. Interplay between Theory and Experiment for Ammonia Synthesis Catalyzed by Transition Metal Complexes. Accounts Chem Res, 2016; 47: 987-995.[DOI]
[47] Yang L, Fan J, Xiao B et al. Unveiling “Sabatier principle” for electrocatalytic nitric oxide reduction on single cluster catalysts: A DFT and machine learning guideline. Chem Eng J, 2023; 468: 143823.[DOI]
[48] Xu G, Cai C, Wang T. Toward Sabatier Optimal for Ammonia Synthesis with Paramagnetic Phase of Ferromagnetic Transition Metal Catalysts. J Am Chem Soc, 2022; 144: 23089-23095.[DOI]
[49] Si P, Jiang W, Wang H et al. The high nitrogen pressure synthesis of manganese nitride. Chinese Phys L, 2012; 29: 128101.[DOI]
[50] Laassiri S, Zeinalipour-Yazdi CD, Catlow CRA et al. The potential of manganese nitride based materials as nitrogen transfer reagents for nitrogen chemical looping. Appl Catal B-Environ, 2018; 223: 60-66.[DOI]
[51] Shan N, Huang C, Lee RT et al. Manipulating the geometric and electronic structures of manganese nitrides for ammonia synthesis. Chem Cat Chem, 2020; 12: 2233-2244.[DOI]
[52] Wang B, Yin X, Wang P et al. Chemical looping ammonia synthesis at atmospheric pressure benefiting from synergistic effect of Mn-and Fe-based nitrogen carriers. Int J Hydrogen Energ, 2023; 48: 2705-2717.[DOI]
[53] Fu E, Gong F, Wang S et al. Ni-Mn-N derived composite nitrogen carriers for enhanced chemical looping ammonia production. Fuel Process Technol, 2023; 252: 107971.[DOI]
[54] Wang S, Gong F, Zhou Q et al. Transition metal enhanced chromium nitride as composite nitrogen carrier for sustainable chemical looping ammonia synthesis. Appl Catal B-Environ, 2023; 339: 123134.[DOI]
[55] Yang S, Zhang T, Yang Y et al. Molybdenum-based nitrogen carrier for ammonia production via a chemical looping route. Appl Catal B-Environ, 2022; 312: 121404.[DOI]
[56] Gregory DH, Hargreaves JSJ, Hunter SM. On the regeneration of Co3Mo3N from Co6Mo6N with N2. Catal L , 2011; 141: 22-26.[DOI]
[57] Brown S, Jiang C, Wang Q et al. Evidence of ammonia synthesis by bulk diffusion in cobalt molybdenum particles in a CLAS process. Catal Commun, 2022; 167: 106438.[DOI]
[58] Chang F, Guan Y, Chang X et al. Alkali and alkaline earth hydrides-driven N2 activation and transformation over Mn nitride catalyst. J Am Chem S, 2018; 140: 14799-14806.[DOI]
[59] Yamamoto H, Miyaoka H, Hino S et al. Recyclable hydrogen storage system composed of ammonia and alkali metal hydride. Int J Hydrogen Energ, 2009; 34: 9760-9764.[DOI]
[60] Yu P, Chua YS, Cao H et al. Hydrogen storage over alkali metal hydride and alkali metal hydroxide composites. J Energ Chem, 2014; 23: 414-419.[DOI]
[61] Guan Y, Liu C, Wang Q et al. Transition-Metal-Free Barium Hydride Mediates Dinitrogen Fixation and Ammonia Synthesis. Angew Chem Int Ed Engl, 2022; 61: e202205805.[DOI]
[62] Sørensen RZ, Hummelshøj JS, Klerke A et al. Indirect, Reversible High-Density Hydrogen Storage in Compact Metal Ammine Salts. J Am Chem S, 2008.[DOI]
[63] Gao W, Guo J, Wang P et al. Production of ammonia via a chemical looping process based on metal imides as nitrogen carriers. Nat Energ, 2018; 3: 1067-1075.[DOI]
[64] Feng S, Gao W, Wang Q et al. A multi-functional composite nitrogen carrier for ammonia production via a chemical looping route. J Mater Chem, A 2021; 9: 1039-1047.[DOI]
[65] Yamaguchi T, Shinzato K, Yamamoto K et al. Pseudo catalytic ammonia synthesis by lithium-tin alloy. Int J Hydrogen Energ, 2020; 45: 6806-6812.[DOI]
[66] Pereira RJL, Hu W, Metcalfe IS. Impact of gas-solid reaction thermodynamics on the performance of a chemical looping ammonia synthesis process. Energ Fuels, 2022; 36: 9757-9767.[DOI]
[67] Burrows L, Gao PX, Bollas GM. Thermodynamic feasibility analysis of distributed chemical looping ammonia synthesis. Chem Eng J, 2021; 426: 131421.[DOI]
[68] Liu L, Li Z, Li Z et al. Heterogeneous reaction kinetics of a perovskite oxygen carrier for chemical looping combustion coupled with oxygen uncoupling. Chem Eng J, 2021; 417: 128054.[DOI]
[69] Su M, Ma J, Tian X et al. Reduction kinetics of hematite as oxygen carrier in chemical looping combustion. Fuel Proc Technol, 2017; 155: 160-167.[DOI]
[70] Miya K, Otomo J. Improvements in reaction kinetics and stability of ilmenite as oxygen carrier by surface modification with calcium titanate in redox cycles of chemical-looping systems. Chem Eng J, 2017; 327: 257-267.[DOI]
[71] Goto Y, Daisley A, Hargreaves J. Towards anti-perovskite nitrides as potential nitrogen storage materials for chemical looping ammonia production: Reduction of Co3ZnN, Ni3ZnN, Co3InN and Ni3InN under hydrogen. Catal Today, 2021; 364: 196-201.[DOI]
[72] Gálvez ME, Frei A, Halmann M et al. Ammonia production via a two-step Al2O3/AlN thermochemical cycle. 2. Kinetic analysis. Ind Eng Chem Res, 2007; 46: 2047-2053.[DOI]
[73] Gálvez ME, Halmann M, Steinfeld A. Ammonia production via a two-step Al2O3/AlN thermochemical cycle. 1. Thermodynamic, environmental, and economic analyses. Ind Eng Chem Res, 2007; 46: 2042-2046.[DOI]
[74] Gálvez ME, Hischier I, Frei A et al. Ammonia production via a two-step Al2O3/AlN thermochemical cycle. 3. Influence of the carbon reducing agent and cyclability. Ind Eng Chem Res, 2008; 47: 2231-2237.[DOI]
[75] Chen Y, Wei J, Duyar MS et al. Carbon-based catalysts for Fischer-Tropsch synthesis. Chem Soc Rev, 2021; 50: 2337-2366.[DOI]
[76] Fischer N, Claeys M. In situ characterization of Fischer-Tropsch catalysts: a review. J Phys D Appl Phys, 2020.[DOI]
[77] Lin B, Heng L, Fang B et al. Ammonia Synthesis Activity of Alumina-Supported Ruthenium Catalyst Enhanced by Alumina Phase Transformation. ACS Catal, 2019; 9: 1635-1644.[DOI]
[78] Feng M, Zhang Q, Wu Y et al. Using Coal Coke for N-Sorption with an Al-based Nitrogen Carrier during Chemical Looping Ammonia Generation. Energ Fuels, 2020; 34: 12527-12534.[DOI]
[79] Zhang Q, Wu Y, Gao Y et al. High-performance mesoporous (AlN/Al2O3) for enhanced NH3 yield during chemical looping ammonia generation technology. Int J Hydrogen Energ, 2020; 45: 9903-9913.[DOI]
[80] Xiong C, Wu Y, Feng M et al. High thermal stability Si-Al based N-carrier for efficient and stable chemical looping ammonia generation. Appl Energ, 2022; 323: 119519.[DOI]
[81] Wu Y, Jiang G, Zhang H et al. Fe2O3, a cost effective and environmentally friendly catalyst for the generation of NH3 - a future fuel - using a new Al2O3-looping based technology. Chem Commun (Camb), 2017; 53: 10664-10667.[DOI]
[82] Liu Z, Yu Q, Wang H et al. Selecting nitrogen carriers used for chemical looping ammonia generation of biomass and H2O by thermodynamic method. Int J Hydrogen Energ, 2023; 48: 4035-4051.[DOI]
[83] Belokopytov YV, Kuznetsov VA, Kholyavenko KM et al. An infrared study of surface properties of metal oxides: I. The interaction of ammonia with the surface of Cr2O3. J Catal, 1976; 44: 1-4.[DOI]
[84] Shi L, Yin Y, Wu H et al. Controllable synthesis of a hollow Cr2O3 electrocatalyst for enhanced nitrogen reduction toward ammonia synthesis. Chinese J Chem Eng, 2021; 41: 358-365.[DOI]
[85] Michalsky R, Pfromm PH. Chromium as reactant for solar thermochemical synthesis of ammonia from steam, nitrogen, and biomass at atmospheric pressure. Sol Energ, 2011; 85: 2642-2654.[DOI]
[86] Michalsky R, Pfromm PH. An Ionicity Rationale to Design Solid phase Metal Nitride Reactants for Solar Ammonia Production. J Phys Chem C, 2012; 116: 23243-23251.[DOI]
[87] McEnaney JM, Singh AR, Schwalbe JA et al. Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energ Environ Sci, 2017; 10: 1621-1630.[DOI]
[88] Brown DE, Edmonds T, Joyner RW et al. The genesis and development of the commercial BP doubly promoted catalyst for ammonia synthesis. Catal L, 2014; 144: 545-552.[DOI]
[89] Li K, Andersen SZ, Statt MJ et al. Enhancement of lithium-mediated ammonia synthesis by addition of oxygen. Sci, 2021; 374: 1593-1597.[DOI]
[90] Manna S, Antonchick AP. Catalytic Transfer Hydrogenation Using Biomass as Hydrogen Source. Chem Sus Chem, 2018; 12: 3094-3098.[DOI]
[91] Zhang P, Guo Y, Chen J et al. Streamlined hydrogen production from biomass. Nat Catal, 2018; 1: 332-338.[DOI]
[92] Pan X, Ma J, Hu X et al. Energy and economic analysis of a hydrogen and ammonia co-generation system based on double chemical looping. Chinese J Chem Eng, 2021; 36: 190-198.[DOI]
[93] Zhao Y, Zhao Y, Yi Q et al. Highly flexible and energy-efficient process for converting coke-oven gas and pulverized coke into methanol and ammonia using chemical looping technology. Energ Convers Manage, 2021; 248: 114796.[DOI]
[94] Tang J, Kang L, Liu Y. Design and optimization of a clean ammonia synthesis system based on biomass gasification coupled with a Ca-Cu chemical loop. Ind Eng Chem Res, 2022; 61: 8128-8140.[DOI]
[95] Tang J, Kang L, Liu Y. Design and Optimization of a Clean Ammonia Synthesis System Based on Biomass Gasification Coupled with a Ca-Cu Chemical Loop. Ind Eng Chem Res, 2022; 61: 8128-8140.[DOI]
[96] Fang J, Xiong C, Feng M et al. Utilization of carbon-based energy as raw material instead of fuel with low CO2 emissions: Energy analyses and process integration of chemical looping ammonia generation. Appl Energ, 2022; 312: 118809.[DOI]
[97] Sarafraz MM, Christo FC. Sustainable three-stage chemical looping ammonia production (3CLAP) process. Energ Conv Manag, 2021; 229: 113735.[DOI]
[98] Weng Q, Toan S, Ai R et al. Ammonia production from biomass via a chemical looping-based hybrid system. J Clean Prod, 2021; 289: 125749.[DOI]
Copyright © 2024 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.
Brief of Corresponding Author(s)
Qingjie Guo He is a professor in Qingdao University of Science and Technology. In the past five years, he has presided over numerous projects, including the National Key Research and Development Program, Joint Key Fund of the National Natural Science Foundation of China, Major Project of Ningxia Key Research and Development Program. Qingjie Guo has published 442 papers in domestic and international chemical engineering journals, including 271 papers in international chemical engineering and energy journals. He is focus on chemical reaction Engineering (fluidization engineering), renewable energy, particuology technique, energy and environmental engineering, energy and environmental materials, as well as capture and utilization technology of greenhouse gas, carbon dioxide.2021 and 2022 were consecutively selected as the top 100,000 scientists in the world, with an h-index of 43. |

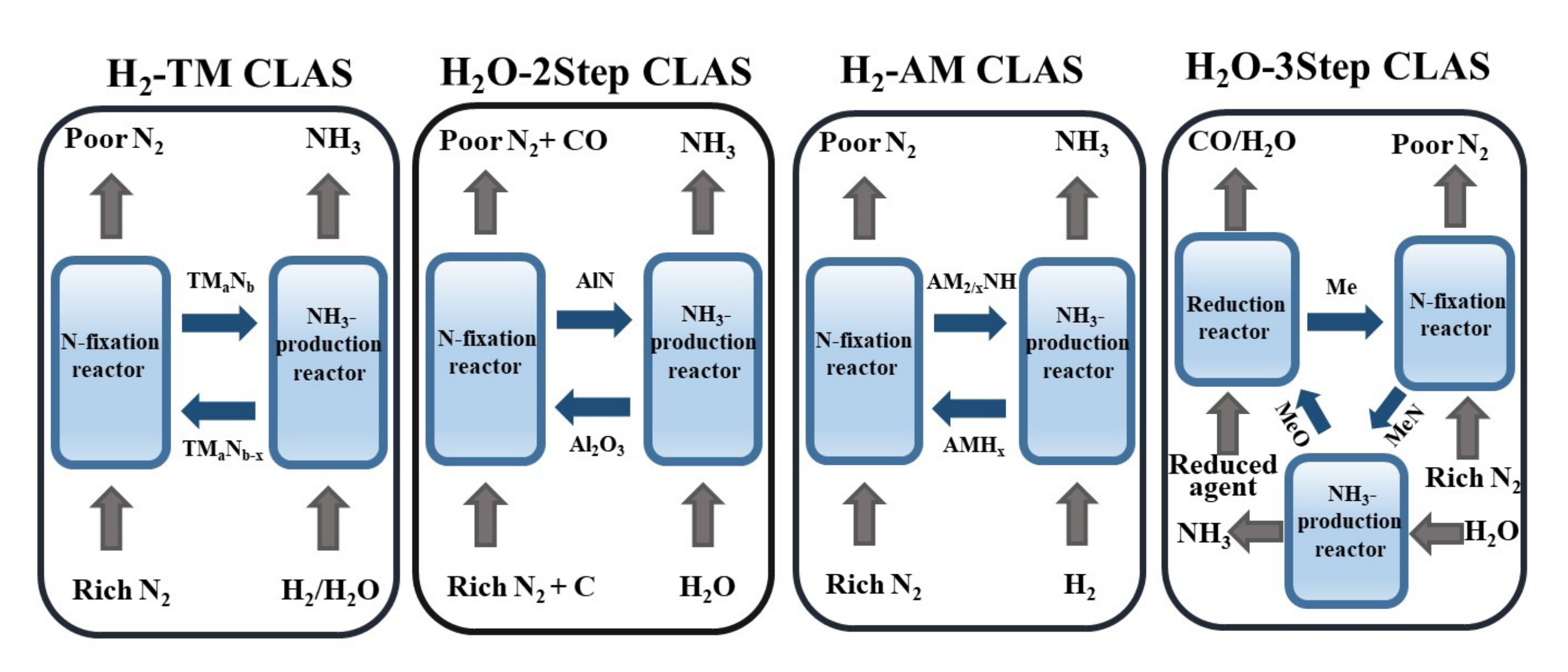
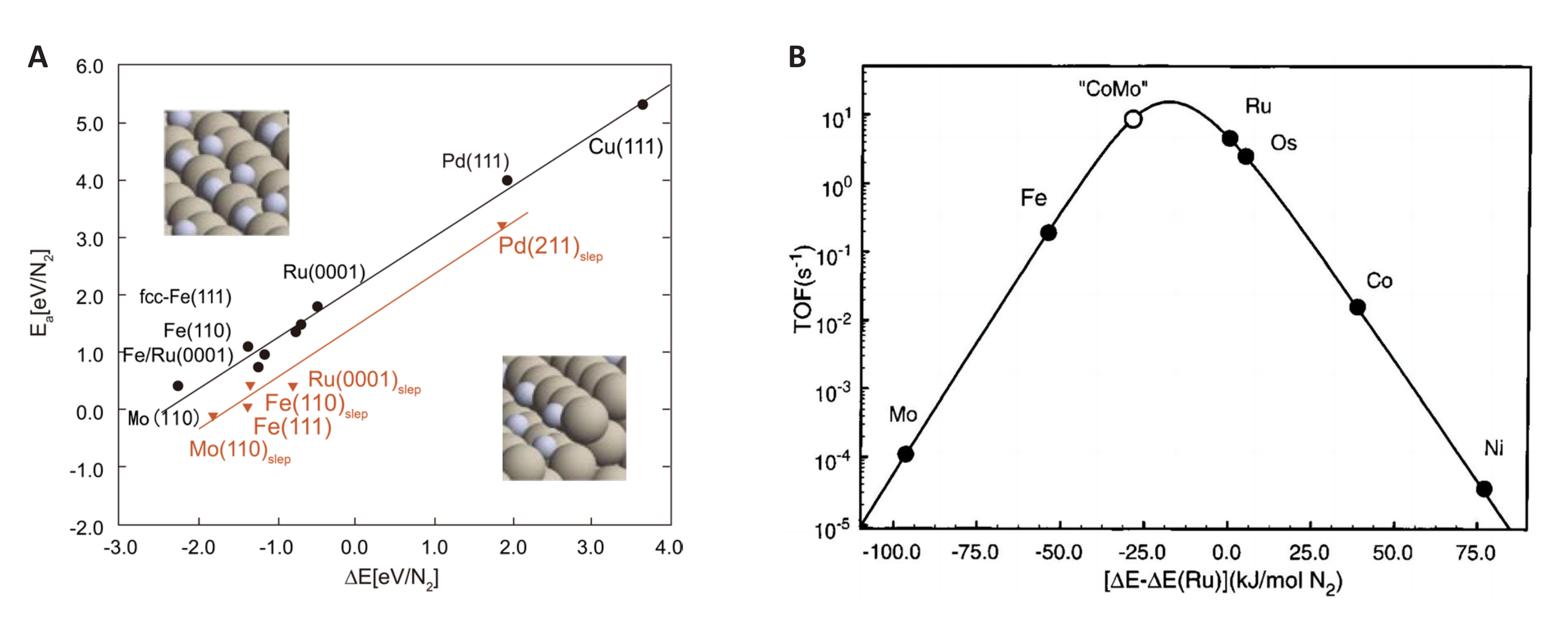
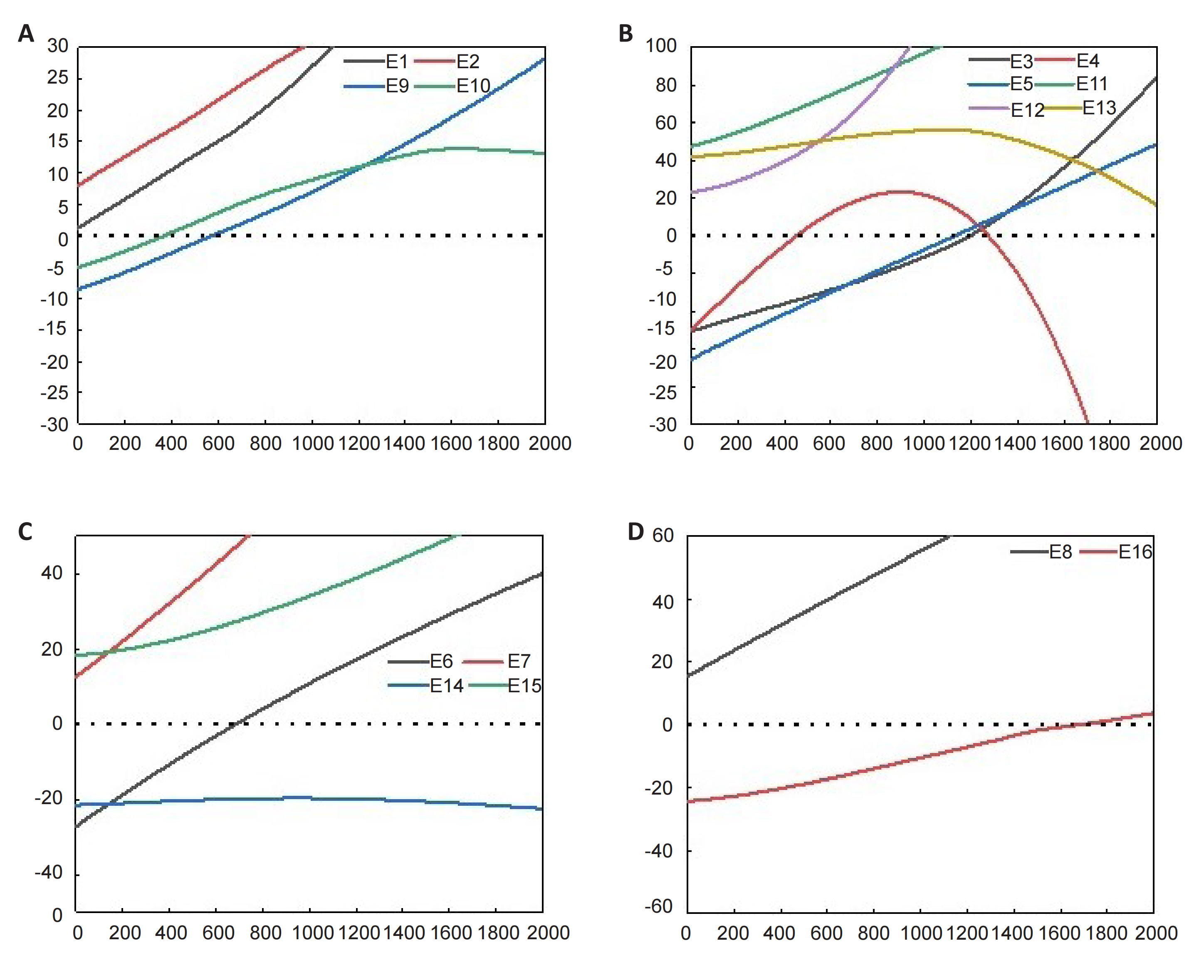
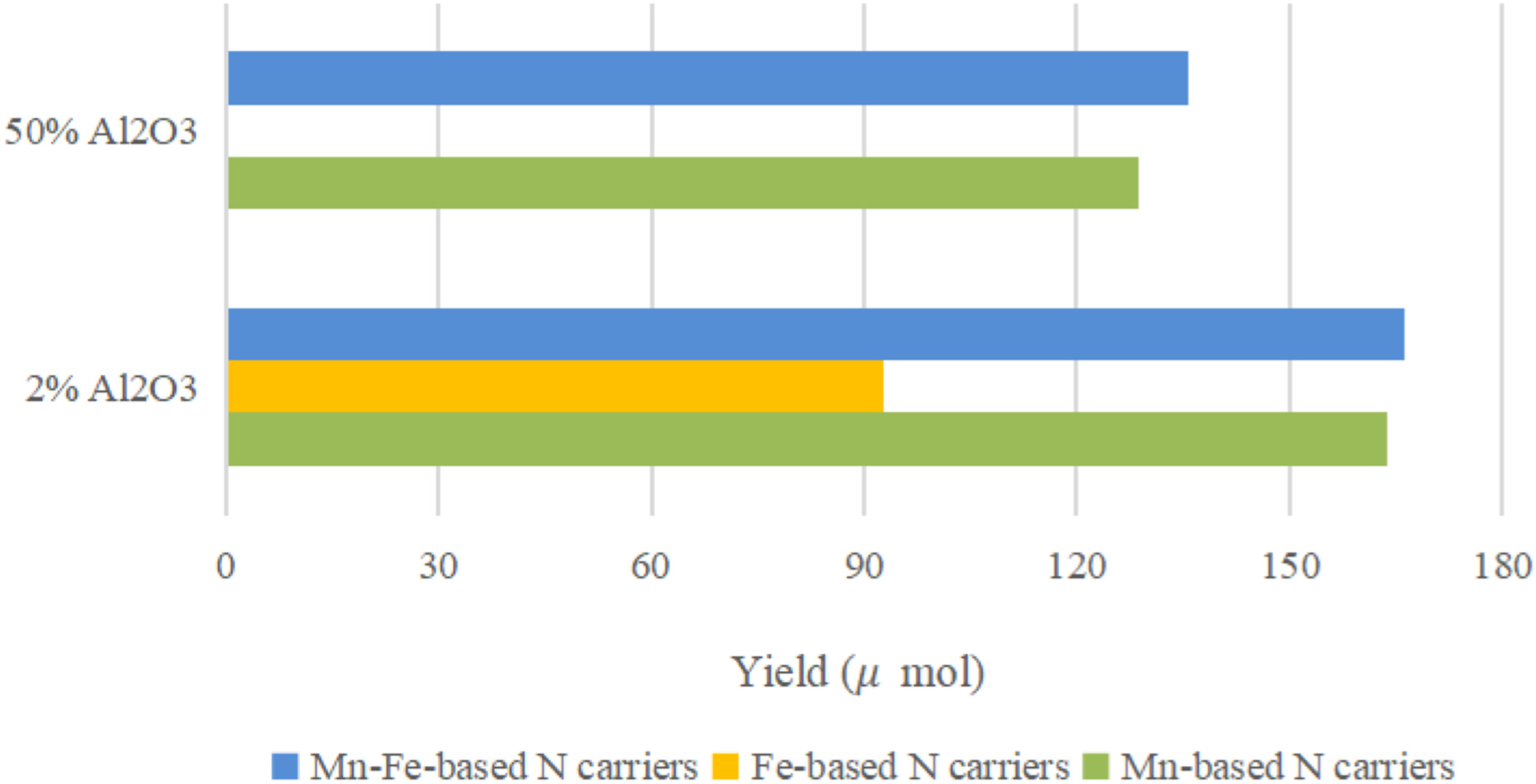

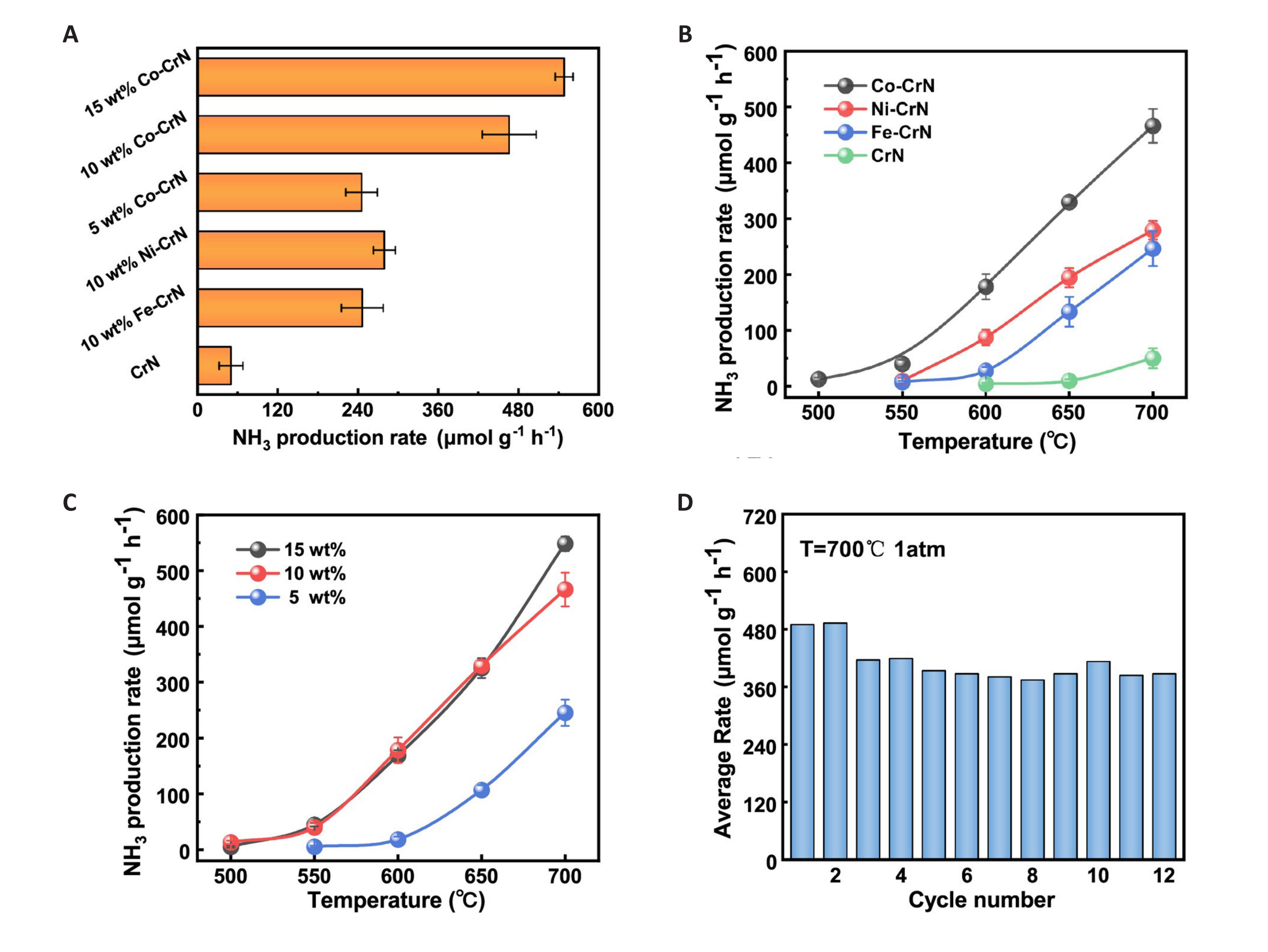
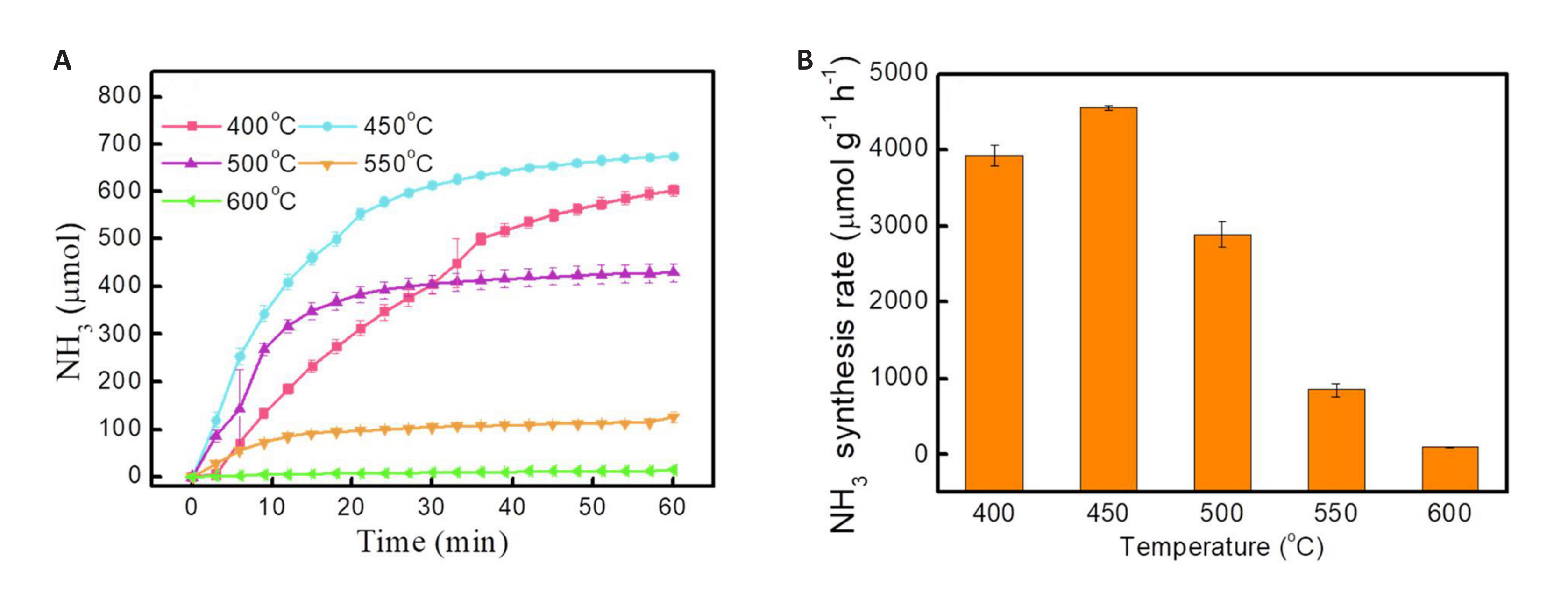
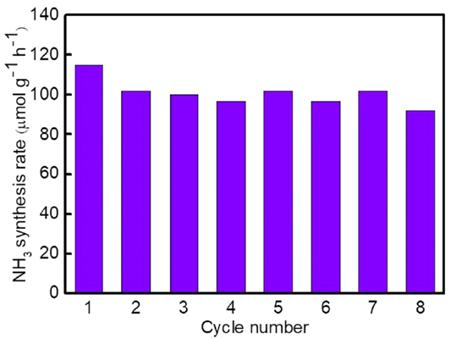
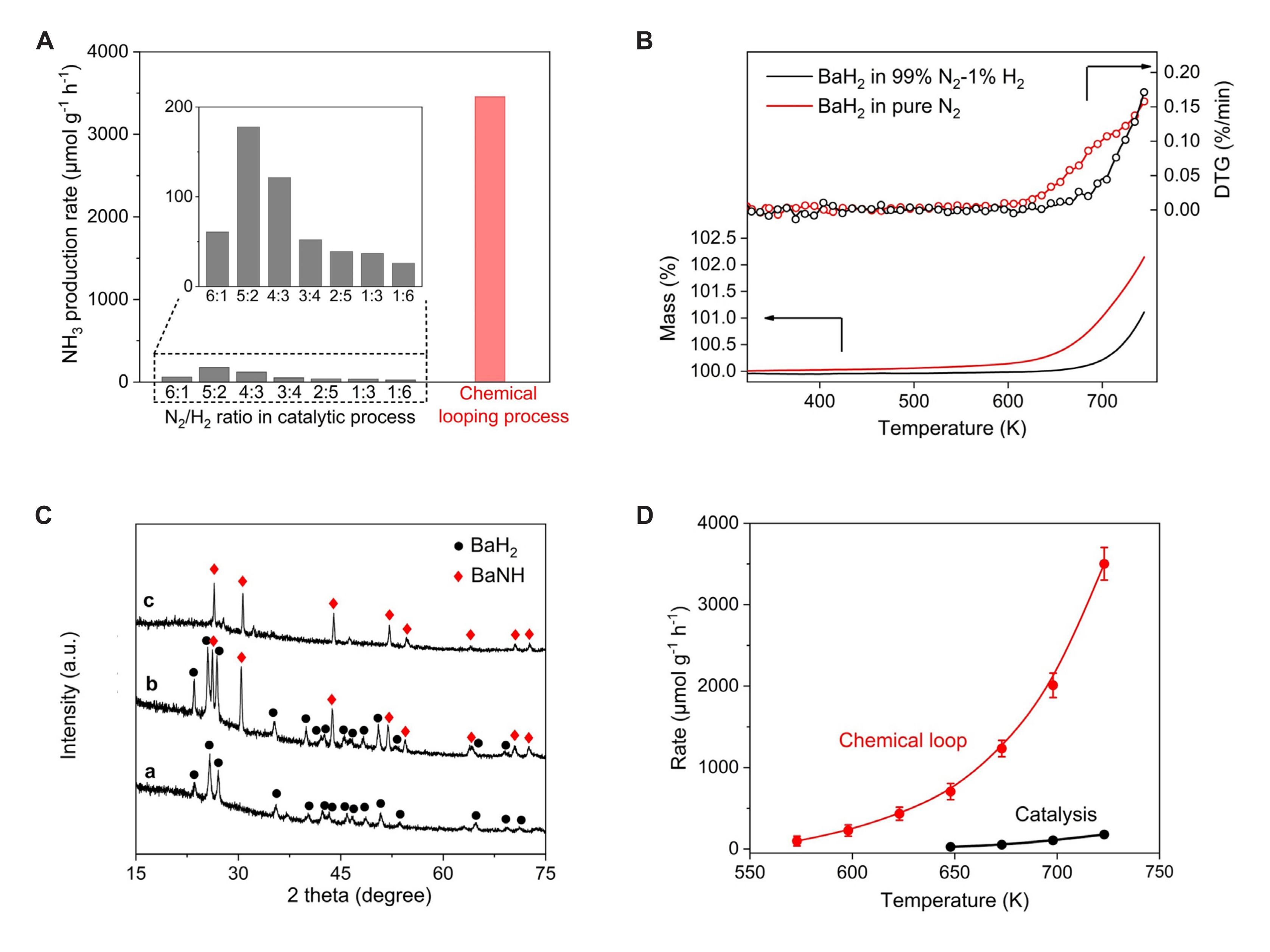
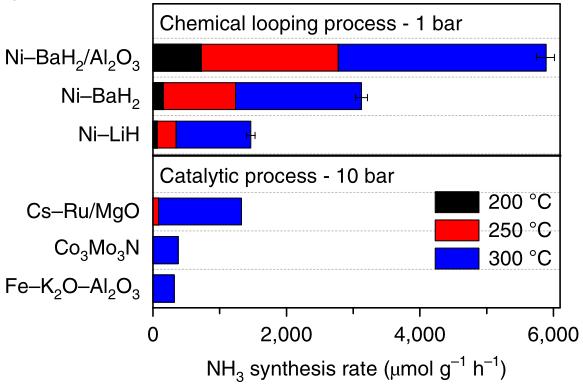
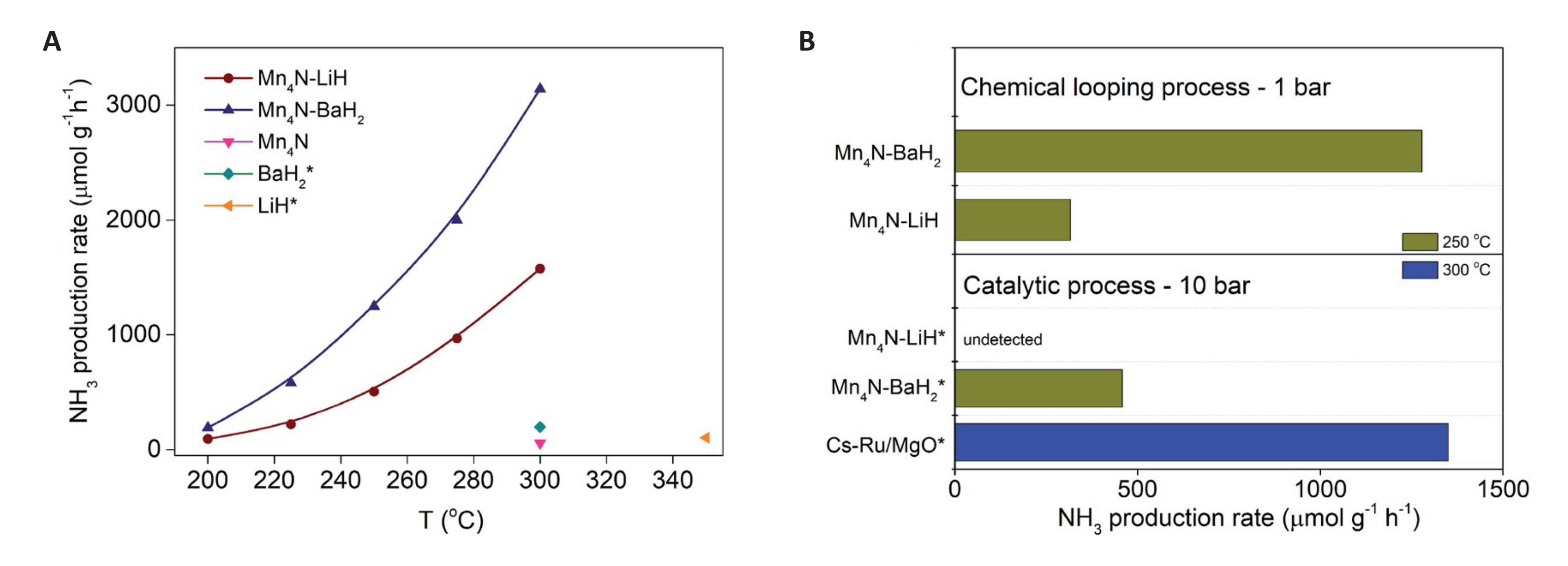
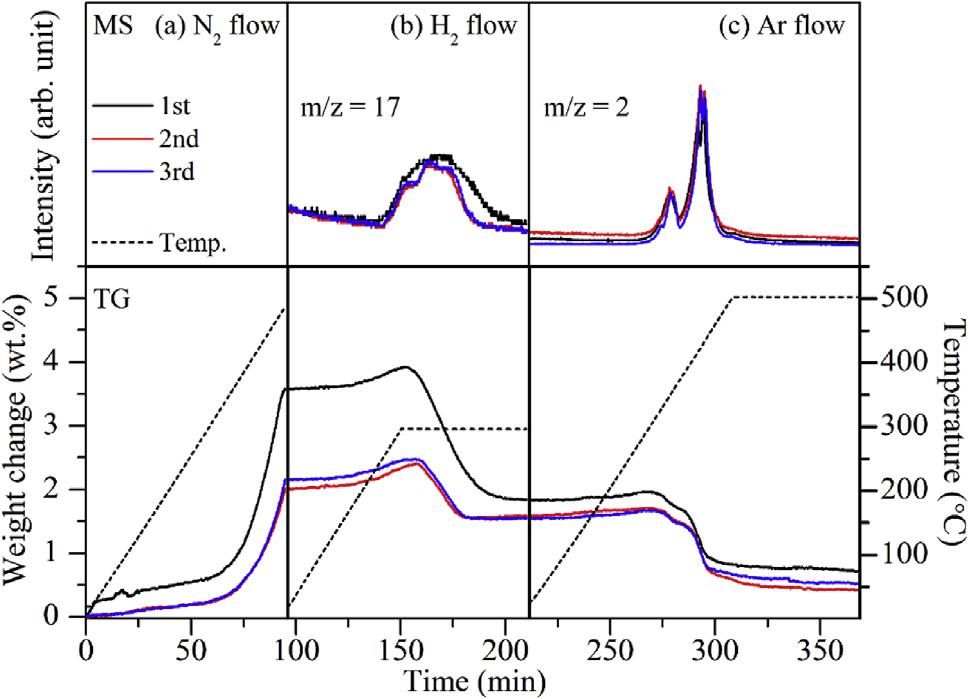
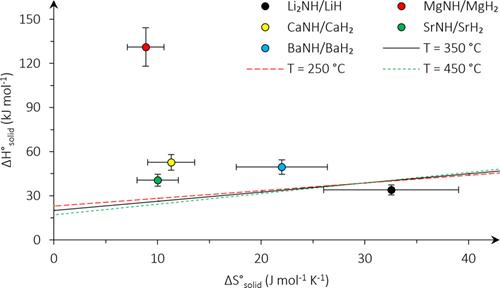
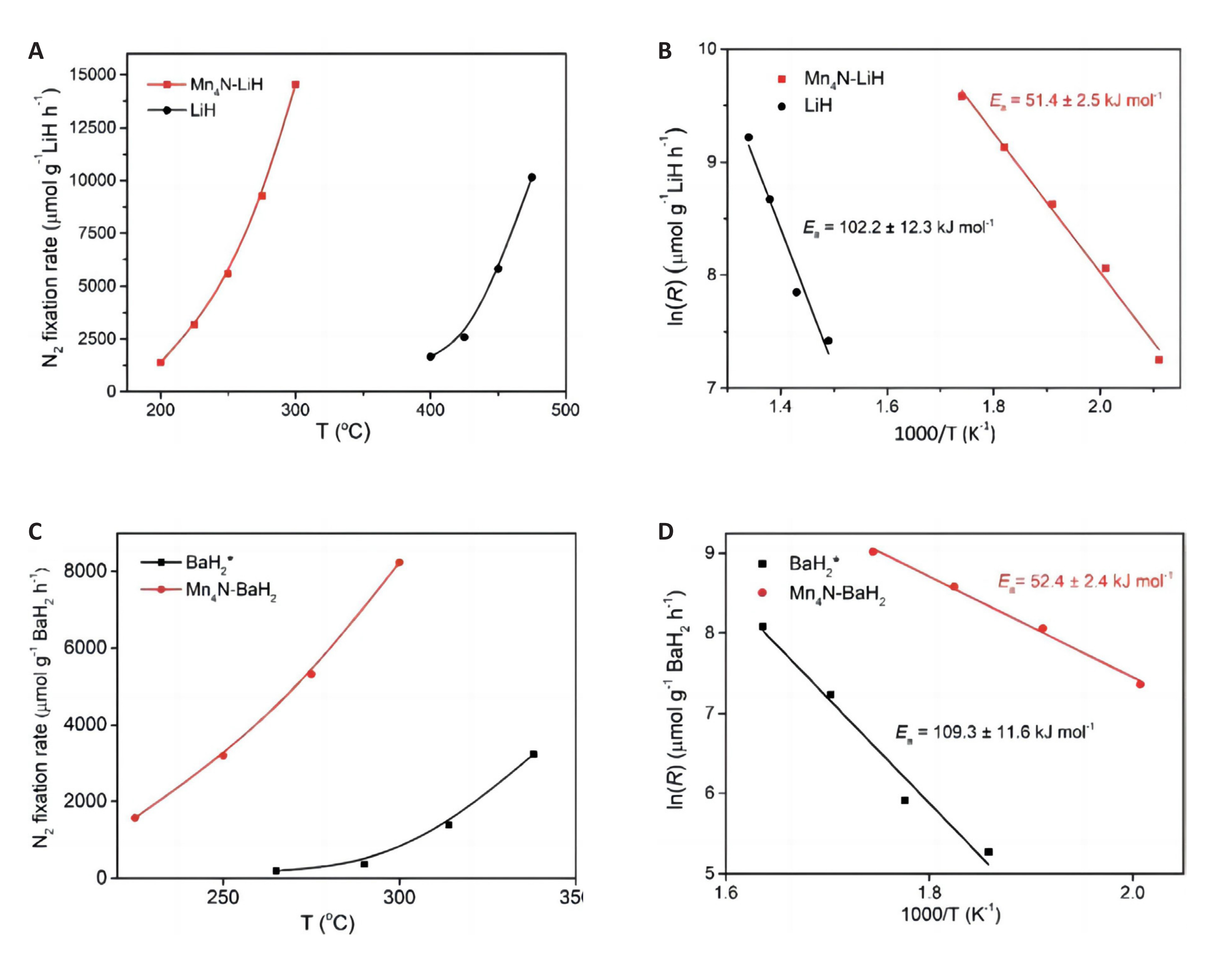
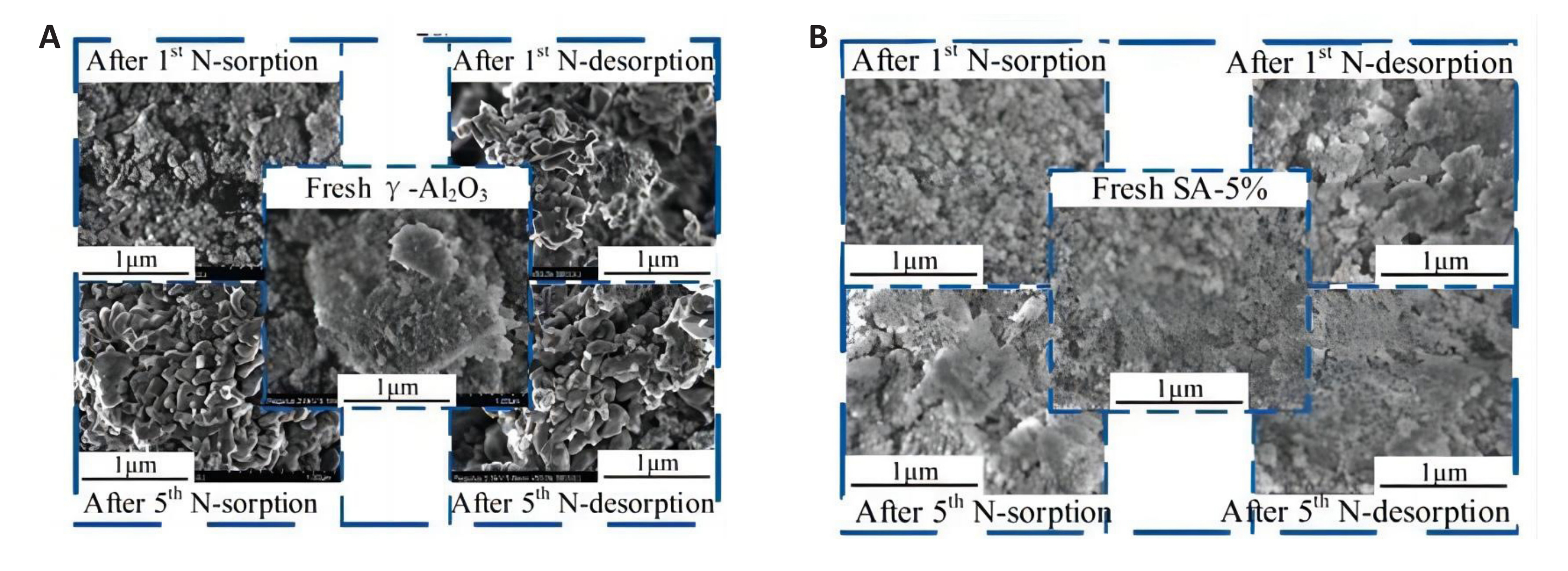
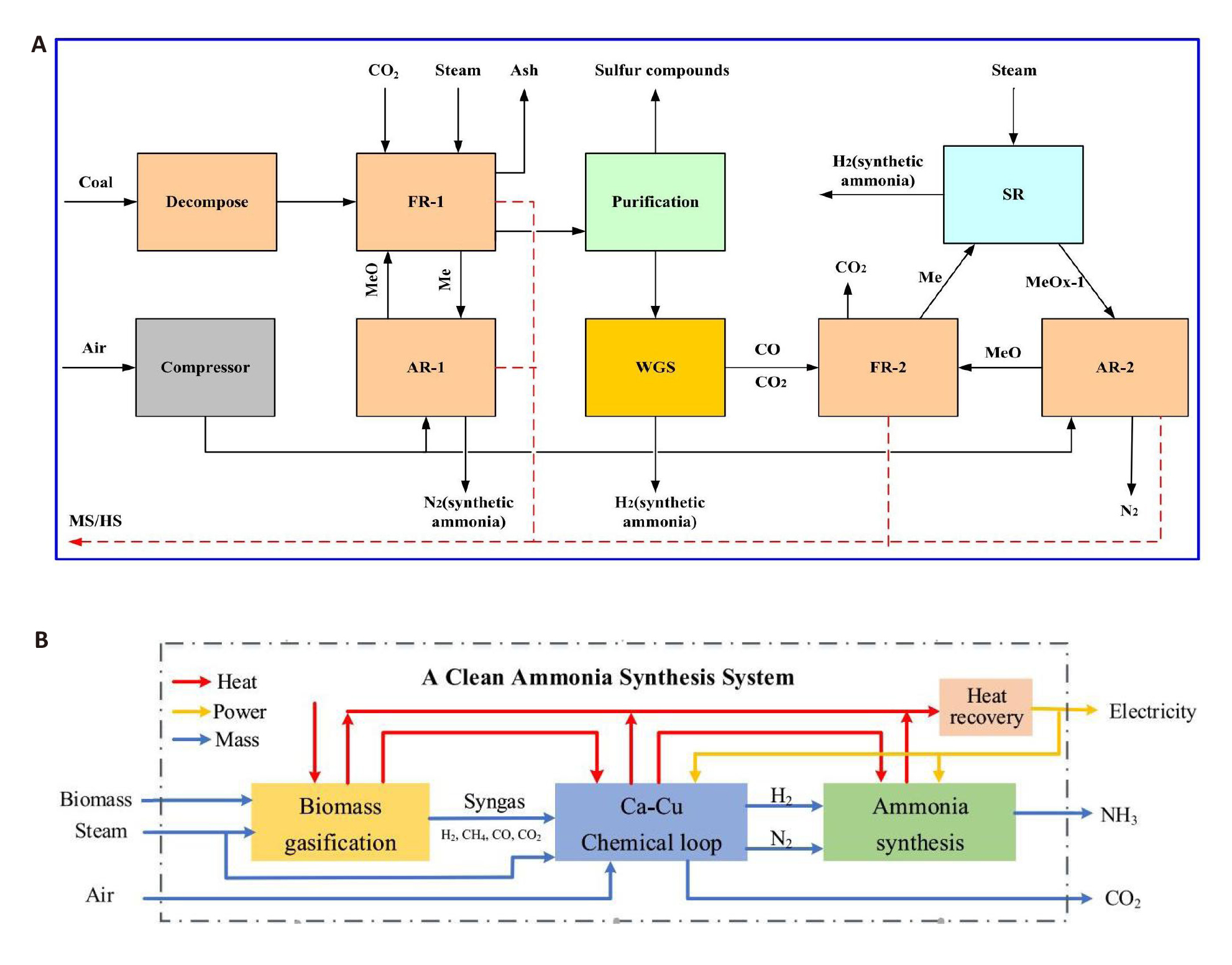
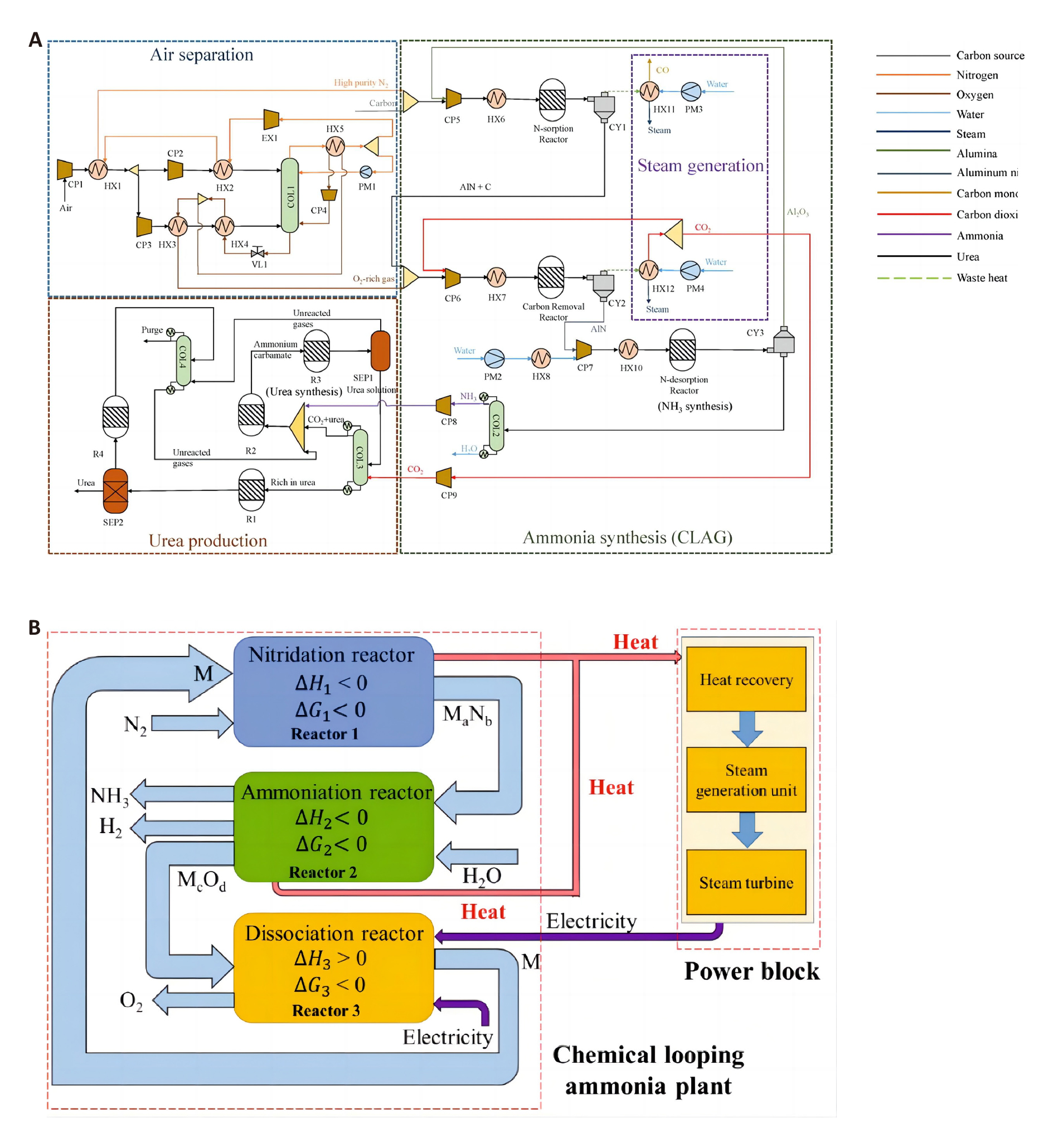
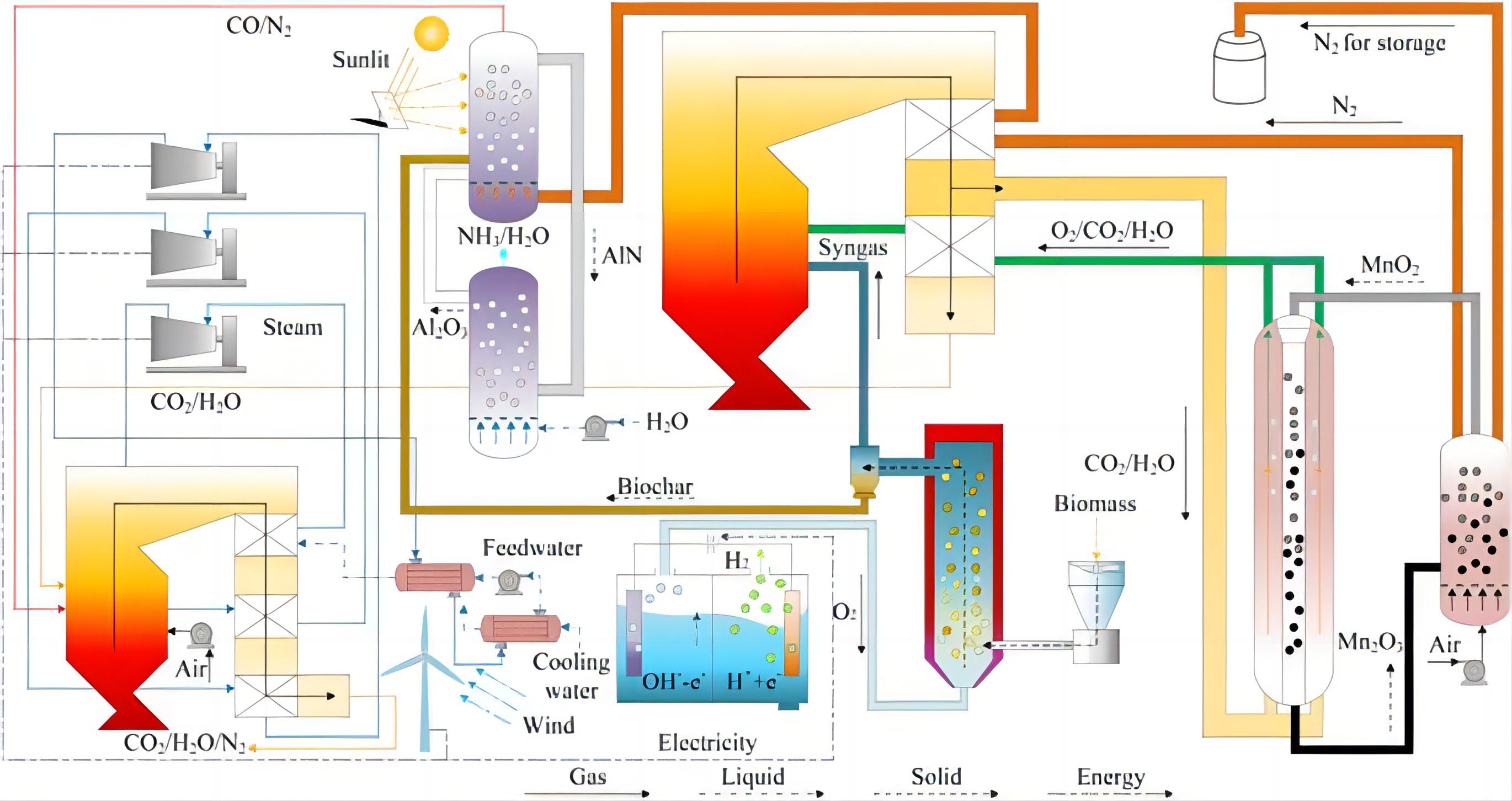




 Copyright ©
Copyright ©